Abstract
The inner ear is one of the most complex structures in the mammalian body. Embedded within it are the hearing and balance sensory organs that contain arrays of hair cells that serve as sensors of sound and acceleration. Within the sensory organs, these hair cells are prototypically arranged in regular mosaic patterns. The development of such complex, yet precise, patterns require the coordination of differentiation, growth, and morphogenesis, both at the tissue and cellular scales. In recent years, there is accumulating evidence that mechanical forces at the tissue, the cellular, and the subcellular scales coordinate the development and organization of this remarkable organ. Here, we review recent works that reveal how such mechanical forces shape the inner ear, control its size, and establish regular cellular patterns. The insights learned from studying how mechanical forces drive the inner ear development are relevant for many other developmental systems in which precise cellular patterns are essential for their function.
Main text
Introduction
The inner ear is an intricate structure that contains some of the most uniquely organized tissues within the mammalian body plan. These include the spiral-shaped cochlea, the three semicircular canals, and the regular patterns of hair cells (HCs) in the hearing and balance sensory organs. How such cellular structures emerge during embryonic development has fascinated scientists for centuries. The development of the inner ear involves the coordination of cellular differentiation with dramatic morphological changes. Much of the research of inner ear development has focused on the genetic and molecular aspects of this process, identifying the specific genes, proteins, and signaling pathways involved in each developmental stage (1, 2, 3). However, it remains unclear how the information from the genetic and molecular levels is converted to physical processes that shape the complex tissue morphologies and precise cellular patterns observed. To close this gap, several recent works have focused on understanding how mechanical forces drive the development of the inner ear and how they are regulated at the molecular level. In this review, we discuss some of the main works that provided insights into the role of mechanical forces in shaping the unique inner ear structures. After providing a short overview of the mammalian inner ear structure, we discuss three main topics: 1) how mechanical forces drive the formation of the spiral-shaped cochlea, 2) how growth control regulated by mechanical forces control the size of the sensory organs, and 3) how mechanical forces drive the precise checkerboard-like pattern of HCs within the hearing sensory organ, the organ of Corti.
The structure of the mammalian inner ear
The inner ear is a complex three-dimensional structure that holds together several distinct sensory organs for hearing and balance. The sensory hearing organ, the organ of Corti, is located within the spiral-shaped cochlea. The five balance sensory organs that detect linear and angular accelerations are distributed between the three semicircular canals (the three cristae), the utricle, and the saccule (the two maculae) (Fig. 1 a). All six sensory organs are epithelial layers that consist of sensory HCs and different types of nonsensory supporting cells (SCs), arranged into mosaic patterns. In the maculae and cristae, also known as the vestibular sensory epithelia, HCs and SCs are distributed in a salt-and-pepper pattern, with each HC surrounded by SCs (Fig. 1 b). In the organ of Corti, the cells are arranged in four rows of HCs interspersed by SCs, forming a periodic checkerboard-like pattern. These four rows are divided into three outer HC rows and one inner HC row and are separated by a single row of boundary SCs called inner pillar cells (PCs) (Fig. 1 c).
Figure 1.
The structure of the mammalian inner ear. (a) A schematic showing the main sensory regions within the inner ear. (b) Zoom in on the sensory domain of the utricular macula. HCs (red) and SCs (green) are distributed in a salt-and-pepper pattern. (c) A schematic of the organ of Corti. HCs and SCs are organized in an ordered checkerboard-like pattern. The three outer HC rows and one inner HC row are marked in red, and the different types of SCs are labeled and marked in green. To see this figure in color, go online.
Shaping a spiral cochlea
The cochlea is the only spiral-shaped organ in the adult mammalian body, but it is certainly not a unique structure in nature. Spiral morphology is ubiquitous in organisms of all scales, such as in the shell of a snail, the horn of a goat, the flower petals of a rose, and even in microorganisms such as spirochetes. It is believed that many of these organisms benefit in some way from utilizing this specific morphology. For the cochlea, it has long been thought that the spiral morphology serves mainly for compactness of the cochlear tube. However, recent studies argue that the spiral structure has an auditory importance, contributing to an increased sensitivity for detection of lower frequencies (4) and better sound localization along the cochlea (5). Although it is clear that the structure of the cochlea has functional benefits, the mechanisms that shape it are just starting to be uncovered.
