Abstract
Stem cell factor (SCF) is an essential cytokine during development and is necessary for gametogenesis, hematopoiesis, mast cell development, stem cell function, and melanogenesis. Here, we measure SCF concentration and distribution in adult humans and mice using gene expression analysis, tissue staining, and organ protein lysates. We demonstrate continued SCF expression in many cell types and tissues into adulthood. Tissues with high expression in adult humans included stomach, spleen, kidney, lung, and pancreas. In mice, we found high SCF expression in the esophagus, ovary, uterus, kidney, and small intestine. Future studies may correlate our findings of increased, organ-specific SCF concentrations within adult tissues with increased risk of SCF/CD117-related disease.
Keywords: Stem cell factor, CD117, immunohistochemistry, c-kit
Graphical Abstract
INTRODUCTION
Stem cell factor (SCF, Gene ID: 4254) is an essential hematopoietic cytokine during mammalian development and is associated with multiple pathologies in adults. SCF, also known as Kit Ligand, Steel factor, and Mast Cell Growth factor, is the only known ligand of the tyrosine kinase receptor CD117/c-kit. The SCF gene is located on chromosome 12 (12q21.32) in humans, and the translated protein can exist as a membrane-bound protein (NM_003994 at 5460 bp) or a soluble protein (NM_003994 at 5460 bp) if exon 6 is spliced, leading to its secretion. Both isoforms are bioactive but vary in effectiveness at inducing CD117 kinase phosphorylation 1. In mice, SCF is encoded in the Steel locus (Sl) while CD117 is encoded in the White locus W, with both being located on chromosome 10. Similar to the human variant, murine SCF is found as a membrane bound-protein (NM_013598.3) and as a soluble protein (NM_001347156.1) with the cleavage site located on exon 7 to produce the secreted form2. Although murine and human SCF are highly conserved, the charged patches of the protein that function to bind the receptor are different, altering the binding affinity of the murine SCF to the human CD117 receptor3
Signaling of SCF through its receptor CD117 is necessary during development for processes such as gametogenesis, hematopoiesis, mast cell development, melanogenesis, stem cell survival, and stem cell differentiation 4. The absence of SCF or CD117 causes death in utero during the perinatal period due to severe anemia, emphasizing the importance of the SCF/CD117 signaling axis during development 4,5. Mice with mutations causing the deletion of the membrane-bound SCF isoform (at the Sl) display severe anemia, reduced number of tissue mast cells, sterility, and are white3,4. This deletion demonstrates that soluble SCF is insufficient in maintaining the CD117 signaling axis, verifying that both forms of SCF are needed for normal hematopoiesis, and these isoforms contribute to different cell signaling pathways 5. However, little is known regarding SCF expression into adulthood.
The purpose of this study was to characterize SCF expression in adult tissues. We evaluated the SCF gene and protein expression in various locations throughout the body to determine potential correlations between SCF expression in normal function as well as during disease progression. Regions of higher SCF expression could correlate with an increased risk of SCF/CD117 mutations leading to disease. Further, we compared SCF expression in mice and humans to evaluate the mouse model and its use in studying SCF function.
METHODS
SCF Gene Expression Analysis
Human (KITLG) and murine (Kitlg) SCF gene expression data for various tissues were mined from the EMBL—European Bioinformatics Institute Gene Expression Atlas 6 and the NIH GenBank 7. All the studies mined performed RNA sequencing to generate the expression values. Mined expression values for organs that were included in two or more data sets were graphed into heat maps using GraphPad Prism 7.0.
Immunohistochemistry for SCF Protein Expression
Immunohistochemistry was performed on tissue microarrays (TMAs) to determine the percentage of SCF positive cells and compare SCF expression between major organs in both mouse and human samples. Mouse (AMS544) and human (BN1002b) TMAs were purchased from Biomax (Maryland, USA). For murine TMAs, all tissues were from 2-month-old mice. The human TMA tissues were collected from subjects ranging in age from 1 month to 75 years. Tissues were deparaffinized in xylene then rehydrated in decreasing ethanol percentages from 100% to 70%. Antigens were retrieved by incubation in 0.05% citraconic anhydride pH 7.4 in a steamer at 98 °C for 45 min. Samples were cooled, washed in phosphate-buffered saline (PBS), and then blocked in 1% bovine serum albumin (BSA) for 30 min at room temperature. TMAs were incubated with primary antibody, Anti-stem cell factor (RRID: AB_2131619, Millipore, Massachusetts USA), at a 1:500 dilution in PBS containing 1% BSA overnight at 4 °C. Tissues were washed in PBS and incubated with the secondary antibody, Anti-rabbit-HRP at 1:100 dilution (RRID: AB_2167272, Jackson Laboratory, Maine, USA) at room temperature for 1 hour in BSA solution. Samples were washed in PBS and visualized with freshly made 3,3’-Diaminobenzidine solution, counter-stained with hematoxylin, and then dehydrated in increasing percentages of ethanol from 70%-100% followed by xylene. Slides were then mounted with mounting medium and scanned at 20X using the Hamamatsu Photonics Nanozoomer Slide Scanner in the Virtual Microscopy Core. To determine the percentage of SCF in tissues, staining was quantified using the VisioPharm digital pathology analysis software. A region of interest (ROI) was drawn around the tissue, and we used custom-designed apps to determine the percent positive area in the ROI to the total area.
