Supplemental Digital Content is available in the text.
Keywords: Alzheimer disease, blood pressure, cerebrovascular disorders, dementia, hemodynamics, meta-analysis, stroke
Abstract
Research links high blood pressure variability (BPV) with stroke and cerebrovascular disease, however, its association with cognition remains unclear. Moreover, it remains uncertain which BP-derived parameter (ie, variability or mean) holds more significance in understanding vascular contributions to cognitive impairment. We searched PubMed, Embase, PsycINFO, and Scopus and performed a meta-analysis of studies that quantified the association between resting BPV with dementia or cognitive impairment in adults. Two authors independently reviewed all titles, abstracts, and full-texts and extracted data, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-Analysis of Observational Studies in Epidemiology guidelines. Study quality was assessed using the (modified) Newcastle-Ottawa Scale. A multilevel meta-analysis was used, which included effect sizes for both BPV and mean BP, with a combined end point of dementia or cognitive impairment as primary outcome. In the primary analysis, 54 effect sizes were extracted from 20 studies, with a total analytical sample of n=7 899 697. Higher systolic BPV (odds ratio [OR], 1.25 [95% CI, 1.16–1.35]), mean systolic pressure (OR, 1.12 [95% CI, 1.02–1.29]), diastolic BPV (OR, 1.20 [95% CI, 1.12–1.29]), and mean diastolic pressure (OR, 1.16 [95% CI, 1.04–1.29]) were associated with dementia and cognitive impairment. A direct comparison showed that mean BP effect sizes were less strong than BPV effect sizes (OR, 0.92 [95% CI, 0.87–0.97], P<0.01), indicating that the relative contribution of BPV exceeded that of mean BP. Methodological and statistical heterogeneity was high. Secondary analyses were less consistent as to whether BPV and mean BP were differentially associated with dementia subtypes and cognitive domains. Future studies are required to investigate BPV as a target for dementia prevention.
High blood pressure (BP) during mid-life is widely recognized as a modifiable risk factor for late-life dementia.1,2 Subsequently, lowering high BP with antihypertensive medication during mid-life is a recommended strategy to prevent dementia.3,4 Yet, several uncertainties remain that hamper clinical guidelines for the management of BP to maintain brain health, including optimal BP targets in mid- to late-life and the choice of antihypertensive drug(s).5 The inconsistency in findings raises the possibility that BP-related factors beyond absolute BP level or treat-to-target BP could be important for dementia prevention and early intervention.
A body of empirical work indicates that oscillations in BP between consecutive measures hold additional prognostic significance, alongside mean BP level, for the risk of cardiovascular diseases and subclinical target organ damage.6,7 Previous meta-analyses have reported associations of high BP variability (BPV) with stroke and cerebral small vessel disease (CSVD), underscoring the importance of BPV to brain health.8–10 An association between BPV with dementia and cognitive impairment was reported as part of a larger meta-analysis on BP and cognition but was limited to only 2 studies.11
Evaluating the current evidence regarding BPV and cognitive function may inform evidence-based clinical practice regarding BP management to preserve brain health. Therefore, the objective of this review is to quantify the association between intraindividual BPV with the risk of dementia or cognitive impairment. A second objective is to compare the magnitude of the association between BPV and cognitive outcomes with the effect sizes for mean BP.
Methods
The authors declare that all supporting data are available within the article and its Data Supplement. The protocol of this systematic review was registered with the International Prospective Register of Systematic Reviews (CRD42017081977) and published.12 The study followed the Meta-Analysis of Observational Studies in Epidemiology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.13,14
Sources and Search Strategy
A comprehensive search of PubMed (Medline), Embase, PsycINFO, and Scopus without language restriction was performed from database inception to April 20, 2021 (Data Supplement). Two reviewers (R.A.A. de Heus and M. Opozda) independently screened titles and abstracts to assess eligibility. Full text was evaluated if eligibility was not clear from the abstract. Inconsistencies were resolved by consulting a third reviewer (P.J. Tully). Articles and conference abstracts of case-control studies, prospective cohorts, database registries, cross-sectional studies, and (secondary analyses of) randomized controlled trials were eligible for inclusion. A hand-search was performed of the articles selected for full-text review and of narrative reviews,15,16 supplementing the electronic search. Where necessary, we contacted authors of relevant articles to request additional data.
Eligibility Criteria
Studies were considered eligible if they investigated an adult sample (≥18 years), examined BPV using repeated measurements of BP at rest, assessed a prespecified cognitive outcome (see below), and reported the association between BPV and study outcome(s), or could provide additional analyses. No restriction was placed on sample size or length of follow-up. Reporting the association between mean BP and study outcome(s) was not a prespecified inclusion criterion. Because the field is lacking a gold standard for quantifying intraindividual BPV, all common metrics were eligible, prioritizing the coefficient of variation where studies reported multiple metrics (Data Supplement).17 Studies including persons with baseline dementia were excluded if they did not report the association of BPV with cognition separately for those without dementia. Studies in patients with recent stroke, Parkinson disease, receiving hemodialysis or renal denervation, revascularization, or facing orthostatic challenge were ineligible.
Outcomes
The primary outcome was the odds for dementia or cognitive impairment attributable to BPV or mean BP. Studies that reported incident dementia, cognitive impairment, a composite of dementia or cognitive impairment, or compared dementia and nondementia groups were included. This approach was chosen to maximize the number of studies in the primary analysis and because dementia and cognitive impairment represent a continuum of the same syndrome. The definition of dementia was criterion-referenced and was based on International Classification of Disease criteria, Diagnostic and Statistical Manual of Mental Disorders criteria, an adjudicated expert panel or the prescription of antidementia drugs, inclusive of any dementia, Alzheimer disease, Vascular dementia, or mixed cause. The definition of cognitive impairment was any of the following definitions that were standardized within studies: criterion-referenced diagnosis of mild cognitive impairment,18,19 a cognitive test score below a predefined, clinical cutoff point, a predefined between-assessment decline, or a score below age and sex appropriate normative data, all based on standardized tests of global cognitive function or assessing specific cognitive domains. Studies using self-reported measures were ineligible.
The secondary outcomes were other effect sizes reporting on the association between BPV and cognition. This included (standardized) mean cognitive function scores in the lowest versus highest group of BPV or mean BP (eg, quartiles) and conversely (standardized) mean differences in BPV and mean BP when grouped by cognitive function. Furthermore, effect sizes of β/r family reporting the correlation between BPV and cognition on a continuous scale were extracted for analyses.
Data Extraction
Data were independently extracted by 3 reviewers (R.A.A. de Heus, M. Opozda, and E.J.L. Lee) and verified by a fourth reviewer (P.J. Tully). We extracted information pertaining to study identification (first author, year, country, and study name), design characteristics (design, population, sample size, and follow-up), population characteristics (age, sex, education, use of antihypertensive medication, and comorbidities), characteristics of BP(V) (measurements, timing, interval, setting, device, and metrics), dementia adjudication (criteria, subtypes, consensus panel, and number of end points), cognitive testing (tests used, domains assessed, and criteria for impairment or decline), effect sizes (most adjusted effect sizes), and list of adjusted covariates. When studies reported multiple metrics of BPV, we prioritized the methods that adjusted for mean BP level (eg, coefficient of variation instead of SD). The association of mean BP with study outcomes was extracted when available. Effect sizes of mean BP for dementia or cognitive impairment were standardized to 10/5 mm Hg increase as not all studies reported the SD of mean BP. In instances where different levels of adjustment were made for mean and BPV data, we prioritized data from the same model to ensure equivalence in covariate adjustment.
