Abstract
Background:
Maintaining functional status is important to older adults with cancer but data are limited on how systemic treatments affect functional status. We systematically reviewed changes in functional status during systemic cancer treatments and identified characteristics associated with functional decline and improvement.
Methods:
We searched PubMed, Embase, Web of Science, and Cochrane Register of Controlled Trials for articles examining characteristics associated with functional change in older adults during systemic cancer treatment published in English from database inception to January 11, 2019 (PROSPERO CRD42019123125). Findings were summarized with descriptive statistics. We used Fisher’s exact tests to compare study characteristics between older adult- and non-older adult-specific studies.
Results:
We screened 15,244 titles/abstracts and 519 full texts. The final analysis included 44 studies, which enrolled >8,400 patients; 39% of studies focused on older adults (1 study enrolled adults age ≥60, 10 studies ≥65, 6 studies ≥70). Almost all studies (98%) utilized patient-reported outcomes to measure functional status; only 20% utilized physical performance tests. Reporting of functional change was heterogeneous with 48% reporting change scores. Older adult-specific studies were more likely to analyze functional change dichotomously (29% versus 4%, P=0.008). Functional decline ranged widely from 6% to 90%. The most common patient characteristics associated with functional decline were older age (n=7 studies), worse performance status (n=4), progressive disease status (n=4), pain (n=4), anemia (n=4), and worse nutritional status (n=4). Twelve studies examined functional improvement and identified 11 unique associated characteristics.
Conclusions:
Functional decline is increasingly recognized as an important outcome in older adults with cancer but definitions and analyses are heterogeneous, leading to a wide range of prevalence. To identify patients at highest risk of functional decline during systemic cancer treatments, trials need to routinely analyze functional outcomes and measure characteristics associated with decline (e.g., nutrition).
Keywords: geriatric oncology, functional status, functional decline, systemic therapy, chemotherapy
INTRODUCTION
Older adults with cancer are at increased risk for treatment toxicity and functional impairment,1–4 resulting in increased healthcare utilization and mortality.5–10 Maintaining functional status (FS) during cancer treatment is critically important to patients. More than 70% of older patients with cancer report that they would not choose a treatment that results in functional impairment, even if it improves survival.11 Despite the importance of functional outcomes to older adults, cancer clinical trials rarely capture the full impact of treatment on FS. Instead, trials focus on narrow definitions of treatment toxicity using provider-reported adverse events,12 which do not capture FS or changes over time. As a result, there are limited data on how cancer treatments affect FS in older adults, hindering delivery of goal-concordant care.
Understanding how FS may change during systemic cancer treatment (e.g., chemotherapy, immunotherapy, targeted therapy) and which patient characteristics are associated with these changes can inform shared decision-making to individualize cancer care. Identifying which patients are at highest risk of functional decline is necessary to weigh the potential benefits and harms of treatment options, better inform patient and caregiver anticipatory guidance, and allow for early introduction of tailored interventions to prevent functional impairment such as exercise and rehabilitation programs.13–15
Given the rising recognition of the importance of FS in older adults with cancer16 and explosion of new cancer treatments, an increasing number of studies have examined characteristics associated with FS change during treatment. While a prior systematic review examined the prognostic and predictive value of FS at baseline,10 no review has systematically synthesized the literature on changes in FS during systemic cancer treatment. Therefore, we aimed to examine changes in FS during systemic cancer treatments with a focus on older adults and identify patient characteristics associated with functional decline and improvement.
Methods
Search strategy and selection criteria
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines.17 With a medical librarian (DC), we searched PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials for articles examining changes in FS during systemic cancer treatment among adults age ≥65 published in English between database inception and January 11, 2019. Full search terms are shown in the Supplemental Table and included: “neoplasms,” “cancer,” “malignancy,” or “tumor,” AND “chemotherapy,” “immunotherapy,” or “antineoplastic,” AND “functional status,” “functional decline,” “physical function,” “mobility,” “daily living activity,” or “activities of daily living.” This systematic review is registered with PROSPERO (CRD42019123125).
Studies were evaluated using these inclusion criteria: 1) study included patients age ≥65 with any cancer type; 2) participants received systemic cancer therapy; 3) FS quantitatively measured using physical performance tests, patient-reported outcomes (PROs; e.g., instrumental activities of daily living [IADL]), physical well-being as part of a quality of life (QOL) measure, physical activity (e.g., step count), and/or clinician-reported performance status (PS); 4) FS measured at ≥2 time points (one before or during treatment such that change in FS during treatment could be ascertained); 5) FS analyzed as an outcome; 6) study reported an analysis of associations between patient characteristics and change in FS; and 7) study published in English. Studies of systemic therapy and other treatment modalities (e.g., surgery) were only included if they reported results separately for patients who received systemic therapy. Of note, studies of concurrent chemoradiation were allowed since chemoradiation is the standard of care for some cancer types (e.g., head and neck). Additionally, studies of FS interventions (e.g., exercise) were required to have control arms to allow evaluation of the effect of systemic cancer treatment on FS. Exclusion criteria included: 1) studies of hormonal therapy, radiation, or surgery alone; 2) articles that did not report original data; and 3) full text unavailable.
All identified articles were imported into Covidence (Veritas Health Innovation) and duplicates were removed. At each step below, discrepancies were resolved by consensus (KPL, MLW). Two investigators independently screened titles and abstracts for eligibility. This evaluation was then repeated for full text review. The final list of included full texts was used for data extraction.
Data extraction and quality appraisal
A standardized template for data extraction was pilot tested (KPL, MLW). Two independent investigators extracted data for each study. Extracted data included first author, publication year, journal, geographic region, study design, intervention and control arm (if applicable), key inclusion criteria, sample size, age distribution, cancer treatment(s), measure(s) of FS, time points assessed, definition of change in FS, key findings, and characteristics associated with functional change.
Two independent investigators performed appraisal of study quality using the National Heart, Lung, and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, which consists of 14 criteria.18 Example criteria included clearly defined research question, study population, inclusion criteria, and exposure and outcome measures. Participation rate and loss to follow-up were also considered. Meeting each criteria earned one point and a summary score was calculated.
Data analysis
Descriptive statistics were used to summarize study characteristics including cancer type, measures of FS used (patient-reported outcome, clinician-reported, physical performance test), assessment time points, and analytic approach. Fisher’s exact tests were used to compare study characteristics between older adult-specific and non-older adult specific studies. Characteristics associated with functional decline and improvement were summarized. No meta-analysis was planned a priori given the heterogeneity in measures used to assess FS, cancer populations studied, and analytic methods.
RESULTS
Study characteristics
We screened 15,244 titles/abstracts and 519 full texts (Supplemental Figure). The final analysis included 44 studies,1, 2, 19–60 which were published from 1991-2019 (Figure 1) and enrolled more than 8,400 patients with cancer. Seventeen studies (39%) focused on older adults (Table 1) while 27 (61%) included adults of all ages (Table 2). Among the older adult-specific studies, which increased in number in recent years (Figure 1), one study enrolled only adults age ≥60,19 ten enrolled only adults age ≥65,1, 2, 20–27 and six enrolled only adults age ≥70.28–33 A quarter of studies enrolled a heterogeneous population of patients with a solid or hematologic malignancy and a quarter enrolled patients with lung cancer. The next most common cancer types were breast cancer (16%) and hematologic malignancies (16%). The majority of studies evaluated FS during chemotherapy (84%) with only five studies32, 33, 36, 59, 60 including targeted therapy and two studies54, 59 including immunotherapy.
Figure 1.
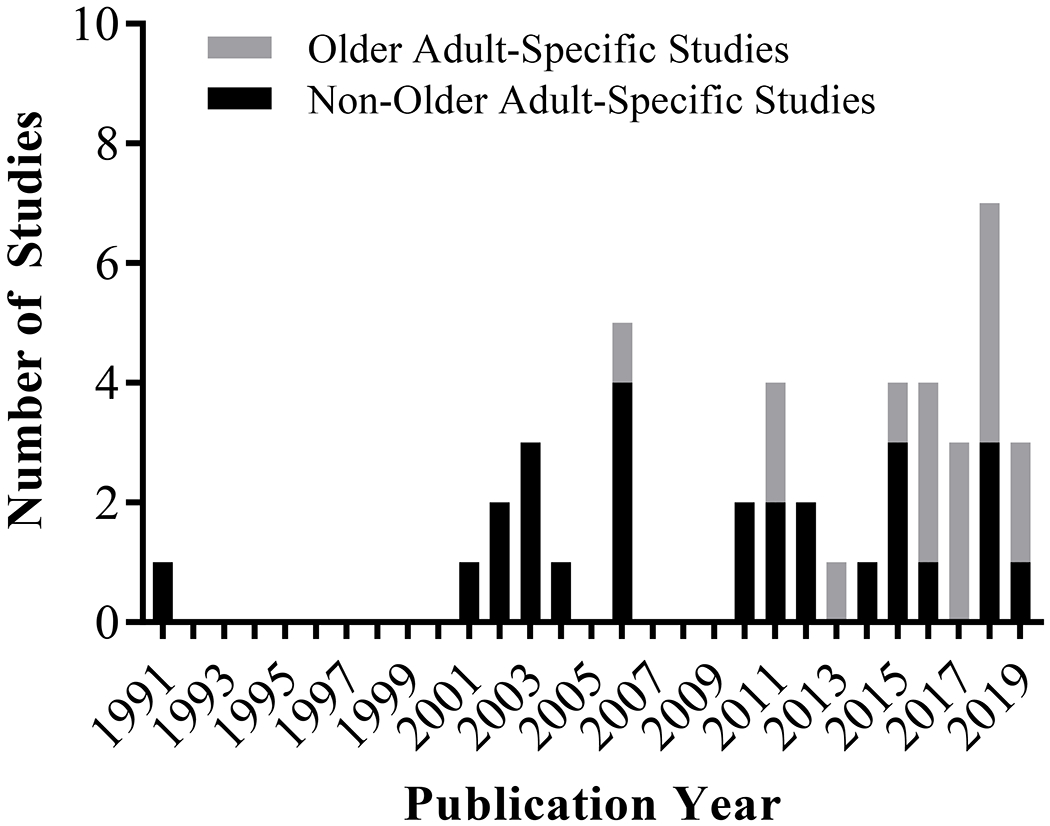
Included studies by publication year and study type (older adult-specific versus non-older adult-specific study). Systematic review included publications from database inception through January 11, 2019.
