Abstract
A comparison of delayed versus immediate inoculation of culture medium for the diagnosis of trichomonosis was conducted. The sensitivities of the two methods were 100 and 97.4%, respectively. Delayed inoculation of culture medium for women without evidence of trichomonosis on direct microscopic examination is a valid diagnostic procedure.
Trichomonosis is an extremely prevalent sexually transmitted disease (STD) worldwide. Although it is not a reportable disease, its annual incidence in the United States is estimated at three million cases (10). Vaginitis due to Trichomonas vaginalis has been associated with transmission and acquisition of human immunodeficiency virus and preterm birth (3, 6). To date, however, control efforts for trichomonosis are lacking in the United States despite the availability of diagnostic tests and the ease of treatment with a single dose of metronidazole. Currently, microscopic examination of vaginal fluid (wet preparation) is the most often used diagnostic test for trichomonosis in women. It is inexpensive and relatively easy to interpret and provides immediate results to facilitate patient treatment. However, the wet preparation has limited sensitivity compared to culture (5, 9, 12).
Although a commercially available culture test for T. vaginalis exists (2), the cost of routinely inoculating culture medium may be prohibitive, especially since over half of the women with infections can be immediately diagnosed by wet preparation. We propose an alternative, practical method of screening for trichomonosis, that is, delayed inoculation of culture medium for those women who are wet preparation negative for T. vaginalis.
Women who presented at the Jefferson County Department of Health STD Clinic with a new problem were eligible for participation in this study. Patients were enrolled and examined by either of two study nurses after giving oral consent. The study was approved by the institutional review boards of the University of Alabama at Birmingham and the Jefferson County Department of Health.
Patients were examined in accordance with the standard clinic protocol. Vaginal specimens were obtained by means of a speculum examination for pH determination (ColorpHast indicator strips; EM Science, Gibbstown, N.J.), a “whiff” test, wet-preparation microscopy, and Gram staining. Two additional vaginal specimens were obtained by using Dacron swabs. One of these was used to immediately inoculate a culture pouch for T. vaginalis (In Pouch TV test; BioMed Diagnostics Inc., San José, Calif.) (2). The second swab was placed either into a glass tube containing 0.25 ml of normal saline (the first 100 patients) or into an empty glass tube (the last 50 patients). After 15 to 20 min at room temperature, this second specimen was inoculated into a second culture pouch. Endocervical specimens were obtained for culture of gonorrhea, plated directly onto modified Thayer-Martin medium, and incubated in 3 to 5% CO2. Endocervical specimens for chlamydia culture were placed into transport medium, refrigerated, and transported to the laboratory within 24 h.
Cultures for T. vaginalis were incubated at 35°C and examined daily by light microscopy (magnification, ×100) for 5 days. Cultures were considered positive when motile trichomonads were identified within the pouch. The presence of bacterial vaginosis was determined by use of the criteria of Amsel et al. (1) Cultures for gonorrhea and chlamydia (microtiter plates) were processed by standard methods (8, 11). Trichomonosis was defined as the presence of motile trichomonads as determined either by direct wet-mount examination of vaginal fluid or by a positive culture.
Statistical comparisons were made by using the Epi Info 6 software program (4). Fisher’s exact test or the chi-square test was used to compare categorical variables, and Wilcoxon’s test was used to compare continuous variables.
One hundred fifty women were enrolled in the study between March and May of 1998. The overall prevalence of trichomoniasis was 26%. No significant differences were noted between women with and without T. vaginalis with regard to age, mean number of sexual partners in the past 6 months, vaginal symptomatology, presence of vaginal discharge noted during pelvic examination, or presence of gonorrhea, chlamydia, or bacterial vaginosis. The mean pH of the vaginal fluid was higher among women with trichomoniasis than among those without (5.87 versus 5.42; P = 0.01).
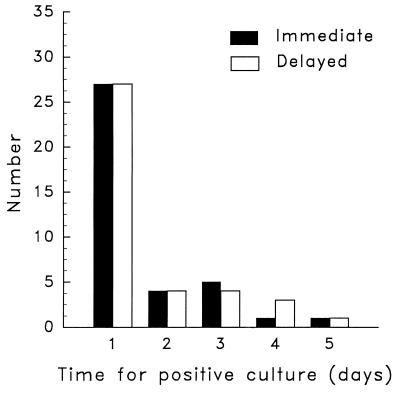
Among the 39 women with trichomonosis, 25 (64%) were identified by wet-preparation microscopy while the remaining 14 were identified only by means of culture. Those women who had wet preparations positive for trichomonas were more likely to have vaginal symptoms than those who were positive by culture only (20 of 25 [80%] versus 7 of 14 [50%]; P = 0.07). The former group also had a significantly higher mean vaginal fluid pH than the latter (6.1 versus 5.5; P = 0.02). There was no significant difference in sensitivity between cultures inoculated by the delayed method and those with immediate inoculation (39 of 39 [100%] versus 38 of 39 [97.4%]). The single discrepant result was from a patient whose delayed culture became positive after 5 days of incubation. The mean time to positivity with the two methods was the same (1.5 days) (Fig. 1). There was also no difference in sensitivity between cultures derived from swabs stored in saline prior to the delayed culture and those derived from swabs that were kept in dry tubes (28 of 28 [100%] versus 11 of 11 [100%]). All of the positive cultures were also wet preparation positive.