SCF Enzyme-Linked Immunosorbent Assay (ELISA)
To determine the concentration of SCF in major murine tissues, organs were harvested from four male and four female C57BL/6 mice (ISMR_JAX:000664, Jackson Laboratory) at 12 weeks of age. The Institutional Animal Care and Committee at the Wake Forest University School of Medicine approved the use of these animals. Mice were anesthetized with isoflurane, and organs were perfused using a 3mL 25G syringe containing PBS. The vena cava was cut to allow drainage, and PBS was injected into the heart until it turned white. Organs were harvested and immediately put in cold T-Per lysis buffer (Thermo Fisher Scientific, Massachusetts, USA) at 1 g tissue/20 mL. Samples were kept on ice and homogenized with Miltenyi Biotec (Bergisch Gladbach, Germany) gentleMACS M tubes using the "Protein" setting for ~1 min on the Miltenyi gentleMACS Octo Dissociator with Heaters. Homogenates were spun at 4,000 RPM for 5 min, and the supernatant was aliquoted and stored at −80 °C until further use. Protein samples were prepared and analyzed according to the R&D systems (Minnesota, USA) Mouse SCF Quantikine ELISA Kit (MCK00, Thermo Fisher Scientific). The plate was scanned at 450nm using a Molecular Devices EMax Precision Microplate Reader. Sample concentrations were determined using a standard curve and graphed in GraphPad Prism 7.0.
RESULTS
SCF Gene Expression in Humans and Mice
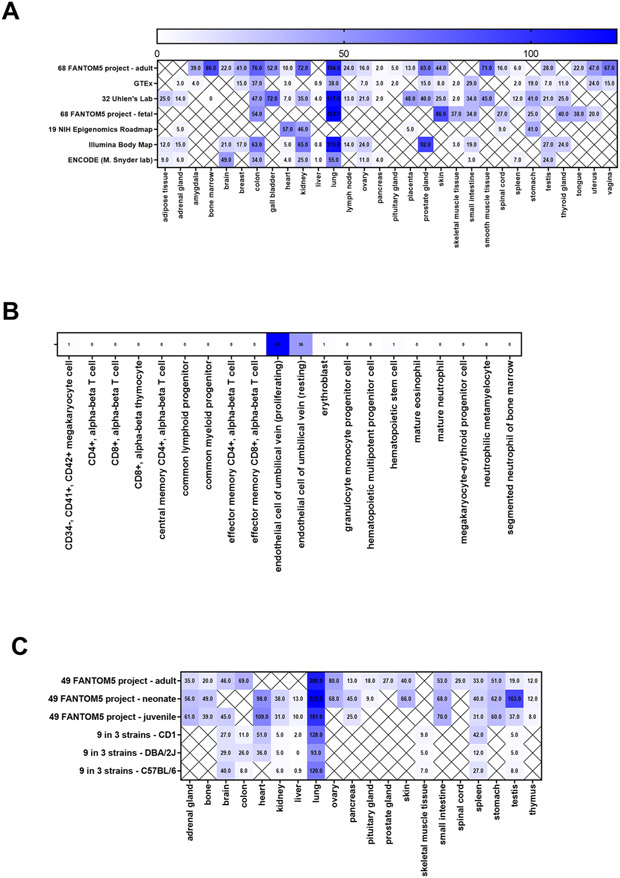
The SCF/CD117 signaling axis and its function are well known in early development; however, less is known about its function during adulthood. Thus, we investigated SCF expression in various adult human and murine organs. To examine SCF gene expression, data were mined from EMBL Gene Expression Atlas and NIH GenBank. The data sets demonstrated SCF gene expression levels in various tissues and stem cells (Figure 1). Highest mean SCF (KITLG) gene expression levels for humans were seen in the lung (92.0 TPM), prostate gland (53.0 TPM), bone marrow (43.0 TPM), skin (40.7 TPM), and colon (51.8 TPM) (Figure 1A). In human stem cells, the highest gene expression of SCF was found in the proliferating (91 TPM) and resting endothelial cells (36 TPM) of the umbilical cord in pregnant women, which is not surprising as SCF is essential in development (Figure 1B). SCF expression was also found in the megakaryocyte (1 TPM), erythroblasts (0.8 TPM), and hematopoietic stem cells (1 TPM). Gene expression of SCF (Kitlg) in normal murine tissue showed the highest mean levels of SCF in the lung (157.8 TPM), testis (39.5 TPM), heart (73.5 TPM), colon (28.5 TPM), ovary (74.0 TPM), skin (53.0 TPM), small intestine (63.7 TPM), stomach (57.7 TPM), and adrenal gland (50.7 TPM) (Figure 1C). These results were comparable to the lung in humans; however, the SCF expression in the murine model lacked a high expression in the prostate gland, skin, and bone marrow.
Figure 1. SCF gene expression in human and murine tissues.
(A-B) SCF (KITLG) expression in normal tissues (A) and bone marrow progenitor cells (B) using data mined from the EMBL—European Bioinformatics Institute Gene Expression Atlas and the NIH GenBank. (C) SCF (Kitlg) expression in normal murine tissues based on data mined from the EMBL—European Bioinformatics Institute Gene Expression Atlas.
