Abstract
Background:
The goal of this study was to determine the relationship between postoperative weight change and breast cancer-related lymphedema.
Methods:
In this cohort study, 1,161 women underwent unilateral breast surgery for breast cancer from 2005 to 2020 and were prospectively screened for breast cancer-related lymphedema. Arm volume measurements were obtained via an optoelectronic perometer preoperatively, postoperatively, and in the follow-up setting every 6–12 months. Mean follow-up from preoperative baseline was 49.1 months. The main outcome was breast cancer-related lymphedema, defined as a relative volume change of the ipsilateral arm of ≥10% at least 3 months after surgery.
Results:
92 patients (7.9%) developed breast cancer-related lymphedema. Net weight loss vs. net weight gain from baseline to last follow-up was not protective against developing BCRL (HR 1.38; 95% CI 0.89 – 2.13; p=0.152).
Conclusion:
Although weight loss may be recommended as part of an individualized lifestyle management program for overall health, weight loss alone may not decrease the risk of developing BCRL.
Keywords: Breast cancer, breast cancer-related lymphedema, lymphedema, weight change
Precis:
Weight loss, when compared to weight gain, does not decrease the risk of BCRL (HR 1.38; 95% CI 0.89 – 2.13; p=0.152).
Background
Advancements in breast cancer (BC) diagnosis and treatment have dramatically increased long-term survival in recent years1. As a result, there is a growing need to better understand how BC treatments affect patients throughout survivorship. One significant complication of BC treatment is breast cancer-related lymphedema (BCRL); approximately one in five individuals treated for BC will develop BCRL2. BCRL results from protein-rich fluid accumulating in the interstitial space, leading to regional swelling3. Patients treated for BC are at lifelong risk of developing BCRL, which is an incurable disease that necessitates stressful, time-consuming and expensive treatment4. Because of the significant impact that BCRL has on patient quality of life, understanding the causes of this disease has become increasingly important.
Various studies have identified risk factors of BCRL, such as axillary lymph node dissection (ALND)3,5–10, number of positive lymph nodes3,5–8,11, regional lymph node irradiation (RLNR)3,9–13, being overweight (BMI ≥ 25 kg/m2) or obese (BMI ≥ 30 kg/m2) at breast cancer diagnosis3,5,17,6,8–11,14–16 and cellulitis8–10,18,19. BCRL risk is lifelong, and timing of highest risk of BCRL development depends on BC treatment received. For example, peak BCRL risk is later for those who undergo SLNB than those who undergo ALND (36–48 months vs. 6–24 months post-operatively)2. While a high preoperative BMI is a well-established risk factor, the impact of overall postoperative weight loss or gain is unknown. Of the few studies that have analyzed the relationship between postoperative weight change and BCRL, they are limited by small sample size8, self-reported lymphedema6,8, self-reported weight/BMI8, retrospective assessment of lymphedema8, lack of presurgical baseline arm volumes6,8,20, and failure to assess the effect of weight loss and weight gain separately6,16,20. Because of these shortcomings, we sought to assess the impact of net weight loss vs. net weight gain on BCRL development in our cohort of 1,161 women who experienced a weight change.
Methods
Lymphedema assessments
At Massachusetts General Hospital, patients diagnosed with breast cancer are prospectively screened for BCRL. To screen for and diagnose BCRL, we consider objective arm volume measurements in tandem with patient-reported outcome measures and clinical examinations. To obtain objective arm measurements, we utilize an optoelectronic Perometer. The Perometer uses infrared light beams to measure arm volume, which has been demonstrated to be a reliable and valid way to measure the arm and thus assess lymphedema21–23. Our group’s measurement and screening protocol has been previously published24. Patients undergo bilateral arm measurements preoperatively, postoperatively, and every 6–12 months coinciding with their oncology follow-up visits. We strive to screen patients for as long as possible, with a current median follow-up time of 32 months.
