Abstract
Health-related quality of life (HRQoL) is an important consideration in managing patients. Spleen aminopeptide oral lyophilized powder (SAOLP) has been used to enhance cellular immunity in a patient. This multicenter, randomized, double-blind, placebo-controlled clinical trial was designed to evaluate the safety and efficacy of SAOLP for improving HRQoL in patients with breast cancer. Patients diagnosed with advanced breast cancer were included, and were administered SAOLP or placebo 4 mg qd for two cycles. The primary endpoint was improvement in HRQoL on day 42 measured by the EORTC QLQ-C30 and EORTC QLQ-BR23. Secondary endpoints included immunologic function, improvement in HRQoL on day 21 and 84, objective response rate, disease control rate, BMI and adverse events. On day 42, on the EORTC QLQ-C30 or EORTC QLQ-BR23, scores on the functional scales and QoL scale were significantly higher and scores on symptom scales were significantly lower in patients who received SAOLP compared to placebo (P < 0.05). On day 84, the number of CD3, CD4 and CD8 cells were significantly higher in patients who received SAOLP. There were no significant differences in objective response rate, disease control rate or BMI. SAOLP may improve HRQoL and the immune response in patients with advanced breast cancer, represents a convenient and safe adjuvant therapy.
Keywords: breast cancer, clinical trial, immune response, quality of life, spleen aminopeptide oral lyophilized powder
Introduction
In the United States (US), in 2020, there will be approximately 1 806 590 new cancer diagnoses and 606 520 cancer-related deaths [1]. On 1 January 2030, an estimated 22.1 million Americans will be living with a cancer history, an increase from the 16.9 million Americans who were living with a cancer history on 1 January 2019. In the US, in 2019, the most prevalent cancers among males included prostate (3 650 030), colon and rectum (776 120), and melanoma of the skin (684 470), and the most prevalent cancers among females included breast (3 861 520), uterine corpus (807 860), and colon and rectum (768 650). Among cancer survivors in the US in 2019, 56% received their diagnosis during the last decade, and 64% were aged ≥65 years [2]. Across all stages, survival rates were highest for prostate cancer (98%), melanoma of the skin (92%) and breast cancer (women) (90%) [1] During 2012–2016, the incidence of breast cancer in the US increased 0.3% per year, driven by an increased incidence of local stage and hormone receptor-positive disease [3].
Treatment for breast cancer includes surgery, radiotherapy and cytotoxic and endocrine drugs. Clinically, the goal of breast cancer treatment is to remove all visible cancer and manage the disease over the long term. Patient health-related quality of life (HRQoL), defined as the patient-perceived psychosocial, emotional and physical outcomes of medical intervention [4], is another important aspect of breast cancer treatment [5,6]. In particular, patient HRQoL should be considered when a treatment approach is unlikely to improve survival, as in some metastatic cancers. The American Society of Clinical Oncology, National Institutes of Health and the National Cancer Institute recommend that patients should only be exposed to treatments without evidence of survival benefits if they can reasonably expect an improvement in HRQoL. Accordingly, HRQoL evaluation is increasingly recognized as an important outcome measure in clinical trials [7].
Spleen aminopeptide oral lyophilized powder (SAOLP), which consists of peptides and nucleotides extracted from fresh pig spleen [8], has been used to enhance cellular immunity and immune homeostasis in pediatric patients with respiratory infection [9] or immune disorders [10,11], and in adult patients with hepatitis B [12]. In patients with gastrointestinal tumors, SAOLP administration was associated with improved HRQoL compared to placebo [13]. This multicenter, randomized, double-blind, placebo-controlled clinical trial was designed to evaluate the safety and efficacy of SAOLP for improving HRQoL and immune response during chemotherapy in Chinese patients with breast cancer.
Methods
Study design
This was a multicenter, randomized, double-blind, placebo-controlled, parallel-arm clinical trial. Patients were randomized by the random number method to receive SAOLP or placebo, which were provided as matching white powders with a similar taste. Patients, physicians and nurses were blinded to the treatment allocation.
Study participants
Patients diagnosed with breast cancer based on biopsy or histology attending the Beijing Friendship hospital affiliated with Capital Medical University, Xiyuan Hospital, China Academy of Chinese medical sciences and Tianjin Medical University general hospital between August 2017 and June 2019 were eligible for this study.
Inclusion criteria were (1) Eastern Cooperative Oncology Group performance status of 0–2; (2) aged 18–75 years; (3) tumor stage IV, irrespective of whether the patient had undergone surgery; (4) maximum tumor diameter ≥10 mm on computed tomography (CT) and MRI; (5) adequate hematological, hepatic and renal function; and (6) expected overall survival of >3 months.
Exclusion criteria were (1) participated in another clinical trial in the past 3 months; (2) allergic to SAOLP or its constituents; (3) taking digitalis; or (4) psychopathic tendencies, pregnant or lactating.
Ethical issues
The trial was conducted according to the Declaration of Helsinki and the Ethical Guidelines for Clinical Research. The trial protocol was approved by an independent ethics committee at the Beijing Friendship Hospital. This trial was registered with the Chinese Clinical Trial Registry at http://www.chictr.org.cn (ChiCTR-IPR-17013733). All patients provided written informed consent to participate in the trial and authorized publication of this article.
Treatment
Breast cancer patients received standard therapy recommended by the NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Patients were administered SAOLP or placebo (Fu Ketuo, Zhejiang Feng; State Medical Permit No. H20068132) 4 mg qd po. on the first day of chemotherapy for two cycles. SAOLP was manufactured according to the Standard for Quality Management of Chinese Medicinal Materials (GAP) (2002).
Patients who were intolerant to SAOLP or showed SAOLP-related or chemotherapy-related toxicity or disease deterioration were transferred to the emergency department. Patients treated for <3 weeks were only included in the safety analyses.
Outcome measures
Patients’ baseline characteristics were recorded before chemotherapy. The primary endpoint was the improvement in patient HRQoL on day 42. Secondary endpoints included immunologic function (CD3, CD4, CD8), improvement in patient HRQoL on day 21 and 84, objective response rate (ORR), disease control rate (DCR), BMI and adverse events.
