Abstract
Wolfram syndrome (WS) is an ultra-rare progressive neurodegenerative disorder defined by early-onset diabetes mellitus and optic atrophy. The majority of patients harbour recessive mutations in the WFS1 gene, which encodes for Wolframin, a transmembrane endoplasmic reticulum protein. There is limited availability of human ocular and brain tissues, and there are few animal models for WS that replicate the neuropathology and clinical phenotype seen in this disorder. We, therefore, characterised two wfs1 zebrafish knockout models harbouring nonsense wfs1a and wfs1b mutations. Both homozygous mutant wfs1a−/− and wfs1b−/− embryos showed significant morphological abnormalities in early development. The wfs1b−/− zebrafish exhibited a more pronounced neurodegenerative phenotype with delayed neuronal development, progressive loss of retinal ganglion cells and clear evidence of visual dysfunction on functional testing. At 12 months of age, wfs1b−/− zebrafish had a significantly lower RGC density per 100 μm2 (mean ± standard deviation; 19 ± 1.7) compared with wild-type (WT) zebrafish (25 ± 2.3, p < 0.001). The optokinetic response for wfs1b−/− zebrafish was significantly reduced at 8 and 16 rpm testing speeds at both 4 and 12 months of age compared with WT zebrafish. An upregulation of the unfolded protein response was observed in mutant zebrafish indicative of increased endoplasmic reticulum stress. Mutant wfs1b−/− zebrafish exhibit some of the key features seen in patients with WS, providing a versatile and cost-effective in vivo model that can be used to further investigate the underlying pathophysiology of WS and potential therapeutic interventions.
Subject terms: Genetics, Eye diseases
Introduction
Wolfram syndrome (WS) is a neurodegenerative disorder defined historically by a cluster of clinical manifestations, namely, diabetes insipidus, diabetes mellitus, optic atrophy and sensorineural deafness (DIDMOAD)1–3. It is now well established that a significant proportion of patients will develop additional neurological and psychiatric deficits such as ataxia, epilepsy and depression, renal tract abnormalities, and in some cases infertility4–6. The majority of patients with WS harbour recessive mutations within the WFS1 gene (4p16, OMIM 606201), which encodes for the transmembrane endoplasmic reticulum (ER) protein Wolframin1,7. Wolframin is abundantly expressed in retinal, neuronal and muscle tissues8. It is a multifunctional protein that regulates a host of cellular functions including the dynamic interaction with mitochondria at mitochondria-associated membranes (MAMs)9,10. It negatively regulates the ER stress sensor ATF6α in β-islet cells through ubiquitination and proteasome-mediated degradation of the protein11. Wolframin also has roles in calcium ion homeostasis9,12–15, proinsulin modification16, the regulation of the cell cycle17, and calpain activation18.
The majority of experimental animal studies have been performed on mouse models of WS with a focus on the development of diabetes and ER stress, which is a key pathway implicated in the loss of β-islet cells19. A knockout mouse with disruption of exon 2 in the Wfs1 gene developed progressive β-cell loss and glucose intolerance20. It has been suggested that an alternative glucose clearing pathway through the urine exist in mice protecting them from a full diabetic phenotype20,21. Testing of visual function of the exon 2, Wfs1 knockout mouse showed only mild changes with no differences in visual function compared with the early-onset optic atrophy phenotype seen in patients with WS22. A rat model of WS has recently been characterised (Wfs1-ex5-KO232) with features of diabetes mellitus and a reduction in brain medullary volume, mirroring the neurodegeneration observed in patients with WS23. This rat model also developed retinal gliosis and cataracts with optic nerve volume reductions and disturbed myelin structure23. A more basic Drosophila model with knockdown of wfs1 has been generated that showed behavioural deficits, neurodegeneration and a reduced lifespan24.
Visual loss in WS is progressive, starting in early childhood, and it is an important cause of registrable blindness in children and young adults25. Research into WS has been limited by the lack of access to diseased human tissues, in particular retinal, optic nerve and brain samples. Zebrafish models are frequently used to study inherited ocular and central nervous system disorders as the embryos are amenable to germline genetic manipulation or more transient regulation of gene expression with morpholinos. Another distinct advantage is that the embryos are optically transparent, allowing easy visualisation of neuronal development in vivo, and there is great similarity in anatomical structures with humans. Using a number of whole-system and live imaging techniques, it is also possible to monitor and quantify changes during early development that would otherwise be more technically challenging and costly in murine models. Morpholinos have been used to transiently knockdown genes in zebrafish thought to be involved in the regulation of β-cell mass and the development of type 2 diabetes mellitus, including wfs126. To our knowledge, no zebrafish with germline genetic knockout of wfs1 has been reported that replicates the clinical phenotype seen in patients with WS.
Here, we describe a novel zebrafish model of WS and examine the role played by Wolframin in early development and neurodegeneration. Due to a genome duplication, WFS1 has two orthologues in zebrafish, namely, wfs1a and wfs1b. Our findings indicate that wfs1b−/− mutant zebrafish show disturbed neuronal development and progressive loss of retinal ganglion cells (RGCs) with impaired visual function.
Results
wfs1a−/− and wfs1b−/− zebrafish models
The sequences of the zebrafish orthologues wfs1a and wsf1b were compared with the human WFS1 gene. The wfs1a and wfs1b sequences had 53.19% and 53.97% sequence homology with the human WFS1 gene, respectively (Fig. S1A,B). The expression of wfs1 in wild-type (WT) zebrafish was examined using reverse transcriptase PCR (RT-PCR). wfs1b is expressed at the one-cell stage suggesting possible maternal expression and it is constitutively expressed. wfs1a expression begins at a later time point only at 24 h post-fertilisation (hpf) (Fig. S1C). Quantitative RT-PCR (qRT-PCR) was used to determine the expression levels of the zebrafish orthologues wfs1a and wfs1b in tissue lysates from 4-month-old zebrafish. wfs1a is more highly expressed in muscle compared with wfs1b. wfs1b is highly expressed in the eye and the brain, whereas wfs1a shows low levels of expression in these two tissues (Fig. S1D,E).
Zebrafish with germline mutations in wfs1a and wfs1b were crossed to create single homozygous wfs1a−/− and wfs1b−/− lines, and a double knockout wfs1a−/−b−/− zebrafish (Fig. S2).
