Abstract
Purpose:
To determine classification criteria for birdshot chorioretinitis.
Design:
Machine learning of cases with birdshot chorioretinitis and 8 other posterior uveitides.
Methods:
Cases of posterior uveitides were collected in an informatics-designed preliminary database, and a final database was constructed of cases achieving supermajority agreement on diagnosis, using formal consensus techniques. Cases were split into a training set and a validation set. Machine learning using multinomial logistic regression was used on the training set to determine a parsimonious set of criteria that minimized the misclassification rate among the infectious posterior/panuveitides. The resulting criteria were evaluated on the validation set.
Results:
One thousand sixty-eight cases of posterior uveitides, including 207 cases of birdshot chorioretinitis, were evaluated by machine learning. Key criteria for birdshot chorioretinitis included a multifocal choroiditis with: 1) the characteristic appearance a bilateral multifocal choroiditis with cream-colored or yellow-orange, oval or round choroidal spots (“birdshot” spots); 2) absent to mild anterior chamber inflammation; and 3) absent to moderate vitreous inflammation; or multifocal choroiditis with positive HLA-A29 testing and either: 1) classic “birdshot spots” or 2) characteristic imaging on indocyanine green angiography. Overall accuracy for posterior uveitides was 93.9% in the training set and 98.0% (95% confidence interval 94.3, 99.3) in the validation set. The misclassification rates for birdshot chorioretinitis were 10% in the training set and 0% in the validation set.
Conclusions:
The criteria for birdshot chorioretinitis had a low misclassification rate and appeared to perform sufficiently well for use in clinical and translational research.
PRECIS
Using a formalized approach to developing classification criteria, including informatics-based case collection, consensus-technique-based case selection, and machine learning, classification criteria for birdshot chorioretinitis were developed. Key criteria included multifocal choroiditis with yellow-orange, oval or round choroidal spots (“birdshot” spots), absent to minimal anterior chamber inflammation, and absent to moderate vitritis; or multifocal choroiditis with positive HLA-A29 testing and either “birdshot” spots or characteristic indocyanine green imaging. The resulting classification criteria had a low misclassification rate.
In 1980 Ryan and Maumenee described a new uveitic disease, birdshot chorioretinitis (BSCR), characterized by vitritis, multifocal choroiditis, and retinal vascular leakage with no to minimal anterior segment inflammation.1 The following year Gass described additional cases, which he called “vitiliginous chorioretinitis” due to the appearance of the choroidal lesions.2 Clinically the choroidal lesions of BSCR are described as multifocal, cream-colored or yellow-orange, and ovoid. Histologically, BSCR is characterized by multifocal choroidal inflammation with mononuclear inflammatory cells and retinal vascular “cuffing”.3 Patients present with complaints of floaters, blurred vision, flashing lights, vibrating vision, loss of peripheral vision, and/or acquired nyctalopia.4 Vision is lost either due to macular edema or diffuse retinal damage with photoreceptor loss.5–9 Given sufficient time, macular edema will occur in the majority of BSCR patients.6 The diffuse retinal damage manifests as visual field loss or an abnormal electroretinogram (ERG).7–9 Assessment of the peripheral visual field is important, as the earliest field loss is peripheral, manifested as a relative constriction on Goldmann perimetry or loss of peripheral field on relevant automated field testing (e.g. the Humphrey P-60 program), which can be missed by automated perimetry sampling only the central 24 or 30 degrees of field. One study suggested that at presentation automated perimetry of the central field was abnormal in ~1/3 of patients, whereas an abnormal peripheral field was present in ~75% of patients; and loss of peripheral field correlated with an abnormal ERG.8 This diffuse retinal damage can be monitored with either visual fields including the peripheral retina or ERG.8,9 The choroidal lesions of BSCR may be hyperfluorescent on fluorescein angiography but often are not well delineated by this modality. Conversely, indocyanine green (ICG) angiography demonstrates the lesions as multifocal hypofluorescent spots and may detect the lesions better than clinical examination, especially early in the disease when the lesions seen on ophthalmoscopy may be difficult to discern. Fluorescein angiography often demonstrates vascular leakage.4,5 Optical coherence tomography demonstrates the choroidal lesions, macular edema (when present), and disruption of the ellipsoid zone of the retina.5 Enhanced depth OCT imaging of the choroid demonstrates hypo-reflective zones in ~64% of patients.10 Fundus autofluorescence is abnormal (hypo-autofluorescence) in 79% of eyes, and the most frequent finding is peripapillary confluent hypo-autofluorescence in ~73%.11 Even patients with good visual acuity may have decreased contrast sensitivity and decreased quality of vision. Among the various symptoms, nyctalopia is most associated with an impaired quality of life.12,13
