Abstract
To achieve the goal of the World Health Organization to eliminate viral hepatitis as a major public health threat by 2030, the Chinese Society of Infectious Diseases and the Chinese Society of Hepatology convened an expert panel in 2019 to update the guidelines for the prevention and treatment of chronic hepatitis B (CHB). The current guidelines cover recent advances in basic, clinical, and preventive studies of CHB infection and consider the actual situation in China. These guidelines are intended to provide support for the prevention, diagnosis, and treatment of CHB.
Keywords: Hepatitis B, Chronic, Guidelines, Prevention, Treatment
A Chinese expert panel led by the Chinese Society of Infectious Diseases and the Chinese Society of Hepatology developed the first edition of the guidelines for the prevention and treatment of chronic hepatitis B (CHB) in 2005, which were updated in 2010 and 2015, respectively. Over the past 4 years, significant progress has been made in basic and clinical research on chronic infection with the hepatitis B virus (HBV), both at home and abroad. The guidelines were updated again to standardize the prevention, diagnosis, and treatment of CHB and help to meet the goal to eliminate viral hepatitis as a major public health threat by 2030 that was proposed by the World Health Organization (WHO) in 2016.
The present guidelines aim to help clinicians make informed decisions for the prevention, diagnosis, and treatment of CHB; however, they are not intended to be mandatory standards and will probably not cover and solve all the issues associated with the diagnosis and treatment of CHB. Therefore, clinicians should use the best clinical evidence and their expertise, experience, and available medical resources to develop a comprehensive and reasonable diagnosis and treatment plan that addresses individual needs.
The quality of evidence in this guideline is divided into three levels: A, B, and C, and the strength of the recommendations is categorized into two levels, 1 and 2, as given in Table 1. (revised according to the Grades of Recommendation, Assessment, Development, and Evaluation classification system).
Table 1. Evidence level and recommendation strength.
| Level | Descriptions |
|---|---|
| Level of evidence | |
| High quality (A) | Further research will probably not change confidence in this assessment. |
| Medium quality (B) | Further research will probably have a significant impact on confidence in this assessment. |
| Low quality (C) | Further research will probably affect and change this assessment. |
| Recommended level | |
| Strong (1) | Quality of the evidence, possible outcomes, and potential results of prevention, diagnosis and treatment are all considered, with expected benefits outweighing estimated costs. |
| Weak (2) | Quality of evidence varies substantially, which results in a less certain recommendation, with expected benefits not necessarily outweighing estimated costs. |
| Terminology | Definition |
|---|---|
| Chronic HBV infection | HBsAg, or HBV DNA seropositive, or both for ≥6 months. |
| CHB | Chronic liver disease caused by persistent HBV infection for ≥6 months. |
| HBV reactivation | Increase in HBV DNA ≥1 lg IU/mL from baseline, or detection of HBV DNA in patients with negative baseline HBV DNA, or HBsAg seroconversion from negative to positive in HBsAg-negative/anti-HBc-positive patients who receive immunosuppressive therapy or chemotherapy. |
| HBeAg clearance | Loss of HBeAg in HBeAg-positive patients. |
| HBeAg seroconversion | Loss of HBeAg and anti-HBe seroconversion from negative to positive in HBeAg-positive patients. |
| Resolved hepatitis B | Patients have a history of acute or CHB but are currently negative for HBsAg, positive or negative for anti-HBs, positive for anti-HBc, with undetectable HBV DNA and normal serum ALT levels. |
| Virological breakthrough | Increase in HBV DNA >1 lg IU/mL from nadir during treatment, or reversion to positive following conversion to negative, with or without elevated ALT, in patient with good compliance to NAs therapy, as confirmed 1 month later using the same reagent. |
| Virological relapse | Serum HBV DNA >2,000 IU/mL following the withdrawal of treatment in patients with virological response, confirmed 1 month later. |
| Drug resistance | |
| Genotypic resistance | Genetic mutations that confer resistance to HBV detected during antiviral therapy. |
| Phenotypic resistance | Decreased susceptibility (determined by in vitro testing) to antiviral drugs, which is associated with genotypic resistance. |
| Cross-resistance | Drug-resistant mutations that arise for one antiviral drug that can show resistance to other antiviral drugs (either one or several). |
| Multidrug resistance | Drug resistance to at least two different classes of NAs. |
ALT, alanine aminotransferase; NAs, nucleos(t)ide analogs; HBeAg, hepatitis B e-antigen; anti-HBe, hepatitis B e-antibody; CHB, chronic hepatitis B; HBV, hepatitis B virus.
Epidemiology and prevention
Epidemiology
HBV is a global epidemic; however, its prevalence varies significantly between different regions. According to WHO, approximately 257 million people live with chronic HBV infection, 68% of whom live in Africa and the Western Pacific.1 In 2015, approximately 887,000 people died from HBV infection-related diseases worldwide, and cirrhosis and hepatocellular carcinoma (HCC) accounted for 52% and 38% of the deaths, respectively. The prevalence of hepatitis B surface antigen (HBsAg) in the general population in Southeast Asia and the Western Pacific was 2% (39 million cases) and 6.2% (115 million cases), respectively. The endemicity of HBV in Asia is heterogeneous, and most of the region has a moderate to high prevalence of HBV infection, except for in a few low endemic areas.
In 2014, the Chinese Center for Disease Control and Prevention conducted a national seroepidemiological survey of hepatitis B among people aged 1–29 years. The results showed that the prevalence of HBsAg in people aged 1–4, 5–14, and 15–29 years was 0.32%, 0.94%, and 4.38%, representing a decrease of 96.7%, 91.2%, and 55.1%, respectively compared with the 1992 survey.2 It was estimated that the current prevalence of HBsAg in the general population was from 5% to 6% in China, and approximately 70 million people were chronically infected by HBV, which included approximately 20–30 million patients with CHB.3
HBV is transmitted from mother-to-child or through blood (which includes minor wounds on the skin and mucous membranes) and sexual contacts. Mother-to-child transmission is the most common route of transmission in China, which accounts for 30–50% of HBV infections.4 It mainly occurs during the perinatal period through exposure to blood and body fluids of HBV-positive mothers. Maternal HBV DNA levels are closely correlated with the risk of HBV infection in infants. Mother-to-child transmission is more probable to occur in children born to HBeAg-positive mothers with high HBV DNA levels.5 HBV is mainly transmitted through blood and sexual contact in adults. Patients with a history of drug injection, immunosuppressive therapy, blood transfusion or on hemodialysis, patients with hepatitis C virus (HCV) or human immunodeficiency virus (HIV) infection, family members of HBsAg-positive people, healthcare workers and public safety workers at risk of occupational exposure to blood or body fluids in the work environment, prisoners, and diabetic patients who have not been vaccinated against hepatitis B have a higher risk of HBV infection.6 Due to the strict screening for HBsAg and HBV DNA in blood donors and the adoption of safe injection practices, transmission through blood transfusion or blood products is rare. In addition, transmission could occur through damaged skin or mucous membranes, such as pedicures, tattoos, piercings, accidental exposure of healthcare workers during their work, and sharing of razors or toothbrushes.6 Unprotected sexual contact with persons infected with HBV carries a high-risk of HBV infection, in particular, for those with multiple sexual partners and who are homosexual men.7
HBV does not spread through the respiratory or digestive tracts. Therefore, HBV cannot be transmitted via normal exposure in schools, workplaces, and other group settings, for example, working in the same office (which includes sharing computers and other office supplies), contact through shaking hands and hugging, living in the same dormitory, dining in the same restaurant, toilet sharing, and other nonblood exposure contacts. No epidemiological or experimental studies have found that HBV can be transmitted via blood-sucking insects (e.g., mosquitoes and bedbugs).8
Prevention
Protecting susceptible people
HBV vaccination is the most effective measure to prevent HBV infections. Vaccination mainly targets newborns, followed by infants,9 previously unvaccinated children and adolescents aged <15 years, and high-risk groups.7–10
The hepatitis B vaccine is administered in a series of three doses; the first dose is received immediately after birth, the second after 1 month, and the final dose at 6 months. The HBV vaccine should be administered as soon as possible after birth. The vaccine is administered by intramuscular injection into the deltoid muscle of the upper arm or the anterolateral aspect of the thigh for newborns and the middle deltoid muscle of the upper arm for children and adults. Newborns who suffer from severe diseases, such as very low birth weight, severe birth defects, severe asphyxia, and respiratory distress syndrome, should receive the first dose of hepatitis B vaccine as soon as possible after their vital signs have stabilized.
The dosing regimen for hepatitis B vaccine for newborns is: (1) 10 µg of recombinant yeast hepatitis B vaccine per injection, regardless of whether the mother is HBsAg-positive or not, and (2) recombinant Chinese hamster ovary (commonly known as CHO) cell hepatitis B vaccine, 10 µg for newborns of HBsAg-negative mothers or 20 µg for newborns of HBsAg-positive mothers per injection.
It is recommended that adults should be vaccinated with three doses of 20 µg recombinant yeast hepatitis B vaccine or 20 µg recombinant CHO cell hepatitis B vaccine. For immunocompromised people or non-responders, the dose (e.g., 60 µg) and the number of injections should be increased; for those who do not respond to the three-dose series (0, 1, and 6 months), one additional dose of 60 µg or three doses of 20 µg hepatitis B vaccine could be administered. Serum anti-HBs should be tested 1–2 months after the second-round vaccination. If there was no response, another dose of 60 µg recombinant yeast hepatitis B vaccine should be administered. The protection conferred by vaccination with hepatitis B vaccine generally lasts for ≥30 years in responders.11 Therefore, anti-HBs monitoring or booster immunization is not required for the general population; however, anti-HBs should be monitored for high-risk groups or immunocompromised people. Another dose of the hepatitis B vaccine should be administered if anti-HBs levels were <10 mIU/mL.
Women who have not been infected with HBV can safely receive the hepatitis B vaccine during pregnancy.12,13 In addition to the standard vaccination procedure, the accelerated vaccination schedule (0, 1, and 2 months) has been proven to be feasible and efficacious.14
Accidentally-exposed people refer to those whose damaged skin or mucous membranes have encountered the blood or body fluids from HBsAg-positive or HBsAg-unknown sources, or who have been stabbed by needles that are contaminated by such blood or body fluids.
Management of the sources of infection
People who have been identified as HBsAg-positive for the first time should be reported to local disease control and prevention centers, as required, if they meet the reporting standards for infectious diseases. In addition, it is recommended that their family members are tested for serum HBsAg, anti-HBs, and anti-hepatitis B core (HBc). Susceptible people should be vaccinated against HBV. The infectivity of HBV-infected people is mainly determined by serum HBV DNA levels, instead of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin levels. It is recommended that HBV infection markers should be tested during health examinations and medical activities that do not involve school admission or job recruitment. The aim is to facilitate early diagnosis and early treatment and to mitigate the harm caused by HBV infection. For the follow-up of people with chronic HBV infection, refer to section XV, “Monitoring and follow-up management of people with chronic HBV infection.” Individuals with chronic HBV infection should avoid sharing dental appliances, razors, syringes, and blood collection needles. In addition, they are not allowed to donate blood, organs, or sperm, and it is suggested that they receive regular medical follow-ups. Their family members or sexual partners should be vaccinated against hepatitis B as soon as possible.
Blocking transmission routes
The promotion of safe injection (which includes blood collection needles and tools for acupuncture and moxibustion) is critical and standard precaution principles for hospital infection management should be followed. The equipment used in service industries, including hairdressing, shaving, pedicuring, puncturing, and tattooing, should be strictly disinfected. People whose sexual partners are HBsAg-positive should receive the HBV vaccine or use condoms. When the health condition of the sexual partner is unknown, condoms must be used to prevent HBV and other blood-borne or sexually transmitted diseases. For pregnant women who are HBsAg-positive, amniocentesis should be avoided whenever possible to ensure the placenta is intact and to minimize the chance of the newborn’s exposure to maternal blood.
Recommendation 1: Hepatitis B vaccination for newborns
Infants born to HBsAg-negative mothers should receive 10 µg of recombinant yeast hepatitis B vaccine as soon as possible within 12 h of birth, the second dose at 1 month, and the third dose at 6 months (A1).
Infants born to HBsAg-positive mothers should receive 100 IU of hepatitis B immunoglobulin (HBIG) and concurrent recombinant yeast HBV vaccine (10 µg) at different sites as soon as possible within 12 h of birth, followed by the second and third doses of HBV vaccine at 1 and 6 months, respectively. Infants born to HBsAg-positive mothers should be tested for HBsAg and anti-HBs 1–2 months after the third dose of hepatitis B vaccine. If HBsAg is negative and anti-HBs levels are <10 mIU/mL, three additional doses of hepatitis B vaccine can be given according to the 0, 1, and 6 months immunization program. If HBsAg is positive, it means that the vaccination has failed, and the infants should be monitored regularly (A1).
Premature infants and low-weight infants born to mothers with unknown HBsAg status should receive the first dose of hepatitis B vaccine and HBIG as soon as possible within 12 h of birth; after 1 month, three doses of hepatitis B vaccine can be administered according to the 0, 1 and 6 month immunization program (A1).
Newborns who are vaccinated with hepatitis B vaccine and HBIG within 12 h of birth can breastfeed from HBsAg-positive mothers (B1).
Recommendation 2: Catch-up vaccination should be administered for children who have not been vaccinated with the hepatitis B vaccine or who have not completed the full vaccination series. The interval between the first dose and the second dose should be ≥28 days, and the interval between the second dose and the third dose should be ≥60 days (A1).
Recommendation 3: For immunocompromised or nonresponsive adults, the vaccination dose (e.g., 60 µg) and the number of doses should be increased; for those who do not respond to the three-dose procedure (0, 1, and 6 months), one additional dose of 60 µg or three doses of 20 µg hepatitis B vaccine should be administered, and serum anti-HBs should be tested 1–2 months after the second-round vaccination. If there is still no response, another dose of 60 µg recombinant yeast hepatitis B vaccine should be administered (A1).
Recommendation 4: People who are accidentally exposed to HBV should be treated as follows:
Gently squeeze around the wound to drain blood from the wound, rinse the wound with 0.9% NaCl solution, and then treat the wound with disinfectant (A1).
HBV DNA and HBsAg should be tested immediately and rechecked after 3–6 months (A1).
No treatment is required for those who have been vaccinated with hepatitis B vaccine and are known to be positive for anti-HBs (≥10 mIU/mL). For those who have not been vaccinated against hepatitis B or those who have been vaccinated against HBV but have anti-HBs levels <10 mIU/mL or unknown anti-HBs levels, 200–400 IU HBIG and concurrent hepatitis B vaccine (20 µg) should be administered immediately at different sites, followed by second and third doses of hepatitis B vaccine (20 µg) 1 and 6 months later (A1).
Recommendation 5: HBsAg, anti-HBc, and anti-HBs should be screened for during health examinations or medical treatments that do not involve school admission or job recruitment. HBsAg, anti-HBc, and anti-HBs should be screened in high-risk groups, pregnant women, and patients who receive antitumor treatment (chemotherapy or radiotherapy) or immunosuppressive agents or direct-acting antiviral (DAA) therapy for HCV, and in HIV-infected people. Hepatitis B vaccination is recommended for those who are negative for all the three HBV markers (B1).
Etiology
HBV belongs to the Hepapadnaviridae family. It is an enveloped DNA virus with a genome length of approximately 3.2 kb and contains a partially double-stranded circular DNA. Its genome encodes HBsAg, HBc antigen (HBcAg), hepatitis B e antigen (HBeAg), viral polymerase, and HBV X protein (HBx). HBV is highly resistant but can be inactivated at 65°C for 10 h, at 100°C for 10 min, or by high-pressure steam. In addition, HBV can effectively be inactivated by ethylene oxide, glutaraldehyde, peroxyacetic acid, and iodophors.
HBV enters liver cells by binding to sodium-taurocholate cotransporting polypeptide (commonly referred to as NTCP) on the liver cell membrane.15 After HBV invasion of liver cells, the partially double-stranded circular DNA uses the negative strand as a template and extends the positive strand into the cell nucleus to repair the nick in the positive strand, which forms a covalently closed circular DNA (cccDNA). cccDNA plays an important role in chronic infection because it has a long half-life and is difficult to completely remove from the body. HBV can integrate into the host genome. HBV uses cccDNA as a template to transcribe viral mRNAs with different lengths, which include the 3.5 kb pregenomic RNA (commonly referred to as pgRNA) that can be released into the peripheral blood. Serum HBV RNA levels can reflect the activity of cccDNA in liver tissue and might be related to virological responses and the prognosis of patients.16–18 HBV can be divided into at least nine genotypes, from A to I;19 genotypes B and C are the most prevalent in China. The incidence of mother-to-child transmission in HBV patients with type B and C was higher than that of other genotypes, and genotype C is associated with earlier progression to HCC. HBV genotypes are associated with disease progression and responses to interferon-α (IFN-α) therapy.20–22 The response rate of HBeAg-positive patients to IFN-α treatment was higher in type B than in type C and higher in type A than in type D.23
Natural history and pathogenesis
Natural history
The natural history of HBV infection depends mainly on the interaction between the virus and the host. The age when HBV infection is acquired is one of the most critical factors that determine whether the HBV infection will become chronic. The risk of chronic HBV infection in newborns and infants aged <1 year is 90%.24 Most people with HBV infection in China are infected during the perinatal period or infancy. The world has achieved significant success in blocking HBV mother-to-child transmission.25 A universal immunization program that combines hepatitis B vaccine and HBIG has been adopted for newborns of HBsAg-positive mothers in China; however, approximately 5–7% of newborns are still infected with HBV due to mother-to-child transmission. This occurs in 7–11% of HBeAg-positive pregnant women and 0–1% in HBeAg-negative pregnant women.26,27
In general, the natural history of chronic HBV infection is divided into four phases based on its natural progression,28–30 namely the immune tolerance phase (chronic HBV carrier state), immune clearance phase (HBeAg-positive CHB), immune control phase (inactive HBsAg carrier state), and reactivation phase (HBeAg-negative CHB) (Table 2). For more details, refer to section IX, “Clinical Diagnosis”. Not all patients with chronic HBV infection will experience all four phases. Patients who are infected with HBV during adolescence and adulthood usually experience no immune tolerance phase and directly enter the immune clearance phase.
