Abstract
Intercellular adhesion is the key to multicellularity, and its malfunction plays an important role in various developmental and disease-related processes. Although it has been intensively studied by both biologists and physicists, a commonly accepted definition of cell-cell adhesion is still being debated. Cell-cell adhesion has been described at the molecular scale as a function of adhesion receptors controlling binding affinity, at the cellular scale as resistance to detachment forces or modulation of surface tension, and at the tissue scale as a regulator of cellular rearrangements and morphogenesis. In this review, we aim to summarize and discuss recent advances in the molecular, cellular, and theoretical description of cell-cell adhesion, ranging from biomimetic models to the complexity of cells and tissues in an organismal context. In particular, we will focus on cadherin-mediated cell-cell adhesion and the role of adhesion signaling and mechanosensation therein, two processes central for understanding the biological and physical basis of cell-cell adhesion.
Introduction
The basic unit of living systems is the cell, which gives rise to unicellular colonies and multicellular organisms. In multicellular organisms, cells are assembled into tissues (1), the formation of which depends on cell-cell adhesion complexes that couple cells to each other. Cell-cell adhesion plays essential roles in organismal development and homeostasis, such as tissue compaction (2), cell sorting (3), and cell migration (4), and misregulation of cell-cell adhesion is a hallmark of many developmental disorders and diseases (5, 6, 7).
Specific cell-cell adhesion receptors help two cells to interact and recognize each other (8). Among them, the cadherin family of cell-cell adhesion receptors was most intensively studied in the past and was shown to be essential for the formation and maintenance of tissues in countless organisms (9). Cadherins function by mechanically coupling cells to each other and modulating a wide array of effector processes that range from the regulation of the cytoskeleton to gene expression. Cadherin adhesion complexes typically consist of hundreds of proteins, some of which change their conformation and stoichiometry under mechanical stress, thereby linking the interacting surfaces of cells to their cytoskeleton and giving cells the ability to sense and respond to extracellular and intracellular signals (10).
Cell-cell adhesion is a complex and dynamic process. For years, physicists have been trying to measure and model cell-cell contacts, and biologists have identified new components, functions, and regulators of the cell-cell adhesion machinery. This led to various descriptions and interpretations of cell-cell adhesion as, for instance, the adhesion energy of molecular interactions at adhesive interfaces (11,12) or the resistance to cell-cell detachment forces (13,14). Moreover, adhesion-mediated cell-cell contact formation was proposed to be driven by the balance of interfacial/surface tensions, which again depend on tension exerted by the actomyosin cortex and its modulation via adhesion receptor signaling and the binding of adhesion molecules over the contact (15, 16, 17, 18). In this review, we will summarize and discuss recent progress in defining cell-cell adhesion at multiple scales by both experiment and theory, predominantly focusing on the role of classical cadherins (generally referred to as cadherins) therein.
The Toolbox of Adhesion
Biological components of cell-cell adhesion
Cadherin adhesion complex
Cadherin adhesion complexes are protein assemblies consisting of cadherin adhesion receptors and their cytoplasmic interactors, such as catenins (19). Classical cadherins, such as E-cadherin (cdh1) and N-cadherin (cdh2), consist of an ectodomain of five repetitive extracellular cadherin (EC) subdomains with rigidity-providing Ca2+-binding pockets in between those domains, a single-pass transmembrane domain, and a cytoplasmic tail. The ectodomains of cadherins of opposing cells interact by binding in trans over the contact, first by engaging in EC1-EC2 interactions, leading to the formation of intermediate fast binding X-dimers, followed by strand swapping to form the so-called S-dimers. Cadherins also interact in cis with other cadherins on the same cell surface, a process important for cadherin clustering (20,21). Intracellularly, the cadherin cytoplasmic tail interacts with adaptor proteins, such as p120- and β-catenins. They directly bind to subdomains in the cadherin tail and recruit other molecules, such as ɑ-catenins, which, by binding to filamentous actin (F-actin), connect cadherins to the actomyosin cytoskeleton (10). As new contacts form, cadherins, catenins, and hundreds of other components and interaction partners of the cadherin adhesion complex get recruited to the contact (19), where they control the establishment, strength, and stability of the contact by regulating cadherin clustering, turnover, and cytoskeletal anchoring. The cadherin adhesion complex also regulates downstream signaling mediators, which again modulate cytoskeletal organization and other cellular functions.
Actin cortex
The actin cortex is a thin, contractile F-actin network tethered to the plasma membrane shaping animal cells. The actin cortex can readily adapt to the microenvironment by rapidly turning over. Besides actin, the cortex contains various actin-binding proteins, such as actin nucleators (e.g., Arp2/3 and formins), which assemble and disassemble the F-actin network, actin cross-linkers, and motor proteins (most prominently myosin II), which can both pull and cross-link actin filaments. The coaction of these different proteins regulates the actin network architecture and function, thereby defining the mechanical properties of the cortex (22).
Cell membrane
The cell membrane (plasma membrane) is a phospholipid bilayer surrounding the cell, and forms the border between the interior and exterior of the cell. The cell membrane has a dynamically changing heterogeneous composition and structure. In particular, transient nanodomains of distinct lipid compositions were proposed to function as organizational hubs for recruiting proteins and thereby spatially restricting and modulating their activity (23,24).
Glycocalyx
The glycocalyx (pericellular matrix) is a carbohydrate-rich meshwork covering the cell membrane and consisting primarily of glycopolymer chains decorated with bulky glycoproteins. Depending on the cell type, the glycocalyx can extend up to several micrometers from the cell membrane (25) and is thought to modulate cell-cell adhesion by physically keeping the cell membranes (and adhesion molecules therein) of adjacent cells at a distance.
Extracellular matrix
The extracellular matrix (ECM) is a three-dimensional network composed of proteoglycans (proteins with polysaccharide chains), fibrous proteins, and water, which is locally secreted by cells, connecting and surrounding them. The ECM supports cells structurally and regulates their activities. Cell-ECM adhesion is mediated through ECM receptors, mainly integrins (26).
Junctions
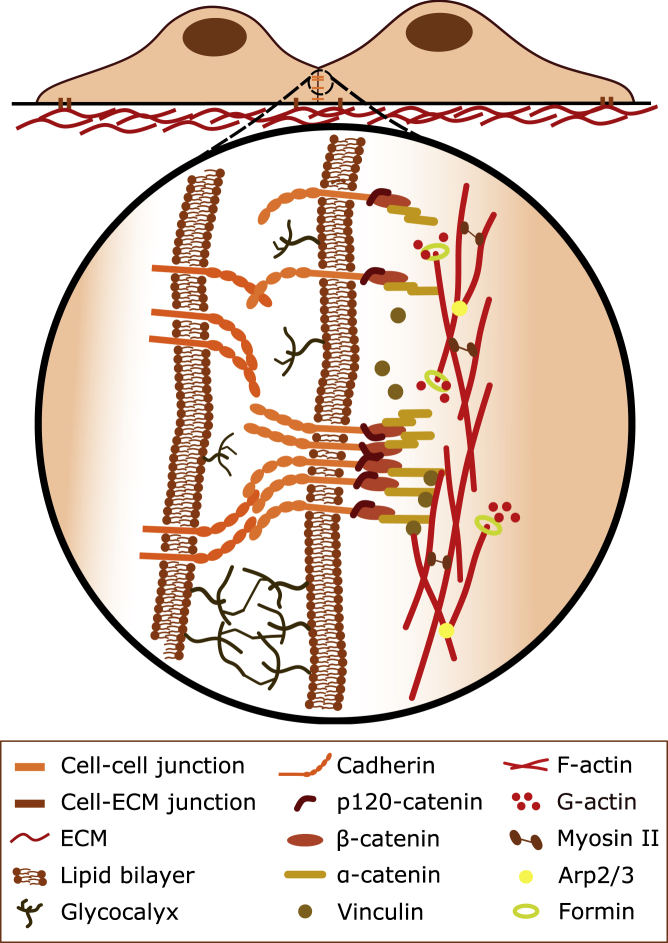
Junctions are cellular structures/multiprotein complexes that connect neighboring cells or cells with the ECM and are connected through adaptor proteins to the cytoskeleton (8). Most common cell-cell junctions are adherens junctions (containing cadherins), tight junctions, and gap junctions. Junctions experience mechanical forces and can convert those into biochemical signals in a process called mechanotransduction, which leads to changes in cell signaling and adhesion (Fig. 1; (14)).
