Abstract
Sarcopenia is an age-related disease with an increased risk of mortality. It is emerging that low serum 25-hydroxyvitamin D [25(OH)D] affects the sarcopenic state in general, but in rheumatoid arthritis (RA), these associations are not understood although the prevalence of vitamin D insufficiency is high in RA. We conducted a cross-sectional study of older female outpatients from our cohort (KURAMA) database. We measured skeletal muscle mass, handgrip strength, and gait-speed to diagnose severe sarcopenia. The serum 25(OH)D concentration was measured using electrochemiluminescence immunoassay. A total of 156 female patients with RA (sarcopenia:44.9%, severe sarcopenia: 29.5%, and without sarcopenia: 25.6%) were enrolled. Classification of vitamin D status at a cutoff point of median 25(OH)D concentration revealed that low 25(OH)D status was associated with a high prevalence of severe sarcopenia and with low measured values of muscle mass, handgrip, and gait speed. Furthermore, multivariable logistic regression analysis identified that low 25(OH)D status was associated with a high prevalence of severe sarcopenia (OR 6.00; 95% CI 1.99–18.08).The same association was observed when the cut-off value was set at 20 ng/ml. In components of sarcopenia, both low physical performance and muscle mass were associated with low 25(OH)D status. In conclusion, vitamin D status was inversely associated with severe sarcopenia, low physical performance, and low skeletal muscle mass. Modification of vitamin D status including vitamin D supplementation should be investigated as a therapeutic strategy for sarcopenic patients with RA.
Subject terms: Rheumatology, Risk factors
Introduction
Sarcopenia is an age-related disease characterized by poor physical performance and reduced muscle mass and strength, and one of the most important and challenging problems in an aging society. Sarcopenia has multifactorial etiology including malnutrition, aging, infrequent exercise and diseases such as cancer, diabetes mellitus, COPD and autoimmune diseases1; it is closely related to increased risk of mortality2,3. Although some therapeutic interventions are established such as encouragement of protein intake and physical exercise in the general population4,5, there still remain unknown aspects of sarcopenia in patients with debilitating diseases.
RA is an inflammatory autoimmune disease characterized by increased morbidity due to joint destruction together with extra-articular manifestations and increased mortality. RA patients exhibit far more sarcopenia than the general population6 and loss of physical performance such as walking and grip strength is a well-known risk factor of mortality7. Severe sarcopenia, which requires more intensive interventions to achieve improvements in physical performance8,9, is highly prevalent in RA patients compared to the general populations10,11. There have been conflicting results as to whether Disease Modifying Anti-Rheumatic Drugs (DMARDs) and biological agents improve the sarcopenic state in RA patients10,12; there is also little evidence on co-adjuvant therapies such as nutritional interventions in these patients.
Recently, vitamin D supplementation is receiving much attention as a potential therapeutic intervention for preventing sarcopenia in the general population together with protein supplementation and exercise. Vitamin D may well ameliorate the sarcopenic state via its role in muscle cell regulation, anti-inflammatory pathways and/or immunomodulatory responses13, as vitamin D status is associated with muscle strength and physical performance14,15. Indeed, meta-analyses and RCTs have shown that vitamin D supplementation improves limb strength in the community-dwelling elderly16,17. Interestingly, over 70% of RA patients exhibit vitamin D insufficiency18; these findings suggest the importance of adequate vitamin D provision on sarcopenia in RA populations as in general populations. However, it remains unclear whether serum vitamin D concentration affects the sarcopenic state in RA patients.
To elucidate the clinical association between vitamin D deficiency and sarcopenia in elderly patients with RA, we performed a cross-sectional study using the cohort study database, KURAMA cohort, established in 2011 for the storage of clinical data and specimens obtained from RA patients.
Methods
Study design and participants
We conducted a cross-sectional study in female RA outpatients from the Kyoto University Rheumatoid Arthritis Management Alliance (KURAMA) cohort database, which has been described in detail elsewhere19,20. We recruited older RA participants who visited the Kyoto University Hospital from May 2014 to December 2014 and who were over 60 years of age. All participants fulfilled the diagnostic criteria of the ACR/EULAR RA classification21. Data for 156 out of 248 participants was subjected to the planned analyses. 10 were excluded because of incomplete data set (lack of preserved serum or parameters of sarcopenia-related factors) and 82 were excluded because of the use of vitamin D supplementation (calcitriol such as eldecalcitol and alfacalcidol).
Ethics
The study protocol and procedures were approved by the Medical Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (Approval number: R0357) and complied with the principles of the Declaration of Helsinki. We obtained written informed consent was obtained from all patients of this study, which included the use of human blood samples and data.