Table 2. Stages of chronic HBV infection.
| Markers |
Immune tolerance phase (chronic HBV carrier status) |
Immune clearance phase (HBeAg-positive CHB) |
Immune control phase (inactive HBsAg carrier status) |
Reactivation phase (HBeAg-negative CHB) |
|---|---|---|---|---|
| HBV serological markers | ||||
| HBsAg (IU/mL) | >1×104 | + | <1×103 | + |
| anti-HBs | − | − | − | − |
| HBeAg | + | + | − | +/− |
| anti-HBe | − | − | + | +/− |
| anti-HBc | + | + | + | + |
| HBV DNA (IU/mL) | >2×107 | >2×104 | <2×103 | ≥2×103 |
| ALT | Normal | Persistent or recurrent increase | Normal | Persistent or recurrent increase |
| Liver pathology | No obvious necroinflammation or fibrosis | Obvious necroinflammation, or fibrosis, or both | No or mild inflammation, with varying degrees of fibrosis | Obvious necroinflammation, or fibrosis, or both |
CHB, chronic hepatitis B; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen.
Spontaneous HBeAg seroconversion might occur during the immune clearance phase, with an annual incidence of 2–15%. Patients with age <40 years, elevated ALT, HBV genotype A and genotype B had higher incidence.28,31 After HBeAg seroconversion, the HBsAg clearance rate was 0.5–1.0% annually.32 Ten years after HBsAg disappeared, cccDNA was still detectable in the liver of approximately 14% of these patients.33 Patients with either age >50 years, cirrhosis, or concomitant HCV or hepatitis D virus (HDV) infections can still progress to HCC even if HBsAg has disappeared; however, the incidence rate is low.34
The annual incidence of cirrhosis in CHB patients without antiviral therapy is 2–10%,35 and risk factors include host (older age, male, age >40 years when HBeAg seroconversion occurs, and persistently elevated ALT levels36,37), the virus (HBV DNA >2,000 IU/mL), persistently positive HBeAg status,38 genotype C, coinfection with HCV, HDV, or HIV, and other liver injury-inducing factors (e.g., alcohol or obesity).35 The annual incidence of decompensated cirrhosis from compensated cirrhosis is 3–5%, and the 5-year survival rate of the patients with decompensated cirrhosis is 14–35%.35 The annual incidence of HCC in patients with HBV infection without cirrhosis is 0.5–1.0%.35 The annual incidence of HCC in patients with cirrhosis is 3–6%.39–41 Also, cirrhosis, diabetes, immediate relatives with HCC, high serum HBsAg levels, and exposure to aflatoxin are all associated with a high incidence of HCC.35,42–46 Lower HBsAg levels often suggest that hosts have exerted good immune control over HBV replication and infection. Studies have shown that even if HBeAg is negative and HBV DNA levels are low, patients with higher HBsAg levels (≥1,000 IU/mL) are still at a higher risk of HCC, regardless of genotype B or C.45,46
Pathogenesis
The pathogenesis of chronic HBV infection is complicated and is not fully understood. HBV does not directly kill liver cells. The immune response caused by the virus is the main mechanism that leads to liver cell damage and necroinflammation. Persistent or recurrent necroinflammation is an important factor for patients with chronic HBV infection progressing to cirrhosis and even HCC.
The nonspecific (innate) immune response plays an important role in the initial stages of HBV infection and initiates the subsequent specific (adaptive) immune response.47,48 HBV uses its proteins, such as HBeAg and HBx, to interfere with antiviral signaling pathways that involve Toll-like receptors and retinoic acid-inducible gene I-like receptors; therefore, the level of the nonspecific immune response is suppressed. CHB patients often present with decreased frequencies of myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) in the peripheral blood. The ability of mDCs to mature and the ability of pDCs to produce IFN-α are significantly impaired, which make it more difficult for the body to eliminate the virus and induce HBV-specific T lymphocytes, which impedes viral elimination.
The HBV-specific immune response plays a leading role in HBV clearance.49 Major histocompatibility complex class I-restricted CD8+ cytotoxic T lymphocytes can induce the apoptosis of infected hepatocytes and can secrete IFN-γ and suppress the expression and replication of HBV genes in liver cells in a noncytolytic fashion.50 During chronic infection, HBV-specific T cells are susceptible to apoptosis. Their ability to produce cytokines and proliferate is significantly impaired, and their functions become exhausted, which might be one of the mechanisms that lead to persistent HBV infection.51 Currently, it is believed that there are large amounts of HBsAg in serum and liver tissue, and the lack, or dysfunction, or both of HBsAg-specific cytotoxic T lymphocytes is an important cause of immune tolerance in patients with chronic HBV infection.52
Laboratory tests
Serological testing for HBV
Traditional serum markers for HBV include HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc, and anti-HBc immunoglobulin (Ig)M. Serum HBsAg can be produced by cccDNA-derived mRNA or an HBV DNA sequence that is integrated into the human genome. HBsAg positivity indicates HBV infection. Anti-HBs is a protective antibody, and anti-HBs positivity indicates immunity to HBV, which can be seen in people with a resolved hepatitis B infection or who have been inoculated with the hepatitis B vaccine. Anti-HBc-IgM positivity is mostly found in patients with acute hepatitis B. Anti-HBc-IgM is usually mildly positive in patients with reactivation of chronic HBV infection. The main subtype of anti-HBc is IgG. The IgG subtype is positive in most HBV cases, whether the virus is eliminated or not.
Recently, the quantitative detection of HBsAg has been widely used in clinical practice. Its level is indicative of the stage of the disease and the risk of disease progression. In addition, it can be used to guide the use of recombinant human IFN and peginterferon-α (peg-IFN-α).
Virological testing for HBV
HBV DNA quantification
This is mainly used to assess the level of virus replication in HBV-infected patients. In addition, it can be used as a crucial component to select the indications and assess the efficacy of antiviral therapy. During antiviral therapy, obtaining a sustained virological response could significantly control the progression of cirrhosis and lower the risk of HCC.53,54 The quantitative detection of HBV DNA utilizes real-time quantitative polymerase chain reaction; however, the detection limit varies between manufacturers’ reagents.
HBV genotyping
To date, at least nine HBV genotypes (A to I) and one undefined genotype (J) have been identified. Some genotypes are further divided into subtypes. The detection of HBV genotypes could help to predict the efficacy of IFN and determine the disease prognosis.55–58
Detection of resistant mutants
HBV is a highly mutable virus. During reverse transcription and replication, a mutation might occur to one or more nucleotides during replication, due to the lack of proofreading ability in RNA polymerase and reverse transcriptase. HBV can naturally mutate during chronic persistent infection. Viral mutation can be induced by antiviral treatment. In both cases, mutated HBV might become less susceptible to antiviral drugs.59 The appropriate detection of drug-resistant mutant strains could help clinicians to identify drug resistance and adjust the treatment plan as required. Commonly used clinical tests for drug resistance include gene sequencing that uses reverse transcriptase and reverse hybridization-based line probe assays (INNO-LIPA kit).
Detection of new HBV markers
Anti-HBc quantification (qAnti-HBc)
A novel double-antigen sandwich enzyme-linked immunosorbent assay (commonly known as ELISA) can quantitatively determine serum anti-HBc levels. In natural history studies, the quantitative (q)anti-HBc levels in patients during the immune clearance and reactivation phases were significantly higher than those in the immune tolerance and low replication phases.60,61 The baseline qAnti-HBc levels of HBeAg-positive CHB patients could predict the efficacy of peg-IFN-α and nucleos(t)ide analogues (NAs).62,63 In addition, qAnti-HBc levels are strongly correlated with ALT levels and are associated with the degree of necroinflammation in liver tissue in patients with normal ALT levels.64
HBV RNA quantification
HBV RNA levels are related to the transcriptional activity of cccDNA in liver cells, and further study is required to assess the risk of recurrence after withdrawal of NAs.65,66 Its use is limited, because the detection methods used by different research teams are different.
Hepatitis B core-related antigen (HBcrAg)
This is a composite marker that contains HBcAg, HBeAg, and p22. It is related to the transcriptional activity of cccDNA in liver cells. Studies have examined its usefulness to determine disease progression, predict the antiviral efficacy of peg-IFN-α and NAs, and assess the risk of HBV recurrence and HCC development with the discontinuation of treatment.67–70
Serum biochemical testing71
ALT and AST
Serum ALT and AST levels can partially reflect the degree of liver cell injury. An increase in ALT levels in patients with long-term virus suppression requires further tests to identify the possible causes.72
Total bilirubin
This is related to the production, uptake, metabolism, and excretion of bilirubin. Bilirubin elevation is caused by liver cell damage, intrahepatic and extrahepatic biliary obstruction, abnormal bilirubin metabolism, and hemolysis. In patients with liver failure, total bilirubin levels might be >171 µmol/L or could increase by >7.1 µmol/L daily.
Serum albumin
This reflects the synthetic functions of the liver. Patients with cirrhosis and liver failure present with decreased serum albumin levels. In addition, serum albumin levels are affected by nutritional status.
Prothrombin activity time (PT), prothrombin activity (PTA), and international normalized ratio
These indicators reflect the synthetic functions of coagulation factors in the liver and are valuable to predict disease progression and prognosis.
Serum γ-glutamyl transferase (GGT)
The serum GGT in healthy people mainly comes from the liver. This enzyme is significantly increased in alcoholic liver disease, drug-induced liver disease, cholangitis, and intrahepatic and extrahepatic cholestasis.
Serum alkaline phosphatase (ALP)
This is not a liver-specific indicator, although cholestasis can stimulate ALP synthesis. If ALP levels are elevated, elevated GGT or ALP isoenzyme levels are required to confirm whether ALP elevation has occurred in the liver. The dynamic changes in ALP levels are often used clinically to assess disease progression, prognosis, and efficacy.
Alpha-fetoprotein (AFP) and its isoform AFP-L3
AFP is an important indicator for the diagnosis of HCC. The magnitude and dynamic changes in AFP increase, and its correlation with the increase and decrease of ALT and AST levels require special attention. In addition, clinical manifestations and liver imaging examinations need to be carried out to offer a comprehensive analysis.73
Protein induced by vitamin K absence or antagonist-II (PIVKA-II)
This is known as des-γ-carboxy prothrombin (DCP) and it is another important indicator for the diagnosis of HCC, and can be used to complement AFP.74
Noninvasive tests for liver fibrosis
AST to platelet ratio index (APRI)
APRI is an index that has been developed based on the data from chronic HCV infections to assess the degree of HCV-related liver fibrosis. The formula for APRI is as follows: [upper limit of normal (ULN) of AST/AST×100]/platelet count (×109/L). An APRI score ≥2 indicates the presence of cirrhosis and an APRI score <1 suggests the absence of cirrhosis in adults. However, recent studies have suggested that this index is less accurate to evaluate the degree of HBV-related liver fibrosis.75–77
Fibrosis 4 (FIB-4) score
FIB-4 was developed based on the data from chronic HCV infections and is used to assess the degree of HCV-related liver fibrosis. The formula for FIB-4 is as follows: age (years)×AST (IU/L)/[platelet count (×109/L)×√ALT (IU/L)]. A FIB-4 value ≥3.25 or a Metavir score ≥F3 suggest the presence of liver fibrosis; a FIB-4 value <1.45 suggests the absence of liver fibrosis. Recent studies have shown that an FIB-4 value ≥0.25 has a 97% specificity for the diagnosis of fibrosis in people with chronic HBV infection,76 and an FIB-4 value ≤0.70 has a negative predictive value of 96% for the exclusion of hepatitis B cirrhosis in people aged >30 years.78
Other indicators
Extracellular matrix components, such as hyaluronic acid, type III procollagen peptide, type IV collagen, and laminin, can indicate the presence of liver fibrosis, but no generally accepted cutoffs are available for clinical application. GGT to platelet ratio (GPR) [=GGT/GGT ULN/platelet count (×109/L)×100] and red cell distribution width-platelet ratio (RPR) [=red cell distribution width (%)/platelet count (×109/L)] are composed of routine lab indicators, but stable diagnostic cutoffs need to be defined.79,80 Serum Golgi glycoprotein 73 (commonly referred to as GP73) used in combination with AST and GGT levels can reflect moderate to severe liver inflammation.81 Serum chitinase 3-like protein 1 (commonly referred to as CHI3L1 or YKL-40) can predict moderate to severe liver fibrosis in patients with normal or mildly elevated ALT levels.82,83
Liver stiffness measurement
Liver stiffness can be measured using transient elastography (TE), ultrasound-based acoustic radiation force impulse (ARFI), and magnetic resonance elastography (MRE). ARFI includes two techniques, namely point shear wave elastography (p-SWE) and 2D shear wave elastography (2D-SWE). ARFI and MRE are still undergoing clinical research.
TE has been approved for use in the USA, Europe, and the Asia-Pacific region. It can accurately identify advanced liver fibrosis and early cirrhosis,84,85 but its results are susceptible to liver necroinflammation, cholestasis, and severe steatosis. TE results should be interpreted in combination with ALT and bilirubin levels.86–88 The combined use of TE and other serological indicators could improve diagnostic accuracy.84,89–91 A multicenter study carried out by Chinese researchers suggested a cutoff of 21.3 kPa for the diagnosis of hepatitis B cirrhosis (specificity: 95%; positive likelihood ratio: 8.5), a cutoff of 12.4 kPa for the diagnosis of advanced liver fibrosis (specificity: 95%; positive likelihood ratio: 11.8), a cutoff of 9.1 kPa for diagnosis of significant liver fibrosis (specificity: 95%; positive likelihood ratio: 6.4); a cutoff value of 8.2 kPa for the exclusion of cirrhosis (sensitivity: 95%; positive likelihood ratio: 0.07), and a cutoff value of 5.8 kPa for the exclusion of advanced liver fibrosis (sensitivity: 95%; positive likelihood ratio: 0.10).92 For guidance on the clinical application of TE, see “Expert consensus on the use of transient elastography for diagnosing liver fibrosis (2018 update).”85
Imaging diagnosis
The aim of imaging examination in patients with chronic HBV infection is to monitor clinical progression by the identification of signs of cirrhosis and portal hypertension, and to detect and differentiate space-occupying lesions to make an early diagnosis of HCC.93,94
Abdominal US
Abdominal US is the most commonly used liver imaging technique, because it offers a noninvasive, inexpensive, real-time imaging solution that is easy to repeat. US can demonstrate the size, shape, and parenchymal echogenicity of the liver and spleen, and can determine the calibrator and blood flow of the portal, splenic, and hepatic veins. In addition, it can identify the presence and severity of ascites which suggests the presence of cirrhosis and portal hypertension. Regular US surveillance is essential for the identification of early HCC. Contrast-enhanced ultrasonography can be used to better differentiate the nature of space-occupying lesions. The downside of US is that the image quality and results are susceptible to several factors, such as equipment performance, gas in the gastrointestinal tract, and the operator’s skills.
Computerized tomography (CT)
In patients with CHB, CT is mainly used to investigate the imaging changes of the liver to determine the presence of cirrhosis and portal hypertension and identify/differentiate the space-occupying lesions. Contrast-enhanced multiphase CT scanning has a high sensitivity and specificity for the diagnosis of HCC.
Magnetic resonance imaging (MRI)
MRI is a preferred imaging modality for the liver due to its avoidance of radiation exposure. In general, MRI with enhanced multiphase scans and with hepatobiliary-specific contrast agents is outperformed to contrast-enhanced CT in differentiating benign from malignant space-occupying lesions in the liver.
Pathological diagnosis
The aim of liver biopsy in people with chronic HBV infection is to evaluate the degree of necroinflammation and fibrosis, to determine the presence or absence of cirrhosis, to exclude other liver diseases, thereby providing information on the diagnosis, prognosis, and efficacy assessment.
The main pathological features of CHB are portal and periportal necroinflammation and fibrosis. The inflammatory cells infiltrating the portal area are predominantly lymphocytes with a few plasma cells and macrophages. The aggregation of inflammatory cells often destroys the limiting plate and leads to interface hepatitis (previously called piecemeal necrosis). Degeneration, necrosis (e.g., spotted, bridging, and confluent necrosis) and apoptosis of hepatocytes can be seen in the lobules. Ground glass hepatocytes and apoptotic bodies can be formed by apoptotic hepatocytes, which is proportional to inflammation activity. Chronic necroinflammation in the liver can cause the excessive deposition of extracellular matrix, especially collagen, which results in fibrosis that is manifested by varying degrees of portal fibrous expansion and fibrous septum formation. Masson’s trichromatic staining and reticulin staining can help to determine the degree of liver fibrosis and lobular disarray. Then, cirrhosis develops, which is, by definition, the combination of diffuse fibrosis and regenerative nodules (pseudolobules). In addition, immunohistochemical staining for HBsAg and HBcAg as well as in situ hybridization or PCR for HBV DNA or cccDNA in liver tissue can be exploited.
The scoring systems developed by Knodell, Scheuer, Metavir, or Ishak are widely used to grade hepatic necroinflammation and stage fibrosis in persons with chronic HBV infection.95–98 The Laennec system further subclassifies Metavir stage 4 (cirrhosis) into stages 4A, 4B, and 4C, based on the size of the regenerative nodules and the thickness of fibrous septa.99 In addition, Chinese researchers have proposed a histopathological grading and staging system for viral hepatitis B.100 Comparisons of the various grading and staging systems are shown in Tables 3 and 4.
