Abstract
Background
Globally, the number of infected women of childbearing age living with human immunodeficiency virus (HIV) and conceiving on antiretroviral therapy (ART) is increasing. Evidence of ART safety at conception and during pregnancy and adverse pregnancy outcomes remains conflicting. The Promoting Maternal and Infant Survival Everywhere (PROMISE) 1077 breastfeeding (BF) and formula feeding (FF) international multisite trials provide an opportunity to examine the impact of ART at conception on pregnancy outcomes with subsequent pregnancies.
Methods
The PROMISE 1077BF/1077FF trials were designed to address key questions in the management of HIV-infected women who did not meet clinical guidelines for ART treatment during the time of the trials. After the period of risk of mother-to-child transmission was over, women were randomized to either continue or discontinue ART. We compared subsequent pregnancy outcomes of nonbreastfeeding women randomized to continue ART following delivery, or breastfeeding women randomized to continue ART following breastfeeding cessation who conceived while on ART to women randomized to discontinue ART, who restarted ART after pregnancy was diagnosed.
Results
Pregnancy outcomes of 939 subsequent pregnancies of 826 mothers were recorded. The intention-to-treat analyses showed increased incidence of low birth weight (<2500 g) for women who conceived while on ART (relative risk, 2.65 [95% confidence interval {CI}, 1.20–5.81]), and also a higher risk of spontaneous abortion, stillbirth, or neonatal death (hazard ratio, 1.40 [95% CI, .99–1.98]) compared to women who restarted ART after they were found to be pregnant during trial follow-up.
Conclusions
We found an increased risk for adverse pregnancy outcomes in women conceiving on ART, emphasizing the need for improved obstetric and neonatal care for this group.
Clinical Trials Registration
Keywords: pregnancy outcomes, conceiving on ART, HIV
Globally more women living with HIV conceive on antiretroviral therapy (ART). This study reports an increased risk of low birth weight, spontaneous abortion, stillbirth, and neonatal death for women conceiving on ART compared to those not on ART at conception.
Universal antiretroviral therapy (ART) for all pregnant and breastfeeding women living with human immunodeficiency virus (HIV) was offered since 2012 (Option B+) and rolled out more broadly since 2014 based on World Health Organization (WHO) recommendations [1]. Increasingly, more HIV-infected women of childbearing age are conceiving while on ART [2]. Evidence on the risk for adverse pregnancy outcomes with ART use at conception and during pregnancy is conflicting [3–5]. A meta-analysis of 11 studies by Uthman et al demonstrated a significantly increased risk of preterm delivery (<37 weeks), very preterm delivery (<32 or 34 weeks), and low birth weight (LBW, <2500 g) when conceiving on ART compared to initiating ART during pregnancy [6]. The magnitude of the association of preterm delivery for women who conceived while on ART was stronger in low- and middle-income countries (LMICs) compared to high-income countries. Preterm infants in LMICs, in addition, have an increased mortality risk [7]. However, studies of ART at conception have been observational and are subject to bias. Further research is necessary to understand these risks and to identify safest maternal ART regimens for optimized pregnancy and infant outcomes.
Clinical trial data from the international multisite Promoting Maternal and Infant Survival Everywhere (PROMISE) trials provide an opportunity to study this question using robust data from varied settings. The PROMISE 1077 breastfeeding (BF) and 1077 formula feeding (FF) trials were designed to test the relative efficacy and safety of various proven antiretroviral (ARV) regimens for perinatal HIV prevention of HIV among women who did not meet in-country guidelines for treatment at the time of the trial. The PROMISE 1077 highly active antiretroviral therapy standard (HS) study, conducted in 8 countries, randomized 1653 asymptomatic nonbreastfeeding women living with HIV with CD4 count >400 cells/μL who started ART during pregnancy to continue or discontinue ART within 42 days after delivery [8]. Subsequent pregnancies occurred in 277 (17%) women [9]. Spontaneous abortions and stillbirths were significantly more common among women in the continue ART arm, compared to the discontinue arm. This study raises further questions around the risks of conception on ART.
The PROMISE 1077 BF and FF trials were conducted in 14 sites in 7 LMICs in India, southern Africa, and eastern Africa; and offer the opportunity to examine subsequent pregnancy outcomes in a larger cohort [10]. HIV-infected pregnant women with CD4 counts of at least 350 cells/μL were evaluated on ART perinatal HIV prevention regimens for maternal safety and efficacy in a randomized controlled trial. There were multiple comparisons at different time points: antenatal, postpartum, and postbreastfeeding, evaluating primary outcomes of safety and efficacy [10, 11]. This study evaluated subsequent pregnancy outcomes for PROMISE women who, after the period of risk of mother-to-child transmission was over, were randomized to either continue ART and were on ART at conception or discontinued ART and only restarted ART after they were found to be pregnant (ART reinitiation in pregnancy).
