Abstract
Objective
We investigated the role of blood pressure, vessel wall stiffness [pulse wave velocity (PWV)] and subclinical atherosclerosis markers [carotid intima-media thickness (cIMT), popliteal vessel wall thickness (pVWT)] as mediators of the association of obesity with OA.
Methods
We used cross-sectional data from a subset of the population-based NEO study (n = 6334). We classified clinical hand and knee OA by the ACR criteria, and structural knee OA, effusion and bone marrow lesions on MRI (n = 1285). cIMT was assessed with ultrasonography. pVWT was estimated on knee MRI (n = 1285), and PWV by abdominal velocity-encoded MRIs (n = 2580), in subpopulations. Associations between BMI and OA were assessed with logistic regression analyses, adjusted for age, sex and education. Blood pressure, cIMT, pVWT and PWV were added to the model to estimate mediation.
Results
The population consisted of 55% women, with a mean (s.d.) age of 56(6) years. Clinical hand OA was present in 8%, clinical knee OA in 10%, and structural knee OA in 12% of participants. BMI was positively associated with all OA outcomes. cIMT partially mediated the association of BMI with clinical hand OA [10.6 (6.2; 30.5)%], structural knee OA [3.1 (1.9; 7.3)%] and effusion [10.8 (6.0; 37.6)%]. Diastolic blood pressure [2.1 (1.6; 3.0)%] minimally mediated the association between BMI and clinical knee OA. PWV and pVWT did not mediate the association between BMI and OA.
Conclusions
cIMT and diastolic blood pressure minimally mediated the association of BMI with OA. This suggests that such mediation is trivial in the middle-aged population.
Keywords: OA, blood pressure, vessel wall stiffness, atherosclerosis, mediation analyses
Rheumatology key messages
To which extent CVD explains the association between obesity and OA has not been investigated.
Carotid intima-media thickness minimally mediated the associations of BMI with OA.
Mediation of the association between BMI and OA by preclinical CVD measures was trivial.
Introduction
Rheumatic musculoskeletal disorders (RMDs) are among the leading causes of disability in the middle-aged population. One of the most common RMDs is OA, which affects over three hundred million people globally. While the prevalence and burden of OA has already surged in the past decade [1], it is expected to increase even further in the coming years due to population ageing and an increasing prevalence of obesity [2]. Together with age and sex, obesity is a major risk factor for OA. Increased body weight results in an increase in mechanical stress, which plays a large role in the risk of OA [3–5]. However, increased mechanical loading does not fully explain the association between obesity and OA, which is apparent from the association of obesity with non-weightbearing joints such as the hand [6, 7].
Obesity is associated with a broad spectrum of systemic effects due to the release of proinflammatory mediators such as adipokines and lipids, resulting in metabolic dysregulation [8]. The role of obesity-related metabolic factors in OA has been of increasing interest in OA research, with a particular focus on the association between cardiovascular disease (CVD) and OA. While some suggest that both disorders might be due to a common pathway of chronic low-grade inflammation, others have suggested a causal relationship between the two. Recent meta-analyses have compiled the evidence on CVD incidence and risk factors in OA patients and showed an increased CVD risk in patients with OA compared with controls [9]. An explanation for this association may be an OA-related decrease in physical activity [10, 11]. In contrast, a reverse causal direction has also been proposed. OA might result from atherosclerotic vascular changes, resulting in a compromised blood flow with detrimental effects on the subchondral bone and on nutrient supply to the cartilage [12]. A recent systematic review of the currently available evidence concluded that an association between vascular pathology and risk of hand and knee OA may be present. However, findings varied and different results were obtained for the investigated OA phenotypes [13].
Moreover, to which extent CVD risk factors may actually explain the association between obesity and OA has not been investigated. Therefore, we aimed to assess the potential role of blood pressure, vessel wall stiffness and multiple subclinical atherosclerosis markers as mediators of the association between obesity and OA in a middle-aged population.
Materials and methods
Study population
The Netherlands Epidemiology of Obesity (NEO) study is a population-based, prospective cohort study, designed to investigate pathways that lead to obesity-related diseases and conditions. Detailed description of study design and data collection has been described elsewhere [14]. In short, men and women between 45 and 65 years with a self-reported BMI ≥27 kg/m2 living in the greater area of Leiden (The Netherlands) were eligible to participate. This resulted in an oversampling of individuals with overweight or obesity, to ensure an adequate number of responses from individuals with higher BMI. In addition, all inhabitants between 45 and 65 years from one municipality (Leiderdorp) were invited to participate in the NEO study irrespective of their BMI, allowing for a reference BMI distribution comparable to the general Dutch population [15]. In total, 6671 participants were included in the NEO study cohort. The Medical Ethical Committee of the Leiden University Medical Center (LUMC) approved the design of the study. All participants gave their written informed consent. The present study is a cross-sectional analysis of baseline measurements. We excluded participants with a missing physical examination (n = 14) and who reported to have concomitant other rheumatic diseases (n = 323).
Questionnaires
Participants completed standardized questionnaires on demographic and medical information, among which a history of inflammatory rheumatic diseases and CVD, and pain in hands and knees on most days of the last month. In addition, participants were asked to list any current medication, which was verified during the study visit.