Figure 1.
Cells can undergo adhesions with other cells and the extracellular matrix (ECM) via junctions. Cadherins mediate specific cell-cell adhesions via trans interactions in the extracellular space, where glycocalices act as a repulsive barrier. Cadherins indirectly bind to the underlying actomyosin cortex via β- and ɑ-catenins. Mechanosensitive cadherin adhesion complexes can change their binding strength to the actin cortex by cis clustering and by recruiting adaptor proteins such as vinculin. These complexes can also lead to local changes in actomyosin contractility by regulating the architecture of the cortex.
Mechanical characterization of cell-cell adhesion
Mechanical stress
Mechanical stress (Pascal, Pa) is equivalent to the force per surface area (Newton per square meter, N/m2) on an object applied by a neighboring object. At intercellular contacts, tensile stress and compressive stress act normally to the contact area. Tensile stress occurs when cells are pulled away from each other (Fig. 2 A), whereas compressive stress exists when cells are squeezed toward each other. In comparison, shear stress arises when forces act parallel to the contact area, as in the case of cells that move alongside each other. Furthermore, mechanical stress is equal to the mechanical energy per volume (Joule per cubic meter, J/m3).
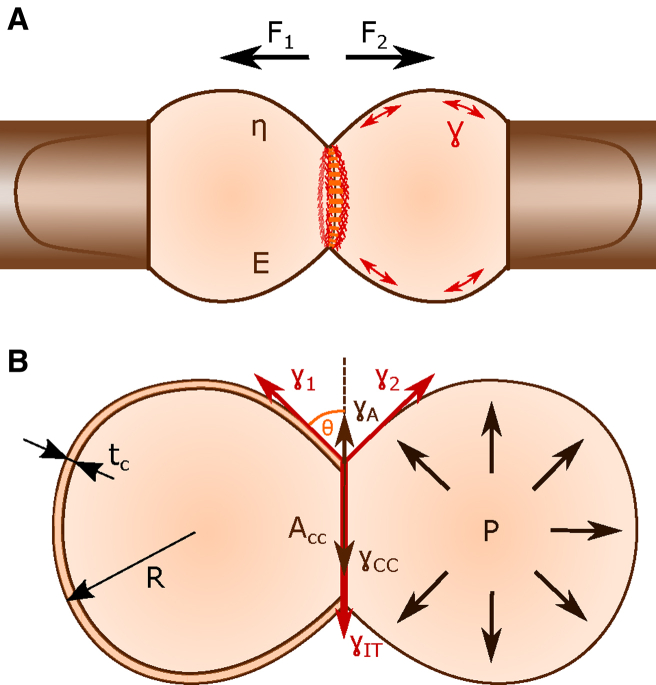
Figure 2.
(A) A schematic representation of dual pipette aspiration (DPA) is shown. Applied detachment forces, F1 + F2, on suspended cells with a given viscoelasticity (viscosity, η, and Young’s modulus, E) forming a contact, where E-cadherin and actin accumulate at the contact rim. (B) Radius, R, and the cortex thickness, tC, define the cortical tensions, γ1 and γ2, of the connected cells. For γ1 = γ2 = γ, cortical tensions at the contact-free area are counteracted by the interfacial tension, γIT = 2 × γ × cos(θ), at the cell-cell adhesion area, ACC. The interfacial tension, γIT, is determined by the difference in magnitude between the cortical tension of both cells at the cell-cell interface, 2∗γCC, and the adhesion tension, γA, acting in antiparallel directions. The cortical tension is in balance with the internal cellular pressure, P.
Cortical tension
Cortical tension (Joule per square meter, J/m2) is the tension generated mainly by myosin motors contracting the thin actin cortex coupled to the cell membrane (27). Cortical tension is modulated by the composition and architecture of the actin cortex (22). Cortical tension must be in balance with the internal cellular pressure, thereby together controlling the cell shape. Cortical tension tends to decrease the surface and the contact area of a cell (Fig. 2 B).
Surface tension
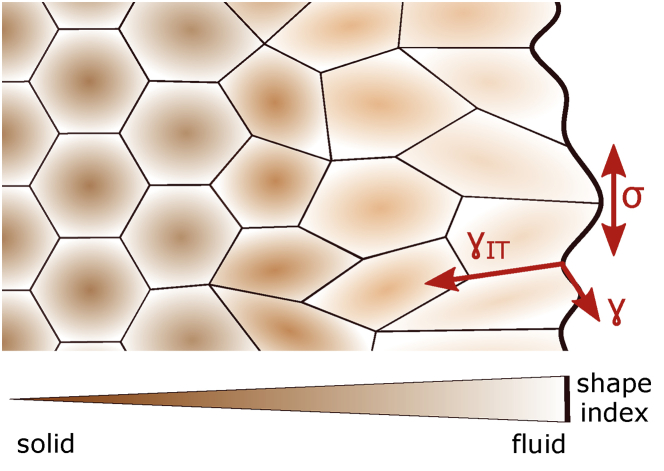
Analogous to water droplets, the surface tension (Joule per square meter, J/m2), as energy per surface area, acts to minimize the surface area of cells (28). Cortical tension together with the typically lower tension of the plasma membrane are the main regulators of cell surface tension. The concept of surface tension can also be applied to describe the mechanical properties of tissues. An aggregate of cells develops tissue surface tension, resulting from the difference in adhesion between cells of the aggregate and their surroundings (Fig. 3; (3)).
Figure 3.
The tissue surface tension, σ, at the tissue edge results from the difference between the interfacial tension, γIT, at the cell-cell contact and the cortical tension, γ, at the contact-free surface. It minimizes the contact-free surface area by smoothing the tissue edge. Interfacial tension also contributes to determining the cell shape index, an indicator of tissue fluidity: cells within the cluster typically display more regular hexagonal shapes, are densely packed by surrounding neighbors, and thus behave more solid-like. Cells at the tissue edge, in contrast, are more elongated and mobile, and thus show a fluid-like behavior.
Cell-cell interfacial tension
Cell-cell interfacial tension (Joule per square meter, J/m2) is the tension that is developed between two cells, described by the energy per contact area. The cell-cell interfacial tension is increased by the cortical tension, which shrinks the contact area, and decreased by adhesion tension because of the binding of adhesion molecules, which increases the contact area (Fig. 2 B; (18,29)).
Adhesion tension
Adhesion tension (Joule per square meter, J/m2) is the total energy per unit area released when two cells come into contact (Fig. 2 B). The total adhesion energy (Joule, J) is given by the integral of the adhesion tension on the interaction area. Sometimes, the adhesion energy is translated as the detachment force (Newton, N), which determines the total work (Newton meter, Nm) needed to separate two objects.
Cellular traction forces
Cellular traction forces (Newton, N) are in-plane pulling forces applied by adherent cells on substrates. They are generated by actomyosin contraction transmitted through the cell-matrix adhesion complexes to the ECM.
Cell-Cell Contact Formation: from Molecules to Cells and Tissues
In the following section, we summarize and discuss how cell-cell adhesion is described by integrating biological components with quantitative terms inspired by polymer physics. We start with descriptions of cell-cell adhesion based on molecular interactions at the contacting membranes and then move on to descriptions on the cellular and tissue/organismal scale.
