Abstract
The coronavirus disease (COVID-19) has once again reminded us of the significance of host immune response and consequential havocs of the immune dysregulation. The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) inflicts severe complications to the infected host, including cough, dyspnoea, fever, septic shock, acute respiratory distress syndrome (ARDs), and multiple organ failure. These manifestations are the consequence of the dysregulated immune system, which gives rise to excessive and unattended production of pro-inflammatory mediators. Elevated circulatory cytokine and chemokine levels are accompanied by spontaneous haemorrhage, thrombocytopenia and systemic inflammation, which are the cardinal features of life-threatening cytokine storm syndrome in advanced COVID-19 diseases. Coronavirus hijacked NF-kappa B (NF-κB) is responsible for upregulating the expressions of inflammatory cytokine, chemokine, alarmins and inducible enzymes, which paves the pathway for cytokine storm. Given the scenario, the systemic approach of simultaneous inhibition of NF-κB offers an attractive therapeutic intervention. Targeted therapies with proteasome inhibitor (VL-01, bortezomib, carfilzomib and ixazomib), bruton tyrosine kinase inhibitor (acalabrutinib), nucleotide analogue (remdesivir), TNF-α monoclonal antibodies (infliximab and adalimumab), N-acetylcysteine and corticosteroids (dexamethasone), focusing the NF-κB inhibition have demonstrated effectiveness in terms of the significant decrease in morbidity and mortality in severe COVID-19 patients. Hence, this review highlights the activation, signal transduction and cross-talk of NF-κB with regard to cytokine storm in COVID-19. Moreover, the development of therapeutic strategies based on NF-κB inhibition are also discussed herein.
Keywords: COVID-19, SARS-CoV-2, NF-κB, Cytokine storm, Novel coronavirus, Inflammation, Severe acute respiratory syndrome
Abbreviations: ACE2, angiotensin-converting enzyme II; AT1R, activation of angiotensin-1-receptor; ADAM17, metalloprotease 17; BLC, B-lymphocyte chemoattractant; BAFFR, B-cell activating factor receptor; CD40+, cluster of differentiation 40; COX-2, cyclooxygenase-2; CXCL, C-X-C- motif chemokine; ALI, acute lung injuries; c-AMP, cyclic adenosine phosphate; CDC, Center for Disease Control and Prevention; CoV, coronavirus; CASP3, caspase 3; COVID-19, coronavirus disease 2019; CXCL C-X-C, motif chemokine ligand; CRP C, reactive protein; CTLs, cytotoxic T lymphocytes; DAMP, damage-associated molecular patterns (DAMP); ERK1/2, extracellular signal-regulated kinases; TNF, Tumour Necrosis Factor; LPS, lipopolysaccharides; MAPK, mitogen-activated protein kinase; RSV, respiratory syncytial virus; iNOS, nitric oxide synthase; SARS, Severe Acute Respiratory Syndrome; IL, Interleukin; NF-κB, nuclear factor kappa B cells; NO, nitric oxide; SMD, SOD, superoxide dismutase; TLR-4, Toll-like receptor-4; IP-10, interferon-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; MIG, monokine induced by interferon-γ; MIP-1α and MIP-1β, macrophage inflammatory protein 1α and 1β, respectively; NK, natural killer; Th, helper T cells; VEGF, vascular endothelial growth factor; PTMs, post-translational modifications; IL-1R, Interleukin 1 receptor (IL-1R); TNFR, tumour necrosis factor receptor; LTβR, Lymphotoxin-β receptor; RANK, receptor activator of NF-κB; IKK, IκB kinase; NEMO, regulatory subunit IKKγ; PKR, threonine-derived kinases; JAK, Janus Kinase; STAT3, signal transduction activator of transcription factor 3; STING, stimulator of interferon genes; MAPKs, mitogen-activated protein kinases; JNK, Jun amino-terminal kinases; CCL, chemokine ligand; PAMPs, pathogen-associated molecular patterns; GSDMD, Gasdermin
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients present a wide range of clinical features, including moderate febrility, cough, septic shock, acute respiratory distress syndrome (ARDs), progressive respiratory epithelial damage and multiple organ failure [1], [2]. A close comparison between the clinical manifestation of severely ill cases to patients with mild symptoms of coronavirus disease 2019 (COVID-19) represents hyperactivated and dysregulated systemic inflammation [3], [4]. In addition, elevated cytokine and chemokine levels are significantly higher in critically ill patients, suggestive of a life-threatening syndrome known as “cytokine storm” [5], [6].
The recent pandemic of SARS-CoV-2 has once again reminded us of the significance of host immune response and consequential havocs of immune dysregulation. The term “cytokine storm” was coined more than two decades ago to define medical conditions associated with the use of muromonab-CD3 (OKT3) for allograft rejection [7]. This term was alternatively used in literature for engraftment syndrome of acute graft-versus-host disease after allogeneic hematopoietic stem-cell transplantation [8]. The gradual increase in the chimeric antigen receptor (CAR) T-cell therapy over the years further strengthened the narrative of cytokines storm [9], [10], [11], [12]. Cytokine storm can be broadly classified as a systemic inflammatory syndrome, responsible for epithelial damage, capillary leakage and multi-organ tissue damage. It can be induced by numerous endo- and exogenous factors, such as bacterial and viral infection, immunotherapies and autoimmune disorders [13], [14], [15]. From a historical perspective, cytokine storm was suspected of contributing to the lethality of the 1918–1919 influenza pandemic. Lately, scientists reconstructed the H1N1 virus from the 1918 pandemic and evaluated its effect on animal models. The inoculation of strain resulted in pulmonary inflammation, acute lung injury and loss of alveolar function [16]. Hence, it was hypothesized that the host immune response towards the pathogen recognition but not the pathogen itself contributes to multiorgan dysfunction and cytokine storm in severe acute respiratory syndrome (SARS) and COVID-19 [17].
The nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) was discovered almost three decades ago. Since then, it has been used as an inducible transcription model due to its numerous pathophysiological effects and therapeutic applications [18], [19]. Nonetheless, the functional diversity of NF-κB still raises concerns about how a small group of signalling mediators integrates a wide range of stimulus into cell type- and stimulus-specific responses. Studies have confirmed that NF-κB does not work alone; instead, its cross-talk interaction with other signalling pathways orchestrates diverse NF-κB responses [20], [21]. Furthermore, the pathogenesis of critically ill COVID-19 cases has been associated with the NF-κB signalling pathway [22], [23]. These implications are based on the studies carried out during the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) pandemics. Numerous viral proteins, including nucleocapsid, spike, SARS-CoV-2 non-structural protein (nsp) 1, nsp3a, and nsp7a can hyperactivate the NF-κB, which propagates cytokines storm and contributes to the multi-organ damage leading to a high fatality rate [24], [25]. Based on the facts and current COVID-19 scenario, the inhibition of the NF-κB signalling pathway as a therapeutic intervention seems to be an inevitable hypothesis for future studies.
2. NF-κB pathways; canonical and non-canonical
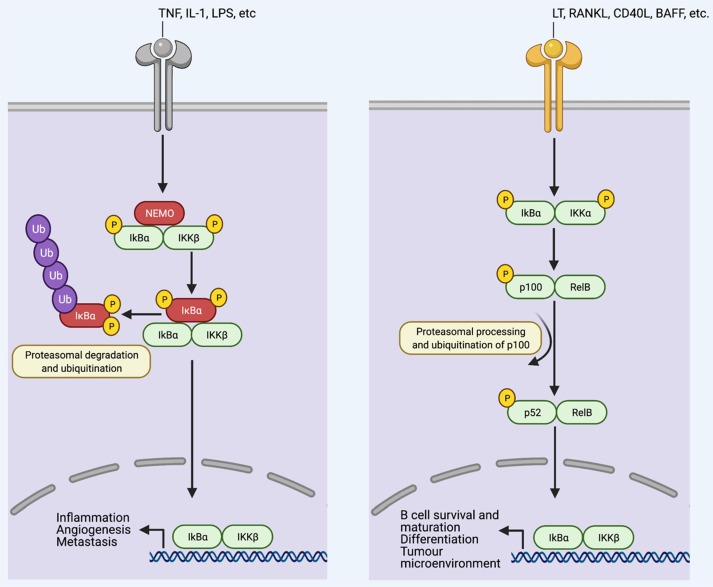
Transcription factors of NF-κB consist of RelA (p65), RelB, and c-Rel in mammals. Two precursor proteins, including NF-κB1(p105) and NF-κB2 (p100) are also part of Rel homology, which are processed and converted to p50 and p52, respectively [26], [27]. Rel homology domain coexists in all the NF-κB proteins, which has a significant role in DNA binding and dimerization. Under the resting condition, negative regulatory IκB proteins are bound with NF-κB dimers, which confine the dimers to the cytoplasm [28], [29]. Upon receiving the stimulus, the IκB kinase (IKK) complex degrades IκB proteins through phosphorylation [30]. Once phosphorylated, IκB proteins are prone to proteasomal degradation and ubiquitination, resulting in the release of bound NF-κB dimers and translocation to the nucleus (Fig. 1 ) [20], [29]. The transcriptional activity of NF-κB is further regulated by post-translational modifications (PTMs) [31], [32].
Fig. 1.
Canonical and non-canonical NF-κB pathways. Under resting states, inhibitory IκB proteins are bound to NF-κB dimers, which restrict the complexes of NF-κB to the cytoplasm. Stimulus-dependent degradation takes place through phosphorylation of IκB proteins by IκB kinase (IKK) complex, which consists of two catalytically active kinases, IKKα and IKKβ, and the regulatory subunit IKKγ (NEMO). Once phosphorylated, IκB proteins undergo extensive ubiquitination and proteasomal degradation, which sets the bound NF-κB dimers free for nuclear translocation. NF-κB signalling is divided into canonical and non-canonical pathways. The induction of the canonical pathway, which is represented as TNFR1 signalling (left), mainly involves physiological NF-κB stimulus, including tumour necrosis factor (TNF), IL‑1 and Toll-like receptor ligands, such as lipopolysaccharide (LPS). Stimulus-induced IκBα phosphorylation takes place in IKKβ- and NEMO-dependent manner, which allows the p65 and p50-containing heterodimers to translocate into the nucleus. The activation of this pathway significantly upregulates the expression of genes involved in inflammation, angiogenesis, cell proliferation and metastasis. On the contrary, the non-canonical pathway (right) can be stimulated by specific TNF family cytokines, including lymphotoxin β (LT-β), B cell-activating factor of the TNF family (BAFF), CD40+ligand (CD40+ L) and receptor activator of NF‑κB ligand (RANKL). IKKα-derived p100 phosphorylation takes place in RelB dependent manner that results in the production of transcriptionally active p52-RelB complexes and activation of this pathway. The non-canonical pathway is involved in the induction of genes associated with B cell survival and maturation, tumour microenvironment, differentiation, secondary lymphoid organ development and maintenance.
