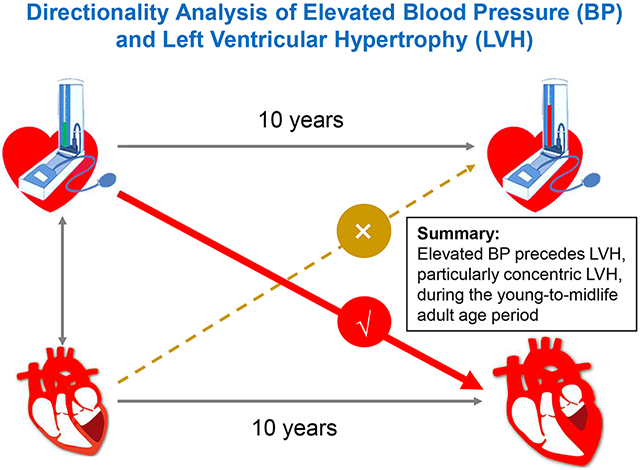
Abstract
This study assessed the temporal relationship of elevated blood pressure (BP) with left ventricular hypertrophy (LVH) and geometric changes in a longitudinal cohort of adults. Left ventricular mass index (LVMI), relative wall thickness (RWT) and BP were measured at two time points 4.1-14.9 years apart between 2000 and 2016 among 984 adults (677 Whites and 307 Blacks, 41.1% males, age range=24.2-56.7 years) in the Bogalusa Heart Study cohort. Cross-lagged path analysis models were used to examine the temporal relationship of BP with LVMI and RWT in subjects who did not take antihypertensive medications (n=693). The cross-lagged path coefficients did not differ significantly between race and sex groups. In the combined sample, the path coefficients from baseline systolic BP to follow-up LVMI/RWT were significantly greater than the path coefficients from baseline LVMI/RWT to follow-up systolic BP (0.111 vs −0.005 for LVMI, p=0.010 for difference; 0.146 vs 0.004 for RWT, p=0.002 for difference). Hypertensive subjects at baseline had a significantly higher incidence rate of concentric LVH at follow-up compared to normotensive subjects (19.4% vs 9.7%, p<0.001 for difference), but incident eccentric LVH did not show such a difference between hypertensive and normotensive subjects (5.4% vs 4.4%, p=0.503 for difference). Diastolic BP showed similar results to those of systolic BP. In conclusion, the findings on these one-directional paths provide strong and fresh evidence that elevated BP precedes the development of LVH, especially concentric LVH, during the young-to-midlife adult age period.
Keywords: blood pressure, left ventricular mass, geometric remodeling, temporal relationship, longitudinal analysis
Graphical Abstract
INTRODUCTION
Left ventricular hypertrophy (LVH) manifested as an enlarged chamber and thickened walls is an independent predictor of adverse cardiovascular events such as heart failure, ischemic heart disease and sudden cardiac arrest (1,2). Among cardiovascular risk factors, hypertension is considered as the most harmful determinant for the development of LVH in the general population (3–6), especially concentric LVH (5–7). There are also longitudinal epidemiologic studies showing that baseline LVH leads to subsequent incident hypertension (8–10).
The strong association between elevated blood pressure (BP) levels and LVH suggests that this relationship is likely bidirectional based on cardiac and vascular hemodynamic mechanisms. The concept that elevated BP increases left ventricular mass (LVM) leading to LVH and left ventricular geometric changes has been well established (3–7). Elevated BP levels play a key role in this process through chronic hemodynamic burden and central pressure overload (11). On the other hand, cardiac mechanics, including increased cardiac output and central blood volume, accelerates the rate of progression in systolic BP levels (12–14). These bi-directional relations may perpetuate a vicious cycle of accelerated hypertension by LVH and in turn further increases in LVM. The interaction between hypertension and LVH suggests that elevated BP and increased LVM influence each other in a bidirectional manner during human development and aging process (15). To date, this temporal relationship is inadequately understood. In particular, it is largely unknown whether the answer to this “chicken-egg” question is different by age periods of early life, young- and mid-adulthood, and older age during the development of hypertension and LVH.
In the present study we aimed to examine the temporal relationship of elevated BP with LVH and geometric changes during the age period from young adulthood to midlife. This aim was achieved by leveraging a longitudinal cohort of adults in the Bogalusa Heart Study.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. Part of the Bogalusa Heart Study data are publicly available at https://biolincc.nhlbi.nih.gov/studies/bhs.
Study Cohort
The Bogalusa Heart Study is a series of long-term epidemiologic studies in a semi-rural biracial (65% white and 35% black) community in Bogalusa, Louisiana. It focuses on the early natural history of cardiovascular disease and risk factors from childhood (16). In the community of Bogalusa, 984 adults (677 Whites and 307 Blacks, 41.1% males, age range=24.2-56.7 years) were examined twice 4.1-14.9 years apart for LVM, relative wall thickness (RWT) and cardiovascular risk factors in the 2000-2004 baseline and 2008-2016 follow-up surveys.