EORTC QLQ-C30
The official Croatian translation of the European Organisation for Research and Treatment of Cancer (EORTC) [14] QLQ-C30 was used to assess cancer-specific HRQoL during the previous week [17]. The EORTC QLQ-C30 was administered on day 21, day 42 and day 84.
The EORTC QLQ-C30 consists of 30 questions, including five functional scales, nine symptom scales, and a global health status/quality of life scale (GHS/QoL). Responses to 28 questions are assessed on a 4-point Likert-type scale (‘1=‘not at all’, 2=‘a little’, 3=‘quite a bit’ and 4=‘very much’). Responses to two questions are assessed on a 7-point scale Likert-type scale ranging from 1=‘very poor’ to 7=‘excellent’.
Baseline EORTC QLQ-C30, EORTC QLQ-BR23 and immune response
The EORTC QLQ-BR23 was used with the EORTC QLQ-C30 to assess breast cancer-specific HRQoL during the previous week, except for the sexual items, which were during the past 4 weeks. The EORTC QLQ-BR23 was administered on day 21, day 42 and day 84.
The EORTC QLQ-BR23 consists of 23 questions, including four functional scales and four symptom scales. Responses to questions are assessed on a 4-point Likert-type scale.
Data were collected through face-to-face interviews and review of patients’ medical charts. Researchers collecting data were provided two days of training and were continuously supervised by the principal investigator. A pre-test was performed on 20 patients to provide feedback on the EORTC QLQ-C30 and EORTC QLQ-BR23 and ensure they were applicable to the trial population.
Immunologic function
The number of CD3+, CD4+ and CD8+ T lymphocytes and the CD4+/CD8+ ratio were measured by flow cytometry to evaluate patients’ immune response. A decrease in CD3+ T lymphocytes indicates severe suppression of cellular immune function. CD4 and CD8 are two important T lymphocyte subsets. A decrease in CD4+ T lymphocytes indicates a decrease in lymphocyte function and B cell antibody production. A decrease in the CD4+/CD8+ ratio reflects a reduction in the health of the immune system.
Evaluation of tumor response
CT was performed 4 weeks before study initiation and 9 weeks after the end of treatment to evaluate tumor size and status. Response rates were assessed by investigators according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). ORR was defined as the percentage of patients who had a complete response and partial response. DCR was defined as the percentage of patients who had a complete response, partial response, and stable disease.
Adverse events
Adverse events were reported based on the Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
Sample size was determined according to previous observations using the same primary outcome measure. Fifteen participants per arm were required to detect a treatment effect with 80% power and a two-sided significance level of 5% on the physical functioning score on the EORTC QLQ-C30 and EORTC QLQ-BR23. Accounting for a 15% withdrawal and dropout rate, 36 subjects, 18 per arm, were needed to ensure statistically significant results.
Continuous data are reported as mean, SD, median, minimum and maximum. Categorical variables are reported as number of cases and percentage. Standardized EORTC QLQ-C30 and EORTC QLQ-BR23 scores were calculated according to the following formulae: (1) for functional items, standardized score=[1 − (crude score−1)/range] × 100; 2) for general health and symptomatic items, standardized score=[(crude score − 1) × range] × 100. Thus, for functional and general health items, a higher standardized score indicated better patient HRQoL. In contrast, for symptomatic items, a higher standardized score indicated worse patient HRQoL. Standardized EORTC QLQ-C30 and EORTC QLQ- BR23 scores on day 21, day 42 and day 84 for patients treated with chemotherapy and SAOLP or placebo were compared with the two-sample independent t test. Statistical analyses were performed with SPSS v20.0 (IBM SPSS Inc., Chicago, Illinois, USA) using a two-sided analysis. P < 0.05 was considered statistically significant.
Results
Patient characteristics
This study included 36 patients with breast cancer diagnosed based on biopsy or histology. All patients had stage IV ductal adenocarcinoma of the breast. Thirty patients underwent surgery, 28 patients received preoperative first-line chemotherapy, 16 patients received endocrine treatment and 10 patients received radiotherapy.
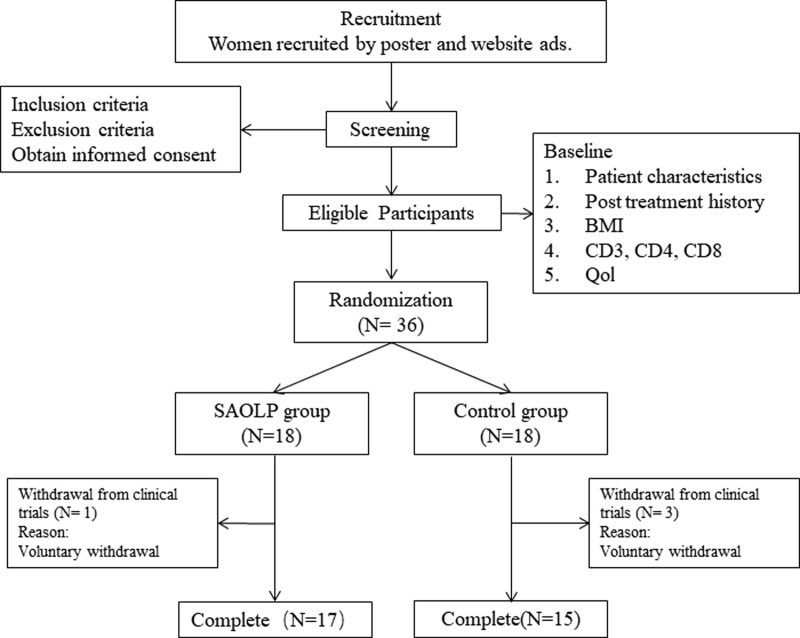
Eighteen patients each were randomized to receive SAOLP or placebo. Four patients were lost to follow-up and did not have data relevant to the primary outcome measure; therefore, data from 32 patients were included in the final analysis (Fig. 1).
Fig. 1.
Flow chart showing patient selection.
The demographic and baseline characteristics of the included patients are summarized in Table 1. Patients had a median age of 55.4 years (range 49.2–63.7). 22.2% of patients received first-line chemotherapy, and 77.8% of patients received second- or later-line chemotherapy.
Table 1.