Intercellularly, NF-κB activating pathway can be subdivided into canonical and non-canonical pathways [33], [34]. Overall, the majority of physiological NF-κB stimuli originating from cytokine receptors, including interleukin 1 (IL-1) receptor (IL-1R) [35], [36], antigen- and pattern-recognition receptors, and toll-like receptor 4 (TLR4), can induce canonical pathway [37], [38]. Canonical pathway relies on IKKβ kinase and NF-κB essential modulator (NEMO) subunit for the IκBα phosphorylation and p65-heterodimers nuclear translocation [39] (Fig. 1). In contrast, activation of non-canonical pathway is selective and responds to discrete stimulus including ligands of tumour necrosis factor receptor (TNFR) superfamily members such as lymphotoxin-β receptor (LTβR) [40], B-cell activating factor receptor (BAFFR) [41], cluster of differentiation 40 (CD40+) and receptor activator of NF-κB (RANK) [42], [43], [44]. The non-canonical pathway relies on p100 associated RelB phosphorylation by IKKα, which leads to the p100 proteasomal processing and production of p52-RelB complexes [26], [34] (Fig. 1). Generally, a canonical pathway is more functional in immune responses related to infections, physical and chemical injuries [45], [46]. Whereas, non-canonical pathway serves as a supplementary signalling axis and supports the canonical pathway in modulating actions required by the adaptive immune system [33], [34], [47].
3. SARS-CoV-2 activated NF-κB pathway
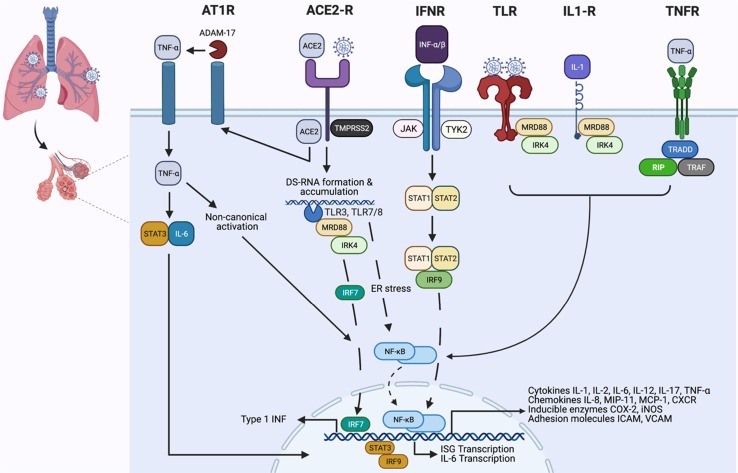
Pro-inflammatory cytokines, oxidative stress, bacterial and viral infections have the potential to elicit NF-κB activation [48]. These instigating factors are responsible for NF-κB-derived generalized or/and specific immune responses [26], [49]. Activated NF-κB participates in a wide range of cell signalling pathways involved in cell differentiation, proliferation, survival, cellular communication, and immunomodulation [18], [50], [51]. Hence, it is not surprising that dysfunction of NF-κB is linked with inflammatory and autoimmune disorders, metabolic diseases, and progression of cancers [52], [53], [54]. Likewise, high fatality rates in viral infections are also linked with excessive NF-κB activation. Studies conducted during SARS-CoV and MERS-CoV outbreaks highlighted viral proteins (nsp1, nsp3a, nsp7a, spike, and nucleocapsid) with the potential to overdrive NF-κB transcriptional activity [24], [55]. It is noteworthy that SARS-CoV-2 shares the replication and NF-κB activation pathways with stranded positive RNA genomes of coronaviridae [56]. The most crucial step in the replication of a positive-stranded RNA virus is the production of a negative-stranded copy of the genome [57]. The negative strand serves as a template for the viral RNA-dependent RNA polymerase for genome replication. Multiplication of SARS-CoV-2 begins the production and accumulation of dsRNA (called transcriptive intermediate) in the host [58], [59]. Interferon (INF)-induced dsRNA-dependent protein kinase (PKR) plays two significant roles at this point. Initially, the threonine-derived kinases (PKR) prompt an innate immune response, terminating the translation processes to prevent viral replication in infected cells. Secondly, dsRNA bound PKR activates IκB kinase (IKK), which triggers the IκBα and IKKβ degradation with subsequent release of NF-κB and activation of the canonical pathway [60], [61]. Additionally, PKR upregulates the expressions of TNF-α, which consequently ends up in the activation of the non-canonical pathway (Fig. 2 ) [39], [62]. Altogether, this produces rapid and short-acting inflammatory responses by activating the canonical pathway [63], [64]. In contrast, non-canonical pathway activation leads to dawdling yet long-lasting production of pro-inflammatory mediators [39], [65].
Fig. 2.
Activation of NF-κB pathway and its cross-talk with cell signalling pathways in cytokine storm. Angiotensin-converting enzyme 2 (ACE2) receptor serves as a binding and entry site for SARS-CoV-2. Upon binding, SARS-CoV-2 activates the serine protease TMPRSS2, allowing viral access into the host cell through endocytosis. While inside the endosomes, single-stranded viral RNA activates the two toll-like receptors (TLR), including TLR7 and TLR8. As the double-stranded RNA starts to accumulate due to the viral replication, it gets quickly identified by TLR3, resulting in the activation of TLR7/8 and TLR3. Activation of these receptors, followed by the recruitment of signal transfer proteins MyD88, IRAK, IKKε and TRAF6, upregulate the transcription of the interferon-regulator factor (IRF) family. Activation of TLR7/8- and TLR3-derived IKK (IκB kinases) pave the pathway for the phosphorylation of inhibitory IκBa, followed by ubiquitination and proteasomal degradation, which results in the translocation of NF-κB heterodimer complexes to the nucleus. The final activation sequence involves the NF-κB cross-talk with numerous cytokine and TLR-mediated cell signalling pathways that depend upon the binding of TNFα and IL-1 to TLR4. Subsequently, the spike proteins of SARS-CoV-2 have been shown to induce TLR4-mediated and endoplasmic reticulum (ER) stress-induced NF-κB activation. Moreover, IFN-α/β binding with IFNR dimer receptors activates the JAK-STAT pathway, which renders the production and subsequent nuclear translocation of STAT1, STAT2 and IRF9 complex. The complex potentiates the transcriptional activity of NF-κB and IFN-stimulated genes (ISG). Additionally, activation of angiotensin-1-receptor (AT1R) imparts pro-inflammatory cytokine activity to angiotensin II, which is capable of inducing NF-κB, disintegrin and metalloprotease 17 (ADAM17). Altogether, activation of NF-κB, disintegrin and ADAM17 promotes the production of TNF- α and epidermal growth factor receptor (EGFR) ligands, which promote the self-activating of NF-κB cycle. Hyperactivation of NF-κB upregulates the expression of multiple genes involved in the production of inflammatory cytokine, chemokines, adhesion molecules, and acute-phase proteins.
4. Cross-talk interactions between NF-κB and non-NF-κB pathways
Janus Kinase (JAK) and signal transduction activator of transcription factor 3 (STAT3) are required for the complete activation of NF-κB [66], [67]. Pro-inflammatory IL-6 serves as a major stimulator of the JAK-STAT pathway [68], [69]. The binding of IL-6 allows phosphorylated STAT3 to translocate into the nucleus and promote the reduction of IFN-γ [22], [70]. Studies have indicated that NF-κB and JAK-STAT activation has the potential to induce IL-6 amplifier response (IL-6 Amp) in COVID-19. IL-6 Amp reciprocally promotes multi-inflammatory responses by reactivating the NF-κB via STAT3 [71], [72], [73] (Fig. 2). It is worth mentioning that IL-6 also serves as a significant cellular senescence marker that tends to increase progressively with aging [74], [75]. As part of the viral replication process, DNA damage is almost inevitable in the elderly age group and advanced COVID-19 cases. At this point, damaged host DNA losses control of the stimulator of interferon genes (STING), which generally serves as a key player in host defence against pathogens [76], [77], [78]. Dysregulated STING induces INF regulatory factor 3 (IRF 3), bolsters IFN-β production, and activates NF-κB canonical pathway [79], [80]. IFN-β production produces delayed yet long-lasting detrimental effects via aggravating multiple innate immune responses in infected hosts [79], [80].
Similarly, coronavirus hijacked mitogen-activated protein kinases (MAPKs) is another major cell signalling pathway involved in the hyperactivation of NF-κB [81], [82]. MAPK pathway consists of serine-threonine kinases; these functional proteins relay, amplify and integrate signals from a diverse range of stimuli that allows the human body to withstand oxidative stress and inflammation [83], [84]. Jun amino-terminal kinases (JNK), P38, and extracellular signal-regulated kinases (ERK1/2) are involved in acute respiratory viral infections induced pathogenesis [85], [86]. A late study by Park, et al. [87] suggested that P38-MAPK and their cross-talk interaction with NF-κB activate the renin-angiotensin system, which results in the excessive production of angiotensin II (ANG II). Moreover, the activation of NF-κB by P38-MAPK upregulates the expression of TNF-α and IL-1β [88]. Consequently, this cross-talk interaction increases systemic levels of Ang II, TNF-α, and IL-1β, which collectively promote thromboembolism by creating an imbalance between prothrombic and fibrinolytic cascades [34], [89]. Additionally, activation of angiotensin-1-receptor (AT1R) imparts pro-inflammatory cytokine activity to Ang II, which is capable of inducing NF-κB, disintegrin and metalloprotease 17 (ADAM17) [90]. Altogether, activation of NF-κB, disintegrin, and ADAM17 promotes the production of TNF-α and epidermal growth factor receptor (EGFR) ligands which propagates the NF-κB self-activating cycle [22], [91] (Fig. 2).
5. NF-κB cascade and cytokine storm
Exacerbation of inflammatory immune responses is common and life-threatening clinical manifestations observed in critical care patients [1], [92], [93]. Cytokines storm is a commonly used term to describe virus-induced elevated systemic levels of cytokines and chemokines including IL-1α, IL-7, IL-8, IL-9, IL-10, G-CSF, GM-CSF, IFN- γ, IP-10, MCP-1, MIP-1β, PDGF, TNF-α, and VEGF [94], [95]. Elevated levels of these pro-inflammatory cytokines play a significant role in the morbidity and mortality of SARS-CoV-2 infections [1], [93]. Cytokines storm can be characterized into three distinct immunotypes based on its CD+ cell activation and relative severity. Activation of CD4+ T and effector CD8+ T cells of immunotype I takes place in severe disease. Moreover, submucosal macrophages, mast cells, monocytes, and dendritic cells are also hyperactivated in immunotype-I [96], [97]. Due to the lesser extent of CD4+ T activation and memory B cells, only intermediate clinical manifestations are observed in immunotype-II [98]. Whereas, due to mere lymphocyte activation and mild clinical symptoms, immunotype-III has the least clinical significance.
