Abstract
Objective
To evaluate the effect of upadacitinib (UPA) monotherapy vs MTX on patient-reported outcomes (PROs) in patients with RA who were MTX-naïve or who had an inadequate response to MTX (MTX-IR).
Methods
PROs from the SELECT-EARLY and SELECT-MONOTHERAPY randomized controlled trials were evaluated at Weeks 2 and 12/14. Patients were ≥18 years of age with RA symptoms for ≥6 weeks (SELECT-EARLY, MTX-naïve) or diagnosed RA for ≥3 months (SELECT-MONOTHERAPY, MTX-IR) and received UPA monotherapy (15 or 30 mg) or MTX. PROs included Patient Global Assessment of Disease Activity (PtGA), pain visual analogue scale, HAQ Disability Index (HAQ-DI), morning stiffness duration/severity, Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue (SELECT-EARLY), health-related quality of life (HRQOL) by the 36-iem Short Form Health Survey and Work Productivity and Activity Impairment (WPAI; SELECT-EARLY). Least square mean (LSM) changes and proportions of patients reporting improvements greater than or equal to the minimum clinically important differences and normative values were determined.
Results
In 945 MTX-naïve and 648 MTX-IR patients, UPA monotherapy (15 mg, 30 mg) vs MTX resulted in greater reported LSM changes from baseline at Weeks 12/14 in PtGA, pain, HAQ-DI, morning stiffness duration/severity, FACIT-F (SELECT-EARLY), HRQOL and WPAI (SELECT-EARLY). These changes were statistically significant with both doses of UPA vs MTX at Weeks 12/14 in both RCTs. Improvements were reported as early as week 2. Compared with MTX, more UPA-treated MTX-naïve and MTX-IR patients reported improvements greater than or equal to the minimum clinically important differences and scores greater than or equal to normative values.
Conclusion
Among MTX-naïve and MTX-IR patients with active RA, UPA monotherapy at 15 or 30 mg for 12/14 weeks resulted in statistically significant and clinically meaningful improvements in pain, physical function, morning stiffness, HRQOL and WPAI compared with MTX alone.
Clinical trial registration number
SELECT-EARLY (NCT02706873) and SELECT-MONOTHERAPY (NCT02706951) are registered with ClinicalTrials.gov.
Keywords: RA, outcome measures, inflammation, DMARDs, quality of life
Rheumatology key messages
Upadacitinib monotherapy (15 mg or 30 mg daily) rapidly improved PROs compared with methotrexate.
Significant improvement between upadacitinib and methotrexate were reported in Week 2 in PROs at Weeks 12/14.
Upadacitinib monotherapy resulted in clinically meaningful improvements in PROs in RA patients with inadequate response to methotrexate.
Introduction
RA is a chronic, inflammatory joint disease associated with substantial clinical burden and reduced health-related quality of life (HRQOL) [1–3]. Patients with RA experience pain, fatigue and impaired physical function, all of which contribute to the substantial negative impact of RA on HRQOL and can lead to impaired work productivity [1, 3, 4]. Current standard-of-care therapies for RA include conventional synthetic DMARDs (csDMARDs) such as MTX, biologic DMARDs (bDMARDs) such as anti-TNF agents and targeted synthetic DMARDs (tsDMARDs) such as Janus kinase (JAK) inhibitors [5, 6]. Although MTX continues to be the first-line therapy for RA, clinical studies indicate that the rate of MTX persistence ranges from 50–90% at 1 year and 25–79% at 5 years [7]. In addition, ∼30% of patients in a US RA registry discontinued or withdrew treatment within 1–2 years and the most common reason was intolerance or toxicity to MTX [8, 9]. Common adverse effects leading to discontinuation of MTX included elevated liver enzymes, fatigue, alopecia, loss of appetite, stomach pain, mouth sores, diarrhoea and myelosuppression [9]. Discontinuation of MTX can lead to poorer clinical and functional outcomes, as biologics are often prescribed in combination with MTX due to better responses over monotherapy and/or immunogenicity [5]. Thus there is a need for monotherapies that work effectively without MTX.
Upadacitinib (UPA), a selective JAK1 inhibitor, [10] is approved for the treatment of adults with moderately to severely active RA with an inadequate response to MTX (MTX-IR) [11, 12] and has shown superior efficacy as monotherapy over MTX in both MTX-naïve [13] and MTX-IR patients [14]. The recommended dose of UPA for the treatment of moderately to severely active RA in adults is 15 mg once daily [11, 12]. Substantial improvements in patient-reported outcomes (PROs) have been reported with UPA in combination with MTX in patients with inadequate responses to csDMARDs or bDMARDs [15, 16], but the impact of UPA monotherapy on PROs remains to be established. Thus the objective of this analysis was to evaluate the effect of UPA monotherapy vs MTX on PROs in MTX-naïve and MTX-IR patients with moderately to severely active RA.
Materials and methods
Study design and participants
The study designs for SELECT-EARLY (NCT02706873) and SELECT-MONOTHERAPY (NCT02706951) phase 3 randomized controlled trials (RCTs) have been described in detail elsewhere [14, 17]. Patients in both trials were ≥18 years of age with RA symptoms for ≥6 weeks (SELECT-EARLY) or diagnosed RA for ≥3 months (SELECT-MONOTHERAPY). MTX-naïve patients had no prior MTX use or ≤3 weekly doses of MTX and completed a 4 week MTX washout period before being randomized 1:1:1 to receive UPA (15 mg or 30 mg) daily or MTX weekly. MTX-IR patients received oral or parenteral MTX for ≥4 months at a stable dose for ≥4 weeks before enrolment and had active RA when they were randomized 1:1:1 to blindly discontinue MTX and receive UPA 15 mg or 30 mg daily as monotherapy plus placebo MTX or continue their previous dose of MTX monotherapy as a blinded study drug plus UPA placebo. This study evaluated the effect of UPA monotherapy vs MTX on PROs during the first 12 or 14 weeks of the SELECT-EARLY and SELECT-MONOTHERAPY RCTs, respectively. These studies comply with the Declaration of Helsinki, locally appointed ethics committees approved the research protocol and patients provided informed consent before participation.
PROs
PROs were collected as secondary outcomes to evaluate the impact of UPA monotherapy (15 mg or 30 mg) on patients’ symptoms and HRQOL at various study visits per protocol. PROs in both RCTs included Patient Global Assessment of Disease Activity (PtGA) visual analogue scale (VAS), pain VAS, HAQ Disability Index (HAQ-DI) [18], severity and duration of morning stiffness VAS [19] and 36-Item Short Form Health Survey (SF-36) [20, 21], including physical (PCS) and mental component summary (MCS) and eight domain scores [physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role-emotional (RE) and mental health (MH)]. In SELECT-EARLY, patients were also evaluated using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) [22] and Work Productivity and Activity Impairment (WPAI) [23] questionnaires. Work productivity was reported for employed patients.