Protein Expression of SCF in the Human TMA
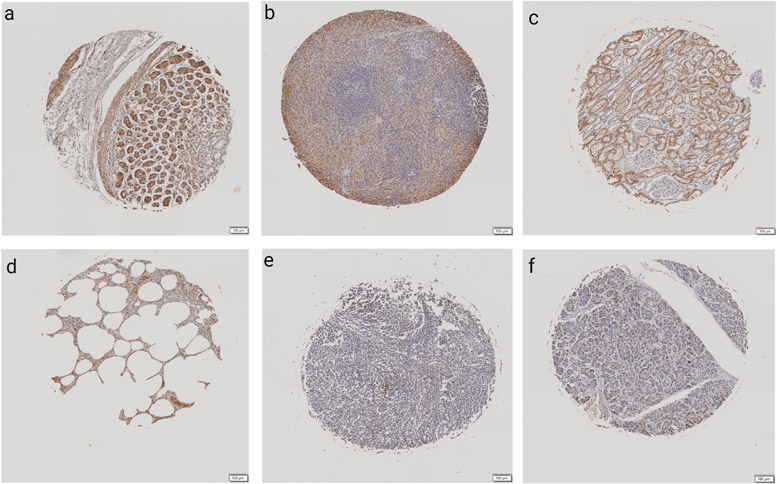
Human TMAs were stained for SCF and analyzed to measure the percentage of SCF positive area to the area of total ROI. The stomach had the highest mean percent SCF positive area at 27.19%, followed by spleen (13.33%), kidney (11.26%), lung (7.91%), lymph node (7.32%), and pancreas (2.69%) (Table 1). Within the gastric mucosa, chief cells predominated in immunoreactivity with fewer parietal cells. The gastric muscularis was mildly immunoreactive, and occasional lymphocytes were moderately immunoreactive. Within the spleen, red pulp was moderately to markedly immunoreactive, with fewer positive endothelial cells, stromal cells, and lymphocytes primarily within the follicular corona. The cortical kidney demonstrated moderate immunoreactivity within the proximal and distal convoluted tubules, with an absence of staining within glomeruli; the medulla was not examined. Within the lung, endothelial cells, alveolar macrophages, and type I pneumocytes were moderately immunoreactive. Within the pancreas, mesenchymal stromal cells, pancreatic exocrine acinar cells, and rare endothelial cells were immunoreactive. When we separated the TMA into women and men, the SCF percentage in the top 5 tissues changed. In women, the tissues with the highest percent SCF positive area were the spleen (24.49%), lung (19.48%), lymph node (15.57%), ovary (14.52%), and liver (3.29%); while in men, the stomach (36.95%), kidney (28.00%), spleen (20.33%), and pancreas (5.68%) had the highest SCF tissue expression. The staining of the top 6 tissues with the highest SCF staining can be seen in Figure 2.
Table 1: Human SCF Protein Expression in TMA Tissues from Men and Women.
Mean percent and range of percent SCF positive cells of total cells within the ROI. NAT= normal tissue adjacent to tumor, N= total number of tissues tested
| ORGAN | SCF MEAN (RANGE) COMBINED |
SD | N | WOMEN (N) | MEN (N) |
|---|---|---|---|---|---|
| BONE | 2.969 (2.96-0.427) | 2.56 | 5 | 2 | 3 |
| BREAST-NAT | 0.4827 (0.0361-1.32) | 0.14 | 5 | 5 | - |
| CEREBRUM | 0.3137 (0.0361-1.32) | 0.57 | 5 | 5 | - |
| CERVIX | 0.8093 (0.423-1.20) | 0.55 | 2 | 2 | - |
| CERVIX-NAT | 0.2292 (0.105-0.354) | 0.18 | 2 | 2 | - |
| COLON | 1.952 (0.855-4.54) | 1.50 | 5 | - | 5 |
| ESOPHAGUS | 2.456 (0.438-5.66) | 1.97 | 5 | 1 | 4 |
| KIDNEY | 11.26 (1.91-28.0) | 10.38 | 5 | - | 5 |
| LUNG | 7.916 (2.29-19.5) | 7.84 | 5 | 1 | 4 |
| LYMPH NODE | 7.321 (1.80-15.6) | 5.16 | 5 | 1 | 4 |
| OVARY | 3.93 (0.373-14.5) | 5.98 | 5 | 5 | - |
| PANCREAS | 4.487 (1.55-9.50) | 3.33 | 5 | 2 | 3 |
| PROSTATE | 0.6791 (0.0797-0.987) | 0.35 | 5 | - | 5 |
| RECTUM | 2.033 (0.658-2.95) | 0.84 | 5 | 1 | 4 |
| SKIN | 2.534 (1.53-3.58) | 0.92 | 5 | 1 | 4 |
| SMALL INTESTINE | 3.327 (1.66-5.25) | 1.51 | 5 | 1 | 4 |
| SPLEEN | 13.33 (1.21-24.50) | 9.26 | 5 | 1 | 4 |
| STOMACH | 27.19 (0.0797-36.9) | 14.02 | 5 | - | 5 |
| TESTIS | 2.18 (0.064-5.00) | 14.02 | 5 | - | 5 |
| THYMUS GLAND | 2.209 (1.41-3.83) | 1.82 | 5 | 1 | 4 |
| UTERUS-NAT | 0.35 | 0.00 | 1 | 1 | - |
Figure 2. Human SCF TMA representative images for both male and female organs.
Immunohistochemistry was performed on the human tissue microarrays, stained for SCF, and analyzed by VisioPharm to compare percent SCF positive cells to total cells within the ROI. From left to right are representative images of the six highest SCF expressing tissues: stomach (a), spleen (b), kidney (c), lung (d), lymph node (e), and pancreas (f). Scale bars are equal to 100 μm.