FIG. 1.
Comparison of the mean numbers of days for positive culture results for T. vaginalis between delayed and immediate inoculation methods.
Trichomonosis is a neglected health care problem in the United States. In public health clinics, the prevalence of trichomonosis remains astonishingly high (9). As rates of bacterial STDs decline, control efforts for trichomonosis should be considered. Although wet-preparation examination of vaginal fluid is inexpensive, requiring only a microscope and trained personnel, the sensitivity of this test is limited (5, 9, 12). Thus, a complementary test is required for screening of at-risk populations. Delayed inoculation of a culture pouch for T. vaginalis permits collection of the specimen during the initial pelvic examination and use of the culture medium if the on-site interpretation of the wet preparation is negative. This allows selective use of the culture medium which is likely to be cost effective, especially in high-prevalence populations. In low-prevalence populations, the cost savings would be less significant. The viability of the organisms for a short period of time is not unexpected, considering previous reports of survival on fomites, particularly if they are moist (7).
In summary, we have demonstrated that T. vaginalis remains viable for up to 20 min on vaginal swab specimens, permitting delayed inoculation of culture medium if needed. This type of diagnostic approach may be of particular value in the screening women who are at high risk for STDs.
Acknowledgments
This work was supported by NIH Sexually Transmitted Disease Cooperative Research Centers grant A1-94-16 and by BioMed Diagnostics Inc.
We gratefully acknowledge the assistance of Bari Cotton with patient enrollment and Mia Oliver with laboratory assistance.
REFERENCES
- 1.Amsel R, Totten P A, Spiegel C A, Chen KCS, Eschenbach D, Holmes K K. Non-specific vaginitis: diagnostic criteria and microbial and epidemiological associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 2.Borchardt K A, Smith R F. An evaluation of an InPouch™TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med. 1991;67:149–152. doi: 10.1136/sti.67.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotch M F, Pastorek J G, Nugent R P, Hillier S L, Gibbs R S, Martin D H, Eschenbach D A, Edelman R, Carey J C, Regan J A, Krohn M A, Klebanoff M A, Rao A V, Rhoads G G. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Transm Dis. 1997;24:361–362. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Dean A G, Dean J A, Columbeer D. EpiInfo, version 6: a word processing database and statistics program for epidemiology on microcomputers. Atlanta, Ga: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 5.Krieger J N, Tam M R, Stevens C E, Nielsen I O, Hale J, Kiviat N B, Holmes K K. Diagnosis of trichomoniasis. JAMA. 1988;259:1223–1227. doi: 10.1001/jama.259.8.1223. [DOI] [PubMed] [Google Scholar]
- 6.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, Goeman J, Behets F, Batter V, Alary M. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Lossick J S. Epidemiology of urogenital trichomoniasis. In: Honigberg B M, editor. Trichomonads parasitic in humans. New York, N.Y: Springer; 1989. pp. 311–323. [Google Scholar]
- 8.Mårdh P A, Danielsson D. Neisseria gonorrhoeae. In: Holmes K K, Mårdh P A, Sparling P F, Wiesner P J, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill, Inc.; 1990. pp. 903–916. [Google Scholar]
- 9.Schwebke J R, Morgan S C, Pinson G B. Validity of self-obtained vaginal specimens for diagnosis of trichomoniasis. J Clin Microbiol. 1997;35:1618–1619. doi: 10.1128/jcm.35.6.1618-1619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparks, J. M. Vaginitis. J. Reprod. Med. 36:745–752. [PubMed]
- 11.Stamm W E, Mårdh P A. Chlamydia trachomatis. In: Holmes K K, Mårdh P A, Sparling P F, Wiesner P J, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill, Inc.; 1990. pp. 917–925. [Google Scholar]
- 12.Wølner-Hanssen P J, Krieger N, Stevens C E, Kiviat N B, Koutsky L, Critchlow C, De Roven T, Hillier S, Holmes K K. Clinical manifestations of vaginal trichomoniasis. JAMA. 1989;261:571–576. doi: 10.1001/jama.1989.03420040109029. [DOI] [PubMed] [Google Scholar]