Table 3. Comparisons of major grading standards for liver inflammation.
| Score | Knodell’s scoring system |
Score | Scheuer’s scoring system |
|||
|---|---|---|---|---|---|---|
| Periportal inflammation with or without bridging necrosis | Intralobular degeneration and focal necrosis | Portal inflammation | Portal/periportal activity | Intralobular activity | ||
| 0 | None | None | None | 0 | None or minimal | None |
| 1 | Mild piecemeal necrosis | Mild (acidophilic bodies, ballooning degeneration or scattered foci, or both of hepatocellular necrosis in <⅓ of lobules or nodules) | Mild (a few inflammatory cells in <⅓ of portal tracts) | 1 | Portal inflammation alone | Inflammation but no necrosis |
| 3 | Moderate piecemeal necrosis (involving <50% of the circumference of most portal tracts) | Moderate (involving ⅓–⅓ of lobules or nodules) | Moderate (increased inflammatory cells in ⅓–⅔ of portal tracts) | 2 | Mild piecemeal necrosis | Focal necrosis or acidophilic bodies |
| 4 | Marked piecemeal necrosis (involving >50% of the circumference of most portal tracts) | Marked (involving >⅔ of lobules or nodules) | Marked (dense inflammatory cells in >⅔ of portal tracts) | 3 | Moderate piecemeal necrosis | Marked focal cell damage |
| 5 | Moderate piecemeal necrosis plus bridging necrosis | – | – | 4 | Marked piecemeal necrosis | Damage includes bridging necrosis |
| 6 | Marked piecemeal necrosis plus bridging necrosis | – | – | |||
| 10 | Multilobular necrosis | – | – | |||
| Score | Wang Tailing’s scoring system |
Score | Ishak’s scoring system |
||||
|---|---|---|---|---|---|---|---|
| Portal/periportal area | Intralobular area | Portal inflammation | Periportal or periseptal interface inflammation | Focal necrosis, apoptosis, or focal inflammation | Confluent necrosis | ||
| 0 | No inflammation | No inflammation | 0 | None | None | None | None |
| 1 | Portal inflammation | Degeneration and a few necrotic foci | 1 | Mild, involving some or all portal areas | Mild (focal, involving few portal areas) | ≤1 foci per 10× objective | Focal confluent necrosis |
| 2 | Mild piecemeal necrosis | Degeneration, spotted or focal necrosis or acidophilic bodies | 2 | Marked, involving some or all portal areas | Mild/moderate (focal, involving most portal areas) | 2–4 foci per 10× objective | Zone 3 necrosis in some areas |
| 3 | Moderate piecemeal necrosis | Degeneration, severe necrosis, or bridging necrosis | 3 | Moderate to severe, involving all portal areas | Moderate (continuous around <50% of tracts or septa) | 5–10 foci per 10× objective | Zone 3 necrosis in most areas |
| 4 | Marked piecemeal necrosis | Bridging necrosis involving multiple lobules | 4 | Severe, involving all portal areas | Severe (continuous around >50% of tracts or septa) | >10 foci per 10× objective | Zone 3 necrosis and occasional portal-central bridging necrosis |
| 5 | – | – | – | Zone 3 necrosis and multiple portal-central bridging necrosis | |||
| 6 | – | – | – | Panacinar or multiacinar necrosis | |||
–, not applicable.
Table 4. Comparisons of major staging standards for liver fibrosis.
| Knodell’s scoring system | Scheuer’s scoring system | Metavir’s scoring system | Wang Tailing’s scoring system | Ishak’s scoring system | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | No fibrosis | 0 | No fibrosis | 0 | No fibrosis | 0 | No fibrosis | 0 | No fibrosis |
| 1 | Fibrous expansion of portal areas | 1 | Fibrous expansion of portal areas | 1 | Fibrous expansion of portal areas, without fibrous septa | 1 | Fibrous expansion of portal areas | 1 | Fibrous expansion of some portal areas, with or without short fibrous septa |
| 2 | – | 2 | Periportal fibrosis or portal-portal fibrous septa but intact architecture | 2 | Fibrous expansion of portal areas, with few fibrous septa | 2 | Periportal fibrosis, with fibrous septa but intact architecture | 2 | Fibrous expansion of most portal areas, with or without short fibrous septa |
| 3 | Marked bridging (portal-portal as well as portal-central) | 3 | Fibrosis with architectural distortion but no obvious cirrhosis | 3 | Numerous septa, with architectural distortion, but no cirrhosis | 3 | Fibrous septa with architectural distortion but no obvious cirrhosis | 3 | Fibrous expansion of most portal areas with occasional portal-portal bridging |
| 4 | Cirrhosis | 4 | Probable or definite cirrhosis | 4 | Cirrhosis | 4 | Early or definite cirrhosis | 4 | Fibrous expansion of most portal areas with marked bridging (portal-portal as well as portal-central ) |
| 5 | Marked bridging (portal-portal, or portal-central, or both) with occasional nodules (incomplete cirrhosis) | ||||||||
| 6 | Probable or definite cirrhosis | ||||||||
–, not applicable.
Computer image analysis can be used to determine the collagen proportional area of the stained sections of liver tissue. Quantitative assessment of liver fibrosis (qFibrosis) based on two-photon second harmonic generation can automatically measure the collagen area and the morphological features in unstained liver tissue sections, with high reproducibility and accuracy.101 Chinese researchers recently developed a P-I-R classification for liver fibrosis. Based on the width and shape of fibrous septa, this system qualitatively subdivides fibrosis with an Ishak score ≥3 into predominantly progressive (P), intermediate (I), and predominantly regressive (R) liver fibrosis. Therefore, the merit of this classification is to judge the dynamic trend of liver fibrosis evolution.102
Clinical diagnosis
According to the results of serological, virological, biochemical, imaging, and pathological tests as well as other auxiliary examinations, chronic HBV infection can be classified into:
Chronic HBV carrier state
This is also known as HBeAg-positive chronic HBV infection.103,104 Patients are in the immune tolerance phase and are generally younger. They are positive for HBeAg, with high levels of HBV DNA (usually >2×107 IU/mL) and serum HBsAg (usually >1×104 IU/mL). However, serum ALT and AST levels are persistently normal (three follow-ups within 1 year, ≥3 months apart), and liver biopsy shows no obvious necroinflammation or fibrosis. In the absence of liver biopsy, factors such as age, HBV DNA level, HBsAg level, noninvasive liver fibrosis tests, and imaging examination should be considered to help make a diagnosis.
HBeAg-positive CHB
Patients are in the immune clearance phase and are seropositive for HBsAg and HBeAg. HBV DNA levels are high (usually >2×104 IU/mL). ALT levels are persistently or intermittently abnormal, or liver biopsy reveals obvious necroinflammation, or fibrosis, or both (≥G2/S2).
Inactive HBsAg carrier state105,106
This is also known as HBeAg-negative chronic HBV infection. Patients are in the immune control phase. They are positive for HBsAg and anti-HBe and negative for HBeAg. HBV DNA levels are <2,000 IU/mL, and HBsAg levels are <1,000 IU/mL. ALT and AST levels are persistently normal (three follow-ups within 1 year, ≥3 months apart). Imaging examination shows no signs of cirrhosis. Liver biopsy shows a histological activity index score <4, or lesions are mild using other semi-quantitative scoring systems.
HBeAg-negative CHB
This is the reactivation phase. Patients are positive for HBsAg and persistently negative for HBeAg, which is often accompanied by anti-HBe positivity. HBV DNA levels are usually ≥2,000 IU/mL. ALT levels are persistently or intermittently abnormal, or liver biopsies show obvious necroinflammation, or fibrosis, or both (≥G2/S2).
Occult HBV infection (OBI)107
Patients are negative for HBsAg in serum but positive for HBV DNA in serum or liver tissue, or both. A total of 80% of OBI patients might be seropositive for anti-HBs, and anti-HBe or anti-HBc, or both, which is designated as seropositive OBI; however, 1–20% of OBI patients are seronegative for all serological indicators, which is designated as seronegative OBI. Its mechanism has not been determined. One possible explanation is that HBsAg disappears after apparent (acute or chronic) HBV infection, and HBV DNA levels are usually very low in the serum or liver tissue, without obvious liver tissue damage. Another possibility is mutations in the S gene region of HBV, which makes HBsAg undetectable by currently available commercial kits. Serum HBV DNA levels are usually high, which might be accompanied by significant liver histopathological changes. These patients can transmit HBV to recipients through blood transfusion or organ transplantation and might experience reactivation of HBV if they are immunosuppressed.
Hepatitis B cirrhosis108,109
The diagnosis of hepatitis B cirrhosis should meet the first and the second criteria of the following pathological diagnosis or the first and the third criteria of the following clinical diagnosis.
Patients are HBsAg-positive, or HBsAg-negative, anti-HBc positive and have a clear history of chronic HBV infection (presence of HBsAg >6 months), and other causes have been excluded.
Liver biopsy suggests cirrhosis.
Patients meet ≥2 of the following five criteria, and noncirrhotic portal hypertension has been excluded: (1) imaging examination shows signs of cirrhosis, or portal hypertension, or both; (2) endoscopy shows esophagogastric varices; (3) liver stiffness measurements suggest cirrhosis; (4) blood biochemical tests show reduced albumin levels (<35 g/L), or prolonged PT, or both (>3 s longer than controls); and (5) routine blood tests show platelet count <100×109/L.
Clinically, cirrhosis is divided into the compensated stage and decompensated stage based on the presence or absence of serious complications, such as ascites, esophagogastric variceal bleeding, and hepatic encephalopathy: (1) compensated cirrhosis; patients who are pathologically or clinically diagnosed with cirrhosis but have never had serious complications, such as ascites, esophagogastric variceal bleeding, or hepatic encephalopathy, could be diagnosed as having compensated cirrhosis and most of them have Child-Pugh A liver function; and (2) decompensated cirrhosis; if patients with cirrhosis develop serious complications, such as ascites, esophagogastric variceal bleeding, or hepatic encephalopathy, they are diagnosed as decompensated cirrhosis.110 Most of them have Child-Pugh B or C liver function.
Recently, to more accurately predict the progression, death risk, or treatment effect of patients with cirrhosis, some researchers have suggested dividing cirrhosis into five stages,111 between which stages 1 and 2 are compensated cirrhosis, and stages 3–5 are decompensated cirrhosis: stage 1, no varicose veins or ascites; stage 2, varicose veins, no bleeding or ascites; stage 3, ascites, no bleeding, with or without varicose veins; Stage 4, bleeding, with or without ascites; and stage 5, sepsis.
Due to the advances in antiviral therapy, many patients with decompensated cirrhosis could be reversed to compensated cirrhosis following treatment. The reversal is characterized by improved liver cell function, such as higher albumin levels, shorter PT than before, the disappearance of serious complications, such as ascites and hepatic encephalopathy, and long-term survival without liver transplantation. This phenomenon is called recompensation of cirrhosis; however, there is no accurate definition and uniform diagnostic criteria for this concept.
Treatment goals
Treatment goals are to maximize long-term inhibition of HBV replication,6,112,113 alleviate the degree of hepatocyte inflammation, necrosis, and hyperplasia of liver fibrous tissue, delay and reduce the occurrence of liver failure, decompensation of liver cirrhosis, HCC and other complications, and to improve the quality of patients’ life as well as prolong their survival time. For eligible patients, the therapy aims are “clinical cure”.
Clinical cure (or functional cure):6,112,114–116 HBsAg remains negative (with or without the presence of anti-HBs), HBV DNA is undetectable, and liver biochemical indicators are normal following withdrawal of the treatment. However, because cccDNA remains in the nuclei of liver cells, patients are still at risk of HBV reactivation and HCC development.
Indications for antiviral therapy
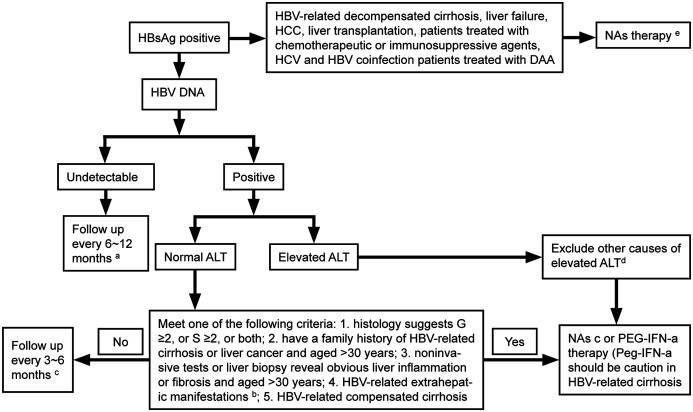
Patients should be assessed for the risk of disease progression to determine whether to start antiviral therapy based on a comprehensive analysis of serum HBV DNA levels, ALT levels, the severity of liver disease, as well as their age, family history, and concomitant diseases.6,112,113 Dynamic assessment is more meaningful than a single test (Fig. 1).
Fig. 1. Flow chart of antiviral therapy for patients with chronic HBV infection.
(a) Follow-up tests: virological test, liver biochemical test, AFP test, PIVKA-II test, abdominal ultrasound, and liver stiffness measurement. (b) HBV-related extrahepatic manifestations: glomerulonephritis and vasculitis. (c) Follow-up criteria for patients with HBV-related decompensated cirrhosis during NA treatment: perform a routine blood test, liver biochemical test, renal function test, virological test, and test for blood ammonia, AFP, and PIVKA-II, and abdominal ultrasound every 3 months. If necessary, perform enhanced CT or MRI. (d) Other causes of ALT elevation: infection by other pathogens, history of drug or poison use, history of alcohol use, lipid metabolism disorders, autoimmune disorders, liver congestion or vascular diseases, inherited metabolic liver disease, systemic diseases. (e) NAs-ETV, TDF, TAF. HbsAg, hepatitis B surface antigen; HBV, hepatitis B virus; ALT, alanine aminotransferase; HCC, hepatocellular carcinoma; NAs, nucleos(t)ide analogs; peg-IFN-α, pegylated interferon-α.
For patients with chronic HBV infection with positivity for serum HBV DNA, antiviral therapy is indicated if their ALT levels are persistently abnormal (>ULN) and other causes of ALT elevation have been excluded.
Other causes of ALT elevation include infection by other pathogens, drug-induced liver injury, alcoholic hepatitis, nonalcoholic steatohepatitis, autoimmune liver disease, and systemic diseases that involve the liver. In addition, the possibility of drug-induced temporarily normal ALT levels should be ruled out.
For patients with clear evidence of cirrhosis, antiviral treatment should be initiated if HBV DNA can be detected, regardless of ALT levels and HBeAg status. For patients with decompensated cirrhosis, antiviral therapy is indicated if HBV DNA is undetectable but HBsAg is positive.
Patients with seropositive HBV DNA and normal ALT levels are at a high risk of disease progression and antiviral therapy is indicated if they meet any of the following criteria: (1) obvious liver inflammation on liver biopsy (≥G2) or fibrosis (≥S2); (2) persistently normal ALT levels (tested every 3 months for 12 months) but with a family history of cirrhosis or liver cancer, and >30 years of age; (3) persistently normal ALT levels (tested every 3 months for 12 months), no family history of cirrhosis or liver cancer, but >30 years of age with noninvasive tests for liver fibrosis or liver biopsy revealing obvious liver inflammation or fibrosis; a (4) HBV-related extrahepatic manifestations (e.g., glomerulonephritis, vasculitis, polyarteritis nodosa, and peripheral neuropathy).
Recommendation 6: If serum HBV DNA is positive, ALT levels are persistently abnormal (>ULN), and other causes have been excluded, antiviral therapy is indicated (B1).
Recommendation 7: Antiviral therapy is indicated for HBV-related compensated cirrhosis patients with positive serum HBV DNA and HBV-related decompensated cirrhosis patients with positive HBsAg (A1).
Recommendation 8: If patients are seropositive for HBV DNA with normal ALT levels, antiviral therapy is indicated when they meet any one of the following criteria: (1) liver biopsy suggests obvious inflammation, or fibrosis, or both (G ≥2, or S ≥2, or both) (A1); (2) a family history of HBV-related cirrhosis or liver cancer and age >30 years-old (B1); (3) noninvasive tests or liver biopsy revealing obvious liver inflammation or fibrosis in those with persistently normal ALT levels and age >30 years-old (A1); and (4) HBV-related extrahepatic manifestations (e.g., HBV-related glomerulonephritis) (B1).
NAs treatment
Efficacy and safety of NAs
Entecavir (ETV)
Numerous studies have shown that the use of ETV is safe and highly effective to suppress virus replication and reduce liver inflammation.117–119 Long-term treatment with ETV can improve histological changes in patients with cirrhosis,120,121 significantly reduce the incidence of cirrhosis-related complications and HCC, and reduce liver-related and all-cause mortality.53,122 The 5-year cumulative probability of ETV resistance was 1.2% in treatment-naïve patients with CHB but increased to 51% in CHB patients who were resistant to lamivudine (LAM).123
Tenofovir disoproxil fumarate (TDF)
Multicenter clinical studies of TDF treatment for CHB patients have shown that it can strongly inhibit virus replication and has low rates of resistance.124,125 A study of TDF usage for 8 years showed that there were 41 cases of virological breakthroughs, 29 cases (70%) of which were due to poor compliance. In total, 59% of patients with virological breakthroughs continued to receive TDF treatment and achieved virological responses. Further nucleic acid sequencing did not identify TDF-related resistance.126 Long-term treatment with TDF can significantly improve liver histology and reduce the incidence of HCC.127,128
In total, 90 CHB patients who were ETV resistant and serum HBV DNA levels >60 IU/mL were equally randomized to receive TDF alone or in combination with ETV for 48 weeks. Clearance of HBV DNA (<15 IU/mL) was achieved in 73% and 71%, respectively. HBV DNA levels decreased by 3.66 lg IU/mL and 3.74 lg IU/mL from baseline, respectively. Overall, six and three patients retained their baseline resistance mutations in TDF alone and in combination with ETV, respectively. Both regimens demonstrated a favorable safety profile.129 Several studies into TDF therapy for patients previously treated with NAs for 48–168 weeks showed that TDF resulted in virological responses in 70–98% of LAM-resistant, adefovir dipivoxil (ADV)-resistant, ETV-resistant, or multidrug-resistant patients. In addition, virological response rates increased during treatment.129–139
Tenofovir alafenamide fumarate tablets (TAF)
In a global phase III clinical trial, 581 HBeAg-positive CHB patients (which excluded patients with decompensated cirrhosis) received TAF treatment for 48 weeks. HBV DNA levels were <29 IU/mL in 64% of patients and ALT levels returned to normal in 72% of patients. HBeAg seroconversion occurred in 10% of patients, and HBsAg disappeared in 1% of patients. After treatment for 96 weeks, HBV DNA levels were <29 IU/mL in 73% of patients, and ALT levels returned to normal in 75% of patients. HBeAg seroconversion occurred in 18% of patients and HBsAg disappeared in 1% of patients. In 285 HBeAg-negative CHB patients (which excluded patients with decompensated cirrhosis) that received TAF treatment for 48 weeks, HBV DNA levels were <29 IU/mL in 94% of patients and ALT levels returned to normal in 83% of patients. No patients experienced HBsAg loss. Following treatment for 96 weeks, HBV DNA levels were <29 IU/mL in 90% of patients and ALT levels returned to normal in 81% of patients. HBsAg loss occurred in <1% of patients.140–142
During the 96-week treatment, headache (12%), nausea (6%), and fatigue (6%) were the most common adverse events.142 Following 96 weeks of treatment, patients that received TAF had significantly smaller decreases in bone mineral density than those that received TDF in the hip (−0.33% vs. −2.51%; p<0.001) and lumbar spine (−0.75% vs. −2.57%; p<0.001), as well as a significantly smaller median change in estimated glomerular filtration rate (eGFR) (−1.2 vs. −4.8 mg/dL; p<0.001).
Other drugs
Telbivudine (LdT) can improve eGFR but it is associated with high rates of resistance.113 LdT exhibits favorable efficacy and safety in blocking mother-to-child transmission (refer to the section “Recommendations on antiviral therapy for special populations”).