Quality Assessment
The risk of bias within each study was assessed independently by 2 reviewers (R.A.A. de Heus and M. Opozda) using modified versions of the Newcastle-Ottawa Scale, for cross-sectional, case-control, and cohort designs.20 Discrepancies were resolved by consulting a third reviewer (P.J. Tully). Adjudication of the strength of evidence for the hypothesis that high BPV increases the risk for dementia or cognitive impairment was made according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, with GRADE Profiler 3.6.1.21
Deviations From Protocol
Based on preliminary data extraction and performing a parallel review on BPV and CSVD, several changes were instigated from the published protocol.10,12 Our adjudication of the primary outcome was expanded, combining dementia and cognitive impairment together, opting to analyze the categories separately in ancillary analysis. Second, we included cross-sectional studies to estimate the association between BPV with dementia or cognitive impairment, opting to analyze different study designs separately in ancillary analysis. Also, we excluded studies assessing beat-to-beat BPV, as this metric likely represents a different physiological mechanism compared with 24-hour, day-to-day, and visit-to-visit BPV.22,23
Statistical Analysis
Data pertaining to the likelihood of dementia or cognitive impairment were pooled as odds ratio (OR) with 95% CIs. A multilevel meta-analysis was used using the metafor package in R version 3.5.2.24 Compared with a traditional meta-analysis, a multilevel meta-analysis accounts for the dependence in effect sizes within a study (eg, between BPV and mean BP from which BPV is often calculated; and dependence of systolic and diastolic BP).10 Thus a single study could contribute up to 4 effect sizes for each analysis (systolic BP and BPV, diastolic BP and BPV). A mixed-effects model, with a random intercept per study, tested fixed effect moderators for BP type (diastolic versus systolic) and measure (mean versus variability). Random-effects models (inverse-variance method) were used under the assumption of high sampling variability between studies, different BPV metrics, and cognitive function outcomes.25 Statistical heterogeneity was evaluated with the I2 statistic and methodological heterogeneity was explored with meta-regression in Comprehensive Meta-Analysis software.26 The presence of publication bias was evaluated with the test of Egger,27 Begg-Mazumdar,28 and the Duval and Tweedie trim-and-fill funnel plot.29
Separate analyses considered key methodological and descriptive characteristics that might modulate the association between BP and dementia cognitive impairment and the different dementia subtypes (Data Supplement). The standardized mean difference between groups of BPV or cognitive impairment groups (dementia or impairment versus no dementia or impairment) were modeled with RevMan 5.3, analyzing cognitive function or BPV, respectively.30 Comprehensive Meta-Analysis software was used for the analysis of r family effect sizes showing the linear association between BP(V) measures and cognitive function.26,31
Results
Study Selection and Characteristics
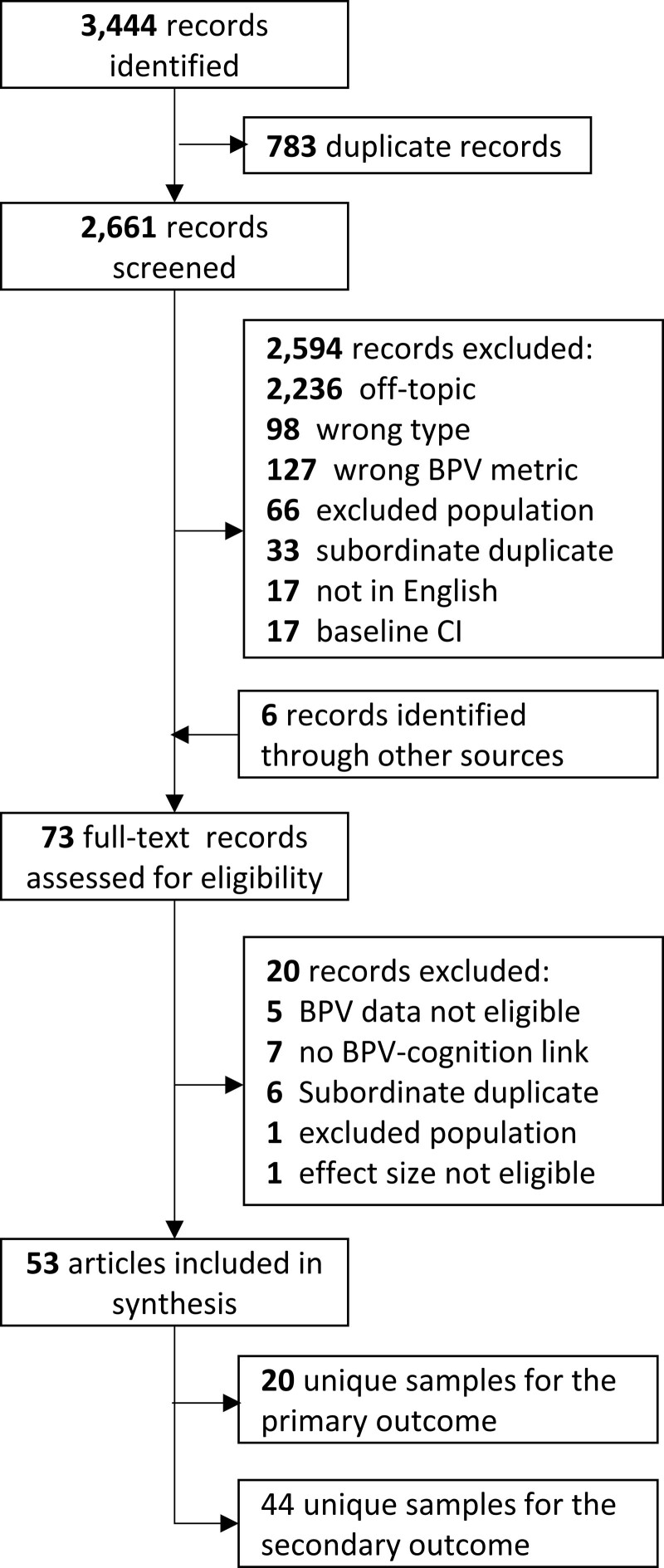
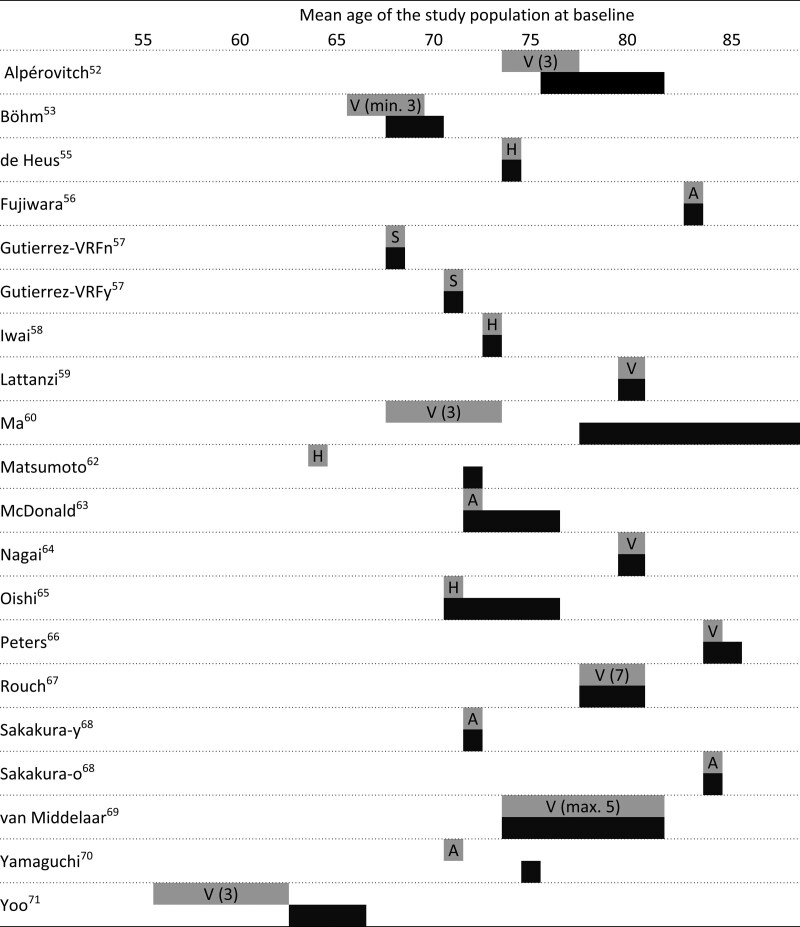
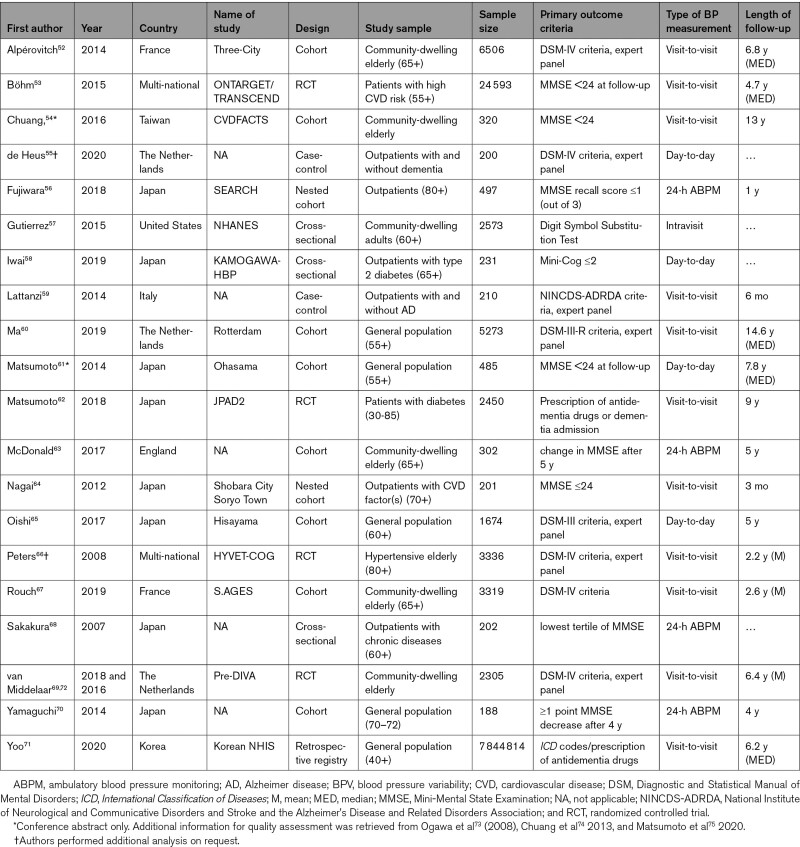
After duplicate removal, 2661 records were screened, from which 53 were retained (Figure 1). Reasons for exclusion after full-text review are described in Table S1 in the Data Supplement.32–51 Twenty unique studies samples met the inclusion criteria for the primary outcome (analytical n=7 899 679).52–71 These comprised 8 cohort studies (n=18 067), 2 nested cohort studies (n=698), one nationwide registry database (n=7 844 814), 4 randomized controlled trials analyzed as a cohort (n=32 684), 3 cross-sectional studies (n=3006), and 2 case-control studies (n=410). The timing and interval of exposure and outcome assessment are depicted in Figure 2. Studies’ participants had a mean age of 73±7 years and 58±13% women. Two study samples comprised a population of type II diabetes patients and 3 studies comprised patients with high CVD risk or hypertension. Eleven studies assessed visit-to-visit office BPV, 4 studies 24-hour ambulatory BPV, 4 studies day-to-day home BPV, and 1 study intravisit office BPV. Characteristics are presented in the Table and additional information in Tables S2 through S4.
