Abstract
Long and short sleep duration are associated with elevated blood pressure (BP), possibly through effects on molecular pathways that influence neuroendocrine and vascular systems. To gain new insights into the genetic basis of sleep-related BP variation, we performed genome-wide gene by short or long sleep duration interaction analyses on four BP traits (systolic BP, diastolic BP, mean arterial pressure, and pulse pressure) across five ancestry groups using 1 degree of freedom (1df) interaction and 2df joint tests. Primary multi-ancestry analyses in 62,969 individuals in stage 1 identified 3 novel loci that were replicated in an additional 59,296 individuals in stage 2, including rs7955964 (FIGNL2/ANKRD33) showing significant 1df interactions with long sleep duration and rs73493041 (SNORA26/C9orf170) and rs10406644 (KCTD15/LSM14A) showing significant 1df interactions with short sleep duration (Pint < 5×10−8). Secondary ancestry-specific two-stage analyses and combined stage 1 and 2 analyses additionally identified 23 novel loci that need external replication, including 3 and 5 loci showing significant 1df interactions with long and short sleep duration, respectively. Multiple genes mapped to our 26 novel loci have known functions in sleep-wake regulation, nervous and cardiometabolic systems. We also identified new gene by long sleep interactions near five known BP loci (≤1Mb) including NME7, FAM208A, MKLN1, CEP164, and RGL3/ELAVL3 (Pint < 5×10−8). This study indicates that sleep and primary mechanisms regulating BP may interact to elevate BP level, suggesting novel insights into sleep-related BP regulation.
Introduction
Hypertension (HTN), including elevations in systolic blood pressure (SBP) and/or diastolic blood pressure (DBP), is a major risk factor for cardiovascular diseases, stroke, renal failure and heart failure1. The heritability of HTN is estimated to be 30–60% in family studies2, 3. Recent well-powered large genome-wide association studies (GWAS) of blood pressure (BP) have identified over 1,000 loci; however, in total these explain less than 3.5% of BP variation4–16. Gene-environment (G×E) interaction analyses have been proposed to explain additional heritability and identified novel loci for traits associated with cardiometabolic diseases17–19.
Long and short sleep durations are associated with elevated BP, possibly through effects on molecular pathways that influence neuroendocrine and vascular systems20, 21. Recent multi-ancestry interaction analyses between genetic variants and sleep duration (gene-sleep for short) on blood lipid traits have identified novel loci and potentially distinct mechanisms for short- and long-sleep associated dyslipidemia, and suggest a modification effect of sleep-wake exposures on lipid biology19. We hypothesize that differences in sleep duration may also modify the effect of genetic factors on BP. Assessment of genetic association studies accounting for potential gene-sleep interactions may help identify novel BP loci and reveal new biological mechanisms that can be explored for treatment or prevention of HTN.
Within the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Gene-Lifestyle Interactions Working Group22, we investigate gene-sleep interactions on BP traits in 122,265 individuals from five ancestry groups. We utilize both the 1 degree of freedom (df) test of interaction effect and the 2df joint test of main and interaction effects23, and identify novel loci for BP.
Materials and methods
We performed genome-wide meta-analysis of gene-sleep interactions on four BP traits (SBP, DBP, mean arterial pressure [MAP], and pulse pressure [PP]) in 30 cohorts of five ancestry groups in two stages (Supplementary Notes). Stage 1 discovery analyses included 62,969 individuals of European (EUR), African (AFR), Asian (ASN), Hispanic (HIS), and Brazilian (BRZ) ancestries from 16 studies (Supplementary Tables 1–3). Stage 2 replication analyses included 59,296 individuals of EUR, AFR, ASN and HIS ancestries from 14 additional studies (Supplementary Tables 4–6). We examined long total sleep time (LTST) and short total sleep time (STST) separately as lifestyle exposures. Given the heterogeneity of age, sleep duration and BP levels across cohorts and ancestry groups, as well as differences in how sleep duration was assessed (Supplementary Tables 2 and 5), we followed procedures used in prior research19 to categorize 20% of each sample as long sleepers and 20% as short sleepers based on responses to questionnaires, accounting for age and sex variability within each cohort (Supplementary Methods).
The overall study design is described in Fig. 1. Gene-sleep interaction analyses were performed adjusting for age, sex, population structure, and other cohort-specific covariates in each ancestry of each cohort. Since BMI is associated with both sleep and BP24, 25, we performed additional interaction analyses adjusted for BMI to identify genetic loci through biological pathways independent of obesity. Inverse-variance weighted meta-analysis for the 1 degree of freedom (df) interaction term (Pint), and 2 df joint fixed-effects meta-analysis of the combined main and interaction effects (Pjoint) using Manning et al’s method23 were performed across multi-ancestry in stage 1 and stage 2 separately. Secondary ancestry-specific meta-analyses were performed restricted to EUR and AFR groups. We performed extensive study-level and meta-level quality controls as described in Supplementary Methods.
Fig. 1.