Morphological assessment of wfs1a−/− and wfs1b−/− zebrafish
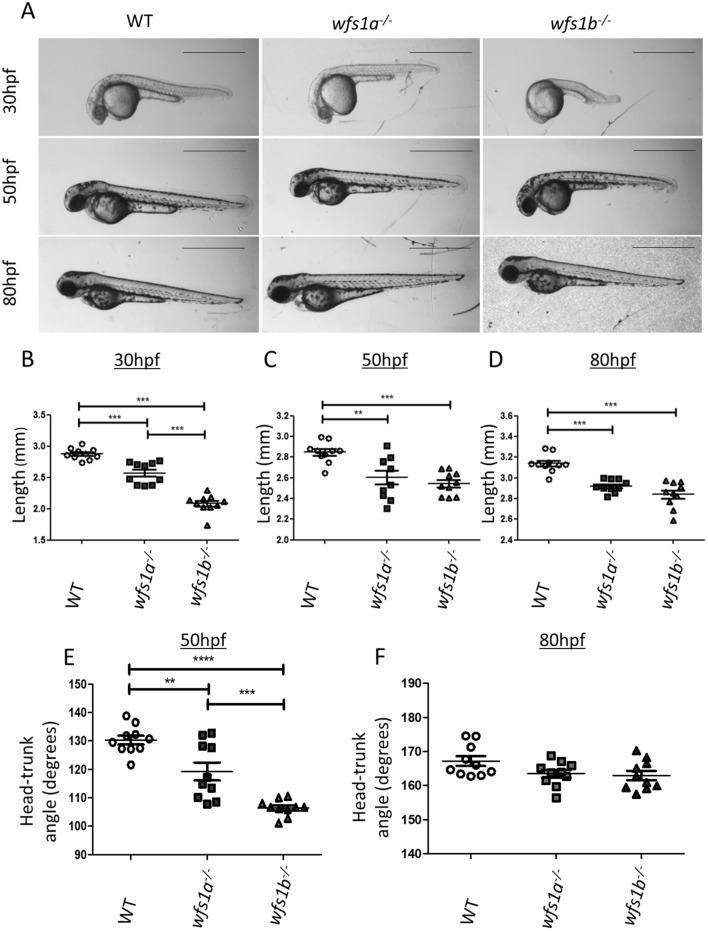
The early embryonic development of wfs1a−/− and wfs1b−/− zebrafish was assessed morphologically at 30, 50 and 80 hpf (Fig. 1A). Developing wfs1a−/− and wfs1b−/− embryos were significantly shorter compared with WT embryos at all time points (Fig. 1B–D). This developmental delay was accompanied by a significant change in head-trunk angle at 50 hpf, which was restored by 80 hpf (Fig. 1E,F). There were no significant differences in eye area between wild-type (WT), wfs1a−/− and wfs1b−/− embryos at 80 hpf when normalised to length (Fig. S5).
Figure 1.
Phenotypic analysis of wfs1a−/− and wfs1b−/− zebrafish. (A) Zebrafish were imaged at 30, 50 and 80 hpf. Scale bars represent 1 mm. (B–D) Zebrafish length at 30 hpf (B), 50 hpf (C) and 80 hpf (D). (E,F) Zebrafish head-trunk angles at 50 hpf (E) and 80 hpf (F). Zebrafish length and head tail angle was measured using ImageJ and data plots represent mean ± SEM (n = 10). Statistical significance was determined by One-Way ANOVA with Bonferroni multiple comparisons. **p < 0.01; ***p < 0.001; hpf hours post-fertilisation, SEM standard error of the mean.
Heat shock response of wfs1a−/− and wfs1b−/− zebrafish
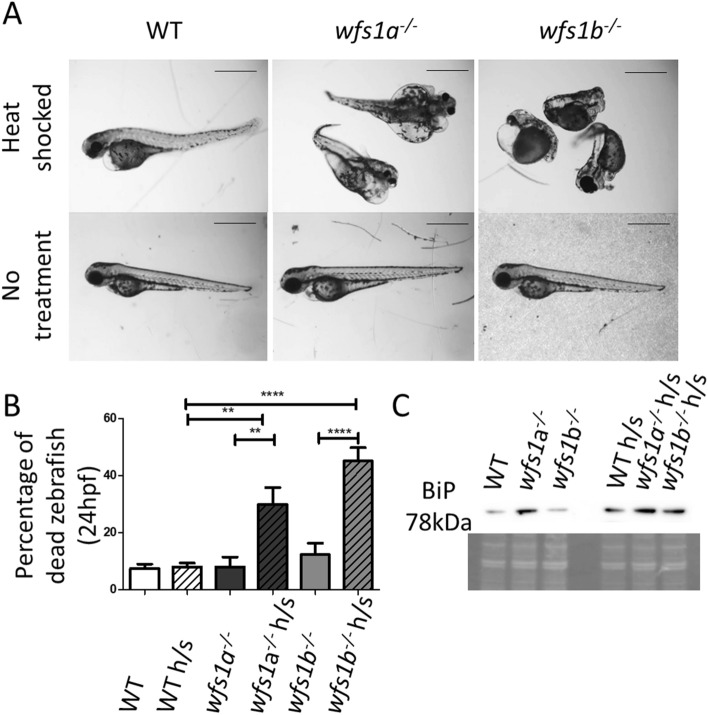
wfs1a−/− and wfs1b−/− zebrafish were heat shocked to increase the amount of unfolded proteins in the ER and to examine for aberrations in the unfolded protein response (UPR), which is a hallmark of ER stress. Heat shocking resulted in severe morphological changes in the tail curvature and eye development, and in the formation of prominent cardiac oedema (Fig. 2A). Significantly higher levels of death were observed in the wfs1a−/− and wfs1b−/− zebrafish compared to WT (Fig. 2B). Heat shock treatment of wfs1a−/− and wfs1b−/− zebrafish stimulated the UPR, as evidenced by increased BiP, which is the master regulatory of the UPR (Fig. 2C). Although BiP expression seemed increased in wfs1a−/− zebrafish without heat shock treatment, this was not a consistent finding in repeat experiments. Quantitative PCR analysis of BiP expression in tissue lysates at 48 hpf showed no significant differences between WT, wfs1a−/− and wfs1b−/− zebrafish (Fig. S6).
Figure 2.
Effects of the unfolded protein response in wfs1a−/− and wfs1b−/− zebrafish. Treated zebrafish were heat shocked for 1 h at 6 hpf (groups of 50 embryos). (A) Representative images of the morphological effects for each genotype at 80 hpf. Scale bar = 1 mm. (B) Percentage of dead zebrafish at 24 hpf. Data plots represent mean ± SEM (n = 5 groups of 50). Statistical significance was determined by One-Way ANOVA with Bonferroni multiple comparisons. (C) Immunoblot of BiP in untreated and heat shocked zebrafish showing an upregulation of BiP in response to heat shock. Coomassie staining demonstrates equal loading. *p < 0.05; **p < 0.01; ****p < 0.0001; h/s: heat shock treated.
Neuronal development of wfs1a−/− and wfs1b−/− zebrafish
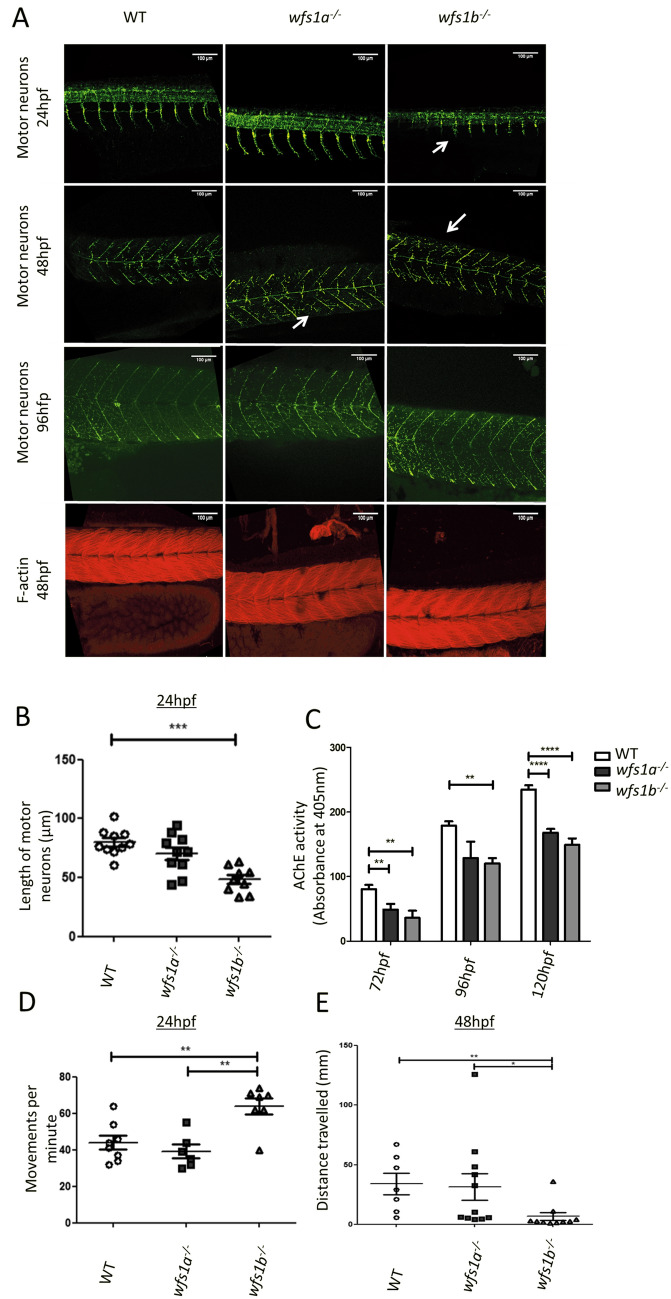
Whole-mount immunofluorescence studies of zebrafish tail motor neurons were performed to visualise neuronal development in wfs1a−/− and wfs1b−/− zebrafish. Shorter or absent motor neurons were observed in the tail region in both wfs1a−/− and wfs1b−/− zebrafish (Fig. 3A). The length of the motor neurons within the tail of wfs1b−/− zebrafish was significantly shorter compared with WT zebrafish at 24 hpf (p < 0.005, Fig. 3B). At 48 hpf, there was no significant difference in the length of motor neurons between wfs1a−/−, wfs1b−/− and WT zebrafish.