To quantify arm volume changes, we utilize the relative volume change (RVC) equation25. The RVC assesses changes in the ipsilateral arm since preoperative baseline while controlling for any changes in volume of the contralateral arm. In short, the RVC = [(A2U1/(U2A1) – 1], where A1 and A2 are the ipsilateral arm volumes at preoperative (baseline) and postoperative assessments, respectively, and U1 and U2 are the contralateral arm volumes at preoperative and postoperative assessments, respectively. In this study, BCRL was defined as an RVC ≥ 10% occurring ≥ 3 months after definitive breast surgery. Patients were censored after development of BCRL.
Patient population
From 2005 to 2020, patients undergoing treatment for primary BC were screened for BCRL. All women were recruited at the time of initial presurgical consultation in our multidisciplinary breast oncology clinic. Each patient had a preoperative bilateral arm measurement, at least one postoperative bilateral arm measurement ≥ 3 months after surgery, and a weight recorded at the preoperative visit and all postoperative visits. Women who underwent bilateral breast surgery were excluded from analysis, providing us with a cohort of 1,409 patients. However, the 67 women who maintained weight from preoperative baseline to last follow up were removed as the primary aim of the study was to examine effect of weight gain or loss. Additionally, patients with ductal carcinoma in situ (DCIS) (n=175) or stage IV breast cancer (n=6) were excluded, resulting in a cohort of 1,161 women. In this cohort, 649 (55.9%) experienced a net weight gain from preoperative baseline to last follow-up (weight at last follow up was greater than preoperative weight) while 512 (44.1%) experienced a net weight loss (weight at last follow up was less than preoperative weight). For those who developed BCRL, last follow-up was defined as the visit in which the patient developed BCRL. For those who did not develop BCRL, last follow-up was defined as the last visit.
Statistical Methods
Chi Square tests, t-tests, and Wilcoxon rank-sum tests were used, as appropriate, to compare categorical and continuous treatment-related risk factors between the cohort of patients who lost weight and those who gained weight. Univariate Cox proportional hazards regression models were performed on the BCRL outcome for the net weight change variable and for each potential covariate. Covariates included preoperative age, race (white vs. non-white), preoperative BMI, surgery type, nodal surgery type, RLNR, and adjuvant chemotherapy. Women were excluded if BCRL (defined as RVC ≥ 10% ≥ 3 months after definitive breast surgery) occurred before receipt of RLNR or adjuvant chemotherapy. If a covariate was significant (p<0.05) in the unadjusted analysis, it was included in the subsequent multivariable model with the rate of weight change variable.
The multivariable model was assessed with the Kolmogorov-type supremum test, based on cumulative sums of martingale residuals, using a significance threshold of 0.01. Kaplan-Meier survival curves were generated in the two cohorts and a log-rank test was used to determine if there was a statistically significant difference in BCRL-free survival between the two groups. Hypothesis tests were two-sided, and the significance threshold was set to 0.05. All statistical analyses were performed using SAS/STAT version 9.4.
Results
Demographics
From 2005 to 2020, 1,161 patients underwent unilateral breast cancer surgery, were prospectively screened for BCRL, and experienced a weight change from preoperative baseline to their last follow-up. Among these women, 92 (7.9%) developed breast cancer-related lymphedema. The median time to onset of lymphedema was 18.5 months after definitive breast surgery (range: 3.0 to 115.0 months). The mean net weight change experienced by the 92 women who developed BCRL was −1.25 lbs (range: −31 to 29 lbs). The mean BMI at baseline for the entire cohort was 26.6 kg/m2, while the mean weight was 155 pounds. Regarding treatment, 28.1% underwent ALND, 32.0% underwent RLNR, and 39.3% received adjuvant chemotherapy. Demographic and treatment-related variables are further described and compared in the cohort of women who gained weight (n=649) vs. those who lost weight (n=512). The two cohorts differed in terms of net weight change, preoperative weight, BMI, and age. The mean (range) weight change from preoperative baseline to last follow-up was +8.2 lbs (+0.1 to +49.0) for those who gained weight and −8.7 lbs (−81.0 to −0.1) for those who lost weight (Table 1).
