Abstract
Both substrate stiffness and surface topography regulate cell behavior through mechanotransduction signaling pathways. Such intertwined effects suggest that engineered surface topographies might substitute or cancel the effects of substrate stiffness in biomedical applications. However, the mechanisms by which cells recognize topographical features are not fully understood. Here we demonstrate that the presence of nanotopography drastically alters cell behavior such that neurons and stem cells cultured on rigid glass substrates behave as if they were on soft hydrogels. With atomic force microscopy, we show that rigid nanotopography resembles the effects of soft hydrogels in reducing cell stiffness and membrane tension. Further, we reveal that nanotopography reduces focal adhesions and cell stiffness by enhancing the endocytosis and the subsequent removal of integrin receptors. This mechanistic understanding will support the rational design of nanotopography that directs cells on rigid materials to behave as if they were on soft ones.
Keywords: nanotopography, substrate stiffness, mechanotransduction, integrin, endocytosis, membrane curvature
Graphical Abstract

The stiffness of substrates has been shown to affect a wide range of cell behaviors such as stem cell differentiation,1–3 aging,4 and cancer cell invasion.5 In response to substrate stiffness, cells remodel their actin cytoskeleton and adapt their own stiffness and membrane tension.6,7 Underlying these changes is the mechanotransduction machinery that exerts cellular traction forces onto extracellular materials through integrin receptors, whose activation is force-dependent.8 Rigid substrates can withstand high traction forces for enhanced integrin activation, which results in increased signaling cascades in mechanotransduction. From the application point of view, most human tissues exhibit low stiffness (e.g., Young’s modulus ~1 kPa for brain tissues9) while many biomedical materials are high stiffness (e.g., Young’s modulus ~110 GPa for titanium implants). This stiffness disparity increases foreign body responses10–12 and causes cell behaviors on rigid materials to differ from those in native soft microenvironments.13,14 To meet this challenge, extensive efforts have been dedicated to developing soft materials, such as hydrogels, for interfacing with cells and tissues.15 However, soft materials do not meet all the demands of biodevices, such as implants that ought to provide mechanical support16,17 and bioelectronic devices that rely on noble metals.18
Alongside stiffness, surface topography is another important physical property of extracellular materials. It has been shown that nanoscale surface topography influences cell behaviors, such as adhesion,19 alignment,20–22 migration,23 and differentiation.24–26 Some studies revealed that nanoscale surface topography reduces actin stress fibers and focal adhesions,27–29 which are key components of the mechanotransduction machinery. Other studies reported that nanotopography affects the activity of yes-associated protein (YAP), a nuclear regulator involved in mechanotransduction.30,31 The documented effects of stiffness and nanotopography on mechanotransduction suggest that it may be possible to engineer surface topography to offset or reduce the stiffness-induced effects on cells. However, surface topography is defined by a high-dimensional space of features, such as domain size, shape, height, and spacing. The molecular mechanisms underlying how cells recognize topographical features to modulate mechanotransduction are not well understood. This hinders the rational design of surface topography.
In this work, we focus on the effects of nanotopography, and we compare, from behavioral to molecular levels, the cellular responses separately induced by stiffness and nanotopography. We demonstrate that nanotopography can substantially modulate cellular responses to rigid glass substrates such that they are similar to responses to soft hydrogels. Furthermore, we reveal that a key mechanism of topography sensing involves the endocytosis of integrin receptors, which is significantly enhanced by nanotopography-induced membrane deformation.
RESULTS AND DISCUSSION
Rigid Nanopillars Accelerate Neurite Outgrowth in Ways Similar to Soft Hydrogels.
We first compared how nanotopography and substrate stiffness modulate the development of embryonic neurons. Quartz has a Young’s modulus around 72 GPa, whereas the polyacrylamide hydrogels used in comparison have a Young’s modulus of 1.04 kPa (named 1 kPa hydrogel). Accurate topographical features of quartz nanopillars were fabricated by electron beam lithography and reactive ion etching (Figure 1a and Figure S1).32 Unlike vertical posts made of elastic materials,2,33 these quartz nanopillars cannot be bent by cells.34 We began with nanopillars similar to those used in earlier studies (200 nm diameter, 1 μm pitch, and 3 μm height). These nanopillars induced membrane curvatures as illustrated by the CellMask fluorescence imaging (Figure S2). By comparing cells on nanopillar areas and cells on flat areas of the same culture, we excluded variabilities associated with surface treatment and culture conditions.
Figure 1.

Cell behaviors on quartz nanotopography are similar to those on soft hydrogels. (a) Scanning electron microscopy (SEM) images of quartz nanopillars. The upper image shows an array of d200p1h3 nanopillars, and the lower image shows a single nanopillar. (b) Bright field and corresponding anti-MAP2 fluorescence images of E18 rat hippocampal neurons after 20 h of culture on flat quartz (flat), nanopillar quartz (nanopillar), and 1 kPa hydrogel (hydrogel) surfaces. Upper images show neurons on a quartz substrate that contains both flat areas and nanopillar areas. White dashed lines indicate the border between the flat and the nanopillar areas. Lower images show neurons cultured on three different surfaces. (c) Percentages of hippocampal neurons with at least one neurite longer than 35 μm (n = 193, 297, and 178 cells in flat, nanopillar, and hydrogel groups, respectively; mean ± s.e.m.). (d) Representative images of ALP staining and Oil Red O staining of hMSCs for osteogenic differentiation and adipogenic differentiation, respectively, on three different surfaces. (e) Percentages of hMSCs with positive ALP staining and positive Oil Red O staining, respectively (for ALP staining, n = 3856, 953, and 1353 cells in flat, nanopillar, and hydrogel groups, respectively; for Oil Red O staining, n = 5152, 5069, and 3819 cells in flat, nanopillar, and hydrogel groups, respectively; mean ± s.e.m.). P value in comparison to flat surface was determined by unpaired two-tailed t test. Scale bars, 2 μm (a, upper), 1 μm (a, lower), 20 μm (b), and 50 μm (d).
