Abstract
Nutrient transition metals are required cofactors for many proteins to perform functions necessary for life. As such, the concentration of nutrient metals is carefully maintained to retain critical biological processes while limiting toxicity. During infection, invading bacterial pathogens must acquire essential metals, such as zinc, manganese, iron, and copper from the host to colonize and cause disease. To combat this, the host exploits the essentiality and toxicity of nutrient metals by producing factors that limit metal availability, thereby starving pathogens or accumulating metals in excess to intoxicate the pathogen in a process termed “nutritional immunity”. As a result of inflammation, a heterogeneous environment containing both metal replete and deplete niches is created, whereby nutrient metal availability may play an underappreciated role in regulating immune cell function during infection. How the host manipulates nutrient metal availability during infection, and the downstream implication that nutrient metals and metal sequestering proteins have on immune cell function is discussed in this review.
The concentration of nutrient transition metals is carefully maintained to avoid both deficiency and toxicity, as nutrient metals such as zinc, manganese, iron, and copper are required cofactors for many proteins that are critical for life. Therefore, pathogens have evolved strategies to acquire essential transition metals from the host to colonize and cause disease. To combat pathogens, the host either accumulates metals in excess to intoxicate the pathogen or produces factors that sequester and starve the pathogen of essential metals through a process termed ‘nutritional immunity’ [1, 2]. By exploiting the essentiality and toxicity of nutrient metals, the distribution of metals are altered dramatically through systemic and local changes that modulate the accessibility of metals to invading pathogens. However, immune cells must also operate in these same environments, therefore, changes in metal concentrations play an underappreciated but valuable role in regulating immune cell function during infection. This review focuses on the strategies by which the host manipulates nutrient metal availability, and the downstream implications that nutrient metal and metal sequestering proteins have on immune cell function.
Metal sequestering proteins during inflammation
S100 Proteins
S100 proteins are EF-hand calcium-binding proteins, and a subset of these proteins are released extracellularly and play a key antimicrobial role in host defense through metal sequestration. The S100 protein complex calprotectin is a heterodimer of S100A8 and S100A9 that binds and sequesters zinc, manganese, iron, and nickel [3–5]. It is highly abundant in neutrophils making up nearly 50% of the cytosolic protein content [6], and is therefore one of the most abundant immune proteins at the host-pathogen interface. As a result, calprotectin has broad antimicrobial activity toward multiple important human pathogens including Staphylococcus aureus [7, 8], Acinetobacter baumannii [9], Clostridioides difficile [10], Candida albicans [11], Aspergillus fumigatus [12] Helicobacter pylori [13], and Mycobacterium tuberculosis [14]. In addition, there is increasing evidence that other S100 proteins contribute to nutritional immunity. For example, S100A7, psoriasin, is highly expressed by keratinocytes [15] and may play a critical role in regulating metal availability among the microbiota of the skin through its capacity to bind zinc [16]. S100A7 is selectively antibacterial toward harmful bacteria such as Escherichia coli, Pseudomonas aeruginosa, and S. aureus, while apparently having no effect on commensal bacteria [17]. The human S100A12, calgranulin C [18], is expressed by monocytes, neutrophils [19], and keratinocytes [20, 21] and exhibits zinc-dependent antimicrobial activity against P. aeruginosa, C. albicans, and E. coli [22]. In addition, it has been suggested that the binding of copper to S100A12 provides antiparasitic activity through the generation of superoxide [20, 23].
Metallothioneins
Metallothioneins are a family of metal-binding proteins that bind zinc and copper in cells [24, 25], but also alleviate metal toxicity by sequestering heavy metals such as cadmium and mercury [26]. Excess zinc induces metallothionein expression, while zinc deplete conditions cause metallothioneins to release Zn as a means to balance the intracellular zinc pool in response to cellular redox and energy states [27, 28]. Metallothionein 1 (MT1) and metallothionein 2 (MT2) cooperatively sequester zinc and readily release only one zinc ion, which is distinct from metallothionein 3 (MT3) that non-cooperatively binds zinc and assumes and “open conformation” that readily releases all bound zinc [29–31]. The literature on metallothionein 4 (MT4) is scarce; however, it is postulated that MT4 may function as a copper-thionein [25]. Intracellularly, metallothioneins regulate zinc availability in the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, lysosomes, cytosol, and potentially zincosomes [32–36]. In addition, metallothioneins have been detected extracellularly, and evidence suggests involvement in actively modulating extracellular cues [37–39]. In support for active secretion of metallothioneins, MT3 interacts with proteins involved in multiple cellular processes including secretion, which would enable targeting to the extracellular milieu [40]. Whether metallothioneins are actively secreted or passively released requires further study.
Iron sequestering proteins
Iron is an essential trace element that is most abundant within erythrocytes, complexed to heme moieties in hemoglobin. To limit the production of free radicals in healthy individuals, iron in the plasma is sequestered by transferrin or stored within macrophages, hepatocytes, and intestinal enterocytes. During inflammation, increased production of hepcidin by the liver, neutrophils, and macrophages inhibits iron secretion thereby facilitating a precipitous drop in free extracellular iron. In addition, the immune protein lactoferrin is released by neutrophils through degranulation and neutrophil extracellular trap (NET) formation, and elicits direct antimicrobial activity by sequestering iron from bacterial pathogens such as S. aureus [41, 42], P. aeruginosa, Burkholderia cenocepacia [43], and the parasite Pneumocystis carinii [44, 45].
The importance of the struggle for iron is highlighted by the arms race between host and pathogen. A primary strategy bacterial pathogens employ to acquire iron is the secretion of an arsenal of low molecular weight iron-binding compounds called siderophores that chelate environmental iron with an extraordinarily high affinity, whereupon the iron-siderophore complex is taken up by bacterial receptors (covered in-depth in other reviews [46, 47]). In response, neutrophils secrete lipocalin-2 to sequester bacterial siderophores, preventing their uptake by bacterial cells, and thus, iron starving pathogens like E. coli during infection [48]. Some pathogens, such as Bacillus anthracis and Salmonella Typhimurium, produce ‘stealth siderophores’ that contain structural modifications to preclude lipocalin-2 binding [49, 50]. Finally, much of the iron in the host is in the form of heme. As such, activation of pathogen recognition receptors triggers the secretion of cytokines, such as interleukin (IL)-6 and IL-22, that cause the liver to produce haptoglobin and hemopexin to sequester free hemoglobin and heme during infection [51–53].
Ceruloplasmin
While during infection many nutrient metals are reduced or restricted, a gradual rise in serum copper is a common hallmark of infection, regardless of the causative agent [54, 55]. This elevation in copper is likely attributed to the cuproprotein ceruloplasmin, which accounts for 95% of the copper content in serum. Coinciding with a role during infection, ceruloplasmin is strongly induced during infection [56] with a marked increase in protein levels [57–59]. It is possible that the increased abundance of ceruloplasmin delivers copper to the site of infection, as monocytes, granulocytes, and lymphocytes contain ceruloplasmin receptors [60]. However, a study assessing ceruloplasmin-deficient mice revealed a disruption in iron homeostasis, not copper [61], which suggests that as a ferroxidase, ceruloplasmin may help mobilize iron away from infected tissues.
Metal mobilization to combat pathogens in the phagosome
Upon engaging many pathogens, professional phagocytes internalize the pathogen into phagosomal compartments. In an effort to prevent intracellular replication within the phagosome, host phagocytes mobilize the subcellular distribution of metals to both exploit the essentiality and toxicity of nutrient metals. Zinc [62, 63] and copper [64–67] are actively accumulated within the phagosomal compartment through a process referred to as the ‘brass dagger’, while iron, manganese, and magnesium are depleted by natural resistance-associated macrophage protein 1 (NRAMP1) [68–70]. The mechanisms by which the host transports metals in the phagosome is covered in-depth in a previous review [71].
The influence of nutrient metal on immune cells
Zinc
The role of zinc in regulating immune cells is complex and the functional outcomes of zinc replete or deplete conditions likely vary depending on the cell type. Zinc deplete conditions increase the overall number of granulocytes and monocytes in circulation while attenuating lymphopoiesis and erythropoiesis [72] and promoting monocyte maturation [73]. In addition, stimulation with lipopolysaccharide (LPS) decreases intracellular zinc levels within dendritic cells, which is required for dendritic cell maturation and antigen presentation [74]. These findings suggest that zinc deplete environments may heighten the inflammatory potential; but this is not always the case as zinc supplementation increases the number of peritoneal macrophages in a Trypanosoma cruzi infection model [75].