Figure 1.
Flow diagram of included studies. BPV indicates blood pressure variability; and CI, cognitive impairment.
Figure 2.
Schematic overview of included studies for the primary analysis. X axis represents time in years and each study is presented at the mean age of the study population at baseline. Chuang et al54 (2016) and Matsumoto et al62 (2018) are missing from this overview because these studies (abstract only) did not report the mean age of the study population. A indicates ambulatory blood pressure measurements (24-h); H, home blood pressure measurements; S, single-visit (within-visit BP variability); and V, visit-to-visit variability (number presents number of visits.
Table.
Characteristics of Included Studies for the Primary Analysis
Forty-seven records, comprising 43 unique study samples (analytical n=7 915 946) reported any of the secondary outcomes.52–59,61,63–65,68–72,76–107 Seventeen studies reported standardized mean difference in BPV between groups of cognitive function, 17 studies reported standardized mean difference in cognitive function between groups of BPV, and 23 studies reported the linear association between BPV with cognitive function. Characteristics are presented in Tables S4 and S5.
Study Quality and GRADE Rating
Quality assessment is presented in Tables S6 through S8. Overall quality was deemed good in 16 studies, fair in one study and poor in 3 studies. In all studies, BP was assessed with reliable methods. Eight studies did not adjust their analyses of BPV for mean BP. There was evidence of publication bias for systolic BPV, based on funnel plot asymmetry and Egger test (P=0.023; Table S9 and Figure S1). GRADE rating of the quality of evidence was very low (Table S10).
BPV and Dementia or Cognitive Impairment
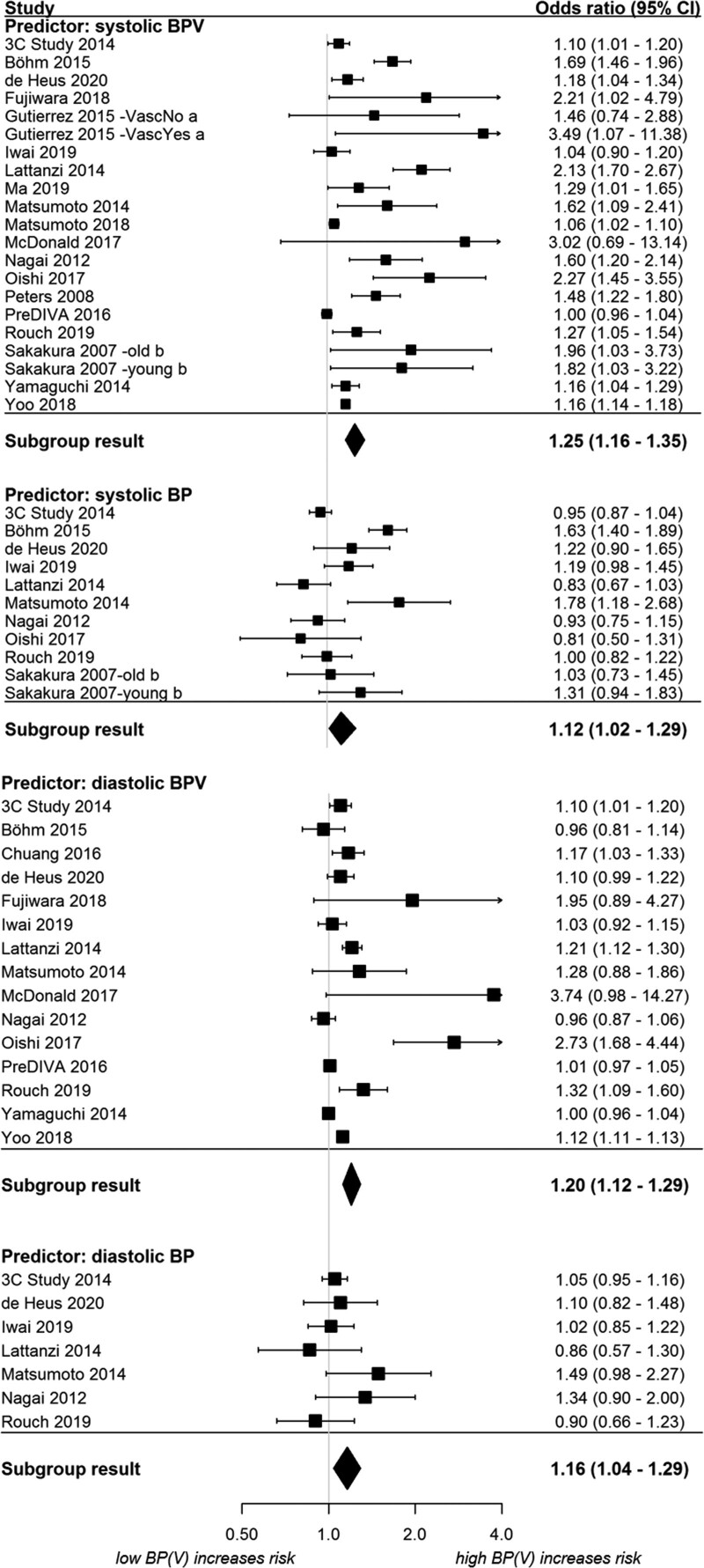
Fifty-four effect sizes retrieved from 20 studies were included in the multilevel model (Figure 3). The model included 21 systolic BPV, 11 mean systolic BP, 15 diastolic BPV, and 7 mean diastolic BP effect sizes. Higher systolic BPV was significantly associated with an increase in dementia/cognitive impairment (OR, 1.25 [95% CI, 1.16–1.35]; I2=87%), as was mean systolic BP (OR, 1.12 [95% CI, 1.02–1.29]; I2=82%). Similar results were found for diastolic BPV (OR, 1.20 [95% CI, 1.12–1.29]; I2=83%) and mean diastolic BP (OR, 1.16 [95% CI, 1.04–1.29]; I2=3%). When effect sizes were directly compared in the multilevel meta-analysis, the association of mean BP with the primary outcome was less strong compared with the association of BPV with the primary outcome (OR, 0.92 [95% CI, 0.87–0.97]; P<0.01 for comparison). Diastolic effect sizes were also less strong than systolic effect sizes in a direct comparison including both BPV and mean BP (OR, 0.96 [95% CI, 0.95–0.98]; P<0.001 for comparison). Overall, heterogeneity was high.
Figure 3.