There is a strong genetic risk to BSCR, which is associated with the HLA type HLA-A29 with most studies reporting that ~90 % to 95% of patients with BSCR are HLA-A29-positive.14–18 Early studies suggested that the association was with the subtype HLA-A29.2, but subsequent studies have reported cases which are HLA-A29.1 positive as well.16–18 Although HLA-A29 is a risk factor, BSCR is a complex disorder and not a Mendelian one. HLA-A29 is present in 7% to 8% of the Caucasian population,14,15,18 and BSCR is a rare disease, accounting for only ~7% of posterior uveitides, which themselves account for only ~5% to 20% of all uveitides.19–23 If one generously estimates that BSCR accounts for ~1% of all uveitides in the United States, then the estimated prevalence is ~1/100,000 population. The proportion of people who possess HLA-A29 that develop BSCR would then be estimated as ~2–3/10,000. Hence other, unknown, environmental factors must contribute to the pathogenesis. Nevertheless, the reported occurrence of chorioretinal lesions in HLA-A29 transgenic mice24 suggests that HLA-A29 may be involved in pathogenesis in an as yet undetermined fashion, perhaps via aberrant antigen presentation.
Although the macular edema of BSCR responds to corticosteroid therapy, it typically relapses at doses of prednisone <15mg/day; a dose too high for long-term use.6,25 Conversely, immunosuppression is associated with an estimated ~85% reduction in macular edema, suggesting that if treatment is needed, immunosuppression should be used from the outset.6 Short-term therapies do not prevent loss of visual field and ERG,8,26 whereas immunosuppression can reverse visual field loss (although not always normalize it).8,27 Intermediate-term studies suggest that immunosuppression of patients with BSCR has a high success rate including preserved acuity, improving (or normal) visual fields, and successful corticosteroid-sparing (≤7.5 mg/day).27,28
The Standardization of Uveitis Nomenclature (SUN) Working Group is an international collaboration, which has developed classification criteria for 25 of the most common uveitides using a formal approach to development and classification. Among the diseases studied was birdshot chorioretinitis.29–35
Methods
The SUN Developing Classification Criteria for the Uveitides project proceeded in four phases as previously described: 1) informatics, 2) case collection, 3) case selection, and 4) machine learning.31–34
Informatics.
As previously described, the consensus-based informatics phase permitted the development of a standardized vocabulary and the development of a standardized, menu-driven hierarchical case collection instrument.31
Case collection and case selection.
De-identified information was entered into the SUN preliminary database by the 76 contributing investigators for each disease as previously described.36,37 Cases in the preliminary database were reviewed by committees of 9 investigators for selection into the final database, using formal consensus techniques described in the accompanying article.36,37 Because the goal was to develop classification criteria,38 only cases with a supermajority agreement (>75%) that the case was the disease in question were retained in the final database (i.e. were “selected”).36,37
Machine learning.
The final database then was randomly separated into a learning set (~85% of the cases) and a validation set (~15% of the cases) for each disease as described in the accompanying article.37 Machine learning was used on the learning set to determine criteria that minimized misclassification. The criteria then were tested on the validation set; for both the learning set and the validation set, the misclassification rate was calculated for each disease. The misclassification rate was the proportion of cases classified incorrectly by the machine learning algorithm when compared to the consensus diagnosis. For BSCR, the diseases against which it was evaluated included: acute posterior multifocal placoid pigment epitheliopathy (APMPPE), multifocal choroiditis with panuveitis (MFCPU), multiple evanescent white dot syndrome (MEWDS), punctate inner choroiditis (PIC), serpiginous choroiditis, sarcoidosis-associated posterior uveitis, syphilitic posterior uveitis, and tubercular (TB) uveitis.
The study adhered to the principles of the Declaration of Helsinki. Institutional Review Boards (IRBs) at each participating center reviewed and approved the study; the study typically was considered either minimal risk or exempt by the individual IRBs.