Clinical assessment
Body weight (kg) and total body fat (%) were measured by bioelectrical impedance balance (TBF-310; Tanita Europe BV, Amsterdam, The Netherlands). BMI was calculated from measured body weight and height (kg/m2). Brachial blood pressure was measured three times with five min rest between consecutive measurements, in a seated position on the right arm using a validated automatic oscillometric device (OMRON, Model M10-IT, Omron Health Care Inc, IL, USA), from which the mean systolic and diastolic blood pressure were calculated. In participants using antihypertensive mediation, we adjusted for the systematic negative bias introduced by the antihypertensive treatment by adding a constant of 15 mmHg to the measured mean systolic blood pressure and 10 mmHg to the diastolic blood pressure, to account for potential shrinkage bias [16]. In addition, physical examination of the hands and knees was performed by trained research nurses, using a standardized scoring form. Of both hands, bony and soft swellings and deformities of the distal interphalangeal, proximal interphalangeal, metacarpophalangeal and first carpometacarpal joints were assessed. Regarding the knees, presence of bony swellings, pain of the bony margins and warmth upon palpation, crepitus and movement restriction were assessed. Hand and knee OA was defined according to the ACR clinical classification criteria [17, 18].
Magnetic resonance imaging of the knee
A random sample of 1285 participants without contra-indications (most notably metallic devices, claustrophobia or a body circumference of >1.70 m) underwent MRI of the right knee. Imaging was performed on a MR system operating at a 1.5 T field strength (Philips, Medical Systems, Best, The Netherlands), using a dedicated knee coil and a standardized scanning protocol as described earlier [19].
All MRI images were analysed using the validated knee OA scoring system (KOSS) [20] as described previously [19]. Structural knee OA was defined when a definite osteophyte and full thickness cartilage loss was present, or one of these features with at least two of the following: subchondral bone marrow lesions (BML), cyst, meniscal subluxation, maceration or degenerative tear, or partial thickness cartilage loss, according to modified criteria by Hunter et al. [21]. In addition, BMLs and joint effusion (grade 2 or higher vs smaller or absent) were investigated separately.
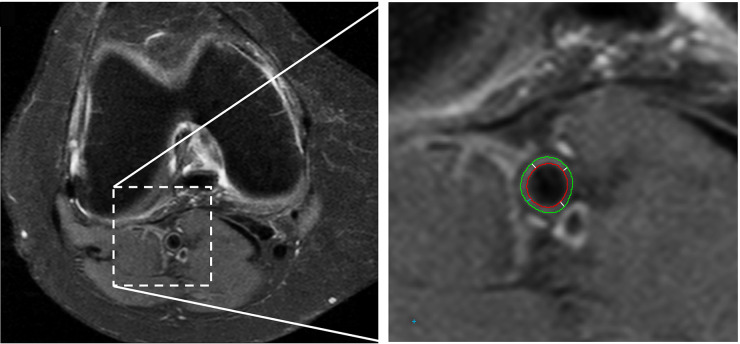
Popliteal vessel wall thickness (VWT) was assessed on axial fat-suppressed proton density images [repetition time TR/echo time (TE) 3225/15; echo train length 6, 4 mm slice thickness; 0.8 mm interslice gap] with a 150–160 mm field of view. The VesselMASS software package, developed at our institution [22], was used for semi-automated detection of the luminal and outer boundaries of the vessel wall on five consecutive slices (see Fig. 1). The popliteal VWT was calculated as the average perpendicular distance between the luminal and outer boundaries measured at 100 positions along the vessel wall circumference, and averaged over the five consecutive slices. The popliteal VWT could not be assessed in 10% of participants due to insufficient quality of the images.
Fig. 1.
Axial MR image of the knee used for assessment of the popliteal vessel wall thickness
The right image shows an enlargement of the popliteal artery area. The green line indicates the outer vessel wall boundary, the red line indicates the lumen. At each analysed slice, the vessel wall thickness is calculated as the average distance between the outer and inner vessel boundary (white lines).
Carotid intima-media thickness
Carotid intima-media thickness (IMT) (mm) was measured by ultrasonography of the far wall of the left and right common carotid arteries along a 15 mm long section 10 mm proximal to the bifurcation, with the participant in supine position. The distal common carotid arteries were visualized with a 7.5–10 MHz linear-array transducer (Art.Lab version 2.1, Esaote, Maastricht, The Netherlands) in B-mode setting. A wall track system was used to detect the lumen-intima and media-adventitia boundaries. Carotid IMT data was missing in 1% of participants.
Aortic pulse wave velocity
Pulse wave velocity (PWV) (m/second) of the aorta was assessed in a random sample of n = 2580 participants (without overlap with the group receiving a knee MRI) without contraindications for MRI. PWV was determined on a 1.5 T field strength whole‐body MRI scanner (Philips, Best, the Netherlands) using velocity‐encoded MRI. PWV was calculated by the ratio of the aortic path length between the measurement sites and the transit‐time of the propagating systolic pulse wave between the measurement sites. Data were analysed using in-house software (MASS and FLOW). Data was missing in 3% of participants due to insufficient coverage or quality of the scans.