Neurons are one of the softest cell types in the human body, and substrate stiffness has been shown to affect neuronal development.14,35,36 We measured neurite outgrowth in response to three types of surfaces: rigid flat quartz, rigid nanopillar quartz, and soft flat hydrogel surfaces. 20 h after plating, embryonic E18 hippocampal neurons on flat quartz surfaces were mostly round with short extensions, consistent with previous studies using flat rigid substrates.35 In contrast, a significant fraction of neurons on nanopillar surfaces had long neurites in the same culture (Figures 1b and S3). Hippocampal neurons cultured on hydrogels also grew out long neurites after 20 h, agreeing with previous reports using soft substrates.14,36 Statistical analysis shows that the percentage of neurons having at least one neurite longer than 35 μm is significantly higher on nanopillars (24.7%) and 1 kPa hydrogels (19.0%) than on flat quartz (0.8%) (Figure 1c). These results demonstrate that nanotopography accelerates neurite outgrowth. We found that nanopillars of different dimensions also accelerated neurite outgrowth (Figure S4). A systematic study of how nanopillar dimensions affect cell behavior is presented in a later section.
Rigid Nanopillars Bias Stem Cell Differentiation in Ways Similar to Soft Hydrogels.
We then compared how nanotopography and stiffness modulate stem cell differentiation. Human mesenchymal stem cells (hMSCs) can differentiate into osteoblasts or adipocytes. Previous studies have separately shown that nanotopography and stiffness affect the differentiation efficiency of hMSCs into osteogenic or adipogenic lineages.1,29,37,38 In particular, microscale topographical regulation of stem cell differentiation has been well established.39,40 Here we compared hMSC differentiation outcomes on rigid flat quartz, rigid nanopillar quartz, and soft flat hydrogel surfaces. We first differentiated hMSCs toward the osteogenic lineage. Fourteen days after the induction of differentiation, hMSCs were stained for alkaline phosphatase (ALP). As shown in Figure 1d, there was much less ALP staining on nanopillar and soft hydrogel surfaces compared to flat quartz surfaces. Quantitative analysis shows a dramatic reduction of ALP-positive cells from 67.3% on flat quartz to 40.5% on nanopillar quartz surfaces, which is close to 33.9% on 1 kPa hydrogel surfaces (Figure 1e).
We also examined the differentiation of hMSCs toward the softer adipogenic lineage. Six days after the induction of differentiation, cells were stained with Oil Red O. We found that more cells were committed to the adipogenic lineage (positively stained in red) on nanopillars and hydrogels than on flat quartz surfaces (Figure 1d). Quantitative analysis confirms that hMSCs were differentiated into adipocytes with higher efficiency on nanopillars (23.7%) and hydrogels (26.3%) than on flat quartz surfaces (17.5%) (Figure 1e). These results regarding neurite outgrowth and hMSC differentiation demonstrate the possibility of engineering surface topography to make cells on rigid quartz substrates behave like those on soft hydrogels.
Rigid Nanopillars Reduce Cell Stiffness and Membrane Tension in Ways Similar to Soft Hydrogels.
It is known that mammalian cells adjust their own mechanics according to the stiffness of extracellular materials.6,7,41 We utilized atomic force microscopy (AFM) to compare how nanotopography and substrate stiffness affect cell stiffness and membrane tension. Contact-mode AFM shows that cells on flat quartz had visible cytoskeletal fibers surrounding the nucleus, but cells on nanopillars exhibited a drastically smaller shape with no discernible stress fibers (Figure 2a). For the cell stiffness measurement, we used a large spherical probe and applied small indentations (<500 nm) to ensure that a representative elasticity of the entire cell was obtained, where the elasticity of the cell cortex made the major contribution.42 Cell stiffness was extracted by fitting the Hertz indentation model to force–separation curves obtained from each cell (Figure 2b,c and Figure S5a,b). We found that cells on quartz nanopillars were significantly softer than cells on flat quartz with a 46% reduction of Young’s modulus and were comparable in stiffness to cells on 1 kPa hydrogels (Figure 2d). We next measured membrane tension by pulling tethers from the cell membrane using a sharp AFM probe. Membrane tethers are lipid nanotubes formed between the AFM probe and the cell surface, which can be stretched by the AFM probe to produce a constant force until rupture. The tether rupture force is proportional to the square root of the membrane tension43 and can be measured as the difference between the force plateaus before and after the rupture (Figure 2e and Figure S5c–e). From our measurements, the average tether force for cells on quartz nanopillars was 15% lower than on flat surfaces and is similar to that on 1 kPa hydrogel substrates (Figure 2f). These AFM measurements clearly demonstrate that cells on nanopillar quartz and soft hydrogels are much softer than cells on flat quartz surfaces.
Figure 2.