All subjects in this study gave informed consent for each survey. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
General Examinations
Standardized protocols were used by trained staff members in all surveys (16). Height and weight were measured in duplicate, and the mean values were used for analysis. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were obtained on right arm in a relaxed sitting position after a rest of 5 minutes by 2 trained observers (3 times each) between 8:00 AM and 10:00 AM. The mean values of the 6 readings were used for analysis. BP was measured using mercury sphygmomanometers, and the first and fifth Korotkoff phases were used to determine SBP and DBP, respectively, in the two baseline surveys and the 2008-2010 follow-up survey. Omron HEM-907XL oscillometric BP monitor (Omron Healthcare, Japan) was used to measure BP in the last 2013-16 follow-up survey. The accuracy of this electronic BP device has been extensively assessed in many studies (17,18). In this study cohort, the difference in BP readings by Omron HEM–907XL (n=505) and mercury BP device (n=479) in the follow-up surveys was not significant for SBP (p=0.738) and DBP (p=0.280), with adjustment for age, race, sex, BMI, heart rate, smoking and alcohol drinking; the difference in the annual rate of change in BP was not significant either for SBP (p=0.817) and DBP (p=0.302). Hypertension was defined as SBP/DBP>=130/80 mmHg or use of anti-hypertensive medications.
Echocardiographic Measurements
Left ventricular (LV) dimensions were assessed by 2-dimensional guided M-mode echocardiography with 2.25- and 3.5-MHz transducers according to American Society of Echocardiography recommendations (19). Parasternal long- and short-axis views were collected for measuring LV end-diastolic and end-systolic measurements in duplicate, and the means were calculated. LVM was calculated from a necropsy-validated formula on the basis of a thick-wall prolate ellipsoidal geometry (20). To take body size into account, LVM was indexed for body height (m2.7) as LVM index (LVMI). RWT was calculated as septal wall thickness plus posterior wall thickness divided by LV end-diastolic diameter (21). The presence of LVH was defined by LVMI >46.7 g/m2.7 in women and >49.2 g/m2.7 in men; LV geometry was considered concentric when RWT>0.42 (22). Four patterns of LV geometry were defined: 1) normal LV geometry (normal RWT with no LVH), 2) concentric remodeling (CR, increased RWT but no LVH), 3) eccentric hypertrophy (EH, normal RWT with LVH), and 4) concentric hypertrophy (CH, increased RWT with LVH) (21–24).
Statistical Analysis
The longitudinal changes in BP and LVM measured at multiple time points can be modeled using a cross-lagged panel design. The cross-lagged panel analysis is a form of path analysis that simultaneously examines reciprocal, longitudinal relationships among inter-correlated variables (25–27). A simplified, conceptual version of the model used in the current analysis is presented in figures and tables below. The path with ρ1 describes the effect of baseline LVMI/RWT on subsequent BP, and the path with ρ2 describes the effect of baseline BP on subsequent LVMI/RWT. Prior to cross-lagged path analysis, the baseline and follow-up values of BP, LVMI and RWT were adjusted for age, BMI, heart rate, smoking and alcohol drinking by regression residual analyses and then standardized with Z-transformation (mean=0, SD=1) by race and sex groups. Pearson correlation coefficients of the Z-transformed quantitative variables of BP, LVMI and RWT at baseline and follow-up were calculated, adjusted for follow-up years. The cross-lagged path coefficients (ρ1 and ρ2) were estimated simultaneously based on the correlation matrix using the maximum likelihood method by the program LISREL version 8.52 (28). The validity of model fitting was indicated by root mean square residual (RMR) and comparative fit index (CFI). A significant path coefficient (ρ1 or ρ2) suggests their respective directionality of the pathways. We also performed stratified analyses by race, sex and follow-up duration groups. The difference between subgroups in ρ1 and ρ2 derived from the standardized variables (Z-scores) was tested using Fisher’s Z-test as described in our previous study (29).
RESULTS
Table 1 summarizes descriptive data of study variables at baseline and follow-up by race and sex. The mean levels of continuous variables were compared between subgroups, adjusting for age (except age itself). Most variables at baseline and follow-up differed significantly between sexes; BP and hypertension showed significant race differences; BMI had significant race differences (Blacks>Whites) in females.
Table 1.