This secondary post hoc analysis evaluates rates of spontaneous abortion, stillbirth, LBW, and neonatal death by randomized arm among women with subsequent pregnancies. Associations of these outcomes with exposure to specific ART regimens were also determined. This PROMISE study is the largest cohort to be examined with longitudinal randomized ARV data available.
METHODS
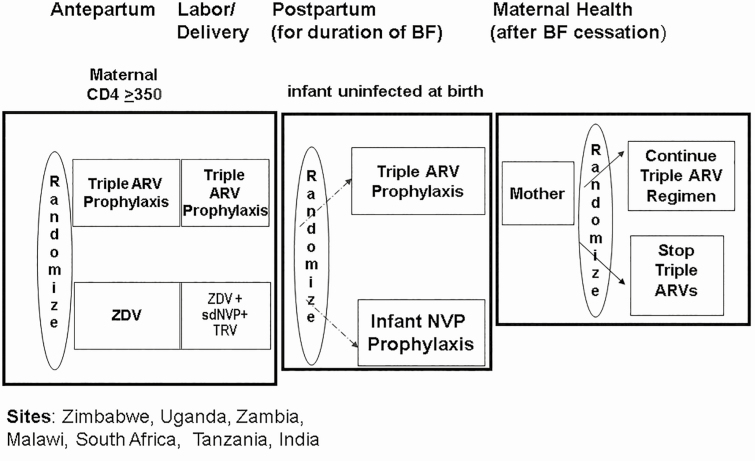
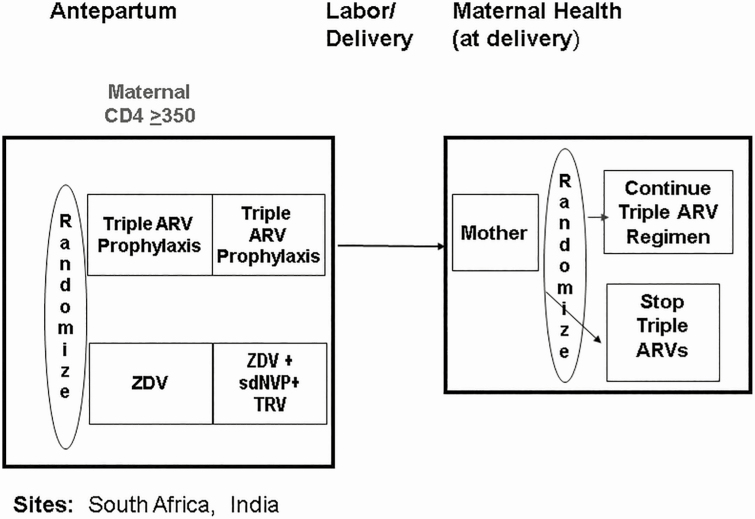
PROMISE 1077BF and 1077FF (Figures 1 and 2) was conducted at 14 sites in 7 countries (India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe). The study included open-label, parallel randomization components to address questions in the management of HIV-infected women with CD4 T-cell counts ≥350 cells/μL and who did not meet clinical guidelines for ART initiation at the time of the study while also evaluating infant ARV prophylaxis during breastfeeding [10]. The postpartum preferred study maternal ART regimen was tenofovir, emtricitabine or lamivudine, and lopinavir/ritonavir (LPV/r). Regimens not provided by the study were allowed if they met the definition for combined ART of at least 3 or more drugs from 2 or more ARV classes. The trial was performed in settings where breastfeeding was common, but allowed enrollment of both breastfeeding and formula-feeding mothers. The postpartum randomizations differed by breastfeeding status. Formula-feeding women were randomized to continue or discontinue ART at delivery. Breastfeeding women and their infants were randomized to maternal ART or daily infant nevirapine prophylaxis (no maternal ART) shortly after delivery. Another randomization at cessation of breastfeeding compared the effects on maternal health of continuing vs discontinuing ART. Follow up of all maternal participants continued until 96 weeks after the last delivery; infants were followed for 2 years. Maternal postpartum follow-up visits were around 1, 6, and 14 weeks after delivery and then every 12 weeks. Pregnancy tests were done when clinically indicated and for all women on efavirenz beginning around 14 weeks postpartum and subsequently every 12 weeks. Data presented include all women randomized postpartum.
Figure 1.
Overall design of the Promoting Maternal and Infant Survival Everywhere (PROMISE) 1077BF trial including the antepartum, postpartum and maternal health components with 3 randomizations (n = 3490). Sites: Zimbabwe, Uganda, Zambia, Malawi, South Africa, Tanzania, and India. Abbreviations: ARV, antiretroviral; BF, breastfeeding; NVP, nevirapine; sdNVP, single-dose nevirapine; TRV, Truvada; ZDV, zidovudine.
Figure 2.
Overall design of the Promoting Maternal and Infant Survival Everywhere PROMISE 1077FF trial including the antepartum and maternal health components with 2 randomizations (n = 284). Sites: South Africa and India. Abbreviations: ARV, antiretroviral; sdNVP, single-dose nevirapine; TRV, Truvada; ZDV, zidovudine.