Statistical analysis
In the NEO study there is an oversampling of participants with a BMI ≥27 kg/m2. In the present analyses, we aimed to make inferences on associations in the general population. To represent distributions and associations in the general population correctly, adjustment for this oversampling was made by weighting all individuals towards the BMI distribution of participants from the Leiderdorp municipality (n = 1671) [23] whose BMI distribution was similar to the general Dutch population [15]. All results were based on weighted analyses, using probability weights. Consequently, results apply to a population-based study without oversampling.
The clinical hand and knee OA outcome groups were mutually exclusive groups, while overlap between clinical and structural knee OA was allowed (co-occurrence in n = 62). The control group was defined as having no clinical hand or knee OA, nor structural knee OA.
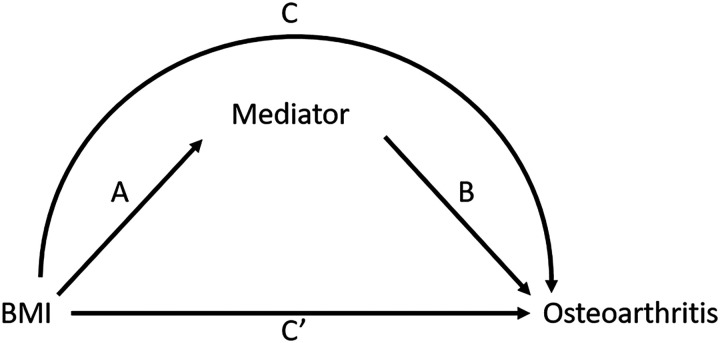
We investigated the mediating role of systolic and diastolic blood pressure, carotid IMT, popliteal VWT and aortic PWV by examining the total, direct and indirect effects according to the method by Baron and Kenny [24] as outlined in Fig. 2. We checked the fulfilment of the four assumptions of the Baron-Kenny framework, by assessing the association between: (i) BMI and OA with logistic regression analyses (total effect C); (ii) BMI and mediator with linear regression analyses (indirect effect A); (iii) mediator and OA with logistic regression analyses (indirect effect B); and (iv) if the association between BMI and OA attenuated after adding the mediator to the model (direct effect C’). Fulfilment of the assumptions was based on the size of the effect estimate in the regression analysis rather than statistical significance. Furthermore, the assumption of no exposure-mediator interaction was checked by adding an interaction term of the independent variable and mediator to the model of the total association. No statistical significance evidence (P <0.05) of interaction was found. For the models fulfilling all assumptions for mediation, we calculated the percentage mediation with the Stata package medeff. All analyses were adjusted for age, sex and education. Continuous variables (BMI, blood pressure, carotid IMT, popliteal VWT and aortic PWV) were standardized by rescaling them to a mean of zero and a standard deviation of one, to ensure a similar interpretation of the estimated effect. Therefore, the regression coefficient can be interpreted as the association with the dependent variable per standard deviation of the independent variable. Pearson correlation coefficients were calculated to examine the pairwise associations between the different potential mediating variables. We considered 0–0.19 as very weak, 0.2–0.39 as weak, 0.40–0.59 as moderate, 0.6–0.79 as strong and 0.8–1 as very strong correlations.
Fig. 2.
Causal diagram illustrating mediation analysis
Path C represents the total effect of obesity on OA. Path A and B represent the indirect effect via atherosclerosis. Path C’ is the direct effect of obesity on OA, controlled for atherosclerosis.
Several sensitivity analyses were performed. We repeated all analyses substituting BMI for total body fat. Furthermore, we repeated all analyses with exclusion of participants with a history of CVD. Lastly, we compared the percentage mediation calculated by medeff with calculation by generalized structural equation modelling with the Stata command gSEM, and with calculation according to the Sobel method [25]. Stata v.14.1 (StataCorp LP, College Station, TX, USA) was used for all analyses.
Results
Population characteristics
The study population consisted of 6334 participants with a mean (s.d.) age of 56 (6) years, of whom 55% were women (Table 1). Mean (s.d.) BMI was 26 (4) kg/m2. Clinical hand OA was present in 8% of participants, clinical knee OA in 10%. Structural knee OA was defined in 12% of participants, and bone marrow lesions (BML) and effusion in 32% and 12% of participants, respectively. The control group consisting of participants without clinical or structural OA comprised 76% of the study population. Mean (s.d.) systolic blood pressure was 134 (19) and diastolic blood pressure 85 (12). Mean (s.d.) carotid IMT was 0.62 (0.09) mm, popliteal VWT was 0.53 (0.05) mm, and aortic PWV was 6.56 (1.30) m/second.
Table 1.