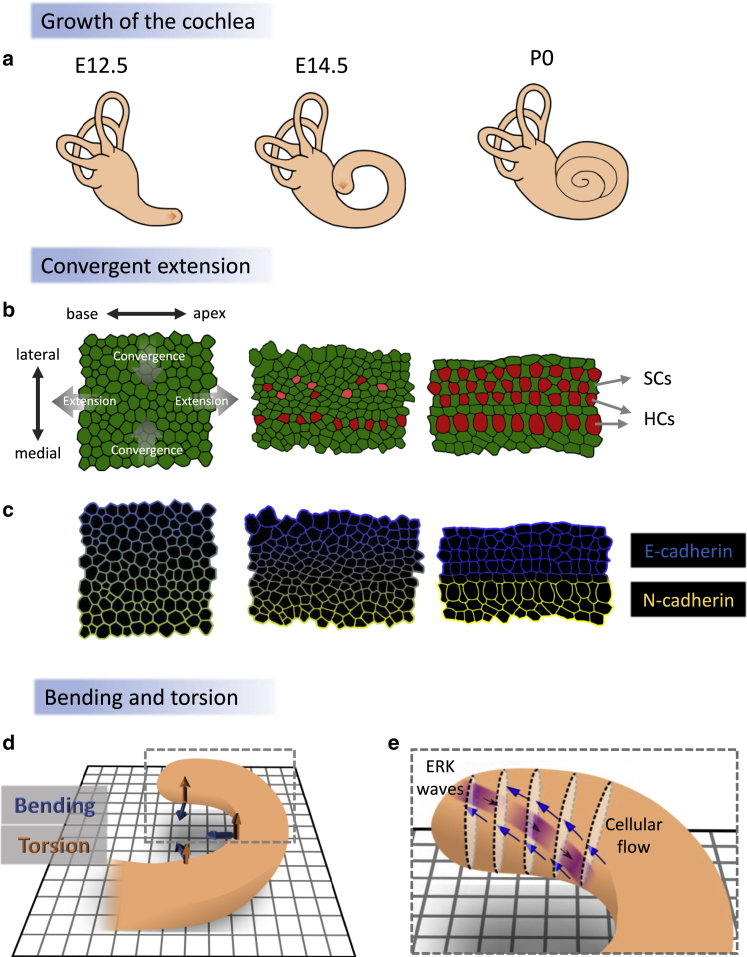
The cochlea begins its development as a short bent tube at the edge of the inner ear. During development, it extends and coils to form its three-dimensional spiral shape (Fig. 2 a). The extension of the cochlea is mainly driven by cell growth; however, it is also accompanied by convergence in the directions perpendicular to the elongation, a process known as convergent extension (CE) (Fig. 2 b; (6,7)). Although convergence during extension is expected in an elastic body, it is not obvious that a complex tissue such as the cochlea should exhibit such a behavior. Thus, it is important to understand what maintains and regulates the elastic properties at the tissue level and the local organization at the cellular level.
Figure 2.
Developmental mechanisms controlling the growth of the cochlea. (a) Schematics of three developmental stages during the growth of the mouse cochlea corresponding to embryonic day 12.5 (E12.5), embryonic day 14.5 (E14.5), and postembryonic day 0 (P0). Arrow marks the growth and extension of the cochlear tip. (b) A schematic of a CE process in the organ of Corti. As the cochlea extends along the base/apex axis, it converges in the medial-lateral axis. This process occurs in parallel to the differentiation into HCs and SCs. (c) During development, N-cadherin and E-cadherins gradually localize medial to the inner HC region and lateral to the inner HC region, respectively. (d) A schematic of the bending and torsion forces during the development of the cochlea. (e) A schematic of the tip of the developing cochlea, showing retrograding ERK waves that propagate from the tip of the cochlea backward in a diagonal manner (purple arrows), whereas cellular flow propagates in the opposite direction (blue arrows). To see this figure in color, go online.