Figure 3.
Neuronal development in wfs1a−/− and wfs1b−/− zebrafish. (A) Immunofluorescence of motor neurons (SV-2 stained using anti-SV2 antibody in green) and muscle fibres (F-actin stained using phalloidin in red). Shorter or missing neurons are highlighted with white arrows. (B) Quantification of the length of motor neurons in 24 hpf zebrafish (WT n = 10; wfs1a n = 11; wfs1b n = 9). For each fish, 9–10 neurons were measured and the average length was calculated. (C) Acetylcholine esterase (AChE) activity assay of developing zebrafish larvae (3–5 dpf). (D) Coiling response of zebrafish embryos at 24 hpf. The average movement per fish per minute was calculated from ~ 15 embryos (WT n = 8; wfs1a n = 6; wfs1b n = 7) (Supplementary Video 1). (E) Quantification of the touch response of zebrafish embryos at 48 hpf. The distance travelled was recorded in response to tactile stimulation (WT n = 8; wfs1a n = 11; wfs1b n = 10) (Supplementary Video 2). Data plots represent mean ± SEM. Statistical significance was calculated using One-way ANOVA with Bonferroni’s multiple comparison tests. **p < 0.01; ***p < 0.001; ****p < 0.0001; dpf days post-fertilisation.
Phalloidin staining of filamentous (F)-actin showed normal structural integrity of the tail musculature. At 4 days post-fertilisation (dpf), the neuronal axonal segments that were absent along the myosepta during early development were seen to extend in the appropriate location (Fig. 3A). Reduced acetylcholinesterase (AChE) activity was observed at 3, 4 and 5 dpf (Fig. 3C).
To determine whether the observed disruption in neuronal development was reflected at a functional level, the motor behaviours of the zebrafish were tested using coiling and touch response assays. At 24 hpf, zebrafish perform spontaneous coiling movements (coiling response). A significant increase in the occurrence of these coiling movements was observed in wfs1b−/− zebrafish compared with wfs1a−/− and WT zebrafish (Fig. 3D, Supplementary Video 1). By 48 hpf, zebrafish have developed the ability to respond to tactile stimulation by swimming rapidly away from the applied stimulus (touch response assay). The touch response in terms of distance travelled was significantly decreased in wfs1b−/− zebrafish compared with wfs1a−/− and WT zebrafish (Fig. 3E, Supplementary Video 2).
Retinal structure and visual function in wfs1a−/− and wfs1b−/− zebrafish
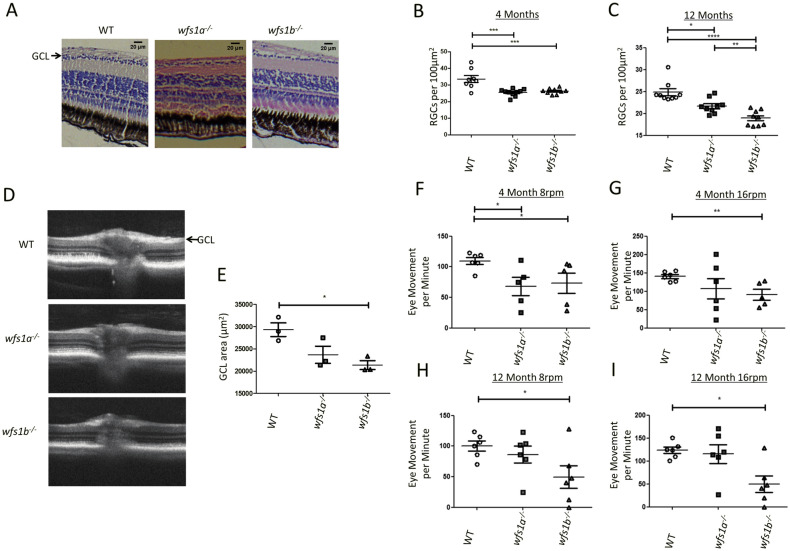
Histological analysis of wfs1a−/− and wfs1b−/− zebrafish retinas revealed a significant loss of RGCs at 4 months of age (Fig. 4A,B). The RGC density per 100μm2 (mean ± standard deviation) was 34 ± 5.9 for WT zebrafish, 26 ± 2.1 for wfs1a−/− zebrafish, 27 ± 1.7 for wfs1b−/− zebrafish. At 12 months of age, wfs1b−/− zebrafish had a significantly lower RGC density (19 ± 1.7) compared with WT zebrafish (25 ± 2.3) (Fig. 4C). Optical coherence tomography (OCT) imaging showed thinner RGC layers in wfs1a−/− and wfs1b−/− zebrafish compared with WT zebrafish at 12 months of age (Fig. 4D). The GCL area was significantly thinner in wfs1b−/− zebrafish compared with WT zebrafish (Fig. 4E). A non-significant trend was observed for wfs1a−/− zebrafish compared with WT zebrafish.
Figure 4.
Retinal ganglion cell count and visual function in wfs1a−/− and wfs1b−/− zebrafish. (A) Representative images of WT, wfs1a−/− and wfs1b−/− retinal sections at 4 months of age (scale bar = 20 µm). For RGC counts, 6 boxes of the same area (100 μm2) were used with 3 boxes on either side of the optic nerve. The number of RGC cell bodies were counted and averaged. (B) RGC count per 100 μm2 at 4 months of age (WT n = 8; wfs1a−/− n = 10; wfs1b−/− n = 9). (C) RGC count per 100 μm2 at 12 months of age (WT n = 10; wfs1a−/− n = 9; wfs1b−/− n = 9). Data plots represent mean ± SEM. Statistical significance was calculated using the Kruskal–Wallis test (One-way ANOVA on ranks). (D) Optical coherence tomography (OCT) images of retinal cross-sections from 12-month-old zebrafish showing significant thinning of the ganglion cell layer (GCL) in wfs1a−/− and wfs1b−/− zebrafish (arrow). (E) Measurement of GCL area (WT mean = 29,393 ± 2653 μm2; wfs1a−/− mean = 23,688 ± 3332 μm2; wfs1b−/− mean = 21,363 ± 1,737 μm2; n = 3 for all 3 groups). Data plots represent mean ± SEM. Statistical significance was calculated using One-way ANOVA with Bonferroni’s multiple comparison tests. (F–I) Optokinetic response (OKR) of 4- and 12-month-old fish tested at 8 rpm and 16 rpm. Videos were recorded of fish eye tracking and the movements were manually counted (Supplementary video 3). Data plots represent mean ± SEM. Statistical significance was calculated using One-way ANOVA with Bonferroni’s multiple comparison tests. *p < 0.05; **p < 0.01; ***p < 0.005; ****p < 0.001; rpm: revolutions per minute.
