Abstract
Nonalcoholic steatohepatitis (NASH) is a leading cause of chronic liver disease, affecting 1.5%–6.5% of the world population. Currently, there are no FDA-approved drugs to treat this disease. Accumulating evidence suggests that metabolically hazardous visceral fat contributes to NASH progression by releasing fatty acids and proinflammatory mediators. Therefore, targeting adipose tissue to reduce adipose inflammation may provide an effective strategy to treat NASH. Another strategy is to target specific inflammatory mediators that are produced by adipose tissue and contribute to NASH progression. In this issue of the JCI, Liu, Xiang, et al. demonstrate that secreted protein acidic and rich in cysteine-like protein 1 (SPARCL1) was highly upregulated in adipose tissue and played a role in exacerbating NASH progression in a mouse model of NASH. Thus, inhibition of SPARCL1 may provide another attractive strategy to tackle NASH.
Nonalcoholic fatty liver disease
The global prevalence of nonalcoholic fatty liver disease (NAFLD) has risen to astronomical proportions over the last several decades, totaling approximately 25% of the general population (1). Nonalcoholic steatohepatitis (NASH), which is a more severe stage of NAFLD, affects 1.5%–6.5% of the world population and that figure is predicted to dramatically rise in the years to come (1). One of the major reasons for this increase in NAFLD and NASH relates to sedentary lifestyles with excessive caloric intake and the associated upsurge of obesity. Lifestyle modifications, including dietary changes and increased physical activity, represent an effective first line of treatment for NASH (2). It has been shown that 5% weight loss by lifestyle intervention has a 10% probability for NASH resolution and that this probability further increases to 90% when 10% weight loss is attained (3). Weight reduction by bariatric surgery has also proven to be an effective treatment, as 1 year after surgery, NASH resolution and improved fibrosis was obtained in 85% and 34% of patients, respectively (4). Furthermore, weight loss and lifestyle modification have also been shown to ameliorate NASH in lean patients, who often have excessive fat at ectopic sites, such as the waist and neck, but are not overweight or obese as per their body mass index (5). This information adds to the notion that visceral fat is particularly metabolically hazardous and contributes to NASH progression. However, the exact molecular mechanisms by which adipose tissue dysfunction contributes to the development of NAFLD remain obscure.
Role of adipose tissue in NAFLD
Adipose tissue consists of white adipose tissue (WAT) and brown adipose tissue (BAT), the former being the predominant type and commonly referred to as “fat.” WAT is composed of multiple cellular populations interwoven in a matrix of vascularized connective tissue, including adipocytes, adipocyte precursors, endothelial cells, fibroblasts, and various types of immune cells. These cell populations are not static and can substantially change during obesity, which is believed to contribute to NAFLD progression. Among lean adults and those with obesity, the number of adipocytes remain roughly constant and the adipocyte turnover rate is not altered by obesity. This relatively fixed feature implies that adipocyte hypertrophy (enlargement of adipocytes) is the predominant contributor to adult obesity, whereas adipocyte hyperplasia (increase in adipocyte numbers) associates more so with childhood obesity (6, 7). Hypertrophic adipocytes have impaired cellular functions that contribute to insulin resistance (IR), which is the pathophysiological hallmark of NAFLD (8). At early stages of obesity, adipocyte hypertrophy contributes to IR through an impaired insulin-regulated glucose transporter, GLUT4, a process that is independent of inflammation (9). At later stages, however, adipose tissue inflammation is the major contributor to systemic IR. The release of proinflammatory cytokines can directly affect the insulin signaling pathway or stimulate inflammatory pathways, including the c-Jun N-terminal kinase (JNK) pathway and I-κ B kinase β (IKKβ)/NF-κB pathway that disrupt the insulin signaling pathways (10). Contrarily, adipose tissue can also release antiinflammatory adipokines, including adiponectin, which is downregulated during NASH (11), and neuregulin 4 (Nrg4), which is hepatoprotective and can attenuate diet-induced NASH in mice (12).
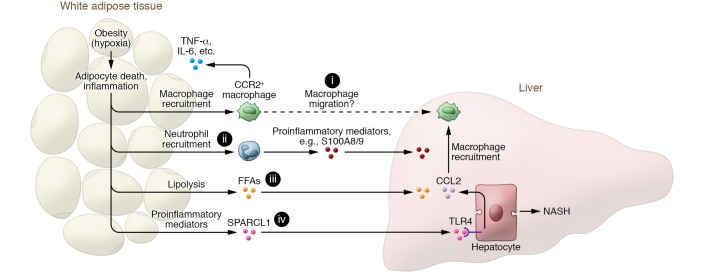
Accumulating evidence suggests that adipocyte death and adipose tissue inflammation play an important role in triggering liver injury and inflammation, and NAFLD progression (13). It is generally accepted that during obesity, adipocyte hypertrophy creates local areas of hypoxia within the WAT that induce adipocyte necrosis, which promotes macrophage recruitment (14) (Figure 1). The infiltrated macrophages produce a variety of inflammatory cytokines, including TNF-α and IL-6, and chemokines such as CCL2, exacerbating tissue inflammation (15, 16) (Figure 1). Our group recently provided direct evidence linking adipocyte death and liver inflammation, showing that selective, acute adipocyte cell death in mice rapidly induces liver injury and inflammation by promoting CCR2-dependent macrophage infiltration and lipolysis (17) (Figure 1). Transplantation of adipose tissue macrophages from obese mice also promotes neutrophil recruitment and macrophage activation in NASH mouse models, highlighting the role of adipose tissue inflammation in NAFLD progression (18). E-selectin, a key adhesion molecule for neutrophils, is highly upregulated in adipose tissues of NASH patients and of mice with NASH induced by high-fat diet (HFD) feeding plus adenovirus-Cxcl1 (AdCxcl1) overexpression compared with those with fatty liver (19). Such induction of E-selectin mediates neutrophil recruitment in adipose tissue, thereby accelerating NASH progression partially via the production of proinflammatory mediators such as S100A8 and S100A9 (19) (Figure 1).
