Abstract
Atrial fibrillation affects almost 60 million adults worldwide. Atrial fibrillation is associated with a high risk of cardiovascular morbidity and death as well as with social, psychological and economic burdens on patients and their families. Social determinants — such as race and ethnicity, financial resources, social support, access to health care, rurality and residential environment, local language proficiency and health literacy — have prominent roles in the evaluation, treatment and management of atrial fibrillation. Addressing the social determinants of health provides a crucial opportunity to reduce the substantial clinical and non-clinical complications associated with atrial fibrillation. In this Review, we summarize the contributions of social determinants to the patient experience and outcomes associated with this common condition. We emphasize the relevance of social determinants and their important intersection with atrial fibrillation treatment and outcomes. In closing, we identify gaps in the literature and propose future directions for the investigation of social determinants and atrial fibrillation.
Atrial fibrillation (AF) is the most commonly encountered cardiac rhythm disorder, affecting nearly 60 million adults worldwide in 2019 and contributing to substantial social and medical burdens1,2. AF is associated with myriad cardiovascular and non-cardiovascular outcomes, including well-recognized outcomes such as heart failure, myocardial infarction, ischaemic stroke and death, as well as frailty, cognitive decline and high health-care utilization3. Social determinants of health contribute to the recognition, evaluation, treatment and outcomes of diverse disease states but have had limited examination in AF. Given their relevance to multiple risk factors for cardiovascular disease and AF, social determinants of health have substantive roles in the patient experience of AF, access to care, health outcomes and social burden.
Social determinants of health are the circumstances into which an individual is born and in which the individual lives, works and plays, and which together influence health and health care4,5. Social determinants of health include race and ethnicity, financial resources, social support, access to health care, residential environment, proficiency in the local language and health literacy. Each of these factors has a prominent role in provider-level evaluation and management of all diseases, including AF, as well as in how individuals experience their health conditions (Fig. 1). Despite their fundamental importance, to date, critical evaluation of social determinants of health in relation to AF has been limited.
Fig. 1 |. Social determinants of atrial fibrillation.
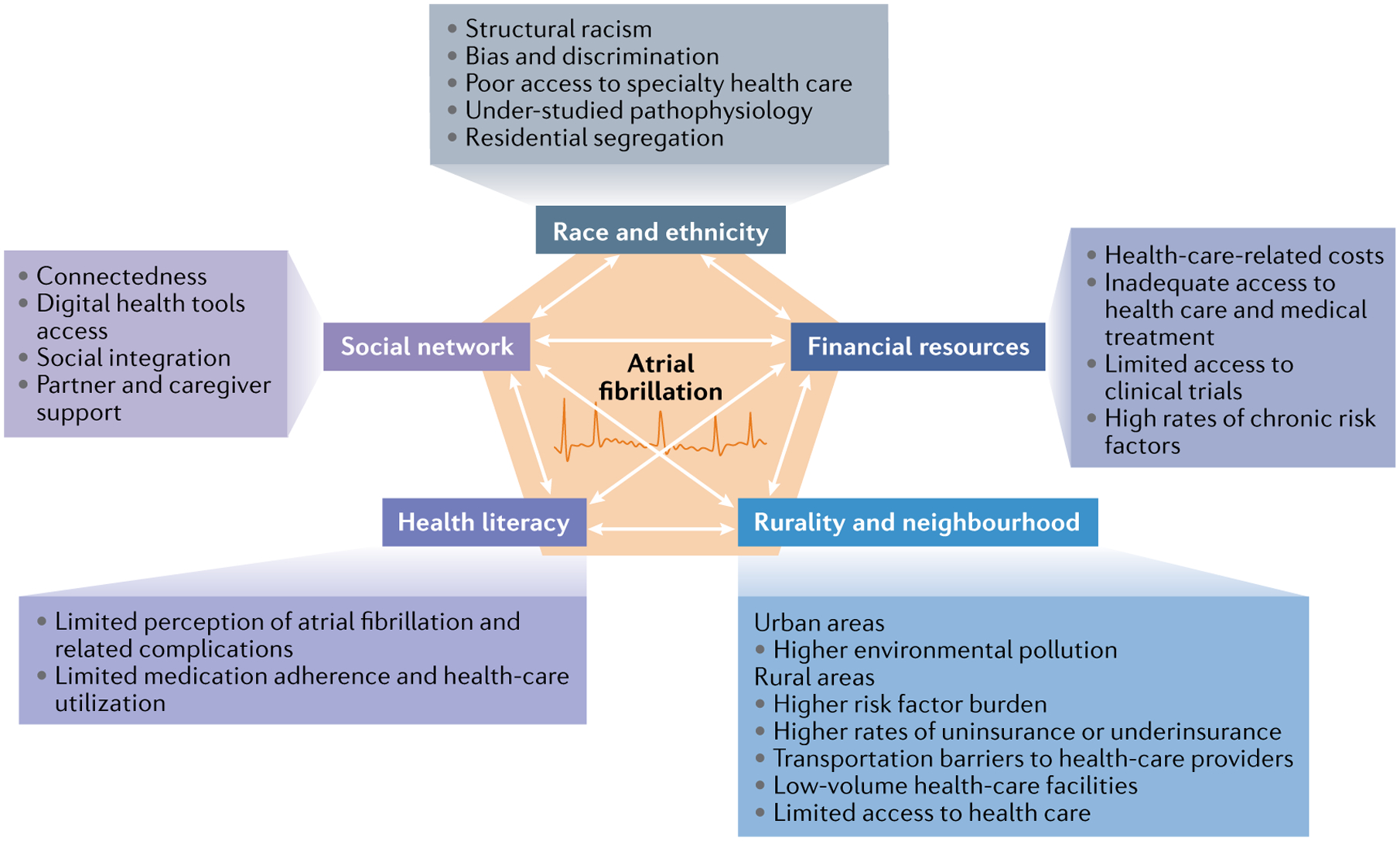
The figure represents the broad, interconnected social determinants and the proposed mechanisms by which they might influence atrial fibrillation incidence, treatment and outcomes. These social determinants include race and ethnicity, financial resources, rurality and neighbourhood residence, health literacy and social networks.
In this Review, we define the multiple contributions of social determinants to the patient experience and outcomes associated with AF, identify gaps and challenges in the literature on the associations between AF and social determinants, and articulate future directions to address these deficits. We further discuss the relevance and importance of integrating social determinants in clinical trials and observational studies on AF to advance the achievement of equitable outcomes in this condition. Although much of the literature cited in this Review is based on studies led in the USA, mostly because of the racial/ethnic, sociodemographic and geographical diversity of the country, we include data from other regions to highlight that addressing the social determinants of health is crucial to the global prevention and management of AF.
Race and ethnicity
Race and ethnicity have been the most studied social determinants of cardiovascular health. Prominent research consistently demonstrates the influence of pervasive racial bias and discrimination both within and beyond medical care6–9. Racial inequities in health and health care have, therefore, been widely described across cardiovascular conditions, including AF10–12. Despite the availability of studies aimed at addressing these disparities, in the USA, the rate of death from cardiovascular disease remains 20% higher in Black Americans than in white Americans13 (Box 1). How race and ethnicity influence AF is observed across the multiple dimensions of the disease, extending from AF recognition to clinical evaluation and management, as well as to short-term and long-term outcomes14,15 (Fig. 2).
Box 1 |. US definitions of racial and ethnic groupsa.
White: a person having origins in any of the original peoples of Europe, the Middle East or North Africa.
Black or African American: a person having origins in any of the Black racial groups of Africa.
Hispanic or Latino: a person of Cuban, Puerto Rican, Central or South American, or other Spanish culture or origin, regardless of race.
American Indian or Alaska Native: a person having origins in any of the original peoples of North and South America (including Central America) and who maintains tribal affiliation or community attachment.
Asian: a person having origins in any of the original peoples of the Far East, Southeast Asia or the Indian subcontinent including, for example, Cambodia, China, India, Japan, Korea, Malaysia, Pakistan, the Philippine Islands, Thailand and Vietnam.
As defined by the US Census Bureau, 2020.
Fig. 2 |. Determinants of racial and ethnic inequities across the atrial fibrillation care continuum.
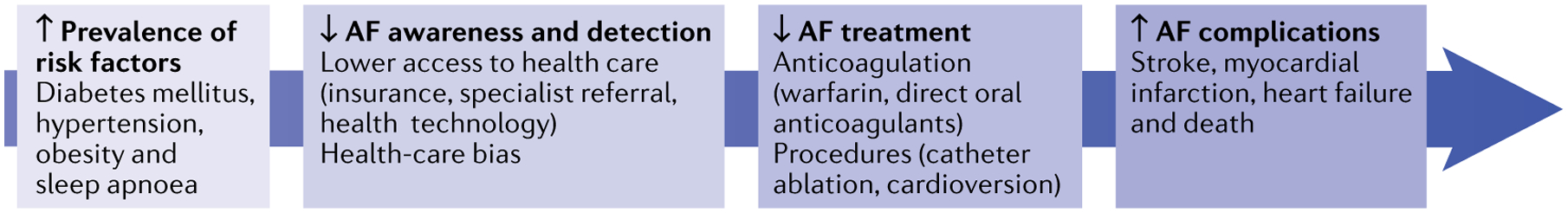
The figure represents where inequities in racial and ethnic groups under-represented in medicine affect the continuum of atrial fibrillation (AF) development and management. These inequities include high prevalence of risk factors for AF, poor awareness and detection of AF, low access to AF treatment and a high prevalence of AF-related complications.
