Abstract
A histologic hallmark of primary SS (pSS) is lymphocytic infiltration of the salivary and lacrimal glands, in particular by CD4+ T and B cells. In the early stages of the disease, infiltrates are dominated by CD4+ T cells, while B cell accumulation occurs at later stages. Activated T cells contribute to pathogenesis by producing pro-inflammatory cytokines and by inducing B cell activation, which results in the establishment of a positive feedback loop. In the inflamed glandular tissues, many different CD4+ effector subsets are present, including IFN-γ-producing Th1 cells, IL-17-producing Th17 cells and IL-21-producing T follicular helper cells. In blood from pSS patients, frequently observed abnormalities of the T cell compartment are CD4+ T cell lymphopenia and enrichment of circulating follicular helper T (Tfh) cells. Tfh cells are critical mediators of T cell–dependent B cell hyperactivity and these cells can be targeted by immunotherapy. Inhibition of T cell activation, preferably early in the disease process, can mitigate B cell activity and may be a promising treatment approach in this disease.
Keywords: SS, lymphocytes, cytokines, T cells, immunotherapy, biologic therapies, histopathology, biomarkers
Rheumatology key messages
CD4+ T cells are critically involved in pSS pathogenesis.
Tfh cells are consistently found to be enriched in blood and likely facilitate B cell hyperactivity.
Inhibition of T cell–B cell interaction is a promising treatment strategy for pSS.
Introduction
Primary SS (pSS) is a systemic autoimmune disease characterized by lymphocytic infiltration of the salivary and lacrimal glands. In addition to the exocrine glands, many other organs can be affected by the disease as well [1]. Hyperactivity of B cells is thought to play a central role in the pathogenesis of pSS [2]. Available evidence strongly indicates that this B cell hyperactivity is mediated by T cells [3, 4]. T cells may also be involved in a loss of self-tolerance and they secrete many pro-inflammatory cytokines associated with local inflammation in pSS, including IFN-γ, IL-17 and IL-21 [5, 6].
In pSS patients, T cells form a large part of the lymphocytic infiltrates observed in salivary and lacrimal gland tissues, particularly in the earlier stages of disease [7]. The infiltrated T cells are mostly CD4-expressing ‘helper’ T cells [8]. However, lymphocytic infiltration and loss of glandular structure are not directly related to the loss of glandular function, which suggests that (intrinsic) defects in epithelial cells contribute to the disease as well.
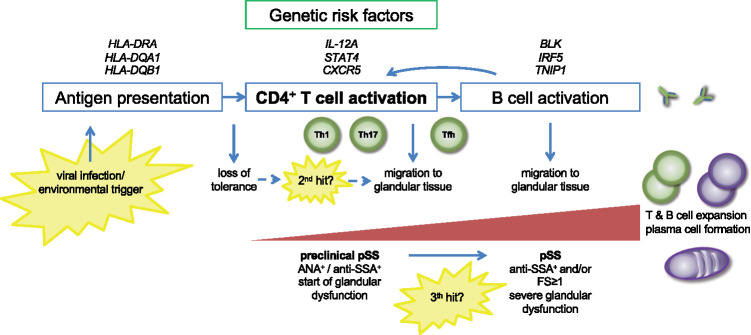
CD4+ T cells recognize antigens presented by antigen-presenting cells via class II MHC molecules. Similar to other systemic autoimmune diseases, the strongest genetic risk haplotypes for pSS were identified within the HLA-DR and HLA-DQ regions [9]. These risk haplotypes may lead to inadequate control of reactivity towards self-antigens and escape of autoreactive T cells from negative selection. HLA class II risk loci are associated with anti-SSA/-SSB autoantibody presence in pSS [10]. In the majority of patients, these autoantibodies are already present years before the onset of clinical symptoms, which suggests that the induction of autoantibody-producing plasma cells by (autoreactive) T cells occurs in a preclinical stage of the disease (Fig. 1) [11]. Besides aberrant thymic T cell selection, alternative explanations for the observed autoreactivity are cross-reactivity between foreign and self-antigens or mimicry with microbial antigens [12, 13].
Fig. 1.
Proposed role of CD4+ T cells in primary Sjögren’s syndrome (pSS) pathogenesis
Antigen is presented to CD4+ T cells via MHC class II (HLA) molecules, resulting in CD4+ T cell activation. Risk loci in HLA-DR and HLA-DQ regions, associated with pSS, may be involved in a loss of tolerance to self-antigens. Depending on the type of antigen and additional environmental cues, differentiation of naïve cells into Th1 cells, Th17 cells and Tfh cells is induced. IL-12A and STAT4 risk variants may contribute to enhanced Th1 cell differentiation. A second, local hit may induce migration of effector CD4+ T cells to salivary and/or lacrimal gland tissues. This stage is clinically reflected in features suggestive of pSS, without the presence of focal periductal infiltrates and without evident signs of B cell hyperactivity. A third hit is probably required to establish a positive feedback loop between T cells and B cells, resulting in T cell–dependent B cell hyperactivity. In this stage, typical features associated with pSS become evident.
CD4+ T cells in pSS patients have been extensively studied over the years, particularly in blood. Th1 cells were the first CD4+ effector subset to be recognized and associated with autoimmunity. Initial studies observed that in inflamed glandular tissue of pSS patients, the majority of CD4+ T cells expressed IFN-γ [8, 9], consistent with a Th1 cell phenotype. Additionally, the role of more recently identified subsets, including Th17 cells and follicular helper T (Tfh) cells, has been assessed in numerous autoimmune diseases, including pSS (reviewed by Patel and Kuchroo [14] and Vinuesa and Linterman [15]). In this review we summarize our current knowledge of the contribution of CD4+ T cells in pSS pathogenesis and briefly appraise the role of CD8+ T cells in the disease. Furthermore, T cell–targeting therapies for pSS will be discussed.
CD4+ T cell lymphopenia
A typical laboratory finding in pSS is a decrease in CD4+ T cell numbers in the blood, which can even lead to CD4+ T cell lymphopenia [16]. Although its origin and implications remain largely unclear, CD4+ T cell lymphopenia is a predictive factor of lymphoma development in pSS (reviewed by Nocturne and Mariette [17]). A recent study showed that lower CD4+ T cell counts were significantly correlated with higher systemic disease activity scores [18], as measured by the EULAR SS disease activity index (ESSDAI) [19]. The same study also showed that decreased CD4+ T cell numbers in blood were associated with increased numbers of lymphocytes, including CD4+ T cells, in minor salivary glands (MSGs), favouring the hypothesis that this lymphopenia in blood is a result of CD4+ T cell migration to inflamed tissues [18]. However, direct evidence for this hypothesis is lacking. Another possible explanation for CD4+ T cell lymphopenia in pSS is the increased differentiation rate of naïve T cells into effector T cells, which have a more rapid turnover than the usually long-lived naïve cells [20]. Because of CD4+ T cell lymphopenia, comparisons between the numbers of circulating CD4+ T cell subsets in pSS patients and healthy controls (HCs) are difficult to interpret. For this reason, many studies have assessed the frequencies of these subsets to identify changes in the CD4+ T cell compartment in pSS.
