Abstract
Introduction: Medical comorbidities (MCs) represent a significant burden in terms of more frequent hospitalizations and overall lower life expectancy among people with severe mental disorders, such as schizophrenia and related psychotic disorders (SZ) and bipolar disorder (BD). The present article aims to compare the prevalence of MCs and to examine the associated characteristics as marital status, job occupation, level of education, and living arrangements, between BD and SZ patients.
Methods: One-hundred-eight-one patients with MCs (85/47% had BD and 96/53% had SZ) were recruited retrospectively from the Acute Inpatients Psychiatry Unit of Policlinico Tor Vergata, Rome, between January-2017 and December-2020. MCs were: cardiovascular diseases (CVD), bacterial infections, mycoses, viral diseases, neoplasms, musculoskeletal, respiratory tract, urological and male genital, gynecological, neurological, gastrointestinal, metabolic syndrome, nutritional, and metabolic diseases.
Results: BD had more MC than SZ (36.2 vs. 28.2%, respectively, p = 0.04). CVD and metabolic MC were more common among BD (51.8 vs.34.4%; 51.8 vs.35.3%; p = 0.018; p = 0.039; respectively), while viral diseases were more frequent in SZ (13.5 vs.3.5%, p = 0.035). Hypertension was common in both psychiatric illnesses (81.8% BD vs. 65.6% SZ, p = 0.18). Obesity was the most frequent metabolic disease in both BD and SZ (75% BD vs. 73.5% SZ, p = 0.91), followed by diabetes mellitus (52.3% BD vs. 55.9% SZ, p = 0.93), metabolic syndrome (54.5% BD vs. 47.1% SZ, p = 0.67) and dysthyroidism (47.7% BD vs. 25.7% SZ, p = 0.093). After performing a binary logistic regression analysis, only two MCs showed a statistically significant association: patients with SZ had an OR of 2.01 [CI 95% (1.00–4.01)] for CVD compared to BD; on the other hand, patients with BD had an OR of 16.57 [CI 95% (3.58–76.77)] for gynecological diseases compared to SZ patients.
Conclusions: MCs are common among people with severe mental illness, especially CVD and metabolic diseases, highlighting the need for a more collaborative relationship between general medical providers and psychiatrists.
Keywords: bipolar disorder, schizophrenia, cardiovascular diseases, dysmetabolic diseases, viral diseases, medical comorbidities
Introduction
Medical comorbidity (MC) is defined as the co-occurrence of multiple diseases within the same person, at the same time, regardless of the causality that links them (1). People with severe mental illness, such as bipolar disorder (BD) or schizophrenia and related psychotic disorders (SZ), have a higher prevalence of MC and a greater mortality rate, losing up to 4 years of life compared to the general population. Furthermore, the combination of mental and medical disorders is linked to an increased functional impairment, greater symptom burden, and, eventually, to a higher weight on healthcare costs (2).
A wide range of risk factors may concur to cause MCs. These might mostly be ascribed to psychiatric medications side effects (mood stabilizers, antidepressants, antipsychotics), lifestyle choices (diet, smoking, alcohol, substance abuse, exercise), and social marginalization (3). Furthermore, a consistent number of authors focused on a metabolic-inflammatory-mood pathway, suggesting that an immune system dysfunction may increase the morbidity and mortality of these patients (4). In particular, reduced levels of glutathione and antioxidant defense have been observed in first-onset psychosis (5).
Recent studies demonstrate also an interesting link between MCs and the complex and dynamical set of microorganisms, collectively referred to as the microbiota. Microbiota can have a pathogenic role in psychiatric disorders because of its modulatory action in the bidirectional communication system between the brain and intestine (widely known as the gut-brain-microbiota axis) (6). Although, there are no studies that analyze possible alterations of the microbiota during a manic episode, there is some evidence showing an altered permeability of the gastrointestinal barrier (7, 8), which can cause compositional variations of the intestinal microbiota.
Kessler et al. reported that the co-occurrence of medical and mental disorders has to be considered as the rule rather than the exception, presenting significant clinical and public health concerns (9). Similarly, Hert et al. assumed that different physical conditions are observed with increased frequency, such as stroke, hypertension, obesity, diabetes mellitus (DM), human immunodeficiency virus (HIV), and hepatitis (2). Patients with SZ are significantly more prone to have at least one MC compared to the general population, ranging from hypothyroidism to chronic obstructive pulmonary disease, diabetes, viral diseases, fluid/electrolyte disorders, and nicotine abuse/dependence (10). On the other hand, Sinha et al. in their comprehensive review found a high prevalence of MCs involving multiple organs in patients with BD, suggesting screening each patient for the added risk of correlated diseases like migraine and asthma (11).
Since MCs represent a significant burden in terms of more frequent hospitalizations and overall lower life expectancy (2), there is an urgent need to better define such correlation in patients with severe mental illnesses. For this reason, the present article aims to compare the prevalence of MC between BD and SZ and examine the associated characteristics such as social status, job occupation, level of education, and living status.
Materials and Methods
This was a single-center, double-arm, retrospective cohort study. Nine hundred and sixty-five patients were admitted to the tertiary care center for treatment of the psychiatric disorder (Psychiatry Unit, Policlinico Tor Vergata, Rome, Italy) between January 2017 (the starting day of inpatient acute psychiatric service) and December 2020. Inclusion criteria were: diagnosis of BD or SZ [according to the International Classification of Disease, 9th Revision (ICD-9) (12)] and presence of MCs. Exclusion criteria were: comorbidity of psychiatric disorders and pregnancy. Diagnoses of MCs were made using the ICD-9. The presence of metabolic syndrome (MetS) was considered only if defined according to The US National Cholesterol Education Programme Adult Treatment Panel III (ATP III), meaning of at least three of the following criteria: waist size of at least 102 cm for men and at least 88 cm for women; triglycerides of at least 150 mg/dl; HDL cholesterol level of <40 mg/dl for men and <50 mg/dl for women; blood pressure of more than 130 mmHg systolic or 85 mmHg diastolic fasting glucose of more than 100 mg/dl (13).