One crucial element that affects the elastic properties of the tissue is cellular adhesion. It has been shown that a proper CE process in the murine cochlea relies on the adherens junction molecules E-cadherin and N-cadherin (8,9). Both E-cadherin and N-cadherin are dynamically distributed within the organ of Corti with increasing expression during development (Fig. 2 c). Interfering with the formation of adherens junctions by knocking out p120-catenin leads to the reduction of E-cadherin and N-cadherin expression and dramatically affects CE, resulting in shorter and wider cochleae. Interestingly, E-cadherin and N-cadherin are expressed in different compartments within the organ of Corti; whereas N-cadherin is predominantly expressed in the region medial to the PCs (toward the inner HC region), E-cadherin is expressed in the region lateral to the PCs (toward the outer HC region). These distributions demarcate a boundary between the inner and outer HC regions of the organ of Corti, with increased boundary sharpness as development progresses (Fig. 2 c). Such compartmentalization driven by cadherins has been associated with differential adhesion, in which cells tend to stick with cells expressing the same cadherin type (10, 11, 12).
As the cochlea extends, cells in the tissue elongate in the direction of extension, and this creates local contractile stresses that need to be resolved. In many epithelial tissues, such stresses are known to be relaxed by cellular rearrangements such as intercalations (13). Two recent articles have directly shown using live explant imaging that such intercalations occur at the organ of Corti (14,15). These intercalations occur more often at earlier developmental stages in which CE is more prominent. Additionally, intercalations at the apical side of the tissue may be accompanied by movement of the nuclei that regulate the stratification of the cells in the organ of Corti. Interestingly, it has been suggested that cells from the sensory epithelium actively migrate in the direction of elongation by sending biased protrusions at the basal side of the tissue. Thus, CE of the cochlea is driven by multiple active reorganization processes.
Although CE can account for the elongation, it cannot account by itself for the coiling of the cochlea. For coiling to occur, there needs to be both bending forces that drive increased curvature and out-of-plane forces that drive torsion (Fig. 2 d). A recent work provides a potential clue for what drives the out-of-plane extension of the cochlea (16). The study reveals the existence of ERK waves propagating from the apex to the base, albeit with diagonal inclination (i.e., from the roof of the apex to the floor of the base; Fig. 2 e). Interestingly, these ERK waves are correlated with active cellular flows observed in the opposite direction (from the base to the apex; Fig. 2 e). In earlier works, the authors have shown that propagating waves of ERK activation drive long-range collective cell migration via a mechanochemical feedback in cell culture (17,18). It is suggested that these ERK waves in the cochlea mechanically drive cellular flow that contributes to both the extension and the torsional growth of the cochlea.
Additional evidence for the contribution of cellular flows to cochlear bending was the recent observation of active shear movement across the organ of Corti (15). It has been observed that cellular layers along the medial-lateral axis of the cochlear duct slide with respect to each other at different velocities (see expanded discussion on this work in Rearrangements at the organ of Corti). Whether this motion is a contributor for cochlear bending or affected by it is yet to be determined.
Interplay between growth control and mechanical forces
Given the structural complexity of the inner ear, it is natural to wonder how the total size and the relative proportions of different compartments within the inner ear are accurately determined. For example, what makes the cochlea stop elongating at a specific length, and how do the vestibular sensory epithelia grow into their characteristic sizes? The questions of how the growth and size of organs are controlled during development are still largely open questions. Mechanisms of growth control have mostly been studied in Drosophila (reviewed in (19)). However, recent works have begun to shed some light on the underlying mechanisms of growth and size control in the inner ear.