Protein Expression of SCF in Mice
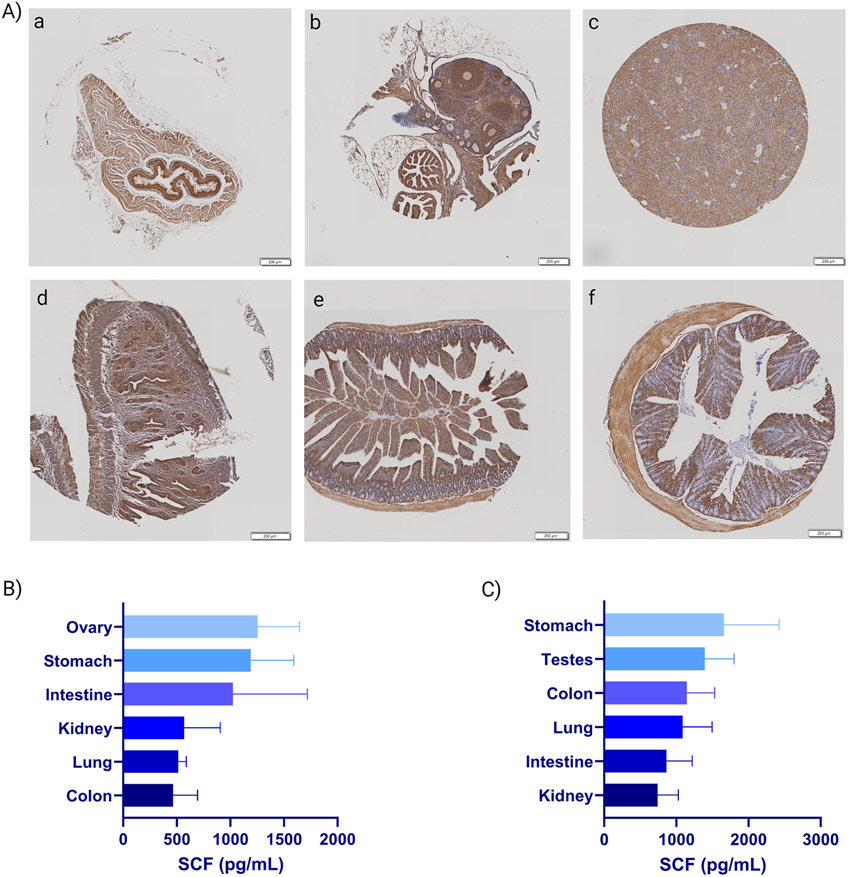
A murine TMA was also stained for SCF to examine the percentage of SCF positive area (Table 2). The top 6 tissues with the highest mean percentage of SCF positive area were ordered differently in mice compared to humans with the esophagus (35.61%), ovary (32.85%), kidney (27.50%), small intestine (25.09%), and colon (23.78%) having the highest SCF expression (Figure 3). As expected, SCF positivity was associated with cell type within different organs. Within and adjacent to the esophagus, predominantly keratinizing squamous epithelium, myocytes, and brown fat were positive. The majority of immunopositive cells within the ovary were granulosa cells, with fewer stromal cells and endothelial cells. The cortical kidney examined was largely immunopositive within the proximal and distal convoluted tubular epithelium with an absence of positivity within the glomeruli; the medulla was not examined. The small intestine was largely immunopositive within the mucosa, with enterocytes representing a large portion of positive cells. Interestingly, rare, markedly immunopositive cells were scattered within the intra-epithelial space. Although the cell lineage could not be determined, this cell type may represent interepithelial mast cells within the intestine of mice, although other cell types could not be ruled out 8. Similar to the small intestine, enterocytes were strongly immunopositive within the large intestine, with weaker immunopositivity within the myocytes in the muscularis. Throughout all tissue examined, endothelial cells were sporadically positive. The TMA examined had only one female sample for most organs preventing comparison between sexes. Our data demonstrate that the murine samples have the ovary, stomach, and small intestine in the top 10, so we cannot discount the mouse as a comparable model for SCF function in adulthood. However, if studying tissues such as the spleen, the mouse might not be the best model for SCF/CD117 function, as shown in this study to have a much lower protein expression level than in humans.
Table 2: SCF Protein Expression in TMA Organs for Male and Female Mice.
Mean percent and range of percent SCF positive cells of total cells within the ROI. N= total number of tissues tested. ND= Not Determined as only single tissue availability.
| ORGAN | SCF MEAN (RANGE) COMBINED |
SD | N | FEMALE (N) | MALE (N) |
|---|---|---|---|---|---|
| BLADDER | 20.69 (16.29-25.08) | 6.21 | 2 | 1 | 1 |
| BRAIN | 3.581 (1.807-6.794) | 2.79 | 3 | 1 | 2 |
| CEREBELLUM | 21.87 (14.54-32.10) | 9.13 | 3 | 1 | 2 |
| COLON | 23.78 (19.40-28.16) | 6.19 | 2 | 1 | 1 |
| ESOPHAGUS | 35.61 | ND | 1 | - | 1 |
| EYE | 14.18 | ND | 1 | - | 1 |
| HEART | 16.73 (11.50-23.08) | 5.87 | 3 | 1 | 2 |
| KIDNEY | 27.5 (14.00-37.38) | 12.11 | 3 | 1 | 2 |
| LUNG | 7.514 (2.832-15.64) | 7.06 | 3 | 1 | 2 |
| OVARY | 32.86 | ND | 1 | 1 | - |
| PANCREAS | 1.433 (1.004-2.162) | 0.63 | 3 | 1 | 2 |
| PROSTATE | 16.42 (15.87-16.96) | 0.77 | 2 | - | 2 |
| SALIVARY GLAND | 3.813 (1.473-8.104) | 3.72 | 3 | 1 | 2 |
| SKIN | 5.288 (2.409-6.865) | 2.50 | 3 | 1 | 2 |
| SMALL INTESTINE | 25.09 (18.82-31.35) | 8.86 | 2 | 1 | 1 |
| SPLEEN | 5.331 (3.985-7.052) | 1.57 | 3 | 1 | 2 |
| STOMACH | 19.83 (13.09-27.76) | 7.41 | 3 | 1 | 2 |
| STRIATED MUSCLE | 4.471 (2.904-5.960) | 1.53 | 3 | 1 | 2 |
| TESTIS | 14.52 (12.05-16.99) | 3.49 | 2 | - | 2 |
| THYMUS | 3.264 (3.190-3.338) | 0.10 | 2 | 1 | 1 |
| UTERUS | 28.2 (26.91-29.48) | 1.82 | 2 | 2 | - |
Figure 3. Murine SCF tissue expression in TMA and ELISA.