Characteristics of the weighted NEO study population
| All n = 6334 | |
|---|---|
| General patient characteristics | |
| Age (year) | 56 (6) |
| Sex (% women) | 55 |
| Education (% high) | 46 |
| Body mass index (kg/m2) | 26 (4) |
| History of cardiovascular disease (%) | 6 |
| Antihypertensive medication (% users) | 23 |
| Exposure variables | |
| Systolic blood pressure (mmHg)a | 134 (19) |
| Diastolic blood pressure (mmHg)a | 85 (12) |
| Carotid intima-media thickness (mm) | 0.62 (0.09) |
| Popliteal artery vessel wall thickness (mm)b | 0.53 (0.05) |
| Aortic pulse wave velocity (m/s)b | 6.56 (1.30) |
| OA phenotypes | |
| Clinical hand OA (%) | 8 |
| Clinical knee OA (%) | 10 |
| Structural knee OA (%)c | 12 |
| Bone marrow lesions (%)c | 32 |
| Effusion (%)c | 12 |
Results are based on analyses weighted towards the BMI distribution of the general population (n = 6334). Numbers represent mean (s.d.) unless otherwise specified. a>Blood pressure was adjusted for antihypertensive medication use when applicable (systolic +15 mmHg, diastolic +10 mmHg). bPopliteal VWT (n = 1095) and PWV (n = 2382) measurements are performed in MRI subpopulations. cPercentage of participants who underwent knee MRI (n = 1285).
Correlation between blood pressure and atherosclerosis measures
Systolic and diastolic blood pressure were moderately (r = 0.40) to weakly (r = 0.34) correlated to aortic PWV, respectively. Carotid IMT and PWV were weakly correlated (r = 0.22); no correlation was present between carotid IMT and popliteal VWT (Supplementary Table S1, available at Rheumatology online).
Mediation of the association between obesity and OA
No exposure-mediator interaction was observed when interaction terms between BMI and the potential mediators were added to the logistic regression analyses between BMI and OA. Tables 2 and 3 show the associations of BMI with clinical hand and knee OA, and with structural knee OA, effusion and BML, respectively. In both tables, the second column shows the total effect between BMI and OA (path C). The third column presents the indirect effect between the independent variable (hypertension or atherosclerosis marker) via path A. In the fourth column, the direct effect between BMI and OA is given (path C′), as well as the indirect effect of hypertension or the atherosclerosis marker on OA, adjusted for BMI (path B).
Table 2.
Preclinical CVD markers as mediators in the association of BMI with clinical OA
| Independent variable | Dependent variable |
|||
|---|---|---|---|---|
| Total effect C | Indirect effect A | Direct effect C’ Indirect effect B | Mediation % (95% CI) | |
| Clinical hand OA OR (95% CI) | Mediator β (95% CI) | Clinical hand OA OR (95% CI) | ||
| BMI | 1.22 (1.08, 1.37) | 0.21 (0.18, 0.25) | 1.22 (1.08, 1.38) | NA |
| Systolic BP | 0.98 (0.84, 1.14) | |||
| BMI | 1.22 (1.08, 1.37) | 0.28 (0.25, 0.32) | 1.22 (1.08, 1.38) | NA |
| Diastolic BP | 0.98 (0.84, 1.15) | |||
| BMI | 1.21 (1.07, 1.36) | 0.23 (0.19, 0.27) | 1.19 (1.05, 1.34) | 10.6 (6.2, 30.5) |
| Carotid IMT | 1.09 (0.94, 1.25) | |||
| BMI | 1.56 (1.17, 2.08) | 0.01 (−0.06, 0.09) | 1.55 (1.16, 2.07) | NA |
| Popliteal VWT | 1.14 (0.84, 1.55) | |||
| BMI | 1.41 (1.15, 1.73) | 0.05 (−0.01, 0.11) | 1.41 (1.15, 1.73) | NA |
| Aortic PWV | 1.04 (0.81, 1.33) | |||
| Clinical knee OA OR (95% CI) | Mediator β (95% CI) | Clinical knee OA OR (95% CI) | ||
| BMI | 1.46 (1.32, 1.62) | 0.21 (0.17, 0.24) | 1.46 (1.31, 1.62) | NA |
| Systolic BP | 1.02 (0.90, 1.15) | |||
| BMI | 1.46 (1.32, 1.62) | 0.27 (0.24, 0.31) | 1.45 (1.31, 1.62) | 2.1 (1.6, 3.0) |
| Diastolic BP | 1.03 (0.91, 1.15) | |||
| BMI | 1.46 (1.32, 1.62) | 0.24 (0.20, 0.27) | 1.47 (1.33, 1.62) | NA |
| Carotid IMT | 0.97 (0.86, 1.09) | |||
| BMI | 1.20 (0.88, 1.64) | 0.03 (−0.04, 0.11) | 1.21 (0.89, 1.64) | NA |
| Popliteal VWT | 0.95 (0.74, 1.24) | |||
| BMI | 1.37 (1.12, 1.67) | 0.05 (−0.00, 0.11) | 1.37 (1.12, 1.67) | NA |
| Aortic PWV | 0.96 (0.76, 1.21) | |||
Results are based on analyses weighted towards the BMI distribution of the general population. Due to analyses in subpopulation and control definitions, numbers included in the analyses vary; numbers included are provided Supplementary Fig. 1, available at Rheumatology online. Continuous variables were standardized (mean 0, s.d. 1), s.d. BMI=4, s.d. systolic BP=19, s.d. diastolic BP=12, s.d. carotid IMT=0.09, s.d. popliteal VWT=0.05, s.d. aortic PWV=1.30. Analyses were adjusted for age, sex and education. BP: blood pressure; IMT: intima media thickness; NA: not applicable; OR: odds ratio; PWV: pulse wave velocity; VWT: vessel wall thickness.