Effector CD4+ T cell subsets in blood and salivary gland tissue of pSS patients
Various CD4+ T cell subsets can be discriminated by surface molecule expression, such as CD45RA/CD45RO for differentiation between naïve and memory cells and expression of chemokine receptors for recognition of different effector subsets. Also, in vitro cytokine production can be used for phenotyping. However, the use of different definitions makes it difficult to compare various studies.
Th1/Th2 cells
One of the first studies that investigated Th1/Th2 balance in matched blood and MSG tissue samples showed that serum levels of IFN-γ were decreased while the number of IFN-γ+ cells in MSG tissue was increased in pSS compared with non-SS sicca patients [21]. No differences in Th2 cell activity, assessed by IL-4 protein levels in serum and the number of IL-4+ cells in the glands, were observed. Subsequent studies showed that neither numbers nor frequencies of Th1 and Th2 cells in the blood from pSS patients were aberrant [22, 23]. However, support for local involvement of Th1 cells has been substantiated by a more recent study showing that local IFN-γ (type II IFN) activity, assessed by the detection of IFN‐inducible guanylate binding protein 1, was associated with the degree of CD45+ infiltration in the MSGs of pSS patients [24]. Th1 cells are likely attracted to the salivary glands via secretion of the pro-inflammatory chemokines CXCL9 and CXCL10 by ductal epithelial cells. These chemokines are the ligands for the CXCR3 receptor on Th1 cells [25]. While Th1 cell–related mRNA transcripts (e.g. IFN-γ) were detected in glandular tissue of the vast majority of pSS patients, Th2 cell–related transcripts seem to be present only in patients with strong B cell accumulation [26]. Furthermore, Th1 cell–related mRNA transcripts (e.g. IFN-γ, T-bet) were more abundant in the MSG tissue of pSS patients without germinal centres (GCs), while Th2 cell–related mRNA transcripts (GATA3 and IL-4) were almost exclusively detected in GC-positive pSS patients [27]. Although IL-4 and GATA3 are Th2 cell–associated molecules, IL-4-producing T cells within B cell follicles have phenotypic characteristics of Tfh cells (see below) [28]. Therefore it is tempting to speculate that the cells responsible for higher IL-4 levels in GC-positive patients are in fact Tfh cells.
While the presence of IFN-γ-producing Th1 cells within lymphocytic infiltrates in pSS is evident, little is known about their contribution to hyposalivation and/or destruction of the acinar and ductal epithelium in vivo. Evidence from in vitro studies with cultured intestinal epithelial cells suggests that IFN-γ can alter tight junction function and increase permeability across the epithelium (reviewed by Walsh et al. [29]). Alterations in tight junction components were also observed in MSGs of pSS patients and in vitro exposure of acinar cells to IFN-γ could mimic these alterations [30]. In addition to an effect on tight junctions, IFN-γ could also induce Fas-mediated apoptosis in salivary gland epithelial cell (SGEC) line cultures [31]. Together, these results suggest that accumulation of IFN-γ-producing Th1 cells in the exocrine glands may contribute to epithelial cell damage and, consequently, diminished saliva secretion.
IL-17-producing cells
The greatest evidence for a pathogenic role of Th17/IL-17-producing cells comes from mouse models of pSS (reviewed by Verstappen et al. [32]). In different models, IL-17 knockout mice were protected from disease development [33, 34]. In human pSS, IL-17 protein and mRNA is increased in MSG tissue of pSS compared with non-SS sicca patients [35–37]. In peripheral blood, frequencies of Th17 cells (defined as CD4+CD45RA−FoxP3−CXCR5−CXCR3−CCR4+CCR6+ cells) were increased at least in pSS patients with moderate to high disease activity [3, 23]. In addition, mRNA levels of the Th17 cell–associated transcription factor RAR-related orphan receptor (ROR)-γt and its co-activator Transcriptional coactivator with PDZ-binding motif (TAZ) were higher in circulating memory CD4+ T cells from pSS patients compared with HCs [38]. On the other hand, when Th17 cells were defined by in vitro IL-17 production, most studies did not find aberrant numbers and/or frequencies of these cells in the blood of pSS patients [22, 23, 39].
In addition to a typical pattern of chemokine receptor expression by Th17 cells, all IL-17-producing T cells express the C-type lectin CD161 [40, 41]. However, not all CD161+ T cells produce IL-17. In blood from pSS patients, the percentages of both CD161+RORγt+ (Th17-like) cells and CD161+RORγt− cells were increased compared with HCs [42]. The percentages of CD161+RORγt+ cells correlated with the presence of anti-SSA/-SSB autoantibodies and IgG levels in serum, but not with ESSDAI scores [42]. CD161 functions as a homing factor to mucosal tissues and as a costimulatory receptor in the context of TCR stimulation [43]. CD161+ T cells were present in MSG tissue of pSS patients with a focus score ⩾1 and a considerable part of these cells (∼40%) co-expressed HLA-DR, indicating an activated phenotype [42]. Whether these local CD161+ T cells produce IL-17 and/or IFN-γ is not known. Another subset of Th17-like cells, i.e. IL-17-producing CD4−CD8− ‘double negative’ T cells, was also expanded in peripheral blood and MSG tissue of pSS patients [44]. These cells were mostly unconventional TCRγδ+ T cells, which suggests that activation occurs in an MHC-independent manner. The presence of various types of IL-17-producing cells in the glandular tissue may contribute to local inflammation, likely via the pro-inflammatory effects of IL-17 on epithelial cells (e.g. induction of MMP secretion, dysregulation of tight junction proteins) and support of ectopic lymphoid tissue formation (reviewed by Verstappen et al. [32]). However, studying the contribution of Th17 cells to pSS pathogenesis in humans is complicated by their plasticity. Th17 cells may readily develop into various subsets, including Th1 cells and peripherally induced Treg cells. We have previously suggested that plasticity towards Th17.1 cells, co-expressing IL-17/IFN-γ (and CCR6/CXCR3), may enhance their pathogenicity [32]. In conclusion, the pathogenic role of IL-17-producing cells observed in mouse models of SS has only been partly confirmed in human pSS and needs further investigation.