Organ growth can be regulated by intrinsic factors, such as genetic regulation, as well as extrinsic factors, such as external forces. One question that is starting to get more focus is how the mechanical feedback from the environment affects growth. This mechanism is probably best associated with the growth of plants. For example, it is well known that the growth of roots is drastically affected by the mechanical feedback from the soil in the ground (20,21). Although this mechanism is less familiar in the animal kingdom, new works are starting to reveal such examples.
Such a mechanism was recently shown in the otic vesicle of the developing zebrafish. The otic vesicle is a sac-like structure filled with fluid and surrounded by epithelium that develops into the labyrinth of the inner ear during embryonic development (2). In a recent work, it was argued that the growth of the otic vesicle in zebrafish is controlled by hydraulic pressure (22). During development, fluid is transported into the lumen of the otic vesicle and gradually increases its internal pressure. In turn, the hydraulic pressure downregulates the flow into the vesicle. This feedback on hydraulic pressure pushes on the surrounding epithelium, which causes the otic vesicle to expand and leads to cell growth (Fig. 3 a).
Figure 3.
Growth control mechanisms in the inner ear. (a) Schematic of how hydraulic pressure regulates growth in the otic vesicle of the zebrafish. Accumulating hydraulic pressure (arrows) drives the expansion of the lumen and promotes cell growth while simultaneously downregulating itself by restricting flow into the lumen. (b) Schematic of elastic pressure regulates growth in the utricular macula. Macula growth is restricted by elastic pressure applied by the surrounding tissue (arrows). To see this figure in color, go online.
Although the aforementioned mechanism for growth control argues that hydraulic pressure induces growth of the otic vesicle, an opposite effect in which growth is restricted by external pressure is observed in the macula of the utricle in mice. The macula begins as a small patch of prosensory cells that grows and expands during development, until reaching its target size. In a recent work (23), it was shown that the growth of the macula is restricted and, finally, arrested because of elastic forces applied on the macula by the surrounding tissue (Fig. 3 b). The applied pressure increases the cellular density at the bulk of the macula, which in turn leads to activation of the Hippo pathway and exclusion of the transcriptional Hippo pathway regulator Yap from the nucleus. This exclusion of Yap from the nucleus inhibits cellular proliferation. Thus, regulation of growth by the Hippo pathway is induced by external elastic pressure to control utricle size.
Interestingly, Yap was shown to regulate cellular proliferation in the prosensory domain of the organ of Corti as well (24). Although the role of mechanical forces was not discussed in this work, it might be that the expression of Yap is regulated by these forces. It would be interesting to test whether stresses generated during CE regulate growth through the Hippo pathway or via some other mechanisms.
Rearrangements at the organ of Corti
Regular mosaic cellular patterns with alternating cell fates are ubiquitous in a variety of tissues. Some examples are the Drosophila ommatidia, avian oviduct, fish retina, and more. In the mammalian inner ear, all sensory epithelia are arranged in mosaic cellular patterns of HCs and SCs; however different compartments exhibit different patterns. Whereas in the vestibular sensory epithelia, HCs and SCs are arranged in a partially disordered salt-and-pepper pattern, in the organ of Corti, the pattern is highly ordered, forming four rows of HCs and SCs arranged in a precise checkerboard-like pattern. HCs and SCs in each sensory epithelium emerge from the prosensory epithelium via Notch-mediated lateral inhibition (25, 26, 27). In a lateral inhibition process, each cell tries to adopt the primary fate while inhibiting its neighbor from doing the same (Fig. 4 a). This local competition leads to the selection of cells with primary fates surrounded by cells with the secondary fate. Lateral inhibition can be used to select a single primary fate from a small group of cells, to generate a row of alternating fates, or to produce a salt-and-pepper pattern in a two-dimensional (2D) field of cells (Fig. 4 b). In general, although lateral inhibition is capable of producing patterns of alternating fates, these patterns are often disordered as they depend on local stochastic processes (25,26). In the context of the inner ear, the primary fates are HCs, and the secondary fates are SCs (28,29). Although the patterns produced by lateral inhibition might align to some extent with the patterns observed in the vestibular sensory epithelia, the final pattern in the organ of Corti is significantly more organized than the patterns expected from simple lateral inhibition models. This raises the question of what processes drive the more regular pattern observed in the organ of Corti.