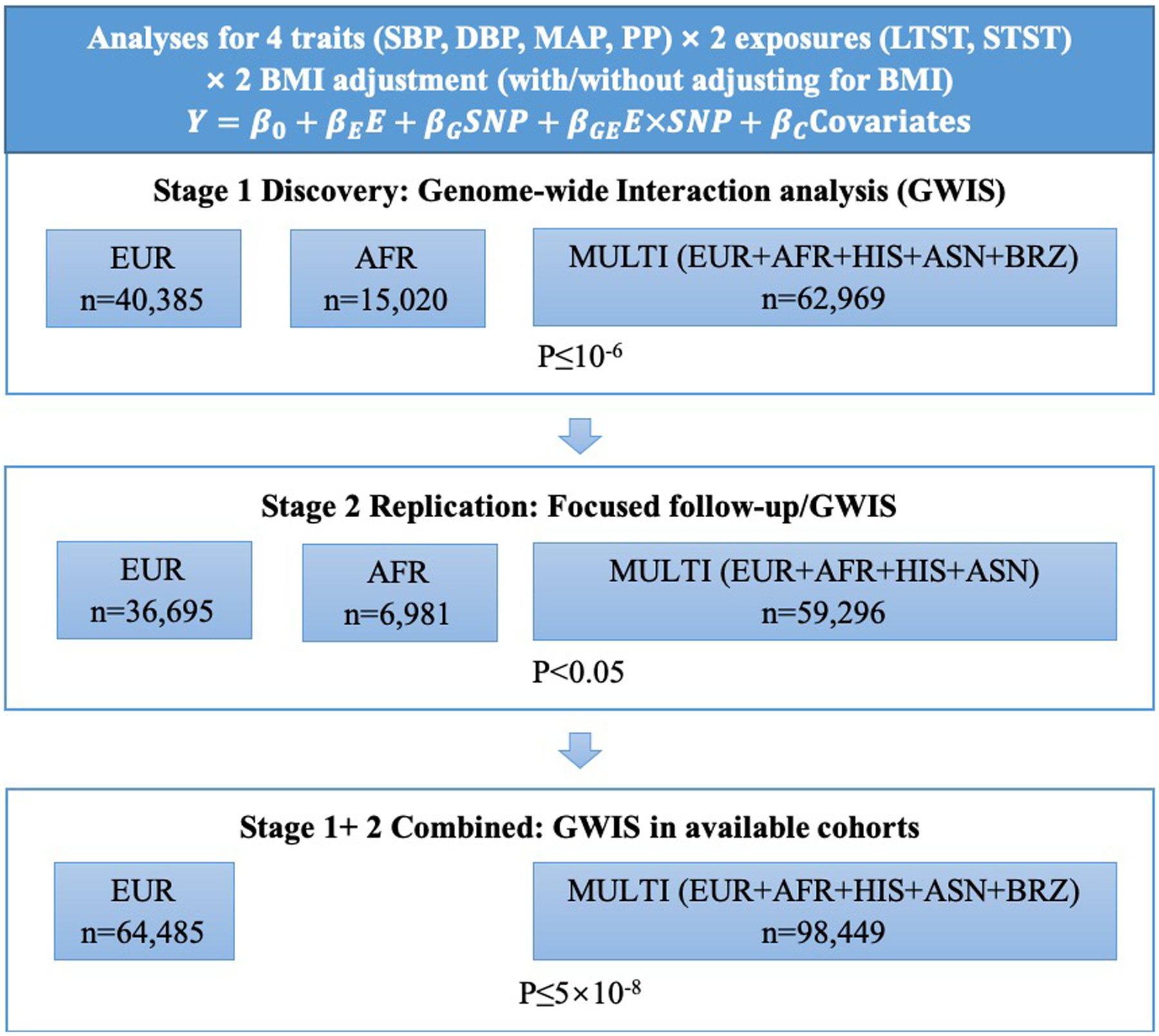
Study overview.
Genetic variants with Pint or Pjoint <10−6 in stage 1 were followed up in stage 2 replication analyses and subsequently meta-analyzed with stage 1 summary statistics. The replication significance threshold was defined as stage 2 P<0.05 and stage 1 + 2 P<5×10−8, with consistent directions of association effects. To maximize the statistical power, we also performed genome-wide combined stage 1 and 2 meta-analyses in multi-ancestry and EUR groups. Additional novel loci were reported at a stricter significant threshold (P<3.125×10−9), accounting for 2 independent BP traits, 2 exposures, 2 tests (joint and interaction), with and without BMI adjustment. Novel loci replicated in two-stage analyses and additional loci identified in combined analyses were followed up for associations with relevant traits and bioinformatics analyses.
This work was approved by the Institutional Review Board of Washington University in St. Louis and complies with all relevant ethical regulations. For each of the participating cohorts, the appropriate ethics review board approved the data collection and all participants provided informed consent.
Results
Genome-wide gene-sleep interaction analyses
The Miami and QQ plots of stage 1 discovery analyses in multi-ancestry, EUR and AFR groups are provided in Supplementary Figs 1–6. 1,976 genetic variants with Pint or Pjoint<10−6 were followed up for replication analyses. Of these, 1,081 variants were available in stage 2 cohorts and passed quality control, of which 268 variants showed Pjoint or Pint <0.05.
