Abstract
Objectives
We aimed to conduct a systematic review and meta-analysis on the incidence and prevalence of SSc covering the entire literature.
Methods
This study followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement of 2009. We conducted a systematic search in MEDLINE, Web of Science and Embase to identify articles reporting incidence and/or prevalence of SSc. Two authors conducted the search, reviewed articles for inclusion and extracted relevant data. We used random-effects models to estimate the pooled prevalence and incidence of SSc and performed subgroup analyses by sex, case definition and region to investigate heterogeneity. We explored the association between calendar period and reported estimates using meta-regression.
Results
Among 6983 unique records identified, we included 61 studies of prevalence and 39 studies of incidence in the systematic review. The overall pooled prevalence of SSc was 17.6 (95% CI 15.1, 20.5) per 100 000 and the overall pooled incidence rate of SSc was 1.4 (95% CI 1.1, 1.9) per 100 000 person-years. We observed significant regional variations in reported estimates; studies conducted in North America reported considerably higher estimates than other regions. The pooled incidence and prevalence in women were five times higher than in men. More recent studies reported higher estimates than older ones.
Conclusion
In this comprehensive review of the incidence and prevalence of SSc across the world, there was large heterogeneity among estimates, which should be taken into consideration when interpreting the results.
Keywords: systemic sclerosis, systematic review, meta-analysis, incidence, prevalence
Rheumatology key messages
Incidence and prevalence estimates of SSc vary considerably between studies.
There is a temporal trend towards increased incidence and prevalence over calendar period.
This increase is likely due to increased awareness and improvement in diagnostic methods and criteria.
Introduction
SSc is an autoimmune rheumatic disease characterized by microvascular damage and generalized fibrosis in the skin and visceral organs. SSc has a broad spectrum of clinical manifestations, varying from RP and fatigue to more serious complications such as pulmonary arterial hypertension and lung fibrosis [1–4]. SSc, like other autoimmune diseases, is overrepresented in women compared with men [5]. Due to the multidimensional nature of its pathophysiology and manifestations, the diagnostic criteria of SSc have significantly evolved over time. In 1980, the ARA proposed preliminary criteria for the classification of SSc known as the ACR 1980 criteria [6]. They were followed by a new set proposed by LeRoy in 1988 [7], which were revised in 2001 by LeRoy and Medsger and divided the disease into three subsets: diffuse cutaneous, limited cutaneous and limited [8]. A collaboration between the ACR and the EULAR in 2013 yielded new classification criteria that proved to be superior to ACR’s 1980 criteria in terms of sensitivity and specificity [9]. The ACR/EULAR 2013 criteria considered new knowledge and techniques in autoimmunity and nailfold capillaroscopy, and new insights on the importance of vascular abnormalities as opposed to the previous focus on the presence of fibrosis.
SSc has throughout the literature been described as a rare disease. Reports on its occurrence differ greatly with respect to geographic region, the criteria the diagnosis was based on, population size and study design. A systematic review and meta-analysis on SSc incidence and prevalence has recently been published [10]. Because only studies published between 2006 and 2016 were included, any potential temporal trends and the impact of changes in diagnostic criteria over time on SSc incidence and prevalence could not be assessed. More recent studies tend to report higher estimates than older ones; SSc incidence in Finland was 0.4 per 100 000 person-years in 1990 [11] and 4.4 in 2010 [12]. Significant regional variations have also been reported where studies in Europe and the USA reported incidence and prevalence estimates 5–10 times higher than in Asia [13–17]. Moreover, no meta-analysis on the incidence of SSc exists.
Herein, we conducted a systematic review and meta-analysis to estimate the incidence and prevalence of SSc, overall and by sex, SSc case definition and geographic region, without imposing any restriction on calendar period of published studies.
Methods
This study followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement of 2009 [18].
Eligibility criteria
We considered studies that reported (i) the methods of diagnosing SSc [International Classification of Diseases (ICD) codes, calendar period relevant classification criteria or doctor’s opinion], (ii) a clearly defined denominator (population-based, hospital-based or outpatient clinic-based) and (iii) the incidence rate and/or prevalence of SSc (point prevalence and/or period prevalence).
Information sources
We conducted a systematic search in three databases, MEDLINE, Web of Science and Embase, for articles published in English with no restriction on publication year. Our search was last updated on 20 October 2020.
Search
We used keywords for SSc, incidence, prevalence and epidemiology in each database, in addition to corresponding MeSH terms and Emtree terms in MEDLINE and Embase, respectively. Our search strategy is presented in Supplementary Data S1, available at Rheumatology online. In addition, reference lists of review articles were screened to find studies that our search may have missed. Furthermore, we screened abstracts submitted to the two main rheumatology conferences between 2014 and 2018, the EULAR Congress and ACR Meeting. We contacted the first authors of relevant abstracts by e-mail to request additional data.
Study selection
We imported records retrieved through our search to the EndNote software where duplicates were removed. Two authors (M.B., M.H.) screened the titles and abstracts of the remaining records. Full texts of remaining relevant studies were assessed for eligibility, as well as additional data received from ACR/EULAR abstract authors. With regard to the meta-analyses, for studies reporting multiple prevalence estimates for several time periods or years, we included the most recent estimate, or the one covering the whole study period in the meta-analysis of incidence. If multiple estimates corresponding to different criteria were reported, we considered the estimate corresponding to the most recent criteria. For studies overlapping with one another in terms of denominator, the most recent study with the broadest denominator was considered. We considered estimates using capture–recapture when reported, and crude estimates before adjusting for sex and/or age when reported. We considered estimates using the adult population as a denominator when reported. If multiple estimates for different, independent populations, such as different cities or native groups, were reported, we regarded them as separate studies. For the study by Robinson et al. [19], we considered prevalence estimates requiring at least one inpatient stay or at least two ambulatory encounters. For the study by Fan et al. [20], we considered prevalence and incidence estimates requiring at least two medical claims for SSc.
Data items
We extracted the following information from eligible articles:
Study design, country, calendar period, publication year, percentage of women, denominator size, person-years, the number of prevalent and/or incident cases, and case definition.
Overall prevalence and incidence estimates as well as estimates stratified by sex.
Synthesis of results
We used random-effects models to pool incidence rates (using log transformation) and prevalence proportions (using logit transformation) across studies. We used the I2 statistic to assess heterogeneity among studies. When denominator size or number of prevalent cases were not reported in primary studies, we calculated them using other reported measures or contacted authors for additional information. If incidence studies reported mean annual incidence without providing person-time denominator, we calculated the person-time denominator using other reported measures. In studies reporting estimates stratified by sex, the person-time denominator for each sex, if not provided, was calculated using other reported measures. We examined publication bias using funnel plots and Egger’s test. We performed the statistical analyses using the package ‘meta’ [21] in R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Additional analyses
We performed subgroup analyses by case definition and geographic region to explore heterogeneity of pooled incidence and prevalence. In terms of case definition, we grouped studies into ACR 1980, LeRoy 1988, LeRoy and Medsger 2001, ACR/EULAR 2013, ICD codes and Other/Doctor’s opinion. Other/Doctor’s opinion included studies using different scoring systems to identify SSc cases or other inclusion criteria specific for each study as well as studies where SSc diagnosis was made based on doctor’s opinion without specifying the criteria used. Studies identifying SSc patients as those fulfilling multiple classification criteria were grouped under the most recent criteria. For the subgroup analysis of geographic region, we grouped studies according to continent: Africa, Asia, Europe, North America, Oceania and South America. We evaluated the impact of calendar period on prevalence and incidence by random-effects meta-regression. To test the impact of studies with small denominator size on the pooled prevalence estimates, we performed a sensitivity analysis where studies with a denominator smaller than 10 000 were excluded.