Treg cells
Conflicting data exist about the involvement of Treg cells in pSS [39, 45–47]. Because the developmental pathways of Th17 and Treg cells are reciprocal, increased frequencies of Th17 cells are often accompanied by reduced frequencies of Treg cells. This Th17/Treg balance seems to be disturbed in several autoimmune conditions [48]. However, such an imbalance is not evident in pSS patients. The discrepancy between various studies on the numbers and frequencies of Treg cells may be partly explained by their definition. Not all studies discriminate between CD45RA+naïve (thymus-derived) Treg cells and CD45RA− memory Treg cells, which comprise mostly peripherally derived Treg cells. Treg cells can be adequately identified by high expression levels of the IL-2 receptor alpha chain (CD25) and the transcription factor FoxP3 [49]. FoxP3 expression is highly associated with suppressor activity [50]. CD25 expression alone has been used in many studies to identify Treg cells in pSS patients, but CD25 can be upregulated by all CD4+ T cells upon activation. In addition to CD25 and FoxP3, Treg cells can express chemokine receptors that may overlap with effector subsets. CXCR5-expressing Treg cells, for example, are considered as regulatory counterparts of Tfh cells (both subsets will be discussed further below).
Despite the existence of conflicting data, a recent study showed that in various systemic autoimmune diseases, including pSS, frequencies of activated memory Treg cells were increased while frequencies of naïve Treg cells were unchanged [51]. We also found that the frequencies of memory Treg cells were increased in pSS patients compared with HCs [3]. In this cohort, most pSS patients had moderate–high systemic disease activity (ESSDAI > 5). In contrast, in our inception cohort, with shorter disease duration and on average lower disease activity scores, we did not find a significant change in memory Treg cell frequencies in pSS compared with non-SS sicca patients (unpublished data). These data suggest that memory Treg cell frequencies are related to disease activity, possibly as a consequence of excessive T cell activation in patients with more severe disease. Correspondingly, higher frequencies of activated memory Treg cells were present in IFN-positive pSS patients compared with IFN-negative patients and HCs and these IFN-positive patients exhibited significantly higher ESSDAI scores than IFN-negative patients [52].
FoxP3-expressing cells were also studied in MSG tissue of pSS patients by immunohistochemistry. The frequency of FoxP3+ cells correlated positively with the biopsy focus score [37, 53]. Whether Treg cells in salivary glands of pSS patients exhibit full suppressive capacity is unknown. Two studies investigated the suppressive capacity of CD4+CD25high T cells in blood from pSS patients, but with conflicting results [45, 54]. In conclusion, the frequencies of (memory) Treg cells are increased in the blood and tissue of pSS patients, in particular in patients with high disease activity, but the functional capacity of these cells in pSS remains ambiguous.
Tfh cells and follicular regulatory T (Tfr) cells
Although the necessity of T cell help for antibody responses was described decades ago, the recognition of a dedicated subset of B cell helper T cells (Tfh cells) followed much later. First, the chemokine receptor CXCR5, promoting migration to B cell follicles, was linked to Tfh cells [55]. Subsequently it was revealed that Tfh cell differentiation is driven by the transcription factor Bcl-6 and that activated Tfh cells express high levels of Inducible T-cell COStimulator (ICOS) and PD-1 [15]. Tfh cells facilitate T cell–dependent B cell responses, mainly by secretion of IL-21. This cytokine is a key driver of B cell activation and differentiation towards plasma cells [56]. Increased frequencies of Tfh cells have been associated with several B cell–mediated autoimmune diseases [57]. Also in pSS, frequencies of Tfh cells are increased in blood and glandular tissue [58–61]. We found in separate cohorts that the frequencies of circulating Tfh (cTfh) cells, defined as CD4+CD45RA−CXCR5+PD-1+ cells (Fig. 2), were increased in pSS patients compared with HCs [3, 23]. This increase was already present at the time of diagnosis and the frequencies of activated Tfh cells correlated positively with ESSDAI scores [62, 63].
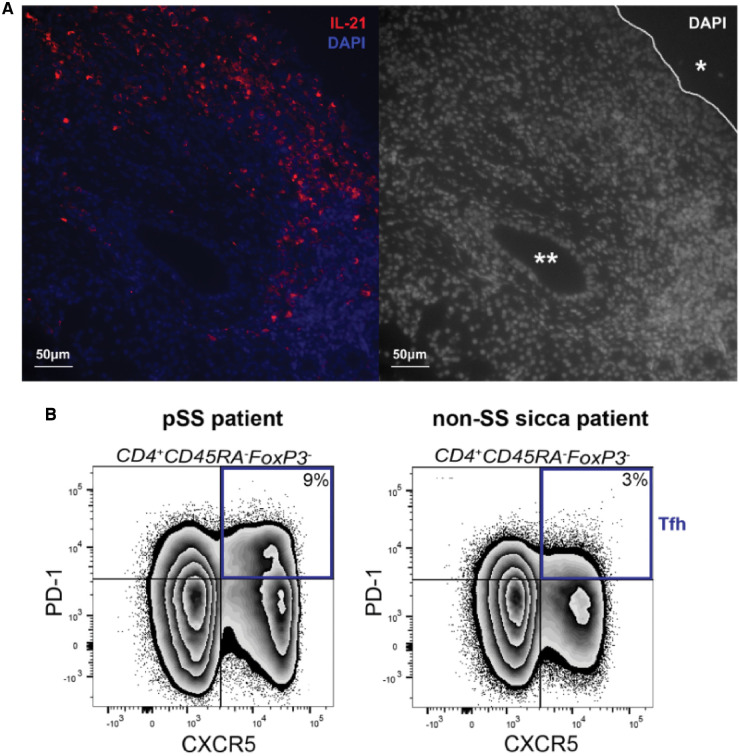
Fig. 2.
Characteristic features of pSS in the tissue and blood involve Tfh cells and IL-21
(A) Immunofluorescent staining of IL-21 protein in inflamed parotid gland tissue of a pSS patient. 4′,6-diamidino-2-phenylindole was used to image the nuclei. *Excretory duct. **Striated duct. (B) A representative example of a flow cytometric analysis of circulating T cells illustrates the increase in Tfh cells, defined as CD4+CD45RA−FoxP3− CXCR5+PD-1+ cells, in pSS patients compared with non-SS sicca patients.