Table 1.
Demographics and treatment-related characteristics
| Entire Cohort (n=1,161) | Gained Weight (n=649) | Lost Weight (n=512) | p-value | |
|---|---|---|---|---|
| Preoperative weight (lbs)* | 155 (92 – 340) | 157.7 (92 – 340) | 167.8 (97.5 – 322) | <0.001 |
| Net weight change (lbs)* | -- | 8.2 (0.1 – 49.0) | −8.7 (−81.0 – −0.1) | <0.001 |
| Preoperative BMI (kg/m2)* | 26.6 (15.8 – 58.9) | 27.1 (15.8 – 58.4) | 29.0 (18.4 – 58.9) | <0.001 |
| Age (years)* | 56.6 (27.0 – 85.9) | 55.5 (27.2 – 85.9) | 58.0 (27.0 – 82.3) | <0.001 |
| Non-white | 118 (10.2%) | 66 (10.2%) | 52 (10.2%) | |
| Lumpectomy | 924 (79.6%) | 512 (78.9%) | 412 (0.5%) | |
| None | 53 (4.6%) | 32 (4.9%) | 21 (4.1%) | |
| Lymph nodes sampled** | 2 (0 – 43) | 3 (0 – 41) | 2 (1 – 43) | 0.395 |
| Lymph nodes malignant** | 0 (0 – 39) | 0 (0 – 39) | 0 (0 – 32) | 0.834 |
| Unknown | 4 (0.3%) | -- | 4 (0.8%) | |
| RLNR | 372 (32.0%) | 209 (2.2%) | 163 (31.8%) | 0.944 |
| Neoadjuvant chemotherapy | 150 (12.9%) | 89 (13.7%) | 61 (11.9%) | 0.520 |
| Adjuvant chemotherapy | 456 (39.3%) | 255 (39.3%) | 201 (39.3%) | 0.999 |
| Adjuvant hormonal therapy | 932 (80.3%) | 525 (80.9%) | 407 (79.5%) | 0.602 |
| Maximum follow-up (months since baseline)* | 49.1 (1.2, 178.2) | 48.7 (1.2, 151.7) | 49.5 (1.4, 178.2) | 0.630 |
| Developed BCRL | 92 (7.9%) | 43 (6.6%) | 49 (9.6%) | 0.083 |
| Time to onset of BCRL (months since surgery)** | 18.5 (3.0 – 115.0) | 20.7 (3.0 – 110.2) | 16.2 (3.4 – 115.0) | 0.684 |
Mean or
median with (range) is shown.
p-value compares variables between those who gained weight vs. those who lost weight.
Abbreviations: body mass index (BMI); axillary lymph node dissection (ALND); sentinel lymph node biopsy (SLNB); regional lymph node radiation (RLNR); breast cancer-related lymphedema (BCRL).
Impact of Net Weight Change on BCRL
We assessed the impact of net weight loss (from preoperative baseline to last follow-up) vs. net weight gain on BCRL development (n=1,161). When controlling for type of surgery (mastectomy vs. lumpectomy), nodal surgery type (ALND vs. SLNB and ALND vs. no nodal surgery), RLNR, and adjuvant chemotherapy, net weight loss, when compared to net weight gain, was not protective against BCRL development (HR 1.38; 95% CI 0.89 – 2.13; p=0.152). Additionally, the Kaplan-Meier survival curve demonstrates that the BCRL-free survival in the cohort of women who lost weight is not significantly different from the BCRL-free survival of those who gained weight (p = 0.077) (Figure 1).
Figure 1.