Data analyses
Least square mean (LSM) changes from baseline to week 12 (SELECT-EARLY) or from baseline to week 14 (SELECT-MONOTHERAPY) were calculated based on an analysis of covariance and mixed effect repeated measures model, respectively. LSM changes from baseline to week 2 were calculated for PtGA, pain, HAQ-DI and severity and duration of morning stiffness.
The percentage of patients reporting improvements in PRO scores from baseline to week 2/12 (SELECT-EARLY) or to week 2/14 (SELECT-MONOTHERAPY) greater than or equal to the minimum clinically important difference (MCID) or scores greater than or equal to normative values at Weeks 12/14 were determined for the UPA and MTX treatment groups. MCIDs were defined as a reduction of ≥10 mm in PtGA [24, 25] and pain VAS [24], a reduction of ≥0.22 units in the HAQ-DI [24, 25], an increase of ≥4.0 points in the FACIT-F [24], proxied at a reduction of 1/2 s.d. of the mean baseline value for morning stiffness duration, a reduction of ≥1 for morning stiffness severity, an increase of ≥2.5 points in the SF-36 PCS and MCS [24, 25], an increase of ≥5.0 points for SF-36 domain scores [25] and an improvement of ≥7 points from baseline for WPAI [26]. Normative values were defined as HAQ-DI ≤0.25 [27], FACIT-F ≥43.6 [28], SF-36 PCS and MCS ≥50 [24, 25] and SF-36 domains according to an age- and gender-matched US normative population matched to each protocol [29]. Non-responder imputation was used when PRO data were missing. Comparisons between groups were made using chi-squared tests with statistical significance at the 5% level.
For each PRO, the incremental number needed to treat (NNT) to report clinically meaningful improvement from baseline (≥MCID) was calculated as the reciprocal of the response rate difference between the UPA and MTX groups.
Results
Study population
A total of 945 MTX-naïve patients and 648 MTX-IR patients with active RA were included in these analyses (Table 1). Among MTX-naïve patients, 314, 317 and 314 patients received MTX, UPA 15 mg and UPA 30 mg, respectively. Of the MTX-IR patients, 216, 217 and 215 received MTX, UPA 15 mg and UPA 30 mg, respectively. Patient demographics were similar across treatment cohorts and between the two patient populations (Table 1). At least 89% of patients in both patient populations had a Clinical Disease Activity Index score >22, indicating the presence of active disease. A major difference between the two populations was the duration of RA; MTX-naïve patients had a mean disease duration of 2.6–2.9 years (median 0.5–0.6) vs 5.8–7.5 years (median 2.7–4.2) in MTX-IR patients across the three treatment groups. Approximately 50% of MTX-naïve patients had disease <6 months, whereas 39–45% of MTX-IR patients had disease ≥5 years. Among MTX-naïve patients, 92.5% had no prior MTX exposure. Among MTX-IR patients, the duration of prior MTX therapy ranged from 3.3 to 3.8 years (median 1.7–2.2) across the three treatment groups.
Table 1.
Patient demographics and baseline characteristics
| Variable | MTX-naïve |
MTX-IR |
||||
|---|---|---|---|---|---|---|
| MTX (n = 314) | UPA 15 mg (n = 317) | UPA 30 mg (n = 314) | MTX (n = 216) | UPA 15 mg (n = 217) | UPA 30 mg (n = 215) | |
| Age, years, mean (s.d.) | 53.3 (12.9) | 51.9 (12.6) | 54.9 (12.6) | 55.3 (11.1) | 54.5 (12.2) | 53.1 (12.7) |
| Female, n (%) | 240 (76.4) | 241 (76.0) | 240 (76.4) | 179 (82.9) | 174 (80.2) | 170 (79.1) |
| Race, n (%) | ||||||
| White | 256 (81.5) | 256 (80.8) | 254 (80.9) | 176 (81.5) | 173 (79.7) | 180 (83.7) |
| Black | 12 (3.8) | 8 (2.5) | 13 (4.1) | 11 (5.1) | 15 (6.9) | 9 (4.2) |
| Asian | 37 (11.8) | 35 (11.0) | 34 (10.8) | 24 (11.1) | 24 (11.1) | 21 (9.8) |
| Other | 9 (2.9) | 18 (5.7) | 13 (4.1) | 5 (2.3) | 5 (2.3) | 5 (2.3) |
| Duration of RA, years, mean (s.d.) [median] | 2.6 (5.1) [0.5] | 2.9 (5.4) [0.5] | 2.8 (5.6) [0.6] | 5.8 (6.6) [2.7] | 7.5 (8.9) [3.6] | 6.5 (7.0) [4.2] |
| Duration of prior MTX therapy, years, mean (s.d.) | – | – | – | 3.3 (3.9) | 3.8 (4.8) | 3.8 (4.3) |
| CDAI >22, n (%)a | 281 (94.0) | 282 (93.7) | 279 (92.1) | 180 (90.0) | 192 (91.9) | 180 (89.1) |
| Seropositive for RF, n (%)a | 232 (73.9) | 251 (79.4) | 234 (74.5) | 151 (69.9) | 155 (71.4) | 151 (70.2) |
