Abstract
Introduction/objectives:
Diabetic Kidney Disease (DKD) is the leading cause of end-stage kidney disease. Despite optimal glycemic control and blood pressure management, progression to DKD cannot be halted in some patients. We aimed to find the association of modifiable and non-modifiable risk factors and comorbid conditions in patients with DKD.
Methods:
Retrospective medical record review of adult patients with diabetes mellitus (DM) was performed who visited our internal medicine office between January 1, 2020 and December 31, 2020.
Results:
Among 728 patients with DM, 471 (64.7%) patients had DKD, and 257 (35.3%) patients were without DKD. Among the group of patients with DKD, the majority were in CKD stage G1A2 (34.6%), followed equally by G2A2 and G3aA1 (16.8% each). Mean age of the patients with DKD was significantly greater than the patients without DKD (69.4 years vs 62.2 years; P < .001). For each unit increase in age, there was a 7.8% increase in the odds of DKD (95% CI 5.3-10.4; P < .001). Women had 2.32 times greater odds of DKD (95% CI, 1.41-3.81; P = .001). We found decreased odds of DKD for those who consumed alcohol moderately (OR 0.612, 95% CI 0.377-0.994; P < .05). Significantly higher frequencies of associations of several comorbid medical conditions were seen in patients with DKD compared to the patients without DKD, such as hypertension (91.9% vs 75.6%), hyperlipidemia (86.6% vs 78.2%), coronary artery disease (39.3% vs 16.8%), cerebrovascular accidents (13.4% vs 7.4%), congestive heart failure (12.9% vs 4.1%), carotid artery stenosis (11.3% vs 2.6%), aortic aneurysm (5.4% vs 2.0%), peripheral artery disease (10.8% vs 3.5%), gout (12.4% vs 5.5%), and osteoarthritis (41.4% vs 31.2%).
Conclusions:
In patients with diabetes, increasing age, female sex, and lack of moderate alcohol consumption were associated with increased odds of DKD. Higher frequencies of association of hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accidents, congestive heart failure, carotid artery stenosis, aortic aneurysm, peripheral artery disease, gout, and osteoarthritis were also seen in patients with DKD.
Keywords: diabetic kidney disease, diabetes mellitus, chronic kidney disease, renal involvement in diabetes
Introduction
Diabetic Kidney Disease (DKD) is the leading cause of end-stage kidney disease (ESKD). It begins as microalbuminuria, which is characterized by moderately increased urine albumin-to-creatinine ratio (UACR) of 30 to 300 mg/g; then progresses to macroalbuminuria, or severely increased UACR of greater than 300 mg/g; and to renal insufficiency in the form of elevated serum creatinine level and lowering of estimated glomerular filtration rate (eGFR) leading in to chronic kidney disease (CKD) stages 3a, 3b, 4, and finally 5, or end stage kidney disease (ESKD). About 25% to 40% of patients with diabetes mellitus (DM) develop DKD.1,2 In the developed world, DKD accounts for about 50% of cases of ESKD. 3 The rates of DKD continue to rise in the high-risk groups, such as middle-aged African Americans, Native Americans, and Hispanics. 3 Despite optimal glycemic control and optimal blood pressure management with guideline directed medical therapy, the progression to CKD and ESKD cannot be halted in some patients. 4
DKD has a complex and multifactorial pathophysiology which includes metabolic factors, such as intracellular signaling pathways, oxidative stress, hypoxia, dysregulated autophagy, epigenetic changes, and hemodynamic factors, which result in kidney inflammation and fibrosis.4,5
Several non-modifiable risk factors contribute to DKD, such as genetics, gender, age, age at onset, and duration of diabetes. 6 Strong and independent association of baseline body-mass-index (BMI) and young age have been reported as factors associated with faster progression to DKD. 7 Similarly, baseline glycosylated hemoglobin (HbA1c), systolic blood pressure, proteinuria and serum uric acid in conjunction with vascular comorbidities are also found to be strongly and independently associated with faster DKD progression. 2 These observations have opened avenues to investigate and modify additional risk factors that may influence the progression to DKD. Factors that have been reported, include family history of DKD, personal history of cigarette smoking history, suboptimal blood pressure control, and elevated plasma lipid levels. 2 These group of risk factors have been identified as the ones that offer the greatest risk of DKD development and progression. 2 Similarly, several other modifiable risk factors have been suggested that have a strong effect on the risk of DKD, such as chronic low-grade inflammation, advanced glycation end products, and lack of physical activity. 6
A comprehensive approach for prevention and management of DKD depends on modification and mitigation of the risk factors, such as combined targeted therapies for hyperglycemia, hypertension, albuminuria, hyperlipidemia, and judicious use of renoprotective agents.8-11 Much emphasis is given to microalbuminuria and UACR, but the UACR categorization lacks the necessary specificity and sensitivity, and estimates of declining GFR are compromised by methodological limitations for eGFRs in the normal-to-high range. 2
There is a general lack of consensus among the available studies in terms of a comprehensive list of both non-modifiable and modifiable risk factors that are associated with DKD. Identification and efficient management of the modifiable risk factors may improve the prognosis of diabetic patients at risk of DKD.
We aimed to find the association of modifiable and non-modifiable risk factors and comorbid conditions in patients with DM with and without DKD. We hypothesized that certain risk factors, such as age, race, gender, tobacco use, recreational use of drugs, elevated BMI, use of non-steroidal anti-inflammatory drugs (NSAIDs) and other nephrotoxic drugs, lack of use of renoprotective agents; and certain comorbid medical conditions, such as hypertension, atherosclerotic cardiovascular disease, hyperlipidemia, obesity, gout, autoimmune disorders, mental health disorders, and suboptimal glycemic control, would be associated with DKD.