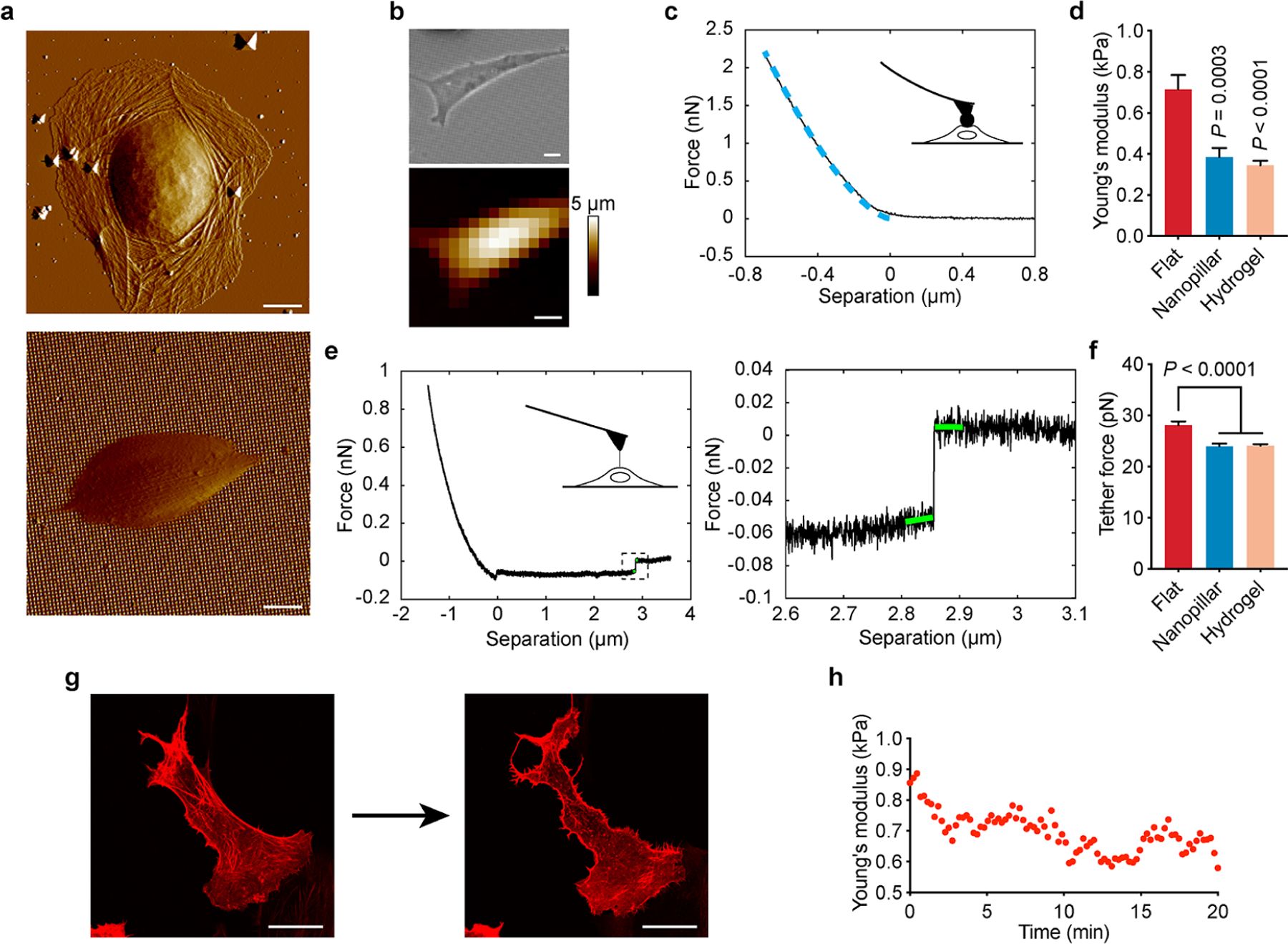
Quartz nanotopography reduces cell stiffness and membrane tension in ways similar to soft hydrogels. (a) Contact-mode AFM deflection images of U2OS cells show different cell shapes on flat quartz (upper) and d200p1h3 nanopillar quartz (lower) surfaces. (b) Optical image (upper) and AFM topographic map (lower) of a U2OS cell on the d200p1h3 nanopillar surface. (c) Representative AFM force–separation curve obtained by AFM indentation. The blue dashed line indicates the Hertz fit used to calculate Young’s modulus. (d) Quantitative analysis of the Young’s modulus of cells obtained by AFM indentation on flat quartz, d200p1h3 nanopillars, and 1 kPa hydrogel (n = 20, 20, and 21 cells in flat, nanopillar, and hydrogel groups, respectively; mean ± s.e.m). (e) Representative AFM retraction curve for pulling membrane tethers (right) and zoom-in view of a tether rupture event (left) in the retraction curve. The difference in the force before and after the rupture (indicated by green lines) was used to calculate the tether force. (f) Quantitative analysis of tether force obtained from cells on flat quartz, nanopillar quartz, and 1 kPa hydrogel surfaces (n = 21, 21, 22 cells in flat, nanopillar, and hydrogel groups, respectively; mean ± s.e.m.). (g) Fluorescence images of stress fibers (transfected with LifeAct-RFP) in a U2OS cell before and after 20 min of blebbistatin treatment. (h) Young’s modulus of a U2OS cell measured by AFM indentation over the 20 min blebbistatin treatment. P value in comparison to flat surfaces was determined by unpaired two-tailed t test (d, f). Scale bars, 10 μm (a, b), 20 μm (g).