Women who became pregnant during follow-up in the PROMISE study, including women with >1 subsequent pregnancy, remained in the study and the pregnancy outcomes of all pregnancies were recorded. Pregnancy outcomes included live births, live births followed by a neonatal death (≤28 days), ectopic pregnancies, induced and spontaneous abortions (<20 weeks), and stillbirths. Women receiving ART as part of the study continued to receive their study drugs, following additional informed consent. Women on an LPV/r regimen received a dose increase in the third trimester. Women not on a study ART regimen were treated according the local standard-of-care ART regimen. Data collected from women with subsequent pregnancies included demographic, clinical, and laboratory data. Summary data of women included age, gravidity, and parity at the time of the estimated conception date of the first subsequent pregnancy. Pregnancy data included pregnancy complications, birth weight, gestational age at delivery, and type of delivery. Gestational age was based on an ordered hierarchal approach using ultrasound, clinical examination, or last menstrual period date. Diagnosis of subsequent pregnancy was based on urine or blood test or clinical examination. Body mass index was calculated based on the last maternal height and weight measured before the conception date. WHO clinical stage at baseline was the last classification given to a mother at or before the estimated conception date, and CD4 and plasma HIV RNA levels were the last available values. History of alcohol or smoking as reported at PROMISE entry and the hepatitis B surface antigen result prior to the first randomization were recorded. Summary data of infants include birth weight, sex, congenital abnormalities, and Apgar scores. Poor pregnancy outcomes included spontaneous abortion, stillbirth, neonatal death, and LBW.
In mid-2015, a change in the PROMISE protocols occurred due to the results of the START study, which demonstrated a significant benefit to receiving ART for people with high CD4 counts [12]. On 7 July 2015, PROMISE sites were notified that all maternal PROMISE participants should be recommended to take ART, breaking the randomizations. Analyses done by randomization arm in this study are thus limited to conceptions before 7 July 2015.
Ethics Approval
The conduct of the study was approved by the respective local and collaborating institutional review boards at each site. Written informed consent was obtained from all participating women.
Statistical Analysis
Multiple imputation was used to include pregnancies in the analysis with known pregnancy outcomes but missing expected delivery date, gestational age, and last menstrual period, while also accounting for the uncertainty in the missing term lengths. Gestational age was used to estimate each pregnancy’s conception date, which determined the treatment designation in the analyses. The probability integral transform was used in the multiple imputation of missing conception dates.
Two types of analyses were conducted of pregnancy outcomes among women with repeat pregnancies: (1) by-arm analyses in which data were restricted to pregnancies before 7 July 2015; and (2) time-to-event analyses that included all observed subsequent pregnancies. Both types evaluated the risk of spontaneous abortion, stillbirth, and neonatal death while the by-arm analyses also separately evaluated the risk of LBW among live births. The first type of analysis used generalized estimating equations to account for multiple repeat pregnancies, and was conducted with 3 strategies: (1) intention-to-treat; (2) excluding crossovers (those whose treatment at the time of estimated conception differed from the treatment assigned at randomization); and (3) as treated (analysis based on recorded regimen at estimated conception). The second analysis used Cox proportional hazards regression, clustered for multiple repeat pregnancies, and adjusted for country and previous adverse pregnancy outcome on study. The Cox models used ART exposure as a time-varying covariate, and regimens were grouped in 2 different ways: (1) a simple indicator for any ARV (compared to no ARVs) at a given time and (2) categorized based on the regimens. All analyses were conducted using SAS version 9.4 software. Results were considered inconclusive if the 95% confidence interval (CI) included the null hypothesis of no difference.
RESULTS
The 1077BF and 1077FF trials in breastfeeding and formula-feeding settings began enrollment in April and May 2011, respectively. Baseline characteristics by randomization arm for mothers are shown in Table 1. The last column in Table 1 includes all women with subsequent pregnancies. ART regimens at conception were approximately 30.8% protease inhibitor (PI) based, 10.1% nonnucleoside reverse transcription inhibitor (NNRTI) based, and 0.4% nucleoside reverse transcription inhibitor (NRTI) only. The NNRTI-based regimen was primarily tenofovir, emtricitabine, or lamivudine, and efavirenz. Pregnancy outcomes by subsequent pregnancy number are shown in Table 2. In total, there were 939 subsequent pregnancies recorded. Gestational ages were unknown in 36%–64% of subsequent pregnancy outcomes that progressed to the second half of pregnancy. Therefore, a meaningful analysis with gestational age as an outcome was not possible, and the analyses focused on spontaneous abortions, stillbirths, neonatal deaths, and birth weight outcomes.
Table 1.