Materials and Methods
Study Selection
This study was a retrospective chart review study of the existing electronic medical records of our patients who visited our internal medicine office for the management of diabetes mellitus and other comorbid medical diagnoses between January 1, 2020 and December 31, 2020. The inclusion criteria were adult patients of age 18 years or older, with diabetes mellitus type I or type II. The study was reviewed and approved by the Institutional Review Board of the Cooper University Health Care (CUHC), Camden, New Jersey, USA. This study was fully compliant with the ethical standards set forth by the CUHC Institutional Review Board.
Data Collection
We collected the following data for each patient from the existing electronic medical records: duration of diabetes, age, gender, race, cigarette smoking, moderate alcohol intake (defined as up to 1 drink a day for women and up to 2 drinks a day for men), heavy alcohol intake (defined as more than 14 drinks per week for men or >4 drinks per occasion; more than 7 drinks per week for women or >3 drinks per occasion; and more than 7 drinks per week for all adults 65 years and above), recreational drug use, history of gestational diabetes mellitus, family history of diabetes mellitus; comorbid medical conditions, such as hypertension, hyperlipidemia, hypothyroidism, coronary artery disease, cerebral vascular accident, carotid artery stenosis, peripheral arterial disease, aortic aneurysm, congestive heart failure, obesity, overweight, chronic obstructive pulmonary disease, chronic kidney disease, obstructive sleep apnea, depression, osteoarthritis, gout, autoimmune disease, and maintenance hemodialysis status. We also collected the data on blood pressure, BMI, lipid profile, HbA1c, UACR; and use of medications, such as statin, aspirin, angiotensin converting enzyme inhibitor (ACE-I), angiotensin II receptor blocker (ARB), metformin, insulin, sulfonylurea, dipeptidyl peptidase-4 (DPP4) inhibitors, sodium-glucose co-transporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP1) agonist, and thiazolidinedione.
Statistical Analysis
Collected data was entered into a Microsoft Excel (2016, Redmond, Washington, USA) spreadsheet. Statistical analysis was done by using SPSS (Statistical Package for the Social Sciences, version 15.01, IBM, Armonk, New York, USA). There were no scientific studies that had conducted similar comprehensive association as per the aims of our study, hence we did not have a baseline sample size for comparison. We calculated an approximate sample size of under 1000 subjects based on the observation that in our internal medicine office, approximately 20 000 or greater number of patients receive care annually. Among them, approximately 10% have DM, and of those, approximately 50% patients follow up in the office on a regular basis.
The patients were divided into 2 groups: first group comprised of patients with DKD, defined as patients with DM and evidence of microalbuminuria (UACR 30-299 mg/g) or macroalbuminuria (UACR ≥300 mg/g) and any eGFR (CKD stages G1A2, G1A3, G2A2, G2A3, G3aA1, G3aA2, G3aA3, G3bA1, G3bA2, G3bA3, G4A1, G4A2, G4A3, G5A1, G5A2, and G5A3). The second group was represented by patients without DKD, defined as patients with DM and normal UACR <30 mg/g as well as normal eGFR. We compared the data between the 2 groups and studied their association with DKD in order to find any significant difference. Group characteristics (demographic, clinical) of the study population were compared using independent t-test, or Wilcoxon rank sum test for continuous data, and Fisher’s exact test, or Chi-square test for categorical data. Logistic regression analysis was carried out to evaluate potential risk factors that were predictive of DKD. Significant predictors from the univariate analysis were the independent variables in the multiple variable logistic regression. This model also provided the odds ratios and 95% confidence interval (CI) for the full model and pairwise comparisons. Receiver operating characteristic (ROC) curves were calculated, where appropriate, with area under the ROC curve and cut points for continuous predictors. In this study, significance was defined as a P < .05.
Results
A total of 728 patients were included in this study. Four hundred and seventy one (64.7%) patients had DKD, and 257 (35.3%) patients were without DKD. Among the group of patients with DKD, the majority were in CKD stage G1A2 (34.6%), followed by G2A2 and G3aA1, equally (16.8% each) (Table 1) (Figure 1). Although we could not deduce the exact onset of DM in the majority of our patients in both the groups due to transition into electronic medical records and lack of documentation, nevertheless follow-up documentation revealed that all the patients had DM for at least 9 years, or greater, in both the groups.
Table 1.
Distribution of Patients with Diabetic Kidney Disease.
| CKD stage (n = 471) | A1 | A2 | A3 | |
|---|---|---|---|---|
| G1, n (%) | 187 (39.7) | 0 (0.0) | 163 (34.6) | 24 (5.1) |
| G2, n (%) | 98 (20.8) | 0 (0.0) | 79 (16.8) | 19 (4.0) |
| G3a, n (%) | 117 (24.9) | 79 (16.8) | 34 (7.2) | 4 (0.9) |
| G3b, n (%) | 47 (10.0) | 8 (1.7) | 32 (6.8) | 7 (1.5) |
| G4, n (%) | 19 (4.0) | 1 (0.2) | 8 (1.7) | 10 (2.1) |
| G5, n (%) | 3 (0.6) | 0 (0.0) | 0 (0.0) | 3 (0.6) |
Abbreviations: A1, UACR < 30 mg/g; A2, UACR 30 to 299 mg/g; A3, UACR ≥ 300 mg/g; CKD, chronic kidney disease; G1, eGFR > 90 mL/min/1.73m2; G2, eGFR 60 to 90 mL/min/1.73m2; G3a, eGFR 45 to 59 mL/min/1.73m2; G3b, eGFR 30 to 44 mL/min/1.73m2; G4, eGFR 15 to 29 mL/min/1.73m2; G5, eGFR <15 mL/min/1.73m2.
Figure 1.
