Abstract
Anemia is a globally widespread condition in women and is associated with reduced economic productivity and increased mortality worldwide. Here we map annual 2000–2018 geospatial estimates of anemia prevalence in women of reproductive age (15–49 years) across 82 low- and middle-income countries (LMICs), stratify anemia by severity and aggregate results to policy-relevant administrative and national levels. Additionally, we provide subnational disparity analyses to provide a comprehensive overview of anemia prevalence inequalities within these countries and predict progress toward the World Health Organization’s Global Nutrition Target (WHO GNT) to reduce anemia by half by 2030. Our results demonstrate widespread moderate improvements in overall anemia prevalence but identify only three LMICs with a high probability of achieving the WHO GNT by 2030 at a national scale, and no LMIC is expected to achieve the target in all their subnational administrative units. Our maps show where large within-country disparities occur, as well as areas likely to fall short of the WHO GNT, offering precision public health tools so that adequate resource allocation and subsequent interventions can be targeted to the most vulnerable populations.
Subject terms: Risk factors, Diseases
Geospatial estimates of the prevalence of anemia in women of reproductive age across 82 low-income and middle-income countries reveals considerable heterogeneity and inequality at national and subnational levels, with few countries on track to meet the WHO Global Nutrition Targets by 2030.
Main
Anemia occurs when the number of healthy red blood cells is insufficient to meet the body’s physiological needs for oxygen delivery to the brain, heart, muscles and other vital tissues. Hemoglobin is the primary oxygen-carrying molecule within red blood cells, so anemia is most typically measured in terms of hemoglobin content of the blood rather than red blood cell volume1,2. Anemia can reduce cognitive and physical capacities and is associated with reduced economic productivity3,4 and increased morbidity and all-cause mortality5. Maternal iron deficiency can lead to adverse pregnancy and newborn outcomes, including stillbirth, low birth weight and infant mortality6, and anemia in pregnancy has been suggested as a potential marker of increased risk of major hemorrhage7 and a risk factor for maternal death8.
Causes of anemia can be divided into three non-mutually exclusive pathways: blood loss, increased red blood cell destruction and inadequate red blood cell production. Blood loss can be acute due to events such as injuries, maternal hemorrhage or surgery, or it can be chronic, due to conditions such as gastrointestinal disorders, helminthic infections, bleeding disorders or abnormal uterine bleeding9,10. Increased red blood cell destruction happens either as a consequence of abnormal red blood cell structure, such as in thalassaemia or sickle cell disease, or because of external mechanical, immune or infectious factors11. Inadequate production of red blood cells can happen when the bone marrow itself is depressed, such as in HIV12 or some malignancies; because there are hormonal imbalances, such as with chronic inflammation;13 or due to increased demand (such as during pregnancy), nutrient malabsorption or inadequate supply of red blood cell building blocks, such as protein, iron, vitamin A14, folate or vitamin B-12 (ref. 15) Iron deficiency is often thought of as the most common cause of anemia, which is true but also misleading, because absolute and/or functional iron deficiency can arise as a consequence of any of the three pathways and, therefore, as a consequence of multiple different causes. Women of reproductive age (WRA; ages 15–49 years) are at particularly increased risk of iron deficiency and, therefore, anemia, compared to men, due to physiological changes such as menstruation (blood loss pathway), pregnancy (inadequate production pathway due to increased demand) and bleeding in childbirth16,17. Additionally, unequal household food allocation can make WRA vulnerable to anemia as they might not have access to iron-rich foods17.
Anemia continues to affect millions of women worldwide and remains concentrated in LMICs as defined by the Global Burden of Disease (GBD) Socio-Demographic Index (SDI)18. In 2019, 30.1% of WRA were estimated to have anemia globally, with wide geographical variation18, and dietary iron deficiency was among the highest-ranking conditions in both prevalence and years lived with disability (YLDs) among WRA in LMICs19. The WHO has set a GNT to reduce anemia in WRA by 50% by 2025 (refs. 2,20); this target and other related WHO GNTs have since been extended to 2030 (ref. 21). In October 2019, the percentage of WRA with anemia was officially added as an indicator to track progress toward the Sustainable Development Goal (SDG) 2.2 to end all forms of malnutrition by 2030 (refs. 22,23). Although the WHO provides national-level anemia estimates and tracking tools, available reports do not show the subnational heterogeneity needed to inform within-country planning, annual changes to track progress or anemia severity stratifications20,24. Maps of comparable estimates across space and time at policy-relevant administrative levels are vital to identify the most vulnerable populations, track progress toward international anemia goals and provide decision-makers and policy-makers with tools to aid targeted interventions.
This study is part of a series using high-spatial-resolution estimates to map progress toward the WHO GNTs25–27. To perform this study, we compiled an extensive geo-positioned dataset from 218 surveys representing over 3 million women. Using Bayesian model-based geostatistics and the assumption that locations with similar socioeconomic and environmental patterns and proximity in time and space would have similar anemia levels, we produced estimates for all areas across 82 LMICs, even where data were sparse. The geospatial nature of our estimates also allows for the flexibility to aggregate to different (and sometimes changing) boundaries and catchment areas over the observation period.
Here we present annual geospatial estimates from 2000 to 2018 of prevalence and absolute counts of anemia of WRA (non-pregnant and pregnant combined), stratified by severity and aggregated to first-level (for example, provinces) and second-level (for example, districts) administrative units and national levels across 82 LMICs. Overall anemia was defined as <12 g dl−1 for non-pregnant WRA and <11 g dl−1 for pregnant WRA28. Anemia severity categories are defined by the WHO: mild anemia (11.0–11.9 g dl−1 for non-pregnant WRA; 10.0–10.9 g dl−1 for pregnant WRA), moderate anemia (8.0–10.9 g dl−1 for non-pregnant WRA; 7.0–9.9 g dl−1for pregnant WRA) and severe anemia (<8.0 g dl−1 for non-pregnant WRA; <7.0 g dl−1 for pregnant WRA). We also discuss our results in light of public health problem thresholds: no public health problem (<5% overall anemia prevalence), low public health problem (5–19.9% overall anemia prevalence), medium public health problem (20–39.9% overall anemia prevalence) and high public health problem (≥40% overall anemia prevalence)28. We show annualized rates of change (AROCs) between 2000 and 2018 and estimate the probability of achieving the WHO GNT by 2025 and 2030 based on recent trends. Additionally, we provide subnational disparity analyses. These estimates can aid in focusing attention on exemplars of progress, highlighting subnational inequalities and identifying locations requiring further investments. The full suite of outputs from the analysis are publicly available on the Global Health Data Exchange (http://ghdx.healthdata.org/record/ihme-data/global-anemia-prevalence-geospatial-estimates-2000-2019) and via our interactive data visualization tool (https://vizhub.healthdata.org/lbd/aneamia).
Results
Prevalence and trends of overall anemia
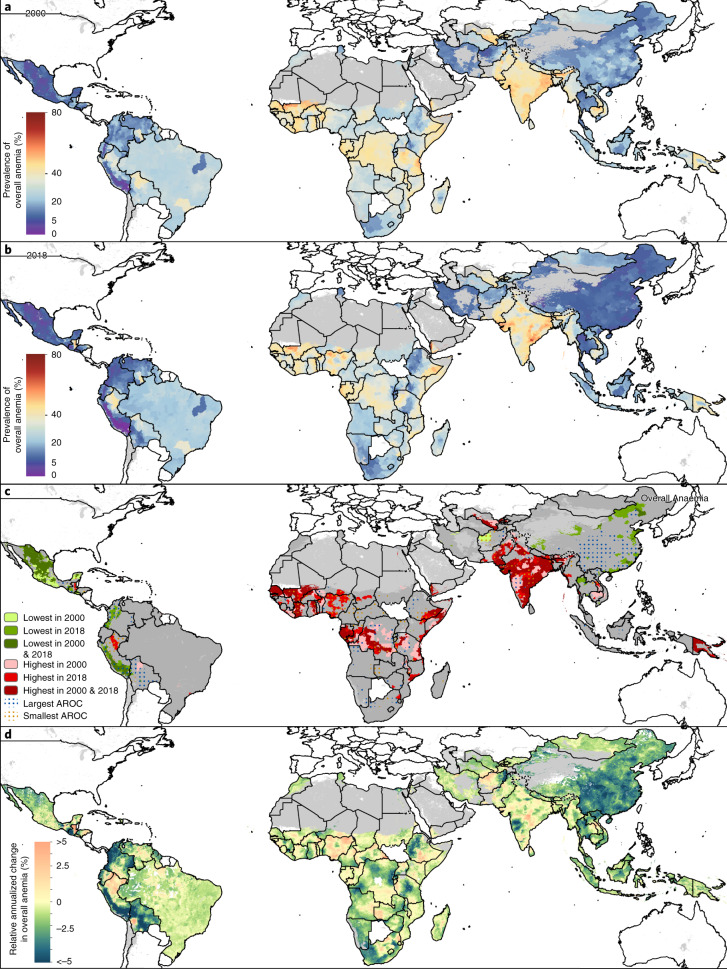
The prevalence of overall anemia among WRA varied broadly across LMICs (Fig. 1a,b). In 2018, anemia prevalence was highest in West African, Middle Eastern and South Asian countries, including Gambia (50.3% (95% uncertainty interval: 43.3–57.5)), Senegal (47.3% (43.4–50.1)), Mali (47.6% (45.8–49.4)), Yemen (57.4% (50.9–63.8)) and India (49.9% (47.2–52.4)). The lowest national-level anemia prevalence in 2018 was found in Central America and the Caribbean, Andean South America and East Asia, including El Salvador (8.2% (3.6–16.1)), Colombia (9.2% (4.5–17.0)), Mexico (10.4% (7.3–15.3)) and China (11.1% (9.1–13.1)).
Fig. 1. Prevalence and AROCs of overall anemia in WRA (2000–2018).
a, b, Prevalence of overall anemia among WRA (ages 15–49) at the second administrative unit (for example, district) level in 2000 (a) and 2018 (b). c, Overlapping population-weighted highest and lowest (10th and 90th deciles) prevalence and AROCs between 2000 and 2018. Largest AROC indicates where largest decreases in overall anemia prevalence from 2000 to 2018 occurred, whereas smallest AROC indicates where the largest increases (or smallest decreases or stagnation) in overall anemia prevalence from 2000 to 2018 occurred. d, Weighted annualized percentage of change of overall anemia prevalence in WRA from 2000 to 2018. Maps reflect administrative boundaries, land cover, lakes and population; gray-colored grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’, whereas white-colored grid cells were not included in this analysis42–47.
Gradual declines on a global scale indicate that little progress was seen in reducing anemia on a more local scale. Across the 82 LMICs, overall anemia among WRA decreased from 35.6% (25.7–46.9) in 2000 to 31.6% (25.2–39.1) in 2018. High levels of anemia remained widespread in 2018, with just over half (56.1%; 46 of 82) of LMICs with 20–39.9% prevalence of mean national-level overall anemia in 2018. On a subnational scale, 80 (97.6%) LMICs had at least one second administrative-level unit (hereafter ‘district’), and 38 (46.3%) LMICs had a majority of districts with 20–39.9% mean overall anemia prevalence. Over a quarter of LMICs (25.6%; 21 LMICs) had >40% mean national-level anemia in 2018, whereas 76 (92.7%) had at least one district, and 22 (26.8%) LMICs had most of their districts, with >40% mean overall anemia prevalence.
Anemia was at unacceptable levels (>5% prevalence)28 in 99.7% (21,868 of 21,917) of districts across LMICs in 2000 and 98.9% (21,686 of 21,917) in 2018. (Fig. 1a,b). In 2000, 30.7% (6,725 of 21,917), 48.9% (10,726 of 21,917) and 20.1% (4,417 of 21,917) of subnational districts had low (5–19.9%), medium (20–39.9%) and high (≥40%) public health threat levels of anemia among WRA28, respectively (Extended Data Table 1). Global shifts led to 37.7% (8,273 of 21,917), 43.5% (9,523 of 21,917) and 17.7% (3,881 of 21,917) of districts having low, medium and high public health problem levels in anemia prevalence among WRA, respectively, in 2018. Only two countries (Peru and Ecuador) had districts that maintained levels below 5% prevalence of overall anemia in both 2000 and 2018. In Peru, 36 of 195 (19.5%) districts had overall anemia prevalence levels <5% in both 2000 and 2018, such as in San Román (Puno) in the south (2.3% (1.1–4.3) in 2000; 0.4% (0.2–0.7) in 2018); in Ecuador, only one of 223 (0.4%) districts mean estimates achieved <5% prevalence in both years: the centrally located Cevallos (Tungurahua) (4.4% (1.0–12.5) in 2000; 4.6% (1.2–11.4) in 2018). Although Mexico had 16 districts and Iran had two districts below public health problem levels (<5%) in 2000, these districts exceeded 5% overall anemia prevalence in 2018. In 2018, only nine LMICs had at least one district with no public health problem in anemia (<5%), including Bolivia (two of 114 districts), Colombia (13 of 1,065 districts), Ecuador (one of 223 districts), El Salvador (six of 266 districts), Guatemala (87 of 354 districts), Mexico (25 of 2,454 districts), Thailand (one of 928 districts) and Uganda (two of 203 districts). Peru has seen great success reducing childhood stunting29, in part due to its targeted focus on those most in need—the poor, the more disadvantaged and rural populations—and some of this progress is mirrored in its low rates of anemia as demonstrated by half of its districts (57.9%; 113 of 195) having less than 5% mean overall anemia prevalence in 2018.
With few exceptions, we see that countries with the subnational units with the best anemia prevalence rates in 2000 continue to have administrative units that perform well in 2018, and likewise for countries with the worst-performing subnational units. To illustrate where these high and low pockets continue to be most pervasive and how their rates of change contribute to maintaining this relative status, we overlaid the highest and lowest deciles for prevalence (Fig. 1a,b) and AROCs (Fig. 1d) for overall anemia among WRA across LMICs to simultaneously show the best- and worst-performing districts as defined by both of these measures over the study period (Fig. 1c). Much of Central and South America had districts with the lowest levels of prevalence of overall anemia in 2000 and 2018, with some areas experiencing the largest decreases over time (largest AROCs), including in western Colombia and central and southern Peru. Much of Mexico and El Salvador, as well as districts in western Honduras, central Ecuador and select districts in eastern Brazil, also had among the lowest prevalence levels in both years, whereas western Bolivia and western Guatemala experienced some of the largest declines in the period that led to their place among the lowest decile of anemia prevalence in 2018. Within these same countries, however, there were also districts with the highest prevalence levels and/or largest increases or stagnating trends in anemia (smallest AROC) between 2000 and 2018. Districts in southern Mexico, eastern Honduras, eastern Venezuela and eastern Colombia had among the lowest prevalence levels in 2000, but increases pushed these districts out of the lowest prevalence decile by 2018. Eastern Guatemala, eastern Ecuador and northern Bolivia had among the highest prevalence levels in both 2000 and 2018. In Asia, northern Vietnam and large stretches of China experienced some of the largest declines and had the lowest levels of anemia prevalence. Districts throughout Uzbekistan, Pakistan, India and Papua New Guinea and in northern Myanmar, however, saw the highest consistent prevalence, and the centers of Laos and India and parts of Afghanistan experienced among the largest increases or stagnating trends (smallest AROC). Several African countries had among the highest levels of anemia in both years, including Senegal, Mali, Côte d’Ivoire, eastern Ghana, southern Benin, central Niger, Nigeria, Gabon, Democratic Republic of the Congo, Tanzania, Kenya, Ethiopia, Somalia, Malawi, Mozambique, Zimbabwe and Egypt, and the belt across the Sahel witnessed some of the worst stagnation. No African districts ranked among the lowest decile of anemia prevalence, but there were areas in Ethiopia, Tanzania, Democratic Republic of the Congo, South Africa and a few other select districts that experienced some of the fastest decreases.
Overall, 71 (86.6%) LMICs experienced decreases in mean anemia prevalence in most of their districts over the 2000–2018 period, and seven (8.5%) LMICs (Cape Verde, China, Kyrgyzstan, Malaysia, Namibia, Tunisia and Turkmenistan) had annualized improvements (declines) in all districts. Increases in overall anemia prevalence were experienced in the majority of districts in nine LMICs (Burundi, Central African Republic, Côte d’Ivoire, Gabon, Gambia, Nigeria, Republic of the Congo, Tajikistan and Yemen), and no countries experienced increases in all their districts. Many countries experienced extreme differences in their rates of change across their subnational units: 57 (69.5%) LMICs had at least 2.5% annualized decreases and increases across their districts, whereas 18 (22.0%) LMICs had districts with at least 5% AROC in both directions.
Prevalence and trends of anemia by severity
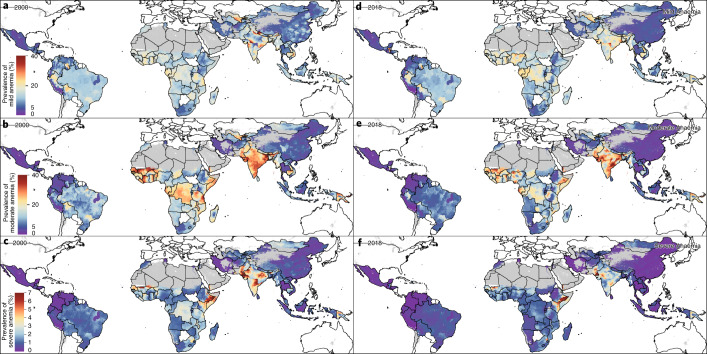
Mean prevalence of moderate and severe anemia had reduced in the majority (84.1%; 18,441 of 21,917) of districts across LMICs between 2000 and 2018 (Fig. 2). In almost a quarter of the districts in which moderate and severe anemia had declined (24.5%; 4,526 of 18,441 across 79 LMICs), mild anemia had increased, indicating a downward shift in severity levels over the populations. Among these, three-quarters (76.0%; 3,476 of 4,562) saw decreases in overall anemia, suggesting an overall shift toward normal levels of hemoglobin regardless of the historical baseline and in spite of the observed increased prevalence of mild anemia. This is further corroborated by the remaining 13,915 districts, which experienced decreases in moderate, severe and mild anemia. Among the districts that saw increases in prevalence of moderate and severe anemia (15.9%; 3,476 of 21,917), 91.3% (3,175 of 3,476 in 57 LMICs) experienced increases in overall anaemia, indicating a population-wide shift toward reduced hemoglobin levels. This was seen particularly in Yemen and Nigeria, where 81.7% (272 of 333) and 68.8% (533 of 775) of their districts, respectively, saw increases in overall, moderate and severe anemia. In contrast, only 276 districts saw increases in moderate and severe anemia but decreases in overall anemia, possibly indicating a subpopulation that has been left behind while the majority trend is toward non-anemic hemoglobin levels. Of note, Papua New Guinea and Burkina Faso experienced this divergent trend where 11.5% (10 of 87) and 11.1% (5 of 45) of their districts, respectively, saw increases in the prevalence of moderate and severe anemia while overall anemia decreased. Our stratified maps of the highest- and lowest-decile districts for prevalence and AROC for mild, moderate and severe anemia offer a detailed view of these shifts in severity across and within LMICs over time (Extended Data Fig. 1).
Fig. 2. Prevalence of anemia in WRA by severity in LMICs (2000 and 2018).
a–f, Prevalence of anemia stratified by severity among WRA (ages 15–49) at the second administrative unit (for example, district) level. Prevalence of mild anemia among WRA in 2000 (a) and 2018 (d). Prevalence of moderate anemia among WRA in 2000 (b) and 2018 (e). Prevalence of severe anemia among WRA in 2000 (c) and 2018 (f). See Supplementary Table 7 for the cutoffs defining mild, moderate and severe anemia. Maps reflect administrative boundaries, land cover, lakes and population; gray-colored grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’, whereas white-colored grid cells were not included in this analysis42–47.