Figure 1. Adipose tissue inflammation promotes NASH progression via proinflammatory mediators, including SPARCL1.
Adipocyte hypertrophy during obesity can cause adipose tissue hypoxia and subsequent adipocyte death and adipose tissue inflammation, which further promote the recruitment of macrophages and neutrophils in adipose tissue. The infiltrated CCR2+ macrophages produce cytokines (such as TNF-α, IL-6, etc.) that perpetuate adipose tissue inflammation and promote hepatic macrophage infiltration (i) and liver inflammation. Recruitment of neutrophils in adipose tissue (ii), which is dependent on E-selectin, accelerates NASH progression by producing many inflammatory mediators (such as S100A8 and S100A9). In addition, adipose tissue inflammation activates adipocyte lipolysis to release free fatty acids (FFAs) (iii), leading to liver injury and inflammation. Finally, dysregulated adipose tissue can produce many proinflammatory mediators, including SPARCL1 (iv) whose function in NASH progression is reported by Liu, Xiang, et al. (22). Notably, SPARCL1 stimulates hepatocytes to produce CCL2 that induces hepatic macrophage recruitment and liver inflammation, thereby promoting NASH progression. Inhibition of SPARCL1 has therapeutic potential for the treatment of NASH.
SPARCL1 in adipose-liver interplay during NASH
Although many studies suggest that adipose tissue–liver crosstalk plays an important role in promoting NASH (20, 21), a full understanding of how this crosstalk contributes to NAFLD progression is missing. In this issue of the JCI, Liu, Xiang, et al. (22) performed bulk RNA-sequencing analysis of epididymal WAT from mice with different stages of NAFLD, including nonalcoholic fatty liver (NAFL) and NASH that were defined by short-term (12 weeks) and long-term (28 weeks) feeding, respectively, with a high-fat, high-fructose, and high-cholesterol (HFHC) diet. The researchers found that secreted protein acidic and rich in cysteine-like protein 1 (SPARCL1) was highly upregulated in WAT of NASH mice, compared with healthy mice or mice with NAFL. In human cohorts, plasma levels of SPARCL1 were also elevated in patients afflicted with NASH. Notably, plasma levels correlated with NASH severity. The authors further proposed a SPARCL1–alanine aminotransferase–aspartate aminotransferase (SPARCL1-ALT-AST) model that improved the accuracy of identifying NASH patients compared with the ALT-AST model used in standard liver function tests. The detrimental role of SPARCL1 in promoting NAFLD progression was demonstrated by experiments in which mice were injected with recombinant SPARCL1 protein or in which Sparcl1 was transiently overexpressed. Further experiments with deletion of the Sparcl1 gene, knockdown of Sparcl1 expression in WAT, or treatment with a SPARCL1-neutralizing antibody also supported the harmful function of SPARCL1 in the pathogenesis of NASH. Mechanistically, SPARCL1 induced liver inflammation by binding to Toll-like receptor 4 (TLR4) on hepatocytes. Subsequently, NF-κB signaling induced C-C motif chemokine ligand 2 (CCL2) to promote hepatic macrophage recruitment (Figure 1).
The study by Liu, Xiang, et al. (22) convincingly describes the pathogenic roles of SPARCL1 in NASH. Yet, insufficient evidence was provided to support the idea that WAT remains the main source of SPARCL1 during NASH. Although the authors show that expression of Sparcl1 was higher in adipose tissues compared with skeletal muscle, liver, kidney, spleen, and testis of unchallenged mice, no comparative data were reported for diet-induced NASH mice. Furthermore, it was previously found that Sparcl1 levels might be higher in other organs, such as the lung (in mice) or the stomach (in humans), and that although Sparcl1 is not expressed in murine livers, it is moderately expressed in human livers (23). The authors tried to specifically delete Sparcl1 in WAT by injecting AAV8-shRNA targeting Sparcl1 into inguinal WAT depots, which only partially reduced tissue Sparcl1 expression and serum SPARCL1. Further studies with tissue-specific Sparcl1-knockout mice may help define the major sources of SPARCL1 in NASH mice. In addition, as indicated by the very low ALT and AST levels of the HFHC-fed control groups reported throughout the study (22), the HFHC diet failed to induce a high degree of liver damage. It would therefore be interesting to investigate the role of SPARCL1 in other NASH models.