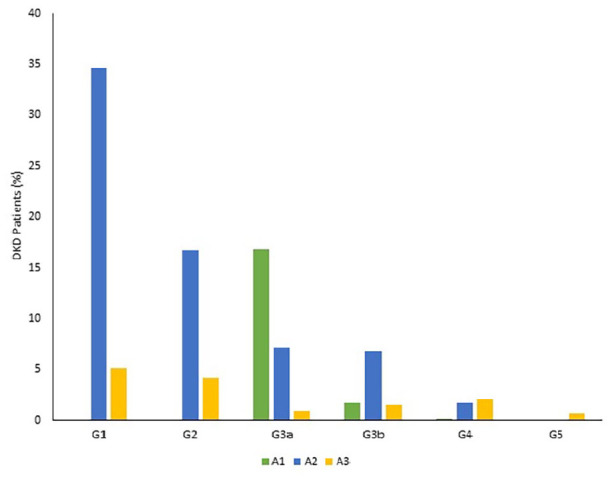
Renal involvement in patients with diabetic kidney disease.
The mean age of the patients with DKD was significantly higher than the patients without DKD (69.4 vs 62.2 years; P < .001) (Table 2). There were more females in the DKD group, and more males in the group without CKD, however the difference was not statistically significant. The majority of the patients in the DKD group were Whites (69.4%), followed by Blacks (15.1%), Hispanics (4.8%), Asians (3.8%), and other races (7.0%). Although the majority of the patients in the group without DKD were Whites (53.9%) as well, followed by Blacks (17.3%), Hispanics (10.1%), Asians (11.8%), and other races (6.8%), there were significantly higher proportion of Whites, and lower proportions of Blacks, Hispanics and Asians in the DKD group compared to the group without DKD (P < .001) (Table 2).
Table 2.
Baseline Characteristics.
| Variable | Variable | Patients with DKD (n = 471) | Patients without DKD (n = 257) | P |
|---|---|---|---|---|
| Age | Years, mean (SD) | 69.4 (11.8) | 62.2 (14.4) | <.001 |
| Sex | Male, n (%) | 225 (47.8) | 140 (54.5) | .111 |
| Female, n (%) | 246 (52.2) | 117 (45.5) | ||
| Race | White, n (%) | 327 (69.4) | 140 (53.9) | <.001 |
| Black, n (%) | 71 (15.1) | 44 (17.3) | ||
| Hispanic, n (%) | 22 (4.8) | 26 (10.1) | ||
| Asian, n (%) | 18 (3.8) | 30 (11.8) | ||
| Other, n (%) | 33 (7.0) | 17 (6.8) | ||
| Social | Tobacco use, n (%) | 152 (32.3) | 70 (27.3) | .197 |
| Moderate alcohol use, n (%) | 129 (27.4) | 111 (43.2) | <.001 | |
| Heavy alcohol use, n (%) | 2 (0.4) | 1 (0.4) | .000 | |
| Recreational drug use, n (%) | 10 (2.2) | 5 (2.0) | .000 | |
| Vitals | Systolic BP (mmHg), mean (SD) | 131 (17.2) | 129 (15.1) | .164 |
| Diastolic BP (mmHg), mean (SD) | 74 (9.9) | 77 (8.7) | <.001 | |
| Weight | BMI (kg/m2), mean (SD) | 29.9 (8.1) | 31.3 (9.4) | .063 |
| Lab Values | HbA1c (%), mean (SD) | 7.1 (1.2) | 7.2 (1.4) | .388 |
| Total cholesterol (mg/dL), mean (SD) | 149.8 (38.8) | 160.7 (48.0) | .005 | |
| LDL (mg/dL), mean (SD) | 75.9 (31.9) | 86.3 (32.2) | <.001 | |
| HDL (mg/dL), mean (SD) | 48.6 (15.7) | 48.1 (12.9) | .737 | |
| TG (mg/dL), mean (SD) | 132.3 (64.9) | 137.3 (92.8) | .502 | |
| eGFR (ml/min/1.73m2), mean (SD) | 57.6 (11.6) | 97.2 (14.1) | <.001 | |
| UACR <30 mg/g, n (%) | 88 (18.7) | 257 (100) | <.001 | |
| UACR = 30-299 mg/g, n (%) | 316 (67.2) | 0 (0) | ||
| UACR ≥300 mg/g, n (%) | 67 (14.1) | 0 (0) | ||
| Comorbidities | Hypertension, n (%) | 433 (91.9) | 194 (75.6) | <.001 |
| Hyperlipidemia, n (%) | 408 (86.6) | 201 (78.2) | .003 | |
| CAD, n (%) | 185 (39.2) | 43 (16.8) | <.001 | |
| CVA, n (%) | 63 (13.4) | 19 (7.4) | .012 | |
| CHF, n (%) | 61 (12.9) | 11 (4.1) | <.001 | |
| Carotid stenosis, n (%) | 53 (11.3) | 7 (2.6) | <.001 | |
| Aortic aneurysm, n (%) | 25 (5.4) | 5 (2.0) | .019 | |
| PAD, n (%) | 51 (10.8) | 9 (3.5) | <.001 | |
| Hypothyroidism, n (%) | 89 (18.8) | 44 (17.3) | .650 | |
| Obesity, n (%) | 203 (43.0) | 137 (53.5) | .013 | |
| Overweight, n (%) | 241 (51.1) | 127 (49.3) | .670 | |
| OSA, n (%) | 76 (16.1) | 56 (21.6) | .110 | |
| Gout, n (%) | 58 (12.4) | 14 (5.5) | .002 | |
| Depression, n (%) | 81 (17.2) | 59 (22.9) | .104 | |
| COPD, n (%) | 43 (9.1) | 19 (7.7) | .549 | |
| Osteoarthritis, n (%) | 195 (41.4) | 80 (31.2) | .011 | |
| Autoimmune disease, n (%) | 35 (7.5) | 17 (6.8) | .747 | |
| Family history of DM, n (%) | 190 (40.3) | 126 (48.9) | .043 | |
| Medications | Diet and exercise only, n (%) | 128 (21.7) | 49 (19.1) | .041 |
| Insulin only, n (%) | 55 (11.6) | 5 (1.8) | <.001 | |
| OHA only, n (%) | 261 (55.4) | 174 (67.8) | <.001 | |
| OHA + Insulin, n (%) | 53 (11.3) | 29 (11.3) | .557 | |
| Aspirin, n (%) | 263 (55.9) | 105 (40.8) | <.001 | |
| Statin, n (%) | 396 (84.4) | 188 (73.2) | .002 | |
| ACE-I or ARB, n (%) | 294 (62.4) | 151 (58.9) | .400 | |
| Metformin, n (%) | 230 (48.9) | 182 (71.0) | <.001 | |
| Insulin, n (%) | 91 (19.4) | 35 (13.8) | .071 | |
| Sulfonylurea, n (%) | 124 (26.3) | 67 (26.2) | .969 | |
| DPP4-I, n (%) | 71 (15.1) | 24 (9.2) | .027 | |
| SGLT2-I, n (%) | 28 (5.9) | 25 (9.6) | .123 | |
| GLP1-RA, n (%) | 31 (6.5) | 26 (10.3) | .117 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; DPP4-I, dipeptidyl peptidase-4 inhibitor; eGFR, estimated glomerular filtration rate; GLP1-RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycosylated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OHA, oral hypoglycemic agent; OSA, obstructive sleep apnea; PAD, peripheral arterial disease; SD, standard deviation; SGLT2-I, sodium-glucose co-transporter-2 inhibitor; TG, triglycerides; UACR, urine albumin-to-creatinine ratio.