Association of blood pressure(variability) (BP(V)) with dementia and cognitive impairment following multilevel meta-analysis. Odds ratio (OR) for mean blood pressure is presented by 10 (systolic) or 5 (diastolic) mm Hg. OR for BPV is presented per 1-unit change in the BPV metric. aEffect size reported separately for group with and without vascular risk factors or disease. bEffect sizes reported separately for young and old group.
Meta-Regression and Subgroup Analyses
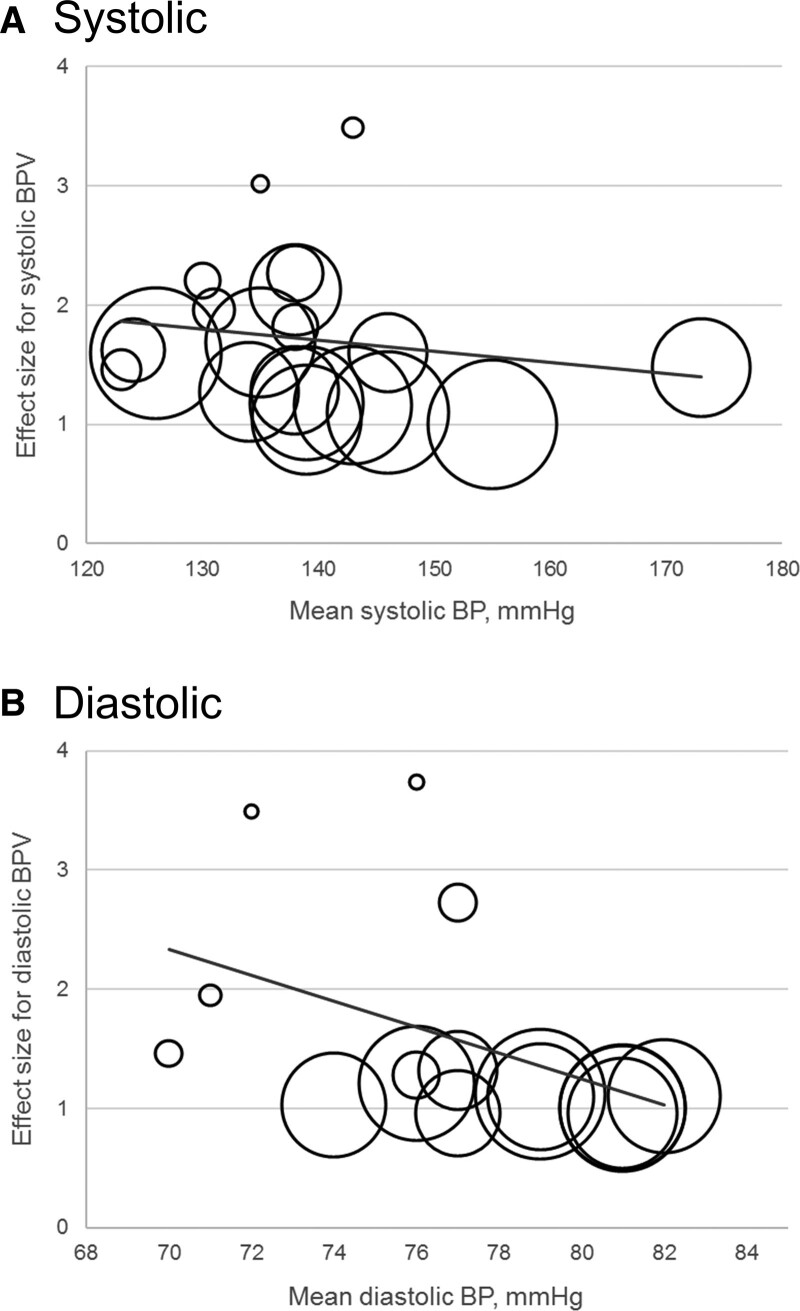
Results of the meta-regression on the primary outcomes are presented in Table S11. Higher mean BP of the study population was associated with an attenuation in association, and thus lower effect sizes, for both systolic BPV (coefficient, −0.003 [95% CI, −0.005 to −0.001]) and diastolic BPV (coefficient, −0.030 [95% CI, −0.039 to −0.021]; Figure 4). In addition, effect sizes for BPV were associated with lower age, female sex, low education, shorter interval between consecutive BP measures, shorter total interval of BP measurements, lower body mass index, and diabetes.
Figure 4.
Bubble plots of meta-regression result for mean blood pressure (BP) as covariate. The size of the bubble reflects the weight of the study. Line represents fitted meta-regression line. BPV indicates BP variability.
Subgroup analyses by methodological characteristics are presented in Figures S2 and S3. Systolic BPV analyses indicated heterogeneity for study quality, follow-up length, BP measurement (oscillometric versus other), BP measurement interval, and study region. Studies including only patients with hypertension were heterogenous for systolic BPV compared with other studies. For diastolic BPV, there was evidence of heterogeneity between study designs and type of BPV metric.
The analysis stratified by subtypes of the primary outcome supported the main findings, indicating an association between BPV and risk of dementia (any type), Alzheimer disease, vascular dementia, cognitive impairment, and cognitive decline (Figure S4). However, the main finding of stronger effect sizes for BPV compared with mean BP was not supported in these subtypes, with the exception of Alzheimer disease (P<0.01).
Secondary Outcomes
Results of the secondary outcomes analyses were generally consistent with the primary outcome analysis (Figures S5–S7), although evidence was sparse. Comparing BP(V) between groups of cognitive function indicated higher systolic BPV in those with cognitive impairment, with no difference observed for mean BP. Conversely, we observed lower general cognitive function in those with high BPV compared with low BPV. In addition, general cognitive function and BPV were associated in studies reporting β/r effect sizes, although for diastolic BPV this was only a trend. Associations between cognition and mean BP in secondary analyses were inconsistent, with mean systolic BP associated with improved memory and attention/executive/psychomotor indices.
Discussion
This systematic review and meta-analysis showed that elevated BPV is associated with a higher risk of dementia and cognitive impairment. Findings were generally consistent across dementia subtypes and general cognitive impairment, although published data were sparse for secondary outcomes. These findings are derived from observational studies of generally good quality but with high heterogeneity and evidence of publication bias in the retained articles. As such, the GRADE rating and strength of evidence were very low for the primary outcome, which tempers the conclusions that can be drawn.
Our findings emerge in the context of past research documenting associations between cognitive function and impaired BP regulation, such as circadian variation and orthostatic hypotension,95,108 as well as previous systematic reviews relating BPV to neurological outcomes, including acute stroke, transient ischemic attack, CSVD, and dementia.8–11 Here, multilevel meta-analysis modeling demonstrated that dementia and cognitive impairment were more consistently associated with BPV than with mean BP. This contrasts with our previous finding showing BPV contributes to CSVD risk but no more than mean BP.10 Besides methodological differences, this discrepancy can be partly explained by the timing of BP and outcome assessment. CSVD is known to be primarily induced by hypertension,109 whereas the link between cardiovascular risk and cognitive impairment is strongest in mid-life, becoming more ambiguous at late-life.110 In addition, it is likely that the association between BPV and cognitive impairment is only partly attributable to CSVD, involving other pathways such as Alzheimer pathology, hypoxia and blood-brain barrier dysfunction. Conversely, neurodegeneration in brain regions involved in autonomic control might lead to high BPV,111 as indicated by heterogeneity in effect sizes for diastolic but not systolic BPV when study designs were compared. However, this is not supported by evidence demonstrating intact baroreflex function in early dementia, hinting towards normal BP regulation.112 Indeed, definitive answers on the direction of causality between BP regulation and dementia are currently lacking.35
Our review analyzed an extensive spectrum of cognitive outcomes. There were sparse published data for vascular dementia, mixed dementia, cognitive decline, and domain-specific cognitive function. As such, the association between BPV and secondary outcomes was less clear. The putative association between BP with dementia appears strongest for vascular dementia often as a result of cerebrovascular disease.5 Yet brain imaging studies indicate that the majority of dementia cases, including Alzheimer disease, have a mix of neurodegenerative and vascular-type pathology evident (eg, amyloid-β, lacunes of vascular origin).113 The combination of neurodegenerative and vascular pathologies further underscores how BP only partly explains the neurodegenerative processes preceding dementia and that BP may work in concert with other nonvascular and vascular risk factors. Previously, we raised the possibility that the interaction between BPV and white matter hyperintensities leads to impairments in processing speed and executive function,102 with BPV especially impacting periventricular white matter pathways.10
The lack of consensus on BPV measurement and quantification contributes substantial heterogeneity between studies.17 Different BPV metrics were pooled separately in ancillary analyses and demonstrated generally consistent results. Likewise, there was no evidence of heterogeneity between intravisit, 24-hour ambulatory BP monitoring, home BP, and visit-to-visit variability. BP measurement intervals of 6 months or less conferred a higher risk for the primary outcome than did BP intervals greater than 6 months. Short- and long-term BPV are hypothesized to both reflect arterial reflex and compliance and dosing/titration of antihypertensive medications.114 Previously meta-analytic findings indicate that both short- and long-term BPV are associated with cardiovascular outcomes and mortality.7
Strengths of this study include the multilevel approach, the large pooled analytical sample, and extensive ancillary analyses. Several limitations temper the results of this review including evidence of publication bias. The retained studies were primarily undertaken in older aged adults (mean age 55–84 years) which may explain the difference in effect sizes observed for BPV and mean BP. Inclusion of such wide-ranging age groups may introduce other biases in the analyses, such as selection and attrition bias.115 In addition, our review was marked by significant heterogeneity even when limiting analyses to high-quality studies, implicating methodological and population characteristics as a source of between study heterogeneity.
