Abstract
Primary hepatic carcinosarcoma (HCS) is an extremely rare malignant tumor of the liver that contains carcinomatous and sarcomatous components. The diagnosis, treatment, and prognosis of HCS pose great challenges to clinicians. Herein, we present a case of HCS in a 67-year-old man with unique pathological manifestation. Preoperative magnetic resonance imaging showed a malignant lesion in the right liver and a small sub-focus in the left liver. Radical treatment was performed, including excision of the right posterior lobe of the liver, thrombectomy of the right posterior portal vein, and radiofrequency ablation of lesions in the left liver. The specimens were confirmed to be HCS by pathological examinations, which revealed a combination of poorly differentiated hepatocellular carcinoma, moderately differentiated cholangiocellular carcinoma, and spindle cell sarcoma. Transhepatic arterial chemotherapy and embolization was performed after surgery. Unfortunately, pulmonary metastasis occurred 1.5 months later, which meant a poor prognosis. In this report, we discuss the clinicopathological characteristics of this case and factors that affected surgical outcomes, which may add some ideas for the future diagnosis and treatment of HCS patients.
Keywords: Liver, carcinosarcoma, tumor, magnetic resonance imaging, diagnosis, case report
Introduction
Primary hepatic carcinosarcoma (HCS) is defined as a malignant neoplasm that contains a mixture of carcinomatous (either hepatocytic or cholangiocytic) and sarcomatous components. 1 While it has been proven that carcinosarcomas can originate from a variety of organs, primary carcinosarcomas of the liver are very rare. As far as we know, no more than 60 cases have been reported in the English language literature to date. Currently, there is poor recognition of HCS tumors, their epidemiology, and risk factors of the disease; furthermore, uniform diagnosis and treatment plans have not yet been established. In this report, we present a case of HCS that showed a unique manifestation upon pathological analysis of sarcomatous components and epithelial components containing combined hepatocellular carcinoma (HCC) and cholangiocellular carcinoma (CCC) that were closely intermingled. The reporting of this study conforms to CARE guidelines. 2
Case report
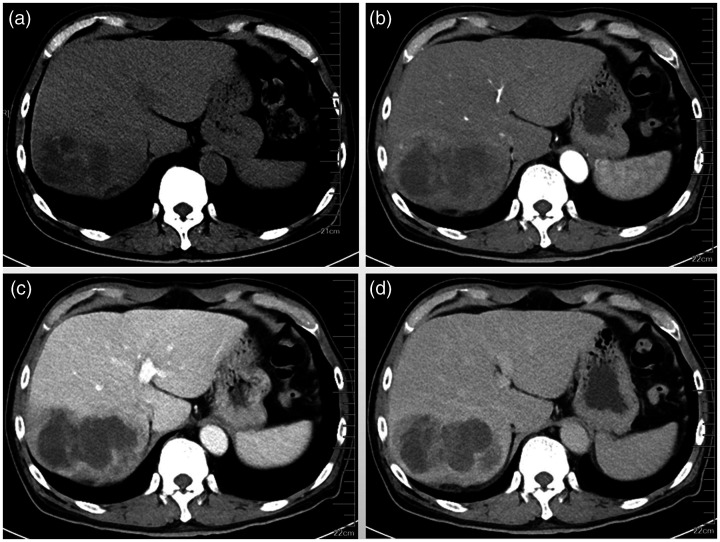
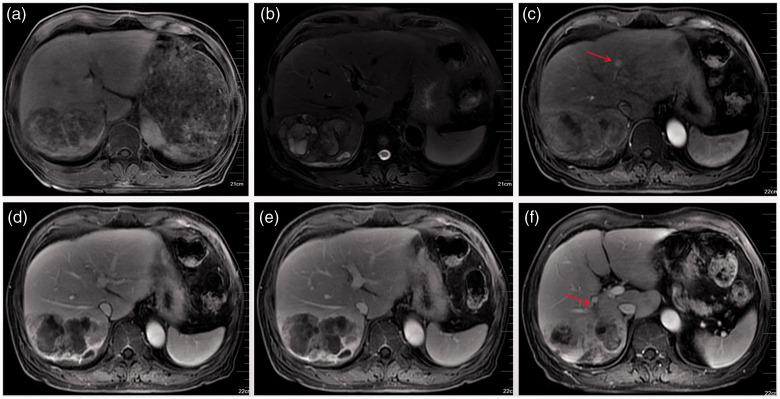
