Abstract
Since December 2019, coronavirus diseases-2019 (COVID-19) dispersed into 200 countries and affected more than 70 million people. The clear picture of the SARS-CoV-2 infection is still under investigation. In this review, we evaluated whether C-reactive protein biomarker is able to predict the clinical outcomes or correlated with the severity of COVID-19 disease. The databases MEDLINE, Hinari, Google Scholar, and Google search were used to find potential studies published from COVID-19 epidemic until May 2021. A format prepared in Microsoft Excel spreadsheet was used to extract the appropriate details from each original report. For further review, the extracted data were exported to STATA/MP version 16.0 software. Keywords including “COVID-19,” “SARS-CoV-2,” and “C-reactive protein,” among others were used to search relevant articles. Only studies which reported the average C-reactive protein value and COVID-19 disease stage outcomes were included. Twenty articles were included in the review. All studies found considerably higher level of C-reactive protein in patients with severe COVID-19 as compared to mildly infected patients. This review evidenced that it is still there for a given biomarker to early identify the state of progression in asymptomatic and/or mildly infected individuals into severe disease; the level of C-reactive protein may be used in predicting the likelihood of disease progression. Findings from this review showed level of C-reactive protein is a good biomarker to predict the severity of COVID-19 disease. Although COVID-19 researches are at the early stages, investigation of C-reactive protein levels throughout the disease course may have paramount importance for clinicians in early detection of severe manifestations and subsequently improve the prognosis. However, further large-scale studies are required to confirm these findings.
Keywords: COVID-19, C-reactive protein, severity prediction systematic review
Introduction
From December 2019 to March 2020, COVID-19 has been thought as epidemic in China. 1 It has then spread into more than 200 countries, and declared as a global epidemic. 2 Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), an etiologic agent, showed strong infectivity as compared to other coronaviruses. Despite that fatality rate is relatively lower than SARS-CoV and Middle East respiratory syndrome (MERS)-CoV, the increasing number of COVID-19-related mortality needs especial attention. 3 The infection with SARS-CoV-2 exhibits different clinical characteristics; the majority of patients exhibit a clinical feature, including fever, headache, cough, myalgia, diarrhea, haemoptysis, and dyspnea. However, some individuals develop severe form of the disease, such as acute respiratory distress syndrome (ARDS). 4 There is also a possibility that non-severe COVID-19 patients develop severe disease. Severely infected patients are usually admitted in the intensive care unit (ICU), while others in the isolation ward.5,6 Therefore, a reliable and convenient biomarker that can reasonably estimate the severity of COVID-19 disease is required.
Recent studies showed that C-reactive protein (CRP) is positively correlated with the severity of different infections. CRP is a plasma protein produced by the liver cells, called hepatocytes, and its production can be induced by various inflammatory mediators like IL-6. In addition to being a biomarker of acute inflammation, it has recently shown to be associated with chronic inflammations, such as cardiovascular diseases and Type II diabetes mellitus. 7 Also, the early expansion of plasma CRP level is shown to increase the likelihood of developing plasma leakage. Hence, CRP level could early predict COVID-19-associated severe pneumonia. 8 In this regard, although there are blood markers that appear to be linked with the degree of severity and mortality, the level of CRP was sharply increased in severely SARS-CoV-2 infected patients. 9 The pathological, physiological, and diagnostic methods of COVID-19 are in the fact finding stage. 10 Upon examination, the clinical features can be interpreted more clearly through the use of biological markers like CRP. Therefore, investigation of the CRP level might have paramount importance for early diagnosis and appropriate management of COVID-19-related complications. This article aims to explore CRP in the context of COVID-19 pathogenesis and assess how its level changes with the severity of the disease.
Methods and materials
Search strategy
This systematic review was conducted to assess the correlation between CRP level and COVID-19 disease among infected patients. A comprehensive literature search was done on PubMed/MEDLINE, Hinari, Google scholar, and Google search to identify articles that discussed CRP and its clinical implications for COVID-19 in agreement with Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA) guidelines. 11 Besides the searching of unpublished thesis, searches from some research centers and library were done. All of the searches were restricted to English language. The date of search was 27–30 April 2021 from all databases.