Diagnosis of sarcopenia and estimation of the related parameters
We measured muscle mass, muscle strength and physical performance to assess sarcopenia status in the study population, as previously described10,22. In brief, skeletal muscle mass was measured by bioelectrical impedance analysis (BIA) (Inbody 720: Biospace Co., Ltd., Seoul, Korea). Skeletal muscle index (SMI) was computed from the limb skeletal muscle mass in kilograms divided by the square of height in meters (kg/m2). Handgrip strength was measured using JAMAR digital hand dynamometer (Patterson Medical, Bolingbrook, IL). Gait speed was also measured by the 6-m walking speed using a portable gait rhythmogram (MG-M1110: LSI Medience Co., Tokyo, Japan) Regarding muscle strength and gait speed, the mean value of duplicate measurements was used for analysis.
Diagnosis of sarcopenia and severe sarcopenia were based on the criteria of the Asian Working Group for Sarcopenia (AWGS) 201923. Briefly, sarcopenia was defined as low muscle mass with low muscle strength or with low physical performance; severe sarcopenia was defined as low muscle mass with both low muscle strength and low physical performance. Cut-offs values for low muscle mass, low muscle strength, and low physical performance were SMI < 5.7 kg/m2, handgrip strength < 18 kg, and gait speed < 1.0 m/s, respectively.
Evaluation of 25(OH)D concentration and the clinical parameters
Serum 25(OH)D, an established biomarker reflecting vitamin D status, was measured using electrochemiluminescence immunoassay (LSI Medience Co., Tokyo, Japan). This laboratory used an external quality control provided by DEQAS , and the intra-assay CV varied between 2.64 and 5.65% across the range of 25(OH) concentration, between 12.87 and 59.16 ng/ml. MNA-SF (Mini Nutritional Assessment Short-Form) was collected to assess nutritional state24. RA disease activity and physical dysfunction were assessed by a 28-Joint RA Disease Activity Score (DAS28-ESR), the doctor or patient Visual Analogue Scale (Dr. or PT-VAS), Steinbrocker’s stage and class and the health assessment questionnaire disability index (HAQ). We also reviewed the information regarding current RA therapeutics including methotrexate, prednisolone, and biological agents (TNF inhibitors: n = 33, IL-6 receptor inhibitor: n = 12, CTLA4-immunoglobulin: n = 10). Other epidemiologic and anthropometric variables including age, duration of RA disease and body mass index (BMI) were extracted from the KURAMA database. Information on falls and fractures in the last year was collected from all subjects by a self-reported questionnaire form. The use of osteoporosis medication was obtained from the electronic medical record.
Statistical analysis
We present continuous variables as the mean (standard deviation (SD)) or as the median (interquartile range (IQR)) and categorial variables as numbers (%). To compare baseline characteristics according to 25(OH)D status, we divided participants into the following two groups by the median of serum 25(OH)D concentration: lower status group (25(OH)D: 5.9–16.0 ng/ml); higher status group (25(OH)D: 16.1–32.1 ng/ml). We then performed a Mann–Whitney’s U test or a Fisher’s exact test for continuous variables and categorical variables, respectively. To explore the relationship between 25(OH)D status and severe sarcopenia, univariate and multivariate logistic regression analyses were conducted. 25(OH)D status was used as either a binary variable as described above, a binary variable with a cutoff value of 20 ng/ml, which value is used for an indicator of bone health25, or a continuous variable in multivariate analysis. In multivariate analyses, we constructed the following multiple models by incorporating significant variables in the univariate analysis and clinically relevant variables including RA therapeutics10,26: model 1 was adjusted for 25(OH)D status, age, and body mass index; model 2 was adjusted for variables in model 1 plus nutrition status (MNA-SF) and RA-related factors (DAS28-ESR, Stage 3 and 4 vs. 1 and 2, HAQ, and therapeutics (use of prednisolone, biologics, and methotrexate)); model 3 was adjusted for variables in model 2 plus the prevalence of osteoporosis medication, as RANKL inhibitors can exert a positive effect on muscle strength27. We also adopted multivariate logistic regression analysis using the same models for each component of severe sarcopenia (low muscle mass, low muscle strength, and low physical performance). JMP 15.2.0 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses; a value of P < 0.05 was considered significant.
Results
Participant characteristics of this study are provided in Table 1. Data for 156 female RA patients with a mean (SD) age of 69.7 (6.7) was subjected to the following analysis. The median (IQR) serum 25(OH)D concentration was 16.0 (12.8–19.2), which represents a lower concentration than that in the general population28,29 and accords with that in other RA studies18. As for RA-related factors, the average (SD) duration of RA was 16.1 years (12.7). Disease activity of RA (DAS28-ESR) was generally low under the following therapeutics: methotrexate in 67.3%, prednisolone in 27.6%, and biological agent in 35.3%, but about two-thirds of the patients had advanced joint deformity or destruction as assessed by a Steinbrocker’s stage of 3 (21.1%) or 4 (46.8%).