Patients who did more than one recovery within the study timeframe were considered unitary. 181/965 (18.8%) patients met the inclusion criteria and were divided into two arms: 85/181 (47%) were classified as BD and 96/181 (53%) as SZ, with at least one MC.
The data were collected by three psychiatrists at the time of the discharge from the original medical records on the medical informative system.
Baseline sociodemographic data, such as gender, age, employment status (i.e., employed -. homemakers and students - or unemployed), educational level (i.e., elementary school, middle school, high school, university), and marital status (i.e., married or cohabiting, divorced, widowed, single) were collected. Medical comorbidities, such as cardiovascular diseases (CVD), bacterial infections and mycosis, viral diseases, neoplasms, musculoskeletal diseases, respiratory tract diseases, urological and genital diseases, gynecological diseases, neurological diseases, gastrointestinal diseases, nutritional and metabolic diseases were recorded. The rate of readmission was aggregated between the two groups.
Statistical Analysis
The Kolmogorov-Smirnov test for the goodness of fit was performed to assess the normality of the distribution of all the tested variables. Continuous normal variables were expressed as mean ± standard deviation. Continuous non-normal variables were expressed as median and 95% confidential interval (CI). Demographic and clinical characteristics were analyzed using chi-square tests with Yates' correction and Odds Ratio for categorical variables and Student's t-tests or Wilcoxon signed-rank test for continuous variables as appropriate. Logistic regression analyses were performed using gender, age, educational level, employment status, and diagnostic group (BD and SZ) as covariates; the dependent variables were MCs. Statistical analysis was performed using MedCalc 18.2.1 software (MedCalc, Mariakerke, Belgium). P < 0.05 were considered statistically significant, and all p-values were calculated using a two-tailed significance level.
Results
181/965 (18.8%) patients (age mean: 49 ± 13 years; women: 51.4%) met inclusion criteria and were divided into two arms: 85/181 (47%) BD with MCs (age mean: 52.2 ± 13 years; women: 57.7%) and 96/181 (53%) SZ with MC (age mean: 45.6 ± 13 years; women: 45.8%).
Sociodemographic Characteristic
Parental-family living was more common in SZ rather than BD (51.0 vs.21.2%; p < 0.001), like also unemployment (90.6 vs.69.4.2%; p = 0.0006) and single-marital status (64.5 vs.30.6%; p < 0.001). Regarding the educational level, most people with BD had higher school license (40%), while in people affected by SZ middle school license was more prevalent (55.2%). The complete sociodemographic data on the 181 patients was given in Table 1.
Table 1.
Sociodemographic information of the sample.
|
BD
(n = 85) |
SZ
(n = 96) |
Statistics | |||
|---|---|---|---|---|---|
| Chi-square * | p-value ** | Odds Ratio (CI 95%) | |||
| Age (SD) | 52.2 (13.0) | 45.6 (13.0) | |||
| Gender | |||||
| Women | 49 (57.7%) | 44 (45.8%) | 1.12 | 0.29 | 1.6 (0.91–2.95) |
| Men | 36 (42.4%) | 52 (54.2%) | |||
| Employment status | |||||
| Currently employed | 26 (30.6%) | 9 (9.4%) | 11.68 | 0.0006 | 4.26 (1.86–9.74) |
| Not currently employed | 59 (69.4%) | 87 (90.6%) | |||
| Marital Status | |||||
| Single | 26 (30.6%) | 62 (64.6%) | 19.52 | <0.0001 | 0.24 (0.13–0.49) |
| Married/Cohabiting | 38 (44.7%) | 18 (18.8%) | 9.9 | 0.016 | 3.5 (1.80–6.83) |
| Divorced | 15 (17.6%) | 15 (15.6%) | 0.03 | 0.87 | 1.16 (0.53–2.54) |
| Widowed | 6 (7.1%) | 1 (1%) | 2.92 | 0.087 | 7.22 (0.85–61.2) |
| Living arrangements | |||||
| Independent | 25 (29.4%) | 29 (30.2%) | 0.002 | 0.96 | 0.96 (0.51–1.82) |
| Living with parents | 18 (21.2%) | 49 (51.1%) | 15.99 | 0.0001 | 0.26 (0.13–0.50) |
| Living with own family | 42 (49.4%) | 17 (17.7%) | 17.77 | <0.0001 | 4.54 (2.31–8.91) |
| Educational level | |||||
| Elementary school | 7 (8.2%) | 12 (12.5%) | 0.48 | 0.49 | 0.63 (0.24–1.68) |
| Middle school | 32 (37.6%) | 53 (55.2%) | 4.9 | 0.027 | 0.49 (0.27–0.89) |
| High school | 34 (40.1%) | 26 (27.1%) | 2.84 | 0.092 | 1.80 (0.96–3.35) |
| University | 12 (14.1%) | 5 (5.2%) | 3.22** | 0.073 | 2.99 (1.01–8.88) |
degree of freedom = 1.
Yates correction was applied due to the low numerosity of the sample size.
Medical Comorbidities
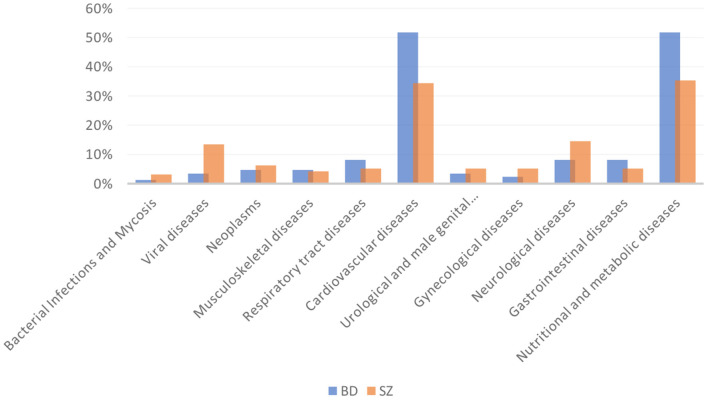
BD patients had an overall higher prevalence of MCs compared to SZ (36.2 vs.28.2%, respectively, p = 0.04). In particular, CVD and metabolic diseases were more frequent among BD than SZ (51.8 vs.34.4%; 51.8 vs.35.4%; p = 0.018; p = 0.039; respectively). The most common CVD in both psychiatric illnesses was hypertension (defined by a clinic systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg) (36/44 [81.8%] BD vs. 21/33 [63.6%] SZ). The prevalence of viral diseases was higher among SZ than BD (13.5 vs.3.5%, p = 0.035). Among nutritional and metabolic diseases, obesity was the most frequent disease in both BD and SZ (75% BD vs. 73.5% SZ), followed by DM (52.3%BD vs. 55.9% SZ), MetS (54.5% BD vs. 47.1% SZ), and dysthyroidism (47.7% BD vs. 25.7% SZ). No significant differences were found among the other MCs between the two arms of the study. The prevalence of MCs by category is shown in Table 2 and Figure 1. The incidence of specific medical illnesses is shown in Table 3.