Further underlying the necessity for zinc, many pro-inflammatory signaling pathways require zinc. For example, zinc associates with LPS and influences its fluidity, causing it to more effectively induce cytokine production in human peripheral blood mononuclear cells (PBMCs) [76]. In addition, LPS causes a transient spike of intracellular free zinc in mouse and human monocytes/macrophages, and this effect is required for activation of p38 mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [77]. As a result, zinc supplementation enhances LPS-mediated tumor necrosis factor-α (TNF-α) production by monocytes and PBMCs [76, 77]; however, this does not apply for all conditions as zinc depletion may also increase TNF-α production by monocytes in response to LPS [73]. Zinc is also necessary for NLRP3 inflammasome activation [78], where the addition of zinc leads to enhanced levels of IL-1β [76, 78].
Zinc homeostasis has a profound effect on immune cell function that can be cell specific during infection. In response to pathogens, zinc depletion impairs phagocytosis of bacteria by neutrophils [79], but high levels of intracellular zinc amplify the respiratory burst [80]. While zinc is required for the formation of NETs [81], high levels of zinc inhibits NET formation and degranulation [82]. By contrast, zinc supplementation enhances phagocytosis of E. coli, S. aureus, [83, 84], Trypanosoma musculi [85], and Candida krusei [86] by primary macrophages with no effect on RAW264.7 macrophage-like cells [87] or bone marrow-derived macrophages [88]. Interestingly, zinc depletion also increases phagocytosis by macrophages [79]. This suggests that zinc concentrations, whether low or high, affects phagocytosis similarly; however, the effect is opposite when comparing neutrophils and macrophages. Coinciding with enhanced phagocytosis by primary macrophages following zinc supplementation, zinc replete conditions promote a stronger respiratory burst by NADPH oxidase in response to E. coli [73] and S. aureus [89]. This brings up an interesting dichotomy. Following phagocytosis, zinc is mobilized into the phagosome to intoxicate the pathogen [62, 63]; however, high levels of zinc in the phagosome could lower the capacity for the NADPH oxidase to generate reactive oxygen species [90]. Adding further complications, macrophages obtained from E. coli-infected rats that have been supplemented with zinc produce higher levels of reactive oxygen species than rats not receiving zinc [91]. But, supplementation of zinc in vitro inhibits the reactive oxygen species production by macrophages isolated from rats undergoing septicemia [91]. This suggests that the mechanisms by which zinc homeostasis is maintained in vivo during infection is cell-specific and delicately balanced to maintain optimal immune cell function.
Metallothioneins also play a critical role in regulating zinc availability and immune cell function. A majority of the work assessing metallothionein function has occurred in T cells and this has been covered in-depth in a previous review [92]. In macrophages, metallothioneins play a critical role in regulating M1/M2 skewing. Mice lacking MT1 and MT2 fail to strongly induce TNFα in response to LPS, suggesting MT1 and MT2 are required for a pro-inflammatory (M1) macrophage response [93]. Additionally, these mice exhibit gross defects in antigen-presentation, expression of MHCII and co-stimulatory molecules, and cytokine production [94]. In response to the pro-inflammatory cytokine, granulocyte macrophage colony stimulating factor (GM-CSF), a pronounced induction of MT2 and mild upregulation of MT1 is observed, which curtails the intracellular growth of Histoplasma capsulatum by sequestering zinc [34]. In contrast, the anti-inflammatory cytokine, IL-4, strongly induces MT3 [95], which is required for an anti-inflammatory (M2) macrophage response [96]. In addition, MT3 expression elevates the labile zinc pool within the macrophage and facilitates the intracellular survival of H. capsulatum [95]. An immunological role for MT4 has not been identified.
Iron/Heme
Hepcidin is a master regulator of iron homeostasis. Inflammatory cytokines, such as IL-1, IL-6, and IL-22, as well as pathogen-associated molecular patterns (PAMPs), such as LPS, induce hepcidin expression and secretion [97–99], thereby blocking cellular export of iron by ferroportin [100]. As a result, macrophages and monocytes withhold higher levels of iron intracellularly. While this iron withholding strategy is beneficial to starve extracellular pathogens of iron, it also has a significant impact on immune cell function. Accumulation of intracellular iron affects the antimicrobial functions of macrophages via inhibition of IFN-γ-inducible effector pathways [101, 102]. In addition, accumulation of intracellular iron impairs the expression of inducible nitric oxide synthase (iNOS) [29, 103, 104], which renders macrophages less capable of clearing infections with the intracellular pathogens Salmonella enterica and typhimurium [105, 106], Chlamydia pneumoniae [107], C. albicans [108], and Legionella pneumophila [109]. In addition, macrophages are critical in recycling iron to meet the metal needs for erythropoiesis; however, delivery of iron for erythropoiesis is blunted by hepcidin during inflammation. The depletion of red blood cells limits the production of the hormone erythropoietin that inhibits NF-κB activation in inflammatory macrophages and reduces expression of iNOS, TNF-α, IL-6, and IL-12, and impairs clearance of S. Typhimurium [110]. The inhibitory effect of intracellular iron accumulation is not limited to macrophages as the antibacterial activity of neutrophils are similarly impaired, including having reduced phagocytosis [111–113], reactive oxygen species production [114], and NET formation [115].
Iron exists mostly in the form of heme in the host. Heme is a necessary cofactor for NADPH oxidase [116], which is critical for producing reactive oxygen species that elicit oxidative stress upon pathogens in the phagosome. In addition, it has been postulated that heme is necessary to sense whether pathogens are alive or dead within the phagosome. Engagement of pathogens by pattern recognition receptors induces the expression of heme oxygenase-1 (HO-1) [117, 118]. The catabolism of heme by HO-1 produces carbon monoxide that diffuses across the cellular membrane and accesses the heme-containing respiratory complexes of bacteria, thereby inducing the bacteria to produce ATP [119]. As a result, the produced ATP is sensed by the P2X7 purinergic receptor of macrophages promoting NLRP3 activation and IL-1β secretion, which is not observed in response to dead bacteria [119]. Mycobacteria can sense carbon monoxide production by the macrophage and subvert this sensory mechanism by converting to a ‘dormant state’ [120].
Heme has also been demonstrated to play a critical role in inducing monocyte differentiation into erythrophagocytic macrophages [121, 122] and contributing to polarization of macrophages. Retention of iron by ferritin has been associated with the polarization of macrophages toward microbicidal function, while the polarization of macrophages toward tissue repair is associated with enhanced iron secretion and heme catabolism [123]. Specifically, heme catabolism likely contributes to a positive feedback loop where in response to LPS, catabolism by HO-1 promotes IL-10 secretion [124], thereby inducing HO-1 expression via MAPK [125] and signal transducer and activator of transcription 3 (STAT3) [126]. However, lowering intracellular heme-iron in macrophages via NRAMP1 in response to the intracellular pathogen S. Typhimurium can have the opposite effect and induce a more inflammatory state through the expression of TNF-α, IL-6, and IL-12, while introducing exogenous iron promotes expression of IL-10 [127]. Underscoring the importance of iron and heme, over 60% of the genes associated with iron homeostasis are differentially expressed between inflammatory and anti-inflammatory macrophages, which argues that the availability of heme-iron and metabolism are closely linked to macrophage polarization [123]. This suggests that iron and heme may play a central role in detecting the presence of live pathogens at the site of infection, and in the overall inflammatory state of the immune response by regulating macrophage polarization.
While essential for immune cell function, heme can be weaponized by some pathogens to suppress the immune response. The intraerythrocytic protozoan parasite Plasmodium, the causative agent of malaria, catabolizes hemoglobin as an essential source of energy [128]. However, the liberation of heme by this process is toxic to Plasmodium species. Since Plasmodium species are unable to secrete free heme and do not possess a heme oxygenase, they instead aggregate the heme into an insoluble crystal called hemozoin [129]. Monocytes and macrophages internalize but fail to degrade hemozoin, resulting in impaired functionality, even though these cells remain viable. Phagocytes that accumulate hemozoin show a significant reduction in subsequent phagocytosis [130], generation of reactive oxygen species by the respiratory burst [131], and reduced antimicrobial activity toward ingested E. coli, S. aureus, and C. albicans [132]. In addition, hemozoin accumulation severely impairs the activity of protein kinase C [133], limits antigen presentation [134], reduces secretion of IL-6 [135], and promotes the release of TNF-α [135–137], IL-1β [136], nitric oxide [138, 139], and macrophage-inhibitory proteins 1α and 1β [137].