Nanotopography has been shown to reduce actin stress fibers.27,30 To confirm the importance of actin fibers in modulating cell mechanics, we continuously monitored cell stiffness by AFM indentation while the cell was being treated by blebbistatin that disrupts actin stress fibers without affecting cell shape (Figure 2g). In situ AFM measurement of cells on flat quartz surfaces shows that blebbistatin treatment effectively reduced the cell stiffness (Figure 2h).
Nanotopography Inhibits YAP Activity When Cell Area and Shape Are Controlled.
We found that the presence of nanopillars on quartz substrates drastically decreased cell areas and YAP activity (measured by YAP nucleus/cytosol ratios) (Figure 3a,b and Figures S6 and S7), agreeing with previous nanotopography studies.29,30 For these measurements, cells were cultured at a low density to reduce cell–cell contact which is known to affect cell size and YAP activity.44 Previous studies have demonstrated that reducing cell size was sufficient to inhibit YAP activity.44–46 To determine whether nanotopography can affect the YAP activity independently of its effect on cell size, we employed a bioprinting method to precisely control cell size and shape. The bioprinting method uses ultraviolet light-induced cleavage to specify areas that are later coated with cell adhesion molecules such as gelatin.47 Unexposed areas are covered with polyethylene glycol to prevent cell adhesion. Hydrogel is not compatible with bioprinting and is not included here.
Figure 3.

Nanotopgraphy inhibits YAP activity when cell area and shape are constrained. (a) Quantitative analysis of cell areas on flat quartz, nanopillar quartz, and 1 kPa hydrogel surfaces (n = 291, 51, 63 cells in flat, nanopillar, and hydrogel groups, respectively; mean ± s.e.m.). (b) Quantitative analysis of YAP nucleus/cytosol ratios on flat quartz, nanopillar quartz, and 1 kPa hydrogel surfaces (n = 291, 51, 63 cells in flat, nanopillar, and hydrogel groups, respectively; mean ± s.e.m.). (c) Fluorescence images of hMSCs cultured on flat and d200p1h3 nanopillar surfaces in 2000 μm2 square areas confined by bioprinting. Cell shape was revealed by CellMask staining (red), and YAP localization was examined with anti-YAP immunostaining (green) and Hoechst staining (blue for nuclei). Dashed lines indicate the bioprinting areas. (d) Quantitative analysis of YAP nucleus/cytosol ratios for cells confined to 2000 μm2 square areas (n = 14 and 26 cells in flat and nanopillar groups, respectively; mean ± s.e.m.). P value in comparison to flat surface was determined by unpaired two-tailed t test. Scale bars: 10 μm (c).
We printed 2000 μm2 square areas on both flat and nanopillar quartz surfaces. Co-staining of YAP, the nucleus, and the cell membrane shows that YAP localization was generally more cytosolic on nanopillars than on flat quartz surfaces when the cell size and the shape were the same (Figure 3c,d). We note that when the two-dimensional spreading area was controlled to be the same, cells on nanopillars had larger total membrane contact areas than those on flat surfaces because the plasma membrane wrapped around vertical nanopillars. On flat surfaces, the membrane contact area is positively correlated with YAP activity.41 The observation that cells on nanopillars had a lower YAP activity despite having larger membrane contact areas further indicates that nanopillars can inhibit YAP activity through mechanisms independently of cell area.
Nanotopography Reduces Focal Adhesions by Enhancing the Endocytosis of Integrin Receptors.
To understand the topographical effect at the molecular level, we first confirmed that nanotopography modulates actin stress fibers and focal adhesions as previously reported,27–29 by immunostaining key protein components including filamentous actin (F-actin), integrin β1, vinculin, paxillin, and phosphorylated focal adhesion kinase (Figure 4a,b and Figure S8). On flat quartz surfaces, thick actin fibers were anchored on large focal adhesion patches, identified as elongated patches of integrin β1, vinculin, paxillin, or phosphorylated focal adhesion kinase. In contrast, on d200p1h3 nanopillar surfaces, actin fibers were much thinner, and focal adhesion proteins mostly were either diffusive or appeared as small puncta. Confocal microscope images projected in the z-direction confirmed that the observed reduction of focal adhesions and stress fibers was not due to the limited imaging depth when cells were cultured on nanopillars (Figure S9). We quantified the number of large focal adhesions (>1 μm2 in U2OS cells48) that are primarily responsible for force generation and substrate stiffness sensing. U2OS cells had about 20 large focal adhesions per cell when cultured on flat quartz surfaces, but they lost nearly all large focal adhesions on nanopillars (Figure 4c, Figure S8). This dramatic reduction of focal adhesions and stress fibers on nanopillars likely explains the reduction of cell stiffness on these surfaces.
Figure 4.