Selection of NAs
Antivirals with high potency and low risk of resistance (ETV, TDF, and TAF) should be the preferred NAs for treatment-naïve patients. ADV and LAM are not recommended for antiviral therapy for HBV-infected patients.
For patients who are being treated with nonpreferred drugs, it is recommended that they switch to antivirals with high potency and low risk of resistance to further reduce the risk of resistance. For patients that receive ADV, it is recommended that they switch to ETV, TDF, or TAF. For patients that receive LAM or LdT, it is recommended that they switch to TDF, TAF, or ETV. For patients that are resistant to LAM or LdT, it is recommended that they switch to TDF or TAF. For patients that are resistant to ADV, it is recommended that they switch to ETV, TDF, or TAF.143 For patients that are being treated with ADV in combination with LAM/LdT, it is recommended that they switch to TDF or TAF.
Prevention and management of NA resistance
Treatment-naïve patients
Potent antivirals with minimal resistance, such as ETV, TDF, and TAF are recommended.
During treatment
HBV DNA levels should be quantified regularly to detect virological breakthroughs and initiate save therapy as soon as possible (Table 5). For patients who develop resistance to NAs, IFN-α is associated with low response rates.
Table 5. Recommendations for salvage therapy for resistance to NAs.
| Types of resistance | Recommended drugs |
|---|---|
| LAM- or LdT-resistant | Switch to TDF or TAF |
| ADV-resistant, never used LAM or LdT before | Switch to ETV, TDF, or TAF |
| ADV-resistant and LAM/LdT-resistant | Switch to TDF or TAF |
| ETV-resistant | Switch to TDF or TAF |
| ETV-resistant and ADV-resistant | ETV combined with TDF, or ETV combined with TAF |
LAM, lamivudine; LdT, telbivudine; ADV, adefovir dipivoxil; ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide fumarate.
Monitoring of NA treatment
Detection of the following relevant indicators at baseline before treatment
(1) major biochemical markers, such as ALT, AST, bilirubin, and albumin; (2) major virological and serological markers, such as HBV DNA quantification, HBsAg, HBeAg, and anti-HBe; (3) blood routine serum creatinine levels, blood phosphorus levels, and renal tubular function should be tested if required; (4) noninvasive tests for liver fibrosis, such as liver stiffness measurement; and (5) when ETV and TDF are used in patients with creatinine clearance <50 mL/min, the doses of both drugs should be adjusted. There is no recommended dose for TAF when it is used in patients with creatinine clearance <15 mL/min who are not receiving hemodialysis. In other cases, no dose adjustment is required.
Patient compliance
It should be closely monitored, which includes dosage, method of use, missed medication or self-discontinuation, to ensure that patients understand the risks that might result from unwarranted discontinuation and improve their compliance.
Prevention and management of rare adverse events
Although NAs are generally safe and well-tolerated, serious adverse events such as renal insufficiency (TDF, ADV), hypophosphatemic bone disease (TDF, ADV), myositis/rhabdomyolysis (LdT), and lactic acidosis (ETV, LdT) can occur in rare cases, and require attention. It is recommended that physicians make detailed inquiries into the medical history before treatment to reduce the risk. Patients should be closely monitored if they present with significant increases in serum levels of creatinine, creatine kinase, or lactate dehydrogenase during treatment, which could be accompanied by clinical manifestations, such as general deterioration, myalgia, muscle weakness, and bone pain. If they are diagnosed with renal insufficiency, myositis, rhabdomyolysis, or lactic acidosis, the existing regimen should be withdrawn and changed to other treatment interventions.
Monitoring and management of drug resistance
The use of potent antiviral drugs with minimal resistance has resulted in significantly reduced rates of resistance that could arise from long-term treatment of NAs. If HBV DNA levels increased >2 lg IU/mL from nadir during treatment and the potential of poor compliance has been ruled out, salvage therapy should be initiated promptly, and drug resistance should be tested for.
IFN-α treatment
Peg-IFN-α and IFN-α have been approved for CHB treatment in China.
Regimens and efficacy of peg-IFN-α therapy
Initial monotherapy with peg-IFN-α
In several multicenter, randomized, controlled clinical trials, HBeAg-positive CHB patients were treated with peg-IFN-α-2a or peg-IFN-α-2b for 48 weeks (180 g/week). HBV DNA levels were <2,000 IU/mL in 30% of the patients and HBeAg seroconversion occurred in 30.75–36.3% of patients at week 24 after drug discontinuation (or 68.4% of patients with HBsAg <1,500 IU/mL at week 12 of treatment and baseline ALT >2×ULN). In addition, HBsAg seroconversion occurred in 2.3–3% of patients at week 24 after drug discontinuation; HBsAg clearance was achieved in 11% of patients after 3 years of drug withdrawal.113,144–146 In HBeAg-negative patients with chronic HBV infections (60% Asians) treated with peg-IFN-α-2a for 48 weeks, HBV DNA levels were <2,000 IU/mL in 43% and 42% of patients at 24 and 48 weeks after drug withdrawal, respectively. HBsAg clearance was achieved in 3%, 8.7%, and 12% of patients 24 weeks, 3 years, and 5 years after drug withdrawal.113,146
At week 24 of peg-IFN-α therapy, if HBV DNA levels decreased by ≤2 lg IU/mL and HBsAg levels were >20,000 IU/mL in HBeAg-positive patients or decreased by <1 lg IU/mL in HBeAg-negative patients, it is recommended to stop peg-IFN-α treatment and switch to NAs therapy.112,116,146
Combined treatment of peg-IFN-α and NAs
The combined use of peg-IFN-α in eligible CHB patients treated with NAs could achieve clinical cure in some patients.116,146 HBsAg clearance was high after combined treatment in patients with low levels of HBsAg (<1,500 IU/mL) before treatment and who experienced a rapid decline of HBsAg levels during treatment (HBsAg <200 IU/mL or a decrease of >1 lg IU/mL at week 12 or 24).147–151 However, further research is required to determine baseline conditions, the optimal course of treatment, and sustained virological responses of combination therapy.
Peg-IFN-α further reduces the incidence of HBV-related liver cancer
In a study of CHB patients that were treated with peg-IFN-α or ETV, a 5-year follow-up showed that none of the patients treated with peg-IFN-α developed HCC within 5 years; however, two and one patients treated with ETV developed HCC at years 4 and 5, respectively. There was no significant difference in the cumulative HCC incidence between the observed and the predicted cases for patients treated with ETV (p=0.36).152 In another retrospective study that included 682 patients treated with NAs and 430 patients treated with IFN-α alone or in combination with NAs, a total of 31 patients developed HCC at a median follow-up of 5.41 years and the 10-year cumulative incidence of HCC was significantly lower in patients treated with IFN-α than those treated with NAs (2.7% vs. 8.0%, p<0.001).146,153 The role of peg-IFN-α in reducing the incidence of HBV-related liver cancer requires further research.
Predictive factors for the efficacy of peg-IFN-α-based antiviral therapy
Pretreatment predictors: HBV DNA levels <2×108 IU/mL, high ALT levels (2–10×ULN) or liver tissue necroinflammation ≥G2, genotype A or B, low baseline HBsAg (<25,000 IU/mL),6,112,113,146,154,155 high levels of anti-HBc at baseline (qAnti-HBc),62,63 signal transducer and activator of transcription 4 (commonly known as STAT4) rs7574865 polymorphism at baseline156 are predictors for favorable outcomes of IFN-based therapy. HBV DNA levels and HBsAg levels at 12 weeks of peg-IFN-α therapy and their dynamic changes could be used to predict the efficacy of peg-IFN-α.146
Management of adverse events of peg-IFN-α therapy6,112,113
Influenza-like syndrome
If patients complain of fever, headache, myalgia, or fatigue, peg-IFN-α could be injected before sleep or taking nonsteroidal anti-inflammatory agents along with the IFN-α.
Myelosuppression
If neutrophil count ≤0.75×109/L and/or platelet count <50×109/L develops, peg-IFN-α dosage should be reduced and cell counts retested after 1–2 weeks. If cell counts return to normal, the initial dosage should be restored. If neutrophil count ≤0.5×109/L or platelet count <25×109/L, or both, the peg-IFN-α should be discontinued. Granulocyte colony-stimulating factor (commonly known as G-CSF) or granulocyte-macrophage colony-stimulating factor (commonly known as GM-CSF) could be used for those with significantly decreased neutrophil counts.
Mental disorders
If patients experience depression, delusions, severe anxiety, or other mental disorders, peg-IFN-α should be immediately withdrawn, and patients could seek consultations with psychiatrists and psychologists if required.
Autoimmune diseases
Some patients might develop autoantibodies, and few patients develop thyroid disease, diabetes, decreased platelet count, psoriasis, vitiligo, rheumatoid arthritis, and systemic lupus erythematosus-like syndrome. In such cases, patients should have consultations with physicians with related expertise, and treatment should be discontinued for patients with severe symptoms.
Other rare adverse events
For other rare adverse events, which include retinopathy, interstitial pneumonia, hearing loss, kidney damage, and cardiovascular complications, peg-IFN-α therapy should be discontinued.
Contraindications for peg-IFN-α therapy6,112,113
Absolute contraindications
Pregnancy or intention to be pregnant in the short-term, history of mental illness (e.g., history of schizophrenia or severe depression), uncontrolled epilepsy, decompensated cirrhosis, uncontrolled autoimmune diseases, severe infection, retinal disease, heart failure, chronic obstructive pulmonary diseases, and other underlying diseases.
Relative contraindications
These include thyroid disease, a history of depression, uncontrolled diabetes, hypertension, and heart disease.
Recommendation 9: HBeAg-positive CHB patients should be treated with ETV, TDF, or TAF. If HBV DNA levels are below the detection limit, ALT levels return to normal, and HBeAg seroconversion occurs after 1 year of treatment, patients should receive consolidation treatment for at least 3 years (followed up every 6 months). Treatment could be discontinued if HBV DNA remains undetectable, and ALT normalization and HBsAg loss are maintained. A longer duration of treatment might reduce recurrence (A1).
Recommendation 10: HBeAg-positive CHB patients could be treated with peg-IFN-α. At week 24 of treatment, if HBV DNA decrease <2 lg IU/mL and HBsAg levels >20,000 IU/mL, it is recommended that peg-IFN-α therapy should be discontinued and changed to NA treatment (A1). For patients who responded effectively, the course of treatment is 48 weeks, and it might be extended according to the patient’s condition but it should not exceed 96 weeks (B1).
Recommendation 11: HBeAg-negative CHB patients should be treated with ETV, TDF, or TAF. Discontinuation and subsequent follow-up are advised if HBsAg loss and undetectable HBV DNA are achieved (A1).
Recommendation 12: HBeAg-negative CHB patients could be treated with peg-IFN-α. At week 12 of treatment, if HBV DNA decreased <2 lg IU/mL or HBsAg decreased <1 lg IU/mL, it is recommended that peg-IFN-α treatment should be discontinued and changed to NA treatment (B1). For patients who responded effectively, the course of treatment is 48 weeks, and it might be extended according to the patient’s condition, but it should not exceed 96 weeks (B1).
Recommendation 13: For HBV-related compensated cirrhosis patients, long-term treatment with ETV, TDF, or TAF, or peg-lFN-α therapy is recommended. The adverse events of peg-lFN-α must be closely monitored (A1).
Recommendation 14: For HBV-related decompensated cirrhosis patients, long-term treatment with ETV or TDF is recommended (A1). TAF therapy could be used if necessary (C1).
Other treatments
Anti-HBV therapy can reduce the incidence of HBV-related complications, HBV-related liver cancer, and improve the survival rate of patients, which is the most important treatment for chronic HBV infection. In addition, other treatment options include anti-inflammatory, antioxidant, hepatoprotective, anti-fibrosis, and immunomodulatory interventions.
Anti-inflammatory, antioxidant, and hepatoprotective treatment
The necroinflammation of liver cells caused by HBV infection is an important pathological and physiological process of disease progression. Glycyrrhizic acid, silymarin, polyunsaturated lecithin, and bicyclol have anti-inflammatory, antioxidant, and hepatoprotective effects, and might reduce inflammatory damage of the liver. For patients with obvious liver inflammation or significantly elevated ALT levels, these agents could be used where appropriate, but it is not advised to use more than two of them in combination.
Antifibrosis treatment
Several antifibrosis Chinese medicine prescriptions, such as Anluohuaxian wan, Fufang Biejiaruangan tablets, Fuzhenghuayu capsules, have shown protective effects against fibrosis in animal experiments and clinical studies.157–161 They could be used where appropriate for patients with obvious fibrosis or cirrhosis. However, multicenter randomized controlled studies are required to further define their treatment course of and long-term efficacy.
Monitoring and follow-up management of people with chronic HBV infection6,112,113
Management of chronic HBV carriers and inactive HBsAg carriers
Because chronic HBV carriers are in the immune tolerance phase, they do not have or only have mild inflammatory activity in the liver, patients in this phase respond poorly to antiviral treatment. Therefore, antiviral therapy is not recommended. However, it is emphasized that some patients in the immune tolerance phase might enter the immune clearance phase and develop hepatitis flares. Inactive HBsAg carriers are in the immune control phase, but they might progress to HBeAg-negative CHB and are at risk of developing HCC in long-term follow-up.
Therefore, for chronic HBV carriers and inactive HBsAg carriers, it is recommended that they should undergo routine blood tests, biochemical tests, virological tests, AFP tests, abdominal ultrasound, and noninvasive tests for liver fibrosis every 6–12 months. A liver biopsy is recommended if necessary. Antiviral therapy should start if they meet the indications for such treatment.
Monitoring during antiviral therapy
Regular monitoring during antiviral therapy aims to monitor the efficacy of antiviral therapy, compliance of patient, drug resistance, and adverse events.
Patients treated with peg-IFN-α
Routine blood tests (every 1–2 weeks in the 1st month of treatment, then once a month after measurements become stable), liver biochemical tests (once a month), thyroid function and blood glucose tests (once every 3 months), quantitative tests of HBV DNA, HBsAg, HBeAg and anti-HBe (every 3 months), liver stiffness measurement (every 6 months), abdominal ultrasound and AFP tests (every 6 months for patients without cirrhosis, and every 3 months for patients with cirrhosis). Enhanced CT or enhanced MRI for early detection of HCC may be performed if necessary.
NAs
Routine blood tests, liver biochemical tests, HBV DNA quantification, serological markers, and liver stiffness measurement should be performed every 3 to 6 months; abdominal ultrasound and AFP test should be performed every 6 months for patients without cirrhosis, and every 3 months for patients with cirrhosis. Enhanced CT or enhanced MRI for early detection of HCC may be performed if necessary. For patients treated with TDF, blood phosphorus levels and renal function should be tested every 6 to 12 months. Early renal tubular injury may be monitored if feasible.
Follow-up after the end of antiviral therapy
The purpose of follow-up of patients after the end of treatment is to evaluate the long-term efficacy of antiviral therapy, monitor disease progression and the development of HCC. Therefore, regardless of patient’s response to antiviral treatment, biochemical tests of the liver, and HBV DNA quantification should be performed once a month within the first 3 months after drug withdrawal, every 3 months thereafter, and every 6 months one year after drug withdrawal. Patients without cirrhosis need to undergo abdominal ultrasound and AFP test every 6 months, and patients with cirrhosis need to be tested every 3 months, and if necessary, enhanced CT or enhanced MRI may be performed for early detection of HCC.
Recommendations on antiviral therapy for special populations
Patients with poor response
CHB patients
After treatment with ETV, TDF, or TAF for 48 weeks, if HBV DNA is >2×103 IU/mL, and excluding compliance and detection errors, NA-based treatment regimens should be adjusted (those using ETV could be switched to TDF or TAF,162,163 and those using TDF or TAF could be switched to ETV), or combination therapy could be used (ETV combined with TDF or TAF). Peg-IFN-α therapy can also be used in combination.
Patients with HBV-related cirrhosis
After treatment with ETV, TDF, or TAF for 24 weeks, if HBV DNA is >2×103 IU/mL, and excluding compliance and detection errors, NA-based treatment regimens could be adjusted (those using ETV could be switched to TDF or TAF, and those using TDF or TAF could be switched to ETV), or combination therapy might be used (ETV combined with TDF or TAF).
Patients treated with chemotherapeutic or immunosuppressive agents
Chemotherapy or immunosuppressive therapy in CHB patients might cause HBV reactivation and might lead to liver failure or even death in severe cases. HBV reactivation occurs after antitumor therapy in approximately 20–50% of HBsAg-positive, anti-HBc-positive tumor patients, and in 8–18% of HBsAg-negative, anti-HBc-positive tumor patients.164,165 Prophylactic antiviral therapy can significantly reduce the incidence of hepatitis B reactivation.166,167 ETV, TDF, or TAF, which are potent antivirals with minimal resistance, are recommended.168–170
All patients that receive chemotherapy or immunosuppressive therapy should be routinely screened for HBsAg and anti-HBc before starting treatment.
HBsAg-positive patients should be given NA therapy (usually 1 week) before or when they start receiving immunosuppressive or chemotherapeutic agents.6,171,172
In addition, HBsAg-negative, anti-HBc-positive patients need prophylactic antiviral therapy if they are positive for HBV DNA;112 if HBV DNA is negative, ALT levels, HBV DNA, and HBsAg should be monitored every 1–3 months. Antiviral therapy should be initiated immediately when HBV DNA or HBsAg becomes positive.112,173
For HBsAg-negative and anti-HBc-positive patients, the use of B-cell monoclonal antibodies or hematopoietic stem cell transplantation is associated with a high-risk of HBV reactivation.174,175 The prophylactic use of antiviral drugs is recommended.112,165,168,176,177
For patients with CHB or cirrhosis that are treated with chemotherapy and immunosuppressants, the course of NA treatment, follow-up monitoring, and drug withdrawal are the same as those for patients with CHB or cirrhosis that receive no chemotherapy or immunosuppressants. For patients with chronic HBV infection in the immune tolerance or immune control phases, or HBsAg-negative, anti-HBc-positive patients that require the prophylactic use of NAs, treatment with ETV, TDF, or TAF should be continued for 6–12 months after the end of chemotherapy and immunosuppressive therapy.6,168,178
For patients that receive B-cell monoclonal antibodies or undergo hematopoietic stem cell transplantation, NAs could be discontinued at least 18 months after the end of immunosuppressive therapy.179,180 HBV could recur or even worsen after NAs are withdrawn. Patients should be followed up for 12 months, during which HBV DNA should be monitored every 1–3 months.