Manganese
Compared to zinc and iron, much less is known about how manganese influences immune cell function. Increasing concentrations of manganese enhances the attachment of neutrophils to different extracellular matrixes [140, 141], suggesting that manganese availability may influence chemotaxis and cellular motility. In addition, higher levels of manganese also heighten degranulation [142], the respiratory burst, and killing of E. coli in vitro [143]. In macrophages, manganese mediates host defense against DNA viruses by heightening the sensitivity of the DNA sensor cGAS and its downstream adaptor protein STING [144], as well as promoting dendritic cell and macrophage maturation [145]. Despite the many beneficial effects of manganese in amplifying antimicrobial functions, mice fed a high manganese diet are more susceptible to systemic S. aureus infections with increased bacterial burdens in the heart; a phenotype not observed during C. difficile or A. baumannii infections [146]. While the authors demonstrate that manganese is utilized by S. aureus to detoxify reactive oxygen species to protect against neutrophil killing [146], this does not fully explain the heart-specific nature of the phenotype and suggests that manganese may alter other aspects of the immune response that are particularly important within the niche of the heart.
Copper
Similar to manganese, the role of copper in regulating immune cell function during infection is incompletely understood. Copper also plays an important role in regulating neutrophil function. A deficiency in copper substantially decreases the numbers of circulating neutrophils [147, 148] as well as reduces their capacity to generate reactive oxygen species [149–151]; however phagocytosis is unaffected by reduced copper [151]. Similarly, an impaired respiratory burst compromises the capacity for macrophages to kill C. albicans [152] and S. typhimurium [153] during copper deficiency. By contrast, heightened dietary copper leads to an unsustainable immune response with enhanced reactive oxygen species production by macrophages and neutrophils, impaired phagocytosis [154] and NET formation [44], and increased degranulation [155] and mitochondrial-mediated apoptosis [44, 154].
The intersection between DAMP activity and nutritional immunity
Many metal sequestering proteins have pleotropic roles in the immune response by acting as a damage-associated molecular pattern (DAMP) and/or opsonin during infection. For example, calprotectin not only binds nutrient metals but is also a potent DAMP that activates toll-like receptor 4 (TLR4) [156], receptor for advanced glycation end products (RAGE) [157], and CD33 [158]. As a result, calprotectin acts as a powerful chemoattractant for myeloid cells during inflammation [159, 160]. The biological effects of calprotectin are not limited to infection, as heightened expression of S100A8 and S100A9 and subsequent DAMP activity has been identified as a critical promoter of multiple cancers including acute myeloid leukemia [158, 161, 162], pancreatic ductal adenocarcinoma [163], and breast cancer [164]. In addition to responding to and regulating zinc availability, metallothioneins can act as a chemokine [165]; although, a specific receptor has not been identified. A diverse array of immunological stressors rapidly induce the expression of metallothioneins, which may support the role of extracellular metallothioneins as a DAMP that is recognized as an early indication of infection or tissues damage. Lactoferrin can exhibit iron-independent antibacterial activity by directly interacting with the cell membrane of bacteria like Bacillus subtilis [166], Klebsiella pneumoniae [167], and S. epidermidis [168], disrupting LPS in the outer membrane of Gram-negative bacteria [169], and causing damage to the cell wall of fungal pathogens like C. albicans [170, 171]. However, lactoferrin seems to support the growth of beneficial commensal bacteria such as Bifidobacteria and Lactobacillus [172]. In addition, lactoferrin possesses many anti-inflammatory functions by suppressing pro-inflammatory signaling through TL4 [173], CD14 [174], and L-selectin [175]. Even though the molecular events are less clear, lipocalin-2 seems to regulate proliferation, apoptosis [176, 177], and pyroptosis [178]. While lipocalin-2 has primarily been studied in the context of nutritional immunity during infection, metastatic cancer cells can express lipocalin-2 to acquire iron in metal deplete environments. Cancer metastases in the cerebrospinal fluid express high levels of lipocalin-2 and cognate receptor SCL22A17 as a means of acquiring iron and sustaining proliferation [179]. Ceruloplasmin levels are elevated during inflammation and infection; however, the effects on disease pathogenesis are unclear. It has been suggested that ceruloplasmin may play a critical role in preventing excessive oxidative damage to the host by binding myeloperoxidase, thereby inhibiting production of the oxidant hypochlorous acid by neutrophils [180, 181]. A better understanding of the pleiotropic functions of metal sequestering proteins is necessary to fully elucidate the complex interactions during inflammation.
Concluding Remarks
The role of metals at the host-pathogen interface is complicated. While significant progress has been made to determine how zinc and iron/heme regulate immune cell function, much less is understood regarding how other metals, such as manganese and copper, are influencing immune cell function. As such, a significant gap exists in the complete understanding of metal biology at the host-pathogen interface. Additionally, much of the work assessing how nutrient transition metal availability affects the immune cell response to pathogens occur in extreme metal excess or deplete environments. However, most healthy individuals do not experience uniform alterations in metal abundance, and during infection metal is not uniformly distributed. As a result, conclusions derived from extreme metal excess and deplete environments may not accurately reflect what is occurring at the host-pathogen interface. One intriguing possibility is that like cytokines, chemokines, metabolites, DAMPs, and PAMPs establish gradients that influence immune cell function in vivo, nutrient metal may play an underappreciated role in regulating immune cell function during infection. Furthermore, how the host mobilizes metals during infection may change depending on the pathogen and site of infection. Advancements made in characterizing the spatial and temporal distribution of metal during infection are necessary to elucidate how metals are influencing immune cell function at the host-pathogen interface.
Adding to the complexity of the host-pathogen interface, bacterial pathogens like S. aureus [8, 182–188], A. baumannii [9, 189–194], and C. difficile [195, 196] express multiple metal acquisition systems and siderophores that would also influence metal availability. While much of the focus has been on how these bacterial strategies of metal acquisition influence bacterial survival and growth within the host, it is relatively unknown how these changes in metal availability affect immune cell function. It is possible that these bacterial-induced changes in metal availability are a signal indicating infection, thereby activating the immune response, or are a strategy employed by bacteria to skew immune cell function in a manner advantageous to the pathogen by dampening or enhancing inflammation. How bacterial-induced changes in metal availability influences immune cell function at the host-pathogen interface provides an intriguing future avenue of research.
Furthermore, it is unclear how metal sequestering proteins like calprotectin, metallothionein, lactoferrin, lipocalin-2, and ceruloplasmin alter metal availability for immune cells at the host-pathogen interface. Whether immune cells can scavenge nutrient metals from metal binding proteins or sequestering of nutrient metals from pathogens also starves host-cells for those same metals is unclear. In addition, the possible synergistic effects between the diverse functions of metal-binding proteins on immune cells is not known. For example, whether metal binding by calprotectin alters or synergizes with its DAMP activity and the downstream effects this has on host cell function is unknown. Further studies assessing the full pleiotropic functions of these metal-binding proteins are necessary to truly appreciate the complex role of metals and their effects on immune cells at the host-pathogen interface.
Figure 1. The effect of nutrient metal on immune cell function.