The terms “C Reactive Protein,” “Protein, C-Reactive,” “C-reactive protein,” “S protein,” “severe acute respiratory syndrome 2,” “2019-nCoV spike glycoprotein,” “coronavirus disease 2019 virus spike glycoprotein,” “severe acute respiratory syndrome coronavirus 2 spike glycoprotein,” “SARS-CoV-2 S protein,” “SARS-CoV-2 spike protein,” “COVID-19 virus spike protein,” “2019-nCoV spike protein,” “coronavirus disease 2019 virus spike protein,” “COVID-19 virus S protein,” “S protein, SARS-CoV-2” “Sprotein, COVID-19 virus,” and “COVID-19 virus spike glycoprotein” were used in various combinations as primary search keywords.
Inclusion and exclusion criteria
Studies were included if they have stated a correlation between CRP and the severity of COVID-19, while consensus documents, commentaries, and studies with no particular report on the role of CRP in COVID-19 have been excluded.
Study selection
All studies identified through different databases were combined, managed, and exported using Endnote software version X9.2 (Thomson Reuters, Philadelphia, PA, USA). All duplicated studies were removed and full text of the articles was searched using Endnote software and manually. Screening for eligibility of the individual articles was assessed independently by two reviewers (G.Y.Y. and S.A.) through review from title, abstract, and full text. The discrepancy between reviewers were managed through discussion and consultation with a third reviewer (F.Y.A. and G.W.A.).
Data extraction
Data were ascertained and obtained on a standardized form used to record relevant items and entered into a database (Microsoft excel). The following data were extracted and recorded: study characteristics, authors, year of publication, country, study design, average CRP value, and patient category. The extracted data were checked by another reviewer for accuracy.
Quality assessment
The modified version of a quality assessment tool for prevalence studies that is validated in previous study was used to assess qualities of included studies. Two reviewers (G.Y.Y. and S.A.) independently assess the quality of the included studies. This review addressed key domains: type of biomarker (CRP), level of the biomarker, correlation with severity of the disease, number of patients investigated, and outcomes. The discrepancy between two reviewers was solved through discussion and there are articles that were included after consensus. The quality assessment tool measures a total of nine questions. Based on the value of the quality assessment tool, the highest score from all questions were declared as having low risk of bias. Overall scores 0–3, 4–6, and 7–9 were declared as low, moderate, and high risk of bias, respectively 12 (Table 1).
Table 1.
Quality assessment of individual studies including in the review, 2020.
| Author (reference) | 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Target group representative | Study population representative | Census or random | Low non-response bias | Primary data collection | Acceptable case definition | Appropriate instrument measurement | Same model of data collection were used | Proper calculation of prevalence | Total “yes” | Overall risk of bias | |
| Li et al. 27 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Potempa et al. 14 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Han et al. 20 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 1 |
| Sadeghi-Haddad-Zavareh et al. 16 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Wang et al. 25 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 8 | 1 |
| Pepys 18 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Wang 10 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 1 |
| Kermali et al. 6 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 1 |
| Ali et al. 19 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Pan et al. 22 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Acar et al. 21 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Velavana et al. 17 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Stringer et al. 23 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 1 |
| Zhou et al. 3 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Statsenko et al. 24 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 1 |
| Luo et al. 13 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Cui et al. 26 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 1 |
| Chen et al. 15 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Kazemi et al. 28 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 0 |
| Chen et al. 8 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 1 |
Statistical analysis
It was impossible to do an appropriate meta-analysis because of lack of research data among the studies on this subject.
Registration
The study was registered with PROSPERO, number CRD42020202065.
