Abstract
Febrile gastroenteritis in five healthy persons was associated with the consumption of vacuum-packed cold-smoked rainbow trout containing Listeria monocytogenes. L. monocytogenes isolates from the incriminated fish product lot and the stool samples were all of serotype 1/2a and were indistinguishable by pulsed-field gel electrophoresis employing AscI and SmaI.
Listeria monocytogenes is a food-borne pathogen causing listeriosis mainly in immunocompromised patients (5, 8, 13). The predominant clinical forms of listeriosis are infections of the central nervous system, sepsis, abortion, and stillbirth. A diarrheal form of disease due to the ingestion of foods contaminated with L. monocytogenes in previously healthy persons has also been reported (3, 7, 11, 12). However, in only one previous case was L. monocytogenes cultured from the implicated food, in that case from chocolate milk, characterized with pulsed-field gel electrophoresis (PFGE), and found to be identical to the isolates from patients (3).
Vacuum-packed salmon and rainbow trout have previously been associated with cases of listeriosis, but not with noninvasive gastroenteritis (4). We report febrile gastroenteritis in five previously healthy persons associated with the consumption of vacuum-packed cold-smoked rainbow trout (Onchorhyncus mykiss) containing high levels of L. monocytogenes.
Two couples, aged between 39 and 52 years, and a 3-year-old child dined together, consuming a meal including cold-smoked rainbow trout. They had no known underlying diseases. All of them fell ill with gastroenteritis within the next 27 h, experiencing nausea, abdominal cramps, and diarrhea. Three of the adults had fever (38.6 to 39.2°C). Other reported symptoms were vomiting, headache, fatigue, and arthralgia. The symptoms lasted 3 to 4 days. The child was admitted to a hospital for one night because of vomiting, diarrhea, and fever. Based on patient interviews and a questionnaire on the types and quantities of the foods consumed, the cold-smoked rainbow trout seemed to be a very likely vehicle of the pathogen that caused the food poisoning.
The patients had eaten the cold-smoked rainbow trout shortly after buying it from a retail store. The fish product had been vacuum packed 17 days prior to consumption. This retail store was visited, and the temperature of the display cabinet for smoked fish products was measured. At the bottom of the cabinet the temperature was 4.6°C, but at the top it was 11.6°C.
A vacuum-packed cold-smoked rainbow trout sample from the same production lot was then obtained from the same retail store and analyzed for L. monocytogenes. Analyses were performed both by selective enrichment according to the method of The Nordic Committee on Food Analyses (10) and by quantitative analysis for L. monocytogenes. The quantitative analysis was performed with Listeria enrichment broth, before selective supplements were added in serial dilutions on Listeria-selective Oxford agar.
Stool samples taken on the day after the onset of the symptoms were analyzed only for Salmonella, Shigella, Campylobacter, and Yersinia. Two additional stool swabs were obtained from one of the couples a week after the onset of the symptoms, and these were analyzed for L. monocytogenes by selective enrichment by using the medium described above for the fish sample.
Serotyping of L. monocytogenes isolates was performed according to the serotyping scheme of Seeliger and Höhne (14). Commercial Listeria antisera (Denka Seiken, Tokyo, Japan) were used according to the manufacturer’s instructions with the exception of the incubation temperature of the 0.2% brain heart infusion agar tubes, which was lowered to 26°C instead of 30°C.
The L. monocytogenes isolates were grown overnight in 5 ml of brain heart infusion broth at 37°C. DNA isolation was performed as described by Maslow et al. (9) with the modifications described previously (2). Two rare-cutting restriction enzymes, AscI and SmaI (New England Biolabs, Beverly, Mass.), were used according to the manufacturer’s recommendations. The samples were run through a 1.0% (wt/vol) agarose gel (SeaKem Gold; FMC Bioproducts, Rockland, Maine) in 0.5× TBE (45 mM Tris, 4.5 mM boric acid [pH 8.3], and 1 mM sodium EDTA) at 200 V at 10°C by using a Gene Navigator system with a hexagonal electrode (Pharmacia, Uppsala, Sweden). Macrorestriction fragments were resolved with pulse times ramping linearly from 1 to 35 s over 18 h for AscI fragments and from 1 to 18 s over 18 h for SmaI fragments. Lambda ladder PFG marker I and low-range PFG marker (New England Biolabs) were used as fragment size markers.