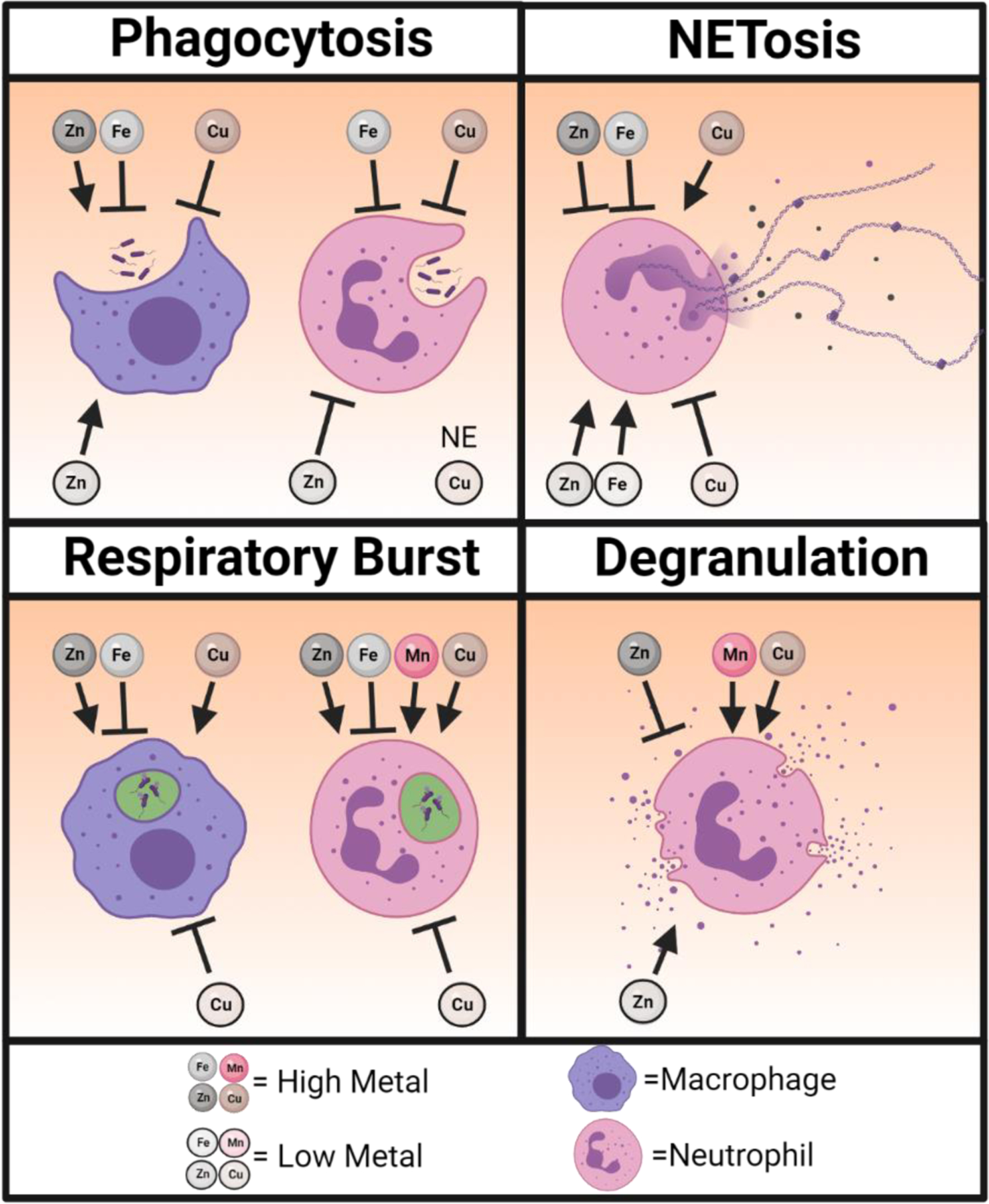
A graphical representation showing how nutrient metal (zinc = Zn, iron = Fe, manganese = Mn, copper = Cu) excess and deplete conditions alter neutrophil and macrophage antimicrobial functions in response to pathogens. Arrows represent conditions that enhance and blocked lines represent conditions that impair the indicated immune cell function. No effect (NE) on phagocytosis by neutrophils has been observed in copper deplete conditions. Created with BioRender.com.
Figure 2. Nutrient metals modulate signal transduction in immune cells.
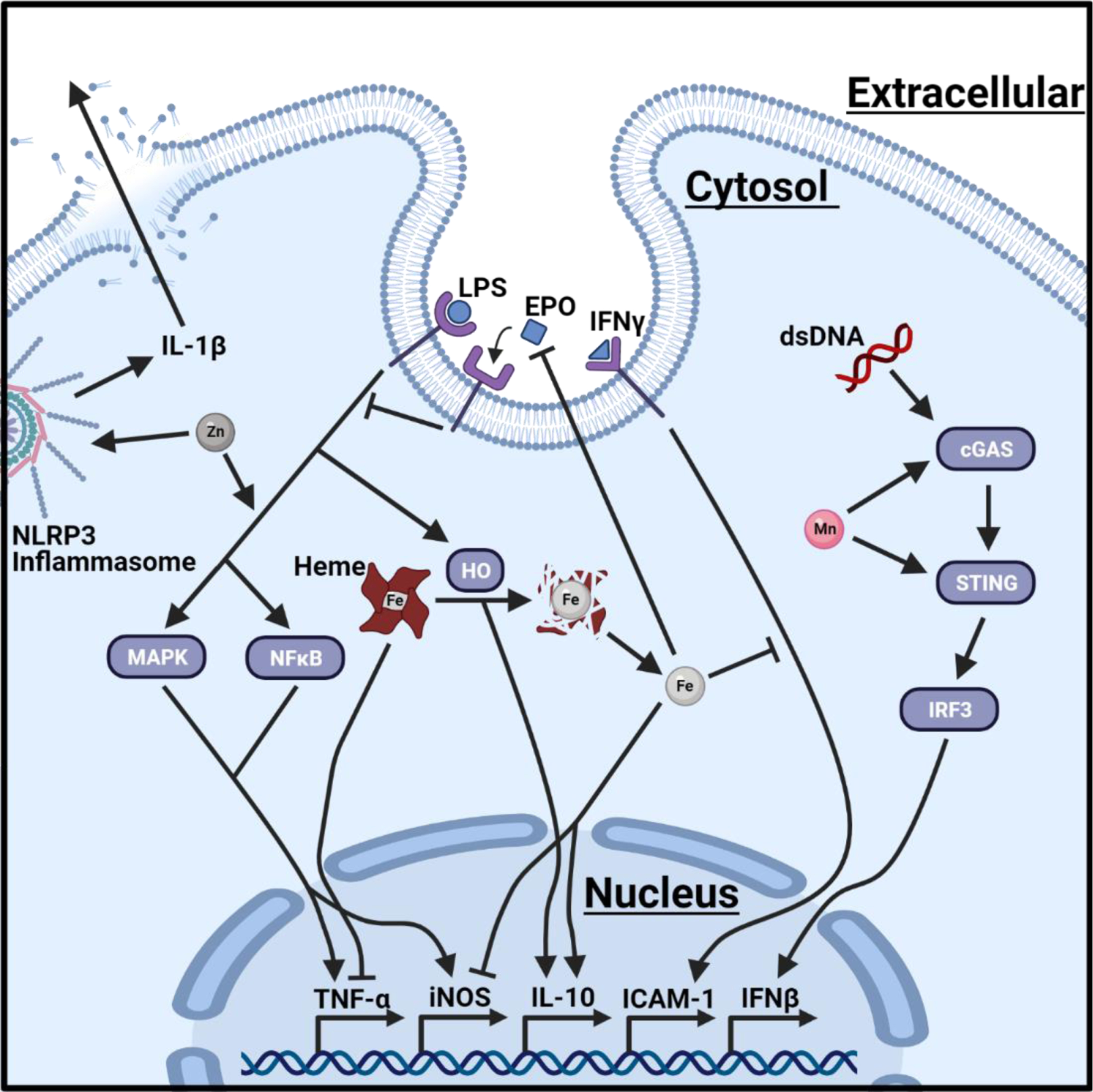
A graphical illustration of how intracellular levels of nutrient metals can influence signal transduction. Increased levels of cytosolic zinc (Zn) enhance IL-1β production from NLRP3 inflammasomes and LPS-mediated NF-κB and MAPK signaling. Elevated concentration of cytosolic manganese (Mn) heightens the sensitivity of cGAS and STING to double stranded DNA (dsDNA), thereby promoting the production of type-1 interferon (IFNβ). Accumulation of cytosolic heme suppresses the expression of TNF-α. LPS signaling promotes heme oxygenase (HO)-mediated degradation of heme, which reduces intracellular heme levels but produces carbon monoxide that activates IL-10. Heme degradation also increases intracellular concentrations of iron (Fe), which restricts pro-inflammatory signaling by impairing IFN-γ inducible effector pathways, including the intercellular adhesion molecule 1 (ICAM-1), and simultaneously repressing iNOS and promoting IL-10. In addition, increased intracellular storage of iron indirectly alters immune cell activation by reducing erythropoiesis, which prevents production of the hormone erythropoietin (EPO). Created with BioRender.com.