Table 1.
Older-adult specific studies meeting inclusion criteria and primary functional status results (N=17).
| Age inclusion criteria | Study/Quality assessment scorea | Study design | Sample (cancer type(s), stage) | Overall N (systemic therapy n) | Age (age 65+ n if available or age summary statistic – mean/median) | Systemic cancer treatment(s) | Primary measure(s) of function status | Assessment time points | Definition of functional change | Change in functional status during treatment | Association of patient characteristicsb with functional status change |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age ≥60 | Klepin 201619/8 | Single center observational cohort (US) | AML | 49 | Mean age 70 (SD 2); 57.1% age 60-69, 34.7% 70-79, 8.2% ≥80 | Chemo | Pepper Assessment Tool for Disability (ADL, IADL, mobility), SPPB, grip strength | Pretx (hospitalization for induction chemo), 8 wks after discharge | Decline using standard cutoffs | -IADL dependence worsened (1.4 baseline vs 2.1 follow-up, p<0.001) -SPPB worsened (7.5 vs 5.9, p=0.02) -Grip strength declined (men: 38.9 vs 34.2, p<0.001; women: 24.5 vs 21.8, p=0.007) |
-Unfavorable cytogenetic risk score (p=0.05) and receipt of the most intense chemo (cytarabine/daunorubicin/etoposide) (p=0.03) were associated with ADL decline -Higher BMI (p=0.03) and unfavorable cytogenetic risk score (p=0.01) were associated with IADL decline -Depressive symptoms at baseline and follow-up were associated with decline in SPPB score (p=0.03) -Receipt of the most intense chemo (p=0.02) was associated with decline in SPPB gait speed -Higher baseline cognition score was associated with improvement in SPPB balance (p=0.05) -Remission status was not associated with functional decline |
| Age ≥65 | Doni 201120/9 | Multicenter observational cohort (Italy) | All types, all stages | 578 | Mean age 72.6 (SD 5.0), median 71.9 (range 64.9-100) | Chemo | ADL, ECOG PS | Pretx, before each cycle (for at least 12 wks) | Longitudinal modeling | -Mean ADL score worsened up to wk 20 -Progressive worsening of ECOG PS starting from wks 11-13 (pretx: 49% ECOG 0, 41% ECOG 1, 9.8% ECOG ≥2; at follow-up: 46.6% ECOG 0, 42.2% ECOG 1, 11.1% ECOG ≥2) |
-At wk 12, hemoglobin change of at least 1 g/dl was associated with ADL decline (p<0.05) -Stage and PS were not associated |
| Age ≥65 | Gajra 201821/10 | Multicenter RCT (US) | Breast, stage I-III | 145 | Median age 71; 40% age 65-69, 54% 70-80, 6% >80 | Adjuvant chemo (AC, CMF) vs capecitabine | Subjective Significance Questionnaire (self-reported change in physical condition), EORTC QLQ-C30 PF | Midtx; 1, 12, 18, 24 mos after EOT | Self-report worsening physical condition | -At mid-treatment, 25% reported worse, 49% same, and 26% improved physical condition -At 1 yr, 6% reported worse, 37% same, 57% improved physical condition |
-Low preference for chemo was associated with worse physical condition at mid-treatment (p=0.005) but not at the other time points -No association between chemo preference and EORTC QLQ-C30 PF |
| Age ≥65 | Goodwin 20061/10 | Multicenter observational cohort (US) | Solid tumor, all stages | 26 | Mean age 71 (SD 5, range 65-82) | Chemo | Short Functional Dependence Scale ADL and IADL | Pretx, chemo visits 1-9 | Longitudinal modeling | -Not reported | -Older age, cancer type, surgery, radiation, and lower hemoglobin were associated with higher functional dependence across visits |
| Age ≥65 | Hurria 201922/10 | Multicenter RCT (US) | Breast, stage I-III | 256 | Mean age 71.9 (SD 4.7, range 65-85) | Chemo | EORTC-QLQ-C30 PF | Pretx; posttx; 12 mos after chemo initiation | -Decline from pretx to posttx: ≥10 point decrease from pretx to posttx, -Resilience (only those declined): returned to within 10 points of pretx PF -Decline at 12 mos: ≥10 point decrease from pretx to 12 mos after -Resistance to decline: <10 point decrease from pretx to posttx and 12 mos after |
-Decline from pretx to posttx: 42% (median decline 20 points, range 11.7-73.3) -Resilience at 12 mos: 47% recovered -Decline at 12 mos: 30% (median decline 20 points, range 13.1-53.3) -Resistance to decline: 49.6% |
-Decline from pretx to posttx was associated with baseline fatigue (OR 2.37, p=0.02). Comorbidity was not associated at posttx. -Resilience at 12 mos was associated with being married (OR 2.52, p=0.04), having <4 positive nodes (OR 3.57, p=0.048), and no pre-treatment appetite loss (OR 3.65, p=0.02) -Decline at 12 mos was associated with being unmarried (OR 1.98, p=0.01) and pretreatment dyspnea (OR 2.37, p=0.007). Fatigue, having <4 positive nodes, and older age were not associated with decline at 12 mos. -Resistance to decline was associated with no pretreatment fatigue (OR 2.49, p=0.01) and no dyspnea (OR 1.94, p=0.03). Comorbidity was not associated. |
| Age ≥65 | Manokumar 201623/9 | Single center observational cohort (Canada) | Prostate, stage IV | 36 | First line: Mean age 77.3 (SD 4.5); Second line: Mean age 71.4 (SD 6.2) | Docetaxel | OARS-IADL, TUG, timed chair stands, handgrip strength, falls (≥1) | Pretx, q3 mos until posttx | Change score | 1st-line chemo -IADL: 21% improved, 52% stable, 28% declined -TUG: 22% improved, 39% stable, 39% declined -Timed Chair Stands: 17% improved, 42% stable, 42% declined -Handgrip strength: 11% improved, 61% stable, 29% declined -Falls: 38% experienced one fall |
-Vulnerable Elders Survey (VES-13) score ≥3 was associated with greater increase in timed chair stand score |
| Age ≥65 | Miaskowski 201724/9 | Multicenter observational cohort (US) | Breast, GI, GYN, lung; all stages | 363 | Mean age between 70.7 to 72.7 (SD 5.4-6.0) | Chemo | SF-12 PCS | Baseline (chemo within the prior 4 wks), 1 wk and 2 wks post-chemo, then repeat for the subsequent cycle | Change score | -Three classes based on latent class analysis (above, below, and well below the normative norm of individuals aged 65-74) -PCS score remained relatively stable over 2 cycles of chemo |
-Unemployment (p<0.001), lower income (p=0.002), and a history of heart disease (p=0.001) were associated with being in the below and well below classes -Exercise on a regular basis (p<0.001), self-reported back pain (p<0.001), lower hemoglobin (p=0.002), and self-reported depression (p=0.028) were associated with being in the well below class -Age, gender, cancer diagnosis, time since cancer diagnosis, number of metastatic sites, number and types of prior cancer treatments, and chemo cycle length were not associated with latent class membership |
| Age ≥65 | Rier 20182/11 | Single center observational cohort study (Netherlands) | All types, all stages | 142 | Median age 72 (range 69-78) | Chemo | IADL | Pretx, midtx, posttx | IADL independence: ≥8 points; IADL decline: ≥3 points decline at posttx or ≥2 points decline at 1 year posttx | -IADL independence: Pretx (63.9%), posttx (56.3%) -IADL decline: 11.5% |
-Age (p=0.05), impaired cognition (0.05), refractory or progressive disease at posttx (vs complete remission, p=0.003), and severe sarcopenia (vs normal, p=0.05) were associated with IADL decline (all univariable analyses) -None were associated with decline on multivariable analysis |
| Age ≥65 | Verelst 201125/11 | Multicenter RCT (Netherlands) | Multiple myeloma, all stages | 284 | Median age 72 (range 65-84) | Melphalan/prednisone, melphalan/prednisone/thalidomide | EORTCQLQ-C30 PF | Pretx, cycle 3 (3 mos), cycle 8 (9 mos), 12 mos, 18 mos | Change score | -EORTC QLQ-C30 improved in both arms over time, though this was in favor of the 2-drug regimen early during induction phase | -Female sex was associated with lower scores on EORTC QLQ-30 PF (p=0.003) |