Identification of Tfh cells within glandular tissue is more complicated. Detection by immunohistochemistry using CXCR5 expression is impeded because of the abundance of B cells that also express this receptor. Quantification of these cells by flow cytometry is hampered by the fact that when biopsies are processed into cell suspensions using enzymatic digestion, CXCR5 expression is lost. Also Bcl-6, although essential for Tfh cell induction, is not suitable for Tfh cell identification in lymphoid tissues [64], because Bcl-6 is probably downregulated in human Tfh cells after antigen exposure [65]. A recent study that analysed MSG cell suspensions by flow cytometry therefore defined Tfh cells as PD-1+ICOS+ cells. Their results indicate that this phenotype represents ∼9% of the total CD4+ T cells [63]. The presence of glandular Tfh cells is further supported by the significant amount of IL-21 protein and mRNA in the salivary glands of pSS patients (Fig. 1) (reviewed by Kwok et al. [6]), although this cytokine can also be produced by other T cell subsets, such as Th17 cells and peripheral helper T cells. Peripheral helper T cells (CXCR5−PD-1high) express Tfh cell–related factors, including IL-21, CXCL13 and ICOS, but lack CXCR5 expression. The frequencies of peripheral helper T cells were increased in blood from pSS patients compared with HCs [3, 66, 67].
Whether glandular Tfh cells are formed locally or whether these cells differentiate in secondary lymphoid tissues and subsequently migrate to inflamed glandular tissues is unknown, but most likely both routes are active. An essential cytokine for Tfh cell differentiation is IL-6, which is elevated in the blood and salivary gland tissue of pSS patients [26, 68, 69]. Importantly, in vitro experiments with SGEC lines derived from pSS patients showed that epithelial cells can promote differentiation of CD4+naive T cells into Tfh cells via upregulation of ICOS-L and IL-6 [70], supporting the possibility of local formation in the salivary glands. At the same time, a relatively large fraction of the circulating Tfh cells in pSS patients expresses CXCR3 (unpublished observations), which enables migration to the inflamed salivary glands where CXCL10 is produced [25].
In addition to Tfh cells, their regulatory counterparts, i.e. Tfr cells, have been identified on the basis of their simultaneous expression of FoxP3 and CXCR5 [71]. These cells are able to control Tfh cell proliferation and B cell activation in secondary (and probably also tertiary) lymphoid tissues (reviewed by Sage and Sharpe [72]). Although Tfr cells, similar to Tfh cells, mainly exert their functions within lymphoid tissue, low numbers of circulating Tfr (cTfr) cells can also be found in blood. Two studies have shown that not only cTfh cells but also cTfr cells are enriched in blood from pSS patients [62, 63]. These cTfr cells were even more increased than cTfh cells, resulting in a significantly higher cTfr:cTfh ratio. Tfr cells were also present within the MSG tissue in majority of the pSS patients [63]. In human lymph nodes, Tfr cells are mainly located at the border between the T cell zone and the B cell follicle and are rarely found within the GC [64]. A similar exclusion of Tfr cells was seen in ectopic GCs in the salivary gland tissue of pSS patients [73]. By their positioning at the T cell/B cell border, Tfr cells can control the input and/or output of the GC reaction by interacting with B cells and Tfh cells trafficking into and out of the GC. Fonseca et al. [74] showed that most cTfr cells in the peripheral blood of HCs have a naïve-like phenotype and lack B cell suppressive capacity. However, cTfr cells were absent from the thymus and generated in peripheral lymphoid tissues. Their increased frequency in pSS patients may reflect ongoing T cell differentiation in secondary lymphoid organs.
A different ‘Tfh-like’ subset, defined by CCR9 expression, was also increased in blood from pSS patients, and small numbers of these cells were found in the MSG tissue of these patients [75, 76]. CCR9+ T cells share phenotypic and functional features with Tfh cells, and in HCs they typically exert their function at mucosal sites [76]. CCR9+ T cells have heterogeneous effector functions in vitro, and both cells from pSS patients and HCs are able to secrete various cytokines, including IL-21, and induce IgG production by B cells [75]. CCR9+ T cells can migrate towards the chemokine CCL25, which is produced in inflamed salivary gland tissue of pSS patients. CCL25 levels increased with disease severity and an influx of CCR9+ T cells may contribute to local B cell activation [75]. Although Tfh cells and CCR9+ T cells share the capacity to produce IL-21, the numbers of CCR9+ T cells in the blood and glandular tissue of pSS patients are essentially lower than Tfh cells and their relative contribution to humoral immune activation in addition to Tfh cells remains to be established.
Together, the available evidence shows that different CD4+ T cell subsets with B helper capacity are enriched in the blood and salivary gland tissue of pSS patients, supporting B cell hyperactivity. This B cell hyperactivity may contribute to the disease process and disease activity by autoantibody formation and pro-inflammatory cytokine production.
TCR specificities in pSS patients
To date, it is not known whether infiltrated (effector) T cells recognize autoantigens, salivary gland–specific proteins, microbial peptides or even other targets within the inflamed glandular tissue. T cells can be activated locally by professional antigen-presenting cells, but also by SGECs. In the inflamed glandular lesions of pSS patients, SGECs aberrantly express HLA-DR and B7 (CD80/CD86) costimulatory molecules, particularly in response to IFN-γ [77, 78]. Single-cell analysis of glandular T cells showed that TCR sequence diversity in the salivary gland was reduced and that there were more clonal expansions in the salivary glands of pSS patients compared with blood [79]. A more restricted local TCR repertoire in pSS was also observed by single-cell analysis of Th1 and Th17 cells isolated from salivary gland tissues of pSS patients compared with non-SS sicca patients [80]. In addition, Joachims et al. [79] showed that expanded clones of memory CD4+ T cells in the salivary glands displayed sequence similarity both within expanded clones of the same individual and among individual patients, indicating local shared antigen recognition. They also observed that an increased frequency of clonal expansions within the glands was correlated with decreased unstimulated salivary flow and increased salivary gland fibrosis. Based on these findings the authors hypothesized that damage to the salivary glands may depend on the expansion of self-reactive T cells that recognize exocrine gland–specific antigens. This damage is likely mediated by cytokine production, e.g. IFN-γ. This hypothesis is supported by an experimental mouse model in which mice were immunized with M3 muscarinic acetylcholine receptor (M3R) peptides to induce SS. In this model, M3R-specific T cells produce large amounts of IFN-γ and IL-17 [81]. When M3R-immunized mice were treated with an antagonistic altered M3R peptide ligand (that harbours an amino acid substitution at the TCR contact site), anergy of CD4+ M3R-reactive T cells was induced and sialoadenitis was suppressed [82]. Thus, at least in an experimental model, recognition of local antigen by CD4+ T cells may result in T cell expansion, pro-inflammatory cytokine production and consequently gland dysfunction. Together, the available evidence suggests that at least a proportion of CD4+ T cells expand locally after antigen recognition in the salivary glands and these antigens may be shared between individuals. The dominant antigens that are recognized remain to be elucidated.