Highlights.
During inflammation, nutrient metals are redistributed to starve and/or intoxicate invading pathogens.
The availability of nutrient metals influences the response of innate immune cells to pathogens during infection.
Metal sequestering proteins have pleotropic functions at the host-pathogen interface that dictate disease outcome.
Outstanding Questions.
What role do concentration gradients of nutritional metals in the tissue play in regulating innate immune cell function during infection?
Are immune cells capable of scavenging nutrient metals from metal binding proteins in metal deplete environment during infection or do metal binding proteins also starve host-cells for nutrient metals?
Do synergistic effects exist between metal sequestration and the diverse function of metal-binding proteins and what implications does this have on immune cell function and disease outcome?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinberg ED (1975) Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA 231 (1), 39–41. [DOI] [PubMed] [Google Scholar]
- 2.Hood MI and Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10 (8), 525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damo SM et al. (2013) Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci USA 110 (10), 3841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashige TG et al. (2015) Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol 11 (10), 765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakashige TG et al. (2017) Nickel Sequestration by the Host-Defense Protein Human Calprotectin. J Am Chem Soc 139 (26), 8828–8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgeworth J et al. (1991) Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem 266 (12), 7706–13. [PubMed] [Google Scholar]
- 7.Corbin BD et al. (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319 (5865), 962–5. [DOI] [PubMed] [Google Scholar]
- 8.Kehl-Fie TE et al. (2013) MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect Immun 81 (9), 3395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hood MI et al. (2012) Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8 (12), e1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zackular JP et al. (2016) Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med 22 (11), 1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urban CF et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5 (10), e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark HL et al. (2016) Zinc and Manganese Chelation by Neutrophil S100A8/A9 (Calprotectin) Limits Extracellular Aspergillus fumigatus Hyphal Growth and Corneal Infection. J Immunol 196 (1), 336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaddy JA et al. (2014) The host protein calprotectin modulates the Helicobacter pylori cag type IV secretion system via zinc sequestration. PLoS Pathog 10 (10), e1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dow A et al. (2021) Zinc limitation triggers anticipatory adaptations in Mycobacterium tuberculosis. PLoS Pathog 17 (5), e1009570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broome AM et al. (2003) S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem 51 (5), 675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodersen DE et al. (1999) Zinc-binding site of an S100 protein revealed. Two crystal structures of Ca2+-bound human psoriasin (S100A7) in the Zn2+-loaded and Zn2+-free states. Biochemistry 38 (6), 1695–704. [DOI] [PubMed] [Google Scholar]
- 17.Glaser R et al. (2005) Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol 6 (1), 57–64. [DOI] [PubMed] [Google Scholar]
- 18.Moroz OV et al. (2009) The crystal structures of human S100A12 in apo form and in complex with zinc: new insights into S100A12 oligomerisation. J Mol Biol 391 (3), 536–51. [DOI] [PubMed] [Google Scholar]
- 19.Guignard F et al. (1995) Identification and characterization of a novel human neutrophil protein related to the S100 family. Biochem J 309 (Pt 2), 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottsch JD et al. (1999) Calgranulin C has filariacidal and filariastatic activity. Infect Immun 67 (12), 6631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottsch JD et al. (1999) Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol 91 (1), 34–40. [DOI] [PubMed] [Google Scholar]
- 22.Cole AM et al. (2001) Calcitermin, a novel antimicrobial peptide isolated from human airway secretions. FEBS Lett 504 (1–2), 5–10. [DOI] [PubMed] [Google Scholar]
- 23.Moroz OV et al. (2003) Structure of the human S100A12-copper complex: implications for host-parasite defence. Acta Crystallogr D Biol Crystallogr 59 (Pt 5), 859–67. [DOI] [PubMed] [Google Scholar]
- 24.Maret W and Vallee BL (1998) Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci USA 95 (7), 3478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tio L et al. (2004) Functional differentiation in the mammalian metallothionein gene family: metal binding features of mouse MT4 and comparison with its paralog MT1. J Biol Chem 279 (23), 24403–13. [DOI] [PubMed] [Google Scholar]
- 26.Piotrowski JK et al. (1974) Binding of cadmium and mercury by metallothionein in the kidneys and liver of rats following repeated administration. Arch Toxicol 32 (4), 351–60. [DOI] [PubMed] [Google Scholar]
- 27.Jiang LJ et al. (1998) The ATP-metallothionein complex. Proc Natl Acad Sci USA 95 (16), 9146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maret W (1994) Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci USA 91 (1), 237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melillo G et al. (1997) Functional requirement of the hypoxia-responsive element in the activation of the inducible nitric oxide synthase promoter by the iron chelator desferrioxamine. J Biol Chem 272 (18), 12236–43. [DOI] [PubMed] [Google Scholar]
- 30.Palumaa P et al. (2002) Brain-specific metallothionein-3 has higher metal-binding capacity than ubiquitous metallothioneins and binds metals noncooperatively. Biochemistry 41 (19), 6158–63. [DOI] [PubMed] [Google Scholar]
- 31.Wang H et al. (2006) Solution structure and dynamics of human metallothionein-3 (MT-3). FEBS Lett 580 (3), 795–800. [DOI] [PubMed] [Google Scholar]
- 32.Levadoux M et al. (1999) Nuclear import of metallothionein requires its mRNA to be associated with the perinuclear cytoskeleton. J Biol Chem 274 (49), 34961–6. [DOI] [PubMed] [Google Scholar]
- 33.Qin Y et al. (2011) Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci USA 108 (18), 7351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian Vignesh K et al. (2013) Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39 (4), 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wellenreuther G et al. (2009) The ligand environment of zinc stored in vesicles. Biochem Biophys Res Commun 380 (1), 198–203. [DOI] [PubMed] [Google Scholar]
- 36.Ye B et al. (2001) Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci USA 98 (5), 2317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynes MA et al. (1993) Immunomodulatory activities of extracellular metallothionein. I. Metallothionein effects on antibody production. Toxicology 85 (2–3), 161–77. [DOI] [PubMed] [Google Scholar]
- 38.Lynes MA et al. (1990) Extracellular metallothionein effects on lymphocyte activities. Mol Immunol 27 (3), 211–9. [DOI] [PubMed] [Google Scholar]
- 39.Youn J et al. (1995) Immunomodulatory activities of extracellular metallothionein. II. Effects on macrophage functions. J Toxicol Environ Health 45 (4), 397–413. [DOI] [PubMed] [Google Scholar]
- 40.El Ghazi I et al. (2010) New proteins found interacting with brain metallothionein-3 are linked to secretion. Int J Alzheimers Dis 2011, 208634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhimani RS et al. (1999) Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J Appl Microbiol 86 (1), 135–44. [DOI] [PubMed] [Google Scholar]
- 42.Hammerschmidt S et al. (1999) Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect Immun 67 (4), 1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berlutti F et al. (2005) Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int J Immunopathol Pharmacol 18 (4), 661–70. [DOI] [PubMed] [Google Scholar]
- 44.Cirioni O et al. (2000) Inhibition of growth of Pneumocystis carinii by lactoferrins alone and in combination with pyrimethamine, clarithromycin and minocycline. J Antimicrob Chemother 46 (4), 577–82. [DOI] [PubMed] [Google Scholar]
- 45.Weinberg GA (1994) Iron chelators as therapeutic agents against Pneumocystis carinii. Antimicrob Agents Chemother 38 (5), 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer LD and Skaar EP (2016) Transition Metals and Virulence in Bacteria. Annu Rev Genet 50, 67–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheldon JR et al. (2016) Iron Acquisition Strategies of Bacterial Pathogens. Microbiol Spectr 4 (2). [DOI] [PubMed] [Google Scholar]