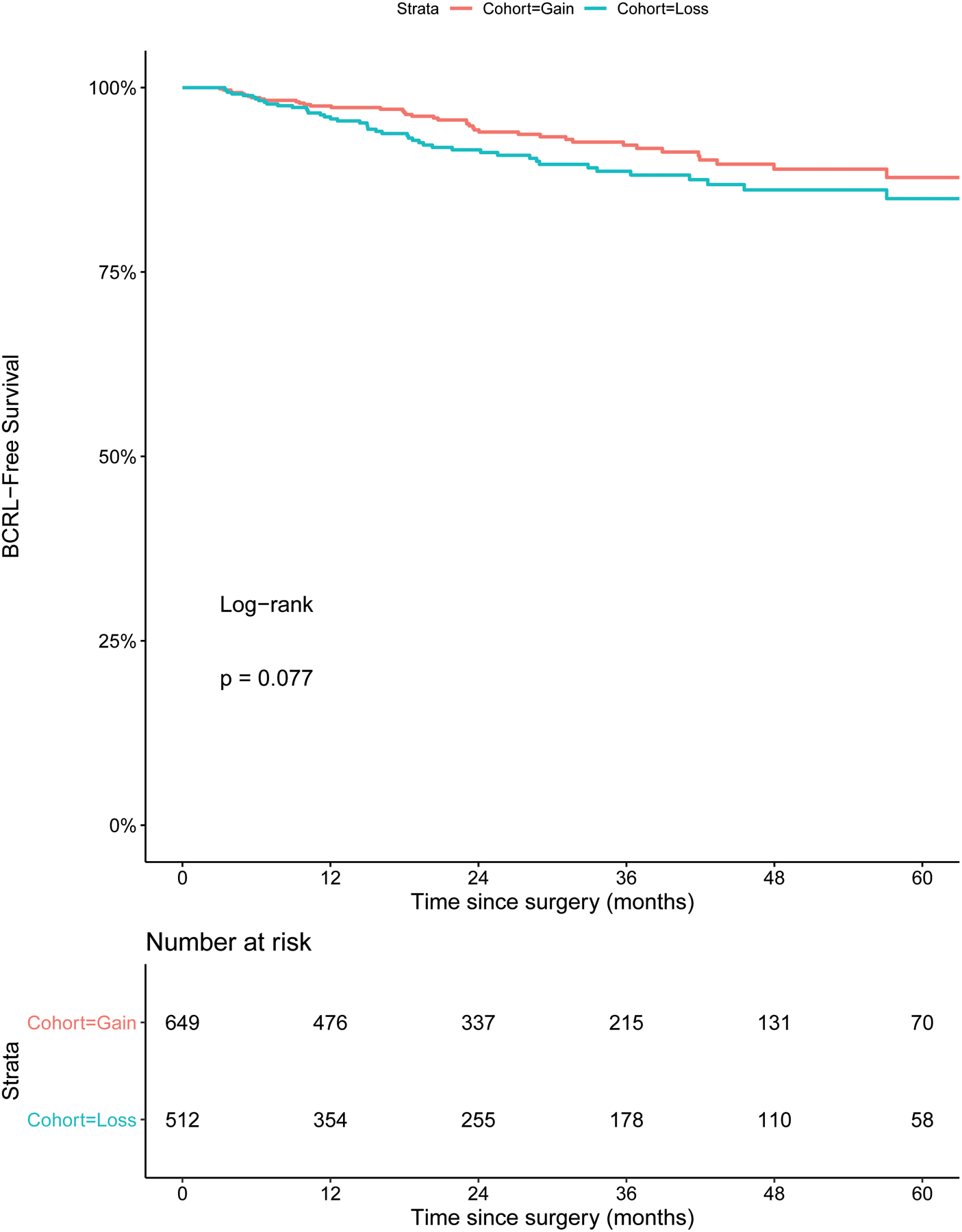
Kaplan-Meier curves displaying the predicted percentage of patients still BCRL-free at various times since surgery, for patients who had an overall net weight gain from baseline to last follow-up and for patients who had an overall net weight loss from baseline to last follow-up. Abbreviations: BCRL = breast cancer-related lymphedema.