Involvement of CD8+ T cells in the pathogenesis of pSS
Although the majority of T cells within the glandular infiltrates of pSS patients are CD4+ cells, CD8+ T cells are also present. Part of these CD8+ T cells show an activated phenotype, as reflected in higher expression levels of HLA-DR. Increased proportions of HLA-DR+ T cells were associated with higher disease severity [18]. Also in the blood of anti-SSA+ pSS patients, increased HLA-DR expression by both CD4+ and CD8+ T cells was observed and the frequencies of HLA-DR-expressing activated CD4+ and CD8+ T cells in blood correlated with ESSDAI scores [18]. Furthermore, the proportion of activated CD8+ T cells in blood was associated with a multi-omic-based disease signature of pSS, which was based on whole blood transcriptomes, serum proteomes and peripheral immunophenotyping [83]. The expression of CXCR3 by activated CD8+ T cells in pSS patients may be important for their migration to the inflamed salivary glands. Indeed, in mice it was shown that after viral infection, recruitment of activated CD8+ T cells to salivary gland tissue was dependent on CXCR3 [84]. We speculate that chronic antigen stimulation and systemic inflammation, reflected as higher ESSDAI scores, results in the activation of CD8+ T cells in secondary lymphoid organs, CXCR3 upregulation and consequent migration to the salivary glands. Whether CD8+ T cells, in turn, contribute to glandular dysfunction or systemic disease activity is unknown.
T cell–targeting treatment of pSS patients
As indicated previously, CD4+ T cell activation is needed for the establishment of B cell (hyper)activation in pSS. Restriction of T cell–dependent B cell hyperactivity might therefore be an important target for the treatment of pSS patients. Abatacept is a biologic DMARD that binds to CD80/86 on antigen-presenting cells (including B cells). Consequently, it impairs CD28-mediated T cell activation. The first open-label study on the effects of abatacept in pSS showed that blood CD4+ T cell numbers, adjusted for disease duration, increased following treatment. This partial recovery of lymphopenia may be clinically beneficial, as CD4+ T cell lymphopenia is associated with systemic disease activity and lymphomagenesis [17, 18, 85]. Abatacept treatment also reduced Treg cell numbers in MSG tissue, along with an increase in stimulated saliva production, adjusted for disease duration [86]. A second open-label study showed that saliva production rates stabilized over the treatment period (24 weeks). This second study also found that abatacept treatment significantly improved systemic disease activity scores, as measured by ESSDAI [87]. Additionally, we showed that abatacept selectively reduced the percentages and numbers of cTfh cells and memory Treg cells to levels seen in HCs [3]. Furthermore, abatacept treatment resulted in decreased ICOS expression by the remaining cTfh cells, which correlated significantly with the reduction in ESSDAI scores [3]. In RA patients, the frequency of cTfh cells at baseline was an independent predictor of response to abatacept [88]. In pSS patients, treatment with abatacept had not only significant effects on cTfh cells, but also on B cell activity, reflected in decreased serum autoantibody levels, frequencies of circulating plasmablasts and protein levels of Bruton’s tyrosine kinase in B cells [3, 89]. The effects of abatacept on B cell activity in pSS provide strong evidence that T cells and B cells act in a positive feedback loop. Consistent with this notion, B cell depletion therapy with rituximab had significant effects on the CD4+ T cell compartment in pSS patients [23, 90]. In particular, levels of cTfh cells and Th17 cells were reduced by rituximab, and this reduction in cTfh cells was associated with the decrease in ESSDAI scores over time [23].
In contrast to the targeted biologic DMARDs, conventional DMARDs (cDMARDs) often have broad immunosuppressive effects. Several cDMARDs, in particular CSA and LEF, exert inhibitory effects on T cell activation and proliferation (reviewed by van der Heijden et al. [91]). Although topical ophthalmic use of CSA for dry eye disease, associated with pSS, is supported by the literature (reviewed by Ramos-Casals et al. [92]), evidence of the efficacy of systemic CSA in pSS patients is lacking. There is evidence that LEF may be effective in pSS [93], and the combined efficacy of LEF and HCQ is currently under investigation.
In addition to abatacept and LEF, several other immunomodulatory treatments that target T cells directly or indirectly are now under investigation in pSS. Recently a clinical trial with low-dose IL-2 therapy in 190 pSS patients was completed. The rationale for such an approach is to restore the balance between effector T cells and Treg cells. Indeed, the number of Treg cells in the blood increased significantly after treatment, but clinical efficacy was lacking [39]. An important limitation of this study is that 89% of patients were concomitantly treated with immunosuppressants. Better insight into the role of Treg cells in pSS is necessary to support the use of Treg-targeted treatment in this disease. Other biologic/synthetic DMARDs that are currently under investigation and may affect T cell activation include anti-IL-6R treatment with tocilizumab, anti-CD40 treatment with CFZ533, anti-ICOSL treatment with AMG557 and JAK1 inhibition with filgotinib. The clinical efficacy as well as the effects of these treatments on T (and B) cells in pSS patients are eagerly awaited.
Conclusion
Current evidence suggests that CD4+ T cells, and perhaps also CD8+ T cells, can contribute significantly to local and systemic inflammation in pSS (Table 1). In particular, T cell subsets that support B cell function, e.g. Tfh and Tfh-like cells, appear to play a major role in pSS, either in the glandular tissue itself, at distinct inflamed sites or in secondary lymphoid organs. We presume that interruption of T cell–B cell interaction is crucial for successful treatment of this disease. If T cell activation can be impaired by treatment, preferably early in the course of disease, excessive B cell activation and damage to glandular tissue by B and T cell–derived pro-inflammatory cytokines may be attenuated.
Table 1.