Table 1.
Characteristics of this study population.
| Characteristics | RA patients |
|---|---|
| (N = 156) | |
| Age, mean (SD), years | 69.7 (6.7) |
| Body composition and physical activity variables | |
| Body mass index, mean (SD), kg/m2 | 22.0 (3.6) |
| Skeletal mass index, mean (SD), kg/m2 | 5.64 (0.83) |
| Handgrip strength-dominant, mean (SD), kg | 14.5 (7.2) |
| Gait speed, mean (SD), m/s | 0.95 (0.29) |
| Sarcopenia (+), n (%) | 70 (44.9) |
| Severe sarcopenia (+), n (%) | 46 (29.5) |
| Any fall in the previous year, n (%) | 25 (16.2) |
| Any fracture in the previous year, n (%) | 7 (4.6) |
| Osteoporosis medication, n (%) | 45 (28.9) |
| MNA-SF, mean (SD) | 12.0 (2.0) |
| RA disease characteristics | |
| Duration, mean (SD), years | 16.1 (12.7) |
| DAS28-ESR, mean (SD) | 2.96 (0.98) |
| HAQ score, mean (SD) | 0.83 (0.74) |
| Stage*, mean (SD) | 3.01 (1.10) |
| Stage 1, n (%) | 21 (13.4) |
| Stage 2, n (%) | 29 (18.6) |
| Stage 3, n (%) | 33 (21.1) |
| Stage 4, n (%) | 73 (46.8) |
| Class*, mean (SD) | 1.82 (0.60) |
| Current RA medications | |
| Methotrexate use, n (%) | 105 (67.3) |
| Prednisolone use, n (%) | 43 (27.6) |
| Biological agent use, n (%) | 55 (35.3) |
| Laboratory data | |
| Serum 25(OH)D, median (IQR), ng/ml | 16.0 (12.8–19.2) |
| CRP, median (IQR), mg/dL | 0.1 (0.075–0.30) |
Data are presented as the mean (standard deviation (SD)) or as the median (interquartile range (IQR)) for continuous variables, and as numbers (%) for categorial variables.
RA rheumatoid arthritis, MNA-SF Mini Nutritional Assessment Short-Form, DAS28 disease activity score using 28 joints, VAS visual analogue scale, HAQ health assessment questionnaire.
*Steinbrocker's classification.
Of these participants, on the basis of the diagnostic criteria of sarcopenia in AWGS 201923, 46 (29.5%) were determined to have severe sarcopenia and 70 (44.9%) were determined to have sarcopenia. Regarding the components of sarcopenia, mean (SD) of SMI, handgrip strength and gait speed were 5.64 (0.83), 14.5 (7.2) and 0.95 (0.29), respectively. Under these conditions, 50.6%, 67.3%, and 50.6% of the participants fulfilled the criterion for low muscle mass, low muscle strength (handgrip strength), or poor physical performance (gait speed), respectively.
Comparison of clinical characteristics according to 25(OH)D status
To determine whether serum 25(OH)D status was associated with RA-related factors and sarcopenia in the RA population, we first divided participants into two groups (lower/higher) at a cut-off point of the median serum 25(OH)D concentration and then compared their characteristics. As for the components of sarcopenia, skeletal muscle mass, handgrip strength and gait speed were significantly lower in the low 25(OH)D group (Table 2). The prevalence of severe sarcopenia was significantly higher in the low 25(OH)D group, which was also in the case in sarcopenia. Regarding RA-related factors, as in other reports30,31, DAS28-ESR, the degree of current disease activity, tended to be higher in the low 25(OH)D group. In the lower 25(OH)D group, the % of patients on prednisolone use was significantly higher (35.9% vs. 19.2%).
Table 2.
Characteristics of participants by serum 25(OH)D status.