Figure 4.
Regulatory and mechanical processes driving organized patterning of HCs in the organ of Corti. (a) Schematic of how Notch-mediated lateral inhibition between two cells drives the differentiation into distinct cell types. An initial small difference in Notch activity between the cells is amplified through the lateral inhibition feedback loop to produce one primary fate cell and one secondary cell. (b) Lateral inhibition in a one-dimensional and 2D random lattice of cells results in a salt-and-pepper pattern of primary fate cells (red), each surrounded by secondary fate cells (green). (c) Schematic of how differential adhesion between two types of cells drives alternating patterns. Preferred adhesion between cells of different types over adhesion between cells of the same type (adhesion energies marked by E) drives pattern of alternating cell types. (d) Application of differential adhesion as described in (c) to a salt-and-pepper lattice results in a checkerboard-like pattern, albeit with defects. (e) Shear-induced crystallization drives precise patterning of HCs in the organ of Corti. (f) A schematic describing how shear-induced crystallization drives rigid spheres into a crystalline organization. (g) Shear motion is a potential mechanism for the observed displacement of the apical protrusions of SCs toward the apex. To see this figure in color, go online.
One mechanism that has been suggested as a contributor for the organization of HCs and SCs is differential adhesion. This mechanism is based on the idea that different cell types would express cell adhesion molecules that promote heterotypic adhesion between different cell types and suppress homotypic adhesion between the same cell types (Fig. 4 c). Note that this type of differential adhesion is opposite to the differential adhesion driven by cadherins mentioned in Shaping a spiral cochlea, in which cells of the same type adhere to each other. Such a mechanism has been proposed to underlie the checkerboard-like pattern of the luminal surface of the avian oviduct (30). A similar mechanism has been suggested to underlie the checkerboard-like pattern of HCs and SCs in the organ of Corti when it was found that cell adhesion molecules from the nectin family were differentially expressed in HCs and SCs (31). It was found that during the development of the organ of Corti, nectin-1 and nectin-3 are exclusively expressed in HCs and SCs, respectively. Moreover, nectin-1 and nectin-3 were shown to preferentially bind to each other, suggesting enhanced adhesion between HCs and SCs. Reinforcing this possibility, knockout experiments of nectin-3 resulted in defects of the cellular pattern such as direct contact between outer HCs and detachment of inner HCs from the PC row. However, more realistic simulations of the differential adhesion model showed that this mechanism was not sufficient to produce the observed ordered pattern when starting from a disordered salt-and-pepper distribution of HCs and SCs (Fig. 4 d; (15)). Moreover, the defects observed in nectin mutants were relatively mild, and the overall pattern of HCs and SCs was maintained, further implying that differential adhesion is not the whole story.
The fact that HCs and SCs in the organ of Corti are postmitotic (3) and are very rarely trans-differentiating (32) strongly suggests that cells dynamically reorganize after differentiation. Early evidence of cellular reorganization was found in the chick basilar papilla, the avian hearing sensory epithelium (33). Similar to the mammalian inner ear, the initial pattern of HCs and SCs in the basilar papilla is determined by lateral inhibition. By quantifying the spatiotemporal pattern characteristics, such as relative number of HCs and SCs and average number of neighboring cells, the initial pattern was shown to gradually develop into a regular pattern, indicating that additional rearrangements of HCs and SCs may take place. Similar morphological analyses on the mammalian organ of Corti also indicated that there are significant reorganization processes taking place as the number of SCs neighboring each HC decreased with developmental stage, and the hexagonal ordering of HCs increased gradually (15). Interestingly, this transition from disordered to ordered pattern follows the developmental gradient from base to apex (3) so that different positions along the base-apex axis correspond to different developmental stages.