Analysis of social factors revealed that there was significantly less use of moderate alcohol consumption in the DKD group compared to the group without DKD (27.4% vs 43.2%; P < .001). About a third of the patients in the DKD group and a quarter of the patients in the group without DKD used tobacco products (32.3% vs 27.3%), and only a very small proportion of patients used alcohol heavily, or used recreational drugs in both the groups. The differences in the tobacco use, heavy alcohol use and recreational drug use were not statistically significant between the 2 groups (Table 2).
We found that although the mean systolic blood pressure of the patients in the DKD group was higher than the group without DKD (131 vs 129 mmHg), the difference was not statistically significant. On the other hand, the mean diastolic blood pressure of the patients in the DKD group was significantly lower than the group without DKD (74 vs 77 mmHg; P < .001). The mean BMI of the patients in the DKD group was also lower than the patients without DKD (29.9 vs 31.3 kg/m2), however the difference was not statistically significant (Table 2).
Analysis of the relevant laboratory studies showed that the mean HbA1c of the patients with DKD was slightly lower than the group without DKD (7.1% vs 7.2%), but the difference was not statistically significant. The mean total cholesterol level and the mean LDL cholesterol level were significantly lower in the patients with DKD compared to the group without DKD (149.8 vs 160.7 mg/dL, and 75.9 vs 86.3 mg/dL, respectively) (Table 2). There were no significant differences in the mean HDL cholesterol and mean triglyceride levels between the 2 groups (Table 2). Patients in the DKD group had significantly lower mean eGFR compared to the group without DKD (57.6 vs 97.2 mL/min/1; P < .001). The majority of the patients with DKD had their UACR in the microalbuminuria range (67.2%) (Table 2).
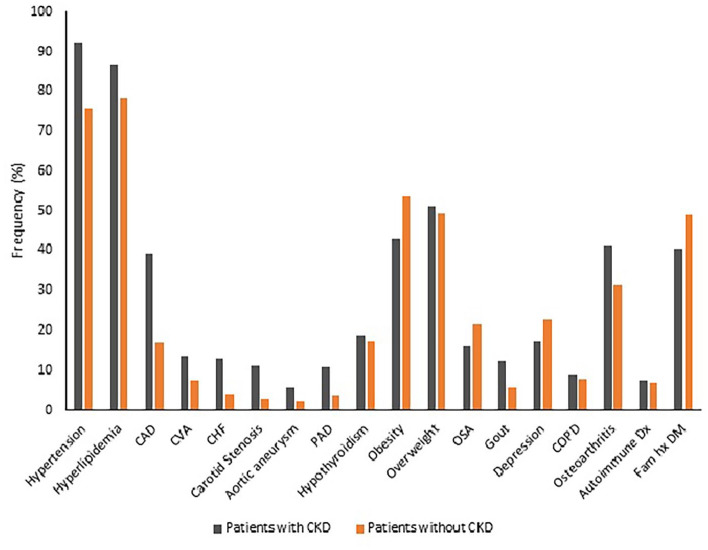
We found significantly higher frequencies of association of several comorbid medical conditions in patients with DKD compared to the group of patients without DKD, such as hypertension (91.9% vs 75.6%), hyperlipidemia (86.6% vs 78.2%), coronary artery disease (39.2% vs 16.8%), cerebrovascular accidents (13.4% vs 7.4%), congestive heart failure (12.9% vs 4.1%), carotid artery stenosis (11.3% vs 2.6%), aortic aneurysm (5.4% vs 2.0%), peripheral artery disease (10.8% vs 3.5%), gout (12.4% vs 5.5%), and osteoarthritis (41.4% vs 31.2%). We also found significantly lower frequency of association of obesity in patients with DKD compared to the group of patients without DKD (43.0% vs 53.5%). The frequency of association of family history of diabetes mellitus was also significantly lower in patients with DKD compared to the group of patients without DKD (40.3% vs 48.9%). There were no significant differences in the frequencies of association of some comorbid conditions between the 2 groups, such as hypothyroidism, overweight, obstructive sleep apnea, depression, chronic obstructive pulmonary disease, and autoimmune disease (Table 2; Figure 2).