Nanotopography reduces focal adhesions by enhancing the endocytosis of integrin. (a) Fluorescence images of integrin β1 and F-actin in U2OS cells on flat quartz surfaces. (b) Fluorescence images of integrin β1 and F-actin in U2OS cells on nanopillar surfaces. (c) Quantification of large focal adhesion patches (>1 μm2) in U2OS cells based on the immunostaining of integrin β1. (d) Fluorescence images of endocytosed FM 1–43 dye in U2OS cells on flat and nanopillar surfaces. The images were obtained by summing the intensity of each pixel of the confocal images over a 12-μm depth with a 500 nm incremental step. (e) Quantitative analysis of endocytic vesicles in U2OS cells on flat quartz and nanopillar quartz surfaces (n = 48 and 30 cells in flat and nanopillar groups, respectively; mean ± s.e.m.). (f) Fluorescence images of integrin β1 affected by temperature-mediated endocytosis. Integrin β1 in U2OS cells was labeled with immunostaining (red). (g) Quantitative analysis of focal adhesion patches (>1 μm2) as a result of temperature-mediated endocytosis (n = 10 cells for all groups; mean ± s.e.m.). (h, i) Fluorescence images of paxillin (green) and F-actin (red) with and without an endocytosis inhibitor MDC treatment, on flat (h) and nanopillar (i) quartz surfaces, respectively. (j) Quantitative analysis of focal adhesion patches on flat and nanopillar surfaces before and after MDC treatment (n = 20, 34, 27, and 28 cells in flat (control), flat (MDC), nanopillar (control), and nanopillar (MDC) groups, respectively; mean ± s.e.m.). Nanopillars for these experiments had a d200p2.5h1 configuration. P values in comparison to flat surface (e), starting point group (g), or control groups (j) were determined by unpaired two-tailed t test. Scale bars, 20 μm (a, b, h, i), 10 μm (d, f), 4 μm (insets in (i)).
Integrin receptors play a key role in sensing substrate stiffness. Clathrin-dependent endocytosis (CME) has been shown to be the primary pathway for removing integrin receptors from the cell surface,49 which also causes the disassembly of focal adhesions.50 Recently, we showed that nanotopography enhances clathrin-mediated endocytosis (CME) by locally curving the cell membrane.51 Based on these studies, we hypothesize that nanotopography-enhanced endocytosis may be responsible for the reduction of focal adhesions on nanotopography.
We first demonstrated that cells on nanopillar quartz surfaces had more enhanced total endocytosis than cells on flat quartz surfaces. To measure endocytosis, cells were incubated with a membrane impermeable FM 1–43 dye for 15 min before the dye was washed out. As it is difficult to discern individual endocytic vesicles in small cells for endocytosis measurements, we used d200p2.5h1 (200 nm diameter, 2.5 μm pitch, and 1 μm height) nanopillars that do not reduce cell size as much as d200p1h3 nanopillars do. Z-stacks of confocal fluorescence images revealed that cells on nanopillar areas had much more fluorescence puncta than those on flat quartz surfaces (Figure 4d). Quantitative analysis confirms that cells on nanopillars had substantially more endocytosed vesicles labeled with FM 1–43 than those on flat quartz surfaces (Figure 4e).
U2OS cells primarily express integrin β1 and β5 isoforms. Substrates in this study were coated with gelatin, which is a binding ligand for integrin β1, but not integrin β5. Therefore, integrin β1 is the primary force receptor for the formation of focal adhesions on the substrates in this study. We followed an established protocol for a pulse-chase experiment to demonstrate that the activation of CME processes causes the removal of integrin β1 from the cell surface and subsequently the disassembly of focal adhesions.50 Briefly, cells were maintained at 4 °C to inhibit endocytosis and then stained with an antibody that recognizes the extracellular domain of integrin. Cells were then brought to 37 °C to recover endocytosis for 20 or 90 min, while control cells were maintained on ice during the same periods to inhibit endocytosis. We found that cells maintained on ice for 20 or 90 min retained a similar number of focal adhesions as 0 min, whereas cells maintained at 37 °C for 90 min had much fewer large integrin patches with the concurrent appearance of endosome-like spots in the intra-cellular domain (Figure 4f,g). Therefore, the activation of integrin endocytosis results in the disassembly of focal adhesions.
Finally, we demonstrated that blocking CME resulted in recovery of stress fibers and focal adhesions on nanopillar surfaces. We chose monodansylcadaverine (MDC) as a CME inhibitor. MDC is more specific than other endocytic inhibitors such as PitStop and does not cause observable cell morphology changes.52 Cells were treated with either 20 μM MDC or blank solutions for 30 min before they were fixed and stained for F-actin and paxillin. On flat quartz surfaces, MDC-treated cells and control cells had similar amounts of stress fibers and large focal adhesions (Figure 4h). On nanopillars, MDC-treated cells had much more stress fibers and large focal adhesions than control cells (Figure 4i). Quantification of focal adhesions shows that MDC treatment of cells on nanopillars significantly recovered focal adhesions (Figure 4j). These results indicate that the enhanced removal of integrin receptors by endocytosis is a key mechanism underlying the reduction of focal adhesions on nanopillar substrates. We noted that MDC treatment only partially recovered focal adhesions, which either indicates that there are additional topography-sensing mechanisms or that our MDC treatment did not sufficiently block integrin endocytosis induced by nanopillars, as the applied MDC concentration is limited by its cytotoxicity.53
Nanotopographical Configurations Fine-Tune Cellular Mechanotransduction.
To further support the hypothesis that nanotopography modulates mechanotransduction through membrane curvature-mediated endocytosis (Figure 5a), we systematically examined how nanotopographical configurations affect cell area and YAP activity. In this study, we varied nanopillar pitch from 1 to 5 μm, height from 1 to 3 μm, and diameter from 200 to 1000 nm (Figure 5b, Table S1). As references to different substrate stiffness, we made a series of polyacrylamide hydrogels ranging from 1 to 14 kPa (Table S2).
Figure 5.