Targeting adipose tissue to treat NASH
Besides lifestyle modifications, including dietary changes and increased physical activity, there are currently no approved drugs to treat NASH. Pharmacotherapies to treat NASH should, however, also target the interplay between adipose tissue and the liver. Since a positive correlation between adipose tissue inflammation and NASH severity is documented in patients, targeting adipose inflammation may curb NASH progression (24). The E-selectin/neutrophil/S100A8/S100A9 axis in adipose tissues, which has been suggested to promote NASH progression in mice (19), is one potential therapeutic target for NASH treatment. As previously stated, dysregulated adipose tissues during NAFLD secrete a large number of adipokines, inflammatory mediators, and soluble proteins, and many of them play an important role in promoting NASH (20, 21). Therefore, specifically targeting some of these mediators, such as SPARCL1 as identified by Liu, Xiang, et al. (22), may provide another attractive strategy to tackle NASH. Further identification of the molecular mechanisms driving adipose tissue–liver interplay may, therefore, be the basis for the development of therapies to treat NASH.
Conclusion
Collectively, the data presented by Liu, Xiang, et al. (22) reveal a function of SPARCL1, a protein for which association with adipose tissue or NAFLD was previously unappreciated. The authors document a pathogenic effect of SPARCL1 that promotes the progression of NAFL to NASH in diet-induced mouse models. The clinical relevance of this molecule should, however, be further confirmed, particularly because expression differences have been reported between humans and mice (23).
Acknowledgments
The authors’ laboratory was supported by the intramural program of the NIAAA, NIH.
Version 1. 10/15/2021
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(20):e153640. https://doi.org/10.1172/JCI153640.
See the related article at Sparcl1 promotes nonalcoholic steatohepatitis progression in mice through upregulation of CCL2.
Contributor Information
Robim M. Rodrigues, Email: Robim.Marcelino.Rodrigues@vub.be.
Yukun Guan, Email: yukun.guan@nih.gov.
Bin Gao, Email: bgao@mail.nih.gov.
References
- 1.Younossi Z, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Gomez M, et al. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Vilar-Gomez E, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Lassailly G, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379–388. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Mohan S. Non-alcoholic fatty liver disease in lean subjects: characteristics and implications. J Clin Transl Hepatol. 2017;5(3):216–223. doi: 10.14218/JCTH.2016.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 7.Choe SS, et al. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugianesi E, et al. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42(5):987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 9.Kim JI, et al. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol. 2015;35(10):1686–1699. doi: 10.1128/MCB.01321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makki K, et al. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savvidou S, et al. Low serum adiponectin levels are predictive of advanced hepatic fibrosis in patients with NAFLD. J Clin Gastroenterol. 2009;43(8):765–772. doi: 10.1097/MCG.0b013e31819e9048. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, et al. Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J Clin Invest. 2017;127(12):4449–4461. doi: 10.1172/JCI96324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, et al. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol Immunol. 2021;18(1):73–91. doi: 10.1038/s41423-020-00579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun K, et al. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korf H, et al. Macrophages as key players during adipose tissue-liver crosstalk in nonalcoholic fatty liver disease. Semin Liver Dis. 2019;39(3):291–300. doi: 10.1055/s-0039-1687851. [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, et al. Adipocyte death preferentially induces liver injury and inflammation through the activation of chemokine (C-C motif) receptor 2-positive macrophages and lipolysis. Hepatology. 2019;69(5):1965–1982. doi: 10.1002/hep.30525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bijnen M, et al. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut. 2018;67(7):1317–1327. doi: 10.1136/gutjnl-2016-313654. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues RM, et al. E-selectin-dependent inflammation and lipolysis in adipose tissue exacerbate steatosis-to-NASH progression via S100A8/9. Cell Mol Gastroenterol Hepatol. doi: 10.1016/j.jcmgh.2021.08.002. [published online August 11, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azzu V, et al. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology. 2020;158(7):1899–1912. doi: 10.1053/j.gastro.2019.12.054. [DOI] [PubMed] [Google Scholar]
- 21.Cordeiro A, et al. Does adipose tissue inflammation drive the development of non-alcoholic fatty liver disease in obesity? Clin Res Hepatol Gastroenterol. 2020;44(4):394–402. doi: 10.1016/j.clinre.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, et al. Sparcl1 promotes nonalcoholic steatohepatitis progression in mice through upregulation of CCL2. J Clin Invest. 2021;131(20):e144801. doi: 10.1172/JCI144801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klingler A, et al. Species-, organ- and cell-type-dependent expression of SPARCL1 in human and mouse tissues. PLoS One. 2020;15(5):e0233422. doi: 10.1371/journal.pone.0233422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.du Plessis J, et al. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology. 2015;149(3):635–648. doi: 10.1053/j.gastro.2015.05.044. [DOI] [PubMed] [Google Scholar]