Abstract
Purpose
The incidence of COVID-19 vaccine-associated hypermetabolic lymphadenopathy (VAHL) is high following the administration of the first and second BNT162b2 vaccine doses. The impact of this finding on [18F]FDG PET-CT interpretation and its correlation with the induced humoral immunity have been reported. Assuming the amnestic immune response is different following the third vaccine dose, we aimed to explore the incidence of VAHL over time after the third BNT162b2 dose administration, and its relevance to [18F]FDG PET-CT interpretation in oncologic patients.
Methods
A total of 179 consecutive oncologic patients that underwent [18F]FDG PET-CT after a third BNT162b2 vaccine dose were included. The presence of VAHL was assessed. On VAHL-positive scans, the SUVmax, number, location, and size of the “hot” nodes were recorded. The median time interval between vaccination and imaging was 8 (IQR, 5–14) days.
Results
The incidences of all-grade VAHL and grade 3–4 VAHL were 47.5% and 8.9%, respectively. VAHL was identified on 82.5% of studies performed within the first 5 days from vaccination. Grade 3–4 VAHL was observed on 28.1% of studies performed within the first 5 days from vaccination, but was not detected on studies performed more than 5 days from vaccination. Separation between VAHL and malignant lymphadenopathy was not possible in only 2 of the 179 study patients. On a multivariable logistic regression, independent predictors of grade 3–4 VAHL were short time interval between vaccination and imaging (Pv < 0.01), younger age (Pv < 0.01), and lower BMI (Pv = 0.03).
Conclusion
VAHL is commonly identified on [18F]FDG PET-CT performed within the first 5 days from the third BNT162b2 vaccine dose administration. High-grade VAHL is unlikely to be observed on a scan performed 6 days or longer from vaccination, and is even less likely in older and obese patients.
Keywords: COVID-19, Vaccination, Lymphadenopathy, Immune response, Oncologic imaging
Introduction
Vaccination against SARS-CoV-2 has become one of the main strategies to control the evolving COVID-19 pandemic. Since late 2020, mass COVID-19 vaccination campaigns are being conducted around the world. In Israel, the two-dose regimen of the Pfizer BNT162b2 mRNA vaccine [1], given 21 days apart, was the most common vaccination regimen [2].
Emerging new SARS-CoV-2 variants [3] as well as evidences of lower vaccine-induced immunogenicity in some patient populations [4–6] are among the causes that raised a need to consider a third vaccine dose administration. The administration of a third BNT162b2 dose has started in Israel during July 2021, first in patients > 60 years and later in younger populations. As of the end of August 2021, more than 1.92 million people have received the third vaccine dose [7].
During the mass vaccination with the first and second vaccine doses, our group and others reported the high incidence of the BNT162b2 vaccine-associated hypermetabolic lymphadenopathy (VAHL) on [18F]FDG PET-CT studies. Following the second vaccine dose, we observed VAHL in 45.8% of the studies [8]. Eshet et al. reported that VAHL persisted in 29% of patients between 7 and 10 weeks after the second BNT162b2 dose [9]. Following the administration of the Moderna mRNA-1273 vaccine, Skawran et al. reported a VAHL incidence of 72% [10]. The finding of VAHL was found relevant when interpreting [18F]FDG PET-CT of oncologic patients [8, 10–13], more commonly in patients with breast cancer, lymphoma, and malignancies of the upper limb, when “hot” axillary nodes may reflect malignant involvement [8].
In a study our group conducted on patients with hematologic malignancy, we presented the positive correlation between the imaging finding of VAHL and the post-vaccination antibody titers. We concluded that VAHL on imaging may reflect a potent germinal center response in lymph nodes draining the vaccine injection site [14]. The relationship between VAHL and immune status was observed by Eifer et al. as well [15].