Table 3.
Preclinical CVD markers as mediators in the association of BMI with structural knee OA
| Independent variable | Dependent variable |
|||
|---|---|---|---|---|
| Total effect C | Indirect effect A | Direct effect C’ Indirect effect B | Mediation % (95% CI) | |
| Structural knee OA OR (95% CI) | Mediator β (95% CI) | Structural knee OA OR (95% CI) | ||
| BMI | 1.58 (1.24, 2.03) | 0.29 (0.21, 0.38) | 1.67 (1.33, 2.12) | NA |
| Systolic BP | 0.81 (0.64, 1.02) | |||
| BMI | 1.58 (1.24, 2.03) | 0.35 (0.27, 0.43) | 1.65 (1.30, 2.09) | NA |
| Diastolic BP | 0.87 (0.70, 1.09) | |||
| BMI | 1.58 (1.23, 2.03) | 0.33 (0.25, 0.40) | 1.56 (1.19, 2.06) | 3.1 (1.9, 7.3) |
| Carotid IMT | 1.03 (0.80, 1.34) | |||
| BMI | 1.50 (1.13, 1.99) | 0.00 (−0.07, 0.08) | 1.50 (1.13, 1.99) | NA |
| Popliteal VWT | 1.09 (0.85, 1.38) | |||
| Effusion OR (95% CI) | Mediator β (95% CI) | Effusion OR (95% CI) | ||
| BMI | 1.46 (1.14, 1.88) | 0.30 (0.22, 0.37) | 1.48 (1.13, 1.94) | NA |
| Systolic BP | 0.96 (0.74, 1.26) | |||
| BMI | 1.46 (1.14, 1.88) | 0.34 (0.27, 0.41) | 1.54 (1.19, 2.01) | NA |
| Diastolic BP | 0.84 (0.63, 1.11) | |||
| BMI | 1.44 (1.12, 1.86) | 0.33 (0.26, 0.40) | 1.40 (1.06, 1.84) | 10.8 (6.0, 37.6) |
| Carotid IMT | 1.12 (0.82, 1.52) | |||
| BMI | 1.40 (1.06, 1.84) | 0.03 (−0.03, 0.09) | 1.38 (1.04, 1.82) | NA |
| Popliteal VWT | 1.29 (1.00, 1.67) | |||
| BML OR (95% CI) | Mediator β (95% CI) | BML OR (95% CI) | ||
| BMI | 1.05 (0.87, 1.27) | NA | NA | NA |
| Systolic BP | ||||
| BMI | 1.05 (0.87, 1.27) | NA | NA | NA |
| Diastolic BP | ||||
| BMI | 1.06 (0.88, 1.28) | NA | NA | NA |
| Carotid IMT | ||||
| BMI | 0.97 (0.79, 1.20) | NA | NA | NA |
| Popliteal VWT | ||||
Results are based on analyses weighted towards the BMI distribution of the general population. Due to analyses in subpopulation and control definitions numbers included in the analyses vary; numbers included are provided Supplementary Fig. 1, available at Rheumatology online. Continuous variables were standardized (mean 0, s.d. 1), s.d. BMI=5, s.d. systolic BP=20, s.d. diastolic BP=12, s.d. carotid IMT=0.09, s.d. popliteal VWT=0.05. Analyses were adjusted for age, sex and education. BML: bone marrow lesion; BP: blood pressure; IMT: intima media thickness; NA: not applicable; OR: odds ratio; VWT: vessel wall thickness.
Clinically defined hand and knee OA
After adjusting for age, sex and education, a positive association of BMI with clinically defined hand and knee OA was observed (Table 2). Furthermore, BMI was positively associated with systolic and diastolic blood pressure. The association of BMI with clinical hand did not attenuate after adding systolic or diastolic blood pressure to the model. In other words, we observed no mediation of the association between BMI and clinical hand OA by blood pressure. The association between BMI and clinical knee OA attenuated from 1.46 (1.32; 1.62) to 1.45 (1.31; 1.62) upon adding diastolic blood pressure to the model, representing 2.1% (1.6; 3.0) mediation.
BMI was positively associated with carotid IMT, and carotid IMT was weakly associated with clinical hand OA with an OR of 1.09 (0.94; 1.25). Carotid IMT mediated the association of BMI with clinical hand OA with 10.6% (6.2; 30.5). No association between carotid IMT and clinical knee OA was observed; hence, no mediation was found.
No associations between BMI and popliteal VWT, and between BMI and aortic PWV were observed. Therefore, mediation of the association between BMI and OA by popliteal VWT and aortic PWV was deemed to be absent.
Structurally defined knee OA
BMI was associated with structurally defined knee OA and effusion, but not with BMLs. Systolic blood pressure and diastolic blood pressure were not positively associated with structural knee OA and the mediation assumptions were not fulfilled. In addition, no attenuation of the association between systolic blood pressure and effusion was observed upon addition of the mediator to the model; hence, mediation was deemed absent.