A 67-year-old male patient visited the endocrinology department with increased blood sugar for 5 years. During a routine abdominal computed tomography (CT) examination, an abnormal shadow was incidentally found in the right liver. Further magnetic resonance imaging (MRI) of the mass revealed signs of a malignant liver tumor. Therefore, he was transferred to our hepatobiliary surgery department for further treatment. Since the onset of disease, the patient had no obvious discomfort, except for weight loss of approximately 8 kg within 4 months. He had a history of chronic viral hepatitis B for 5 years and is regularly treated with tenofovir at 300 mg/day. Blood test results are summarized in Table 1. The levels of alpha-fetoprotein, serum carbohydrate antigen (CA) 19-9, total bilirubin, and direct bilirubin were abnormally elevated. Abdominal CT scanning of the tumors was then performed. Plain scanning revealed a giant hypointense mass (10 × 7 × 6 cm) involving the right liver that was oval-like, irregular, and with septations inside (Figure 1a). On dynamic CT scanning, the tumor edge showed irregular ring-like enhancement with strengthened septations, moderate enhancement in arterial phase (Figure 1b), continued enhancement in portal vein phase (Figure 1c), but attenuation in the equilibrium phase (Figure 1d). On MRI, the lesion appeared as an inhomogeneous high and low intensity on T1-weighted images (Figure 2a) and T2-weighted images (Figure 2b) with septations inside. On enhanced MRI scanning, the arterial phase of the enhancement scan was slightly inhomogeneously intensified, there was no signal enhancement in the inner part, and slight edge and separation enhancement (Figure 2c), with the enhancement range gradually increasing in the portal vein phase and attenuating in the equilibrium phase (Figure 2d,e). A sub-focus with a diameter of approximately 8 mm was found in the lateral segment of the left liver, which presented as slightly enhanced (Figure 2c). The tumor was accompanied by an embolus in the right posterior portal vein (Figure 2f).
Table 1.
Laboratory test results of the patient with primary hepatic carcinosarcoma.
| Items | Factors | Values | Reference range |
|---|---|---|---|
| Tumor markers | AFP (ng/mL) | 13.880 | <7 |
| CA199 (U/mL) | 41.840 | <34 | |
| Liver function tests | ALT (U/L) | 16.7 | 9–50 |
| AST (U/L) | 21.0 | 15–40 | |
| T-Bil (μmol/L) | 27.8 | 0–23 | |
| D-Bil (μmol/L) | 7.4 | 0–4 | |
| Viral markers | HBsAg (IU/mL) | 250.00 | <0.05 |
| HBcAb (s/co) | 6.08 | <1 | |
| Quantitative detection of hepatitis B virus nucleic acid (IU/mL) | 6.90E + 01 | <20 |
AFP, alpha-fetoprotein; CA19-9, carbohydrate antigen 19-9; ALT, alanine aminotransferase; AST, aspartate aminotransferase; T-Bil, total bilirubin; D-bil, direct bilirubin; HBsAg, Hepatitis B surface antigen; HBcAb, Hepatitis B core antibody.
Figure 1.
Computed tomography scans, non-enhanced and enhanced imaging. (a) Plain computed-tomography showed a heterogeneous hypoattenuating lesion in segments 7 and 8 of the liver; contrast-enhanced images showed a peripheral ring-like enhancement mass with reinforced septations. (b) Arterial; (c) portal; and (d) equilibrium phases of imaging.
Figure 2.