Key findings describing changes in the CD4+ T cell compartment of patients with pSS
| Finding | Reference |
|---|---|
| Blood | |
| CD4+ T cell lymphopenia is a predictive factor of lymphoma development and is associated with higher systemic disease activity and with increased numbers of lymphocytes in MSGs of pSS patients. | [17, 18, 85] |
| Different subtypes of IL-17-producing CD4+ T cells are enriched in peripheral blood from at least a subgroup of pSS patients. | [3, 23, 38, 42] |
| Frequencies of memory Treg cells are increased in peripheral blood from pSS patients, at least in patients with moderate–high disease activity. | [3, 51, 52] |
| Frequencies of circulating Tfh cells are increased in pSS patients compared with non-SS sicca controls and healthy individuals. Frequencies of activated cTfh cells (CD4+CD45RA−CXCR5+PD-1+ICOS+) correlate with systemic disease activity. | [3, 23, 62, 63] |
| Circulating Tfr cells are enriched in peripheral blood from pSS patients, resulting in a higher cTfr:cTfh ratio in pSS patients compared with healthy individuals. | [62, 63] |
| The proportion of CCR9+ ‘Tfh-like’ cells is increased in peripheral blood from pSS patients compared with healthy individuals. | [75] |
| Tissue | |
| IFN-γ-producing CD4+ T cells (Th1 cells) are present within lymphocytic infiltrates and IFN-γ (type II IFN) activity is associated with the degree of CD45+ infiltration in MSGs of pSS patients. | [21, 24] |
| IL-17 protein and mRNA is increased in MSG tissue of pSS patients compared with non-SS sicca controls. | [35–37] |
| The frequency of FoxP3+ cells in MSGs correlates positively with the biopsy focus score. | [37, 53] |
| Tfh-like cells (CD4+PD-1+ICOS+) make up a significant part of the T cell infiltrate in MSGs of pSS patients and likely form a major source of IL-21. | [63] |
| The TCR repertoire of glandular CD4+ T cells indicates local antigen recognition (and expansion) by these cells. | [79] |
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: F.K. and H.B. have received unrestricted research grants from Bristol-Myers Squibb. F.K. has received consulting fees from Bristol‐Myers Squibb (<$10 000). H.B. has received consulting fees and/or honoraria from Bristol‐Myers Squibb (<$10 000). The other authors have declared no conflicts of interest.
References
- 1.Brito-Zerón P, Baldini C, Bootsma H. et al. Sjögren syndrome. Nat Rev Dis Prim 2016;2:16047. [DOI] [PubMed] [Google Scholar]
- 2.Kroese FGM, Abdulahad WH, Haacke E. et al. B-cell hyperactivity in primary Sjögren’s syndrome. Expert Rev Clin Immunol 2014;10:483–99. [DOI] [PubMed] [Google Scholar]
- 3.Verstappen GM, Meiners PM, Corneth OBJ. et al. Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjögren’s syndrome. Arthritis Rheumatol 2017;69:1850–61. [DOI] [PubMed] [Google Scholar]
- 4.Corneth OBJ, de Bruijn MJW, Rip J. et al. Enhanced expression of Bruton’s tyrosine kinase in B cells drives systemic autoimmunity by disrupting T cell homeostasis. J Immunol 2016;197:58–67. [DOI] [PubMed] [Google Scholar]
- 5.Roescher N, Tak PP, Illei GG.. Cytokines in Sjogren’s syndrome. Oral Dis 2009;15:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwok SK, Lee J, Yu D. et al. A pathogenetic role for IL-21 in primary Sjogren syndrome. Nat Rev 2015;11:368–74. [DOI] [PubMed] [Google Scholar]
- 7.Voulgarelis M, Tzioufas AG.. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nat Rev 2010;6:529–37. [DOI] [PubMed] [Google Scholar]
- 8.Skopouli FN, Fox PC, Galanopoulou V. et al. T cell subpopulations in the labial minor salivary gland histopathologic lesion of Sjögren’s syndrome. J Rheumatol 1991;18:210–4. [PubMed] [Google Scholar]
- 9.Lessard CJ, Li H, Adrianto I. et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nat Genet 2013;45:1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottenberg J-E, Busson M, Loiseau P. et al. In primary Sjögren’s syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum 2003;48:2240–5. [DOI] [PubMed] [Google Scholar]
- 11.Theander E, Jonsson R, Sjöström B. et al. Prediction of Sjögren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol 2015;67:2427–36. [DOI] [PubMed] [Google Scholar]
- 12.Stathopoulou EA, Routsias JG, Stea EA. et al. Cross-reaction between antibodies to the major epitope of Ro60 kD autoantigen and a homologous peptide of Coxsackie virus 2B protein. Clin Exp Immunol 2005;141:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szymula A, Rosenthal J, Szczerba BM. et al. T cell epitope mimicry between Sjögren’s syndrome antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol 2014;152:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel DD, Kuchroo VK.. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity 2015;43:1040–51. [DOI] [PubMed] [Google Scholar]
- 15.Vinuesa CG, Linterman MA, Yu D. et al. Follicular helper T cells. Annu Rev Immunol 2016;34:335–68. [DOI] [PubMed] [Google Scholar]
- 16.Mandl T, Bredberg A, Jacobsson LTH. et al. CD4+ T-lymphocytopenia—a frequent finding in anti-SSA antibody seropositive patients with primary Sjögren’s syndrome. J Rheumatol 2004;31:726–8. [PubMed] [Google Scholar]
- 17.Nocturne G, Mariette X.. Sjogren syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol 2015;168:317–27. [DOI] [PubMed] [Google Scholar]
- 18.Mingueneau M, Boudaoud S, Haskett S. et al. Cytometry by time-of-flight immunophenotyping identifies a blood Sjögren’s signature correlating with disease activity and glandular inflammation. J Allergy Clin Immunol 2016;137:1809–21.e12. [DOI] [PubMed] [Google Scholar]
- 19.Seror R, Ravaud P, Bowman SJ. et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis 2010;69:1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Boer RJ, Perelson AS.. Quantifying T lymphocyte turnover. J Theor Biol 2013;327:45–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Woerkom JM, Kruize AA, Wenting-van Wijk MJG. et al. Salivary gland and peripheral blood T helper 1 and 2 cell activity in Sjögren’s syndrome compared with non-Sjögren’s sicca syndrome. Ann Rheum Dis 2005;64:1474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikker A, Moret FM, Kruize AA. et al. IL-7 drives Th1 and Th17 cytokine production in patients with primary SS despite an increase in CD4 T cells lacking the IL-7Rα. Rheumatology 2012;51:996–1005. [DOI] [PubMed] [Google Scholar]
- 23.Verstappen GM, Kroese FGM, Meiners PM. et al. B cell depletion therapy normalizes circulating follicular TH cells in primary Sjögren syndrome. J Rheumatol 2017;44:49–58. [DOI] [PubMed] [Google Scholar]
- 24.Hall JC, Baer AN, Shah AA. et al. Molecular subsetting of interferon pathways in Sjögren’s syndrome. Arthritis Rheumatol 2015;67:2437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa N, Ping L, Zhenjun L. et al. Involvement of the interferon-γ-induced T cell-attracting chemokines, interferon-γ-inducible 10-kd protein (CXCL10) and monokine induced by interferon-γ (CXCL9), in the salivary gland lesions of patients with Sjögren’s syndrome. Arthritis Rheum 2002;46:2730–41. [DOI] [PubMed] [Google Scholar]