Descriptive data of the longitudinal cohort by race and sex
| Variable | White |
Black |
P for Race Difference |
|||
|---|---|---|---|---|---|---|
| Male (N=285) | Female (N=392) | Male (N=119) | Female (N=188) | Male | Female | |
| Baseline | ||||||
| Age (year) | 36.2 (4.8) | 35.4 (4.6)* | 36.7 (4.6) | 35.2 (5.0)* | 0.285 | 0.603 |
| Smokers, n (%) | 78 (27.4) | 97 (24.7) | 51 (42.9) | 62 (33.0) | 0.002 | 0.038 |
| Drinkers, n (%) | 95 (33.3) | 69 (17.6)* | 75 (63.0) | 44 (23.4)* | <0.001 | 0.099 |
| BMI (kg/m2) | 28.6 (5.3) | 27.6 (6.7) | 28.8 (7.0) | 31.7 (8.5)* | 0.804 | <0.001 |
| Heart rate (beat/min) | 67.6 (8.2) | 70.9 (9.1)* | 70.2 (9.7) | 71.2 (10.1) | 0.007 | 0.702 |
| SBP (mmHg) † | 114 (9) | 108 (9)* | 120 (14) | 114 (12)* | <0.001 | <0.001 |
| DBP (mmHg) † | 78 (7) | 72 (7)* | 79 (10) | 76 (9)* | <0.001 | <0.001 |
| Hypertension, n (%) ‡ | 135 (47.4) | 94 (24.0)* | 76 (63.9) | 78 (41.5)* | 0.003 | <0.001 |
| Follow-up | ||||||
| Age (year) | 46.7 (5.3) | 46.2 (5.3) | 46.5 (5.4) | 45.7 (5.6) | 0.622 | 0.391 |
| Smokers, n (%) | 66 (23.2) | 99 (25.3) | 50 (42.0) | 48 (25.5)* | <0.001 | 0.943 |
| Drinkers, n (%) | 115 (40.4) | 120 (30.6)* | 55 (46.2) | 42 (22.3)* | 0.276 | 0.038 |
| BMI (kg/m2) | 30.3 (5.7) | 29.7 (7.2) | 30.4 (7.6) | 33.8 (8.6)* | 0.963 | <0.001 |
| Heart rate (beat/min) | 68.7 (10.0) | 72.4 (9.5)* | 72.0 (10.1) | 72.3 (10.5) | 0.003 | 0.959 |
| SBP (mmHg) † | 121 (12) | 115 (13)* | 127 (16) | 119 (17)* | <0.001 | <0.001 |
| DBP (mmHg) † | 81 (8) | 76 (9)* | 83 (10) | 79 (11)* | 0.001 | <0.001 |
| Hypertension, n (%) ‡ | 198 (69.5) | 188 (48.0)* | 96 (80.7) | 135 (71.8) | 0.021 | <0.001 |
Data are presented in means (SD) or n (%).
Sex difference within racial groups:
p<0.05
BMI=body mass index; SBP=systolic blood pressure; DBP=diastolic blood pressure
, BP in participants who did not take anti-hypertensive medications
, Hypertension was defined as SBP/DBP>=130/80 mmHg or taking anti-hypertensive medications.
In Table 2, LVM had significant sex difference in both races, and LVMI had significant sex difference only in Whites at baseline and follow-up. Significant race differences in females were noted for LVM, LVMI, RWT and prevalence of LVH at baseline, and greater race differences were noted for both sexes in these variables at follow-up. Prevalence of CH at baseline and follow-up differed significantly between races in both males and females. Among 881 participants who did not have LVH at baseline, incidence rates at follow-up were 12.9% in Whites and 29.9% in Blacks (p<0.001 for difference) for LVH, 4.2% in Whites and 6.1% in Blacks (p=0.218 for difference) for EH, and 8.7% in Whites and 23.8% in Blacks (p<0.001 for difference) for CH.
Table 2.
Left ventricular measures of the longitudinal cohort by race and sex
| Variable | White |
Black |
P for Race Difference |
|||
|---|---|---|---|---|---|---|
| Male (N=285) | Female (N=392) | Male (N=119) | Female (N=188) | Male | Female | |
| Baseline | ||||||
| Age (year) | 36.2 (4.8) | 35.4 (4.6)* | 36.7 (4.6) | 35.2 (5.0)* | 0.285 | 0.603 |
| LVM (g) | 157 (44) | 113 (36)* | 156 (51) | 120 (41)* | 0. 695 | <0.001 |
| LVMI (g/m2.7) | 33.0 (9.0) | 29.9 (9.0)* | 33.8 (10.9) | 32.3 (10.2) | 0.260 | <0.001 |
| RWT (cm) | 0.33 (0.07) | 0.31 (0.06) | 0.34 (0.07) | 0.34 (0.08) | 0.040 | 0.001 |
| LVH, n (%) | 27 (9.5) | 30 (7.7) | 15 (12.6) | 31 (16.5) | 0.347 | 0.001 |
| CR, n (%) | 26 (9.1) | 20 (5.1)* | 11 (9.2) | 16 (8.5) | 0.969 | 0.111 |
| EH, n (%) | 23 (8.1) | 22 (5.6) | 8 (6.7) | 19 (10.1) | 0.643 | 0.048 |
| CH, n (%) | 4 (1.4) | 8 (2.0) | 7 (5.9) | 12 (6.4) | 0.012 | 0.007 |
| Follow-up | ||||||
| Age (year) | 46.7 (5.3) | 46.2 (5.3) | 46.5 (5.4) | 45.7 (5.6) | 0.622 | 0.391 |
| LVM (g) | 184 (49) | 138 (50)* | 205 (76) | 144 (42)* | 0.001 | <0.001 |
| LVMI (g/m2.7) | 38.9 (9.8) | 36.8 (14.1)* | 44.6 (16.4) | 38.5 (10.4) | <0.001 | <0.001 |
| RWT (cm) | 0.41 (0.07) | 0.41 (0.08) | 0.43 (0.07) | 0.41 (0.08) | 0.008 | 0.004 |
| LVH, n (%) | 51 (17.9) | 57 (14.5) | 41 (34.5) | 64 (34.0) | <0.001 | <0.001 |
| CR, n (%) | 86 (30.2) | 101 (25.8) | 35 (29.4) | 41 (21.8) | 0.879 | 0.300 |
| EH, n (%) | 19 (6.7) | 17 (4.3) | 8 (6.7) | 16 (8.5) | 0.984 | 0.042 |
| CH, n (%) | 32 (11.2) | 40 (10.2) | 33 (27.7) | 48 (25.5) | <0.001 | <0.001 |
Data are presented in means (SD) or n (%).