(A) Murine tissue microarrays were stained for SCF and analyzed by VisioPharm. Percent SCF positive cells were compared to total cells within the ROI. Shown from left to right, representative images from the TMA of the six highest expressing tissues: esophagus (a), ovary (b), kidney (c), uterus (d), small intestine (e), and colon (f). Scale bar represents 200 μm. (B-C) An ELISA was performed to obtain SCF concentration in various female (B) and male (C) murine tissues and is shown as mean ± SD (n=4).
To further examine SCF expression in mice, an ELISA was performed on tissues collected from both males and females (Figure 3 B and C). The data demonstrated that for female mice, the ovary (1254.89 pg/mL) had the highest average concentration of SCF compared to stomach (1188.39 pg/mL), intestine (1024.641 pg/mL), kidney (34.28 pg/mL), lung (575.73 pg/mL), and colon (465.73 pg/mL). In male mice, stomach (1494.81 pg/mL) had the highest average concentration of SCF compared to testes (1394.61 pg/mL), colon (1293.15 pg/mL), lung (422.31 pg/mL), intestine (443.98 pg/mL), and kidney (472.31 pg/mL). While the ovary was the second highest SCF protein expressing tissue from the TMA, stomach and testis were far below the kidney, small intestine, and colon. Using these three methods of detecting SCF expression, differences were detected between gene expression, protein expression, and protein concentration. This demonstrates the importance of performing more than one assay to obtain a complete picture.
Discussion
SCF is an essential cytokine during development with its distribution well characterized 3,9. However, little is understood about the function and distribution of SCF in adults. In this study, we compared SCF expression in a variety of human and murine tissues. Here, we show that both murine and human SCF gene expression was high in the lung and skin. Additionally, human prostate, bone marrow, and colon tissues had high SCF gene expression, whereas murine testis, heart, ovary, and small intestine demonstrated high SCF gene expression. Similarly, there was limited conserved SCF protein expression between mice and humans, with only the kidney and stomach having high SCF staining across the species. In human samples, the spleen, pancreas, and lung also showed high SCF protein expression, while in mice, we observed the highest expression in the esophagus, ovary, uterus, and small intestine. Further analysis of murine tissue via ELISA showed differences in SCF expression when compared to immunohistochemistry. The ovary was a top SCF expressing tissue for both the ELISA and TMA. We demonstrated that SCF protein levels in the stomach were high for both male and female mice in the ELISA but were lower than the kidney, small intestine, and colon in the TMA. It is not surprising that we measured differences in the magnitude of SCF expression and concentration. The TMA represents a section of the entire tissue, while the ELISA measures the whole organ concentration. Our data demonstrated that many cell types and tissues continue to express SCF into adulthood, and that the murine model should be carefully considered when studying certain SCF diseases as tissue SCF expression may not translate well. We show that SCF expression is conserved in adult organs also reported to have SCF-related disease pathology. In addition, tissues known to require SCF for normal function were also the tissues that highly expressed SCF in our study. Organs such as the lung, skin, bone, stomach, spleen, kidney, and reproductive organs all require SCF for normal function. Studies show that dysregulation of SCF in these locations causes dysfunction and disease 10.
The lung demonstrated the highest SCF gene and protein expression for both humans and mice. In normal function, stable SCF/CD117 expression plays a vital role in maintaining the lung tissue structure and integrity, particularly the alveolae 11. Pathologically, an increase of SCF leads to pulmonary fibrosis and is implicated in lung cancers 12. SCF plays an important role in remodeling and fibrosis of the lung, and alveolar fibroblasts from patients with diffuse interstitial fibrosis secrete increased SCF 13. The SCF/CD117 axis plays a direct role in pulmonary fibrosis by recruiting CD117 positive bone marrow cells to sites of lung based on decreased fibrosis in mice with SCF deficiency 12. Accordingly, SCF neutralization attenuated airway remodeling and collagen deposition in a murine asthma model reducing fibrosis 14. In addition to its effects on fibrosis, approximately 70% of small cell lung cancer tumors express SCF. Both SCF and CD117 are important for the survival and growth of these tumors, and small cell lung cancer cells expressing the defective KIT gene show a loss of cell survival and growth 15,16. Thus, while stable SCF/CD117 expression is important for maintaining lung structure and function, an imbalance can lead to diseases such as fibrosis and cancer.
In addition to lungs, skin demonstrated conserved SCF expression for both humans and mice. SCF is a known regulator of melanocytes and keratinocytes. In mice with homozygous defects in either SCF (Kitlg) or CD117 (Kit), melanocytic stem cells have decreased survival, preventing hair pigmentation 17,18. Melanocyte growth and survival are regulated by keratinocyte SCF secretion. SCF neutralization disrupts the keratinocyte-melanocyte communication leading to melanocyte death 17. Conversely, SCF injection causes activation of the adult human epidermal melanocytes and increases the proliferation and immunoreactivity of melanocytes 19. As a regulator of melanocyte survival, SCF signaling disruption can induce melanomas, with 20% of malignant melanomas affecting hand palms and foot soles (acral melanomas) and mucosal melanomas being associated with activating mutations of KIT20. Our data demonstrate that even low expression of SCF in tissues, such as the skin, can control the normal function and pathology.