Table 2.
Number of medical comorbidities in BD and SZ.
| BD | SZ | Statistics | |||
|---|---|---|---|---|---|
| Chi-square * | p-value | Odds Ratio (CI 95%) | |||
| Bacterial Infections and Mycosis | 1 (1.2%) | 3 (3.1%) | 0.15 | 0.70 | 0.37 (0.038–3.62) |
| Viral diseases | 3 (3.5%) | 13 (13.5%) | 4.43 | 0.035 | 0.23 (0.064–0.85) |
| Neoplasms | 4 (4.7%) | 6 (6.3%) | 0.016 | 0.90 | 0.74 (0.20–2.72) |
| Musculoskeletal diseases | 4 (4.7%) | 4 (4.2%) | 0.035 | 0.85 | 1.14 (0.28–4.69) |
| Respiratory tract diseases | 7 (8.2%) | 5 (5.2%) | 0.27 | 0.61 | 1.63 (0.50–5.35) |
| Cardiovascular diseases | 44 (51.8%) | 33 (34.4%) | 5.55 | 0.018 | 2.05 (1.13–3.73) |
| Urological and genital diseases | 3 (3.5%) | 5 (5.2%) | 0.035 | 0.85 | 0.67 (0.15–2.87) |
| Gynecological diseases | 2 (2.4%) | 5 (5.2%) | 0.37 | 0.54 | 0.44 (0.083–2.32) |
| Neurological diseases | 7 (8.2%) | 14 (14.6%) | 1.21 | 0.27 | 0.52 (0.20–1.37) |
| Gastrointestinal diseases | 7 (8.2%) | 5 (5.2%) | 0.27 | 0.61 | 1.63 (0.50–5.35) |
| Nutritional and metabolic diseases | 44 (51.8%) | 34 (35.4%) | 4.27 | 0.039 | 1.96 (1,08–3,55) |
degree of freedom = 1.
Figure 1.
Percentage of medical comorbidities in BD and SZ.
Table 3.
Incidence of specific medical illnesses.
| BD | SZ | |
|---|---|---|
| Hypertension | 36 (42.4%) | 21 (21.9%) |
| Obesity | 33 (38.8%) | 25 (26%) |
| Diabetes | 23 (27.1%) | 19 (19.8%) |
| Metabolic syndrome | 24 (28.2%) | 16 (16.7%) |
| Dysthyroidism | 21 (24.7%) | 9 (9.4%) |
| HIV | 2 (2.4%) | 5 (5.2%) |
After performing a binary logistic regression analysis, using age, gender, educational level, employment status, and diagnostic group (BD or SZ) as covariates, only two MCs showed a statistically significant association. In particular, patients with SZ had an OR of 2.01 [CI 95% (1.00–4.01)] for CVD compared to BD; on the other hand, patients with BD had an OR of 16.57 [CI 95% (3.58–76.77)] for gynecological diseases compared to SZ patients. Results by category are shown in Table 4.
Table 4.
Logistic regression results.
| BD coefficient | SZ coefficient | Statistics | ||
|---|---|---|---|---|
| p-value | Odds Ratio (CI 95%) | |||
| Bacterial Infections and Mycosis | 0.16 | – | 0.90 | 1.18 (0.08–16.84) |
| Viral diseases | 1.09 | – | 0.14 | 2.98 (0.71–12.57) |
| Neoplasms | 0.045 | – | 0.95 | 1.05 (0.22–5.05) |
| Musculoskeletal diseases | 0.0022 | – | 1.00 | 1.023 (0.21–4.75) |
| Respiratory tract diseases | – | 0.53 | 0.43 | 1.69 (0.46–6.20) |
| Cardiovascular diseases | – | 0.70 | 0.049 | 2.01 (1.00–4.01) |
| Urological and genital diseases | 0.94 | – | 0.29 | 2.57 (0.45–14.77) |
| Gynecological diseases | 2.81 | – | 0.0003 | 16.57 (3.58–76.77) |
| Neurological diseases | 0.30 | – | 0.59 | 1.35 (0.45–4.03) |
| Gastrointestinal diseases | – | 0.42 | 0.57 | 1.53 (0.36–6.57) |
| Nutritional and metabolic diseases | 0.50 | – | 0.14 | 0.61 (0.32–1.18) |
Discussion
Parental leaving, unemployment and single-marital status were more common among SZ than BD patients (51.0 vs.21.2%; 90.6 vs.69.4.2%; 64.5 vs.30.6%). Impairment for SZ patients in areas of intimate relationships, occupational activity, and living situations was described in different other studies (14, 15).
The present study findings are consistent with the results of Brekke, which reported that people with SZ are having impairment in vocational ability, lack of adequate social competence, and necessary social skills in the workplace (16).
On the contrary, the episodic course of illness combined with above-average educational skills would explain why patients with BD were more motivated to seek treatment, as well as more able to engage in the treatment process compared to SZ individuals (17). This results in a stronger incentive to maintain contact with the services and therefore achieve a better quality of life.
The prevalence of overall MCs among people with BD (36.2% BD vs. 28.2% SZ, p = 0.04) was slightly inferior compared to those from prior studies, whose ranges were of almost 40–50%, with CVD, metabolic, respiratory, and musculoskeletal diseases being the medical categories mostly involved (18, 19). Although people with mental disorders have a higher percentage of risk factors for cancer (smoking, obesity, reduced physical activities), some studies have shown the paradoxical finding that, apparently, they also have a lower risk of cancer (20).