Results
Two hundred fifty-seven cases of birdshot chorioretinitis were collected, and 207 (81%) achieved supermajority agreement on the diagnosis during the “selection” phase and were used in the machine learning phase These cases of BSCR were compared to cases of posterior uveitides, including 82 cases of APMPPE, 122 cases of serpiginous choroiditis, 51 cases of MEWDS, 138 cases of MFCPU, 144 cases of PIC, 12 cases of sarcoid posterior uveitis, 35 cases of syphilitic posterior uveitis, and 277 cases of tubercular posterior uveitis (including 96 cases of serpiginous-like tubercular choroiditis). The details of the machine learning results for these diseases are outlined in the accompanying article.34 The characteristics of cases with birdshot chorioretinitis are listed in Table 1. The classification criteria developed after machine learning are listed in Table 2. Key features of the criteria include: 1) a bilateral multifocal choroiditis with the characteristic appearance, namely multifocal cream-colored or yellow-orange, round or ovoid choroidal spots (“birdshot spots”; Figure 1); 2) absent to minimal anterior chamber inflammation; 3) absent to mild vitritis; and 3) the exclusion of sarcoidosis; or a positive test for HLA-A29 and either 1) the characteristic “birdshot spots”; or 2) the characteristic choroidal lesions on indocyanine green angiography. The overall accuracies for posterior uveitides were 93.9% in the learning set and 98.0% (95% confidence interval 94.3, 99.3) in the validation set.34 The misclassification rate for BSCR in the learning set was 10%, and in the validation set 0%. The disease with which it most often was confused in the learning set was MFCPU.
Table 1.
Characteristics of Cases with Birdshot Chorioretinitis
| Characteristic | Result |
|---|---|
| Number cases | 207 |
| Demographics | |
| Age, median, years (25th 75th percentile) | 51 (45, 58) |
| Gender (%) | |
| Men | 39 |
| Women | 61 |
| Race/ethnicity (%) | |
| White, non-Hispanic | 92 |
| Black, non-Hispanic | 0 |
| Hispanic | 1 |
| Asian, Pacific Islander | 0 |
| Other | 0 |
| Missing | 7 |
| Uveitis History | |
| Uveitis course (%) | |
| Acute, monophasic | 1 |
| Acute, recurrent | 1 |
| Chronic | 95 |
| Indeterminate | 3 |
| Laterality (%) | |
| Unilateral | 0 |
| Unilateral, alternating | 0 |
| Bilateral | 100 |
| Ophthalmic examination | |
| Keratic precipitates (%) | |
| None | 100 |
| Anterior chamber cells (%) | |
| Grade 0 | 83 |
| ½+ | 11 |
| 1+ | 5 |
| ≥2+ | 0 |
| Anterior chamber flare (%) | |
| Grade 0 | 93 |
| 1+ | 6 |
| 2+ | 1 |
| ≥3+ | 0 |
| Iris (%) | |
| Normal | 100 |
| Intraocular pressure (IOP), involved eyes | |
| Median, mm Hg (25th, 75th percentile) | 15 (14, 18) |
| Proportion patients with IOP>24 mm Hg either eye (%) | 2 |
| Vitreous cells (%) | |
| Grade 0 | 13 |
| ½+ | 16 |
| 1+ | 36 |
| 2+ | 32 |
| 3+ | 3 |
| 4+ | 0 |
| Vitreous haze (%) | |
| Grade 0 | 49 |
| ½+ | 19 |
| 1+ | 21 |
| 2+ | 11 |
| ≥3+ | 0 |
| Chorioretinal lesion characteristics | |
| Lesion number (%) | |
| Unifocal (1 lesion) | 0 |
| Paucifocal (2–4) | 0 |
| Multifocal (≥5) | 96 |
| Missing | 4 |
| Lesion shape & character (%) | |
| Ameboid or serpentine | 0 |
| Oval or round | 96 |
| Placoid | 0 |
| Atrophic | 2 |
| Punctate | 0 |
| Wedge-shaped | 0 |
| Missing | 2 |
| Lesion location (%) | |
| Posterior pole involved | 68 |
| Mid-periphery and periphery only | 32 |
| Typical lesion size (%) | |
| <250 μm | 31 |
| 250–500 μm | 40 |
| >500 μm | 24 |
| Missing | 5 |
| Other features (%) | |
| Retinal vascular sheathing | 17 |
| Retinal vascular leakage | 33 |
| Choroidal neovascularization | 0 |
| Laboratory data (%) | |
| HLA-A29 positive* | 89 |
184 of 186 cases tested (99%) were positive.
Table 2.