Discussion
Although a high preoperative BMI is a well-established risk factor for BCRL, little is known regarding the relationship between overall weight changes and BCRL development. Women undergoing treatment for breast cancer often experience significant weight changes throughout and beyond BC treatment26,27,36,28–35. Therefore, we analyzed data from a cohort of 1,161 women who were treated for primary breast cancer. To our knowledge, this is the largest cohort study investigating the relationship between net weight change and BCRL development. In addition to the large sample size, this study utilized preoperative assessments on all patients, objective measures of arm volume, weight measurements at all visits, and a mean follow-up of 49.1 months.
Our main findings suggest that weight loss does not decrease the risk of BCRL (Table 2). This counteracts the belief held by some that weight loss may be protective against developing BCRL. This belief may have been perpetuated as being overweight or obese at time of BC diagnosis is a risk factor for BCRL3,5,17,6,8–11,14–16. Given this, the hypothesis amongst some clinicians and researchers is that losing weight would therefore be protective against BCRL development. To our knowledge, there have been three studies examining the effect of weight loss in women with BCRL, two of which found a beneficial effect of weight loss on BCRL outcomes37–39. However, note that these cohorts already developed BCRL, as opposed to being at-risk for BCRL. Shaw et al found a significant correlation between weight loss and a reduction in excess arm volume (r: 0.423; P = .002) in 64 women with BCRL randomized to weight reduction through diet or to control group38. In another study by the same group analyzing data from 21 women with BCRL randomized to oral or written dietary advice vs control, there was a significant reduction in swollen arm volume at the end of the 12-week period (P = .003)37. Conversely, results from the Women in Steady Exercise Research (WISER) randomized clinical trial suggest that weight loss does not decrease arm volume difference in patients with BCRL39. In this study, 351 overweight breast cancer survivors with BCRL were randomized to one of four groups: control group, exercise group, weight loss group, or combined exercise and weight loss group. The authors found that engagement in 12 months of an exercise or weight loss program did not have an effect on BCRL outcomes.
Table 2.
Impact of weight change from preoperative baseline to last follow-up on BCRL development: multivariable analysis (n=1,161)
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Net weight loss vs. net weight gain | 1.45 (0.96, 2.18) | 0.078 | 1.38 (0.89, 2.13) | 0.152 |
| Baseline BMI (kg/m2) | 1.04 (1.01, 1.07) | 0.003 | 1.04 (1.01, 1.07) | 0.005 |
| Age at baseline (years) | 1.01 (0.99, 1.03) | 0.232 | -- | -- |
| Race (white vs. non-white) | 0.87 (0.44, 1.74) | 0.701 | -- | -- |
| Mastectomy vs. lumpectomy | 2.49 (1.64, 3.80) | <0.001 | 1.02 (0.61, 1.70) | 0.955 |
| ALND vs. SLNB | 4.47 (2.88, 6.95) | <0.001 | 2.77 (1.37, 5.60) | 0.005 |
| ALND vs. no nodal surgery | 4.22 (1.03, 17.3) | 0.045 | 2.41 (0.53, 10.9) | 0.256 |
| RLNR vs. no RLNR | 4.08 (2.59, 6.42) | <0.001 | 2.47 (1.21, 5.04) | 0.013 |
| Adjuvant chemotherapy (+/− neoadjuvant chemotherapy) vs. no adjuvant chemotherapy | 1.50 (0.99, 2.26) | 0.055 | 0.66 (0.41, 1.05) | 0.080 |
Abbreviations: BMI, body mass index; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; sx, surgery; RLNR, regional lymph node irradiation; HR, hazard ratio; CI, confidence interval.