This numerical divergence could emerge from the differences in exclusion criteria and the study design. In particular, this might be related to the comparison between acute inpatients and chronic lithium-dependent outpatients and between observational and randomized controlled trials (18).
A reason for explaining the higher prevalence of MCs in BD compared to SZ patients could be found, other than the already known adverse effects of unhealthy lifestyle and psychotropic medications, in a new link between common MCs and pathological pathways in BD. Studies have shown that in BD inflammation and oxidative stress pathways might play a key role in the co-occurrence of neuroprogression and MCs (21, 22).
Furthermore, patients with SZ are very often affected by thought disorders and executive dysfunctions, so that they become unable to look after their own health. This weighs not only on their psychological conditions but also causes them to procrastinate the diagnosis of their “hidden” medical illnesses (3).
As previously shown by other studies, CVD was the most common MCs among both BD and SZ patients (51.8 vs.34.4%; p = 0.001). This may be explained by the fact that, in addition to the conventional CVD risk factors (i.e., obesity, smoking, raised blood cholesterol), other predisposing factors, such as physical inactivity, unhealthy diet, and low socioeconomic status, are very common among these patients.
Evidence from previous studies reported increased rates of “any cardiovascular illness” such as angina, stroke, myocardial infarction, hypertension, in patients with BD (23–25). For instance, McEvoy et al. found out that coronaropathy was 2–3.6-fold higher in patients with SZ (26), while cerebrovascular diseases seemed to be 2.1–3.3-fold higher in patients with BD compared to the general population (27, 28). To this end, it is easy to understand how almost 25% of patients with BD and 33% of patients with SZ die for CVD (29).
In line with this, Puntervold et al. showed in an autoptic study the prevalence of undiagnosed diseases, with cardiovascular illnesses being more frequent in SZ patients compared to controls (30).
The authors pointed out how people with such psychiatric conditions had difficulties in taking care of themselves and potentially explaining the higher prevalence of diagnosed MC in BD in the present series.
Among CVD, hypertension was the most frequent disease in both BD and SZ (36/44 [81.8%] vs. 21/33 [63.6%]). This finding is in line with previous studies showing the prevalence of CVD in severe mental illnesses ranging 35%-61% in BD vs. 19%-58% in SZ (31, 32).
In a very large study involving 25,339 people with BD and a control population of 113,698 individuals, Johannessen et al. showed an increased rate of hypertension among those with BD compared with both the control population and subjects with SZ (33). Interestingly, the authors highlighted how medications might surely have impacted the development of hypertension. However, the prevalence of MCs was also to be correlated to an unhealthy lifestyle and high levels of sedentary behavior, abuse of substance or alcohol, and mental health symptoms such as memory impairment, difficulties in taking appointments with the doctor, reduce motivation. Furthermore, clinicians often had wrong beliefs about these people, underestimating their capabilities of taking care of themselves (34). Regarding this, Chen et al. found out that people suffering from SZ, who were employed or lived in a highly urbanized area, had a decreased risk of sudden cardiac death (35).
For these reasons, a more consistent screening for those with BD and SZ should be implemented as quickly as possible, in order to prevent worse cardiovascular scenarios from occurring and therefore to reduce the overall mortality.
In the present series, patients with BD have a higher prevalence of endocrine, nutritional and metabolic diseases (51.8 vs.35.4%; p = 0.039). Consistent with our findings, literature reports how obesity, DM, MetS, and dysthyroidism may be associated with multiple risk factors, including unhealthy lifestyles (36, 37) psychotropic medications (particularly atypical antipsychotics, affecting between 15 and 72% of patients) (38–40), and abuse of recreational drugs.
When it comes to BD, different studies suggest that these patients tend to be more overweight than people with SZ and consequently have a higher risk of MCs, only because of their medical treatment (e.g., lithium, anticonvulsants, etc.) but also the possible comorbid binge-eating disorders, depressive episodes, low exercise and a diet full of carbohydrate (41–43). In this regard, more recent works show that patients with elevated Body Mass Index (BMI) have been associated with more frequent and longer depressive episodes (42, 44), poor response to treatment suicidal ideation, and a history of suicide attempts (45, 46) comorbid anxiety disorders (44, 47), and functional impairment (47, 48).
Obesity is also associated with MetS, which confers a 5- to 6-fold increased risk of developing DM and a 3- to 6-fold increased risk of coronary heart diseases. Among SZ, percentages vary between 19.4 and 68%, depending on age, gender, MetS criteria, and ethnicity (2) Mitchell et al., in a meta-analysis of 77 publications, showed a MetS prevalence of 32.5% among adults with SZ (49).
Furthermore, different studies regarding MetS and BD, reported a percentage of 22–30% (50–52). Comparing the data obtained with the Italian literature, no statistically significant differences were reported. Rossi et al. reported MetS prevalence rates in subjects with BD and SZ of 36.4% and 30.6%, respectively (53). Similarly, Maina et al. highlighted the presence of MetS in 27.9% in 185 patients with BD (54).
Important in the prevalence of Mets are lifestyle and behavioral patterns, such as cigarette smoking, substance abuse, physical inactivity, and overeating, which lead to insulin resistance and, frequently, in overweight. Also, specific psychotropic medications (increasing MetS dysregulations) and genetic vulnerability, and pathophysiological mechanisms play a role in MetS. In fact, in a study carried out by Maina et al. on 70 subjects with BD, a significant increase in the MetS prevalence, over a period of 2 years, from 28.6 to 44.3%, was found out, especially in patients treated with atypical antipsychotics (55).
Regarding diabetes mellitus, both in people with BD and with SZ, the prevalence is 2- to 3-fold higher compared to the general population (56, 57). The reason for the increased risk of DM in these patients is multifactorial and includes medical treatment, genetic factors, and lifestyle. Among psychotropic medications, in addition to second-generation antipsychotics olanzapine and clozapine (58, 59), lithium and valproic acid are known to induce weight gain and dysregulation in glucose metabolism (43, 60).