Nucleocapsid and spike protein-induced NF-κB activation significantly increase the turnover of pro-inflammatory cytokines [25], [99]. More robust immune responses to viral infection were observed in aged macaques than the younger group. Comprehensive genomic analyses confirmed that hyper-immune response in the aged group was due to the upregulated differential expression of NF-κB [100]. Activated NF-κB is pivotal for the full-blown cytokine storm as it upregulates the expression and production of inflammatory cytokines and chemokines (TNF-α, IL-1β, IL-6, IL-8, and MCP-1) involved acute inflammatory response [25], [99]. Inflammatory phenotypes with NF-κB down signalling molecules not only damage the alveolar epithelium but prolong activation can lead to complete loss of alveolar function [101]. Pulmonary epithelium transcriptome analysis of deceased COVID-19 patients exhibited upregulated NF-κB expression, accompanied with oedema, pulmonary inflammation with destructed epithelial lining [102].
Spike protein subunit 1 (CoV2-S1) of SARS-CoV-2 has been identified as a potent cytokine storm inducer in COVID-19. This subunit has a high binding affinity towards the ACE2 receptor, which empowers CoV2-S1 to activate NF-κB more aggressively than CoV-S1 [103], [104]. Owing to its ability to induce the NF-κB, CoV2-S1 exponentially increases the production of IL-1β, TNF-α, IL-6, and CCL2 [105]. Thus, an outburst of pro-inflammatory cytokines and chemokines starts with the interaction of CoV2-S1 with human ACE2 receptors. This cross-interaction is only possible with subsequent activation of endoplasmic reticulum (ER) stress, unfolded protein response (UPR), and MAP kinase signalling pathways (Fig. 2)[104], [106]. Once CoV2-S1-induced NF-κB activation is achieved, inflammatory signalling pathways go haywire and peripheral blood mononuclear cells (PBMC) starts producing chemokine ligand subfamilies including chemokine ligand (CCL) 2, CCL7, CCL8, CCL24, CCL20, CCL13, CCL3, along with C-X-C motifs (CXC) chemokine ligand such as (CXCL) 2 and CXCL10 [105]. As the production of IL-1β increases, it gives rise to a spontaneous upsurge of IL1R1, MYD88, IRAK1, TRAF6, NF-κBIA, NF-κB1, and RELA. Additionally, NF-κB derived transcriptional gene expressions reciprocate with the downstream signalling molecules of TLR4; CD14, MYD88, IRAK1, TRAF6, TIRAP, and TICAM, which initiates and promote the vicious self-activating cycle of the NF-κB pathway (Fig. 2) [105], [107].
6. Clinical and laboratory findings
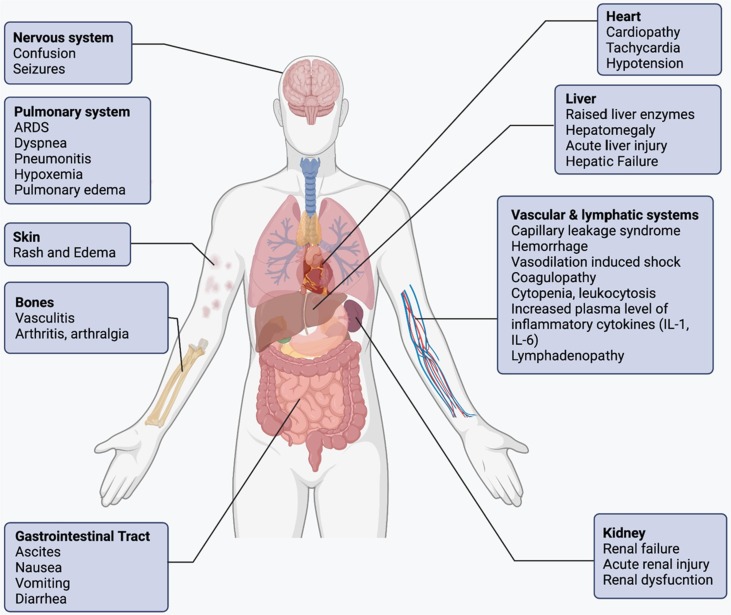
Cytokine storm is a compendium of several immune dysregulations categorized by generalized systemic inflammation, multi-organ failures, pulmonary and extrapulmonary clinical manifestations (Fig. 3 ) [108]. Though the initial symptoms of cytokine storm may differ depending upon the triggering factor, however later stages of the syndrome present overlapping clinical manifestations [109], [110]. The presence of fever is predominant in almost all the patients, while a degree of fever from mild to high grade depends upon the severity of cytokine storm [111]. Furthermore, a patient may also present rash, headache, fatigue, anorexia, arthralgia, myalgia, and neuropsychiatric findings (Fig. 3). The cellular damage caused by the high cytokine plasma concentrations and immune cell-mediated responses paves the pathway for these clinical features [112], [113]. As the patient reaches the advanced stage of syndrome, systemic intravascular coagulation is often presented with imbalanced haemostatics, peripheral venous block, haemorrhagic stroke, and hypotensive vasodilatory shock [113]. These are often accompanied by pulmonary symptoms, including dyspnoea, cough, tachypnoea, and ARDs, which may require mechanical ventilation due to the low oxygen saturation [114]. Spontaneous haemorrhages are very common due to thrombocytopenia, hyper inflammation, and coagulopathy. The patients at the advanced stage start presenting organ-specific manifestations, including takotsubo-like cardiomyopathy, acute renal and hepatic failure [115], [116]. Surprisingly, patients may also mimic the symptoms of high-dose IL-2 treated cancer patients, such as capillary leak syndrome, anasarca with renal inefficiency, and endothelial-cell death [117]. Effector T cells-induced neurotoxicity syndrome is the driving force of neurological symptoms. Although neurological manifestations take several weeks to present but once presented, they persist for an extended period [109].
Fig. 3.
Clinical features and laboratory findings are essential for the accurate diagnosis of cytokine storm in COVID-19. Although, an extensive list of clinical manifestations and laboratory abnormalities are observed in a cytokine storm. Nonetheless, all cases exhibit increased cytokine levels and acute systemic inflammatory symptoms are often accompanied by secondary organ damage, including renal failure, hepatic dysfunction and acute respiratory distress syndrome (ARDS).
The C-reactive protein, which serves as a classical non-specific inflammatory marker, is generally elevated and corresponds to the severity of the syndrome [118], [119]. Abnormalities in the blood count, including leukopenia, thrombocytopenia, leucocytosis, and anaemia are present in nearly all patients with cytokine storm [120], [121]. Moreover, hypertriglyceridemia, along with elevated ferritin and d-dimer levels, have been frequently observed in laboratory findings. The aberration in the blood composition is often linked with cytokine-stimulated changes in the production and release of the cells from bone marrow [120]. In addition, immune-mediated destruction and chemokine-induced migration are also responsible for the changes in circulating cell counts. The complete list of elevated cytokines is given in Table 1 , but elevated levels of chemokines induced by INF-γ, IL-6, IL-10, and IL-2 receptors are almost present in the majority of the patients [111].
Table 1.
Common inflammatory mediators and immune cells involved in cytokine storm.
| Mediato | Origin | Action, function and mechanism |
|---|---|---|
| Cytokines and growth factors | ||
| IL-1 | Macrophages, epithelial cells; pyroptotic cells | Pro-inflammatory alarmin cytokine; pyrogenic activity, activates macrophage and Th17 cell [190] |
| IL-2 | T cells | Effector T-cell and regulatory T-cell growth factor [191] |
| IL-6 | Macrophages, T cells, endothelial cells | Pyrogenic cytokine with pro-inflammatory activity, stimulate acute-phase reactions and antibody production[192] |
| IL-9 | Th9 cells | Defence against helminth infections, activates mast cells, association with type I interferon in COVID-19 [125], [193] |
| IL-10 | Regulatory T cells, Th9 cells | Anti-inflammatory cytokine; inhibit Th1 and cytokine release[194] |
| IL-12 | Dendritic cells, macrophages | Th1 pathway activation; induce Th1 cells for INF-γ release; activate CTLs and NK cells; show synergism with IL-18 [195] |
| IL-17 | Th17 cells, NK cells, group 3 innate lymphoid cells | Activate and propagate neutrophilic inflammation; protect against infections[196] |
| IL-18 | Monocytes, macrophages, dendritic cells | Alarmin cytokine with pro-inflammatory function; activation of Th1 pathway, exhibit synergism with IL-12 [197] |
| IL-33 | Macrophages, dendritic cells, mast cells, epithelial cells | Pro-inflammatory cytokine with alarmin function; potentiates Th1 and Th2 cells, NK cells, CTLs, and mast cells [198] |
| INF-γ | Th1, CTLs, group 1 innate lymphoid and NK cells | Pro-inflammatory cytokine; macrophages stimulation [125], [168] |
| TNF | Macrophages, T cells, NK cells, mast cells | Pro-inflammatory cytokine with pyrogenic function, increase vascular permeability[168] |
| GM-CSF | Th17 cells | Pro-inflammatory cytokine [199] |
| VEGF | Macrophages | Promotes angiogenesis [200], [201] |
| Chemokines | ||
| IL-8 (CXCL8) | Macrophages, epithelial cells | Chemotactic agent of neutrophils [202], [203] |
| MIG (CXCL9) | Monocytes, endothelial cells, keratinocytes | INF-stimulated chemokine; employment of Th1 cells, NK cells, plasmacytoid dendritic cells [24], [105] |
| IP-10 (CXCL10) | Monocytes, endothelial cells, keratinocytes | Recruitment of macrophages, Th1 cells, NK cells; INF-induced chemokine [204], [205] |
| MCP-1 (CCL2) | Macrophages, dendritic cells, cardiac myocytes | Chemotactic agent of Th2 cells, monocytes, dendritic cells, basophils [206], [207] |
| MIP-1α (CCL3) | Monocytes, neutrophils, dendritic cells, NK cells, mast cells | Chemotactic agent of macrophages, Th1 cells, NK cells, eosinophils, dendritic cells; pyrogenic function [207] |
| MIP-1β (CCL4) | Macrophages, neutrophils, endothelium | Recruitment of macrophages, Th1 cells, NK cells, dendritic cells [208] |
| BLC (CXCL13) | B cells, follicular dendritic cells | Recruitment of B cells, CD4+ T cells, dendritic cells [209] |
| Plasma proteins | ||
| CRP | Hepatocytes | Monomeric CRP increases IL-8 and MCP-1 production; IL-6 induced upregulated expression of CRP [186], [210] |
| Complement | Hepatocytes, other cells | Amplify tissue damage in cytokine storm; suppression of complement system abrogates pathophysiological effects of cytokine storm [211], [212] |
| Ferritin | Ubiquitous | Primary intracellular storage site of iron [210], [213] |
Note: BLC B-lymphocyte chemoattractant; COVID-19 coronavirus disease 2019; CRP C-reactive protein; CTLs cytotoxic T lymphocytes; CXCL C-X-C motif chemokine ligand; GM-CSF granulocyte–macrophage colony-stimulating factor; IP-10 interferon-inducible protein 10; MCP-1 monocyte chemoattractant protein 1; MIG monokine induced by interferon-γ; MIP-1α and MIP-1β macrophage inflammatory protein 1α and 1β, respectively; NK natural killer; Th helper T cells and VEGF vascular endothelial growth factor.