Baseline Characteristics
| By Arm Analysesa (Conceptions Prior to 7 July 2015 Only) | Time-to-Event Analysesb (All Conceptions) | ||||
|---|---|---|---|---|---|
| Randomized at Delivery | Randomized After Breastfeeding | ||||
| Characteristic | ART at Conception (n = 97) | ART Reinitiation in Pregnancy (n = 121) | ART at Conception (n = 41) | ART Reinitiation in Pregnancy (n = 41) | All Mothers (N = 760) |
| Age, y, at estimated conception | |||||
| No. | 96 | 121 | 41 | 41 | 755 |
| No. missing | 1 | 0 | 0 | 0 | 5 |
| Min–Max | 19–41 | 19–39 | 21–37 | 21–39 | 19–43 |
| Median (Q1–Q3) | 27 (24–31) | 28 (25–32) | 28 (25–32) | 25 (23–28) | 28 (24–31) |
| Country | |||||
| India | 2 (2) | 3 (2) | 3 (7) | 3 (7) | 39 (5) |
| Malawi | 35 (36) | 43 (36) | 15 (37) | 17 (41) | 240 (32) |
| South Africa | 26 (27) | 26 (21) | 9 (22) | 4 (10) | 188 (25) |
| Tanzania | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 10 (1) |
| Uganda | 21 (22) | 23 (19) | 8 (20) | 10 (24) | 135 (18) |
| Zambia | 0 (0) | 5 (4) | 0 (0) | 0 (0) | 15 (2) |
| Zimbabwe | 11 (11) | 20 (17) | 6 (15) | 7 (17) | 133 (18) |
| Race or ethnic group | |||||
| Black African | 95 (98) | 118 (98) | 38 (93) | 38 (93) | 719 (95) |
| Indian | 2 (2) | 3 (2) | 3 (7) | 3 (7) | 40 (5) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Time, mo, between first PROMISE pregnancy outcome date and subsequent conception | |||||
| No. | 96 | 121 | 41 | 41 | 755 |
| No. missing | 1 | 0 | 0 | 0 | 5 |
| Min–Max | 1–46 | 2–45 | 6–44 | 4–39 | 1–57 |
| Median (Q1–Q3) | 16 (11–25) | 18 (11–24) | 22 (16–30) | 23 (16–28) | 22 (13–34) |
| BMI, kg/m2, at or before estimated conception | |||||
| No. | 95 | 121 | 41 | 41 | 753 |
| No. missing | 2 | 0 | 0 | 0 | 7 |
| Min–Max | 18–49 | 19–37 | 19–37 | 16–41 | 16–49 |
| Median (Q1–Q3) | 26 (23–29) | 26 (23–29) | 26 (22–28) | 25 (23–27) | 26 (23–29) |
| WHO stage at or before estimated conception | |||||
| Clinical stage I | 84 (88) | 112 (93) | 35 (85) | 36 (88) | 683 (90) |
| Clinical stage II | 11 (11) | 8 (7) | 6 (15) | 4 (10) | 61 (8) |
| Clinical stage III | 1 (1) | 1 (1) | 0 (0) | 1 (2) | 9 (1) |
| Clinical stage IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0) |
| CD4 count, cells/μL at or before estimated conception | |||||
| No. | 96 | 121 | 41 | 41 | 755 |
| Min–Max | 350–1545 | 306–1568 | 531–1545 | 350–1297 | 216–1908 |
| Median (Q1–Q3) | 818 (648–952) | 600 (485–729) | 771 (654–952) | 710 (519–843) | 692 (533–885) |
| Plasma HIV RNA, copies/mL, at or before estimated conception | |||||
| No. | 96 | 121 | 41 | 41 | 755 |
| Min–Max | 20–89 755 | 20–975 501 | 20–27 372 | 30–203 421 | 20–975 501 |
| Median (Q1–Q3) | 40 (40–1052) | 3726 (473–13 113) | 40 (40–40) | 565 (200–9498) | 200 (40–5277) |
| <400 | 62 (65) | 27 (22) | 37 (90) | 15 (37) | 413 (55) |
| History of alcohol or smoking at PROMISE entry | |||||
| Yes, one | 21(22) | 16 (13) | 4 (10) | 8 (20) | 114 (15) |
| Yes, both | 1 (1) | 1 (1) | 0 (0) | 1 (2) | 11 (1) |
| Hepatitis B–positive antigen result prior to first randomization in PROMISE | 7 (7) | 8 (7) | 5 (12) | 1 (2) | 36 (5) |
| Gravida prior to first subsequent pregnancy | |||||
| 1–2 | 52 (54) | 59 (49) | 22 (54) | 27 (66) | 413 (54) |
| 3–4 | 39 (40) | 53 (44) | 15 (37) | 13 (32) | 296 (39) |
| ≥5 | 6 (6) | 9 (7) | 4 (10) | 1 (2) | 51 (7) |
| Cohort participationc, No. | |||||
| A | 58 | 121 | 0 | 0 | … |
| B | 0 | 0 | 20 | 23 | … |
| A + B | 39 | 0 | 21 | 18 | … |
Data are presented as no. (%) unless otherwise indicated. Induced abortions, ectopic, or other nonviable pregnancies, and missing outcomes were excluded. Variables reported “at or before estimated conception” were based on the average of imputed term lengths for mothers with missing gestational ages.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; PROMISE, Promoting Maternal and Infant Survival Everywhere; Q1, first quartile; Q3, third quartile; WHO, World Health Organization.
aCharacteristics for mothers analyzed in the by-arm analyses, limited to those with estimated conception dates after the comparison group randomization, and prior to the protocol change on 7 July 2015.
bCharacteristics for all PROMISE mothers with subsequent pregnancies.
cMothers could be randomized after delivery (A) and/or after breastfeeding (B) cessation. A total of 39 mothers were overlaps, undergoing both randomizations.