Result
Study selection and identification
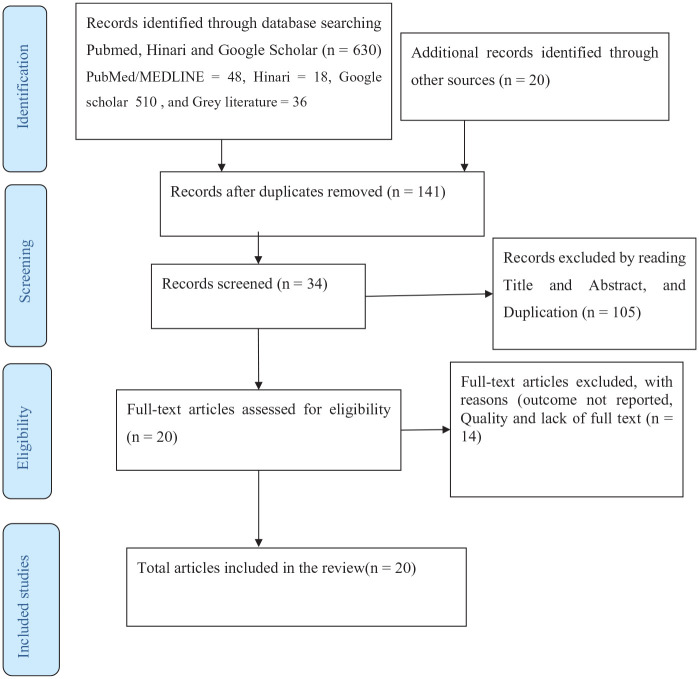
This systematic review includes published articles on CRP among COVID-19 patients starting from the epidemic until May 2021. A total of 630 articles were identified through database search. The removal of duplicates leaves the articles into 141. Further screening was applied through reading the title and abstract, another 105 duplicated articles were excluded. Out of the remaining 34 articles, 14 were again excluded with reasons. Eventually, 20 potential articles were included for qualitative and quantitative synthesis, as summarized in PRISMA flow diagram3,8–10,13,15,17,20,22,25,27 (Figure 1).
Figure 1.
PRISMA flow diagram of article selection for systematic review of CRP level and severity of COVID-19.
Characteristics of included studies
Ten of the 15 included studies have been done in China,10,19,20,29 1 study was from Vietnam, 2 studies from United States,14,26 2 studies from Iran,16,28 1 study from United Arab Emirates (UAE), 24 1 study from Turkey, 21 another from United Kingdom and China.3,8–10,13,15,18,22,23,25,27 Except one case series study, all of the included studies are retrospective cohort. A total of 15,434 study participants were included in the review. The studies contain a minimum and maximum sample size of 25 and 4514 participants, respectively6,17 (Table 2).
Table 2.
Characteristics of studies included for systematic review on the level of CRP and severity of COVID-19 disease, 2020.
| S. No | Author | Country | Study design | Sample size |
|---|---|---|---|---|
| 1 | Li et al. 27 | China | Retrospective | 25 |
| 2 | Han et al. 20 | China | Retrospective | 185 |
| 3 | Wang et al. 25 | China | Case series | 209 |
| 4 | Wang 10 | China | Retrospective | 27 |
| 5 | Kermali et al. 6 | United Kingdom | Retrospective | 4514 |
| 6 | Pan et al. 22 | China | Retrospective | 124 |
| 7 | Velavana et al. 17 | Vietnam | Retrospective | 2652 |
| 8 | Zhou et al. 3 | China | Retrospective | 123 |
| 9 | Luo et al. 13 | China | Retrospective | 298 |
| 10 | Chen et al. 15 | China | Retrospective | 249 |
| 11 | Chen et al. 8 | China | Retrospective | 76 |
| 12 | Sadeghi-Haddad-Zavareh et al. 16 | Iran | Retrospective | 429 |
| 13 | Potempa et al. 14 | United States | Retrospective | 140 |
| 14 | Pepys 18 | United Kingdom | Expert review | Not available |
| 15 | Ali et al. 19 | China | Retrospective | 63 |
| 16 | Acar et al. 21 | Turkey | Retrospective | 148 |
| 17 | Stringer et al. 23 | United Kingdom | Retrospective | 1835 |
| 18 | Statsenko et al. 24 | United Arab Emirates | Retrospective | 560 |
| 19 | Cui et al. 26 | United States | Retrospective | 2707 |
| 20 | Kazemi et al. 28 | Iran | Retrospective | 1070 |
CRP: C-reactive protein.