Classification Criteria for Birdshot Chorioretinitis
| Criteria ([#’s 1, 2, and 3] OR # 4) |
| 1. Characteristic bilateral multifocal choroiditis on ophthalmoscopy |
| a. Multifocal cream-colored or yellow-orange, oval or round choroidal lesions (“birdshot spots”) |
| AND |
| 2. Absent to mild anterior chamber inflammation |
| a. Absent to mild anterior chamber cells AND |
| b. No keratic precipitates AND |
| c. No posterior synechiae |
| AND |
| 3. Absent to moderate vitritis |
| OR |
| 4. Multifocal choroiditis with |
| a. Positive HLA-A29 test AND either (b. or c.) |
| b. Characteristic “birdshot” spots (multifocal cream-colored or yellow-orange, oval or round choroidal lesions) on ophthalmoscopy OR |
| c. Characteristic indocyanine green angiogram (multifocal hypofluorescent spots) without characteristic “birdshot” spots on ophthalmoscopy |
| Exclusions |
| 1. Positive serologic test for syphilis using a treponemal test |
| 2. Evidence of sarcoidosis (either bilateral hilar adenopathy on chest imaging or tissue biopsy demonstrating non-caseating granulomata)* |
| 3. Evidence of intraocular lymphoma on diagnostic vitrectomy or tissue biopsy |
Possible sarcoidosis should be evaluated with chest imaging at a minimum.
Figure 1.
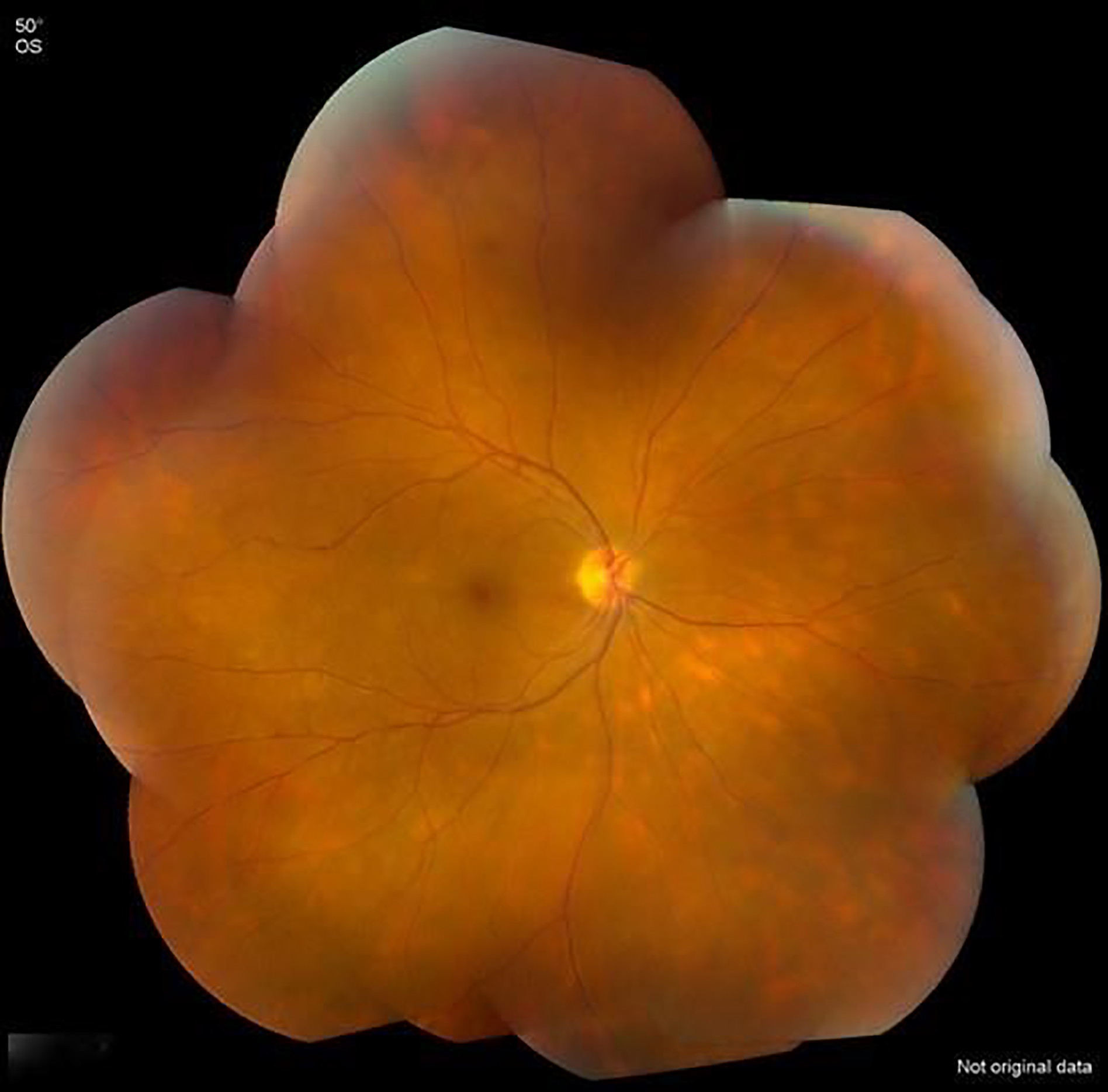
Fundus photograph of a case of birdshot chorioretinitis, demonstrating multifocal yellow-orange, ovoid, choroidal spots.