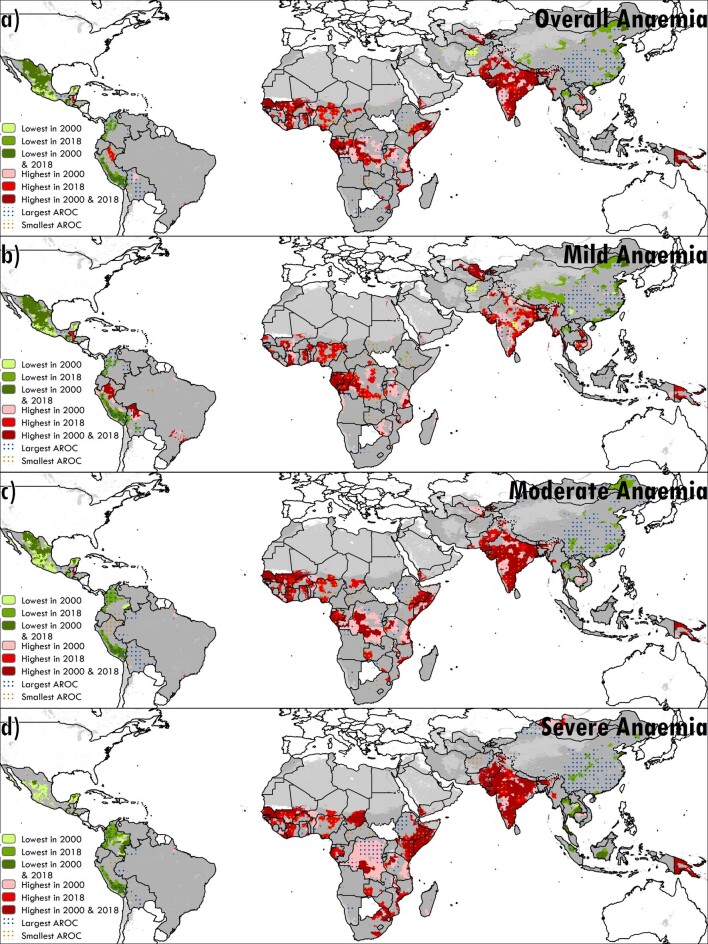
Extended Data Fig. 1. Highest- and lowest-performing second administrative-level units stratified by anaemia severity in women of reproductive age across LMICs (2000–2018).
a–d, Overlapping population-weighted highest and lowest deciles of prevalence and weighted annualised rates of change (AROC) by severity at the second administrative level between 2000 and 2018 for overall (a), mild (b), moderate (c), and severe anaemia (d) among WRA. Maps reflect administrative boundaries, land cover, lakes, and population; grey-coloured grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as “barren or sparsely vegetated”, while white-coloured grid cells were not included in this analysis42–47.
Absolute and relative geographic inequalities of anemia
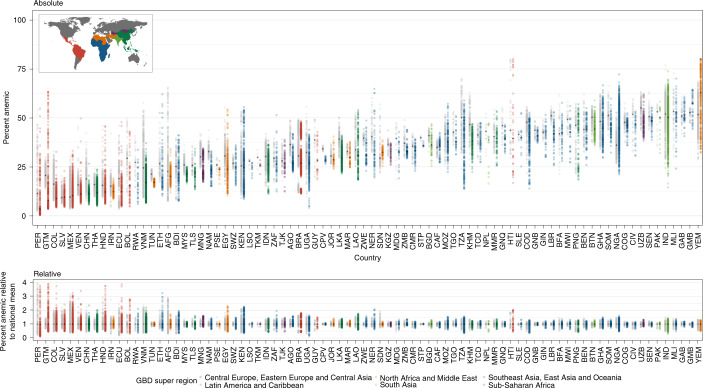
In addition to the overall trend toward lower levels of anemia prevalence, the heterogeneity of district-level anemia prevalence and, thus, subnational inequality has decreased over the last two decades. By plotting the absolute geographic inequalities (Fig. 3a), we show the range of overall anemia prevalence among each country’s districts in 2000 and 2018. Subnational inequalities between districts with the highest and lowest anemia prevalence in each country have increased in most (65.9%; 54 of 82) LMICs over the study period. Absolute inequalities among districts as well as national median anemia prevalence increased in six countries during the period from 2000 to 2018: Yemen (2.4-fold to 2.6-fold difference; 51.7% (34.0–68.6%) to 63.0% (50.9–74.3%) national median prevalence); Gambia (1.2-fold to 1.5-fold difference; 52.7% (30.4–74.7%) to 637.4% (47.4–66.2%)); Nigeria (2.0-fold to 3.4-fold difference; 36.1% (17.4–58.4%) to 44.8% (37.2–66.2%)); Central African Republic (1.3-fold to 1.6-fold difference; 35.0% (19.5–53.5%) to 36.2% (20.4–54.2%)); El Salvador (3.3-fold to 5.0-fold difference; 9.2% (4.6–16.8%) to 9.5% (3.1–21.4%)); and Gabon (1.4-fold to 1.5-fold difference; 51.0% (32.2–70.5%) to 51.2% (33.8–67.9%)). Although absolute inequalities had also increased in the other 48 LMICs, national median anemia prevalence decreased in these countries, indicating select exemplar districts that made progress and/or districts that were left behind in national progress. Overall, 28 LMICs reduced absolute inequalities as well as their national median anemia prevalence; most notably, China had reduced absolute inequalities from 5.6-fold to 4.7-fold across its districts, reducing its national median from 18.8% (10.2–30.9%) to 11.4% (4.4–22.7%) between 2000 and 2018. In 2000, 19 LMICs experienced ≥3-fold difference in overall anemia, and six LMICs experienced ≥6-fold difference in overall anemia (Afghanistan, Ecuador, Iran, Mexico, Peru and Vietnam); in 2018, 30 LMICs had ≥3-fold difference, and 11 LMICs had ≥6-fold difference, across districts (Bolivia, Colombia, Ecuador, Ethiopia, Guatemala, Honduras, Kenya, Mexico, Peru, Uganda and Venezuela) (Supplementary Table 10).
Fig. 3. Geographical inequality in overall anemia among WRA across 82 countries for 2000 and 2018.
a, Absolute inequalities: range of overall anemia estimates in WRA in second administrative-level units within 82 LMICs. b, Relative inequalities: range of ratios of overall anemia estimates in WRA in second administrative-level units relative to country means (administrative level/country level). Each dot represents a second administrative-level unit. The lower bound of each bar represents the second administrative-level unit with the lowest overall anemia in WRA in each country. The upper end of each bar represents the second administrative-level unit with the highest overall anemia in WRA in each country. Thus, each bar represents the extent of geographic inequality in overall anemia in WRA estimated for each country. Bars indicating the range in 2018 are colored according to their GBD super-region48 (Extended Data Fig. 3). Gray bars indicate the range in overall anemia in WRA in 2000. The black diamond in each bar represents the median and mean overall anemia in WRA estimated across second administrative-level units in each country and year for the absolute (median) and relative (mean) inequalities plots. A colored bar that is shorter than its gray counterpart indicates that geographic inequality has narrowed.
Our relative inequality plot shows the relative deviation of each country’s districts from their national mean anemia prevalence (Fig. 3b). To elucidate these within-country differences, consider that, in 2000, overall anemia prevalence varied across the national level by as much as 5.8-fold (9.5% (6.4%–13.7%) in El Salvador; 55.5% (41.4%–69.4%) in Gabon), and, in 2018, overall anemia varied by as much as 7.0-fold at the national level (8.2% (3.5%–16.3%) in El Salvador; 57.4% (51.4%–63.5%) in Yemen). Within-country relative inequalities in overall anemia increased in 63 LMICs between 2000 and 2018, with some of the most apparent deviations in Guatemala, Venezuela, Colombia, Ecuador, Bolivia, Thailand, Ethiopia, Egypt and Tajikistan; 19 LMICs experienced decreases in relative inequalities, including Iran, Vietnam, Palestine and Sudan. Although many of the countries with large subnational disparities in anemia prevalence could use the results from this study to efficiently target precision public health interventions where they are most needed, there is a second set of countries that had low subnational inequalities and high national prevalence, indicating a pervasive problem where ubiquitous intervention coverage is warranted. In 2018, among the 21 countries that qualified as high public health problems with a national mean overall anemia prevalence above 40%, four of these countries had low relative inequalities ranging from 75% to 125% of the national median: Gabon, Guinea-Bissau, Republic of Congo and Senegal.
Population size, severity and disability burden of anemia
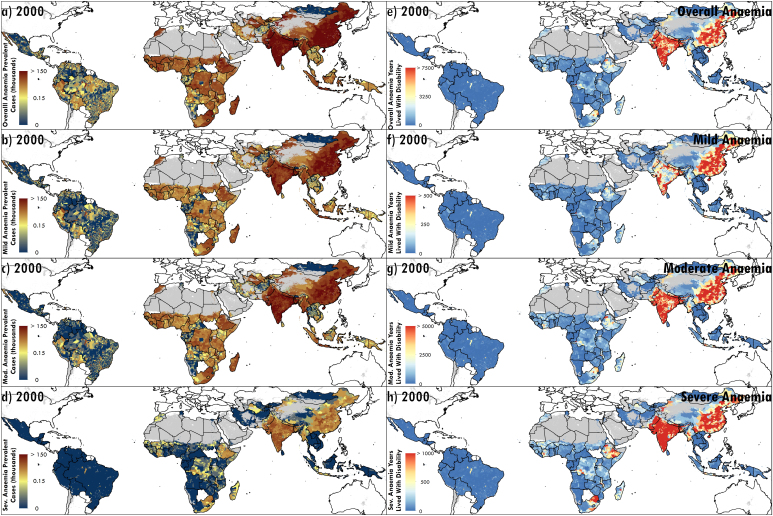
Of the estimated 1.2 billion WRA across the 82 LMICs represented by our analysis in 2000, we estimate that 378.3 million (95% uncertainty interval: 308.0–456.0) (32.8% (26.7–39.5)) of WRA were anemic (Extended Data Fig. 2a). Of these, 178.4 million (134.6–231.8) or 47.2% (43.7–50.8) were categorized as having mild anemia, whereas 182.4 million (138.1–234.5) or 48.2% (44.9–51.4) were moderate anemia cases, and 17.4 million (11.2–26.3) or 4.6% (3.6–5.8) were severe anemia cases (Extended Data Fig. 2b–d). In 2018, of the 1.5 billion WRA represented by our analysis, 449.1 million (382.4–526.9) (30.4% (25.9–35.6)) were estimated to be anemic—224.8 million (180.9–275.7) (50.1% (47.3–52.3%)) with mild cases of anemia, 208.1 million (173.1–247.3) (46.3% (45.3–46.9)) with moderate cases of anemia, and 16.1 million (12.2–21.3) (3.6% (3.2-4.0)) with severe cases of anemia (Fig. 4a–d).
Extended Data Fig. 2. Counts and years lived with disability (YLDs) by anaemia severity among WRA in 2000.
a–d, Number of WRA across 82 LMICs with overall (a), mild (b), moderate (c), and severe anaemia (d) in 2000 by second administrative-level units. e–h, Number of years lived with disability (YLDs) among women of reproductive age (WRA) attributable to overall (e), mild (f), moderate (g), and severe anaemia (h) in 2000 by second administrative-level units. Maps reflect administrative boundaries, land cover, lakes, and population; grey-coloured grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as “barren or sparsely vegetated”, while white-coloured grid cells were not included in this analysis42–47.
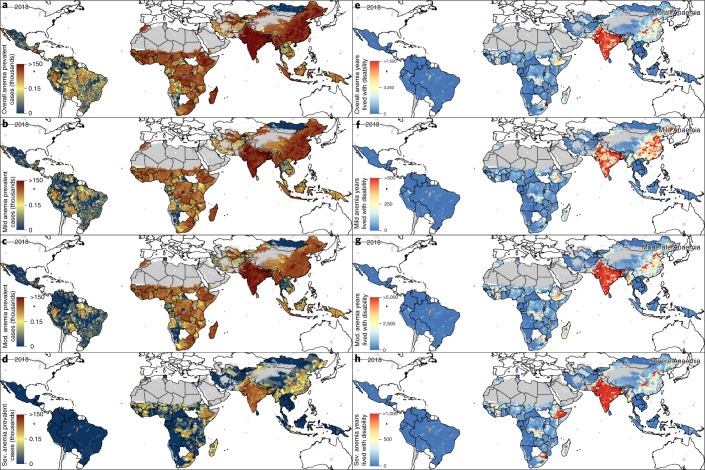
Fig. 4. Counts and YLDs by anemia severity among WRA across LMICs in 2018.
a–d, Number of WRA across 82 LMICs with overall (a), mild (b), moderate (c) and severe (d) anemia in 2018 by second administrative-level units. e–h, Number of YLDs among WRA attributable to overall (e), mild (f), moderate (g) and severe (h) anemia in 2018 by second administrative-level units. Maps reflect administrative boundaries, land cover, lakes and population; gray-colored grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’, whereas white-colored grid cells were not included in this analysis42–47.
A large proportion of anemic WRA were concentrated in a few countries in 2018; 83.0% (81.0–85.3) of overall anemia occurred in the Asian (61.7% (60.9–62.9)) and sub-Saharan African (21.3% (20.1–22.4)) regions (Fig. 4a). An estimated 59.6% (55.6–63.2) of anemic WRA, amounting to an estimated 267.5 million (241.5–293.2) cases across LMICs, lived in just four countries in 2018: India (181.3 million (171.4–190.2) cases; 40.4% (36.1–44.8) of anemia burden), China (39.5 million (32.3–46.9); 8.8% (8.9–8.5)), Pakistan (23.8 million (15.3–32.6); 5.3% (4.0–6.2)) and Nigeria (23.0 million (22.5–23.5); 5.1% (5.9–4.5)). In 2018, we estimated that 65.2% (14,292 of 21,917) of districts contained fewer than 5,000 anemic WRA, 14.6% (3,199 of 21,917) with 5,000–14,999, 12.4% (2,716 of 21,917) with 15,000–49,999, 4.5% (991 of 21,917) with 50,000–150,000 and 1.7% (377 of 21,917) with 150,000–250,000, and 1.7% (374 of 21,917) had more than 250,000 WRA with any severity of anemia (Supplementary Table 12). The 374 districts that had more than 250,000 anemic WRA each were in 22 LMICs: Angola, Bangladesh, Brazil, Burkina Faso, Cameroon, China, Côte d’Ivoire, Democratic Republic of the Congo, Ethiopia, Ghana, Haiti, India, Indonesia, Madagascar, Morocco, Myanmar, Nepal, Pakistan, Peru, South Africa, Tanzania and Togo. Across the 1,545 first administrative-level units (hereafter ‘provinces’) in the 82 LMICs, 66 provinces located in 12 LMICs (Angola, Bangladesh, Brazil, China, Ethiopia, India, Indonesia, Myanmar, Nepal, Nigeria, Pakistan and South Africa) each had 1 million or more anemic WRA in 2018. All five of the provinces with the highest estimated number of WRA with anemia in 2018 were in India and Pakistan: Uttar Pradesh in India (29.0 million (26.3–31.5)), Bihar in India (15.8 million (14.4–17.1)), West Bengal in India (15.5 million (14.4–16.6)), Maharashtra in India (15.4 million (13.4–17.2)) and Punjab in Pakistan (12.6 million (6.8–18.8)).
Stratifying by severity, an estimated 57.9% (55.1–60.9) of moderately or severely anemic WRA lived in only three countries in 2018: India (103.4 million (94.2–112.7) cases; 46.1% (41.9–50.8%) of moderate or severe WRA anemia cases), Pakistan (13.4 million (8.5–19.0) cases; 6.0% (4.6–7.1%)) and China (13.0 million (10.2–16.5) cases; 5.8% (5.5–6.1%)) (Fig. 4c,d). We found that 133 districts had more than 250,000 WRA with moderate or severe anemia in 2018, located in nine LMICs: Bangladesh (two districts), Brazil (one district), China (one district), Côte d’Ivoire (one district), Democratic Republic of the Congo (one district), India (118 districts), Nepal (one district), Pakistan (seven districts) and Peru (one district). The five provinces with the highest estimated numbers of moderate or severe WRA in 2018 were also all in India and Pakistan: Uttar Pradesh in India (16.7 million (14.7–19.0)), Bihar in India (9.4 million (8.4–10.5)), Maharashtra in India (8.2 million (6.7–9.8)), West Bengal in India (8.1 million (7.2–9.2)) and Punjab in Pakistan (6.9 million (3.7–10.6)).
Multiplying counts in each anemia severity category with the appropriate disability weights from the GBD study30 allowed us to visualize where the majority of YLDs (attributable burden) due to anemia among WRA have been most concentrated in LMICs and how it has reduced over time (Extended Data Fig. 2e–h and Fig. 4e–h). Overall anemia contributed 12.7 million (5.9–22.2) YLDs in 2000, with 0.7 million (0.3–1.1), 9.4 million (4.7–15.4) and 2.6 million (0.9–5.7) YLDs from mild, moderate and severe anaemia, respectively (Extended Data Fig. 2e–h). By 2018, YLDs had increased to 14.0 million (9.0–20.4) overall; mild, moderate and severe anemia increased to 0.8 million (0.5–1.3), 10.7 million (7.2–15.1) and reduced to 2.4 million (1.3–4.1) YLDs, respectively (Fig. 4e–h). In 2018, 0.7% (145 of 21,917) of districts each contributed more than 15,000 YLDs due to overall anemia among WRA; these districts were in just nine LMICs: Bangladesh, Brazil, China, Côte d’Ivoire, Democratic Republic of the Congo, India, Nepal, Pakistan and Peru. Districts with over 5,000 YLDs attributed to overall anemia among WRA (3.1% (677 of 21,917)) were in 33 LMICs. The three countries with the most YLDs from overall anemia among WRA in 2018 were India (6.43 million (5.80–7.11) YLDs), Pakistan (0.85 million (0.53–1.21) YLDs) and China (0.83 million (0.65–1.06) YLDs). In 2018, 532 of 21,917 districts across 30 LMICs contributed more than half of YLDs (7.0 million (4.8–9.6)) attributed to overall anemia across the 82 LMICs in this analysis.
Between 2000 and 2018, the majority of districts across LMICs experienced reductions in estimates of YLDs attributable to moderate anemia (54.1%; 11,859 of 21,917 districts) and severe anemia (67.9%; 14,876 of 21,917 districts) among WRA (Extended Data Fig. 2g,h and Fig. 4g,h). This progress in reducing YLDs due to moderate and severe anemia was especially evident in China (1.74 million (1.40–2.17) YLDs in 2000 and 0.74 million (0.57–0.95) in 2018; declines in 359 of 364 districts). In 10,078 districts located across all 82 LMICs, however, YLDs from moderate anemia increased, including in 12 countries where all districts experienced increases: Burkina Faso, Chad, Côte d’Ivoire, Guinea-Bissau, Jordan, Mali, Pakistan, São Tomé and Príncipe, Senegal, Sierra Leone, Somalia and Yemen. The YLDs from severe anemia increased in 7,061 districts across 79 LMICs, including in Yemen (332 of 333 districts), Burkina Faso (44 of 45 districts), Chad (53 of 55 districts) and Jordan (48 of 52 districts). The district with the largest increase in YLDs from moderate anemia was Bangalore (Karnataka) in India, with 23,003 (7,665–44,041) YLDs in 2000 and 43,497 (30,063–57,816) YLDs in 2018. The largest increase in YLDs from severe anemia was in Bijnor (Uttar Pradesh) in India, with 791 (282–1,627) YLDs in 2000 and 6,884 (4,767–9,476) YLDs in 2018.