Magnetic resonance imaging. (a) T1-weighted images; (b) T2-weighted images. After enhancement, the mass presented as an irregular ring-like enhancement. (c) Arterial phase, a sub-focus located in the lateral segment of the left liver (red arrow) was found. (d) Portal phase; (e) equilibrium phase; (f) embolus in branches of the right posterior portal vein (red arrow).
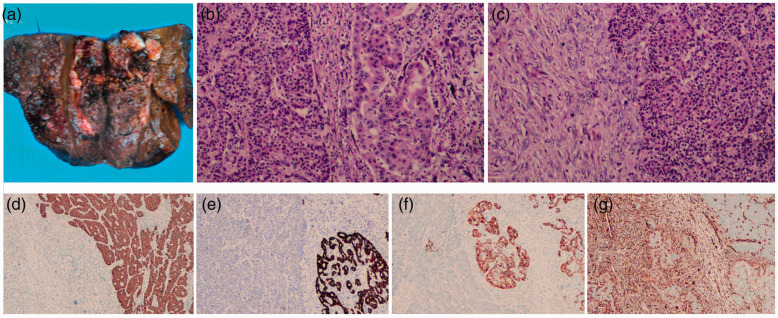
The patient was diagnosed with atypical HCC and radical resection of the tumors was recommended to prolong survival. During laparotomy, the liver showed pink sclerosis, and the tumor was located in the right posterior hepatic segments. No extrahepatic lesions were found. Thus, a right posterior hepatectomy with removal of the right posterior portal venous thrombus, and ultrasound-guided radiofrequency ablation of the sub-focus were performed. On pathology, the resected lesion was macroscopically of the multi-nodular type and had a size of 10 × 8 × 6.5 cm in the largest cross-section. The cut surface of the tumor was grayish-white and grayish-red with a central necrotic area (Figure 3a).
Figure 3.
Pathological images. (a) Surgically resected specimen showing a multiple nodular-type mass with a central necrotic area. Hematoxylin–eosin (HE) staining (magnification: 100×) (b–c). (b) The poorly differentiated hepatocellular carcinoma (HCC; left), sarcomatous component (middle), and moderately differentiated cholangiocellular carcinoma (CCC; right). (c) There was a clear boundary between spindle and epithelial cells. Immunohistochemical staining (magnification: 40×) (d–g). (d) HepPar-1 was positive in the HCC component; (e–f) CK7 and CK19 were positive in the CCC component; (g) Vimentin was positive in the sarcomatous component.
Microscopically, the tumor was composed of both carcinomatous and sarcomatous elements, with histological features of a collision tumor containing HCC, CCC, and undifferentiated spindle cell sarcoma. Unequivocal glandular structures comprising atypical gland cells with pleomorphic hyperchromatic nuclei and prominent nucleoli were interpreted as moderately differentiated CCC (Figure 3b). Additionally, carcinomatous cells with nestlike distribution, obvious atypia, high nucleus:cytoplasm ratios, and hyperchromatic nuclei were found, suggesting poorly differentiated HCC (Figure 3b). There was a clear boundary between the two elements. The sarcomatous component, which occupied approximately 20% of the whole tumor, consisted of long spindle-shaped cells with elongated nuclei arranged in bundle patterns (Figure 3c). There was a clear boundary between the carcinomatous and sarcomatous components (Figure 3c). The tumor was 2 cm from the closest liver resection margin, and the surrounding liver showed nodular cirrhotic changes. Cutting edges of the tumor were negative. On immunostaining, part of the epithelial component with a nested growth pattern was positive for HepPar-1 (Figure 3d), CKpan, arginase-1, and focally positive for glypican 3, which was suggestive of HCC, while the other part of the epithelial component with glandular architecture was positive for CK7 (Figure 3e) and CK19 (Figure 3f), and negative for arginase-1 and glypican 3, suggesting CCC. Both epithelial components were negative for vimentin. Sarcomatous cells were positive for vimentin (Figure 3g) and negative for cytokeratins 7 and 19, epithelial membrane antigen, and CD34.