More direct evidence for the reorganization processes came from live imaging of cochlear explants that confirmed that cells in the organ of Corti reorganize during development (14,15). A significant amount of intercalations were observed during early stages of development, right after differentiation. Additionally, some SCs were extracted from the epithelium, in processes termed delaminations. Both of these processes reposition cells with respect to their neighbors and therefore modify the cellular pattern.
Intercalations and delaminations serve as the elementary local processes for reorganization; however, if these processes are not coordinated, an ordered pattern cannot form. Surprisingly, in early stages of development, significant shear motion was observed across the medial-lateral axis of the organ of Corti, namely the cells in the outer HC rows were displaced with respect to each other such that cells further away from the inner HCs move faster (Fig. 4 e). This shear motion was suggested to be driven by the active motion of the Hensen cells that border the organ of Corti on its lateral side. Additionally, outer HCs were gradually compressed toward the PCs, leading to squeezing out of SCs toward the lateral border. Thus, the shear and compression drive the intercalations and delaminations in such a way that gives rise to the final organization.
To better understand how this process gives rise to the transition from disordered to ordered patterning of HCs and SCs, a mechanical model of the organ of Corti was developed (15). The mechanical model, which is based on the 2D vertex model, is used to show that shear forces on HCs and local repulsion between HCs are sufficient to drive an initially disordered salt-and-pepper pattern into an almost perfect hexagonal mosaic pattern through a coordinated series of intercalations and delaminations (Fig. 4, e and f). Interestingly, this process of ordered patterning is analogous to the classical process of shear-induced crystallization observed in granular and polymer physics. It was also shown that incorporating differential adhesion between HCs and SCs in the model enabled further refinement of the pattern. This suggests that mechanical forces are the main driver for the perfect organization of HCs and SCs in the organ of Corti.
Overall, the picture that emerges is the following: The patterning of the organ of Corti begins with lateral inhibition that generates a disordered salt-and-pepper pattern. Then, coordinated intercalations and delaminations driven by shear forces lead to reorganization via a mechanism analogous to shear-induced crystallization. Finally, the pattern is refined by differential adhesion between HCs and SCs. Interestingly, the shear motion within the organ of Corti could also explain the classical observation of the unique three-dimensional geometry of SCs (34,35): The apical surface of each SC, which is connected to the cellular body by a long protrusion, is significantly displaced with respect to the position of cell body (Fig. 4 g). This displacement likely arises from the shear flow of HCs, which drags the apical surface toward the apex, whereas the main body of SCs is still attached to the basement membrane.
Discussion and future directions
With such unique structures, and several sensory epithelia exhibiting different patterns, the inner ear is an outstanding model to study the role of mechanical forces in tissue morphogenesis and developmental patterning processes. Moreover, the inner ear is a system in which different scales are strongly coupled, namely that the tissue scale morphogenesis is strongly coupled to the local cellular scale. For example, cochlear CE at the tissue scale produces compressional forces that act on single HCs and affect their local organization. However, even with the major advancements in the field, many questions remain open as to how morphogenesis at each of these scales occurs and how these scales are coordinated. At the tissue scale, additional efforts must be made to clarify the molecular mechanisms that coordinate mechanical forces, cellular motility, and growth control. At the cellular scale, the differences that lead to different patterning in the different sensory epithelia are not well understood.
Even in the organ of Corti, in which a great deal is already known about the processes that drive cellular organization, there are several open questions that need to be addressed. For example, it is not yet known what drives the precise patterning of the inner HC row. Although shear motion drives the organization of the three outer HC rows, the same mechanism probably do not apply for the inner HC row, as shear motion is not well defined for a single line of cells. Furthermore, it is not clear how the number of HC rows is so finely tuned. Although it is known that the number of rows is strongly coupled to Notch signaling (36), stochasticity implies that there is a missing fine-tuning mechanism that sets the exact number of cells required for the formation of three outer HCs and one inner HCs row. Finally, planar cell polarity pathways have been implicated in coordinating the patterning of the organ of Corti (37). However, it remains unclear how these pathways interact with the mechanical and adhesion forces between cells and how these contribute to morphogenesis.