Our primary two-stage multi-ancestry analyses replicated 3 novel loci (r2<0.1 and >1Mb from any previously identified BP locus) using the 1df interaction test. Among these loci, rs7955964 (FIGNL2/ANKRD33) showed significant 1df interactions with LTST, while rs73493041 (SNORA26/C9orf170) and rs10406644 (KCTD15/LSM14A) revealed significant 1df interactions with STST (Pint<5×10−8; Table 1). The regional association plots are shown in Supplementary Fig. 7. Consistent directions of main and interaction effects were observed across different cohorts (Fig. 2). Note that since rs7955964 and rs10406644 are common only in AFR and HIS cohorts (minor allele frequency [MAF] > 1%), the association effects may be driven by African ancestral alleles. With and without adjustment for BMI showed similar association results at those three loci (Table 1). Using 2df joint test we identified 9 loci that were within 1Mb from previously reported BP loci (Pjoint<5×10−8; Supplementary Table 7). However, none of these loci showed nominal significant 1df interaction association with LTST or STST (Pint>0.05).
Table 1.
Replicated novel BP loci identified incorporating gene-sleep interactions with and without BMI adjustment.
| Exposure | rsID | Gene(s) | Chr: position (Build 37) | Alleles (E/A) | EAF | Trait | Stage | BMI adjustment | N | β SNP | SESNP | β Int | SEInt | PJoint | PInt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTST | rs7955964 | FIGNL2, ANKRD33 | 12:52281279 | A/T | 0.896 | MAP | 1 | Without BMI | 18583 | −0.400 | 0.290 | 2.711 | 0.582 | 1.75×10−5 | 1.34×10−6 |
| with BMI | 18583 | −0.462 | 0.279 | 2.768 | 0.546 | 2.48×10−6 | 2.10×10−7 | ||||||||
| 2 | Without BMI | 12335 | −0.621 | 0.289 | 2.163 | 0.632 | 2.52×10−3 | 5.49×10−4 | |||||||
| with BMI | 12327 | −0.519 | 0.281 | 2.210 | 0.613 | 1.72×10−3 | 2.66×10−4 | ||||||||
| 1+2 | Without BMI | 29985 | −0.517 | 0.208 | 2.505 | 0.433 | 1.11×10−7 | 4.40×10 −9 | |||||||
| with BMI | 29957 | −0.500 | 0.201 | 2.577 | 0.413 | 6.74×10 −9 | 2.94×10 −10 | ||||||||
| STST | rs73493041 | SNORA26, C9orf170 | 9:89849304 | T/C | 0.959 | DBP | 1 | Without BMI | 36858 | −0.725 | 0.229 | 2.336 | 0.471 | 4.65×10−6 | 3.6×10−7 |
| with BMI | 36858 | −0.723 | 0.219 | 2.235 | 0.456 | 5.16×10−6 | 5.18×10−7 | ||||||||
| 2 | Without BMI | 24413 | −0.763 | 0.321 | 1.888 | 0.705 | 5.44×10−3 | 9.43×10−3 | |||||||
| with BMI | 24385 | −0.704 | 0.335 | 1.875 | 0.704 | 1.09×10−2 | 1.27×10−2 | ||||||||
| 1+2 | Without BMI | 61271 | −0.724 | 0.185 | 2.213 | 0.387 | 3.62×10 −8 | 1.30×10 −8 | |||||||
| with BMI | 61243 | −0.709 | 0.182 | 2.132 | 0.381 | 7.15×10−8 | 2.58×10 −8 | ||||||||
| rs10406644 | KCTD15, LSM14A | 19:34595645 | A/G | 0.095 | PP | 1 | Without BMI | 15021 | 0.542 | 0.275 | −3.194 | 0.605 | 1.26×10−6 | 1.35×10−7 | |
| with BMI | 12921 | 0.565 | 0.306 | −3.382 | 0.677 | 5.23×10−6 | 4.81×10−7 | ||||||||
| 2 | Without BMI | 11401 | 1.142 | 0.587 | −2.702 | 1.163 | 4.59×10−2 | 2.02×10−2 | |||||||
| with BMI | 11373 | 1.102 | 0.582 | −2.533 | 1.155 | 6.08×10−2 | 2.83×10−2 | ||||||||
| 1+2 | Without BMI | 26422 | 0.648 | 0.249 | −3.067 | 0.536 | 1.39×10−7 | 7.59×10 −9 | |||||||
| with BMI | 24294 | 0.678 | 0.271 | −3.135 | 0.584 | 8.56×10−7 | 4.35×10 −8 |
Fig. 2.