- 26.Ohyama Y, Nakamura S, Matsuzaki G. et al. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum 1996;39:1376–84. [DOI] [PubMed] [Google Scholar]
- 27.Maehara T, Moriyama M, Hayashida J-N. et al. Selective localization of T helper subsets in labial salivary glands from primary Sjögren’s syndrome patients. Clin Exp Immunol 2012;169:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King IL, Mohrs M.. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med 2009;206:1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh SV, Hopkins AM, Nusrat A.. Modulation of tight junction structure and function by cytokines. Adv Drug Deliv Rev 2000;41:303–13. [DOI] [PubMed] [Google Scholar]
- 30.Ewert P, Aguilera S, Alliende C. et al. Disruption of tight junction structure in salivary glands from Sjögren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum 2010;62:1280–9. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Helu RF, Dimitriou ID, Kapsogeorgou EK. et al. Induction of salivary gland epithelial cell injury in Sjogren’s syndrome: in vitro assessment of T cell-derived cytokines and Fas protein expression. J Autoimmun 2001;17:141–53. [DOI] [PubMed] [Google Scholar]
- 32.Verstappen GM, Corneth OBJ, Bootsma H. et al. Th17 cells in primary Sjögren’s syndrome: pathogenicity and plasticity. J Autoimmun 2018;87:16–25. [DOI] [PubMed] [Google Scholar]
- 33.Voigt A, Esfandiary L, Wanchoo A. et al. Sexual dimorphic function of IL-17 in salivary gland dysfunction of the C57BL/6.NOD-Aec1Aec2 model of Sjögren’s syndrome. Sci Rep 2016;6:38717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin X, Rui K, Deng J. et al. Th17 cells play a critical role in the development of experimental Sjogren’s syndrome. Ann Rheum Dis 2015;74:1302–10. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen CQ, Hu MH, Li Y. et al. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: findings in humans and mice. Arthritis Rheum 2008;58:734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai A, Sugawara Y, Kuroishi T. et al. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjogren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol 2008;181:2898–906. [DOI] [PubMed] [Google Scholar]
- 37.Katsifis GE, Rekka S, Moutsopoulos NM. et al. Systemic and local interleukin-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis. Am J Pathol 2009;175:1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng J, Yu S, Zhao H. et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol 2017;18:800–12. [DOI] [PubMed] [Google Scholar]
- 39.Miao M, Hao Z, Guo Y. et al. Short-term and low-dose IL-2 therapy restores the Th17/Treg balance in the peripheral blood of patients with primary Sjögren’s syndrome. Ann Rheum Dis 2018;77:1838–40. [DOI] [PubMed] [Google Scholar]
- 40.Cosmi L, De Palma R, Santarlasci V. et al. Human interleukin 17–producing cells originate from a CD161+ CD4+ T cell precursor. J Exp Med 2008;205:1903–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maggi L, Santarlasci V, Capone M. et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol 2010;40:2174–81. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L, Nocturne G, Haskett S. et al. Clinical relevance of RORγ positive and negative subsets of CD161+CD4+ T cells in primary Sjögren’s syndrome. Rheumatology 2017;56:303–12. [DOI] [PubMed] [Google Scholar]
- 43.Fergusson JR, Smith KE, Fleming VM. et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep 2014;9:1075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alunno A, Bistoni O, Bartoloni E. et al. IL-17-producing CD4-CD8- T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren’s syndrome. Ann Rheum Dis 2013;72:286–92. [DOI] [PubMed] [Google Scholar]
- 45.Gottenberg J, Lavie F, Abbed K. et al. CD4 CD25 regulatory T cells are not impaired in patients with primary Sjögren’s syndrome. J Autoimmun 2005;24:235–42. [DOI] [PubMed] [Google Scholar]
- 46.Liu M-F, Lin L-H, Weng C-T. et al. Decreased CD4+CD25+bright T cells in peripheral blood of patients with primary Sjögren’s syndrome. Lupus 2008;17:34–9. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Li X, Qian L. et al. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjögren’s syndrome. J Rheumatol 2007;34:2438–45. [PubMed] [Google Scholar]
- 48.Noack M, Miossec P.. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 2014;13:668–77. [DOI] [PubMed] [Google Scholar]
- 49.Miyara M, Yoshioka Y, Kitoh A. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899–911. [DOI] [PubMed] [Google Scholar]
- 50.Fontenot JD, Rasmussen JP, Williams LM. et al. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 2005;22:329–41. [DOI] [PubMed] [Google Scholar]
- 51.Miyara M, Chader D, Sage E. et al. Sialyl Lewis × (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci USA 2015;112:7225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maria NI, van Helden-Meeuwsen CG, Brkic Z. et al. Association of increased Treg cell levels with elevated indoleamine 2,3-dioxygenase activity and an imbalanced kynurenine pathway in interferon-positive primary Sjögren’s syndrome. Arthritis Rheumatol 2016;68:1688–99. [DOI] [PubMed] [Google Scholar]
- 53.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos NM. et al. Foxp3+ T-regulatory cells in Sjögren’s syndrome. Am J Pathol 2008;173:1389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szodoray P, Papp G, Horvath IF. et al. Cells with regulatory function of the innate and adaptive immune system in primary Sjögren’s syndrome. Clin Exp Immunol 2009;157:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bryant VL, Ma CS, Avery DT. et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol 2007;179:8180–90. [DOI] [PubMed] [Google Scholar]
- 56.Moens L, Tangye SG.. Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front Immunol 2014;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014;41:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simpson N, Gatenby PA, Wilson A. et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010;62:234–44. [DOI] [PubMed] [Google Scholar]
- 59.Szabo K, Papp G, Barath S. et al. Follicular helper T cells may play an important role in the severity of primary Sjogren’s syndrome. Clin Immunol 2013;147:95–104. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Y, Lutalo PM, Thomas JE. et al. Circulating T follicular helper cell and regulatory T cell frequencies are influenced by B cell depletion in patients with granulomatosis with polyangiitis. Rheumatology 2014;53:621–30. [DOI] [PubMed] [Google Scholar]
- 61.Brokstad KA, Fredriksen M, Zhou F. et al. T follicular-like helper cells in the peripheral blood of patients with primary Sjögren’s syndrome. Scand J Immunol 2018;88:e12679. [DOI] [PubMed] [Google Scholar]