| Age ≥65 | Wong 201826/10 | Multicenter observational cohort (US) | Breast, GI, GYN, lung; all stages | 363 | Mean age 71.4 (SD 5.5) | Chemo | SF-12 PCS | Baseline (chemo within the prior 4 wks), 1 wk and 2 wks post-chemo, then repeat for the subsequent cycle | Longitudinal modeling | -PCS scores decreased slightly (0.21 points, p<0.01) at each subsequent assessment | -Higher morning fatigue (p=0.04) and lower enrollment PCS scores (p=0.01) were associated with decrease in PCS score over time -Age, sex, ethnicity, education, marital status, living alone, employment status, child care responsibilities, BMI, smoking status, hemoglobin, KPS, comorbidity, regular exercise, cancer type, time since cancer diagnosis, prior cancer treatments, metastatic disease, chemotherapy toxicity index, cycle length, evening fatigue, morning energy, evening energy, pain, anxiety, and attentional function were not associated |
| Age ≥65 | Xue 201527/7 | Single center RCT (China) | NSCLC, stage IIIB-IV | 24 | Mean age 73 (SD 5.3, range 65-83) | Chemo | EORTC QLQ-C30 PF | Pretx (day 1 prior to chemo); 7d, 21d, 42d, 63d post-chemo | Change score | -No change in functioning scale among function-independent and mildly function-impaired patients -For function-dependent patients, PF improved |
-Worse baseline functional status was associated with improvement in PF |
| Age ≥70 | Chakiba 201928/11 | Multicenter observational cohort (France) | Colon, pancreatic, stomach, ovarian, bladder, prostate, lung, NHL, cancer of unknown primary; all stages | 292 | Median age 77 (range 70-93); 36% age 70-75, 35% 76-80, 22% 81-85, 7% >85 |
Chemo | ADL | Pretx, before cycle 2 | Decline: 0.5 point decrease | -16% declined, 10% improved, 73% stable ADL score | -Abnormal G8 (≤14) was associated with functional decline (OR 4.3, 95% CI 1.28-14.92; p=0.018) in multivariable model -Age, sex, tumor type, stage, neutrophil count, platelet count, creatinine clearance, albumin, and CRP were not associated |
| Age ≥70 | Fiteni 201629/10 | Multicenter RCT (France) | NSCLC, stage III-IV | 361 | Mean age 77.1 (range 70-88); 49.9% age <77, 50.1% ≥77 | Carboplatin/paclitaxel vs gemcitabine vs vinorelbine | EORTC QLQ-C30 PF | Pretx, 6 and 18 wks | PF MCID: 5 points; time to deterioration | -Median time to deterioration: doublet chemo 2.04 mos (95% CI 1.87-3.88) vs monotherapy 1.71 (95% CI 1.58-1.91), p=0.01 | -Doublet chemo was associated with longer time to PF deterioration (HR 0.57, 95% CI 0.42-0.78, p=0.008) -Female sex (HR 1.53, 95% CI 1.09-2.15, p=0.01) and CCI score >2 (HR 1.50, 95% CI 1.08-1.12, p=0.002) were associated with shorter time to PF deterioration -Age, PS, smoking status, BMI, MMSE, ADL, stage, and histology were not associated with time to PF deterioration |
| Age ≥70 | Hoppe 201330/12 | Multicenter observational cohort (France) | Colon, pancreatic, stomach, ovarian, bladder, prostate, lung, non-Hodgkin lymphoma, unknown primary origin, all stages | 364 | Median age 77.3 (range 70-93) | Chemo | Katz ADL | Pretx, before cycle 2 | ADL decline: decrease of ≥0.5 in total score | -Decline: 16.7% (median 0.5 points, range 0.5-3) -Improvement: 10.7% (median 0.5 points, range 0.5-2.5) |
-High GDS-15 (OR 2.16, p=0.03) and low IADL (OR 2.87, p=0.04) were associated with decline -Age, sex, PS, weight loss, BMI, leukocytes, platelet count, creatinine, CRP, hemoglobin, albumin, tumor type, disease extension, CIRS-G, MAX2 index, ADL, MNA, MMSE, and GUG were not associated with decline |
| Age ≥70 | Kenis 201731/11 | Multicenter observational cohort (Belgium) | Breast, CRC, ovarian, lung, prostate, hematologic malignancy; all stages | 439 | Mean age 75 (range 70-95) | Chemo | Katz ADL, Lawton IADL | Pretx, 2-4 mos after chemo | ADL decline: change of ≥2 in total score, IADL decline: change of ≤1 in total score | -ADL decline: 19.9% -IADL decline: 41.3% |
-Abnormal nutritional status (OR 2.02, p=0.007) and baseline IADL dependency (OR 1.76, p=0.037) were associated with ADL decline -Disease status (progression/relapsed vs new diagnosis; OR 0.59, p=0.014) was associated with IADL decline -Age, geriatric screening tools, ECOG PS, fall, pain, polypharmacy, living situation, comorbidity, fatigue, baseline ADL, cognition, depression, cancer type, and treatment were not associated with both ADL and IADL decline |
| Age ≥70 | Morikawa 201832/8 | Single center observational cohort (Japan) | NSCLC, stage III-IV | 18 | Median age 74.5 (range 70-82) | Chemo, targeted therapy | Physical activity (accelerometer) | Pretx (prior to hospitalization); during hospitalization; 1, 2, 3 wks after discharge | Hospitalization-associated physical inactivity: decreased mean daily steps both during hospitalization and during the 1st wk as compared with mean daily steps at baseline | -50% walked fewer daily steps during hospitalization and did not recover to baseline level at 1 wk after discharge | -Cachexia and longer hospitalization (≥8 vs <8 days) were associated with hospitalization-associated physical inactivity |
| Age ≥70 | Naito 201733/8 | Single center observational cohort (Japan) | NSCLC, stage III-IV | 30 | Median age 74 (range 70-82) | Chemo, targeted therapy | Barthel ADL | Pretx, each hospital visit | Disabling event defined as decrease in Barthel ADL >10 points; disability free survival | -90% were disabled at the cutoff date -Disabling events: stair climbing (100%), morbidity (96%), bathing (89%), toilet use (56%), and transferring (41%) |
-Cachexia was associated with shorter disability-free survival (7.5 vs 17.1 mos, p<0.05) and longer post-disability survival (2.5 vs 0.7 mos, p<0.05) |
Abbreviations: ADL, activities of daily living; AC, cyclophosphamide/doxorubicin; AML, acute myeloid leukemia; BMI, body mass index; chemo, chemotherapy; CIRS-G, Cumulative Illness Rating Scale-Geriatrics; CMF, cyclophosphamide/methotrexate/fluorouracil; CRC, colorectal cancer; CRP, C-reactive protein; d, day; ECOG, Eastern Cooperative Oncology Group; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; EOT, end of treatment; GDS-15, Geriatric Depression Scale-15; GI, gastrointestinal; GUG, Timed Get Up and Go; GYN, gynecological; IADL, instrumental activities of daily living; KPS, Karnofsky Performance Status; MCID, minimal clinically important difference; midtx, midtreatment; MMSE, Mini-Mental State Examination; MNA, Mini-Nutritional Assessment; mo, month; mos, months; NHL, non-Hodgkin lymphoma; OARS, Older American’s Resource Scale; PCS, Physical Component Summary; PF, physical functioning; posttx, posttreatment; pretx, pretreatment; PS, performance status; RCT, randomized controlled trial; NSCLC, non-small cell lung cancer; SD, standard deviation; SF, short-form; vs, versus; wk, week; wks, weeks; TUG, Timed Up and Go; tx; treatment; yr, year; yrs, years
Quality assessment performed using the National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, which consists of 14 criteria.
We listed patient characteristics that are associated with functional change as well as those that are not associated. We did not list patient characteristics if they were only included as covariates without a reported result.
Table 2.
Non-older adult specific studies meeting inclusion criteria and primary functional status results (N=27).