In order to investigate the effects on visual function, optokinetic response (OKR) tests were performed at 4 and 12 months of age (Fig. 4F–I, Supplementary Video 3). Zebrafish eye movements were analysed in response to a rotating black and white grating at different rotations per minute (rpm). The OKR for wfs1b−/− zebrafish was significantly reduced at 8 and 16 rpm testing speeds at both 4 and 12 months of age. In comparison, the OKR for wfs1a−/− zebrafish was not significantly different compared with WT zebrafish at 4 months of age (16 rpm) and 12 months of age (8 and 16 rpm).
Fertility of wfs1a−/− and wfs1b−/− zebrafish
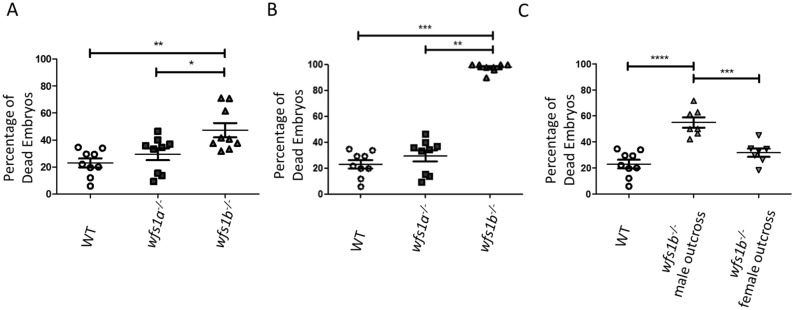
A significantly higher number of unfertilised embryos was observed in wfs1b−/− zebrafish that were < 9 months old compared with wfs1a−/− and WT zebrafish (Fig. 5A). In more aged zebrafish (> 9 months old), a further drop in fertility was observed for wfs1b−/− zebrafish, with a mean of 97.8% of embryos remaining unfertilised (Fig. 5B). When wfs1b−/− zebrafish were outcrossed with WT zebrafish, a high percentage of dead embryos were seen in the wfs1b−/− male outcross, but not in the wfs1b−/− female outcross (Fig. 5C). Double mutants (wfs1a−/−b−/−) were unable to breed indicating significant infertility.
Figure 5.
Fertility of wfs1a−/− and wfs1b−/− zebrafish. (A) Percentage of dead embryos at 24 hpf produced from adults < 9 months (n = 9). (B) Percentage of dead embryos at 24 hpf produced from adults > 9 months (n = 9). (C) Percentage of dead embryos at 24 hpf in randomly selected embryos from wfs1b−/− zebrafish (male or female) that were outcrossed to WT (controls n = 9, wfs1b−/− outcrosses n = 7). A total of 50 randomly selected embryos were placed in E3 medium, incubated overnight and any dead embryos were determined the next morning. Data plots represent mean ± SEM. Statistical significance was determined by One-Way ANOVA with Bonferroni multiple comparisons. **p < 0.01; ***p < 0.001; ****p < 0.0001.
The significantly higher number of unfertilised embryos in wfs1b−/− knockouts could be due to fertilised embryos dying within the first 24 h. To determine if that was the case, eggs were collected at 8 hpf and unfertilised embryos were removed. The percentage of dead embryos was then assessed at 24 hpf. No significant difference was observed between fertilised controls (mean = 5.6%), wfs1a−/− knockouts (mean = 6.4%), and wfs1b−/− knockouts (mean = 11.1%) (Fig. S7).
Discussion
Zebrafish has been successfully applied to characterize disease mechanisms for rare inherited diseases and they have also proven useful as in vivo models for therapeutic screening. This is of particular relevance to WS caused by recessive WFS1 mutations, which is a syndrome characterized by progressive visual loss from early childhood secondary to the irreversible loss of RGCs and optic nerve degeneration. As the zebrafish eye is comparable to the human eye27,28, and optic atrophy is a defining clinical feature of WS29, we investigated the morphological and functional characteristics in embryos and adult fish established from two mutant lines carrying stop codon mutations in wfs1a and wfs1b, which are the orthologues of WFS1 in zebrafish.
Homozygous mutant wfs1a−/− and wfs1b−/− embryos showed significant developmental delay as judged by their morphology. This has been corroborated in culture models where impaired cell cycle progression has been observed in β-islet cells of a wfs1 knockout mouse16. Cell cycle delay, especially in the early stages, could slow the development of the zebrafish embryo by reducing the rate of cell division. In addition to changes in the cell cycle, a greater susceptibility to apoptosis was observed in β-islet cells from this mutant mouse model, which was linked to an increased ER stress response17. We observed increased death rates in both wfs1a−/− and wfs1b−/− zebrafish and upregulation of the UPR as a response to heat shock treatment, pointing towards an increased ER stress response30. Changes in the UPR pathway have been well characterized in rat, mouse and Drosophila knockout models, as well as in human cell models of WS17,23,24,31. It will be important to explore as part of future mechanistic studies whether wfs1b−/− zebrafish exhibit increased apoptosis and its contribution to the development of the neurodegenerative phenotype, in particular the loss of RGCs and visual dysfunction.
Over 50% of patients with WS will develop significant neurological complications, in particular cerebellar ataxia, peripheral neuropathy and spasticity due to pyramidal tract dysfunction5. As part of our phenotyping protocol, we investigated whether neuromuscular development was impaired in wfs1a−/− and wfs1b−/− zebrafish. Both models showed impairment in early development of motor neurons, but this defect was more pronounced in the wfs1b−/− zebrafish. The dorsal growth along the vertical myoseptum was delayed with motor neurons not extending along a number of myosepta until later in development (4 dpf). Similar results were obtained in Wfs1 shRNA-transfected mouse cortical neurons, which showed an improvement in mature neuron growth after an initial delay during early development9. It should be stressed that the developmentally delayed mutant embryos were not developed to the same stage prior to analysing motor neurons and measuring axon length in the tail region. Additional experiments comparing mutant and WT zebrafish that have reached similar developmental time points will provide further insight into the contribution of developmental delay to the observed neuronal defects.
The descending motor axons are affected in a subgroup of patients with WS resulting in spasticity4–6. In this study, we measured axon length in the tail region to assess general neuronal development in mutant zebrafish and to first establish that the defects in locomotion are indeed neural in origin and not due to the other factors, such as improper muscle development. The neural circuit for locomotion in the zebrafish is well characterised. It is controlled by the reticulospinal network in the hind brain, which is primarily composed of a pair of neurons, namely, the Mauthner (M) cells and the spiral fibre neurons. The M cells and its associated motor neurons running towards the tail form an intricate network that is activated in response to different types of stimuli (touch, sound and visual), ultimately controlling the movement of the zebrafish. Thus, it was important to look at the motor neurons in the tail region first to detail any possible defects and to confirm that the defects are neural in origin. Further work is needed to conduct more extensive analysis of the reticulospinal network in wfs1b−/− zebrafish.