Sex difference within racial groups:
p<0.05
LVM(I)=left ventricular mass (index); RWT=relative wall thickness; LVH= left ventricular hypertrophy; CR=concentric remodeling; CH=concentric hypertrophy; EH=eccentric hypertrophy
Figure 1 provides detailed parameter information on the cross-lagged path analysis model of SBP with LVMI and RWT in subjects who did not take anti-hypertensive medications, adjusting for age, race, sex, smoking, alcohol drinking, BMI, heart rate and follow-up years. The path coefficients from baseline SBP to follow-up LVMI and RWT were significant, but the path coefficients from baseline LVMI and RWT to follow-up SBP were not. The tracking correlations of SBP from baseline to follow-up were higher than the tracking correlations of LVMI and RWT and the synchronous correlations of SBP with LVMI and RWT at baseline. A good model fit to the observed data was indicated by CFI=0.93/0.97 and RMR=0.03/0.02 for LVMI/RWT according to the criteria CFI>0.90 and RMR<0.05. As presented in Supplement Table S1 and S2, differences in path coefficients of SBP with LVMI and RWT between subgroups were not significant. Path coefficients of DBP with LVMI and RWT were similar to those of SBP (Figure S1).
Figure 1.
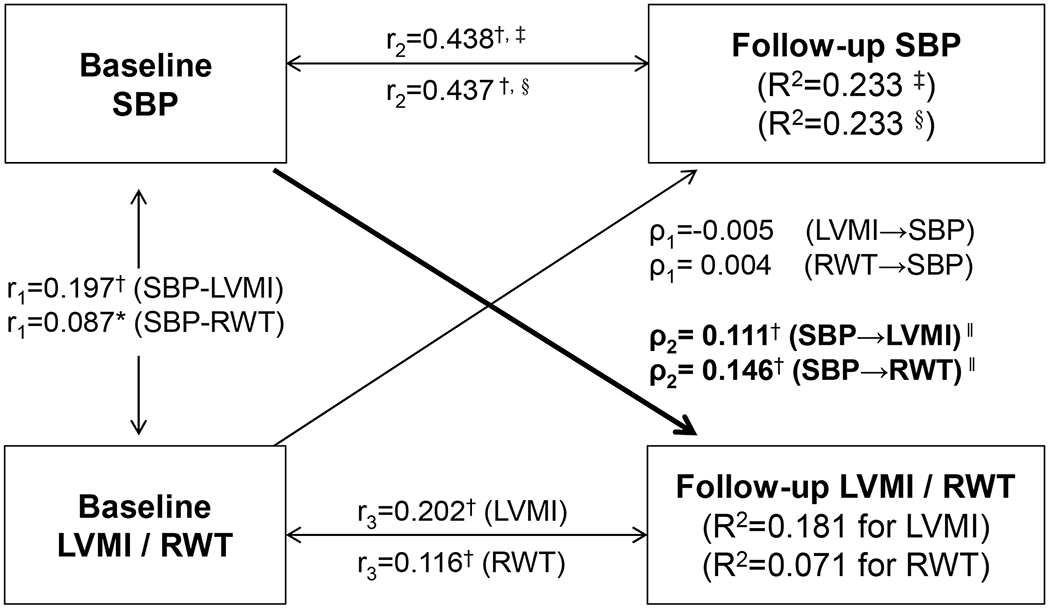
Cross-lagged path analysis models of systolic blood pressure (SBP) with left ventricular mass index (LVMI) and relative wall thickness (RWT) in subjects who did not take anti-hypertensive medications
ρ1, ρ2 = cross-lagged path coefficients; r1 = synchronous correlations; r2, r3 = tracking correlations; R2 = variance explained; comparative fit index (CFI)=0.93/0.97 for LVMI/RWT; Root mean square residual (RMR)=0.03/0.02 for LVMI/RWT
Models with adjustment for age, race, sex, smoking, alcohol drinking, BMI, heart rate and follow-up years
Coefficients different from 0: * p<0.05, † p<0.01
‡, LVMI was included in the model; §, RWT was included in the model.
‖, P for difference between ρ1 and ρ2: p=0.005 for LVMI, p<0.001 for RWT
In Figure 2, covariates-adjusted means of follow-up LVMI (panel A) and RWT (panel B) showed significant increasing trends across quartiles of baseline SBP in subjects who did not take anti-hypertensive medications at baseline. In contrast, follow-up SBP levels did not show significant trends across quartiles of baseline LVMI (panel C) and RTW (panel D) in subjects who did not take anti-hypertensive medications at follow-up. In Figure S2, the relationships of LVMI, RWT with DBP in their respective baseline and follow-up values were similar to those with SBP.
Figure 2.