Patients’ baseline demographic and clinical characteristics
| Characteristics | SAOLP group | Control group | Total | statistic(χ2/t) | P value | |
|---|---|---|---|---|---|---|
| Age | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 3.07 | 0.004 |
| Mean (SD) | 60.1 (7.5) | 50.7 (10.7) | 55.4 (10.3) | |||
| Median (Q1,Q3) | 61.4 (54.2,65.1) | 50.9 (43.4, 55.2) | 54.3 (49.2,63.7) | |||
| Min, Max | 47.4, 73.7 | 34.6, 72.7 | 34.6, 73.7 | |||
| BMI | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | −0.53 | 0.598 |
| Mean (SD) | 23.5 (2.9) | 24.1 (2.9) | 23.8 (2.9) | |||
| Median (Q1,Q3) | 23.6 (20.7,24.9) | 23.8 (22.6,25.0) | 23.8 (22.5,25.0) | |||
| Min, Max | 19.4, 30.1 | 19.2, 31.2 | 19.2, 31.2 | |||
| ECOG | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.83 | 0.411 |
| Mean (SD) | 0.9 (0.4) | 0.8 (0.4) | 09 (0.4) | |||
| Median (Q1,Q3) | 1.0 (1.0,1.0) | 1.0 (1.0,1.0) | 1.0 (1.0,1.0) | |||
| Min, Max | 1.0, 2.0 | 0.0, 1.0 | 0.0, 2.0 | |||
| First line | NO,% | 16 (88.9) | 12 (66.7) | 28 (77.8) | 1.45 | 0.229 |
| YES,% | 2 (11.1) | 6 (33.3) | (22.2) |
ECOG: Eastern Cooperative Oncology Group.
EORTC QLQ-BR23
There were no significant differences in baseline EORTC QLQ-C30 (Table 2), EORTC QLQ-BR23 (Table 3) or immune response in patients who received SAOLP or placebo (Table 4).
Table 2.
Baseline EORTC QLQ-C30 scores
| Indicators | SAOLP group | control group | total | statistic(t) | P value | |
|---|---|---|---|---|---|---|
| PF (physical function) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.07 | 0.794 |
| Mean (SD) | 1.7 (0.3) | 1.7 (0.3) | 1.7 (0.3) | |||
| Median (Q1,Q3) | 1.7 (1.6,1.8) | 1.6 (1.6,2) | 1.6 (1.6,1.8) | |||
| Min, Max | 1.0, 2.6 | 1.2, 2.2 | 1.0, 2.6 | |||
| RF (role function) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.08 | 0.778 |
| Mean (SD) | 1.6 (0.6) | 1.6 (0.6) | 1.6 (0.6) | |||
| Median (Q1,Q3) | 1.3 (1,2) | 1.5 (1.0,2.0) | 1.5 (1.0,2.0) | |||
| Min, Max | 1.0,2.5 | 1.0,2.5 | 1.0,2.5 | |||
| EF (emotional functioning) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.25 | 0.617 |
| Mean (SD) | 1.6 (0.6) | 1.7 (0.5) | 1.6 (0.6) | |||
| Median (Q1,Q3) | 1.5 (1.0,2.3) | 1.8 (1.3,2.3) | 1.8 (1,2.3) | |||
| Min, Max | 1, 2.8 | 1, 2.5 | 1.0, 2.8 | |||
| CF (cognitive function) | N(Nmiss) | 18(0) | 18(0) | 36(0) | 0.15 | 0.702 |
| Mean (SD) | 1.5 (0.5) | 1.5 (0.5) | 1.5 (0.5) | |||
| Median (Q1,Q3) | 1.5 (1.0,2.0) | 1.5 (1.0,2.0) | 1.5 (1.0,2.0) | |||
| Min, Max | 1.0,2.5 | 1.0,2.5 | 1.0,2.5 | |||
| SF (social function) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.86 | 0.355 |
| Mean (SD) | 2.2 (0.6) | 2.1 (0.5) | 2.1 (0.6) | |||
| Median (Q1,Q3) | 2.0 (2.0,3.0) | 2 (2.0,2.0) | 2 (2.0,2.5) | |||
| Min, Max | 1,3 | 1,3 | 1,3 | |||
| QL (quality) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 2.62 | 0.106 |
| Mean (SD) | 3.9 (0.9) | 4.3 (0.8) | 4.1 (0.9) | |||
| Median (Q1,Q3) | 4.0 (3.0,5.0) | 4.0 (4.0,5.0) | 4.0 (3.3,5.0) | |||
| Min, Max | 2.5,5.0 | 3.0, 5.5 | 2.5, 5.5 | |||
| FA (fatigue) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | ||
| Mean (SD) | 1.9 (0.5) | 1.8 (0.3) | 1.8 (0.5) | |||
| Median (Q1,Q3) | 1.8 (1.3,2.0) | 1.7 (1.7,2.0) | 1.7 (1.7,2.0) | |||
| Min, Max | 1.0, 3.0 | 1.3, 2.3 | 1.0, 3.0 | |||
| NV (nausea and vomiting) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.41 | 0.52 |
| Mean (SD) | 1.2 (0.3) | 1.4 (0.6) | 1.3 (0.5) | |||
| Median (Q1,Q3) | 1.0 (1.0,1.5) | 1.0 (1.0,2.0) | 1.0 (1.0,1.5) | |||
| Min, Max | 1.0, 2.0 | 1.0, 3.0 | 1.0, 3.0 | |||
| PA (pain) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.92 | 0.339 |
| Mean (SD) | 1.9 (0.6) | 1.7 (0.6) | 1.8 (0.6) | |||
| Median (Q1,Q3) | 1.5 (1.5,2.5) | 1.5 (1.0,2.0) | 1.5 (1.5,2.0) | |||
| Min, Max | 1.0, 3.0 | 1.0, 3.0 | 1.0, 3.0 | |||
| DY (dyspnea) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 1 | 0.337 |
| Mean (SD) | 1.7 (0.5) | 1.5 (0.5) | 1.6 (0.5) | |||
| Median (Q1,Q3) | 2.0 (1.0,2.0) | 1.5 (1.0,2.0) | 2.0 (1.0,2.0) | |||
| Min, Max | 1.0,2.0 | 1.0,2.0 | 1.0,2.0 | |||
| AP (appetite loss) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.13 | 0.722 |
| Mean (SD) | 1.9 (0.5) | 1.8 (0.5) | 1.9 (0.5) | |||
| Median (Q1,Q3) | 2.0 (2.0,2.0) | 2.0 (2.0,2.0) | 2.0 (2.0,2.0) | |||
| Min, Max | 1.0,3.0 | 1.0,3.0 | 1.0,3.0 | |||
| CO (constipation) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 2.99 | 0.084 |
| Mean (SD) | 1.7 (0.5) | 1.4 (0.6) | 1.5 (0.6) | |||
| Median (Q1,Q3) | 2.0 (1.0,2.0) | 1.0 (1.0,2.0) | 1.5 (1.0,2.0) | |||
| Min, Max | 1.0,2.0 | 1.0,3.0 | 1.0,3.0 | |||
| DI (diarrhea) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.73 | 0.393 |
| Mean (SD) | 1.2 (0.4) | 1.3 (0.6) | 1.3 (0.5) | |||
| Median (Q1,Q3) | 1.0 (1.0,1.0) | 1.0 (1.0,2.0) | 1 (1,1) | |||
| Min, Max | 1.0,2.0 | 1.0,3.0 | 1.0,3.0 | |||
| FI (financial difficulties) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 3.43 | 0.064 |
| Mean (SD) | 2.6 (0.5) | 2.2 (0.6) | 2.4 (0.6) | |||
| Median (Q1,Q3) | 3.0 (2.0,3.0) | 2.0 (2.0,3.0) | 2.0 (2.0,3.0) | |||
| Min, Max | 2.0,3.0 | 1.0,3.0 | 1.0,3.0 |
Table 3.