Another limitation is that several studies defined visit-to-visit BPV using BP measurements that were taken during follow-up, introducing bias due to informative censoring.109 Likewise, some studies adjusted BPV analyses for mean BP, which may lead to an attenuation of effect sizes due to over-adjustment or multicollinearity. These are inherent limitations of the original studies, which were not designed to prospectively assess BPV independent of mean BP. Pooled analyses are prone to aggregation of study-level biases, and therefore, an individual participant data meta-analysis might reduce methodological heterogeneity and offer new insights on the role of BPV in dementia risk.
Perspectives
In summary, this systematic review and meta-analysis showed that high BPV was associated with an increased risk of dementia and cognitive impairment, although the strength of evidence was low. The relative contribution of BPV to the risk of dementia and cognitive impairment exceeded that of mean BP in primarily older adult samples. Further investigation is warranted concerning the mechanisms through which BPV may confer heightened dementia risk over mean BP, and the potential of BPV as a target for dementia prevention.
Sources of Funding
This investigation was supported by grants from the Alzheimer’s Drug Discovery Foundation (RC-201711-2014067) and Dementia Australia Research Foundation (82221-58921), awarded to P.J. Tully, C. Tzourio, L.J. Launer, K.J. Anstey, R. Peters, D.Turnbull, and A.D. Vincent and by a fellowship grant from Alzheimer Nederland awarded to R.A.A. de Heus (grant number WE.15-2019-06). K.J. Anstey is funded by ARC Fellowship FL190100011. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- CSVD
- cerebral small vessel disease
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- OR
- odds ratio
A list of all VARIABLE BRAIN Consortium participants is given in the Data Supplement.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.17797.
For Sources of Funding and Disclosures, see page 1486.
Contributor Information
Rianne A.A. de Heus, Email: rianne.deheus@radboudumc.nl.
Christophe Tzourio, Email: christophe.tzourio@u-bordeaux.fr.
Emily Jo Lynn Lee, Email: emilyjolynn.lee@student.adelaide.edu.au.
Melissa Opozda, Email: melissa.opozda@adelaide.edu.au.
Andrew D. Vincent, Email: andrew.vincent@adelaide.edu.au.
Kaarin J. Anstey, Email: k.anstey@unsw.edu.au.
Albert Hofman, Email: ahofman@hsph.harvard.edu.
Kazuomi Kario, Email: kkario@jichi.ac.jp.
Simona Lattanzi, Email: alfierelattanzisimona@gmail.com.
Lenore J. Launer, Email: launerl@nia.nih.gov.
Yuan Ma, Email: yuanma@hsph.harvard.edu.
Rajiv Mahajan, Email: rajiv.mahajan@adelaide.edu.au.
Simon P. Mooijaart, Email: s.p.mooijaart@lumc.nl.
Michiaki Nagai, Email: nagai10m@r6.dion.ne.jp.
Ruth Peters, Email: r.peters@imperial.ac.uk.
Deborah Turnbull, Email: deborah.turnbull@adelaide.edu.au.
Yuichiro Yano, Email: yyano@jichi.jp.
Jurgen A.H.R. Claassen, Email: jurgen.claassen@radboudumc.nl.
Novelty and Significance
What Is New?
High blood pressure variability may be a predictor for the risk of dementia or cognitive impairment.
The relative contribution of variability in blood pressure exceeded that of mean blood pressure.
What Is Relevant?
Variability might be a novel blood pressure-derived parameter to be taken into account in hypertension management.
Blood pressure variability might be a future target to prevent dementia.
Summary
In this meta-analysis, that included 20 studies for the primary outcome, both a higher mean level of blood pressure as well as a higher degree of blood pressure variability were associated with greater odds for dementia or cognitive impairment. Effect sizes for blood pressure variability were larger than effect sizes for mean blood pressure.
References
- 1.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 2.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, Bosch J, O’Donnell MJ, Canavan M. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323:1934–1944. doi: 10.1001/jama.2020.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124:1025–1044. doi: 10.1161/CIRCRESAHA.118.313260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1 [DOI] [PubMed] [Google Scholar]
- 7.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Li M, Xie SH, Oyang YT, Yin M, Bao B, Chen ZY, Yin XP. Visit-to-visit systolic blood pressure variability and stroke risk: a systematic review and meta-analysis. Curr Med Sci. 2019;39:741–747. doi: 10.1007/s11596-019-2100-9 [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, Papatheodorou S. Blood pressure variability and cerebral small vessel disease: a systematic review and meta-analysis of population-based cohorts. Stroke. 2020;51:82–89. doi: 10.1161/STROKEAHA.119.026739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tully PJ, Yano Y, Launer LJ, Kario K, Nagai M, Mooijaart SP, Claassen JAHR, Lattanzi S, Vincent AD, Tzourio C; Variability in Blood Pressure and Brain Health Consortium †; Variability in Blood Pressure and Brain Health Consortium. Association between blood pressure variability and cerebral small-vessel disease: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e013841. doi: 10.1161/JAHA.119.013841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou YN, Tan CC, Shen XN, Xu W, Hou XH, Dong Q, Tan L, Yu JT. Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 209 prospective studies. Hypertension. 2020;76:217–225. doi: 10.1161/HYPERTENSIONAHA.120.14993 [DOI] [PubMed] [Google Scholar]
- 12.VARIABLE BRAIN consortium. The association between blood pressure variability (BPV) with dementia and cognitive function: a systematic review and meta-analysis protocol. Syst Rev. 2018;7:163. doi: 10.1186/s13643-018-0811-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai M, Hoshide S, Dote K, Kario K. Visit-to-visit blood pressure variability and dementia. Geriatr Gerontol Int. 2015;15Suppl 1:26–33. doi: 10.1111/ggi.12660 [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Tully PJ, Hofman A, Tzourio C. Blood pressure variability and dementia: a state-of-the-art review. Am J Hypertens. 2020;33:1059–1066. doi: 10.1093/ajh/hpaa119 [DOI] [PubMed] [Google Scholar]
- 17.Stergiou GS, Parati G, Vlachopoulos C, Achimastos A, Andreadis E, Asmar R, Avolio A, Benetos A, Bilo G, Boubouchairopoulou N, et al. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement: current status and future directions - position statement of the European Society of Hypertension working group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2016;34:1665–1677. doi: 10.1097/HJH.0000000000000969 [DOI] [PubMed] [Google Scholar]
- 18.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from The National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. doi: 10.1111/joim.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells G, Shea B, O’Connor D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Accessed July 20, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 21.Schunemann H, Brozek J, Guyatt G, Oxman A. editors: The GRADE Working Group. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. 2020Hamilton, Ontario: McMaster University. www.gradepro.org [Google Scholar]
- 22.Rickards CA, Tzeng YC. Arterial pressure and cerebral blood flow variability: friend or foe? A review. Front Physiol. 2014;5:120. doi: 10.3389/fphys.2014.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Zhou J, Kavousi M, Lipsitz LA, Mattace-Raso F, Westerhof BE, Wolters FJ, Wu JW, Manor B, Ikram MK, et al. Lower complexity and higher variability in beat-to-beat systolic blood pressure are associated with elevated long-term risk of dementia: The Rotterdam Study. Alzheimers Dement. 2021;17:1134–1144. doi: 10.1002/alz.12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assink M, Wibbelink CJM. Fitting three-level meta-analytic models in r: a step-by-step tutorial. Quant Meth Psychol. 2016;12:154–174. [Google Scholar]