Table 2.
Pregnancy Outcomes for All Subsequent Pregnancies in Promoting Maternal and Infant Survival Everywhere (PROMISE)
| Subsequent Pregnancy Numbera | ||||
|---|---|---|---|---|
| Pregnancy Outcome | First (n = 837) | Second (n = 97) | Third (n = 5) | Total (N = 939) |
| Ectopic or other nonviable pregnancy | 11 (1) | 0 (0) | 0 (0) | 11 (1) |
| Induced abortion | 64 (8) | 10 (13) | 1 (33) | 75 (9) |
| Spontaneous abortion (<20 wk) | 100 (13) | 6 (8) | 0 (0) | 106 (12) |
| Stillbirth (≥20 wk) | 25 (3) | 0 (0) | 0 (0) | 25 (3) |
| Live birth | 558 (72) | 57 (72) | 2 (67) | 617 (72) |
| Live birth followed by neonatal death (≤28 d) | 19 (2) | 6 (8) | 0 (0) | 25 (3) |
| Missing data, No. | 60 | 18 | 2 | 80 |
Data are presented as no. (%) unless otherwise indicated. Outcomes are from 826 mothers, from any pregnancy that occurred after the first Promoting Maternal and Infant Survival Everywhere (PROMISE) pregnancy.
aFirst, second, third subsequent pregnancies on PROMISE.
Subsequent pregnancy birth weights for the entire PROMISE follow-up subsequent pregnancy cohort are shown in Table 3. Only live births were included in this analysis. Birthweights were available for 465 (72%) infants, of which 60 (13%) of the pregnancies resulted in LBW infants (<2500 g). Table 4 shows subsequent pregnancy birth weights by comparison group and randomization arm for women with a conception date before 7 July 2015. There were 11 (17%) LBW infants among women in the ART at conception group, vs 5 (7%) in the ART reinitiated in pregnancy group. Among women randomized after cessation of breastfeeding, there were 8 (29%) LBW infants in the ART at conception group vs 4 (14%) in the ART reinitiated in pregnancy group.
Table 3.
Birth Weights Among Infants Born From a Subsequent Pregnancy in Promoting Maternal and Infant Survival Everywhere (PROMISE)
| Subsequent Pregnancy Numberb | ||||
|---|---|---|---|---|
| Birth Weighta | First (n = 577) | Second (n = 63) | Third (n = 2) | Total (N = 642) |
| Very low birth weight (<1500 g) | 5 (1) | 2 (4) | 0 (0) | 7 (2) |
| Low birth weight (≥1500 g to <2500 g) | 46 (11) | 7 (14) | 0 (0) | 53 (11) |
| Not low birth weight (≥2500 g) | 363 (88) | 40 (82) | 2 (100) | 405 (87) |
| Live birth with missing birth weight | 163 (28) | 14 (22) | 0 | 177 (28) |
Data are presented as no. (%) unless otherwise indicated.
Outcomes are from 600 mothers, from any live birth that occurred after the first Promoting Maternal and Infant Survival Everywhere (PROMISE) pregnancy.
aPercentages for known birth weights out of the total number of nonmissing observations.
bFirst, second, and third subsequent pregnancies on PROMISE.
Table 4.
Birth Weights Among Infants Born From a Subsequent Pregnancy With a Conception Date Before 7 July 2015, by Comparison Group, Promoting Maternal and Infant Survival Everywhere (PROMISE)
| Comparison Group and Randomization Arm | |||||
|---|---|---|---|---|---|
| Randomized at Delivery | Randomized After Breastfeeding | ||||
| Birth Weight | ART at Conception (n = 90 | ART Reinitiation in Pregnancy (n = 105) | ART at Conception (n = 39) | ART Reinitiation in Pregnancy (n = 39) | Total (N = 237) |
| Very low birth weight (<1500 g) | 0 (0) | 1 (1) | 2 (7) | 0 (0) | 3 (2) |
| Low birth weight (≥1500 g to <2500 g) | 11 (17) | 4 (6) | 6 (22) | 4 (14) | 20 (13) |
| Not low birth weight (≥2500 g) | 52 (83) | 63 (93) | 19 (70) | 24 (86) | 137 (86) |
| Live birth with missing birth weight, No. | 27 | 37 | 12 | 11 | 77 |
Data are presented as no. (%) unless otherwise indicated. Outcomes are from 184 mothers in comparison group randomization at delivery and 71 mothers in randomization after breastfeeding, with 34 mothers overlapping. The birth weights shown are limited to pregnancies with estimated conception dates before the protocol change on 7 July 2015.