Assuming that the amnestic immune response following a third vaccine dose may differ from the response elicited by the first and second vaccine doses, we hypothesized that the characteristics of VAHL following the third dose are different from those previously reported. We therefore conducted this study to explore the incidence of VAHL over time after the third COVID-19 vaccine administration and its relevance to [18F]FDG PET-CT interpretation of oncologic patients.
Methods
Patient population
After receiving the consent of the institutional ethical committee, all oncologic patients over 16 years of age that underwent whole-body [18F]FDG PET-CT in our department were interviewed regarding their COVID-19 vaccination status. Between August 1 and August 25, 2021, a total of 179 consecutive patients reported they had received a third BNT162b2 vaccine dose before imaging, and were included in the study. For each patient, we recorded the following parameters in the dataset: (1) the time interval between vaccination and imaging, and the vaccination site; (2) age, sex, and body mass index (BMI); (3) the indication for PET-CT, including type of malignancy and types of systemic anti-cancer therapies received during the 3 months prior to imaging.
Table 1 summarizes the characteristics of the study cohort.
Table 1.
Patient characteristics
| Variable | All patients | |
|---|---|---|
| (n = 179) | ||
| General | Female | 93 (52%) |
| Age (years) | 71.5 (65–78) | |
| BMI (kg/m2) | 26.1 (23.4–29.6) | |
| Primary malignancy | Hematologic malignancies | 32 (18%) |
| Lower gastrointestinal cancers | 27 (15%) | |
| Breast cancer | 26 (15%) | |
| Lung cancers | 24 (13%) | |
| Skin cancers and sarcomas | 16 (9%) | |
| Hepatobiliary-pancreatic cancers | 14 (8%) | |
| Gynecological cancers | 13 (7%) | |
| Genitourinary cancers | 10 (6%) | |
| Upper gastrointestinal cancers | 8 (5%) | |
| Head and neck cancers | 4 (2%) | |
| Others | 5 (3%) | |
| PET-CT indication | Staging | 36 (20%) |
| Monitor response to therapy | 70 (39%) | |
| Recurrence detection | 20 (11%) | |
| Follow-up (with NED) | 53 (30%) | |
| Recent therapy | Any systemic anti-cancer therapy | 70 (39%) |
| Chemotherapy | 38 (21%) | |
| Biologic therapy | 33 (18%) | |
| Immunotherapy | 13 (7%) |
Categorical variables are reported as frequency and percentage. Continuous variables are reported as median and IQR. BMI, body mass index; NED, no evidence of disease
Imaging and categorization of lymphadenopathy
[18F]FDG PET-CT studies were performed on PET-CT scanners (GE Healthcare; DISCOVERY 690 and DISCOVERY MI; 7 to 8 frames; frame time 1.5–3 min), according to our standard protocol, with the administration of a diluted oral contrast agent and injection of 3.7 MBq/kg [18F]FDG approximately 60 min prior to the study. Final PET-CT interpretation was carried out by at least one nuclear medicine specialist with PET-CT experience of at least 8 years.
For each patient, the presence or absence of “hot” axillary or supraclavicular lymph nodes ipsilateral to the vaccine injection site was recorded. Based on the interpretation that appeared in the PET-CT report, the “hot” nodes were further categorized as benign vaccine-associated, malignant, or equivocal. VAHL was recorded if the lymphadenopathy was reported as benign vaccine-associated. In case the hypermetabolic lymphadenopathy could not be confidently categorized as neither benign vaccine-associated nor malignant, the case was recorded as equivocal. The type and site of the primary tumor, the stage of the disease, the presence and location of other abnormal findings (mainly malignant lymphadenopathy in other nodal stations), and findings on previous studies were all considered when interpreting the nature of the hypermetabolic lymphadenopathy.