| Anti-CCP antibody positive, n (%)a | 236 (75.2) | 258 (81.4) | 230 (73.7) | 153 (70.8) | 159 (73.3) | 151 (70.6)b |
| Tender joint count (of 68), mean (s.d.) | 26.4 (16.2) | 25.4 (14.4) | 25.2 (15.0) | 25.2 (16.0) | 24.5 (15.1) | 24.8 (15.2) |
| Swollen joint count (of 66), mean (s.d.) | 16.9 (10.6) | 16.9 (10.4) | 15.7 (9.7) | 16.9 (11.5) | 16.4 (10.9) | 16.9 (10.2) |
| PtGA, mm, mean (s.d.) | 65.8 (21.5) | 66.6 (22.0) | 64.9 (21.6) | 59.6 (21.8) | 62.2 (22.3)c | 59.4 (22.8) |
| Pain VAS, mm, mean (s.d.) | 65.7 (21.5) | 68.4 (20.6) | 65.3 (21.5) | 62.5 (21.3) | 62.3 (22.5)c | 61.9 (22.1) |
| HAQ-DI, mean (s.d.) | 1.6 (0.7) | 1.6 (0.7) | 1.5 (0.7) | 1.5 (0.7) | 1.5 (0.7)c | 1.5 (0.7) |
| FACIT-F, mean (s.d.) | 26.6 (11.7) | 26.4 (11.9) | 27.8 (11.1) | – | – | – |
| AM stiffness, mean (s.d.) | ||||||
| Duration, min | 128.5 (134.2) | 168.9 (227.5) | 136.4 (166.5) | 153.0 (221.7) | 144.2 (215.1) | 133.9 (152.7) |
| Severityd | 6.3 (2.3) | 6.6 (2.3) | 6.4 (2.2) | 6.0 (2.2) | 5.9 (2.4) | 5.9 (2.4) |
| SF-36 summary score, mean (s.d.) | ||||||
| PCS | 33.1 (7.5) | 32.7 (7.7) | 33.7 (7.2) | 33.3 (7.3) | 33.3 (7.9) | 33.9 (7.8)e |
| MCS | 43.2 (10.8) | 42.5 (10.6) | 43.3 (11.6) | 45.1 (11.0) | 44.1 (11.3) | 44.5 (11.5)e |
| SF-36 domains, mean (s.d.) | ||||||
| Physical functioning | 31.8 (9.3) | 31.7 (9.3) | 33.0 (9.4) | 33.0 (9.0) | 32.7 (9.6) | 32.9 (9.3)e |
| Role-physical | 33.9 (8.2) | 33.6 (8.6) | 34.4 (8.5) | 35.6 (8.2) | 34.6 (8.2) | 35.7 (8.6)e |
| Bodily pain | 34.0 (7.2) | 33.0 (6.8) | 34.3 (6.9) | 35.0 (6.8) | 35.3 (6.7) | 35.4 (7.5)e |
| General health | 40.2 (9.1) | 39.7 (9.6) | 39.6 (8.7) | 38.7 (7.6) | 38.1 (8.5) | 39.1 (8.7)e |
| Vitality | 40.9 (9.7) | 40.0 (9.2) | 41.6 (9.2) | 41.4 (9.0) | 41.4 (9.3) | 42.0 (9.0)e |
| Social functioning | 38.0 (10.6) | 37.9 (10.3) | 38.8 (10.8) | 40.0 (10.2) | 39.6 (10.3) | 40.1 (10.2)e |
| Role-emotional | 38.5 (11.5) | 37.9 (11.7) | 38.2 (12.4) | 41.2 (11.5) | 39.8 (11.3) | 39.8 (12.0)e |
| Mental health | 41.1 (10.3) | 40.4 (10.5) | 41.5 (11.0) | 42.5 (10.6) | 41.5 (10.9) | 42.2 (10.9)e |
| SF-6D health utility index scores, mean (s.d.) | 0.54 (0.11) | 0.53 (0.10) | 0.55 (0.10) | 0.56 (0.10) | 0.56 (0.10) | 0.57 (0.11) |
| WPAI activity impairment, mean (s.d.) | 61.9 (26.2) | 64.7 (25.3) | 61.8 (24.4) | – | – | – |
| WPAI overall work impairment,f mean (s.d.) | 55.5 (31.3) | 59.8 (30.6) | 53.2 (29.0) | – | – | – |
Percentages calculated on non-missing values.
n = 214, one missing value.
n = 216.
Assessed on a numeric scale of 1–10 with 10 indicating the worst level.
n = 214.
Calculated only for employed subjects.
AM, morning; CDAI, Clinical Disease Activity Index; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; HAQ-DI, Health Assessment Questionnaire Disability Index; MCS, Mental Component Summary; MTX-IR, inadequate response to methotrexate; PCS, Physical Component Summary; PtGA, Patient’s Global Assessment of Disease Activity; SF-36, 36-Item Short Form Health Survey; UPA, upadacitinib; VAS, visual analogue scale; WPAI, Work Productivity and Activity Impairment Questionnaire; y, years.
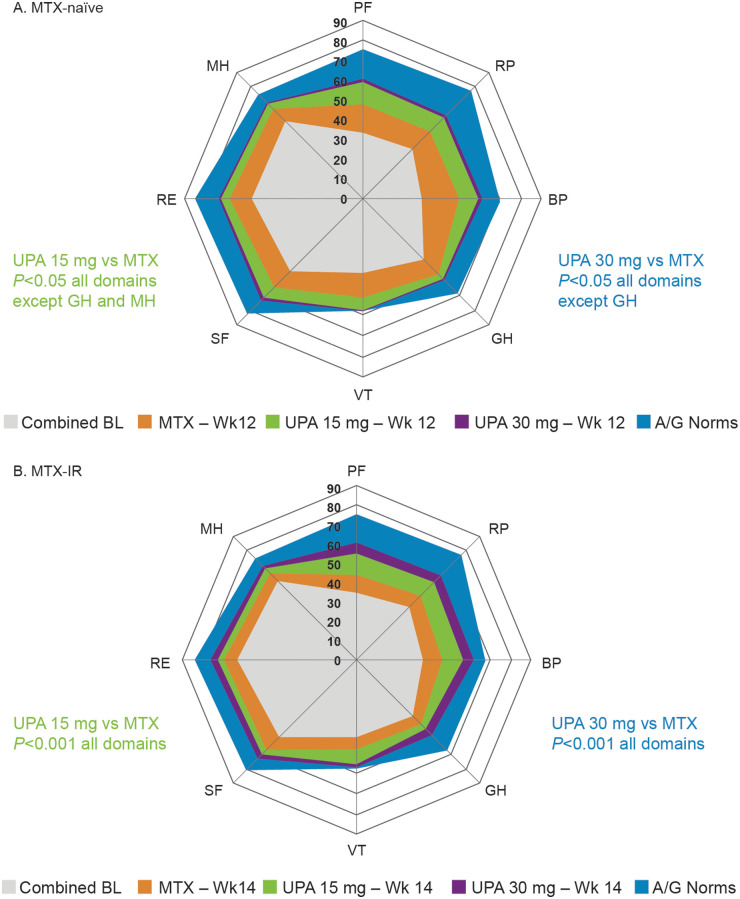
Baseline PRO scores were similar among treatment groups within each protocol (Table 1). At baseline, MTX-naïve patients reported substantially reduced HRQOL based on SF-36 domain scores compared with age- and gender-matched normative values (Fig. 1A). Baseline 6-dimension Short Form (SF-6D) utility scores, based on mean scores across all eight domains [30, 31] were well matched: 0.54 in the MTX group, 0.53 in the UPA 15 mg group and 0.55 in the UPA 30 mg group compared with 0.76 for the normative population age and gender matched to the SELECT-EARLY RCT population as a benchmark comparison. Likewise, baseline scores across all SF-36 domains were significantly reduced in MTX-IR patients compared with age- and gender-matched normative values (Fig. 1B). Baseline SF-6D utility scores in MTX-IR patients were 0.56 in the MTX group, 0.56 in the UPA 15 mg group and 0.57 in the UPA 30 mg group compared with 0.76 for the normative population age and gender matched to the SELECT-MONOTHERAPY RCT population.