Results
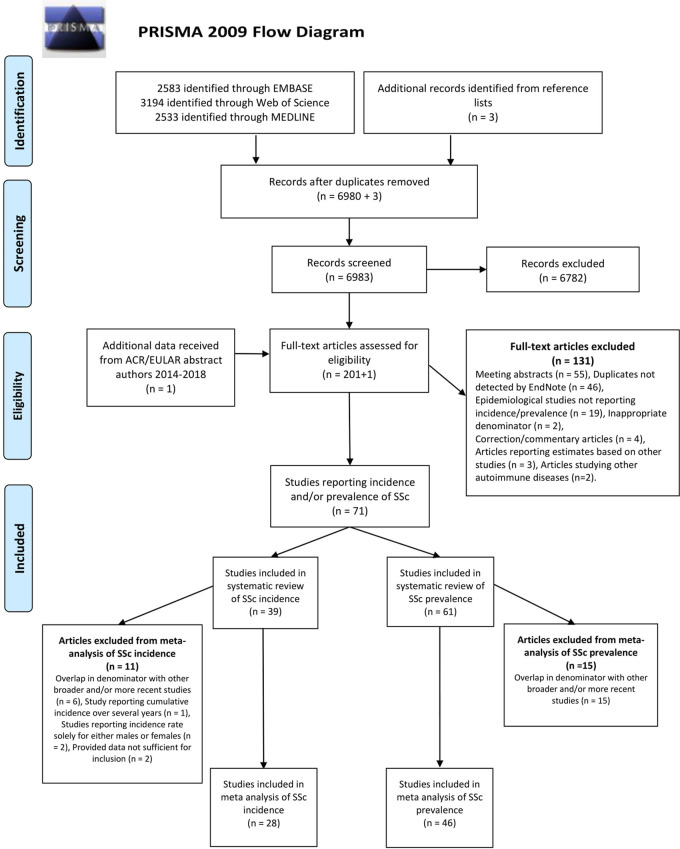
The PRISMA 2009 flow diagram (Fig. 1) summarizes the screening and study selection process. Of the 6983 unique records identified in the three databases, we excluded 6721 based on abstract and title. The full text of the remaining records (n = 201), as well as additional data received from one ACR/EULAR abstract author, were read and assessed for eligibility. We excluded 131 articles due to the following: meeting abstracts (n = 55), duplicates not previously detected by EndNote (n = 46), studies not reporting incidence/prevalence (n = 19), inappropriate denominator (n = 2), correction/commentary articles (n = 4), articles reporting estimates based on other studies (n = 3) and articles studying other autoimmune diseases (n = 2). The exclusion of the 55 meeting abstracts was mainly due to duplicates and later published full-length articles already included. Thus, we identified 71 studies reporting incidence and/or prevalence estimates of SSc. SSc prevalence was reported in 61 studies while incidence was reported in 39 studies. Table 1 summarizes the characteristics of the studies.
Fig. 1.
PRISMA 2009 flow diagram for process and outcome of the study selection strategy
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Summary of characteristics of studies reporting incidence and/or prevalence of SSc
| Author year | Country | Case definition method | Prevalence calendar period | Incidence calendar period |
|---|---|---|---|---|
| Abbot 2020 [22] | Australia | ACR/EULAR 2013 | 2018 | N/A |
| Airò 2007 [23] | Italy | ACR 1980 and/or LeRoy and Medsger 2001 | g | N/A |
| Airò 2020 [15] | Italy | ACR 1980 or ACR/EULAR 2013 | g | g |
| Alamanos 2005 [24] | Greece | ACR 1980 | 2002 | 1981-2002 |
| Allcock 2004 [25] | UK | ACR 1980 or a | 2000 | N/A |
| Anagnostopoulos 2010 [26] | Greece | ACR 1980 | 2007–2008 | N/A |
| Andréasson 2014 [27] | Sweden | ACR/EULAR 2013 | 2010 | 2006–2010 |
| Arias-Nuñez 2008 [28] | Spain | ACR 1980 and/or LeRoy and Medsger 2001 | 2006 | 1988–2006 |
| Arnett 1996 [17] | USA | ACR 1980 | 1990–1994 | N/A |
| Bajraktari 2013 [29] | Kosovo | ACR 1980 | 2010 | g |
| Barnabe 2012 [30] | Canada | ICD codes | 2007 | N/A |
| Bauer 2013 [31] | USA | LeRoy 1988 | 2010 | 1980–2010 |
| Bernatsky 2009 [32] | Canada | ICD codes | 2003 | N/A |
| Butt 2018 [33] | Denmark | ICD codes | N/A | 1995–2015 |
| Çakır 2012 [34] | Turkey | ACR 1980 | ? | N/A |
| Chandran 1995 [35] | Australia | Doctor’s opinion | g | N/A |
| Ciaffi 2021 [36] | Italy | Doctor’s opinion | 2016 | 2016 |
| Eason 1981 [37] | New Zealand | ACR 1980 | N/A | 1970–1979 |
| Eaton 2010 [38] | Denmark | ICD codes | g | N/A |
| El Adssi 2013 [39] | France | ACR 1980 and/or LeRoy and Medsger 2001 | 2006 | N/A |
| Elfving 2016 [12] | Finland | ACR/EULAR 2013 | N/A | 2010 |
| Englert 2005 [40] | Australia | ACR 1980 | 1991 | N/A |
| Englert 1999 [41] | Australia | ACR 1980 or a | 1988 | N/A |
| Fan 2020 [20] | USA | ICD codes | 2011–2016 | 2011–2016 |
| Fernández-Ávila 2020 [42] | Colombia | ICD codes | 2012–2016 | N/A |
| Fretheim 2020 [43] | Norway | ACR 1980 and/or ACR/EULAR 2013 | 2013 | N/A |
| Furst 2012 [16] | USA | ICD codes | 2008 | 2003–2008 |
| García Rodrígues 2019 [44] | UK | Doctor’s opinion | 2012 | 2000–2012 |
| Geirsson 1994 [45] | Iceland | ACR 1980 | 1990 | 1975–1990 |
| Hoffmann-Vold 2012 [46] | Norway | ACR 1980 and/or LeRoy and Medsger 2001 | g | N/A |
| Horimoto 2017 [47] | Brazil | LeRoy and Medsger 2001 or ACR/EULAR 2013 | 2014 | 2014 |
| Hvidberg 2020 [48] | Denmark | ICD codes | 2013 | N/A |
| Kaipiainen-Seppanen 1996 [11] | Finland | ACR 1980 or CREST | N/A | 1990 |
| Kanecki 2017 [49] | Poland | ICD codes | 2012 | 2008–2012 |
| Kang 2018 [13] | South Korea | ACR 1980 | g | 2008–2013 |
| Kim 2020 [14] | South Korea | ACR 1980 | 2016 | N/A |
| Kuo 2016 [50] | Taiwan | ICD codes | 2010 | N/A |
| Kuo 2011 [51] | Taiwan | ICD codes | g | 2002–2007 |
| Kurland 1969 [52] | USA | Doctor’s opinion | g | g |
| Laing 1997 [53] | USA | ACR 1980 or b | N/A | 1985–1991c |
| Le Guern 2004 [54] | France | ACR 1980 and/or LeRoy and Medsger 2001 | 2001 | N/A |
| Lo Monaco 2011 [55] | Italy | LeRoy and Medsger 2001 | 1999–2007 | 1999–2007 |
| Madu 2019 [56] | Botswana | Doctor’s opinion | N/A | 2008–2015 |
| Maricq 1989 [57] | USA | ACR 1980 | 1985 | N/A |
| Mayes 2003 [58] | USA | ACR 1980 or d | 1989–1991 | 1989–1991 |
| Medsger 1971 [59] | USA | Doctor’s opinion | N/A | 1947–1968 |
| Medsger 1978 [60] | USA | Doctor’s opinion | N/A | 1963–1968c |