Components of cellular mechanotransduction can be fine-tuned in a wide range by varying nanotopographical geometry. (a) Schematic illustration of mechanotransduction-related cellular mechanisms on flat and nanostructured substrates. (b) Schematic illustration indicating the diameter, pitch, and height of nanopillars. (c, d) Quantitative analyses of cell area (c) and YAP nucleus/cytosol ratio (d) of hMSCs on 200 nm-diameter nanopillars with varied pitches and heights (n = 15 to 291 cells, Table S3; mean ± s.e.m.). (e, f) Quantitative analyses of cell areas (e) and YAP nucleus/cytosol ratios (f) of hMSCs on nanopillars with varied diameters and heights and a constant diameter/pitch ratio (n = 15 to 291 cells, Table S3; mean ± s.e.m.). P value in comparison to the flat surface was determined by unpaired two-tailed t test (c-f).
We first varied the curved membrane area by changing the pitch and the height while keeping the nanopillar diameter constant. As shown in Figure 5c, the cell areas of hMSCs on flat quartz surfaces were substantially larger than those on hydrogels and quartz nanopillars. For the hydrogel group, cell areas decreased when the hydrogel stiffness was reduced, illustrating the stiffness effect.6,38,41 For nanopillars of the same height (either 1 or 3 μm), averaged cell areas decreased as the curved membrane area per substrate area was increased by reducing the pitch from 5 to 1 μm (Figure 5c). For nanopillars of the same spacing, averaged cell areas decreased as the height of nanopillars increases from 1 to 3 μm to increase curved membrane areas. Furthermore, the YAP nucleus/cytoplasm ratios decreased as the curved membrane area was increased by either reducing the pitch or increasing the height (Figure 5d).
Next, we varied the nanopillar diameters. We varied the pitch along with the diameter to keep a constant diameter/pitch ratio for efficient membrane wrapping. We found that 200-nm-diameter nanopillars induced a stronger reduction of cell area and YAP activity than 500-nm-diameter and 1000-nm-diamter nanopillars with the same height (Figure 5e,f). This observation agrees with our hypothesis based on nanotopography-enhanced CME, since we previously showed that CME was more enhanced by 200-nm-diameter nanopillars than by thicker ones.51 Measurements using U2OS cells revealed similar effects (Figure S10).
CONCLUSIONS
In this work, we demonstrate that nanotopography can drastically modulate cellular mechanotransduction such that cells respond to rigid nanopillar substrates (72 GPa) in ways similar to their responses to soft hydrogels (1–14 kPa). We reveal that nanotopography-induced membrane curvatures enhance the endocytosis of integrin receptors, which leads to fewer integrin receptors on the cell surface and subsequently the disassembly of focal adhesions and stress fibers. Extensive studies have indicated that soft substrates do not provide sufficient resistance to traction forces generated by stress fibers, whereas we and others have shown that nanotopographies induce membrane curvatures. Interestingly, although substrate stiffness and surface topography initially act in different ways, the two physical cues converge on modulating integrin receptors. These findings will support the rational design of surface topography for such purposes as making cells on a rigid implant behave as if the implant had a stiffness similar to native tissues.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH Grants 1R35GM141598 and 1R01GM128142. X.L. thanks the Banting Postdoctoral Fellowships program, administered by the Government of Canada. L.H.K. thanks support from the Carlsberg Foundation. We thank W. Zhao and A.F. McGuire of the B.C. group at Stanford University for help on nanofabrication, advice on experiments, and comments on the manuscript; L. Kaplan of the B.C. group for help on dissection; C.D. Lindsey of the S.C. Heilshorn group and A.J. Price of the A.R. Dunn group at Stanford University for sharing reagents and protocols; Y. Yang of the B.C. group and J. Wang of the J. Puglisi group at Stanford University for comments on the manuscript; M. Dong at Aarhus University and V.M. Weaver at UC San Francisco for advice on the project; and Stanford Nanofabrication Facility and Stanford Nano Shared Facilities for help on nanofabrication. Part of this work was performed at the Stanford Cell Sciences Imaging Facility, supported by Award 1S10OD021514-01 from the National Center for Research Resources.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.nanolett.1c01934.