Fig. 1.
Change in SF-36 domain over time relative to age- and gender-matched normative values
(A) SF-36 domain scores at baseline and week 12 in MTX-naïve patients vs age- and gender-matched normative values. (B) SF-36 domain scores at baseline and week 14 in MTX-IR patients vs age- and gender-matched normative values. A/G, age/gender; BL, baseline; BP, bodily pain; GH, general health; MH, mental health; MTX-IR, inadequate response to MTX; PF, physical functioning; RE, role-emotional; RP, role-physical; SF, social functioning; SF-36, 36-Item Short Form Health Survey; UPA, upadacitinib; VT, vitality; Wk, week.
Improvements at week 2: MTX-naïve and MTX-IR patients
Compared with MTX, UPA treatment (both doses) resulted in significant LSM changes (P < 0.01) from baseline as early as week 2 in PtGA, pain VAS, HAQ-DI and morning stiffness duration/severity in MTX-naïve and MTX-IR patients (Table 2). In the MTX-naïve group, significantly more UPA-treated vs MTX-treated patients reported improvements greater than or equal to the MCID in PtGA, pain VAS, HAQ-DI and morning stiffness duration/severity. In the MTX-IR group, more UPA-treated vs MTX-treated patients reported improvements greater than or equal to the MCID in PtGA, pain VAS, HAQ-DI and morning stiffness severity.
Table 2.
PROs at week 2
| PRO measures | MTX-naïve |
MTX-IR |
||||
|---|---|---|---|---|---|---|
| MTX (n = 314) | UPA 15 mg (n = 317) | UPA 30 mg (n = 314) | MTX (n = 216) | UPA 15 mg (n = 217) | UPA 30 mg (n = 215) | |
| LSM change from baseline (95% CI) | ||||||
| PtGA (mm) | −8.59 (−11.10, −6.07) | −20.02*(−22.51, −17.53) | −25.39*(−27.91, −22.87) | −4.60 (−7.71, −1.49) | −13.93*(−17.02, −10.85) | −16.76*(−19.82, −13.69) |
| Pain VAS (mm) | −8.71 (−11.26, −6.16) | −19.71*(−22.23, −17.18) | −24.38*(−26.93, −21.83) | −4.74 (−7.67, −1.81) | −14.37*(−17.28, −11.47) | −19.52*(−22.41, −16.63) |
| HAQ-DI | −0.19 (−0.25, −0.14) | −0.44*(−0.50, −0.39) | −0.50*(−0.55, −0.44) | −0.16 (−0.22, −0.09) | −0.34*(−0.41, −0.28) | −0.43*(−0.50, −0.37) |
| AM stiffness | ||||||
| Duration (min) | −10.00 (−25.66, −5.65) | −42.20*(−57.66, −26.74) | −74.57*(−90.16, −58.98) | −12.84 (−31.71, 6.04) | −59.78*(−78.43, −41.12) | −51.13*(−69.80, −32.47) |
| Severitya | −1.01 (−1.26, −0.76) | −1.99*(−2.24, −1.75) | −2.46*(−2.70, −2.21) | −0.82 (−1.11, −0.54) | −1.52*(−1.80, −1.23) | −1.86*(−2.14, −1.58) |
| Patients reporting improvements greater than or equal to the MCID, n (%) | ||||||
| PtGA (mm) | 134 (42.7) | 186 (58.7)* | 190 (61.1)* | 71 (32.9) | 111 (51.4)* | 127 (59.1)* |
| Pain VAS (mm) | 129 (41.1) | 198 (62.5)* | 202 (65.0)* | 68 (31.5) | 117 (54.2)* | 141 (65.6)* |
| HAQ-DI | 132 (42.0) | 203 (64.0)* | 201 (64.6)* | 77 (35.6) | 114 (52.8)* | 125 (58.1*) |
| AM stiffness | ||||||
| Duration (min) | 27 (8.6) | 60 (19.0)* | 65 (20.8)* | 18 (8.4) | 34 (15.7)** | 30 (14.0) |
| Severitya | 162 (51.8) | 235 (74.4)* | 231 (73.8)* | 104 (48.4) | 128 (59.0)** | 147 (68.7)* |
Assessed on a numeric scale of 1–10 with 10 indicating the worst level.
P < 0.01 for UPA vs MTX,.
P < 0.05 for UPA vs MTX.
AM, morning; HAQ-DI, Health Assessment Questionnaire Disability Index; LSM, least squares mean; MCID, minimum clinically important difference; MTX-IR, inadequate response to MTX; PRO, patient-reported outcome; PtGA, Patient’s Global Assessment of Disease Activity; UPA, upadacitinib; VAS, visual analogue scale.