Detailed laboratory workup and improved clinical outcomes of immunosuppressants in severely ill cases highlight the role of cytokine storm in COVID-19 [122], [123], [124]. An abnormal elevation of immunological markers is observed almost in all the COVID-19 patients. However, it is difficult to predict whether these markers are elevated due to the hyperimmune response, inability to hamper viral replication, or pre-existing underlining comorbidities [125]. In most cases, cytokine levels (e.g., INF-α, INF-γ, and TNF-α) are directly proportional to the nasopharyngeal viral load. The viral loads in the severe cases are higher than moderate ones, suggesting that the viral burden is positively related to the hyperactive immune response [125]. In addition, the presence of autoantibodies for type I INF and erroneous type 1 INF immunity in COVID-19 patients refer to the incompetent antiviral response [126], [127]. Surprisingly, there is a vast difference between the signs and symptoms of asymptomatic patients; who are effectively capable of handling the virus compared to the severe COVID-19 cases that are powerless in controlling the virus categorically. From this, we can infer that host immune dysregulation plays a significant role in the pathogenesis in a majority of the cases. A multi-organ inflammatory syndrome is a prominent feature of COVID-19, which coincides with cytokines storm completely by definition [123], [128]. Numerous commodities, including chronic hypertension, diabetes, and obesity, worsen the disease prognosis since these diseases are capable of harbouring an underlining chronic inflammatory state, which lowers the damage threshold and hastens the organ dysfunction from hyper-exaggerated immune response [129], [130].
Numerous immune effector cells participate in the production of soluble inflammatory mediators (Table 1) [1], [131]. Nevertheless, classical markers of systemic inflammation CD4+ and CD8+ T cells mainly contribute to the immune-activation and pathophysiology of cytokine storm [98], [132], [133]. Lymphopenia is another distinctive feature of cytokine storm in COVID-19, which is surprisingly not observed in any other cytokine storm disorder. It is currently unclear whether the lymphopenia observed in COVID-19 is due to tissue infiltration or destruction of lymphocytes [134], [135]. In addition, thromboembolic events are observed more commonly in COVID-19-associated cytokine storm as compared to other cytokine storm disorders [136], [137], [138]. It is noteworthy to mention that compete for workup for bacterial or viral infection, hepatic and renal laboratory findings are required for the complete assessment and evaluation of the severity of cytokine storm. Nevertheless, the assessment of the severity of cytokine storm can be based on elevated serum inflammatory biomarkers levels, including glycoprotein 130 (gp130) and INF-γ [139]. Additionally, IL-1receptor antagonists (IL1RA) and a separate grading scale used in CAR T-cell therapy can be employed to accurately evaluate cytokine storm severity in COVID-19 patients [109], [140], [141].
7. Cellular proptosis
Lymphopenia is a common attribute of severe COVID-19 disease and based on new findings, NF-κB-induced cellular proptosis is mainly accountable for this life-threatening clinical manifestation [142]. In general, cellular proptosis promotes pro-apoptotic and cytokine-releasing adaptive mechanisms [143], [144]. According to Chen, et al. [145], the viroporin 3a protein of SARS-CoV has the potential to induce NLRP3 inflammasome, which increases the production of IL-1β. Similarly, in COVID-19 patients, the increased level of IL-1β along with low blood count indicates the activation of systemic cell pyroptosis. Once a sufficient amount of extracellular pathogen-associated molecular patterns (PAMPs) are recognized by TLRs, activation of the NF-κB pathway takes place, which results in the upregulation of numerous inflammasome including NLRP3, proIL-1β, and proIL-18 [146]. Successively, activation of caspase-1 occurs as NLRP3 oligomerizes and interacts with the pro-caspase-1 via adaptor protein ASC to form a multiprotein complex [147]. The activation of caspase-1 subsequently employees and breaks down the members of the Gasdermin family (GSDMD). In the next step, GSDMD undergoes polymerization, which results in the further breakdown of IL-1β and IL-18 into their respective active states [148]. Once activated, IL-1β and IL-18 are secreted into the extracellular spaces, which employees pro-inflammatory mediators capable of potentiating and aggravating existing inflammatory response [149]. Furthermore, GSDMD activated fragments pierce through the phospholipid bilayer membrane, resulting in cellular matrix and cytokines release. Through these mechanisms, numerous immunomodulatory molecules such as oxidized phospholipids and cellular matrix are released from the intracellular environment, which serves as damage-associated molecular patterns (DAMP) [150]. As the name suggests, DAMP consists of specific molecular patterns that act as alarming signals similar to PAMPs [150]. Once recognized by nucleotide-binding oligomerization domain-leucine-rich repeats (NOD-LRR) and pyrin domain-containing protein 3 (NLRP3), DAMP amplifies the inflammatory response, which promotes sudden and progressive pyroptosis [151].
8. Putative role of sex hormones and X-chromosome
The immune responses of the elderly patients to SARS-CoV-2 infection are usually sluggish due to the immunosenescence [152]. This allows the virus to replicate in the host freely and aggressively. Moreover, aging-related dysregulation of the RAS system increase ACE2 shedding and Ang II/ADAM17 activity [153], [154]. The Ang II constitutively binds to the AT1R causing the NF-κB derived vasoconstriction and systemic inflammation [75], [152]. Innate immunity propagates NF-κB-derived inflammaging, while due to the immunosenescence, the adaptive immune response constantly decreases in the elderly age group [155], [156]. A decline in the steroidal hormones oestradiol and testosterone in postmenopausal women and aged men prolong NF-κB activation resulting in higher levels of TNF-α and IL-6 with increased incidences of pulmonary damage. Subsequently, a decrease in the glutathione levels and increase in the oxidative stress affords the NF-κB activation through TLR, which adds up to the severity of COVID-19 in the higher age group [75], [157]. Apart from these age-related risks, the young and middle-aged population is also vulnerable to cytokine storm in COVID-19 depending upon their lifestyle, genetic predisposition, smoking habits, epigenetic dysregulation, and autoimmune disease family history [158], [159].
The epidemiological studies construed that the severity and duration of cytokine storm in COVID-19 varies among different genders [160], [161]. According to the data, the mortality rate among the male patients was noted higher than female patients. A plausible explanation of this erraticism among both genders lies in X-chromosomes [162], [163]. It is noteworthy that X-chromosomes encode the majority of the immune regulatory gene and henceforth, it is not surprising it confers more active immune cells to the female gender [157], [160]. Consequently, their innate immunity allows them to quickly recognize the invader, clear the viral load and avoid sustained activation of inflammatory signalling pathways [152]. Besides that, the principal sex hormone estrogen attenuates the NF-κB pathway and suppresses the production of cytokines in female patients. The periovulatory dosages of estrogen have been shown to effectively inhibit the production of IL-6, IL-8, and TNF-α in menopausal COVID-19 patients [164], [165].
9. Therapeutics
Considering the pleiotropic nature of cytokine storm syndrome and involvement of numerous cytokines/chemokine, the question arises of which cytokine/chemokine should be targeted as a therapeutic intervention [166]. In this respect, special consideration should be given to the triggering factors responsible for the upregulation of signalling molecules with positive autocrine and paracrine feedback mechanisms [167]. Few studies have suggested that cytokine and chemokine cocktails significantly induce cell necrosis and cytopathic effects in SARS-CoV-2 induced peripheral blood mononuclear cells (PBMCs). However, when tested individually, IL-6, IL-18, IFN-γ, IL-15, TNF-α, IL-1, IL-1β, and IL-2 did not exhibit cytopathic effect at giving concentrations [168]. Due to these synergistic and feedback-loop effects, it is impossible to select, identify and inhibit one cytokine or chemokine from these intricate cascades. In this scenario, a systemic approach of simultaneous inhibition of multiple cytokines can offer an attractive therapeutic intervention [169]. As NF-κB serves as an immune relay switch for the cytokine storm, it is hypothesized that NF-κB pathway inactivation will simultaneously inhibit the release of multiple pro-inflammatory cytokines, chemokines, and adhesion molecules [66], [105].
Thus far, numerous studies have been discussed in this review with mounting shreds of evidence that NF-κB plays a pivotal role in cytokine storm propagation. Hence, the identification of therapeutic strategies corresponding to NF-κB will be instrumental in managing the morbidity and mortality of COVID-19 [169], [170]. Similar approaches were proposed in a study carried out by DeDiego, et al. [24] to demonstrate the role of the NF-κB-mediated inflammation in SARS-CoV infection. The animal model (mice) used in the study exhibited lower plasma levels of inflammatory cytokines (TNF-α and IL-6) and chemokines (CCL2, CCL5, CXCL10) when NF-κB activation was purposely inhibited by experimental drugs including CAPE, resveratrol, Bay11-7082, and parthenolide. These drugs were highly variant in their NF-κB inhibitory mechanisms; nevertheless, all compounds suppressed the production of pro-inflammatory mediators involved in cytokine storm at non-cytotoxic concentrations. The notion of inhibiting NF-κB was further supported by Roschewski, et al. [171] study, in which acalabrutinib, bruton tyrosine kinase inhibitor and TLR7/8-induced TNF-α transcription inhibitor were employed as empirical therapies in advanced staged COVID-19 patients. Administered drug categorically inhibited the NF-κB at the p65 phosphorylation stage, which resulted in the overall reduction of C-reactive protein, IL-6 plasma level, lymphopenia alleviation, and improved oxygen saturation.