- 25.Taylor KS, Heneghan CJ, Stevens RJ, Adams EC, Nunan D, Ward A. Heterogeneity of prognostic studies of 24-hour blood pressure variability: systematic review and meta-analysis. PLoS One. 2015;10:e0126375. doi: 10.1371/journal.pone.0126375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2. 2013Englewood, NJ: Biostat [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 29.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 30.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen. 2014. The Nordic Cochrane Centre, The Cochrane Collaboration [Google Scholar]
- 31.Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol. 2005;90:175–181. doi: 10.1037/0021-9010.90.1.175 [DOI] [PubMed] [Google Scholar]
- 32.Gamaldo AA, Weatherbee SR, Allaire JC. Exploring the within-person coupling of blood pressure and cognition in elders. J Gerontol B Psychol Sci Soc Sci. 2008;63:P386–P389. doi: 10.1093/geronb/63.6.p386 [DOI] [PubMed] [Google Scholar]
- 33.Leritz EC, Salat DH, Milberg WP, Williams VJ, Chapman CE, Grande LJ, Rudolph JL, Schnyer DM, Barber CE, Lipsitz LA, et al. Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology. 2010;24:199–208. doi: 10.1037/a0018108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos WB, Matoso JM, Maltez M, Gonçalves T, Casanova M, Moreira IF, Lourenço RA, Monteiro WD, Farinatti PT, Soares PP, et al. Spectral analyses of systolic blood pressure and heart rate variability and their association with cognitive performance in elderly hypertensive subjects. J Hum Hypertens. 2015;29:488–494. doi: 10.1038/jhh.2014.119 [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Akiguchi I, Oiwa K, Hayashi M, Imai K. Twenty-four-hour blood pressure changes in the course of lacunar disease. Cerebrovasc Dis. 2001;11:100–106. doi: 10.1159/000047620 [DOI] [PubMed] [Google Scholar]
- 36.Cicconetti P, Monteforte G, Thau F, Lorido A, Durante M, Piccirillo G, Cacciafesta M, Marigliano V. Cognitive assessment in the elderly with new mild systolic hypertension. Arch Gerontol Geriatr. 1998Suppl 675–78.18653153 [Google Scholar]
- 37.Efimova NY, Chernov VI, Efimova IY, Lishmanov YB. Influence of antihypertensive therapy on cerebral perfusion in patients with metabolic syndrome: relationship with cognitive function and 24-h arterial blood pressure monitoring. Cardiovasc Ther. 2015;33:209–215. doi: 10.1111/1755-5922.12136 [DOI] [PubMed] [Google Scholar]
- 38.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890 [DOI] [PubMed] [Google Scholar]
- 39.Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31:780–786. doi: 10.1161/01.hyp.31.3.780 [DOI] [PubMed] [Google Scholar]
- 40.Mossello E, Pieraccioli MC, Zanieri S, Fedeli A, Belladonna M, Nesti N, Marchionni N, Masotti G, Ungar A. Ambulatory blood pressure monitoring in older nursing home residents: diagnostic and prognostic role. J Am Med Dir Assoc. 2012;13:760.e1–760.e5. doi: 10.1016/j.jamda.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 41.Ramirez AJ, Parati G, Castiglioni P, Consalvo D, Solís P, Risk MR, Waissman P, di Rienzo M, Mancia G, Sanchez RA. Elderly hypertensive patients: silent white matter lesions, blood pressure variability, baroreflex impairment and cognitive deterioration. Curr Hypertens Rev. 2011;7:80–87. [Google Scholar]
- 42.Yaneva-Sirakova T, Traykov L, Petrova J, Gruev I, Vassilev D. Screening for mild cognitive impairment in patients with cardiovascular risk factors. Neuropsychiatr Dis Treat. 2017;13:2925–2934. doi: 10.2147/NDT.S144264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dore GA, Elias MF, Crichton GE, Robbins MA. Age modifies the relation between intraindividual measurement-to-measurement variation in blood pressure and cognitive function: the Maine-Syracuse Study. J Hypertens. 2018;36:268–276. doi: 10.1097/HJH.0000000000001510 [DOI] [PubMed] [Google Scholar]
- 44.Geijselaers SLC, Sep SJS, Claessens D, Schram MT, Van Boxtel MPJ, Henry RMA, Verhey FRJ, Kroon AA, Dagnelie PC, Schalkwijk CG, et al. The role of hyperglycemia, insulin resistance, and blood pressure in diabetes-associated differences in cognitive performance - the Maastricht Study. Diab Care. 2017;40:1537–1547. [DOI] [PubMed] [Google Scholar]
- 45.Gunstad J, Keary TA, Spitznagel MB, Poppas A, Paul RH, Sweet LH, Hoth KF, Haley AP, Forman DE, Cohen RA. Blood pressure and cognitive function in older adults with cardiovascular disease. Int J Neurosci. 2009;119:2228–2242. doi: 10.3109/00207450903139713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SH, Han K, Cho H, Park YM, Kwon HS, Kang G, Yoon KH, Kim MK. Variability in metabolic parameters and risk of dementia: a nationwide population-based study. Alzheimers Res Ther. 2018;10:110. doi: 10.1186/s13195-018-0442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagai M, Hoshide S, Nishikawa M, Masahisa S, Kario K. Visit-to-visit blood pressure variability in the elderly: associations with cognitive impairment and carotid artery remodeling. Atherosclerosis. 2014;233:19–26. doi: 10.1016/j.atherosclerosis.2013.11.071 [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, Kawanami T, Kato T. Impact of nocturnal heart rate variability on cerebral small-vessel disease progression: a longitudinal study in community-dwelling elderly Japanese. Hypertens Res. 2015;38:564–569. doi: 10.1038/hr.2015.38 [DOI] [PubMed] [Google Scholar]
- 49.Sible IJ, Nation DA; Alzheimer’s Disease Neuroimaging Initiative. Long-term blood pressure variability across the clinical and biomarker spectrum of alzheimer’s disease. J Alzheimers Dis. 2020;77:1655–1669. doi: 10.3233/JAD-200221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okonkwo OC, Cohen RA, Gunstad J, Poppas A. Cardiac output, blood pressure variability, and cognitive decline in geriatric cardiac patients. J Cardiopulm Rehabil Prev. 2011;31:290–297. doi: 10.1097/HCR.0b013e318220a817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruebner RL, Laney N, Kim JY, Hartung EA, Hooper SR, Radcliffe J, Furth SL. Neurocognitive dysfunction in children, adolescents, and young adults with CKD. Am J Kidney Dis. 2016;67:567–575. doi: 10.1053/j.ajkd.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 52.Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues JF, Richard-Harston S, Tzourio C. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimers Dement. 2014;105 SupplS330–S337. doi: 10.1016/j.jalz.2013.05.1777 [DOI] [PubMed] [Google Scholar]
- 53.Böhm M, Schumacher H, Leong D, Mancia G, Unger T, Schmieder R, Custodis F, Diener HC, Laufs U, Lonn E, et al. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension. 2015;65:651–661. doi: 10.1161/HYPERTENSIONAHA.114.04568 [DOI] [PubMed] [Google Scholar]
- 54.Chuang SY, Cheng HM, Yip BS, Pan WH, Chen CH. Greater visit-to-visit variability was associated with cognitive function impairment in an elderly population: Prospective study. Eur Heart J. 2016;37:741–742.26685144 [Google Scholar]
- 55.de Heus RAA, Reumers SFI, van der Have A, Tumelaire M, Tully PJ, Claassen JAHR. Day-to-Day Home Blood Pressure Variability is Associated with Cerebral Small Vessel Disease Burden in a Memory Clinic Population. J Alzheimers Dis. 2020;74:463–472. doi: 10.3233/JAD-191134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujiwara T, Hoshide S, Kanegae H, Eguchi K, Kario K. Exaggerated blood pressure variability is associated with memory impairment in very elderly patients. J Clin Hypertens (Greenwich). 2018;20:637–644. doi: 10.1111/jch.13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutierrez J, Marshall RS, Lazar RM. Indirect measures of arterial stiffness and cognitive performance in individuals without traditional vascular risk factors or disease. JAMA Neurol. 2015;72:309–315. doi: 10.1001/jamaneurol.2014.3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwai K, Ushigome E, Matsumoto S, Kitagawa N, Ushigome H, Yokota I, Asano M, Hamaguchi M, Yamazaki M, Fukui M. Home blood pressure is associated with cognitive impairment among elderly patients with type 2 diabetes: KAMOGAWA-HBP study. Diab Vasc Dis Res. 2019;16:506–512. doi: 10.1177/1479164119847479 [DOI] [PubMed] [Google Scholar]