Another source of SCF and CD117 expression in the skin is within mast cells. In peripheral tissues, activation of mast cells and eosinophils by CD117 results in degranulation concomitant release of pro-inflammatory cytokines21. As cells mature, most immune cells lose SCF/CD117 expression except for natural killer cells, mast cells, and some dendritic cells, which retain expression. Spleen and bone marrow are also important sites for immune system regulation as most lymphocytes are produced in the bone marrow, and the spleen is where blood cells are replenished, and lymphocytes are stored and filtered. In both bone marrow and spleen, our data demonstrate increased SCF expression in humans compared with mice. In the spleen, several cell types express SCF, including red pulp endothelial cells, perivascular stromal cells, and central arteriolar cells in the white pulp 22,23. During development, the fetal spleen is a major site for hematopoiesis before initiation of bone marrow hematopoiesis, and SCF is required for the development and differentiation of erythroid cells 24. As the spleen matures, hematopoietic stems cells become rarer and, in adults, SCF/CD117 promotes hematopoietic stem cell mobilization and differentiation of erythroid cells. We expected the spleen to have higher concentrations of SCF as its one of the main sites of murine hematopoiesis 25.
Beyond species specificity, our data further indicates a discrepancy in tissue distribution between the sexes. When breaking these data into men and women, high expression is seen in women for spleen, lung, lymph node, ovary, and pancreas, while in men, the highest expression is found in the stomach, kidney, spleen, and pancreas. However, due to the small number of female samples available for both human and murine TMAs, we cannot draw any conclusions on the role sex may play in the tissue distribution of SCF. The high expression of SCF in hormone-dependent organs, such as the ovary, prostate, and bone marrow, indicated potential crosstalk between SCF and hormone signaling. SCF/CD117 is required for the regulation of ovarian and testes steroidogenesis. In ovaries, soluble SCF caused suppression of follicle-stimulating hormone-induced estradiol production and aromatase mRNA expression; however, this suppression did not affect follicle-stimulating hormone-induced progesterone production 26. In the testes, SCF stimulates CD117 activity in Leydig cells, increasing the steroidogenic activity and testosterone production 27. In the prostate, SCF/CD117 signaling is regulated by estrogens in both neoplastic and non-neoplastic male reproductive organs 28.
SCF/CD117 is expressed in several tissues involved in the reproductive tract for both males and females, and SCF and CD117 in these organs are hormonally regulated 29. SCF expression in ovaries and testis is crucial in germ cell proliferation, migration, and survival in embryos and adulthood. Mice with the mutation at the W locus are sterile, causing issues with both ovarian and sperm development 30-32. Further, during the estrous reproductive cycle, SCF rises, and CD117 decreases, demonstrating that SCF is not only involved in germ cell development but also in the follicular development of adult females. In both murine and human females, SCF is found on many cell types of the reproductive system, including granulosa cells and oocytes, where it is needed for normal fertility function. Studies suggest that SCF signaling from the granulosa cells to the oocytes controls early follicle development 26. In the breast, SCF is expressed in the ductal epithelium, but the role of SCF and CD117 in mammary gland function is unknown 29. Conversely, the SCF/CD117 signaling axis is known to regulate breast and ovarian cancers as well as testicular and prostate cancers 33.
In male reproductive organs, SCF is expressed in a variety of cell types. Prostate progenitor cells require SCF for proliferation and survival 33. With the importance of SCF in proliferation, the SCF/CD117 signaling increases during prostate cancer progression and may be involved in bone metastasis 22,34,35. In the testes, SCF is expressed in the Sertoli cells and may also be a secretory product. SCF promotes and sustains the development of male germ cells, and germ cell survival is dependent on the SCF/CD117 axis to prevent apoptosis. Further, the SCF receptor CD117 is associated with early stages of spermatogenesis and decreases in expression as the cycle enters meiosis 27,29,36. Thus, SCF plays a vital role in reproductive organ function and pathogenesis.
In the remaining organs- bone, kidney, stomach, and small intestine- that demonstrated high SCF expression in our study, less is known about the role of SCF in normal function and disease. In the bone, SCF expression stimulates proliferation and differentiation of hematopoietic stem cells and mobilization of peripheral blood cells. Bone cells expressing SCF include megakaryocytes, perivascular cells, endothelial cells, pericytes, mesenchymal stem cells, and stromal cells 5,22,37, which is further confirmed by our gene expression data. In the kidney, SCF regulates renal function during acute kidney injury 38. In the stomach and small intestine, SCF can activate CD117 in gastrointestinal stromal tumors, which often have gain-of-function mutations in CD117, resulting in oncogenesis 34. Based on our data demonstrating the high levels of SCF expression in these tissues, the potential role of SCF in their function should be further studied.
Our data demonstrate that the SCF/CD117 signaling axis continues to be present in mature organs. This signaling axis may play a partial role in the initiation and progression of malignancies and other disorders. We have compared SCF expression between humans and mice to ascertain which organs can be well represented by the murine model. Understanding the location and expression of SCF in various adult organs will help to understand disease progression better and to develop interventions and treatments for these various human diseases and malignancies.
ACKNOWLEDGEMENTS
This work was supported by research funding from the National Cancer Institute at the National Institutes of Health (grant R00 CA175291 to BAK). The Wake Forest School of Medicine Virtual Microscopy Core is funded in part by a NIH/NCATS Grant UL1 TR001420.