Administration of proteasome inhibitor VL-01 can be a possible therapeutic intervention in severe COVID-19 cases. Results from H5N1 and lipopolysaccharide- (LPS) induced cytokine storm animal models support this recommendation [74], [172], [173]. In these studies, Balb/c mice have been exposed to highly pathogenic avian H5N1 influenza A virus through the intranasal route. Within 72 h of inoculation, an outburst of RANTES, KC (neutrophil-activating protein-3), IL-1β, IL-6, TNF-α, and Macrophage inflammatory protein-1 beta (MIP-1β) were reported. Interestingly, the VL-01 treatment not only decreased the production of cytokines and chemokines but in addition, it also increased the survival rate of the animal model significantly as compared to the control group. Similar results were observed when VL-01 was tested in the LPS-induced acute liver injury mice model. Interestingly, all model with elevated circulatory cytokine and chemokines resembles the laboratory findings of critical stage COVID-19 patients. The results from two independent models demonstrated the inhibitory potential of proteasome inhibitors (Bortezomib, Carfilzomib, or Ixazomib) against NF-κB nuclear translocation. Moreover, these studies validated the potency and effectiveness of proteasome inhibitors in the management of cytokine storm in COVID-19 [172].
A recent adaptive clinical trial (ACTT-1) supported the use of nucleotide analogue remdesivir in COVID-19 management. The nucleotide analogue inhibits RNA-dependent RNA polymerase, deceases dsRNA-induced NF-κB activation, prevents the replication of the virus, and significantly mitigates the cytokine storm in severe COVID-19 cases. Due to these activities, remdesivir treated patients showed a quicker recovery time than the control group [174].
Although N-acetylcysteine belongs to an entirely different class of drugs, nevertheless it shares its cytokine storm alleviation mechanism with remdesivir. N-acetylcysteine comprehensively inhibits the NF-κB activation by downregulating IκB phosphorylation and completely blocks the TNF-α-mediated NF-κB activation [175]. In addition, due to its potential to act as a prodrug to L-cysteine, it serves as a precursor to the biological antioxidant glutathione. Hence careful use of N-acetylcysteine can also replenish the depleted glutathione stores and reduce the spontaneous outburst of reactive oxygen species (ROS). Redox-sensitive transcription factors, including NF-κB and activator protein-1, get activated from ROS and oxidative stress, which results in the upregulated expression of IL-1β, IL-6, IL-8, and TNF-α. Hence N-acetylcysteine has the tenacity to reduce the direct and indirect activation of the NF-κB pathway [176], [177]. Previously, the addition of N-acetylcysteine with standard therapy has been shown to reduce pulmonary inflammation and improve oxidative stress at the dose of 1200 mg/day [178]. Furthermore, the same dose of N-acetylcysteine was effective in reducing the expression of IL-8, IL-6, and TNF-α in influenza (A and B) and respiratory syncytial virus-induced alveolar type II cells [179]. Based on these results, the addition of 1200 mg/day oral N-acetylcysteine to the regimen of COVID-19 patients seems like a plausible therapeutic intervention to potentially prevent the development of the cytokine storm syndrome and ARDS in severe cases [180]. In an ongoing phase IV clinical trial, N-acetylcysteine is reported to significantly improve the clinical features of critically ill COVID-19 patients [181].
An alternative strategy to block the NF-κB non-canonical pathway with TNF-α monoclonal antibodies (infliximab and adalimumab) has shown effectiveness in COVID-19 management [182], [183]. The ongoing clinical trials have given interesting revelations about the anti-TNF-α treatment regimen. COVID-19 patients (n = 536) treated with anti-TNF-α drugs for pre-existing inflammatory bowel disease (IBD) exhibited mild symptoms and were treated as outdoor patients. Only 15% (n = 84) required hospitalisation, while only 2% (n = 10) needed intensive care or ventilator or had death as outcome [184]. Similarly, empirical use of exogenous estrogen and testosterone hormone improved clinical outcomes of menopausal women and aged men with COVID-19. Hormone therapy decreases lung injury and improves the alveolar function by blocking NF-κB canonical and non-canonical pathways [75], [157].
Conclusively, the RECOVERY trial served as a gold standard study that wholeheartedly supported the use of NF-κB pathway inhibitors in critical stage COVID-19 patients. In this study, dexamethasone was given to the patients on mechanical ventilator support with severe respiratory complications. Astoundingly, the death rate plunged sharply; additionally, the number of days of ventilator support was remarkably reduced, while the recovery rate among the treated group improved significantly [124]. Meta-analysis of seven randomized trials concluded that patients treated with corticosteroids (dexamethasone, hydrocortisone, or methylprednisolone) showed lower mortality as compared to those who received usual care or placebo [185]. These results are coherent with the observational study, which suggested that COVID-19 patients with elevated CRP respond significantly to corticosteroid therapy [186]. Dexamethasone is a widely practiced drug in clinical settings due to its potent anti-inflammatory activity. Over the years, numerous anti-inflammatory mechanisms of action have been proposed and little is known that down-regulation of NF-κB transcriptional activity and increased IkB expression in the cytoplasm potentially contribute to its anti-inflammatory activities [187], [188], [189].
10. Conclusion and future directions
Inflammation is a coping strategy of the immune system to protect the body from microbial infections and mechanical injuries. However, prolong and uncontrolled inflammation results in loss of cellular function and secondary organ dysfunction. Nevertheless, inflammation is an evolutionarily acceptable process since it allows overcoming the instigating factor and increases the survival rate of the host. The activation of NF-κB by SARS-CoV-2 propagates acute systemic inflammation by increasing circulatory cytokine and chemokine levels in advanced COVID-19 patients. Elevated cytokine levels are accompanied by pulmonary effusion, alveoli destruction, capillary leakage and secondary organ dysfunction, which are differential signs and symptoms of life-threatening cytokine storm syndrome. In this scenario, the systemic approach of simultaneous inhibition of NF-κB with proteasome inhibitor, bruton tyrosine kinase inhibitors, nucleotide analogues, TNF-α monoclonal antibodies, N-acetylcysteine and corticosteroids offers an attractive therapeutic intervention. Cytokine storm associated with idiopathic multicentric Castleman's disease or CAR T-cell therapy were considered deadly conditions in the past, but targeted therapeutics approaches have turned these life-threatening events into manageable states. Recent advances in multi-omic profiling and tailored immunomodulatory therapies are expected to bring continued improvements in COVID-19 treatment prognosis.
Authors' contribution
Ali Attiq conceptualized the study, collected and analyzed the data and drafted the manuscript. Lui Jin Yao, Sheryar Afzal and Mansoor Ali Khan participated in the study design, preparation of illustrations, data collection and tabulation. All authors listed have made a substantial, direct and intellectual contribution to the work and approved it for publication
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pennisi M., Lanza G., Falzone L., Fisicaro F., Ferri R., Bella R. SARS-CoV-2 and the nervous system: from clinical features to molecular mechanisms. Int. J. Mol. Sci. 2020;21(15):5475. doi: 10.3390/ijms21155475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. Resp. Medi. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu T., Huang R., Zhu L., Wang J., Cheng J., Zhang B., Zhao H., Chen K., Shao H., Zhu C. Epidemiological and clinical features of asymptomatic patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92(10):1884–1889. doi: 10.1002/jmv.25944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatenoud L., Ferran C., Bach J.-F. The anti-CD3-induced syndrome: a consequence of massive in vivo cell activation. Superantigens. 1991:121–134. doi: 10.1007/978-3-642-50998-8_9. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara J.M., Abhyankar S., Gilliland D. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant. Proc. 1993:1216–1217. [PubMed] [Google Scholar]
- 9.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay K.A. Cytokine release syndrome and neurotoxicity after CD 19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br. J. Haematol. 2018;183(3):364–374. doi: 10.1111/bjh.15644. [DOI] [PubMed] [Google Scholar]
- 11.Acharya U.H., Dhawale T., Yun S., Jacobson C.A., Chavez J.C., Ramos J.D., Appelbaum J., Maloney D.G. Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy. Expert Rev. Hematol. 2019;12(3):195–205. doi: 10.1080/17474086.2019.1585238. [DOI] [PubMed] [Google Scholar]
- 12.Magee M.S., Snook A. Challenges to chimeric antigen receptor (CAR)-T cell therapy for cancer. Discov. Med. 2014;18(100):265–271. [PMC free article] [PubMed] [Google Scholar]
- 13.Fajgenbaum D.C., June C.H. Cytokine storm. NEJM. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerlach H. Agents to reduce cytokine storm, F1000. Research. 2016;5 doi: 10.12688/f1000research.9092.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark I.A. The advent of the cytokine storm. Immun. Cell. Biol. 2007;85(4):271. doi: 10.1038/sj.icb.7100062. [DOI] [PubMed] [Google Scholar]
- 16.Kash J.C., Tumpey T.M., Proll S.C., Carter V., Perwitasari O., Thomas M.J., Basler C.F., Palese P., Taubenberger J.K., García-Sastre A. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443(7111):578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J. Clin. Inves. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasparakis M., Luedde T., Schmidt-Supprian M. Dissection of the NF-κB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13(5):861–872. doi: 10.1038/sj.cdd.4401870. [DOI] [PubMed] [Google Scholar]
- 19.Li F., Zhang J., Arfuso F., Chinnathambi A., Zayed M.E., Alharbi S.A., Kumar A.P., Ahn K.S., Sethi G. NF-κB in cancer therapy. Arch. Toxicol. 2015;89(5):711–731. doi: 10.1007/s00204-015-1470-4. [DOI] [PubMed] [Google Scholar]
- 20.Oeckinghaus A., Hayden M.S., Ghosh S. Cross-talk in NF-κB signaling pathways. Nat. Immunol. 2011;12(8):695. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 21.Attiq A., Jalil J., Husain K., Ahmad W. Raging the War Against Inflammation With Natural Products. Front. Pharmacol. 2018;9(976) doi: 10.3389/fphar.2018.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021;394(3):561–567. doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C., Castaño-Rodriguez C., Perlman S., Enjuanes L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88(2):913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Q.J., Ye L.B., Timani K.A., Zeng Y.C., She Y.L., Ye L., Wu Z.H. Activation of NF-κB by the full-length nucleocapsid protein of the SARS coronavirus. Acta. Biochimica et Biophysica Sinica. 2005;37(9):607–612. doi: 10.1111/j.1745-7270.2005.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayden M.S., Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21(2):223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamal A., Husein A., Bihari C., Kumar V. Ubiquitin ligase TRUSS augments the expression of interleukin-10 via proteasomal processing of NF-κB1/p105 to NF-κB/p50. Cell. Signal. 2020;75 doi: 10.1016/j.cellsig.2020.109766. [DOI] [PubMed] [Google Scholar]