AChE is expressed in the central nervous system, peripheral cholinergic neurons and muscle fibres of the zebrafish embryo32,33. By measuring its activity in homogenates of zebrafish embryos, this assay provides an indirect measure of AChE, which correlates with the amount of neuronal tissue and the overall developmental stage in zebrafish34. A decrease in AChE activity was found in both wfs1a−/− and wfs1b−/− zebrafish, with the latter manifesting a more severe reduction. Phalloidin staining of filamentous (F)-actin showed normal structural integrity of the muscle fibres in the tail musculature, indicating that neuronal outgrowth is impaired without an underlying primary muscle fibre disorder. To determine whether the observed disruption in neuronal development was functionally relevant, the motor behaviours of the zebrafish were quantified using the coiling and touch response assays35,36. Both responses were significantly disrupted in wfs1b−/− zebrafish, consistent with a developmental delay, and indicating that a lack of Wolframin likely affects neuronal development, contributing to the neurological deficits seen in patients with WS. Defective mitophagy has been implicated as the mechanism by which neuronal development is delayed in neurons from Wfs1-deficient mice, with shRNA silencing of the mitophagy-related proteins PINK1 and Parkin correcting this defect9. Further investigation of mitochondrial function and mitophagy in our zebrafish model is needed to provide a better understanding of the mechanisms involved in delayed neuronal development.
A pathological hallmark of WS is progressive RGC loss resulting in optic atrophy and visual failure in affected patients37. A significant reduction in RGC density was observed in both wfs1a−/− and wfs1b−/− models, with the loss of RGCs being more prominent in wfs1b−/− zebrafish at 12 months implying a more severe degenerative process compared with the wfs1a−/− zebrafish. OCT imaging confirmed marked thinning of the RGC layer and this was correlated with the reduced visual function recorded using the OKR in wfs1b−/− zebrafish. Our data show that zebrafish lacking the wfs1b orthologue is an attractive model that successfully recapitulates the progressive RGC loss and visual dysfunction seen in patients with WS. Research into this relatively rare inherited form of optic atrophy has been limited by the lack of human tissues and the wfs1b−/− zebrafish will be a useful resource to dissect the disease mechanisms that precipitate RGC loss in this disorder.
WS is a multisystemic neurodegenerative disorder and reduced fertility has also been reported in some patients38,39. Although this observation needs to be investigated further, our data indicate that the fertility of adult fish lacking wfs1b is impaired with an increased number of unfertilised embryos. When adults older than 9 months of age were outcrossed with WT zebrafish, they were able to produce viable offspring. However, the wfs1b−/− males still exhibited a significantly higher percentage of unfertilised offspring. Consistent with our findings, male knockout mice with deleted Wfs1 gene have reduced fertility due to abnormal sperm morphology and reduced number of spermatogenic cells40.
Wolframin plays an important role in early zebrafish development and wfs1b−/− zebrafish exhibit a more severe neurological and ocular phenotype compared with the wfs1a−/− zebrafish. This could be due to their tissue-specific expression patterns with wfs1b being constitutively expressed with high levels in the eye and brain compared with wfs1a, which is predominantly expressed in muscle. Interestingly, skeletal muscle seems to be spared in WS with myopathy not being reported in patients with confirmed pathogenic WFS1 mutations5.
In summary, we have characterized two wfs1 zebrafish knockout models with the wfs1b−/− zebrafish recapitulating some of the key ocular and neurological deficits observed in patients with WS. This zebrafish model will be a valuable tool to further investigate the pathophysiology in WS and the pathways that could potentially be modulated to delay or stop the neurodegenerative process driving cellular loss in this disorder. WS is an important cause of blindness in children and young adults. There are currently no effective treatments available and the progressive loss of RGCs and visual dysfunction observed in the wfs1b−/− zebrafish provide powerful readouts for drug screening and investigating new therapeutic interventions.
Methods
All the methods have been reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org).
Zebrafish maintenance
All zebrafish procedures were performed under Home Office UK licence regulations and approved by the Newcastle University Animal Welfare and Ethical Review Board. Fish strains used in this study include AB, sa10021 (wfs1a) and sa16422 (wfs1b). Embryos were collected from breeding pairs of zebrafish and grown for up to 5 days in E3 medium (5 mM NaCl, 0.17 mM KCl, 10 mM HEPES, 0.33 mM MgSO4, 0.33 mM CaCl2 0.00002% methylene blue) placed in an incubator at 27.5 °C.
Zebrafish strains
Two heterozygote mutant lines were purchased from the European Zebrafish Resource Centre (EZRC). The wfs1a (wfs1asa1002141) line had a G > A nonsense mutation at amino acid (aa) 692 resulting in a TAG stop codon (Fig. S3). The wfs1b (wfs1bsa1642241) line had a G > A nonsense mutation at aa 493 resulting in a TGA stop codon. The F2 lines obtained from the EZRC were outcrossed twice prior to experimental work. The lines were inbred to create two homozygous lines, wfs1a−/− and wfs1b−/− (Fig. S2). This was confirmed by Sanger sequencing of isolated genomic DNA (Fig. S2A,B). Unless stated, experimental crosses were performed with homozygous knockouts to remove the confounding factor of maternal RNA. Zebrafish were group mated with 3 males and 3 females per large breeding tank. Double knockout mutants were also derived (Fig. S2C). Experimental blinding was not performed. However, prior to analysis, data was blinded to minimise potential bias. AB control lines were used in all experiments (https://zfin.org/action/genotype/view/ZDB-GENO-960809-7).
Zebrafish imaging
Imaging was carried out using bright field microscopy. Images were taken on a Leica MZ16F stereomicroscope with a Leica DFC420 C camera attachment on the Leica Application Suite V3 program. Zebrafish measurements were performed using ImageJ. A micrometer image at each magnification was used to set the scale. Zebrafish length measurements at 30 hpf used straight lines from head to tail. At 50 hpf and 80 hpf, measurements were taken from the first muscle somite to the tip of the tail. Head-trunk angles were assessed and quantified using the angle tool from ImageJ as described previously42. The segmented line tool in ImageJ was used to measure axon length (Fig. S4).
Sequencing
Genomic DNA was isolated using the Hotshot method43. DNA was amplified by PCR using the following primers:wsf1a_F ACCCCAATCAGACACACCTT, wsf1a_R ATCGAGTCCAGAGTCGCAGT, wfs1b_F AGCCATACCTCTACTTTCTCCT and wfs1b_R AGATGCACACTGTTACGATCA using Mytaq (Bioline). PCR reactions were purified by ExoFastAP reaction to remove any excess nucleotides that could interfere with Sanger sequencing. The purified products were then subjected to a BigDye terminator cycle sequencing reaction v3.1 (Applied Biosystems). The big dye reaction was purified by ethanol precipitation, resuspended in HiDi (Applied Biosystems) and then sequenced using a capillary electrophoresis on a 3130xl Genetic Analyser (Applied Biosystems).
Immunofluorescence of whole-mount zebrafish
Zebrafish were manually dechorionated and euthanized in 4 mg/ml buffered tricaine methanesulfonate (MS222), diluted 1:1 in system water. Whole-mount staining was performed as previously described44. Mouse anti-SV2 antibody was applied at a 1:200 dilution (Developmental Studies Hybridoma Bank) and Alexa Fluor™ 488 (ThermoFisher) anti-mouse secondary antibody was used at a 1:1000 dilution. F-actin staining was performed using Alexa Fluor™ 594 Phalloidin (ThermoFisher) at a 1:1000 dilution. Zebrafish were imaged on a Nikon A1R confocal microscope at a 20× objective (NA 0.75) and z-stack images were obtained.