Nanofabrication and surface functionalization of nanopillar quartz substrates, preparation and surface functionalization of polyacrylamide hydrogels, cell culture, isolation, and culture of hippocampal neurons, staining and imaging of hippocampal neurons, differentiation of stem cells, staining and imaging of stem cells, AFM measurement, staining and imaging of cell area and YAP, bioprinting, staining imaging of focal adhesions and stress fibers, imaging of total endocytosis, temperature-mediated endocytosis inhibition, and drug-induced endocytosis inhibition (PDF)
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.nanolett.1c01934
REFERENCES
- (1).Engler AJ; Sen S; Sweeney HL; Discher DE Matrix elasticity directs stem cell lineage specification. Cell 2006, 126 (4), 677–689. [DOI] [PubMed] [Google Scholar]
- (2).Sun YB; Yong KMA; Villa-Diaz LG; Zhang XL; Chen WQ; Philson R; Weng SN; Xu HX; Krebsbach PH; Fu JP Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater 2014, 13 (6), 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hadden WJ; Young JL; Holle AW; McFetridge ML; Kim DY; Wijesinghe P; Taylor-Weiner H; Wen JH; Lee AR; Bieback K; Vo BN; Sampson DD; Kennedy BF; Spatz JP; Engler AJ; Choi YS Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (22), 5647–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Engler AJ; Carag-Krieger C; Johnson CP; Raab M; Tang HY; Speicher DW; Sanger JW; Sanger JM; Discher DE Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci 2008, 121 (22), 3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Levental KR; Yu HM; Kass L; Lakins JN; Egeblad M; Erler JT; Fong SFT; Csiszar K; Giaccia A; Weninger W; Yamauchi M; Gasser DL; Weaver VM Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139 (5), 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Solon J; Levental I; Sengupta K; Georges PC; Janmey PA Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J 2007, 93 (12), 4453–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tee SY; Fu JP; Chen CS; Janmey PA Cell shape and substrate rigidity both regulate cell stiffness. Biophys. J 2011, 100 (5), L25–L27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Li J; Springer TA Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (18), 4685–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wells RG Tissue mechanics and fibrosis. Biochim. Biophys. Acta, Mol. Basis Dis 2013, 1832 (7), 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Salatino JW; Ludwig KA; Kozai TDY; Purcell EK Glial responses to implanted electrodes in the brain. Nat. Biomed Eng 2017, 1 (11), 862–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sridharan A; Rajan SD; Muthuswamy J Long-term changes in the material properties of brain tissue at the implant-tissue interface. J. Neural Eng 2013, 10 (6), 066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lacour SP; Courtine G; Guck J Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater 2016, 1 (10), 16063. [Google Scholar]
- (13).Jacot JG; McCulloch AD; Omens JH Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys. J 2008, 95 (7), 3479–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Tanaka A; Fujii Y; Kasai N; Okajima T; Nakashima H Regulation of neuritogenesis in hippocampal neurons using stiffness of extracellular microenvironment. PLoS One 2018, 13 (2), No. e0191928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zhang YS; Khademhosseini A Advances in engineering hydrogels. Science 2017, 356 (6337), eaaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gao X; Fraulob M; Haiat G Biomechanical behaviours of the bone-implant interface: a review. J. R. Soc., Interface 2019, 16 (156), 20190259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Smeets R; Stadlinger B; Schwarz F; Beck-Broichsitter B; Jung O; Precht C; Kloss F; Grobe A; Heiland M; Ebker T Impact of dental implant surface modifications on osseointegration. BioMed Res. Int 2016, 2016, 6285620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Merrill DR Materials considerations of implantable neuroengineering devices for clinical use. Curr. Opin. Solid State Mater. Sci 2014, 18 (6), 329–336. [Google Scholar]
- (19).Chen WQ; Villa-Diaz LG; Sun YB; Weng SN; Kim JK; Lam RHW; Han L; Fan R; Krebsbach PH; Fu JP Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano 2012, 6 (5), 4094–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ding YF; Sun JR; Ro HW; Wang Z; Zhou J; Lin NJ; Cicerone MT; Soles CL; Lin-Gibson S Thermodynamic underpinnings of cell alignment on controlled topographies. Adv. Mater 2011, 23 (3), 421–425. [DOI] [PubMed] [Google Scholar]
- (21).Jacchetti E; Di Rienzo C; Meucci S; Nocchi F; Beltram F; Cecchini M Wharton’s Jelly human Mesenchymal Stem Cell contact guidance by noisy nanotopographies. Sci. Rep 2015, 4, 3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sun JR; Ding YF; Lin NJ; Zhou J; Ro H; Soles CL; Cicerone MT; Lin-Gibson S Exploring cellular contact guidance using gradient nanogratings. Biomacromolecules 2010, 11 (11), 3067–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kwon KW; Park H; Song KH; Choi JC; Ahn H; Park MJ; Suh KY; Doh J Nanotopography-guided migration of T cells. J. Immunol 2012, 189 (5), 2266–2273. [DOI] [PubMed] [Google Scholar]
- (24).Wu YN; Law JBK; He AY; Low HY; Hui JHP; Lim CT; Yang Z; Lee EH Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomedicine 2014, 10 (7), 1507–1516. [DOI] [PubMed] [Google Scholar]
- (25).Teo BKK; Wong ST; Lim CK; Kung TYS; Yap CH; Ramagopal Y; Romer LH; Yim EKF Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 2013, 7 (6), 4785–4798. [DOI] [PubMed] [Google Scholar]
- (26).Antonini S; Meucci S; Parchi P; Pacini S; Montali M; Poggetti A; Lisanti M; Cecchini M Human mesenchymal stromal cell-enhanced osteogenic differentiation by contact interaction with polyethylene terephthalate nanogratings. Biomed Mater. 2016, 11 (4), 045003. [DOI] [PubMed] [Google Scholar]