- 28.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017;2(1):1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birbach A., Gold P., Binder B.R., Hofer E., de Martin R., Schmid J.A. Signaling molecules of the NF-κB pathway shuttle constitutively between cytoplasm and nucleus. J. Biol. Chem. 2002;277(13):10842–10851. doi: 10.1074/jbc.M112475200. [DOI] [PubMed] [Google Scholar]
- 30.Pontoriero M., Fiume G., Vecchio E., de Laurentiis A., Albano F., Iaccino E., Mimmi S., Pisano A., Agosti V., Giovannone E. Activation of NF-κB in B cell receptor signaling through Bruton's tyrosine kinase-dependent phosphorylation of IκB-α. J. Mol. Med. 2019;97(5):675–690. doi: 10.1007/s00109-019-01777-x. [DOI] [PubMed] [Google Scholar]
- 31.Hirata Y., Takahashi M., Morishita T., Noguchi T., Matsuzawa A. Post-translational modifications of the TAK1-TAB complex. Int. J. Mol. Sci. 2017;18(1):205. doi: 10.3390/ijms18010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan K., Wang K., Li P. The role of post-translational modifications in cardiac hypertrophy. J. Cell. Mole. Med. 2019;23(6):3795–3807. doi: 10.1111/jcmm.14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun S.C. The non-canonical NF-κB pathway. Immunol. Rev. 2012;246(1):125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017;17(9):545. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verstrepen L., Bekaert T., Chau T.-L., Tavernier J., Chariot A., Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-κB: variations on a common theme. Cell. Mol. Life Sci. 2008;65(19):2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muzio M., Polentarutti N., Bosisio D., Kumar P.M., Mantovani A. Toll-like receptor family and signalling pathway. Biochem. Soc. Trans. 2000;28(5):563–566. doi: 10.1042/bst0280563. [DOI] [PubMed] [Google Scholar]
- 37.Hayden M.S., Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T., Akira S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Dorrington M.G., Fraser I.D. NF-κB signaling in macrophages: dynamics, crosstalk, and signal integration. Front. Immunol. 2019;10:705. doi: 10.3389/fimmu.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dejardin E., Droin N.M., Delhase M., Haas E., Cao Y., Makris C., Li Z.-W., Karin M., Ware C.F., Green D.R. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity. 2002;17(4):525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 41.Jellusova J., Miletic A.V., Cato M.H., Lin W.-W., Hu Y., Bishop G.A., Shlomchik M.J., Rickert R.C. Context-specific BAFF-R signaling by the NF-κB and PI3K pathways. Cell Rep. 2013;5(4):1022–1035. doi: 10.1016/j.celrep.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.W. Zhang, Q. Shi, X. Xu, H. Chen, W. Lin, F. Zhang, X. Zeng, X. Zhang, D. Ba, W. He, Aberrant CD40-induced NF-κB activation in human lupus B lymphocytes, 2012. [DOI] [PMC free article] [PubMed]
- 43.Baccam M., Woo S.-Y., Vinson C., Bishop G.A. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: involvement of NF-κB, AP-1, and C/EBP. J. Immunol. 2003;170(6):3099–3108. doi: 10.4049/jimmunol.170.6.3099. [DOI] [PubMed] [Google Scholar]
- 44.Luo G., Li F., Li X., Wang Z.G., Zhang B. TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol. Med. Report. 2018;17(5):6605–6611. doi: 10.3892/mmr.2018.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Attiq A., Jalil J., Husain K. Annonaceae: breaking the wall of inflammation. Front. Pharmacol. 2017;8:752. doi: 10.3389/fphar.2017.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attiq A., Jalil J., Husain K., Mohamad H.F., Ahmad A. Luteolin and apigenin derived glycosides from Alphonsea elliptica abrogate LPS-induced inflammatory responses in human plasma. J. Ethnopharmacol. 2021;275 doi: 10.1016/j.jep.2021.114120. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto Y., Gaynor R.B. IκB kinases: key regulators of the NF-κB pathway. Trends Biochem. Sci. 2004;29(2):72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Attiq A., Jalil J., Husain K., Ahmad W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018;9:976. doi: 10.3389/fphar.2018.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attiq A., Jalil J., Husain K., Jamal J.A., Ismail E.N. A new prenylated benzoquinone from Cyathocalyx pruniferus abrogates LPS-induced inflammatory responses associated with PGE2, COX-2 and cytokines biosynthesis in human plasma. Inflammopharmacology. 2021 doi: 10.1007/s10787-021-00807-w. [DOI] [PubMed] [Google Scholar]
- 50.Gerondakis S., Grossmann M., Nakamura Y., Pohl T., Grumont R. Genetic approaches in mice to understand Rel/NF-κB and IκB function: transgenics and knockouts. Oncogene. 1999;18(49):6888–6895. doi: 10.1038/sj.onc.1203236. [DOI] [PubMed] [Google Scholar]
- 51.Mattson M., Meffert M. Roles for NF-κB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 52.Courtois G., Gilmore T. Mutations in the NF-κ B signaling pathway: implications for human disease. Oncogene. 2006;25(51):6831–6843. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 53.Baker R.G., Hayden M.S., Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 2018;18(5):309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 55.Oeckinghaus A., Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009;1(4) doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang H., Lyu Y., Hou F. SARS-CoV-2 infection and the antiviral innate immune response. J. Mol. Cell. Biol. 2020;12(12):963–967. doi: 10.1093/jmcb/mjaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020:1–16. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hillen H.S., Kokic G., Farnung L., Dienemann C., Tegunov D., Cramer P. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584(7819):154–156. doi: 10.1038/s41586-020-2368-8. [DOI] [PubMed] [Google Scholar]
- 59.Luo R., Delaunay-Moisan A., Timmis K., Danchin A. SARS-CoV-2 biology and variants: anticipation of viral evolution and what needs to be done, Wiley Online. Library. 2021 doi: 10.1111/1462-2920.15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia M.A., Gallego P., Campagna M., González-Santamaría J., Martínez G., Marcos-Villar L., Vidal A., Esteban M., Rivas C. Activation of NF-kB pathway by virus infection requires Rb expression. PLoS One. 2009;4(7) doi: 10.1371/journal.pone.0006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meusel T.R., Kehoe K.E., Imani F. Protein kinase R regulates double-stranded RNA induction of TNF-α but not IL-1β mRNA in human epithelial cells. J. Immunol. 2002;168(12):6429–6435. doi: 10.4049/jimmunol.168.12.6429. [DOI] [PubMed] [Google Scholar]
- 62.Ma C.-H., Wu C.-H., Jou I.-M., Tu Y.-K., Hung C.-H., Hsieh P.-L., Tsai K.-L. PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes. Redox biology. 2018;14:72–81. doi: 10.1016/j.redox.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1(6) doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shih R.-H., Wang C.-Y., Yang C.-M. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front. Mol. Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen I.C., Wilson J.E., Schneider M., Lich J.D., Roberts R.A., Arthur J.C., Woodford R.-M.T., Davis B.K., Uronis J.M., Herfarth H.H. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of non-canonical NF-κB signaling. Immunity. 2012;36(5):742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hariharan A., Hakeem A.R., Radhakrishnan S., Reddy M.S., Rela M. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology. 2020:1–10. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu X., Chen J., Wang L., Ivashkiv L.B. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukoc. Biol. 2007;82(2):237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 68.Jin S., Mutvei A., Chivukula I., Andersson E., Ramsköld D., Sandberg R., Lee K., Kronqvist P., Mamaeva V., Östling P. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene. 2013;32(41):4892–4902. doi: 10.1038/onc.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe S., Mu W., Kahn A., Jing N., Li J.H., Lan H.Y., Nakagawa T., Ohashi R., Johnson R.J. Role of JAK/STAT pathway in IL-6-induced activation of vascular smooth muscle cells. Am. J. Nephrol. 2004;24(4):387–392. doi: 10.1159/000079706. [DOI] [PubMed] [Google Scholar]
- 70.Ma J.F., Sanchez B.J., Hall D.T., Tremblay A.M.K., Di Marco S., Gallouzi I.E. STAT 3 promotes IFN γ/TNF α-induced muscle wasting in an NF-κB-dependent and IL-6-independent manner. EMBO Mol. Med. 2017;9(5):622–637. doi: 10.15252/emmm.201607052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2020 doi: 10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murakami M., Harada M., Kamimura D., Ogura H., Okuyama Y., Kumai N., Okuyama A., Singh R., Jiang J.-J., Atsumi T. Disease-association analysis of an inflammation-related feedback loop. Cell Rep. 2013;3(3):946–959. doi: 10.1016/j.celrep.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 73.Atsumi T., Singh R., Sabharwal L., Bando H., Meng J., Arima Y., Yamada M., Harada M., Jiang J.-J., Kamimura D. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 2014;74(1):8–14. doi: 10.1158/0008-5472.CAN-13-2322. [DOI] [PubMed] [Google Scholar]
- 74.Kojima H., Inoue T., Kunimoto H., Nakajima K. IL-6-STAT3 signaling and premature senescence. Jak-stat. 2013;2(4) doi: 10.4161/jkst.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swärd P., Edsfeldt A., Reepalu A., Jehpsson L., Rosengren B.E., Karlsson M.K. Age and sex differences in soluble ACE2 may give insights for COVID-19. Critical Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-02942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berthelot J.-M., Lioté F. COVID-19 as a STING disorder with delayed over-secretion of interferon-beta. EBioMedicine. 2020;56 doi: 10.1016/j.ebiom.2020.102801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vargas T.R., Benoit-Lizon I., Apetoh L. Rationale for stimulator of interferon genes–targeted cancer immunotherapy. Eur. J. Cancer. 2017;75:86–97. doi: 10.1016/j.ejca.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X., Liu B., Tang L., Su Q., Hwang N., Sehgal M., Cheng J., Ma J., Zhang X., Tan Y. Discovery and mechanistic study of a novel human-stimulator-of-interferon-genes agonist, ACS. Infect. Diseas. 2019;5(7):1139–1149. doi: 10.1021/acsinfecdis.9b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang R., Wang C., Jiang Q., Lv M., Gao P., Yu X., Mu P., Zhang R., Bi S., Feng J.-M. NEMO–IKKβ are essential for IRF3 and NF-κB activation in the cGAS–STING pathway. J. Immunol. 2017;199(9):3222–3233. doi: 10.4049/jimmunol.1700699. [DOI] [PubMed] [Google Scholar]