| Study/Quality assessment scorea | Study design | Sample (cancer type(s), stage) | Overall N (systemic therapy n) | Age (age 65+ n if available or age summary statistic – mean/median) | Systemic cancer treatment(s) | Primary measure(s) of functional status | Assessment time points | Definition of functional status change | Change in functional status during treatment | Association of patient characteristicsb with functional status change |
|---|---|---|---|---|---|---|---|---|---|---|
| Alibhai 201534/10 | Multicenter observational cohort (Canada) | AML | 237 | 59% age <60 (median age 52.9); 41% ≥60 (median age 69.7); range 21-81 | Induction daunorubicin, cytarabine | 2MWT, chair stands, grip strength, IADL | Pretx; 4-6, 9-12, 13-16 wks; 6, 8, 10, 12 mos | 2MWT MCID: 59 ft, grip strength MCID: 4.5 kg (MCID not reported for chair stands and IADL) | -2MWT (p<0.001), chair stands (p<0.001), and IADLs (p=0.003) improved -No change in grip strength |
-Younger age was associated with greater recovery in chair stands (p=0.048) -Gender, smoking status, baseline PS, and hemoglobin were not associated with change in physical function |
| Bergman 199135/9 | Single center observational cohort (Sweden) | SCLC, all stages | 62 | Mean age 66 (range 36-80); 35% age ≥70 | Chemo | Sickness Impact Profile physical index (ambulation, body care/movement, mobility) | Pretx; 3, 6, 9, 12 mos | Correlation with physical index change score | -Body care/movement subscale worsened 5 points at 3 mos and 6.1 points at 6 mos (p<0.05); no difference at 12 mos -No change in ambulation or mobility |
-Characteristics associated with worsening physical index: worsening ECOG/WHO PS, EORTC pain, EORTC appetite (p<0.01); tumor non-response, cisplatin-containing regimen, neutropenia (p<0.05) -Age, sex, depression, hair loss, and nausea were not associated |
| Bezjak 200636/13 | Multicenter RCT (global) | NSCLC, stage IIIB-IV | 425 | Median age 61 | Erlotinib vs placebo | EORTC QLQ-C30 PF | Pretx, q4 wks during tx, 4 wks posttx, q12 wks until EOT | PF decline: ≥10 point decrease, improvement: ≥10 point increase, stable: <10 point change | -Erlotinib: 51% declined, 31% improved, 18% stable PF | -Only treatment with erlotinib was associated with improvement in PF (p=0.006) -Age, sex, ethnicity, PS, prior treatment, histology, and smoking history were not associated |
| Chen 200337/9 | Single center observational cohort (US) | Solid tumor, non-leukemic hematologic malignancy, all stages | 37 | Mean age 75.6 (range 70-87); 38% age 70-74, 54% 75-80, 8% 81-87 | Chemo | IADL, ECOG PS | Pretx, EOT (or at 6 mos) | Change score | -Mean 1.44 point decline in IADL score (p=0.04) -No change in ECOG PS |
-Severe chemotherapy toxicities were associated with decline in IADL (change score −2.93 vs −0.17; p=0.03) and ECOG PS (change score 0.56 vs −0.11; p = 0.03) |
| de Jong 200638/12 | Multicenter observational cohort (Netherlands) | Breast, stage I-III | 157 | Mean age 47.3 (SD 8.8, range 25-70) | Adjuvant CMF or doxorubicin-containing regimen | Multidimensional Fatigue Inventory reduced activity subscale | Cycle 1, 3, 5; 4 and 12 wks after last cycle | Longitudinal modeling | -Activity level stable during study period | -Older age and not having children were associated with lower activity level over time -Mastectomy (vs lumpectomy), longer duration of radiation, and fewer total chemotherapy treatments were associated with lower activity level -Chemotherapy type, marital status, education, having a job, hemoglobin, and time between surgery and chemotherapy were not associated with change in activity level |
| Dodd 200139/10 | Multicenter RCT (US) | All types, all stages | 93 | Mean age 55.4 (SD 14.6) | Chemo (RCT of two mouthwashes for mucositis) | Patient-reported KPS | Pretx, end of cycle 3 | Longitudinal modeling | -Pretx mean KPS 84.8 -End of cycle 3 mean KPS 82.7 |
-Pretx KPS, older age, worse pain, and worse fatigue were associated with worse KPS at the end of cycle 3 -Sleep insufficiency was not associated |
| Dodd 201040/8 | Multicenter RCT (US) | Breast, stage I-III | 112 | Mean age 50 (SD 9.3) | Chemo (RCT of exercise intervention during chemo, after chemo, vs usual care) | Patient-reported KPS | Before cycle 2, EOT, 1 yr | Association with symptom clusters (pain, fatigue, sleep disturbance, depression) at each assessment | -Not reported | -Before cycle 2, All Low symptom cluster was associated with better KPS vs Moderate cluster (p=0.002) -At EOT, All Low cluster was associated with better KPS vs all other clusters (p<0.0001) -At 1 yr, Mild cluster associated with better KPS vs Moderate and All High clusters (p<0.005) |
| Doorenbos 200641/10 | Multicenter RCT (US) | Solid tumor, all stages | 237 | Mean age 60 (SD 10, range 31-87) | Chemo (RCT of cognitive behavioral theory guided intervention vs usual care) | SF-36 PF | Baseline; 10, 20, 32 wks | Longitudinal modeling | -Baseline mean PF 64.2 (SD 29.5), wk 20 mean PF 70.6 (SD 28.1) -At wk 20, women with breast cancer had higher PF than women with lung cancer (p=0.001); men with lung cancer had higher PF than women with lung cancer (p=0.02) |
-At wk 20, patients with a higher number of chronic health conditions (vs low number) benefited more from the effect of the intervention on PF (p=0.02) -Lower depressive symptoms and lower symptom limitations were associated with increased intervention effect on PF -Age, sex, stage, and tumor type were not found to mediate intervention effect on PF |
| Fallowfield 200242/12 | Multicenter RCT (global) | Solid tumor or non-myeloid hematologic malignancies, all stages | 375 | Epoetin arm: Mean age 58.1 (SD 14.2, range 18-84) Placebo arm: Mean age 59.2 (SD 14.3, range 21-88) |
Non-platinum chemo (RCT of epoetin alfa vs placebo) | CLAS daily activities, SF-36 PCS | Randomization; 4, 16, 28 wks | Change score at last available assessment | -Mean CLAS daily activities change score: Epoetin arm 7.78 vs placebo arm −1.96 (p<0.04). -No difference in mean SF-36 PCS change score by arm: Epoetin arm 1.27 vs placebo arm 0.05 (p=0.33) |
-Higher hemoglobin level was associated with higher CLAS daily activities and SF-36 PCS over time (p<0.01) -Higher baseline endogenous erythropoietin was associated lower CLAS daily activities over time (p<0.05) -Lower reticulocyte count and pre-study transfusion dependency were associated with higher SF-36 PCS over time (p<0.05) -Older age was associated with lower SF-36 PCS score over time (p<0.01) -Age, race, and disease progression were not associated with change in CLAS daily activities -Sex and disease progression were not associated with change in SF-36 PCS |
| Frodin 201143/9 | Single center observational cohort (Sweden) | Myeloma, lymphoma, testicular, sarcoma, AML; all stages | 96 | Mean age 54 (SD 12) | auto-SCT | EORTC QLQ-C30 PF | Pretx; wkly during wks 1-4; monthly during mos 2, 3; 6 mos; q6 mos up to yr 3 | Longitudinal modeling | -At wk 2, 42% declined in PF -PF improved back to baseline by month 2 |
-At wk 2, a diagnosis of myeloma (vs lymphoma) was associated was better PF (p=0.001) |
| Frodin 201544/10 | Single center observational cohort (Sweden) | Any hematologic malignancy, all stages | 94 | Mean age 48 | Allogenic SCT, reduced intensity conditioning | EORTC QLQ-C30 PF | Pretx, wkly during wks 1-4, monthly during mos 2, 3; 6 mos; q6 mos up to yr 3 | Change score | -Pretx PF mean: 81 -At 3 wks, change score −36 (p<0.05 compared with pretx) -At 3 mos, change score −20 (p<0.05) -At 1 yr, change score −8 -At 3 yrs, change score −5 |
-Extensive chronic GVHD was associated with worse PF compared with limited chronic GVHD and no chronic GVHD at 1.5, 2, and 2.5 yrs (all p<0.01) |
| Gaston-Johansson 201545/11 | Single center observational cohort (US) | Breast, all stages | 30 | Mean age 52.7 (SD 10.2, range 32-72) | Chemo | FACT-Breast physical and functional well-being | Pretx, midpoint, EOT | Longitudinal modeling | -Physical and functional well-being decreased at midpoint and EOT compared with pretx (all p<0.001) | -Worst pain intensity was associated with worse functional well-being at EOT -No association with age or stage |
| Given 200246/10 | Multicenter RCT (US) | Solid tumor and NHL, all stages | 113 | Mean age 58 (SD 10.5) | Chemo (RCT of nursing symptom management intervention vs usual care) | SF-36 Physical role functioning | Baseline (within 8 wks of chemo initiation); 10, 20 wks | Longitudinal modeling | -Intervention arm: Mean physical role functioning score 11 (SD 22) at baseline, 50 (SD 41) at 20 wks -Control arm: Mean physical role functioning score 11 (SD 22) at baseline, 31 (SD 36) at 20 wks |
-Intervention was associated with improved physical role functioning at 20 wks (mean score 50 vs 31) -Diagnosis of breast cancer (vs non-breast cancer) was associated with improved physical role functioning at 20 wks |
| Greimel 200647/10 | Multicenter RCT (Germany, Austria) | Ovarian, stage IIB-IV | 416 | Mean age 56.6 (SD 10.1) | Cisplatin/paclitaxel vs carboplatin/paclitaxel | EORTC QLQ-C30 PF | Pretx; cycle 2, 4; EOT; q6 mos | Longitudinal modeling | -Mean PF change score 9.4 (carboplatin/paclitaxel) vs 1.7 (cisplatin/paclitaxel) | -Carboplatin/paclitaxel arm had better EOT PF -Characteristics associated with worse PF over time: anemia, neurotoxicity, GI toxicity, older age -Hematologic toxicity, pain, and treatment arm were not associated with change in PF over time |
| Kim 201048/8 | Single center observational cohort (Korea) | Diffuse large B cell or follicular lymphoma, all stages | 32 | Mean age 55.9 (range 21-79) | Chemo | SF-36 PCS | Pretx, 2nd visit during chemo, last visit after cycle 6 | PCS score <40 | -Pretx: 12 patients had a PCS score <40 −2nd visit: 12 patients had a score <40 -Last visit: 5 patients had a score <40 |
-Neuropathy at 2nd visit was associated with lower PCS score at the last visit (PCS score 44.5 for neuropathy vs 49.9 for non-neuropathy, p=0.02) |
| Kinsey 201849/12 | Multicenter RCT (global) | NSCLC, stage III-IV | 236 | 37.5% age ≥ 65 | Chemo | Stair climb power | Pretx, day 84 | % Loss | ≥10% loss: 31% 0 to <10% loss: 18% ≥0 to <10% gain: 10%, ≥10% gain: 31% |