Baseline EORTC QLQ-BR23 scores
| Indicators | SAOLP group | Control group | Total | Statistic(t) | P value | |
|---|---|---|---|---|---|---|
| BRBI (body image) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.19 | 0.666 |
| Mean (SD) | 1.9 (0.6) | 2 (0.5) | 1.9 (0.5) | |||
| Median (Q1,Q3) | 1 (3.3,0) | 1 (3,0) | 2 (1.6,2) | |||
| Min, Max | 1, 3.3 | 1, 3 | 1,3.3 | |||
| BRSFF (sexual functioning) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.98 | 0.323 |
| Mean (SD) | 1.6 (0.5) | 1.4 (0.5) | 1.5 (0.5) | |||
| Median (Q1,Q3) | 1 (2,0) | 1 (2,0) | 1 (1,2) | |||
| Min, Max | 1, 2 | 1, 2 | 1, 2 | |||
| BRSEE (sexual enjoyment) | N (Nmiss) | 11 (7) | 8 (10) | 36 (0) | 0.73 | 0.394 |
| Mean (SD) | 1.9 (0.3) | 2 (0) | 1.9 (0.2) | |||
| Median (Q1,Q3) | 1 (2,0) | 2 (2,0) | 2 (2,2) | |||
| Min, Max | 1,2 | 2, 2 | 1, 2 | |||
| BRFU (future perspective) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.03 | 0.871 |
| Mean (SD) | 2.5 (0.6) | 2.5 (0.5) | 2.5 (0.6) | |||
| Median (Q1,Q3) | 2 (4,0) | 2 (3,0) | 2 (2,3) | |||
| Min, Max | 2,4 | 2,3 | 2,4 | |||
| BRST (systemic therapy side effects) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.41 | 0.521 |
| Mean (SD) | 1.6 (0.3) | 1.5 (0.3) | 1.6 (0.3) | |||
| Median (Q1,Q3) | 1.1 (2.3,0) | 1 (2.1,0) | 1.5 (1.3,1.9) | |||
| Min, Max | 1.1, 2.3 | 1, 2.1 | 1, 2.3 | |||
| BRBS (breast symptoms) | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 0.19 | 0.662 |
| Mean (SD) | 1.6 (0.4) | 1.6(0.4) | 1.6 (0.4) | |||
| Median (Q1,Q3) | 1 (2,0) | 1 (2.3,0) | 1.6 (1.3,2) | |||
| Min, Max | 1, 2 | 1, 2.3 | 1, 2.3 | |||
| BRAS (arm symptoms) | N (Nmiss) | 11 (7) | 9 (9) | 20 (16) | 0.2 | 0.657 |
| Mean (SD) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | |||
| Median (Q1,Q3) | 1 (2.7,0) | 1 (2.3,0) | 1.7 (1.3,2) | |||
| Min, Max | 1, 2.7 | 1, 2.3 | 1, 2.7 | |||
| BRHL (upset by hair loss) | N (Nmiss) | 14 (4) | 11(7) | 27 (9) | 0.14 | 0.705 |
| Mean (SD) | 2 (0.6) | 1.9 (0.5) | 2 (0.5) | |||
| Median (Q1,Q3) | 1 (3,0) | 1(3,0) | 2 (2,2) | |||
| Min, Max | 1, 3 | 1, 3 | 1, 3 |
Table 4.