- 80.Abe T., Barber G.N. Cytosolic-DNA-mediated, STING-dependent pro-inflammatory gene induction necessitates canonical NF-κB activation through TBK1. J. Virol. 2014;88(10):5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saccani S., Pantano S., Natoli G. p38-dependent marking of inflammatory genes for increased NF-κB recruitment. Nat. Immunol. 2002;3(1):69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 82.Ulivi V., Giannoni P., Gentili C., Cancedda R., Descalzi F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J. Cell. Biochem. 2008;104(4):1393–1406. doi: 10.1002/jcb.21717. [DOI] [PubMed] [Google Scholar]
- 83.Santarpia L., Lippman S.M., El-Naggar A.K. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16(1):103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kyriakis J.M., Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 2012;92(2):689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 85.Cheng Y., Sun F., Wang L., Gao M., Xie Y., Sun Y., Liu H., Yuan Y., Yi W., Huang Z. Virus-induced p38 MAPK activation facilitates viral infection. Theranostics. 2020;10(26):12223. doi: 10.7150/thno.50992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Panteva M., Korkaya H., Jameel S. Hepatitis viruses and the MAPK pathway: is this a survival strategy? Virus Res. 2003;92(2):131–140. doi: 10.1016/s0168-1702(02)00356-8. [DOI] [PubMed] [Google Scholar]
- 87.Park J.-K., Fischer R., Dechend R., Shagdarsuren E., Gapeljuk A., Wellner M., Meiners S., Gratze P., Al-Saadi N., Feldt S. p38 Mitogen-activated protein kinase inhibition ameliorates angiotensin II–Induced Target Organ Damage. Hypertension. 2007;49(3):481–489. doi: 10.1161/01.HYP.0000256831.33459.ea. [DOI] [PubMed] [Google Scholar]
- 88.Kułdo J.M., Westra J., Ásgeirsdóttir S.A., Kok R.J., Oosterhuis K., Rots M.G., Schouten J.P., Limburg P.C., Molema G. Differential effects of NF-κB and p38 MAPK inhibitors and combinations thereof on TNF-α-and IL-1β-induced pro-inflammatory status of endothelial cells in vitro. Am. J. Physiol. Cell Physiol. 2005;289(5):C1229–C1239. doi: 10.1152/ajpcell.00620.2004. [DOI] [PubMed] [Google Scholar]
- 89.Battagello D.S., Dragunas G., Klein M.O., Ayub A.L., Velloso F.J., Correa R.G. Unpuzzling COVID-19: tissue-related signaling pathways associated with SARS-CoV-2 infection and transmission. Clin. Sci. 2020;134(16):2137–2160. doi: 10.1042/CS20200904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Devaux C.A., Rolain J.-M., Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Y.M., Xu G., Wang B., Liu B.C. Cytokine storm syndrome in coronavirus disease 2019: A narrative review. J. Intern. Med. 2020 doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet. Infect. Dise. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zawawi A., Naser A.Y., Alwafi H., Minshawi F. Profile of Circulatory Cytokines and Chemokines in Human Coronaviruses: A Systematic Review and Meta-Analysis. Front. Immunol. 2021;12:1453. doi: 10.3389/fimmu.2021.666223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y., Zhang C., Huang F., Yang Y., Wang F., Yuan J., Zhang Z., Qin Y., Li X., Zhao D. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Nat. Sci. Rev. 2020;7(6):1003–1011. doi: 10.1093/nsr/nwaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., Debnath O., Thürmann L., Kurth F., Völker M.T. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38(8):970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 97.Kritas S., Ronconi G., Caraffa A., Gallenga C., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents. 2020;34(1):9–14. doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 98.Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., Kuri-Cervantes L., Pampena M.B., D'Andrea K. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508) doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y., Kong L., Fang X., Zheng H., Wu Z. Up-regulation of IL-6 and TNF-α induced by SARS-coronavirus spike protein in murine macrophages via NF-κB pathway. Virus Res. 2007;128(1–2):1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smits S.L., De Lang A., Van Den Brand J.M., Leijten L.M., Van Ijcken W.F., Eijkemans M.J., Van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2) doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang J., Hume A.J., Abo K.M., Werder R.B., Villacorta-Martin C., Alysandratos K.-D., Beermann M.L., Simone-Roach C., Lindstrom-Vautrin J., Olejnik J. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell. Stem. Cell. 2020;27(6):962–973. doi: 10.1016/j.stem.2020.09.013. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.M.R. Islam, A. Fischer, A Transcriptome analysis identifies potential preventive and therapeutic approaches towards COVID-19, 2020.
- 103.Hölscher C. Review 2:“ SARS-CoV-2 Spike protein promotes hyper-inflammatory response that can be ameliorated by Spike-antagonistic peptide and FDA-approved ER stress and MAP kinase inhibitors in vitro”. Rapid. Rev. COVID-19. 2020 [Google Scholar]
- 104.Hsu A.C., Wang G., Reid A.T., Veerati P.C., Pathinayake P.S., Daly K., Mayall J.R., Hansbro P.M., Horvat J.C., Wang F. SARS-CoV-2 Spike protein promotes hyper-inflammatory response that can be ameliorated by Spike-antagonistic peptide and FDA-approved ER stress and MAP kinase inhibitors in vitro. bioRxiv. 2020 [Google Scholar]
- 105.Kircheis R., Haasbach E., Lueftenegger D., Heyken W.T., Ocker M., Planz O. NF-κB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.598444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin T., Lee J.E., Kang J.W., Shin H.Y., Lee J.B., Jin D.I. Endoplasmic reticulum (ER) stress and unfolded protein response (UPR) in mammalian oocyte maturation and preimplantation embryo development. Int. J. Mol. Sci. 2019;20(2):409. doi: 10.3390/ijms20020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sohn K.M., Lee S.-G., Kim H.J., Cheon S., Jeong H., Lee J., Kim I.S., Silwal P., Kim Y.J., Paik S. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J. Korean Med. Sci. 2020;35(38) doi: 10.3346/jkms.2020.35.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee D.W., Santomasso B.D., Locke F.L., Ghobadi A., Turtle C.J., Brudno J.N., Maus M.V., Park J.H., Mead E., Pavletic S. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. NEJM. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ye Q., Wang B., Mao J. Cytokine storm in COVID-19 and treatment. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun X., Wang T., Cai D., Hu Z., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., Wang J. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Templin C., Ghadri J.R., Diekmann J., Napp L.C., Bataiosu D.R., Jaguszewski M., Cammann V.L., Sarcon A., Geyer V., Neumann C.A. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. NEJM. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 116.Tsao C.W., Strom J.B., Chang J.D., Manning W.J. COVID-19–associated stress (Takotsubo) cardiomyopathy. Circ. Cardiovasc. Imaging. 2020;13(7) doi: 10.1161/CIRCIMAGING.120.011222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwartzentruber D.J. Guidelines for the safe administration of high-dose interleukin-2. J. Immunother. 2001;24(4):287–293. doi: 10.1097/00002371-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 118.Chandrashekara S. C-reactive protein: An inflammatory marker with specific role in physiology, pathology, and diagnosis. Int. J. Rheum. Clin. Immunol. 2014;2(S1) [Google Scholar]
- 119.Pérez-Prieto D., Portillo M.E., Puig-Verdié L., Alier A., Martínez S., Sorlí L., Horcajada J.P., Monllau J.C. C-reactive protein may misdiagnose prosthetic joint infections, particularly chronic and low-grade infections. Int. Orthop. 2017;41(7):1315–1319. doi: 10.1007/s00264-017-3430-5. [DOI] [PubMed] [Google Scholar]
- 120.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Grupp S.A., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaur S., Bansal R., Kollimuttathuillam S., Gowda A.M., Singh B., Mehta D., Maroules M. The looming storm: blood and cytokines in COVID-19. Blood Rev. 2020;100743 doi: 10.1016/j.blre.2020.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA. Int. Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 123.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 124.R.C. Group Dexamethasone in hospitalized patients with Covid-19—preliminary report. NEJM. 2020 [Google Scholar]
- 125.Lucas C., Wong P., Klein J., Castro T.B., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K., Hodeib S., Korol C. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baradaran A., Ebrahimzadeh M.H., Baradaran A., Kachooei A.R. Prevalence of comorbidities in COVID-19 patients: A systematic review and meta-analysis. Arch. Bone Joint Surg. 2020;8(Suppl 1):247. doi: 10.22038/abjs.2020.47754.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., Abosalif K.O., Ahmed Z., Younas S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infec. Pub. Heal. 2020 doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu Z., Cai T., Fan L., Lou K., Hua X., Huang Z., Gao G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease, 2019. Int. J. Infect. Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Caricchio R., Gallucci M., Dass C., Zhang X., Gallucci S., Fleece D., Bromberg M., Criner G.J. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021;80(1):88–95. doi: 10.1136/annrheumdis-2020-219720. [DOI] [PubMed] [Google Scholar]
- 134.Fathi N., Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol. Int. 2020;44(9):1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal. Transduct. Target. Ther. 2020;5(1):1–3. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.-D., Sacco C., Alexia B. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klok F., Kruip M., Van der Meer N., Arbous M., Gommers D., Kant K., Kaptein F., van Paassen J., Stals M., Huisman M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Teachey D.T., Lacey S.F., Shaw P.A., Melenhorst J.J., Maude S.L., Frey N., Pequignot E., Gonzalez V.E., Chen F., Finklestein J. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.N.C. Institute, 2009. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 142.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020 doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yap J.K., Moriyama M., Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J. Immunol. 2020;205(2):307–312. doi: 10.4049/jimmunol.2000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.N. Duran, W.J. Favaro, Pyroptosis: Physiological roles in viral infection, arXiv preprint arXiv:2006.15777, 2020.
- 145.Chen I.-Y., Moriyama M., Chang M.-F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-TeKippe E., Taxman D.J., Guthrie E.H., Pickles R.J., Ting J.P.-Y. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fernandes-Alnemri T., Wu J., Yu J., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., Alnemri E. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell. Death. Differ. 2007;14(9):1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 149.Man S.M., Karki R., Kanneganti T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017;277(1):61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pandolfi F., Altamura S., Frosali S., Conti P. Key role of DAMP in inflammation, cancer, and tissue repair. Clin. Ther. 2016;38(5):1017–1028. doi: 10.1016/j.clinthera.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 151.M. Yang, Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection, Available at SSRN 3527420, 2020.