Pregnancy
Women of childbearing age or women that are preparing to become pregnant should be screened for HBsAg, and HBV DNA should be tested for HBsAg-positive patients.181 Peg-IFN-α treatment should be initiated before pregnancy if antiviral therapy is indicated, and antiviral therapy should be completed 6 months before pregnancy. Reliable contraceptive measures should be taken during treatment. If peg-IFN-α is not indicated or fails, TDF could be used for antiviral therapy. For patients diagnosed with CHB for the first time during pregnancy, treatment indications are the same as those for other CHB patients, and TDF could be used for antiviral therapy. Pregnant women with CHB who started taking antiviral drugs before or during pregnancy should continue antiviral therapy after delivery, and based on virological responses, decide whether to continue the original treatment regimen or change to other NAs or peg-IFN-α.
For patients with unexpected pregnancy during antiviral therapy, it is recommended that they continue their pregnancy if they are taking TDF. Patients do not have to terminate the pregnancy if they are taking ETV, and it is suggested that they switch to TDF instead. If pregnant patients are receiving IFN-α treatment, they should be informed (and their family members) of the associated risks, and whether or not to continue the pregnancy should be decided by patients themselves; if the pregnancy choice is to continue, patients should change to TDF treatment.
High levels of serum HBV DNA is a high-risk factor for mother-to-child transmission. If HBV DNA levels are >2×105 IU/mL in the second and third trimesters of pregnancy,182 the patients should be informed of the associated risks and their consent should be obtained to start antiviral therapy at 24–28 weeks of the pregnancy using TDF or LdT.183,184 Breastfeeding is not a contraindication for TDF therapy.185,186 Pregnant women taking NAs at the immune tolerance phase could discontinue NAs immediately after delivery or continue for another 1–3 months. Approximately 17.2–62% of patients might develop hepatitis flares after discontinuation, which usually occurs within 24 weeks.187–189 Postpartum monitoring is needed. Blood biochemical indexes for the liver and HBV DNA should be rechecked 4–6 weeks after delivery. If the biochemical markers for the liver are normal, they should be followed up every 3 months until 6 months after delivery. If patients develop hepatitis flares, antiviral therapy is indicated.
Fertility issues for male patients that receive antiviral therapy: Male patients treated with IFN-α might consider having children 6 months after drug withdrawal. No evidence is currently available that indicates NA therapy harms sperm in male patients that receive NA therapy. Male patients should be informed of the potential risks when they consider having children.
Pediatric patients
If children with HBV infection are in the immune tolerance phase, antiviral therapy is not considered. Antiviral therapy should be initiated as soon as possible for children with chronic hepatitis or cirrhosis. Antiviral therapy for children with CHB can significantly inhibit HBV DNA replication and increase the chance of ALT normalization and HBeAg seroconversion.190 However, the safety and resistance issues associated with long-term treatment should be concerned.112,191,192
The drugs approved by the USA’s Food and Drug Administration (FDA) for the treatment of pediatric patients include IFN-α (aged ≥1 year), ETV (aged ≥2 years), and TDF (aged ≥2 years and body weight ≥10 kg).6,190 China has approved TAF for adolescents (aged ≥12 years and body weight ≥35 kg). Peg-IFN-α-2a could be used in CHB children aged ≥5 years.6
HBeAg-positive CHB patients with elevated ALT levels should be treated with a finite course of IFN-α or peg-IFN-α-2a therapy to achieve HBeAg conversion.178,193 Alternatively, they could be treated with ETV, TDF, or TAF. The recommended dose of IFN-α for pediatric patients is 3–6 million U/m2, three times a week. The maximum dose should not exceed 10 million U/m2, and the recommended duration of treatment is 24–48 weeks. Peg-IFN-α-2a should be dosed at 180 µg/1.73 m2, with a treatment course of 48 weeks.194 The doses of ETV, TDF, or TAF refer to the recommendations of the USA’s FDA and WHO, and drug labels (Table 6).8,190,193
Table 6. Recommended doses of NAs for children.
| Drug | Body weight (kg) | Dose (mg/d) |
|---|---|---|
| ETV (aged ≥2 years, and body weight ≥10 kg; use adult dose if body weight >30 kg) | 10–11 | 0.15 |
| >11–14 | 0.20 | |
| >14–17 | 0.25 | |
| >17–20 | 0.30 | |
| >20–23 | 0.35 | |
| >23–26 | 0.40 | |
| >26–30 | 0.45 | |
| > 30 | 0.50 | |
| TDF | ||
| Aged ≥2 years and body weight ≥17 kg, who can take tablets orally | 17–22 | 150 |
| 22–28 | 200 | |
| 28–35 | 250 | |
| ≥35 | 300 | |
| Aged ≥2 years and body weight ≥10 kg; those who cannot take tablets orally can be given powder using a special spoon (40 mg/spoon) | 10–12 | 80 (2 spoons) |
| 12–14 | 100 (2.5 spoons) | |
| 14–17 | 120 (3 spoons) | |
| 17–19 | 140 (3.5 spoons) | |
| 19–22 | 160 (4 spoons) | |
| 22–24 | 180 (4.5 spoons) | |
| 24–27 | 200 (5 spoons) | |
| 27–29 | 220 (5.5 spoons) | |
| 29–32 | 240 (6 spoons) | |
| 32–34 | 260 (6.5 spoons) | |
| 34–35 | 280 (7 spoons) | |
| ≥35 | 300 (7.5 spoons) | |
| TAF (aged ≥12 years) | ≥35 | 25 |
ETV, entecavir; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate.
For children with CHB or cirrhosis that receive therapy with IFN-α or peg-IFN-α-2a but fail to achieve HBeAg seroconversion or HBeAg-negative status, NAs should be used for treatment.178
Patients with renal injury
High-risk factors for renal injury include ≥1 of the following: decompensated cirrhosis; eGFR <60 mL/min; poorly controlled hypertension; proteinuria; uncontrolled diabetes; active glomerulonephritis; concomitant use of nephrotoxic drugs; or history of solid organ transplantation.112 When there are high-risk factors for renal injury, the changes in renal function should be monitored during the use of any NA. If ADV or TDF is used for treatment, serum creatinine and blood phosphorus levels should be monitored regularly, regardless of the presence or absence of high-risk factors for renal injury.195,196
For patients with chronic kidney disease, renal insufficiency, or who receive renal replacement therapy, ETV or TAF is recommended as the first-line anti-HBV therapy; LdT could also be chosen for antiviral therapy, where appropriate. ADV or TDF is not recommended.142,197 When TAF is used in patients without HIV coinfection, no dose adjustment is necessary when eGFR is ≥15 mL/min; however, the doses of other NAs currently on the market need to be adjusted when eGFR is <50 mL/min. Dosing adjustments should refer to drug labels.
ETV or TAF could be used as prophylactic or therapeutic drugs for HBsAg-positive patients that receive kidney transplantation. Because of the increased risk of rejection, kidney transplant patients should avoid IFN-α or peg-IFN-α therapy.
HBV-related glomerulonephritis could be treated with NA therapy, and ETV or TAF is recommended.198
For patients that have been treated with ADV or TDF, it is recommended that they should change to ETV or TAF if kidney or bone disease occurs, or if other high-risk factors are involved.197
Recommendation 15: For CHB patients, after 48 weeks of treatment with ETV, TDF, or TAF, of HBV DNA is >2×103 IU/mL, NAs treatment regimens should be adjusted when excluding compliance and detection errors. Those using ETV could change to TDF or TAF, and those using TDF or TAF could change to ETV, or combination therapy might be used (ETV combined with TDF or TAF) (C2). Peg-IFN-α therapy could be used in combination (B1).
For patients with HBV-related cirrhosis, after treatment with ETV, TDF, or TAF for 24 weeks, of their HBV DNA is >2×103 IU/mL, and excluding compliance and detection errors, NAs treatment regimens could be adjusted. Those using ETV could change to TDF or TAF, and those using TDF or TAF could change to ETV, or combination therapy might be used (ETV combined with TDF or TAF) (C2).
Recommendation 16: All patients that receive chemotherapy or immunosuppressive therapy should be routinely screened for HBsAg and anti-HBc before starting treatment (A1). For HBsAg-positive patients, antiviral therapy should be given 1 week before or when they start to receive immunosuppressive therapy or chemotherapy (A1), and ETV, TDF, or TAF should be used (B1). For HBsAg-negative and anti-HBc-positive patients, antiviral therapy with ETV, TDF, or TAF is recommended if they receive B-cell monoclonal antibodies or undergo hematopoietic stem cell transplantation (B1).
Recommendation 17: When patients with chronic HBV infection attempt pregnancy or have antiviral indications during pregnancy, TDF could be used when informed consent has been obtained (B1).
Recommendation 18: For patients with unexpected pregnancy during antiviral therapy it is recommended that they continue their pregnancy if they are taking TDF. Patients do not have to terminate the pregnancy if they are taking ETV and it is suggested that they change to TDF instead (B1). If patients are receiving IFN-α treatment, they (and their family members) should be informed of the associated risks, and whether or not to continue the pregnancy should be decided by patients themselves; if they decide to continue, they should change to TDF treatment (C2).
Recommendation 19: If HBV DNA levels are >2×105 IU/mL during the second and third trimesters of pregnancy, patients should be informed of the associated risks and could start antiviral therapy at 24–28 weeks of pregnancy using TDF or LdT when they give informed consent (A1). Pregnant women in the immune tolerance phase should stop medication immediately after delivery or after 1–3 months of treatment. Breastfeeding is not a contraindication for TDF treatment (C2). Blood biochemical indexes for the liver function and HBV DNA levels should be tested at least every 3 months until 6 months after delivery after drug withdrawal. Patients that develop hepatitis flares should start antiviral therapy immediately (A2).
Recommendation 20: For children with advanced liver disease or cirrhosis, antiviral therapy should be given in time, but safety and resistance issues associated with long-term antiviral treatment should be considered. IFN-α treatment could be considered for children aged ≥1 year, ETV or TDF for children aged ≥2 years, peg-IFN-α-2a for children aged ≥5 years. and TAF treatment for children aged ≥12 years (A1).
Recommendation 21: For patients with chronic kidney disease, renal insufficiency, or who receive renal replacement therapy, ETV or TAF is recommended as the first-line anti-HBV therapy. Alternatively, LdT could be used for antiviral therapy, where appropriate. ADV or TDF is not recommended (B1). The changes in renal function should be monitored during the use of any NAs for CHB patients at a high-risk of kidney injury. For patients that have been treated with ADV or TDF, it is recommended that they switch to ETV or TAF if they develop kidney or bone disease or present with other high-risk factors (B1).
Patients with coinfection of HBV and HCV
All people that are HBsAg-positive should be screened for anti-HCV. If they are positive, they need further testing for HCV RNA. Those who test positive for HCV RNA should receive DAA therapy. These patients are at risk of HBV reactivation. Therefore, it is recommended that they receive antiviral therapy with ETV, TDF, or TAF during and within 3 months after the end of anti-HCV therapy and they should be closely monitored.112
In addition, HBsAg-negative and anti-HBc-positive patients are at risk of HBV reactivation during treatment of hepatitis C with DAAs. Serum HBV DNA levels and HBsAg should be monitored monthly. If HBsAg seroreversion occurs, antiviral therapy is recommended.112
Recommendation 22: When a DAA is used to treat HCV in patients with coinfection of HCV and HBV, NA treatment should be administered to prevent HBV reactivation if HBsAg is positive. NA treatment could be withdrawn 12 weeks after the end of DAA treatment (B2). HBsAg-negative, anti-HBc-positive patients should be closely monitored for HBV DNA and HBsAg levels during DAA therapy. If HBsAg seroreversion occurs, NA therapy is recommended (B2).
Patients with coinfection of HBV and HIV
Antiretroviral therapy (commonly referred to as ART) should be initiated as soon as possible if there is no contraindication for anti-HIV therapy, regardless of CD4+T lymphocyte count. HIV/HBV co-infected patients should be treated for both viral infections at the same time. The highly active anti-retroviral therapy (HAART) regimen should include two drugs active against HBV, preferably TDF or TAF + LAM or emtricitabine (FTC) (TDF+FTC and TAF+FTC are available as a mixture). HBV-related markers, such as HBV DNA, liver biochemical indicators, and liver imaging should be monitored during treatment. For HIV/HBV co-infected patients, a treatment regimen for hepatitis B that contains only one NA against HBV is not recommended (TDF, LAM, ETV, LdT, ADV).199,200
The following should be noted for patients with renal insufficiency: (1) if the creatinine clearance rate is <60 mL/min, avoid use of TDF; and (2) if the creatinine clearance rate is between 30 mL/min and 50 mL/min, TAF+ (FTC or LAM) could be considered in the regimen. However, TAF has not been approved for use yet in patients with a creatinine clearance rate <30 mL/min. Finally, (3) when TDF/TAF cannot be used, ETV should be added to the HAART regimen. For pregnant women with HIV/HBV co-infection, the recommended regimen should include LAM (FTC)+TDF.201
Recommendation 23: For people who are co-infected with HBV and HIV, it is recommended that a combination of antiviral drugs effective for both HIV and HBV be chosen (A1).
Patients with HBV-related liver failure
Patients with HBV-related acute, subacute, acute-on-chronic, and chronic liver failure are associated with high mortality. If HBsAg is positive, antiviral therapy is indicated.
Anti-HBV therapy can improve the long-term prognosis of HBV-related acute-on-chronic liver failure (ACLF).202,203 Several clinical studies have confirmed that both ETV and LAM can effectively reduce the mortality of ACLF.204,205 Meta-analyses have shown that ETV is superior to LAM for the treatment of HBV-related ACLF.205,206 Small cohort clinical studies have found that HBV-related ACLF can benefit from the use of LdT and TDF.207,208 Compared with TDF, TAF can reduce nephrotoxicity and maintain antiviral efficacy,142 but no definite clinical evidence is available to support the use of TAF for liver failure. Early rapid reduction of HBV DNA levels is key to treatment.204,207 If HBV DNA levels can be reduced by 2 lg IU/mL within 2–4 weeks, survival can be improved.206,207 Fast-acting NAs with high potency and minimal resistance (ETV, TDF, or TAF) should be chosen for antiviral therapy.209 Antiviral therapy should be continued for long periods after patients with liver failure recover.
Recommendation 24: For patients with HBV-related acute, subacute, acute-on-chronic, and chronic liver failure, it is recommended that they should receive antiviral therapy with ETV, TDF, or TAF if they are positive for HBsAg (A1).
Patients with HBV-related HCC
HBV DNA-positive HCC patients that receive anti-HBV treatment could reduce the recurrence of HCC after surgery and improve the overall survival rate.210–216 Fast-acting NAs with high potency (ETV, TDF, or TAF) should be chosen for antiviral therapy. Patients without contraindications could use IFN-α.
HCC patients that are HBsAg-positive but HBV DNA-negative might experience HBV reactivation when undergoing liver resection, transcatheter arterial chemoembolization, radiotherapy, or systemic chemotherapy.217–221 ETV, TDF, or TAF are recommended for antiviral therapy.
Recommendation 25: Patients with HBV-related HCC are recommended to use ETV, TDF or TAF for antiviral therapy if they are positive for HBsAg (A1).
Liver transplant patients
For patients that undergo liver transplantation for HBV-related diseases (including liver failure and HCC), anti-HBV regimens should be rationally selected to reduce the risk of reinfection of HBV in the transplanted liver. The design of treatment regimens mainly depends on the major risk factor for reinfection, i.e. HBV DNA levels before transplantation.
A negative HBV DNA quantification test before transplantation suggests a low risk of reinfection. High potency NAs with minimal resistance, such as ETV, TDF, or TAF, should be used as early as possible before surgery to prevent HBV reactivation. There is no need to use HBIG after surgery.222,223 Positive HBV DNA testing before transplantation suggests a high-risk of reinfection. NAs with high potency and minimal resistance should be used as early as possible before surgery to reduce HBV DNA levels; HBIG should be injected intravenously during the anhepatic phase. After surgery, low-dose HBIG should be used in combination with NAs for 0.5–1.0 year, followed by the use of NAs alone.222,224,225 A recent study found that a shortened course of HBIG was effective in patients treated with ETV.226 If patients have already used other NAs, HBV DNA should be closely monitored to detect drug resistance and adjust the regimen if necessary. In addition, hepatitis B vaccination was reported to prevent recurrence after liver transplantation, but its clinical use remains controversial.227
Recommendation 26: For patients that undergo liver transplantation for HBV-related infections, it is recommended that they start antiviral therapy with ETV, TDF, or TAF before transplantation if they are positive for HBsAg (A1).
Unsolved clinical issues
Identify and evaluate new markers that could be used to differentiate the various stages of the natural course of chronic HBV infections.
Define the value of new serum markers, such as anti-HBc levels during the treatment planning for patients with normal ALT levels.
Define the value of noninvasive tests for liver fibrosis in initiating treatment, evaluating the efficacy, and predicting long-term outcome.
Assess the effect of long-term treatment with different NAs on the reversal of cirrhosis and the incidence of HCC.
Identify clinical indicators and biological markers that could guide the safe discontinuation of NAs.
Develop innovative drugs aimed at a clinical cure (functional cure) and evaluate their synergy and combined use with existing drugs.
Use real-world data (e.g., long-term follow-up cohorts or medical insurance databases) to evaluate the safety, efficacy, and cost-effectiveness of marketed drugs and provide evidence for clinical and public health decisions.
Innovate the management of CHB, enhance the ability to detect, diagnose, and cure CHB, and reduce mortality related to hepatitis B.
Acknowledgments
We would like to thank all the committee members of the Chinese Society of Infectious Diseases and the Chinese Society of Hepatology, Chinese Medical Association for reviewing and commenting on the current guidelines. We are also indebted to colleagues who make valuable suggestions for these guidelines.
Abbreviations
- ACLF
acute-on-chronic liver failure
- ADV
adefovir dipivoxil
- AFP
alpha-fetoprotein
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- APRI
AST to platelet ratio index
- AST
aspartate aminotransferase
- cccDNA
covalently closed circular DNA
- CHB
chronic hepatitis B
- CT
Computerized tomography
- DAA
direct-acting antiviral
- ETV
entecavir
- GFR
glomerular filtration rate
- GGT
γ-glutamyl transferase
- HBV
hepatitis B virus
- HBcAg
hepatitis B core antigen
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IFN
interferon
- Ig
immunoglobulin
- LAM
lamivudine
- LdT
telbivudine
- MRI
Magnetic resonance imaging
- PT
prothrombin activity time
- PTA
prothrombin activity
- TAF
tenofovir alafenamide fumarate tablets
- TE
transient elastography
- TDF
tenofovir disoproxil fumarate
- ULN
upper limit of normal
- WHO
World Health Organization
Authors (sorted by strokes of last name)
Guiqiang Wang, Fusheng Wang, Hui Zhuang, Taisheng Li, Sujun Zheng, Hong Zhao, Zhongping Duan, Jinlin Hou, Jidong Jia, Xiaoyuan Xu, Fuqiang Cui, Lai Wei.