Baseline immune response
| Indicators | SAOLP | Placebo | Total | Statistic(t) | P value | |
|---|---|---|---|---|---|---|
| CD3 | N (Nmiss) | 18 (0) | 18 (0) | 36(0) | 0.53 | 0.597 |
| Mean (SD) | 68.6 (10.4) | 66.9 (9.5) | 67.8 (9.9) | |||
| Median (Q1,Q3) | 66.6 (59.4,76.3) | 63.8 (59.4,77.2) | 65.5 (59.4,76.7) | |||
| Min, Max | 57.6, 89.7 | 54.4, 84.3 | 54.4, 89.7 | |||
| CD4 | N (Nmiss) | 18 (0) | 18 (0) | 36 (0) | 1.19 | 0.244 |
| Mean (SD) | 37.3 (8.3) | 34.5 (5.7) | 35.9 (7.2) | |||
| Median (Q1,Q3) | 34.4 (32.4,43.6) | 34.4 (30.2,39.6) | 34.4 (30.7,39.8) | |||
| Min, Max | 28.7, 63.0 | 26.9, 44.2 | 26.9, 63.0 | |||
| CD8 | N (Nmiss) | 18 (0) | 18 (0) | 36(0) | −1.49 | 0.145 |
| Mean (SD) | 25.4 (9.9) | 29.8 (8.0) | 27.6 (9.1) | |||
| Median (Q1,Q3) | 24.2 (20.1,28.9) | 29.8 (22.3,36.5) | 26.1 (20.3,35.8) | |||
| Min, Max | 10.9, 50.5 | 18.4, 43.6 | 10.9 (50.5) |
EORTC QLQ-C30 on day 21, day 42 and day 84
On day 21, the score on the global health status/QoL scale was significantly higher in patients who received SAOLP compared to placebo. There were no significant differences in scores on the functional scales or the symptom scales. On day 42, scores on the five functional scales (physical, role, cognitive, emotional and social) and the global health status/QoL scale were significantly higher in patients who received SAOLP compared to placebo (P < 0.05). Scores on six of the symptom scales (fatigue, pain, dyspnea, loss of appetite, diarrhea and financial difficulties) were significantly lower in patients who received SAOLP compared to placebo (P < 0.05). On day 84, scores on the five functional scales (physical, role, cognitive, emotional and social) and the global health status/QoL scale were significantly higher in patients who received SAOLP compared to placebo (P < 0.05). Scores on the nine symptom scales (fatigue, pain, nausea and vomiting, dyspnea, loss of appetite, insomnia, constipation, diarrhea and financial difficulties) were significantly lower in patients who received SAOLP compared to placebo (Table 5).
Table 5.
EORTC QLQ-C30 scores on day 21, day 48 and day 84
| Indicators | Placebo (day 21) | SAOLP (day 21) | Statistic(t) (day 21) | P value (day 21) | Placebo (day 42) | SAOLP (day 42) | Statistic(t) (day 42) | P value (day 42) | Placebo (day 84) | SAOLP (day 84) | Statistic(t) (day84) | P value (day 84) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PF (physical function) | N (Nmiss) | 17 (1) | 17 (1) | 1.21 | 0.235 | 17 (1) | 15 (3) | 6.95 | <0.001 | 17 (1) | 15 (3) | 7.75 | <0.001 |
| Mean (SE) | 78.4(2.0) | 74.9(2.1) | 83.1(2.1) | 58.7(2.9) | 84.7(1.9) | 57.3(3.1) | |||||||
| RF (role function) | N (Nmiss) | 17 (1) | 17 (1) | 1.6 | 0.119 | 17 (1) | 15 (3) | 4.12 | <0.001 | 17 (1) | 15 (3) | 8.36 | <0.001 |
| Mean (SE) | 82.4 (5.0) | 71.6 (4.5) | 87.3 (4.2) | 58.9 (5.6) | 90.2 (3.5) | 48.9 (7.0) | |||||||
| EF (emotional functioning) | N (Nmiss) | 17 (1) | 17 (1) | 0.93 | 0.362 | 17 (1) | 15 (3) | 6.47 | <0.001 | 17 (1) | 15 (3) | 6.76 | <0.001 |
| Mean (SE) | 82.4 (4.1) | 76.5 (4.9) | 93.6 (2.4) | 60.6 (4.7) | 94.6 (2.1) | 60.0 (4.9) | |||||||
| CF (cognitive function) | N (Nmiss) | 17 (1) | 17 (1) | 1.94 | 0.061 | 17 (1) | 15 (3) | 2.94 | 0.006 | 17 (1) | 15 (3) | 6.19 | <0.001 |
| Mean (SE) | 83.3 (3.2) | 74.5 (3.2) | 92.2 (3.2) | 76.7 (4.2) | 92.2 (2.9) | 63.3 (3.7) | |||||||
| SF (social function) | N (Nmiss) | 17(1) | 17 (1) | 1.71 | 0.096 | 17 (1) | 15 (3) | 5.6 | <0.001 | 17 (1) | 15 (3) | 6.42 | <0.001 |
| Mean (SE) | 68.6 (4.7) | 58.8 (3.2) | 79.4 (3.7) | 46.7 (4.7) | 80.4 (3.6) | 40.0 (5.3) | |||||||
| QL (quality) | N (Nmiss) | 17 (1) | 17 (1) | 2.56 | 0.015 | 17 (1) | 15 (3) | 10.53 | <0.001 | 17 (1) | 15 (3) | 13.59 | <0.001 |
| Mean (SE) | 60.8 (2.7) | 50.0 (3.2) | 78.9 (2.4) | 37.2 (3.2) | 81.4 (1.5) | 32.8 (3.4) | |||||||
| FA (fatigue) | N (Nmiss) | 17 (1) | 17 (1) | −1.21 | 0.233 | 17 (1) | 15 (3) | −6.72 | <0.001 | 17 (1) | 15 (3) | −8.52 | <0.001 |
| Mean (SE) | 26.1 (3.9) | 32.0 (2.8) | 13.1 (3.0) | 51.1 (4.9) | 9.8 (2.3) | 62.2 (6.0) | |||||||
| NV (nausea and vomiting) | N (Nmiss) | 17 (1) | 17 (1) | −1.37 | 0.18 | 17 (1) | 15 (3) | −1.92 | 0.0 64 | 17 (1) | 15 (3) | −2.47 | 0.019 |
| Mean (SE) | 6.9 (2.9) | 13.7 (4.1) | 4.9 (2.8) | 15.6 (5.0) | 4.9 (2.4) | 16.7 (4.3) | |||||||
| PA (pain) | N (Nmiss) | 17 (1) | 17 (1) | −0.32 | 0.754 | 17 (1) | 15 (3) | −2.3 | 0.029 | 17 (1) | 15 (3) | −3.39 | 0.002 |
| Mean(SE) | 21.6(4.7) | 23.5(4.1) | 14.7(3.7) | 30.0(5.7) | 11.8(4.0) | 34.4(5.5) | |||||||
| DY (dyspnea) | N (Nmiss) | 17 (1) | 17 (1) | −0.68 | 0.28 | 17 (1) | 15 (3) | −2.67 | 0.012 | 17 (1) | 15 (3) | −3.68 | 0.001 |