Discussion
The classification criteria developed by the SUN Working Group for BSCR have a low misclassification rate, indicating good discriminatory performance against other posterior uveitides.
In 2006 Levinson et al36 proposed research criteria for birdshot chorioretinitis from an international consensus conference. These criteria were compared to a small number of uveitis cases of all classes and a good performance was suggested. The SUN Criteria for BSCR developed here are similar to but not identical with the previously proposed criteria for BSCR. Differences include: 1) the number of lesions required; 2) the “supportive criteria” as used by the international consensus conference; and the 3) international consensus conference’s blanket exclusion of infectious, neoplastic, or other inflammatory diseases (suggesting the need for extensive laboratory investigations). The SUN BSCR Criteria required ≥5 spots (as opposed to 3), as all cases of BSCR with information entered were multifocal (≥5 spots). The SUN BSCR Criteria were more focused in their exclusions, making them easier to use. Syphilis is in the differential diagnosis of posterior uveitides, and sarcoidosis may present with a multifocal choroiditis similar in appearance to BSCR.37,38 The SUN BSCR Criteria also added indocyanine green (ICG) angiographic evidence of a multifocal choroiditis (Figure 2) if the patient was HLA-A29-positive, as rare cases of BSCR can present with uncertain findings on clinical examination and have a classic ICG angiogram. However, because other posterior uveitides can have multifocal hypofluorescent spots on ICG, these patients are required to be HLA-A29-positive.
Figure 2.

Indocyanine green angiogram of a case of birdshot chorioretinitis, demonstrating multifocal, hypofluorescent choroidal spots.
In the machine learning, HLA-A29 emerged as an important criterion for BSCR, in part as a consequence of the retrospective nature of the data collection and typical clinical practices: HLA-A29 data were available for most cases with BSCR but not on other patients. Given the population frequency of HLA-A29 and the proportion of cases of posterior uveitis that are BSCR, a strategy of using HLA-A29 to define BSCR in the absence of considerations of morphology has a positive predictive value of only 47%.19 Nevertheless, in those situations where the differential diagnosis is limited in number but there are similar chorioretinal morphologies (e.g. intraocular lymphoma, sarcoidosis-associated multifocal choroiditis), HLA-A29 testing may be of great value with positive predictive and negative values >90%.19 Some investigators have championed the idea that HLA-A29 should be required for the diagnosis of BSCR, and in their case series all cases will be HLA-A29-positive. However, most case series have reported that HLA-A29 is present in 90% to 95% of cases, suggesting that it is a tightly linked risk factor, similar to that of HLA-B27 and ankylosing spondylitis. Future studies demonstrating differences in the characteristics and course between HLA-A29-positive and HLA-A29-negative cases with BSCR could lead to a revision of the criteria.
These criteria were designed to be used at patient presentation in order to facilitate enrollment in prospective studies or clinical trials. There are case reports suggesting that with successful treatment the clinical findings (including the birdshot spots) can resolve,39, 40 so that evaluation of a treated patient might require evaluation of the presentation findings, possibly including fundus photography and ICG angiography, in order to correctly classify the patient.