| 25(OH)D concentration (range, ng/ml) | Lower status (n = 78) | Higher status (n = 78) | P value |
|---|---|---|---|
| 5.9–16.0 | 16.1–32.1 | ||
| Age, mean (SD), year | 70.4 (6.9) | 69.1 (6.4) | 0.21 |
| Body mass index, mean (SD), kg/m2 | 21.7 (3.5) | 22.2 (3.6) | 0.29 |
| Factors associated with sarcopenia | |||
| Skeletal mass index, mean (SD), kg/m2 | 5.45 (0.90) | 5.83 (0.69) | 0.0036 |
| Handgrip strength-dominant, mean (SD), kg | 13.1 (7.6) | 16.0 (6.5) | 0.0094 |
| Gait speed, mean (SD), m/s | 0.88 (0.30) | 1.02 (0.27) | 0.0025 |
| Sarcopenia (+), n (%) | 44 (56.4) | 26 (33.3) | 0.0036 |
| Severe sarcopenia (+), n (%) | 34 (43.6) | 12 (15.4) | < 0.0001 |
| Osteoporosis medication, n (%) | 41 (35.0) | 36 (29.8) | 1.00 |
| MNA-SF, mean (SD) | 11.9 (2.1) | 12.0 (1.9) | 0.69 |
| RA disease characteristics | |||
| Disease duration, mean (SD), year | 16.6 (13.6) | 15.6 (11.7) | 0.63 |
| DAS28-ESR, mean (SD) | 3.11 (1.04) | 2.81 (0.90) | 0.055 |
| CRP, median (IQR), mg/dL | 0.1 (0–0.4) | 0.1 (0.1–0.3) | 0.44 |
| HAQ, mean (SD) | 1.00 (0.79) | 0.67 (0.66) | 0.0096 |
| Stage, mean (SD) | 2.99 (1.10) | 3.04 (1.10) | 0.77 |
| Stage 4, n (%) | 35 (44.9) | 38 (48.7) | 0.63 |
| Stage 3 and 4, n (%) | 53 (68.0) | 53 (68.0) | 1.00 |
| Stage 2, 3 and 4, n (%) | 67 (85.9) | 68 (87.2) | 0.81 |
| Class, mean (SD) | 1.91 (0.65) | 1.73 (0.53) | 0.060 |
| Current therapeutic agent | |||
| Methotrexate use, n (%) | 49 (62.8) | 56 (71.8) | 0.23 |
| Biological agent use, n (%) | 29 (37.2) | 26 (33.3) | 0.61 |
| Prednisolone use, n (%) | 28 (35.9) | 15 (19.2) | 0.019 |
RA patients are divided into the following two groups by median of serum 25(OH)D: lower status group (25(OH)D: 5.9–16.0 ng/ml) and higher status group (25(OH)D: 16.1–32.1 ng/ml). Data are presented as the mean (± standard deviation) or as the median (interquartile range (IQR)) for continuous variables, and as numbers (%) for categorial variables.
RA rheumatoid arthritis, MNA-SF Mini Nutritional Assessment Short-Form, DAS28 disease activity score using 28 joints, VAS visual analogue scale, HAQ health assessment questionnaire.
Low 25(OH)D status is independently and positively associated with a high prevalence of severe sarcopenia
Although it is emerging that low 25(OH)D concentrations affect the sarcopenia state in the general population14,15, in a RA population these associations are not well understood. We therefore performed univariate and multivariate regression analyses with the presence of severe sarcopenia as the dependent variable. Univariate logistic regression analyses revealed that low 25(OH)D status was significantly associated with a high prevalence of severe sarcopenia (OR 4.25; 95% CI 1.99–9.09) (Table 3 left). Age, BMI, DAS28-ESR, Stage, HAQ score, the use of prednisolone and nutritional status (MNA-SF) were also associated with severe sarcopenia. We then conducted multivariate logistic regression analyses to clarify whether low 25(OH)D status independently contributes to severe sarcopenia cross-sectionally. Among the factors that were significant in the univariate analysis, we excluded the HAQ score from covariates in the multivariate analyses because this score might be a result, not a cause, of sarcopenia. We then determined that low 25(OH)D status was independently associated with high prevalence of severe sarcopenia in model 1 adjusted for age, BMI, 25(OH)D status (OR 4.42; 95% CI 1.80–10.8) (Table 3 middle). In other models adjusted for RA-related and nutritional factors (model 2) or adjusted for factors that may affect 25(OH) D including osteoporosis medication (model 3), the same significant relationships still remained. When 25(OH)D was modeled as a continuous variable, lower 25(OH)D concentrations were also significantly associated with severe sarcopenia in all models (OR 0.91; 95% CI 0.84–0.99: model 1) (Supplementary Table S1). Similarly, when 25(OH)D was used as a binary variable with a cutoff value of 20 ng/ml, lower 25(OH)D was also significantly associated with severe sarcopenia in all models (OR 3.87; 95% CI 1.14–13.1: model 1) (Supplementary Table S2).
Table 3.