Guideline development group (sorted by strokes of last name)
Yan Wang, Guiqiang Wang, Fusheng Wang, Hong You, Qin Ning, Hong Ren, Hui Zhuang, Jie Li, Taisheng Li, Lanjuan Li, Wenhong Zhang, Xinxin Zhang, Lungen Lu, Yu Chen, Sujun Zheng, Qinghua Meng, Hong Zhao, Yuemin Nan, Zhongping Duan, Jinlin Hou, Huiying Rao, Jidong Jia, Xiaoyuan Xu, Xinhua Weng, Hong Tang, Fuqiang Cui, Jie Peng, Chongwen Si, Ying Han, Qing Xie, Xiaoguang Dou, Lai Wei.
References
- 1. WHO. Global hepatitis report 2017. Available from: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 2.Cui F, Shen L, Li L, Wang H, Wang F, Bi S, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23(5):765–772. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97(3):230–238. doi: 10.2471/blt.18.219469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Liu H, Wang Y, Hao R, Li Z, Song H. The next step in controlling HBV in China. BMJ. 2013;347:f4503. doi: 10.1136/bmj.f4503. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Zhu FC, Liu JX, Zhai XJ, Chang ZJ, Yan L, et al. The maternal viral threshold for antiviral prophylaxis of perinatal hepatitis B virus transmission in settings with limited resources: a large prospective cohort study in China. Vaccine. 2017;35(48):6627–6633. doi: 10.1016/j.vaccine.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018;67(1):1–31. doi: 10.15585/mmwr.rr6701a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection 2015. Available from: https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ [PubMed]
- 9.Action plan for the prevention and treatment of viral hepatitis in China (2017-2020) Chin J Viral Dis. 2018;8(01):1–5. doi: 10.16505/j.2095-0136.2018.0001. [DOI] [Google Scholar]
- 10.World Health Organization Hepatitis B vaccines: WHO position paper, July 2017 - Recommendations. Vaccine. 2019;37(2):223–225. doi: 10.1016/j.vaccine.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214(1):16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 12.Levy M, Koren G. Hepatitis B vaccine in pregnancy: maternal and fetal safety. Am J Perinatol. 1991;8(3):227–232. doi: 10.1055/s-2007-999384. [DOI] [PubMed] [Google Scholar]
- 13.Moro PL, Zheteyeva Y, Barash F, Lewis P, Cano M. Assessing the safety of hepatitis B vaccination during pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 1990-2016. Vaccine. 2018;36(1):50–54. doi: 10.1016/j.vaccine.2017.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheffield JS, Hickman A, Tang J, Moss K, Kourosh A, Crawford NM, et al. Efficacy of an accelerated hepatitis B vaccination program during pregnancy. Obstet Gynecol. 2011;117(5):1130–1135. doi: 10.1097/AOG.0b013e3182148efe. [DOI] [PubMed] [Google Scholar]
- 15.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;3:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giersch K, Allweiss L, Volz T, Dandrilz M, Luetgehetmann M. Serum HBV pgRNA as a clinical marker for cccDNA activity. J Hepatol. 2017;66(2):460–462. doi: 10.1016/j.jhep.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Lu FM, Wang J, Zhuang H. Potential clinical significance of HBV RNA virus-like particle. Zhonghua Gan Zang Bing Za Zhi. 2016;24(9):641–642. doi: 10.3760/cma.j.issn.1007-3418.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu FM, Dou XG, Zhang WH, Wang FS. Clinical significance of serum HBVRNA measurement in chronic hepatitis B patients. Zhonghua Gan Zang Bing Za Zhi. 2018;34(05):934–938. doi: 10.3969/j.issn.1001-5256.2018.05.005. [DOI] [Google Scholar]
- 19.McNaughton AL, D’Arienzo V, Ansari MA, Lumley SF, Littlejohn M, Revill P, et al. Insights from deep ssequencing of the HBV genome-unique, tiny, and misunderstood. Gastroenterology. 2019;156(2):384–399. doi: 10.1053/j.gastro.2018.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: recent advances. J Gastroenterol Hepatol. 2011;26(Suppl 1):123–130. doi: 10.1111/j.1440-1746.2010.06541.x. [DOI] [PubMed] [Google Scholar]
- 21.Livingston SE, Simonetti JP, Bulkow LR, Homan CE, Snowball MM, Cagle HH, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology. 2007;133(5):1452–1457. doi: 10.1053/j.gastro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, Liu CJ, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97(4):265–272. doi: 10.1093/jnci/dji043. [DOI] [PubMed] [Google Scholar]
- 23.Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection a review. JAMA. 2018;319(17):1802–1813. doi: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- 24.Indolfi G, Easterbrook P, Dusheiko G, Siberry G, Chang MH, Thorne C, et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4(6):466–476. doi: 10.1016/s2468-1253(19)30042-1. [DOI] [PubMed] [Google Scholar]
- 25.Nayagam S, Thursz M, Sicuri E, Conteh L, Wiktor S, Low-Beer D, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16(12):1399–1408. doi: 10.1016/s1473-3099(16)30204-3. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Yao N, Chen T, Fu S, Wu Y, Feng Y, et al. Prevalence of mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis. J Hepatol. 2019;70(1):E123–E124. doi: 10.1016/s0618-8278(19)30217-8. [DOI] [Google Scholar]
- 27.Chinese Society of Infectious Diseases CMA Guidelines for prevention and treatment of mother-to-child transmission of hepatitis B virus in China. Zhonghua Gan Zang Bing Za Zhi. 2019;37(7) doi: 10.3760/cma.j.issn.1000-6680.2019.07.002. [DOI] [Google Scholar]
- 28.Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29:100–107. doi: 10.1111/j.1478-3231.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 29.Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18(11):827–844. doi: 10.1038/s41573-019-0037-0. [DOI] [PubMed] [Google Scholar]
- 30.Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46(2):395–401. doi: 10.1002/hep.21724. [DOI] [PubMed] [Google Scholar]
- 31.Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol. 2003;18(3):246–252. doi: 10.1046/j.1440-1746.2003.02976.x. [DOI] [PubMed] [Google Scholar]
- 32.Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116(12):829–834. doi: 10.1016/j.amjmed.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 33.Chu CM, Liaw YF. Prevalence of and risk factors for hepatitis B viremia after spontaneous hepatitis B surface antigen seroclearance in hepatitis B carriers. Clin Infect Dis. 2012;54(1):88–90. doi: 10.1093/cid/cir755. [DOI] [PubMed] [Google Scholar]
- 34.Chu CM, Liaw YF. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther. 2010;15(2):133–143. doi: 10.3851/imp1497. [DOI] [PubMed] [Google Scholar]
- 35.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology. 2010;51(2):435–444. doi: 10.1002/hep.23348. [DOI] [PubMed] [Google Scholar]
- 37.Park BK, Park YN, Ahn SH, Lee KS, Chon CY, Moon YM, et al. Long-term outcome of chronic hepatitis B based on histological grade and stage. J Gastroenterol Hepatol. 2007;22(3):383–388. doi: 10.1111/j.1440-1746.2007.04857.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46(1):45–52. doi: 10.1016/j.jhep.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Chu CM, Liaw YF. Hepatitis B virus-related cirrhosis: natural history and treatment. Semin Liver Dis. 2006;26(2):142–152. doi: 10.1055/s-2006-939752. [DOI] [PubMed] [Google Scholar]
- 40.Chen YC, Chu CM, Yeh CT, Liaw YF. Natural course following the onset of cirrhosis in patients with chronic hepatitis B: a long-term follow-up study. Hepatol Int. 2007;1(1):267–273. doi: 10.1007/s12072-007-5001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35(6):1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 42.McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135(9):759–768. doi: 10.7326/0003-4819-135-9-200111060-00006. [DOI] [PubMed] [Google Scholar]
- 43.Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21(1):77–82. doi: 10.1002/hep.1840210114. [DOI] [PubMed] [Google Scholar]
- 44.Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23(1):47–58. doi: 10.1055/s-2003-37590. [DOI] [PubMed] [Google Scholar]
- 45.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology. 2013;57(2):441–450. doi: 10.1002/hep.26041. [DOI] [PubMed] [Google Scholar]
- 46.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142(5):1140–1149.e3. doi: 10.1053/j.gastro.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Zhang JY, Wang LF, Wang FS. Immunopathogenesis and prognostic immune markers of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2012;27(2):223–230. doi: 10.1111/j.1440-1746.2011.06940.x. [DOI] [PubMed] [Google Scholar]
- 48.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut. 2012;61:6–17. doi: 10.1136/gutjnl-2012-302056. [DOI] [PubMed] [Google Scholar]
- 49.Isogawa M, Tanaka Y. Immunobiology of hepatitis B virus infection. Hepatol Res. 2015;45(2):179–189. doi: 10.1111/hepr.12439. [DOI] [PubMed] [Google Scholar]
- 50.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 51.Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61(12):1754–1764. doi: 10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 52.Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66(2):398–411. doi: 10.1016/j.jhep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Hou JL, Zhao W, Lee C, Hann HW, Peng CY, Tanwandee T, et al. Outcomes of Long-term Treatment of Chronic HBV Infection With Entecavir or Other Agents From a Randomized Trial in 24 Countries. Clin Gastroenterol Hepatol. 2020;18(2):457–467.e421. doi: 10.1016/j.cgh.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Kim JH, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Low-Level Viremia and the Increased Risk of Hepatocellular Carcinoma in Patients Receiving Entecavir Treatment. Hepatology. 2017;66(2):335–343. doi: 10.1002/hep.28916. [DOI] [PubMed] [Google Scholar]
- 55.Wiegand J, Hasenclever D, Tillmann HL. Should treatment of hepatitis B depend on hepatitis B virus genotypes? A hypothesis generated from an explorative analysis of published evidence. Antivir Ther. 2008;13(2):211–220. [PubMed] [Google Scholar]
- 56.Ni YH, Chang MH, Wang KJ, Hsu HY, Chen HL, Kao JH, et al. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology. 2004;127(6):1733–1738. doi: 10.1053/j.gastro.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 57.Chu CJ, Hussain M, Lok ASF. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology. 2002;122(7):1756–1762. doi: 10.1053/gast.2002.33588. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe K, Takahashi T, Takahashi S, Okoshi S, Ichida T, Aoyagi Y. Comparative study of genotype B and C hepatitis B virus-induced chronic hepatitis in relation to the basic core promoter and precore mutations. J Gastroenterol Hepatol. 2005;20(3):441–449. doi: 10.1111/j.1440-1746.2004.03572.x. [DOI] [PubMed] [Google Scholar]
- 59.Rajoriya N, Combet C, Zoulim F, Janssen HLA. How viral genetic variants and genotypes influence disease and treatment outcome of chronic hepatitis B. Time for an individualised approach? J Hepatol. 2017;67(6):1281–1297. doi: 10.1016/j.jhep.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Jia W, Song LW, Fang YQ, Wu XF, Liu DY, Xu C, et al. Antibody to Hepatitis B Core Antigen Levels in the Natural History of Chronic Hepatitis B: A Prospective Observational Study. Medicine. 2014;93(29):e322. doi: 10.1097/md.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song LW, Liu PG, Liu CJ, Zhang TY, Cheng XD, Wu HL, et al. Quantitative hepatitis B core antibody levels in the natural history of hepatitis B virus infection. Clin Microbiol Infect. 2015;21(2):197–203. doi: 10.1016/j.cmi.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Fan R, Sun J, Yuan Q, Xie Q, Bai X, Ning Q, et al. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues. Gut. 2016;65(2):313–320. doi: 10.1136/gutjnl-2014-308546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou FQ, Song LW, Yuan Q, Fang LL, Ge SX, Zhang J, et al. Quantitative Hepatitis B Core Antibody Level Is a New Predictor for Treatment Response In HBeAg-positive Chronic Hepatitis B Patients Receiving Peginterferon. Theranostics. 2015;5(3):218–226. doi: 10.7150/thno.10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou J, Song L, Zhao H, Yan L, Ma A, Xie S, et al. Serum hepatitis B core antibody as a biomarker of hepatic inflammation in chronic hepatitis B patients with normal alanine aminotransferase. Sci Rep. 2017;7(1):2747. doi: 10.1038/s41598-017-03102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol. 2016;65(4):700–710. doi: 10.1016/j.jhep.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 66.Fan R, Zhou B, Xu M, Tan D, Niu J, Wang H, et al. Association Between Negative Results From Tests for HBV DNA and RNA and Durability of Response After Discontinuation of Nucles(t)ide Analogue Therapy. Clin Gastroenterol Hepatol. 2020;18(3):719–727.e717. doi: 10.1016/j.cgh.2019.07.046. [DOI] [PubMed] [Google Scholar]
- 67.Wong DK, Seto WK, Cheung KS, Chong CK, Huang FY, Fung J, et al. Hepatitis B virus core-related antigen as a surrogate marker for covalently closed circular DNA. Liver Int. 2017;37(7):995–1001. doi: 10.1111/liv.13346. [DOI] [PubMed] [Google Scholar]
- 68.Martinot-Peignoux M, Lapalus M, Maylin S, Boyer N, Castelnau C, Giuily N, et al. Baseline HBsAg and HBcrAg titres allow peginterferon-based ‘precision medicine’ in HBeAg-negative chronic hepatitis B patients. J Viral Hepat. 2016;23(11):905–911. doi: 10.1111/jvh.12565. [DOI] [PubMed] [Google Scholar]
- 69.Honda M, Shirasaki T, Terashima T, Kawaguchi K, Nakamura M, Oishi N, et al. Hepatitis B Virus (HBV) Core-Related Antigen During Nucleos(t)ide Analog Therapy Is Related to Intra-hepatic HBV Replication and Development of Hepatocellular Carcinoma. J Infect Dis. 2016;213(7):1096–1106. doi: 10.1093/infdis/jiv572. [DOI] [PubMed] [Google Scholar]
- 70.Chuaypen N, Posuwan N, Payungporn S, Tanaka Y, Shinkai N, Poovorawan Y, et al. Serum hepatitis B core-related antigen as a treatment predictor of pegylated interferon in patients with HBeAg-positive chronic hepatitis B. Liver Int. 2016;36(6):827–836. doi: 10.1111/liv.13046. [DOI] [PubMed] [Google Scholar]
- 71.Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112(1):18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 72.Wang K, Lin W, Kuang Z, Fan R, Liang X, Peng J, et al. Longitudinal Change of Body Mass Index Is Associated With Alanine Aminotransferase Elevation After Complete Viral Suppression in Chronic Hepatitis B Patients. J Infect Dis. 2019;220(9):1469–1476. doi: 10.1093/infdis/jiz326. [DOI] [PubMed] [Google Scholar]
- 73.Amaddeo G, Cao Q, Ladeiro Y, Imbeaud S, Nault JC, Jaoui D, et al. Integration of tumour and viral genomic characterisations in HBV-related hepatocellular carcinomas. Gut. 2015;64(5):820–829. doi: 10.1136/gutjnl-2013-306228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2015;21(13):3928–3935. doi: 10.3748/wjg.v21.i13.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wai CT, Cheng CL, Wee A, Dan YY, Chan E, Chua W, et al. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. 2006;26(6):666–672. doi: 10.1111/j.1478-3231.2006.01287.x. [DOI] [PubMed] [Google Scholar]
- 76.Kim WR, Berge T, Asselah T, Flisiak R, Fung S, Gordon SC, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64(4):773–780. doi: 10.1016/j.jhep.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Dong XQ, Wu Z, Zhao H, Wang GQ, China Hep BRFA Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in chinese hepatitis B patients. J Viral Hepat. 2019;26(2):297–307. doi: 10.1111/jvh.13031. [DOI] [PubMed] [Google Scholar]
- 78.Sonneveld MJ, Brouwer WP, Chan HLY, Piratvisuth T, Jia JD, Zeuzem S, et al. Optimisation of the use of APRI and FIB-4 to rule out cirrhosis in patients with chronic hepatitis B: results from the SONIC-B study. Lancet Gastroenterol Hepatol. 2019;4(7):538–544. doi: 10.1016/s2468-1253(19)30087-1. [DOI] [PubMed] [Google Scholar]
- 79.Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65(8):1369–1376. doi: 10.1136/gutjnl-2015-309260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen YP, Hu XM, Liang XE, Huang LW, Zhu YF, Hou JL. Stepwise application of fibrosis index based on four factors, red cell distribution width-platelet ratio, and aspartate aminotransferase-platelet ratio for compensated hepatitis B fibrosis detection. J Gastroenterol Hepatol. 2018;33(1):256–263. doi: 10.1111/jgh.13811. [DOI] [PubMed] [Google Scholar]
- 81.Yao M, Wang L, You H, Wang J, Liao H, Yang D, et al. Serum GP73 combined AST and GGT reflects moderate to severe liver inflammation in chronic hepatitis B. Clin Chim Acta. 2019;493:92–97. doi: 10.1016/j.cca.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 82.Wang L, Liu T, Zhou J, You H, Jia J. Changes in serum chitinase 3-like 1 levels correlate with changes in liver fibrosis measured by two established quantitative methods in chronic hepatitis B patients following antiviral therapy. Hepatol Res. 2018;48(3):E283–E290. doi: 10.1111/hepr.12982. [DOI] [PubMed] [Google Scholar]
- 83.Yan L, Deng Y, Zhou J, Zhao H, Wang G, China Hep BRFA Serum YKL-40 as a biomarker for liver fibrosis in chronic hepatitis B patients with normal and mildly elevated ALT. Infection. 2018;46(3):385–393. doi: 10.1007/s15010-018-1136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.European Association for the Study of the Liver, Asociacion Latinoamericana para el Estudio del Higado EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Chinese Foundation for Hepatitis Prevention and Control; Chinese Society of Infectious Disease and Chinese Society of Hepatology, Chinese Medical Association; Liver Disease Committee of Chinese Research Hospital Association Consensus on clinical application of transient elastography detecting liver fibrosis: a 2018 update. Zhonghua Gan Zang Bing Za Zhi. 2019;27(3):182–191. doi: 10.3760/cma.j.issn.1007-3418.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 86.Chen YP, Liang XE, Dai L, Zhang Q, Peng J, Zhu YF, et al. Improving transient elastography performance for detecting hepatitis B cirrhosis. Dig Liver Dis. 2012;44(1):61–66. doi: 10.1016/j.dld.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 87.Chen YP, Liang XE, Zhang Q, Peng J, Zhu YF, Wen WQ, et al. Larger biopsies evaluation of transient elastography for detecting advanced fibrosis in patients with compensated chronic hepatitis B. J Gastroenterol Hepatol. 2012;27(7):1219–1226. doi: 10.1111/j.1440-1746.2012.07122.x. [DOI] [PubMed] [Google Scholar]