- 48.Flo TH et al. (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432 (7019), 917–21. [DOI] [PubMed] [Google Scholar]
- 49.Abergel RJ et al. (2006) Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc Natl Acad Sci USA 103 (49), 18499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raffatellu M et al. (2009) Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5 (5), 476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakamoto K et al. (2017) IL-22 Controls Iron-Dependent Nutritional Immunity Against Systemic Bacterial Infections. Sci Immunol 2 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres VJ et al. (2006) Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol 188 (24), 8421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mikkelsen JH et al. (2020) The human protein haptoglobin inhibits IsdH-mediated heme-sequestering by Staphylococcus aureus. J Biol Chem 295 (7), 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garduno Espinosa J et al. (1991) Response of serum zinc and copper to inflammatory stimulation in the rat with chronic liver damage. Arch Invest Med (Mex) 22 (3–4), 289–94. [PubMed] [Google Scholar]
- 55.Milanino R et al. (1993) Copper and zinc body levels in inflammation: an overview of the data obtained from animal and human studies. Agents Actions 39 (3–4), 195–209. [DOI] [PubMed] [Google Scholar]
- 56.Eckersall PD et al. (1996) The acute phase response of acid soluble glycoprotein, alpha(1)-acid glycoprotein, ceruloplasmin, haptoglobin and C-reactive protein, in the pig. Vet Immunol Immunopathol 51 (3–4), 377–85. [DOI] [PubMed] [Google Scholar]
- 57.Cernat RI et al. (2011) Serum trace metal and ceruloplasmin variability in individuals treated for pulmonary tuberculosis. Int J Tuberc Lung Dis 15 (9), 1239–45, i. [DOI] [PubMed] [Google Scholar]
- 58.Kocyigit A et al. (1998) Alterations of serum selenium, zinc, copper, and iron concentrations and some related antioxidant enzyme activities in patients with cutaneous leishmaniasis. Biol Trace Elem Res 65 (3), 271–81. [DOI] [PubMed] [Google Scholar]
- 59.Novikova I and Zlotnikova M (2011) Ceruloplasmin plasma levels in patients with severe forms of herpes infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155 (4), 361–6. [DOI] [PubMed] [Google Scholar]
- 60.Kataoka M and Tavassoli M (1985) Identification of ceruloplasmin receptors on the surface of human blood monocytes, granulocytes, and lymphocytes. Exp Hematol 13 (8), 806–10. [PubMed] [Google Scholar]
- 61.Harris ZL et al. (1999) Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA 96 (19), 10812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Botella H et al. (2011) Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10 (3), 248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDevitt CA et al. (2011) A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7 (11), e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achard ME et al. (2012) Copper redistribution in murine macrophages in response to Salmonella infection. Biochem J 444 (1), 51–7. [DOI] [PubMed] [Google Scholar]
- 65.Osman D et al. (2010) Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem 285 (33), 25259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner D et al. (2005) Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol 174 (3), 1491–500. [DOI] [PubMed] [Google Scholar]
- 67.White C et al. (2009) A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem 284 (49), 33949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gruenheid S et al. (1997) Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med 185 (4), 717–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jabado N et al. (2000) Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med 192 (9), 1237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunrath O and Bumann D (2019) Host resistance factor SLC11A1 restricts Salmonella growth through magnesium deprivation. Science 366 (6468), 995–999. [DOI] [PubMed] [Google Scholar]
- 71.Sheldon JR and Skaar EP (2019) Metals as phagocyte antimicrobial effectors. Curr Opin Immunol 60, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.King LE and Fraker PJ (2002) Zinc deficiency in mice alters myelopoiesis and hematopoiesis. J Nutr 132 (11), 3301–7. [DOI] [PubMed] [Google Scholar]
- 73.Dubben S et al. (2010) Cellular zinc homeostasis is a regulator in monocyte differentiation of HL-60 cells by 1 alpha,25-dihydroxyvitamin D3. J Leukoc Biol 87 (5), 833–44. [DOI] [PubMed] [Google Scholar]
- 74.Kitamura H et al. (2006) Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol 7 (9), 971–7. [DOI] [PubMed] [Google Scholar]
- 75.Brazao V et al. (2008) Zinc supplementation increases resistance to experimental infection by Trypanosoma cruzi. Vet Parasitol 154 (1–2), 32–7. [DOI] [PubMed] [Google Scholar]
- 76.Wellinghausen N et al. (1996) Stimulation of human peripheral blood mononuclear cells by zinc and related cations. Cytokine 8 (10), 767–71. [DOI] [PubMed] [Google Scholar]
- 77.Haase H et al. (2008) Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol 181 (9), 6491–502. [DOI] [PubMed] [Google Scholar]
- 78.Brough D et al. (2009) Pannexin-1-dependent caspase-1 activation and secretion of IL-1beta is regulated by zinc. Eur J Immunol 39 (2), 352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ercan MT and Bor NM (1991) Phagocytosis by macrophages in zinc-deficient rats. Int J Rad Appl Instrum B 18 (7), 765–8. [DOI] [PubMed] [Google Scholar]
- 80.Freitas M et al. (2010) Zinc activates neutrophils’ oxidative burst. Biometals 23 (1), 31–41. [DOI] [PubMed] [Google Scholar]
- 81.Hasan R et al. (2013) Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun 19 (3), 253–64. [DOI] [PubMed] [Google Scholar]
- 82.Kuzmicka W et al. (2020) Zinc Supplementation Modulates NETs Release and Neutrophils’ Degranulation. Nutrients 13 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nowak JE et al. (2012) Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr Crit Care Med 13 (5), e323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sheikh A et al. (2010) Zinc influences innate immune responses in children with enterotoxigenic Escherichia coli-induced diarrhea. J Nutr 140 (5), 1049–56. [DOI] [PubMed] [Google Scholar]
- 85.Humphrey PA et al. (1997) Hepatic cells’ mitotic and peritoneal macrophage phagocytic activities during Trypanosoma musculi infection in zinc-deficient mice. J Natl Med Assoc 89 (4), 259–67. [PMC free article] [PubMed] [Google Scholar]
- 86.Salvin SB et al. (1987) The effect of dietary zinc and prothymosin alpha on cellular immune responses of RF/J mice. Clin Immunol Immunopathol 43 (3), 281–8. [DOI] [PubMed] [Google Scholar]
- 87.Triboulet S et al. (2014) Analysis of cellular responses of macrophages to zinc ions and zinc oxide nanoparticles: a combined targeted and proteomic approach. Nanoscale 6 (11), 6102–14. [DOI] [PubMed] [Google Scholar]
- 88.Gao H et al. (2017) Metal transporter Slc39a10 regulates susceptibility to inflammatory stimuli by controlling macrophage survival. Proc Natl Acad Sci USA 114 (49), 12940–12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mayer LS et al. (2014) Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics 6 (7), 1288–95. [DOI] [PubMed] [Google Scholar]
- 90.Prasad AS et al. (2004) Antioxidant effect of zinc in humans. Free Radic Biol Med 37 (8), 1182–90. [DOI] [PubMed] [Google Scholar]
- 91.Srinivas U et al. (1989) Superoxide production of peritoneal macrophages in experimental gram-negative sepsis; influence of in vitro and in vivo supplements of zinc. APMIS 97 (8), 682–8. [DOI] [PubMed] [Google Scholar]
- 92.Subramanian Vignesh K and Deepe GS Jr. (2017) Metallothioneins: Emerging Modulators in Immunity and Infection. Int J Mol Sci 18 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kanekiyo M et al. (2002) Metallothionein modulates lipopolysaccharide-stimulated tumour necrosis factor expression in mouse peritoneal macrophages. Biochem J 361 (Pt 2), 363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sugiura T et al. (2004) Dysfunction of macrophages in metallothionein-knock out mice. J UOEH 26 (2), 193–205. [DOI] [PubMed] [Google Scholar]
- 95.Subramanian Vignesh K et al. (2016) IL-4 Induces Metallothionein 3- and SLC30A4-Dependent Increase in Intracellular Zn(2+) that Promotes Pathogen Persistence in Macrophages. Cell Rep 16 (12), 3232–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chowdhury D et al. (2019) Metallothionein 3 Controls the Phenotype and Metabolic Programming of Alternatively Activated Macrophages. Cell Rep 27 (13), 3873–3886 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Armitage AE et al. (2011) Hepcidin regulation by innate immune and infectious stimuli. Blood 118 (15), 4129–39. [DOI] [PubMed] [Google Scholar]