-Taxane (vs non-taxane therapy, p=0.023) and prior smoking (vs current use, p=0.027) were associated with functional decline -Prior weight loss, disease response, lean body mass, ECOG PS, disease stage, age, gender, and comorbidities were not associated with functional decline |
| Land 200450/12 | Multicenter RCT (US, Canada) | Breast, stage I-II | 160 | 50.6% age ≤ 49, 32.5% 50-59, 16.9% ≥ 60 |
Chemo | SF-36 return to normal activity | Pretx, start of each chemo cycle (several time points from wk 3-52) | Change score | -Return to normal activity score did not change during chemotherapy | -Lumpectomy and radiation (vs mastectomy) were associated with lower return to normal activity score (1.11 points lower, p=0.02) -Chemotherapy (CMF vs AC), tamoxifen, surgery, tumor size, and age were not associated with return to normal activity score |
| Mohamedali 201251/10 | Single center observational cohort (Canada) | AML | 103 | Younger: Median age 52.0 (range 21-59) Older: Median age 69.7 (range 60-80) |
Induction daunorubicin, cytarabine | EORTC-QLQ-C30 PF, handgrip strength, timed chair stands, 2MWT | Time of dx; 4-5 wks, 8-10 wks, 12-16 wks (after each cycle of chemo) | Change scores; MCID 10 points | -No statistically significant change in EORTC PF -Handgrip strength decreased |
-Older patients had greater magnitude of decline in handgrip strength (younger: 30.7 to 28.0; older: 31.1 to 25.0) -Younger patients had improved chair stands over time (22.3 to 29.0, p<0.001) but older adults did not (20.3 to 19.8, p=0.36) -Both groups improved on the 2MWT (p<0.001) -Younger patients had less decline or greater improvement in all three PF tests over time compared to older patients |
| Morita 200352/10 | Multicenter RCT (Japan) | NSCLC, stage IIIB-IV | 377 | Median age 61 (range 35-75) | Chemo | QOL-ACD physical well-being, ECOG PS | Pretx; day 8, 15, 22 | Change scores | -Maximum decrease in score for each domain was observed at wk 1 -More severe deterioration of PS was observed in wks 1 and 2, while a noticeable number of patients experienced improved PS in wk 4 |
-Nausea/vomiting (p<0.001), anorexia (p<0.001), diarrhea (p<0.001), and PS deterioration (p=0.001) were associated with decline of physical well-being -Age, gender, and treatment arm were not associated |
| Oechsle 201153/9 | Single center observational cohort (Germany) | All types, stage II | 53 | Median age 58 (range 29-76) | Chemo | Questionnaire for Measurement of Habitual Physical Activity (work, sports, leisure indices), EORTC-QLQ-C30 PF, International Physical Activity questionnaire | Baseline; 4 wks | Change score | -Work index: decreased from 2.25 to 0.56 (p<0.001) -Sports index: decreased from 2.91 to 2.47 (p<0.001) -Leisure time index: increased from 2.81 to 3.01 (p<0.01) -Median time of sportive activities decreased (1.6 hour to 0.8 hour, p<0.01) -No change in EORTC-QLQ-C30 PF |
-Sports index prior to cancer diagnosis was higher among men (p<0.05) but no difference in sports index during chemo by gender -No gender differences in EORTC QLQ-C30 PF |
| Revicki 201254/9 | Multicenter RCT (global) | Melanoma, stage III-IV | 676 | Mean age 56.2 (SD 57) | Ipilimumab/gp100 vs ipilimumab alone vs gp100 alone | EORTC-QLQ-C30 PF | Pretx; 12 wks | No change: 0–5 points; a little change: 5–10 points; moderate change: 10–20 points; very much >20 points | Change scores: -Ipilimumab plus gp100: −6.2 -Ipilimumab alone: −5.1 -Gp100 alone: −10.1 |
-Older and younger patients (<65 vs ≥ 65 yrs) had similar PF decline in the ipilimumab plus gp100 and ipilimumab alone groups |
| Shallwani 201655/8 | Single center observational cohort (Canada) | NSCLC, stage IIIA-IV | 47 | Mean age 63.3 (SD 12.2) | Chemo | SF-36 PCS, 6MWT, 1 minute chair rise test, grip strength | Pretx, post-cycle 2 | SF-36 MCID: 5 units; 6MWT MCID: 54 meters. Longitudinal modeling |
-SF-36 PCS worsened overall: Pretx 40.8, posttx 38; p=0.02. 20% had clinically significant improvements; 33% deteriorated −6MWT worsened overall: Pretx (454.5), posttx (414.3); p<0.01. 9% had clinically significant improvements; 14% deteriorated -Chair rise test stable: Pretx (21.4), posttx (20.7); p<0.25 -Grip strength worsened: Pretx (27.4), posttx (26.5); p=0.03 |
-Nutritional status, fatigue, and 6MWT were not associated with SF-36 PCS |
| Timilshina 201956/9 | Multicenter observational cohort (Canada) | AML | 71 | Median age 52 (IQR 41-58) | Intensive chemo | EORTC QLQ-C30 PF, grip strength, 10 timed chair stands | Pretx; 11 time points over 3 yrs | Recovery defined as reaching 1 MCID unit for each outcome (EORTC QLQ-C30 MCID: 10, FACT-F MCID: 4, grip strength MCID: 4.5 kg, 6MWT MCID: 54 meters, timed chair stand MCID: 3.4 seconds) | -EORTC QLQ-C30 PF unchanged: Baseline (80.6), 12 mos (85.2), 24 mos (82.5), 36 mos (90.0) -EORTC QLQ-C30 PF: 72% returned to normal at 1 yr and 77% at 3 yrs -Grip strength unchanged: Baseline (30.3), 12 mos (31.2), 24 mos (31.8), 36 mos (32.0) -Grip strength: 50% returned to normal at 1 yr and 54% at 3 yrs -Chair stands/min improved: Baseline (25.6), 12 mos (35.0), 24 mos (39.0), 36 mos (40.7); p=0.002 -Chair stands: 44% returned to normal at 1 yr and 64% at 3 yrs |
-Older age (time x age interaction, p=0.01) and male gender (time x gender interaction, p=0.002) were associated with slower recovery in timed chair stands -Age and gender were not associated with recovery for other functional status measures |
| Verdonck-de Leeuw 201457/10 | Single center observational cohort (Netherlands) | HNSCC, all stages (curative only) | 164 | Median age 59 (range 40-84) | Chemoradiation (generally cisplatin) | EORTC QLQ-C30 PF | Pretx (1 wk before tx); 6 wks; 6, 12, 18, 24 mos post-RT | Change score | -EORTC QLQ-C30 worsened in the first 6 wks, then improved in survivors -EORTC QLQ-C30 worsened in non-survivors |
-Comorbidity (p=0.03) and non-survivors were associated with lower EORTC QLQ-C30 PF over time |
| Watters 200358/10 | Single center observational cohort (Canada) | Breast, all stages | 65 (45 young, 25 old) | Old (65+): mean age 70 (SD 5, range 65-80) Young (>65): mean age 55 (SD 6, range 31-64) |
5-FU, doxorubicin, cyclophosphamide | EORTC QLQ-C30 PF, SF-36 PF, KPS, handgrip strength | Pretx; prior to cycle 3; 3 wks post-cycle 6; 6 mos, 12 mos | Change score | -EORTC QLQ-C30 and SF-36 PF were lower at completion of chemo (p<0.01 and p<0.05, respectively) -EORTC QLQ-C30 and SF-36 PF were similar from baseline to follow-up -KPS declined significantly by completion of chemo (92 +/− 6 vs. 85 +/− 11, p<0.001) -KPS did not differ from baseline to follow-up -No change in handgrip strength |
-Younger age was associated with greater decline in EORTC QLQ-C30 PF (p<0.05) -Age was not associated with changes in SF-36 PF |
| Williamson 201859/10 | Single center observational cohort (US) | Lung, all stages | 101 | Mean age 64.5 (SD 11.6) | Chemo, immunotherapy, targeted therapy, combination | FACT-L TOI (physical/functional well-being) | Baseline (study entry), 6 wks, 12 wks | Change score | -Baseline: 51.37 −6 wks: 33.17 −12 wks: 44.55 |
-Lower baseline physical/functional well-being (p<0.001), being unmarried (p=0.017), non-Hispanic White race (p=0.004), higher internalized stigma (p=0.045) were associated with lower physical/functional well-being at 6 wks -Lower baseline physical/functional well-being (p<0.001) was associated with lower physical/functional well-being at 12 wks -Age, sex, education, smoking history, months since diagnosis, cancer stage/type, prior surgery, prior chemotherapy, and constrained disclosure (avoidance of or discomfort about disclosing one’s cancer status to others) were not associated with physical/functional well-being at 6 and 12 weeks |
| Yang 201860/10 | Single center observational cohort (Taiwan) | NSCLC, stage IIIB-IV | 344 | Gefitinib: mean age 63.7 (SD 11.2); erlotinib: mean age 61.9 (SD 12.8); afatinib: mean age 60.8 (SD 10.2) |
Gefitinib, erlotinib, afatinib | WHOQOL-BREF physical, mobility, daily activities | Baseline; q2-4 wks during tx up to 25 mos (no specific time point) | Change score | -Physical, mobility, and daily activities scores were lower in the afatinib arm | -ECOG PS 2-4 (vs 0-1) was associated with worse scores on physical, mobility, and daily activities (p<0.001) -EGFR exon 19 deletion was associated with worse scores on physical (p<0.05), mobility (p<0.05), and daily activities (p<0.01) -Brain metastasis was associated with worse scores on mobility (p<0.05) -Disease progression was associated with worse scores on physical, mobility, and daily activities (p<0.001) -Afatinib (vs gefitinib) was associated with worse scores on physical (p<0.05), mobility (p<0.01), and daily activities (p<0.01) -Sex, education, employment, marital status, comorbidities, and recurrence were not associated with any decline |
Abbreviations: 2MWT, two-minute walk test; 6MWT, six-minute walk test; 5-FU, fluorouracil; AC, cyclophosphamide/doxorubicin; AML, acute myeloid leukemia; chemo, chemotherapy; CLAS, Cancer Linear Analogue Scale; CMF, cyclophosphamide/methotrexate/fluorouracil; d, day; ECOG, Eastern Cooperative Oncology Group; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; EOT, end of treatment; FACT-L TOI, Functional Assessment of Cancer Therapy-Lung Trial Outcome Index;GI, gastrointestinal; HNSCC, head and neck squamous cell carcinoma; IADL, instrumental activities of daily living; MCID, minimal clinically important difference; mo, month; mos, months; NHL, non-Hodgkin lymphoma; PCS, Physical Component Summary; PF, physical functioning; posttx, posttreatment; pretx, pretreatment; PS, performance status; QOL-ACD, Quality of Life Questionnaire for Cancer Patients Treated with Anti-Cancer Drugs; RCT, randomized controlled trial; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; SCT, stem cell transplant; SD, standard deviation; SF, short-form; vs, versus; wk, week; wks, weeks; tx; treatment; WHO, World Health Organization; WHOQOL-BREF, World Health Organization Quality-of-Life Brief; yr, year; yrs, years
Quality assessment performed using the National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, which consists of 14 criteria.