Carotid IMT attenuated the association between BMI and structural knee OA from 1.58 (1.23; 2.03) to 1.56 (1.19; 2.06), representing 3.1% (1.9; 7.3) mediation. In addition, the association between BMI and effusion was mediated by carotid IMT with 10.8% (6.0; 37.6). Similar to the results described above, BMI was not associated with popliteal VWT in the knee MRI subpopulation.
Sensitivity analyses
We substituted BMI for total body fat and repeated all analyses, which showed similar results (Supplementary Tables S2 and S3, available at Rheumatology online). In addition, repeating the analyses without participants with a history of CVD resulted in similar findings (Supplementary Tables S4 and S5, available at Rheumatology online). We compared the calculation of the percentage mediation by the medeff command with gSEM and the Sobel method. All three methods yielded similar percentages mediation. However, the observed confidence intervals varied and were generally broader using gSEM compared with medeff (Supplementary Table S6, available at Rheumatology online).
Discussion
In the present population-based study, we examined the role of blood pressure, vessel wall stiffness, carotid IMT and popliteal VWT as mediators of the association of BMI with clinically defined hand and knee OA, and structural knee OA. As expected, we observed that BMI was positively associated with all OA outcomes. A small attenuation of the estimated effect of the association between BMI and clinical knee OA was observed when diastolic blood pressure was added to the model. Furthermore, carotid IMT minimally mediated the associations of BMI with clinical hand OA, structural knee OA, and effusion. No evidence for mediation by PWV or popliteal VWT was observed in any of the associations.
We present novel findings, as the mediating role of preclinical CVD markers in the association between obesity and OA has not previously been investigated. The associations between these markers and OA (pathway B) have been reported, offering a comparison with the present results. We observed that a small proportion of the association of BMI with OA was mediated by carotid IMT. Although this could represent a chance finding, we observed this for multiple phenotypes: clinical hand OA, structural knee OA, as well as effusion. In addition, this is in line with previous results [26]. Furthermore, other atherosclerosis measures have been investigated previously. While a positive association between arterial calcifications and hand OA was shown [27], in knee OA, contrasting results have been found [26, 28]. Also, a positive association of carotid plaques was observed with hand OA, but not knee OA [26, 27].
We did not observe an association of BMI with popliteal VWT. Hence, no mediation of the association between BMI and OA by popliteal VWT was found. Because carotid IMT and popliteal VWT are both measures of atherosclerosis, we anticipated similar results. The unexpected discrepancy might be explained by the very limited variation in popliteal VWT in our population. Moreover, in 10%, the quality of the knee MRIs was not sufficient to measure the popliteal VWT, and the resulting missingness was not completely at random. Rather, we observed that insufficient quality MRIs occurred more often in participants with a higher BMI, implying that the popliteal VWT scores were missing at random. However, it is unlikely that being missing is related to the popliteal VWT measurements, as we did not take the VWT into account in the decision to discard the MRIs. Therefore, it is doubtful if selection bias has occurred. Moreover, even if being missing might have distorted the estimated effect, this influence is unlikely so strong as to cause the observed null association. However, previous research does suggest a positive association between popliteal VWT and generalized OA [20]. In addition, in a population-based cohort without knee disorders, popliteal VWT was negatively associated with cartilage volume, but not BMLs, in cross-sectional and longitudinal analyses [29, 30].
Blood pressure played no relevant mediating role in the association of BMI with OA in our population. Current evidence on the association between blood pressure and OA is inconclusive. In the Rotterdam Study, a population-based cohort comparable to the NEO study, no association between hypertension and hand OA was observed after adjustment for BMI [31]. In the Framingham study, the association of hypertension with OA was questionable [32]. In contrast to this, in a community-based study with only women, hypertension was associated with painful interphalangeal joint OA, even after adjustment for BMI [33]. The results from longitudinal studies are equally contradictory [32, 34]. Similarly, some studies have shown a positive association between hypertension and radiographic knee OA [35–37], while others found no associations [38, 39]. In addition, we found no mediation by aorta PWV, which is in line with a lack of association of PWV with OA observed previously [40, 41].
Our study has notable strengths, among which is the large sample size of the study population. Furthermore, the design of the NEO study, aiming to investigate obesity-related conditions in the middle-aged general population, enabled the study of many of the previously investigated preclinical CVD markers. Often, the various measures have been investigated separately, which hinders comparisons. Moreover, in contrast to most studies, we investigated both clinically defined hand and knee OA according to the ACR criteria, as well as the more frequently investigated structural knee OA phenotype. However, our study also has some limitations. The MRIs have been performed in a subpopulation, reducing the number of participants in whom structural knee OA could be measured, and limiting the number of popliteal VWT and aorta PWV measurements, resulting in a loss of power. To limit the effect this loss of power may have on our conclusions, we focused on the size of the observed effects rather than on statistical significance. Furthermore, perhaps as a result of the relatively healthy and young study population, we observed a narrow distribution of carotid IMT and popliteal VWT, which might have resulted in an underestimation of the mediating effect. In addition, our study has a cross-sectional design, which hinders causal interpretations.