Figure 2.
Frequencies of associated medical diagnoses.
Analysis of medication management for DM and additional medication use showed that more than half of the patients in both the groups were managed by one or more oral hypoglycemic agents (OHA) (55.4% in the DKD group vs 67.7% in patients without DKD), however there were significantly lesser number of patients in the group with DKD compared to the group without DKD who were managed only with one or more than one OHA (P < .001). Also, there were significantly more patients with DKD who were managed only with diet and exercise compared to the group of patients without DKD (24.7% vs 18.1%, P < .001) (Table 2). Patients who were treated with insulin only represented only a small fraction in both the groups, nevertheless significantly more patients with DKD were treated with insulin only compared to the group without DKD (8.6% vs 2.8%, P < .001). About one-tenth of the patients in both the groups received combined insulin and OHA treatment, and the difference was not statistically significant (Table 2).
Among the various classes of medications, the majority of the patients were treated with metformin in both the groups, however there were significantly lower proportion of patients in the DKD group who received metformin compared to the group without DKD (48.9% vs 71.0%, P < .001). Sulfonylureas were the next commonly used medication in both the groups (26.3% in DKD group vs 26.2% in the group without DKD) followed by insulin’s (19.4% in DKD group vs 13.8% in the group without DKD), however there were no statistically significant differences between the 2 groups. A significantly greater proportion of patents with DKD were treated with DPP4-I compared to the group without DKD (15.1% vs 9.2%, P < .05). A lower proportion of patients with DKD received SGLT2-I compared to the group without DKD (5.9% vs 9.6%), however the difference was not statistically significant. Similarly, a lower proportion of patients with DKD received GLP1-RA compared to the group without DKD (6.5% vs 10.3%), and the difference was not statistically significant, as well (Table 2). Among other medications, significantly greater proportions of patients with DKD received statins and aspirin compared to the group without DKD (84.4% vs 73.2%, P < .05; and 55.9% vs 40.8%, P < .001; respectively). Use of ACE-I and ARB was comparable between the 2 groups (Table 2).
Logistic regression analysis showed significant findings only for age, sex, and alcohol use (Table 3). We found that for each unit increase in age, there was a 7.8% increase in the odds of DKD (95% confidence interval (CI) 5.3-10.4; P < .001). Women had 2.32 times of greater odds of DKD (95% CI, 1.41-3.81; P = .001). Also, there were decreased odds of DKD for those who consumed alcohol moderately (OR 0.612, 95% CI 0.377-0.994; P < .05) (Table 2).
Table 3.
Influence of Risk Factors on Diabetic Kidney Disease.
| Risk factor | B | P | Exp(B) | 95% C.I. for Exp(B) | Upper |
|---|---|---|---|---|---|
| Lower | |||||
| Age (years) | 0.075 | .000 | 1.078 | 1.053 | 1.104 |
| Sex female | 0.840 | .001 | 2.316 | 1.407 | 3.814 |
| White vs Black | −0.275 | .397 | 0.759 | 0.401 | 1.436 |
| White vs Hispanic | −0.437 | .378 | 0.646 | 0.245 | 1.704 |
| White vs Asian | −0.505 | .300 | 0.603 | 0.202 | 1.568 |
| White vs Other races | −0.143 | .756 | 0.867 | 0.351 | 2.141 |
| BMI (Kg/M2) | 0.014 | .321 | 1.014 | 0.986 | 1.043 |
| Tobacco use | −0.015 | .952 | 0.985 | 0.597 | 1.624 |
| Moderate alcohol use | −0.491 | .047 | 0.612 | 0.377 | 0.994 |
| Hypertension | 0.327 | .363 | 1.387 | 0.685 | 2.810 |
| Hyperlipidemia | −0.114 | .741 | 0.892 | 0.455 | 1.752 |
| CAD | 0.361 | .188 | 1.435 | 0.838 | 2.458 |
| CVA | −0.489 | .181 | 0.613 | 0.299 | 1.255 |
| CHF | 0.348 | .421 | 1.416 | 0.607 | 3.306 |
| PAD | 0.476 | .318 | 1.609 | 0.632 | 4.094 |
| Carotid artery stenosis | 0.820 | .065 | 2.271 | 0.949 | 5.432 |
| Aortic aneurysm | 0.503 | .412 | 1.653 | 0.497 | 5.503 |
| Hypothyroidism | −0.018 | .950 | 0.982 | 0.555 | 1.736 |
| COPD | −0.756 | .079 | 0.469 | 0.202 | 1.092 |
| Osteoarthritis | −0.092 | .695 | 0.912 | 0.577 | 1.443 |
| Gout | 0.446 | .281 | 1.563 | 0.694 | 3.518 |
| No medication vs Insulin | 0.355 | .520 | 1.426 | 0.484 | 4.205 |
| No medication vs OHA | −0.116 | .736 | 0.891 | 0.455 | 1.745 |
| No Med vs Insulin+OHA | −0.377 | .437 | 0.686 | 0.266 | 1.774 |
| Statin | 0.404 | .226 | 1.497 | 0.779 | 2.879 |
| Aspirin | −0.073 | .761 | 0.930 | 0.582 | 1.486 |
| Metformin | −0.349 | .231 | 0.706 | 0.399 | 1.249 |
| DPP4-I | 0.103 | .778 | 1.109 | 0.541 | 2.27 |
Abbreviations: CAD, Coronary artery disease; CHF, Congestive heart failure; COPD, Chronic obstructive pulmonary disease; CVA, Cerebrovascular accident; DPP4-I, Dipeptidyl peptidase-4 inhibitor; OHA, Oral hypoglycemic agent; PAD, Peripheral arterial disease.