- (27).Lou HY; Zhao WT; Li X; Duan LT; Powers A; Akamatsu M; Santoro F; McGuire AF; Cui Y; Drubin DG; Cui BX Membrane curvature underlies actin reorganization in response to nanoscale surface topography. Proc. Natl. Acad. Sci. U. S. A 2019, 116 (46), 23143–23151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kuo CW; Chueh DY; Chen PL Investigation of size-dependent cell adhesion on nanostructured interfaces. J. Nanobiotechnol 2014, 12, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Loye AM; Kinser ER; Bensouda S; Shayan M; Davis R; Wang R; Chen Z; Schwarz UD; Schroers J; Kyriakides TR Regulation of mesenchymal stem cell differentiation by nano-patterning of bulk metallic glass. Sci. Rep 2018, 8, 8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Hansel CS; Crowder SW; Cooper S; Gopal S; Joao Pardelha da Cruz M; de Oliveira Martins L; Keller D; Rothery S; Becce M; Cass AEG; Bakal C; Chiappini C; Stevens MM Nanoneedle-mediated stimulation of cell mechanotransduction machinery. ACS Nano 2019, 13 (3), 2913–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Song LQ; Wang K; Li Y; Yang Y Nanotopography promoted neuronal differentiation of human induced pluripotent stern cells. Colloids Surf., B 2016, 148, 49–58. [DOI] [PubMed] [Google Scholar]
- (32).Li X; Matino L; Zhang W; Klausen L; McGuire AF; Lubrano C; Zhao WT; Santoro F; Cui BX A nanostructure platform for live-cell manipulation of membrane curvature. Nat. Protoc 2019, 14 (6), 1772–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Fu JP; Wang YK; Yang MT; Desai RA; Yu XA; Liu ZJ; Chen CS Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 2010, 7 (9), 733–U95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Santoro F; Zhao WT; Joubert LM; Duan LT; Schnitker J; van de Burgt Y; Lou HY; Liu BF; Salleo A; Cui LF; Cui Y; Cui BX Revealing the cell-material interface with nanometer resolution by focused ion beam/scanning electron microscopy. ACS Nano 2017, 11 (8), 8320–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Barnes AP; Polleux F Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci 2009, 32, 347–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Chang TY; Chen C; Lee M; Chang YC; Lu CH; Lu ST; Wang DY; Wang A; Guo CL; Cheng PL Paxillin facilitates timely neurite initiation on soft-substrate environments by interacting with the endocytic machinery. eLife 2017, 6, No. e31101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wen JH; Vincent LG; Fuhrmann A; Choi YS; Hribar KC; Taylor-Weiner H; Chen SC; Engler AJ Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater 2014, 13 (10), 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Qian WY; Gong LQ; Cui X; Zhang ZJ; Bajpai A; Liu C; Castillo AB; Teo JCM; Chen WQ Nanotopographic regulation of human mesenchymal stem cell osteogenesis. ACS Appl. Mater. Interfaces 2017, 9 (48), 41794–41806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Abagnale G; Steger M; Nguyen VH; Hersch N; Sechi A; Joussen S; Denecke B; Merkel R; Hoffmann B; Dreser A; Schnakenberg U; Gillner A; Wagner W Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 2015, 61, 316–326. [DOI] [PubMed] [Google Scholar]
- (40).Ahn EH; Kim Y; Kshitiz; An SS; Afzal J; Lee S; Kwak M; Suh KY; Kim DH; Levchenko A Spatial control of adult stem cell fate using nanotopographic cues. Biomaterials 2014, 35 (8), 2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yeung T; Georges PC; Flanagan LA; Marg B; Ortiz M; Funaki M; Zahir N; Ming WY; Weaver V; Janmey PA Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 2005, 60 (1), 24–34. [DOI] [PubMed] [Google Scholar]
- (42).Vargas-Pinto R; Gong H; Vahabikashi A; Johnson M The effect of the endothelial cell cortex on atomic force microscopy measurements. Biophys. J 2013, 105 (2), 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Krieg M; Helenius J; Heisenberg CP; Muller DJ A bond for a lifetime: Employing membrane nanotubes from living cells to determine receptor-ligand kinetics. Angew. Chem., Int. Ed 2008, 47 (50), 9775–9777. [DOI] [PubMed] [Google Scholar]
- (44).Dupont S; Morsut L; Aragona M; Enzo E; Giulitti S; Cordenonsi M; Zanconato F; Le Digabel J; Forcato M; Bicciato S; Elvassore N; Piccolo S Role of YAP/TAZ in mechanotransduction. Nature 2011, 474 (7350), 179–83. [DOI] [PubMed] [Google Scholar]
- (45).von Erlach TC; Bertazzo S; Wozniak MA; Horejs CM; Maynard SA; Attwood S; Robinson BK; Autefage H; Kallepitis C; del Río Hernández A; Chen CS; Goldoni S; Stevens MM Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat. Mater 2018, 17 (3), 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Kilian KA; Bugarija B; Lahn BT; Mrksich M Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A 2010, 107 (11), 4872–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Strale PO; Azioune A; Bugnicourt G; Lecomte Y; Chahid M; Studer V Multiprotein printing by light-induced molecular adsorption. Adv. Mater 2016, 28 (10), 2024. [DOI] [PubMed] [Google Scholar]
- (48).Legerstee K; Geverts B; Slotman JA; Houtsmuller AB Dynamics and distribution of paxillin, vinculin, zyxin and VASP depend on focal adhesion location and orientation. Sci. Rep 2019, 9, 10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Bridgewater RE; Norman JC; Caswell PT Integrin trafficking at a glance. J. Cell Sci 2012, 125 (16), 3695–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Chao WT; Kunz J Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009, 583 (8), 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Zhao WT; Hanson L; Lou HY; Akamatsu M; Chowdary PD; Santoro F; Marks JR; Grassart A; Drubin DG; Cui Y; Cui BX Nanoscale manipulation of membrane curvature for probing endocytosis in live cells. Nat. Nanotechnol 2017, 12 (8), 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Guo S; Zhang X; Zheng M; Zhang X; Min C; Wang Z; Cheon SH; Oak MH; Nah SY; Kim KM Selectivity of commonly used inhibitors of clathrin-mediated and caveolae-dependent endocytosis of G protein-coupled receptors. Biochim. Biophys. Acta, Biomembr 2015, 1848 (10), 2101–2110. [DOI] [PubMed] [Google Scholar]
- (53).Gilad GM; Gilad VH Cytotoxic effects of monodansylcadaverine and methylamine in primary cultures of rat cerebellar neurons. Int. J. Dev. Neurosci 1986, 4 (5), 401–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