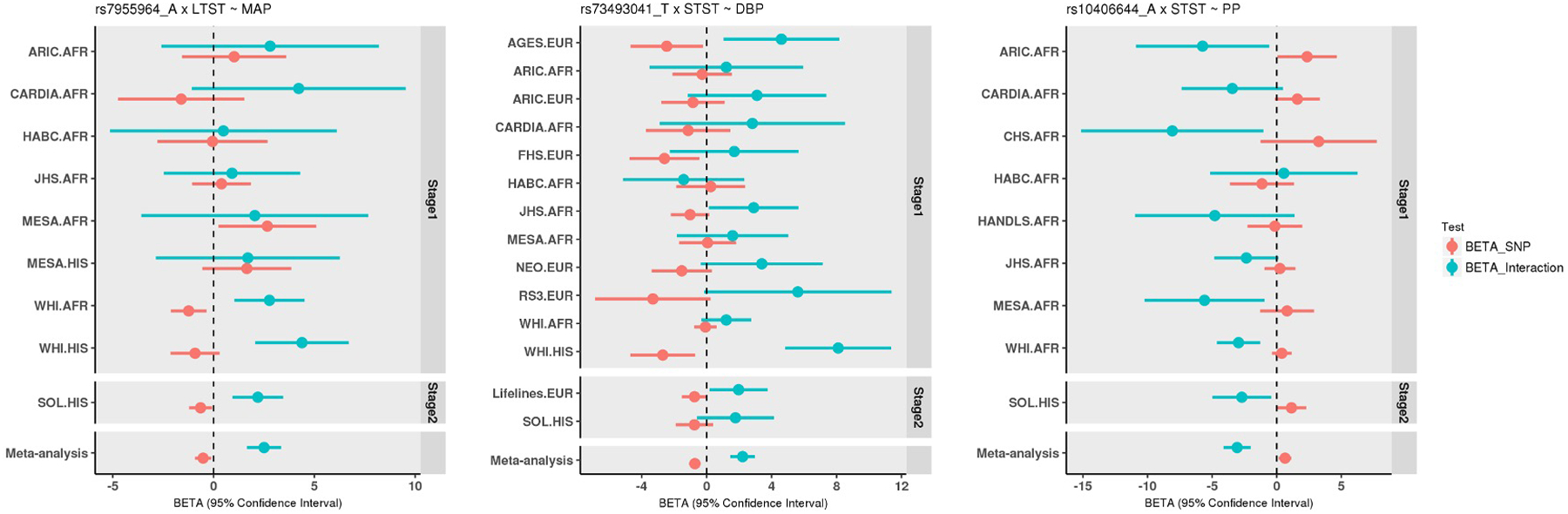
Forest plots of 3 replicated novel loci identified in genome-wide gene- sleep 1df interaction analyses in multi-ancestry population.
In secondary ancestry-specific two-stage analyses restricted to EUR or AFR individuals, the 1df interaction test did not identify any significant locus. The 2df joint test in EUR group identified 1 locus near previously reported BP loci (≤1Mb; Supplementary Table 7). The 2df joint test in AFR group identified 3 novel loci after incorporating interactions with LTST (stage 1 Pjoint <5×10−8 and stage 2 Pjoint<0.05, with consistent directions of main effects), including rs111887471 (TRPC3/KIAA1109), rs114043188 (ANK), and rs138967672 (RP11–322L20.1/RP11–736P16.1) (Supplementary Table 8 and Supplementary Fig. 8). However, these 3 variants did not survive our formal replication criteria of stage 1+2 P<5×10−8, possibly reflecting heterogeneity between discovery and replication cohorts.
Genome-wide combined stage 1 and stage 2 meta-analyses (Miami and QQ plots in Supplementary Figs 9–12) additionally identified 20 loci near previously reported BP loci (≤1Mb) and 56 unreported loci (>1Mb from previously reported BP) with Pjoint or Pint<5×10−8 (Supplementary Tables 9–11). Loci near known BP genes, including NME7, FAM208A, MKLN1, CEP164, and RGL3/ELAVL37–11 showed significant 1df interactions with LTST (Pint<5×10−8; Supplementary Table 9). Among the 56 unreported loci, 20 loci passed a stricter significance threshold accounting for multiple comparisons (Pjoint or Pint <3.125×10−9; Supplementary Table 10 and Supplementary Fig 13). Among these 20 novel loci, 3 loci showed significant 1df interaction with LTST and 5 loci showed genome-wide significant 1df interactions with STST (Pint<5×10−8; Supplementary Table 10). Replication in independent datasets is needed to validate those unreported loci. We also looked up the previously validated 362 BP loci4–15 and 113 sleep duration loci26 in the combined analyses, but none of these showed significant 1df interactions after accounting for multiple comparisons (Pint>10−4; Supplementary Tables 12–15).
We estimated the variance of BP traits explained by 3 replicated and 23 additional novel loci (3 in AFR two-stage analyses and 20 in combined analyses) using the R package VarExp27 (Supplementary Table 16). The 3 replicated novel variants together explained 0.002–0.039% and 0.304–0.939% of BP variation in EUR and AFR, respectively. The other 23 variants additionally explained 0.230–0.419% and 0.806–2.597% of BP variation in EUR and AFR. Given the limited sample sizes in the AFR group, the estimation of BP variation in AFR is likely inflated.
Associations with other relevant traits
We looked up the associations between the 3 replicated and 23 additional novel loci with cardiovascular diseases, stroke, chronic kidney disease, and self-reported and objective (derived from 7-day accelerometry) sleep traits using publicly available genome-wide summary statistics from large GWAS26, 28–37 (Supplementary Tables 17–22). One of the replicated loci rs73493041 (SNORA26/C9orf170) was associated with self-reported chronotype (morningness vs eveningness) (P=9.1×10−6; Supplementary Table 21). Among the other novel loci, rs17036094 (PSRC1/MYBPHL) was associated with coronary artery disease and myocardial infarction (P≤0.005; Supplementary Table 18), and rs140526840 (FSTL5) was associated with chronic kidney disease (P=0.006; Supplementary Table 20),
Bioinformatics analyses
All of the 26 novel variants were mapped to intergenic or intronic regions using HaploReg38, including 4 in promoter histone marks, 11 in enhancer histone marks, 10 in DNAse, 3 altered the binding sites of regulatory proteins and 2 conserved elements (Supplementary Table 23).