Discussion
We found increasing age, female sex and lack of moderate alcohol consumption as the major risk factors for DKD, and we found several significant risk associations. An increase in age by a year increased the odds of DKD by 7.8% in our patients. Advancing age has an impact in the eGFR and it is believed that increasing age reduces eGFR by 3.5 mL/min/1.73 m2 per year in both diabetic and non-diabetic individuals. Süleymanlar et al 12 found that for every 10-year increase in age among subjects older than 30 years with or without DM, the odds of DKD ranged from 1.45 to 2.18. Estimates suggest that after the age of 40, kidney filtration begins to fall by approximately 1% per year. 13 A much higher increase in the odds of DKD by 7.8% per year of advancing age in our patients clearly reflects that age happens to be an independent risk factor for DKD. Our findings align with the studies that have found that advanced age, or increasing age, increases the risk of DKD, both in type-1 DM and type-2 DM, irrespective of the duration of DM.2,6,14-18 Studies have also shown that an early decline in eGFR by greater than 3.5 mL/min/1.73 m2 per year prior to a decline in eGFR below 60 mL/min/1.73 m2, has been linked to the development of ESKD in type-1 DM.2,14 Gall and associates followed 191 White patients in an outpatient setting for a median period of 5.8 years who were aged under 66 years and had type-2 DM with normal UACR (<30 mg/24 h). They found that increasing age contributed to an increase in the relative risk of development of incipient or overt diabetic nephropathy by 1.07 (1.02-1.12). 15 The Australian Diabetes, Obesity, and Lifestyle Study included 11 247 adults aged 25 years and older and identified age as an independent risk factors for albuminuria. 16 In the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT-EDIC) Study, de Boer and associates examined 1279 participants between the ages of 13 and 39 years who had type-1 DM and followed them for development of microalbuminuria and decline in eGFR. They reported a decline in the eGFR by an average of 0.34 ml/min/1.73 m2 each year over 8 years of follow-up. They also found that greater rate of decline in eGFR was associated with greater age. 17 Increasing age remains a major risk factor for the development of DKD as observed in our study.
In our study, women with DM had 2.32 times greater odds of DKD. Although sex has been reported as a risk factor for DKD, so far it is the male sex that has been implicated as the risk factor. 2 For instance, in the UKPDS 74, Retnakaran and associates found male sex as an independent risk factor for DKD. They also reported that males were more likely to follow an albuminuric pathway to a decline in eGFR, whereas females were more likely to follow a non-albuminuric pathway. 19 In fact female sex has not been reported as a risk factor for DKD in several studies.15,18,20,21 In the DCCT-EDIC study, female sex was associated with reduced risk for progression from moderate albuminuria to severe albuminuria and even ESKD. 20 Gall et al 15 reported an increased relative risk of 2.6 (CI 1.2-54) for incipient diabetic nephropathy (persistent microalbuminuria) and overt diabetic nephropathy (persistent macroalbuminuria) for male sex in type-2 DM. Hovind and associates followed 286 (216 adults) newly diagnosed patients with type-1 DM for a median follow-up of 18.0 years. They reported that being male was associated with an increase in the relative risk by 2.41 (CI 1.43-4.06) for the development of persistent microalbuminuria. 21 The finding of female sex as an independent risk factor for DKD contrasts the reports of the available literature, which is a unique finding of our study.
An increase in the odds of DKD in patients who lacked moderate consumption of alcohol was another major finding in our study. Consumption of alcohol affects many parts of our body, including the kidneys. Studies have shown that consumption of moderate amounts of alcohol, such as 1 or 2 drinks, daily or every now and then, is associated with reduced risk of coronary heart disease and CKD.22-26 Moderate amount of alcohol consumption is defined as 1 drink per day which quantifies to the equivalent amounts of either one 12–ounce bottle of beer, or 1 glass of wine, or 1 ounce of hard liquor. However, excessive drinking, such as more than 4 drinks a day, can worsen the kidney function. 22 Our findings align with the reports of several studies that have shown that moderate alcohol consumption was associated with a decreased risk of CKD. Fan et al 23 reported in their review after an in-depth analysis of major studies related with the influence of alcohol in development of CKD as well as in patients with CKD, and found a protective effect of light-to-moderate alcohol consumption. Lai and associates followed 45 200 adults for a mean duration of 8.5 years. After adjusting for the baseline demographics and comorbidities, they reported that participants who were social and regular drinkers had a significantly decreased risk of incidence of CKD (social drinking: adjusted hazard ratio (AHR): 0.85; 95% CI, 0.74-0.97; regular-drinking: AHR: 0.85; 95% CI, 0.74-0.98). 24 Similarly, Lee et al 25 reported that alcohol consumption was associated with lesser decline in kidney function over 12 years among the general population. In the Atherosclerosis Risk in Communities (ARIC) study, Hu and associates followed 12 692 participants aged 45 to 64 years for a median follow-up of 24 years and reported 3664 cases of incident CKD. They found that the participants who drank ≤1 drink per week, 2 to 7 drinks per week, 8 to 14 drinks per week, and ≥15 drinks per week had, a 12% (HR: 0.88; 95% CI, 0.79-0.97), 20% (HR: 0.80; 95% CI, 0.72-0.89), 29% (HR: 0.71; 95% CI, 0.62-0.83), and 23% (HR: 0.77; 95% CI, 0.65-0.91) lower risk of CKD, respectively, compared with those who never drank alcohol. 26 Although the impact of alcohol on kidney function is largely unknown, several possible protective mechanisms of alcohol on kidney function have been proposed. It is well known that the risk factors that lead to atherosclerotic cardiovascular disease (ASCVD) are also associated with the development of CKD in patients with DM or without DM. Alcohol consumption elevates high-density lipoprotein cholesterol concentration,27,28 improves insulin sensitivity, 29 reduces serum insulin concentrations, enhances insulin sensitivity index,30,31 and reduces platelet aggregation rate as well as fibrinolysis.27,28 Similarly, the anti-oxidative effects of ethanol and polyphenol are well known. Ethanol improves polyphenol absorption, thereby contributing to the bioavailability.32-34 Additionally, the anti-inflammatory effect of alcohol has been demonstrated by the observation of increased serum interleukin-10 levels and decreased serum interleukin-16 levels in individuals who consume alcohol moderately. 35 Although we do not support consumption of alcohol, nevertheless we found that lack of moderate amount of alcohol consumption was a major factor associated with the development of DKD in our patients.