We listed patient characteristics that are associated with functional change as well as those that are not associated. We did not list patient characteristics if they were only included as covariates without a reported result.
Quality assessment
Using the NHLBI Quality Assessment Tool, the mean quality assessment score was 9.86 (range 7 to 13; Tables 1 and 2). The most common reasons for lower study quality were lack of participation rate reporting, lack of sample size justification, loss to follow-up ≥20%, and lack of adjustment for confounders. Outcome assessors were often not blinded to the patient’s exposure status (e.g., demographics). Only 59% of studies adjusted for key potential confounders in their analyses between patient characteristics and FS change.
Measures of FS
Almost all studies (98%) used PROs to measure FS (Table 3). The most commonly used PRO was the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC QLQ-C30) physical functioning scale61 (34% of studies). Patient-reported ADL (20%) and IADL (16%) were commonly used as well. Physical performance was tested in 20% of studies with grip strength, chair stands, and walking tests as the most common. Traditional oncology measures of PS (e.g., Eastern Cooperative Oncology Group PS) were uncommon among included studies. There were no statistically significant differences in FS measures used in older adult-specific versus non-older adult-specific studies.
Table 3.
Measures of functional status, assessment time points, and analytic approach by study type.
| Characteristic | Overall No. (%) (N=44) | Older adult-specific studies No. (%) (n=17) | Non-older adult-specific studies No. (%) (n=27) | p-valuea |
|---|---|---|---|---|
| Measures of functional status | ||||
| Patient-reported outcomeb | 43 (98) | 17 (100) | 26 (96) | 1.00 |
| EORTC QLQ-C30 | 15 (34) | 5 (11) | 10 (37) | |
| ADL | 9 (20) | 7 (16) | 2 (7) | |
| IADL | 7 (16) | 5 (11) | 2 (7) | |
| Physical performance testb | 9 (20) | 3 (18) | 6 (22) | 1.00 |
| Grip strength | 8 (18) | 3 (18) | 5 (19) | |
| Walking test | 6 (14) | 3 (19) | 3 (11) | |
| Chair stands | 5 (11) | 1 (12) | 4 (15) | |
| Clinician-reported | 4 (9) | 1 (6) | 3 (11) | 1.00 |
| ECOG PS | 3 (7) | 1 (6) | 2 (7) | |
| KPS | 1 (2) | 0 (0) | 1 (4) | |
| Assessment time points | ||||
| Pretreatment and 1 follow-up | 9 (20) | 3 (18) | 6 (22) | 0.91 |
| Pretreatment and 2 follow-ups | 6 (14) | 3 (18) | 3 (11) | |
| Pretreatment and ≥3 follow-ups | 22 (50) | 8 (47) | 14 (52) | |
| Other assessment schedulec | 7 (16) | 3 (18) | 4 (15) | |
| Analytic approach | ||||
| Change score between two assessments | 21 (48) | 6 (35) | 15 (56) | 0.008 |
| Longitudinal analysis | 14 (32) | 3 (18) | 11 (41) | |
| Dichotomous functional decline | 6 (14) | 5 (29) | 1 (4) | |
| Time to deterioration | 2 (5) | 2 (12) | 0 (0) | |
| Other analysisd | 1 (2) | 1 (6) | 0 (0) |
Abbreviations: ADL, activities of daily living; ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; IADL, instrumental activities of daily living; KPS, Karnofsky Performance Status.
Fisher’s exact test comparing older adult-specific studies and non-older adult-specific studies.
Only the three most common measures of functional status within each subgroup are shown.
Other assessment schedule: Initial assessment occurred after initiation of systemic therapy.
Other analysis: Association with patient-reported change in physical condition.
Assessment time points and analytic approach to FS
Most studies assessed FS prior to starting systemic therapy and at 1 (20% of studies), 2 (14%), or ≥3 (50%) follow-up time points, while 16% of studies performed the first assessment after therapy had started (Table 3). Analyses of FS change were heterogeneous with many reporting change scores between two assessments (48% of studies). Only 32% of studies utilized longitudinal methods to examine trajectories of FS over ≥2 time points. The remaining studies analyzed FS change dichotomously by defining cutoff scores for decline and/or improvement (14% of studies), time to deterioration (5%), or association with patient-reported change (2%). Older adult-specific studies were more likely to analyze FS change dichotomously (29% of older adult-specific studies versus 4% of non-older adult-specific studies, P=0.008).
Changes in FS and its associated characteristics
Among studies that reported the percentage of patients who developed functional decline, results ranged widely from 6%21 to 90%33 depending on the cancer type, treatment, measure of FS, and timing of assessments (Tables 1 and 2). Functional improvement occurred between 9%55 and 57%21 of patients. The most common patient characteristics associated with functional decline during systemic cancer therapy (Table 4) were older age (n=7 studies1, 2, 38, 39, 42, 47, 51), worse PS (n=435, 39, 52, 60), progressive disease status (n=42, 31, 35, 60), pain (n=424, 35, 39, 45), anemia (n=41, 20, 24, 47), and worse nutritional status (n=42, 31–33). Definitions for these characteristics are listed in Table 4.
Table 4.
Common characteristics associated with functional decline during systemic cancer treatment (N=36 studies that examined functional decline).
| Characteristic | Total no. of studies examining characteristic and functional decline | No. of studies reporting an association with functional decline | No. of studies reporting no association | No. of studies including characteristic as a covariate without reporting association | Characteristic variable detailsa |
|---|---|---|---|---|---|
| Age | 25 | Older age: 71, 2, 38, 39, 42, 47, 51 Younger age: 158 |
1222, 24, 26, 28–31, 35, 45, 49, 52, 59 | 519, 20, 44, 55, 57 | -Categorical age: 65-69 vs 70-74 vs 75-841 25-45 vs 46-7038 <60 vs ≥6051 <65 vs ≥6558 -Continuous age42 -Age details not specified2,39,47 |
| Worse performance status | 11 | 435, 39, 52, 60 | 620, 26, 29–31, 49 | 119 | -ECOG PS: 0-1 vs 2-460 -Continuous KPS score39 -Change in ECOG/WHO PS35,52 |
| Progressive disease status | 8 | 42, 31, 35, 60 | 419, 42, 49, 60 | -Refractory/progressive disease at treatment completion vs complete remission2 -Disease progression/relapse vs new diagnosis31 -Tumor response: Partial/complete response vs progressive/stable disease35 -Disease progression: Yes vs no60 |
|
| Pain | 7 | 424, 35, 39, 45 | 326, 31, 47 | -Back pain: Yes vs no24 -EORTC QLQ-C30 pain: Quite a bit/very much vs not at all/a little35 -Quality of Life-Cancer pain score39 -Pain-O-Meter worst pain intensity score45 |
|
| Anemia | 7 | 41, 20, 24, 47 | 326, 30, 38 | -Categorical hemoglobin level: 7.3-10.9, 11.0-12.9, 13.0-13.9, 14.0-15.61 -Continuous hemoglobin level24 -Hemoglobin change ≥1 g/dl20 -Anemia CTCAE grade 0-2 vs 3-447 |
|
| Worse nutritional status | 6 | 42, 31–33 | 230, 49 | -Sarcopenia: Severe (low muscle mass, slow gait speed, and low handgrip strength) vs none2 -Mini-Nutritional Assessment-Short Form score: ≤11 vs >1131 -Cancer cachexia: Unintentional weight loss >5% in last 6 mos (or >2% if BMI <20 kg/m2) or presence of muscle depletion vs no cachexia32,33 |
Abbreviations: BMI, body mass index; CTCAE, Common Terminology Criteria for Adverse Events; ECOG, Eastern Cooperative Oncology Group; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; dl, deciliter; g, gram; kg, kilogram; KPS, Karnofsky Performance Status; m, meter; mos, months; PS, performance status; vs, versus; WHO, World Health Organization.
Characteristic variable details shown for studies that reported an association with functional decline.
Only twelve studies19, 22, 27, 29, 34, 40–43, 46, 51, 56 examined characteristics associated with functional improvement. Three articles34, 51, 56 reporting results from a study of acute myeloid leukemia found that younger age was associated with greater improvement in timed chair stands during intensive chemotherapy. Lower symptom burden was associated with greater improvement in FS in three studies.22, 40, 41 Additional characteristics associated with greater functional improvement included female sex,56 being married,22 baseline functional dependence,27 better cognition,19 lack of depression,41 higher hemoglobin,42 cancer type,43, 46 and less than four positive nodes (in a breast cancer study).22 In a randomized controlled trial of a cognitive behavioral therapy intervention, worse comorbidity was associated with greater improvements in FS in the intervention arm.41
DISCUSSION
Over almost three decades of research from 1991-2019, we identified only 44 studies that included older adults that rigorously examined patient characteristics associated with FS change during systemic cancer treatment. While the increasing number of studies in more recent years is promising, especially the increasing number of older adult-specific studies, the relative lack of studies examining FS as a longitudinal outcome highlights the importance of synthesizing the existing data and the ongoing need to add this patient-centered outcome to cancer clinical trials and observational cohort studies.