| Meyer 2016 [61] | France | ACR 1980 and/or LeRoy and Medsger 2001 | 2008 | N/A |
| Michet 1985 [62] | USA | ACR 1980 | g | g |
| Peláez-Ballestas 2018 [63] | Latin America | Doctor’s opinion | ? | N/A |
| Peláez-Ballestas 2011 [64] | Mexico | Doctor’s opinion | ? | N/A |
| Piga 2016 [65] | Italy | ICD codes | g | N/A |
| Quintana 2016 [66] | Argentina | ACR/EULAR 2013 | g | N/A |
| Radić 2010 [67] | Croatia | ACR 1980 | 2008 | N/A |
| Repae | Greece | ACR/EULAR 2013 | 2015 | g |
| Roberts-Thomson 2006 [68] | Australia | ACR 1980 or a | 2002 | 1993–2002 |
| Roberts-Thomson 2001 [69] | Australia | ACR 1980 or a | g | g |
| Robinson 2008 [19] | USA | ICD codes | 2001–2002 | N/A |
| Rosa 2011 [70] | Argentina | ACR 1980 and/or LeRoy and Medsger 2001 | 2004 | 1999–2004 |
| Royle 2018 [71] | UK | Read codes (NHS) | 2013 | 1994–2013 |
| Sardu 2012 [72] | Italy | ICD codes | g | N/A |
| See 2013 [73] | Taiwan | ICD codes | g | g |
| Silman 1988 [74] | UK | Doctor’s opinion | 1986 | 1980–1985 |
| Silman 1990 [75] | UK | Doctor’s opinion | 1987 | N/A |
| Sipek Dolnicar 2013 [76] | Slovenia | LeRoy and Medsger 2001 | N/A | 2007–2009 |
| Steen 1997 [77] | USA | ACR 1980 or f | N/A | 1963–1982 |
| Tamaki 1991 [78] | Japan | ACR 1980 | 1988 | N/A |
| Thompson 2002 [79] | Canada | ACR 1980 or CREST | 1996 | N/A |
| Valter 1997 [80] | Estonia | ACR 1980 | ? | N/A |
| Vonk 2009 [81] | Netherlands | ACR 1980 and LeRoy and Medsger 2001 | 2007 | 2005–2006 |
| Yu 2013 [82] | Taiwan | ICD codes | g | g |
Prevalence calendar period and incidence calendar period refer to the estimates used in the meta-analyses.
One major criterion, sclerodactyly and at least two of the following minor criteria: RP, oesophageal dysmotility, calcinosis, telangiectasia or an elevated ANA titre.
Sclerodactyly and one or more other features of the CREST syndrome.
Included only in the analyses stratified by sex.
A rheumatologist diagnosis of SSc, sclerodactyly and at least two other features of the CREST syndrome.
Argyro Repa, Rheumatology Department, University Hospital Crete, Greece.
RP, sclerodactyly, telangiectasias or calcinosis and one organ involvement characteristic of SSc, including oesophageal hypomotility, small bowel hypomotility, pulmonary arterial hypertension or scleroderma renal crisis.
Reported but not included in the overall meta-analyses. N/A: not available; ICD: International Classification of Diseases; NHS: UK National Health Service; ? Prevalence calendar period was not stated clearly.
SSc prevalence
Of the 61 studies reporting prevalence of SSc, we included 46 studies (58 independent populations) in the overall meta-analysis. Fifteen studies were excluded due to overlap in denominator with other broader and/or more recent studies. Prevalence ranged from 3.1 to 144.5 per 100 000 individuals. The pooled prevalence was 17.6 (95% CI 15.1, 20.5) per 100 000, I2 = 100% (supplementary Fig. S1, available at Rheumatology online). There were signs of publication bias when the funnel plot was examined visually (supplementary Fig. S2, available at Rheumatology online), but Egger’s test indicated otherwise (P = 0.19).
Stratification by sex
There were 23 studies (30 independent populations) presenting prevalence estimates stratified by sex. The pooled prevalence among men was 6.0 (95% CI 4.8, 7.5) per 100 000, I2 = 97% (supplementary Fig. S3, available at Rheumatology online) and 28.0 (95% CI 23.1, 33.9) per 100 000, I2 = 99% among women (supplementary Fig. S4, available at Rheumatology online).
Subgroup analyses
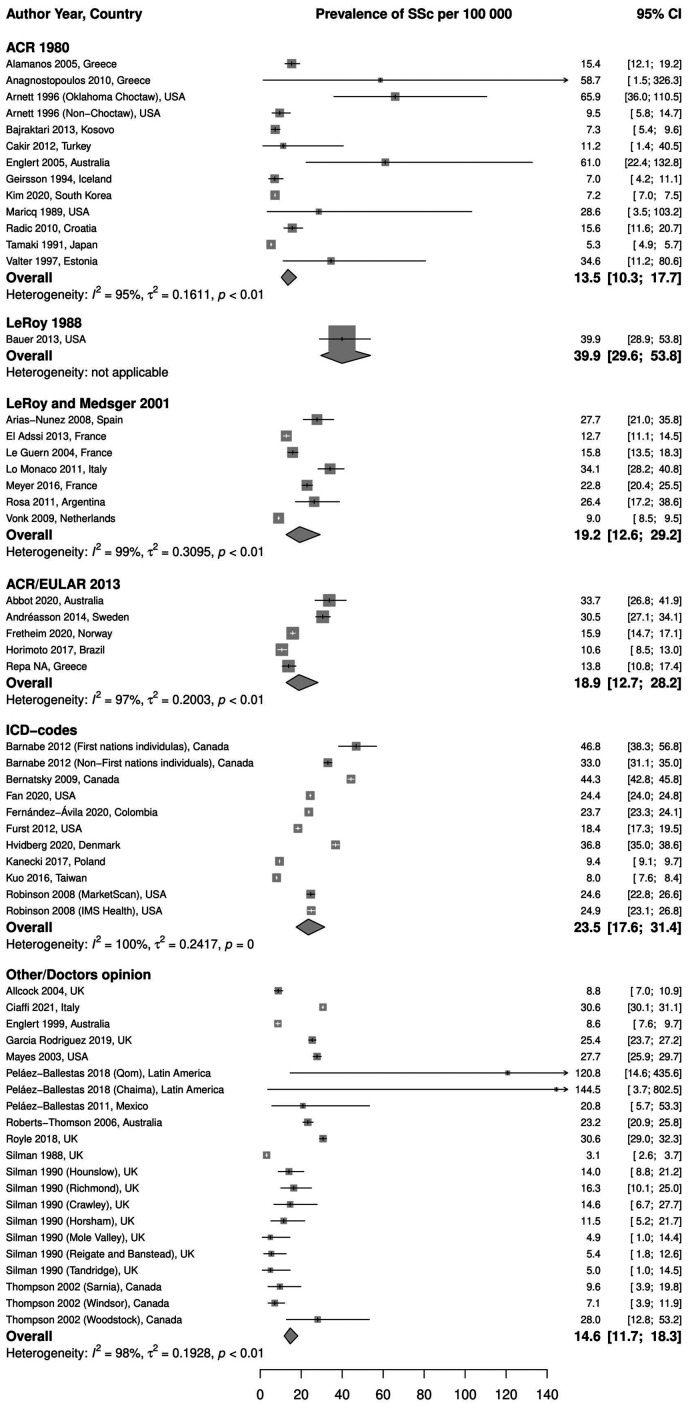
Stratification by case definition. A pooled prevalence of 13.5 (95% CI 10.3, 17.7) per 100 000, I2 = 95%, the lowest among case definitions, was estimated from 12 studies (13 independent populations) that used ACR 1980 criteria. One study used LeRoy 1988 criteria and reported a prevalence of 39.9 (95% CI 29.6, 53.8) per 100 000. The pooled prevalence of studies using LeRoy and Medsger 2001 criteria (seven studies) was 19.2 (95% CI 12.6, 29.2) per 100 000, I2 = 99%. The most recent criteria, ACR/EULAR 2013, were used in five studies; the pooled prevalence was 18.9 (95% CI 12.7, 28.2) per 100 000, I2 = 97%. The highest pooled prevalence was observed in the nine studies (11 independent populations) using ICD codes to identify SSc patients: 23.5 (95% CI 17.6, 31.4) per 100 000, I2 = 100%. The remaining 12 studies (21 independent subgroups) were grouped under Other/Doctor’s opinion, and the pooled prevalence was 14.6 (95% CI 11.7, 18.3) per 100 000, I2 = 98%. Fig. 2 illustrates these results.