Abbreviation: ART, antiretroviral therapy.
Table 5 shows the analyses of LBW in the ART at conception vs ART reinitiation in pregnancy groups for those randomized at delivery and after breastfeeding. The analysis combining the groups randomized at delivery and after breastfeeding by intention to treat and excluding crossovers both showed increased risk of LBW delivery in the ART at conception group with relative risks (RR) of 2.65 (95% CI, 1.20–5.81) and 2.94 (95% CI, 1.24–6.98), respectively. The as-treated analysis RR was 2.47 (95% CI, 1.00–6.14). When analyzed separately as randomized at delivery and after breastfeeding, the results were inconclusive since the 95% CIs included both increased and decreased relative risks.
Table 5.
Risk of Low Birth Weight Among the On Antiretroviral Therapy (ART) at Conception and the ART Reinitiation in Pregnancy Randomization Groups by Analysis Type
| Groupa and Analysis Type | Average Countb of LBW ART-c | Average Count of LBW ART-p | Percentageb With LBW ART-c | Percentage With LBW ART-p | Relative Risk (95% CI) Comparing ART-c to ART-p |
|---|---|---|---|---|---|
| Groups A + B | |||||
| ITT | 16 | 7 | 21.2% | 8.0% | 2.65 (1.20–5.81) |
| Excluding crossovers | 14 | 7 | 26.6% | 9.0% | 2.94 (1.24–6.98) |
| As treated | 14 | 9 | 22.3% | 9.0% | 2.47 (1.00–6.14) |
| Group A | |||||
| ITT | 11 | 6 | 17.9% | 8.0% | 2.24 (.98–5.11) |
| Excluding crossovers | 9 | 6 | 23.2% | 9.1% | 2.56 (.98–6.71) |
| As treated | 9 | 8 | 18.8% | 9.0% | 2.08 (.76–5.72) |
| Group B | |||||
| ITT | 10 | 3 | 25.0% | 12.3% | 2.03 (.71–5.79) |
| Excluding crossovers | 8 | 3 | 28.9% | 13.4% | 2.15 (.60–7.62) |
| As treated | 8 | 5 | 26.9% | 14.5% | 1.86 (.51–6.80) |
Only live birth outcomes were analyzed. LBW is defined as <2500 g.
Abbreviations: ART-c, antiretroviral therapy at conception; ART-p, reinitiation of antiretroviral therapy during pregnancy; CI, confidence interval; ITT, intention-to-treat; LBW, low birth weight.
aRandomization groups: A, randomization at delivery; B, randomization after breastfeeding.
bAverage counts are across 1000 imputations. Since dates were imputed and determine the treatment assignment, the treatment assignment could change across imputations. Percentages are based on the average counts of LBWs across the imputations.
Table 6 shows the result from the time-to-event analysis with a time-varying ART exposure indicator. Compared to ART reinitiation in pregnancy, the hazard rate of spontaneous abortion, stillbirth, or neonatal death among mothers in the ART at conception group was higher (hazard ratio [HR], 1.40 [95% CI, .99–1.98]). Table 6 also includes results with time-varying ART by regimen category. Comparing the ART at conception to the ART reinitiation in pregnancy groups, including NNRTI without a PI, had a higher hazard rate of spontaneous abortion, stillbirth, or neonatal death (HR, 1.48 [95% CI, 1.02–2.14]). Mothers on ART at conception including LPV/r or on an NRTI-only regimen had higher hazard rates for the composite poor pregnancy outcomes, but the result was inconclusive. Country of residence did not have a large overall effect on the HRs.
Table 6.
Hazard Ratios for Adverse Pregnancy Outcomes Comparing Time-varying Antiretroviral Therapy (ART) Exposure or Time-varying Regimen Group to No ART
| Endpoint | ART Exposure and Regimen Group | Hazard Ratio (95% CI) |
|---|---|---|
| Spontaneous abortion, stillbirth, or neonatal death | No ART | Ref |
| On ART at conception | 1.40 (.99–1.98) | |
| ART including boosted/nonboosted PI | 1.24 (.79–1.93) | |
| ART including NNRTI with no PI | 1.48 (1.02–2.14) | |
| Only NRTIs | 3.11 (.73–13.33) |
Results are based on 1000 imputations of missing gestational ages. Live births followed by neonatal deaths within 28 days were censored at the time of birth. Model was adjusted for country and for whether the mother’s first Promoting Maternal and Infant Survival Everywhere pregnancy resulted in a spontaneous abortion, stillbirth, neonatal death, or low birth weight (<2500 g).