The role of molecular interactions over the contact
For describing cell-cell adhesion on the molecular scale, biomimetic systems such as phospholipid membranes and vesicles were initially used. Here, adhesion is described based on the formation of specific molecular bonds and the role that the plasma membrane and the glycocalyx play therein. The theoretical basis for such description of cell-cell adhesion was first established by Bell (30), arguing that, aside from weak electrical forces between two cell membranes, attractive forces, generated by the specific binding of integral membrane proteins, must be considered to explain cell-cell detachment forces. This was soon followed by the identification of cadherin adhesion receptors capable of mediating attractive forces between cells (31). On the experimental side, various biomimetic systems were established that allowed controlling the identity, density, and mobility of adhesion molecules on surfaces. Specifically, giant vesicles and planar membranes decorated with adhesion molecules (attractive forces) and polymer cushions (repellent forces—inspired by glycocalyx) were employed to mimic interactions between two cells (32). On the theoretical side, various frameworks were developed to explain different aspects of adhesion in those biomimetic settings. They showed that the distance of an adhering vesicle to the contacting membrane is determined by the minimum of the free adhesion energy (11,12). At high receptor concentrations, contacts formed a homogenous tight adhesion zone, whereas at low receptor concentrations, contacts were composed of tight adhesion domains conferring strong adhesion separated by weak adhesion domains containing glycocalyx, corresponding to two minima of the free energy (33). Using a thermodynamic framework in which the adhesion energy depends on both the gain of enthalpy by the formation of bonds and the cost of entropy through the immobilization of receptors and suppression of membrane fluctuations, adhesion domains were predicted to preferentially localize to the rim of vesicle-bilayer contacts (34). This configuration is a result of bond dynamics, receptor crowding, and slowed-down diffusion upon adhesion molecule binding. These predictions were subsequently confirmed by experimental observations in a physiological context showing that cadherin adhesion molecules preferentially accumulate at the rim of cell-cell contacts (Fig. 2 A; (35,36)).
Biomimetic studies were also crucial for unraveling the role of cadherin clustering and mobility in cell-cell adhesion. Cadherins are known to form nanoclusters, which increase the cooperativity and stability of those molecules (37). Cadherin clustering depends on cis interactions of cadherins within the same cell and does not necessarily require cadherin trans binding given that cadherin ectodomains can form those clusters without engaging in trans interactions over the contact (21). Changing the ability of cadherins to engage in cis clustering through membrane fluctuations was further found to influence their ability to form trans bonds, which are required for nucleation and growth of adhesion domains in model membranes (38). In a cellular context, intracellular interactions of cadherin nanoclusters with the cortical actomyosin network were shown to be critical for cadherin-mediated contact formation by decreasing the mobility of those clusters within the membrane (39). Yet, biomimetic studies predicted that some mobility of adhesion receptors is still required to form stronger contacts by allowing diffusion of those receptors into the contact zone and thus increasing their likelihood to participate in bond formation (33).
Finally, through biomimetic, single-molecule, and cell culture studies, the sensitivity of adhesion molecules to mechanical forces was shown to be a critical determinant of cell-cell adhesion strength. In contacts between bilayers and vesicles carrying mobile adhesion proteins, adhesion sites were found to enlarge and become more immobile in response to a pulling force at the contact as a result of the acquisition of new bonds at edges of already-dense sites or condensation of existing bonds (33). In addition to those general effects on adhesion site assembly, mechanical forces also affect the bonds between individual adhesion receptors. Typically, molecular interactions between adhesion receptors are studied by atomic force microscopy at the millisecond timescale, which is well below the timescale of molecular off-rates at which bond dissociation occurs even if no external force is applied (30). Atomic force microscopy measurements of cadherin bonds revealed that detachment forces between cadherins typically range from a few tens to hundreds of pN (40) and that the bond strength of cadherins depends on the type of cadherin and its specific off-rate. The analysis of detachment forces further showed that cadherin molecules preferably form homotypic bonds, with, for instance, homotypic E-cadherin bonds being stronger than homotypic N-cadherin bonds (41). Moreover, cadherin bonds also become more resistant to detachment with increased loading, a phenomenon explained by cadherin ectodomains forming X-dimers that function as catch bonds (42), increasing bond lifetime as a function of pulling force (43,44).
Collectively, biomimetic studies using model membranes and vesicles, together with single-molecule studies probing the characteristics of adhesion molecules, paved the way for understanding the molecular and physical processes by which cell-cell contacts are initiated and maintained. In particular, they provided insight into the role of several cell structures and processes, such as the glycocalyx and membrane fluctuations, for cell-cell contact formation, which is still difficult to rigorously address in a more physiological cell setting. By stepwise increasing the complexity of biomimetic assays—e.g., by encapsulating cytoskeletal components within vesicles to study the interaction between adhesion molecules and the cytoskeleton—those reconstituted systems might become even more powerful and provide a platform for systematically analyzing cell-cell adhesion independently from the specific features of entire cells, tissues, or organisms.
The role of intercellular forces arising at the contact
In the following section, we discuss how experimental and theoretical studies of cell-cell adhesion forces on the cellular scale provided insight into the role of cell mechanics in cell-cell adhesion and contact formation. It is well established that most biological tissues are viscoelastic, behaving predominantly elastic at short timescales and viscous at long timescales (45). Consequently, cells have been modeled as solid elastic spheres or viscous liquid droplets depending on the specific cellular process studied. Assuming that the contacting cells behave as solid elastic spheres able to establish short-interaction-range adhesion, the Johnson-Kendall-Roberts (JKR) model used in polymer adhesion was applied to describe cell-cell contact detachment. The model permits the adhesion energy to be determined based on the pulling force needed to detach two spherical objects and their harmonic mean radii. For measuring detachment forces between contacting cells in the nN range, the dual pipette aspiration (DPA) technique is most commonly used (Fig. 2 A; (46)). Interestingly, the detachment force measured by DPA for nonspecific adhesion between culture cells displaying high elasticity could be well explained using the JKR model (47). However, for other cell types that display lower elasticity, only an extended version of the JKR model, in which cells are represented as thin shells with liquid cores that could be deformed as pulling forces were applied, was able to recapitulate experimental data (48,49).
The advantage of those coarse-grained theoretical models of cell detachment forces over the molecular-interaction-based theoretical models described in the previous chapter is the inclusion of the mechanical properties of cells. However, a caveat of taking detachment forces as a proxy for adhesion energy is the observation that cells can respond to mechanical forces by modulating their adhesion apparatus and thus adhesive properties. For instance, pulling on the contact zone increases E-cadherin and actin recruitment (Fig. 2 A; (50)), and applied forces can alter the mechanical properties of the cell cytoskeleton (51). Given that the detachment forces are thought to depend on mechanical properties of the actomyosin cortex of the adhering cells, such as its thickness, stiffness, and contractility (52); the equilibrium adhesion energy would be expected to change when detachment forces are applied.
The linkage of cadherins to the actomyosin cortex plays a central role for mechanosensation at cell-cell contacts (Fig. 1; (10)). Anchorage of cadherins to the actomyosin cortex is mediated by various molecules, including β-catenin, ɑ-catenin, and vinculin, and strengthens under force, a behavior characteristic of catch bonds (53). Specifically, whereas a single β-catenin/ɑ-catenin heterodimer forms a slip bond with F-actin, cooperativity of several heterodimers results in a catch-bond behavior (54). This is due to several β-catenin/ɑ-catenin heterodimers mediating longer-lasting contacts with F-actin, thereby allowing the tension-mediated unfolding of ɑ-catenin (55), which in turn reveals cryptic binding sites to vinculin, a molecule directly linking the cadherin/catenin complex to the actin cytoskeleton (56). This internal amplification mechanism, together with the observation that vinculin itself forms a catch bond with F-actin (57), provides an explanation for the mechanosensitivity of cadherin-mediated cell-cell contact sites.