Abbreviations:
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- PBS
phosphate-buffered saline
- ROI
region of interest
- SCF
stem cell factor
- Sl
Steel locus
- TMA
tissue microarray
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest, and the sponsors had no involvement in the design, collection, analysis, or interpretation of data nor in writing or submission of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sasaki T, Mizuochi C, Horio Y, Nakao K, Akashi K, Sugiyama D: Regulation of hematopoietic cell clusters in the placental niche through SCF/Kit signaling in embryonic mouse. J Cell Sci, The Company of Biologists, 2010, 123:e1.1–e1. [DOI] [PubMed] [Google Scholar]
- 2.Dehbashi M, Kamali E, Vallian S: Comparative genomics of human stem cell factor (SCF). Mol Biol Res Commun, Shiraz University of Medical Sciences, 2017, 6:1–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Ashman LK: The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol 1999, 31:1037–1051. [DOI] [PubMed] [Google Scholar]
- 4.Roskoski R: Signaling by Kit protein-tyrosine kinase - The stem cell factor receptor [Internet]. Biochem. Biophys. Res. Commun 2005. [cited 2017 Dec 19], pp. 1–13. Available from: https://ac.els-cdn.com/S0006291X05017596/1-s2.0-S0006291X05017596-main.pdf?_tid=565f80da-e4da-11e7-a21a-00000aab0f6b&acdnat=1513701386_db4f02846665ee64d87d2a22f36df9af [DOI] [PubMed] [Google Scholar]
- 5.Broudy VC: Stem Cell Factor and Hematopoiesis. 1997. [cited 2018 Feb 22],. Available from: http://www.bloodjournal.org/content/bloodjournal/90/4/1345.full.pdf [PubMed] [Google Scholar]
- 6.Petryszak R, Keays M, Tang YA, Fonseca NA, Barrera E, Burdett T, Füllgrabe A, Fuentes AM-P, Jupp S, Koskinen S, Mannion O, Huerta L, Megy K, Snow C, Williams E, Barzine M, Hastings E, Weisser H, Wright J, Jaiswal P, Huber W, Choudhary J, Parkinson HE, Brazma A: Expression Atlas update—an integrated database of gene and protein expression in humans, animals and plants. Nucleic Acids Res [Internet] 2016. [cited 2019 Aug 19], 44:D746–D752. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26481351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW: GenBank. Nucleic Acids Res [Internet], Oxford University Press, 2013. [cited 2019 Aug 19], 41:D36–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23193287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel P, Janke L, Gravano DM, Lu M, Sawant D V., Bush D, Shuyu E, Vignali DAA, Pillai A, Rehg JE: Globule Leukocytes and Other Mast Cells in the Mouse Intestine. Vet Pathol [Internet], SAGE Publications Inc., 2018. [cited 2021 Jun 29], 55:76–97. Available from: https://pubmed.ncbi.nlm.nih.gov/28494703/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster BM, Zaidi D, Young TR, Mobley ME, Kerr BA, Kerr B: CD117/c-kit in Cancer Stem Cell-Mediated Progression and Therapeutic Resistance. 2018. [cited 2018 Feb 28],. Available from: 10.1101/256099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lennartsson J, Rönnstrand L: Stem cell factor receptor/c-Kit: From basic Science to clinical implications. Physiol Rev [Internet], American Physiological Society; Bethesda, MD, 2012. [cited 2021 Feb 15], 92:1619–1649. Available from: www.ensembl.org [DOI] [PubMed] [Google Scholar]
- 11.Lindsey JY, Ganguly K, Brass DM, Li Z, Potts EN, Degan S, Chen H, Brockway B, Abraham SN, Berndt A, Stripp BR, Foster WM, Leikauf GD, Schulz H, Hollingsworth JW: c-Kit is essential for alveolar maintenance and protection from emphysema-like disease in mice. Am J Respir Crit Care Med, American Thoracic Society, 2011, 183:1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 12.Ding L, Dolgachev V, Wu Z, Liu T, Nakashima T, Wu Z, Ullenbruch M, Lukacs NW, Chen Z, Phan SH: Essential role of stem cell factor-c-Kit signalling pathway in bleomycin-induced pulmonary fibrosis. J Pathol [Internet] 2013. [cited 2019 Jun 23], 230:205–214. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23401096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fireman E, Kivity S, Shahar I, Reshef T, Mekori YA: Secretion of stem cell factor by alveolar fibroblasts in interstitial lung diseases. Immunol Lett, Elsevier, 1999, 67:229–236. [DOI] [PubMed] [Google Scholar]
- 14.Dolgachev VA, Ullenbruch MR, Lukacs NW, Phan SH: Role of stem cell factor and bone marrow-derived fibroblasts in airway remodeling. Am J Pathol, American Society for Investigative Pathology Inc., 2009, 174:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krystal GW, Hines SJ, Organ CP: Autocrine growth of small cell lung cancer mediated by coexpression of c-kit and stem cell factor. Cancer Res 1996, 56:370–376. [PubMed] [Google Scholar]
- 16.Maulik G, Bharti A, Khan E, Broderick RJ, Kijima T, Salgia R: Modulation of c-Kit/SCF pathway leads to alterations in topoisomerase-I activity in small cell lung cancer. J Environ Pathol Toxicol Oncol, J Environ Pathol Toxicol Oncol, 2004, 23:237–251. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi SI, Kunisada T, Era T, Sakakura T, Nishikawa SI: In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: Two distinct waves of c-kit-dependency during melanocyte development. EMBO J 1991, 10:2111–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grichnik JM, Burch JA, Burchette J, Shea CR: The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. J Invest Dermatol, Nature Publishing Group, 1998, 111:233–238. [DOI] [PubMed] [Google Scholar]