| Mean (SE) | 17.6 (4.2) | 21.6 (4.0) | 9.8 (3.8) | 24.4 (3.9) | 5.9 (4.3) | 26.7 (3.6) | |||||||
| AP (appetite loss) | N (Nmiss) | 17 (1) | 17 (1) | −0.65 | 0.518 | 17 (1) | 15 (3) | −2.91 | 0.007 | 17 (1) | 15 (3) | −2.23 | 0.033 |
| Mean (SE) | 25.5 (4.5) | 29.4 (3.9) | 13.7 (4.1) | 28.9 (3.0) | 19.6 (4.1) | 33.3 (4.6) | |||||||
| CO (constipation) | N (Nmiss) | 17 (1) | 17 (1) | 0.34 | 0.734 | 17 (1) | 15 (3) | −1.4 | 0.171 | 17 (1) | 15 (3) | −2.3 | 0.029 |
| Mean (SE) | 13.7 (4.1) | 17.8 (4.0) | 5.9 (3.2) | 13.3 (4.4) | 3.9 (2.7) | 15.6 (4.4) | |||||||
| DI (diarrhea) | N (Nmiss) | 17 (1) | 17 (1) | −0.79 | 0.434 | 17 (1) | 15 (3) | −2.05 | 0.049 | 17 (1) | 15 (3) | −2.61 | 0.014 |
| Mean (SE) | 5.9 (3.2) | 9.8 (3.8) | 2.0 (2.0) | 11.1 (4.2) | 0 (0) | 13.3 (5.4) | |||||||
| FI (financial difficulties) | N (Nmiss) | 17 (1) | 17 (1) | 0.3 | 0.765 | 17 (1) | 15 (3) | −2.06 | 0.048 | 17 (1) | 15 (3) | −4.67 | <0.001 |
| Mean (SE) | 49.0 (4.2) | 47.1 (5.0) | 41.2 (3.5) | 57.8 (7.6) | 35.3 (4.5) | 71.1 (6.4) |
EORTC QLQ-BR23 on day 21, day 42 and day 84
On day 21, scores on two of the functional scales (body image and future perspective) were significantly higher in patients who received SAOLP compared to placebo (P < 0.05). On day 42, scores on two of the functional scales (body image and future perspective) were significantly higher and scores on two of the symptom scales (systemic therapy side effects and breast symptoms) were significantly lower in patients who received SAOLP compared to placebo (P < 0.05). On day 84, scores on two of the functional scales (body image and future perspective) were significantly higher and scores on three of the symptom scales (systemic therapy side effects, breast symptoms and arm symptoms) were significantly lower in patients who received SAOLP compared to placebo (P < 0.05) (Table 6).
Table 6.
EORTC QLQ-BR23 scores on day 21, day 48 and day 84
| Indicators | SAOLP (day 21) | Placebo (day 21) | Statistic(t) (day 21) | P value (day 21) | SAOLP (day 42) | Placebo (day 42) | Statistic(t) (day 42) | P value (day 42) | SAOLP (day 84) | Placebo (day 84) | Statistic(t) (day84) | P value (day 84) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRBI (body image) | N (Nmiss) | 17 (1) | 17 (1) | 2.1 | 0.044 | 17 (1) | 15 (3) | 6.98 | <0.001 | 17 (1) | 15 (3) | 7.08 | <0.001 |
| Mean (SE) | 77.9 (4.3) | 64.2 (4.9) | 83.8 (4.0) | 45.0 (3.8) | 85.8 (3.3) | 46.7 (4.6) | |||||||
| BRSFF (sexual functioning) | N (Nmiss) | 17 (1) | 17 (1) | −1.02 | 0.315 | 17 (1) | 15 (3) | −1.37 | 0.181 | 17 (1) | 15 (3) | −1.37 | 0.181 |
| Mean (SE) | 82.4 (4.2) | 88.2 (4.0) | 83.3 (4.0) | 91.1 (3.9) | 83.3 (4.0) | 91.1 (3.9) | |||||||
| BRSEE (sexual enjoyment) | N (Nmiss) | 10 (8) | 6 (12) | 0.76 | 0.458 | 10 (8) | 10 (8) | −1.9 | 0.074 | 11 (7) | 10 (8) | −1.53 | 0.144 |
| Mean (SE) | 70.0 (3.3) | 66.7 (0) | 73.3 (4.4) | 86.7 (5.4) | 75.8 (4.7) | 86.7 (5.4) | |||||||
| BRFU (future perspective) | N (Nmiss) | 17 (1) | 17 (1) | 2.58 | 0.015 | 17 (1) | 15 (3) | 4.36 | <0.001 | 17 (1) | 15 (3) | 6.14 | <0.001 |
| Mean (SE) | 58.8 (3.5) | 45.1 (4.0) | 66.7 (5.0) | 35.6 (5.1) | 68.6 (4.5) | 22.2 (6.2) | |||||||
| BRST (systemic therapy side effects) | N (Nmiss) | 17 (1) | 17 (1) | −0.47 | 0.641 | 17 (1) | 15 (3) | −2.92 | 0.007 | 17 (1) | 15 (3) | −4 | <0.001 |
| Mean (SE) | 18.2 (2.3) | 19.9 (2.7) | 12.6 (3.0) | 25.1 (3.0) | 11.5 (2.3) | 26.7 (3.0) | |||||||
| BRBS (breast symptoms) | N (Nmiss) | 17 (1) | 17 (1) | −0.42 | 0.675 | 16 (2) | 15 (3) | −4.07 | <0.001 | 17 (1) | 15 (3) | −5.21 | <0.001 |
| Mean (SE) | 19.6 (2.7) | 21.6 (3.8) | 12.0 (2.7) | 30.6 (3.7) | 8.3 (2.3) | 30.0 (3.6) | |||||||
| BRAS (arm symptoms) | N (Nmiss) | 17 (1) | 17 (1) | −0.68 | 0.502 | 17 (1) | 15 (3) | −1.98 | 0.0 57 | 17 (1) | 15 (3) | −2.72 | 0.011 |
| Mean (SE) | 17.6 (2.7) | 20.3 (2.7) | 15.7 (2.5) | 23.7 (3.2) | 14.4 (2.3) | 25.2 (3.3) | |||||||
| BRHL (upset by hair loss) | N (Nmiss) | 12 (6) | 12 (6) | −0.69 | 0.496 | 8 (10) | 10 (8) | −1.56 | 0.138 | 11 (7) | 11 (7) | −1.78 | 0.091 |
| Mean (SE) | 33.3 (7.1) | 38.9 (3.7) | 29.2 (11.7) | 50.0 (7.5) | 24.2 (9.1) | 42.4 (4.7) |
Immune response on day 21, day 42 and day 84
On day 21, there were no significant differences in the number of CD3, CD4 or CD8 cells or the CD4/CD8 ratio in patients who received SAOLP or placebo. On day 42, the number of CD3, CD4 and CD8 cells were significantly higher in patients who received SAOLP compared to placebo (P < 0.05). On day 84, the number of CD3, CD4 and CD8 cells and the CD4/CD8 ratio were significantly higher in patients who received SAOLP compared to placebo (P < 0.05) (Table 7).