Measured cell-cell detachment forces not only might change because of mechanosensitive feedback but also are dependent on the main direction of forces applied to the contact (normal or shear forces). Recent work suggests the direction of force to have different effects on cell-cell contacts: during Drosophila embryonic axis elongation, normal forces on cell-cell junctions, exerted by a medial actomyosin network within the apex of epithelial cells, increase E-cadherin levels and thus cell-cell adhesion, whereas shear forces through a junctional actomyosin network decrease E-cadherin levels (58). Such differential effects of normal versus shear forces might explain why detachment forces can vary depending on the specific measurement methods used, such as centrifugation, shear flow, or DPA.
In addition to cell-cell detachment force measurements, intercellular forces were determined by measuring traction forces of adhering cells through traction force microscopy (59) and micropillar arrays (60), both of which allow the extraction of intercellular forces on the basis of the two-dimensional force balance (61,62). Those intercellular forces were found to positively correlate with cadherin levels at cell-cell contacts (63). Likewise, for endothelial cell doublets on a defined spreading area, intercellular forces linearly increased with cell-cell contact size (62). In contrast, epithelial cells grown on a free spreading area showed no apparent scaling between intercellular forces and cell-cell contact size (61), suggesting that the relation of contact size and intercellular forces is highly context dependent.
The analysis of traction forces might also give important insights into the interplay between cell-cell and cell-matrix adhesions. In migrating cell clusters, traction forces dominate at the edge (64,65) and intercellular stresses increase toward the center of the cluster as a result of traction forces of the outwardly moving cells being transmitted as intercellular forces to the trailing cells behind (66,67). Recently, the interplay between cell-cell-adhesion-mediated intercellular and cell-ECM-adhesion-mediated intracellular tension was found to be responsible for cell monolayers displaying either contractile or extensile behavior (68), suggesting that the nature of active forces in tissues depends on the cross talk between cell-cell and cell-ECM adhesion. In line with this, knockout of E-cadherin in epithelial cells caused a crossover from extensile to contractile tissue behavior along with relocalization of vinculin from cell-cell to cell-ECM contacts and an increase in cell-ECM adhesion (68). Thus, the strength of cell-cell adhesion—and, with that, the tissue behavior—strongly depends on the interactions with the extracellular environment and the adaptation of intracellular contractility.
Collectively, the analysis of cell-cell detachment forces was instrumental in identifying the adhesion energy and thus cell-intrinsic adhesion of adherent cells when separated. However, to understand the discrepancies in the adhesive behavior of different cell types, more parametric tests and models need to be developed to incorporate effects of cell viscoelasticity, contractility, and adhesion receptor mobility. In particular, changes in the distribution of adhesion molecules at heterogeneous cell-cell contact sites and the effect of cytoskeletal rearrangements that occur upon force application need to be quantified and incorporated in future computational models. Finally, the observation that intracellular bonds, linking the adhesion complex to the actomyosin cytoskeleton, break first when cell-cell contacts are being separated suggests that deadhesion and adhesion energies might be different (18,29). Current models of cell-cell detachment, however, do not distinguish between the two. In line with this, recent observations showed that experimentally measured detachment forces are higher than theoretically predicted on the basis of the adhesion energy, pointing at the possibility that cell-cell detachment forces might depend more on dissipative processes associated with the detachment process rather than the adhesion energy (69). Emerging tools for determining cell-cell adhesion forces, such as Förster resonance energy transfer sensors to measure endogenous molecular forces (70,71), DNA-based fluorescent force probes (72), oil droplets decorated with cadherin receptor ligands (73), and pressure probes that deform with local stresses (74), might lead to a deeper understanding of intercellular adhesion.
The role of interfacial tension in cell aggregates
In analogy to liquids minimizing their surface area through surface tension as a result of the cohesion of their constituent molecules, the surface tension of cells and tissues is used as a proxy for cell-cell adhesion strength. In the following section, we discuss how cell-cell adhesion can be interpreted by the extent of surface tension, how surface tension is determined by tensions at different cellular interfaces, and how those surface/interfacial tensions were used in various models explaining cell/tissue shape changes and cell sorting. Originally, tissue surface tension was assumed to be determined by the adhesion energy, for instance, emerging from cadherin binding over the contact, a view supported with experiments in cell aggregates, which showed cadherin expression levels to linearly correlate with tissue surface tension (15,75). Subsequent work showed that, in addition or as an alternative to adhesion energy, tissue surface tension critically depends on the function of cortical actomyosin tension (17,59) and its modulation at cell-cell contacts (Fig. 3; (36,76)). Cortical tension is modulated not only by the binding of cadherin adhesion molecules over the contact (18) but also by unbound cadherins not engaged in trans binding, suggesting that a dynamic interplay between cadherins and the cortical actomyosin network determines the balance of interfacial tensions and thus surface tension of tissues (69).
At the cell-cell contact interface, interfacial tension is determined by both adhesion tension (a negative tension as a result of adhesion molecules binding over the contact), which expands the contact area, and cortical tension, which reduces it (Fig. 2 B). At contact-free interfaces, in contrast, surface tension is predominantly determined by cortical tension. Notably, cortical tension can differ at contact-free and adhering interfaces. Studies on zebrafish germ layer progenitor cells suggest that tissue surface tension arises from the difference between the two (77). This difference in tensions between the cell-cell versus contact-free interfaces is due to adhesion receptor signaling changing the actomyosin cortex, and thus cortical tension, at the cell-cell interface rather than adhesion tension lowering cell-cell interfacial tension (36). In line with adhesion receptors lowering cortical tension at the cell-cell contact are observations showing that E-cadherin-mutant mouse embryos fail in reducing myosin II from cell-cell contacts (78). Likewise, downregulation of C-cadherin in Xenopus embryonic aggregates prevents proper reduction of actin from contacts (79). This suggests that adhesion receptor signaling reduces cortical tension at contacts by both diminishing myosin II activity and/or localization and modifying cortical actin density and organization. The molecular composition of the signaling cascade downstream of cadherin adhesion receptors modulating the actomyosin cortex is not yet entirely clear. The actin-severing protein cofilin was found to colocalize with E-cadherin at punctate adherens junctions (80), whereas other studies reported that interaction of the cadherin adhesion complex through ɑ-E-catenin with actin inhibits cofilin binding in vitro (81). Similarly, the branched actin nucleator Arp2/3 was proposed to be not only suppressed at nascent contacts through ɑ-E-catenin (81) but also recruited to cortical actin underlying cell-cell contacts (80,82). Moreover, the linear actin nucleator formin was shown to be recruited to adherens junctions by ɑ-E-catenin (83). These data suggest that cadherin adhesion receptors affect the cortical actin cytoskeleton by dynamically recruiting different types of actin nucleators, which could potentially control cortical tension by regulating actin filament length (84), and network density (85). Changes in cortical actin at cell-cell contacts might feed back on cortical myosin II recruitment given that, for instance, in mouse oocytes, cortical Arp2/3 enrichment leads not only to cortex thickening but also to myosin II depletion and, consequently, reduction in cortical tension (86).