Race, ethnicity and AF incidence.
Despite a preponderance of clinical risk factors for AF, such as hypertension, diabetes mellitus, obstructive sleep apnoea and obesity, in individuals from under-represented racial and ethnic groups in the USA, multiple analyses have shown lower rates of AF in Black Americans, Hispanic or Latino (further referred to as Hispanic) Americans, Asian Americans and Native Americans than in white Americans16–19. In the ARIC study20, a biracial, community-based cohort of >15,000 participants conducted in four US cities, the lifetime risk of developing AF over 25 years of follow-up was ~33% among white Americans compared with ~20% among Black Americans. Lower rates of AF in Black Americans than in white Americans were also observed in a separate analysis using 48-h electrocardiography monitoring of 1,193 participants in the ARIC cohort (adjusted OR 0.49, 95% CI 0.24–0.99)21. Examining a large sample of Medicare beneficiaries with implanted devices (pacemaker, cardioverter–defibrillator or loop recorder) in the USA from 2009 to 2015 identified a higher incidence of AF in white individuals (17.6 per 100 person-years, 95% CI 17.4–17.9) than in Black individuals (12.2 per 100 person-years, 95% CI 11.5–13.1)22. Similarly, in an analysis of the MESA study23, a diverse, community-based sample of participants across the USA, white participants had a higher incidence of hospitalization for AF (adjusted incidence rate (IR) 11.23, 95% CI 9.82–12.84) than Hispanic (IR 6.07, 95% CI 4.71–7.84), Chinese (IR 3.94, 95% CI 2.54–6.11) or Black (IR 5.77, 95% CI 4.75–7.02) participants.
The higher prevalence of risk factors for AF in racial/ethnic groups under-represented in the clinical literature (such as Black, Hispanic or American Indian/Alaska Native individuals) but decreased observed prevalence of AF compared with white American individuals in the USA has previously been termed a paradox24. Researchers have assessed whether genetic factors (for example, European ancestry)25 or structural cardiac factors, such as increased atrial ectopy in white individuals26, might explain the differences in AF across racial and ethnic groups, although neither factor seems to fully explain this racially based variation. Other studies have examined social factors as the driver of these differences. In the REGARDS study27, a US observational study of >30,000 Black American and white American participants, the likelihood of Black participants being aware of having AF was one -third of that of white participants (OR 0.32, 95% CI 0.20–0.52). An analysis of the MESA study28 published in 2020 found similar rates of AF incidence across all racial and ethnic groups with the use of 14-day ambulatory continuous rhythm monitoring to detect AF instead of clinical detection. This finding suggests that differential clinical recognition of AF by providers might partly explain the racial/ethnic differences observed in AF incidence rather than true biological or genetic differences between racial/ethnic groups.
The aetiology of the paradox in the observed associations between race/ethnicity and AF is complex and incompletely understood. A partial explanation for the lower incidence and prevalence of AF in Black, Hispanic or American Indian/Alaska Native individuals in the USA than in white individuals is limited ascertainment owing to structural racism and decreased access to general and specialty health care among racial/ethnic groups under-represented in medicine. Nevertheless, with few exceptions, adequately powered studies have shown a higher incidence and prevalence of AF in white individuals than among individuals of other races/ethnicities in the USA26–28. Other possible explanations include varying rates of premature death by race/ethnicity that result in differential exposure to AF-related risk factors and therefore incidence of AF, a condition consistently associated with old age1. Furthermore, although traditional clinical risk factors for AF, such as hypertension, diabetes and obesity, are known to be more common in racial/ethnic groups under-represented in medicine, social factors that protect against AF might be present in these racial/ethnic groups, and further study of this possibility is warranted.
Race, ethnicity and AF management.
For decades, the cornerstone of AF management has been oral anticoagulation, which has been shown to reduce the risk of stroke by up to 70% in patients with AF29. Despite the clear benefits of stroke prevention with anticoagulant therapy, using either warfarin or direct oral anticoagulants (DOACs), research has demonstrated racial/ethnic inequities in the initiation, adherence and quality of this therapy30–35.
Before 2010, warfarin was the only anticoagulant therapy available for the prevention of ischaemic stroke in individuals with AF, and several studies showed racial inequities in the use of this drug. An analysis of a large, hospital-based registry of patients with AF and heart failure in the USA showed that Black patients hospitalized with heart failure were less likely to be prescribed guideline-based warfarin therapy at discharge than white patients (OR 0.76, 95% CI 0.69–0.85)36. A retrospective cohort analysis of 98,053 patients in the US Veterans Health Administration demonstrated that white patients with AF receiving warfarin had a higher percentage of time in the therapeutic range than Black patients (62.3% versus 55.8%; P < 0.001)32. This racial difference in time in the therapeutic range was confirmed in a larger Veterans Health Administration analysis that demonstrated that time in the therapeutic range was higher in white individuals (0.57 ± 0.21) than in Black individuals (0.49 ± 0.23; P < 0.001) during their first year of receiving warfarin therapy37. These racial differences in time in the therapeutic range were sustained in the long term37.
Few analyses have examined racial and ethnic differences in the initiation of DOAC therapy, but those studies that have point towards similar racial/ethnic disparities to those found with warfarin therapy10,11. DOACs, which were approved for stroke prevention in AF in 2008 by the EMA and in 2010 by the FDA, have demonstrated improved safety, effectiveness and treatment adherence compared with warfarin, but inequities exist in their use38–40. In the second cohort of the ORBIT-AF II, a retrospective US-wide registry of patients with AF from 2013 to 2016, Black individuals with AF had lower rates of DOAC initiation than white individuals, even after adjusting for clinical and socioeconomic factors (OR 0.73, 95% CI 0.55–0.95)10. Racial inequities in DOAC prescription were further confirmed in an analysis of >40,000 medically insured patients with AF aged ≥65 years in the USA, showing that DOAC therapy was less likely to be initiated in Black patients than in white patients (adjusted OR 0.75, 95% CI 0.66–0.85)11. Notably, even as DOAC use has increased over time and overall warfarin use is trending down, data suggest that compared with white patients, Black patients are more likely to be prescribed warfarin than more contemporary DOACs41. For example, a large analysis of patients with ischaemic stroke in the Florida Puerto Rico AF Stroke Study41 showed that Black patients with AF were more likely than white patients to be discharged from the hospital with a prescription of warfarin therapy (OR 1.22, 95% CI 1.07–1.40).
Several AF cohort, registry and insurance claims database studies have similarly confirmed that Black patients are less likely to have adequate anticoagulation than white patients10,30,37,42,43. Additionally, researchers observed that among US Medicare beneficiaries, Black patients with AF were more likely to discontinue anticoagulation within 1 year of diagnosis than white patients (OR 1.45, 95% CI 1.25–1.72)11. A 2019 analysis of a national, hospital-based registry of patients with AF in the USA found that Hispanic patients (90.2%) were less likely than white patients (93.7%) or Black patients (94.3%) to be discharged with a prescription for any anticoagulant (P < 0.01)44. Finally, a 2020 analysis of the PINNACLE registry, the largest cardiovascular outpatient registry in the USA, determined that American Indian and Alaska Native individuals were significantly less likely to be treated with anticoagulation than individuals of other races/ethnicities (OR 0.84, 95% CI 0.77–0.93)45.
Together with disparities in oral anticoagulant therapy, racial differences have been observed for cardiac rhythm-control strategies. In a longitudinal US-based AF registry, white patients were more likely to receive antiarrhythmic medications or to undergo interventional procedures, such as cardioversion and catheter ablation, than Black patients43,46. Potential reasons for the treatment inequities include health-care provider bias, structural racism and limited access to health care. Furthermore, Black, Hispanic or American Indian/Alaska Native individuals have been poorly represented in US and international clinical trials and observational studies for AF, particularly in interventional studies47. Figure 3 summarizes the proportion of individuals of white and racial/ethnic groups under-represented in medicine in major AF clinical trials conducted globally. The poor enrolment rates of individuals of racial/ethnic groups under-represented in medicine limits the generalizability of trial findings and suggests selection biases in clinical trial development and recruitment.
Fig. 3 |. Racial/ethnic representation in clinical trials and observational studies of atrial fibrillation.