Logistic analysis for RA patients with severe sarcopenia.
| Variables | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (1 year) | 1.15 (1.08–1.22) | < 0.0001 | 1.17 (1.09–1.25) | < 0.0001 | 1.21 (1.10–1.33) | < 0.0001 | 1.21 (1.10–1.33) | < 0.0001 |
| Body mass index (1 kg/m2) | 0.76 (0.67–0.86) | < 0.0001 | 0.72 (0.62–0.84) | < 0.0001 | 0.76 (0.60–0.95) | 0.0085 | 0.76 (0.60–0.95) | 0.010 |
| Low 25(OH)D status (≦16.0 ng/ml) | 4.25 (1.99–9.09) | < 0.0001 | 4.42 (1.80–10.8) | 0.0007 | 5.92 (1.98–17.7) | 0.0006 | 6.00 (1.99–18.08) | 0.0006 |
| DAS28-ESR | 1.58 (1.09–2.31) | 0.015 | 1.03 (0.62–1.74) | 0.90 | 1.23 (0.86–1.76) | 0.88 | ||
| Stage (3, 4 vs. 1, 2) | 4.44 (1.74–11.4) | 0.0005 | 4.33 (1.33–14.08) | 0.0010 | 4.40 (1.35–14.32) | 0.0097 | ||
| HAQ | 4.03 (2.19–7.41) | < 0.0001 | ||||||
| Methotrexate use | 0.58 (0.28–1.19) | 0.14 | 2.18 (0.66–7.22) | 0.19 | 2.18 (0.66–7.22) | 0.19 | ||
| Prednisolone use | 2.91 (1.39–6.11) | 0.0049 | 2.45 (0.74–8.06) | 0.09 | 2.45 (0.74–8.06) | 0.13 | ||
| Biological agents use | 0.47 (0.21–1.03) | 0.058 | 0.70 (0.22–2.21) | 0.52 | 0.70 (0.22–2.21) | 0.54 | ||
| MNA-SF | 0.70 (0.59–0.85) | < 0.0001 | 0.91 (0.66–1.24) | 0.54 | 0.90 (0.66–1.24) | 0.53 | ||
| Osteoporosis medication (+) | 1.72 (0.82–3.59) | 0.15 | 1.22 (0.40–3.72) | 0.73 | ||||
Results of univariate (left) and multivariate (right) logistic analyses for independent variables associated with severe sarcopenia. Model 1 was adjusted for vitamin D status, age, and body mass index. Model 2 was adjusted for variables in model 1 plus nutrition status (MNA-SF), and RA-related factors (DAS28-ESR, Stage, HAQ, and therapeutics (use of prednisolone, biologics, and methotrexate)). Model 3 was adjusted for variables in model 2 plus the prevalence of osteoporosis medication.
RA rheumatoid arthritis, DAS28 disease activity score using 28 joints, HAQ health assessment questionnaire, MNA-SF Mini Nutritional Assessment Short-Form.
25(OH)D status associates both with physical performance and skeletal muscle mass
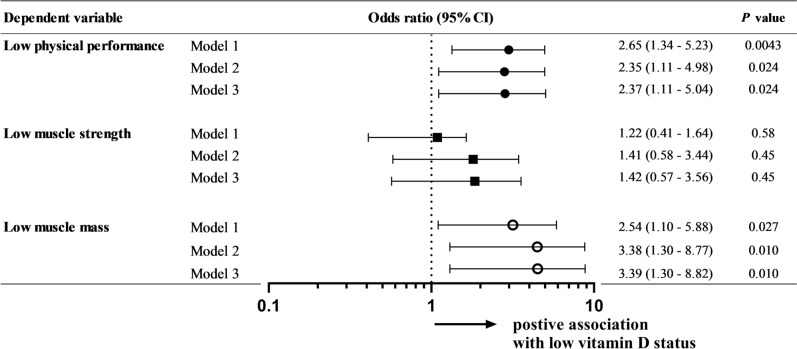
We then conducted further multivariate analyses to clarify which components of severe sarcopenia were most strongly associated with low serum 25(OH)D status. Regarding the components of severe sarcopenia, both low physical performance and low skeletal muscle mass were significantly associated with low serum 25(OH)D status in model 1 (physical performance: OR 2.65; 95% CI 1.34–5.23, skeletal muscle mass: OR 2.54; 95% CI 1.10–5.88) (Fig. 1). The same associations were maintained in the other models (model 2 and 3). Other variables are described in supplementary Table S3. There was no significant association with muscle strength. Low serum 25(OH)D is therefore independently associated with a high prevalence of severe sarcopenia and with both low physical performance and low skeletal muscle index mass among the measures used to assess sarcopenia.
Figure 1.
Associations between vitamin D status and components of sarcopenia. Results of multivariate logistic analyses for independent variables associated with components of sarcopenia. This forest plot represents the odds ratio and 95% confidence interval (CI) for each sarcopenia-related component in each adjusted model. Model 1 was adjusted for vitamin D status, age, and body mass index. Model 2 was adjusted for variables in model 1 plus nutrition status (MNA-SF) and RA-related factors (DAS28-ESR, Stage, HAQ, and therapeutics (use of prednisolone, biologics, and methotrexate)). Model 3 was adjusted for variables in model 2 plus the prevalence of osteoporosis medication.