Among the 3 replicated novel loci, rs73493041 (SNORA26/C9orf170) was an eQTL for GAS1 in suprapubic skin using GTEx (v8)39 (Supplementary Table 24). Using PLINK pruning and SNPsea40, rs7955964 (SNORA26/C9orf170) was mapped to a region of 10 genes (Supplementary Table 25), including ANKRD33 and NR4A1, implicated in sleep-wake control regulation and the neurovascular system41, 42. Rs10406644 (KCTD15/LSM14A) was mapped to a region overlapping with 9 genes (Supplementary Table 26), including KCTD15 and CHST8, previously associated with adiposity traits and involved in neurodevelopmental and neuropsychiatric diseases43–45 (see Discussion).
Among the other 23 novel loci, 4 variants showed strong eQTL evidence across various tissues such as blood and adipose tissue (Supplementary Table 24). 14 loci were mapped to genes with known functions in cardiac and nervous systems (e.g., TRPC346, RYR247, ANK248, GJA449 and SORT150) and associated with other cardiometabolic (e.g., HTR1A51, PSRC152, PSKH153), inflammatory (e.g., IL3354), cognition (e.g., FRMD4A55) and psychiatric traits (e.g., NFATC356) (Supplementary Tables 25 and 26).
In total, 11 novel loci harbored genes implicated in Mendelian syndromes such as ventricular tachycardia and cryptogenic cirrhosis. 13 loci harbored one or more genes with potential drug targets (Supplementary Tables 25 and 26).
We performed tissue and pathway enrichment analyses using annotated genes under novel association regions using FUMA57 (Supplementary Tables 27 and 28). Genes under the association regions in gene-LTST interaction analyses were enriched in multiple artery and cardiac muscle related pathways (Supplementary Table 29).
Discussion
We performed gene-sleep interaction analyses on BP using 122,265 individuals from 5 ancestry groups in 30 studies, using both a 1df test of interaction effect and a 2df joint test of main and interaction effects. Following a two-stage design, we identified 3 novel loci that were replicated in additional samples, including rs7955964 (FIGNL2/ANKRD33) showing significant interactions with LTST, and rs73493041 (SNORA26/C9orf170) and rs10406644 (KCTD15/LSM14A) showing significant interactions with STST (Pint<5×10−8). Secondary analyses additionally identified 3 novel loci with weak replication evidence in AFR groups using 2df joint test. Combined stage 1 and 2 analyses identified another 20 novel loci after accounting for multiple comparisons (Pjoint or Pint<3.125×10−9), which require external replication. The associations were largely unchanged after additionally adjusting for BMI. Collectively, these 26 loci explained 0.23–0.43% of BP variation in EUR and 1.33–2.96% BP variation in AFR groups.
The emergence of novel loci after considering gene-sleep interactions suggests an important modifying role of sleep on BP regulation, which involves both central and peripheral regulation (including the brain, adrenal glands, kidneys, and vasculature). Insufficient or short sleep can increase BP through effects on elevating sympathetic nervous system activity and altering hypothalamic-pituitary-adrenal (HPA) axis activities, leading to hormonal changes, endothelial dysfunction, insulin resistance, and systemic inflammation20, 58. The mechanisms underlying the association between long sleep duration and BP are less well understood, and may reflect circadian misalignment in a 24-hour period, including disrupted sleep-wake cycle, a misalignment of internal biological clocks with the external environment, and desynchronized central and peripheral clocks in tissues relevant for BP control59. The importance of circadian control of BP is evident by the normal nocturnal decline (“dipping”) in BP. Non-dipping of BP, associated with increased mortality, is observed with both sleep disturbances and abnormalities of sodium transport in the kidney60, 61. Our data suggest that sleep and renal and neuro-endocrine control of BP may interact to influence susceptibility to HTN. The novel loci found by gene-LTST and gene-STST interaction analyses were distinct, supporting the different mechanisms of short and long sleep modifying BP. Similarly, in prior gene-sleep interaction analyses for blood lipids, LTST and STST each also modified gene effects in a non-overlapping pattern19.
Primary two-stage multi-ancestry analyses identified 3 novel loci. At rs7955964 (FIGNL2/ANKRD33), ANKRD33 is expressed in retinal photoreceptors and the pineal gland and acts as a transcriptional repressor for CRX-activated photoreceptor gene regulation41. Given the importance of light in the central regulation of circadian rhythms, long sleep- a circadian disruptor- may interact with this gene to influence BP60. Additionally, NR4A1 (also at rs7955964) is a member of the nuclear hormone receptors, which regulate neurohormonal systems including dopamine and norepinephrine and cardiac stress responses42. At rs10406644 (KCTD15/LSM14A), CHST8 and KCTD15 are associated with adiposity traits43, 44 and GPI functions in glucose metabolism and immune system pathways62, 63. Short sleep may exacerbate weight gain and metabolic dysfunction64, thus amplifying effects of this locus on BP. KCTD15 belongs to a gene family involved in neurodevelopmental and neuropsychiatric diseases45. Rs73493041 (SNORA26/C9orf170) was an eQTL for GAS1, a pleiotropic regulator of cellular homeostasis and widely expressed in the central nervous system65, 66. This variant was also significantly associated with self-reported chronotype, an indicator of circadian preference (P=9.1×10−6; Supplementary Table 20).