The median age of the cases with BSCR was 51 years, suggesting that many patients with BSCR will be in an age group at risk for intraocular lymphoma, and intraocular lymphoma has been reported occasionally to present with an appearance similar to BSCR.41 Intraocular lymphoma accounts for ~1.5% of cases of “uveitis” presenting in older patients and 10% of such cases undergoing diagnostic vitrectomy,42 so that routine vitrectomy to exclude lymphoma would be unreasonable. In this situation, HLA-A29 testing may be valuable, as noted above, and an atypical appearance for BSCR may prompt a diagnostic vitrectomy in clinical care.
The presence of any of the exclusions in Table 2 suggests an alternate diagnosis, and the diagnosis of BSCR should not be made in their presence. In prospective studies many of these tests will be performed routinely, and the alternative diagnoses excluded. However, in retrospective studies based on clinical care, not all of these tests may have been performed. In these studies the presence of an exclusionary criterion excludes BSCR, but the absence of such testing does not always exclude the diagnosis of BSCR if the criteria for the diagnosis are met. Nevertheless, because sarcoidosis may present as a multifocal choroiditis with a clinical picture very similar to BSCR, its exclusion through at least routine chest imaging should be performed.37,38
Classification criteria are employed to diagnose individual diseases for research purposes.35 Classification criteria differ from clinical diagnostic criteria, in that although both seek to minimize misclassification, when a trade-off is needed, diagnostic criteria typically emphasize sensitivity, whereas classification criteria emphasize specificity,35 in order to define a homogeneous group of patients for inclusion in research studies and limit the inclusion of patients without the disease in question that might confound the data. The machine learning process employed did not explicitly use sensitivity and specificity; instead it minimized the misclassification rate. Because we were developing classification criteria and because the typical agreement between two uveitis experts on diagnosis is moderate at best,33 the selection of cases for the final database (“case selection”) included only cases which achieved supermajority agreement on the diagnosis. As such, some cases which clinicians would diagnose with birdshot chorioretinitis may not be so classified by classification criteria.
In conclusion, the criteria for birdshot chorioretinitis outlined in Table 2 appear to perform sufficiently well for use as classification criteria in clinical research.34
Grant support:
Supported by grant R01 EY026593 from the National Eye Institute, the National Institutes of Health, Bethesda, MD, USA; the David Brown Fund, New York, NY, USA; the Jillian M. And Lawrence A. Neubauer Foundation, New York, NY, USA; and the New York Eye and Ear Foundation, New York, NY, USA.
Footnotes
Writing committee: Douglas A. Jabs, MD, MBA2,3; Antoine P. Brezin, MD4; Ralph D. Levinson, MD5; Peter McCluskey, MD6; Neal Oden, PhD7; Alan G. Palestine, MD8; Russell W. Read, MD, PhD9; Jennifer E. Thorne, MD, PhD2,3; Brett E. Trusko, PhD, MBA10; Albert Vitale, MD11; Susan E. Wittenberg, MD12
Affiliations: 1Members of the SUN Working Group are listed online at ajo.com. From 2the Department of Epidemiology, the Johns Hopkins University Bloomberg School of Public Health, and 3the Wilmer Eye Institute, the Department of Ophthalmology, the Johns Hopkins University School of Medicine, Baltimore, MD, USA; 4Department of Ophthalmology, University of Paris V – Hôpital Cochin, Paris, France; 5the UCLA Stein Eye Institute and the Department of Ophthalmology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 6the UCLA Stein Eye Institute and the Department of Ophthalmology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 7the Emmes Company, LLC, Rockville, MD, USA; 8the Department of Ophthalmology, University of Colorado School of Medicine, Aurora, Co, USA; 9the Department of Ophthalmology and Visual Sciences, University of Alabama at Birmingham, Birmingham, AL, USA; 10the Department of Medicine, Texas A&M University, College Station, TX, USA; 11the Department of Ophthalmology, the University of Utah School of Medicine, Salt Lake City, UT, USA; 12Houston Eye Associates, Houston, TX, USA.