This systematic review identified a substantial amount of heterogeneity between studies in how FS is measured, when it is assessed during systemic cancer treatment, and how it is analyzed, limiting direct comparisons between studies. For example, functional decline was identified in 6% of women age ≥65 with breast cancer in a trial of adjuvant therapy using patient-reported worsening physical condition.21 In contrast, functional decline was identified in 90% of adults age ≥70 with advanced non-small cell lung cancer receiving chemotherapy using ADLs.33 Study populations also differed widely in cancer types and specific treatments evaluated. However, the vast majority of studies included only chemotherapy, revealing a gap in understanding the functional impact of immunotherapy and targeted therapy, which are key components of modern cancer care.
FS was most commonly assessed using PROs (98% of studies), such as the EORTC QLQ-C30.61 The wide spread use of PRO measures to assess FS among patients with cancer mirrors the broader surge of PROs to assess symptoms and adverse events during routine cancer care and in trials.62–65 Advantages of PRO FS measures in clinical care include the ability to assess FS outside of busy clinic visits (e.g., previsit questionnaire), remotely without an in-person component (which is increasingly important during the COVID-19 pandemic), and with potentially fewer resources compared to conducting a physical performance test. PROs also allow FS to be more easily studied longitudinally in clinical trials or observational cohort studies where FS is not the primary outcome. Compared to physical performance tests, PROs are more representative of the patient perspective about their FS. Furthermore, patient-reported functional decline in ADLs is a strong predictor of overall survival among older adults with cancer.31
Several challenges existed in the analysis of FS changes during systemic treatment. There were no uniform definitions for clinically meaningful functional decline or improvement. Despite half of the included studies measuring FS at ≥3 follow-up assessments, over 60% only analyzed data from two time points, effectively ignoring informative patient-centered information. To illustrate, 48% of studies analyzed longitudinal FS as a change score between two assessments and 14% used a threshold definition of a dichotomous functional decline outcome. While these types of analytic approaches may assist in clinical interpretation (e.g., percent of patients who functionally decline after one cycle of chemotherapy30), they do not capture FS trajectories that may include both declines and improvements. In contrast, Hurria et. al22 combined more nuanced changes in FS over time and clinically applicable results by conducting four analyses using dichotomous outcomes to examine: Functional decline at the end of adjuvant breast cancer chemotherapy, functional decline at twelve months, improvement of FS among those with decline, and resistance to decline. This study exemplifies an alternative analytic approach that examines several time points as well as several definitions of FS change.
Older age, which was defined differently across studies, was the most common characteristic associated with functional decline. However, because many studies did not adjust for comorbidity, which is more common among older adults66 and associated with functional decline,24,29,49,5 there may have been residual confounding. We also found that worse PS, pain, anemia, and worse nutrition were associated with functional decline, highlighting the importance of assessing and addressing these concerns. Evaluation of these characteristics and other domains important to older adults (e.g., cognition) through geriatric assessment67 is necessary to comprehensively risk stratify patients. Geriatric assessment results can guide recommendations to address modifiable risk factors via supportive care interventions (e.g., physical therapy for worse PS) and co-management with a multidisciplinary team (e.g., palliative medicine for pain, dietician for malnutrition).68
Some older adults with cancer do not experience functional decline during systemic treatment, some experience decline but later improve, and some never improve. Resilience refers to the process of adapting well in the face of a stressor,69 or simply the ability to recover to baseline.22, 70 Understanding characteristics associated with functional improvement can guide conversations regarding cancer treatment as patients may be more willing to undergo treatment if they are likely to recover. Future studies should evaluate the underlying mechanisms by which these characteristics lead to functional improvement, which can guide development of interventions.
There are several limitations to this systematic review. First, our review focused on the effects of systemic cancer therapy on FS changes and did not examine surgery or radiation. Second, we only included studies that reported results separately for patients who received systemic therapy. Therefore, studies that examined FS changes in a heterogeneous sample of patients with cancer receiving a variety of treatments were excluded. Additionally, we focused on studies examining patient characteristics associated with functional decline and improvement rather than a comprehensive review of the prevalence of functional change. Lastly, a meta-analysis was not possible due to heterogeneity of patient populations, FS measures, time points, and analytic approaches.
Future studies of cancer treatments in older adults, particularly beyond chemotherapy, must include serial FS measures and analyze these data to determine which older patients are at highest risk of functional decline and which may be more resilient. Measurement of characteristics associated with functional decline such as nutritional status, which are absent from many trials, is equally important. This is especially critical given the rapidly evolving treatment landscape and need to understand how newer therapies impact older adults. Many cancer trials already contain a collection of valuable, unanalyzed FS information as part of broader QOL questionnaires. These data have the potential to greatly expand the knowledge base on FS changes during cancer treatment and inform shared decision-making with information on this important patient-centered outcome.
Furthermore, the development of risk prediction tools such as risk scores or nomograms would make information about characteristics associated with FS change more clinically accessible for patient care and shared decision-making. Examples of successful translations of research data into clinical practice include the Cancer and Aging Research Group chemotherapy toxicity calculator71, 72 and ePrognosis.org collection of prognostic indices.73, 74 Studies of FS changes among older adults with the same cancer type are also needed to make results less heterogeneous and more clinically applicable.
In conclusion, there is increasing recognition of change in FS as an important outcome among older patients with cancer receiving systemic treatment. However, definitions and analyses of functional decline and improvement are heterogeneous, leading to a wide range of prevalence. To better understand functional decline and improve outcomes in this vulnerable population, measures of FS outcomes need to be incorporated with traditional oncology outcomes.
Supplementary Material
Supplemental Figure. Study selection.
REFERENCES
- 1.Goodwin JA, Coleman EA, Shaw J. Short Functional Dependence Scale: development and pilot test in older adults with cancer. Cancer Nurs 2006; 29: 73–81. [DOI] [PubMed] [Google Scholar]
- 2.Rier HN, Jager A, Meinardi MC et al. Severe sarcopenia might be associated with a decline of physical independence in older patients undergoing chemotherapeutic treatment. Support Care Cancer 2018; 26: 1781–1789. [DOI] [PubMed] [Google Scholar]
- 3.Crombag MBS, de Vries Schultink AHM, van Doremalen JGC et al. Age-Associated Hematological Toxicity in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Docetaxel in Clinical Practice. Drugs Aging 2019; 36: 379–385. [DOI] [PubMed] [Google Scholar]
- 4.Lund CM, Vistisen KK, Dehlendorff C et al. Age-dependent differences in first-line chemotherapy in patients with metastatic colorectal cancer: the DISCO study. Acta Oncol 2018; 57: 1445–1454. [DOI] [PubMed] [Google Scholar]
- 5.DuMontier C, Liu MA, Murillo A et al. Function, Survival, and Care Utilization Among Older Adults With Hematologic Malignancies. J Am Geriatr Soc 2019; 67: 889–897. [DOI] [PubMed] [Google Scholar]
- 6.Liu MA, DuMontier C, Murillo A et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019; 134: 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang V, Zhao S, Boscardin J et al. Functional Status and Survival After Breast Cancer Surgery in Nursing Home Residents. JAMA Surg 2018; 153: 1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maione P, Perrone F, Gallo C et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the Multicenter Italian Lung Cancer in the Elderly study. J Clin Oncol 2005; 23: 6865–6872. [DOI] [PubMed] [Google Scholar]
- 9.Bruijnen CP, van Harten-Krouwel DG, Koldenhof JJ et al. Predictive value of each geriatric assessment domain for older patients with cancer: A systematic review. Journal of Geriatric Oncology 2019; 10: 859–873. [DOI] [PubMed] [Google Scholar]
- 10.Couderc A-L, Boulahssass R, Nouguerède E et al. Functional status in a geriatric oncology setting: A review. Journal of Geriatric Oncology 2019; 10: 884–894. [DOI] [PubMed] [Google Scholar]
- 11.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002; 346: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 20 Feb 2019.
- 13.Fairman CM, Focht BC, Lucas AR, Lustberg MB. Effects of exercise interventions during different treatments in breast cancer. J Community Support Oncol 2016; 14: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buffart LM, Sweegers MG, May AM et al. Targeting Exercise Interventions to Patients With Cancer in Need: An Individual Patient Data Meta-Analysis. J Natl Cancer Inst 2018; 110: 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stout NL, Baima J, Swisher AK et al. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005–2017). Pm r 2017; 9: S347–s384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohile SG, Dale W, Somerfield MR et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018; 36: 2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health National Heart Lung and Blood Institute. Study Quality Assessment Tools: Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 1 Jun 2020.