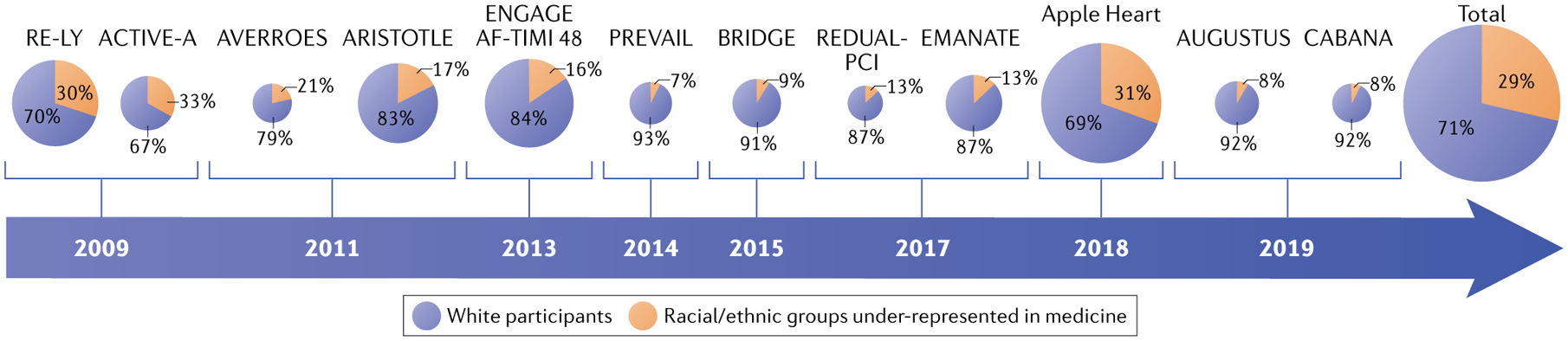
Chart illustrating the proportion of white participants (blue) versus participants from racial and ethnic groups under-represented in medicine (orange) in clinical trials and observational studies of atrial fibrillation and anticoagulation conducted internationally between 2009 and 2019 (REFS38,39,131–140). The year of publication of the study is shown in the arrow. The sizes of the individual pie charts indicate the relative overall size of the trial or study, except for the total chart.
Race, ethnicity and AF outcomes.
Underdiagnosis of AF as well as lower receipt of guideline-based therapies might contribute to observed differences in AF outcomes according to race. Previous research has demonstrated that Black patients with AF have higher rates of ischaemic stroke and cardiovascular morbidity, including myocardial infarction and heart failure, than white patients12,14,48. An analysis of the ARIC study concluded that Black participants with AF had double the incidence rates of stroke (21.4 versus 10.2 per 1,000 person-years; P < 0.01) and death (106.0 versus 55.9 per 1,000 person-years; P < 0.01) than white participants12. Disparities in stroke risk and mortality have also been described in Hispanic Americans with AF, including in a study of a national US registry of 24,000 participants that found 5.2 (95% CI 4.6–5.8) stroke cases per 100 person-years for white Americans, 12.2 (95% CI 8.0–18.5) for Black Americans and 10.6 (95% CI 6.0–18.7) for Hispanic Americans49. The racial and ethnic differences in the risk of ischaemic stroke in patients with AF served as the impetus for researchers to develop a refined CHA2DS2-VASc stroke risk score that added an additional point for Black race and reported significantly improved stroke prediction compared with the previous score50. Nevertheless, it has been argued that adding a point to capture the social construct of race without a known biological association to the outcome of interest cannot be heralded as the solution to addressing inequities in AF51,52. Instead, suggesting that Black race is an independent risk factor for stroke diminishes the contribution of centuries of structural racism or historical, cultural, institutional and interpersonal discrimination, neighbourhood segregation and underinsurance experienced by Black, Hispanic and American Indian/Alaska Native individuals in the USA, which have led to persistent health inequities53,54.
Financial resources
Individual-level and household-level income constitute robust social determinants of health55. High income and wealth are associated with improved health-care access, provider decision-making56,57 and clinical outcomes in patients with AF58–61. Conversely, lower income at both the individual and household levels has been related to higher rates of cardiovascular risk factors, adverse cardiac events and death in patients with AF62,63. We summarize in the next sections the associations between wealth and improved health-care access, treatment and outcomes in patients with AF.
Financial resources and AF incidence.
Longitudinal cohort studies have found that AF incidence is higher with worse socioeconomic position, as measured by income, educational status and employment status20,64,65. In a large, community-based, observational cohort study in the USA, AF incidence was higher among unemployed individuals than in those who were employed (OR 1.73, 95% CI 1.15–2.58) after adjusting for sociodemographic and economic factors64. Similarly, in the ARIC study, AF incidence was higher among participants with lower house hold income than in participants with higher house hold income (HR 1.45, 95% CI 1.27–1.67)65. In a New York City-based cohort study of 4,556 individuals, higher socioeconomic status, a variable that included neighbourhood-level income, was associated with a borderline, although not significantly, lower risk of AF (HR 0.99, 95% CI, 0.98–1.00)66. By contrast, a nationwide analysis from a longitudinal, community-based study in Sweden demonstrated that lower socioeconomic status was associated with a decreased incidence of AF (HR 0.79, 95% CI 0.77–0.81) and showed no association between neighbourhood socioeconomic deprivation and AF incidence (HR 0.98, 95% CI 0.95–1.01)67. Finally, a multi-cohort study of participants from Finland, Sweden and the UK who did not have AF found that those with longer working hours had a 1.4-fold increased risk of AF compared with those working standard hours (HR 1.42, 95% CI 1.13–1.80)68.
Financial resources and AF management.
Oral anticoagulation remains under-prescribed globally for the prevention of stroke in AF, particularly in low-income countries62. Just as patient-level clinical factors and provider-level knowledge and comfort can influence anticoagulant prescribing patterns, so too can the patients’ financial status. For example, among patients with AF in Sweden, higher socioeconomic status was associated with an increased likelihood of DOAC prescription rather than warfarin prescription in those in the highest-income quartile compared with those in the lowest-income quartile (OR 1.23, 95% CI 1.16–1.31)69. In addition, a Canadian study demonstrated that high neighbourhood income was associated with higher rates of DOAC prescription than warfarin prescription (OR 1.50, 95% CI 1.33–1.69)56. The same study showed that the addition of a DOAC to the Canadian drug formulary in 2012 led to the disappearance of this income-related prescribing disparity (OR 0.93, 95% CI 0.82–1.05)56. A retrospective study in Scotland of patients with AF who survived a stroke found that residing in neighbourhoods with higher socioeconomic deprivation was associated with a lack of prescription of oral anticoagulation (OR 0.59, 95% CI 0.57–0.76)70. A US-based insurance claims study of 35,000 individuals with newly diagnosed AF found that individuals who were eligible for Medicaid insurance had a higher likelihood of discontinuation of anticoagulant therapy within 1 year of initiation than those not eligible for Medicaid (OR 1.94, 95% CI 1.38–2.70)71.
In addition to preventing thromboembolic events, guidelines for the treatment of AF include managing comorbid conditions and achieving rate and rhythm control72. For patients who have medication-resistant AF or have intolerance to AF medications, treatment of AF via catheter-based ablation can reduce symptoms and improve quality of life72,73. Nonetheless, data show disparities in ablation referral depending on the economic status of the patient. A study of >16,000 patients in the USA admitted for AF catheter ablation revealed that recipients of ablation more often had private insurance (HR 1.17, 95% CI 1.11–1.22) and higher household incomes (HR 1.21, 95% CI 1.16–1.27, for the highest-income versus the lowest-income quartile) than non-recipients of ablation57. Data on the effects of socioeconomic status on receipt of other non-pharmacological AF therapies, such as percutaneous or surgical closure of the left atrial appendage, are limited and these effects require further investigation. Most of the available trials on these catheter-based or surgical therapies for AF do not specify participant income level or insurance status, limiting the generalizability of trial findings to economically diverse populations. The inclusion of social risk factors in AF clinical trials and registries has so far been limited and marks an important area for future study.
Financial resources and AF outcomes.
Individuals with AF can present with a range of symptoms secondary to differing patterns of arrhythmia, disease aetiology and comorbid conditions. The most debilitating of these symptoms is stroke, which studies have demonstrated to be more common in individuals with AF and low income. In a Canadian study of 166,742 individuals with AF aged ≥65 years, the patients in the lowest – Income quintile had an increased risk of hospitalization for stroke (HR 1.18, 95% CI 1.12–1.23) and fatal haemorrhage (HR 1.28, 95% CI 1.11–1.48) compared with those in the highest-income quintile61. A large Danish study also found that individuals with AF with higher socioeconomic status had lower all-cause mortality than those of lower socioeconomic status (HR 0.64, 95% CI 0.61–0.68), after adjusting for stroke risk and anticoagulant use74. This finding was supported by a single-centre, retrospective study in the USA that demonstrated that individuals in the lowest quartile of socioeconomic status had significantly higher mortality than those in the highest quartile (OR 1.3, 95% CI 1.1–1.5)75. Although a large insurance claims database study of >300,000 individuals with AF from the USA did not find a significant association between the risk of stroke and the patient income level, the study revealed a graded, inverse association between income level and the rates of AF-related hospitalization58. Individuals with AF who were in the lowest-income quintile had a higher risk of hospital admission for heart failure (HR 1.17, 95% CI 1.05–1.30) and myocardial infarction (HR 1.18, 95% CI 0.98–1.41) than patients with AF in the highest-income quintile58.