Two other studies have examined the effect of weight gain or fluctuation on BCRL development in cohorts at risk, however, both studies carry significant limitations. In a previous study by our team, postoperative weight fluctuation data from 787 patients undergoing treatment for BC was analyzed in a retrospective cohort study16. Though this study was strong in terms of utilizing preoperative assessments, objective arm volume measurements, and having a large sample size, we did not assess weight gain separately from weight loss. In other words, we analyzed the effect of the absolute value of weight changes. We found that cumulative absolute fluctuation in weight from preoperative assessment significantly increased risk of developing BCRL on multivariable analysis. However, from this result, it remains unclear as to whether it was the weight gain, weight loss, or both, that increased risk of BCRL.
In one study that found an association between weight gain and BCRL development, 260 women treated for breast cancer were followed for 20 years, making this one of the longest-term follow-up studies in BCRL8. At 20 years postoperatively, the only factors found to be associated with BCRL were history of arm infection and post-treatment weight gain. No change in weight or weight loss was not found to be associated with BCRL. However, this study presents with significant limitations including but not limited to small sample size, lack of objective preoperative arm volume measurement, self-report of BCRL, and failure to assess weight fluctuations over time.
An imperative area of discussion is that weight loss in this study does not provide a protective effect on BCRL risk. This is a similar point to that made by the authors of the WISER trial39. Weight loss is recommended for patients who are overweight at breast cancer diagnosis, through diet and exercise interventions. Such weight loss imparts significant cardiovascular, pulmonary and musculoskeletal health benefits, and this should continue to be prioritized for these patients. However, the findings of this study suggest that these patients should also be screened vigilantly for BCRL, understanding that weight loss is not protective against BCRL. We may postulate that perhaps it is the improvement in tissue extensibility or decrease in fibrosis in the area of surgery, or improved kinematics of the shoulder with exercise that may impart a protective effect on BCRL risk. Further research is required in this area to better elucidate exercise effects which may protect patients from BCRL.
Of note, our study is not without limitations. Because all patients were screened for BCRL primarily at their naturally occurring oncology follow-up visits, patients were not measured at regular, pre-determined intervals. To most accurately determine the impact of postoperative weight fluctuations on BCRL risk, it would be imperative to weigh patients at frequent, regular intervals for at least five years postoperatively, considering the majority of cases of BCRL occur within the first five years postoperatively2. In addition to this major limitation, we did not collect information on the lifestyle habits of each patient throughout their treatment for breast cancer. This information would have been especially useful for the cohort of women who lost weight, as it may have helped to elucidate whether one’s weight loss was attributed to healthy habits such as exercise, or if it was related to chemotherapy or other factors related to breast cancer management. A final yet significant limitation is that we were unable to assess the impact of weight maintenance on BCRL development due to the small number of patients (n=67) in this category.
It is imperative for providers to continue to prioritize overall health maintenance to maximize health outcomes after breast cancer treatment. This may include recommendation for weight loss in patients who are overweight, however, the clinician should be aware that weight loss is not protective against BCRL development. Screening for BCRL is imperative for early diagnosis and timely treatment, and weight changes should be incorporated with the goal of overall health maintenance rather than in an effort to protect against BCRL.
Acknowledgements:
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Funding Statement:
The project was supported by Award Number R01CA139118 (AG Taghian) and Award Number P50CA08393 (AG Taghian) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This program is supported by the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema (AG Taghian), the Heinz Family Foundation (AG Taghian), and the Olayan-Xefos Family Fund for Breast Cancer Research (AG Taghian).
Footnotes
Conflict of Interest Statement: Alphonse Taghian is on the Scientific Advisory Board of Puretech Health and a previous Consultant in VisionRT. AGT has been loaned equipment from ImpediMed for use in investigator-initiated clinical trials. Cheryl Brunelle is on the Scientific Advisory Board of Puretech Health. These associations are unrelated to this manuscript. For the remaining authors none were declared.
References
- 1.American Cancer Society: Breast Cancer Facts & Figures. 2017–2018. https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html.