Furthermore, less than half of people with BD and DM in the National Health and Nutrition Examination Survey were able to reach the glycemic control goal, considering that BD patients have high rates of treatment non-adherence and recurrence (61).
In respect to thyroid diseases, our sample is in line with findings reported in the literature, showing a higher prevalence of thyroid hypofunction among patients with BD than patients with SZ. The prevalence of dysthyroidism among people with BD might surely be related to lithium's direct antithyroid effects, whose prevalence ranges from 14 to 17 % (62). For instance, Joffe et al. evaluated 42 patients with BD who received at least 3 months of lithium treatment: 19% of them required thyroid replacement or showed evidence of subclinical hypothyroidism (63). As Lambert et al. highlighted, the average 4-year risk of developing hypothyroidism was higher for lithium (8.8%) compared to quetiapine (8.3%), lamotrigine (7.1%), valproate (7.02%), aripiprazole (7%), carbamazepine (6.7%), risperidone (6.5%), olanzapine (6.4%), and oxcarbazepine (6.3%). Nevertheless, the risk of hypothyroidism has to be considered concrete regardless of the treatment. Recent evidence shows in fact that impaired levels of thyroid hormone were evident across all BD medications, highlighting the need for a thyroid screen irrespective of the medication used (64).
In our sample patients with SZ have a high prevalence of viral diseases (13.5% SZ vs. 3.5%BD, p = 0.035). In particular, HIV was the most prevalent [5/13 (35.5%)], followed by hepatitis B virus [4/13 (30.8%)] and hepatitis C virus [4/13 (30.8%)]. This could be linked to the higher frequency of substance abuse, sexual risky behaviors (e.g., sex without a condom, trading sex for money and drugs), and a reduced knowledge about HIV-related safety concerns (65). Furthermore, patients with both HIV and psychiatric conditions are more at risk of developing metabolic issues, as, other than antipsychotic therapies, also antiretroviral medicines may enhance the occurrence of metabolic abnormalities and ultimately of CVD (66).
After performing the binary logistic regression analysis, only two MCs showed a statistically significant association.
CVD predicts the diagnosis of SZ (OR 2.01), independently from socio-demographic characteristics. C VD increased the mortality rate of SZ both including personal-related factors (metabolic syndrome, sedentary behavior, tobacco smoking) and drug-related factors (67), with a 90% higher mortality rate for CVD comparing with the general population (68). However, guidelines still do not advocate for a routine check of CVD in these patients (67). The present study suggests that screening for CVD in SZ may be helpful for early diagnosis and potentially for reducing mortality.
Gynecological diseases significantly predict the diagnosis of bipolar disorder (OR 16.57). Although this data may be affected by gender distribution, our spread is quite similar (female BD 57.7% vs. SZ 45.8%). Moreover, endometriosis and polycystic ovary syndrome (PCOS) are more frequent in BD (69).
Endometriosis affects nearly 10–15% of women and is one of the main causes of female infertility (70). As reported by Pope et al. in a review, 16.7% of the women with endometriosis met the criteria for one of the forms of bipolar disorder, compared to only 2.7% of women in the comparison group (71).
Endometriosis has been reported to have significant psychosocial implications, such as depression, increased stress, and anxiety, which can also influence its clinical evaluation and the success of interventions (71).
PCOS is a common disorder, affecting up to 15–20% of reproductive age women, which manifests as hirsutism, menstrual irregularity, subfertility (72). A large amount of literature has shown that women with PCOS have a poor quality of life (73–75) and different medical disorders such as elevated testosterone level (in the cerebrospinal fluid of SZ patients) (76), obesity (77), insulin resistance (78) and inflammation (72). Nevertheless, further research is needed to strengthen these findings.
This study suffers from some limitations—notably related to its retrospective design. Firstly, since many health professionals have contributed to the collection of medical records, patients' clinical history might have been taken with less strict criteria than those achievable with a prospective design. Secondly, the present work is a single-center study. This may bias the induction of our results to other centers. Thirdly, psychiatric comorbidities that could also weigh on the overall clinical picture, such as substance use disorder, were not considered as the study was focused only on medical comorbidities. Moreover, although some patients had more than one medical comorbidity, they were considered separately and only once in the statistical analysis to make results more understandable and applicable. Since we did not set medical variables, such as DM or nutritional and metabolic diseases, as dependent variables in the logistic regression, we cannot exclude for these latter to also predict CVDs in people with BD and SZ. Lastly, this is a cross-sectional study, and the lack of follow-up data does not allow to make any causal inferences. For these reasons, extra caution must be taken when generalizing these findings.
Conclusions
In summary, regarding sociodemographic features, parental-family living, unemployment, and single-marital status were more common among patients with SZ. Thus, it seems essential to implement an action plan that seeks to promote the autonomy of these individuals and their insertion into society.
Overall MCs were more common among BD patients, especially cardiovascular and dysmetabolic diseases, while viral diseases had a higher prevalence among SZ patients. Both CVD and gynecological diseases predict SZ and BD, respectively. Medications, lifestyle choices (such as unhealthy diet, smoking, alcohol and substance abuse), and social marginalization could be considered as the most common risk factors. Thus, psychiatrists and primary healthcare providers should promptly identify these clinical risk factors, in order to give patients an appropriate, integrative and complete medical treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AM: manuscript drafting, patients information collection, and statistical analysis. GD, AS, and CN: manuscript critical revision. TJ and RS: patients information collection and literature review. EB: patients information collection. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- MCs
Medical comorbidities
- SZ
Schizophrenia and related psychotic disorders
- BD
Bipolar disorder
- CVD
Cardiovascular diseases
- DM
Diabetes mellitus
- MetS
Metabolic Syndrome
- HIV
human immunodeficiency virus
- PCOS
Polycystic ovary syndrome.