Conflict of Interest: Douglas A. Jabs: none; Antoine P. Brezin: none; Ralph D. Levinson: none; Peter McCluskey: none; Neal Oden: none; Alan G. Palestine: none; Russell W. Read: none; Jennifer E. Thorne: Dr. Thorne engaged in a portion of this research as a consultant and was compensated for the consulting service; Brett E. Trusko: none; Albert Vitale: none; Susan E. Wittenberg: none.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ryan SJ, Maumenee AE. Birdshot retinochoroidopathy. Am J Ophthalmol 1980; 89:31–45. [DOI] [PubMed] [Google Scholar]
- 2.Gass JD. Vitiliginous chorioretinitis. Arch Ophthalmol 1981;99:1778–87. [DOI] [PubMed] [Google Scholar]
- 3.Gaudio PA, Kaye DB, Crawford JB. Histopathology of birdshot retinochoroidopathy. Br J Ophthalmol 2001;86:1439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monnet D, Brezin AP, Holland GN, Yu F, Mahr A, Gordon LK, Levinson RD. Longitudinal cohort study of patients with birdshot chorioretinopathy. I. Baseline clinical characteristics. Am J Ophthalomol 2006;141:135–42. [DOI] [PubMed] [Google Scholar]
- 5.Monnet D, Levinson RD, Holland GN, Haddad L, Yu F, Brezin AP. Longitudinal cohort study of patients with birdshot chorioretinopathy. III. Macular imaging at baseline. Am J Ophthalmol 2007;144:818–28. [DOI] [PubMed] [Google Scholar]
- 6.Thorne JE, Jabs DA, Peters GB, Hair D, Dunn JP, Kempen JH. Birdshot retinochoroidopathy: ocular complications and visual impairment. Am J Ophthalmol 2005;140:45–51. [DOI] [PubMed] [Google Scholar]
- 7.Gordon LK, Monnet D, Holland GN, Brezin AP, Yu F, Levinson RD. Longitudinal cohort study of patients with birdshot chorioretinopathy. IV. Visual field results at baseline. Am J Ophthalmol 2007;144:829–37. [DOI] [PubMed] [Google Scholar]
- 8.Thorne JE, Jabs DA, Kedhar SR, Peters GB, Dunn JP. Loss of visual field among patients with birdshot chorioretinopathy. Am J Ophthalmol 2008:145:23–8. [DOI] [PubMed] [Google Scholar]
- 9.Sobrin L, Lam BL, Liu M, Feuer WJ, Davis JL. Electroretinographic monitoring in birdshot chorioretinopathy. AM J Ophthalmol 2005;140:52–64. [DOI] [PubMed] [Google Scholar]
- 10.Boni C, Thorne JE, Spaide RF, et al. Choroidal findings in eyes with birdshot chorioretinitis using enhanced-depth optical coherence tomography. Invest Ophthalmol Vis Sci 2016;57:591–9. [DOI] [PubMed] [Google Scholar]
- 11.Boni C, Thorne JE, Spaide RF, et al. Fundus autofluorescence in eyes with birdshot chorioretinitis. Invest Ophthamol Vis Sci 2017;58:4015–25. [DOI] [PubMed] [Google Scholar]
- 12.Kappel PJ, Monnet D, Yu F, Brezin AP, Levinson RD, Holland GN. Contrast sensitivity among patients with birdshot chorioretinopathy. Am J Ophthalmol 2009;147:351–6. [DOI] [PubMed] [Google Scholar]
- 13.Levinson RD, Du Z, Luo L, et al. Longitudinal cohort study of patients with birdshot chorioretinopathy. V. Quality of life at baseline. Am J Ophthalmol 2009;147:346–50. [DOI] [PubMed] [Google Scholar]
- 14.Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol 1982:94:147–58. [DOI] [PubMed] [Google Scholar]
- 15.Baarsma GS, Priem HA, Kilstra A. Association of birdshot retinochoroidopathy and HLA-A29 antigen. Curr Eye Res 1990;9:suppl 63–8. [DOI] [PubMed] [Google Scholar]
- 16.Le Hoang P, Ozdemir N, Benhamou A, et al. HLA-A29.2 subtype associated with birdshot retinochoroidopathy. Am J Ophthalmol 1992;113:33–5. [DOI] [PubMed] [Google Scholar]
- 17.Levinson RD, Rajalinngam R, Park MS, et al. Human leukocyte antigen A29 subtypes associated with birdshot retinochoroidopathy. Am J Ophthalmol 2004;138:631–4. [DOI] [PubMed] [Google Scholar]
- 18.Brezin AP, Monnet DS, Cohen JH, Levinson RD. HLA-A29 and birdshot chorioretinopathy. Ocul Immunol Inflamm 2011;19:397–400. [DOI] [PubMed] [Google Scholar]
- 19.Zamecki KJ, Jabs DA. HLA-typing in uveitis: use and misuse. Am J Ophthalmol 2010;149:189–93. [DOI] [PubMed] [Google Scholar]
- 20.Wakefield D, Dunlop I, McCluskey PJ, Penny R. Uveitis: aetiology and disease associations in an Australian population. Aust NZ Ophthalmol 1986;14:181–7. [DOI] [PubMed] [Google Scholar]