Prospects of meeting 2030 WHO GNT
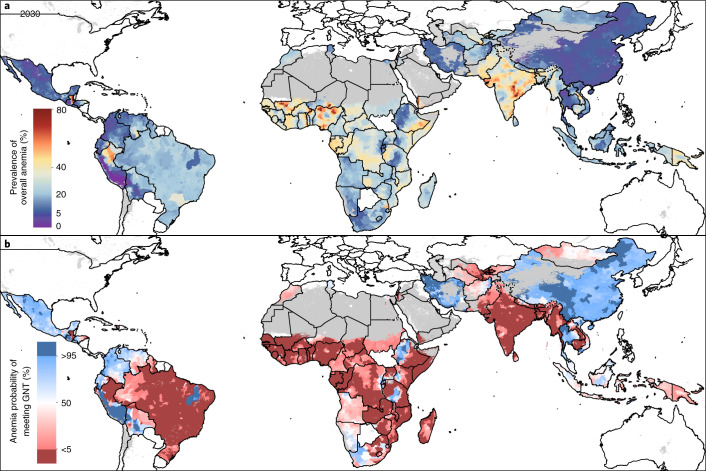
We applied the estimated AROCs to the final year of our estimates to predicted anemia prevalence estimates for the year 2030 (Fig. 5a). In 2018, 29 of 21,917 districts had >80% mean prevalence of overall anemia; if current trends continue, 100 districts across Guatemala (11 districts), Haiti (seven districts), India (two districts), Nigeria (four districts) and Yemen (76 districts) are estimated to reach >80% mean prevalence for overall anemia among WRA by 2030. Subnational inequalities in Guatemala are expected to continue, and, although 17 northeastern districts are projected to reach >75% prevalence by 2030, 179 southwestern districts are expected to reduce to below 5% prevalence, considered acceptable levels of anemia. Including Guatemala, we estimate that districts in 15 LMICs will have less than 5% prevalence in overall anemia by 2030: Afghanistan (1 of 399 districts), Bolivia (16 of 117), China (2 of 364), Colombia (123 of 1,065), Ecuador (1 of 223), El Salvador (32 of 266), Guatemala (179 of 354), Honduras (13 of 298), Mexico (31 of 2,454), Peru (120 of 195), Rwanda (3 of 30), Thailand (28 of 928), Uganda (4 of 203), Venezuela (1 of 338) and Vietnam (1 of 710). Based on current projections, we expect that 21 LMICs will maintain high national levels of overall anemia (≥40%) in 2030; on a subnational scale, 16.4% (3,594 of 21,917) of districts located in 61 LMICs are estimated to have ≥40% anemia prevalence in 2030 if existing trajectories continue.
Fig. 5. Prevalence for overall anemia among WRA in 2030 and probability of achieving the WHO GNT for overall anemia by 2030.
a, Predicted prevalence of overall anemia among WRA in 2030 by second administrative-level units. b, Probability of achievement of the WHO GNT to reduce overall anemia in WRA by 50% by the year 2030, with the year 2012 as a baseline, by second administrative-level units. Maps reflect administrative boundaries, land cover, lakes and population; gray-colored grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’ or were not included in this analysis42–47.
Assuming that recent trends persist, and using the year 2012 (the year that WHO GNTs were established) as a baseline, we estimated the probability of subnational units across LMICs achieving the WHO GNT to relatively reduce anemia by 50% by the year 2030 (Fig. 5b). By 2030, only three of the 82 (3.7%) LMICs in this analysis are expected to achieve the target of 16.2% at a national scale with a high probability (>95% posterior probability): China, Iran and Thailand. Subnationally, however, no countries have a high probability of meeting the WHO GNT for anemia in all provinces, nor in all their districts, by the target year. About a third (31.7%; 26 of 82) of LMICs have a high probability (>95%) of meeting the target in at least one district, whereas only three LMICs (Guatemala, Iran and Peru) have a high probability of meeting the goal in most districts. We expect far more LMICs to have a low probability (<5% posterior probability) of achieving the target nationally and subnationally. By 2030, 64.6% (53 of 82) of LMICs have a low probability (<5%) of meeting the WHO GNT nationally, whereas 21.2% (18) have a low probability in all provinces, and four LMICs (Gabon, Gambia, Senegal and Togo) have a low probability of meeting the target in all their districts. Although 15 (18.3%) LMICs have a >50% probability of achieving the WHO GNT by 2030 nationally, five (6.1%) LMICs have >50% probability of achieving the target in all their province-level units, and only Tunisia has >50% probability of meeting the goal in all its district-level units by 2030.
Large inequalities in achieving the WHO GNT are expected to continue, and 56.1% (46 of 82) of LMICs are predicted to have districts with both >50% and <50% probability of meeting the goal by 2030. We estimate that 20 LMICs have districts with both high probability (>95%) and low probability (<5%) of achieving the WHO GNT by 2030.
Discussion
Marginal declines in anemia prevalence among WRA in LMICs have left individuals, populations and nations at risk of reduced economic productivity3,4, increased all-cause mortality5 and increased potential for adverse outcomes for mothers and newborns31. Although most district-level units (80.5%; 17,651 of 21,917 districts) decreased their prevalence between 2000 and 2018, the overall prevalence among the 82 LMICs in our analysis has only declined, from 35.6% (95% uncertainty interval: 25.9–46.6) to 31.6% (25.7–38.2) in the nearly 20-year period. Even for the many countries with overall improvements in reducing anemia prevalence, our results highlight enduring disparities across global geographic regions and within select countries and subnational locations that have stagnated or fallen behind the general improvements of their neighbors. Although three LMICs (China, Iran and Thailand) have a high probability of meeting the WHO GNT of reducing anemia among WRA by 50% by the year 2030, no LMIC is predicted to meet the target in all provinces or all districts. Most LMICs (64.6%; 53 LMICs) have a low probability (<5%) of meeting the target even on a national scale. Broad inequalities are expected to continue into 2030; we estimate that 20 LMICs have districts with a high probability of meeting the target as well as districts with a low probability of meeting the target. Furthermore, population growth during this period has led to substantial increases in the number of WRA affected by anemia in various locations. Although the overall number of prevalence of anemia in WRA has decreased, growing populations have caused the number of anemic WRA to increase from 378.3 million to 449.1 million, with the largest increases in Central Asia and western, central and eastern sub-Saharan Africa (54.7% increase: 20.2–31.3 million; 88.0% increase: 24.6–46.2 million; 53.1% increase: 7.6–11.6 million; and 51.8% increase: 20.9–31.8 million, respectively), offsetting the large decreases seen in East Asia and Andean South America (44.1% decrease: 70.9–39.6 million and 13.7% decrease: 5.6–4.8 million, respectively).
The multitude of different diseases and injuries, nutritional and behavioral risk factors and sociodemographic factors that can lead to anemia mandate inter- and multi-sectorial approaches involving stakeholders and actors in the public and private sectors and coordination across food systems and health-related sectors if large-scale reductions in anemia prevalence are to be achieved2,16. GBD 2019 estimated the top-ranked global causes of anemia in WRA to be, in order, dietary iron deficiency; thalassaemia trait; sickle cell trait; menstrual disorders; endocrine, metabolic, blood and immune disorders; and malaria19, although the specific cause composition varied by country and age group. Regardless of anemia prevalence levels, the WHO recommends a diet with adequate bioavailable iron and iron folate and micronutrient fortification of rice and flours where they are major staples16. Intermittent or daily iron and folic acid supplementation is recommended for WRA depending on pregnancy and postpartum status, menstruation, tuberculosis diagnosis and population-level prevalence, with key prevalence thresholds of 20% and 40%16. Research suggests that multiple micronutrient supplementation for pregnant women in LMICs might provide additional benefits of reducing low-birth-weight outcomes, small-for-gestational-age outcomes and preterm birth outcomes32. Universal antenatal hemoglobin testing can help identify anemic women early, providing time to investigate causality and eliminate anemia before delivery33. In endemic areas, malaria control has demonstrated over 25% and 60% reduction in overall anemia and severe anaemia, respectively16. Countries with high levels of anemia and malaria34, such as Mali, Democratic Republic of the Congo, Papua New Guinea, Pakistan and India, might benefit from increased malaria control efforts. Proper water and sanitation, including safe water and education on hand-washing and hygienic disposal of fecal matter, can reduce infection risks and related nutritional losses2. Additionally, the association between intestinal helminths and anemia, due to nutritional theft and direct blood loss, has led the WHO35 to recommend de-worming pregnant women in helminth-endemic areas. LMICs with co-distribution of helminths36 and high prevalence of anemia include Nigeria, Madagascar, Bangladesh and Papua New Guinea. A variety of intervention delivery platforms could be used, including regular routine antenatal care visits, community health workers and community-based social marketing16. Strategies and delivery platforms should be context-specific and tailored for populations based on the local culture and disease burden; these estimates provide policy-makers the opportunity to ‘aim to ensure the most vulnerable members of the populations are reached’16. For those with chronic conditions, such as sickle cell disease, thalassaemia, inflammatory bowel disease, endocrine disorders or chronic kidney disease, more nuanced and potentially more intensive treatments are likely to be required to manage the underlying disease and reduce anemia burden.
Future research could cross-reference our estimates with implemented policies by location to determine effective strategies and exemplars of progress to further aid policy-makers and decision-makers. Although the models used in this study are not inherently inferential, the complex, yet still relatively predictable, pathways that lead to anemia suggest that those populations with a high burden of anemia are also highly likely to have a high burden of the diseases that cause anemia and are likely to be suffering from multiple simultaneous deprivations of nutrition, economics, health systems and overall resilience. We have seen success, as evidenced in Peru, in using targeted programs to reach those most in need, and understanding where they might be is a prerequisite toward analogous future campaigns against anemia and many other inequitable global health crises. These maps thus provide a roadmap to identifying the most vulnerable populations in the world and can be viewed concurrently with our previous work tracking progress and/or predictions of meeting other WHO GNTs—including geospatial annual estimates of exclusive breastfeeding25, childhood overweight and wasting37 and childhood stunting, wasting and underweight26,27—as well as estimates of child diarrhea38, child mortality39, malaria34, inherited blood disorders (for example, sickle cell diseases40), helminths36 and food system sustainability41 to gain a more complete view of the needs of specific countries and communities.
Although this study sheds light on the varied levels of anemia across countries, the unequal levels within them and the varied rates of progress that have led them to their status, it is not without limitations. Most notably, the accuracy of these estimates is predicated on the quality and the quantity of the underlying data. We have invested substantial effort in building a geo-located database of over 3 million women for the purpose of this analysis, but large gaps in both the spatial and temporal data coverage remain. Supplementary Figs. 6 and 7 show the number of years of data underpinning each administrative level-one and level-two unit in the analysis, and Supplementary Figs. 1–5 illustrate the spatial resolution and temporal location of this data. The uncertainty of these estimates, shown in Supplementary Figs. 10–13, is largely driven by the consistency and volume of the data and, at times, can be quite high. Our validation analysis shows that our model is well-calibrated with minimal bias and good coverage of the 95% prediction intervals, demonstrating that the uncertainty of the estimates is appropriate given the data. To improve the precision of these estimates, increases in data collection and reporting will be needed, and the uncertainty maps provide a starting point for adaptive sampling techniques that can target areas that we uncertainly estimate to have high risk.
Combined with the lack of necessary data that would be needed to perform high-resolution mapping of the conditions that cause anemia, our analysis and some of its limitations underscore the challenges in large-scale global reduction of anemia. Venous sampling of whole blood followed by assessment via automated hematology analyzers is considered the gold standard measurement, but most population-based surveys use capillary samples and the HemoCue colorimetric point-of-care tool to measure hemoglobin concentration and assess population prevalence of anemia. There are documented differences in the concentration of hemoglobin in venous blood samples compared to capillary blood samples, but the direction and consistency of the error introduced by capillary measurement has not been definitively established. We did not have sufficient data to stratify by the mode of assessment in each country at the local level. In addition, we did not estimate anemia by underlying cause, which limits the precision with which we can make specific statements about likely appropriateness of specific interventions for specific locations, although we do expect the epidemiology of anemia to track with the underlying causes of anemia. Similarly, prevalence and count maps of all-anemia burden can be used to target hotspots but are not sufficient to determine the best course of treatment for those communities. Neither the uncertainty from resampling polygonal data to point data, nor the uncertainty from modeled covariates, were accounted for in our models. Uncertainty plots of the outputs in our models can be found in Supplementary Figs. 10–13 and 16. We expect that propagating the uncertainty from the resampling and the modeled covariates would increase the overall uncertainty in our estimates. In contrast, if we were able to incorporate the assessment technique (venous versus capillary) or the processing technique, we expect that accounting for these possible confounders would decrease the uncertainty of these estimates.
The large global burden of anemia continues to underline the need for high-resolution estimates to track progress toward international targets and to aid policy-makers in targeting interventions and scarce resources. The recent addition of anemia reduction as a target for the Sustainable Development Goal 2 further highlights the global importance of the issue22,23. This study details the subnational trends in anemia prevalence in WRA across 82 LMICs, broken down by severity, and highlights the local differences in burden and progress within and between countries. The results and the interactive visualizations presented in this study provide an unprecedented opportunity for policy-makers and health institutes to examine the variation in anemia prevalence and its historical progress within their communities and can aid targeting of further data collection, limited resources and interventions to populations most in need.
Methods
Overview
This study implemented continuous geostatistics modeling of mild, moderate and severe anemia prevalence over time, from which local-, administrative- and national-level estimates of prevalence, counts and all other estimated quantities were derived. An ensemble approach using a Bayesian generalized linear mixed effects model was used to embed non-linear algorithmically predicted mean functions within a Gaussian process framework, assumed to have a correlated space–time covariance structure. We sampled 1,000 draws from an approximate posterior distribution of this model and generated annual prevalence estimates for mild, moderate, and severe anemia prevalence of WRA (ages 15–49 years) on an approximate 5 × 5-km grid over 82 LMICs from 2000 to 2018 and performed population-weighted aggregation of these gridded estimates to administrative and national levels. Countries were selected for inclusion in this study using the SDI, a summary measure of development that combines education, fertility and poverty18. Selected countries were in the low, lower-middle and middle SDI quintiles, with several exceptions (Supplementary Table 3). China, Malaysia and Turkmenistan were included despite high-middle SDIs for geographic continuity with other included countries. Albania, Bosnia-Herzegovina, North Korea and Moldova were excluded due to geographic discontinuity and lack of available survey data. Of this set of countries, we did not generate estimates for 26 countries, as no survey data could be sourced (Supplementary Table 4).
Data
Surveys and hemoglobin data
We extracted each individual woman’s hemoglobin concentrations (g L−1), age, pregnancy status, smoking status and elevation from household series, including the Demographic and Health Surveys, the Multiple Indicator Cluster Surveys, the Living Standards Measurement Study and the Core Welfare Indicators Questionnaire, among other country-specific child health and nutrition surveys. Included across our models were 218 geo-referenced household surveys from 2000 to 2018 representing over 3 million WRA. Each individual woman’s record was associated with a cluster, a group of neighboring households or a ‘community’ that acted as a primary sampling unit in the survey design. The 218 surveys with hemoglobin, pregnancy, smoking and elevation data included geographic coordinates or precise place names for each cluster within that survey. In the absence of geographic coordinates for each cluster, we assigned data to the smallest available administrative areal unit in the survey (polygon) while accounting for the survey sample design49. Boundary information for these administrative units was obtained as shapefiles either directly from the surveys or by matching to shapefiles in the Global Administrative Unit Layers database42 or the Database of Global Administrative Areas (GADM)50. In select cases, shapefiles provided by the survey administrator were used, or custom shapefiles were created based on survey documentation. Using methods from our previous works38, these areal data were resampled to point locations using a population-weighted sampling approach over the relevant areal unit with the number of locations set proportionally to the number of grid cells in the area and the total weights of all the resampled points summing to 1. In addition, some data sources did not contain hemoglobin concentrations and, instead, reported only the anemia severity category. These severity categories were used directly, whereas hemoglobin concentrations were adjusted and thresholded as described in the following section.
Select data sources were excluded for the following reasons: missing survey weights for areal data, missing sex or age variable, incomplete sampling (for example, only women aged 20–24 years measured) or untrustworthy data (as determined by the survey administrator or by inspection). Data availability plots for anemia by country, data type and year can be found in Supplementary Figs. 1–5.
Hemoglobin adjustments and anemia severity
For the purpose of defining anemia severity status, hemoglobin concentrations are often first adjusted for individual smoking status and residential elevation28. Many data sources provide some combination of raw hemoglobin, smoking-adjusted hemoglobin, elevation-adjusted hemoglobin and smoking- and elevation-adjusted hemoglobin concentrations. Wherever possible, this study started with the raw hemoglobin concentrations and performed both smoking and elevation adjustments, as suggested by the WHO. If only partially adjusted (either only smoking-adjusted or elevation-adjusted), we performed the second adjustment, and, if only completely adjusted hemoglobin concentrations were available, we used those. The elevation adjustments are shown in Supplementary Table 5, and the smoking adjustments are shown in Supplementary Table 6.
Once the hemoglobin concentrations had been doubly adjusted for smoking and elevation, they were then thresholded into non-anemic, mild anaemia, moderate anaemia or severe anaemia categories using the WHO definitions shown in Supplementary Table 7. Some data sources reported only the anemia severity categories, which were then used directly in the modeling stage. After classification into anemia severity categories, individual-level data observations were then collapsed to cluster-level totals for the number of WRA sampled and total number of WRA who were determined to be mildly, moderately or severely anemic.
Temporal resolution
We estimated the prevalence of mild, moderate and severe anemia annually from 2000 to 2018 using a model that allowed us to account for data points measured across survey years and, as such, allows us to predict at monthly or finer temporal resolutions. We were limited, however, both computationally and by the temporal resolution of covariates and, thus, have produced annual estimates (Supplementary Table 8 and Supplementary Fig. 8).
Spatial covariates
A variety of socioeconomic and environmental variables were used to predict anemia. Where available, the finest spatio-temporal resolution of gridded datasets was used. These covariates were selected based on their potential to be predictive for anemia and the pathways to anemia, including certain nutritional deficiencies, according to literature review and plausible hypothesis as to their influence. Acquisition of temporally dynamic datasets, where possible, was prioritized to closely align with our observations and to predict the changing dynamics of the anemia severity indicators.
We used covariate-driven predictive models to leverage strength from locations with observations to the entire spatial-temporal domain. Several 5 × 5-km raster layers of putative socioeconomic and environmental correlates of anemia were compiled and used as covariates across the 82 LMICs in the modeling domain (Supplementary Table 8 and Supplementary Fig. 8). These covariates were selected based on their potential to be predictive for anemia and the pathways to anemia, including certain nutritional deficiencies, according to literature review and plausible hypothesis as to their influence. Acquisition of temporally dynamic datasets, where possible, was prioritized to closely align with our observations and to predict the changing dynamics of the anemia severity indicators. Of the 19 covariates included, 12 were temporally dynamic and were re-formatted as a mid-year estimate or synoptic mean for each year in the estimation period. These included average diurnal temperature range, average potential evapotranspiration, average daily mean rainfall (precipitation), outdoor air pollution (PM2.5), educational attainment in WRA (ages 15–49 years), enhanced vegetation index, tasselled cap brightness, prevalence of underweight (ages 0–5 years), Healthcare Access and Quality Index, fertility, urbanicity and population. The remaining seven covariate layers were static throughout the study period and were applied uniformly across all modeling years; these covariates included growing season length, irrigation, nutritional yield for vitamin A, nutritional yield for zinc, nutritional yield for iron, distance to rivers and lakes and travel time to nearest settlement with more than 50,000 inhabitants.