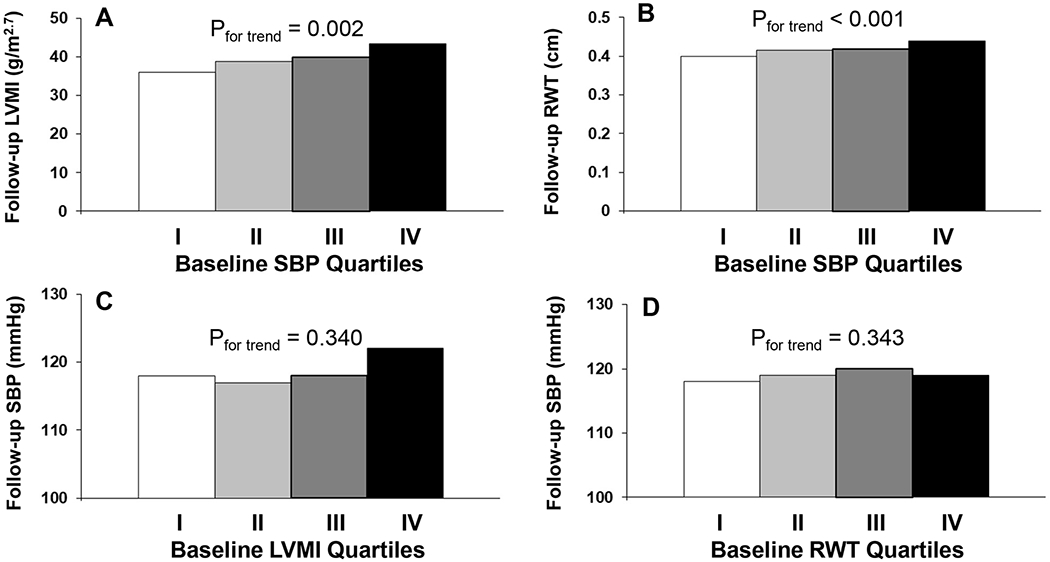
Covariates-adjusted left ventricular mass index (LVMI), relative wall thickness (RWT) and systolic blood pressure (SBP) at follow-up by quartiles of their cross-matched baseline values in subjects who did not take anti-hypertensive medications at baseline or follow-up
Covariates included age, race, sex, BMI, heart rate, smoking, alcohol drinking and follow-up years.
Figure 3 shows associations of baseline hypertension with incidence rates of LVH and remodeling patterns at follow-up. Baseline hypertension was significantly associated with incident LVH. Notably, baseline hypertension was significantly associated with incident CH but not with incident EH at follow-up. In contrast, among subjects who did not have baseline hypertension, LVH vs normal LVMI at baseline was associated with incident hypertension at follow-up (67.6% vs 46.0%, p=0.014 for difference); CH vs normal LVMI at baseline was associated with incident hypertension at follow-up (87.5% vs 46.7%, p=0.022 for difference). It is worth mentioning that the sample size of LVH groups was small with baseline hypertension excluded. There were 23 incident hypertensive cases among 34 participants who had LVH at baseline (23/34=67.6%) and 7 incident hypertensive cases among 8 participants who had CH at baseline (7/8=87.5%).
Figure 3.
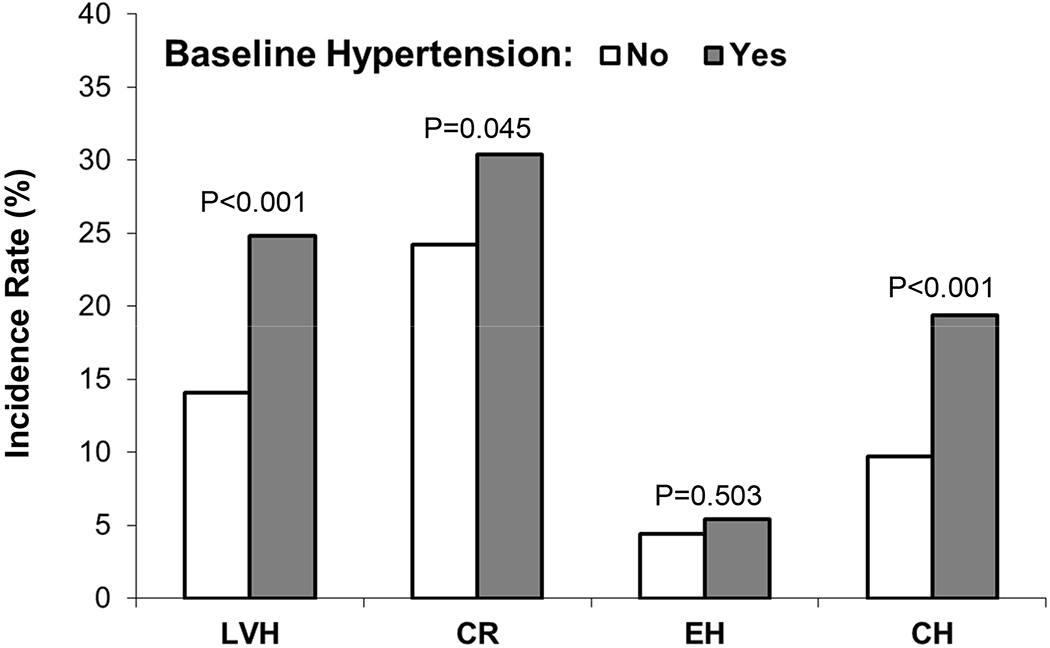
Incidence rates of LVH and geometric remodeling patterns at follow-up by baseline hypertension status
LVH=left ventricular hypertrophy; CR=concentric remodeling; EH=eccentric hypertrophy; CH=concentric hypertrophy
DISCUSSION
In the current study, we examined the temporal relationship of BP with LVMI and LV geometric changes in the community-based longitudinal cohort of adults from the Bogalusa Heart Study. The central findings are that high baseline BP precedes alterations in follow-up LVMI and that elevated baseline BP was associated with concentric LVH, but not with eccentric LVH, at follow-up. These unidirectional temporal relations were noted consistently in Black-White race and sex subgroups.