CRP and COVID-19 disease progression status
The clinical importance of CRP toward COVID-19 was demonstrated by one of the retrospective single-center study in China. The study showed that the majority of patients with severe stage showed significantly higher level of CRP compared to the non-severe cohort (100 vs 9.65 mg/L). 13 Another retrospective cohort revealed the higher level of CRP among severe clinical manifestations on CT scan than those with moderate and mild manifestation 15 In addition, a study done in Vietnam evidenced all COVID-19 patients irrespective of the stage of the disease displayed a higher level of CRP. 17 In a separate study from China, individuals died of COVID-19 exhibit higher CRP level (85.3 mg/L) than improved and discharged (53.5 mg/L). 20
A study in the United States showed CRP level was a straightforward, quick, and cost-effective tool to estimate the extent of tissue damage that is occurring in COVID-19 patients. 14 In addition, a study in Turkey concluded that inflammatory parameters, such as CRP, among others, were associated with disease severity and could be used as potentially important risk factors for COVID-19 progression. 21
Moreover, another study in China retrospectively assessed the different biomarkers that could result in poor prognosis among COVID-19 death individuals. Along with other markers, the level of CRP was showed to increase in 84.6% of individuals of the last test before death as compared to the first-time test. 27 Therefore, except one article that showed the higher level of CRP in all COVID-19 patients, all other studies showed a sharp increase in the CRP level among severe patients than mild and moderately infected individuals (Table 3).
Table 3.
Summary of different studies showing levels of C-reactive protein (CRP) in patients with COVID-19, 2020.
| Author | Country | Patient category | CRP value (mg/L) | comment |
|---|---|---|---|---|
| Li et al. | China | First test | 52.9 | In 85%–100% of dead patients, CRP was found increased before death indicating that there is a high inflammatory reaction in COVID-19 patients. CRP can be a good indicator of disease progression and the decline of lymphocytes counts |
| Last test | 96.2 | |||
| Han et al. | China | Mild | 4.25 | CRP level was increased among poor prognostic patients |
| Severe | 33.4 | |||
| Wang et al. | China | Mild | 12.1 | Patients with severe disease had increased levels of CRP compared with non-severe patients. CRP was highly associated with the aggravation of non-severe COVID-19 patients, which is evidenced from regression analysis |
| Severe | 43.8 | |||
| Wang | Mild | 1.52 | Increased CRP levels were correlated with severe COVID-19 presentation | |
| Severe | 105 | |||
| Kermali et al. | United Kingdom | Mild | 33.2 | Among others, CRP showed higher levels in patients with severe COVID-19 patients compared to their non-severe counterparts |
| Severe | 57.9 | |||
| Pan et al. | China | Discharge | 53.5 | CRP level was found significantly correlated with death than discharged improved patients. |
| Death | 85.8 | |||
| Velavana et al. | Vietnam | All cases | Increased | Clinicians must consider the serum levels of CRP, which may be applicable in risk stratification to predict severe and fatal COVID-19 in admitted patients |
| Severe cases | Increased | |||
| Zhou et al. | China | Mild | 0.46 | CRP levels extremely varies between mild and severe COVID-19 patients |
| Severe | 1.46 | |||
| Luo et al. | China | Mild | 9.65 | Compared to survivors, non-survivors showed a significantly increased CRP level, and thereby identified as independent predictors of poor disease outcome. |
| Severe | 100 | |||
| Chen et al. | China | Mild manifestation on CT scan | 11.47 | Among others, increased CRP levels were all associated with the development of ARDS |
| Moderate manifestation on CT scan | 22.94 | |||
| Severe manifestation on CT scan | 34.8 | |||
| Chen et al. | China | Mild | 12.47 | Higher level of plasma CRP was linearly correlated to the severity of COVID-19 disease. |
| Severe | 23 | |||
| Potempa et al. | United States | Non-severe | 28.7 | CRP blood levels correspond with acute phase response activation, making them a straightforward, quick, and cost-effective tool to estimate the extent of tissue damage that is occurring in that patient |
| Severe | 47.6 | |||
| Sadeghi-Haddad-Zavareh et al. | Iran | Non-severe | Slight increase | In severe cases, CRP levels were substantially higher than in non-severe cases. CRP could be utilized as an independent predictor in predicting the severity of COVID-19, according to an analysis of the ROC curve. |
| Severe | Increased | |||
| Pepys | United Kingdom | – | – | CRP has not yet been identified in COVID-19 lesions, but given the significant cell damage and amount of circulating CRP, it must be there. |
| Ali et al. | Deceased (65%) | >100 | They discovered that CRP levels in deceased individuals were significantly increased, indicating hepatic impairment. | |