Travel time to nearest settlement, nutritional yield for vitamin A, nutritional yield for iron and nutritional yield for zinc were selected because of their potential to be predictive for anemia and the pathways to anemia. Fertility, malaria incidence, population, outdoor air pollution and prevalence of underweight were selected for inclusion in modeling owing to their correlation with a wide variety of health-related outcomes. Average daily mean temperature, average daily mean rainfall, irrigation, land cover, multi-source weighted-ensemble precipitation and tassled cap brightness were selected for their correlation with a variety of crop yields. In addition, the stacking methodology used in this study boosts the predictive performance of individual covariates by leveraging non-linear and high-order interactions among the covariates and generally performs better when given a variety of covariates.
Analysis
Geostatistical model
To model the full distribution of possible indicators of anemia status—that is, all, mild, moderate and severe anemia—we used an ordinal modeling approach51 to estimate the relative proportion of each indicator.
We implemented a continuation ratio model to estimate the prevalence of three categories: mild, moderate and severe. We first modeled the proportion of all anemia within a Bayesian hierarchical framework using logistic regression with a spatially and temporally explicit generalized linear mixed effects model. Second, we modeled the probability of being mildly anemic conditional on being anemic (that is, being mildly, moderately or severely anemic) using the same Bayesian modeling framework. Finally, we modeled the probability of being severely anemic conditional on being either moderately or severely anemic. The estimates from the two conditional models were combined with the all-anemia estimates to compute the marginal prevalence of mild, moderate and severe anemia.
For each modeling region, at each cluster, d, where , and time t, where , the prevalence of all anemia was modeled using the observed number of WRA in cluster d who were found to be anemic as a binomial count, Cd, among an observed sample of Nd:
For indices d,i and t, *(index) is the value of * at the index. The annual prevalence of all anemia, pi,t, in spatial location i, in time t, was modeled as a linear combination of the three submodels (generalized additive model, boosted regression trees and lasso regression), rasterised covariate values, Xi,t, a correlated spatio-temporal random effect term Zi,t, country random effects , with one unstructured country random effect fit for each country in the modeling region (Extended Data Fig. 3) and all sharing a common variance parameter, γ2, and an independent nugget random effect, , with variance parameter σ2. Coefficients βh in the three submodels h∈1,2,3 represent their respective predictive weighting in the logit-link, whereas the joint structured process, Zi,t, accounts for residual spatio-temporal autocorrelation among individual data points that remain after accounting for the predictive effect of the submodel covariates, the country-level random effect, , and the nugget, . The spatio-temporal residual process, Zi,t, was modeled as a three-dimensional Gaussian process in space–time centerd at 0 and with a covariance matrix constructed from a Kronecker product of spatial and temporal covariance kernels. The spatial covariance, , was modeled using an isotropic and stationary Matérn function52 and the temporal covariance, , as an annual autoregressive (AR1) function over the 19 years represented in the model. In the stationary Matérn function, the covariance between two spatial locations that are Euclidean distance D apart is a function of Γ, the gamma function, Kv, the modified Bessel function of the second kind of order , a scaling parameter and ω2, the marginal variance. The scaling parameter, κ, is defined to be , where δ is the range parameter (interpreted to be the approximate distance at which the correlation between two locations drops to 0.1), and v is a scaling constant, which is set to 2 rather than fit from the data. The number of rows and the number of columns of the spatial Matérn covariance matrix are both equal to the number of spatial mesh points for a given modeling region. The Matérn kernel is a practical and common choice that can flexibly model a wide variety of spatial surfaces and allows for fitting or selection of the smoothness of the surface, helping to avoid unrealistic over-smoothing52. For the temporal kernel, we chose to use an AR1 process owing to its stability, which aligns well with the observed relatively slow and smooth changes in anemia prevalence across time, and for its interpretability. In the AR1 function, ρ is the temporal correlation between adjacent time steps, taken to be single years in this study, and k and j are time steps. The number of rows and the number of columns of the AR1 covariance matrix are both equal to the number of temporal mesh points (19). The number of rows and the number of columns of the space–time covariance matrix, , for a given modeling region are equal (the number of spatial mesh points × the number of temporal mesh points). Previous sensitivity analyses on these models showed these modeling choices to be generally quite robust37,53.
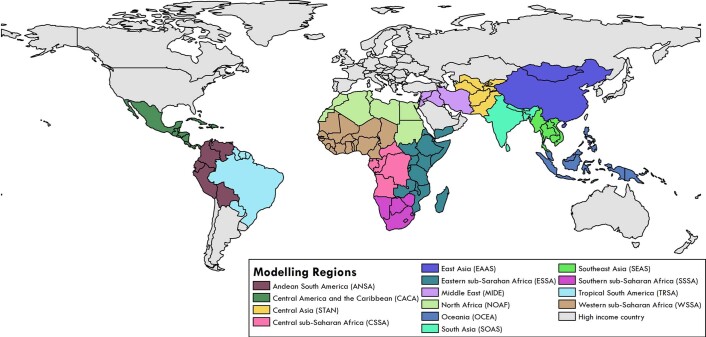
Extended Data Fig. 3. Modelling regions.
Modelling regions48 were based on geographic and Socio-demographic Index (SDI) regions from the Global Burden of Disease18, defined as Andean South America, Central America and the Caribbean, Central sub-Saharan Africa (SSA), East Asia, Eastern SSA, Middle East, North Africa, Oceania, Southeast Asia, South Asia, South SSA, Central Asia, Tropical South America, and Western SSA. Regions in grey (Stage 3) were not included in our models due to high-middle and high SDI. Only 82 low- and middle-income countries (LMICs) were included in this study.
This approach leverages the residual correlation structure to more accurately predict prevalence estimates for locations with no data while also propagating the dependence in the data through to uncertainty estimates54. The posterior distributions were fit using computationally efficient and accurate approximations in R-INLA55 (integrated nested Laplace approximation) with the stochastic partial differential equations (SPDE)56 approximation to the spatio-temporal Gaussian process using R version 3.5.1. The SPDE approach using INLA was demonstrated elsewhere, including the estimation of health indicators, particulate air matter and population age structure56. Uncertainty intervals were generated from 1,000 draws (that is, statistically plausible candidate maps)57 created from the posterior-estimated distributions of modeled parameters.
Mesh construction
We constructed the finite elements mesh for the SPDE approximation to the Gaussian process regression using a simplified polygon boundary (in which coastlines and complex boundaries were smoothed) for each of the regions within our model. We set the inner mesh triangle maximum edge length (the mesh size for areas over land) to be 0.75 degrees and the buffer maximum edge length (the mesh size for areas over the ocean) to be 5.0 degrees58. An example finite elements mesh constructed for eastern sub-Saharan Africa mesh can be found in Supplementary Fig. 7.
Post-estimation
To transform grid cell-level estimates into a range of information useful to a wide constituency of potential users, these estimates were aggregated at first and second administrative units specific to each country and at national levels40. Although the models can predict all locations covered by available raster covariates, all final model outputs for which land cover was classified as ‘barren or sparsely vegetated’ on the basis of Moderate Resolution Imaging Spectroradiometer satellite data (2013) were masked59. Areas where the total population density was fewer than ten individuals per 1 × 1-km grid cell in 2015 were also masked in the final outputs. To compute the YLDs, we applied the corresponding disability weights from the GBD study30 on prevalence estimates of the severity bins (mild, moderate and severe anemia) and summed to get the total YLDs for all anemia.
Model validation
Models were validated using spatially stratified five-fold out-of-sample cross-validation. To replicate real-world missingness in the datasets and to fairly assess model performance in areas far from observed data, acknowledging the spatial correlation inherent in the observation, holdout folds were created by combining sets of all data falling within first administrative-level units. Validation was performed by calculating bias (mean error), variance (root-mean-square error), 95% data coverage within prediction intervals and correlation between observed data and predictions. All validation metrics were calculated on the out-of-sample predictions from the five-fold cross-validation. All validation procedures and corresponding results are provided in Supplementary Tables 17–19 and Supplementary Figs. 20–22.
In-sample metrics
To assess the in-sample performance of our models and compare to national-level estimates produced by the GBD study, we generated a suite of diagnostic plots for anemia estimates in each of the regions and countries modeled. To explore residual error over space and time, absolute error (data minus predicted posterior mean estimates at the corresponding grid cells) was produced.
Metrics of predictive validity
To assess the predictive validity of our estimates, we validated our models using spatially stratified five-fold out-of-sample cross-validation60. To construct each spatial fold, we used a modified bi-tree algorithm to spatially aggregate data points. This algorithm recursively partitions two-dimensional space, alternating between horizontal and vertical splits on the weighted data sample size medians, until the data contained within each spatial partition are of a similar sample size. The depth of recursive partitioning is constrained by the target sample size within a partition and the minimum number of clusters or pseudo-clusters allowed within each spatial partition (in this case, a minimum sample size of 500 was used). These spatial partitions are then allocated to one of five folds for cross-validation. For validation, each geostatistical model was run five times, each time holding out data from one of the folds, generating a set of out-of-sample predictions for the held-out data. A full suite of out-of-sample predictions over the entire dataset was generated by combining the out-of-sample predictions from the five cross-validation runs.
Using these out-of-sample predictions, we then calculated mean error (or bias), root-mean-squared error (RMSE, which summarizes total variance), coefficient of variation (defined to be the standard deviation divided by the mean and multiplied by 100, which is a measure of relative variability) and 95% coverage of our predictive intervals (the proportion of observed out-of-sample data that fall within our predicted 95% credible intervals) aggregated up to different administrative levels (levels 0, 1 and 2) as defined by the GADM50. Administrative level 0 (admin 0) borders correspond to national boundaries; administrative level 1 (admin 1) borders generally correspond to regions, provinces or state-level boundaries within a country; and administrative level 2 (admin 2) borders correspond to the next finer subdivision, often districts, within regions. These metrics are summarized in Supplementary Tables 13–15 and Supplementary Figs. 15–17 and are calculated across all regions. Included in the sample tables for comparison are the same metrics calculated on in-sample predictions.
Sensitity analysis
We ran four five-fold cross-validation holdout in-sample experiments, using different combinations of covariates and random effects:
Raw covariates + Gaussian process:
Raw covariates:
Stacking predictions as covariates:
Stacking covariates + Gaussian process (standard model):
The summary error measures for all models are shown in Supplementary Figs. 20 and 21 to demonstrate how adding stackers or the Gaussian process individually change predictive capacity on administrative level 1 and 2, respectively. Across the two levels of aggregation and all four validation metrics, the models with a Gaussian process outperformed those without, as they had smaller RMSE and greater correlation. For the standard model, which used both the stacking covariates and the Gaussian process, the in-sample RMSE and correlation were 0.053 and 0.078 and 0.87 and 0.77 at administrative levels 1 and 2, respectively. For the raw covariates model with the Gaussian process, RMSE = 0.069 and 0.084, and the correlation = 0.71 and 0.63; for the model that used raw covariates only, RMSE = 0.066 and 0.091, and the correlation = 0.55 and 0.43; and for the stacked covariates model, RMSE = 0.056 and 0.079, and the correlation = 0.85 and 0.75, at administrative levels 1 and 2, respectively.
Projections
To compare our estimated rates of improvement in all-anemia prevalence over the 19-year period across different locations, and to assess if locations are on track to meet the WHO GNT for anemia given historical rates of improvement, we performed a simple projection using estimated AROCs applied to the final year of our estimates. Both AROCs and projections were calculated at the draw level to construct uncertainty estimates for both.
For all-anemia prevalence, we calculated AROCs at each administrative-level unit (a) by calculating the AROC between each pair of adjacent years, t:
We then calculated a weighted AROC for all-anemia by taking a weighted average across the years, where more recent AROCs were given more weight in the average. We defined the weights to be:
where γ may be chosen to give varying amounts of weight across the years. Using the weights and the AROCs between consecutive years, the average AROC across the duration of the study was calculated:
Finally, we calculated the projections (Proj) by applying the 7 years of the AROC (from 2018 to 2025) to our mean 2018 prevalence estimates. The projection was performed in logit-space (consistent with the AROC calculation) to ensure that the projected estimates range between 0 and 1:
This projection scheme is analogous to the methods used in the GBD 2017 measurement of progress and projected attainment of health-related SDGs18. The exponential power in the weighting scheme was chosen to match that used by the GBD study, which selects this parameter using an out-of-sample predictive validation framework. Our projections assume that areas will sustain the current AROC, and the precision of the AROC estimates is dependent on this assumption and the uncertainty from the all-anemia annual prevalence estimates.
Post-estimation calibration to national and subnational estimates
To leverage national-level data that were included in GBD 2017 (ref. 18) but were outside the scope of our current geospatial modeling framework, and to ensure alignment between this study’s estimates and GBD 2017 estimates, we performed a post hoc calibration to each of our 1,000 candidate maps. For each posterior draw, we calculated population-weighted grid cell aggregations at the level of GBD estimates (at national or subnational level) and compared these estimates in each year to the analogous and available GBD 2017 estimates from 2000 to 2017. We defined the raking factor to be the ratio between the GBD 2017 estimates and our current estimates and linearly interpolated raking factors in each country between the available years. Finally, we multiplied each of our grid cells in a country-year by its associated raking factor. This ensures alignment between our geospatial estimates and GBD 2017 estimates while preserving our estimated within-country geospatial and temporal variation.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-021-01498-0.
Supplementary information
Supplementary Figs. 1–21, Supplementary Tables 1–18, GATHER compliance, Supplementary Data, Supplementary Covariates, Supplementary Methods, Model Results, Model Validation and Supplementary References
Acknowledgements
This work was primarily supported by grant OPP1132415 from the Bill & Melinda Gates Foundation. Lucas Guimarães Abreu acknowledges support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – Código de Financiamento 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (404710/2018-2 and 310797/2019-5), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) and Pró-Reitoria de Pesquisa (PRPq) of Universidade Federal de Minas Gerais. Olatunji Adetokunboh acknowledges the Department of Science and Innovation and National Research Foundation for its support. Syed Aljunid acknowledges the Department of Health Policy and Management, Faculty of Public Health, Kuwait University and International Centre for Casemix and Clinical Coding, Faculty of Medicine, National University of Malaysia for the approval and support to participate in this research project. Alaa Badawi acknowledges support by the Public Health Agency of Canada. Josip Car acknowledges their post at Imperial College London, which is supported by the NIHR NW London Applied Research Collaboration. Felix Carvalho acknowledges UID/MULTI/04378/2019 and UID/QUI/50006/2019 support, with funding from FCT/MCTES through national funds. Vera Marisa Costa acknowledges her grant (SFRH/BHD/110001/2015), received by Portuguese national funds through Fundação para a Ciência e Tecnologia (FCT), IP, under the Norma Transitória DL57/2016/CP1334/CT0006. Jan-Walter De Neve acknowledges support by the Alexander von Humboldt Foundation. Kebede Deribe acknowledges support by the Wellcome Trust (grant no. 201900/Z/16/Z) as part of his International Intermediate Fellowship. Sagnik Dey acknowledges support from IIT Delhi for the Institute Chair position. Marcel Ausloos, Adrian Pana and Claudiu Herteliu are partially supported by a grant from the Romanian National Authority for Scientific Research and Innovation, CNDS-UEFISCDI, project number PN-III-P4-ID-PCCF-2016-0084. Claudiu Herteliu is partially supported by a grant co-funded by the European Fund for Regional Development through the Operational Program for Competitiveness, project ID P_40_382. Claudiu Herteliu and Adrian Pana are partially supported by a grant from the Romanian National Authority for Scientific Research and Innovation, CNDS-UEFISCDI, project number PN-III-P2-2.1-SOL-2020-2-0351. Praveen Hoogar wholeheartedly thanks the Centre for Bio Cultural Studies (CBiCS), Directorate of Research, Manipal Academy of Higher Education (MAHE), Manipal. Sheikh Mohammed Shariful Islam is funded by a Fellowship from the National Heart Foundation of Australia and NHMRC. Oommen John is the recipient of an UIPA scholarship, UNSW, Sydney. Md. Nuruzzaman Khan acknowledges Jatiya Kabi Kazi Nazrul Islam University, Bangladesh. Yun Jin Kim acknowledges support by the Research Management Centre, Xiamen University Malaysia (grant no. XMUMRF/2020-C6/ITM/0004). Kewal Krishan acknowledges support by a DST PURSE grant and the UGC Centre of Advanced Study (CAS II) awarded to the Department of Anthropology, Panjab University. Ben Lacey acknowledges support from the NIHR Oxford Biomedical Research Centre and the BHF Centre of Research Excellence, Oxford. Iván Landires is member of the Sistema Nacional de Investigación (SNI), which is supported by the Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT), Panamá. Alberto Ortiz acknowledges their research, which is supported by FRIAT, ISCIIII FEDER funds RETIC REDINREN RD016/0009. George Patton acknowledges support from an NHMRC research fellowship. Maarten J. Postma reports various grants and honoraria from public and private partners and shareholdership in two health economics consultancies. Rajesh Kumar Rai received financial support from the West Bengal State Department of Health and Family Welfare, India (memo no. 114-P&B/ HFW-27011/114/2019-NHM SEC). Milena Santric-Milicevic acknowledges the support received from the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract no. 175087). Feng Sha acknowledges support from the Shenzhen Science and Technology Program (grant no. KQTD20190929172835662). Aziz Sheikh acknowledges Health Data Research UK. Adithi Shetty acknowledges Kasturba Medical College, Mangalore and Manipal Academy of Higher Education for the support and encouragement. B. Suresh Kumar Shetty acknowledges Kasturba Medical College, Mangalore and Manipal Academy of Higher Education, Manipal for the encouragement and support provided. Mohammad Reza Sobhiyeh acknowledges the clinical research development center of Imam Reza Hospital Kermanshah University of Medical Sciences for their wise advice. Riaz Uddin is supported by the Alfred Deakin Postdoctoral Research Fellowship. Charles Shey Wiysonge’s work is supported by the South African Medical Research Council. Sojib Bin Zaman received a scholarship from the Australian Government Research Training Program in support of his academic career. Yunquan Zhang was supported by the Key Research Center for Humanities and Social Sciences in Hubei Province (Hubei University of Medicine) (grant no. 2020ZD001).
Extended data
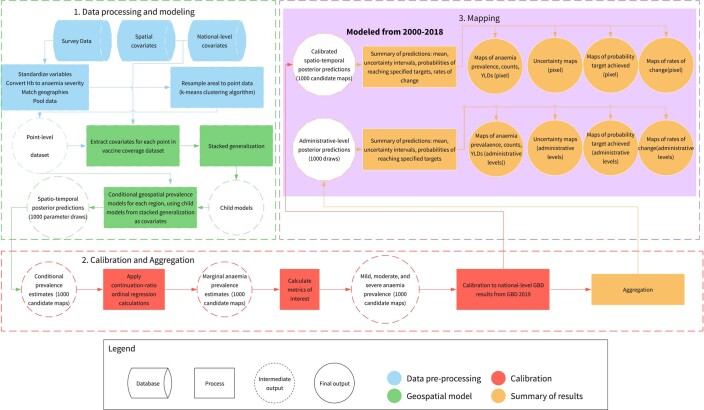
Extended Data Fig. 4. Modeling process flow diagram.
The geospatial modelling process consists of three main sections. First, all available survey data that can be referenced to a coordinate/point (for example, survey cluster) or small polygon (administrative) unit are compiled, Hb measurements are converted to anaemia severity, data matched to polygons are resampled into pseudo-points, covariates are merged onto the point-level dataset, a series of conditional geospatial model is fit using stacked generalization child models as main effects is fit for each geographic region, and 1000 posterior predictions are sampled from the fitted model. Second, the 1000 parameter draws are projected into 1000 5x5km pixel conditional prevalence candidate maps, converted into marginal anaemia severity prevalence maps, calibrated to GBD 2019 estimates, and aggregated to administrative levels. Lastly, the 1000 calibrated pixel and aggregated draws are summarized into estimates of anaemia prevalence, counts, YLDs, and the associated uncertainty, probabilities of meeting targets, and rates of change. See the Methods for more details.