AF can adversely affect quality of life, a main patient-reported outcome of AF. In a retrospective analysis of data from a large, US-based regional health-care system, individuals in the lowest-income quartile reported poorer health-related quality of life, as measured by the Atrial Fibrillation Effect on Quality-of-life score, than those in the highest-income quartile (68.2 ± 21.4 for income less than US $20,000 versus 81.9 ± 17.0 for income more than $100,000; P = 0.04)60. This study also found that individuals in the lower-income category were significantly more likely to report poorer mental health, as measured by the 12-item Short Form Survey.
Rurality and neighbourhood factors
Rurality and AF.
A growing body of literature demonstrates differences in cardiovascular health and health outcomes between individuals residing in rural settings and those residing in urban settings76–78. Several factors contribute to poorer health outcomes in rural communities, including an increased burden of AF risk factors, such as old age, tobacco use, physical inactivity, diabetes mellitus, obesity and hypertension, as well as decreased access to health-care services79. For example, in the USA, individuals living in rural areas were more likely to smoke and were less likely to quit smoking than their urban counterparts80,81. In addition, data from a large, US-based cardiology practice registry revealed that individuals residing in rural areas were less likely to receive advice to quit smoking than individuals living in urban areas (OR 0.92, 95% CI 0.88–0.95; P < 0.001)82. These risk factors might result in a higher incidence and lower awareness of having AF in individuals living in rural areas83.
Individuals residing in rural communities also more frequently have social factors that can have deleterious effects on cardiovascular health, including lower levels of income, education, food security and employment76. When considering education, the ARIC study found that AF incidence was higher in individuals with lower incomes and educational levels, independently of rurality20. Of interest, when examining the lifetime risk of AF in this study, the opposite pattern was observed, with a higher lifetime risk of AF with higher education and income levels20. Similar patterns were observed in a nationwide study from Denmark, in which men (HR 0.85, 95% CI 0.76–0.96) and women (HR 0.62, 95% CI 0.50–0.77) in the highest-education group had a lower incidence of AF than those in the lowest-education group84.
Compared with urban residents, rural residents have multiple factors related to health-care delivery that impede access to timely, high-quality care, including transportation barriers, further driving distances to health-care services, lower-volume health-care facilities, increased hospital closings, higher rates of uninsurance or underinsurance, physician and nursing shortages, and limited access to clinical specialists76. When AF care is available in rural areas, it can be of limited quality69. In a nationwide Swedish study of patients with AF, researchers found that patients residing in rural areas were half as likely to be initiated on DOAC therapy than those in urban areas (OR 0.48, 95% CI 0.45–0.51)69. Furthermore, a national study of Canadian primary care practices found a higher likelihood of inappropriate DOAC dosing for AF in rural primary care practices than in urban primary care clinics (OR 2.1, 95% CI 1.7–2.6)85.
Studies from some, but not all, countries have demonstrated that patients with AF residing in rural areas have increased risks of AF complications compared with their urban counterparts. An analysis of a 2012–2014 US inpatient registry, in which nearly 60 million individuals (20% of the overall study population) resided in rural neighbourhoods, revealed that patients with AF admitted to rural hospitals had a higher risk of death than those admitted to urban hospitals (OR 1.17, 95% CI 1.04–1.32)77. By contrast, in a Canadian study of >25,000 adults with AF, clinical outcomes were similar between patients residing in rural and those residing in urban areas: 7.8% of patients in both groups died, with a similar rate of stroke or systemic embolism in patients residing in rural (3.2%) versus urban (2.8%) areas (OR 0.92, 95% CI 0.77–1.11)78.
Despite the available data, the interventions that might attenuate the observed rural disparities in AF incidence and treatment remain unclear. A systematic review of interventions to improve cardiovascular disease management in individuals residing in rural areas found substantial variability in approaches (organizational, educational and telehealth), heterogeneous results and unclear improvements in health outcomes and mortality86. Similarly, major research gaps remain about effective strategies to increase disease awareness, treatment adherence and outcomes in individuals with AF residing in rural areas. One potential strategy to improve outcomes in rural settings is the use of clinical decision support tools. Whereas previous studies have examined the role of these tools in improving treatment access, including in patients with AF87,88, a cluster-randomized controlled trial published in 2020 examining the use of computerized clinical decision support tools for the management of AF in rural versus urban settings did not show improved outcomes or safety compared with usual care89.
Neighbourhood factors and AF.
The literature examining the relationship between neighbourhood factors and health is highly complex and heterogeneous; studies have tended to be observational and to have methodological challenges90,91. In addition, whether adjusting for coexisting factors, such as income and education level, accounts for confounders or over-adjusts for potential mediators is controversial. Nonetheless, neighbourhood socioeconomic level has been associated with differences in AF incidence, management and outcomes. Neighbourhood socioeconomic deprivation can result in poorer access to health care and sustaining resources, such as healthy food and safe spaces to exercise, resulting in poor health. One example is a US study of nearly 250,000 Medicare beneficiaries, in which neighbourhood greenness had a protective association with the risk of AF, but the association was not significant after accounting for cardiovascular disease risk factors (OR 0.94, 95% CI 0.87–1.00)92. A 2015 Swedish study found that individuals with AF residing in neighbourhoods of high socioeconomic status were more likely to be prescribed oral anticoagulation than those residing in neighbourhoods of low socioeconomic status93. Another Swedish study showed that in adjusted analyses, individuals with AF living in neighbourhoods of low socioeconomic status had a higher risk of death (HR 1.49, 95% CI 1.13–1.96)94.
In the USA, individuals residing in counties with lower income levels and higher proportions of under-represented racial and ethnic groups are exposed to higher levels of environmental pollutants, with the associated lower life expectancy, compared with individuals residing in counties with higher income levels95,96. Studies of cohorts, regions and countries support associations between air pollution and incident AF97–99. In a study of >5 million residents in Ontario, Canada, high levels of multiple air pollutants were significantly associated with an increased risk of new-onset AF, including fine particulate matter of ≤2.5 μm in diameter (PM2.5; HR 1.03, 95% CI 1.01–1.04 per interquartile range increment), nitrogen dioxide (HR 1.02, 95% CI 1.01–1.03) and ozone (HR 1.01, 95% CI 1.00–1.02)98. Analogously, in a Danish cohort study, exposure to road traffic noise was associated with an adjusted 6% higher risk of incident AF (adjusted IR 1.06, 95% CI 1.00–1.12) for every 10-dB increase in road traffic noise100. Another study of 31,414 adults with AF in a large health-care system in a US region with high industrial activity demonstrated an association between the highest quartile of PM2.5 levels and the risk of ischaemic stroke (HR 1.20, 95% CI 1.00–1.44) relative to the lowest quartile101. Although at an individual level, the relative risks associated with air pollution exposure are small, given the ubiquitous and disparate exposures at the population level, these risks are consequential.
Health literacy
Health literacy is the “capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions”102. Low health literacy is widely prevalent worldwide and is adversely associated with heterogeneous risk factors, diseases and health outcomes, in addition to greater health-care utilization and costs103. Limited English proficiency, a correlate of health literacy in English-speaking countries, is designated by individuals having a primary language other than English and challenges with English reading, writing or verbally communicating104. For example, approximately 36% of the US adult population is estimated to have limited health literacy and 8% to have limited English proficiency105. In 2018, 49% of foreign-born adults in the UK reported speaking a language other than English as their primary language106. AF is a complex condition that demands ascertainment of the condition, education about the disease, symptom recognition, treatment adherence and access to specialty health care for potentially complex treatments and medications. Therefore, limited health literacy and/or limited local language proficiency (LLLP) are likely to influence many of the individuals’ experiences with AF.
Health literacy, language proficiency and AF awareness.
Low health literacy is associated with reduced knowledge of AF, its treatment and related outcomes. Research indicates that individuals with limited health literacy have decreased understanding of AF and the importance of anticoagulation for stroke prevention107,108. A study of an ethnically diverse US cohort of 12,500 patients with AF from 2006 to 2009 reported that patients with inadequate health literacy were less likely to be aware of their AF diagnosis than patients with adequate health literacy (prevalence ratio 0.96, 95% CI 0.94–0.98)109. A study of an ethnically and linguistically diverse sample of patients in the USA who were taking warfarin from 2002 to 2003 found that nearly 40% of participants had inaccurate perceptions of stroke and only 33% of participants were able to describe a symptom or sign of stroke110. Although, to our knowledge, health literacy and LLLP have not been included as factors in AF clinical trials or registry-based studies, their importance is underscored by an international survey of physicians in which 46% of respondents considered their patients to be unable to explain AF adequately111.
Health literacy, language proficiency and AF treatment.
AF requires self-management of symptom recognition and adherence to medications. In individuals taking warfarin therapy, health literacy has been related to less time in the therapeutic range, even when adjusting for demographic factors112,113. An Australian study identified that individuals with poor anticoagulation control were more likely to have limited health literacy (OR 4.0, 95% CI 2.1–7.4) than referents with superior anticoagulation control, as defined by warfarin monitoring112. Although few studies have examined the effect of LLLP on medication adherence, a single-centre, retrospective study of 3,770 patients with AF in the USA further identified that individuals with LLLP were less likely to maintain time in the therapeutic range than those with local language proficiency, even after adjustment for sociodemographic and clinical factors113.