Given the high prevalence of HTN in African Americans, there is a critical need to identify modifiable risk factors. Notably, African Americans have poorly controlled HTN as well as circadian abnormalities in BP regulation67. They also have a higher prevalence of short and long sleep duration compared to individuals of European ancestry68, 69, likely due to combinations of social-environmental exposures and genetic and epigenetic susceptibility70. In AFR specific gene-LTST analyses we identified 3 unreported loci, including two loci mapped to TRPC3 and ANK2 with known functions in cardiac ion (Na+ and Ca2+) homeostasis46, 48. These associations in AFR may reflect differences in BP control with individuals of African ancestry having greater sodium sensitivity71, with BP effects amplified by disrupted circadian rhythm regulation due to long sleep61. While intriguing, these associations were observed in African Americans (Pjoint<5×10−8) and the only available replication sample was individuals of African ancestry from the UK (Pjoint<0.05). There may be a systematic difference of the admixture structure between the discovery and replication cohorts. Future analyses using larger AFR samples will be needed to validate these findings and further identify the potential for sleep disturbances to interact with BP regulatory systems in AFR who are at increased risk for HTN-associated morbidities.
Although we did not observe significant 1df interactions with sleep duration on the 362 previously reported BP or 101 sleep duration variants (Supplementary Tables 12–15), we identified 30 loci within 1Mb from previously reported BP regions (Supplementary Tables 7 and 9). Among those, variants in/near NME7, FAM208A, MKLN1, CEP164, and RGL3/ELAVL3 showed significant 1df interactions with LTST (Pint<5×10−8; Supplementary Table 9). Some of these genes have known functions in neuronal systems, including MKLN1 regulating the internalization and transport of the GABAA receptor72, 73 and ELAVL3 encoding a neural-specific RNA-binding protein involved in neuronal differentiation and maintenance74.
In this study we defined short and long sleep duration using self-reported questionnaires, which can result in misclassification75, potentially reducing statistical power. Although we used a within cohort approach for harmonizing sleep duration that accounted for age and sex differences across cohorts, there may be systematic residual differences in sleep assessments that resulted in heterogeneity across our samples. Future work using objective measurements (e.g., polysomnography and actigraphy data) may provide further insight into sleep-related BP mechanisms.
Some of our most interesting findings - and ones with high potential public health impact due to the burden of extreme sleep duration and HTN in AFR group. Unfortunately, limited samples of AFR were available for replication. We identified 1,976 variants with significant association effect in gene-sleep interaction analyses in stage 1. However, only 1,081 of those variants were available in stage 2 analyses. Most of the unavailable variants in stage 2 had been identified in non-EUR cohorts and were rare in EUR populations (MAF<1%). Future studies following-up these “missing” variants in diverse groups and additional studies of minority populations are needed to further understand mechanisms for BP regulation that are modulated by sleep. In addition, some of our findings were mapped to large genomic regions covering many genes. Further fine-mapping analyses using sequencing data or biochemistry experiments may further clarify the causal variants.
In summary, we performed a large-scale gene-sleep interaction meta-analyses in multi-ancestry groups. In addition to identifying interactions at a genome-wide significant level near 5 previously reported BP regions, we identified 3 novel loci with formal replication and 23 novel loci that need external replication. Multiple genes showing significant interactions with long or short sleep duration were functional in tissues relevant for BP control, and associated with circadian, metabolic, and neuropsychiatric traits. Prior research indicates that extreme sleep durations (short or long) are strongly associated with cardiovascular disease and mortality through causal and noncausal pathways20, 21. Our data suggest that interactions between sleep and BP-regulating genes may contribute to the increased cardiovascular morbidity observed with extreme sleep duration.
Supplementary Material
Acknowledgments
This project was supported by the US National Heart, Lung, and Blood Institute (NHLBI) R01HL118305. H.W. and S.R. was supported by NHLBI R35HL135818. B.E.C. was supported by NHLBI K01HL135405. A.R.B. was supported by the Intramural Research Program of the National Institutes of Health in the Center for Research on Genomics and Global Health (CRGGH). The CRGGH is supported by the National Human Genome Research Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the Center for Information Technology, and the Office of the Director at the National Institutes of Health (1ZIAHG200362). D.v.H. was supported by the European Commission funded project HUMAN (Health-2013-INNOVATION-1-602757). The CHARGE cohorts was supported in part by NHLBI infrastructure grant HL105756. Study-specific acknowledgments can be found in the Supplementary Notes.
Conflict of Interest
D.O.M.K. is a part time research consultant at Metabolon, Inc. B.M.P. serves on the DSMB of a clinical trial funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. H.J.G. has received research support from the German Research Foundation (DFG), the German Ministry of Education and Research (BMBF), the DAMP Foundation, Fresenius Medical Care, the EU “Joint Programme Neurodegenerative Disorders (JPND) and the European Social Fund (ESF)”. The remaining authors declare no competing interests.