References
- 1.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. (2009) 7:357–63. 10.1370/afm.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hert DEM, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. (2011) 10:52–77. 10.1002/j.2051-5545.2011.tb00014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey DA, Rodriguez M, Northcott C, Vickar G, Shihabuddin L. Schizophrenia: medical illness, mortality, and aging. Int J Psychiatry Med. (2011) 41:245–51. 10.2190/PM.41.3.c [DOI] [PubMed] [Google Scholar]
- 4.Rosenblat JD, McIntyre RS. Are medical comorbid conditions of bipolar disorder due to immune dysfunction? Acta Psychiatr Scand. (2015) 132:180–91. 10.1111/acps.12414 [DOI] [PubMed] [Google Scholar]
- 5.Ventriglio A, Belloma A, Favale D, Bonfitto I, Vitrani G, Sabatino DD, et al. Oxidative stress in the early stage of psychosis. Curr Top Med Chem. (2021). 10.2174/1568026621666210701105839 [DOI] [PubMed] [Google Scholar]
- 6.Rogers GB, Keating DJ, Young RL, Wong ML, LIcinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. (2016) 21:738–48. 10.1038/mp.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilic F, Işik U, Demirdaş A, Doguç DK, Bozkurt M. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J Affect Disord. (2020) 266:37–42. 10.1016/j.jad.2020.01.117 [DOI] [PubMed] [Google Scholar]
- 8.Rudzki L, Szulc A. “Immune gate“ of psychopathology-the role of gut derived immune activation in major psychiatric disorders. Front Psychiatry. (2018) 9:205. 10.3389/fpsyt.2018.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler RC, Berglund P, Chiu WT, Demler O, Heeringa S, Hiripi E, et al. The US National Comorbidity Survey Replication (NCS-R): design and field procedures. Int J Methods Psychiatr Res. (2004) 13:69–92. 10.1002/mpr.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med. (2006) 21:1133–7. 10.1111/j.1525-1497.2006.00563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha A, Shariq A, Said K, Sharma A, Jeffrey Newport D, Salloum IM. Medical Comorbidities in Bipolar Disorder. Curr Psychiatry Rep. (2018) 20:36. 10.1007/s11920-018-0897-8 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization(WHO) . The ICD-10 classification of mental and behavioural disorders. Geneva: World Health Organization. (1993). [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. (2005) 4:198–203. 10.1161/CIRCULATIONAHA.105.169405 [DOI] [PubMed] [Google Scholar]
- 14.Saarni SI, Viertiö S, Perälä J, Koskinen S, Lönnqvist J, Suvisaari J. Quality of life of people with schizophrenia, bipolar disorder and other psychotic disorders. Br J Psychiatry. (2010) 197:386–94. 10.1192/bjp.bp.109.076489 [DOI] [PubMed] [Google Scholar]
- 15.Guedes de Pinho LM, Pereira AMS, Chaves C. Quality of life in schizophrenic patients: the influence of sociodemographic and clinical characteristics and satisfaction with social support. Trends Psychiatry Psychother. (2018) 40:202–9. 10.1590/2237-6089-2017-0002 [DOI] [PubMed] [Google Scholar]
- 16.Brekke JS, Raine A, Ansel M, Lencz T, Bird L. Neuropsychological and psychophysiological correlates of psychosocial functioning in schizophrenia. Schizophr Bull. (1997) 23:19–28. 10.1093/schbul/23.1.19 [DOI] [PubMed] [Google Scholar]
- 17.Morgan VA, Mitchell PB, Jablensky AV. The epidemiology of bipolar disorder: sociodemographic, disability and service utilization data from the Australian National Study of Low Prevalence (Psychotic) Disorders. Bipolar Disord. (2005) 7:326–37. 10.1111/j.1399-5618.2005.00229.x [DOI] [PubMed] [Google Scholar]
- 18.Kemp DE, Sylvia LG, Calabrese JR, Nierenber AA, Thase ME, Reilly-Harrington NA, et al. General medical burden in bipolar disorder: findings from the LiTMUS comparative effectiveness trial. Acta Psychiatr Scand. (2014) 129:24–34. 10.1111/acps.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber NS, Fisher JA, Cowan DN, Niebuhr DW. Psychiatric and general medical conditions comorbid with bipolar disorder in the National Hospital Discharge Survey. Psychiatr Serv. (2011) 62:1152–8. 10.1176/ps.62.10.pss6210_1152 [DOI] [PubMed] [Google Scholar]
- 20.Momen NC, Plana-Ripoll O, Agerbo E, Benros ME, Børglum AD, Christensen MK, et al. Association between mental disorders and subsequent medical conditions. N Engl J Med. (2020) 382:1721–31. 10.1056/NEJMoa1915784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magalhaes PV, Kapczinski F, Nierenber AA, Deckersbach T, Weisinger D, Dodd S, et al. Illness burden and medical comorbidity in the Systematic Treatment Enhancement Program for Bipolar Disorder. Acta Psychiatr Scand. (2012) 125:303–8. 10.1111/j.1600-0447.2011.01794.x [DOI] [PubMed] [Google Scholar]
- 22.Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. (2011) 35:804–17. 10.1016/j.neubiorev.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Martin DJ, Ul-Haq Z, Nicholl BI, Cullen B, Evans J, Gill JM, et al. Cardiometabolic disease and features of depression and bipolar disorder: population-based, cross-sectional study. Br J Psychiatry. (2016) 208:343–51. 10.1192/bjp.bp.114.157784 [DOI] [PubMed] [Google Scholar]
- 24.Wageck AR, Torres FS, Gama CS, Martins DS, Scotton E, Reckziegel R, et al. Cardiovascular risk and bipolar disorder: factors associated with a positive coronary calcium score in patients with bipolar disorder type 1. Braz J Psychiatry. (2018) 40:163–8. 10.1590/1516-4446-2017-2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu SI, Chen SC, Liu SI, Sun FJ, Juang JJ, Lee HC, et al. Relative risk of acute myocardial infarction in people with schizophrenia and bipolar disorder: a population-based cohort study. PLoS ONE. (2015) 10:e0134763. 10.1371/journal.pone.0134763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. (2005) 80:45–53. 10.1016/j.schres.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 27.Osborn DPJ, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom's General Practice Rsearch Database. Arch Gen Psychiatry. (2007) 64:242–9. 10.1001/archpsyc.64.2.242 [DOI] [PubMed] [Google Scholar]