Larvae tracking
At 24 hpf and 27 hpf, zebrafish were imaged using a Leica stereomicroscope with a Chameleon digital camera (CMLN-13s2M) 25 frames per second. Starting at 17 hpf, zebrafish start to perform spontaneous coiling movements (coiling response), which refers to a movement of the tail in the chorion, and this gradually decreases in frequency until it stops altogether at 27 hpf (35, 36). Spontaneous coiling movements were counted per embryo over a period of one minute. Zebrafish tracking was performed as described previously45. Briefly, the 48 hpf touch response was recorded using a Canon legria hfr76 camera at 25 frames per second. Single embryos were placed in E3 medium and then touched on the back of the head with a fine pipette tip. Videos were analysed using Trackmate (ImageJ).
Immunoblot
Zebrafish embryos/tissues were lysed in RIPA buffer with protease inhibitor tablet (Roche) using a Tissue Ruptor. Lysates were maintained at 4 °C for 30 min before centrifugation at 13,000×g for 15 min. The supernatant was quantified with the Bradford assay. 50 μg of protein was loaded according to the NuPAGE Bis–Tris Mini Gels protocol (ThermoFisher) and transferred using iBlot™ 2 Transfer Stacks, PVDF, mini (ThermoFisher) according to manufacturer’s instructions. PVDF membranes were blocked in 5% low-fat dried milk in Tris-buffered saline (TBS) and 0.1% Tween 20 (TBS-T) for 1 h at room temperature, and then incubated with primary antibody (in 5% milk TBS-T), Anti-HSPA5 1:1000 dilution (Abnova PAB2462), overnight at 4 °C. Blots were washed with TBS-T and anti-rabbit polyclonal HRP conjugated antibody (Agilent, Santa Clara, USA) secondary antibody in 5% milk TBS-T was added for 1 h. SeeBlue Plus2 Pre-stained protein standard was used (ThermoFisher). The blots were visualised using Biorad Clarity ECL and imaged with an Amersham Imager 600.
Histology
Adult zebrafish were euthanized in 4 mg/ml buffered tricaine methanesulfonate (MS222), diluted 1:1 in system water, before being decapitated. Their heads were then fixed for 10 days at 4 °C in 4% paraformaldehyde. Decalcification was performed as described previously46. The tissue was dehydrated in increasing grades of ethanol (70%, 90% and 100%), cleared in xylene and impregnated with paraffin wax. Zebrafish heads were embedded in paraffin, sectioned at 4 μm using a Leica RM 2135 microtome (Leica Biosystems) and then subjected to haematoxylin and eosin staining. Images were acquired by light microscopy using an Axio Imager Z1 fluorescence microscope (Zeiss).
Heat shock treatment
The UPR, which is a hallmark of ER stress, is regulated by Wolframin11. Heat shocking increases the amount of unfolded proteins in the ER allowing for the examination of the UPR. For inducing the UPR, zebrafish were heated to 37.5 °C in E3 medium for 1 h. Zebrafish were euthanised in 4 mg/ml buffered tricaine methanesulfonate (MS222), diluted 1:1 in system water. To anesthetise zebrafish, a 1:20 dilution was used in system water. Zebrafish were group mated with 3 males and 3 females per large breeding tank. Zebrafish were exposed to 1 h of heat shock treatment at 37 °C to induce protein misfolding30.
Optokinetic response (OKR)
Zebrafish were anaesthetized, immobilised in a foam holder within a petri dish filled with tank water and then placed into a custom-made optokinetic device47. This device included a 12 cm rotating optokinetic drum with adjustable speeds and stereo microscope (Zeiss Stemi-2000C) c-mounted with a digital SLR camera at 30 fps (Nikon D5100). The distance between the zebrafish eye and the rotating drum was 6.5 cm. When the fish had regained consciousness, the drum was rotated for 30 s clockwise and 30 s anti-clockwise, using a grating width of 0.8 cm. The rotational speeds used were 8 rpm and 16 rpm, corresponding to angular speeds of 48 degrees per second and 96 degrees per second, respectively. The eye movements were counted manually from video recordings.
Optical coherence tomography (OCT)
OCT images were captured using the Bioptigen Envisu R2200 SDOIS (Bioptigen, Inc., Morrisville, USA)48. The zebrafish were anesthetised in a 1:20 tricaine to system water ratio and placed in a rubber holder. For optic nerve imaging, a 1.4 × 1.4 mm perimeter protocol with 1000 A-scans per B-scan with 100 total scans was used. Images were created using ImageJ. The ganglion cell layer (GCL) was defined manually using ImageJ. The combined GCL area was then determined for the region extending from the optic disc to a radius of 400 µm.
RNA extraction and cDNA synthesis
RNA was extracted using a combined method of TRIzol (Invitrogen) and RNeasy kit (Qiagen). About 50 mg of tissue was homogenised in TRIzol and incubated at room temperature for 5 min. 1:5 ratio of chloroform to TRIzol was added and mixed before centrifugation at 12,000×g for 15 min. The aqueous phase was removed and added to 70% ethanol at a 1:1 ratio. This was then added to an RNeasy spin column as per the manufacturer’s instructions for the remainder of the protocol. cDNA synthesis was performed using the Applied Biosystems: High-Capacity cDNA Reverse Transcription Kit with 1000 ng RNA.
Quantitative PCR (qPCR)
qPCR was performed using qPCR iQ™ SYBR® Green Supermix (BioRad) according to the manufacturer’s instructions. The following primers were used with an annealing temperature of 58 °C: (i) wfs1a: wfs1a-qF 5′-TGTGCCCTGTGTGCTCTAC-3′ and wfs1a-qR 5′-GGCAACACAAGTACGGATCA-3′; (ii) wfs1b: wfs1b-qF 5′-CGCCCCGAATCTAAGCTTTT-3′ and wfs1b-qR 5′-GCGGAAGTGTGTGTTTGTCT-3′; and (iii) ef1α: ef1αF 5′-CTGGAGGCCAGCTCAAACAT-3′ and ef1αR 5′-ATCAAGAAGAGTAGTACCGCTAGCATTAC-3′ with an annealing temperature of 58 °C. The reactions were carried out on a CFX96 Touch™ Real-Time PCR Detection System (BioRad) and the results analysed using Bio-Rad CFX Manager. The expression levels of wfs1a and wfs1b were normalised to the housekeeping gene ef1α.
PCR and gel electrophoresis
PCR reactions used MyTaq DNA Polymerase (Bioline) according to the manufacturer’s instructions. The primers used were the same as for the qPCR reactions, in addition to β-actinF 5′-CGAGCTGTCTTCCCATCCA-3′ and β-actin-R 5′-TCACCAACGTAGCTGTCTTTCTG-3′. The PCR products underwent electrophoresis in 1% (w/v) agarose (Bioline) gels made in 1× TAE buffer with Gel red (Merck). Bioline hyperladder IV was used and the gels were imaged using a GelDoc-IT Imaging system (UVP, Upland, USA).