- 59.Lattanzi S, Luzzi S, Provinciali L, Silvestrini M. Blood pressure variability predicts cognitive decline in Alzheimer’s disease patients. Neurobiol Aging. 2014;35:2282–2287. doi: 10.1016/j.neurobiolaging.2014.04.023 [DOI] [PubMed] [Google Scholar]
- 60.Ma Y, Wolters FJ, Chibnik LB, Licher S, Ikram MA, Hofman A, Ikram MK. Variation in blood pressure and long-term risk of dementia: a population-based cohort study. PLoS Med. 2019;16:e1002933. doi: 10.1371/journal.pmed.1002933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsumoto A, Satoh M, Kikuya M, Ohkubo T, Hirano M, Inoue R, Hashimoto T, Hara A, Hirose T, Obara T, et al. Day-to-day variability in home blood pressure is associated with cognitive decline: the Ohasama study. Hypertension. 2014;63:1333–1338. doi: 10.1161/HYPERTENSIONAHA.113.01819 [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto C, Ogawa H, Saito Y, Okada S, Sakuma M, Soejima H, Nakayama M, Doi N, Jinnouchi H, Waki M, et al. The association of visit-to-visit blood pressure and blood glucose variability and incidence of dementia in patients with type 2 diabetes mellitus: Insights from the JPAD2 Cohort Study. Circulation. 2018;138 [Google Scholar]
- 63.McDonald C, Pearce MS, Kerr SR, Newton JL. Blood pressure variability and cognitive decline in older people: a 5-year longitudinal study. J Hypertens. 2017;35:140–147. doi: 10.1097/HJH.0000000000001120 [DOI] [PubMed] [Google Scholar]
- 64.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J Hypertens. 2012;30:1556–1563. doi: 10.1097/HJH.0b013e3283552735 [DOI] [PubMed] [Google Scholar]
- 65.Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, Shibata M, Ohtsubo T, Kitazono T, Kiyohara Y, et al. Day-to-day blood pressure variability and risk of dementia in a General Japanese Elderly Population: The Hisayama Study. Circulation. 2017;136:516–525. doi: 10.1161/CIRCULATIONAHA.116.025667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, et al. ; HYVET investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1 [DOI] [PubMed] [Google Scholar]
- 67.Rouch L, Cestac P, Sallerin B, Benattar-Zibi L, Bertin P, Berrut G, Corruble E, Derumeaux G, Falissard B, Forette F, et al. Visit-to-visit blood pressure variability is associated with cognitive decline and incident dementia: The S.AGES cohort. Circ. 2019;140 [DOI] [PubMed] [Google Scholar]
- 68.Sakakura K, Ishikawa J, Okuno M, Shimada K, Kario K. Exaggerated ambulatory blood pressure variability is associated with cognitive dysfunction in the very elderly and quality of life in the younger elderly. Am J Hypertens. 2007;20:720–727. doi: 10.1016/j.amjhyper.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 69.van Middelaar T, van Dalen JW, van Gool WA, van den Born BH, van Vught LA, Moll van Charante EP, Richard E. Visit-to-visit blood pressure variability and the risk of dementia in older people. J Alzheimers Dis. 2018;62:727–735. doi: 10.3233/JAD-170757 [DOI] [PubMed] [Google Scholar]
- 70.Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, Kawanami T, Kato T. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community-based elderly Japanese. Am J Hypertens. 2014;27:1257–1267. doi: 10.1093/ajh/hpu045 [DOI] [PubMed] [Google Scholar]
- 71.Yoo JE, Shin DW, Han K, Kim D, Lee SP, Jeong SM, Lee J, Kim S. Blood Pressure variability and the risk of dementia: A Nationwide Cohort Study. Hypertension. 2020;75:982–990. doi: 10.1161/HYPERTENSIONAHA.119.14033 [DOI] [PubMed] [Google Scholar]
- 72.Van Middelaar T, Van Dalen JW, Van Gool WA, Moll Van Charante EP, Richard E. The association between visit-to-visit blood pressure variability and cognitive impairment in older people. Eur Stroke J. 2016;1:242. [Google Scholar]
- 73.Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623 [DOI] [PubMed] [Google Scholar]
- 74.Chuang SY, Hsu PF, Chang HY, Bai CH, Yeh WT, Pan HW. C-reactive protein predicts systolic blood pressure and pulse pressure but not diastolic blood pressure: the Cardiovascular Disease Risk Factors Two-Township Study. Am J Hypertens. 2013;26:657–664. doi: 10.1093/ajh/hps095 [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto C, Ogawa H, Saito Y, Okada S, Soejima H, Sakuma M, Masuda I, Nakayama M, Doi N, Jinnouchi H, et al. ; JPAD Trial Investigators; JPAD Trial Investigators. Sex difference in effects of low-dose aspirin on prevention of dementia in patients with type 2 diabetes: a long-term follow-up study of a randomized clinical trial. Diabetes Care. 2020;43:314–320. doi: 10.2337/dc19-1188 [DOI] [PubMed] [Google Scholar]
- 76.Baranowski J, Klęczar K, Sołtysiak M, Widecka K. The association between cognitive decline and short-term blood pressure variability in middle-aged patients with primary hypertension - a pilot study. Arterial Hypertens (Poland). 2018;22:135–142. doi: 10.5603/AH.a2018.0013 [Google Scholar]
- 77.Bellelli G, Pezzini A, Bianchetti A, Trabucchi M. Increased blood pressure variability may be associated with cognitive decline in hypertensive elderly subjects with no dementia. Arch Intern Med. 2002;162:483–484. doi: 10.1001/archinte.162.4.483 [DOI] [PubMed] [Google Scholar]
- 78.Chen C, Lee J, Ko Y, Lee C, Chang Y. Low diastolic blood pressure and high blood pressure variability are risk factors for cognitive decline in elderly adults: a case-control study. Neuropsychiatry. 2018;8:1986–1992. [Google Scholar]
- 79.Cho N, Hoshide S, Nishizawa M, Fujiwara T, Kario K. Relationship between blood pressure variability and cognitive function in elderly patients with good blood pressure control. Am J Hypertens. 2018;31:293–298. doi: 10.1093/ajh/hpx155 [DOI] [PubMed] [Google Scholar]
- 80.Cicconetti P, Costarelia M, Moisè A, Ciotti V, Tafaro L, Monteforte G, Piccirillo G, Cacciafesta M. Blood pressure variability and cognitive function in older hypertensives. Arch Gerontol Geriatr Suppl. 2004;38:63–68. [DOI] [PubMed] [Google Scholar]
- 81.Cohen RA, Poppas A, Forman DE, Hoth KF, Haley AP, Gunstad J, Jefferson AL, Tate DF, Paul RH, Sweet LH, et al. Vascular and cognitive functions associated with cardiovascular disease in the elderly. J Clin Exp Neuropsychol. 2009;31:96–110. doi: 10.1080/13803390802014594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conway KS, Forbang N, Beben T, Criqui MH, Ix JH, Rifkin DE. Relationship between 24-hour ambulatory blood pressure and cognitive function in community-living older adults: the UCSD ambulatory blood pressure study. Am J Hypertens. 2015;28:1444–1452. doi: 10.1093/ajh/hpv042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crichton GE, Elias MF, Dore GA, Torres RV, Robbins MA. Measurement-to-measurement blood pressure variability is related to cognitive performance: the Maine Syracuse study. Hypertension. 2014;64:1094–1101. doi: 10.1161/HYPERTENSIONAHA.114.04282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Epstein NU, Lane KA, Farlow MR, Risacher SL, Saykin AJ, Gao S. Cognitive dysfunction and greater visit-to-visit systolic blood pressure variability. J Am Geriatr Soc. 2013;61:2168–2173. doi: 10.1111/jgs.12542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fioravanti M, Nacca D, Golfieri B, Lucia P, Cugini P. The relevance of continuous blood pressure monitoring in examining the relationship of memory efficiency with blood pressure characteristics. Physiol Behav. 1996;59:1077–1084. doi: 10.1016/0031-9384(95)02259-7 [DOI] [PubMed] [Google Scholar]
- 86.Godai K, Kabayama M, Gondo Y, Yasumoto S, Sekiguchi T, Noma T, Tanaka K, Kiyoshige E, Akagi Y, Sugimoto K, et al. Day-to-day blood pressure variability is associated with lower cognitive performance among the Japanese community-dwelling oldest-old population: The SONIC study. Hypertens Res. 2020;43:404–411. [DOI] [PubMed] [Google Scholar]