The Rho family GTPases Rac, Cdc42, and RhoA play an important role in remodeling the actomyosin cortex at cell-cell adhesion sites. Rac, for instance, is transiently activated by cadherins at the edges of an expanding contact, leading to local activation of the Arp2/3 complex and thus branched actin polymerization (87,88). Activation of both Rac and Cdc42 were observed during the formation of cell aggregates, which contributed to the strengthening of cell-cell contacts (13). Cdc42 was also found to be involved in the initiation of cell-cell adhesion (89), possibly by promoting the formation of E-cadherin-containing filopodia, facilitating contact formation (90). RhoA is recruited to adherens junctions, where it activates cortical actomyosin contractility and recruits formins, promoting linear actin polymerization (91). At nascent contacts, in contrast, RhoA activity is inhibited by Rac, decreasing cortical actomyosin contraction and thus tension (88,92). Yet the exact spatiotemporal regulation and function of Rho family GTPases as signaling effectors of cadherin adhesion receptors in contact formation and maintenance remain to be fully explored.
To explain the effects of interfacial tension regulation by different effector mechanisms, several microscopic mechanical models based on energy minimization and interfacial tension balance were employed describing cell-cell contact dynamics both in vitro and in vivo. For instance, the cellular Potts model, in which each cell is defined as connected pixels, was developed to test the contribution of different levels of adhesion receptor expression in cell-sorting experiments and the role of cell motility therein (93). Later, cortical tension was added to this model to capture the role of differential cell cortical tension in cell sorting (94,95). To more realistically capture the dynamics of confluent tissues on a cellular scale, vertex models, in which cells are defined as polygons whose vertices can move with mechanical forces, were developed. Vertex models were successfully applied for describing various morphogenetic processes, such as boundary formation, epithelial buckling, and wound healing, because of their ability to capture specific cellular processes, such as cell shape changes, divisions, extrusions, and rearrangements, as well as viscoelastic cell properties (96). As a hybrid of vertex models and self-propelled particle models, Voronoi models were recently developed in which not vertices but cell centers are tracked (97). These models were able to incorporate single-cell motility, missing from the vertex models, and predict more diverse shape distributions (98) and cellular rearrangements (99). More recently, vertex and Voronoi models were also used to describe abrupt and drastic changes in tissue material properties that might resemble transitions in states of matter, commonly referred to as phase transitions (100, 101, 102). Interestingly, phase transitions in confluent tissues appear to correlate to a “cell shape index,” a quantity that describes the cell geometry (Fig. 3; (100)). The cell geometry is regulated by the competition between cell-cell adhesion energy and cortical tension. An increase in cell-cell adhesion and a decrease in cortical tension lead to a change in cell shape and in turn to a transition of the whole tissue from solid-like to fluid-like behavior in a process called “unjamming transition”. The unjamming transition is characterized by increased irregularity in cell shapes and reduced number of contacts with neighboring cells, allowing cellular rearrangements (100,102). Recent studies also suggest the unjamming transition to be dominated by cellular traction forces (103). At the level of cell-cell contacts, force-mediated ɑ-catenin clustering was found to trigger a fluid-to-solid phase transition, suggesting that changes in the composition of cadherin adhesion complexes can locally modulate rheological properties of the contact (54). Tissue-scale phase transitions were observed not only in cultures but also within the physiologically relevant context of the developing embryo (104, 105, 106, 107) and in disease-related processes such as wound healing (108) and tumor metastasis (109). Extension of existing vertex models (110) and application of new theoretical frameworks, such as rigidity percolation theory (111), were recently shown to accurately describe tissue phase transitions in nonconfluent embryonic tissues to understand these phenomena mechanistically.
So far, research on interfacial tensions of cells and tissues primarily focused on the role of adhesion tension and cortical tension in regulating interfacial tension. However, other factors might also be involved. Membrane tension, for instance, also contributes to surface tension, although its specific contribution is difficult to determine because the plasma membrane is mechanically coupled to the underlying actomyosin cortex through proteins mediating membrane-to-cortex attachment and thus is difficult to disentangle from cortical tension. Although membrane tension was shown to be typically much smaller than cortical tension, there is increasing evidence in different cell types, such as keratocytes, that suggests membrane tension still significantly contributes to the overall surface tension of those cells (112). In addition to membrane tension, high adhesion tension between dynamically cross-linking components of interacting glycocalices was recently proposed to contribute to tissue surface tension in systems such as chick embryos and various mammalian cell lines, in which surface tension clearly exceeds the theoretically expected values based on cadherin-mediated adhesion and cortical tension alone (113). Finally, external factors, such as the presence of ECM and the osmolarity of the interstitial fluid, were shown to affect interfacial tensions of cells and tissues. ECM interactions can contribute to cell sorting by regulating cell-ECM and cell-cell interfacial tensions in monolayers and surface tension in cell aggregates (68,114), whereas osmolarity was recently demonstrated as an important regulator of tissue surface tension by regulating membrane tension and cortical tension via changes in the internal cellular pressure and volume (115).
Beyond cadherins, comparably little is known about upstream regulators of cell/tissue interfacial tensions. Living tissues have a remarkably diverse cell surface proteome, suggesting that several other of those proteins might be involved in controlling interfacial tensions. For instance, the differential expression of proteins mediating cell repulsion, such as Eph-ephrin receptor-ligand pairs, or signaling receptors, such as leucine-rich repeat family receptors (including Toll-like receptors), were shown to mediate differences in cortical tension, which is important for boundary formation in developing vertebrate and invertebrate embryos (116,117). The potential role of those and many other cell surface proteins in regulating interfacial tensions in different model systems remains to be investigated.
Conclusions and Perspectives
Cell-cell adhesion has been studied for many decades by both biologists and physicists. In those studies, different views of adhesion emerged, which can be roughly categorized as 1) the affinity of molecular bonds, 2) a cohesive force supported by a force-sensing and force-transducing machinery, and 3) the modulation of interfacial tensions through adhesion receptor signaling. These different views are nonexclusive because they simply emphasize different functions of the adhesion apparatus that together define adhesion. In evolution, these different functions seem to have coevolved because, for instance, the core adhesion complex, consisting of cadherins and catenins that bind to F-actin, emerged together with the appearance of metazoans (118). Moreover, cadherins predating this complex already carry intracellular domains that can possibly interact with actin-binding proteins (119), suggesting that cadherin extracellular binding and intracellular signaling could have been directly adapted with the appearance of multicellularity.
Initially, the degree of cell-cell adhesion was thought to correspond to the adhesion strength of cell-cell contacts at steady state. However, observations of cell-cell contacts in their physiological context show that cell-cell adhesion is a rather dynamic process, with the duration and size of cell-cell contacts constantly changing. Contact size and duration represent critical parameters modulating not only the extent by which cells rearrange in cohesive tissues (120) but also the activity of various signaling pathways involved in cell fate specification in embryos (121,122). Recently, cell-cell contact dynamics were shown to be important parameters determining tissue material properties and the transitions between different material phases (45). How those dynamic cell-cell contact properties are regulated on a molecular and cellular scale have only begun to be understood. For example, the size of cell-cell contacts was originally thought to increase with the ratio of cortical tension at the contact-free to the cell-cell interfaces (36). Surprisingly, most recently, this view was challenged by showing that the relationship between cell-cell contact size and cortical tension of the contact-free cortex is nonmonotonic, reversing at high levels of cortical tension because of tension-mediated E-cadherin stabilization, which limits contact expansion (123). Further work is needed to elucidate the relationship between various features of cell-cell contacts to determine their multifaceted functions in multicellular settings.