The association of several comorbid medical conditions, such as hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accidents, congestive heart failure, carotid artery stenosis, aortic aneurysm, peripheral artery disease, gout, and osteoarthritis were significantly higher in our patients with DKD compared to the group of patients without DKD, although multivariate logistic regression analysis did not show significant increase in the odds of DKD. Hypertension is widely known as an important independent risk factor for DKD.16,21 Lower blood pressure levels have been shown to be associated with regression from moderate albuminuria to normal UACR, especially in patients with type-2 DM. 36 Optimizing hypertension management by lowering blood pressure levels reduce risk of progression from moderate albuminuria to severe albuminuria, and ESKD. 20
Hyperlipidemia has been implicated in the pathogenesis of DKD, however experimental models show that elevated levels of total cholesterol and triglycerides were not sufficient to drive overt changes in renal structure and function. 37 On the contrary, prospective cohort study of type-2 DM patients for a median period of 5.8 years showed that elevated total cholesterol levels were associated with increased risk for the development of both moderate and severe increase in the UACR. 15 Similarly, Samsu 38 reported in a review that low levels of total cholesterol and triglycerides were associated with regression from moderate albuminuria to normal UACR. In our study, we found no significant differences in the mean HDL cholesterol and mean triglyceride levels between the patients with DKD and without DKD. Although, in the DCCT-EDIC study, lower LDL cholesterol and triglyceride levels were associated with reduced risk for progression from moderate albuminuria to severe albuminuria or ESKD, 20 our patients with DKD had significantly lower mean total cholesterol level and the mean LDL cholesterol level compared to the group without DKD.
Hyperglycemia, hypertension, dyslipidemia, insulin resistance and cigarette smoking are known initiators and promoters of DKD, as well as risk factors for ASCVD, which include coronary artery disease, cerebrovascular accidents, carotid artery stenosis, aortic aneurysm, and peripheral artery disease. 2 It is estimated that over half of all patients with congestive heart failure have moderate to severe CKD, as both the diseases share similar risk factors, such as age, hypertension, diabetes, and coronary artery disease. 39
Hyperuricemia and gout have been reported as risk factors for CKD. Nakayama and associates followed 138 511 participants younger than 65 years of age without CKD for a mean follow-up period of 4.68 years. They reported fully adjusted hazard ratio of CKD with serum uric acid level of ≥11.0 mg/dL of 3.74 (95% CI, 1.68-8.35). They identified high serum uric acid level as a risk factor for CKD in middle-aged men and women. 40 Osteoarthritis per se has not been implicated as a risk factor for the development of CKD, however use of non-steroidal anti-inflammatory drugs (NSAIDs) to treat osteoarthritis has been shown to be associated with increased incidence of CKD. 41 Our findings of higher association of hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accidents, congestive heart failure, carotid artery stenosis, aortic aneurysm, peripheral artery disease, gout, and osteoarthritis in patients with DKD align with the findings of the aforementioned studies.
There were a few limitations in our study. Being a retrospective chart review study, we had to rely solely on the electronic medical record documentations made by the patient care teams, hence certain items, such as the exact duration of DM, could not been reliably ascertained. The major strength of our study was the availability of a large sample size from 1 primary care office comprising of a small group of primary care physicians in which patients were followed by their specific physicians for a long period who monitored and documented the course of DM and associated comorbidities accurately.
Conclusion
We conclude that in patients with diabetes, increasing age, female sex and lack of moderate alcohol consumption were associated with increased odds of DKD. Higher frequencies of association of hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accidents, congestive heart failure, carotid artery stenosis, aortic aneurysm, peripheral artery disease, gout, and osteoarthritis were also seen in patients with DKD.
Acknowledgments
The authors thank Christine Rickette, RN (study coordinator) for her contribution to this study.
Footnotes
Author Contributions: SR made substantial contributions to the study design, drafting, data acquisition and analysis, and manuscript writing. All authors contributed in data collection and manuscript review. KH analyzed the data. SR contributed in revising the manuscript critically for improved intellectual content, and final approval for the version to be published.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Not applicable. Being a retrospective chart review study the Institutional Review Board waived the need for informed consent.
ORCID iD: Satyajeet Roy  https://orcid.org/0000-0002-1536-3678
https://orcid.org/0000-0002-1536-3678
Data Availability: The authors declare that data supporting the findings of this study are available within the article.