- 28.De Hert M, Schreurs V, Sweers K, Van Eyck D, Hanssens L, Sinko S, et al. Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr Res. (2008) 101:295–303. 10.1016/j.schres.2008.01.028 [DOI] [PubMed] [Google Scholar]
- 29.Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol. (2021) 18:136–45. 10.1038/s41569-020-00463-7 [DOI] [PubMed] [Google Scholar]
- 30.Puntervold OE, Kruckow L, Banner J. Undiagnosed disease in decedents with and without schizophrenia: an autopsy-based case-control study. Nord J Psychiatry. (2021):1–7. 10.1080/08039488.2021.1905877 [DOI] [PubMed] [Google Scholar]
- 31.Correll CU, Frederickson AM, Kane JM, Manu P. Equally increased risk for metabolic syndrome in patients with bipolar disorder and schizophrenia treated with second-generation antipsychotics. Bipolar Disord. (2008) 10:788–97. 10.1111/j.1399-5618.2008.00625.x [DOI] [PubMed] [Google Scholar]
- 32.Holt RI. Diabetes and risk. Diabet Med. (2017) 34:459. 10.1111/dme.13343 [DOI] [PubMed] [Google Scholar]
- 33.Johannessen L, Strudsholm U, Foldager L, Munk-Jørgensen P. Increased risk of hypertension in patients with bipolar disorder and patients with anxiety compared to background population and patients with schizophrenia. J Affect Disord. (2006) 95:13–7. 10.1016/j.jad.2006.03.027 [DOI] [PubMed] [Google Scholar]
- 34.Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. (2010) 51:458–65. 10.1016/S0033-3182(10)70737-4 [DOI] [PubMed] [Google Scholar]
- 35.Chen PH, Tsai SY, Pan CH, Chang HM, Chen YL, Su SS, et al. Age effect on incidence, physical, and psychiatric comorbidity for sudden cardiac death in schizophrenia: effet de l'age sur l'incidence, la comorbidite physique et psychiatrique de la mort cardiaque subite dans la schizophrenie. Can J Psychiatry. (2021) 66:367–75. 10.1177/0706743720948429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elmslie JL, Mann JI, Silverstone JT, Williams SM, Romans SE. Determinants of overweight and obesity in patients with bipolar disorder. J Clin Psychiatry. (2001) 62:486–91. 10.4088/JCP.v62n0614 [DOI] [PubMed] [Google Scholar]
- 37.Vancampfort D, Knapen J, Probst M, Scheewe T, Remans S, De Hert M. A systematic review of correlates of physical activity in patients with schizophrenia. Acta Psychiatr Scand. (2012) 125:352–62. 10.1111/j.1600-0447.2011.01814.x [DOI] [PubMed] [Google Scholar]
- 38.Henderson DC. Weight gain with atypical antipsychotics: evidence and insights. J Clin Psychiatry. (2007) 68 Suppl 12:18–26. [PubMed] [Google Scholar]
- 39.Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. (2012) CD008016. 10.1002/14651858.CD008016.pub2 [DOI] [PubMed] [Google Scholar]
- 40.Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. (2010) 123:225–33. 10.1016/j.schres.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McElroy SL, Frye MA, Suppes T, Dhavale D, Keck PEJr, Levericj GS, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. (2002) 63:207–13. 10.4088/JCP.v63n0306 [DOI] [PubMed] [Google Scholar]
- 42.Fagiolini A, Frank E, Houck PR, Mallinger AG, Swartz HA, Buysse DJ, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry. (2002) 63:528–33. 10.4088/JCP.v63n0611 [DOI] [PubMed] [Google Scholar]
- 43.Keck PE, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry. (2003) 64:1426–35. 10.4088/JCP.v64n1205 [DOI] [PubMed] [Google Scholar]
- 44.Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the american heart association. Circulation. (2015) 132:965–86. 10.1161/CIR.0000000000000229 [DOI] [PubMed] [Google Scholar]
- 45.Fagiolini A, Kupfer DJ, Rucci P, Scott JA, Novick DM, Frank E. Suicide attempts and ideation in patients with bipolar I disorder. J Clin Psychiatry. (2004) 65:509–14. 10.4088/JCP.v65n0409 [DOI] [PubMed] [Google Scholar]
- 46.Dong M, Wang SB, Wang F, Zhang L, Ungvari GS, Ng CH, et al. Suicide-related behaviours in schizophrenia in China: a comprehensive meta-analysis. Epidemiol Psychiatr Sci. (2019) 28:290–9. 10.1017/S2045796017000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruceanu C, Alda M, Rouleau G, Turecki G. Response to treatment in bipolar disorder. Curr Opin Psychiatry. (2011) 24:24–8. 10.1097/YCO.0b013e328341352c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heo M, Pietrobelli A, Wang D, Heymsfield SB, Faith MS. Obesity and functional impairment: influence of comorbidity, joint pain, and mental health. Obesity (Silver Spring). (2010) 18:2030–8. 10.1038/oby.2009.400 [DOI] [PubMed] [Google Scholar]
- 49.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. (2013) 39:306–18. 10.1093/schbul/sbr148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fagiolini A, Frank E, Scott JA, Turkin S, Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. (2005) 7:424–30. 10.1111/j.1399-5618.2005.00234.x [DOI] [PubMed] [Google Scholar]
- 51.Bai YM, Li CT, Tsai SJ, Tu PC, Chen MH, Su TP. Metabolic syndrome and adverse clinical outcomes in patients with bipolar disorder. BMC Psychiatry. (2016) 16:448. 10.1186/s12888-016-1143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Portilla MP, Saiz PA, Benabarre A, Sierra P, Perez J, Rodriguez A, et al. The prevalence of metabolic syndrome in patients with bipolar disorder. J Affect Disord. (2008) 106:197–201. 10.1016/j.jad.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 53.Santini I, Stratta P, D'Onofrio S, De Laurentis I, Santarelli V, Pacitti F, et al. The metabolic syndrome in an Italian psychiatric sample: a retrospective chart review of inpatients treated with antipsychotics. Riv Psichiatr. (2016) 51:37–42. 10.1708/2168.23452 [DOI] [PubMed] [Google Scholar]