Discussion
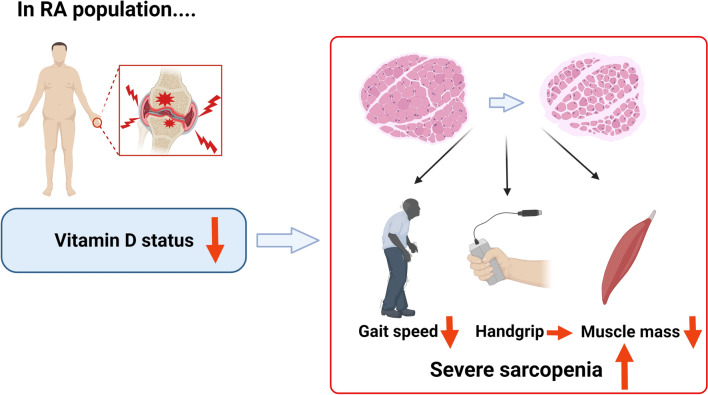
The present study is the first to document a significant association between vitamin D deficiency and severe sarcopenia in a female RA population. Our participants had a high prevalence of severe sarcopenia (29.5%) and vitamin D deficiency (median 16.0 ng/ml); multivariate analyses showed low serum 25(OH)D to be an independent risk for severe sarcopenia (OR 4.42). In addition, among the three components of sarcopenia, decreased gait speed and muscle mass showed significant associations with low serum vitamin D. These findings accord with those in general population studies: low serum vitamin D has a stronger association with lower function on walking than on other physical performance or muscle strength32,33. Our results suggest that vitamin D deficiency may contribute to the development of severe sarcopenia and impaired lower limb performance and skeletal muscle in RA patients, as is the case in the general population (Fig. 2).
Figure 2.
A proposed model of the relationship between vitamin D status, severe sarcopenia and its components in RA patients. In this study, vitamin D deficiency were strongly associated with increased prevalence of severe sarcopenia and impaired lower limb performance and skeletal muscle in RA patients. Modification strategy of vitamin D status including vitamin D supplementation may contribute to the improvement of sarcopenia in RA. This figure was created with BioRender.com (https://biorender.com/). RA rheumatoid arthritis.
Recently, basic studies have provided insight into the mechanism of vitamin D action on skeletal muscle function, including modulation of muscle differentiation, oxidative stress, cellular metabolism and inflammatory condition13,34. The expression of vitamin D receptors in muscle nuclei decreases with aging, which affects muscle differentiation and is implicated in the development of sarcopenia35. Vitamin D deficiency also contributes to skeletal muscle atrophy via dysregulation of cellular metabolism such as oxidative stress and calcium homeostasis resulting in mitochondrial function36,37. Furthermore, vitamin D is well known for its anti-inflammatory and immunomodulatory properties38. It suppresses Th1, Th17 and macrophage cytokines (IL-2, IFN-γ, IL-17, IL-21, TNF-α, IL-6, IL1-β) as well as innate immune responses such as toll-like receptor signaling and antigen presentation39,40; it promotes the expression of Th2 cytokines (IL-4, IL-5, IL-10, IL-13) and the differentiation of regulatory T cells41,42. Given that Th1 and Th17 responses participate in the pathogenesis of RA43, vitamin D might well ameliorate sarcopenia in RA patients partly through attenuating chronic inflammation that results in muscle catabolism as well as through modulating muscle function via multiple physiological pathways13,36,37.
Similarly, epidemiological studies have shown clinical correlations between vitamin D deficiency and sarcopenia. In general populations, low serum vitamin D concentration significantly associates with increased prevalence of sarcopenia and loss of physical performance such as walking speed14,15. Several meta-analyses and RCTs have also shown the positive effects of vitamin D supplementation such as improvement in global muscle strength (especially lower limb muscles) and a decrease in the sit-to-stand time16,17. In RA populations, however, the relationship between sarcopenia and vitamin D status has not been clarified even though the prevalence of sarcopenia and vitamin D insufficiency is markedly higher in the RA population than it is in the general population6,44. The present study provides novel evidence that low serum 25(OH)D is a significant risk factor of severe sarcopenia in RA. Taking these findings together, vitamin D deficiency may well be a candidate co-adjuvant therapeutic target to prevent severe sarcopenia in RA patients; prospective studies of vitamin D supplementation to prevent sarcopenia in RA will be worthwhile.
There are several limitations in the present study. The present cross-sectional study does not imply causation and further prospective investigation is needed. There is also the possibility of selection bias because the study included only female patients older than 60 years, although these are the dominant populations with RA. We excluded vitamin D supplementation as a confounding factor in the present study, which might produce a biased group of subjects: our findings are applicable only to RA patients without supplementation therapy. In addition, there might remain unadjusted confounding variables related to vitamin D status and sarcopenia including dietary profile, seasonal variation and socioeconomic status, and information on falls and fractures was obtained by personal recall.