It is expected that novel molecular, imaging, and mechanical measurements as well as theoretical approaches currently being developed worldwide would significantly improve our understanding of the organization of the inner ear. Such new understanding will provide insights, not only to the development of this unique system, but also to many other developmental systems in which interactions between regulatory circuits and cell mechanics occur over different length and time scales. Moreover, the improved understanding of the processes controlling inner ear development will likely contribute to the development of new therapeutic approaches to hearing loss, such as the ones that rely on regeneration of HCs.
Acknowledgments
We thank Karen Avraham, Shahar Taiber, and Shahar Kassirer for their advice and comments on this work.
This work has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement 682161).
Editor: Stanislav Shvartsman.
References
- 1.Chatterjee S., Kraus P., Lufkin T. A symphony of inner ear developmental control genes. BMC Genet. 2010;11:68. doi: 10.1186/1471-2156-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitfield T.T. Development of the inner ear. Curr. Opin. Genet. Dev. 2015;32:112–118. doi: 10.1016/j.gde.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Basch M.L., Brown R.M., II, Groves A.K. Where hearing starts: the development of the mammalian cochlea. J. Anat. 2016;228:233–254. doi: 10.1111/joa.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manoussaki D., Dimitriadis E.K., Chadwick R.S. Cochlea’s graded curvature effect on low frequency waves. Phys. Rev. Lett. 2006;96:088701. doi: 10.1103/PhysRevLett.96.088701. [DOI] [PubMed] [Google Scholar]
- 5.Huang X., Xu C., Bai L. Is the cochlea coiled to provide sound localization? EPL. 2012;98:58002. [Google Scholar]
- 6.Sutherland A., Keller R., Lesko A. Convergent extension in mammalian morphogenesis. Semin. Cell Dev. Biol. 2020;100:199–211. doi: 10.1016/j.semcdb.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Mark S., Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chacon-Heszele M.F., Ren D., Chen P. Regulation of cochlear convergent extension by the vertebrate planar cell polarity pathway is dependent on p120-catenin. Development. 2012;139:968–978. doi: 10.1242/dev.065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitlon D.S. E-cadherin in the mature and developing organ of Corti of the mouse. J. Neurocytol. 1993;22:1030–1038. doi: 10.1007/BF01235747. [DOI] [PubMed] [Google Scholar]
- 10.Foty R.A., Steinberg M.S. The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg M.S. Differential adhesion in morphogenesis: a modern view. Curr. Opin. Genet. Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Foty R.A., Steinberg M.S. Differential adhesion in model systems. Wiley Interdiscip. Rev. Dev. Biol. 2013;2:631–645. doi: 10.1002/wdev.104. [DOI] [PubMed] [Google Scholar]
- 13.Tetley R.J., Mao Y. The same but different: cell intercalation as a driver of tissue deformation and fluidity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373:20170328. doi: 10.1098/rstb.2017.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driver E.C., Northrop A., Kelley M.W. Cell migration, intercalation and growth regulate mammalian cochlear extension. Development. 2017;144:3766–3776. doi: 10.1242/dev.151761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen R., Amir-Zilberstein L., Sprinzak D. Mechanical forces drive ordered patterning of hair cells in the mammalian inner ear. Nat. Commun. 2020;11:5137. doi: 10.1038/s41467-020-18894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii M., Tateya T., Hirashima T. Retrograde ERK activation waves drive base-to-apex multicellular flow in murine cochlear duct morphogenesis. eLife. 2021;10:e61092. doi: 10.7554/eLife.61092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki K., Kondo Y., Matsuda M. Propagating wave of ERK activation orients collective cell migration. Dev. Cell. 2017;43:305–317.e5. doi: 10.1016/j.devcel.