PROs at week 12: MTX-naïve patients
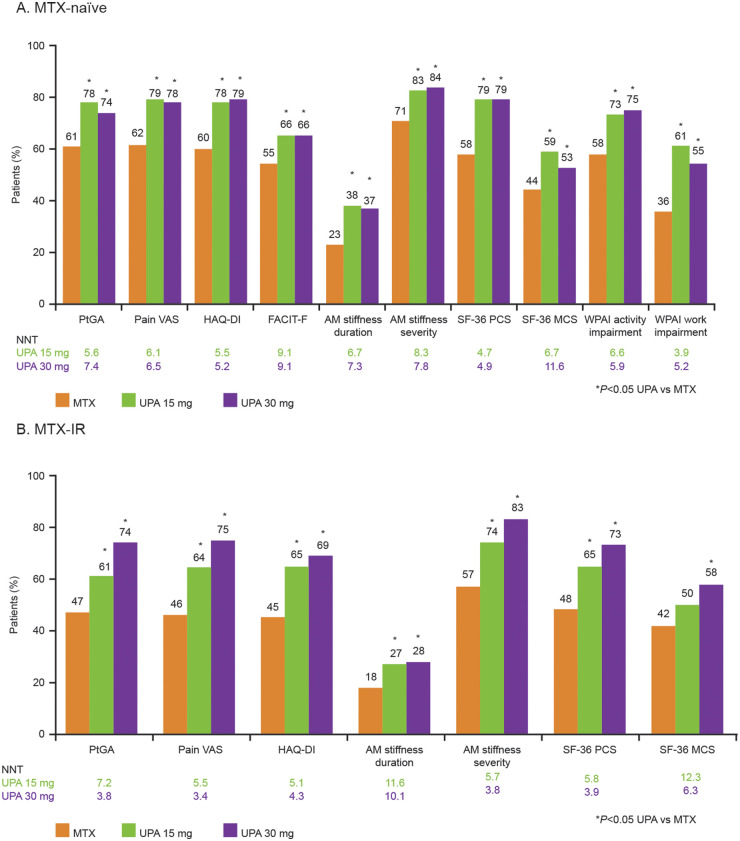
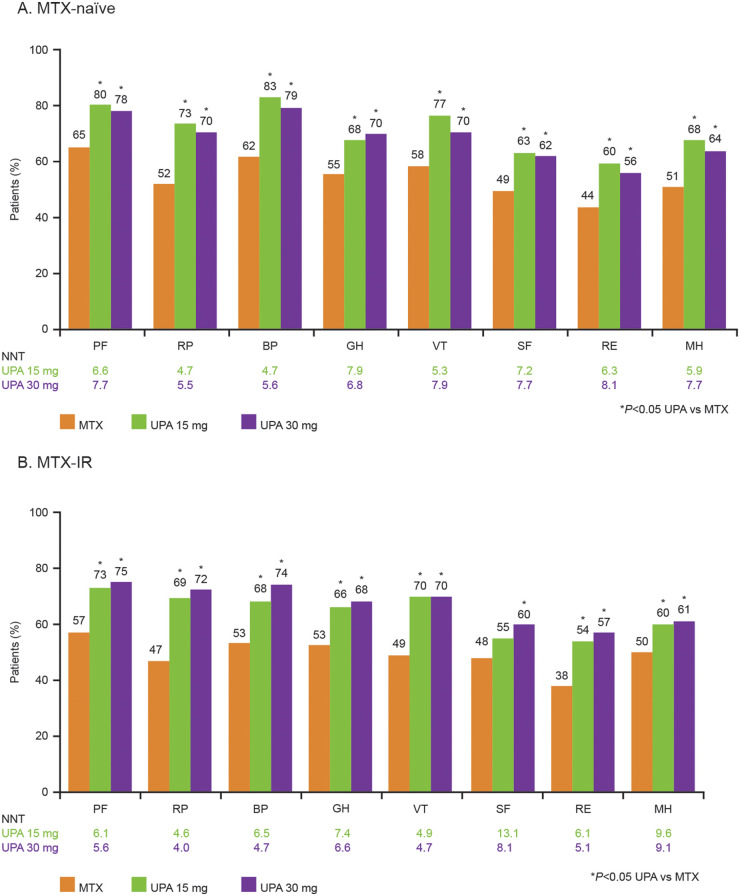
Among MTX-naïve patients treated with UPA 15 or 30 mg, LSM changes at week 12 were significantly greater (P < 0.01) compared with patients receiving MTX alone in PtGA; pain VAS; HAQ-DI; FACIT-F; morning stiffness duration/severity; SF-36 PCS, MCS and all domains; SF-6D health utility index score; WPAI activity impairment and WPAI overall work impairment (Table 3). At week 12, significantly more MTX-naïve patients treated with UPA (both doses) than MTX-treated patients reported improvements greater than or equal to the MCID in PtGA; pain VAS; HAQ-DI; FACIT-F; morning stiffness duration/severity; SF-36 PCS, MCS and all domains; and WPAI activity impairment and overall work impairment (Figs 2A and 3A). Significantly more MTX-naïve patients treated with either dose of UPA reported scores within the range of normative values in the HAQ-DI, FACIT-F and SF-36 PCS and MCS compared with MTX-treated patients at week 12 (Supplementary Fig. 1A, available at Rheumatology online). Compared with MTX, a greater percentage of MTX-naïve patients treated with UPA reported scores greater than or equal to the normative values across the SF-36 domains, except for the GH and MH domains in the 15 mg group and the GH domain in the 30 mg group (Supplementary Fig. 2A, available at Rheumatology online). In MTX-naïve patients, NNTs across all PROs ranged from 4 to 9 for UPA 15 mg and from 5 to 12 for UPA 30 mg.
Table 3.
LSM change from baseline to week 12/14 in PRO scores
| PRO measures, LSM change (95% CI) | MTX-naïve change from baseline to Week 12 |
MTX-IR change from baseline to Week 14 |
||||
|---|---|---|---|---|---|---|
| MTX (n = 314) | UPA 15 mg (n = 317) | UPA 30 mg (n = 314) | MTX (n = 216) | UPA 15 mg (n = 217) | UPA 30 mg (n = 215) | |
| PtGA (mm) | −24.59 (−27.48, −21.71) | −34.90* (−37.66, −32.14) | −38.00* (−40.80, −35.19) | −11.18 (−14.85, −7.50) | −23.40* (−27.05, −19.75) | −29.89* (−33.52, −26.26) |
| Pain VAS (mm) | −25.36 (−28.28, −22.44) | −36.28* (−39.08, −33.49) | −39.67* (−42.51, −36.84) | −13.88 (−17.44, −10.31) | −26.15* (−29.69, −22.60) | −33.18* (−36.70, −29.66) |
| HAQ-DI | −0.51 (−0.58, −0.44) | −0.84* (−0.91, −0.77) | −0.86* (−0.93, −0.79) | −0.32 (−0.41, −0.24) | −0.65* (−0.73, −0.57) | −0.73* (−0.81, −0.64) |
| FACIT-F | 6.80 (5.70, 7.91) | 10.01* (8.94, 11.07) | 9.57* (8.50, 10.64) | – | – | – |
| AM stiffness | ||||||
| Duration (min) | −72.37 (−83.67, −61.06) | −105.97*(−116.87, −95.08) | −111.52*(−122.49, −100.56) | −53.03 (−72.18, −33.88) | −94.56*(−113.57, −75.54) | −102.34*(−121.24, −83.45) |
| Severitya | −3.02 (−3.31, −2.73) | −3.95* (−4.23, −3.67) | −4.05* (−4.33, −3.76) | −1.56 (−1.91, −1.22) | −3.08* (−3.43, −2.74) | −3.55* (−3.90, −3.21) |
| SF-36 summary scores | ||||||
| PCS | 5.77 (4.83, 6.71) | 10.09* (9.19, 10.99) | 10.11* (9.20, 11.01) | 4.32 (3.19, 5.44) | 8.28* (7.17, 9.40) | 10.19* (9.07, 11.30) |
| MCS | 3.86 (2.81, 4.92) | 5.79* (4.78, 6.80) | 5.78* (4.76, 6.80) | 1.88 (0.64, 3.12) | 4.55* (3.33, 5.78) | 4.68*(3.46, 5.91) |
| SF-36 domains | ||||||
| PF | 5.61 (4.56, 6.65) | 10.04* (9.03, 11.04) | 10.05* (9.04, 11.06) | 4.11 (2.90, 5.32) | 8.47* (7.27, 9.67) | 10.27*(9.08, 11.47) |
| RP | 4.67 (3.68, 5.66) | 8.43* (7.48, 9.38) | 8.57* (7.62, 9.52) | 3.58 (2.46, 4.71) | 7.02* (5.91, 8.13) | 8.26*(7.15, 9.37) |
| BP | 7.41 (6.42, 8.39) | 11.87* (10.92, 12.82) | 11.94* (10.99, 12.89) | 4.67 (3.47, 5.87) | 8.89* (7.70, 10.07) | 11.02*(9.84, 12.20) |
| GH | 4.43 (3.49, 5.37) | 6.33* (5.43, 7.23) | 6.83* (5.93, 7.74) | 2.81 (1.68, 3.94) | 5.57*(4.45, 6.69) | 7.05*(5.93, 8.17) |
| VT | 5.54 (4.51, 6.57) | 9.33* (8.33, 10.32) | 8.64* (7.65, 9.64) | 3.13 (1.85, 4.40) | 8.06* (6.80, 9.32) | 8.36*(7.10, 9.61) |
| SF | 4.62 (3.52, 5.71) | 7.66* (6.62, 8.71) | 8.14* (7.09, 9.19) | 3.56 (2.28, 4.83) | 6.26* (5.01, 7.52) | 7.01*(5.76, 8.26) |