LVH represents the most frequent hypertension-mediated organ damage in hypertensive patients (30). BP elevation is generally thought to be the most important risk factor resulting in increased LVM and leading to LVH in the general and hypertensive populations (3–6). Studies have shown that childhood BP and lifetime cumulative burden of increased BP are predictors of LVM measures in later life (4–6). In contrast, another line of evidence from longitudinal studies has been accumulated that an increase in LVM, assessed by echocardiography, can promote, or predict the subsequent development of hypertension (8–10). An important question raised from existing data is whether changes in mechanical properties of increased BP precede an increase in LVM or vice versa. Further, it is largely unknown whether the causal relationship varies by different age periods. The temporal sequence between hypertension and subclinical cardiac organ damage is the central subject of a continuous debate (15).
The hypertension-LVH relationship is likely bidirectional based on hemodynamic mechanisms and previous findings (3–15,31). However, the data from this longitudinal study do not support the notion that increased LVM/RWT predicts elevated BP using the continuous study variables in the cross-lagged path analysis model. It should be noted that hypertension was defined using cut-offs of 140/90 mmHg in the previous studies (8–10) and 130/80 mmHg in the current study. The different definitions of hypertension might result in discrepancies in the findings; another possible explanation is that the current study cohort followed from young adulthood to midlife was younger than previous cohorts (8–10). Therefore, the data on the one-way direction from BP to LVMI/RWT do not preclude the possibility that the path direction and effect size may vary by developmental periods such as early childhood and late adulthood. In fact, we also found in this study significant associations between baseline LVH/CH with incident hypertension, but the numbers at baseline (n=34 for LVH and n=8 for CH) were very small and did not allow us to use 140/90 mmHg to define incident hypertension.
Echocardiography allows identification of different forms of LV geometric remodeling, including eccentric or concentric hypertrophy and disproportionate septal thickness. Although the significance of the different forms is not yet entirely defined, CH, the predominant form in middle-aged and elderly patients with hypertension (7), is considered to carry the highest risk for cardiovascular events (2,32). Previous studies (2,3,7), including ours (5,6,24,33), indicated that elevation in BP and hypertension were more strongly associated with CH. On the other hand, in a large-scale four-year follow-up cohort of the Framingham Heart Study and the Framingham Offspring Study of 1121 men (mean age, 44.4 years) and 1559 women (mean age, 45.6 years), baseline echocardiographic LVM (p=0.01) and RWT (p=0.02) were associated with progression from normotension to hypertension (8). But the results from the Multi-Ethnic Study of Atherosclerosis suggest that LV concentric geometry does not contribute to hypertension onset in an LVM-independent manner (9). In our study, we found that baseline BP was significantly associated with follow-up RWT in the cross-lagged analysis, but not the other way around. Despite the strong evidence in the one-way path from BP to RWT analyzed as continuous variables, we also found in subgroups with a small number of participants that baseline CH was associated with incident hypertension at follow-up as dichotomous variables (7 incident hypertension cases among 8 subjects with baseline CH). Further large studies with robust inference analyses are warranted to determine the directionality between LV geometric remodeling patterns and the development of hypertension.
Blacks outpace other ethnic groups in the United States in terms of prevalence, early onset, and severity of hypertension (34). Blacks had significantly higher values of LVM than Whites in the Multi-ethnic Study of Atherosclerosis cohort (9). In the present study cohort of young and middle-aged adults, Blacks versus Whites showed higher levels of BP, LVMI and RWT, and higher prevalence and incidence rates of LVH and CH. With respect to association parameters, the data are inconsistent in previous studies. In our early cross-sectional analysis in the Bogalusa Heart Study cohort, the odds ratios of hypertension for CH were significantly greater in Blacks (6.21) than in Whites (2.14) (24). The Multi-Ethnic Study of Atherosclerosis longitudinal cohort showed that the relative risks of the highest quartile versus the lowest quartile of baseline LVM for incident hypertension did not differ significantly between Blacks (1.79) and Whites (1.64) (9). Despite the marked Black-White differences in levels and association strength regarding these variables, the temporal parameters, the cross-lagged path coefficients (ρ1 and ρ2), did not differ significantly between Blacks and Whites in this study as shown in Tables S1 and S2. The consistency in the temporal sequence between BP and LVMI/RWT across the races further supports the unidirectional relationship from elevated BP to the development of LVH. More cause-effect inference studies are needed to understand the temporal directionality between BP and LVH/CH in race groups.