Author contributions
S.I.H. and N.J.K. conceived and planned the study. S.B.E., A.L.A, D.L. and K.M.D. identified and obtained data for analysis. S.B.E., A.L.A., D.L. and K.M.D. extracted, processed and geo-positioned the data. D.K.K. carried out the statistical analyses with input from A.O.-Z. and A.L.A. A.O.-Z., N.J.H., M.L.C., M.A.C., J.F.M., A.D., L.P.W., J.D.V., K.E.W., R.C.R. and L.D.-L. provided input on the methods. D.K.K., L.E.S., I.L. and A.C. prepared the figures and tables. M.F.S. served as project manager for the study. D.K.K., L.E.S. and A.O.-Z. wrote the first draft of the manuscript, and all authors contributed to subsequent revisions. All authors provided intellectual input into aspects of this study, specifically as below. Managing the estimation or publications process: Damaris Kinyoki, Aaron Osgood-Zimmerman and Megan Schipp. Writing the first draft of the manuscript: Damaris Kinyoki, Aaron Osgood-Zimmerman, Lauren Schaeffer and Megan Schipp. Primary responsibility for this manuscript focused on applying analytical methods to produce estimates: Damaris Kinyoki, Aaron Osgood-Zimmerman and Natalia V. Bhattacharjee. Primary responsibility for this manuscript focused on seeking, cataloguing, extracting or cleaning data and designing or coding figures and tables: Lauren Schaeffer, Alice Lazzar-Atwood, Dan Lu, Samuel B. Ewald, Katie Donkers, Ian Louterneau and Michael Collison. Providing data or critical feedback on data sources: Damaris Kinyoki, Samuel B. Ewald, Ian Letourneau, Megan Schipp, Mitra Abbasifard, Hedayat Abbastabar, Foad Abd-Allah, Ahmed Abdelalim, Amir Abdoli, Hassan Abolhassani, Ahmed Abualhasan, Abdelrahman Abushouk, Maryam Adabi, Oladimeji Adebayo, Victor Adekanmbi, Olatunji Adetokunboh, Daniel Adeyinka, Shailesh Advani, Sohail Ahmad, Tauseef Ahmad, Sepideh Ahmadi, Muktar Ahmed, Miloud Taki Eddine Aichour, Budi Aji, Oluwaseun Akinyemi, Addis Aklilu, Chisom Akunna, Jacqueline Alcalde-Rabanal, Sheikh Alif, Hesam Alizade, Syed Aljunid, Amir Almasi-Hashiani, Nelson Alvis-Guzman, Saeed Amini, Nahla Anber, Tudorel Andrei, Masresha Anegago, Mina Anjomshoa, Fereshteh Ansari, Ernoiz Antriyandarti, Davood Anvari, Muhammad Aqeel, Jalal Arabloo, Morteza Arab-Zozani, Olatunde Aremu, Mulusew Asemahagn, Seyyed Shamsadin Athari, Seyyede Masoume Athari, Marcel Ausloos, Nefsu Awoke, Beatriz Paulina Ayala Quintanilla, Getinet Ayano, Yared Aynalem, Alaa Badawi, Mohammad Amin Bahrami, Palash Banik, Miguel Barboza, Sanjay Basu, Gholamreza Bazmandegan, Neeraj Bedi, Tariku Tesfaye Bekuma, Suraj Bhattarai, Boris Bikbov, Somayeh Bohlouli, Mahdi Bohluli, Archith Boloor, Shiva Borzouei, Nicola Bragazzi, Dejana Braithwaite, Luis Cámera, Luis Cámera, Joao Mauricio Castaldelli-Maia, Carlos Castañeda-Orjuela, Franz Castro, Souranshu Chatterjee, Vijay Kumar Chattu, Simiao Chen, Vera Costa, Lalit Dandona, Rakhi Dandona, Rajat Das Gupta, Aditya Dash, Kairat Davletov, Kebede Deribe, Meghnath Dhimal, Govinda Dhungana, Mostafa Dianatinasab, Hoa Do, Leila Doshmangir, Andre Duraes, Maysaa El Sayed Zaki, Rajesh Elayedath, Teshome Elema, Babak Eshrati, Khalil Eskandari, Emerito Jose Faraon, Mohammad Fareed, Farshad Farzadfar, Abidemi Fasanmi, Berhanu Feleke, Takeshi Fukumoto, Mohamed Gad, Abhay Gaidhane, Reta Gayesa, Birhan Gebregiorgis, Leake Gebremeskel, Yilma Geramo, Lemma Getacher, Keyghobad Ghadiri, Fatemeh Ghaffarifar, Ahmad Ghashghaee, Nermin Ghith, Ayman Grada, Nachiket Gudi, Arvin Haj-Mirzaian, Ahmed Hasaballah, Syed Shahzad Hasan, Abdiwahab Hashi, Soheil Hassanipour, Hadi Hassankhani, Mohamed Hegazy, Reza Heidari-Soureshjani, Claudiu Herteliu, Praveen Hoogar, Mehdi Hosseinzadeh, Mowafa Househ, Syed Hussain, Usman Iqbal, Sheikh Mohammed Shariful Islam, Vardhmaan Jain, Mihajlo Jakovljevic, Amir Jalali, Tahereh Javaheri, Oommen John, Kimberly Johnson, Jost Jonas, Farahnaz Joukar, Jacek Jozwiak, Zubair Kabir, Leila Kalankesh, Rohollah Kalhor, Ashwin Kamath, Zahra Kamiab, Umsh Kapil, Salah Eddin Karimi, Ayele Semachew Kasa, Gbenga Kayode, Bayew Kelkay, Nauman Khalid, Maseer Khan, Md. Nuruzzaman Khan, Khaled Khatab, Amir Khater, Mona Khater, Abdullah Khoja, Jagdish Khubchandani, Neda Kianipour, Young-Eun Kim, Yun Jin Kim, Adnan Kisa, Sezer Kisa, Soewarta Kosen, Parvaiz Koul, Kewal Krishan, Barthelemy Kuate Defo, G. Anil Kumar, Pushpendra Kumar, Om Kurmi, Dian Kusuma, Deepesh Lad, Dharmesh Lal, Huong Lan Nguyen, Anders Larsson, Savita Lasardo, Savita Lasrado, Kate Legrand, Chi Linh Hoang, Xuefeng Liu, Jaifred Christian Lopez, Hassan Magdy Abd El Razek, Muhammed Magdy Abd El Razek, D. R. Mahadeshwara Prasad, Narayan Mahotra, Shokofeh Maleki, Reza Malekzadeh, Deborah Malta, Deborah Malta, Fariborz Mansour-Ghanaei, Borhan Mansouri, Francisco Martins-Melo, Benjamin Mayala, Medhin Mehari, Man Mohan Mehndiratta, Entezar Mehrabi Nasab, Mulugeta Melku, Walter Mendoza, Ritesh Menezes, Endalkachew Mengesha, Meresa Mengesha, Workua Metekiya, Erkin Mirrakhimov, Hamed Mirzaei, Babak Moazen, Masoud Moghadaszadeh, Dara Mohammad, Naser Mohammad Gholi Mezerji, Abdollah Mohammadian-Hafshejani, Ammas Mohammed, Hayat Mohammed, Salahuddin Mohammed, Shafiu Mohammed, Ali Mokdad, Masoud Moradi, Maziar Moradi-Lakeh, Seyyed Meysam Mousavi, Getaneh Mulu, Mehnaz Munir, Ghulam Mustafa, Mehdi Naderi, Ahamarshan Nagarajan, Shankar Prasad Nagaraju, Bruno Nascimento, Javad Nazari, Ionut Negoi, Ruxandra Negoi, Georges Nguefack-Tsague, Josephine Ngunjiri, Cuong Nguyen, Yeshambel Nigatu, Amin Reza Nikpoor, Chukwudi Nnaji, Vuong Nong, Jean Jacques Noubiap, Bogdan Oancea, Felix Ogbo, Andrew Olagunju, Bolajoko Olusanya, Jacob Olusanya, Ahmed Omar Bali, Obinna Onwujekwe, Mayowa Owolabi, Mahesh P A, Jagadish Rao Padubidri, Keyvan Pakshir, Adrian Pana, Songhomitra Panda-Jonas, Anamika Pandey, Seithikurippu Pandi-Perumal, Deepak Kumar Pasupula, Urvish Patel, Ashish Pathak, Jeevan Pereira, Julia Pescarini, Brandon Pickering, Khem Pokhrel, Maarten Postma, Hadi Pourjafar, Sergio Prada, Hai Quang Pham, Zahiruddin Quazi Syed, Navid Rabiee, Fakher Rahim, Amir Masoud Rahmani, Aashish Rajesh, Kiana Ramezanzadeh, Chhabi Ranabhat, Sowmya J. Rao, Lal Rawal, Reza Rawassizadeh, Bhageerathy Reshmi, Nima Rezaei, Seyed Mohammad Riahi, Jennifer Rickard, Leonardo Roever, Godfrey Rwegerera, Siamak Sabour, Ehsan Sadeghi, Rajesh Sagar, S. Mohammad Sajadi, Nasir Salam, Milena Santric-Milicevic, Sivan Saraswathy, Arash Sarveazad, Brijesh Sathian, Deepak Saxena, David Schwebel, Debarka Sengupta, Subramanian Senthilkumaran, Sadaf Sepanlou, Allen Seylani, Feng Sha, Omid Shafaat, Saeed Shahabi, Masood Shaikh, Mohammed Shaka, Mohammadbagher Shamsi, Morteza Shamsizadeh, Mohammed Shannawaz, Amrollah Sharifi, Adithi Shetty, B. Suresh Shetty, Wondimeneh Shiferaw, Jae Il Shin, Soraya Siabani, Valentin Skryabin, Anna Skryabina, Mohammad Reza Sobhiyeh, Amin Soheili, Emma Spurlock, Emma Spurlock, Chandrashekhar Sreeramareddy, Agus Sudaryanto, Rafael Tabarés-Seisdedos, Amir Taherkhani, Zemenu Tamir, Animut Tamiru, Hirut Teame, Nihal Thomas, Asres Tilahune, Marcos Tovani-Palone, Khanh Tran, Bach Tran Xuan, Rajnish Tripathi, Phuong Truong, Riaz Uddin, Anayat Ullah, Bhaskaran Unnikrishnan, Era Upadhyay, Alireza Vakilian, Pascual Valdez, Pascual Ruben Valdez, Yasser Vasseghian, Bay Vo, Yohannes Wado, Yasir Waheed, Yafeng Wang, Girmay Weldesamuel, Taweewat Wiangkham, Charles Wiysonge, Ali Yadollahpour, Mingyou Yang, Sanni Yaya, Alex Yeshaneh, Yordanos Yeshitila, Mekdes Yilma, Melissa Young, Abdilahi Yousuf, Chuanhua Yu, Syed Saoud Zaidi, Josefina Zakzuk, Mohammad Zamani, Alireza Zangeneh, Mikhail Zastrozhin, Anasthasia Zastrozhina Dejene Zewdie and Arash Ziapour. Developing methods or computational machinery: Damaris Kinyoki, Aaron Osgood-Zimmerman, Muktar Ahmed, Hesam Alizade, Amir Almasi-Hashiani, Nahla Anber, Masresha Anegago, Davood Anvari, Mulusew Asemahagn, Nefsu Awoke, Yared Aynalem, Mohammad Amin Bahrami, Natalia V. Bhattacharjee, Somayeh Bohlouli, Shiva Borzouei, Farah Daoud, Mostafa Dianatinasab, Lucas Earl, Khalil Eskandari, Reza Heidari-Soureshjani, Nathaniel Henry, Mowafa Househ, Amir Khater, Neda Kianipour, Young-Eun Kim, Adnan Kisa, Borhan Mansouri, Fereshteh Mehri, Masoud Moghadaszadeh, Salahuddin Mohammed, Ali Mokdad, Mohsen Naghavi, Rajan Nikbakhsh, Chhabi Ranabhat, Seyed Mohammad Riahi, Alyssa Sbarra, Allen Seylani, Omid Shafaat, Emma Spurlock, Hirut Teame, Rajnish Tripathi, Era Upadhyay, Yasser Vasseghian, Syed Saoud Zaidi, Mikhail Zastrozhin, Anasthasia Zastrozhina and Arash Ziapour. Providing critical feedback on methods or results: Damaris Kinyoki, Lauren Schaeffer, Ian Letourneau, Megan Schipp, Amanuel Abajobir, Nooshin Abbasi, Sima Abbasi, Sherief Abd-Elsalam, Ibrahim Abdollahpour, Aidin Abedi, Biju Abraham, Lucas Abreu, Michael Abrigo, Eman Abu-Gharbieh, Manfred Accrombessi, Adeyinka Adegbosin, Davoud Adham, Pradyumna Agasthi, Mohammad Aghaali, Keivan Ahmadi, Ziyad Al-Aly, Turki Alanzi, Ayinalem Alemu, Biresaw Alemu, Robert Alhassan, Vahid Alipour, Hesham Al-Mekhlafi, Rajaa Al-Raddadi, Fatemeh Amiri, Dickson Amugsi, Robert Ancuceanu, Fereshteh Ansari, Alireza Ansari-Moghaddam, Zelalem Anteneh, Habtamu Areri, Al Artaman, Mengistu Ashebir, Maha Atout, Martin Ayanore, Muluken Ayza, Abbas Azadmehr, Darshan B. Tesleem Babalola, Ashish Badiye, Mohan Bairwa, Adhanom Baraki, Huda Basaleem, Mohsen Bayati, Bayisa Baye, Michelle Bell, Abadi Berhe, Kidanemaryam Berhe, Kidanemariam Berhie, Dinesh Bhandari, Nikha Bhardwaj, Pankaj Bhardwaj, Krittika Bhattacharyya, Zulfiqar Bhutta, Ali Bijani, Antonio Biondi, Minyichil Birhanu, Andre Brunoni, Sharath Burugina Nagaraja, Zahid Butt, Florentino Luciano Caetano Dos Santos, Rosario Cárdenas, Muge Cevik, Wagaye Chanie, Jaykaran Charan, Ken Chin, Mohiuddin Ahsanul Kabir Chowdhury, Berihun Dachew, Henok Dagne, Saad Dahlawi, Haijiang Dai, Hancheng Dai, Jai Das, Claudio Davila-Cervantes, Jan-Walter De Neve, Farah Deeba, Assefa Desalew, Getenet Dessie, Sagnik Dey, Daniel Diaz, Shirin Djalalinia, Fariba Dorostkar, Bereket Duko, Hisham Edinur, Iman El Sayed, Hala Elhabashy, Aisha Elsharkawy, Yasser El-Sherbiny, Daniel Endalew, Sharareh Eskandarieh, Ibtihal Fadhil, Pawan Faris, Medhat Farwati, Nazir Fattahi, Valery Feigin, Seyed-Mohammad Fereshtehnejad, Nataliya Foigt, Artem Fomenkov, Masoud Foroutan, Joel Francis, Richard Franklin, Biniyam Geberemariyam, Hadush Gebremariam, Gebreamlak Gebremeskel, Assefa Ayalew Gebreslassie, Hailay Gesesew, Bradford Gessner, Mansour Ghafourifard, Mahsa Ghajarzadeh, Farhad Ghamari, Syed Amir Gilani, Tiffany Gill, Myron Godinho, Philimon Gona, Davide Guido, Rashid Guled, Yuming Guo, Rachita Gupta, Randah Hamadeh, Demelash Woldeyohannes Handiso, Asif Hanif, Arief Hargono, Md. Mehedi Hasan, Maryam Hashemian, Shoaib Hassan, Khezar Hayat, Fatemeh Heydarpour, Sousan Heydarpour, Hagos Hidru, Ramesh Holla, Sung Hwi Hong, Seyyed Nasrollah Hosseini, Mihaela Hostiuc, Sorin Hostiuc, Peter Hotez, Tanvir Huda, Dawit Huluko, Bing-Fang Hwang, Olayinka Ilesanmi, Irena Ilic, Milena Ilic, Leeberk Inbaraj, M. Mofizul Islam, Chidozie Iwu, Chinwe Iwu, Farhad Jadidi-Niaragh, Mohammad Jahani, Farzad Jalilian, Ravi Jha, Jitendra Jonnagaddala, Ankur Joshi, Ali Kabir, Tanvir Kahlon, Tanuj Kanchan, Neeti Kapoor, Behzad Karami Matin, Gebremicheal Kasahun, Gebrehiwot Kassa, Zemenu Kassa, Getinet Kassahun, Ali Kazemi Karyani, Tibebeselassie Keflie, Peter Keiyoro, Maryam Keramati, Daniel Ketema, Mohammad Khammarnia, Junaid Khan, Ruth Kimokoti, Tufa Kolola, Ali Koolivand, Ai Koyanagi, Vijay Krishnamoorthy, Vaman Kulkarni, Manasi Kumar, Nithin Kumar, Faris Lami, Matthew Laurens, Avula Laxmaiah, Shaun Lee, Sonia Lewycka, Bingyu Li, Shanshan Li, Daiane Machado, Shilpashree Madhava Kunjathur, Phetole Mahasha, Mina Maheri, Abdullah Mamun, Mohammad Ali Mansournia, Md. Dilshad Manzar, Carlos Marrugo Arnedo, Anthony Masaka, Kala Mehta, Wahengbam Meitei, Teferi Mekonnen, Gebrekiros Meles, Abera Mersha, Tomasz Miazgowski, Irmina Maria Michalek, Gk Mini, Andreea Mirica, Maryam Mirzaei, Mehdi Mirzaei-Alavijeh, Sanjeev Misra, Yousef Mohammad, Reza Mohammadpourhodki, Jemal Mohammed, Mohammad Mohseni Bandpei, Alex Molassiotis, Rahmatollah Moradzadeh, Simin Mouodi, Amin Mousavi Khaneghah, Moses Muriithi, Gvs Murthy, Ashraf Nabhan, Mehdi Naderi, Gurudatta Naik, Mukhammad David Naimzada, Vinay Nangia, Jobert Richie Nansseu, Atta Abbas Naqvi, Rawlance Ndejjo, Henok Netsere, Diep Nguyen, Vincent Nwatah, Tafadzwa Nyanhanda, Onome Oghenetega, In-Hwan Oh, Daniel Okello, Abidemi Omonisi, Alberto Ortiz, Eduardo Ortiz-Panozo, Nikita Otstavnov, Stanislav Otstavnov, Abhijit Pakhare, Helena Pangaribuan, Sangram Patel, George Patton, Saeed Pirouzpanah, Meghdad Pirsaheb, Faheem Pottoo, Hadis Pourchamani, Hadi Pourjafar, Hossein Poustchi, Dimas Pribadi, Ata Rafiee, Muhammad Aziz Rahman, Rajesh Kumar Rai, Pradhum Ram, Satish Rao, Prateek Rastogi, Priya Rathi, Lemma Regassa, Robert Reiner, Omid Rezahosseini, Aziz Rezapour, Ana Isabel Ribeiro, Hirbo Roba, Susan Rumisha, Sahar Saeedi Moghaddam, Mohammad Ali Sahraian, Marwa Salem, Hossein Samadi Kafil, Itamar Santos, Nizal Sarrafzadegan, Benn Sartorius, Thirunavukkarasu Sathish, Anbissa Senbeta, Mohammad Shahbaz, Izza Shahid, Ali Shalash, Mehran Shams-Beyranvand, Kiomars Sharafi, Abbas Sheikhtaheri, Ranjitha Shetty, Mika Shigematsu, Rahman Shiri, Reza Shirkoohi, Velizar Shivarov, Sudeep Siddappa Malleshappa, Tariq Jamal Siddiqi, Negussie Sidemo, Balbir