In conclusion, female RA patients with low serum 25(OH)D concentration has a significant risk for a higher prevalence of severe sarcopenia, low physical performance, and low skeletal muscle mass. The interventions to improve serum 25(OH)D concentration including vitamin D supplementation could be beneficial for RA patients with sarcopenia.
Supplementary Information
Acknowledgements
We thank Ms. N. Kitayama and Ms. M. Yoneyama for the support of the patients. We also thank S. Nakagawa and M. Iida for their technical assistance.
Author contributions
H.M. and M.T. (Torii) are responsible for study conception and design. H.M. and M.K contributed to the interpretation of the data, drafted the manuscript, and revised the manuscript. M.H. contributed to interpretation of the data and revised the manuscript. W.Y., Y.F., K.I., E.O., K.M. (Murakami), R.W., K.M. (Murata), H.I., M.T. (Tanaka), H.A., S.M., A.M., and N.I. contributed to supervision of the manuscript for intellectual content. All authors have approved the final manuscript for publication and have agreed to be personally accountable for the authors’ contributions.
Funding
This study (KURAMA cohort study) is supported by AMED (the Japan Agency for Medical Research and Development) under Grant Number JP21ek0410069, JSPS KAKENHI Grant Number JP 19K11165 and a grant from Daiichi Sankyo Co. Ltd. The funder had no role in the design of the study, the collection or analysis of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Competing interests
K.M. (Murata), and M.T. (Tanaka) are members of a department financially supported by Nagahama City, Shiga, Japan, Toyooka City, Hyogo, Japan and five pharmaceutical companies (Tanabe-Mitsubishi, Chugai, UCB Japan, Ayumi and Asahi-Kasei). R.W. receives speaker’s fee from Mitsubishi Tanabe Pharma, Pfizer, Sanofi, AbbVie, Asahi Kasei, Eisai, Eli Lilly, Bristol-Myers Squibb, and Janssen. H.I. receives a research grant and/or speaker fee from Bristol-Myers, Kyocera, and Asahi-Kasei. M.T. (Tanaka) has received research grants and/or speaker fees from AbbVie GK, Asahi Kasei Pharma Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Corp., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Pfizer Inc., UCB Japan Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corp., Novartis Pharma K.K., Taisho Pharma Co., Ltd. S.M. receives grants and/or speaker fees from Daiichi Sankyo, Asahi-Kasei, Chugai, Ayumi, and Tanabe Mitsubishi. A.M. receives speaking fees and/or research grants from Eli Lilly Japan K.K., Ono Pharmaceutical Co., Pfizer Inc., UCB Japan, AbbVie G.K., Asahi Kasei Pharma and Chugai Pharmaceutical Co. Ltd. M.H. receives grants and /or speaker fees from Bristol-Meyers, Eisa, Eli Lilly, Novartis Pharma, and Tanabe Mitsubishi. H.M., M.K., M.T.(Torii), W.Y., Y.F., K.I., E.O., K.M.(Murakami), K.M. (Murata), H.A., and N.I. declare no conflicts of interest. The sponsors had no role in the design of the study, the collection or analysis of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hiroto Minamino and Masao Katsushima.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-99894-6.