This community-based longitudinal cohort provides a unique opportunity to examine the temporal relationship between intercorrelated variables. It also has certain limitations. First, exclusion of hypertensives on pharmacologic treatment may result in a loss of information because these individuals represent a subgroup who, without treatment, would be expected to have the highest BP levels and thus may lead to an underestimation of the BP-to-LVMI/RWT relationship. Second, the relatively small sample size, especially in Blacks, had a limited power to detect weak-to-moderate associations in subgroups and did not allow us to conduct a comparison analysis of the directionality using the old and new definitions for hypertension. Third, the findings from in this study in young and middle-aged adults in a Black-White population may not be generalized to other age and race groups.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
We found one-directional temporal sequence from blood pressure to left ventricular mass index and relative wall thickness.
Hypertensive subjects at baseline had a significantly higher incidence rate of concentric hypertrophy at follow-up compared to normotensive subjects, but incident eccentric hypertrophy did not show such a difference between hypertensive and normotensive subjects.
What Is Relevant?
These findings underscore a one-way path from blood pressure elevation to cardiac hypertrophy and geometric remodeling, and provide new insights regarding the pathogenesis of hypertension-mediated cardiac organ damage and the potential for its reversal by interventions targeting at BP to reduce cardiovascular risk.
Summary
Elevated baseline blood pressure levels preceded subsequent increased left ventricular mass index and relative wall thickness in adults. The results suggest that hemodynamic properties are responsible for the development of cardiac enlargement, particularly concentric hypertrophy, during the age period from young adulthood to midlife.
PERSPECTIVES.
The current study demonstrates that elevated baseline BP levels preceded subsequent increased LVMI and RWT in adults. The results suggest that hemodynamic properties are responsible for the development of cardiac enlargement, particularly CH, during the age period from young adulthood to midlife. Further research is needed to demonstrate the age-related directionality between increased BP and subclinical cardiac damages. The findings of the current study underscore a one-way path from BP elevation to cardiac hypertrophy and geometric remodeling and provide new insights regarding the pathogenesis of hypertension-mediated cardiac organ damage and the potential for its reversal by interventions targeting at BP to reduce cardiovascular risk.
ACKNOWLEDGMENTS
The Bogalusa Heart Study is a joint effort of many investigators and staff members whose contribution is gratefully acknowledged. We especially thank the Bogalusa, LA, school system, and most importantly the children and adults who have participated in this study over many years. We wish to thank the reviewers for their insightful comments.
SOURCES OF FUNDING
This study was supported by grants R03AG060619 from National Institute on Aging, R01HL121230 from the National Heart, Lung and Blood Institute, P20GM109036 from the National Institute of General Medical Sciences of the National Institutes of Health, and 81973147 and 81673271 from National Natural Science Foundation of China. Miaoying Yun is partly supported by the MUC 111 Project from Minzu University of China.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. New Engl J Med. 1990;322:1561–6. [DOI] [PubMed] [Google Scholar]
- 2.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52. [DOI] [PubMed] [Google Scholar]
- 3.Cuspidi C, Sala C, Negri F, Mancia G, Morganti A. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012;26:343–9. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh AK, Francis DP, Chaturvedi N, Kuh D, Mayet J, Hughes AD, Hardy RJ. Cardiovascular Risk Factors from Early Life Predict Future Adult Cardiac Structural and Functional Abnormalities: A Systematic Review of the Published Literature. Cardiol Ther. 2014;2:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y, Li S, Guo Y, Fernandez C, Bazzano L, He J, Mi J, Chen W, the International Childhood Cardiovascular Cohort Consortium Investigators. Life-Course Cumulative Burden of Body Mass Index and Blood Pressure on Progression of Left Ventricular Mass and Geometry in Midlife: The Bogalusa Heart Study. Cir Res. 2020;126:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CC, Sun D, Cen R, Wang J, Li S, Fernandez-Alonso C, Chen W, Srinivasan SR, Berenson GS. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64:1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage DD, Garrison RJ, Kannel WB, Levy D, Anderson SJ, Stokes J, Feinleib M, Castelli WP. The spectrum of left ventricular hypertrophy in a general population sample: the Framingham Study. Circulation. 1987;75(Suppl I):I 26–33. [PubMed] [Google Scholar]
- 8.Post WS, Larson MG, Levy D. Impact of left ventricular structure on the incidence of hypertension. The Framingham Heart Study. Circulation. 1994;90:179–85. [DOI] [PubMed] [Google Scholar]