The cold-smoked rainbow trout sample was found to be positive for L. monocytogenes by selective enrichment and by quantification. The fish sample contained 1.9 × 105 CFU of L. monocytogenes per g. The storage temperature of the fish product at the retail outlet had been around 10°C, rather than the range of 0 to 3°C recommended by the manufacturer. The high storage temperature of the fish product probably allowed L. monocytogenes to grow to such high levels.
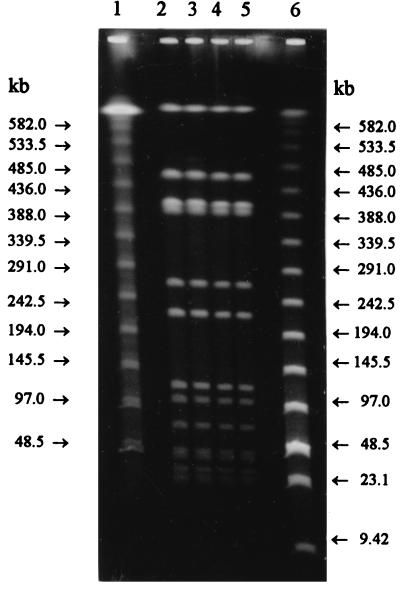
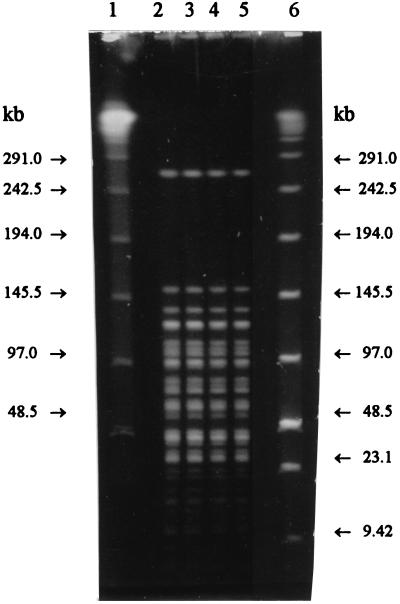
Both of the additional stool swabs were positive for L. monocytogenes. Taken together with the fact that no other enteric pathogens could be found, this strongly suggests that this outbreak of gastroenteritis among five persons was caused by L. monocytogenes. Furthermore, L. monocytogenes isolates from the stool swabs and the cold-smoked rainbow trout were of serotype 1/2a and showed indistinguishable macrorestriction enzyme patterns by PFGE with both restriction enzymes, AscI and SmaI (Fig. 1 and 2).
FIG. 1.
Macrorestriction patterns of L. monocytogenes with restriction enzyme AscI. Lane 1, lambda ladder PFG marker; lane 2, LMU1 (isolated from patient); lane 3, LMU10 (isolated from patient); lane 4, LMK55 (isolated without an enrichment step from cold-smoked rainbow trout); lane 5, LMK65 (isolated through an enrichment step from cold-smoked rainbow trout); lane 6, low-range PFG marker.
FIG. 2.
Macrorestriction patterns of L. monocytogenes with restriction enzyme SmaI. Lane 1, lambda ladder PFG marker; lane 2, LMU1 (isolated from patient); lane 3, LMU10 (isolated from patient); lane 4, LMK55 (isolated without an enrichment step from cold-smoked rainbow trout); lane 5, LMK65 (isolated through an enrichment step from cold-smoked rainbow trout); lane 6, low-range PFG marker.
These results provide additional supporting evidence for previous findings that L. monocytogenes may cause febrile gastroenteritis without invasiveness in healthy persons, if the consumed food is heavily contaminated with L. monocytogenes. They also show that vacuum-packed cold-smoked rainbow trout may contain large numbers of L. monocytogenes and must be considered as a potential source of infection (4, 6). The cold-smoking process does not kill L. monocytogenes (1), and despite the cold storage of the product, growth may occur. The sell-by date is usually around 3 weeks from the packing date; this is clearly too long, especially during the summer months. This kind of product is usually eaten without heat treatment before consumption.