- 152.Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging. 2020;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target, Inten. Care. Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sanghai N., Tranmer G.K. Taming the cytokine storm: repurposing montelukast for the attenuation and prophylaxis of severe COVID-19 symptoms. Drug Discov. Today. 2020 doi: 10.1016/j.drudis.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Salminen A., Huuskonen J., Ojala J., Kauppinen A., Kaarniranta K., Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing. Res. Rev. 2008;7(2):83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 157.R.A. Al-Lami, R.J. Urban, E. Volpi, A.M. Algburi, J. Baillargeon, Sex hormones and novel corona virus infectious disease (COVID-19), Mayo Clin. Proc., Elsevier, 2020. [DOI] [PMC free article] [PubMed]
- 158.Abbas S., Ahmad A., Lacasse A. COVID-19 infection and its deadly cytokine storm in a young obese adult. J. Community Hosp. Intern. Med. Perspect. 2020;10(4):295–298. doi: 10.1080/20009666.2020.1781030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sawalha A.H., Zhao M., Coit P., Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dana P.M., Sadoughi F., Hallajzadeh J., Asemi Z., Mansournia M.A., Yousefi B., Momen-Heravi M. An insight into the sex differences in COVID-19 patients: what are the possible causes? Prehosp. Disaster Med. 2020;35(4):438–441. doi: 10.1017/S1049023X20000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Strope J.D., Chau C.H., Figg W.D. Elsevier; Semin. Oncol.: 2020. Are sex discordant outcomes in COVID-19 related to sex hormones? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Y., Jerkic M., Slutsky A.S., Zhang H. Molecular mechanisms of sex bias differences in COVID-19 mortality. Criti. Care. 2020;24(1):1–6. doi: 10.1186/s13054-020-03118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020;34(2):339–343. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 164.Foresta C., Rocca M., Di Nisio A. Gender susceptibility to COVID-19: a review of the putative role of sex hormones and X chromosome. J. Endocrinol. Invest. 2020;1–6 doi: 10.1007/s40618-020-01383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rachon D., Mysliwska J., Suchecka-Rachon K., Wieckiewicz J., Mysliwski A. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J. Endocrinol. 2002;172(2):387–396. doi: 10.1677/joe.0.1720387. [DOI] [PubMed] [Google Scholar]
- 166.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20(23):6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020;40(1):1–7. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.S. COVID-19 cytokines and the hyperactive immune response: Synergism of TNF-α and IFN-γ in triggering inflammation, tissue damage, and death. bioRxiv. 2020 [Google Scholar]
- 169.Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021:1–7. doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Castiglione V., Chiriacò M., Emdin M., Taddei S., Vergaro G. Statin therapy in COVID-19 infection. EHJ-CVP. 2020;6(4):258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A., Roshon M., Wrzesinski S.H., Desai J.V., Zarakas M.A. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Haasbach E., Pauli E.-K., Spranger R., Mitzner D., Schubert U., Kircheis R., Planz O. Antiviral activity of the proteasome inhibitor VL-01 against influenza A viruses. Antiviral. Res. 2011;91(3):304–313. doi: 10.1016/j.antiviral.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 173.Hamasaki T., Okamoto M., Baba M. Antiviral Activity of the Proteasome Inhibitor VL-01 against Human and Avian Influenza A Viruses. Abstracts/Antiviral Research. 2011;90:A21–A78. doi: 10.1016/j.antiviral.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 174.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S. Remdesivir for the treatment of Covid-19 preliminary report, The. NEJM. 2020 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 175.Oka S.-I., Kamata H., Kamata K., Yagisawa H., Hirata H. N-Acetylcysteine suppresses TNF-induced NF-κB activation through inhibition of IκB kinases. FEBS Lett. 2000;472(2–3):196–202. doi: 10.1016/s0014-5793(00)01464-2. [DOI] [PubMed] [Google Scholar]
- 176.Wu X.Y., Luo A.Y., Zhou Y.R., Ren J.H. N-acetylcysteine reduces oxidative stress, nuclear factor-κB activity and cardiomyocyte apoptosis in heart failure. Mol. Med. Report. 2014;10(2):615–624. doi: 10.3892/mmr.2014.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Conner E.M., Grisham M.B. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12(4):274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 178.Zhang Q., Ju Y., Ma Y., Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: A randomized controlled trial. Medicine. 2018;97(45) doi: 10.1097/MD.0000000000013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mata M., Morcillo E., Gimeno C., Cortijo J. N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV) Biochem. Pharmacol. 2011;82(5):548–555. doi: 10.1016/j.bcp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 180.Assimakopoulos S.F., Marangos M. N-acetyl-cysteine may prevent COVID-19-associated cytokine storm and acute respiratory distress syndrome. Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.ClinicalTrials.gov.2020, Efficacy of n-acetylcysteine (NAC) in preventing COVID-19 from progressing to severe disease. https://clinicaltrials.gov/ct2/show/NCT04419025 (accessed 11 Aug 2020).
- 182.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395(10234):1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Benucci M., Damiani A., Infantino M., Manfredi M., Quartuccio L. Old and new antirheumatic drugs for the treatment of COVID-19. Joint Bone Spine. 2020;87(3):195. doi: 10.1016/j.jbspin.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.SECURE-IBD.database.2020, surveillance epidemiology of coronavirus (COVID-19) under research exclusion. https://covidibd.org/current-data/ (accessed 11 Aug 2020).
- 185.Sterne J.A., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., Cavalcanti A.B. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Keller M.J., Kitsis E.A., Arora S., Chen J.-T., Agarwal S., Ross M.J., Tomer Y., Southern W. Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19. J. Hosp. Med. 2020;15(8):489–493. doi: 10.12788/jhm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Meduri G.U., Muthiah M.P., Carratu P., Eltorky M., Chrousos G.P. Nuclear Factor-ĸB-and Glucocorticoid Receptor α-Mediated Mechanisms in the Regulation of Systemic and Pulmonary Inflammation during Sepsis and Acute Respiratory Distress Syndrome. Neuroimmunomodulation. 2005;12(6):321–338. doi: 10.1159/000091126. [DOI] [PubMed] [Google Scholar]
- 188.Aghai Z.H., Kumar S., Farhath S., Kumar M.A., Saslow J., Nakhla T., Eydelman R., Strande L., Stahl G., Hewitt C. Dexamethasone suppresses expression of Nuclear Factor-kappaB in the cells of tracheobronchial lavage fluid in premature neonates with respiratory distress. Pediatr. Res. 2006;59(6):811–815. doi: 10.1203/01.pdr.0000219120.92049.b3. [DOI] [PubMed] [Google Scholar]
- 189.Yamamoto Y., Gaynor R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 2001;107(2):135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu Q., Kanneganti T.-D. Cutting edge: distinct regulatory mechanisms control pro-inflammatory cytokines IL-18 and IL-1β. J. Immunol. 2017;198(11):4210–4215. doi: 10.4049/jimmunol.1700352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ross S.H., Cantrell D.A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 2018;36:411–433. doi: 10.1146/annurev-immunol-042617-053352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Velazquez-Salinas L., Verdugo-Rodriguez A., Rodriguez L.L., Borca M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019;10(1057) doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S., Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137 doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Couper K.N., Blount D.G., Riley E.M. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 195.Wojno E.D.T., Hunter C.A., Stumhofer J.S. The immunobiology of the interleukin-12 family: room for discovery. Immunity. 2019;50(4):851–870. doi: 10.1016/j.immuni.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Monin L., Gaffen S.L. Interleukin 17 family cytokines: signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb. Perspect. Biol. 2018;10(4) doi: 10.1101/cshperspect.a028522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nakanishi K. Unique action of interleukin-18 on T cells and other immune cells. Front. Immunol. 2018;9:763. doi: 10.3389/fimmu.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cayrol C., Girard J.P. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol. Rev. 2018;281(1):154–168. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 199.Lang F.M., Lee K.M.-C., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;1–8 doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Evans I.M., Kennedy S.A., Paliashvili K., Santra T., Yamaji M., Lovering R.C., Britton G., Frankel P., Kolch W., Zachary I.C. Vascular endothelial growth factor (VEGF) promotes assembly of the p130Cas interactome to drive endothelial chemotactic signaling and angiogenesis. Mol. Cell. Proteomics. 2017;16(2):168–180. doi: 10.1074/mcp.M116.064428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Guo D., Wang Q., Li C., Wang Y., Chen X. VEGF stimulated the angiogenesis by promoting the mitochondrial functions. Oncotarget. 2017;8(44):77020. doi: 10.18632/oncotarget.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lyons M.J., Yoshimura T., McMurray D.N. Interleukin (IL)-8 (CXCL8) induces cytokine expression and superoxide formation by guinea pig neutrophils infected with Mycobacterium tuberculosis. Tuberculosis. 2004;84(5):283–292. doi: 10.1016/j.tube.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 203.Gonzalez-Aparicio M., Alfaro C. Significance of the IL-8 pathway for immunotherapy. Hum. Vaccin. Immunother. 2020;16(10):2312–2317. doi: 10.1080/21645515.2019.1696075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 204.Torres-Vázquez J., Vázquez-Medina M.U., Comoto-Santacruz D.A., Pradillo-Macias M.E., Muñoz-Monroy O.E., Martínez-Cuazitl A. Relationship of IP-10 gene expression to systemic lupus erythematosus activity. Reumatología Clínica (English Edition) 2021 doi: 10.1016/j.reumae.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 205.Nakamura A., Ikeda K., Hamaoka K. Aetiological significance of infectious stimuli in Kawasaki disease. Front. Ped. 2019;7:244. doi: 10.3389/fped.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu S., Liu M., Bennett S., Wang Z., Pfleger K.D., Xu J. The molecular structure and role of CCL2 (MCP-1) and C-C chemokine receptor CCR2 in skeletal biology and diseases. J. Cell. Physiol. 2021 doi: 10.1002/jcp.30375. [DOI] [PubMed] [Google Scholar]
- 207.Khan M.M. Role of cytokines. Immunopharmacology. 2016;Springer:57–92. [Google Scholar]
- 208.Maurer M., Von Stebut E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2004;36(10):1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 209.Kobayashi S., Murata K., Shibuya H., Morita M., Ishikawa M., Furu M., Ito H., Ito J., Matsuda S., Watanabe T. A distinct human CD4+ T cell subset that secretes CXCL13 in rheumatoid synovium. Arthritis Rheum. 2013;65(12):3063–3072. doi: 10.1002/art.38173. [DOI] [PubMed] [Google Scholar]
- 210.Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Polycarpou A., Howard M., Farrar C.A., Greenlaw R., Fanelli G., Wallis R., Klavinskis L.S., Sacks S. Rationale for targeting complement in COVID-19. EMBO Mol. Med. 2020;12(8) doi: 10.15252/emmm.202012642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Java A., Apicelli A.J., Liszewski M.K., Coler-Reilly A., Atkinson J.P., Kim A.H., Kulkarni H.S. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5(15) doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Edeas M., Saleh J., Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]