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Hino N., Rossetti L., Hirashima T. ERK-mediated mechanochemical waves direct collective cell polarization. Dev. Cell. 2020;53:646–660.e8. doi: 10.1016/j.devcel.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Vollmer J., Casares F., Iber D. Growth and size control during development. Open Biol. 2017;7:170190. doi: 10.1098/rsob.170190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bengough A.G., Croser C., Pritchard J. A biophysical analysis of root growth under mechanical stress. Plant Soil. 1997;189:155–164. [Google Scholar]
- 21.Potocka I., Szymanowska-Pułka J. Morphological responses of plant roots to mechanical stress. Ann. Bot. 2018;122:711–723. doi: 10.1093/aob/mcy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosaliganti K.R., Swinburne I.A., Megason S.G. Size control of the inner ear via hydraulic feedback. eLife. 2019;8:e39596. doi: 10.7554/eLife.39596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnedeva K., Jacobo A., Hudspeth A.J. Elastic force restricts growth of the murine utricle. eLife. 2017;6:e25681. doi: 10.7554/eLife.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnedeva K., Wang X., Segil N. Organ of Corti size is governed by Yap/Tead-mediated progenitor self-renewal. Proc. Natl. Acad. Sci. USA. 2020;117:13552–13561. doi: 10.1073/pnas.2000175117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collier J.R., Monk N.A., Lewis J.H. Pattern formation by lateral inhibition with feedback: a mathematical model of delta-notch intercellular signalling. J. Theor. Biol. 1996;183:429–446. doi: 10.1006/jtbi.1996.0233. [DOI] [PubMed] [Google Scholar]
- 26.Shaya O., Sprinzak D. From Notch signaling to fine-grained patterning: modeling meets experiments. Curr. Opin. Genet. Dev. 2011;21:732–739. doi: 10.1016/j.gde.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Binshtok U., Sprinzak D. Modeling the notch response. Adv. Exp. Med. Biol. 2018;1066:79–98. doi: 10.1007/978-3-319-89512-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanford P.J., Lan Y., Kelley M.W. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 29.Petrovic J., Formosa-Jordan P., Giraldez F. Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear. Development. 2014;141:2313–2324. doi: 10.1242/dev.108100. [DOI] [PubMed] [Google Scholar]
- 30.Honda H., Yamanaka H., Eguchi G. Transformation of a polygonal cellular pattern during sexual maturation of the avian oviduct epithelium: computer simulation. J. Embryol. Exp. Morphol. 1986;98:1–19. [PubMed] [Google Scholar]
- 31.Togashi H., Kominami K., Takai Y. Nectins establish a checkerboard-like cellular pattern in the auditory epithelium. Science. 2011;333:1144–1147. doi: 10.1126/science.1208467. [DOI] [PubMed] [Google Scholar]
- 32.Bramhall N.F., Shi F., Edge A.S.B. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014;2:311–322. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodyear R., Richardson G. Pattern formation in the basilar papilla: evidence for cell rearrangement. J. Neurosci. 1997;17:6289–6301. doi: 10.1523/JNEUROSCI.17-16-06289.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim D.J. Functional structure of the organ of Corti: a review. Hear. Res. 1986;22:117–146. doi: 10.1016/0378-5955(86)90089-4. [DOI] [PubMed] [Google Scholar]
- 35.Raphael Y., Lenoir M., Pujol R. The sensory epithelium and its innervation in the mole rat cochlea. J. Comp. Neurol. 1991;314:367–382. doi: 10.1002/cne.903140211. [DOI] [PubMed] [Google Scholar]
- 36.Basch M.L., Brown R.M., II, Groves A.K. Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. eLife. 2016;5:e19921. doi: 10.7554/eLife.19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rida P.C.G., Chen P. Line up and listen: planar cell polarity regulation in the mammalian inner ear. Semin. Cell Dev. Biol. 2009;20:978–985. doi: 10.1016/j.semcdb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]