In all VAHL cases, the number of the “hot” nodes, their location, the size of the biggest “hot” node (short-axis diameter), and the [18F]FDG uptake intensity measured in the “hottest” node (using maximal standardized uptake value (SUVmax)) were recorded. The presence or absence of a regional [18F]FDG uptake (higher than the background deltoid uptake) in the vaccination side reported by the patient was recorded as well. All VAHL cases were graded on the 4 grade scale described in our previous paper [8]: Grade 1, mild uptake intensity (SUVmax < 2.2); Grade 2, moderate uptake intensity (2.2 ≤ SUVmax < 4); Grade 3, high uptake intensity (SUVmax ≥ 4) in normal-size nodes; and Grade 4, high uptake intensity (SUVmax ≥ 4) in enlarged nodes. Grade 3–4 VAHL were considered high grade.
Statistical analysis
Categorical data were described with contingency tables that included frequency and percent. Continuous variables were evaluated for normal distribution and reported as mean ± standard deviation (SD) or as median and interquartile range (IQR). Pearson’s χ2 test and Fisher’s exact test were applied to compare proportions between groups. The Mann–Whitney U test was applied to compare medians of continuous variables between two groups. Univariate and bivariable logistic regression analyses were applied to study the association between possible predictors and VAHL status. A multivariable logistic regression analysis was performed in order to identify independent predictors of VAHL status. Variables with a trend or a significant association with VAHL status, as well as those known to be of important clinical significance, were tested in the multivariable model. A two-sided P value of < 0.05 was considered statistically significant. SPSS software (IBM SPSS Statistics for Windows, version 27, IBM corp., Armonk, NY, USA, 2017) was used for statistical analyses. Plots were generated using the open-source statistics software R (version 4.0.5, R Foundation for Statistical Computing).
Results
VAHL characteristics following the third BNT162b2 vaccine dose
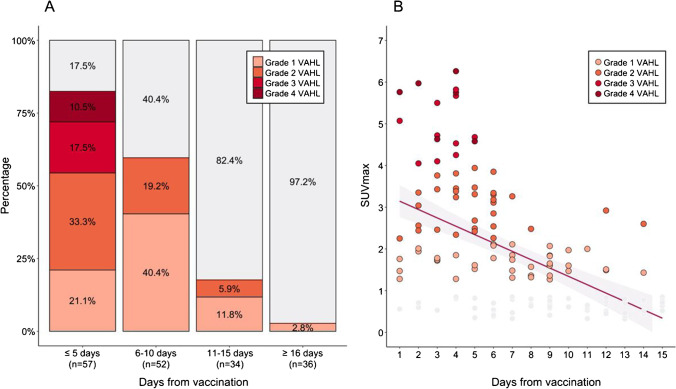
With a median time interval of 8 (IQR, 5–14, range, 1–36) days between vaccination and imaging, 85 (47.5%) of the patients had any-grade VAHL on their [18F]FDG PET-CT studies. Only 16 (8.9%) of the vaccinated patients had high-grade VAHL on imaging. Figure 1A illustrates the proportion of vaccinated patients that had VAHL on imaging and their VAHL grades at four time intervals post vaccination. All-grade VAHL was significantly more common on studies done during the first 5 days from vaccination compared to studies done ≥ 6 days from vaccination (82.5% vs 31.1%, Pv < 0.01). Cases of grade 3–4 VAHL were only detected on scans performed during the first 5 days following vaccination (in 28.1% of studies, compared with 0% of studies done ≥ 6 days from vaccination, Pv < 0.01). Later than day 5 from vaccination, the incidence of VAHL decreased gradually. The incidence of VAHL on studies performed 11–15 days from vaccination was 17.6%, and dropped to 2.8% on studies performed ≥ 16 days from vaccination.
Fig. 1.
Vaccine-associated hypermetabolic lymphadenopathy (VAHL) following the third COVID-19 vaccine dose. A Rates of VAHL and their grade at different time intervals from vaccination. B Distribution of SUVmax measured in VAHL cases over time from vaccination
Table 2 summarizes the grade, SUVmax, number, location, and size of the VAHL cases reported after the third vaccine dose, as well as the rate of increased uptake in the vaccination site. VAHL on scans performed within the first 5 days from vaccination was of higher uptake intensity with significantly higher median SUVmax (3.4 vs 1.9, Pv < 0.01) and involved significantly higher number of “hot” nodes (median 4 vs 3, Pv = 0.03). Supraclavicular involvement in VAHL was observed only on scans performed within 5 days from vaccination (in 15% of cases, compared with 0% of scans performed ≥ 6 days from vaccination, Pv = 0.02).