Cell-cell adhesion is regulated through both intracellular and extracellular cues, possibly involving various feedback loops between them. For instance, myosin II activity was shown not only to increase cytoskeletal anchoring of cadherins (70) but also to slow down actin turnover, which affects E-cadherin mobility at the cell-cell contacts and thus contact expansion (35,123). In turn, the stability of cadherin clusters was shown to regulate actin turnover, suggesting a bidirectional coupling between actin and cadherin dynamics (80). Many questions remain as to the regulation and function of cell-cell adhesion. What distinguishes the adhesion apparatus from the cell cytoskeleton? Does cell-cell adhesion simply function as a molecular linker connecting the cytoskeleton of neighboring cells? That said, could the adhesion complex be regarded as a specialized cytoskeletal component needed for the assembly, dynamic regulation, and coordination of supracellular cytoskeletal networks? Would such supracellular cytoskeletal networks just represent a permutation of intracellular cytoskeletal networks, or would the addition of cell-cell adhesion sites provide emergent features that cannot be found in unconnected cytoskeletal networks? To answser those questions, synthetic approaches for engineering cell-cell contacts might be helpful because they would allow the systematic study of different properties of cell-cell contacts in the presence and absence of cytoskeletal anchoring. Likewise, theoretical models need to be developed to connect molecular-scale interactions and dynamics of adhesion and cytoskeletal molecules to tissue-scale functions of cell-cell adhesion, such as tissue morphogenesis and material properties (124).
Cell-cell adhesion is integral to the evolution of multicellularity. Studying cell-cell adhesion, therefore, provides the basis for understanding how multicellularity has emerged. Although in the past, cell-cell adhesion has been predominantly studied on the basis of the extracellular bindings of adhesion receptors and their affinity and strength, it becomes increasingly clear that the coupling of those receptors to the cytoskeleton is equally important. This highlights two essential and tightly intertwined functions of adhesion: providing selectivity in cellular interactions and regulating the mechanical and biochemical cross talk between neighboring cells. This naturally involves both biochemical and mechanical signals; thus, understanding their interaction through mechanosensation will be indispensable for elucidating the basis of cell-cell adhesion.
Acknowledgments
T.S. acknowledges funding by the research program “The Active Matter Physics of Collective Metastasis,” which is financed by the Dutch Research Council (NWO).
Editor: Stanislav Y. Shvartsman.
Footnotes
Feyza Nur Arslan and Julia Eckert contributed equally to this work.
References
- 1.Miller J.G. Living systems. II. The organism. Quad. Criminol. Clin. 1973;48:92–276. doi: 10.1086/407589. [DOI] [PubMed] [Google Scholar]
- 2.Turlier H., Maître J.-L. Mechanics of tissue compaction. Semin. Cell Dev. Biol. 2015;47–48:110–117. doi: 10.1016/j.semcdb.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecuit T., Lenne P.-F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 4.Collins C., Nelson W.J. Running with neighbors: coordinating cell migration and cell-cell adhesion. Curr. Opin. Cell Biol. 2015;36:62–70. doi: 10.1016/j.ceb.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berx G., van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect. Biol. 2009;1:a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Amraoui A., Petit C. Cadherins as targets for genetic diseases. Cold Spring Harb. Perspect. Biol. 2010;2:a003095. doi: 10.1101/cshperspect.a003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janiszewska M., Primi M.C., Izard T. Cell adhesion in cancer: beyond the migration of single cells. J. Biol. Chem. 2020;295:2495–2505. doi: 10.1074/jbc.REV119.007759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada S., Nelson W.J. Synapses: sites of cell recognition, adhesion, and functional specification. Annu. Rev. Biochem. 2007;76:267–294. doi: 10.1146/annurev.biochem.75.103004.142811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halbleib J.M., Nelson W.J. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 10.Huveneers S., de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J. Cell Sci. 2013;126:403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 11.Bell G.I., Dembo M., Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys. J. 1984;45:1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruinsma R., Behrisch A., Sackmann E. Adhesive switching of membranes: experiment and theory. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 2000;61:4253–4267. doi: 10.1103/physreve.61.4253. [DOI] [PubMed] [Google Scholar]
- 13.Chu Y.-S., Thomas W.A., Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J. Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charras G., Yap A.S. Tensile forces and mechanotransduction at cell-cell junctions. Curr. Biol. 2018;28:R445–R457. doi: 10.1016/j.cub.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg M.S. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 16.Harris A.K. Is cell sorting caused by differences in the work of intercellular adhesion? A critique of the Steinberg hypothesis. J. Theor. Biol. 1976;61:267–285. doi: 10.1016/0022-5193(76)90019-9. [DOI] [PubMed] [Google Scholar]
- 17.Brodland G.W. The differential interfacial tension hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J. Biomech. Eng. 2002;124:188–197. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- 18.Winklbauer R. Cell adhesion strength from cortical tension - an integration of concepts. J. Cell Sci. 2015;128:3687–3693. doi: 10.1242/jcs.174623. [DOI] [PubMed] [Google Scholar]
- 19.Zaidel-Bar R. Cadherin adhesome at a glance. J. Cell Sci. 2013;126:373–378. doi: 10.1242/jcs.111559. [DOI] [PubMed] [Google Scholar]
- 20.Brasch J., Harrison O.J., Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 2012;22:299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson C.J., Vu V.H., Schwartz D.K. Cadherin extracellular domain clustering in the absence of trans-interactions. J. Phys. Chem. Lett. 2019;10:4528–4534. doi: 10.1021/acs.jpclett.9b01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chugh P., Paluch E.K. The actin cortex at a glance. J. Cell Sci. 2018;131:jcs186254. doi: 10.1242/jcs.186254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 24.Truong-Quang B.-A., Lenne P.-F. Membrane microdomains: from seeing to understanding. Front Plant Sci. 2014;5:18. doi: 10.3389/fpls.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarris B.J., Fraser J.R. On the pericellular zone of some mammalian cells in vitro. Exp. Cell Res. 1968;49:181–193. doi: 10.1016/0014-4827(68)90530-2. [DOI] [PubMed] [Google Scholar]
- 26.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salbreux G., Charras G., Paluch E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012;22:536–545. doi: 10.1016/j.tcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. C. 1973;28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 29.Maître J.-L., Heisenberg C.-P. Three functions of cadherins in cell adhesion. Curr. Biol. 2013;23:R626–R633. doi: 10.1016/j.cub.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell G.I. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 31.Takeichi M., Atsumi T., Okada T.S. Selective adhesion of embryonal carcinoma cells and differentiated cells by Ca2+-dependent sites. Dev. Biol. 1981;87:340–350. doi: 10.1016/0012-1606(81)90157-3. [DOI] [PubMed] [Google Scholar]
- 32.Sackmann E. Supported membranes: scientific and practical applications. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 33.Smith A.-S., Sackmann E. Progress in mimetic studies of cell adhesion and the mechanosensing. ChemPhysChem. 2009;10:66–78. doi: 10.1002/cphc.200800683. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt D., Bihr T., Smith A.-S. Crowding of receptors induces ring-like adhesions in model membranes. Biochim. Biophys. Acta. 2015;1853:2984–2991. doi: 10.1016/j.bbamcr.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Engl W., Arasi B., Viasnoff V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat. Cell Biol. 2014;16:587–594. doi: 10.1038/ncb2973. [DOI] [PubMed] [Google Scholar]