Fig. 2.
Meta-analysis of SSc prevalence per 100 000, stratified by case definition method
ICD: International Classification of Diseases.
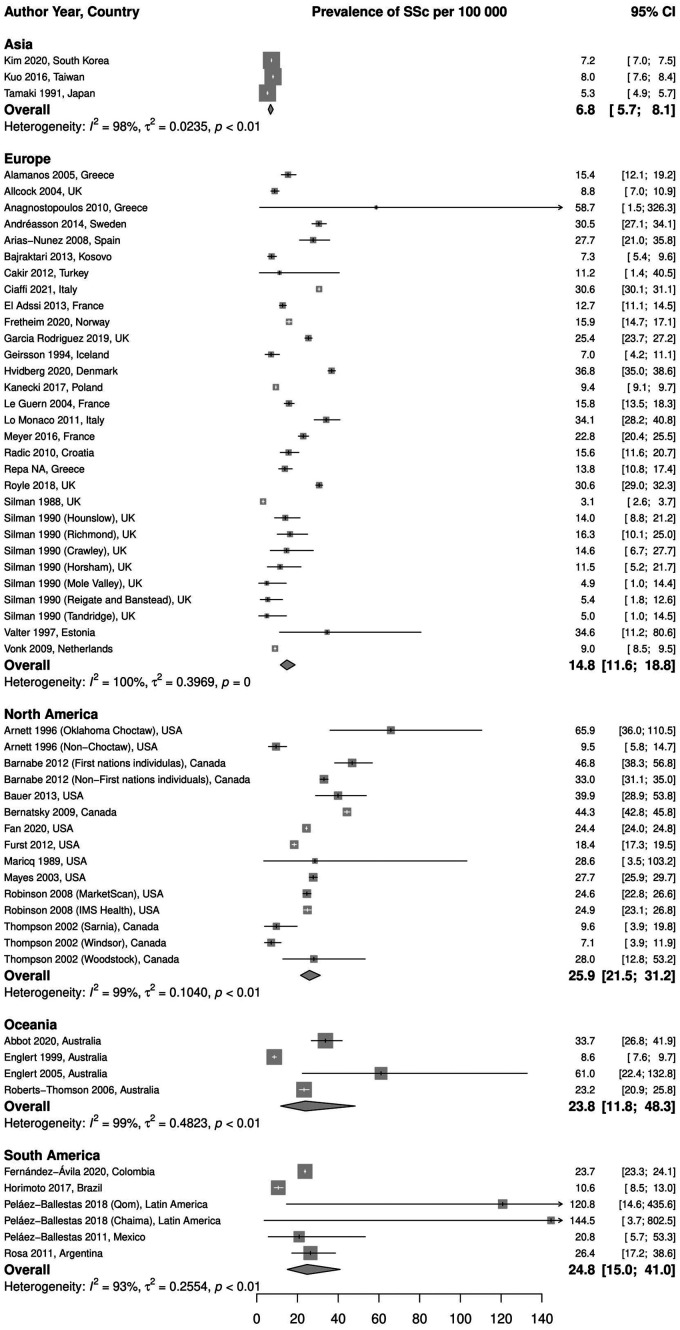
Stratification by geographic region. We found three studies conducted in Asia, with a pooled prevalence estimate of 6.8 (95% CI 5.7, 8.1) per 100 000, I2 = 98%. In Europe, there were 24 studies (30 independent populations) varying in size, design and case definition. The pooled prevalence was 14.8 (95% CI 11.6, 18.8) per 100 000, I2 = 100%. The pooled prevalence estimates of studies conducted in North America (10 studies, 15 independent populations) and Oceania (4 studies) were comparable, 25.9 (95% CI 21.5, 31.2) per 100 000, I2 = 99%, and 23.8 (95% CI 11.8, 48.3) per 100 000, I2 = 99%, respectively. Five studies were conducted in South America (six independent populations), two of them had the highest prevalence estimates observed. The pooled prevalence in South America was also comparable to other regions: 24.8 (95% CI 15.0, 41.0) per 100 000, I2 = 93%. We found no study conducted in Africa reporting SSc prevalence. These results are illustrated in Fig. 3.
Fig. 3.
Meta-analysis of SSc prevalence per 100 000, stratified by region
Meta-regression. Results from the meta-regression of prevalence against calendar period indicates that more recent studies report higher prevalence estimates (P = 0.006) (supplementary Fig. S5, available at Rheumatology online).
Sensitivity analysis. There were four studies (five independent populations) [26, 40, 57, 63] with a denominator smaller than 10 000. All of them reported a prevalence higher than the overall pooled prevalence mentioned above, and they were grouped under ACR 1980 or Other/Doctor’s opinion. After exclusion of them, the overall pooled estimate declined slightly to 16.7 (95% CI 14.3, 19.5) per 100 000, I2 = 100%. The pooled estimate in Europe was hardly affected, while there was no change in North America and Asia. In South America, however, there was a considerable decrease in the pooled estimate to 18.9 (95% CI 11.2, 31.9) per 100 000, I2 = 95%, when the two populations with highest prevalence estimates were excluded. A similar change was noticed in Oceania, 18.8 (95% CI 8.6, 41.1) per 100 000, I2 = 99% (supplementary Figs S6–8, available at Rheumatology online).
SSc incidence
Of the 39 studies reporting incidence of SSc, we included 28 studies in the overall meta-analysis. The exclusion of 11 studies was due to overlap in denominator with other broader and/or more recent studies (n = 6), report of cumulative incidence over several years (n = 1), incidence rate solely for either men or women (n = 2) or insufficient data (n = 2). The pooled incidence rate was 1.4 (95% CI 1.1, 1.9) per 100 000 person-years, I2 = 100% (supplementary Fig. S9, available at Rheumatology online). The funnel plot (supplementary Fig. S10, available at Rheumatology online) indicates a probable publication bias. This visual impression was not confirmed by Egger’s test (P = 0.18).
Stratification by sex
There were 14 studies reporting SSc incidence in men with a pooled incidence of 0.5 (95% CI 0.4, 0.7) per 100 000 person-years, I2 = 96% (supplementary Fig. S11, available at Rheumatology online). Thirteen studies reported SSc incidence in women with a pooled incidence of 2.3 (95% CI 1.8, 2.9) per 100 000 person-years, I2 = 98% (supplementary Fig. S12, available at Rheumatology online).