References
- 1.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A et al. Lifetime risks of cardiovascular disease. The New England journal of medicine 2012; 366(4): 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper RS, Luke A, Zhu X, Kan D, Adeyemo A, Rotimi C et al. Genome scan among Nigerians linking blood pressure to chromosomes 2, 3, and 19. Hypertension 2002; 40(5): 629–633. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension 2000; 36(4): 477–483. [DOI] [PubMed] [Google Scholar]
- 4.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009; 41(6): 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A et al. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41(6): 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Consortium for Blood Pressure Genome-Wide Association S, Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478(7367): 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet 2016; 48(10): 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Kraja AT, Smith JA, Brody JA, Franceschini N, Bis JC et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet 2016; 48(10): 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet 2016; 48(10): 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann TJ, Ehret GB, Nandakumar P, Ranatunga D, Schaefer C, Kwok PY et al. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat Genet 2017; 49(1): 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren HR, Evangelou E, Cabrera CP, Gao H, Ren M, Mifsud B et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet 2017; 49(3): 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 2018; 50(10): 1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri A, Hellwege JN, Keaton JM, Park J, Qiu C, Warren HR et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet 2019; 51(1): 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschini N, Fox E, Zhang Z, Edwards TL, Nalls MA, Sung YJ et al. Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. Am J Hum Genet 2013; 93(3): 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Feng T, Tayo BO, Liang J, Young JH, Franceschini N et al. Meta-analysis of correlated traits via summary statistics from GWASs with an application in hypertension. Am J Hum Genet 2015; 96(1): 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang J, Le TH, Edwards DRV, Tayo BO, Gaulton KJ, Smith JA et al. Single-trait and multi-trait genome-wide association analyses identify novel loci for blood pressure in African-ancestry populations. PLoS Genet 2017; 13(5): e1006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 2012; 44(6): 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung YJ, Winkler TW, de Las Fuentes L, Bentley AR, Brown MR, Kraja AT et al. A Large-Scale Multi-ancestry Genome-wide Study Accounting for Smoking Behavior Identifies Multiple Significant Loci for Blood Pressure. Am J Hum Genet 2018; 102(3): 375–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noordam R, Bos MM, Wang H, Winkler TW, Bentley AR, Kilpelainen TO et al. Multi-ancestry sleep-by-SNP interaction analysis in 126,926 individuals reveals lipid loci stratified by sleep duration. Nat Commun 2019; 10(1): 5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens 2014; 27(10): 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daghlas I, Dashti HS, Lane J, Aragam KG, Rutter MK, Saxena R et al. Sleep Duration and Myocardial Infarction. J Am Coll Cardiol 2019; 74(10): 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao DC, Sung YJ, Winkler TW, Schwander K, Borecki I, Cupples LA et al. Multiancestry Study of Gene-Lifestyle Interactions for Cardiovascular Traits in 610 475 Individuals From 124 Cohorts: Design and Rationale. Circ Cardiovasc Genet 2017; 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning AK, LaValley M, Liu CT, Rice K, An P, Liu Y et al. Meta-analysis of gene-environment interaction: joint estimation of SNP and SNP x environment regression coefficients. Genet Epidemiol 2011; 35(1): 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandner MA, Schopfer EA, Sands-Lincoln M, Jackson N, Malhotra A. Relationship between sleep duration and body mass index depends on age. Obesity (Silver Spring) 2015; 23(12): 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins D, Tareen N, Pan D, Norris K. The relationship between body mass index, blood pressure and pulse rate among normotensive and hypertensive participants in the third National Health and Nutrition Examination Survey (NHANES). Cell Mol Biol (Noisy-le-grand) 2003; 49(8): 1305–1309. [PubMed] [Google Scholar]
- 26.Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, Wang H et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun 2019; 10(1): 1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laville V, Bentley AR, Prive F, Zhu X, Gauderman J, Winkler TW et al. VarExp: estimating variance explained by genome-wide GxE summary statistics. Bioinformatics 2018; 34(19): 3412–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet 2017; 49(9): 1385–1391. [DOI] [PubMed] [Google Scholar]
- 29.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015; 47(10): 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 2018; 50(4): 524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tin A, Marten J, Halperin Kuhns VL, Li Y, Wuttke M, Kirsten H et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat Genet 2019; 51(10): 1459–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teumer A, Li Y, Ghasemi S, Prins BP, Wuttke M, Hermle T et al. Genome-wide association meta-analyses and fine-mapping elucidate pathways influencing albuminuria. Nat Commun 2019; 10(1): 4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 2019; 51(6): 957–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun 2019; 10(1): 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lane JM, Jones SE, Dashti HS, Wood AR, Aragam KG, van Hees VT et al. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet 2019; 51(3): 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Lane JM, Jones SE, Dashti HS, Ollila HM, Wood AR et al. Genome-wide association analysis of self-reported daytime sleepiness identifies 42 loci that suggest biological subtypes. Nat Commun 2019; 10(1): 3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones SE, van Hees VT, Mazzotti DR, Marques-Vidal P, Sabia S, van der Spek A et al. Genetic studies of accelerometer-based sleep measures yield new insights into human sleep behaviour. Nat Commun 2019; 10(1): 1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 2012; 40(Database issue): D930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consortium GT. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 2015; 348(6235): 648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slowikowski K, Hu X, Raychaudhuri S. SNPsea: an algorithm to identify cell types, tissues and pathways affected by risk loci. Bioinformatics 2014; 30(17): 2496–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanuki R, Omori Y, Koike C, Sato S, Furukawa T. Panky, a novel photoreceptor-specific ankyrin repeat protein, is a transcriptional cofactor that suppresses CRX-regulated photoreceptor genes. FEBS Lett 2010; 584(4): 753–758. [DOI] [PubMed] [Google Scholar]