Thus, the tumor was finally diagnosed as HCS (T4N0M0 stage IIIB) with a mix of HCC, CCC, and sarcoma. The patient’s tumor pathological classification and immunohistochemical indexes are shown in Table 2. The patient recovered well and was discharged 11 days later. Transhepatic arterial chemotherapy and embolization was given after surgery. Unfortunately, during the follow-up visit at one and a half months post-surgery, a chest CT scan revealed a single small nodule in the right lung. After comparing the with preoperative CT scans, this nodule was suspected of being metastatic lesions, and the CA19-9 level was elevated to 53.97 U/mL.
Table 2.
Tumor pathological classification and immunohistochemical indexes of the patient.
| Components | CK-P | Vim | Glypican -3 | Hepa-1 | Arginase-1 | Ki-67 | CK7 | CK19 | CD34 | AFP |
|---|---|---|---|---|---|---|---|---|---|---|
| HCC | + | − | + | + | + | +50% | − | − | + | − |
| CCC | + | − | − | − | − | +30% | + | + | − | − |
| Spindle Cells | − | + | − | − | − | +30% | − | − | − | − |
HCC, hepatocellular carcinoma; CCC, cholangiocellular carcinoma; CK, cytokeratin; Vim, vimentin; Glypican-3, phosphatidylinositol proteoglycan 3; AFP, Alpha-fetoprotein.
Discussion
Primary HCS is an exceedingly rare subtype of hepatic tumor that accounts for less than 0.3% of all HCCs. 3 In 2000, the World Health Organization classified HCS as a type of liver mesenchymal tumor and specifically defined it as a malignant tumor containing an intimate mixture of carcinomatous (either HCC or cholangiocarcinoma) and sarcomatous elements. 1 In the most recent version of the World Health Organization classification, carcinosarcoma is included in the category of sarcomatous neoplasms with poorer prognosis. 4 The case presented here is particularly rare owing to the combination of HCC and CCC in the epithelial part of the neoplasm. Therefore, it is important to report the tumor morphology for this relatively unknown malignancy.
Based on the previously published cases in the literature, we summarized the clinical and imaging manifestations of this rare disease. HCS is more common in middle-aged and elderly male patients, most of whom have a history of chronic hepatitis. 5 This shows that the development of HCS may be related to liver cirrhosis as well as hepatitis B virus infection. HCS tumors are usually relatively large when symptoms appear, presenting with nonspecific gastrointestinal symptoms, such as abdominal pain, abdominal distension, and dyspepsia. Laboratory tests can find liver damage and elevated tumor markers such as alpha-fetoprotein and CA19-9, but the probability of HCS cannot be removed if these markers are within the normal range. Due to the different ratios and pathological types of carcinomatous and sarcomatous components in HCS, previous imaging findings have been different. However, most of HCS lesions have presented with necrotic changes. We hypothesize that the sarcomatous elements of these tumors grow rapidly, outpacing the blood supply, which leads to necrosis. Therefore, HCS is always reported to present as heterogeneous low-attenuation lesions with large necrotic cystic portions on plain CT scan, T1-weighted hypointense, T2-weighted hyperintense, and irregular peripheral reinforced masses on enhanced imaging.5,6 Liu et al. reported the typical contrast-enhanced sonography findings of HCS, which were similar to enhanced-CT imaging. 7 Additionally, the enhanced linear, cord-like partitions and nodules in the tumor are caused by the interstitial components of the tumor, which are rare in HCC and CCC. Therefore, patients with these manifestations should be highly vigilant about the possibility of HCS. In our case, enhanced CT revealed irregular ring-like enhancement and continuous progressive reinforcement from outside to inside with hypodense central areas. However, the final diagnosis depends on pathological examination. It is noteworthy that in this case, in addition to sarcomatous components, both HCC and CCC components were concomitantly observed in the tumor.