Table 2.
VAHL characteristics following the third COVID-19 vaccine dose
| All VAHL cases | VAHL cases ≤ 5 days from vaccination | VAHL cases ≥ 6 days from vaccination | Pv | ||
|---|---|---|---|---|---|
| (n = 85) | (n = 47) | (n = 38) | |||
| Grading | Grade 1–2 VAHL | 69 (81%) | 31 (66%) | 38 (100%) | < 0.01* |
| Grade 3–4 VAHL | 16 (19%) | 16 (34%) | 0 (0%) | < 0.01* | |
| Intensity | SUVmax of the “hottest” node | 2.4 (1.7–3.5) | 3.4 (2.0–4.6) | 1.9 (1.5–2.6) | < 0.01* |
| Number | Number of “hot” nodes | 3 (2–5) | 4 (2–6) | 3 (2–4) | 0.03* |
| Number of “hot” nodes > 3 | 39 (46%) | 26 (55%) | 13 (34%) | 0.05* | |
| Location | Axilla – level 1 | 85 (100%) | 47 (100%) | 38 (100%) | > 0.99 |
| Axilla – level 2/3/interpectoral | 35 (41%) | 23 (49%) | 12 (32%) | 0.11 | |
| Supraclavicular | 7 (8%) | 7 (15%) | 0 (0%) | 0.02* | |
| Size | Enlarged lymph node | 11 (13%) | 7 (15%) | 4 (11%) | 0.75 |
| Injection site | Increased uptake | 56 (66%) | 42 (89%) | 14 (37%) | < 0.01* |
Pv refers to the comparison between the cases of VAHL observed ≤ 5 days from vaccination and the cases of VAHL observed ≥ 6 days from vaccination. Categorical variables are reported as frequency and percentage. Continuous variables are reported as median and IQR. VAHL, vaccine-associated hypermetabolic lymphadenopathy; SUVmax, maximum standardized uptake value
The overall 85 cases of VAHL had a median SUVmax of 2.4 (IQR 1.7–3.5). The distribution of SUVmax over time from vaccination is presented in Fig. 1B, illustrating the gradual decrease in [18F]FDG uptake intensity.
In only two cases (1.1%) of the total vaccinated patients, the recent vaccination interfered with PET-CT interpretation: a patient referred for staging of upper limb melanoma, and a patient with an indolent lymphoma.
Predictors of VAHL appearance on PET-CT scans (other than time from vaccination)
On a univariate logistic regression, short time interval since vaccination was found predictive of the imaging identification of any-grade VAHL (OR, 0.73; 95% CI, 0.67–0.80; Pv < 0.01) and of grade 3–4 VAHL (OR, 0.61; 95% CI, 0.47–0.80; Pv < 0.01).
To identify other predictors of VAHL appearance on [18F]FDG PET-CT, bivariable logistic regression analyses were performed (Table 3). Each analysis included two variables: the interval between vaccination and imaging, and the investigated variable. Beyond the time interval variable, younger age was identified as an independent predictor of all-grade VAHL and of high-grade VAHL (Pv = 0.01 for both). BMI showed trends toward significance on the bivariable analyses for all-grade VAHL (Pv = 0.08) and for high-grade VAHL (Pv = 0.10). Types of malignancy and systemic therapies were not found predictive of VAHL.
Table 3.