- 98.Nemeth E et al. (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101 (7), 2461–3. [DOI] [PubMed] [Google Scholar]
- 99.Pigeon C et al. (2001) A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276 (11), 7811–9. [DOI] [PubMed] [Google Scholar]
- 100.Nemeth E et al. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306 (5704), 2090–3. [DOI] [PubMed] [Google Scholar]
- 101.Oexle H et al. (2003) Pathways for the regulation of interferon-gamma-inducible genes by iron in human monocytic cells. J Leukoc Biol 74 (2), 287–94. [DOI] [PubMed] [Google Scholar]
- 102.Weiss G et al. (1992) Iron modulates interferon-gamma effects in the human myelomonocytic cell line THP-1. Exp Hematol 20 (5), 605–10. [PubMed] [Google Scholar]
- 103.Dlaska M and Weiss G (1999) Central role of transcription factor NF-IL6 for cytokine and iron-mediated regulation of murine inducible nitric oxide synthase expression. J Immunol 162 (10), 6171–7. [PubMed] [Google Scholar]
- 104.Weiss G et al. (1994) Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med 180 (3), 969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chlosta S et al. (2006) The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun 74 (5), 3065–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nairz M et al. (2007) The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol 9 (9), 2126–40. [DOI] [PubMed] [Google Scholar]
- 107.Bellmann-Weiler R et al. (2013) Neutrophil gelatinase-associated lipocalin and interleukin-10 regulate intramacrophage Chlamydia pneumoniae replication by modulating intracellular iron homeostasis. Immunobiology 218 (7), 969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mencacci A et al. (1997) Iron overload alters innate and T helper cell responses to Candida albicans in mice. J Infect Dis 175 (6), 1467–76. [DOI] [PubMed] [Google Scholar]
- 109.Paradkar PN et al. (2008) Iron depletion limits intracellular bacterial growth in macrophages. Blood 112 (3), 866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nairz M et al. (2013) Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med 210 (5), 855–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cantinieaux B et al. (1993) Neutrophils from patients with secondary haemosiderosis contain excessive amounts of autotoxic iron. Eur J Haematol 51 (3), 161–5. [DOI] [PubMed] [Google Scholar]
- 112.van Asbeck BS et al. (1984) Deferoxamine enhances phagocytic function of human polymorphonuclear leukocytes. Blood 63 (3), 714–20. [PubMed] [Google Scholar]
- 113.van Asbeck BS et al. (1984) Effect of iron (III) in the presence of various ligands on the phagocytic and metabolic activity of human polymorphonuclear leukocytes. J Immunol 132 (2), 851–6. [PubMed] [Google Scholar]
- 114.Renassia C et al. (2020) Neutrophils from hereditary hemochromatosis patients are protected from iron excess and are primed. Blood Adv 4 (16), 3853–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vollger L et al. (2016) Iron-chelating agent desferrioxamine stimulates formation of neutrophil extracellular traps (NETs) in human blood-derived neutrophils. Biosci Rep 36 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu L et al. (1998) Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci USA 95 (14), 7993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gozzelino R et al. (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50, 323–54. [DOI] [PubMed] [Google Scholar]
- 118.Rushworth SA et al. (2005) Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol 175 (7), 4408–15. [DOI] [PubMed] [Google Scholar]
- 119.Wegiel B et al. (2014) Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest 124 (11), 4926–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shiloh MU et al. (2008) Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3 (5), 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haldar M et al. (2014) Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell 156 (6), 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kohyama M et al. (2009) Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature 457 (7227), 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Recalcati S et al. (2010) Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol 40 (3), 824–35. [DOI] [PubMed] [Google Scholar]
- 124.Otterbein LE et al. (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6 (4), 422–8. [DOI] [PubMed] [Google Scholar]
- 125.Lee TS and Chau LY (2002) Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8 (3), 240–6. [DOI] [PubMed] [Google Scholar]
- 126.Ricchetti GA et al. (2004) Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol 76 (3), 719–26. [DOI] [PubMed] [Google Scholar]
- 127.Fritsche G et al. (2008) Nramp1-functionality increases iNOS expression via repression of IL-10 formation. Eur J Immunol 38 (11), 3060–7. [DOI] [PubMed] [Google Scholar]
- 128.Pandey AV and Tekwani BL (1996) Formation of haemozoin/beta-haematin under physiological conditions is not spontaneous. FEBS Lett 393 (2–3), 189–93. [DOI] [PubMed] [Google Scholar]
- 129.Slater AF et al. (1991) An iron-carboxylate bond links the heme units of malaria pigment. Proc Natl Acad Sci USA 88 (2), 325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schwarzer E et al. (1992) Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med 176 (4), 1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schwarzer E and Arese P (1996) Phagocytosis of malarial pigment hemozoin inhibits NADPH-oxidase activity in human monocyte-derived macrophages. Biochim Biophys Acta 1316 (3), 169–75. [DOI] [PubMed] [Google Scholar]
- 132.Fiori PL et al. (1993) Reduced microbicidal and anti-tumour activities of human monocytes after ingestion of Plasmodium falciparum-infected red blood cells. Parasite Immunol 15 (12), 647–55. [DOI] [PubMed] [Google Scholar]
- 133.Schwarzer E et al. (1993) Phagocytosis of P. falciparum malarial pigment hemozoin by human monocytes inactivates monocyte protein kinase C. Biochim Biophys Acta 1181 (1), 51–4. [DOI] [PubMed] [Google Scholar]
- 134.Schwarzer E et al. (1998) Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect Immun 66 (4), 1601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prada J et al. (1995) Hemozoin differentially modulates the production of interleukin 6 and tumor necrosis factor in murine malaria. Eur Cytokine Netw 6 (2), 109–12. [PubMed] [Google Scholar]
- 136.Pichyangkul S et al. (1994) Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-alpha and interleukin-1 beta. Am J Trop Med Hyg 51 (4), 430–5. [PubMed] [Google Scholar]
- 137.Sherry BA et al. (1995) Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1 alpha, and MIP-1 beta) in vitro, and altered thermoregulation in vivo. J Inflamm 45 (2), 85–96. [PubMed] [Google Scholar]
- 138.Prada J et al. (1996) Effects of Plasmodium vinckei hemozoin on the production of oxygen radicals and nitrogen oxides in murine macrophages. Am J Trop Med Hyg 54 (6), 620–4. [DOI] [PubMed] [Google Scholar]
- 139.Taramelli D et al. (1995) The heme moiety of malaria pigment (beta-hematin) mediates the inhibition of nitric oxide and tumor necrosis factor-alpha production by lipopolysaccharide-stimulated macrophages. Exp Parasitol 81 (4), 501–11. [DOI] [PubMed] [Google Scholar]
- 140.Altieri DC (1991) Occupancy of CD11b/CD18 (Mac-1) divalent ion binding site(s) induces leukocyte adhesion. J Immunol 147 (6), 1891–8. [PubMed] [Google Scholar]
- 141.Bohnsack JF and Zhou XN (1992) Divalent cation substitution reveals CD18- and very late antigen-dependent pathways that mediate human neutrophil adherence to fibronectin. J Immunol 149 (4), 1340–7. [PubMed] [Google Scholar]
- 142.Xu X and Hakansson L (2002) Degranulation of primary and secondary granules in adherent human neutrophils. Scand J Immunol 55 (2), 178–88. [DOI] [PubMed] [Google Scholar]
- 143.Klebanoff SJ et al. (1993) Stimulation of the bactericidal activity of polymorphonuclear leukocytes by manganese. J Leukoc Biol 53 (6), 666–72. [DOI] [PubMed] [Google Scholar]
- 144.Wang C et al. (2018) Manganese Increases the Sensitivity of the cGAS-STING Pathway for Double-Stranded DNA and Is Required for the Host Defense against DNA Viruses. Immunity 48 (4), 675–687 e7. [DOI] [PubMed] [Google Scholar]
- 145.Lv M et al. (2020) Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res 30 (11), 966–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Juttukonda LJ et al. (2017) Dietary Manganese Promotes Staphylococcal Infection of the Heart. Cell Host Microbe 22 (4), 531–542 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Danks DM (1985) Inborn errors of trace element metabolism. Clin Endocrinol Metab 14 (3), 591–615. [DOI] [PubMed] [Google Scholar]