Currently, the histogenetic origin of HCS remains unclear. One theory holds that HCS is the result of the differentiation of pluripotent liver stem cells into carcinomatous and sarcomatous cells.8,9 Thompson et al. proved a monoclonal origin of carcinosarcomas through two independent means of determining clonality. These findings support the single totipotential stem-cell-divergence hypothesis of carcinosarcoma. 10 On the basis of the existence of transitional or transformational zones between cancer and sarcoma, others support that preexisting HCC transforms into mesenchymal element through metaplasia mechanisms.1,8,11 It is believed that HCC cells can dedifferentiate into pluripotent undifferentiated cells, and then differentiate into a more sarcoma-like morphology. According to the findings of Gu et al., most HCS patients had a medical history of hepatitis B virus or cirrhosis, which indirectly supports the “transformed or dedifferentiated” tumor hypothesis. 11
Patients with HCS need to be distinguished from those with the following other diseases: (1) Hepatic sarcomatoid carcinoma: the clinical differentiation depends on pathological examination and immunohistochemistry. If the sarcomatoid area expresses mesenchymal markers, it will be diagnosed as HCS; if the sarcomatoid area expresses epithelioid markers but mesenchymal markers were negative, it will be diagnosed as hepatic sarcomatoid carcinoma. (2) Adult hepatoblastoma: the mixed type of hepatoblastoma is composed of epithelial and mesenchymal components. Fibrous septa and mucoid areas can be seen, especially in osteoid areas. In osteoid tissues, proto-fusiform cells and osteoblast-like cells can show positive CK staining. 12 (3) Primary hepatic carcinoma: HCC enhancement is often characterized by “fast in fast washout,” but its necrosis is smaller than that of HCS; CCC always presented with a “slow in slow washout” pattern of enhancement. (4) Hepatic abscesses: On imaging, the wall of the liver abscess is thick and continuously strengthening, showing a honeycomb-like chamber, and there can be edema of liver parenchyma around the lesion.
The prognosis of HCS cases is worse than that of conventional liver neoplasms. 13 Radical surgery is the primary method of treating carcinosarcomas of the liver.14–16 A study from Japan analyzed 21 reported cases of primary HCS and found that the survival time of patients with surgery and postoperative comprehensive treatment was significantly longer than that of patients without surgery, with the survival time of the former group being up to 30 months. 17 However, because the sarcoma component of HCS possesses highly aggressive features, most patients show recurrence or metastasis soon after surgery. The median survival time for these patients is only approximately 6 months. 8 After analyzing reports published before 2020, Bin et al. found that patients with invasion of adjacent organs, metastasis, or carcinomatous emboli had a poor prognosis. 5 In this report, the HCS tumor was treated with surgical resection and postoperative transhepatic arterial chemotherapy and embolization. Unfortunately, only 39 days after the operation, an early metastasis was discovered in the right lung through a CT examination. Compared with previously reported cases with optimistic postoperative results, we believe that the early metastasis in this case may be related to the complex composition and poor differentiation of primary HCS cells, the large tumor, and tumor embolism. These factors may be risk factors for poor prognosis.
In summary, we report an extremely rare case of primary HCS composed of HCC, CCC, and sarcoma components. Currently, most treatment options for advanced HCS come from the accumulated experience of similar case reports. Radical resection is the best available treatment for HCS. Further investigations are needed to fully identify the risk factors for early recurrence and metastasis after surgery and to determine effective post-surgical therapeutic approaches. Through the introduction of this case, we hope to add some insight into the diagnosis and clinical management of HCS and to help pathologists further identify the clinicopathological characteristics of this rare tumor.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Ethics statement: Because of the retrospective nature of this report and because all data are based on routine testing performed during hospitalization, which was approved by the patient, we did not seek ethical approval for this study. The patient provided written informed consent for publication.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Hebei Provincial Government Clinical Medicine Talents Training and Basic Research Project (grant No. 361003).