Bivariable analyses for all-grade VAHL and grade 3–4 VAHL
| Variable 1 | Variable 2 | All grade VAHL | Grade 3–4 VAHL | ||||
|---|---|---|---|---|---|---|---|
| Pv | OR | 95% CI | Pv | OR | 95% CI | ||
| (Numbers refer to variable 2) | (Numbers refer to variable 2) | ||||||
| Interval between vaccination and imaging (days) | Gender (female) | 0.10 | 1.91 | 0.89–4.11 | 0.31 | 1.85 | 0.57–6.06 |
| Age (years) | 0.01* | 0.93 | 0.89–0.98 | 0.01* | 0.92 | 0.86–0.98 | |
| BMI (kg/m2) | 0.08 | 1.01 | 0.99–1.17 | 0.10 | 0.88 | 0.76–1.03 | |
| Hematologic malignancies | 0.41 | 0.64 | 0.23–1.83 | 0.42 | 0.40 | 0.04–3.77 | |
| Lower gastrointestinal cancers | 0.58 | 0.74 | 0.26–2.10 | 0.72 | 0.74 | 0.14–3.96 | |
| Breast cancer | 0.83 | 1.12 | 0.39–3.25 | 0.38 | 0.37 | 0.04–3.29 | |
| Lung cancers | 0.90 | 1.07 | 0.36–3.22 | 0.62 | 0.65 | 0.12–3.56 | |
| Skin cancers and sarcomas | 0.15 | 0.40 | 0.11–1.39 | 0.43 | 0.41 | 0.04–3.79 | |
| Hepatobiliary-pancreatic cancers | 0.23 | 2.29 | 0.59–8.93 | 0.08 | 4.80 | 0.84–27.32 | |
| Gynecological cancers | 0.23 | 2.67 | 0.53–13.33 | 0.58 | 1.77 | 0.23–13.59 | |
| Genitourinary cancers | 0.94 | 0.94 | 0.19–4.58 | 0.99 | - | - | |
| Upper gastrointestinal cancers | 0.94 | 1.07 | 0.18–6.32 | 0.14 | 5.30 | 0.58–48.46 | |
| Head and neck cancers | 0.65 | 1.89 | 0.12–30.54 | 0.15 | 8.69 | 0.46–162.69 | |
| Active malignancy | 0.50 | 0.74 | 0.31–1.77 | 0.46 | 1.72 | 0.41–7.26 | |
| Recent systemic anti-cancer therapy | 0.30 | 0.66 | 0.30–1.45 | 0.43 | 1.59 | 0.50–5.04 | |
| Recent chemotherapy | 0.77 | 0.87 | 0.35–2.18 | 0.22 | 2.12 | 0.65–6.91 | |
| Recent biologic therapy | 0.41 | 0.68 | 0.27–1.71 | 0.78 | 0.81 | 0.19–3.46 | |
| Recent immunotherapy | 0.57 | 0.64 | 0.14–2.92 | 0.26 | 2.91 | 0.45–18.80 | |
Each parameter was analyzed together with the time interval between vaccination and imaging on a bivariable logistic regression. Analyses were performed for the prediction of all-grade VAHL and grade 3–4 VAHL. The odds ratio (OR) with 95% confidence interval (CI) for each analyzed variable are presented for the prediction of all-grade VAHL and grade 3–4 VAHL. VAHL, vaccine-associated hypermetabolic lymphadenopathy; BMI, body mass index. Active malignancy, this variable was considered positive in cases of treatment-naïve patients, those who received recent anti-cancer therapy, and those with recurrent disease identified on their [18F]FDG PET-CT scan. Recent therapy was considered if given during the 3 months before the scan
On a multivariable logistic regression for grade 3–4 VAHL, days from vaccination (OR, 0.54; 95% CI, 0.38–0.77; Pv < 0.01), age of the patient (OR, 0.90; 95% CI, 0.83–0.97; Pv < 0.01), and the BMI of the patient (OR, 0.83; 95% CI, 0.70–0.98; Pv = 0.03) were all identified as independent predictors. The model is detailed in Table 4. Indeed, patients with grade 3–4 VAHL were younger (median age 65.5 vs 72 years, Pv = 0.01) and their median BMI was significantly lower (23.9 vs 26.1 kg/m2, Pv = 0.05).