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
DISCUSSION
The PROMISE 1077BF and 1077FF randomized trials, conducted in LMICs [13] in sub-Saharan Africa and India, compared maternal health outcomes for women randomized to either continue or discontinue ART after the period of transmission risk for their infant [10]. Some women in PROMISE subsequently became pregnant again during study follow-up, providing a unique opportunity to assess pregnancy outcomes among women who conceived while on ART compared to those who had discontinued ART. The post hoc analyses found that women who were on ART at the time of conception had an increased risk of adverse pregnancy outcomes compared to women not on ART at the time of conception. This included a >2-fold risk of delivering a LBW baby and a 1.4-fold increased risk of having either a spontaneous abortion, stillbirth, or neonatal death. In previous reports, the risk for LBW is increased with more advanced HIV disease [14]. However, the index study population were mainly WHO clinical stage I (>95%) asymptomatic HIV-infected women with high CD4 counts and low viral loads, suggesting that risk remains for all HIV-infected women conceiving on ART. The findings are important in high-HIV-prevalence countries in sub-Saharan Africa as more and more HIV-infected women conceive while receiving lifelong ART.
Our PROMISE findings are similar to some but not all prior published data. In a meta-analysis including 52 cohort studies, Xiao et al found a significant association between HIV infection and LBW and preterm deliveries [15]. However, in a subgroup analysis, women receiving ARVs had a similar risk of LBW compared to those who did not. This finding concurs with a meta-analysis by Kourtis et al that found no association between ARV use during pregnancy and preterm delivery [16]. Kourtis et al did, however, find a significant association between ART initiation prepregnancy or during the first trimester and prematurity, vs initiation in the second or third trimester, which supports our findings [16]. Likewise, the meta-analysis by Uthman et al confirmed a significantly increased risk of preterm delivery, very preterm delivery, and LBW (<2500 g) when conceiving on ART compared to initiating ART during pregnancy [6]. The magnitude of the association of preterm delivery was stronger in the 5 LMICs compared to 5 high-income countries. These findings, which included LBW, small for gestational age (SGA), and preterm delivery warrants their inclusion as outcome criteria in future trials including HIV-infected women. In addition, accurate gestational age determination needs to be a prerequisite to assess the contribution SGA babies in the LBW group.
The related PROMISE 1077HS trial provides data supporting the potential risks of ART exposure at the time of conception. PROMISE 1077HS was conducted in 56 sites in high- and middle-income countries where ART during pregnancy was standard of care as was formula feeding [8]. Women in PROMISE 1077HS were generally healthy with high CD4 counts, 90% had WHO clinical stage I disease and 55% were virally suppressed [9]. There were 227 subsequent pregnancies during follow-up. PROMISE 1077HS did not report the incidence of preterm deliveries and LBW but found a significantly increased risk of stillbirth and/or spontaneous abortion among those women on ART at the time of conception compared to women who had discontinued ART.
Similar to the 1077HS results, the 1077BF/1077FF analyses found an increased risk of spontaneous abortion and stillbirths in subsequent pregnancies for women on ART at conception. In addition, we found an increased risk of LBW and neonatal death. We also noted some differences by type of ART regimen: Women on ART at conception including an NNRTI without a PI had a higher risk of spontaneous abortion, stillbirths, and neonatal deaths. For women on ART including a PI or NRTI-only regimen, the risk was also higher, but with a result that was inconclusive. In contrast, Stringer et al did not find any significant difference in preterm birth by class of preconception ART in a meta-analysis including 3 ART trials [17]. An observational surveillance study from Botswana reported a significantly increased risk of preterm birth for infants born to mothers on LPV/r-based ART compared to those on efavirenz-based ART [18]. These contrasting findings may be related to differences in sample sizes or to differences in other background risk factors for preterm birth among these varied cohorts.
The overall incidence of spontaneous abortion were reported in 12% of this cohort, is similar to population-based rates of 8%–20% and to that reported in the PROMISE 1077HS study (15%) [9, 19]. The slight differences could be explained by higher very early spontaneous abortion detection in the 1077HS trial.
Missing gestational age and birth weight were limitations of this substudy. However, strengths include that the data were collected in a carefully monitored trial and generalizability is increased by the multisite, multicountry design of the PROMISE trial.
Progress toward the Joint United Nations Programme on HIV/AIDS 90-90-90 targets by 2020 has resulted in increasing numbers of HIV-infected women conceiving while on ART [20]. The benefit of ART in improving maternal health and reducing mother-to-child transmission have been proven beyond doubt in large randomized trials [8, 10]. However, the PROMISE findings underscore the need for ongoing prospective monitoring of pregnancy outcomes as new ART regimens are rolled out, which include the recent WHO recommendations (International AIDS Society 2019) for use of dolutegravir-based ART as the first-line treatment regimen in resource-limited settings [21].
The Botswana Tsepamo Birth Outcomes Surveillance Study and the Antiretroviral Pregnancy Registry data serve as examples of combining ongoing research and surveillance during the ARV era [22]. Monitoring for possible adverse pregnancy outcomes related to ART will contribute toward improved care of HIV-infected women and their infants. In addition, the inclusion of pregnant women in trials evaluating new ARV drugs is crucial [23].