Possible explanations for an association between atherosclerosis and OA have been extensively discussed by Bierma-Zeinstra and Waarsing [42]. Due to the long lead-in time of both CVD and OA, as well as the frequent co-occurrence of other morbidities, it is challenging to study the causal direction of the effect. Both directions of the association have been investigated, adding to the controversy on this subject. Moreover, although most studies showed some associations, results of these studies were contradicting with regard to associated joints and type of atherosclerosis measure under investigation, making it difficult to draw a straightforward conclusion [42]. Alternatively, CVD and OA may co-occur as a result of common pathophysiological processes, such as obesity-related altered fat metabolism, activation of the innate immune system or changes to the collagen composition that might affect both joints and vascular structure [42, 43]. Overall, despite the increasing attention for this subject, much of the observed association remains incompletely understood. Further research is warranted to draw clear and robust conclusions.
To conclude, in our population, mediation of the association between BMI and OA by preclinical CVD measures was questionable. Future research is warranted to further elucidate the association between CVD and OA, which perhaps could be explained by an alternative hypothesis such as shared pathophysiological processes.
M.L. was the principal investigator and contributed to design of the study, data acquisition, data analysis and interpretation, and drafting of the article. R.J.vdG., R.dM., F.R.R. and M.K. contributed to study design, data acquisition, data interpretation and critical revision of the article. S.lC. contributed to study design, data analysis and interpretation and critical revision of the article. All authors give final approval of the submitted article.
Supplementary Material
Acknowledgements
The authors thank all individuals who participated in the NEO study, and all participating general practitioners for inviting eligible participants. The authors also thank P.R. van Beelen and all research nurses for collection of the data, P.J. Noordijk and her team for sample handling and storage, and I. de Jonge for data management of the NEO study.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The NEO study is supported by the participating Departments, the Division and the Board of Directors of the Leiden University Medical Centre, and by the Leiden University, Research Profile Area ‘Vascular and Regenerative Medicine’.
Data availability statement
The data underlying this article cannot be shared publicly due to the privacy of the participants of the NEO study and legal reasons (NEO study participants did not sign informed consent to make their data publicly available). The data is available upon request to interested qualified researchers. Data requests should be sent to the NEO Executive Board, which can be contacted via https://www.lumc.nl/org/neo-studie/contact/.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bijlsma JWJ, Berenbaum F, Lafeber FPJG.. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26. [DOI] [PubMed] [Google Scholar]
- 3.Radin EL, Paul IL, Rose RM.. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet 1972;299: 519–22. [DOI] [PubMed] [Google Scholar]
- 4.Davis MA, Ettinger WH, Neuhaus JM.. Obesity and osteoarthritis of the knee: evidence from the National Health and Nutrition Examination Survey (NHANES I). Semin Arthritis Rheum 1990;20:34–41. [DOI] [PubMed] [Google Scholar]
- 5.Visser AW, de Mutsert R, le Cessie S. et al. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis 2015;74:1842–7. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf E, Nelissen RG, Ioan-Facsinay A. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010;69:761–5. [DOI] [PubMed] [Google Scholar]
- 7.Visser AW, Ioan-Facsinay A, de Mutsert R. et al. Adiposity and hand osteoarthritis: the Netherlands Epidemiology of Obesity study. Arthritis Res Ther 2014;16:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng CN, Saltiel AR.. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathieu S, Couderc M, Tournadre A, Soubrier M.. Cardiovascular profile in osteoarthritis: a meta-analysis of cardiovascular events and risk factors. Joint Bone Spine 2019;86:679–84. [DOI] [PubMed] [Google Scholar]
- 10.Kendzerska T, Jüni P, King LK. et al. The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: a population-based cohort study. Osteoarthritis Cartilage 2017;25:1771–80. [DOI] [PubMed] [Google Scholar]
- 11.Hoeven TA, Leening MJG, Bindels PJ. et al. Disability and not osteoarthritis predicts cardiovascular disease: a prospective population-based cohort study. Ann Rheum Dis 2015;74:752–6. [DOI] [PubMed] [Google Scholar]
- 12.Findlay DM. Vascular pathology and osteoarthritis. Rheumatol Oxf Engl 2007;46:1763–8. [DOI] [PubMed] [Google Scholar]
- 13.Hussain SM, Dawson C, Wang Y. et al. Vascular pathology and osteoarthritis: a systematic review. J Rheumatol 2020;47:748–60. [DOI] [PubMed] [Google Scholar]
- 14.de Mutsert R, den Heijer M, Rabelink TJ. et al. The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection. Eur J Epidemiol 2013; 28:513–23. [DOI] [PubMed] [Google Scholar]
- 15.Ministerie van VWS. Hoeveel mensen hebben overgewicht ? 2013. www.rivm.nl/nldemaat (5 August 2018, date last accessed).