- 2.McDuff SGR, Mina AI, Brunelle CL, et al. Timing of Lymphedema After Treatment for Breast Cancer: When Are Patients Most At Risk? Int J Radiat Oncol Biol Phys. 2019;103(1):62–70. doi: 10.1016/j.ijrobp.2018.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7 [DOI] [PubMed] [Google Scholar]
- 4.Fu MR. Breast cancer-related lymphedema: Symptoms, diagnosis, risk reduction, and management. World J Clin Oncol. 2014;5(3):241. doi: 10.5306/wjco.v5.i3.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu MR, Axelrod D, Guth AA, et al. Patterns of obesity and lymph fluid level during the first year of breast cancer treatment: A prospective study. J Pers Med. 2015;5(3):326–340. doi: 10.3390/jpm5030326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SA M, MJ W, KT M, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol. 2008;26(32):5220–5226. doi: 10.1200/JCO.2008.16.3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16(4):775–782. doi: 10.1158/1055-9965.EPI-06-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrek JA, Senie RT, Peters M, Peterrosen P. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377. doi: [DOI] [PubMed] [Google Scholar]
- 9.Gillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg. 2018;7(4):379–403. doi: 10.21037/gs.2017.11.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson CM, Swaroop MN, Horick N, et al. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J Clin Oncol. 2016;34(7):691–698. doi: 10.1200/JCO.2015.61.5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GKD, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: A meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–1972. doi: 10.1245/s10434-009-0452-2 [DOI] [PubMed] [Google Scholar]
- 12.Warren LEG, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: A prospective cohort study. Int J Radiat Oncol Biol Phys. 2014;88(3):565–571. doi: 10.1016/j.ijrobp.2013.11.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilbreath SL, Refshauge KM, Beith JM, et al. Risk factors for lymphoedema in women with breast cancer: A large prospective cohort. Breast. 2016;28(2016):29–36. doi: 10.1016/j.breast.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 14.Wu R, Huang X, Dong X, Zhang H, Zhuang L. Obese patients have higher risk of breast cancer-related lymphedema than overweight patients after breast cancer: a meta-analysis. Ann Transl Med. 2019;7(8):172–172. doi: 10.21037/atm.2019.03.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman SA, Localio AR, Kallan MJ, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2734–2746. doi: 10.1158/1055-9965.EPI-09-1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jammallo LS, Miller CL, Singer M, et al. Impact of body mass index and weight fluctuation on lymphedema risk in patients treated for breast cancer. Breast Cancer Res Treat. 2013;142(1):59–67. doi: 10.1007/s10549-013-2715-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat. 2011;130(3):981–991. doi: 10.1007/s10549-011-1667-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vignes S, Arrault M, Dupuy A. Factors associated with increased breast cancer-related lymphedema volume. Acta Oncol (Madr). 2007;46(8):1138–1142. doi: 10.1080/02841860701403020 [DOI] [PubMed] [Google Scholar]
- 19.Bevilacqua JLB, Kattan MW, Changhong Y, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. 2012;19(8):2580–2589. doi: 10.1245/s10434-012-2290-x [DOI] [PubMed] [Google Scholar]
- 20.Goldberg JI, Wiechmann LI, Riedel ER, Morrow M, Van Zee KJ. Morbidity of sentinel node biopsy in breast cancer: The relationship between the number of excised lymph nodes and lymphedema. Ann Surg Oncol. 2010;17(12):3278–3286. doi: 10.1245/s10434-010-1155-4 [DOI] [PubMed] [Google Scholar]
- 21.Lee MJ, Boland RA, Czerniec S, Kilbreath SL. Reliability and concurrent validity of the perometer for measuring hand volume in women with and without lymphedema. Lymphat Res Biol. 2011;9(1):13–18. doi: 10.1089/lrb.2010.0021 [DOI] [PubMed] [Google Scholar]