- 9.Daichi Shimbo; Paul Muntner; Devin Mann; Graham Barr, R.; Weihong Tang; Post, Wendy: Association of left ventricular hypertrophy with incident hypertension: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011;173:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simone Giovanni de; Devereux Richard B.; Chinali Marcello; Roman Mary J.; Welty Thomas K.; Lee Elisa T.; Howard Barbara V. Left ventricular mass and incident hypertension in individuals with initial optimal blood pressure: the Strong Heart Study. J Hypertens. 2008;26:1868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–9. [DOI] [PubMed] [Google Scholar]
- 12.McEniery CM, Sharon Wallace Y, Maki-Petaja K, McDonnell B, Sharman JE, Retallick C, Franklin SS, Brown MJ, Lloyd RC, Cockcroft JR, Wilkinson IB; on behalf of the ENIGMA Study Investigators. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–6. [DOI] [PubMed] [Google Scholar]
- 13.Schmieder RE, Schobel HP, Messerli FH. Central blood volume: a determinant of early cardiac adaptation in arterial hypertension? J Am Coll Cardiol. 1995;26:1692–8. [DOI] [PubMed] [Google Scholar]
- 14.Lutas EM, Devereux RB, Reis G, Alderman MH, Pickering TG, Borer JS, Laragh JH. Increased cardiac performance in mild essential hypertension. Left ventricular mechanics. Hypertension. 1985;7(6 pt 1):979–88. [DOI] [PubMed] [Google Scholar]
- 15.Cuspidi C, Tadic M, Grassi G. Left ventricular mass and incident hypertension: Missing pieces in the puzzle. J Clin Hypertens (Greenwich, Conn.) 2019;00:1–2. 10.1111/jch.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC, Foster TA, Blonde CV. Cardiovascular risk factors in children: The early natural history of atherosclerosis and essential hypertension. New York, NY: Oxford University Press. 1980:47–123. [Google Scholar]
- 17.Ostchega Y, Nwankwo T, Sorlie PD, Wolz M, Zipf G. Assessing the validity of the Omron HEM-907XL oscillometric blood pressure measurement device in a national survey environment. J Clin Hypertens (Greenwich) 2010;12:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostchega Y, Zhang G, Sorlie P, Hughes JP, Reed-Gillette DS, Nwankwo T, Yoon S. Blood pressure randomized methodology study comparing automatic oscillometric and mercury sphygmomanometer devices: National Health and Nutrition Examination Survey, 2009-2010. Natl Health Stat Report. 2012;(59):1–15. [PubMed] [Google Scholar]
- 19.Sahn DJ, DeMaria AN, Kisslo JO, Weyman AF. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. [DOI] [PubMed] [Google Scholar]
- 20.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. The American journal of cardiology. 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- 21.Foppa M, Duncan BB, Rohde LE. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy?. Cardiovascular ultrasound. 2005;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Simone G, Kitzman DW, Chinali M, Oberman A, Hopkins PN, Rao DC, Arnett DK, Devereux RB. Left ventricular concentric geometry is associated with impaired relaxation in hypertension: the HyperGEN study. Eur Heart J. 2005;26:1039–45. [DOI] [PubMed] [Google Scholar]
- 23.Ganau A, Devereux RB, Roman MJ, De Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–8. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Chen W, Ruan L, Toprak A, Srinivasan SR, Berenson GS. Differential Impact of Elevated Blood Pressure on Left Ventricular Geometry Types in Black and White Young Adults in a Community (from the Bogalusa Heart Study). Am J Cardiol. 2011;107:717–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivimaki M, Feldt T, Vahtera J. Sense of coherence and health: evidence from two cross-lagged longitudinal samples. Social Science & Medicine 2000;50:583–97. [DOI] [PubMed] [Google Scholar]
- 26.Li CC. Path Analysis – A primer. Pacific Grove. CA, Boxwood Press, 1975. [Google Scholar]
- 27.Joreskog K, Sorbom D. LISREL 8.52: Structural Equation Modeling with the SIMPLIS Command Language. Chicago: Scientific Software International; 1993. [Google Scholar]
- 28.Joreskog K, Sorbom D. LISREL 8.52: User’s Reference Guide. Chicago: Scientific Software International; 2001. [Google Scholar]
- 29.Chen W, Srinivasan SR, Berenson GS. Path analysis of metabolic syndrome components in black versus white children, adolescents and adults: The Bogalusa Heart Study. Ann Epidemiol 2008;18:85–91. [DOI] [PubMed] [Google Scholar]
- 30.Rodilla E, Millasseau S, Costa JA, Pascual JM. Arterial destiffening in previously untreated mild hypertensives after 1 year of routine clinical management. Am J Hypertens. 2017;30:510–7. [DOI] [PubMed] [Google Scholar]
- 31.Lund-Johansen P Hemodynamic patterns in the natural history of borderline hypertension. J Cardiovasc Pharmacol. 1986;8(suppl 5):S8–S14. [DOI] [PubMed] [Google Scholar]
- 32.Verdecchia P, Angeli F, Achilli P, Castellani C, Broccatelli A, Gattobigio R, Cavallini C. Echocardiographic left ventricular hypertrophy in hypertension: marker for future events or mediator of events? Curr Opin Cardiol. 2007;22:329–34. [DOI] [PubMed] [Google Scholar]
- 33.Toprak A, Reddy J, Chen W, Srinivasan S, Berenson G. Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the Bogalusa Heart Study). Am J Cardiol. 2009;103:978–84. [DOI] [PubMed] [Google Scholar]
- 34.Pickering TG. Hypertension in blacks. Curr Opin Nephrol Hypertens 1994;3:207–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