Acetylcholine esterase (AChE) activity assay
The motor neuron defects observed in mutant zebrafish led us to investigate whether there was also any difference in the total amount of neuronal tissue in mutant zebrafish compared with WT zebrafish. AChE is a cholinergic enzyme present in the post-synaptic junctions that hydrolyses the neurotransmitter acetylcholine. As AChE is expressed in most neuronal tissues, measuring the total amount of AChE in zebrafish embryo lysates provides an indirect measurement of total neuronal tissue. A modified AChE activity assay was used49. In brief, pooled embryos were homogenized in 0.5 ml ice-cold sodium phosphate buffer (0.1 M, pH 7.4, and 0.1% v/v Triton X-100). Homogenates were centrifuged for 15 min at 4 °C at 10,000×g. After quantification with a BCA assay, 0.3 mM DNTB and 0.45 mM AChE were added to 10 μg of protein and spectrophotometric readings at 405 nm was performed for 10 min.
Fertility experiments
Zebrafish were group mated with 3 WT male and 3 female wfs1b−/− knockouts per large breeding tank and data points were collected from 3 independent matings. Fifty randomly selected embryos were placed in E3 medium and then incubated overnight. The count of dead embryos was determined the following morning.
Supplementary Information
Acknowledgements
PYWM is supported by an Advanced Fellowship Award (NIHR301696) from the UK National Institute of Health Research (NIHR) and a Clinician Scientist Fellowship Award (G1002570) from the UK Medical Research Council (MRC). PYWM also receives funding from Fight for Sight (UK), the Isaac Newton Trust (UK), Moorfields Eye Charity (GR001376), the Addenbrooke’s Charitable Trust, the National Eye Research Centre (UK), the International Foundation for Optic Nerve Disease (IFOND), the NIHR as part of the Rare Diseases Translational Research Collaboration, the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014), and the NIHR Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Abbreviations
- AChE
Acetylcholine esterase
- dpf
Days post-fertilisation
- EZRC
European Zebrafish Resource Centre
- F-Actin
Filamentous actin
- hpf
Hours post-fertilisation
- OA
Optic atrophy
- OCT
Optical coherence tomography
- OKR
Optokinetic response
- qRT-PCR
Quantitative RT-PCR
- RGC
Retinal ganglion cells
- rpm
Revolutions per minute
- RT-PCR
Reverse transcriptase PCR
- UPR
Unfolded protein response
- VEP
Visual evoked potentials
- WS
Wolfram syndrome
- WT
Wild-type
Author contributions
G.C., J.A.S. and P.Y.-W.-M. conceived and designed the study. G.C., F.B., R.P., E.O., M.T. and R.M. acquired, analyzed and interpreted the data. G.C., F.B., R.M., M.M., A.P., J.A.S. and P.Y.-W.-M. drafted and revised the manuscript. All authors reviewed and approved the final manuscript.
Funding
The funding was also provided by Fight for Sight (UK) and the Wellcome Trust.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: J. A. Sayer and P. Yu-Wai-Man.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-99781-0.
References
- 1.Strom TM, Hortnagel K, Hofmann S, Gekeler F, Scharfe C, Rabl W, et al. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (Wolframin) coding for a predicted transmembrane protein. Hum. Mol. Genet. 1998;7(13):2021–2028. doi: 10.1093/hmg/7.13.2021. [DOI] [PubMed] [Google Scholar]
- 2.Wolfram DJWH. Diabetes mellitus and simple optic atrophy among siblings: Report of four cases. Mayo Clin. Proc. 1938;13:715–718. [Google Scholar]
- 3.Barrett TG, Bundey SE, Macleod AF. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995;346(8988):1458–1463. doi: 10.1016/S0140-6736(95)92473-6. [DOI] [PubMed] [Google Scholar]
- 4.Hershey T, Lugar HM, Shimony JS, Rutlin J, Koller JM, Perantie DC, et al. Early brain vulnerability in Wolfram syndrome. PLoS ONE. 2012;7(7):e40604. doi: 10.1371/journal.pone.0040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaussenot A, Bannwarth S, Rouzier C, Vialettes B, Mkadem SA, Chabrol B, et al. Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann. Neurol. 2011;69(3):501–508. doi: 10.1002/ana.22160. [DOI] [PubMed] [Google Scholar]
- 6.Boutzios G, Livadas S, Marinakis E, Opie N, Economou F, Diamanti-Kandarakis E. Endocrine and metabolic aspects of the Wolfram syndrome. Endocrine. 2011;40(1):10–13. doi: 10.1007/s12020-011-9505-y. [DOI] [PubMed] [Google Scholar]
- 7.Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nat. Genet. 1998;20(2):143–148. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Kastner R, Kreczmanski P, Preising M, Diederen R, Schmitz C, Reis D, et al. Expression of the diabetes risk gene Wolframin (WFS1) in the human retina. Exp. Eye Res. 2009;89(4):568–574. doi: 10.1016/j.exer.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagalinec M, Liiv M, Hodurova Z, Hickey MA, Vaarmann A, Mandel M, et al. Role of mitochondrial dynamics in neuronal development: Mechanism for Wolfram syndrome. PLoS Biol. 2016;14(7):e1002511. doi: 10.1371/journal.pbio.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angebault C, Fauconnier J, Patergnani S, Rieusset J, Danese A, Affortit CA, et al. ER-mitochondria cross-talk is regulated by the Ca(2+) sensor NCS1 and is impaired in Wolfram syndrome. Sci. Signal. 2018;11(553):1380. doi: 10.1126/scisignal.aaq1380. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, Ghosh R, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Investig. 2010;120(3):744–755. doi: 10.1172/JCI39678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osman AA, Saito M, Makepeace C, Permutt MA, Schlesinger P, Mueckler M. Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. J. Biol. Chem. 2003;278(52):52755–52762. doi: 10.1074/jbc.M310331200. [DOI] [PubMed] [Google Scholar]
- 13.Takei D, Ishihara H, Yamaguchi S, Yamada T, Tamura A, Katagiri H, et al. WFS1 protein modulates the free Ca(2+) concentration in the endoplasmic reticulum. FEBS Lett. 2006;580(24):5635–5640. doi: 10.1016/j.febslet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Zatyka M, Da Silva XG, Bellomo EA, Leadbeater W, Astuti D, Smith J, et al. Sarco(endo)plasmic reticulum ATPase is a molecular partner of Wolfram syndrome 1 protein, which negatively regulates its expression. Hum. Mol. Genet. 2015;24(3):814–827. doi: 10.1093/hmg/ddu499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Morgia C, Maresca A, Amore G, Gramegna LL, Carbonelli M, Scimonelli E, et al. Calcium mishandling in absence of primary mitochondrial dysfunction drives cellular pathology in Wolfram Syndrome. Sci. Rep. 2020;10(1):4785. doi: 10.1038/s41598-020-61735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharanei S, Zatyka M, Astuti D, Fenton J, Sik A, Nagy Z, et al. Vacuolar-type H+-ATPase V1A subunit is a molecular partner of Wolfram syndrome 1 (WFS1) protein, which regulates its expression and stability. Hum. Mol. Genet. 2013;22(2):203–217. doi: 10.1093/hmg/dds400. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, Ishihara H, Tamura A, Takahashi R, Yamaguchi S, Takei D, et al. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum. Mol. Genet. 2006;15(10):1600–1609. doi: 10.1093/hmg/ddl081. [DOI] [PubMed] [Google Scholar]
- 18.Lu S, Kanekura K, Hara T, Mahadevan J, Spears LD, Oslowski CM, et al. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc. Natl. Acad. Sci. U.S.A. 2014;111(49):E5292–E5301. doi: 10.1073/pnas.1421055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatanaka M, Tanabe K, Yanai A, Ohta Y, Kondo M, Akiyama M, et al. Wolfram syndrome 1 gene (WFS1) product localizes to secretory granules and determines granule acidification in pancreatic beta-cells. Hum. Mol. Genet. 2011;20(7):1274–1284. doi: 10.1093/hmg/ddq568. [DOI] [PubMed] [Google Scholar]