- 36.Maître J.-L., Berthoumieux H., Heisenberg C.-P. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science. 2012;338:253–256. doi: 10.1126/science.1225399. [DOI] [PubMed] [Google Scholar]
- 37.Yap A.S., Gomez G.A., Parton R.G. Adherens junctions revisualized: organizing cadherins as nanoassemblies. Dev. Cell. 2015;35:12–20. doi: 10.1016/j.devcel.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Fenz S.F., Bihr T., Smith A.-S. Membrane fluctuations mediate lateral interaction between cadherin bonds. Nat. Phys. 2017;13:906–913. [Google Scholar]
- 39.Chandran R., Kale G., Mayor S. Distinct actin-dependent nanoscale assemblies underlie the dynamic and hierarchical organization of E-cadherin. Curr. Biol. 2021 doi: 10.1016/j.cub.2021.01.059. Published online February 12, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumgartner W., Hinterdorfer P., Drenckhahn D. Cadherin interaction probed by atomic force microscopy. Proc. Natl. Acad. Sci. USA. 2000;97:4005–4010. doi: 10.1073/pnas.070052697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panorchan P., Thompson M.S., Wirtz D. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J. Cell Sci. 2006;119:66–74. doi: 10.1242/jcs.02719. [DOI] [PubMed] [Google Scholar]
- 42.Dembo M., Torney D.C., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc. R. Soc. Lond. B Biol. Sci. 1988;234:55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- 43.Pittet P., Lee K., Hinz B. Fibrogenic fibroblasts increase intercellular adhesion strength by reinforcing individual OB-cadherin bonds. J. Cell Sci. 2008;121:877–886. doi: 10.1242/jcs.024877. [DOI] [PubMed] [Google Scholar]
- 44.Rakshit S., Zhang Y., Sivasankar S. Ideal, catch, and slip bonds in cadherin adhesion. Proc. Natl. Acad. Sci. USA. 2012;109:18815–18820. doi: 10.1073/pnas.1208349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petridou N.I., Heisenberg C.-P. Tissue rheology in embryonic organization. EMBO J. 2019;38:e102497. doi: 10.15252/embj.2019102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung K.L., Sung L.A., Chien S. Determination of junction avidity of cytolytic T cell and target cell. Science. 1986;234:1405–1408. doi: 10.1126/science.3491426. [DOI] [PubMed] [Google Scholar]
- 47.Chu Y.-S., Dufour S., Pincet F. Johnson-Kendall-Roberts theory applied to living cells. Phys. Rev. Lett. 2005;94:028102. doi: 10.1103/PhysRevLett.94.028102. [DOI] [PubMed] [Google Scholar]
- 48.Pierrat S., Brochard-Wyart F., Nassoy P. Enforced detachment of red blood cells adhering to surfaces: statics and dynamics. Biophys. J. 2004;87:2855–2869. doi: 10.1529/biophysj.104.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brochard-Wyart F., de Gennes P.-G. Unbinding of adhesive vesicles. C. R. Phys. 2003;4:281–287. [Google Scholar]
- 50.Gao X., Acharya B.R., Viasnoff V. Probing compression versus stretch activated recruitment of cortical actin and apical junction proteins using mechanical stimulations of suspended doublets. APL Bioeng. 2018;2:026111. doi: 10.1063/1.5025216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz U.S., Safran S.A. Physics of adherent cells. Rev. Mod. Phys. 2013;85:1327–1381. [Google Scholar]
- 52.Smeets B., Cuvelier M., Ramon H. The effect of cortical elasticity and active tension on cell adhesion mechanics. Biophys. J. 2019;116:930–937. doi: 10.1016/j.bpj.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buckley C.D., Tan J., Dunn A.R. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arbore C., Sergides M., Capitanio M. α-catenin switches between a slip and a cooperative catch bond with F-actin to regulate cell junction fluidity. bioRxiv. 2020 doi: 10.1101/2020.04.15.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yonemura S., Wada Y., Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 56.Huveneers S., Oldenburg J., de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J. Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang D.L., Bax N.A., Dunn A.R. Vinculin forms a directionally asymmetric catch bond with F-actin. Science. 2017;357:703–706. doi: 10.1126/science.aan2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kale G.R., Yang X., Lecuit T. Distinct contributions of tensile and shear stress on E-cadherin levels during morphogenesis. Nat. Commun. 2018;9:5021. doi: 10.1038/s41467-018-07448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris A.K., Stopak D., Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 60.Tan J.L., Tien J., Chen C.S. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maruthamuthu V., Sabass B., Gardel M.L. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc. Natl. Acad. Sci. USA. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Z., Tan J.L., Chen C.S. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. USA. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng M.R., Besser A., Danuser G. Mapping the dynamics of force transduction at cell-cell junctions of epithelial clusters. eLife. 2014;3:e03282. doi: 10.7554/eLife.03282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.du Roure O., Saez A., Ladoux B. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mertz A.F., Che Y., Horsley V. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc. Natl. Acad. Sci. USA. 2013;110:842–847. doi: 10.1073/pnas.1217279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trepat X., Wasserman M.R., Fredberg J.J. Physical forces during collective cell migration. Nat. Phys. 2009;5:426–430. [Google Scholar]
- 67.Tambe D.T., Hardin C.C., Trepat X. Collective cell guidance by cooperative intercellular forces. Nat. Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balasubramaniam L., Doostmohammadi A., Ladoux B. Investigating the nature of active forces in tissues reveals how contractile cells can form extensile monolayers. Nat. Mater. 2021 doi: 10.1038/s41563-021-00919-2. Published online February 18, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aladin D.M.K., Chu Y.S., Thiery J.P. Extracellular domains of E-cadherin determine key mechanical phenotypes of an epithelium through cell- and non-cell-autonomous outside-in signalling. bioRxiv. 2020 doi: 10.1101/2020.03.17.996181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borghi N., Sorokina M., Dunn A.R. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc. Natl. Acad. Sci. USA. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conway D.E., Breckenridge M.T., Schwartz M.A. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao B., Li N., You M. Quantifying tensile forces at cell–cell junctions with a DNA-based fluorescent probe. Chem. Sci. (Camb.) 2020;11:8558–8566. doi: 10.1039/d0sc01455a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campàs O., Mammoto T., Ingber D.E. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods. 2014;11:183–189. doi: 10.1038/nmeth.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan C.J., Hiiragi T. Integration of luminal pressure and signalling in tissue self-organization. Development. 2020;147:dev181297. doi: 10.1242/dev.181297. [DOI] [PubMed] [Google Scholar]
- 75.Foty R.A., Steinberg M.S. The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 76.Youssef J., Nurse A.K., Morgan J.R. Quantification of the forces driving self-assembly of three-dimensional microtissues. Proc. Natl. Acad. Sci. USA. 2011;108:6993–6998. doi: 10.1073/pnas.1102559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manning M.L., Foty R.A., Schoetz E.-M. Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc. Natl. Acad. Sci. USA. 2010;107:12517–12522. doi: 10.1073/pnas.1003743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maître J.-L., Niwayama R., Hiiragi T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat. Cell Biol. 2015;17:849–855. doi: 10.1038/ncb3185. [DOI] [PubMed] [Google Scholar]
- 79.David R., Luu O., Winklbauer R. Tissue cohesion and the mechanics of cell rearrangement. Development. 2014;141:3672–3682. doi: 10.1242/dev.104315. [DOI] [PubMed] [Google Scholar]
- 80.Indra I., Troyanovsky R.B., Troyanovsky S.M. Sensing actin dynamics through adherens junctions. Cell Rep. 2020;30:2820–2833.e3. doi: 10.1016/j.celrep.2020.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen S.D., Kwiatkowski A.V., Nelson W.J. αE-catenin actin-binding domain alters actin filament conformation and regulates binding of nucleation and disassembly factors. Mol. Biol. Cell. 2013;24:3710–3720. doi: 10.1091/mbc.E13-07-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kovacs E.M., Goodwin M., Yap A.S. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 2002;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- 83.Kobielak A., Pasolli H.A., Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chugh P., Clark A.G., Paluch E.K. Actin cortex architecture regulates cell surface tension. Nat. Cell Biol. 2017;19:689–697. doi: 10.1038/ncb3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia S., Lim Y.B., Kanchanawong P. Nanoscale architecture of the cortical actin cytoskeleton in embryonic stem cells. Cell Rep. 2019;28:1251–1267.e7. doi: 10.1016/j.celrep.2019.06.089. [DOI] [PubMed] [Google Scholar]