Singh, Yitagesu Sintayehu, Valentin Skryabin, Anna Skryabina, Shahin Soltani, Muluken Sorrie, Mu’awiyyah Sufiyan, Biruk Taddele, Eyayou Tadesse, Md. Tareque, Abdelghani Tbakhi, Yonas Tefera, Arash Tehrani-Banihashemi, Yohannes Tekalegn, Merhawi Tekle, Berhane Teklehaimanot, Getayeneh Tesema, Takele Tiki, Maria Titova, Jaya Tripathy, Chukwuma Umeokonkwo, Chigozie Uneke, Muhammad Shariq Usman, Marco Vacante, Madhur Verma, Francesco Violante, Yuan-Pang Wang, Nuwan Wickramasinghe, Tewodros Wonde, Ai-Min Wu, Chenkai Wu, Yang Xie, Tomohide Yamada, Vahid Yazdi-Feyzabadi, Paul Yip, Zabihollah Yousefi, Taraneh Yousefinezhadi, Yong Yu, Shamsa Zafar, Sojib Bin Zaman, Maryam Zamanian, Alireza Zandifar, Mikhail Zastrozhin, Anasthasia Zastrozhina, Kaleab Zewdie, Yunquan Zhang, Cong Zhu, Natalia V Bhattacharjee, Nathaniel Henry, Mohsen Naghavi, Rajan Nikbakhsh, Alyssa Sbarra, Hedayat Abbastabar, Foad Abd-Allah, Ahmed Abdelalim, Hassan Abolhassani, Ahmed Abualhasan, Abdelrahman Abushouk, Oladimeji Adebayo, Victor Adekanmbi, Olatunji Adetokunboh, Daniel Adeyinka, Shailesh Advani, Sohail Ahmad, Miloud Taki Eddine Aichour, Budi Aji, Oluwaseun Akinyemi, Addis Aklilu, Chisom Akunna, Jacqueline Alcalde-Rabanal, Sheikh Alif, Syed Aljunid, Nelson Alvis-Guzman, Saeed Amini, Mina Anjomshoa, Muhammad Aqeel, Jalal Arabloo, Morteza Arab-Zozani, Olatunde Aremu, Seyyed Shamsadin Athari, Seyyede Masoume Athari, Marcel Ausloos, Beatriz Paulina Ayala Quintanilla, Getinet Ayano, Alaa Badawi, Palash Banik, Miguel Barboza, Sanjay Basu, Tariku Tesfaye Bekuma, Suraj Bhattarai, Boris Bikbov, Mahdi Bohluli, Archith Boloor, Nicola Bragazzi, Dejana Braithwaite, Luis Cámera, Joao Mauricio Castaldelli-Maia, Carlos Castañeda-Orjuela, Franz Castro, Souranshu Chatterjee, Vijay Kumar Chattu, Simiao Chen, Lalit Dandona, Rakhi Dandona, Rajat Das Gupta, Aditya Dash, Kairat Davletov, Kebede Deribe, Meghnath Dhimal, Hoa Do, Leila Doshmangir, Maysaa El Sayed Zaki, Rajesh Elayedath, Teshome Elema, Emerito Jose Faraon, Mohammad Fareed, Farshad Farzadfar, Abidemi Fasanmi, Berhanu Feleke, Takeshi Fukumoto, Mohamed Gad, Abhay Gaidhane, Reta Gayesa, Birhan Gebregiorgis, Leake Gebremeskel, Yilma Geramo, Lemma Getacher, Ahmad Ghashghaee, Nermin Ghith, Ayman Grada, Nachiket Gudi, Ahmed Hasaballah, Syed Shahzad Hasan, Abdiwahab Hashi, Soheil Hassanipour, Mohamed Hegazy, Claudiu Herteliu, Praveen Hoogar, Mehdi Hosseinzadeh, Syed Hussain, Usman Iqbal, Sheikh Mohammed Shariful Islam, Vardhmaan Jain, Mihajlo Jakovljevic, Tahereh Javaheri, Oommen John, Jost Jonas, Farahnaz Joukar, Jacek Jozwiak, Zubair Kabir, Leila Kalankesh, Rohollah Kalhor, Ashwin Kamath, Salah Eddin Karimi, Ayele Semachew Kasa, Gbenga Kayode, Bayew Kelkay, Nauman Khalid, Maseer Khan, Md. Nuruzzaman Khan, Khaled Khatab, Mona Khater, Abdullah Khoja, Jagdish Khubchandani, Yun Jin Kim, Sezer Kisa, Kewal Krishan, Barthelemy Kuate Defo, G Anil Kumar, Pushpendra Kumar, Om Kurmi, Dian Kusuma, Deepesh Lad, Dharmesh Lal, Huong Lan Nguyen, Anders Larsson, Savita Lasardo, Savita Lasrado, Kate Legrand, Chi Linh Hoang, Xuefeng Liu, Jaifred Christian Lopez, Hassan Magdy Abd El Razek, Muhammed Magdy Abd El Razek, Reza Malekzadeh, Deborah Malta, Fariborz Mansour-Ghanaei, Francisco Martins-Melo, Benjamin Mayala, Medhin Mehari, Entezar Mehrabi Nasab, Mulugeta Melku, Walter Mendoza, Ritesh Menezes, Endalkachew Mengesha, Workua Metekiya, Erkin Mirrakhimov, Hamed Mirzaei, Babak Moazen, Dara Mohammad, Naser Mohammad Gholi Mezerji, Abdollah Mohammadian-Hafshejani, Ammas Mohammed, Shafiu Mohammed, Maziar Moradi-Lakeh, Seyyed Meysam Mousavi, Getaneh Mulu, Mehnaz Munir, Ghulam Mustafa, Ahamarshan Nagarajan, Shankar Prasad Nagaraju, Bruno Nascimento, Javad Nazari, Ionut Negoi, Ruxandra Negoi, Georges Nguefack-Tsague, Josephine Ngunjiri, Cuong Nguyen, Yeshambel Nigatu, Amin Reza Nikpoor, Chukwudi Nnaji, Vuong Nong, Jean Jacques Noubiap, Bogdan Oancea, Felix Ogbo, Andrew Olagunju, Bolajoko Olusanya, Jacob Olusanya, Ahmed Omar Bali, Obinna Onwujekwe, Mayowa Owolabi, Mahesh P A, Jagadish Rao Padubidri, Adrian Pana, Songhomitra Panda-Jonas, Anamika Pandey, Deepak Kumar Pasupula, Urvish Patel, Ashish Pathak, Julia Pescarini, Maarten Postma, Hadi Pourjafar, Sergio Prada, Hai Quang Pham, Zahiruddin Quazi Syed, Fakher Rahim, Amir Masoud Rahmani, Aashish Rajesh, Sowmya J Rao, Lal Rawal, Reza Rawassizadeh, Bhageerathy Reshmi, Nima Rezaei, Jennifer Rickard, Leonardo Roever, Godfrey Rwegerera, Siamak Sabour, Ehsan Sadeghi, Rajesh Sagar, S. Mohammad Sajadi, Nasir Salam, Milena Santric-Milicevic, Sivan Saraswathy, Arash Sarveazad, Brijesh Sathian, Deepak Saxena, David Schwebel, Sadaf Sepanlou, Feng Sha, Saeed Shahabi, Masood Shaikh, Mohammed Shaka, Mohammed Shannawaz, Amrollah Sharifi, Jae Il Shin, Soraya Siabani, Amin Soheili, Emma Spurlock, Chandrashekhar Sreeramareddy, Agus Sudaryanto, Rafael Tabarés-Seisdedos, Animut Tamiru, Nihal Thomas, Asres Tilahune, Marcos Tovani-Palone, Khanh Tran, Bach Tran Xuan, Phuong Truong, Riaz Uddin, Anayat Ullah, Bhaskaran Unnikrishnan, Pascual Valdez, Bay Vo, Yohannes Wado, Yasir Waheed, Girmay Weldesamuel, Taweewat Wiangkham, Charles Wiysonge, Ali Yadollahpour, Sanni Yaya, Yordanos Yeshitila, Mekdes Yilma, Melissa Young, Abdilahi Yousuf, Chuanhua Yu, Josefina Zakzuk, Dejene Zewdie, Muktar Ahmed, Hesam Alizade, Amir Almasi-Hashiani, Nahla Anber, Masresha Anegago, Davood Anvari, Mulusew Asemahagn, Nefsu Awoke, Yared Aynalem, Mohammad Amin Bahrami, Somayeh Bohlouli, Shiva Borzouei, Mostafa Dianatinasab, Khalil Eskandari, Mowafa Househ, Amir Khater, Neda Kianipour, Young-Eun Kim, Adnan Kisa, Borhan Mansouri, Masoud Moghadaszadeh, Salahuddin Mohammed, Ali Mokdad, Chhabi Ranabhat, Seyed Mohammad Riahi, Allen Seylani, Omid Shafaat, Hirut Teame, Rajnish Tripathi, Era Upadhyay, Yasser Vasseghian, Syed Saoud Zaidi, Arash Ziapour and Simon I. Hay. Drafting the work or revising is critically for important intellectual content: Damaris Kinyoki, Lauren Schaeffer, Samuel B Ewald, Katie Donkers, Megan Schipp, Amanuel Abajobir, Mohsen Abbasi-Kangevari, Sherief Abd-Elsalam, Hassan Abolhassani, Lucas Abreu, Ahmed Abualhasan, Eman Abu-Gharbieh, Abdelrahman Abushouk, Oladimeji Adebayo, Victor Adekanmbi, Olatunji Adetokunboh, Daniel Adeyinka, Shailesh Advani, Pradyumna Agasthi, Mohammad Aghaali, Sohail Ahmad, Sepideh Ahmadi, Muktar Ahmed, Miloud Taki Eddine Aichour, Budi Aji, Oluwaseun Akinyemi, Jacqueline Alcalde-Rabanal, Robert Alhassan, Hesam Alizade, Saeed Amini, Dickson Amugsi, Nahla Anber, Robert Ancuceanu, Zelalem Anteneh, Razique Anwer, Muhammad Aqeel, Jalal Arabloo, Morteza Arab-Zozani, Olatunde Aremu, Afsaneh Arzani, Malke Asaad, Mehran Asadi-Aliabadi, Ali Asadi-Pooya, Mohammad Asghari Jafarabadi, Zerihun Ataro, Maha Atout, Marcel Ausloos, Nefsu Awoke, Beatriz Paulina Ayala Quintanilla, Martin Ayanore, Darshan B. Alaa Badawi, Ashish Badiye, Mohan Bairwa, Shankar Bakkannavar, Huda Basaleem, Sanjay Basu, Neeraj Bedi, Michelle Bell, Isabela Bensenor, Abadi Berhe, Kidanemaryam Berhe, Dinesh Bhandari, Krittika Bhattacharyya, Zulfiqar Bhutta, Boris Bikbov, Antonio Biondi, Raaj Kishore Biswas, Moses Bockarie, Somayeh Bohlouli, Shiva Borzouei, Nicola Bragazzi, Dejana Braithwaite, Andre Brunoni, Sharath Burugina Nagaraja, Josip Car, Felix Carvalho, Joao Mauricio Castaldelli-Maia, Carlos Castañeda-Orjuela, Franz Castro, Muge Cevik, Souranshu Chatterjee, Vijay Kumar Chattu, Sarika Chaturvedi, Vera Costa, Baye Dagnew, Tukur Dahiru, Parnaz Daneshpajouhnejad, Rajat Das Gupta, Claudio Davila-Cervantes, Jan-Walter De Neve, Edgar Denova-Gutiérrez, Kebede Deribe, Assefa Desalew, Govinda Dhungana, Daniel Diaz, Isaac Dipeolu, Hoa Do, Leila Doshmangir, Ferry Efendi, Iman El Sayed, Maysaa El Sayed Zaki, Maha El Tantawi, Shaimaa El-Jaafary, Aisha Elsharkawy, Daniel Endalew, Khalil Eskandari, Sharareh Eskandarieh, Medhat Farwati, Abidemi Fasanmi, Nelsensius Fauk, Berhanu Feleke, Seyed-Mohammad Fereshtehnejad, Eduarda Fernandes, Pietro Ferrara, Nataliya Foigt, Artem Fomenkov, Masoud Foroutan, Joel Francis, Marisa Freitas, Takeshi Fukumoto, Mohamed Gad, Abhay Gaidhane, Reta Gayesa, Biniyam Geberemariyam, Birhan Gebregiorgis, Hadush Gebremariam, Tesfay Gebremariam, Gebreamlak Gebremeskel, Leake Gebremeskel, Assefa Ayalew Gebreslassie, Hailay Gesesew, Bradford Gessner, Lemma Getacher, Mansour Ghafourifard, Ahmad Ghashghaee, Nermin Ghith, Tiffany Gill, Myron Godinho, Philimon Gona, Ayman Grada, Mohammed Gubari, Nachiket Gudi, Davide Guido, Yuming Guo, Rajeev Gupta, Ahmed Hasaballah, Maryam Hashemian, Abdiwahab Hashi, Amr Hassan, Shoaib Hassan, Mohamed Hegazy, Claudiu Herteliu, Hagos Hidru, Ramesh Holla, Sung Hwi Hong, Praveen Hoogar, Mowafa Househ, Syed Hussain, Olayinka Ilesanmi, Irena Ilic, Milena Ilic, Usman Iqbal, Sheikh Mohammed Shariful Islam, Chidozie Iwu, Vardhmaan Jain, Mihajlo Jakovljevic, Manthan Janodia, Ravi Jha, Jost Jonas, Nitin Joseph, Jacek Jozwiak, Ali Kabir, Tanvir Kahlon, Leila Kalankesh, Ashwin Kamath, Neeti Kapoor, Gebremicheal Kasahun, Getinet Kassahun, Gbenga Kayode, Tibebeselassie Keflie, Bayew Kelkay, Maryam Keramati, Nauman Khalid, Mohammad Khammarnia, Junaid Khan, Maseer Khan, Md. Nuruzzaman Khan, Khaled Khatab, Amir Khater, Mona Khater, Abdullah Khoja, Neda Kianipour, Young-Eun Kim, Adnan Kisa, Sezer Kisa, Ali Koolivand, Parvaiz Koul, Ai Koyanagi, Kewal Krishan, Barthelemy Kuate Defo, Nuworza Kugbey, Vaman Kulkarni, Manasi Kumar, Om Kurmi, Dian Kusuma, Carlo La Vecchia, Ben Lacey, Deepesh Lad, Huong Lan Nguyen, Iván Landires, Anders Larsson, Savita Lasardo, Savita Lasrado, Avula Laxmaiah, Paul Lee, Shaun Lee, Kate Legrand, Sonia Lewycka, Shanshan Li, Chi Linh Hoang, Jaifred Christian Lopez, Daiane Machado, Shilpashree Madhava Kunjathur, Hassan Magdy Abd El Razek, Muhammed Magdy Abd El Razek, D. R. Mahadeshwara Prasad, Azeem Majeed, Venkatesh Maled, Deborah Malta, Borhan Mansouri, Mohammad Ali Mansournia, Md. Dilshad Manzar, Carlos Marrugo Arnedo, Francisco Martins-Melo, Pallab Maulik, Medhin Mehari, Walter Mendoza, Ritesh Menezes, Tuomo Meretoja, Shabir Mir, Babak Moazen, Masoud Moghadaszadeh, Dara Mohammad, Yousef Mohammad, Abdollah Mohammadian-Hafshejani, Reza Mohammadpourhodki, Jemal Mohammed, Salahuddin Mohammed, Shafiu Mohammed, Mohammad Mohseni Bandpei, Ali Mokdad, Lorenzo Monasta, Maziar Moradi-Lakeh, Paula Moraga, Abbas Mosapour, Amin Mousavi Khaneghah, Mehnaz Munir, Ghulam Mustafa, Ashraf Nabhan, Ahamarshan Nagarajan, Shankar Prasad Nagaraju, Mohsen Naghavi, Mukhammad David Naimzada, Vinay Nangia, Jobert Richie Nansseu, Bruno Nascimento, Smitha Nayak, Vinod Nayak, Javad Nazari, Ionut Negoi, Ruxandra Negoi, Henok Netsere, Georges Nguefack-Tsague, Josephine Ngunjiri, Cuong Nguyen, Yeshambel Nigatu, Rajan Nikbakhsh, Amin Reza Nikpoor, Vuong Nong, Jean Jacques Noubiap, Virginia Nuñez-Samudio, Vincent Nwatah, Bogdan Oancea, Felix Ogbo, Onome Oghenetega, In-Hwan Oh, Seyede Momeneh Ohammadi, Morteza Oladnabi, Andrew Olagunju, Bolajoko Olusanya, Jacob Olusanya, Muktar Omer, Abidemi Omonisi, Obinna Onwujekwe, Alberto Ortiz, Eduardo Ortiz-Panozo, Nikita Otstavnov, Stanislav Otstavnov, Mayowa Owolabi, Mahesh P A, Jagadish Rao Padubidri, Adrian Pana, Songhomitra Panda-Jonas, Helena Pangaribuan, Urvish Patel, Hamidreza Pazoki Toroudi, Saeed Pirouzpanah, Khem Pokhrel, Maarten Postma, Sergio Prada, Hai Quang Pham, Zahiruddin Quazi Syed, Navid Rabiee, Fakher Rahim, Mohammad Hifz Ur Rahman, Pradhum Ram, Chhabi Ranabhat, Satish Rao, Sowmya J. Rao, Lal Rawal, Wasiq Rawasia, Lemma Regassa, Bhageerathy Reshmi, Nima Rezaei, Seyed Mohammad Riahi, Ana Isabel Ribeiro, Daniela Ribeiro, Jennifer Rickard, Leonardo Roever, Luca Ronfani, Morteza Rostamian, Susan Rumisha, Godfrey Rwegerera, Siamak Sabour, Rajesh Sagar, Amirhossein Sahebkar, Mohammad Ali Sahraian, Nasir Salam, Marwa Salem, Hossein Samadi Kafil, Itamar Santos, Milena Santric-Milicevic, Arash Sarveazad, Deepak Saxena, David Schwebel, Subramanian Senthilkumaran, Saeed Shahabi, Izza Shahid, Masood Shaikh, Mohammed Shaka, Mahdi Shamali, Mehran Shams-Beyranvand, Morteza Shamsizadeh, Amrollah Sharifi, Aziz Sheikh, Mika Shigematsu, Jae Il Shin, Reza Shirkoohi, Velizar Shivarov, Sudeep Siddappa Malleshappa, Tariq Jamal Siddiqi, Negussie Sidemo, Surya Singh, Valentin Skryabin, Anna Skryabina, Muluken Sorrie, Chandrashekhar Sreeramareddy, Mu’awiyyah Sufiyan, Iyad Sultan, Takahiro Tabuchi, Eyayou Tadesse, Zemenu Tamir, Animut Tamiru, Hirut Teame, Yohannes Tekalegn, Mohamad-Hani Temsah, Kavumpurathu Thankappan, Nihal Thomas, Maria Titova, Marcos Tovani-Palone, Bach Tran Xuan, Rajnish Tripathi, Jaya Tripathy, Riaz Uddin, Bhaskaran Unnikrishnan, Era Upadhyay, Muhammad Shariq Usman, Marco Vacante, Sahel Valadan Tahbaz, Yasser Vasseghian, Madhur Verma, Francesco Violante, Yasir Waheed, Yafeng Wang, Kinley Wangdi, Nuwan Wickramasinghe, Charles Wiysonge, Tewodros Wonde, Ai-Min Wu, Chenkai Wu, Seyed Hossein Yahyazadeh Jabbari, Sanni Yaya, Vahid Yazdi-Feyzabadi, Tomas Yeheyis, Alex Yeshaneh, Yigizie Yeshaw, Yordanos Yeshitila, Mekdes Yilma, Hebat-Allah Yousof, Syed Saoud Zaidi, Sojib Bin Zaman, Mohammad Zamani, Maryam Zamanian, Alireza Zandifar, Mikhail Zastrozhin, Anasthasia Zastrozhina, Arash Ziapour and Simon I. Hay. Managing the overall research enterprise: Damaris Kinyoki, Aubrey J. Cook, Lalit Dandona, Deborah Malta, Ali Mokdad, Mohsen Naghavi, George Patton, Benn Sartorius, Andrea Werdecker and Simon I. Hay.