Table 4.
A multivariable model for prediction of grade 3–4 VAHL
| Pv | OR | 95% CI | |
|---|---|---|---|
| Interval (days) | < 0.01* | 0.54 | 0.38–0.77 |
| Age (years) | < 0.01* | 0.90 | 0.83–0.97 |
| BMI (kg/m2) | 0.03* | 0.83 | 0.70–0.98 |
A multivariable model for the prediction of grade 3–4 VAHL. The odds ratios (OR) with 95% confidence intervals (CI) of the independent predictors are presented
Discussion
The frequent appearance of VAHL on [18F]FDG PET-CT studies in recently vaccinated oncologic patients has become a major concern in the era of the COVID-19 pandemic [8, 11–13].
The current study demonstrates that the overall incidence of any-grade VAHL following the third COVID-19 vaccine dose is basically similar to that reported following the first and second COVID-19 vaccine doses [8–10, 14–16]. However, VAHL cases following the third dose were found to have shorter duration and low uptake intensity after the first 5 days from vaccination. VAHL following a third vaccination does not usually persist for weeks, and only rarely interferes with imaging interpretation.
In view of these findings, previous published recommendations to postpone imaging to even 6 weeks away from vaccination [13] are not applicable after the third vaccine dose, and we recommend to schedule [18F]FDG PET-CT for oncologic patients starting 6 days from the third BNT162b2 vaccine dose.
Advising oncologic patients to be vaccinated in the arm contralateral to the tumor expected nodal drainage is still relevant in the context of the third COVID-19 vaccination. Actually, the two cases of equivocal reports included in the current study could have both been prevented had the vaccination sites been chosen as recommended [8].
The previously described association between VAHL and immunogenicity [14, 15] may be implied also in the results of the current study. Older age [17] and obesity [18] were both reported to be associated with weaker vaccine-induced immunity. The results of the current study that younger age and lower BMI are independent predictors of high-grade VAHL (which correlates with humoral immunity [14]) further strengthen the association between VAHL and the vaccine-induced immunity.
In this study, no association was found between systemic anti-cancer therapies and VAHL. It is not unlikely that such association does exist, but could not have been found in this study patients, whose anti-cancer therapies were heterogeneous. In particular, the association we previously reported between VAHL and anti-CD20-containing therapies [14] could not have been investigated in the current study. Only four cohort patients received such therapies in the year prior to vaccination, probably due to the published recommendation to postpone COVID-19 vaccination to at least 6 months after completion of anti-CD20-containing treatment [19].
The characteristics of VAHL following the first and second vaccine doses were reported to be different [8, 10, 16]. This probably reflects the difference between the immune response elicited by a first vaccine dose and the amnestic response induced by a booster given 3 weeks later. Unlike the naïve cells involved in the primary immune response following a first vaccination, the memory B cell and T cell responses are different following booster vaccinations [20]. The memory cells have already undergone clonal expansion, differentiation, and affinity maturation, so the amnestic immune response has a minimal lag period. The VAHL characteristics observed in the current study correspond to the lymph node reaction to a booster given several months after a previous dose, probably reflecting the transient lymph node involvement in the tertiary immune response. At this point in the management of the COVID-19 pandemic, it is still unclear whether more vaccine doses will be indicated in the future. In case another dose will be recommended several months apart from a previous dose, it seems possible that the incidence and characteristics of VAHL described in the current study will be the most relevant in clinical practice.