| RE | 4.21 (3.11, 5.32) | 6.39* (5.33, 7.44) | 6.81* (5.75, 7.87) | 2.05 (0.79, 3.31) | 4.82* (3.57, 6.06) | 6.08* (4.83, 7.32) |
| MH | 4.56 (3.49, 5.62) | 6.93* (5.91, 7.94) | 6.77* (5.75, 7.79) | 2.46 (1.21, 3.72) | 5.35* (4.11, 6.58) | 5.67*(4.43, 6.90) |
| SF-6D health utility index score | 0.07 (0.06, 0.09) | 0.13*(0.11, 0.14) | 0.12*(0.11, 0.14) | 0.05 (0.04, 0.07) | 0.10* (0.08, 0.11) | 0.11* (0.10, 0.13) |
| WPAI activity impairment | −18.95 (−21.93, −15.98) | −27.64* (−30.49, −24.79) | −31.21* (−34.08, −28.34) | – | – | – |
| WPAI overall work impairmentb | −9.81 (−15.27, −4.35) | −26.61* (−31.62, −21.59) | −23.52* (−28.91, −18.14) | – | – | – |
Assessed on a numeric scale of 1–10 with 10 indicating the worst level.
Calculated only for employed subjects.
P < 0.01 for UPA vs MTX.
AM, morning; BP, bodily pain; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; GH, general health; HAQ-DI, Health Assessment Questionnaire Disability Index; LSM, least squares mean; MCS, Mental Component Summary; MH, mental health; MTX-IR, inadequate response to MTX; PCS, Physical Component Summary; PF, physical functioning; PRO, patient-reported outcome; PtGA, Patient’s Global Assessment of Disease Activity; RE, role-emotional; RP, role-physical; SF, social functioning; SF-36, 36-Item Short Form Health Survey; UPA, upadacitinib; VAS, visual analogue scale; VT, vitality; WPAI, Work Productivity and Activity Impairment Questionnaire.
Fig. 2.
Patients reporting PRO score improvements greater than or equal to the MCID at week 12 or 14
(A) Percentage of MTX-naïve patients reporting PRO scores greater than or equal to the MCID at week 12. (B) Percentage of MTX-IR patients reporting PRO scores greater than or equal to the MCID at week 14. AM, morning; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; HAQ-DI, Health Assessment Questionnaire Disability Index; MCID, minimum clinically important difference; MCS, Mental Component Summary; MTX-IR, inadequate response to MTX; NNT, number needed to treat; PCS, Physical Component Summary; PRO, patient-reported outcome; PtGA, Patient’s Global Assessment of Disease Activity; SF-36, 36-Item Short Form Health Survey; UPA, upadacitinib; VAS, visual analogue scale; WPAI, Work Productivity and Activity Impairment Questionnaire.
PROs at week 14: MTX-IR patients
In MTX-IR patients treated with either dose of UPA, LSM changes at week 14 were statistically significantly (P < 0.001) greater compared with MTX in the PtGA; pain VAS; HAQ-DI; FACIT-F; morning stiffness duration/severity; SF-36 PCS, MCS and all domain scores; and SF-6D health utility score (Table 3). Except for the SF-36 MCS score in the UPA 15 mg group, significantly more MTX-IR patients treated with UPA (both doses) reported improvements greater than or equal to the MCID across all PROs at week 14 compared with MTX (Fig. 2B). Similarly, a statistically greater number of MTX-IR patients treated with either dose of UPA reported improvements greater than or equal to the MCID in all SF-36 domains compared with MTX, except for the SF domain in the UPA 15 mg group (Fig. 3B). Significantly more MTX-IR patients receiving UPA (both doses) reported scores in the range of normative values for HAQ-DI and SF-36 PCS compared with MTX-treated patients (Supplementary Fig. 1B, available at Rheumatology online). Compared with MTX-treated patients, significantly more patients treated with UPA 30 mg reported scores greater than or equal to the normative values at week 14 across all SF-36 domains, while a statistically greater number of patients treated with UPA 15 mg reported scores greater than or equal to the normative values in the PF, BP, VT and SF domains (Supplementary Fig. 2B, available at Rheumatology online). In MTX-IR patients, NNTs across all PROs ranged from 5 to 12 for UPA 15 mg and from 3 to 10 for UPA 30 mg.
Fig. 3.