Social determinants of health and social factors intersect with health literacy and local language proficiency through multiple pathways5,114. The shared decision-making articulated by AF management guidelines for the initiation of anticoagulation can be challenged by limited health literacy and local language proficiency115. Low health literacy and local language proficiency are associated with decreased patient-centred communication and, in turn, diminished shared decision-making116. Despite the increased recognition of the importance of these social determinants to the patient experience of AF, the relationship between AF, low health literacy or local language proficiency, and shared decision-making requires further study and addition to future AF clinical guidelines117.
Social support and social networks
Social support and AF.
Little investigation has been performed on the association between social support or social isolation and cardiovascular disease, specifically AF5. We include these factors in our Review as a knowledge frontier that merits further exploration. Multiple observations suggest the relevance and influence of social isolation on AF incidence, including by being associated with common risk factors for AF, such as obesity, tobacco use and alcohol consumption118. However, a 2020 analysis of 11,445 participants of the ARIC study who were free from AF at baseline found no significant association between social ties and AF incidence119.
Social integration is associated with a lower incidence of cardiovascular disease and mortality120. A study in the USA found that women without a spouse or a partner had increased cardiovascular mortality than married or partnered women120. Strong social integration seems to be beneficial to health even in individuals with poor socioeconomic and educational status and in older people121–123. Despite the plausibility of a relationship between social isolation and AF incidence, this concept has not been studied. The mechanisms for this potential relationship include the described role of stress on the development of cardiovascular diseases such as AF as well as the specific biological pathways through which stress promotes disease progression120,124,125 (Fig. 4).
Fig. 4 |. Proposed framework for the role of social networks and social support in atrial fibrillation.
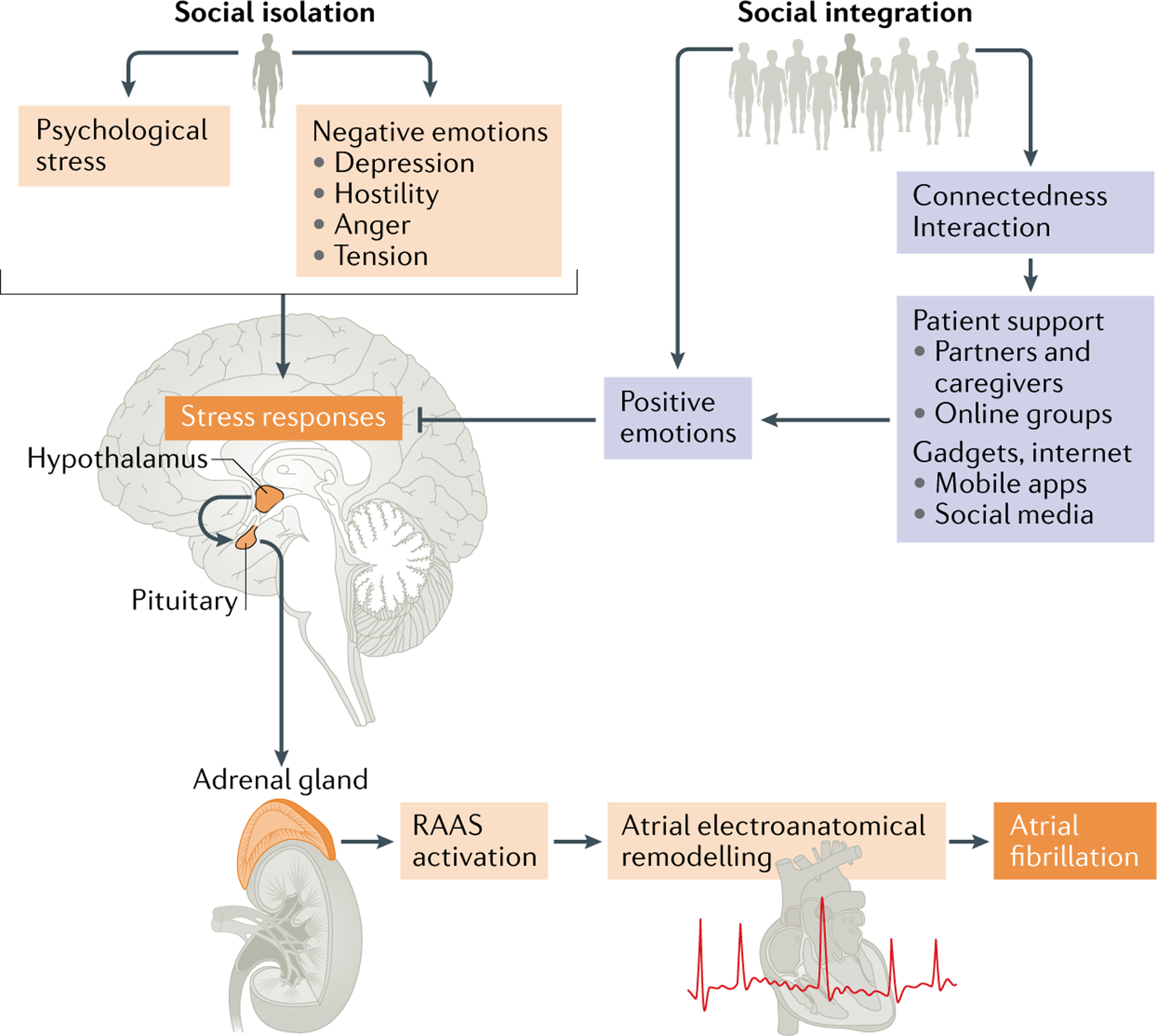
The figure illustrates the pathways through which social isolation and social integration might act to influence atrial fibrillation symptoms and outcomes. Psychological stress has a role in atrial fibrillation initiation through the activation of the autonomic nervous system, hypothalamus–pituitary–adrenal axis and the renin–angiotensin–aldosterone system (RAAS), which might contribute to atrial remodelling. The chain of reactions caused by stress could be attenuated by positive emotions, including those invoked by improved patient support and access to diverse social networks.
Social connectedness and AF.
In an era of broad Internet availability, mobile devices and diverse social media platforms, digital health applications (apps) provide an avenue to increase social engagement. Nevertheless, a digital divide exists in which certain populations, including under-represented racial and ethnic groups, those with low income and education level, rural residents and older adults, have less access to advanced mobile technology126. Furthermore, for those who do have access, many digital apps demand high readability, as evidenced by a review of mobile apps for AF directed at non–health-care providers that determined that these apps had a readability score of 12.1 ± 2.6 grade level (that is, fairly difficult to read)127,128. The researchers further found that all English language apps that included patient education text were written above the recommended 5th–6th grade reading level, that 49% of apps were written above the 12th grade reading level and that 7% of apps were written at a college-grade reading level.
Whether digital health apps can improve social support in patients with AF is unknown. Nevertheless, to date, nearly 200 English and non-English language mobile apps that are related to AF are available in the two leading digital app marketplaces, the Apple App Store and Google Play Store127. Few apps provide patient-level social support through online communities or groups. Beyond the app landscape, a study published in 2019 of 816 adults with AF aged ≥65 years found that 40% reported interest in an online AF patient community129. As these and other data show, opportunities exist to broaden the role of digital apps for AF care to those individuals with traditionally limited access, such as older adults at high risk of social isolation.
Future directions
In this Review, we highlight several important pathways through which social determinants influence AF incidence and outcomes. We also reveal opportunities for further study within each social determinant described, as we summarize below, including the crucial need for clinical trials, registries and cohort studies of AF to include, measure, quantify and report social determinants to improve the interpretation and increase the generalizability of the findings to broader populations (Fig. 5).
Fig. 5 |. Future directions for research on social determinants of atrial fibrillation.