| Recovered (6%) | ||||
| Acar et al. | Turkey | Non-survivor | They concluded that inflammatory parameters, such as CRP, among others were associated with disease severity and could be used as potentially important risk factors for COVID-19 progression. | |
| Survivor | ||||
| Stringer et al. | United Kingdom | Dead | 115 | The most accurate predictor of death was found to be IL-6, with CRP coming in second. |
| Survived | 69 | |||
| Yauhen et al. | UAE | Admitted to ICU | High | Because CRP levels indicate the activity of an inflammatory process, they have a strong predictive value. |
| Not admitted to ICU | Low | |||
| Cui et al. | United States | Participants with Corticosteroid, low risk of death | CRP responder | Inpatient mortality may be predicted by a reduction in CRP of 50% or more within 72 h after starting corticosteroid medication. This could be used as an early indicator for COVID-19 patients’ responsiveness to corticosteroid therapy. |
| Participants with Corticosteroid, high risk of death | CRP non-responder | |||
| Kazemi et al. | Iran | Dead | 85.82 | There’s an association between CRP and illness severity. |
| Improved | 32.99 |
CRP: C-reactive protein; CT: computed tomography; ARDS: acute respiratory distress syndrome; ROC: receiver operating characteristic; ICU: intensive care unit; UAE: United Arab Emirates.
Discussion
The number of infected individuals as well as death due to COVID-19 is rapidly growing across the globe. Eventhough, preparation of several vaccines and drugs are underway, none of these are commercially available. It is clearly known that an appropriate treatment of infected individuals has a great implication in controlling the infectious diseases. Hence, there is an urge of early indicator biomarker of severity in the era of COVID-19 that may help to initiate timely and effective treatment strategies. 10
This systematic review revealed that the average level of CRP in mildly and severely ill patients were 33.27 and 81.28 mg/L, respectively. This indicates there is a net average difference of 48.041 mg/L between the groups. The elevated level of CRP was witnessed in about 85% of severe COVID-19 patients. It is not only indicating severe infection, but also shown to be a progression of poor outcome of severe infection, since an elevated CRP level was observed in 85% of patients before death. 27 This could be suggesting that there is gradual inflammatory reaction in patients with COVID-19.
In addition, the progression of non-severe to severe has also been assessed, and all of them progresses to severe displayed elevated level of CRP. The CRP levels of those who progressed to severe infection and those who remained non-severe form was 43.8 and 12.1 mg/L, respectively. The study also showed that CRP was the only marker which is related to the progression of non-severe COVID-19 disease, specifically in every 1 unit increase in CRP level, the probability of developing severe disease is increased by 5%. 25 The notion that CRP is correlated with diseases severity was supported by another study. The study revealed the higher level of CRP among non-survivors when compared to survivors. 13
In another study, upon admission, the severe COVID-19 patients were split up into discharge group and death event group, and found a significant correlation between CRP and death event.22,28 Likewise, a study demonstrated a progressive increase in the CRP level from mild, moderate, and severe pneumonia. 8 It is now well established that pneumonia is the most common clinical feature of symptomatic SARS-CoV-2 infection. Also, the severe form of pneumonia resulting from excessive inflammation contributed to the loss of lives related to COVID-19. CRP is an indicator of systemic inflammation. Therefore, the level of CRP may clearly show not only the progression of mildly infected individuals but also dictate the recovery or adverse outcome of severe patients. A study in the United Kingdom strongly evidenced that the most accurate predictor of death was found to be IL-6, with CRP coming in second. 23
In conclusion, infection with SARS-CoV-2 is characterized by exuberant inflammatory reaction, particularly of severe form of the disease. It is still there for a given biomarker to early identify the state of progression in asymptomatic and/or mildly infected individuals into severe disease. With this in mind, the level of CRP may be appreciated in predicting the likelihood of disease progression. The initiation of early treatment option could be possible if regular monitoring of its level for both symptomatic and asymptomatic individuals. Furthermore, sharp increment of CRP level from severity to death is also helpful to warn clinicians for transfer of severe patients to intensive care unit.
Limitation of the review
The review lacks meta-analysis which may provide a more powerful evidence than it was only systematic review. On the contrary, most of the studies included in this article are observational.