Patients reporting SF-36 domain score improvements greater than or equal to the MCID at week 12 or 14
(A) Percentage of MTX-naïve patients reporting SF-36 domain scores greater than or equal to the MCID at week 12. (B) Percentage of MTX-IR patients reporting SF-36 domain scores greater than or equal to the MCID at week 14. BP, bodily pain; GH, general health; MCID, minimum clinically important difference; MH, mental health; MTX-IR, inadequate response to MTX; NNT, number needed to treat; PF, physical functioning; RE, role-emotional; RP, role-physical; SF, social functioning; SF-36, 36-Item Short Form Health Survey; UPA, upadacitinib; VT, vitality.
Discussion
Improving PROs is crucial to effect tangible change in disease and patient outcomes. A systematic literature review of clinical studies evaluating the rate of MTX persistence in patients with RA found the persistence rate was 50–94% at year 1 and 25–79% at year 5 [7]. Real-world data indicate that ∼30% of patients in a US RA registry discontinued MTX within 1–2 years of treatment initiation, of which 50% was due to intolerance or safety reasons [8, 9]. Discontinuation of MTX in these patients can lead to poorer clinical outcomes and demonstrates an unmet need for therapies that are effective in patients who do not respond or are intolerant to MTX. UPA monotherapy is clinically effective in this population. Our analysis of PROs in both MTX-naïve and MTX-IR patients strongly demonstrates the additional benefits of UPA monotherapy compared with MTX. The importance of aiming for meaningful improvements in symptoms and impact of RA from the patient perspective is well-established. Trials now acknowledge this and address PROs such as PtGA, pain and physical function. In addition, people with RA have identified fatigue as a persistent burden of disease and one of the more important symptoms that requires improvement so that they can manage daily activities, continue to work and maintain social interactions [32–34].
Compared with an age- and gender-matched normative population, patients enrolled in the SELECT-EARLY and SELECT-MONOTHERAPY RCTs reported reduced HRQOL at baseline and decreased SF-36 domain scores, consistent with significant disease burden. Significant improvements in these PROs were reported in UPA-treated patients as early as week 2, and treatment with UPA (15 mg or 30 mg) monotherapy daily for 12 or 14 weeks resulted in significant and clinically meaningful improvements in physical function, pain, morning stiffness and HRQOL compared with MTX treatment alone in both MTX-naïve and MTX-IR patients with active RA. Improvements in vitality domain scores approached normative values reported for the age- and gender-matched populations in both RCTs. In general, treatment responses between UPA 15 mg and 30 mg doses were similar. By 12/14 weeks, 74–84% of MTX-naïve and 61–83% of MTX-IR patients treated with UPA monotherapy reported clinically meaningful improvements in PtGA, pain, physical function and morning stiffness severity compared with 45–71% of those treated with MTX. These improvements are similar to those recently reported for UPA-treated patients on background csDMARDs, where 71–80% of patients reported improvements in PtGA, pain, physical function and morning stiffness severity [15].
In MTX-naïve patients, improvements in PROs reported for UPA monotherapy were significantly greater than MTX therapy. Similar results have been reported with tofacitinib (5 mg twice daily) monotherapy [35] and baricitinib (4 mg daily) monotherapy [36, 37] in MTX-naïve patients and with tofacitinib monotherapy in csDMARD-IR patients [38]. These findings support monotherapy with a JAK inhibitor, such as UPA, as an option for patients who discontinue treatment because of intolerance, toxicity or inefficacy with MTX.
A noteworthy strength of this study is that data were collected during phase 3 RCTs, which ensured patients were closely followed and PROs were consistently measured. The validated PROs used in this analysis evaluate different aspects of the patient’s experience. The use of the MCID and normative criteria to measure responses translates the data into improvements considered to be clinically meaningful from the patient’s perspective. The randomized trial design mitigates bias that may arise due to unobservable differences between cohorts.
This study also has limitations that should be considered when interpreting the results. PROs were collected at fixed visits, therefore responses were unavailable at other time points. The generalizability of these results may be limited, as patients enrolled in RCTs may differ from patients in the general population or other RA cohorts. The method used to impute missing data (non-responder imputation) assumed that missing PRO scores were associated with non-response, which may underestimate the true rate of response. This analysis was limited to 12/14 weeks, therefore future studies are needed to determine whether these improvements are sustained over the long term in patients with chronic disease.
Conclusion
MTX-naïve and MTX-IR patients with active RA treated with UPA 15 mg or 30 mg monotherapy daily for 12 or 14 weeks, respectively, reported rapid and clinically meaningful improvements in PtGA, pain, physical function, fatigue, morning stiffness, HRQOL and work productivity compared with MTX. UPA monotherapy offers an effective second-line treatment option for patients with RA who have an inadequate response to MTX.
Supplementary Material
Acknowledgement
Medical writing assistance was provided by Joann Hettasch, PhD (Fishawack Facilitate Ltd, part of Fishawack Health) and was funded by AbbVie, North Chicago, IL.
Funding: This work was supported by AbbVie. AbbVie sponsored the study; contributed to the design; participated in the collection, analysis and interpretation of data and in writing, reviewing and approval of the final version. No honoraria or payments were made for authorship.
Disclosure statement: V.S. is a consultant for AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Genentech/Roche, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi and UCB and has participated in advisory boards for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Genentech, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi and UCB. N.T., H.S.C., A.F., J.L.S. and K.D. are employees of AbbVie and may own AbbVie stock or stock options. A.W. is a consultant for AbbVie and has received research support. M.H.B. has received research/grant support from Pfizer, Roche and UCB and consulting fees and travel honoraria from AbbVie, Boehringer Ingelheim, Eli Lilly Galapagos, Gilead, MSD and Pfizer. S.C.R. is a consultant and advisor and serves on speaker bureaus for AbbVie, Celgene, Genentech/Roche, Janssen, Pfizer and UCB. D.G. is an employee of Analysis Group, Inc., which received consulting fees from AbbVie for this study. M.B. is a consultant and advisor, serves on speaker bureaus for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Genentech/Roche, Janssen, Merck, Novartis, Pfizer and Sanofi/Regeneron and is a shareholder of Johnson and Johnson (parent company of Janssen).