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the american Diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetologia. 2012;55:1577-1596. [DOI] [PubMed] [Google Scholar]
- 2. Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 Suppl 2):S39-S62. [DOI] [PubMed] [Google Scholar]
- 3. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Am J Kidney Dis. 2014;64(4):510-533. [DOI] [PubMed] [Google Scholar]
- 4. Sugahara M, Pak WLW, Tanaka T, Tang SCW, Nangaku M. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology. 2021;26(6):491-500. [DOI] [PubMed] [Google Scholar]
- 5. Liang S, Li Q, Zhu HY, et al. Clinical factors associated with the diagnosis and progression of diabetic nephropathy. Cell Biochem Biophys. 2014;70(1):9-15. [DOI] [PubMed] [Google Scholar]
- 6. Harjutsalo V, Groop PH. Epidemiology and risk factors for diabetic kidney disease. Adv Chronic Kidney Dis. 2014; 21(3):260-266. [DOI] [PubMed] [Google Scholar]
- 7. Othman M, Kawar B, El Nahas AM. Influence of obesity on progression of non-diabetic chronic kidney disease: a retrospective cohort study. Nephron Clin Pract. 2009;113(1):c16-c23. [DOI] [PubMed] [Google Scholar]
- 8. Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD). Nephrol Dial Transplant. 2012;27(5):1847-1854. [DOI] [PubMed] [Google Scholar]
- 9. Zou H, Zhou B, Xu G. Correction to: SGLT2 inhibitors: a novel choice for the combination therapy in diabetic kidney disease. Cardiovasc Diabetol. 2018;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab. 2016;27:820-830. [DOI] [PubMed] [Google Scholar]
- 12. Süleymanlar G, Utaş C, Arinsoy T, et al. A population-based survey of chronic REnal disease In Turkey—the CREDIT study. Nephrol Dial Transplant. 2010;26(6):1862-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Chronic kidney disease surveillance system—United States. 2021. Accessed August 14, 2021. https://nccd.cdc.gov/ckd/FactorsOfInterest.aspx?type=Age
- 14. Skupien J, Warram JH, Smiles AM, et al. The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int. 2012;82(5):589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314(7083):783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tapp RJ, Shaw JE, Zimmet PZ, et al. Albuminuria is evident in the early stages of diabetes onset: results from the Australian diabetes, obesity, and lifestyle study (AusDiab). Am J Kidney Dis. 2004;44:792-798. [PubMed] [Google Scholar]
- 17. de Boer IH, Sibley SD, Kestenbaum B, et al. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J Am Soc Nephrol. 2007;18:235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tziomalos K, Athyros VG. Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud. 2015;12(1-2):110-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective diabetes study 74. Diabetes. 2006;55(6):1832-1839. [DOI] [PubMed] [Google Scholar]
- 20. de Boer IH. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171:412-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Kidney Foundation. Drinking alcohol affects your kidneys. 2014. Accessed August 14, 2021. https://www.kidney.org/news/kidneyCare/winter10/AlcoholAffects
- 23. Fan Z, Yun J, Yu S, Yang Q, Song L. Alcohol consumption can be a “Double-Edged Sword” for chronic kidney disease patients. Med Sci Monit. 2019;25:7059-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lai YJ, Chen YY, Lin YK, Chen CC, Yen YF, Deng CY. Alcohol consumption and risk of chronic kidney disease: a nationwide observational cohort study. Nutrients. 2019;11(9):2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee YJ, Cho S, Kim SR. Effect of alcohol consumption on kidney function: population-based cohort study. Sci Rep. 2021;11(1):2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu EA, Lazo M, Rosenberg SD, et al. Alcohol consumption and incident kidney disease: results from the atherosclerosis risk in communities study. J Ren Nutr. 2020;30(1):22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wakabayashi I, Daimon T. Alcohol-independent beneficial cardiometabolic profile of individuals with hyper-HDL cholesterolemia in Japanese men and women. J Clin Lipidol. 2015;9:684-691. [DOI] [PubMed] [Google Scholar]
- 28. Huang S, Li J, Shearer GC, et al. Longitudinal study of alcohol consumption and HDL concentrations: a community-based study. Am J Clin Nutr. 2017;105:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crandall JP, Polsky S, Howard AA, et al. Alcohol consumption and diabetes risk in the Diabetes prevention program. Am J Clin Nutr. 2009;90:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim SH, Abbasi F, Lamendola C, Reaven GM. Effect of moderate alcoholic beverage consumption on insulin sensitivity in insulin-resistant, nondiabetic individuals. Metab Clin Exp. 2009;58:387-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Napoli R, Cozzolino D, Guardasole V, et al. Red wine consumption improves insulin resistance but not endothelial function in type 2 diabetic patients. Metabolism. 2005;54:306-313. [DOI] [PubMed] [Google Scholar]
- 32. Dinu D, Nechifor MT, Movileanu L. Ethanol-induced alterations of the antioxidant defense system in rat kidney. J Biochem Mol Toxicol. 2005;19:386-395. [DOI] [PubMed] [Google Scholar]
- 33. Rodrigo R, Miranda A, Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta. 2011;412:410-424. [DOI] [PubMed] [Google Scholar]
- 34. Rodrigo R, Rivera G, Orellana M, Araya J, Bosco C. Rat kidney antioxidant response to long-term exposure to flavonol rich red wine. Life Sci. 2002;71:2881-2895. [DOI] [PubMed] [Google Scholar]
- 35. Chiva-Blanch G, Urpi-Sarda M, Llorach R, et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: a randomized clinical trial. Am J Clin Nutr. 2012;95:326-334. [DOI] [PubMed] [Google Scholar]
- 36. Yamanouchi M, Furuichi K, Hoshino J, et al. Two-year longitudinal trajectory patterns of albuminuria and subsequent rates of end-stage kidney disease and all-cause death: a nationwide cohort study of biopsy-proven diabetic kidney disease. BMJ Open Diabetes Res Care. 2021;9(1):e002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watson AMD, Gould EAM, Moody SC, et al. Disparate effects of diabetes and hyperlipidemia on experimental kidney disease. Front Physiol. 2020;11:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin. 2008;4(4):387-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakayama S, Satoh M, Tatsumi Y, et al. Detailed association between serum uric acid levels and the incidence of chronic kidney disease stratified by sex in middle-aged adults. Atherosclerosis. 2021;330:107-113. [DOI] [PubMed] [Google Scholar]
- 41. Katsuno T, Togo K, Ebata N, et al. Burden of renal events associated with nonsteroidal anti-inflammatory drugs in patients with osteoarthritis and chronic low back pain: a retrospective database study. Pain Ther. 2021;10(1):443-455. [DOI] [PMC free article] [PubMed] [Google Scholar]