- 62.Verstappen GM, Nakshbandi U, Mossel E. et al. Is the T follicular regulatory/T follicular helper cell ratio in blood a biomarker for ectopic lymphoid structure formation in Sjögren’s syndrome? Arthritis Rheumatol 2018;70:1354–5. [DOI] [PubMed] [Google Scholar]
- 63.Fonseca VR, Romão VC, Agua-Doce A. et al. Blood T follicular regulatory cells/T follicular helper cells ratio marks ectopic lymphoid structure formation and PD-1+ ICOS+ T follicular helper cells indicate disease activity in primary Sjögren’s syndrome. Arthritis Rheumatol 2018;70:774–84. [DOI] [PubMed] [Google Scholar]
- 64.Sayin I, Radtke AJ, Vella LA. et al. Spatial distribution and function of T follicular regulatory cells in human lymph nodes. J Exp Med 2018;215:1531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitano M, Moriyama S, Ando Y. et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity 2011;34:961–72. [DOI] [PubMed] [Google Scholar]
- 66.Wei L, Laurence A, Elias KM. et al. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 2007;282:34605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao DA, Gurish MF, Marshall JL. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017;542:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollard RP, Abdulahad WH, Bootsma H. et al. Predominantly proinflammatory cytokines decrease after B cell depletion therapy in patients with primary Sjogren’s syndrome. Ann Rheum Dis 2013;72:2048–50. [DOI] [PubMed] [Google Scholar]
- 69.Boumba D, Skopouli FN, Moutsopoulos HM.. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren’s syndrome. Br J Rheumatol 1995;34:326–33. [DOI] [PubMed] [Google Scholar]
- 70.Gong Y-Z, Nititham J, Taylor K. et al. Differentiation of follicular helper T cells by salivary gland epithelial cells in primary Sjögren’s syndrome. J Autoimmun 2014;51:57–66. [DOI] [PubMed] [Google Scholar]
- 71.Linterman MA, Pierson W, Lee SK. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 2011;17:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sage PT, Sharpe AH.. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol 2015;36:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pontarini E, Murray Brown W, Croia C. et al. T follicular-helper cells (Tfh) enrichment and T follicular-regulatory cells (Tfr) exclusion from ectopic germinal centers in salivary glands of Sjogren’s syndrome patients. Arthritis Rheumatol 2017;69(Suppl 10):abstract 2706. [Google Scholar]
- 74.Fonseca VR, Agua-Doce A, Maceiras AR. et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci Immunol 2017;2:eaan1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blokland SLM, Hillen MR, Kruize AA. et al. Elevated CCL25 and CCR9-expressing T helper cells in salivary glands of primary Sjögren’s syndrome patients: potential new axis in lymphoid neogenesis. Arthritis Rheumatol 2017;69:2038–51. [DOI] [PubMed] [Google Scholar]
- 76.McGuire HM, Vogelzang A, Ma CS. et al. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity 2011;34:602–15. [DOI] [PubMed] [Google Scholar]
- 77.Moutsopoulos HM, Hooks JJ, Chan CC. et al. HLA-DR expression by labial minor salivary gland tissues in Sjögren’s syndrome. Ann Rheum Dis 1986;45:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manoussakis MN, Dimitriou ID, Kapsogeorgou EK. et al. Expression of B7 costimulatory molecules by salivary gland epithelial cells in patients with Sjögren’s syndrome. Arthritis Rheum 1999;42:229–39. [DOI] [PubMed] [Google Scholar]
- 79.Joachims ML, Leehan KM, Lawrence C. et al. Single-cell analysis of glandular T cell receptors in Sjögren’s syndrome. JCI Insight 2016;1:e85609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voigt A, Bohn K, Sukumaran S. et al. Unique glandular ex-vivo Th1 and Th17 receptor motifs in Sjögren’s syndrome patients using single-cell analysis. Clin Immunol 2018;192:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tahara M, Tsuboi H, Segawa S. et al. RORγt antagonist suppresses M3 muscarinic acetylcholine receptor-induced Sjögren’s syndrome-like sialadenitis. Clin Exp Immunol 2017;187:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asashima H, Tsuboi H, Takahashi H. et al. The anergy induction of M3 muscarinic acetylcholine receptor-reactive CD4+ T cells suppresses experimental sialadenitis-like Sjögren’s syndrome. Arthritis Rheumatol 2015;67:2213–25. [DOI] [PubMed] [Google Scholar]
- 83.Tasaki S, Suzuki K, Nishikawa A. et al. Multiomic disease signatures converge to cytotoxic CD8 T cells in primary Sjögren’s syndrome. Ann Rheum Dis 2017;76:1458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caldeira-Dantas S, Furmanak T, Smith C. et al. The chemokine receptor CXCR3 promotes CD8+ T cell accumulation in uninfected salivary glands but is not necessary after murine cytomegalovirus infection. J Immunol 2018;200:1133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramos-Casals M, Brito-Zeron P, Solans R. et al. Systemic involvement in primary Sjogren’s syndrome evaluated by the EULAR-SS disease activity index: analysis of 921 Spanish patients (GEAS-SS Registry). Rheumatology 2014;53:321–31. [DOI] [PubMed] [Google Scholar]
- 86.Adler S, Korner M, Forger F. et al. Evaluation of histologic, serologic, and clinical changes in response to abatacept treatment of primary Sjogren’s syndrome: a pilot study. Arthritis Care Res (Hoboken) 2013;65:1862–8. [DOI] [PubMed] [Google Scholar]
- 87.Meiners PM, Vissink A, Kroese FG. et al. Abatacept treatment reduces disease activity in early primary Sjogren’s syndrome (open-label proof of concept ASAP study). Ann Rheum Dis 2014;73:1393–6. [DOI] [PubMed] [Google Scholar]
- 88.Nakayamada S, Kubo S, Yoshikawa M. et al. Differential effects of biological DMARDs on peripheral immune cell phenotypes in patients with rheumatoid arthritis. Rheumatology 2018;57:164–74. [DOI] [PubMed] [Google Scholar]
- 89.Corneth OBJ, Verstappen GMP, Paulissen SMJ. et al. Enhanced Bruton’s tyrosine kinase activity in peripheral blood B lymphocytes of autoimmune disease patients. Arthritis Rheumatol 2017;69:1313–24. [DOI] [PubMed] [Google Scholar]
- 90.Ciccia F, Guggino G, Rizzo A. et al. Rituximab modulates IL-17 expression in the salivary glands of patients with primary Sjögren’s syndrome. Rheumatology 2014;53:1313–20. [DOI] [PubMed] [Google Scholar]
- 91.van der Heijden EHM, Kruize AA, Radstake TRDJ. et al. Optimizing conventional DMARD therapy for Sjögren’s syndrome. Autoimmun Rev 2018;17:480–92. [DOI] [PubMed] [Google Scholar]
- 92.Ramos-Casals M, Brito ZP, Siso-Almirall A. et al. Topical and systemic medications for the treatment of primary Sjogren’s syndrome. Nat Rev Rheumatol 2012;8:399–411. [DOI] [PubMed] [Google Scholar]
- 93.van Woerkom JM, Kruize AA, Geenen R. et al. Safety and efficacy of leflunomide in primary Sjogren’s syndrome: a phase II pilot study. Ann Rheum Dis 2007;66:1026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]