Subgroup analyses
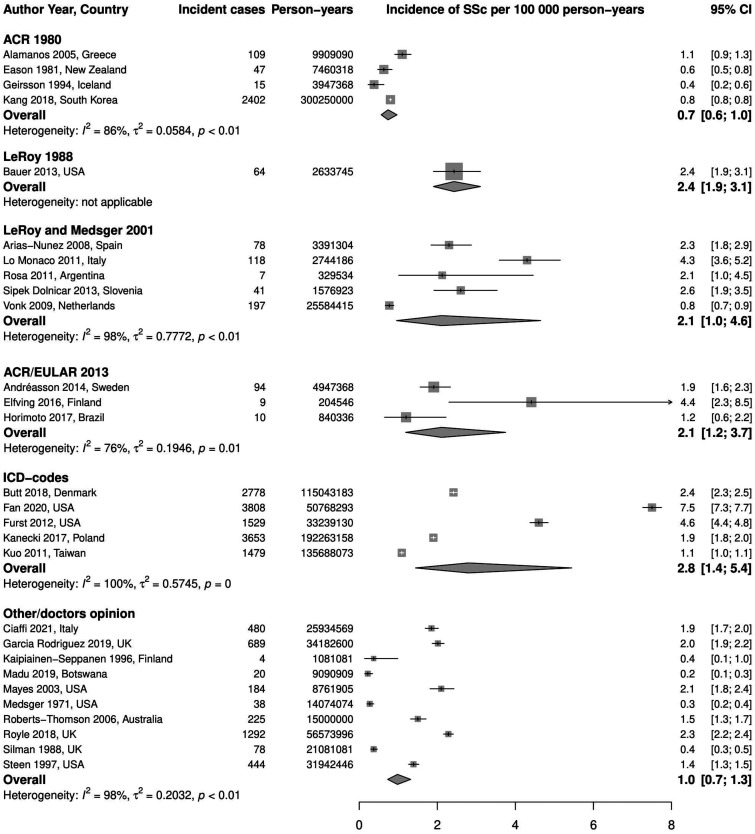
Stratification by case definition. There were four studies using ACR 1980 criteria to define cases; the pooled incidence was 0.7 (95% CI 0.6, 1.0) per 100 000 person-years, I2 = 86%. Only one study used LeRoy 1988 criteria, and the incidence rate was 2.4 (95% CI 1.9, 3.1) per 100 000 person-years. LeRoy and Medsger 2001 criteria were used in five studies giving a pooled incidence of 2.1 (95% CI 1.0, 4.6) per 100 000 person-years, I2 = 98%. A comparable pooled incidence rate was observed in studies using ACR/EULAR 2013 criteria (three studies): 2.1 (95% CI 1.2, 3.7) per 100 000 person-years, I2 = 76%. Five studies used ICD codes to identify SSc cases, the pooled incidence was 2.8 (95% CI 1.4, 5.4) per 100 000 person-years, I2 = 100%, which is the highest rate in this analysis. Ten studies were grouped under Other/Doctor’s opinion and the pooled incidence was 1.0 (95% CI 0.7, 1.3) per 100 000 person-years, I2 = 98%. Fig. 4 demonstrates these results.
Fig. 4.
Meta-analysis of SSc incidence per 100 000 person-years, stratified by case definition method
ICD: International Classification of Diseases.
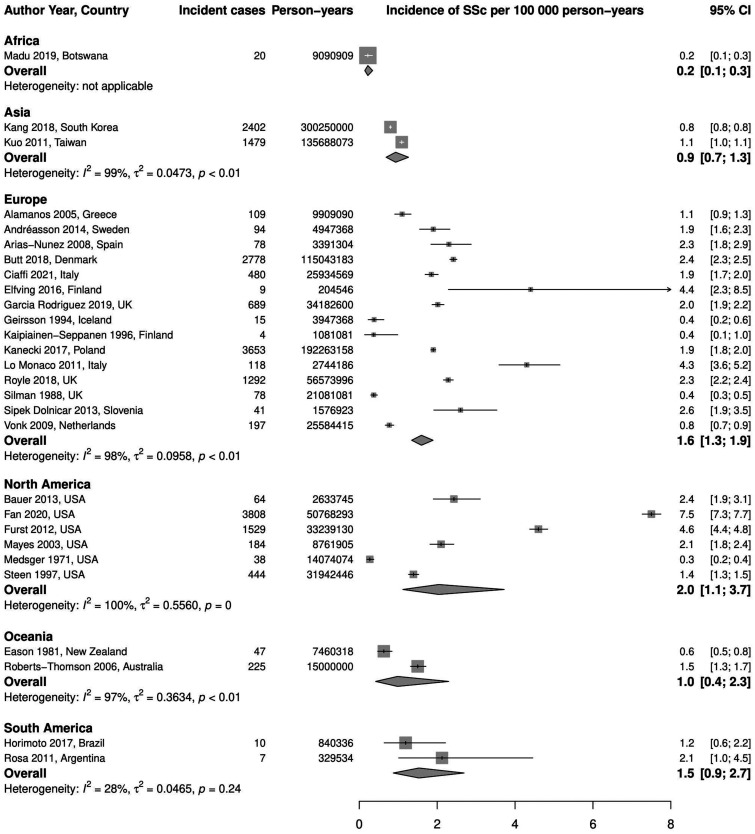
Stratification by geographic region. We found only one study reporting incidence rate of SSc in Africa: 0.2 (95% CI 0.1, 0.3) per 100 000 person-years, which represents the lowest observed incidence rate. In Asia, two studies were found with a pooled incidence of 0.9 (95% CI 0.7, 1.3) per 100 000 person-years, I2 = 99%. Studies in Europe (15 studies) and North America (6 studies) had comparable pooled rates: 1.6 (95% CI 1.3, 1.9) per 100 000 person-years, I2 = 98%, and 2.0 (95% CI 1.1, 3.7) per 100 000 person-years, I2 = 100%, respectively. There were two studies from Oceania, and the pooled incidence was 1.0 (95% CI 0.4, 2.3) per 100 000 person-years, I2 = 97%. Two studies were also conducted in South America with a pooled incidence of 1.5 (95% CI 0.9, 2.7) per 100 000 person-years, I2 = 28%. These results are demonstrated in Fig. 5.
Fig. 5.
Meta-analysis of SSc incidence per 100 000 person-years, stratified by region
Meta-regression. Meta-regression of incidence against calendar period suggests that more recent studies report higher incidence rates (P = 0.002) (supplementary Fig. S13, available at Rheumatology online).
Discussion
In this report, we present the first ever meta-analysis on the incidence of SSc globally, in addition to a meta-analysis on the prevalence of SSc covering the whole available literature in English. The pooled prevalence is 17.6 per 100 000 and the pooled incidence rate is 1.4 per 100 000 person-years. When we excluded studies with small denominator size, the overall pooled prevalence and estimates of the majority of subgroups were hardly affected. These sensitivity analyses proved our results to be robust. This report revealed high I2 values indicating large heterogeneity among included studies due to differences in study design, case definition, geographic region and calendar period.
More recent studies reported higher estimates than older ones. We could attribute this temporal trend to the wider availability of modern diagnostic methods in addition to increased awareness of SSc and more sensitive criteria, rather than a true increase in SSc occurrence. Our subgroup analyses revealed that pooled estimates in the ACR 1980 group were lower than other groups while pooled estimates of LeRoy and Medsger 2001 and ACR/EULAR 2013 were comparable. This observation was confirmed in studies reporting prevalence or incidence estimates using different sets of criteria; estimates corresponding to more recent criteria were higher than estimates corresponding to older ones. In Italy, the prevalence and incidence using ACR 1980 criteria were 25.4 per 100 000 and 3.2 per 100 000 person-years, respectively, while they were 34 and 4.3, respectively, using LeRoy and Medsger 2001 criteria [55]. In Sweden, the incidence of SSc increased from 1.4–1.9 per 100 000 person-years when using ACR/EULAR 2013 criteria instead of ACR 1980 criteria, and the prevalence increased from 23.5–30.5 per 100 000 [27]. We found no study comparing LeRoy and Medsger 2001 with ACR/EULAR 2013 criteria.