References
- 1.Chen LK, et al. Sarcopenia in Asia: consensus report of the asian working group for aarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 2.Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS ONE. 2017;12:e0169548. doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeung SSY, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle. 2019;10:485–500. doi: 10.1002/jcsm.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle. 2015;6:197–207. doi: 10.1002/jcsm.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshimura Y, et al. Interventions for treating sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J. Am. Med. Dir. Assoc. 2017;18:553 e551. doi: 10.1016/j.jamda.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Petermann-Rocha F, et al. Factors associated with sarcopenia: a cross-sectional analysis using UK Biobank. Maturitas. 2020;133:60–67. doi: 10.1016/j.maturitas.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Sokka T, Hakkinen A, Krishnan E, Hannonen P. Similar prediction of mortality by the health assessment questionnaire in patients with rheumatoid arthritis and the general population. Ann. Rheum. Dis. 2004;63:494–497. doi: 10.1136/ard.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer JT, et al. Impacts of high-protein oral nutritional supplements among malnourished men and women with sarcopenia: a multicenter, randomized, double-blinded, controlled trial. J. Am. Med. Dir. Assoc. 2016;17:1044–1055. doi: 10.1016/j.jamda.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. The Lancet. 2019;393:2636–2646. doi: 10.1016/s0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 10.Torii M, et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod. Rheumatol. 2019;29:589–595. doi: 10.1080/14397595.2018.1510565. [DOI] [PubMed] [Google Scholar]
- 11.Wu CH, et al. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: the Tianliao Old People study 04. Geriatr. Gerontol. Int. 2014;14(Suppl 1):69–75. doi: 10.1111/ggi.12233. [DOI] [PubMed] [Google Scholar]
- 12.Giles JT, et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum. 2008;59:807–815. doi: 10.1002/art.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzik KP, Kaczor JJ. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019;119:825–839. doi: 10.1007/s00421-019-04104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, et al. Vitamin D is related to handgrip strength in adult men aged 50 years and over: a population study from the TCLSIH cohort study. Clin. Endocrinol. (Oxf) 2019;90:753–765. doi: 10.1111/cen.13952. [DOI] [PubMed] [Google Scholar]
- 15.Wicherts IS, et al. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 16.Beaudart C, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014;99:4336–4345. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 17.Gkekas NK, et al. The effect of vitamin D plus protein supplementation on sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Maturitas. 2021;145:56–63. doi: 10.1016/j.maturitas.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Furuya T, et al. Prevalence of and factors associated with vitamin D deficiency in 4,793 Japanese patients with rheumatoid arthritis. Clin. Rheumatol. 2013;32:1081–1087. doi: 10.1007/s10067-013-2216-4. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto M, et al. Increase of hemoglobin levels by anti-IL-6 receptor antibody (tocilizumab) in rheumatoid arthritis. PLoS ONE. 2014;9:e98202. doi: 10.1371/journal.pone.0098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minamino H, et al. Urinary sodium-to-potassium ratio associates with hypertension and current disease activity in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res. Ther. 2021;23:96. doi: 10.1186/s13075-021-02479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh JA, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res.. (Hoboken) 2016;68:1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 22.Minamino H, et al. Habitual fish intake negatively correlates with prevalence of frailty among patients with rheumatoid arthritis. Sci. Rep. 2021;11:5104. doi: 10.1038/s41598-021-84479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LK, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M366–372. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 25.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664. doi: 10.1136/bmj.b5664. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, et al. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis: from the CHIKARA study. Clin. Rheumatol. 2020;39:1757–1764. doi: 10.1007/s10067-020-04929-4. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Invest. 2019;129:3214–3223. doi: 10.1172/JCI125915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marozik P, Rudenka A, Kobets K, Rudenka E. Vitamin D status, bone mineral density, and VDR gene polymorphism in a cohort of Belarusian postmenopausal women. Nutrients. 2021 doi: 10.3390/nu13030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okereke OI, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471–480. doi: 10.1001/jama.2020.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haque UJ, Bartlett SJ. Relationships among vitamin D, disease activity, pain and disability in rheumatoid arthritis. Clin. Exp. Rheumatol. 2010;28:745–747. [PubMed] [Google Scholar]
- 31.Rossini M, et al. Vitamin D deficiency in rheumatoid arthritis: prevalence, determinants and associations with disease activity and disability. Arthritis Res. Ther. 2010;12:R216. doi: 10.1186/ar3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toffanello ED, et al. Vitamin D and physical performance in elderly subjects: the Pro.V.A study. PLoS One. 2012;7:e34950. doi: 10.1371/journal.pone.0034950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaes AMM, et al. The association between 25-hydroxyvitamin D concentration, physical performance and frailty status in older adults. Eur. J. Nutr. 2019;58:1173–1181. doi: 10.1007/s00394-018-1634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain SK, Parsanathan R, Achari AE, Kanikarla-Marie P, Bocchini JA., Jr Glutathione stimulates vitamin D regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25-hydroxy-vitamin D levels in blood: a novel approach to treat 25-hydroxyvitamin D deficiency. Antioxid Redox Signal. 2018;29:1792–1807. doi: 10.1089/ars.2017.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bischoff-Ferrari HA, et al. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 36.Ryan ZC, et al. 1alpha,25-dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J. Biol. Chem. 2016;291:1514–1528. doi: 10.1074/jbc.M115.684399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013;98:E509–513. doi: 10.1210/jc.2012-3592. [DOI] [PubMed] [Google Scholar]
- 38.Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020 doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang J, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J. Immunol. 2009;182:4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villaggio B, Soldano S, Cutolo M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin. Exp. Rheumatol. 2012;30:934–938. [PubMed] [Google Scholar]
- 41.Boonstra A, et al. 1alpha,25-dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 42.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 43.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 44.Mouterde G, et al. Association between vitamin D deficiency and disease activity, disability, and radiographic progression in early rheumatoid arthritis: The ESPOIR cohort. J. Rheumatol. 2020;47:1624–1628. doi: 10.3899/jrheum.190795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.