- 20.Ishihara H, Takeda S, Tamura A, Takahashi R, Yamaguchi S, Takei D, et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum. Mol. Genet. 2004;13(11):1159–1170. doi: 10.1093/hmg/ddh125. [DOI] [PubMed] [Google Scholar]
- 21.Terasmaa A, Soomets U, Oflijan J, Punapart M, Hansen M, Matto V, et al. Wfs1 mutation makes mice sensitive to insulin-like effect of acute valproic acid and resistant to streptozocin. J. Physiol. Biochem. 2011;67(3):381–390. doi: 10.1007/s13105-011-0088-0. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet Wersinger D, Benkafadar N, Jagodzinska J, Hamel C, Tanizawa Y, Lenaers G, et al. Impairment of visual function and retinal ER stress activation in Wfs1-deficient mice. PLoS ONE. 2014;9(5):e97222. doi: 10.1371/journal.pone.0097222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plaas M, Seppa K, Reimets R, Jagomae T, Toots M, Koppel T, et al. Wfs1- deficient rats develop primary symptoms of Wolfram syndrome: Insulin-dependent diabetes, optic nerve atrophy and medullary degeneration. Sci. Rep. 2017;7(1):10220. doi: 10.1038/s41598-017-09392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakakibara Y, Sekiya M, Fujisaki N, Quan X, Iijima KM. Knockdown of wfs1, a fly homolog of Wolfram syndrome 1, in the nervous system increases susceptibility to age- and stress-induced neuronal dysfunction and degeneration in Drosophila. PLoS Genet. 2018;14(1):e1007196. doi: 10.1371/journal.pgen.1007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majander A, Bitner-Glindzicz M, Chan CM, Duncan HJ, Chinnery PF, Subash M, et al. Lamination of the outer plexiform layer in optic atrophy caused by dominant WFS1 mutations. Ophthalmology. 2016;123(7):1624–1626. doi: 10.1016/j.ophtha.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hare EA, Yerges-Armstrong LM, Perry JA, Shuldiner AR, Zaghloul NA. Assignment of functional relevance to genes at type 2 diabetes-associated loci through investigation of beta-cell mass deficits. Mol. Endocrinol. 2016;30(4):429–445. doi: 10.1210/me.2015-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoon M, Okawa H, Della Santina L, Wong RO. Functional architecture of the retina: development and disease. Prog. Retin. Eye Res. 2014;42:44–84. doi: 10.1016/j.preteyeres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson R, Tracey-White D, Webster A, Moosajee M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye. 2017;31(1):68–86. doi: 10.1038/eye.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Heredia ML, Cleries R, Nunes V. Genotypic classification of patients with Wolfram syndrome: insights into the natural history of the disease and correlation with phenotype. Genet. Med. 2013;15(7):497–506. doi: 10.1038/gim.2012.180. [DOI] [PubMed] [Google Scholar]
- 30.Lam PY, Harvie EA, Huttenlocher A. Heat shock modulates neutrophil motility in zebrafish. PLoS ONE. 2013;8(12):e84436. doi: 10.1371/journal.pone.0084436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang L, Hua H, Foo K, Martinez H, Watanabe K, Zimmer M, et al. beta-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes. 2014;63(3):923–933. doi: 10.2337/db13-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertrand C, Chatonnet A, Takke C, Yan YL, Postlethwait J, Toutant JP, et al. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7. Gene structure and polymorphism; molecular forms and expression pattern during development. J. Biol. Chem. 2001;276(1):464–474. doi: 10.1074/jbc.M006308200. [DOI] [PubMed] [Google Scholar]
- 33.Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, et al. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 2002;5(2):111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- 34.Koenig JA, Dao TL, Kan RK, Shih TM. Zebrafish as a model for acetylcholinesterase-inhibiting organophosphorus agent exposure and oxime reactivation. Ann. N. Y. Acad. Sci. 2016;1374(1):68–77. doi: 10.1111/nyas.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menelaou E, Husbands EE, Pollet RG, Coutts CA, Ali DW, Svoboda KR. Embryonic motor activity and implications for regulating motoneuron axonal pathfinding in zebrafish. Eur. J. Neurosci. 2008;28(6):1080–1096. doi: 10.1111/j.1460-9568.2008.06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998;37(4):622–632. doi: 10.1002/(SICI)1097-4695(199812)37:4<622::AID-NEU10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Hoekel J, Chisholm SA, Al-Lozi A, Hershey T, Tychsen L, Washington University Wolfram Study Group Ophthalmologic correlates of disease severity in children and adolescents with Wolfram syndrome. J. AAPOS. 2014;18(5):461–415. doi: 10.1016/j.jaapos.2014.07.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haghighi A, Haghighi A, Setoodeh A, Saleh-Gohari N, Astuti D, Barrett TG. Identification of homozygous WFS1 mutations (p.Asp211Asn, p.Gln486*) causing severe Wolfram syndrome and first report of male fertility. Eur. J. Hum. Genet. 2013;21(3):347–351. doi: 10.1038/ejhg.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urano F. Wolfram syndrome: Diagnosis, management, and treatment. Curr. Diab. Rep. 2016;16(1):6. doi: 10.1007/s11892-015-0702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noormets K, Koks S, Kavak A, Arend A, Aunapuu M, Keldrimaa A, et al. Male mice with deleted Wolframin (Wfs1) gene have reduced fertility. Reprod. Biol. Endocrinol. 2009;7:82. doi: 10.1186/1477-7827-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496(7446):494–497. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 43.Meeker ND, Hutchinson SA, Ho L, Trede NS. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques. 2007;43(5):610. doi: 10.2144/000112619. [DOI] [PubMed] [Google Scholar]
- 44.Muller JS, Jepson CD, Laval SH, Bushby K, Straub V, Lochmuller H. Dok-7 promotes slow muscle integrity as well as neuromuscular junction formation in a zebrafish model of congenital myasthenic syndromes. Hum. Mol. Genet. 2010;19(9):1726–1740. doi: 10.1093/hmg/ddq049. [DOI] [PubMed] [Google Scholar]
- 45.O'Connor E, Phan V, Cordts I, Cairns G, Hettwer S, Cox D, et al. MYO9A deficiency in motor neurons is associated with reduced neuromuscular agrin secretion. Hum. Mol. Genet. 2018;27(8):1434–1446. doi: 10.1093/hmg/ddy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul S, Schindler S, Giovannone D, de Millo TA, Mariani FV, Crump JG. Ihha induces hybrid cartilage-bone cells during zebrafish jawbone regeneration. Development. 2016;143(12):2066–2076. doi: 10.1242/dev.131292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toms M, Burgoyne T, Tracey-White D, Richardson R, Dubis AM, Webster AR, et al. Phagosomal and mitochondrial alterations in RPE may contribute to KCNJ13 retinopathy. Sci. Rep. 2019;9(1):3793. doi: 10.1038/s41598-019-40507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toms M, Tracey-White D, Muhundhakumar D, Sprogyte L, Dubis AM, Moosajee M. Spectral domain optical coherence tomography: An in vivo imaging protocol for assessing retinal morphology in adult zebrafish. Zebrafish. 2017;14(2):118–125. doi: 10.1089/zeb.2016.1376. [DOI] [PubMed] [Google Scholar]
- 49.Teixido E, Pique E, Gomez-Catalan J, Llobet JM. Assessment of developmental delay in the zebrafish embryo teratogenicity assay. Toxicol. In Vitro. 2013;27(1):469–478. doi: 10.1016/j.tiv.2012.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.