- 88.Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16(1):36–44. doi: 10.1111/j.1365-2893.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 89.Liang XE, Zhong C, Huang L, Yang S, Zhu Y, Chen Y, et al. Optimization of hepatitis B cirrhosis detection by stepwise application of transient elastography and routine biomarkers. J Gastroenterol Hepatol. 2017;32(2):459–465. doi: 10.1111/jgh.13475. [DOI] [PubMed] [Google Scholar]
- 90.Scott DR, Levy MT. Liver transient elastography (Fibroscan (R)): a place in the management algorithms of chronic viral hepatitis. Antivir Ther. 2010;15(1):1–11. doi: 10.3851/imp1474. [DOI] [PubMed] [Google Scholar]
- 91.Miao L, Yang WN, Dong XQ, Zhang ZQ, Xie SB, Zhang DZ, et al. Combined anluohuaxianwan and entecavir treatment significantly improve the improvement rate of liver fibrosis in patients with chronic hepatitis B virus infection. Zhonghua Gan Zang Bing Za Zhi. 2019;27(7):521–526. doi: 10.3760/cma.j.issn.1007-3418.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jia J, Hou J, Ding H, Chen G, Xie Q, Wang Y, et al. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30(4):756–762. doi: 10.1111/jgh.12840. [DOI] [PubMed] [Google Scholar]
- 93.Fetzer DT, Rodgers SK, Seow JH, Dawkins AA, Joshi G, Gabriel H, et al. Ultrasound Evaluation in Patients at Risk for Hepatocellular Carcinoma. Radiol Clin North Am. 2019;57(3):563–583. doi: 10.1016/j.rcl.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 94.Elsayes KM, Kielar AZ, Chernyak V, Morshid A, Furlan A, Masch WR, et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma. 2019;6:49–69. doi: 10.2147/jhc.S186239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 96.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13(3):372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 97.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24(2):289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 98.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 99.Kim SU, Oh HJ, Wanless IR, Lee S, Han KH, Park YN. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol. 2012;57(3):556–563. doi: 10.1016/j.jhep.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 100.Tailing W, Xia L, Yuanping Z, Jingwen H, Jing Z, Ningzhang L, et al. A Semiquantitative score system for assessment of hepatic inflammation and fibrosisi in chronic viral hepatits. Zhonghua Gan Zang Bin Za Zhi. 1998;6(4):195. doi: 10.3760/j.issn:1007-3418.1998.04.002. [DOI] [Google Scholar]
- 101.Xu S, Wang Y, Tai DCS, Wang S, Cheng CL, Peng Q, et al. qFibrosis: A fully-quantitative innovative method incorporating histological features to facilitate accurate fibrosis scoring in animal model and chronic hepatitis B patients. J Hepatol. 2014;61(2):260–269. doi: 10.1016/j.jhep.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun Y, Zhou J, Wang L, Wu X, Chen Y, Piao H, et al. New Classification of Liver Biopsy Assessment for Fibrosis in Chronic Hepatitis B Patients Before and After Treatment. Hepatology. 2017;65(5):1438–1450. doi: 10.1002/hep.29009. [DOI] [PubMed] [Google Scholar]
- 103.Wong GL. Management of chronic hepatitis B patients in immunetolerant phase: what latest guidelines recommend. Clin Mol Hepatol. 2018;24(2):108–113. doi: 10.3350/cmh.2017.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martin P. Immune-Tolerant Hepatitis B: Maybe a Misnomer but Still Hard to Treat. Hepatology. 2019;69(6):2315–2317. doi: 10.1002/hep.30654. [DOI] [PubMed] [Google Scholar]
- 105.Maimone S, Caccamo G, Squadrito G, Alibrandi A, Saffioti F, Spinella R, et al. A combination of different diagnostic tools allows identification of inactive hepatitis B virus carriers at a single time point evaluation. Liver Int. 2017;37(3):362–368. doi: 10.1111/liv.13246. [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Yang HI, Lee MH, Jen CL, Batrla-Utermann R, Lu SN, et al. Serum Levels of Hepatitis B Surface Antigen and DNA Can Predict Inactive Carriers With Low Risk of Disease Progression. Hepatology. 2016;64(2):381–389. doi: 10.1002/hep.28552. [DOI] [PubMed] [Google Scholar]
- 107.Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71(2):397–408. doi: 10.1016/j.jhep.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 108.Wu X, Zhou J, Xie W, Ding H, Ou X, Chen G, et al. Entecavir monotherapy versus de novo combination of lamivudine and adefovir for compensated hepatitis B virus-related cirrhosis: a real-world prospective multicenter cohort study. Infect Drug Resist. 2019;12:745–757. doi: 10.2147/idr.S185120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH, et al. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol. 2012;18(1):1–21. doi: 10.3350/kjhep.2012.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 111.D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, et al. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Int. 2018;12(Suppl 1):34–43. doi: 10.1007/s12072-017-9808-z. [DOI] [PubMed] [Google Scholar]
- 112.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 113.Chinese Society of Hepatology CMA The guideline of prevention and treatment for chronic hepatitis B:a 2015 update. Zhonghua Gan Zang Bin Za Zhi. 2015;23(12):888–905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mak LY, Seto WK, Fung J, Yuen MF. Novel developments of hepatitis B: treatment goals, agents and monitoring tools. Expert Rev Clin Pharmacol. 2019;12(2):109–120. doi: 10.1080/17512433.2019.1567327. [DOI] [PubMed] [Google Scholar]
- 115.Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313–2324. doi: 10.1016/s0140-6736(18)31865-8. [DOI] [PubMed] [Google Scholar]
- 116.Ning Q, Wu D, Wang GQ, Ren H, Gao ZL, Hu P, et al. Roadmap to functional cure of chronic hepatitis B: An expert consensus. J Viral Hepat. 2019;26(10):1146–1155. doi: 10.1111/jvh.13126. [DOI] [PubMed] [Google Scholar]
- 117.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 118.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354(10):1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 119.Chang TT, Lai CL, Yoon SK, Lee SS, Coelho HSM, Carrilho FJ, et al. Entecavir Treatment for up to 5 Years in Patients with Hepatitis B e Antigen-Positive Chronic Hepatitis B. Hepatology. 2010;51(2):422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 120.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-Term Entecavir Therapy Results in the Reversal of Fibrosis/Cirrhosis and Continued Histological Improvement in Patients with Chronic Hepatitis B. Hepatology. 2010;52(3):886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 121.Xu Y, Zhang YG, Wang X, Qi WQ, Qin SY, Liu ZH, et al. Long-term antiviral efficacy of entecavir and liver histology improvement in Chinese patients with hepatitis B virus-related cirrhosis. World J Gastroenterol. 2015;21(25):7869–7876. doi: 10.3748/wjg.v21.i25.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36(12):1755–1764. doi: 10.1111/liv.13253. [DOI] [PubMed] [Google Scholar]
- 123.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-Term Monitoring Shows Hepatitis B Virus Resistance to Entecavir in Nucleoside-Naive Patients Is Rare Through 5 Years of Therapy. Hepatology. 2009;49(5):1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 124.Hou JL, Gao ZL, Xie Q, Zhang JM, Sheng JF, Cheng J, et al. Tenofovir disoproxil fumarate vs adefovir dipivoxil in Chinese patients with chronic hepatitis B after 48 weeks: a randomized controlled trial. J Viral Hepat. 2015;22(2):85–93. doi: 10.1111/jvh.12313. [DOI] [PubMed] [Google Scholar]
- 125.Liang X, Gao Z, Xie Q, Zhang J, Sheng J, Cheng J, et al. Long-term efficacy and safety of tenofovir disoproxil fumarate in Chinese patients with chronic hepatitis B: 5-year results. Hepatol Int. 2019;13(3):260–269. doi: 10.1007/s12072-019-09943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Y, Corsa AC, Buti M, Cathcart AL, Flaherty JF, Miller MD, et al. No detectable resistance to tenofovir disoproxil fumarate in HBeAg plus and HBeAg- patients with chronic hepatitis B after 8years of treatment. J Viral Hepat. 2017;24(1):68–74. doi: 10.1111/jvh.12613. [DOI] [PubMed] [Google Scholar]
- 127.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/s0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 128.Kim WR, Loomba R, Berg T, Schall REA, Yee LJ, Dinh PV, et al. Impact of Long-Term Tenofovir Disoproxil Fumarate on Incidence of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. Cancer. 2015;121(20):3631–3638. doi: 10.1002/cncr.29537. [DOI] [PubMed] [Google Scholar]
- 129.Lim YS, Byun KS, Yoo BC, Kwon SY, Kim YJ, An J, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in patients with entecavir-resistant chronic hepatitis B with multiple drug failure: results of a randomised trial. Gut. 2016;65(5):852–860. doi: 10.1136/gutjnl-2014-308353. [DOI] [PubMed] [Google Scholar]
- 130.Kim HJ, Cho JY, Kim YJ, Gwak GY, Paik YH, Choi MS, et al. Long-term efficacy of tenofovir disoproxil fumarate therapy after multiple nucleos(t)ide analogue failure in chronic hepatitis B patients. Korean J Intern Med. 2015;30(1):32–41. doi: 10.3904/kjim.2015.30.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suzuki F, Suzuki Y, Hosaka T, Sezaki H, Akuta N, Fujiyama S, et al. Efficacy of long-term tenofovir-based rescue therapy in patients with chronic hepatitis B refractory to nucleoside/nucleotide analogs. J Gastroenterol. 2017;52(5):641–651. doi: 10.1007/s00535-016-1270-5. [DOI] [PubMed] [Google Scholar]
- 132.Zhou J, Liu YY, Lian JS, Pan LF, Yang JL, Huang JR. Efficacy and Safety of Tenofovir Disoproxil Treatment for Chronic Hepatitis B Patients with Genotypic Resistance to Other Nucleoside Analogues: A Prospective Study. Chin Med J (Engl) 2017;130(8):914–919. doi: 10.4103/0366-6999.204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fasano M, Maggi P, Leone A, Volpe A, Fiore JR, Angarano G, et al. Long-term efficacy and safety of switching from lamivudine plus adefovir to tenofovir disoproxil fumarate in virologically suppressed patients. Dig Liver Dis. 2017;49(5):530–534. doi: 10.1016/j.dld.2017.01.140. [DOI] [PubMed] [Google Scholar]
- 134.Rodriguez M, Manuel Pascasio J, Fraga E, Fuentes J, Prieto M, Sanchez-Antolin G, et al. Tenofovir vs lamivudine plus adefovir in chronic hepatitis B: TENOSIMP-B study. World J Gastroenterol. 2017;23(41):7459–7469. doi: 10.3748/wjg.v23.i41.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, et al. Tenofovir disoproxil fumarate (TDF) vs. emtricitabine (FTC)/TDF in lamivudine resistant hepatitis B: A 5-year randomised study. J Hepatol. 2017;66(1):11–18. doi: 10.1016/j.jhep.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 136.Lim YS, Lee YS, Gwak GY, Byun KS, Kim YJ, Choi J, et al. Monotherapy With Tenofovir Disoproxil Fumarate for Multiple Drug-Resistant Chronic Hepatitis B: 3-Year Trial. Hepatology. 2017;66(3):772–783. doi: 10.1002/hep.29187. [DOI] [PubMed] [Google Scholar]
- 137.Lim YS, Gwak GY, Choi J, Lee YS, Byun KS, Kim YJ, et al. Monotherapy with tenofovir disoproxil fumarate for adefovir-resistant vs. entecavir-resistant chronic hepatitis B: A 5-year clinical trial. J Hepatol. 2019;71(1):35–44. doi: 10.1016/j.jhep.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 138.Lee HW, Park JY, Lee JW, Yoon KT, Kim CW, Park H, et al. Long-term Efficacy of Tenofovir Disoproxil Fumarate Monotherapy for Multidrug-Resistant Chronic HBV infection. Clin Gastroenterol Hepatol. 2019;17(7):1348–1355.e2. doi: 10.1016/j.cgh.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 139.Lim YS, Yoo BC, Byun KS, Kwon SY, Kim YJ, An J, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in adefovir-resistant chronic hepatitis B patients with multiple drug failure: results of a randomised trial. Gut. 2016;65(6):1042–1051. doi: 10.1136/gutjnl-2014-308435. [DOI] [PubMed] [Google Scholar]
- 140.Chan HLY, Fung S, Seto WK, Chuang WL, Chen CY, Kim H, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–195. doi: 10.1016/s2468-1253(16)30024-3. [DOI] [PubMed] [Google Scholar]
- 141.Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206. doi: 10.1016/s2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 142.Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatology. 2018;68(4):672–681. doi: 10.1016/j.jhep.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 143.Chinese Society of Hepatology CMA An expert consensus for the adjustment of treatment strategies in paints with chronic hepatitis B treated with non-first-line nucleos(t)ide analogues. Zhonghua Gan Zang Bin Za Zhi. 2019;27(5):343–346. doi: 10.3760/cma.j.issn.1007-3418.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hsu CW, Su WW, Lee CM, Peng CY, Chuang WL, Kao JH, et al. Phase IV randomized clinical study: Peginterferon alfa-2a with adefovir or entecavir pre-therapy for HBeAg-positive chronic hepatitis B. J Formos Med Assoc. 2018;117(7):588–597. doi: 10.1016/j.jfma.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 145.Fengqin H, Yalin Y, Lingying Z, Jia S, Guozhong G, Chen P, et al. Clinical effect and safety of pegylated interferon-α-2b injection (Y shape, 40 kD) in treatment of HBeAg-positive chronic hepatitis B patients. Zhonghua Gan Zang Bin Za Zhi. 2017;25(8):589–596. doi: 10.3760/cma.j.issn.1007-3418.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang WH, Dou XG, Xie Q, Jiang JJ, Chen XY. Consensus on pegylated interferon alpha in treatment of chronic hepatitis B. Zhonghua Gan Zang Bin Za Zhi. 2017;25(9):678–686. doi: 10.3760/cma.j.issn.1007-3418.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu D, Wang P, Han M, Chen Y, Chen X, Xia Q, et al. Sequential combination therapy with interferon, interleukin-2 and therapeutic vaccine in entecavir-suppressed chronic hepatitis B patients: the Endeavor study. Hepatol Int. 2019;13(5):573–586. doi: 10.1007/s12072-019-09956-1. [DOI] [PubMed] [Google Scholar]
- 148.Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: A randomised open-label trial (OSST trial) J Hepatology. 2014;61(4):777–784. doi: 10.1016/j.jhep.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 149.Han M, Jiang J, Hou J, Tan D, Sun Y, Zhao M, et al. Sustained immune control in HBeAg-positive patients who switched from entecavir therapy to pegylated interferon-alpha 2a: 1 year follow-up of the OSST study. Antivir Ther. 2016;21(4):337–344. doi: 10.3851/imp3019. [DOI] [PubMed] [Google Scholar]
- 150.Hu P, Shang J, Zhang W, Gong G, Li Y, Chen X, et al. HBsAg Loss with Peg-interferon Alfa-2a in Hepatitis B Patients with Partial Response to Nucleos(t)ide Analog: New Switch Study. J Clin Transl Hepatol. 2018;6(1):25–34. doi: 10.14218/jcth.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chan HLY, Chan FWS, Hui AJ, Li MKK, Chan KH, Wong GLH, et al. Switching to peginterferon for chronic hepatitis B patients with hepatitis B e antigen seroconversion on entecavir - A prospective study. J Viral Hepat. 2019;26(1):126–135. doi: 10.1111/jvh.13000. [DOI] [PubMed] [Google Scholar]
- 152.Li SY, Li H, Xiong YL, Liu F, Peng ML, Zhang DZ, et al. Peginterferon is preferable to entecavir for prevention of unfavourable events in patients with HBeAg-positive chronic hepatitis B: A five-year observational cohort study. J Viral Hepat. 2017;24:12–20. doi: 10.1111/jvh.12755. [DOI] [PubMed] [Google Scholar]
- 153.Ren P, Cao Z, Mo R, Liu Y, Chen L, Li Z, et al. Interferon-based treatment is superior to nucleos(t)ide analog in reducing HBV-related hepatocellular carcinoma for chronic hepatitis B patients at high risk. Expert Opin Biol Ther. 2018;18(10):1085–1094. doi: 10.1080/14712598.2018.1518423. [DOI] [PubMed] [Google Scholar]
- 154.Lampertico P, Messinger D, Cornberg M, Brunetto M, Petersen J, Kennedy P, et al. A genotype-specific baseline score predicts post-treatment response to peginterferon alfa-2a in hepatitis B e antigen-negative chronic hepatitis B. Ann Gastroenterol. 2018;31(6):712–721. doi: 10.20524/aog.2018.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chan HL, Messinger D, Papatheodoridis GV, Cornberg M, Xie Q, Piratvisuth T, et al. A baseline tool for predicting response to peginterferon alfa-2a in HBeAg-positive patients with chronic hepatitis B. Aliment Pharmacol Ther. 2018;48(5):547–555. doi: 10.1111/apt.14862. [DOI] [PubMed] [Google Scholar]
- 156.Chen H, Sun J, Zhou B, Xie Q, Liang X, Fan R, et al. Variants in STAT4 Associated With Cure of Chronic HBV Infection in HBeAg-positive Patients Treated With Pegylated Interferon-alpha. Clin Gastroenterol Hepatol. 2020;18(1):196–204.e198. doi: 10.1016/j.cgh.2019.04.044. [DOI] [PubMed] [Google Scholar]
- 157.Jiang YF, Ma J, He B, Li NP, Tang W, Gong GZ. The therapeutic effect of Anluohuaxian capsule combined with adefovir dipivoxil on patients with chronic hepatitis B and influence on hepatic histology. Zhonghua Gan Zang Bing Za Zhi. 2012;20(5):344–347. doi: 10.3760/cma.j.issn.1007-3418.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 158.Lin W, Wei L, Yuhua G, Xi C, Fei P, Xueen L, et al. Effect of Anluohuaxianwan on the expression of matrix metalloproteinases and their inhibitors in rat liver with fibrosis. Zhonghua Gan Zang Bin Za Zhi. 2019;27(4):267–273. doi: 10.3760/cma.j.issn.l007-3418.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liang M, Wanna Y, Xiaoqin D, Zhanqing Z, Shibin X, Dazhi Z, et al. Combined anluohuaxianwan and entecavir treatment significantly improve the improvement rate of liver fibrosis in patients with chronic hepatitis B virus infection. Zhonghua Gan Zang Bin Za Zhi. 2019;27(7):521–526. doi: 10.3760/cma.j.issn.1007-3418.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang NH, Yuan GS, Zhou YC, Liu JW, Huang HP, Hu CG, et al. Entecavir combined with Fufang Biejia Ruangan tablet in treatment of chronic hepatitis B patients with liver fibrosis: 96-week efficacy analyses. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(6):775–779. [PubMed] [Google Scholar]