- 148.Koller LD et al. (1987) Immune dysfunction in rats fed a diet deficient in copper. Am J Clin Nutr 45 (5), 997–1006. [DOI] [PubMed] [Google Scholar]
- 149.Boyne R and Arthur JR (1981) Effects of selenium and copper deficiency on neutrophil function in cattle. J Comp Pathol 91 (2), 271–6. [DOI] [PubMed] [Google Scholar]
- 150.Boyne R and Arthur JR (1986) Effects of molybdenum or iron induced copper deficiency on the viability and function of neutrophils from cattle. Res Vet Sci 41 (3), 417–9. [PubMed] [Google Scholar]
- 151.Babu U and Failla ML (1990) Copper status and function of neutrophils are reversibly depressed in marginally and severely copper-deficient rats. J Nutr 120 (12), 1700–9. [DOI] [PubMed] [Google Scholar]
- 152.Babu U and Failla ML (1990) Respiratory burst and candidacidal activity of peritoneal macrophages are impaired in copper-deficient rats. J Nutr 120 (12), 1692–9. [DOI] [PubMed] [Google Scholar]
- 153.Newberne PM et al. (1968) The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhilmurium infection. Br J Exp Pathol 49 (5), 448–57. [PMC free article] [PubMed] [Google Scholar]
- 154.Chen M et al. (2019) Copper Regulates the Susceptibility of Zebrafish Larvae to Inflammatory Stimuli by Controlling Neutrophil/Macrophage Survival. Front Immunol 10, 2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hunt JA et al. (1992) Stimulation of neutrophil movement by metal ions. J Biomed Mater Res 26 (6), 819–28. [DOI] [PubMed] [Google Scholar]
- 156.Vogl T et al. (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13 (9), 1042–9. [DOI] [PubMed] [Google Scholar]
- 157.Yan L et al. (2013) Beneficial effects of quinoline-3-carboxamide (ABR-215757) on atherosclerotic plaque morphology in S100A12 transgenic ApoE null mice. Atherosclerosis 228 (1), 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen X et al. (2013) Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest 123 (11), 4595–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cornish CJ et al. (1996) S100 protein CP-10 stimulates myeloid cell chemotaxis without activation. J Cell Physiol 166 (2), 427–37. [DOI] [PubMed] [Google Scholar]
- 160.Lackmann M et al. (1992) Purification and structural analysis of a murine chemotactic cytokine (CP-10) with sequence homology to S100 proteins. J Biol Chem 267 (11), 7499–504. [PubMed] [Google Scholar]
- 161.Laouedj M et al. (2017) S100A9 induces differentiation of acute myeloid leukemia cells through TLR4. Blood 129 (14), 1980–1990. [DOI] [PubMed] [Google Scholar]
- 162.Karjalainen R et al. (2019) Elevated expression of S100A8 and S100A9 correlates with resistance to the BCL-2 inhibitor venetoclax in AML. Leukemia 33 (10), 2548–2553. [DOI] [PubMed] [Google Scholar]
- 163.Nedjadi T et al. (2018) S100A8 and S100A9 proteins form part of a paracrine feedback loop between pancreatic cancer cells and monocytes. BMC Cancer 18 (1), 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bergenfelz C et al. (2015) S100A9 expressed in ER(−)PgR(−) breast cancers induces inflammatory cytokines and is associated with an impaired overall survival. Br J Cancer 113 (8), 1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yin X et al. (2005) Metallothionein mediates leukocyte chemotaxis. BMC Immunol 6, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oram JD and Reiter B (1968) Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta 170 (2), 351–65. [DOI] [PubMed] [Google Scholar]
- 167.Qiu J et al. (1998) Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc Natl Acad Sci USA 95 (21), 12641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roseanu A et al. (2010) Liposomalization of lactoferrin enhanced its anti-tumoral effects on melanoma cells. Biometals 23 (3), 485–92. [DOI] [PubMed] [Google Scholar]
- 169.Ellison RT 3rd et al. (1988) Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun 56 (11), 2774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nikawa H et al. (1993) The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch Oral Biol 38 (12), 1057–63. [DOI] [PubMed] [Google Scholar]
- 171.Xu YY et al. (1999) In vitro susceptibility of Candida species to lactoferrin. Med Mycol 37 (1), 35–41. [DOI] [PubMed] [Google Scholar]
- 172.Sherman MP et al. (2004) Neonatal small bowel epithelia: enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals 17 (3), 285–9. [DOI] [PubMed] [Google Scholar]
- 173.Haversen L et al. (2002) Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell Immunol 220 (2), 83–95. [DOI] [PubMed] [Google Scholar]
- 174.Baveye S et al. (2000) Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect Immun 68 (12), 6519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baveye S et al. (2000) Lactoferrin inhibits the binding of lipopolysaccharides to L-selectin and subsequent production of reactive oxygen species by neutrophils. FEBS Lett 469 (1), 5–8. [DOI] [PubMed] [Google Scholar]
- 176.Devireddy LR et al. (2001) Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science 293 (5531), 829–34. [DOI] [PubMed] [Google Scholar]
- 177.Nelson AM et al. (2008) Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest 118 (4), 1468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Song E et al. (2017) Lipocalin-2 induces NLRP3 inflammasome activation via HMGB1 induced TLR4 signaling in heart tissue of mice under pressure overload challenge. Am J Transl Res 9 (6), 2723–2735. [PMC free article] [PubMed] [Google Scholar]
- 179.Chi Y et al. (2020) Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science 369 (6501), 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chapman AL et al. (2013) Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J Biol Chem 288 (9), 6465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park YS et al. (2000) Antioxidant binding of caeruloplasmin to myeloperoxidase: myeloperoxidase is inhibited, but oxidase, peroxidase and immunoreactive properties of caeruloplasmin remain intact. Free Radic Res 33 (3), 261–5. [DOI] [PubMed] [Google Scholar]
- 182.Horsburgh MJ et al. (2002) MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol 44 (5), 1269–86. [DOI] [PubMed] [Google Scholar]
- 183.Mazmanian SK et al. (2003) Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299 (5608), 906–9. [DOI] [PubMed] [Google Scholar]
- 184.Skaar EP et al. (2004) Iron-source preference of Staphylococcus aureus infections. Science 305 (5690), 1626–8. [DOI] [PubMed] [Google Scholar]
- 185.Beasley FC et al. (2011) Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect Immun 79 (6), 2345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Drechsel H et al. (1993) Purification and chemical characterization of staphyloferrin B, a hydrophilic siderophore from staphylococci. Biometals 6 (3), 185–92. [DOI] [PubMed] [Google Scholar]
- 187.Konetschny-Rapp S et al. (1990) Staphyloferrin A: a structurally new siderophore from staphylococci. Eur J Biochem 191 (1), 65–74. [DOI] [PubMed] [Google Scholar]
- 188.Sheldon JR et al. (2014) TCA cycle activity in Staphylococcus aureus is essential for iron-regulated synthesis of staphyloferrin A, but not staphyloferrin B: the benefit of a second citrate synthase. Mol Microbiol 92 (4), 824–39. [DOI] [PubMed] [Google Scholar]
- 189.Hesse LE et al. (2019) The Acinetobacter baumannii Znu System Overcomes Host-Imposed Nutrient Zinc Limitation. Infect Immun 87 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Juttukonda LJ et al. (2016) Acinetobacter baumannii Coordinates Urea Metabolism with Metal Import To Resist Host-Mediated Metal Limitation. mBio 7 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alvarez-Fraga L et al. (2018) Pneumonia infection in mice reveals the involvement of the feoA gene in the pathogenesis of Acinetobacter baumannii. Virulence 9 (1), 496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Giardina BJ et al. (2019) Heme uptake and utilization by hypervirulent Acinetobacter baumannii LAC-4 is dependent on a canonical heme oxygenase (abHemO). Arch Biochem Biophys 672, 108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Runci F et al. (2019) Contribution of Active Iron Uptake to Acinetobacter baumannii Pathogenicity. Infect Immun 87 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sheldon JR and Skaar EP (2020) Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog 16 (10), e1008995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Knippel RJ et al. (2020) Clostridioides difficile Senses and Hijacks Host Heme for Incorporation into an Oxidative Stress Defense System. Cell Host Microbe 28 (3), 411–421 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zackular JP et al. (2020) ZupT Facilitates Clostridioides difficile Resistance to Host-Mediated Nutritional Immunity. mSphere 5 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]