Pooled estimates from studies that used ICD codes to ascertain SSc were comparable to the ones in which ACR/EULAR 2013 criteria were used. Butt et al. showed that identification of incident SSc cases through ICD-10 codes in Denmark had a positive predictive value of 94% using ACR/EULAR 2013 criteria as reference [33]. The definition of SSc cases using ICD codes differed among included studies with some studies used rather strict definitions of SSc leading to higher specificity and thus lower prevalence/incidence. Robinson et al. [19] reported considerably lower prevalence when requiring at least one inpatient claim or two or more office or emergency room visits compared with requiring at least one medical claim. Similarly, Fan et al. [20] reported an incidence of 16.4 per 100 000 person-years and a prevalence of 44.1 per 100 000 requiring one medical claim of SSc using ICD codes. These estimates declined to 7.5 and 24.4, respectively, when two medical claims were required.
Stratification by region showed significant variation between different parts of the world. The pooled prevalence in studies from Asia was the lowest as relatively low estimates were consistently reported. For example, SSc prevalence in Taiwan was 8 per 100 000, while it was 34.8 in Sardinia, Italy despite similarities in design, calendar period and case definition method [50, 65]. Likewise, the incidence rate was 1.1 and 4.6 per 100 000 person-years in Taiwan and the USA, respectively [16, 51]. We may therefore conclude that the lower occurrence of SSc in Asia, can not only be explained by methodological differences, and a true difference may be present. In North America, high estimates were seen in the majority of studies despite considerable methodological variations among them, which indicates the occurrence of SSc there may be the highest worldwide. Scarce data from Africa did not allow us to provide estimates of incidence and prevalence for the continent. Interestingly, a higher incidence of SSc was reported in African American women compared with European American women (2.25 vs 1.28 per 100 000 person-years) in the USA [53], and a recent genetic study has identified two African ancestry-predominant HLA alleles that were associated with increased frequency of SSc among African Americans [83].
In Europe, the previously proposed north-to-south gradient [84] where higher latitude indicates lower occurrence seems to be rather present in our analysis despite a few exceptions. Prevalence estimates in Italy (34.8 and 58.6 per 100 000 [15, 65]) and Spain (27.7 per 100 000 [28]) were markedly higher than France (13.2 and 15.8 per 100 000 [39, 54]), the Netherlands (8.9 per 100 000 [81]) and Norway (15.9 per 100 000 [43]). On the contrary, prevalence in Sweden (30.5 per 100 000) seems to not adhere to this gradient despite using similar methodology to the Norwegian study [27]. The prevalence in Denmark was also higher than in Norway: 36.8 per 100 000. Similar results were also observed regarding incidence rates: Italy (4.6 per 100 000 person-years [15]), Spain (2.3 per 100 000 person-years [28]), the Netherlands (0.8 per 100 000 person-years [81]), Sweden (1.9 per 100 000 person-years [27]) and Denmark (2.4 per 100 000 person-years [33]). As expected, the pooled incidence and prevalence estimates for Europe were similar to the overall pooled estimates as more European than studies from other regions were included in our analyses. The pooled prevalence estimates in both South America and Oceania after exclusion of studies with small denominator size were also comparable to the overall pooled estimate.
SSc has consistently been described to be more common in women compared with men. A women-to-men ratio of almost 5:1 was noticed in the majority of studies. Our analyses stratified by sex seem to be in line with that. The pooled prevalence and incidence in women were almost five times higher than the pooled estimates in men (prevalence 28 vs 6.1 per 100 000; incidence 2.3 vs 0.5 per 100 000 person-years).
This report has some limitations. It was restricted to studies published in English, excluding potentially relevant studies in other languages. If incidence and prevalence estimates from some parts of the world were missed, pooled overall and regional estimates may not reflect the true picture of SSc epidemiology. Furthermore, reported estimates from developing regions may be lower than other regions due to the limited accessibility to the modern diagnostic methods. We were unable to account for the broad and overlapping spectrum of methods used to identify patients in primary studies, ranging from primary care practice- to tertiary centre- and register-based studies, differing in their coverage of the studied population. Large heterogeneity was observed among studies as indicated by high I2 values due to variations in design, case definition, calendar period and region. Also, inclusion of studies with large denominators and thus small standard errors led to an overestimation of the I2 statistic [85]. We believe pooled estimates and measures of heterogeneity should be interpreted with caution. A major strength of our study is the comprehensive and broad search strategy, and that we present the first ever meta-analysis of SSc incidence. Compared with a previous systematic review [10], considerably more studies were included in our meta-analysis of SSc prevalence (46 vs 18). This allowed us to conduct sensitivity analyses, subgroup analyses and analyses stratified by sex to explore the impact of different factors on prevalence and incidence estimates.
In conclusion, the incidence and prevalence of SSc vary significantly depending on study settings, acquisition routes, region and calendar period, in addition to the considerably higher occurrence of SSc in women in comparison to men. Our results should therefore be interpreted taking these issues into consideration.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: M.R. reports non-promotional speaker fees from Teva, outside the submitted work. The other authors have declared no conflicts of interest.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1.Jimenez SA, Derk CT.. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med 2004;140:37–50. [PubMed] [Google Scholar]
- 2.Allanore Y, Simms R, Distler O. et al. Systemic sclerosis. Nat Rev Dis Primers 2015;1:15002. [DOI] [PubMed] [Google Scholar]
- 3.Walker UA, Tyndall A, Czirjak L. et al. ; EUSTAR Co-authors. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 2007;66:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denton CP, Khanna D.. Systemic sclerosis. Lancet 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 5.Hughes M, Pauling JD, Armstrong-James L. et al. Gender-related differences in systemic sclerosis. Autoimmun Rev 2020;19:102494. [DOI] [PubMed] [Google Scholar]
- 6.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 7.LeRoy EC, Black C, Fleischmajer R. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 8.LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573–6. [PubMed] [Google Scholar]
- 9.van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 10.Zhong L, Pope M, Shen Y, Hernandez JJ, Wu L.. Prevalence and incidence of systemic sclerosis: a systematic review and meta-analysis. Int J Rheum Dis 2019;22:2096–107. [DOI] [PubMed] [Google Scholar]
- 11.Kaipiainen-Seppanen O, Aho K.. Incidence of rare systemic rheumatic and connective tissue diseases in Finland. J Intern Med 1996;240:81–4. [DOI] [PubMed] [Google Scholar]