- 42.Medzikovic L, de Vries CJM, de Waard V. NR4A nuclear receptors in cardiac remodeling and neurohormonal regulation. Trends Cardiovasc Med 2019; 29(8): 429–437. [DOI] [PubMed] [Google Scholar]
- 43.Comuzzie AG, Cole SA, Laston SL, Voruganti VS, Haack K, Gibbs RA et al. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PLoS One 2012; 7(12): e51954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009; 41(1): 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng X, Aouacheria A, Lionnard L, Metz KA, Soane L, Kamiya A et al. KCTD: A new gene family involved in neurodevelopmental and neuropsychiatric disorders. CNS Neurosci Ther 2019; 25(7): 887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eder P, Probst D, Rosker C, Poteser M, Wolinski H, Kohlwein SD et al. Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex. Cardiovasc Res 2007; 73(1): 111–119. [DOI] [PubMed] [Google Scholar]
- 47.Dabertrand F, Nelson MT, Brayden JE. Ryanodine receptors, calcium signaling, and regulation of vascular tone in the cerebral parenchymal microcirculation. Microcirculation 2013; 20(4): 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashef F, Li J, Wright P, Snyder J, Suliman F, Kilic A et al. Ankyrin-B protein in heart failure: identification of a new component of metazoan cardioprotection. J Biol Chem 2012; 287(36): 30268–30281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vicario N, Zappala A, Calabrese G, Gulino R, Parenti C, Gulisano M et al. Connexins in the Central Nervous System: Physiological Traits and Neuroprotective Targets. Front Physiol 2017; 8: 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen JL, Schroder TJ, Christensen S, Strandbygard D, Pallesen LT, Garcia-Alai MM et al. Identification of the first small-molecule ligand of the neuronal receptor sortilin and structure determination of the receptor-ligand complex. Acta Crystallogr D Biol Crystallogr 2014; 70(Pt 2): 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng JS, Arnett DK, Lee YC, Shen J, Parnell LD, Smith CE et al. Genome-wide contribution of genotype by environment interaction to variation of diabetes-related traits. PLoS One 2013; 8(10): e77442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandhu MS, Waterworth DM, Debenham SL, Wheeler E, Papadakis K, Zhao JH et al. LDL-cholesterol concentrations: a genome-wide association study. Lancet 2008; 371(9611): 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013; 45(11): 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 2016; 48(7): 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambert JC, Grenier-Boley B, Harold D, Zelenika D, Chouraki V, Kamatani Y et al. Genome-wide haplotype association study identifies the FRMD4A gene as a risk locus for Alzheimer’s disease. Mol Psychiatry 2013; 18(4): 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511(7510): 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun 2017; 8(1): 1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res 2012; 35(10): 1012–1018. [DOI] [PubMed] [Google Scholar]
- 59.Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry 2014; 26(2): 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 2018; 119: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M et al. The circadian clock modulates renal sodium handling. J Am Soc Nephrol 2012; 23(6): 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adeva-Andany MM, Perez-Felpete N, Fernandez-Fernandez C, Donapetry-Garcia C, Pazos-Garcia C. Liver glucose metabolism in humans. Biosci Rep 2016; 36(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cascone T, McKenzie JA, Mbofung RM, Punt S, Wang Z, Xu C et al. Increased Tumor Glycolysis Characterizes Immune Resistance to Adoptive T Cell Therapy. Cell Metab 2018; 27(5): 977–987 e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xi B, He D, Zhang M, Xue J, Zhou D. Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev 2014; 18(4): 293–297. [DOI] [PubMed] [Google Scholar]
- 65.Segovia J, Zarco N. Gas1 is a pleiotropic regulator of cellular functions: from embryonic development to molecular actions in cancer gene therapy. Mini Rev Med Chem 2014; 14(14): 1139–1147. [DOI] [PubMed] [Google Scholar]
- 66.Zarco N, Bautista E, Cuellar M, Vergara P, Flores-Rodriguez P, Aguilar-Roblero R et al. Growth arrest specific 1 (GAS1) is abundantly expressed in the adult mouse central nervous system. J Histochem Cytochem 2013; 61(10): 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019; 139(10): e56–e528. [DOI] [PubMed] [Google Scholar]
- 68.Nunes J, Jean-Louis G, Zizi F, Casimir GJ, von Gizycki H, Brown CD et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc 2008; 100(3): 317–322. [DOI] [PubMed] [Google Scholar]
- 69.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep 2007; 30(9): 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barfield R, Wang H, Liu Y, Brody JA, Swenson B, Li R et al. Epigenome-wide association analysis of daytime sleepiness in the Multi-Ethnic Study of Atherosclerosis reveals African-American-specific associations. Sleep 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr 2007; 18(4): 241–247. [PMC free article] [PubMed] [Google Scholar]
- 72.Delto CF, Heisler FF, Kuper J, Sander B, Kneussel M, Schindelin H. The LisH motif of muskelin is crucial for oligomerization and governs intracellular localization. Structure 2015; 23(2): 364–373. [DOI] [PubMed] [Google Scholar]
- 73.Heisler FF, Loebrich S, Pechmann Y, Maier N, Zivkovic AR, Tokito M et al. Muskelin regulates actin filament- and microtubule-based GABA(A) receptor transport in neurons. Neuron 2011; 70(1): 66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogawa Y, Kakumoto K, Yoshida T, Kuwako KI, Miyazaki T, Yamaguchi J et al. Elavl3 is essential for the maintenance of Purkinje neuron axons. Sci Rep 2018; 8(1): 2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jackson CL, Patel SR, Jackson WB 2nd, Lutsey PL, Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep 2018; 41(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