- 86.Chaigne A., Campillo C., Terret M.E. A narrow window of cortical tension guides asymmetric spindle positioning in the mouse oocyte. Nat. Commun. 2015;6:6027. doi: 10.1038/ncomms7027. [DOI] [PubMed] [Google Scholar]
- 87.Kitt K.N., Nelson W.J. Rapid suppression of activated Rac1 by cadherins and nectins during de novo cell-cell adhesion. PLoS One. 2011;6:e17841. doi: 10.1371/journal.pone.0017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamada S., Nelson W.J. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Collins C., Denisin A.K., Nelson W.J. Changes in E-cadherin rigidity sensing regulate cell adhesion. Proc. Natl. Acad. Sci. USA. 2017;114:E5835–E5844. doi: 10.1073/pnas.1618676114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biswas K.H., Hartman K.L., Groves J.T. E-cadherin junction formation involves an active kinetic nucleation process. Proc. Natl. Acad. Sci. USA. 2015;112:10932–10937. doi: 10.1073/pnas.1513775112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Acharya B.R., Wu S.K., Yap A.S. Mammalian diaphanous 1 mediates a pathway for E-cadherin to stabilize epithelial barriers through junctional contractility. Cell Rep. 2017;18:2854–2867. doi: 10.1016/j.celrep.2017.02.078. [DOI] [PubMed] [Google Scholar]
- 92.Wildenberg G.A., Dohn M.R., Reynolds A.B. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 93.Glazier J.A., Graner F. Simulation of the differential adhesion driven rearrangement of biological cells. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 1993;47:2128–2154. doi: 10.1103/physreve.47.2128. [DOI] [PubMed] [Google Scholar]
- 94.Krieg M., Arboleda-Estudillo Y., Heisenberg C.-P. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 95.Glazier J.A., Balter A., Popławski N.J. In: Single-Cell-Based Models in Biology and Medicine. Anderson A.R.A., Chaplain M.A.J., Rejniak K.A., editors. Birkhäuser; 2007. Magnetization to morphogenesis: a brief history of the Glazier-Graner-Hogeweg model; pp. 79–106. [Google Scholar]
- 96.Alt S., Ganguly P., Salbreux G. Vertex models: from cell mechanics to tissue morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372:20150520. doi: 10.1098/rstb.2015.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bi D., Yang X., Manning M.L. Motility-driven glass and jamming transitions in biological tissues. Phys. Rev. X. 2016;6:021011. doi: 10.1103/PhysRevX.6.021011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sánchez-Gutiérrez D., Tozluoglu M., Escudero L.M. Fundamental physical cellular constraints drive self-organization of tissues. EMBO J. 2016;35:77–88. doi: 10.15252/embj.201592374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barton D.L., Henkes S., Sknepnek R. Active vertex model for cell-resolution description of epithelial tissue mechanics. PLoS Comput. Biol. 2017;13:e1005569. doi: 10.1371/journal.pcbi.1005569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bi D., Lopez J.H., Manning M.L. A density-independent rigidity transition in biological tissues. Nat. Phys. 2015;11:1074–1079. [Google Scholar]
- 101.Farhadifar R., Röper J.-C., Jülicher F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 2007;17:2095–2104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 102.Park J.-A., Kim J.H., Fredberg J.J. Unjamming and cell shape in the asthmatic airway epithelium. Nat. Mater. 2015;14:1040–1048. doi: 10.1038/nmat4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saraswathibhatla A., Notbohm J. Tractions and stress fibers control cell shape and rearrangements in collective cell migration. Phys. Rev. X. 2020;10:011016. [Google Scholar]
- 104.Petridou N.I., Grigolon S., Heisenberg C.-P. Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling. Nat. Cell Biol. 2019;21:169–178. doi: 10.1038/s41556-018-0247-4. [DOI] [PubMed] [Google Scholar]
- 105.Mongera A., Rowghanian P., Campàs O. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature. 2018;561:401–405. doi: 10.1038/s41586-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jain A., Ulman V., Pavlopoulos A. Regionalized tissue fluidization is required for epithelial gap closure during insect gastrulation. Nat. Commun. 2020;11:5604. doi: 10.1038/s41467-020-19356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saadaoui M., Rocancourt D., Gros J. A tensile ring drives tissue flows to shape the gastrulating amniote embryo. Science. 2020;367:453–458. doi: 10.1126/science.aaw1965. [DOI] [PubMed] [Google Scholar]
- 108.Tetley R.J., Staddon M.F., Mao Y. Tissue fluidity promotes epithelial wound healing. Nat. Phys. 2019;15:1195–1203. doi: 10.1038/s41567-019-0618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oswald L., Grosser S., Käs J.A. Jamming transitions in cancer. J. Phys. D Appl. Phys. 2017;50:483001. doi: 10.1088/1361-6463/aa8e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim S., Pochitaloff M., Campàs O. Embryonic tissues as active foams. bioRxiv. 2020 doi: 10.1101/2020.06.17.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petridou N.I., Corominas-Murtra B., Hannezo E. Rigidity percolation uncovers a structural basis for embryonic tissue phase transitions. Cell. 2021;184:1914–1928. doi: 10.1016/j.cell.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sens P., Plastino J. Membrane tension and cytoskeleton organization in cell motility. J. Phys. Condens. Matter. 2015;27:273103. doi: 10.1088/0953-8984/27/27/273103. [DOI] [PubMed] [Google Scholar]
- 113.Winklbauer R. Dynamic cell-cell adhesion mediated by pericellular matrix interaction - a hypothesis. J. Cell Sci. 2019;132:jcs231597. doi: 10.1242/jcs.231597. [DOI] [PubMed] [Google Scholar]
- 114.Caicedo-Carvajal C.E., Shinbrot T., Foty R.A. α5β1 integrin-fibronectin interactions specify liquid to solid phase transition of 3D cellular aggregates. PLoS One. 2010;5:e11830. doi: 10.1371/journal.pone.0011830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gabrie Krens S.F., Veldhuis J.H., Heisenberg C.-P. Interstitial fluid osmolarity modulates the action of differential tissue surface tension in progenitor cell segregation during gastrulation. Development. 2017;144:1798–1806. doi: 10.1242/dev.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fagotto F. Tissue segregation in the early vertebrate embryo. Semin. Cell Dev. Biol. 2020;107:130–146. doi: 10.1016/j.semcdb.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 117.Sharrock T.E., Sanson B. Cell sorting and morphogenesis in early Drosophila embryos. Semin. Cell Dev. Biol. 2020;107:147–160. doi: 10.1016/j.semcdb.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 118.Miller P.W., Clarke D.N., Nelson W.J. The evolutionary origin of epithelial cell-cell adhesion mechanisms. Curr. Top. Membr. 2013;72:267–311. doi: 10.1016/B978-0-12-417027-8.00008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abedin M., King N. The premetazoan ancestry of cadherins. Science. 2008;319:946–948. doi: 10.1126/science.1151084. [DOI] [PubMed] [Google Scholar]
- 120.Guirao B., Bellaïche Y. Biomechanics of cell rearrangements in Drosophila. Curr. Opin. Cell Biol. 2017;48:113–124. doi: 10.1016/j.ceb.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Barone V., Lang M., Heisenberg C.-P. An effective feedback loop between cell-cell contact duration and morphogen signaling determines cell fate. Dev. Cell. 2017;43:198–211.e12. doi: 10.1016/j.devcel.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 122.Shaya O., Binshtok U., Sprinzak D. Cell-cell contact area affects notch signaling and notch-dependent patterning. Dev. Cell. 2017;40:505–511.e6. doi: 10.1016/j.devcel.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Slováková J., Sikora M., Heisenberg C.-P. Tension-dependent stabilization of E-cadherin limits cell-cell contact expansion. bioRxiv. 2020 doi: 10.1101/2020.11.20.391284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lenne P.-F., Rupprecht J.-F., Viasnoff V. Cell junction mechanics beyond the bounds of adhesion and tension. Dev. Cell. 2021;56:202–212. doi: 10.1016/j.devcel.2020.12.018. [DOI] [PubMed] [Google Scholar]