- 12.Elfving P, Marjoniemi O, Niinisalo H. et al. Estimating the incidence of connective tissue diseases and vasculitides in a defined population in Northern Savo area in 2010. Rheumatol Int 2016;36:917–24. [DOI] [PubMed] [Google Scholar]
- 13.Kang GW, Jung KH, Lee YS. et al. Incidence, prevalence, mortality and causes of death in systemic sclerosis in Korea: a nationwide population-based study. Br J Dermatol 2018;178:E37–9. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Cho SK, Kim JW. et al. An increased disease burden of autoimmune inflammatory rheumatic diseases in Korea. Semin Arthritis Rheum 2020;50:526–33. [DOI] [PubMed] [Google Scholar]
- 15.Airò P, Regola F, Lazzaroni MG. et al. Incidence and prevalence of systemic sclerosis in Valcamonica, Italy, during an 18-year period. J Scleroderma Relat Disord 2020;5:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furst DE, Fernandes AW, Iorga SR, Greth W, Bancroft T.. Epidemiology of systemic sclerosis in a large US managed care population. J Rheumatol 2012;39:784–6. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Howard RF, Tan F. et al. Increased prevalence of systemic sclerosis in a Native American tribe in Oklahoma. Association with an Amerindian HLA haplotype. Arthritis Rheum 1996;39:1362–70. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. W64. [DOI] [PubMed] [Google Scholar]
- 19.Robinson D, Eisenberg D, Nietert PJ. et al. Systemic sclerosis prevalence and comorbidities in the US, 2001-2002. Curr Med Res Opin 2008;24:1157–66. [DOI] [PubMed] [Google Scholar]
- 20.Fan Y, Bender S, Shi W, Zoz D.. Incidence and prevalence of systemic sclerosis and systemic sclerosis with interstitial lung disease in the United States. J Manag Care Spec Pharm 2020;26:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balduzzi S, Rücker G, Schwarzer G.. How to perform a meta-analysis with R: a practical tutorial. Evid Based Mental Health 2019;22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbot S, McWilliams L, Spargo L. et al. Scleroderma in Cairns: an epidemiological study. Intern Med J 2020;50:445–52. [DOI] [PubMed] [Google Scholar]
- 23.Airò P, Tabaglio E, Frassi M. et al. Prevalence of systemic sclerosis in Valtrompia in northern Italy. A collaborative study of rheumatologists and general practitioners. Clin Exp Rheumatol 2007;25:878–80. [PubMed] [Google Scholar]
- 24.Alamanos Y, Tsifetaki N, Voulgari PV. et al. Epidemiology of systemic sclerosis in northwest Greece 1981 to 2002. Semin Arthritis Rheum 2005;34:714–20. [DOI] [PubMed] [Google Scholar]
- 25.Allcock RJ, Forrest I, Corris PA, Crook PR, Griffiths ID.. A study of the prevalence of systemic sclerosis in northeast England. Rheumatology (Oxford) 2004;43:596–602. [DOI] [PubMed] [Google Scholar]
- 26.Anagnostopoulos I, Zinzaras E, Alexiou I. et al. The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet Disord 2010;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andréasson K, Saxne T, Bergknut C, Hesselstrand R, Englund M.. Prevalence and incidence of systemic sclerosis in southern Sweden: population-based data with case ascertainment using the 1980 ARA criteria and the proposed ACR-EULAR classification criteria. Ann Rheum Dis 2014;73:1788–92. [DOI] [PubMed] [Google Scholar]
- 28.Arias-Nuñez MC, Llorca J, Vazquez-Rodriguez TR. et al. Systemic sclerosis in northwestern Spain: a 19-year epidemiologic study. Medicine (Baltimore) 2008;87:272–80. [DOI] [PubMed] [Google Scholar]
- 29.Bajraktari IH, Berisha I, Berisha M, Saiti V, Bajraktari H.. Incidence, prevalence and clinical manifestations of systemic sclerosis in dukagjini plain. Mater Sociomed 2013;25:14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnabe C, Joseph L, Belisle P. et al. Prevalence of systemic lupus erythematosus and systemic sclerosis in the First Nations population of Alberta, Canada. Arthritis Care Res (Hoboken) 2012;64:138–43. [DOI] [PubMed] [Google Scholar]
- 31.Bauer PR, Schiavo DN, Osborn TG. et al. Influence of interstitial lung disease on outcome in systemic sclerosis: a population-based historical cohort study. Chest 2013;144:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernatsky S, Joseph L, Pineau CA. et al. Scleroderma prevalence: demographic variations in a population-based sample. Arthritis Rheum 2009;61:400–4. [DOI] [PubMed] [Google Scholar]
- 33.Butt SA, Jeppesen JL, Fuchs C. et al. Trends in incidence, mortality, and causes of death associated with systemic sclerosis in Denmark between 1995 and 2015: a nationwide cohort study. BMC Rheumatol 2018;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Çakır N, Pamuk ÖN, Derviş E. et al. The prevalences of some rheumatic diseases in western Turkey: Havsa study. Rheumatol Int 2012;32:895–908. [DOI] [PubMed] [Google Scholar]
- 35.Chandran G, Smith M, Ahern MJ, Roberts-Thomson PJ.. A study of scleroderma in South Australia: prevalence, subset characteristics and nailfold capillaroscopy. Aust N Z J Med 1995;25:688–94. [DOI] [PubMed] [Google Scholar]
- 36.Ciaffi J, Morabito MF, Ruscitti P. et al. Incidence, prevalence and mortality of systemic sclerosis in Italy: a nationwide population-based study using administrative health data. Rheumatol Int 2021;41:129–37. [DOI] [PubMed] [Google Scholar]
- 37.Eason RJ, Tan PL, Gow PJ.. Progressive systemic sclerosis in Auckland: a ten year review with emphasis on prognostic features. Aust N Z J Med 1981;11:657–62. [DOI] [PubMed] [Google Scholar]
- 38.Eaton WW, Pedersen MG, Atladóttir HO. et al. The prevalence of 30 ICD-10 autoimmune diseases in Denmark. Immunol Res 2010;47:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Adssi H, Cirstea D, Virion JM, Guillemin F, de Korwin JD.. Estimating the prevalence of systemic sclerosis in the Lorraine region, France, by the capture-recapture method. Semin Arthritis Rheum 2013;42:530–8. [DOI] [PubMed] [Google Scholar]
- 40.Englert H, Joyner E, Bade R. et al. Systemic scleroderma: a spatiotemporal clustering. Int Med J 2005;35:228–33. [DOI] [PubMed] [Google Scholar]
- 41.Englert H, Small-McMahon J, Davis K. et al. Systemic sclerosis prevalence and mortality in Sydney 1974-1988. Aust N Z J Med 1999;29:42–50. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Ávila DG, Bernal-Macías S, Gutiérrez JM, Rincón DN, Rosselli D.. Prevalence of systemic sclerosis in Colombia: data from the National Health Registry 2012-2016. J Scleroderma Relat Disord 2020;5:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fretheim H, Halse AK, Seip M. et al. Multidimensional tracking of phenotypes and organ involvement in a complete nationwide systemic sclerosis cohort. Rheumatology (Oxford) 2020;59:2920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García Rodríguez LA, González-Pérez A, Michel A, Sáez ME.. Contemporary epidemiology of systemic sclerosis: a population-based cohort study in the United Kingdom. Semin Arthritis Rheum 2019;49:105–11. [DOI] [PubMed] [Google Scholar]
- 45.Geirsson AJ, Steinsson K, Guthmundsson S, Sigurthsson V.. Systemic sclerosis in Iceland. A nationwide epidemiological study. Ann Rheum Dis 1994;53:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann-Vold AM, Midtvedt O, Molberg O, Garen T, Gran JT.. Prevalence of systemic sclerosis in south-east Norway. Rheumatology (Oxford) 2012;51:1600–5. [DOI] [PubMed] [Google Scholar]
- 47.Horimoto AMC, Matos ENN, Costa MRD. et al. Incidence and prevalence of systemic sclerosis in Campo Grande, State of Mato Grosso do Sul, Brazil. Rev Bras Reumatol Engl Ed 2017;57:107–14. [DOI] [PubMed] [Google Scholar]