- 19.Funasaka Y, Boulton T, Cobb M, Yarden Y, Fan B, Lyman SD, Williams DE, Anderson DM, Zakut R, Mishima Y, Halaban R: c-Kit-kinase induces a cascade of protein tyrosine phosphorylation in normal human melanocytes in response to mast cell growth factor and stimulates mitogen-activated protein kinase but is down-regulated in melanomas. Mol Biol Cell 1992, 3:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodman SE, Davies MA: Targeting KIT in melanoma: A paradigm of molecular medicine and targeted therapeutics. Biochem. Pharmacol., Biochem Pharmacol, 2010, pp. 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welker P, Schadendorf D, Artuc M, Grabbe J, Langrish V: Expression of SCF splice variants in human melanocytes and melanoma cell lines: potential prognostic implications. Br J Cancer [Internet], Nature Publishing Group, 2000. [cited 2019 Jan 2], 82:1453–1458. Available from: http://www.nature.com/doifinder/10.1054/bjoc.1999.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster B, Zaidi D, Young T, Mobley M, Kerr B, Foster BM, Zaidi D, Young TR, Mobley ME, Kerr BA: CD117/c-kit in Cancer Stem Cell-Mediated Progression and Therapeutic Resistance. Biomedicines, Multidisciplinary Digital Publishing Institute, 2018, 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inra CN, Zhou BO, Acar M, Murphy MM, Richardson J, Zhao Z, Morrison SJ: A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature, Nature Research, 2015, 527:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan KS, Inoue T, Kulkeaw K, Tanaka Y, Lai MI, Sugiyama D: Localized SCF and IGF-1 secretion enhances erythropoiesis in the spleen of murine embryos. Biol Open, Company of Biologists Ltd, 2015, 4:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppin E, Florentin J, Vasamsetti SB, Arunkumar A, Sembrat J, Rojas M, Dutta P: Splenic hematopoietic stem cells display a pre-activated phenotype. Immunol Cell Biol [Internet], John Wiley and Sons Inc., 2018. [cited 2021 Jun 29], 96:772–784. Available from: /pmc/articles/PMC6379147/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi T, Otsuka F, Nakamura E, Inagaki K, Ogura-Ochi K, Tsukamoto N, Takeda M, Makino H: Regulatory role of kit ligand-c-kit interaction and oocyte factors in steroidogenesis by rat granulosa cells. Mol Cell Endocrinol, Elsevier, 2012, 358:18–26. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso HJ, Figueira MI, Correia S, Vaz CV, Socorro S: The SCF/c-KIT system in the male: Survival strategies in fertility and cancer. Mol Reprod Dev 2014, 81:1064–1079. [DOI] [PubMed] [Google Scholar]
- 28.Figueira MI, Correia S, Vaz CV, Cardoso HJ, Gomes IM, Marques R, Maia CJ, Socorro S: Estrogens down-regulate the stem cell factor (SCF)/c-KIT system in prostate cells: Evidence of antiproliferative and proapoptotic effects. Biochem Pharmacol [Internet], Elsevier Inc., 2016, 99:73–87. Available from: 10.1016/j.bcp.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 29.MI Figueira, Cardoso HJ, Correia S, Maia CJ, Socorro S: The stem cell factor (SCF)/c-KIT system in carcinogenesis of reproductive tissues: What does the hormonal regulation tell us? Cancer Lett, Elsevier, 2017, 405:10–21. [DOI] [PubMed] [Google Scholar]
- 30.Mauduit C, Hamamah S, Benahmed M: Stem cell factor/c-kit system in spermatogenesis. Hum. Reprod. Update 1999, pp. 535–545. [DOI] [PubMed] [Google Scholar]
- 31.Abir R, Fisch B, Jin S, Barnnet M, Kessler-Icekson G, Ao A: Monozygotic twinning is not associated with zona pellucida micromanipulation procedures but increases with high-order multiple pregnancies. 1995,. [DOI] [PubMed] [Google Scholar]
- 32.Smikle CB, Smikle in B, Dandekar P V, Schriock don D, Givens CR: Elevated ovarian follicular fluid stem cell factor concentrations are associated with improved pregnancy rates in in-vitro fertilization cycles. Fertil.STERILITY@. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso HJ, Figueira MI, Socorro S: The stem cell factor (SCF)/c-KIT signalling in testis and prostate cancer. J Cell Commun Signal, Journal of Cell Communication and Signaling, 2017, :1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesner C, Nabha SM, Dos Santos EB, Yamamoto H, Meng H, Melchior SW, Bittinger F, Thüroff JW, Vessella RL, Cher ML, Bonfil RD: C-kit and its ligand stem cell factor: potential contribution to prostate cancer bone metastasis. Neoplasia [Internet], Neoplasia Press, 2008. [cited 2019 Jan 20], 10:996–1003. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18714401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr BA, Miocinovic R, Smith AK, West XZ, Watts KE, Alzayed AW, Klink JC, Mir MC, Sturey T, Hansel DE, Heston WD, Stephenson AJ, Klein EA, Byzova TV., Kerr BA, Miocinovic R, Smith AK, West XZ, Watts KE, Alzayed AW, Klink JC, Mir MC, Sturey T, Hansel DE, Heston WD, Stephenson AJ, Klein EA, Byzova TV: CD117+ cells in the circulation are predictive of advanced prostate cancer. Oncotarget [Internet], Impact Journals, 2015. [cited 2018 Mar 6], 6:1889–1897. Available from: http://www.oncotarget.com/fulltext/2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothschild G, Sottas CM, Kissel H, Agosti V, Manova K, Hardy MP, Besmer P: A role for kit receptor signaling in Leydig cell steroidogenesis. Biol Reprod, Oxford Academic, 2003, 69:925–932. [DOI] [PubMed] [Google Scholar]
- 37.Ding L, Saunders TL, Enikolopov G, Morrison SJ: Endothelial and perivascular cells maintain haematopoietic stem cells. Nature [Internet] 2012. [cited 2018 Mar 1], 481. Available from: https://www.nature.com/articles/nature10783.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bengatta S, Arnould C, Letavernier E, Monge M, De Préneuf HM, Werb Z, Ronco P, Lelongt B: MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol, American Society of Nephrology, 2009, 20:787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]