Conclusion
This systematic review showed that routine investigation of CRP level had paramount contribution for predicting severity of COVID-19 disease and responding timely and appropriately. Moreover, large-scale multicenter studies is required for its precision and accuracy.
Acknowledgments
The authors are thankful for those who supported them in writing this review. Their gratitude goes more specifically to Mr Sofonyas Abebaw Tiruneh who helped them in this work from its beginning.
Footnotes
Author contributions: From the development of the idea through the final approval, all of the authors made important contributions to the work reported. The journal to which the essay had been submitted was agreed upon by all authors.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Getachew Yideg Yitbarek  https://orcid.org/0000-0001-9823-9981
https://orcid.org/0000-0001-9823-9981
Gashaw Walle Ayehu  https://orcid.org/0000-0001-7333-152X
https://orcid.org/0000-0001-7333-152X
References
- 1. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018; 9: 754–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. Coronavirus disease 2019 (COVID-19), 2020, https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 3. Zhou C, Huang Z, Tan W, et al. Predictive factors of severe coronavirus disease 2019 in previously healthy young adults: a single-center, retrospective study. BMC Respir Res 2020; 21: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greeland JR, Michelow MD, Wang L, et al. COVID-19 infection: implications for perioperative and critical care physicians. Anesthesiology 2020; 132: 1346–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Care I, Shang Y, Pan C, et al. Management of critically ill patients with COVID‑19 in ICU: statement from front-line intensive care experts in Wuhan, China. Ann Intensive Care 2020; 10: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kermali M, Khalsa RK, Pillai K, et al. The role of biomarkers in diagnosis of COVID-19—a systematic review. Life Sci 2020; 254: 117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luan YY, Yao YM. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol 2018; 9: 1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen W, Zheng KI, Liu S, et al. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob 2020; 19: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol 2020; 92(11): 2409–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect 2020; 50(4): 332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65(9): 934–939. [DOI] [PubMed] [Google Scholar]
- 13. Luo X, Campus E, Zhou W, et al. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin Infect Dis 2020; 71: 2174–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Potempa LA, Rajab IM, Hart PC, et al. Insights into the use of C-reactive protein as a diagnostic index of disease severity in COVID-19 infections. Am J Trop Med Hyg 2020; 103(2): 561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020; 80(5): e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadeghi-Haddad-Zavareh M, Bayani M, Shokri M, et al. C-reactive protein as a prognostic indicator in covid-19 patients. Interdiscip Perspect Infect Dis 2021; 2021: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis 2020; 95: 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pepys MB. C-reactive protein predicts outcome in COVID-19: is it also a therapeutic target? Eur Heart J 2021; 42(23): 2280–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ali A, Noman M, Guo Y, et al. Myoglobin and C-reactive protein are efficient and reliable early predictors of COVID-19 associated mortality. Sci Rep 2021; 11(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han J, Shi L, Xie Y, et al. Analysis of factors affecting the prognosis of COVID-19 patients and viral shedding duration. Epidemiol Infect 2020; 148: e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Acar E, Demir A, Yıldırım B, et al. The role of hemogram parameters and C-reactive protein in predicting mortality in COVID-19 infection. Int J Clin Pract 2021; 75(7): e14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan F, Yang L, Li Y, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci 2020; 17(9): 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stringer D, Braude P, Myint PK, et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol 2021; 50(2): 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Statsenko Y, Al Zahmi F, Habuza T, et al. Prediction of COVID-19 severity using laboratory findings on admission: informative values, thresholds, ML model performance. BMJ Open 2021; 11(2): e044500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang G, Wu C, Zhang Q, et al. C-reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect Dis 2020; 7(5): ofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cui Z, Merritt Z, Assa A, et al. Early and significant reduction in C-reactive protein levels after corticosteroid therapy is associated with reduced mortality in patients with COVID-19. J Hosp Med 2021; 16(3): 142–148. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical. Int J Infect Dis 2020; 94: 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kazemi E, Soldoozi Nejat R, Ashkan F, et al. The laboratory findings and different COVID-19 severities: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 2021; 20(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson A. You have come a long way baby: five decades of research on the biology of aging from the perspective of a researcher studying aging. Biomed Gerontol 2021; 76: 57–63. [DOI] [PubMed] [Google Scholar]