- 48.Hvidberg MF, Johnsen SP, Davidsen M, Ehlers L.. A nationwide study of prevalence rates and characteristics of 199 chronic conditions in Denmark. Pharmacoecon Open 2020;4:361–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanecki K, Goryński P, Tarka P, Wierzba W, Tyszko P.. Incidence and prevalence of systemic sclerosis (SSc) in Poland - differences between rural and urban regions. Ann Agric Environ Med 2017;24:240–4. [DOI] [PubMed] [Google Scholar]
- 50.Kuo CF, Luo SF, Yu KH. et al. Familial risk of systemic sclerosis and co-aggregation of autoimmune diseases in affected families. Arthritis Res Ther 2016;18:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo CF, See LC, Yu KH, Chou IJ. et al. Epidemiology and mortality of systemic sclerosis: a nationwide population study in Taiwan. Scand J Rheumatol 2011;40:373–8. [DOI] [PubMed] [Google Scholar]
- 52.Kurland LT, Hauser WA, Ferguson RH, Holley KE.. Epidemiologic features of diffuse connective tissue disorders in Rochester, Minn., 1951 through 1967, with special reference to systemic lupus erythematosus. Mayo Clin Proc 1969;44:649–63. [PubMed] [Google Scholar]
- 53.Laing TJ, Gillespie BW, Toth MB. et al. Racial differences in scleroderma among women in Michigan. Arthritis Rheum 1997;40:734–42. [DOI] [PubMed] [Google Scholar]
- 54.Le Guern V, Mahr A, Mouthon L. et al. Prevalence of systemic sclerosis in a French multi-ethnic county. Rheumatology (Oxford) 2004;43:1129–37. [DOI] [PubMed] [Google Scholar]
- 55.Lo Monaco A, Bruschi M, La Corte R, Volpinari S, Trotta F.. Epidemiology of systemic sclerosis in a district of northern Italy. Clin Exp Rheumatol 2011;29:10–4. [PubMed] [Google Scholar]
- 56.Madu PN, Williams VL, Noe MH. et al. Autoimmune skin disease among dermatology outpatients in Botswana: a retrospective review. Int J Dermatol 2019;58:50–3. [DOI] [PubMed] [Google Scholar]
- 57.Maricq HR, Weinrich MC, Keil JE. et al. Prevalence of scleroderma spectrum disorders in the general population of South Carolina. Arthritis Rheum 1989;32:998–1006. [DOI] [PubMed] [Google Scholar]
- 58.Mayes MD, Lacey JV, Beebe-Dimmer J. et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 2003;48:2246–55. [DOI] [PubMed] [Google Scholar]
- 59.Medsger TA, Masi AT.. Epidemiology of systemic sclerosis (scleroderma). Ann Int Med 1971;74:714–21. [DOI] [PubMed] [Google Scholar]
- 60.Medsger TA, Masi AT.. Epidemiology of systemic-sclerosis (scleroderma) among male U. S. veterans. J Chronic Dis 1978;31:73–85. [DOI] [PubMed] [Google Scholar]
- 61.Meyer A, Chifflot H, Chatelus E. et al. Brief report: spatial heterogeneity of systemic sclerosis in France: high prevalence in the Northeast Region. Arthritis Rheumatol 2016;68:1731–7. [DOI] [PubMed] [Google Scholar]
- 62.Michet CJ Jr, McKenna CH, Elveback LR, Kaslow RA, Kurland LT.. Epidemiology of systemic lupus erythematosus and other connective tissue diseases in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc 1985;60:105–13. [DOI] [PubMed] [Google Scholar]
- 63.Peláez-Ballestas I, Granados Y, Quintana R. et al. ; Latin American Study Group of Rheumatic Diseases in Indigenous Peoples (GLADERPO). Epidemiology and socioeconomic impact of the rheumatic diseases on indigenous people: an invisible syndemic public health problem. Ann Rheum Dis 2018;77:1397–404. [DOI] [PubMed] [Google Scholar]
- 64.Peláez-Ballestas I, Sanin LH, Moreno-Montoya J. et al. ; Grupo de Estudio Epidemiológico de Enfermedades Músculo Articulares (GEEMA). Epidemiology of the rheumatic diseases in Mexico. A study of 5 regions based on the COPCORD methodology. J Rheumatol Suppl 2011;86:3–8. [DOI] [PubMed] [Google Scholar]
- 65.Piga M, Casula L, Sanna S. et al. Population-based analysis of hospitalizations for patients with systemic sclerosis in a West-European region over the period 2001-2012. Rheumatol Int 2016;36:73–81. [DOI] [PubMed] [Google Scholar]
- 66.Quintana R, Silvestre AM, Goni M. et al. Prevalence of musculoskeletal disorders and rheumatic diseases in the indigenous Qom population of Rosario, Argentina. Clin Rheumatol 2016;35:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radić M, Kaliterna DM, Fabijanić D, Radić J.. Prevalence of systemic sclerosis in Split-Dalmatia county in Southern Croatia. Clin Rheumatol 2010;29:419–21. [DOI] [PubMed] [Google Scholar]
- 68.Roberts-Thomson PJ, Walker JG, Lu TY. et al. Scleroderma in South Australia: further epidemiological observations supporting a stochastic explanation. Intern Med J 2006;36:489–97. [DOI] [PubMed] [Google Scholar]
- 69.Roberts-Thomson PJ, Jones M, Hakendorf P. et al. Scleroderma in South Australia: epidemiological observations of possible pathogenic significance. Intern Med J 2001;31:220–9. [DOI] [PubMed] [Google Scholar]
- 70.Rosa JE, Soriano ER, Narvaez-Ponce L. et al. Incidence and prevalence of systemic sclerosis in a healthcare plan in Buenos Aires. J Clin Rheumatol 2011;17:59–63. [DOI] [PubMed] [Google Scholar]
- 71.Royle JG, Lanyon PC, Grainge MJ, Abhishek A, Pearce FA.. The incidence, prevalence, and survival of systemic sclerosis in the UK Clinical Practice Research Datalink. Clin Rheumatol 2018;37:2103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sardu C, Cocco E, Mereu A. et al. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. Plos One 2012;7:e32487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.See LC, Kuo CF, Chou IJ, Chiou MJ, Yu KH.. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin Arthritis Rheum 2013;43:381–6. [DOI] [PubMed] [Google Scholar]
- 74.Silman A, Jannini S, Symmons D, Bacon P.. An epidemiological study of scleroderma in the West Midlands. Br J Rheumatol 1988;27:286–90. [DOI] [PubMed] [Google Scholar]
- 75.Silman AJ, Howard Y, Hicklin AJ, Black C.. Geographical clustering of scleroderma in south and west London. Br J Rheumatol 1990;29:93–6. [PubMed] [Google Scholar]
- 76.Sipek Dolnicar A, Rotar Z, Tomsic M.. Incidence of scleroderma spectrum disorders in Slovenia. Clin Exp Rheumatol 2013;31:8–11. [PubMed] [Google Scholar]
- 77.Steen VD, Oddis CV, Conte CG. et al. Incidence of systemic sclerosis in Allegheny County, Pennsylvania. A twenty-year study of hospital-diagnosed cases, 1963-1982. Arthritis Rheum 1997;40:441–5. [DOI] [PubMed] [Google Scholar]
- 78.Tamaki T, Mori S, Takehara K.. Epidemiological study of patients with systemic sclerosis in Tokyo. Arch Dermatol Res 1991;283:366–71. [DOI] [PubMed] [Google Scholar]
- 79.Thompson AE, Pope JE.. Increased prevalence of scleroderma in southwestern Ontario: a cluster analysis. J Rheumatol 2002;29:1867–73. [PubMed] [Google Scholar]
- 80.Valter I, Saretok S, Maricq HR.. Prevalence of scleroderma spectrum disorders in the general population of Estonia. Scand J Rheumatol 1997;26:419–25. [DOI] [PubMed] [Google Scholar]
- 81.Vonk MC, Broers B, Heijdra YF. et al. Systemic sclerosis and its pulmonary complications in The Netherlands: an epidemiological study. Ann Rheum Dis 2009;68:961–5. [DOI] [PubMed] [Google Scholar]
- 82.Yu KH, See LC, Kuo CF, Chou IJ, Chou MJ.. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res (Hoboken) 2013;65:244–50. [DOI] [PubMed] [Google Scholar]
- 83.Gourh P, Safran SA, Alexander T, Boyden SE. et al. HLA and autoantibodies define scleroderma subtypes and risk in African and European Americans and suggest a role for molecular mimicry. Proc Natl Acad Sci USA 2020;117:552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J.. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008;37:223–35. [DOI] [PubMed] [Google Scholar]
- 85.Borenstein M HLV, Higgins JPT, Rothstein HR.. Introduction to meta‐analysis. USA: Wiley, 2009. https://onlinelibrary.wiley.com/doi/book/10.1002/9780470743386. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.