- 16.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR.. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005;24:2911–35. [DOI] [PubMed] [Google Scholar]
- 17.Altman R, Alarcón G, Appelrouth D. et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 1990;33:1601–10. [DOI] [PubMed] [Google Scholar]
- 18.Altman R, Asch E, Bloch D. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29: 1039–49. [DOI] [PubMed] [Google Scholar]
- 19.Visser AW, de Mutsert R, Loef M. et al. The role of fat mass and skeletal muscle mass in knee osteoarthritis is different for men and women: the NEO study. Osteoarthritis Cartilage 2014;22:197–202. [DOI] [PubMed] [Google Scholar]
- 20.Kornaat PR, Ceulemans RYT, Kroon HM. et al. MRI assessment of knee osteoarthritis: knee Osteoarthritis Scoring System (KOSS)–inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol 2005;34:95–102. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DJ, Arden N, Conaghan PG. et al. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage 2011;19:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adame IM, van der Geest RJ, Wasserman BA. et al. Automatic segmentation and plaque characterization in atherosclerotic carotid artery MR images. Magma N Y N 2004;16:227–34. [DOI] [PubMed] [Google Scholar]
- 23.Lumley T. Analysis of compex survey samples. 2004. http://www.jstatsoft.org/v09/i08/paper (5 August 2018, date last accessed).
- 24.Baron RM, Kenny DA.. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82. [DOI] [PubMed] [Google Scholar]
- 25.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol 1982;13:290–312. [Google Scholar]
- 26.Hoeven TA, Kavousi M, Clockaerts S. et al. Association of atherosclerosis with presence and progression of osteoarthritis: the Rotterdam Study. Ann Rheum Dis 2013;72:646–51. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson H, Helgadottir GP, Aspelund T. et al. Hand osteoarthritis in older women is associated with carotid and coronary atherosclerosis: the AGES Reykjavik study. Ann Rheum Dis 2009;68:1696–700. [DOI] [PubMed] [Google Scholar]
- 28.Gielis WP, Welsing PMJ, van Spil WE. et al. A sex-specific association between incident radiographic osteoarthritis of hip or knee and incident peripheral arterial calcifications: 8-year prospective data from Cohort Hip and Cohort Knee (CHECK). Osteoarthritis Cartilage 2017;25:1814–21. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Dawson C, Hanna F, Fairley J, Cicuttini FM.. Association between popliteal artery wall thickness and knee cartilage volume loss in community-based middle-aged women without clinical knee disease. Maturitas 2015;82:222–7. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Novera D, Wluka AE. et al. Association between popliteal artery wall thickness and knee structure in adults without clinical disease of the knee: a prospective cohort study. Arthritis Rheumatol 2015;67:414–22. [DOI] [PubMed] [Google Scholar]
- 31.Dahaghin S, Bierma-Zeinstra SMA, Koes BW, Hazes JMW, Pols HAP.. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann Rheum Dis 2007;66:916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strand MP, Neogi T, Niu J, Felson DT, Haugen IK.. Association between metabolic syndrome and radiographic hand osteoarthritis: data from a community-based longitudinal cohort study. Arthritis Care Res 2018;70:469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Santos M, Judge A, Gulati M. et al. Association of metabolic syndrome with knee and hand osteoarthritis: A community-based study of women. Semin Arthritis Rheum 2019;48:791–8. [DOI] [PubMed] [Google Scholar]
- 34.Marshall M, Peat G, Nicholls E. et al. Metabolic risk factors and the incidence and progression of radiographic hand osteoarthritis: a population-based cohort study. Scand J Rheumatol 2019;48:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura N, Muraki S, Oka H. et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012;20:1217–26. [DOI] [PubMed] [Google Scholar]
- 36.Hart DJ, Doyle DV, Spector TD.. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol 1995;22: 1118–23. [PubMed] [Google Scholar]
- 37.Monira Hussain S, Wang Y, Cicuttini FM. et al. Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: a prospective cohort study. Semin Arthritis Rheum 2014;43:429–36. [DOI] [PubMed] [Google Scholar]
- 38.Davis MA, Ettinger WH, Neuhaus JM.. The role of metabolic factors and blood pressure in the association of obesity with osteoarthritis of the knee. J Rheumatol 1988;15:1827–32. [PubMed] [Google Scholar]
- 39.Niu J, Clancy M, Aliabadi P, Vasan R, Felson DT.. Metabolic syndrome, its components, and knee osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheumatol 2017;69:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saleh AS, Najjar SS, Muller DC. et al. Arterial stiffness and hand osteoarthritis: a novel relationship? Osteoarthritis Cartilage 2007;15:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldsmith GM, Aitken D, Cicuttini FM. et al. Osteoarthritis bone marrow lesions at the knee and large artery characteristics. Osteoarthritis Cartilage 2014;22:91–4. [DOI] [PubMed] [Google Scholar]
- 42.Bierma-Zeinstra SMA, Waarsing JH.. The role of atherosclerosis in osteoarthritis. Best Pract Res Clin Rheumatol 2017;31:613–33. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes GS, Valdes AM.. Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur J Clin Invest 2015;45:405–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of the participants of the NEO study and legal reasons (NEO study participants did not sign informed consent to make their data publicly available). The data is available upon request to interested qualified researchers. Data requests should be sent to the NEO Executive Board, which can be contacted via https://www.lumc.nl/org/neo-studie/contact/.