Table 7.
Immune response on day 21, day 48, and day 84
| Indicators | SAOLP (day 21) | Placebo (day 21) | Statistic(t) | P value | |
|---|---|---|---|---|---|
| CD3 | N (Nmiss) | 17 (1) | 17 (1) | 1.86 | 0.073 |
| Mean (SE) | 70.5 (2.3) | 64.4 (2.4) | |||
| CD4 | N (Nmiss) | 17 (1) | 17 (1) | 2.05 | 0.049 |
| Mean (SE) | 36.7 (1.4) | 32.9 (1.1) | |||
| CD8 | N (Nmiss) | 17 (1) | 17 (1) | 1.25 | 0.219 |
| Mean (SE) | 30.1 (2.6) | 25.9 (2.1) | |||
| CD4/CD8 | N (Nmiss) | 17 (1) | 17 (1) | 0.15 | 0.879 |
| Mean (SE) | 1.38 (0.14) | 1.36 (0.08) | |||
| Indicators | SAOLP (day 42) | Placebo (day 42) | Statistic(t) | P value | |
| CD3 | N (Nmiss) | 17 (1) | 14 (3) | 10.47 | <0.001 |
| Mean (SE) | 76.1 (1.4) | 59.0 (5.0) | |||
| CD4 | N (Nmiss) | 17 (1) | 14 (3) | 6.12 | <0.001 |
| Mean (SE) | 39.2 (1.4) | 29.0 (0.6) | |||
| CD8 | N (Nmiss) | 17 (1) | 14 (3) | 5.52 | <0.001 |
| Mean (SE) | 34.6 (2.3) | 20.5 (0.6) | |||
| CD4/CD8 | N (Nmiss) | 17 (1) | 14 (3) | −2.02 | 0.055 |
| Mean (SE) | 1.22 (0.09) | 1.43 (0.04) | |||
| Indicators | SAOLP (day 84) | Placebo (day 84) | Statistic(t) | P value | |
| CD3 | N (Nmiss) | 17 (1) | 14 (4) | 15.1 | <0.001 |
| Mean (SE) | 77.8 (1.2) | 57.7 (0.4) | |||
| CD4 | N (Nmiss) | 17 (1) | 14 (4) | 5.31 | <0.001 |
| Mean (SE) | 42.0 (1.5) | 29.0 (2.2) | |||
| CD8 | N (Nmiss) | 17 (1) | 14 (4) | 8.82 | <0.001 |
| Mean (SE) | 36.9 (1.8) | 18.9 (0.3) | |||
| CD4/CD8 | N (Nmiss) | 17 (1) | 14 (4) | −2.58 | 0.015 |
| Mean (SE) | 1.20 (0.07) | 1.53 (0.11) |
Objective effective rate and disease control rate
ORR was 7.1% and 8.3% (P = 1.000) in patients who received SAOLP or placebo, respectively. DCR was 100 and 83.3% (P = 0.203) in patients who received SAOLP or placebo, respectively. There were no significant differences in ORR or DCR in patients who received SAOLP or placebo (Table 8).
Table 8.
BMI on day 21, day 48 and day 84
| Indicators | SAOLP | Placebo | Statistic(t) | P value | |
|---|---|---|---|---|---|
| BMI_Day 21 | N (Nmiss) | 17 (1) | 17 (1) | 0.06 | 0.951 |
| Mean (SE) | 23.7 (0.7) | 23.6 (0.7) | |||
| BMI_Day 42 | N (Nmiss) | 17 (1) | 15 (3) | 0.2 | 0.84 |
| Mean (SE) | 23.4 (0.8) | 23.2 (0.7) | |||
| BMI_Day 84 | N (Nmiss) | 17 (1) | 15 (3) | 1.49 | 0.147 |
| Mean (SE) | 23.6 (0.7) | 22.2 (0.5) |
BMI
On day 21, day 42 and day 84, BMI was higher in patients who received SAOLP compared to placebo, but the differences were NS.
Safety
Across all enrolled patients, 47.22% (17/36) of patients reported an adverse event. Most of the adverse events were Grade 1 or 2. Frequent nonhematological toxicities included nausea/vomiting, fatigue and proteinuria. Among patients who received SAOLP, 50% (9/18) of patients reported an adverse event. Among patients who received placebo, 44.4% (8/18) of patients reported an adverse event. No patients reported a serious drug-related adverse event. All adverse events were considered related to chemotherapy.
Discussion
This clinical trial was designed to evaluate the safety and efficacy of SAOLP for improving HRQoL and immune response during chemotherapy in Chinese patients with breast cancer. Breast cancer treatment involves a multimodal approach that includes chemotherapy [15]. Chemotherapy can significantly improve survival and quality of life compared to best supportive care in patients with breast cancer [16–18]. However, adverse effects of chemotherapy in patients with malignant tumors include fatigue, nausea and vomiting, bone marrow suppression, renal toxicity, neurotoxicity and cancer-related cognitive impairment [19]. Accordingly, most patients with breast cancer experience a decline in HRQoL due to tumor burden and treatment effects, such as loss of appetite, reduction in body mass and emotional instability. Improving the quality of life of patients with cancer is essential to decrease psychological distress and enhance coping [20,21]. Disease, such as malignant tumor, may weaken the immune system, which can have a direct effect on treatment outcomes and prognosis. Therapies that manipulate the immune system may achieve long-term cancer regression.