- 161.Ruihu Y, Qin L, Wei C. Efficacy and safety of Fuzhenghuayu capsule for treating liver fibrosis in patients with chronic hepatitis B: a meta-analysis. Zhonghua Gan Zang Bin Za Zhi. 2015;23(4):295–296. doi: 10.3760/cma.j.issn.1007-3418.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 162.Chen J, Zhao SS, Liu XX, Huang ZB, Huang Y. Comparison of the Efficacy of Tenofovir Versus Tenofovir plus Entecavir in the Treatment of Chronic Hepatitis B in Patients With Poor Efficacy of Entecavir: A Systematic Review and Meta-analysis. Clin Ther. 2017;39(9):1870–1880. doi: 10.1016/j.clinthera.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 163.Chaung KT, O’Brien C, Ha NB, Nguyen NH, Trinh HN, Nguyen MH. Alternative Therapies for Chronic Hepatitis B Patients With Partial Virological Response to Standard Entecavir Monotherapy. J Clin Gastroenterol. 2016;50(4):338–344. doi: 10.1097/mcg.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 164.Bozza C, Cinausero M, Iacono D, Puglisi F. Hepatitis B and cancer: A practical guide for the oncologist. Crit Rev Oncol Hematol. 2016;98:137–146. doi: 10.1016/j.critrevonc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 165.Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, et al. Randomized Controlled Trial of Entecavir Prophylaxis for Rituximab-Associated Hepatitis B Virus Reactivation in Patients With Lymphoma and Resolved Hepatitis B. J Clin Oncol. 2013;31(22):2765–2772. doi: 10.1200/jco.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 166.Liu CJ, Chen PJ, Chen DS, Kao JH. Hepatitis B virus reactivation in patients receiving cancer chemotherapy: natural history, pathogenesis, and management. Hepatol Int. 2013;7(2):316–326. doi: 10.1007/s12072-011-9279-6. [DOI] [PubMed] [Google Scholar]
- 167.Choi J, Lim YS. Characteristics, Prevention, and Management of Hepatitis B Virus (HBV) Reactivation in HBV-Infected Patients Who Require Immunosuppressive Therapy. J Infect Dis. 2017;216:S778–S784. doi: 10.1093/infdis/jix178. [DOI] [PubMed] [Google Scholar]
- 168.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT. American Gastroenterological Association Institute Guideline on the Prevention and Treatment of Hepatitis B Virus Reactivation During Immunosuppressive Drug Therapy. Gastroenterology. 2015;148(1):215–351. doi: 10.1053/j.gastro.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 169.Sarmati L, Andreoni M, Antonelli G, Arcese W, Bruno R, Coppola N, et al. Recommendations for screening, monitoring, prevention, prophylaxis and therapy of hepatitis B virus reactivation in patients with haematologic malignancies and patients who underwent haematologic stem cell transplantation-a position paper. Clin Microbiol Infect. 2017;23(12):935–940. doi: 10.1016/j.cmi.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 170.Gentile G, Andreoni M, Antonelli G, Sarmati L. Screening, monitoring, prevention, prophylaxis and therapy for hepatitis B virus reactivation in patients with haematologic malignancies and patients who underwent haematologic stem cell transplantation: a systematic review. Clin Microbiol Infect. 2017;23(12):916–923. doi: 10.1016/j.cmi.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 171.Zhang MY, Zhu GQ, Shi KQ, Zheng JN, Cheng Z, Zou ZL, et al. Systematic review with network meta-analysis: Comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7(21):30661–30677. doi: 10.18632/oncotarget.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang C, Qin B, Yuan Z, Chen L, Zhou HY. Meta-analysis of prophylactic entecavir or lamivudine against hepatitis B virus reactivation. Ann Hepatol. 2016;15(4):501–511. [PubMed] [Google Scholar]
- 173.Liu WP, Xiao XB, Xue M, Wang GQ, Wang XP, Song YQ, et al. Prophylactic Use of Entecavir for Lymphoma Patients With Past Hepatitis B Virus Infection: A Randomized Controlled Trial. Clin Lymphoma Myeloma Leuk. 2019;19(2):103–108. doi: 10.1016/j.clml.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 174.Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, et al. Hepatitis B Reactivation in Patients With Previous Hepatitis B Virus Exposure Undergoing Rituximab-Containing Chemotherapy for Lymphoma: A Prospective Study. J Clin Oncol. 2014;32(33):3736–3743. doi: 10.1200/jco.2014.56.7081. [DOI] [PubMed] [Google Scholar]
- 175.Kusumoto S, Tanaka Y, Suzuki R, Watanabe T, Nakata M, Takasaki H, et al. Monitoring of Hepatitis B Virus (HBV) DNA and Risk of HBV Reactivation in B-Cell Lymphoma: A Prospective Observational Study. Clin Infect Dis. 2015;61(5):719–729. doi: 10.1093/cid/civ344. [DOI] [PubMed] [Google Scholar]
- 176.Buti M, Manzano ML, Morillas RM, Garcia-Retortillo M, Martin L, Prieto M, et al. Randomized prospective study evaluating tenofovir disoproxil fumarate prophylaxis against hepatitis B virus reactivation in anti-HBc-positive patients with rituximab-based regimens to treat hematologic malignancies: The Preblin study. Plos One. 2017;12(9):e0184550. doi: 10.1371/journal.pone.0184550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, et al. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood. 2019;133(2):137–146. doi: 10.1182/blood-2018-04-848044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu WP, Wang XP, Zheng W, Ping LY, Zhang C, Wang GQ, et al. Hepatitis B virus reactivation after withdrawal of prophylactic antiviral therapy in patients with diffuse large B cell lymphoma. Leuk Lymphoma. 2016;57(6):1355–1362. doi: 10.3109/10428194.2015.1116121. [DOI] [PubMed] [Google Scholar]
- 180.Nakaya A, Fujita S, Satake A, Nakanishi T, Azuma Y, Tsubokura Y, et al. Delayed HBV reactivation in rituximab-containing chemotherapy: How long should we continue anti-virus prophylaxis or monitoring HBV-DNA? Leuk Res. 2016;50:46–49. doi: 10.1016/j.leukres.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 181.Chinese Society of Hepatology CMA Consensus on clinical management of hepatitis B virus-infected women of childbearing age. Zhonghua Gan Zang Bin Za Zhi. 2018;26(3):204–208. doi: 10.3760/cma.j.issn.1007-3418.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19(2):E18–E25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 183.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N Engl J Med. 2016;374(24):2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 184.Shang J, Wen Q, Wang CC, Liu K, Bai L, Tang H. Safety and efficacy of telbivudine for chronic hepatitis B during the entire pregnancy: Long-term follow-up. J Viral Hepat. 2017;24:43–48. doi: 10.1111/jvh.12785. [DOI] [PubMed] [Google Scholar]
- 185.Corbett AH, Kayira D, White NR, Davis NL, Kourtis AP, Chasela C, et al. Antiretroviral pharmacokinetics in mothers and breastfeeding infants from 6 to 24 weeks post-partum: results of the BAN Study. Antivir Ther. 2014;19(6):587–595. doi: 10.3851/imp2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Benaboud S, Pruvost A, Coffie PA, Ekouévi DK, Urien S, Arrivé E, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d’Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 2011;55(3):1315–1317. doi: 10.1128/AAC.00514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau DT, et al. Serum Aminotransferase Flares in Pregnant and Postpartum Women With Current or Prior Treatment for Chronic Hepatitis B. J Clin Gastroenterol. 2018;52(3):255–261. doi: 10.1097/mcg.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 188.Nguyen V, Tan PK, Greenup AJ, Glass A, Davison S, Samarasinghe D, et al. Anti-viral therapy for prevention of perinatal HBV transmission: extending therapy beyond birth does not protect against post-partum flare. Aliment Pharmacol Ther. 2014;39(10):1225–1234. doi: 10.1111/apt.12726. [DOI] [PubMed] [Google Scholar]
- 189.ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15(1):37–41. doi: 10.1111/j.1365-2893.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 190.Jonas MM, Lok AS, McMahon BJ, Brown RS, Wong JB, Ahmed AT, et al. Antiviral therapy in management of chronic hepatitis B viral infection in children: A systematic review and meta-analysis. Hepatology. 2016;63(1):307–318. doi: 10.1002/hep.28278. [DOI] [PubMed] [Google Scholar]
- 191.Sokal EM, Paganelli M, Wirth S, Socha P, Vajro P, Lacaille F, et al. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines Consensus of an expert panel on behalf of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Hepatology. 2013;59(4):814–829. doi: 10.1016/j.jhep.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 192.Wong GL, Seto WK, Wong VW, Yuen MF, Chan HL. Review article: long-term safety of oral anti-viral treatment for chronic hepatitis B. Aliment Pharmacol Ther. 2018;47(6):730–737. doi: 10.1111/apt.14497. [DOI] [PubMed] [Google Scholar]
- 193.Komatsu H, Inui A, Fujisawa T. Pediatric hepatitis B treatment. Ann Transl Med. 2017;5(3):37. doi: 10.21037/atm.2016.11.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wirth S, Zhang H, Hardikar W, Schwarz KB, Sokal E, Yang W, et al. Efficacy and Safety of Peginterferon Alfa-2a (40KD) in Children With Chronic Hepatitis B: The PEG-B-ACTIVE Study. Hepatology. 2018;68(5):1681–1694. doi: 10.1002/hep.30050. [DOI] [PubMed] [Google Scholar]
- 195.Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16–34. doi: 10.1111/apt.13659. [DOI] [PubMed] [Google Scholar]
- 196.Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and Meta-analysis. Int Immunopharmacol. 2017;42:168–175. doi: 10.1016/j.intimp.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 197.Grossi G, Loglio A, Facchetti F, Borghi M, Soffredini R, Galmozzi E, et al. Tenofovir alafenamide as a rescue therapy in a patient with HBV-cirrhosis with a history of Fanconi syndrome and multidrug resistance. J Hepatol. 2018;68(1):195–198. doi: 10.1016/j.jhep.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 198.Shah AS, Amarapurkar DN. Spectrum of hepatitis B and renal involvement. Liver Int. 2018;38(1):23–32. doi: 10.1111/liv.13498. [DOI] [PubMed] [Google Scholar]
- 199.Chinese Society of Infectious Disease CMA Guidelines for diagnosis and treatment of AIDS in China (2018 Edition) Zhonghua Xue Ye Xue Za Zhi. 2018;36(2):705–724. doi: 10.3760/cma.j.issn.1000-6680.2018.12.001. [DOI] [Google Scholar]
- 200.Li Y, Xie J, Han Y, Wang H, Zhu T, Wang N, et al. Lamivudine Monotherapy-Based cART Is Efficacious for HBV Treatment in HIV/HBV Coinfection When Baseline HBV DNA <20,000 IU/mL. J Acquir Immune Defic Syndr. 2016;72(1):39–45. doi: 10.1097/qai.0000000000000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li H, Zhang FJ, Lu HZ, Cai WP, Wu H, Sun YT, et al. Expert consensus on the management of patients with HIV infection and chronic kidney disease. Zhongguo Ai Zi Bin Xing Bin. 2017;23(6):578–583. doi: 10.13419/j.cnki.aids.2017.06.30. [DOI] [Google Scholar]
- 202.Yuen MF. Anti-viral therapy in hepatitis B virus reactivation with acute-on-chronic liver failure. Hepatol Int. 2015;9(3):373–377. doi: 10.1007/s12072-014-9569-x. [DOI] [PubMed] [Google Scholar]
- 203.Lisheng J, Yongguo L, Shu C, Yinghua L. Short-term and long-term efficacy of antiviral treatment of patients with HBV-associated acute-on-chronic liver failure. Zhonghua Xue Ye Xue Za Zhi. 2013;29(2):110–113. doi: 10.3969/j.issn.1001-5256.2013.02.009. [DOI] [Google Scholar]
- 204.Sun LJ, Yu JW, Zhao YH, Kang P, Li SC. Influential factors of prognosis in lamivudine treatment for patients with acute-on-chronic hepatitis B liver failure. J Gastroenterol Hepatol. 2010;25(3):583–590. doi: 10.1111/j.1440-1746.2009.06089.x. [DOI] [PubMed] [Google Scholar]
- 205.Zhang Y, Hu XY, Zhong S, Yang F, Zhou TY, Chen G, et al. Entecavir vs lamivudine therapy for naive patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. World J Gastroenterol. 2014;20(16):4745–4752. doi: 10.3748/wjg.v20.i16.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Huang KW, Tam KW, Luo JC, Kuan YC. Efficacy and Safety of Lamivudine Versus Entecavir for Treating Chronic Hepatitis B Virus-related Acute Exacerbation and Acute-on-Chronic Liver Failure A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2017;51(6):539–547. doi: 10.1097/mcg.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 207.Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir Improves the Outcome in Patients with Spontaneous Reactivation of Hepatitis B Presenting as Acute-On-Chronic Liver Failure. Hepatology. 2011;53(3):774–780. doi: 10.1002/hep.24109. [DOI] [PubMed] [Google Scholar]
- 208.Zheng YS, Sun FL, Liu X. Comparative study on the therapeutic effects of telbivudine and entecavir on acute-on-chronic liver failure. J Clin Hepatol. 2011;27(6):641–642+646. [Google Scholar]
- 209.Chinese Society of Infectious Disease CMA Guidelines for Diagnosis and Treatment of Liver Failure (2018 Edition) Zhonghua Xue Ye Xue Za Zhi. 2019;37(1):1–9. doi: 10.3760/cma.j.issn.1000-6680.2019.01.001. [DOI] [Google Scholar]
- 210.Sun P, Dong X, Cheng X, Hu Q, Zheng Q. Nucleot(s)ide Analogues for Hepatitis B Virus-Related Hepatocellular Carcinoma after Curative Treatment: A Systematic Review and Meta-Analysis. Plos One. 2014;9(7):e102761. doi: 10.1371/journal.pone.0102761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, et al. Effect of Antiviral Treatment With Nucleotide/Nucleoside Analogs on Postoperative Prognosis of Hepatitis B Virus-Related Hepatocellular Carcinoma: A Two-Stage Longitudinal Clinical Study. J Clin Oncol. 2013;31(29):3647–3655. doi: 10.1200/jco.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 212.Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, et al. Antiviral Therapy Improves Postoperative Survival in Patients With Hepatocellular Carcinoma A Randomized Controlled Trial. Ann Surg. 2015;261(1):56–66. doi: 10.1097/sla.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 213.Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33(10):1104–1112. doi: 10.1111/j.1365-2036.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 214.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association Between Nucleoside Analogues and Risk of Hepatitis B Virus-Related Hepatocellular Carcinoma Recurrence Following Liver Resection. JAMA. 2012;308(18):1906–1913. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 215.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43(2):233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 216.Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132(7):458–465. doi: 10.1007/s00432-006-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lao XM, Luo G, Ye LT, Luo C, Shi M, Wang D, et al. Effects of antiviral therapy on hepatitis B virus reactivation and liver function after resection or chemoembolization for hepatocellular carcinoma. Liver Int. 2013;33(4):595–604. doi: 10.1111/liv.12112. [DOI] [PubMed] [Google Scholar]
- 218.Jang JW, Choi JY, Bae SH, Kim CW, Yoon SK, Cho SH, et al. Transarterial chemo-lipiodolization can reactivate hepatitis B virus replication in patients with hepatocellular carcinoma. J Hepatol. 2004;41(3):427–435. doi: 10.1016/j.jhep.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 219.Peng JW, Lin GN, Xiao JJ, Jiang XM. Hepatitis B virus reactivation in hepatocellular carcinoma patients undergoing transcatheter arterial chemoembolization therapy. Asia Pac J Clin Oncol. 2012;8(4):356–361. doi: 10.1111/j.1743-7563.2012.01534.x. [DOI] [PubMed] [Google Scholar]
- 220.Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69(3):813–819. doi: 10.1016/j.ijrobp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 221.Yeo W, Lam KC, Zee B, Chan PSK, Mo FKF, Ho WM, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15(11):1661–1666. doi: 10.1093/annonc/mdh430. [DOI] [PubMed] [Google Scholar]
- 222.Maiwall R, Kumar M. Prevention and Treatment of Recurrent Hepatitis B after Liver Transplantation. J Clin Transl Hepatol. 2016;4(1):54–65. doi: 10.14218/jcth.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cholongitas E, Papatheodoridis GV. High Genetic Barrier Nucleos(t)ide Analogue(s) for Prophylaxis From Hepatitis B Virus Recurrence After Liver Transplantation: A Systematic Review. Am J Transplant. 2013;13(2):353–362. doi: 10.1111/j.1600-6143.2012.04315.x. [DOI] [PubMed] [Google Scholar]
- 224.Yi NJ, Choi JY, Suh KS, Cho JY, Baik M, Hong G, et al. Post-transplantation sequential entecavir monotherapy following 1-year combination therapy with hepatitis B immunoglobulin. J Gastroenterol. 2013;48(12):1401–1410. doi: 10.1007/s00535-013-0761-x. [DOI] [PubMed] [Google Scholar]
- 225.Teperman LW, Poordad F, Bzowej N, Martin P, Pungpapong S, Schiano T, et al. Randomized trial of emtricitabine/tenofovir disoproxil fumarate after hepatitis B immunoglobulin withdrawal after liver transplantation. Liver Transpl. 2013;19(6):594–601. doi: 10.1002/lt.23628. [DOI] [PubMed] [Google Scholar]
- 226.Chen G, Liu H, Hu ZQ, Bai JH, Liu QY, Zhao YP, et al. A new scheme with infusion of hepatitis B immunoglobulin combined with entecavir for prophylaxis of hepatitis B virus recurrence among liver transplant recipients. European J Gastroenterol Hepatol. 2015;27(8):901–906. doi: 10.1097/meg.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 227.Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2019;40(1):52–57. doi: 10.3760/cma.j.issn.0253-2727.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]