Conclusions
VAHL is common during the first 5 days following the administration of the third BNT162b2 vaccine dose. High-grade VAHL is unlikely to be observed on [18F]FDG PET-CT performed at least 6 days from inoculation of the third vaccine dose, and is even less likely in older and obese patients. Albeit the low risk of equivocal findings on [18F]FDG PET-CT in the context of the third COVID-19 vaccine dose, oncologic patients should be advised about the timing of imaging and about the site of vaccination, especially those with potential axillary tumor involvement.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COVID-19
Coronavirus disease 2019
- mRNA
Messenger ribonucleic acid
- VAHL
Vaccine-associated hypermetabolic lymphadenopathy
- EqHL
Equivocal hypermetabolic lymphadenopathy
- [18F]FDG
F18-fluorodeoxyglucose
- PET-CT
Positron emission tomography-computed tomography
- SUVmax
Maximum standardized uptake value
- MIP
Maximal intensity projection
- BMI
Body mass index
- Pv
P Value
- IQR
Interquartile range
- OR
Odds ratio
- CI
Confidence interval
- CD20
Cluster of differentiation 20
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors. This retrospective study protocol was approved by the local institutional ethics committee which waived written informed consent (Reference ID 0056–21-TLV).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Oncology—General
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N Engl J Med. 2021;384(19):1866–1868. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, Rahman S, Kim SY, Ko B, Sica RA, Kornblum N. Seroconversion rates following COVID-19 vaccination amongst patients with cancer. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, Morales M, Ziv T, Shorer Arbel Y, Scarfo L, Joffe E. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addeo A, Shah PK, Bordry N, Hudson RD, Albracht B, Di Marco M, Kaklamani V, Dietrich PY, Taylor BS, Simand PF, Patel D. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israel Ministry of health. COVID-19 in Israel - dashboard data. https://datadashboard.health.gov.il/COVID-19/general. Accessed August 25, 2021.
- 8.Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA COVID-19 vaccine: incidence assessed by [18 F] FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48(6):1854–1863. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology. 2021;27:210886. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skawran S, Gennari AG, Dittli M, Treyer V, Muehlematter UJ, Maurer A, Burger IA, Mader C, Messerli O, Grünig H, Gebhard C. [18F] FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol. 2021;22:1–9. doi: 10.1007/s00330-021-08122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Özütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, Domingo-Musibay E, Steinberger D. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients. Radiology. 2021;24:210275. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton RC, Terán AK, Erba PA, Giammarile F. Medical imaging in times of pandemic: focus on the cornerstones of successful imaging. Eur J Nucl Med Mol Imaging. 2021;48(6):1724–1725. doi: 10.1007/s00259-021-05331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, El Homsi M, Feigin KN, Gallagher KM, Hanna EY, Hicks M, Ilica AT, Mayer EL. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021;24:210436. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen D, Krauthammer SH, Cohen YC, Perry C, Avivi I, Herishanu Y, Even-Sapir E. Correlation between BNT162b2 mRNA COVID-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur J Nucl Med Mol Imaging. 2021;48(11):3540–3549. doi: 10.1007/s00259-021-05389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eifer M, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Shams J, Keret N, Gorfine M, Eshet Y. COVID-19 mRNA vaccination: age and immune status and its association with axillary lymph node PET/CT uptake. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treglia G, Cuzzocrea M, Giovanella L, Elzi L, Muoio B. Prevalence and significance of hypermetabolic lymph nodes detected by 2-[18F] FDG PET/CT after COVID-19 vaccination: a systematic review and a meta-analysis. Pharmaceuticals. 2021;14(8):762. doi: 10.3390/ph14080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier DA, Ferreira IA, Kotagiri P, Datir R, Lim E, Touizer E, Meng B, Abdullahi A, Elmer A, Kingston N, Graves B. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;30:1–9. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, Conti L, De Virgilio A, De Marco F, Di Domenico EG, Di Bella O. Obesity may hamper SARS-CoV-2 vaccine immunogenicity. medRxiv. 2021 Jan 1. 10.1101/2021.02.24.21251664
- 19.Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, Tabib Y, Cohen YC, Benyamini N, Beyar-Katz O, Neaman M. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbas AK, Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. Elsevier; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.