Data availability
The findings of this study are supported by data available in public online repositories, data publicly available upon reasonable request of the data provider and data not publicly available owing to restrictions by the data provider. Non-publicly available data were used under licence for the current study but might be available from the authors upon reasonable request and with permission of the data provider. A detailed table of data sources and availability can be found in Supplementary Section 2. The full list of input data sources and output of the analyses is publicly available in the Global Health Data Exchange (http://ghdx.healthdata.org/record/ihme-data/global-anemia-prevalence-geospatial-estimates-2000-2019) and can further be explored via customized data visualization tools (https://vizhub.healthdata.org/lbd/anemia).
Administrative boundaries were retrieved from the Database of Global Administrative Areas50. Land cover was retrieved from the online Data Pool, courtesy of the NASA EOSDIS Land Processes Distributed Active Archive Center, USGS/Earth Resources Observation and Science Center43. Lakes were retrieved from the Global Lakes and Wetlands Database45, courtesy of the World Wildlife Fund and the Center for Environmental Systems Research at the University of Kassel44. Populations were retrieved from WorldPop46,47.
Code availability
This study follows the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER; Supplementary Table 1). All code used for these analyses is publicly available at http://ghdx.healthdata.org/record/ihme-data/global-anemia-prevalence-geospatial-estimates-2000-2019 and https://github.com/ihmeuw/lbd/tree/anemia-lmic-2021.
Competing interests
This study was funded by the Bill & Melinda Gates Foundation. Co-authors employed by the Bill & Melinda Gates Foundation provided feedback on initial maps and drafts of this manuscript. Otherwise, the funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the final report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. R.A. received consultancy/speaker fees from UCB, Sandoz, Abbvie, Zentiva, Teva, Laropharm, CEGEDIM, Angelini, Biessen Pharma, Hofigal, AstraZeneca and Stada. Dr. Bell reports grants from the Wellcome Trust Foundation, grants from the NIH, grants from the Environmental Protection Agency, personal fees from the University of Montana, other from the NIH and grants from the Yale Climate Change and Health Center, all outside of the submitted work. Dr. Gessner reports employment from Pfizer Vaccines, outside of the submitted work. Dr. Islam reports grants from the National Heart Foundation of Australia and grants from the NHMRC, outside of the submitted work. Dr. Jozwiak reports personal fees from Amgen, personal fees from Alab Laboratories, personal fees from Teva, personal fees from Synexus, personal fees from Boehringer Ingelheim and personal fees from Zentiva, outside of the submitted work. Dr. Krishan reports non-financial support from the UGC Centre of Advanced Study, CAS II, Department of Anthropology, Panjab University, outside of the submitted work. Dr. Mendoza reports employment as a program analyst in Population and Development at the United Nations Population Fund-UNFPA Country Office in Peru, which does not necessarily endorse this study. Dr. Pandi-Perumal reports non-financial support from Somnogen Canada and personal fees from royalties, outside of the submitted work. Dr. Postma reports grants and personal fees from MSD, grants and personal fees from GSK, grants and personal fees from Pfizer, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novavax, personal fees from Quintiles, grants from Bayer, grants and personal fees from BMS, grants and personal fees from Astra Zeneca, grants and personal fees from Sanofi, personal fees from Novartis, personal fees from Pharmerit, other from Health-Ecore, other from PAG, other from Asc Academics, grants and personal fees from IQVIA, grants from bioMérieux, grants from the WHO, grants from the European Union, grants and personal fees from Seqirus, grants from FIND, grants from Antilope and grants from DIKTI, LPDP, Budi, all outside of the submitted work. Dr. Rezahosseini reports grants from the Research Foundation of Rigshospitalet and grants from the A.P. Møller Fonden, outside of the submitted work. Dr. Shivarov reports salary from PRAHS, outside of the submitted work. Dr. Uddin reports as having worked as a visiting fellow at Deakin University Institute for Physical Activity and Nutrition (IPAN). IPAN paid for travel (including flights and transportation), accommodations and meals. E.Upadhyay is listed on two patents: ‘A system and method of reusable filters for anti-pollution mask’ (pending) and ‘A system and method for electricity generation through crop stubble by using microbial fuel cells’ (pending). Dr. Wu reports grants from the Ministry of Science and Technology in China, personal fees from HealthKeepers, grants from Suzhou Municipal Science and Technology Bureau and grants from Kunshan Government, outside of the submitted work. Dr. Zhu reports grants from UTHealth Innovation for Cancer Prevention Research Training Program Pre-doctoral Fellowship (Cancer Prevention and Research Institute of Texas, grant no. RP160015), during the conduct of the study.
Footnotes
Editor recognition statement Jennifer Sargent was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. Reviewer recognition statement Nature Medicine thanks Ian Hambleton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Simon I. Hay, Email: sihay@uw.edu
Local Burden of Disease Anaemia Collaborators:
Lauren E. Schaeffer, Alice Lazzar-Atwood, Dan Lu, Samuel B. Ewald, Katie M. Donkers, Ian D. Letourneau, Michael Collison, Megan F. Schipp, Amanuel Abajobir, Sima Abbasi, Nooshin Abbasi, Mitra Abbasifard, Mohsen Abbasi-Kangevari, Hedayat Abbastabar, Foad Abd-Allah, Ahmed Abdelalim, Sherief M. Abd-Elsalam, Amir Abdoli, Ibrahim Abdollahpour, Aidin Abedi, Hassan Abolhassani, Biju Abraham, Lucas Guimarães Abreu, Michael R. M. Abrigo, Ahmed Abualhasan, Eman Abu-Gharbieh, Abdelrahman I. Abushouk, Manfred Mario Kokou Accrombessi, Maryam Adabi, Oladimeji M. Adebayo, Adeyinka Emmanuel Adegbosin, Victor Adekanmbi, Olatunji O. Adetokunboh, Daniel Adedayo Adeyinka, Davoud Adham, Shailesh M. Advani, Pradyumna Agasthi, Mohammad Aghaali, Sohail Ahmad, Tauseef Ahmad, Keivan Ahmadi, Sepideh Ahmadi, Muktar Beshir Ahmed, Miloud Taki Eddine Aichour, Budi Aji, Oluwaseun Oladapo Akinyemi, Addis Aklilu, Chisom Joyqueenet Akunna, Ziyad Al-Aly, Turki M. Alanzi, Jacqueline Elizabeth Alcalde-Rabanal, Biresaw Wassihun Alemu, Ayinalem Alemu, Robert Kaba Alhassan, Sheikh Mohammad Alif, Vahid Alipour, Hesam Alizade, Syed Mohamed Aljunid, Amir Almasi-Hashiani, Hesham M. Al-Mekhlafi, Rajaa M. Al-Raddadi, Nelson Alvis-Guzman, Saeed Amini, Fatemeh Amiri, Dickson A. Amugsi, Nahla Hamed Anber, Robert Ancuceanu, Tudorel Andrei, Masresha Tessema Anegago, Mina Anjomshoa, Fereshteh Ansari, Alireza Ansari-Moghaddam, Zelalem Alamrew Anteneh, Ernoiz Antriyandarti, Davood Anvari, Razique Anwer, Muhammad Aqeel, Jalal Arabloo, Morteza Arab-Zozani, Olatunde Aremu, Habtamu Abera Areri, Al Artaman, Afsaneh Arzani, Malke Asaad, Mehran Asadi-Aliabadi, Ali A. Asadi-Pooya, Mulusew A. Asemahagn, Mohammad Asghari Jafarabadi, Mengistu M. Ashebir, Zerihun Ataro, Seyyede Masoume Athari, Seyyed Shamsadin Athari, Maha Moh’d Wahbi Atout, Marcel Ausloos, Nefsu Awoke, Beatriz Paulina Ayala Quintanilla, Getinet Ayano, Martin Amogre Ayanore, Yared Asmare Aynalem, Muluken Altaye Ayza, Abbas Azadmehr, Darshan B, Tesleem Kayode Babalola, Alaa Badawi, Ashish D. Badiye, Mohammad Amin Bahrami, Mohan Bairwa, Shankar M. Bakkannavar, Palash Chandra Banik, Adhanom Gebreegziabher Baraki, Miguel A. Barboza, Huda Basaleem, Sanjay Basu, Mohsen Bayati, Bayisa Abdissa Baye, Gholamreza Bazmandegan, Neeraj Bedi, Tariku Tesfaye Tesfaye Bekuma, Michelle L. Bell, Isabela M. Bensenor, Kidanemaryam Berhe, Abadi Kidanemariam Berhe, Kidanemariam Alem Berhie, Dinesh Bhandari, Nikha Bhardwaj, Pankaj Bhardwaj, Krittika Bhattacharyya, Suraj Bhattarai, Zulfiqar A. Bhutta, Ali Bijani, Boris Bikbov, Antonio Biondi, Minyichil Birhanu, Raaj Kishore Biswas, Moses John Bockarie, Somayeh Bohlouli, Mahdi Bohluli, Archith Boloor, Shiva Borzouei, Nicola Luigi Bragazzi, Dejana Braithwaite, Andre R. Brunoni, Sharath Burugina Nagaraja, Zahid A. Butt, Florentino Luciano Caetano dos Santos, Luis Alberto Cámera, Josip Car, Rosario Cárdenas, Felix Carvalho, Joao Mauricio Castaldelli-Maia, Carlos A. Castañeda-Orjuela, Franz Castro, Muge Cevik, Wagaye Fentahun Chanie, Jaykaran Charan, Souranshu Chatterjee, Vijay Kumar Chattu, Sarika Chaturvedi, Simiao Chen, Ken Lee Chin, Mohiuddin Ahsanul Kabir Chowdhury, Aubrey J. Cook, Vera Marisa Costa, Elizabeth A. Cromwell, Berihun Assefa Dachew, Henok Dagne, Baye Dagnew, Tukur Dahiru, Saad M. A. Dahlawi, Haijiang Dai, Hancheng Dai, Lalit Dandona, Rakhi Dandona, Parnaz Daneshpajouhnejad, Farah Daoud, Jai K. Das, Rajat Das Gupta, Aditya Prasad Dash, Claudio Alberto Dávila-Cervantes, Kairat Davletov, Farah Deeba, Jan-Walter De Neve, Edgar Denova-Gutiérrez, Kebede Deribe, Assefa Desalew, Getenet Ayalew Dessie, Sagnik Dey, Meghnath Dhimal, Govinda Prasad Dhungana, Mostafa Dianatinasab, Daniel Diaz, Isaac Oluwafemi Dipeolu, Shirin Djalalinia, Hoa Thi Do, Fariba Dorostkar, Leila Doshmangir, Bereket Duko, Andre Rodrigues Duraes, Lucas Earl, Hisham Atan Edinur, Ferry Efendi, Rajesh Elayedath, Teshome Bekele Elema, Hala Rashad Elhabashy, Shaimaa I. El-Jaafary, Iman El Sayed, Maysaa El Sayed Zaki, Aisha Elsharkawy, Yasser Mohamed El-Sherbiny, Maha El Tantawi, Daniel Adane Endalew, Babak Eshrati, Khalil Eskandari, Sharareh Eskandarieh, Ibtihal Fadhil, Emerito Jose A. Faraon, Mohammad Fareed, Pawan Sirwan Faris, Medhat Farwati, Farshad Farzadfar, Abidemi Omolara Fasanmi, Nazir Fattahi, Nelsensius Klau Fauk, Valery L. Feigin, Berhanu Elfu Feleke, Seyed-Mohammad Fereshtehnejad, Eduarda Fernandes, Pietro Ferrara, Nataliya A. Foigt, Artem Alekseevich Fomenkov, Masoud Foroutan, Joel Msafiri Francis, Richard Charles Franklin, Marisa Freitas, Takeshi Fukumoto, Mohamed M. Gad, Abhay Motiramji Gaidhane, Reta Tsegaye Gayesa, Biniyam Sahiledengle Geberemariyam, Birhan Gebresillassie Gebregiorgis, Hadush Gebremariam, Tesfay B. B. Gebremariam, Leake Gebremeskel, Gebreamlak Gebremedhn Gebremeskel, Assefa Ayalew Gebreslassie, Yilma Chisha Dea Geramo, Hailay Abrha Gesesew, Bradford D. Gessner, Lemma Getacher, Keyghobad Ghadiri, Fatemeh Ghaffarifar, Mansour Ghafourifard, Mahsa Ghajarzadeh, Farhad Ghamari, Ahmad Ghashghaee, Nermin Ghith, Syed Amir Gilani, Tiffany K. Gill, Myron Anthony Godinho, Philimon N. Gona, Ayman Grada, Mohammed Ibrahim Mohialdeen Gubari, Nachiket Gudi, Davide Guido, Rashid Abdi Guled, Yuming Guo, Rachita Gupta, Rajeev Gupta, Arvin Haj-Mirzaian, Randah R. Hamadeh, Demelash Woldeyohannes Handiso, Asif Hanif, Arief Hargono, Ahmed I. Hasaballah, Md Mehedi Hasan, Syed Shahzad Hasan, Maryam Hashemian, Abdiwahab Hashi, Shoaib Hassan, Amr Hassan, Soheil Hassanipour, Hadi Hassankhani, Khezar Hayat, Mohamed I. Hegazy, Reza Heidari-Soureshjani, Nathaniel J. Henry, Claudiu Herteliu, Fatemeh Heydarpour, Sousan Heydarpour, Hagos Degefa de Hidru, Chi Linh Hoang, Ramesh Holla, Julia Hon, Sung Hwi Hong, Praveen Hoogar, Seyyed Nasrollah Hosseini, Mehdi Hosseinzadeh, Mihaela Hostiuc, Sorin Hostiuc, Peter J. Hotez, Mowafa Househ, Tanvir M. Huda, Dawit Hoyiso Huluko Huluko, Syed Ather Hussain, Bing-Fang Hwang, Olayinka Stephen Ilesanmi, Irena M. Ilic, Milena D. Ilic, Leeberk Raja Inbaraj, Usman Iqbal, M. Mofizul Islam, Sheikh Mohammed Shariful Islam, Chinwe Juliana Iwu, Chidozie C. D. Iwu, Farhad Jadidi-Niaragh, Mohammad Ali Jahani, Vardhmaan Jain, Mihajlo Jakovljevic, Amir Jalali, Farzad Jalilian, Manthan Dilipkumar Janodia, Tahereh Javaheri, Ravi Prakash Jha, Oommen John, Kimberly B. Johnson, Jost B. Jonas, Jitendra Jonnagaddala, Nitin Joseph, Ankur Joshi, Farahnaz Joukar, Jacek Jerzy Jozwiak, Ali Kabir, Zubair Kabir, Tanvir Kahlon, Leila R. Kalankesh, Rohollah Kalhor, Ashwin Kamath, Zahra Kamiab, Tanuj Kanchan, Umesh Kapil, Neeti Kapoor, Behzad Karami Matin, Salah Eddin Karimi, Ayele Semachew Kasa, Gebremicheal Gebreslassie Kasahun, Zemenu Yohannes Kassa, Gebrehiwot G. Kassa, Getinet Kassahun, Gbenga A. Kayode, Ali Kazemi Karyani, Tibebeselassie S. Keflie, Peter Njenga Keiyoro, Bayew Kelkay, Maryam Keramati, Daniel Bekele Ketema, Nauman Khalid, Mohammad Khammarnia, Md Nuruzzaman Khan, Maseer Khan, Junaid Khan, Khaled Khatab, Amir M. Khater, Mona M. Khater, Abdullah T. Khoja, Jagdish Khubchandani, Neda Kianipour, Young-Eun Kim, Yun Jin Kim, Ruth W. Kimokoti, Sezer Kisa, Adnan Kisa, Tufa Kolola, Ali Koolivand, Soewarta Kosen, Parvaiz A. Koul, Ai Koyanagi, Kewal Krishan, Vijay Krishnamoorthy, Barthelemy Kuate Defo, Nuworza Kugbey, Vaman Kulkarni, G. Anil Kumar, Nithin Kumar, Pushpendra Kumar, Manasi Kumar, Om P. Kurmi, Dian Kusuma, Ben Lacey, Deepesh P. Lad, Dharmesh Kumar Lal, Faris Hasan Lami, Iván Landires, Anders O. Larsson, Savita Lasrado, Matthew B. Laurens, Carlo La Vecchia, Avula Laxmaiah, Paul H. Lee, Shaun Wen Huey Lee, Kate E. LeGrand, Sonia Lewycka, Bingyu Li, Shanshan Li, Xuefeng Liu, Jaifred Christian F. Lopez, Daiane Borges Machado, Shilpashree Madhava Kunjathur, Hassan Magdy Abd El Razek, Muhammed Magdy Abd El Razek, D. R. Mahadeshwara Prasad, Phetole Walter Mahasha, Mina Maheri, Narayan B. Mahotra, Azeem Majeed, Venkatesh Maled, Shokofeh Maleki, Reza Malekzadeh, Deborah Carvalho Malta, Abdullah A. Mamun, Fariborz Mansour-Ghanaei, Borhan Mansouri, Mohammad Ali Mansournia, Md Dilshad Dilshad Manzar, Carlos Alberto Marrugo Arnedo, Francisco Rogerlândio Martins-Melo, Anthony Masaka, Pallab K. Maulik, Benjamin K. Mayala, Medhin Mehari, Man Mohan Mehndiratta, Entezar Mehrabi Nasab, Fereshteh Mehri, Kala M. Mehta, Wahengbam Bigyananda Meitei, Teferi Mekonnen, Gebrekiros Gebremichael Meles, Mulugeta Melku, Walter Mendoza, Ritesh G. Menezes, Meresa Berwo Mengesha, Endalkachew Worku Mengesha, Tuomo J. Meretoja, Abera M. Mersha, Workua Mekonnen Metekiya, Tomasz Miazgowski, Irmina Maria Michalek, G. K. Mini, Shabir Ahmad Mir, Andreea Mirica, Erkin M. Mirrakhimov, Hamed Mirzaei, Maryam Mirzaei, Mehdi Mirzaei-Alavijeh, Sanjeev Misra, Babak Moazen, Masoud Moghadaszadeh, Yousef Mohammad, Dara K. Mohammad, Naser Mohammad Gholi Mezerji, Seyyede Momeneh Mohammadi, Abdollah Mohammadian-Hafshejani, Reza Mohammadpourhodki, Hayat Maeruf Mohammed, Salahuddin Mohammed, Ammas Siraj Mohammed, Shafiu Mohammed, Jemal Abdu Mohammed, Mohammad A. Mohseni Bandpei, Ali H. Mokdad, Alex Molassiotis, Lorenzo Monasta, Masoud Moradi, Maziar Moradi-Lakeh, Rahmatollah Moradzadeh, Paula Moraga, Abbas Mosapour, Simin Mouodi, Seyyed Meysam Mousavi, Amin Mousavi Khaneghah, Getaneh Baye B. Mulu, Mehnaz Munir, Moses K. Muriithi, G. V. S. Murthy, Ghulam Mustafa, Ashraf F. Nabhan, Mehdi Naderi, Ahamarshan Jayaraman Nagarajan, Shankar Prasad Nagaraju, Mohsen Naghavi, Gurudatta Naik, Mukhammad David Naimzada, Vinay Nangia, Jobert Richie Nansseu, Atta Abbas Naqvi, Bruno Ramos Nascimento, Smitha Nayak, Vinod C. Nayak, Javad Nazari, Rawlance Ndejjo, Ionut Negoi, Ruxandra Irina Negoi, Henok Biresaw Netsere, Georges Nguefack-Tsague, Josephine W. Ngunjiri, Cuong Tat Nguyen, Diep Ngoc Nguyen, Huong Lan Thi Nguyen, Yeshambel T. Nigatu, Rajan Nikbakhsh, Amin Reza Nikpoor, Chukwudi A. Nnaji, Vuong Minh Nong, Jean Jacques Noubiap, Virginia Nunez-Samudio, Vincent Ebuka Nwatah, Tafadzwa Nyanhanda, Bogdan Oancea, Felix Akpojene Ogbo, Onome Bright Oghenetega, In-Hwan Oh, Daniel Micheal Okello, Morteza Oladnabi, Andrew T. Olagunju, Jacob Olusegun Olusanya, Bolajoko Olubukunola Olusanya, Ahmed Omar Bali, Muktar Omer Omer, Abidemi E. Emmanuel Omonisi, Obinna E. Onwujekwe, Alberto Ortiz, Eduardo Ortiz-Panozo, Nikita Otstavnov, Stanislav S. Otstavnov, Mayowa O. Owolabi, P. A. Mahesh, Jagadish Rao Padubidri, Abhijit P. Pakhare, Keyvan Pakshir, Adrian Pana, Songhomitra Panda-Jonas, Anamika Pandey, Seithikurippu R. Pandi-Perumal, Helena Ullyartha Pangaribuan, Deepak Kumar Pasupula, Sangram Kishor Patel, Urvish K. Patel, Ashish Pathak, George C. Patton, Hamidreza Pazoki Toroudi, Jeevan Pereira, Julia Moreira Pescarini, Hai Quang Pham, Brandon V. Pickering, Saeed Pirouzpanah, Meghdad Pirsaheb, Khem Narayan Pokhrel, Maarten J. Postma, Faheem Hyder Pottoo, Hadis Pourchamani, Hadi