- 19.Klepin HD, Tooze JA, Pardee TS et al. Effect of Intensive Chemotherapy on Physical, Cognitive, and Emotional Health of Older Adults with Acute Myeloid Leukemia. J Am Geriatr Soc 2016; 64: 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doni L, Perin A, Manzione L et al. The impact of anemia on quality of life and hospitalisation in elderly cancer patients undergoing chemotherapy. Crit Rev Oncol Hematol 2011; 77: 70–77. [DOI] [PubMed] [Google Scholar]
- 21.Gajra A, McCall L, Muss HB et al. The preference to receive chemotherapy and cancer-related outcomes in older adults with breast cancer CALGB 49907 (Alliance). J Geriatr Oncol 2018; 9: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurria A, Soto-Perez-de-Celis E, Allred JB et al. Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J Am Geriatr Soc 2019; 67: 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manokumar T, Aziz S, Breunis H et al. A prospective study examining elder-relevant outcomes in older adults with prostate cancer undergoing treatment with chemotherapy or abiraterone. J Geriatr Oncol 2016; 7: 81–89. [DOI] [PubMed] [Google Scholar]
- 24.Miaskowski C, Wong ML, Cooper BA et al. Distinct Physical Function Profiles in Older Adults Receiving Cancer Chemotherapy. J Pain Symptom Manage 2017; 54: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verelst SG, Termorshuizen F, Uyl-de Groot CA et al. Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQoL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial. Ann Hematol 2011; 90: 1427–1439. [DOI] [PubMed] [Google Scholar]
- 26.Wong ML, Paul SM, Mastick J et al. Characteristics Associated With Physical Function Trajectories in Older Adults With Cancer During Chemotherapy. J Pain Symptom Manage 2018; 56: 678–688 e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue D, Han S, Jiang S et al. Comprehensive geriatric assessment and traditional Chinese medicine intervention benefit symptom control in elderly patients with advanced non-small cell lung cancer. Med Oncol 2015; 32: 114. [DOI] [PubMed] [Google Scholar]
- 28.Chakiba C, Bellera C, Etchepare F et al. The prognostic value of G8 for functional decline. J Geriatr Oncol 2019; 10: 921–925. [DOI] [PubMed] [Google Scholar]
- 29.Fiteni F, Anota A, Bonnetain F et al. Health-related quality of life in elderly patients with advanced non-small cell lung cancer comparing carboplatin and weekly paclitaxel doublet chemotherapy with monotherapy. Eur Respir J 2016; 48: 861–872. [DOI] [PubMed] [Google Scholar]
- 30.Hoppe S, Rainfray M, Fonck M et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol 2013; 31: 3877–3882. [DOI] [PubMed] [Google Scholar]
- 31.Kenis C, Decoster L, Bastin J et al. Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J Geriatr Oncol 2017; 8: 196–205. [DOI] [PubMed] [Google Scholar]
- 32.Morikawa A, Naito T, Sugiyama M et al. Impact of Cancer Cachexia on Hospitalization-associated Physical Inactivity in Elderly Patients with Advanced Non-small-cell Lung Cancer. Asia Pac J Oncol Nurs 2018; 5: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito T, Okayama T, Aoyama T et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non-small-cell lung cancer in Japan: a prospective longitudinal observational study. BMC Cancer 2017; 17: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alibhai SM, Breunis H, Timilshina N et al. Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol 2015; 6: 262–271. [DOI] [PubMed] [Google Scholar]
- 35.Bergman B, Sullivan M, Sorenson S. Quality of life during chemotherapy for small cell lung cancer. I. An evaluation with generic health measures. Acta Oncol 1991; 30: 947–957. [DOI] [PubMed] [Google Scholar]
- 36.Bezjak A, Tu D, Seymour L et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2006; 24: 3831–3837. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Cantor A, Meyer J et al. Can older cancer patients tolerate chemotherapy? A prospective pilot study. Cancer 2003; 97: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 38.de Jong N, Candel MJ, Schouten HC et al. Course of the fatigue dimension “activity level” and the interference of fatigue with daily living activities for patients with breast cancer receiving adjuvant chemotherapy. Cancer Nurs 2006; 29: E1–13. [DOI] [PubMed] [Google Scholar]
- 39.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 2001; 28: 465–470. [PubMed] [Google Scholar]
- 40.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs 2010; 14: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doorenbos A, Given B, Given C, Verbitsky N. Physical functioning: effect of behavioral intervention for symptoms among individuals with cancer. Nurs Res 2006; 55: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fallowfield L, Gagnon D, Zagari M et al. Multivariate regression analyses of data from a randomised, double-blind, placebo-controlled study confirm quality of life benefit of epoetin alfa in patients receiving non-platinum chemotherapy. Br J Cancer 2002; 87: 1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frodin U, Borjeson S, Lyth J, Lotfi K. A prospective evaluation of patients’ health-related quality of life during auto-SCT: a 3-year follow-up. Bone Marrow Transplant 2011; 46: 1345–1352. [DOI] [PubMed] [Google Scholar]
- 44.Frodin U, Lotfi K, Fomichov V et al. Frequent and long-term follow-up of health-related quality of life following allogeneic haematopoietic stem cell transplantation. Eur J Cancer Care (Engl) 2015; 24: 898–910. [DOI] [PubMed] [Google Scholar]
- 45.Gaton-Johansson F, Watkins CC, Kanu IK et al. The Effects of Symptoms on Quality of Life during Chemotherapy in African-American Women with Breast Cancer. J Natl Black Nurses Assoc 2015; 26: 7–16. [PMC free article] [PubMed] [Google Scholar]
- 46.Given B, Given CW, McCorkle R et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncol Nurs Forum 2002; 29: 949–956. [DOI] [PubMed] [Google Scholar]
- 47.Greimel ER, Bjelic-Radisic V, Pfisterer J et al. Randomized study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group comparing quality of life in patients with ovarian cancer treated with cisplatin/paclitaxel versus carboplatin/paclitaxel. J Clin Oncol 2006; 24: 579–586. [DOI] [PubMed] [Google Scholar]
- 48.Kim BJ, Park HR, Roh HJ et al. Chemotherapy-related polyneuropathy may deteriorate quality of life in patients with B-cell lymphoma. Qual Life Res 2010; 19: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 49.Kinsey E, Ajazi E, Wang X et al. Predictors of Physical and Functional Loss in Advanced-Stage Lung Cancer Patients Receiving Platinum Chemotherapy. J Thorac Oncol 2018; 13: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 50.Land SR, Kopec JA, Yothers G et al. Health-related quality of life in axillary node-negative, estrogen receptor-negative breast cancer patients undergoing AC versus CMF chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel Project B-23. Breast Cancer Res Treat 2004; 86: 153–164. [DOI] [PubMed] [Google Scholar]
- 51.Mohamedali H, Breunis H, Timilshina N et al. Older age is associated with similar quality of life and physical function compared to younger age during intensive chemotherapy for acute myeloid leukemia. Leuk Res 2012; 36: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 52.Morita S, Kobayashi K, Eguchi K et al. Influence of clinical parameters on quality of life during chemotherapy in patients with advanced non-small cell lung cancer: application of a general linear model. Jpn J Clin Oncol 2003; 33: 470–476. [DOI] [PubMed] [Google Scholar]
- 53.Oechsle K, Jensen W, Schmidt T et al. Physical activity, quality of life, and the interest in physical exercise programs in patients undergoing palliative chemotherapy. Support Care Cancer 2011; 19: 613–619. [DOI] [PubMed] [Google Scholar]
- 54.Revicki DA, van den Eertwegh AJ, Lorigan P et al. Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual Life Outcomes 2012; 10: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shallwani SM, Simmonds MJ, Kasymjanova G, Spahija J. Quality of life, symptom status and physical performance in patients with advanced non-small cell lung cancer undergoing chemotherapy: an exploratory analysis of secondary data. Lung Cancer 2016; 99: 69–75. [DOI] [PubMed] [Google Scholar]
- 56.Timilshina N, Breunis H, Tomlinson GA et al. Long-term recovery of quality of life and physical function over three years in adult survivors of acute myeloid leukemia after intensive chemotherapy. Leukemia 2019; 33: 15–25. [DOI] [PubMed] [Google Scholar]
- 57.Verdonck-de Leeuw IM, Buffart LM, Heymans MW et al. The course of health-related quality of life in head and neck cancer patients treated with chemoradiation: a prospective cohort study. Radiother Oncol 2014; 110: 422–428. [DOI] [PubMed] [Google Scholar]
- 58.Watters JM, Yau JC, O’Rourke K et al. Functional status is well maintained in older women during adjuvant chemotherapy for breast cancer. Ann Oncol 2003; 14: 1744–1750. [DOI] [PubMed] [Google Scholar]
- 59.Williamson TJ, Choi AK, Kim JC et al. A Longitudinal Investigation of Internalized Stigma, Constrained Disclosure, and Quality of Life Across 12 Weeks in Lung Cancer Patients on Active Oncologic Treatment. J Thorac Oncol 2018; 13: 1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang SC, Lin CC, Lai WW et al. Dynamic changes in quality of life after three first-line therapies for EGFR mutation-positive advanced non-small-cell lung cancer. Ther Adv Med Oncol 2018; 10: 1758834018755072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aaronson NK, Ahmedzai S, Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85. [DOI] [PubMed] [Google Scholar]
- 62.Basch E, Rogak LJ, Dueck AC. Methods for Implementing and Reporting Patient-reported Outcome (PRO) Measures of Symptomatic Adverse Events in Cancer Clinical Trials. Clin Ther 2016; 38: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basch E, Deal AM, Kris MG et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol 2016; 34: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017; 318: 197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basch E, Reeve BB, Mitchell SA et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014; 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fowler H, Belot A, Ellis L et al. Comorbidity prevalence among cancer patients: a population-based cohort study of four cancers. BMC Cancer 2020; 20: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hurria A, Cirrincione CT, Muss HB et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 2011; 29: 1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohile SG, Velarde C, Hurria A et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw 2015; 13: 1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piccirillo JF, Tierney RM, Costas I et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004; 291: 2441–2447. [DOI] [PubMed] [Google Scholar]
- 70.Duan-Porter W, Cohen HJ, Demark-Wahnefried W et al. Physical resilience of older cancer survivors: An emerging concept. J Geriatr Oncol 2016; 7: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurria A, Togawa K, Mohile SG et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011; 29: 3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cancer and Aging Research Group. Chemotherapy toxicity prediction tool. http://www.mycarg.org/Chemo_Toxicity_Calculator. Accessed 30 Jun 2020.
- 73.Yourman LC, Lee SJ, Schonberg MA et al. Prognostic Indices for Older Adults: A Systematic Review. JAMA : the journal of the American Medical Association 2012; 307: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ePrognosis. http://eprognosis.org/. Accessed 30 Jun 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Study selection.