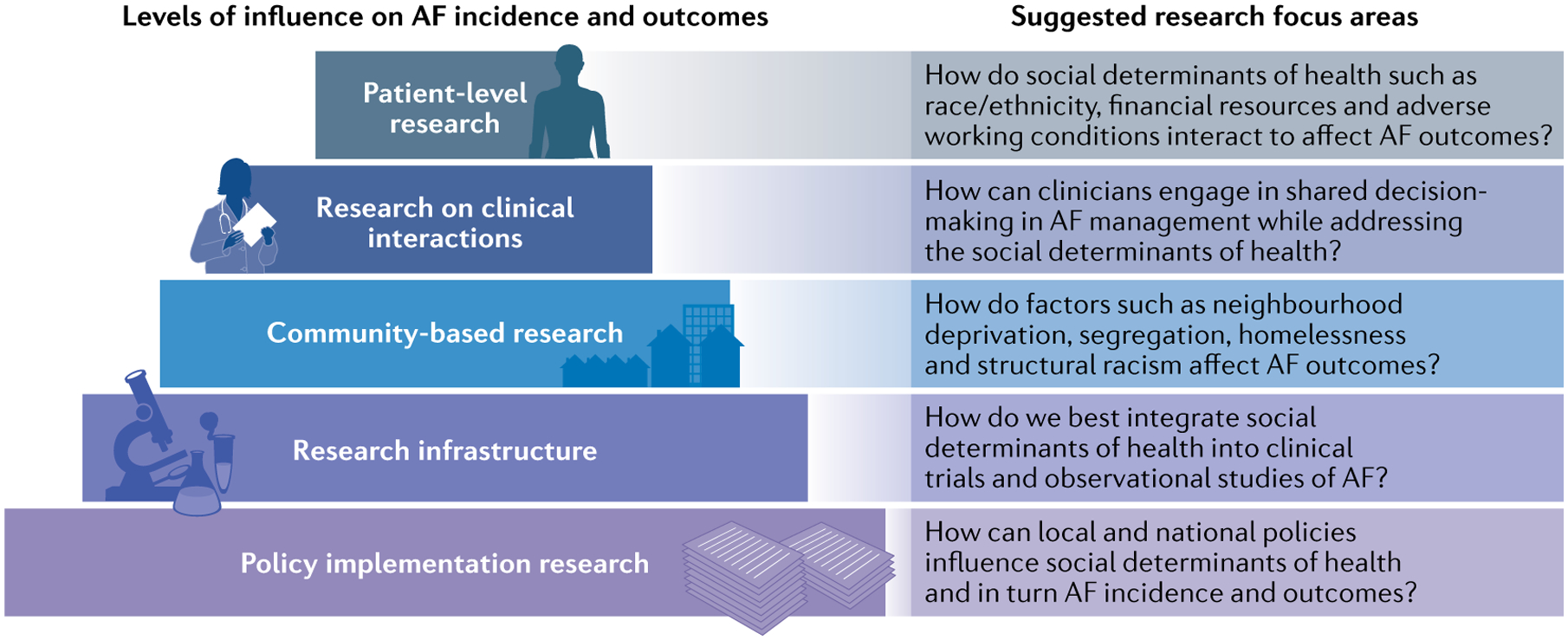
The represented framework emphasizes the separate, yet overlapping, levels of research needed to understand how social determinants of health influence atrial fibrillation (AF) incidence, treatment and outcomes. The levels include: the intrapersonal, patient-level; interpersonal clinical interactions; community level; and broader infrastructure influences on the health of individuals and populations, including research infrastructure and policy implementation. The figure offers a systematic approach to future research as well as suggested focus areas.
Important areas for future study include a deeper understanding of race/ethnicity as a social construct, the interaction with other social determinants and the role that structural racism and provider implicit biases have in the patient experience of AF and in the provider management of AF.
Factors such as access to safe neighbourhoods and healthy food, allostatic load, childhood adverse events, housing instability and homelessness, incarceration, immigration status and adverse working conditions are not fully examined in this Review given the paucity of literature describing associations between these factors and AF incidence and outcomes.
Future studies need to examine how financial resources and health-care coverage, together with the intersection of these factors with other social determinants such as race/ethnicity, education and rurality, impede the efficacy of AF prevention, treatment and associated quality of life.
To address rural inequities in AF, studies into the implementation of mobile-health and telemedicine strategies should be adapted. Furthermore, a gap remains in the literature for several pertinent neighbourhood-level factors, including structural, demographic and environmental factors, that might influence AF treatment and outcomes.
Multiple gaps are present in the study of health literacy, LLLP and AF, despite the importance of these concepts to medication adherence, health-care access, symptom recognition and adverse outcomes. Adaptation and testing of tools with demonstrated utility for chronic disease self-management are essential. Measurement of health literacy and local language proficiency as part of clinical trials, registries and community-based studies will facilitate assessing the role of risk factors in AF. Other areas that merit investigation include longitudinal, patient-centred educational interventions as well as the effect of language-concordant care (in which the patient and clinician speak the same language) on outcomes in individuals with AF and LLLP.
Further exploration of potential protective factors for AF such as spirituality, social support and resilience is warranted. Additionally, little is known about the role that caregivers have in improving AF treatment rates and outcomes130. Furthermore, whether the longitudinal effects of social isolation and pathophysiological and/or psychological health influence AF outcomes requires attention and investigation.
Future research should examine the mediating and intersectional influence of multiple social determinants on structurally disadvantaged populations with AF. Most fundamentally, effective policies and implementation strategies to eliminate the inequitable effect of social determinants of AF need to be researched.
Conclusions
Examining the social determinants of AF provides an opportunity to improve health in individuals with AF. Social determinants — race/ethnicity, financial resources, rurality and residential environment, language proficiency and health literacy, and social support — have a prominent role in the detection, evaluation, treatment and management of AF. Through describing the relevance and importance of integrating social determinants into the prevention, ascertainment, management and further research of AF, we envision a future of more equitable and high-quality care for patients with this increasingly common cardiac condition.
Key points.
Atrial fibrillation is the most common cardiac rhythm disorder, affecting nearly 60 million adults worldwide, and is associated with substantial morbidity and mortality.
Social determinants, such as race/ethnicity, financial resources, social support, rurality and residential environment, and health literacy, have crucial roles in the detection, evaluation, treatment and management of atrial fibrillation.
Despite their fundamental importance, to date, critical evaluation of the social determinants of health in relation to atrial fibrillation has been limited.
Collecting, studying and addressing social determinants of health provides an important opportunity to reduce the substantial social and economic burden of atrial fibrillation and its associated complications.
Important areas for future research include an examination of the intersectional influence of multiple social determinants of health on disadvantaged populations with atrial fibrillation and implementation strategies to eliminate health-care inequities.
Readability score
A score indicating how difficult a text in english is to understand; it can be graded according to us school and higher education level: 5th grade (very easy; easily understood by students aged 11 years), 6th grade (easy; conversational english for consumers), 7th grade (fairly easy), 8th–9th grade (plain english; easily understood by students aged 13–15 years), 10th–12th grade (fairly difficult), College (difficult), College graduate (very difficult) and Professional (extremely difficult).
Allostatic load
The ‘wear and tear’ on the body that accumulates as an individual is exposed to repeated or chronic stress.
Competing interests
U.R.E. has received funding from the Department of Veterans Affairs VISN 4 Competitive Career Development Fund and Health Services Research and Development Service CDA-20-049. J.K. has received funding from the Marie Sklodowska-Curie Actions under the European Commission’s Horizon 2020 research and innovation programme (grant agreement no. 838259). E.J.B. is supported by grants 2R01 HL092577, 1R01 HL141434, 2U54HL120163, 1R01AG066010, 1R01AG066914, 2U54HL120163 and AHA 18SFRN34110082. J.W.M. is supported by grants R33HL144669 and R01HL143010. The other authors declare no competing interests.
References
- 1.Roth GA et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J. Am. Cardiol 76, 2982–3021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lippi G, Sanchis-Gomar F & Cervellin G Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int. J. Stroke 16, 217–221 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Kornej J, Börschel CS, Benjamin EJ & Schnabel RB Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ. Res 127, 4–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marmot M, Friel S, Bell R, Houweling TA & Taylor S Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 372, 1661–1669 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Havranek EP et al. Social determinants of risk and outcomes for cardiovascular disease. Circulation. 132, 873–898 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (National Academies Press, 2003). [PubMed] [Google Scholar]
- 7.Williams DR & Mohammed SA Discrimination and racial disparities in health: evidence and needed research. J. Behav. Med 32, 20–47 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DR & Cooper LA COVID-19 and health equity–a new kind of “herd immunity”. JAMA 323, 2478–2480 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Jones CP Toward the science and practice of anti-racism: launching a national campaign against racism. Ethn. Dis 28, 231–234 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essien UR et al. Association of race/ethnicity with oral anticoagulant use in patients with atrial fibrillation. JAMA Cardiol. 3, 1174–1182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essien UR et al. Race/ethnicity and sex-related differences in direct oral anticoagulant initiation in newly diagnosed atrial fibrillation: a retrospective study of Medicare data. J. Natl Med. Assoc 112, 103–108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnani JW et al. Racial differences in atrial fibrillation-related cardiovascular disease and mortality: the Atherosclerosis Risk in Communities (ARIC) study. JAMA Cardiol. 1, 433–441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dyke M et al. Heart disease death rates among blacks and whites aged ≥35 years – United States, 1968–2015. MMWR Surveill. Summ 67, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugowe FE, Jackson LR & Thomas KL Racial and ethnic differences in the prevalence, management, and outcomes in patients with atrial fibrillation: a systematic review. Heart Rhythm. 15, 1337–1345 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Nanda A & Kabra R Racial differences in atrial fibrillation epidemiology, management, and outcomes. Curr. Treat. Options Cardiovasc. Med 21, 85 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Linares JD et al. Prevalence of atrial fibrillation and association with clinical, sociocultural, and ancestral correlates among Hispanic/Latinos: the Hispanic Community Health Study/Study of Latinos. Heart Rhythm. 16, 686–693 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staerk L, Sherer JA, Ko D, Benjamin EJ & Helm RH Atrial fibrillation: epidemiology, pathophysiology, clinical outcomes. Circ. Res 120, 1501–1517 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez JM et al. Incident atrial fibrillation among American Indians in California. Circulation. 140, 1605–1606 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Dewland TA, Olgin JE, Vittinghoff E & Marcus GM Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 128, 2470–2477 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Mou L et al. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC Study (Atherosclerosis Risk in Communities). Circ. Arrhythmia Electrophysiol 11, e006350 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loehr LR et al. The prevalence of atrial fibrillation on 48-hour ambulatory electrocardiography in African Americans compared to Whites: the Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J 216, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ML et al. Risk of atrial fibrillation in black versus white Medicare beneficiaries with implanted cardiac devices. J. Am. Heart Assoc 8, e010661 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez CJ et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann. Epidemiol 25, 71–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soliman EZ, Alonso A & Goff DC Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 5, 547–556 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Marcus GM et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 122, 2009–2015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen MA et al. Atrial ectopy as a mediator of the association between race and atrial fibrillation. Heart Rhythm. 14, 1856–1861 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meschia JF et al. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke. 41, 581–587 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heckbert SR et al. Differences by race/ethnicity in the prevalence of clinically detected and monitor-detected atrial fibrillation: MESA. Circ. Arrhythm. Electrophysiol 13, e007698 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Pasquale G & Casella G Antithrombotic strategies for atrial fibrillation: on the threshold of changes? Yes. J. Thromb. Haemost 3, 428–432 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Shen AY-J et al. Racial/ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke 39, 2736–2743 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Hellyer JA et al. Impact of baseline stroke risk and bleeding risk on warfarin international normalized ratio control in atrial fibrillation (from the TREAT-AF study). Am. J. Cardiol 119, 268–274 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Rao SR et al. Explaining racial disparities in anticoagulation control: results from a study of patients at the Veterans Administration. Am. J. Med. Qual 30, 214–222 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Rodwin BA et al. Variation in the use of warfarin and direct oral anticoagulants in atrial fibrillation and associated cost implications. Am. J. Med 132, 61–70 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Bhave PD, Lu X, Girotra S, Kamel H & Vaughan Sarrazin MS Race-and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 12, 1406–1412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razouki Z, Ozonoff A, Zhao S & Rose AJ Pathways to poor anticoagulation control. J. Thromb. Haemost 12, 628–634 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Thomas KL et al. Racial differences in the prevalence and outcomes of atrial fibrillation among patients hospitalized with heart failure. J. Am. Heart Assoc 2, e000200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yong C et al. Racial differences in quality of anticoagulation therapy for atrial fibrillation (from the TREAT-AF study). Am. J. Cardiol 117, 61–68 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Connolly SJ et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med 361, 1139–1151 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Granger CB et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med 365, 981–992 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Patel MR et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med 365, 883–891 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Sur NB et al. Disparities and temporal trends in the use of anticoagulation in patients with ischemic stroke and atrial fibrillation. Stroke 50, 452–1459 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lip GYH et al. Determinants of time in therapeutic range in patients receiving oral anticoagulants (a substudy of IMPACT). Am. J. Cardiol 118, 1680–1684 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Golwala H et al. Racial/ethnic differences in atrial fibrillation symptoms, treatment patterns, and outcomes: insights from Outcomes Registry for Better Informed Treatment for Atrial Fibrillation Registry. Am. Heart J 174, 29–36 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Piccini JP et al. Adherence to guideline-directed stroke prevention therapy for atrial fibrillation is achievable: first results from Get With the Guidelines-Atrial Fibrillation (GWTG-AFIB). Circulation. 139, 1497–1506 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Khalid U et al. Treatment of AF in American Indians and Alaska Natives. J. Am. Coll. Cardiol 75, 2749–2750 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Patel N et al. Gender, race, and health insurance status in patients undergoing catheter ablation for atrial fibrillation. Am. J. Cardiol 117, 1117–1126 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Sarraju A, Maron DJ & Rodriguez F Under-reporting and under-representation of racial/ethnic minorities in major atrial fibrillation clinical trials. JACC Clin. Electrophysiol 6, 739–741 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neal WT et al. Sex and racial differences in cardiovascular disease risk in patients with atrial fibrillation. PLoS ONE 14, e0222147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birman-Deych E, Radford MJ, Nilasena DS & Gage BF Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 37, 1070–1074 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Kabra R, Girotra S & Vaughan Sarrazin M Refining stroke prediction in atrial fibrillation patients by addition of African-American ethnicity to CHA2DS2-VASc score. J. Am. Coll. Cardiol 68, 461–470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson AE & Magnani JW Race and stroke risk in atrial fibrillation: the limitations of a social construct. J. Am. Coll. Cardiol 69, 906–907 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Essien UR & Jackson LR Race effects in CVD prediction models. J. Gen. Intern. Med 34, 484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pool LR et al. Longitudinal associations of neighborhood-level racial residential segregation with obesity among blacks. Epidemiology. 29, 207–214 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Buchmueller TC, Levinson ZM, Levy HG & Wolfe BL Effect of the affordable care act on racial and ethnic disparities in health insurance coverage. Am. J. Public Health 106, 1416–1421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makaroun LK et al. Wealth-associated disparities in death and disability in the United States and England. JAMA Intern. Med 177, 1745–1753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sholzberg M et al. The influence of socioeconomic status on selection of anticoagulation for atrial fibrillation. PLoS ONE 11, e0149142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kummer BR et al. Demographic differences in catheter ablation after hospital presentation with symptomatic atrial fibrillation. J. Am. Heart Assoc 22, e002097 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larosa AR et al. Association of household income and adverse outcomes in patients with atrial fibrillation. Heart 106, 1679–1685 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanchate AD, Schwamm LH, Huang W & Hylek EM Comparison of ischemic stroke outcomes and patient and hospital characteristics by race/ethnicity and socioeconomic status. Stroke 44, 469–476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guhl E et al. Association of income and health-related quality of life in atrial fibrillation. Open Heart 6, e000974 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cressman AM et al. Socioeconomic status and risk of hemorrhage during warfarin therapy for atrial fibrillation: a population-based study. Am. Heart J 170, 133–140 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Gebreyohannes EA, Bhagavathula AS, Abebe TB, Seid MA & Haile KT In-hospital mortality among ischemic stroke patients in Gondar University Hospital: a retrospective cohort study. Stroke Res. Treat 2019, 7275063 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Addo J et al. Socioeconomic status and stroke: an updated review. Stroke. 43, 1186–1191 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Soliman EZ, Zhang ZM, Judd S, Howard VJ & Howard G Comparison of risk of atrial fibrillation among employed versus unemployed (from the REasons for Geographic and Racial Differences in Stroke study). Am. J. Cardiol 120, 1298–1301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Misialek JR et al. Socioeconomic status and the incidence of atrial fibrillation in whites and blacks: the Atherosclerosis Risk in Communities (ARIC) study. J. Am. Heart Assoc 3, e001159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shulman E et al. Socioeconomic status and the development of atrial fibrillation in Hispanics, African Americans and non-Hispanic whites. Clin. Cardiol 40, 770–776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zöller B, Li X, Sundquist J & Sundquist K Neighbourhood deprivation and hospitalization for atrial fibrillation in Sweden. Europace. 15, 1119–1127 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Kivimaki M et al. Long working hours as a risk factor for atrial fibrllation: a multi-cohort study. Eur. Heart J 7, 2621–2628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurusamy VK, Brobert G, Vora P & Friberg L Sociodemographic factors and choice of oral anticoagulant in patients with non-valvular atrial fibrillation in Sweden: a population-based cross-sectional study using data from national registers. BMC Cardiovasc. Disord 19, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdul-Rahim AH, Wong J, McAlpine C, Young C & Quinn TJ Associations with anticoagulation: a cross-sectional registry-based analysis of stroke survivors with atrial fibrillation. Heart. 100, 557–562 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Hernandez I et al. Trajectories of oral anticoagulation adherence among Medicare beneficiaries newly diagnosed with atrial fibrillation. J. Am. Heart Assoc 8, e011427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.January CT et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J. Am. Coll. Cardiol 74, 104–132 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Mark DB et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 321, 1275–1285 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hagengaard L et al. Socioeconomic differences in outcomes after hospital admission for atrial fibrillation or flutter. Eur. Hear. J. Qual. Care Clin. Outcomes 7, 295–303 (2021). [DOI] [PubMed] [Google Scholar]
- 75.Kargoli F et al. Socioeconomic status as a predictor of mortality in patients admitted with atrial fibrillation. Am. J. Cardiol 119, 1378–1381 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Harrington RA et al. Call to action: rural health: a presidential advisory from the American Heart Association and American Stroke Association. Circulation. 141, e615–e644 (2020). [DOI] [PubMed] [Google Scholar]
- 77.O’Neal WT, Sandesara PB, Kelli HM, Venkatesh S & Soliman EZ Urban-rural differences in mortality for atrial fibrillation hospitalizations in the United States. Heart Rhythm. 15, 175–179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu C et al. Impact of rural residence on warfarin use and clinical events in patients with non-valvular atrial fibrillation: a Canadian population based study. PLoS ONE 10, e0140607 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alonso A et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J. Am. Heart Assoc 2, e000102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee YH, Ang TFA, Lin HC & Chang YC Rural-urban disparities in smoking patterns among Chinese adults: a social-ecological approach. J. Ethn. Subst. Abuse 10.1080/15332640.2019.1633980 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Coughlin LN et al. Changes in urban and rural cigarette smoking and cannabis use from 2007 to 2017 in adults in the United States. Drug Alcohol. Depend 205, e107699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sardana M et al. Provider-level variation in smoking cessation assistance provided in the cardiology clinics: insights from the NCDR PINNACLE registry. J. Am. Heart Assoc 8, e011412 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frewen J et al. Factors that influence awareness and treatment of atrial fibrillation in older adults. QJM 106, 415–424 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Lunde ED et al. Socioeconomic position and risk of atrial fibrillation: a nationwide Danish cohort study. J. Epidemiol. Community Health 74, 7–13 (2020). [DOI] [PubMed] [Google Scholar]