Pourjafar, Hossein Poustchi, Sergio I. Prada, Dimas Ria Angga Pribadi, Zahiruddin Quazi Syed, Navid Rabiee, Ata Rafiee, Fakher Rahim, Mohammad Hifz Ur Rahman, Muhammad Aziz Rahman, Amir Masoud Rahmani, Rajesh Kumar Rai, Aashish Rajesh, Pradhum Ram, Kiana Ramezanzadeh, Chhabi Lal Ranabhat, Sowmya J. Rao, Satish Rao, Prateek Rastogi, Priya Rathi, Lal Rawal, Wasiq Faraz Rawasia, Reza Rawassizadeh, Lemma Demissie Regassa, Robert C. Reiner, Jr, Bhageerathy Reshmi, Nima Rezaei, Omid Rezahosseini, Aziz Rezapour, Seyed Mohammad Riahi, Daniela Ribeiro, Ana Isabel Ribeiro, Jennifer Rickard, Hirbo Shore Roba, Leonardo Roever, Luca Ronfani, Morteza Rostamian, Susan Fred Rumisha, Godfrey M. Rwegerera, Siamak Sabour, Ehsan Sadeghi, Sahar Saeedi Moghaddam, Rajesh Sagar, Amirhossein Sahebkar, Mohammad Ali Sahraian, S. Mohammad Sajadi, Nasir Salam, Marwa Rashad Salem, Hossein Samadi Kafil, Itamar S. Santos, Milena M. Santric-Milicevic, Sivan Yegnanarayana Iyer Saraswathy, Nizal Sarrafzadegan, Benn Sartorius, Arash Sarveazad, Brijesh Sathian, Thirunavukkarasu Sathish, Deepak Saxena, Alyssa N. Sbarra, David C. Schwebel, Anbissa Muleta Senbeta, Debarka Sengupta, Subramanian Senthilkumaran, Sadaf G. Sepanlou, Allen Seylani, Feng Sha, Omid Shafaat, Saeed Shahabi, Mohammad Shahbaz, Izza Shahid, Masood Ali Shaikh, Mohammed Feyisso Shaka, Ali S. Shalash, Mahdi Shamali, Mehran Shams-Beyranvand, MohammadBagher Shamsi, Morteza Shamsizadeh, Mohammed Shannawaz, Kiomars Sharafi, Amrollah Sharifi, Aziz Sheikh, Abbas Sheikhtaheri, Ranjitha S. Shetty, B. Suresh Kumar Shetty, Adithi Shetty, Wondimeneh Shibabaw Shiferaw, Mika Shigematsu, Jae Il Shin, Rahman Shiri, Reza Shirkoohi, Velizar Shivarov, Soraya Siabani, Sudeep K. Siddappa Malleshappa, Tariq Jamal Siddiqi, Negussie Boti Sidemo, Balbir Bagicha Singh, Surya Singh, Yitagesu Sintayehu, Valentin Yurievich Skryabin, Anna Aleksandrovna Skryabina, Mohammad Reza Sobhiyeh, Amin Soheili, Shahin Soltani, Muluken Bekele Sorrie, Emma Elizabeth Spurlock, Chandrashekhar T. Sreeramareddy, Agus Sudaryanto, Mu’awiyyah Babale Sufiyan, Iyad Sultan, Rafael Tabarés-Seisdedos, Takahiro Tabuchi, Biruk Wogayehu Taddele, Eyayou Girma Tadesse, Amir Taherkhani, Zemenu Tamir, Animut Tagele Tamiru, Md Ismail Tareque, Abdelghani Tbakhi, Hirut Teame, Yonas Getaye Tefera, Arash Tehrani-Banihashemi, Yohannes Tekalegn, Merhawi Gebremedhin Tekle, Berhane Fseha Teklehaimanot, Mohamad-Hani Temsah, Getayeneh Antehunegn Tesema, Kavumpurathu Raman Thankappan, Nihal Thomas, Takele Tiki, Asres Bedaso Tilahune, Mariya Vladimirovna Titova, Marcos Roberto Tovani-Palone, Khanh Bao Tran, Bach Xuan Tran, Rajnish Tripathi, Jaya Prasad Tripathy, Phuong N. Truong, Riaz Uddin, Anayat Ullah, Chukwuma David Umeokonkwo, Chigozie Jesse Uneke, Bhaskaran Unnikrishnan, Era Upadhyay, Muhammad Shariq Usman, Marco Vacante, Alireza Vakilian, Sahel Valadan Tahbaz, Pascual R. Valdez, Yasser Vasseghian, Madhur Verma, Francesco S. Violante, Bay Vo, Yohannes Dibaba Wado, Yasir Waheed, Yafeng Wang, Yuan-Pang Wang, Kinley Wangdi, Girmay Teklay Weldesamuel, Andrea Werdecker, Taweewat Wiangkham, Nuwan Darshana Wickramasinghe, Charles Shey Wiysonge, Tewodros Eshete Wonde, Ai-Min Wu, Chenkai Wu, Yang Xie, Ali Yadollahpour, Seyed Hossein Yahyazadeh Jabbari, Tomohide Yamada, Mingyou Yang, Sanni Yaya, Vahid Yazdi-Feyzabadi, Tomas Y. Yeheyis, Alex Yeshaneh, Yigizie Yeshaw, Yordanos Gizachew Yeshitila, Mekdes Tigistu Yilma, Paul Yip, Melissa F. Young, Zabihollah Yousefi, Taraneh Yousefinezhadi, Hebat-Allah Salah A. Yousof, Abdilahi Yousuf Yousuf, Chuanhua Yu, Yong Yu, Shamsa Zafar, Syed Saoud Zaidi, Zoubida Zaidi, Josefina Zakzuk, Sojib Bin Zaman, Mohammad Zamani, Maryam Zamanian, Alireza Zandifar, Alireza Zangeneh, Mikhail Sergeevich Zastrozhin, Anasthasia Zastrozhina, Dejene Tesfaye Zewdie, Kaleab Alemayehu Zewdie, Yunquan Zhang, Cong Zhu, and Arash Ziapour
Extended data
is available for this paper at 10.1038/s41591-021-01498-0.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-021-01498-0.
References
- 1.Kassebaum NJ, GBD 2013 Anemia Collaborators. The Global Burden of Anemia. Hematol. Oncol. Clin. North Am. 2016;30:247–308. doi: 10.1016/j.hoc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global nutrition targets 2025: anaemia policy brief. https://apps.who.int/iris/handle/10665/148556 (2014) .
- 3.Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J. Nutr. 2001;131:676S–690S. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- 4.Horton S, Ross J. The economics of iron deficiency. Food Policy. 2003;28:51–75. doi: 10.1016/S0306-9192(02)00070-2. [DOI] [Google Scholar]
- 5.Martinsson A, et al. Anemia in the general population: prevalence, clinical correlates and prognostic impact. Eur. J. Epidemiol. 2014;29:489–498. doi: 10.1007/s10654-014-9929-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith ER, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob. Health. 2017;5:e1090–e1100. doi: 10.1016/S2214-109X(17)30371-6. [DOI] [PubMed] [Google Scholar]
- 7.Kavle JA, et al. Association between anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J. Health Popul. Nutr. 2008;26:232–240. [PMC free article] [PubMed] [Google Scholar]
- 8.CHERG Iron Report: Maternal Mortality, Child Mortality, Perinatal Mortality, Child Cognition, and Estimates of Prevalence of Anemia due to Iron Deficiency. Global Health Data Exchange. http://ghdx.healthdata.org/record/cherg-iron-report-maternal-mortality-child-mortality-perinatal-mortality-child-cognition-and
- 9.Davis E. & Sparzak P. B. Abnormal uterine bleeding. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK532913/ (2021). [PubMed]
- 10.Stein J, et al. Anemia and iron deficiency in gastrointestinal and liver conditions. World J. Gastroenterol. 2016;22:7908–7925. doi: 10.3748/wjg.v22.i35.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White NJ. Anaemia and malaria. Malar. J. 2018;17:371. doi: 10.1186/s12936-018-2509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calis JCJ, et al. Erythropoiesis in HIV-infected and uninfected Malawian children with severe anemia. AIDS. 2010;24:2883–2887. doi: 10.1097/QAD.0b013e32833fed27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alamo IG, et al. Characterization of erythropoietin and hepcidin in the regulation of persistent injury-associated anemia. J. Trauma Acute Care Surg. 2016;81:705–712. doi: 10.1097/TA.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semba RD, Bloem MW. The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur. J. Clin. Nutr. 2002;56:271–281. doi: 10.1038/sj.ejcn.1601320. [DOI] [PubMed] [Google Scholar]
- 15.Black AK, Allen LH, Pelto GH, de Mata MP, Chávez A. Iron, vitamin B-12 and folate status in Mexico: associated factors in men and women and during pregnancy and lactation. J. Nutr. 1994;124:1179–1188. doi: 10.1093/jn/124.8.1179. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Nutritional anaemias: tools for effective prevention and control. https://apps.who.int/iris/handle/10665/259425 (2017).
- 17.Girard AW, Self JL, McAuliffe C, Olude O. The effects of household food production strategies on the health and nutrition outcomes of women and young children: a systematic review. Paediatr. Perinat. Epidemiol. 2012;26:205–222. doi: 10.1111/j.1365-3016.2012.01282.x. [DOI] [PubMed] [Google Scholar]
- 18.Dicker D, et al. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1684–1735. doi: 10.1016/S0140-6736(18)31891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute for Health Metrics and Evaluation. GBD Compare. https://vizhub.healthdata.org/gbd-compare/
- 20.World Health Organization. Global targets tracking tool. https://www.who.int/tools/global-targets-tracking-tool (2019).
- 21.WHO/UNICEF Discussion Paper. The extension of the 2025 Maternal, Infant and Young Child nutrition targets to 2030. https://www.who.int/nutrition/global-target-2025/discussion-paper-extension-targets-2030.pdf?ua=1 (2018).
- 22.Sustainable Development Solutions Network. Indicators and a Monitoring Framework. Goal 02. End hunger, achieve food security and improved nutrition, and promote sustainable agriculture. https://indicators.report/goals/goal-2/
- 23.Leone, F. New Indicators on AMR, Dispute Resolution, GHG Emissions Agreed for SDG Framework. IISD SDG Knowledge Hub. https://sdg.iisd.org:443/news/new-indicators-on-amr-dispute-resolution-ghg-emissions-agreed-for-sdg-framework/ (2019).
- 24.World Health Organization. The global prevalence of anaemia in 2011. http://www.who.int/entity/nutrition/publications/micronutrients/global_prevalence_anaemia_2011/en/index.html (2015).
- 25.Bhattacharjee NV, et al. Mapping exclusive breastfeeding in Africa between 2000 and 2017. Nat. Med. 2019;25:1205–1212. doi: 10.1038/s41591-019-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osgood-Zimmerman A, et al. Mapping child growth failure in Africa between 2000 and 2015. Nature. 2018;555:41–47. doi: 10.1038/nature25760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinyoki DK, et al. Mapping child growth failure across low- and middle-income countries. Nature. 2020;577:231–234. doi: 10.1038/s41586-019-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. https://www.who.int/vmnis/indicators/haemoglobin.pdf (2011).
- 29.Akseer N, Vaivada T, Rothschild O, Ho K, Bhutta ZA. Understanding multifactorial drivers of child stunting reduction in Exemplar countries: a mixed-methods approach. Am. J. Clin. Nutr. 2020;112:792S–805S. doi: 10.1093/ajcn/nqaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet396, 1223–1249 (2020). [DOI] [PMC free article] [PubMed]
- 31.Rasmussen K. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality? J. Nutr. 2001;131:590S–601S; discussion 601S–603S. doi: 10.1093/jn/131.2.590S. [DOI] [PubMed] [Google Scholar]
- 32.Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019;3:CD004905. doi: 10.1002/14651858.CD004905.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jose A, et al. Comparison of ferric Carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy—randomised controlled trial. BMC Pregnancy Childbirth. 2019;19:54. doi: 10.1186/s12884-019-2200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss DJ, et al. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000–17: a spatial and temporal modelling study. Lancet. 2019;394:322–331. doi: 10.1016/S0140-6736(19)31097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njua-Yafi C, et al. Malaria, helminths, co-infection and anaemia in a cohort of children from Mutengene, south western Cameroon. Malar. J. 2016;15:69. doi: 10.1186/s12936-016-1111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pullan RL, Brooker SJ. The global limits and population at risk of soil-transmitted helminth infections in 2010. Parasites Vectors. 2012;5:81. doi: 10.1186/1756-3305-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinyoki DK, et al. Mapping local patterns of childhood overweight and wasting in low- and middle-income countries between 2000 and 2017. Nat. Med. 2020;26:750–759. doi: 10.1038/s41591-020-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiner RC, Jr., et al. Mapping geographical inequalities in childhood diarrhoeal morbidity and mortality in low-income and middle-income countries, 2000–2017: analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:1779–1801. doi: 10.1016/S0140-6736(20)30114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burstein R, et al. Mapping 123 million neonatal, infant and child deaths between 2000 and 2017. Nature. 2019;574:353–358. doi: 10.1038/s41586-019-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Malaria Atlas Project. Inherited Blood Disorders. https://malariaatlas.org/research-project/blood-disorders/
- 41.Béné C, et al. Global map and indicators of food system sustainability. Sci. Data. 2019;6:279. doi: 10.1038/s41597-019-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.GeoNetwork. The Global Administrative Unit Layers (GAUL). http://www.fao.org/geonetwork/srv/en/main.home (2015).
- 43.Friedl, M. & Sulla-Menashe, D. MCD12Q1 v006: MODIS/Terra+Aqua Land Cover Type Yearly L3 Global 500 m SIN Grid 10.5067/MODIS/MCD12Q1.006 (2019).
- 44.World Wildlife Fund. Global Lakes and Wetlands Database, Level 3. https://www.worldwildlife.org/pages/global-lakes-and-wetlands-database (2004).
- 45.Lehner B, Döll P. Development and validation of a global database of lakes, reservoirs and wetlands. J. Hydrol. 2004;296:1–22. doi: 10.1016/j.jhydrol.2004.03.028. [DOI] [Google Scholar]
- 46.WorldPop. Open Spatial Demographic Data and Research. https://www.worldpop.org/
- 47.Tatem AJ. WorldPop, open data for spatial demography. Sci. Data. 2017;4:170004. doi: 10.1038/sdata.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray CJ, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 49.Lumley T. Analysis of complex survey samples. J. Stat. Softw. 2004;9:1–19. doi: 10.18637/jss.v009.i08. [DOI] [Google Scholar]
- 50.Global Administrative Areas (GADM). GADM maps and data. http://www.gadm.org
- 51.Ananth CV, Kleinbaum DG. Regression models for ordinal responses: a review of methods and applications. Int. J. Epidemiol. 1997;26:1323–1333. doi: 10.1093/ije/26.6.1323. [DOI] [PubMed] [Google Scholar]
- 52.Stein, M. L. Interpolation of Spatial Data (Springer, 1999).
- 53.Dwyer-Lindgren L, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570:189–193. doi: 10.1038/s41586-019-1200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotez, P. & Ribeiro, P. Model-based Geostatistics (Springer, 2007).
- 55.Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Ser. B (Methodol.) 2009;71:319–392. doi: 10.1111/j.1467-9868.2008.00700.x. [DOI] [Google Scholar]
- 56.Lindgren F, Rue H, Lindström J. An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic partial differential equation approach. J. R. Stat. Soc. Ser. B (Methodol.) 2011;73:423–498. doi: 10.1111/j.1467-9868.2011.00777.x. [DOI] [Google Scholar]
- 57.Patil AP, Gething PW, Piel FB, Hay SI. Bayesian geostatistics in health cartography: the perspective of malaria. Trends Parasitol. 2011;27:246–253. doi: 10.1016/j.pt.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fienberg, S. E. The Analysis of Cross-Classified Categorical Data (Springer, 2007).
- 59.Scharlemann JPW, et al. Global data for ecology and epidemiology: a novel algorithm for temporal Fourier processing MODIS data. PLoS ONE. 2008;3:e1408. doi: 10.1371/journal.pone.0001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts, D. R. et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography10.1111/ecog.02881 (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–21, Supplementary Tables 1–18, GATHER compliance, Supplementary Data, Supplementary Covariates, Supplementary Methods, Model Results, Model Validation and Supplementary References
Data Availability Statement
The findings of this study are supported by data available in public online repositories, data publicly available upon reasonable request of the data provider and data not publicly available owing to restrictions by the data provider. Non-publicly available data were used under licence for the current study but might be available from the authors upon reasonable request and with permission of the data provider. A detailed table of data sources and availability can be found in Supplementary Section 2. The full list of input data sources and output of the analyses is publicly available in the Global Health Data Exchange (http://ghdx.healthdata.org/record/ihme-data/global-anemia-prevalence-geospatial-estimates-2000-2019) and can further be explored via customized data visualization tools (https://vizhub.healthdata.org/lbd/anemia).
Administrative boundaries were retrieved from the Database of Global Administrative Areas50. Land cover was retrieved from the online Data Pool, courtesy of the NASA EOSDIS Land Processes Distributed Active Archive Center, USGS/Earth Resources Observation and Science Center43. Lakes were retrieved from the Global Lakes and Wetlands Database45, courtesy of the World Wildlife Fund and the Center for Environmental Systems Research at the University of Kassel44. Populations were retrieved from WorldPop46,47.
This study follows the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER; Supplementary Table 1). All code used for these analyses is publicly available at http://ghdx.healthdata.org/record/ihme-data/global-anemia-prevalence-geospatial-estimates-2000-2019 and https://github.com/ihmeuw/lbd/tree/anemia-lmic-2021.