- 21.McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. Am J Ophthalmol 1996;121:35–46. [DOI] [PubMed] [Google Scholar]
- 22.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California epidemiology of uveitis study. Ophthalmology 2004;111:491–500. [DOI] [PubMed] [Google Scholar]
- 23.Gritz DC, Schwaber EJ, Wong IG. Complications of uveitis: the Northern California epidemiology of uveitis study. Ocular Immunol Inflamm 2018;26:584–9. [DOI] [PubMed] [Google Scholar]
- 24.Szpak Y, Vieville JC, Tabary T, et al. Spontaneous retinopathy in HLA-A29 transgenic mice. Proc Natl Acad Sci USA 2001;98:2572–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabs DA. Immunosuppression for the uveitides. Ophthalmology 2018;125:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh KT, Christmas NJ, Folk JC. Birdshot retinochoroiditis: long term follow-up of a chronically progressive disease. Am J Ophthalmol 2002;133:622–9. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg NR, LKyu T, Moshier E, Godbold J, Jabs DA. Success with single-agent immunosuppression for multifocal choroidopathies. Am J Ophthalmol 2014;158:1310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomkins-Netzer O, Taylor SR, Lightman S. Long-term clinical and anatomic outcome of birdshot chorioretinopathy. JAMA Ophthalmol 2014;132:57–62. [DOI] [PubMed] [Google Scholar]
- 29.Jabs DA, Rosenbaum JT, Nussenblatt RB, the Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Report of the first international workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jabs DA, Busingye J. Approach to the diagnosis of the uveitides. Am J Ophthalmol 2013;156:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trusko B, Thorne J, Jabs D, et al. Standardization of Uveitis Nomenclature Working Group. The SUN Project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf Med 2013;52:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada AA, Jabs DA. The SUN Project. The future is here. Arch Ophthalmol 2013;131:787–9. [DOI] [PubMed] [Google Scholar]
- 33.Jabs DA, Dick A, Doucette JT, Gupta A, Lightman S, McCluskey P, Okada AA, Palestine AG, Rosenbaum JT, Saleem SM, Thorne J, Trusko B for the Standardization of Uveitis Nomenclature Working Group. Interobserver agreement among uveitis experts on uveitic diagnoses: the Standard of Uveitis Nomenclature experience. Am J Ophthalmol 2018; 186:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Standardization of Uveitis Nomenclature (SUN) Working Group. Development of classification criteria for the uveitides. Am J Ophthalmol 2020;volume:pp. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria. Arthritis Care Res 2015;67(7):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levinson RD, Brezin A, Rothova A, Accorinti M, Holland GN. Research criteria for the diagnosis of birdshot chorioretinopathy: results of an international consensus conference. Am J Ophthalmol 2006;141:185–7. [DOI] [PubMed] [Google Scholar]
- 37.Khurana RN, Parikh JG, Rao NA. Sarcoid choroiditis simulating birdshot chorioretinopathy. Retinal Cases Brief Rep 2008;2:301–3. [DOI] [PubMed] [Google Scholar]
- 38.The Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for sarcoidosis-associated uveitis. Am J Ophthalmol 2020;volume:pp. [DOI] [PubMed] [Google Scholar]
- 39.Leder HA, Galor A, Thorne JE, Jabs DA. Disappearance of classic birdshot spots after immunosuppression with tacrolimus and mycophenolate mofetil. Br J Ophthalmol 2008;92:291. [DOI] [PubMed] [Google Scholar]
- 40.Forooghian F, Gulati N, Jabs DA. Restoration of retinal architecture following systemic immunosuppression in birdshot chorioretinopathy. Ocular Immunol Inflamm 2010;18:470–1 [DOI] [PubMed] [Google Scholar]
- 41.Miserocchi E, Mdoorati G, De Benedetto U, Colucci A, Bandello F. Birdshot retinochoroidopathy masquerading as intraocular lymphoma. Ocular Immunol Inflamm 2012;20:306–8. [DOI] [PubMed] [Google Scholar]
- 42.Chatzistefanou K, Markomichelakis NM, Christen W, Sohelian M, Foster CS. Characteristics of uveitis presenting for the first time in the elderly. Ophthalmology 1998;105:347–52. [DOI] [PubMed] [Google Scholar]