- 85.McAlister FA, Garrison S, Kosowan L, Ezekowitz JA & Singer A Use of direct oral anticoagulants in Canadian primary care practice 2010–2015: a cohort study from the Canadian Primary Care Sentinel Surveillance Network. J. Am. Heart Assoc 7, e007603 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruiz-Pérez I, Bastos Á, Serrano-Ripoll MJ & Ricci-Cabello I Effectiveness of interventions to improve cardiovascular healthcare in rural areas: a systematic literature review of clinical trials. Prev. Med 199, 131–144 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Roshanov PS et al. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 346, f657 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Arts DL, Abu-Hanna A, Medlock SK & Van Weert HCPM Effectiveness and usage of a decision support system to improve stroke prevention in general practice: a cluster randomized controlled trial. PLoS ONE 12, e0170974 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cox JL et al. Integrated management program advancing community treatment of atrial fibrillation (IMPACT-AF): a cluster randomized trial of a computerized clinical decision support tool. Am. Heart J 224, 35–46 (2020). [DOI] [PubMed] [Google Scholar]
- 90.O’Brien DT, Farrell C & Welsh BC Broken (windows) theory: a meta-analysis of the evidence for the pathways from neighborhood disorder to resident health outcomes and behaviors. Soc. Sci. Med 228, 272–292 (2019). [DOI] [PubMed] [Google Scholar]
- 91.Arcaya MC et al. Research on neighborhood effects on health in the United States: a systematic review of study characteristics. Soc. Sci. Med 168, 16–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang K et al. Relationship of neighborhood greenness to heart disease in 249 405 US Medicare beneficiaries. J. Am. Heart Assoc 8, e010258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carlsson AC, Wändell P, Gasevic D, Sundquist J & Sundquist K Neighborhood deprivation and warfarin, aspirin and statin prescription – a cohort study of men and women treated for atrial fibrillation in Swedish primary care. Int. J. Cardiol 187, 547–552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wändell P, Carlsson AC, Gasevic D, Sundquist J & Sundquist K Neighbourhood socio-economic status and all-cause mortality in adults with atrial fibrillation: a cohort study of patients treated in primary care in Sweden. Int. J. Cardiol 202, 776–781 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bennett JE et al. Particulate matter air pollution and national and county life expectancy loss in the USA: a spatiotemporal analysis. PLoS Med. 16, e10002856 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banzhaf S, Ma L & Timmins C Environmental justice: the economics of race, place, and pollution. J. Econ. Perspect 33, 185–208 (2019). [PubMed] [Google Scholar]
- 97.Monrad M et al. Long-term exposure to traffic-related air pollution and risk of incident atrial fibrillation: a cohort study. Env. Health Perspect 125, 422–427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shin S et al. Ambient air pollution and the risk of atrial fibrillation and stroke: a population-based cohort study. Env. Health Perspect 127, e870009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim I-S et al. Long-term exposure of fine particulate matter air pollution and incident atrial fibrillation in the general population: a nationwide cohort study. Int. J. Cardiol 283, 178–183 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Monrad M et al. Residential exposure to traffic noise and risk of incident atrial fibrillation: a cohort study. Env. Int 92–93, 457–463 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Rhinehart ZJ et al. Association of fine particulate matter and risk of stroke in patients with atrial fibrillation. JAMA Netw. Open 3, e2011760 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Institute of Medicine. Health Literacy: A Prescription to End Confusion (National Academies Press, 2004). [PubMed] [Google Scholar]
- 103.US Department of Health and Human Services. HHS.gov. Civil Rights https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/guidance-federal-financial-assistance-recipients-title-vi/index.html (2013).
- 104.Kutner M, Greenberg E, Jin Y, Paulsen C The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy (NCES 2006–483) (US Department of Education, 2006). [Google Scholar]
- 105.Ryan C Language use in the United States: 2011. census.gov https://www2.census.gov/library/publications/2013/acs/acs-22/acs-22.pdf (2013).
- 106.The Migration Observatory. English language use and proficiency of migrants in the UK. https://migrationobservatory.ox.ac.uk/resources/briefings/english-language-use-and-proficiency-of-migrants-in-the-uk/ (2019).
- 107.Obamiro KO, Chalmers L, Lee K, Bereznicki BJ & Bereznicki LRE Anticoagulation knowledge in patients with atrial fibrillation: an Australian survey. Int. J. Clin. Pract 72, e13072 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Konstantinos NA et al. Health literacy and atrial fibrillation: relevance and future directions for patient-centred care. Eur. Cardiol. Rev 12, 52–57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reading SR et al. Health literacy and awareness of atrial fibrillation. J. Am. Heart Assoc 6, e005128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fang MC, Panguluri P, Machtinger EL & Schillinger D Language, literacy, and characterization of stroke among patients taking warfarin for stroke prevention: implications for health communication. Patient Educ. Couns 95, 403–410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aliot E et al. An international survey of physician and patient understanding, perception, and attitudes to atrial fibrillation and its contribution to cardiovascular disease morbidity and mortality. Europace 12, 626–633 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Diug B et al. The unrecognized psychosocial factors contributing to bleeding risk in warfarin therapy. Stroke 42, 2866–2871 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Rodriguez F et al. Limited English proficient patients and time spent in therapeutic range in a warfarin anticoagulation clinic. J. Am. Heart Assoc 2, e00017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schillinger D The intersections between social determinants of health, health literacy, and health disparities. Stud. Health Technol. Inform 269, 22–41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hindricks et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J 5, 373–498 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Pérez-Stable EJ & El-Toukhy S Communicating with diverse patients: how patient and clinician factors affect disparities. Patient Educ. Couns 101, 2186–2194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seaburg L et al. Shared decision making in atrial fibrillation: where we are and where we should be going. Circulation 129, 704–710 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Pantell M et al. Social isolation: a predictor of mortality comparable to traditional clinical risk factors. Am. J. Public Health 103, 2056–2062 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garg PK et al. Associations of anger, vital exhaustion, anti-depressant use, and poor social ties with incident atrial fibrillation: the Atherosclerosis Risk in Communities Study. Eur. J. Prev. Cardiol 28, 633–640 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang S-C et al. Social integration and reduced risk of coronary heart disease in women: the role of lifestyle behaviors. Circ. Res 120, 1927–1937 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Laugesen K et al. Social isolation and all-cause mortality: a population-based cohort study in Denmark. Sci. Rep 8, 4731 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valtorta NK, Kanaan M, Gilbody S, Ronzi S & Hanratty B Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 102, 1009–1016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang W et al. Physical, cognitive, and psychosocial conditions in relation to anticoagulation satisfaction among elderly adults with atrial fibrillation: the SAGE-AF study. J. Cardiovasc. Electrophysiol 30, 2508–2515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Westcott SK et al. Relationship between psychosocial stressors and atrial fibrillation in women over 45 years of age. Am. J. Cardiol 122, 1684–1687 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kivimaki M & Steptoe A Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol 15, 215–229 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Smith B & Magnani JW New technologies, new disparities: the interesection of electronic health and digital health literacy. Int. J. Cardiol 292, 280–282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ayyaswami V et al. Mobile health applications for atrial fibrillation: a readability and quality assessment. Int. J. Cardiol 15, 288–293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kincaid JP, Fishburne RP Jr., Rogers RL & Chissom BS Derivation of new readability formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy Enlisted Personnel. (Institute for Simulation and Training, University of Central Florida, 1975) [Google Scholar]
- 129.Waring ME Characteristics associated with Facebook use and interest in digital disease support among older adults with atrial fibrillation: cross-sectional analysis of baseline data from the Systematic Assessment of Geriatric Elements in Atrial Fibrillation (SAGE-AF) cohort. JMIR Cardio. 3, e15320 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferguson C et al. The caregiver role in thromboprophylaxis management in atrial fibrillation: a literature review. Eur. J. Cardiovasc. Nurs 14, 98–107 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Connolly SJ et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N. Engl. J. Med 360, 2066–2078 (2009). [DOI] [PubMed] [Google Scholar]
- 132.Connolly SJ et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med 364, 806–817 (2011). [DOI] [PubMed] [Google Scholar]
- 133.Giugliano RP et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med 369, 2093–2104 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Holmes DR et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J. Am. Coll. Cardiol 64, 1–12 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Douketis JD et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N. Engl. J. Med 373, 823–833 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cannon CP et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Engl. J. Med 377, 1513–1524 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Ezekowitz MD et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur. Heart J 39, 2959–2971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Perez MV et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med 381, 1909–1917 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lopes RD et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N. Engl. J. Med 380, 1509–1524 (2019). [DOI] [PubMed] [Google Scholar]
- 140.Packer DL et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 321, 1261–1274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