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. Access is provided to anonymized, patient and trial-level data (analysis data sets) as well as other information (e.g. protocols and Clinical Study Reports) from AbbVie-sponsored phase II–IV global interventional clinical trials conducted in patients (completed as of May 2004, for products and indications approved in either the USA or the European Union), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. Access to this clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Radner H, Smolen JS, Aletaha D.. Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Ther 2014;16:R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 3.Strand V, Khanna D.. The impact of rheumatoid arthritis and treatment on patients’ lives. Clin Exp Rheumatol 2010;28(3 Suppl 59):S32–40. [PubMed] [Google Scholar]
- 4.Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M.. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int 2016;36:685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewe RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 6.Singh JA, Saag KG, Bridges SL Jr. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 7.Curtis JR, Bykerk VP, Aassi M, Schiff M.. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol 2016;43:1997–2009. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Wallenstein G, Takiya L. et al. Patterns of methotrexate use and discontinuation in a U.S. rheumatoid arthritis registry. Arthritis Rheumatol 2017;69(Suppl):abstract 1815. [Google Scholar]
- 9.Harrold L, Litman HJ, O’Brien J. et al. Methotrexate treatment patterns in advanced therapy-naive patients with rheumatoid arthritis: clinical characteristics and outcomes of patients in the Corrona registry. Ann Rheum Dis 2019;78:747–8. [Google Scholar]
- 10.Parmentier JM, Voss J, Graff C. et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol 2018;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RINVOQ (upadacitinib) [package insert]. Sligo, Ireland: AbbVie Ireland, 2019. [Google Scholar]
- 12.European Medicines Agency. RINVOQ (upadacitinib): summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq (18 February 2020, date last accessed).
- 13.van Vollenhoven R, Takeuchi T, Pangan A. et al. A phase 3, randomized, controlled trial comparing upadacitinib monotherapy to MTX monotherapy in MTX-naïve patients with active rheumatoid arthritis. Rheumatology 2019;58(Suppl 3):abstract 059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolen JS, Pangan AL, Emery P. et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019;393:2303–11. [DOI] [PubMed] [Google Scholar]
- 15.Strand V, Pope J, Tundia N. et al. Upadacitinib improves patient-reported outcomes in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying antirheumatic drugs: results from SELECT-NEXT. Arthritis Res Ther 2019;21:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strand V, Schiff M, Tundia N. et al. Effects of upadacitinib on patient-reported outcomes: results from SELECT-BEYOND, a phase 3 randomized trial in patients with rheumatoid arthritis and inadequate responses to biologic disease-modifying antirheumatic drugs. Arthritis Res Ther 2019;21:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Vollenhoven R, Takeuchi T, Pangan AL. et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately to severely active rheumatoid arthritis (SELECT-EARLY): a randomized, double-blind, active-comparator, multi-center, multi-country trial. Arthritis Rheumatol 2020;72:1607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruce B, Fries JF.. The Health Assessment Questionnaire (HAQ). Clin Exp Rheumatol 2005;23(5 Suppl 39):S14–8. [PubMed] [Google Scholar]
- 19.Orbai AM, Smith KC, Bartlett SJ, De Leon E, Bingham CO III.. “Stiffness has different meanings, I think, to everyone”: examining stiffness from the perspective of people living with rheumatoid arthritis. Arthritis Care Res 2014;66:1662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 21.Busija L, Pausenberger E, Haines TP. et al. Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care Res 2011;63(Suppl 11):S383–412. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Yount S, Sorensen M. et al. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811–9. [PubMed] [Google Scholar]
- 23.Zhang W, Bansback N, Boonen A. et al. Validity of the work productivity and activity impairment questionnaire–general health version in patients with rheumatoid arthritis. Arthritis Res Ther 2010;12:R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitchen H, Hansen BB, Abetz L, Hojberre L, Strandberg-Larsen M.. Patient-reported outcome measures for rheumatoid arthritis: minimal important differences review. Arthritis Rheumatol 2013;65:abstract 2268. [Google Scholar]
- 25.Strand V, Boers M, Idzerda L. et al. It’s good to feel better but it’s better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol 2011;38:1720–7. [DOI] [PubMed] [Google Scholar]
- 26.Fleischmann R, Weinblatt ME, Schiff M. et al. Patient-reported outcomes from a two-year head-to-head comparison of subcutaneous abatacept and adalimumab for rheumatoid arthritis. Arthritis Care Res 2016;68:907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan E, Sokka T, Hakkinen A, Hubert H, Hannonen P.. Normative values for the health assessment questionnaire disability index: benchmarking disability in the general population. Arthritis Rheumatol 2004;50:953–60. [DOI] [PubMed] [Google Scholar]
- 28.Kavanaugh A, Gladman DD, Edwards CJ. et al. Apremilast, an oral phosphodiesterase 4 inhibitor, is associated with long-term (104-week) improvement in fatigue in patients with psoriatic arthritis: pooled results from 3 phase III, randomized, controlled trials. Ann Rheum Dis 2016;75:1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strand V, Crawford B, Singh J. et al. Use of “spydergrams” to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann Rheum Dis 2009;68:1800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ara R, Brazier J.. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health 2008;11:1131–43. [DOI] [PubMed] [Google Scholar]
- 31.Ara R, Brazier J.. Predicting the short form-6D preference-based index using the eight mean short form-36 health dimension scores: estimating preference-based health-related utilities when patient level data are not available. Value Health 2009;12:346–53. [DOI] [PubMed] [Google Scholar]
- 32.van Tuyl LH, Sadlonova M, Hewlett S. et al. The patient perspective on absence of disease activity in rheumatoid arthritis: a survey to identify key domains of patient-perceived remission. Ann Rheum Dis 2017;76:855–61. [DOI] [PubMed] [Google Scholar]
- 33.Kirwan JR, Newman S, Tugwell PS. et al. Progress on incorporating the patient perspective in outcome assessment in rheumatology and the emergence of life impact measures at OMERACT 9. J Rheumatol 2009;36:2071–6. [DOI] [PubMed] [Google Scholar]
- 34.Minnock P, Kirwan J, Bresnihan B.. Fatigue is a reliable, sensitive and unique outcome measure in rheumatoid arthritis. Rheumatology 2009;48:1533–6. [DOI] [PubMed] [Google Scholar]
- 35.Strand V, Lee EB, Fleischmann R. et al. Tofacitinib versus methotrexate in rheumatoid arthritis: patient-reported outcomes from the randomised phase III ORAL Start trial. RMD Open 2016;2:e000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleischmann R, Schiff M, van der Heijde D. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiff M, Takeuchi T, Fleischmann R. et al. Patient-reported outcomes of baricitinib in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Res Ther 2017;19:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strand V, Kremer J, Wallenstein G. et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther 2015;17:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. Access is provided to anonymized, patient and trial-level data (analysis data sets) as well as other information (e.g. protocols and Clinical Study Reports) from AbbVie-sponsored phase II–IV global interventional clinical trials conducted in patients (completed as of May 2004, for products and indications approved in either the USA or the European Union), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. Access to this clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.