This is, to our knowledge, the first report of febrile gastroenteritis associated with L. monocytogenes where a fish product acted as the vehicle of the pathogen. It also shows the value of selective enrichment for L. monocytogenes in stool samples. Without this enrichment, the Listeria might have been missed. PFGE was a very useful tool in confirming the food-borne origin of L. monocytogenes in the cases of febrile gastroenteritis presented in this study. The study emphasizes the need to keep the possibility of L. monocytogenes gastroenteritis in mind, especially after the presence of other enteropathogens has been ruled out.
Acknowledgments
This work was supported by the Technology Development Center (TEKES).
REFERENCES
- 1.Autio T, Hielm S, Miettinen M, Sjöberg A-M, Aarnisalo K, Björkroth J, Mattila-Sandholm T, Korkeala H. Sources of Listeria monocytogenes contamination in a cold-smoked rainbow trout processing plant detected by pulsed-field gel electrophoresis typing. Appl Environ Microbiol. 1999;65:150–155. doi: 10.1128/aem.65.1.150-155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björkroth J, Ridell J, Korkeala H. Characterization of Lactobacillus sake strains associating with production of ropy slime by randomly amplified polymorphic DNA (RAPD) and pulsed-field gel electrophoresis (PFGE) patterns. Int J Food Microbiol. 1996;31:59–68. doi: 10.1016/0168-1605(96)00964-6. [DOI] [PubMed] [Google Scholar]
- 3.Dalton C B, Austin C C, Sobel J, Hayes P S, Bibb W F, Graves L M, Swaminathan B, Proctor M E, Griffin P M. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997;336:100–105. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 4.Ericsson H, Eklöw A, Danielsson-Tham M-L, Loncarevic S, Mentzing L-O, Persson I, Unnerstad H, Tham W. An outbreak of listeriosis suspected to have been caused by rainbow trout. J Clin Microbiol. 1997;35:2904–2907. doi: 10.1128/jcm.35.11.2904-2907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming D W, Cochi S L, MacDonald K L, Brondum J, Hayes P S, Plikaytis B D, Holmes M B, Audurier A, Broome C V, Reingold A L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985;312:404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- 6.Guyer S, Jemmi T. Behavior of Listeria monocytogenes during fabrication and storage of experimentally contaminated smoked salmon. Appl Environ Microbiol. 1991;57:1523–1527. doi: 10.1128/aem.57.5.1523-1527.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heitmann M, Gerner-Smidt P, Heltberg O. Gastroenteritis caused by Listeria monocytogenes in a private day-care facility. Pediatr Infect Dis J. 1997;16:827–828. doi: 10.1097/00006454-199708000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Linnan M J, Mascola L, Lou X D, Goulet V, May S, Salminen C, Hird D W, Yonekura M L, Hayes P, Weaver R, Audurier A, Plikaytis B D, Fannin S L, Kleks A, Broome C V. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med. 1988;319:823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- 9.Maslow J N, Slutsky A M, Arbeit R D. Application of pulsed-field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 10.NCFA. L. monocytogenes. Detection in foods. Method no. 136. Espoo, Finland: Nordic Committee on Food Analyses; 1990. [Google Scholar]
- 11.Riedo F X, Pinner R W, Tosca M L, Cartter M L, Graves L M, Reeves M W, Weaver R E, Plikaytis B D, Broome C V. A point-source foodborne listeriosis outbreak: documented incubation period and possible mild illness. J Infect Dis. 1994;170:693–696. doi: 10.1093/infdis/170.3.693. [DOI] [PubMed] [Google Scholar]
- 12.Salamina G, Dalle Donne E, Niccolini A, Poda G, Cesaroni D, Bucci M, Fini R, Maldini M, Schuchat A, Swaminathan B, Bibb W, Rocourt J, Binkin N, Salmaso S. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes. Epidemiol Infect. 1996;117:429–436. doi: 10.1017/s0950268800059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlech W F, Lavigne P M, Bortolussi R A, Allen A C, Haldane E V, Wort A J, Hightower A W, Johnson S E, King S H, Nicholls E S, Broome C V. Epidemic listeriosis—evidence for transmission by food. N Engl J Med. 1983;308:203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- 14.Seeliger H P R, Höhne K. Serotyping of Listeria monocytogenes and related species. Methods Microbiol. 1979;13:31–49. [Google Scholar]