- 54.Maina G, D'Ambrosio V, Aguglia A, Paschetta E, Salvi V, Bogetto F. Bipolar disorders and metabolic syndrome: a clinical study in 185 patients. Riv Psichiatr. (2010) 45:34–40. [PubMed] [Google Scholar]
- 55.Salvi V, Albert U, Soreca I, Bogetto F, Maina G. Metabolic syndrome in Italian patients with bipolar disorder: a 2-year follow-up study. J Affect Disord. (2012) 136:599–603. 10.1016/j.jad.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 56.Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. (2004) 47:S67–71. 10.1192/bjp.184.47.s67 [DOI] [PubMed] [Google Scholar]
- 57.McIntyre RS, Konarski JZ, Misener VL, Kennedy SH. Bipolar disorder and diabetes mellitus: epidemiology, etiology, and treatment implications. Ann Clin Psychiatry. (2005) 17:83–93. 10.1080/10401230590932380 [DOI] [PubMed] [Google Scholar]
- 58.Guo JJ, Keck PEJr, Corey-Lisle PK, Li H, Jiang D, Jang R, L'Italien GJ. Risk of diabetes mellitus associated with atypical antipsychotic use among patients with bipolar disorder: A retrospective, population-based, case-control study. J Clin Psychiatry. (2006) 67:1055–61. 10.4088/JCP.v67n0707 [DOI] [PubMed] [Google Scholar]
- 59.Yood MU, DeLorenze G, Quesenberry CPJr, Oliveria SA, Tsai AL, Willey VJ, et al. The incidence of diabetes in atypical antipsychotic users differs according to agent–results from a multisite epidemiologic study. Pharmacoepidemiol Drug Saf. (2009) 18:791–9. 10.1002/pds.1781 [DOI] [PubMed] [Google Scholar]
- 60.Torrent C, Amann B, Sánchez-Moreno J, Colom F, Reinares M, Comes M, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. (2008) 118:4–18. 10.1111/j.1600-0447.2008.01204.x [DOI] [PubMed] [Google Scholar]
- 61.Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. (2004) 27:17–20. 10.2337/diacare.27.1.17 [DOI] [PubMed] [Google Scholar]
- 62.Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Dis. (2016) 4:27. 10.1186/s40345-016-0068-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joffe RT, Kutcher S, MacDonald C. Thyroid function and bipolar affective disorder. Psychiatry Res. (1988) 25:117–21. 10.1016/0165-1781(88)90042-X [DOI] [PubMed] [Google Scholar]
- 64.Lambert CG, Mazurie AJ, Lauve NR, Hurwitz NG, Young SS, Obenchain RL, et al. Hypothyroidism risk compared among nine common bipolar disorder therapies in a large US cohort. Bipolar Disord. (2016) 18:247–60. 10.1111/bdi.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sewell DD. Schizophrenia and HIV. Schizophr Bull. (1996) 22:465–73. 10.1093/schbul/22.3.465 [DOI] [PubMed] [Google Scholar]
- 66.Vergara-Rodriguez P, Vibhakar S, Watts J. Metabolic syndrome and associated cardiovascular risk factors in the treatment of persons with human immunodeficiency virus and severe mental illness. Pharmacol Ther. (2009) 124:269–78. 10.1016/j.pharmthera.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 67.Azad MC, Shoesmith WD, Mamun MA, Abdullah AF, Naing DKS, Phanindranath M, et al. Cardiovascular diseases among patients with schizophrenia. Asian J Psychiatr. (2016) 19:28–36. 10.1016/j.ajp.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 68.Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. (1998) 173:11–53. [DOI] [PubMed] [Google Scholar]
- 69.Chen SF, Yang YC, Hsu CY, Shen YC. Risk of bipolar disorder in patients with polycystic ovary syndrome: A nationwide population-based cohort study. J Affect Disord. (2020) 263:458–62. 10.1016/j.jad.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 70.Carey ET, Martin CE, Siedhoff MT, Bair ED, As-Sanie S. Biopsychosocial correlates of persistent postsurgical pain in women with endometriosis. Int J Gynaecol Obstet. (2014) 124:169–73. 10.1016/j.ijgo.2013.07.033 [DOI] [PubMed] [Google Scholar]
- 71.Pope CJ, Sharma V, Sharma S, Mazmanian D. A Systematic Review of the Association Between Psychiatric Disturbances and Endometriosis. J Obstet Gynaecol Can. (2015) 37:1006–15. 10.1016/s1701-2163(16)30050-0 [DOI] [PubMed] [Google Scholar]
- 72.Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. (2018) 62:318–25. 10.1007/s12020-018-1692-3 [DOI] [PubMed] [Google Scholar]
- 73.Jones GL, Hall JM, Balen AH, Ledger WL. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update. (2008) 14:15–25. 10.1093/humupd/dmm030 [DOI] [PubMed] [Google Scholar]
- 74.Hollinrake E, Abreu A, Maifeld M, Voorhis BJV, Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril. (2007) 87:1369–76. 10.1016/j.fertnstert.2006.11.039 [DOI] [PubMed] [Google Scholar]
- 75.Mansson M, Holte J, Landin-Wilhelmsen K, Dahlgren E, Johansson A, Landén M. Women with polycystic ovary syndrome are often depressed or anxious–a case control study. Psychoneuroendocrinology. (2008) 33:1132–8. 10.1016/j.psyneuen.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 76.Misiak B, Frydecka D, Loska O, Moustafa AA, Samochowiec J, Kasznia J, et al. Testosterone, DHEA and DHEA-S in patients with schizophrenia: A systematic review and meta-analysis. Psychoneuroendocrinology. (2018) 89:92–102. 10.1016/j.psyneuen.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 77.Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ. (2011) 343:d6309. 10.1136/bmj.d6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Owens SJ, Purves Tyson TD, Webster MJ, Weickert CS. Evidence for enhanced androgen action in the prefrontal cortex of people with bipolar disorder but not schizophrenia or major depressive disorder. Psychiatry Res. (2019) 280:112503. 10.1016/j.psychres.2019.112503 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.