ORCID iDs: Ze Liang https://orcid.org/0000-0003-3070-1695
Hongfang Tuo https://orcid.org/0000-0001-9226-2830
Yanhui Peng https://orcid.org/0000-0002-7854-0926
References
- 1.Nomura K Aizawa S andUshigome S.. Carcinosarcoma of the liver. Arch Pathol Lab Med. 2000; 124: 888–890. DOI: 10.5858/2000-124-0888-COTL [DOI] [PubMed] [Google Scholar]
- 2.Gagnier JJ, Kienle G, Altman DG, et al. ; CARE Group. The CARE guidelines: Consensus-based clinical case reporting guideline development. Headache. 2013; 53: 1541–1547. DOI: 10.1136/bcr-2013-201554 [DOI] [PubMed] [Google Scholar]
- 3.Wang QB, Cui BK, Weng JM, et al. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma and carcinosarcoma of the liver. J Gastrointest Surg. 2012; 16: 1715–1726. DOI: 10.1007/s11605-012-1946-y [DOI] [PubMed] [Google Scholar]
- 4.WHO Classification of Tumours Editorial Board. WHO Classification of Tumors: Digestive System Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2019. [Google Scholar]
- 5.Bin F, Chen Z, Liu P, et al. The clinicopathological and imaging characteristics of primary hepatic carcinosarcoma and a review of the literature. J Hepatocell Carcinoma. 2020; 7: 169–180. DOI: 10.2147/JHC.S272768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L andLu L.. Case report: Review of CT findings and histopathological characteristics of primary liver carcinosarcoma. Front Genet. 2021; 12: 638636. DOI: 10.3389/fgene.2021.638636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu LP, Yu XL, Liang P, et al. Characterization of primary hepatic carcinosarcoma by contrast-enhanced ultrasonography: A case report. World J Gastroenterol. 2014; 20: 1630–1634. DOI: 10.3748/wjg.v20.i6.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto T, Kurashima Y, Ohata K, et al. Carcinosarcoma of the liver: Report of a case. Surg Today. 2014; 44: 1161–1170. DOI: 10.1007/s00595-013-0612-7 [DOI] [PubMed] [Google Scholar]
- 9.Fayyazi A, Nolte W, Oestmann JW, et al. Carcinosarcoma of the liver. Histopathology. 1998; 32: 385–387. DOI: 10.1046/j.1365-2559.1998.0401j.x [DOI] [PubMed] [Google Scholar]
- 10.Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol. 1996; 20: 277–285. DOI: 10.1097/00000478-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 11.Gu YJ, Zhu YY, Lu XY, et al. Hepatic carcinosarcoma: Evidence of polyclonal origin based on microsatellite analysis. Pathol Res Pract. 2015; 211: 905–910. DOI: 10.1016/j.prp.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 12.Kasper HU, Longerich T, Stippel DL, et al. Mixed hepatoblastoma in an adult. Arch Pathol Lab Med. 2005; 129: 234–237. DOI: 10.5858/2005-129-234-MHIAA [DOI] [PubMed] [Google Scholar]
- 13.Li J, Liang P, Zhang D, et al. Primary carcinosarcoma of the liver: Imaging features and clinical findings in six cases and a review of the literature. Cancer Imaging. 2018; 18: 7. DOI: 10.1186/s40644-018-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto H, Tanaka A, Kondo F, et al. Carcinosarcoma of the liver. Intern Med. 2010; 49: 2577–2582. DOI: 10.2169/internalmedicine.49.3581 [DOI] [PubMed] [Google Scholar]
- 15.Kurita D, Mokuno Y, Matsubara H, et al. Primary hepatic carcinosarcoma with multimodal treatment. Nagoya J Med Sci. 2018; 80: 423–429. DOI: 10.18999/nagjms.80.3.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YS, Wang TY, Lin JC, et al. Hepatic carcinosarcoma: Clinicopathologic features and a review of the literature. Ann Hepatol. 2013; 12: 495–500. [PubMed] [Google Scholar]
- 17.Yamamoto Y, Ojima H, Shimada K, et al. Long-term recurrence-free survival in a patient with primary hepatic carcinosarcoma: Case report with a literature review. Jpn J Clin Oncol. 2010; 40: 166–173. DOI: 10.1093/jjco/hyp123 [DOI] [PubMed] [Google Scholar]