- 22.Hidding JT, Viehoff PB, Beurskens CHG, van Laarhoven HWM, Nijhuis-van der Sanden MWG, van der Wees PJ. Measurement Properties of Instruments for Measuring of Lymphedema: Systematic Review. Phys Ther. 2016;96(12):1965–1981. doi: 10.2522/ptj.20150412 [DOI] [PubMed] [Google Scholar]
- 23.Stanton AWB, Northfield JW, Holroyd B, Mortime PS, Levick JR. Validation of an opeoelectronic lomb volumeter (perometer). Lymphology. 1997;30(2):77–97. [PubMed] [Google Scholar]
- 24.Brunelle C, Skolny M, Ferguson C, Swaroop M, O’Toole J, Taghian AG. Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the massachusetts general hospital: Lessons learned. J Pers Med. 2015;5(2):153–164. doi: 10.3390/jpm5020153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(5):1436–1443. doi: 10.1016/j.ijrobp.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz AL. Exercise and weight gain in breast cancer patients receiving chemotherapy. Cancer Pract. 2000;8(5):231–237. doi: 10.1046/j.1523-5394.2000.85007.x [DOI] [PubMed] [Google Scholar]
- 27.Saquib N, Flatt SW, Natarajan L, et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: Evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Res Treat. 2007;105(2):177–186. doi: 10.1007/s10549-006-9442-2 [DOI] [PubMed] [Google Scholar]
- 28.Villarini A, Pasanisi P, Raimondi M, et al. Preventing weight gain during adjuvant chemotherapy for breast cancer: A dietary intervention study. Breast Cancer Res Treat. 2012;135(2):581–589. doi: 10.1007/s10549-012-2184-4 [DOI] [PubMed] [Google Scholar]
- 29.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. doi: 10.1200/JCO.2005.01.079 [DOI] [PubMed] [Google Scholar]
- 30.Irwin ML, McTiernan A, Baumgartner RN, et al. Changes in body fat and weight after a breast cancer diagnosis: Influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23(4):774–782. doi: 10.1200/JCO.2005.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoskin PJ, Ashley S, Yarnold JR. Weight gain after primary surgery for breast cancer - effect of tamoxifen. Breast Cancer Res Treat. 1992;22(2):129–132. doi: 10.1007/BF01833342 [DOI] [PubMed] [Google Scholar]
- 32.Goodwin PJ. Weight gain in early-stage breast cancer: Where do we go from here? J Clin Oncol. 2001;19(9):2367–2369. doi: 10.1200/JCO.2001.19.9.2367 [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Lu W, Zheng W, et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 2010;122(3):823–833. doi: 10.1007/s10549-009-0708-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caan BJ, Kwan ML, Hartzell G, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19(10):1319–1328. doi: 10.1007/s10552-008-9203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg MMGA, Winkels RM, Kruif JTCM, et al. Weight change during chemotherapy in breast cancer patients: A meta-analysis. BMC Cancer. 2017;17(1). doi: 10.1186/s12885-017-3242-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atalay C, Kucuk AI. Effect of weight gain during adjuvant chemotherapy on survival in breast cancer. Turkish J Surg. 2015:124–127. doi: 10.5152/ucd.2015.3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. 2007;110(8):1868–1874. doi: 10.1002/cncr.22994 [DOI] [PubMed] [Google Scholar]
- 38.Shaw C, Mortimer P, Judd PA. Randomized controlled trial comparing a low-fat diet with a weight-reduction diet in breast cancer-related lymphedema. Cancer. 2007;109(10):1949–1956. doi: 10.1002/cncr.22638 [DOI] [PubMed] [Google Scholar]
- 39.Winkels RM, Sturgeon KM, Kallan MJ, et al. The women in steady exercise research (WISER) survivor trial: The innovative transdisciplinary design of a randomized controlled trial of exercise and weight-loss interventions among breast cancer survivors with lymphedema. Contemp Clin Trials. 2017;61(May 2017):63–72. doi: 10.1016/j.cct.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]