- 87.Goldstein IB, Shapiro D, La Rue A, Guthrie D. Relationship between 24-hour ambulatory blood pressure and cognitive function in healthy elderly people. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 1998;5:215–224. doi: 10.1076/anec.5.3.215.611 [DOI] [PubMed] [Google Scholar]
- 88.Haring B, Liu J, Salmoirago-Blotcher E, Hayden KM, Sarto G, Roussouw J, Kuller LH, Rapp SR, Wassertheil-Smoller S. Blood pressure variability and brain morphology in elderly women without cardiovascular disease. Neurology. 2019;92:e1284–e1297. doi: 10.1212/WNL.0000000000007135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanemaru A, Kanemaru K, Kuwajima I. The effects of short-term blood pressure variability and nighttime blood pressure levels on cognitive function. Hypertens Res. 2001;24:19–24. doi: 10.1291/hypres.24.19 [DOI] [PubMed] [Google Scholar]
- 90.Keary TA, Gunstad J, Poppas A, Paul RH, Jefferson AL, Hoth KF, Sweet LH, Forman DE, Cohen RA. Blood pressure variability and dementia rating scale performance in older adults with cardiovascular disease. Cogn Behav Neurol. 2007;20:73–77. doi: 10.1097/WNN.0b013e3180335f9f [DOI] [PubMed] [Google Scholar]
- 91.Liu J, Huang YL, Song L, Li CH, Zhao HL, Wang YM, An SS, Li ZF, Chen SH, Wang AX, et al. [Association between long term systolic blood pressure variability index and cognitive function in middle-aged and elderly people]. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44:548–554. doi: 10.3760/cma.j.issn.0253-3758.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 92.Liu Z, Zhao Y, Zhang H, Chai Q, Cui Y, Diao Y, Xiu J, Sun X, Jiang G. Excessive variability in systolic blood pressure that is self-measured at home exacerbates the progression of brain white matter lesions and cognitive impairment in the oldest old. Hypertens Res. 2016;39:245–253. doi: 10.1038/hr.2015.135 [DOI] [PubMed] [Google Scholar]
- 93.Ogliari G, Smit RA, Westendorp RG, Jukema JW, de Craen AJ, Sabayan B. Visit-to-visit blood pressure variability and future functional decline in old age. J Hypertens. 2016;34:1544–1550. doi: 10.1097/HJH.0000000000000979 [DOI] [PubMed] [Google Scholar]
- 94.Osovska NY, Mazur YV, Bereziuk OM, Dmytryshyn SP, Velychkovych MM, Perebetiuk LA, Temna OV, Honcharenko OM, Furman OV, Balatskyi OR. Cardiovascular remodeling in patients with hypertension with different degrees of cognitive impairment. Wiad Lek. 2019;72:670–676. [PubMed] [Google Scholar]
- 95.Paganini-Hill A, Bryant N, Corrada MM, Greenia DE, Fletcher E, Singh B, Floriolli D, Kawas CH, Fisher MJ. Blood pressure circadian variation, cognition and brain imaging in 90+ year-olds. Front Aging Neurosci. 2019;11:54. doi: 10.3389/fnagi.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qin B, Viera AJ, Muntner P, Plassman BL, Edwards LJ, Adair LS, Popkin BM, Mendez MA. Visit-to-visit variability in blood pressure is related to late-life cognitive decline. Hypertension. 2016;68:106–113. doi: 10.1161/HYPERTENSIONAHA.116.07494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rouch L, Cestac P, Sallerin B, Piccoli M, Benattar-Zibi L, Bertin P, Berrut G, Corruble E, Derumeaux G, Falissard B, et al. ; S.AGES investigators. Visit-to-visit blood pressure variability is associated with cognitive decline and incident dementia: the S.AGES Cohort. Hypertension. 2020;76:1280–1288. doi: 10.1161/HYPERTENSIONAHA.119.14553 [DOI] [PubMed] [Google Scholar]
- 98.Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, Sattar N, Jukema JW, Van Osch MJP, Van Der Grond J, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. BMJ. 2013;347::f4600. doi: 10.1136/bmj.f4600 [DOI] [PubMed] [Google Scholar]
- 99.Tadic M, Cuspidi C, Bombelli M, Facchetti R, Mancia G, Grassi G. Relationships between residual blood pressure variability and cognitive function in the general population of the PAMELA study. J Clin Hypertens (Greenwich). 2019;21:39–45. doi: 10.1111/jch.13428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsang S, Sperling SA, Park MH, Helenius IM, Williams IC, Manning C. Blood pressure variability and cognitive function among Older African Americans: introducing a new blood pressure variability measure. Cogn Behav Neurol. 2017;30:90–97. doi: 10.1097/WNN.0000000000000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tully PJ, Dartigues JF, Debette S, Helmer C, Artero S, Tzourio C. Dementia risk with antihypertensive use and blood pressure variability: a cohort study. Neurology. 2016;87:601–608. doi: 10.1212/WNL.0000000000002946 [DOI] [PubMed] [Google Scholar]
- 102.Tully PJ, Debette S, Tzourio C. The association between systolic blood pressure variability with depression, cognitive decline and white matter hyperintensities: the 3C Dijon MRI study. Psychol Med. 2018;48:1444–1453. doi: 10.1017/S0033291717002756 [DOI] [PubMed] [Google Scholar]
- 103.Wijsman LW, de Craen AJ, Muller M, Sabayan B, Stott D, Ford I, Trompet S, Jukema JW, Westendorp RG, Mooijaart SP. Blood pressure lowering medication, visit-to-visit blood pressure variability, and cognitive function in old age. Am J Hypertens. 2016;29:311–318. doi: 10.1093/ajh/hpv101 [DOI] [PubMed] [Google Scholar]
- 104.Yano Y, Griswold M, Wang W, Greenland P, Lloyd-Jones DM, Heiss G, Gottesman RF, Mosley TH. Long-term blood pressure level and variability from midlife to later life and subsequent cognitive change: the ARIC neurocognitive study. J Am Heart Assoc. 2018;7:e009578. doi: 10.1161/JAHA.118.009578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64:983–988. doi: 10.1161/HYPERTENSIONAHA.114.03978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yildirim E, Ermis E, Allahverdiyev S, Ucar H, Yavuzer S, Yavuzer H, Cengiz M. Relationship between blood pressure variability and cognitive function in geriatric hypertensive patients with well-controlled blood pressure. Aging Clin Exp Res. 2020;32:93–98. doi: 10.1007/s40520-019-01141-6 [DOI] [PubMed] [Google Scholar]
- 107.Zhou TL, Kroon AA, van Sloten TT, van Boxtel MPJ, Verhey FRJ, Schram MT, Köhler S, Stehouwer CDA, Henry RMA. Greater blood pressure variability Is associated with lower cognitive performance. Hypertension. 2019;73:803–811. doi: 10.1161/HYPERTENSIONAHA.118.12305 [DOI] [PubMed] [Google Scholar]
- 108.Rawlings AM, Juraschek SP, Heiss G, Hughes T, Meyer ML, Selvin E, Sharrett AR, Windham BG, Gottesman RF. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology. 2018;91:e759–e768. doi: 10.1212/WNL.0000000000006027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Courson H, Leffondré K, Tzourio C. Blood pressure variability and risk of cardiovascular event: is it appropriate to use the future for predicting the present? Eur Heart J. 2018;39:4220. doi: 10.1093/eurheartj/ehy825 [DOI] [PubMed] [Google Scholar]
- 110.Landau SM, Harrison TM. A link between cardiovascular risk management and alzheimer disease is still elusive. JAMA Neurol. 2021;78:524–526. doi: 10.1001/jamaneurol.2021.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens. 2010;4:174–182. doi: 10.1016/j.jash.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 112.de Heus RAA, de Jong DLK, Sanders ML, van Spijker GJ, Oudegeest-Sander MH, Hopman MT, Lawlor BA, Olde Rikkert MGM, Claassen JAHR. Dynamic regulation of cerebral blood flow in patients with alzheimer disease. Hypertension. 2018;72:139–150. doi: 10.1161/HYPERTENSIONAHA.118.10900 [DOI] [PubMed] [Google Scholar]
- 113.Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement. 2018;14:148–156. doi: 10.1016/j.jalz.2017.07.755 [DOI] [PubMed] [Google Scholar]
- 114.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155. doi: 10.1038/nrcardio.2013.1 [DOI] [PubMed] [Google Scholar]
- 115.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.