Notes
Acknowledgments. The Promoting Maternal and Infant Survival Everywhere (PROMISE) team gratefully acknowledges the contributions of the PROMISE mothers and their infants enrolled in the study; as well as the PROMISE staff at the clinical research sites and staff at the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) Central Laboratory at the University of North Carolina, Chapel Hill; the IMPAACT Statistical and Data Management Center at the Center for Biostatistics in AIDS Research, Harvard School of Public Health, and Frontier Science and Technology Research Foundation; and the IMPAACT Operations Center at FHI 360.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases of the NIH under award numbers UM1AI068632 (IMPAACT Leadership and Operations Centre), UM1AI068616 (IMPAACT Statistical Data Management Centre), and UM1AI106716 (IMPAACT Laboratory Centre), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health. Study products were provided free of charge by AbbVie, Gilead Sciences, Boehringer Ingelheim, and ViiV/GlaxoSmithKline.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Available at: https://www.ncbi.nlm.nih.gov/books/NBK327115/. Accessed 3 July 2020. [PubMed]
- 2. Avert. Prevention of mother-to-child transmission (PMTCT) of HIV. Available at: https://www.avert.org/professionls/hiv-programming/prevention/prevention-mother-child. Accessed 16 May 2018.
- 3. Chen JY, Ribaudo HJ, Souda S, et al. . Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis 2012; 206:1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moodley T, Moodley D, Sebitloane M, Maharaj N, Sartorius B. Improved pregnancy outcomes with increasing antiretroviral coverage in South Africa. BMC Pregnancy Childbirth 2016; 16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mofenson LM. Antiretroviral therapy and adverse pregnancy outcome: the elephant in the room. J Infect Dis 2016; 213:10511054. [DOI] [PubMed] [Google Scholar]
- 6. Uthman OA, Nachega JB, Anderson J, et al. . Timing of initiation of antiretroviral therapy and averse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV 2017; 4:e21–30. [DOI] [PubMed] [Google Scholar]
- 7. Katz J, Lee AC, Kozuki N, et al. . CHERG Small-for-Gestational-Age-Preterm Birth Working Group . Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013; 382:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Currier JS, Britto P, Hoffman RM, et al. . 1077HS PROMISE Team . Randomized trial of stopping or continuing ART among postpartum women with pre-ART CD4 ≥ 400 cells/mm3. PLoS One 2017; 12:e0176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffman RM, Brummel SS, Britto P, et al. . PROMISE (Promoting Maternal and Infant Safety Everywhere) 1077HS Team . Adverse pregnancy outcomes among women who conceive on antiretroviral therapy. Clin Infect Dis 2019; 68: 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fowler MG, Qin M, Fiscus SA, et al. . IMPAACT 1077BF/1077FF PROMISE Study Team . Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016; 375:1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flynn PM, Taha TE, Cababasay M, et al. . PROMISE Study Team . Prevention of HIV-1 transmission through breastfeeding: efficacy and safety of maternal antiretroviral therapy versus infant nevirapine prophylaxis for duration of breastfeeding in HIV-1-infected women with high CD4 cell count (IMPAACT PROMISE): a randomized, open-label, clinical trial. J Acquir Immune Defic Syndr 2018; 77:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection (START study). N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Bank. Low and middle income. Available at: https://data.worldbank.org/income-level/low-and-middle-income. Accessed 13 December 2018.
- 14. Wedi CO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV 2016; 3:e33–48. [DOI] [PubMed] [Google Scholar]
- 15. Xiao PL, Zhou YB, Chen Y, et al. . Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015; 15:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS 2007; 21:607–15. [DOI] [PubMed] [Google Scholar]
- 17. Stringer EM, Kendall MA, Lockman S, et al. . Pregnancy outcomes among HIV-infected women who conceived on antiretroviral therapy. PLoS One 2018; 13:e0199555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zash R, Jacobson DL, Diseko M, et al. . Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017; 171:e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. UpToDate. Pregnancy loss (miscarriage): risk factors, etiology, clinical manifestations, and diagnostic evaluation. Available at: https://www.uptodate.com/contents/spontaneous-abortion-risk-factors-etiology-clinical-manifestations-and-diagnostic-evaluation. Accessed 13 December 2018.
- 20. Nachega JB, Sam-Agudu NA, Mefenson LM, et al. . Achieving viral suppression in 90% of people living with human immunodeficiency virus on antiretroviral therapy in low- and middle- income countries: progress, challenges, and opportunities. Clin Infect Dis 2018; 66:1487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. WHO recommends dolutegravir as preferred HIV treatment option in all populations. 2019. Available at: https://www.who.int/news-room/detail/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations. Accessed 2 July 2020.
- 22. Zash R. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Global Health 2018; 6:e804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heyrana K, Byers HM, Stratton P. Increasing the participation of pregnant women in clinical trials. JAMA 2018; 320:2077–8. [DOI] [PubMed] [Google Scholar]