SAOLP consists of polypeptides and nucleotides extracted from the pig spleen, which may enhance the body’s cellular immunity, stimulate the release of a variety of immune molecules and promote antitumor immunity. SAOLP has potential to shorten the course of the disease and improve HRQoL in patients with immune dysfunction [22]. However, the effect of SAOLP during chemotherapy in patients with cancer is unclear. In one study [13], immunity and DCRs were improved in patients with gastrointestinal cancer (n = 60) treated with oxaliplatin and capecitabine combined with SAOLP compared to oxaliplatin and capecitabine combined with placebo. To the authors’ knowledge, the present study is the first randomized, double-blind, placebo-controlled clinical trial evaluating the safety and efficacy of SAOLP for improving HRQoL and immune response during chemotherapy in patients with breast cancer. Data suggest that SAOLP improved HRQoL and immune response in the study population. SAOLP was well tolerated as there were no significant differences in ORR, DCR or adverse events in patients receiving SAOLP compared to placebo.
There were several limitations to our study. First, the sample size was small. Second, SAOLP was only used during two cycles of chemotherapy. Larger, longer-term studies are required to confirm the safety and efficacy of SAOLP for improving HRQoL and immune response in patients with advanced cancer.
Conclusion
SAOLP in combination with chemotherapy may improve HRQoL and immune response in patients with advanced cancer. SAOLP represents a convenient and safe adjuvant therapy.
Acknowledgements
This study was supported by grants from Beijing Key Specialty (Bangwei Cao).
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by J.W., X.M., K.S., S.W., Y.M., Z.M. and B.C. The first draft of the manuscript was written by J.W., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019; 69:363–385. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019; 69:438–451. [DOI] [PubMed] [Google Scholar]
- 4.Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surg Oncol Clin N Am 2018; 27:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Maio M, Perrone F. Quality of life in elderly patients with cancer. Health Qual Life Outcomes 2003; 1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahfouz MA, Almaghrabi MY. Quality of life for elderly breast cancer patients: a new regard. Transl Cancer Res 2020; 9:S122–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. BMJ 1998; 317:771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen XF, Zhou A, Huang Q. An analysis of spleen aminopeptide oral lyophilized powder in the treatment of children after tonsillectomy. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017; 31:1690–1692. [DOI] [PubMed] [Google Scholar]
- 9.Tan TJ, Liu GY, Liao JY, Zeng GQ, Gan L, Shui-Lan LU, et al. The effect of spleen aminopeptide on treatment and prevention of pediatric repeated respiratory infection and its influence on T lymphocyte subset. Med Recapitulate 2015; 21:2257–2258. [Google Scholar]
- 10.Wang W, Yu-Xue LI, Min HU. Efficacy of spleen aminopeptide combined with vitamin A and E in the treatment of children with recurrent respiratory tract infection and its immune function. Clin Misdiagn Misther 2019; 32:21–24. [Google Scholar]
- 11.Boying DU, Gao J, Liu C, Weiwei MA, Qin X, Suwen XU. Observation of the curative effect of Yupingfeng granule and spleen aminopeptide in the children with pneumonia and syndrome of lung-spleen deficiency in recovery stage. Modern J Integr Trad Chin Western Med 2017; 26:3883–3885. [Google Scholar]
- 12.Min M, Huaming S, Hua L. Effects of spleen aminopeptide oral lyophilized powder on immune function of patients with chronic hepatitis B. Modern J Integr Trad Chinese Western Med 2010; 19:1953. [Google Scholar]
- 13.Liang XU, Ming-xing Z, Bang-wei CAO. Therapeutic efficacy of oral lyophilized powder splenic aminopeptide combined with platinum-containing chemotherapy in patients with gastrointestinal tumors. Chin J Clin Oncol Rehabil 2019; 26:1043–1047. [Google Scholar]
- 14.Koller M, Aaronson NK, Blazeby J, Bottomley A, Dewolf L, Fayers P, et al.; EORTC Quality of Life Group. Translation procedures for standardised quality of life questionnaires: The European Organisation for Research and Treatment of Cancer (EORTC) approach. Eur J Cancer 2007; 43:1810–1820. [DOI] [PubMed] [Google Scholar]
- 15.Teraoka S, Sato E, Narui K, Yamada A, Fujita T, Yamada K, et al. Neoadjuvant chemotherapy with anthracycline-based regimen for BRCAness tumors in triple-negative breast cancer. J Surg Res 2020; 250:143–147. [DOI] [PubMed] [Google Scholar]
- 16.Prioli KM, Pizzi LT, Kash KM, Newberg AB, Morlino AM, Matthews MJ, Monti DA. Costs and effectiveness of mindfulness-based art therapy versus standard breast cancer support group for women with cancer. Am Health Drug Benefits 2017; 10:288–295. [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman RA, Partridge AH. Emerging data and current challenges for young, old, obese, or male patients with breast cancer. Clin Cancer Res 2017; 23:2647–2654. [DOI] [PubMed] [Google Scholar]
- 18.Haussmann J, Nestle-Kraemling C, Bölke E, Wollandt S, Speer V, Djiepmo Njanang FJ, et al. Long-term quality of life after preoperative radiochemotherapy in patients with localized and locally advanced breast cancer. Strahlenther Onkol 2020; 196:386–397. [DOI] [PubMed] [Google Scholar]
- 19.Campbell KL, Zadravec K, Bland KA, Chesley E, Wolf F, Janelsins MC. The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: systematic review of randomized controlled trials. Phys Ther 2020; 100:523–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konieczny M, Cipora E, Sygit K, Fal A. Quality of life of women with breast cancer and socio-demographic factors. Asian Pac J Cancer Prev 2020; 21:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mojgan F, Karimollah HT, Moslemi D. Analysis of quality of life in breast cancer survivors using structural equation modelling: the role of spirituality, social support and psychological well-being. Int Health 2020; 12:354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua YC, Wang J. Process of splenic aminopeptide oral freeze-dried powder on patients with immune dysfunction. Eval Anal Drug-Use Hosp China 201919:644–646. [Google Scholar]