Abstract
Cardiovascular disease (CVD) is the leading cause of death globally. Risk assessment is crucial for identifying at-risk individuals who require immediate attention as well as to guide the intensity of medical therapy to reduce subsequent risk of CVD. In the past decade, many risk prediction models have been proposed to estimate the risk of developing CVD. However, in patients with a history of CVD, the current models that based on traditional risk factors provide limited power in predicting recurrent cardiovascular events. Several biomarkers from different pathophysiological pathways have been identified to predict cardiovascular events, and the incorporation of biomarkers into risk assessment may contribute to enhance risk stratification in secondary prevention. This review focuses on biomarkers related to cardiovascular and metabolic diseases, including B-type natriuretic peptide, high-sensitivity cardiac troponin I, adiponectin, adipocyte fatty acid-binding protein, heart-type fatty acid-binding protein, lipocalin-2, fibroblast growth factor 19 and 21, retinol-binding protein 4, plasminogen activator inhibitor-1, 25-hydroxyvitamin D, and proprotein convertase subtilisin/kexin type 9, and discusses the potential utility of these biomarkers in cardiovascular risk prediction among patients with CVD. Many of these biomarkers have shown promise in improving risk prediction of CVD. Further research is needed to assess the validity of biomarker and whether the strategy for incorporating biomarker into clinical practice may help to optimize decision-making and therapeutic management.
Keywords: adipocyte, B-type natriuretic peptide, cardiac troponin, coronary artery disease, fibroblast growth factor, lipocalin, plasminogen activator inhibitor, risk prediction
Introduction
Individuals with stable coronary artery disease (CAD) are at higher risk of recurrent cardiovascular event and mortality than the general population. Preventive strategies and intensive management of cardiovascular risk factors are much needed to improve the prognosis of these patients. Although conventional risk prediction models such as Framingham Risk Score have been developed and widely used to estimate individual's risk for primary prevention of cardiovascular disease (CVD) (1), effective tools for risk assessment in secondary prevention are still missing. The mechanisms underlying the increased risk of recurrent CVD are not fully understood. Existing prediction models that based on traditional risk factors such as age, gender, diabetes status, blood pressure, cholesterol levels, and smoking status may have limited value to risk stratify patients with stable CAD (2).
Circulating biomarkers such as high sensitivity C-reactive protein and cardiac troponin have been playing a crucial role in the diagnosis, risk stratification, and management of patients with several disease conditions including heart failure (HF) and acute coronary syndrome (ACS) (3, 4). Recently, numerous novel biomarkers from different pathophysiological pathways have been found to be associated with cardiovascular risk and may provide important prognostic information (5–7). The combined use of multiple biomarkers has also proven to be useful in the risk stratification of CVD (8). In this review, we focus on the potential utility of various biomarkers from cardiac- and metabolic-related pathways for predicting cardiovascular risk in secondary prevention setting. The reviewed biomarkers include: (i) cardiac-related biomarkers [B-type natriuretic peptide (BNP), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and cardiac troponin I (cTnI)]; and (ii) metabolic-related biomarkers [adiponectin, adipocyte fatty acid-binding protein (A-FABP), heart-type fatty acid binding protein (H-FABP), lipocalin-2, fibroblast growth factor (FGF) 19 and 21, retinol-binding protein 4 (RBP4), plasminogen activator inhibitor-1 (PAI-1), 25-hydroxyvitamin D, and proprotein convertase subtilisin/kexin type 9 (PCSK9)]. These biomarkers are of special interest as they are thought to provide sufficient information for improving cardiovascular risk stratification. Evolving biomarkers such as non-coding RNAs are beyond the scope of this review, although they have shown a potential in this field (9). The potential mechanistic link between biomarkers and CVD are summarized in Table 1.
Table 1.
Potential mechanistic link between CVD and biomarkers.
| Biomarker | Potential link with CVD | References |
|---|---|---|
| Cardiac troponin I | Myocardial injury | (10) |
| BNP/NT-proBNP | Myocardial stretch | (11) |
| Adiponectin | Insulin resistance Altered lipid metabolism Endothelial dysfunction Atherosclerosis Inflammation |
(12, 13) |
| A-FABP | Insulin resistance Altered lipid metabolism Endothelial dysfunction Atherosclerosis Inflammation |
(14, 15) |
| H-FABP | Altered lipid metabolism Myocardial injury |
(16) |
| Lipocalin-2 | Atherosclerosis Plaque instability Vascular remodelling Insulin resistance Inflammation |
(17, 18) |
| FGF-19 | Altered lipid metabolism Altered glucose metabolism Insulin resistance |
(19, 20) |
| FGF-21 | Altered lipid metabolism Altered glucose metabolism Insulin resistance |
(19, 21) |
| RBP4 | Insulin resistance Atherosclerosis Inflammation |
(22, 23) |
| PAI-1 | Thrombus formation Impaired fibrinolysis Insulin resistance Inflammation |
(24) |
| 25-hydroxyvitamin D | Insulin resistance Endothelial dysfunction Atherosclerosis Inflammation |
(25) |
| PCSK9 | Altered lipid metabolism Atherosclerosis |
(26) |
A-FABP, adipocyte fatty acid-binding protein; BNP, B-type natriuretic peptide; FGF, fibroblast growth factor; H-FABP, heart-type fatty acid-binding protein; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAI, plasminogen activator inhibitor; PCSK9, proprotein convertase subtilisin/kexin type 9; RBP, retinol-binding protein.
Characteristics of Biomarker
A biomarker, or biological marker, is broadly defined as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (27). Biomarkers can be classified into four types: diagnostic biomarkers are expected to facilitate the early detection of disease; prognostic biomarkers are used for estimating the likely course of the disease; predictive biomarkers are used to predict patient's response to a particular therapy; therapeutic biomarkers help to identify new therapeutic targets (28). Biomarkers can also be used as a substitute for a clinical endpoint in clinical trials. The desired characteristics of biomarkers vary based on their intended use. For instance, high specificity is required if a biomarker is used for screening purpose. As stated by Morrow and de Lemos, biomarker should fulfill a set of criteria to be clinically useful: (1) it must be accurate, reproducible, easy to obtain and inexpensive; (2) it must provide added value over existing measures; (3) it must aid in clinical decision-making (29).
Statistical Assessments for the Evaluation of Biomarker Performance
Several statistical measures have been proposed for evaluating the utility of a new biomarker. The statistical association between a biomarker and the outcome can be assessed using metrics such as odds ratio, relative risk or hazard ratio. Statistical significance of an association is necessary but insufficient to provide information regarding the clinical contribution or usefulness of a new biomarker (30). Other measures including discrimination, calibration and reclassification are recommended for assessing the incremental contribution of a new biomarker to a conventional risk prediction model.
Discrimination refers to the ability of a biomarker to distinguish individuals who develop a disease from those who do not (31). The area under the receiver operating characteristic (AUC), which is equivalent to the c statistic, is the most used measure of model discrimination (32). The AUC is the probability that a randomly chosen individual with the disease has a higher predicted risk than a randomly chosen individual without the disease. Values for AUC range from 0.5 (no discrimination) to 1.0 (perfect discrimination). In general, the AUC > 0.7 indicates a good model. The increase in AUC can also be used to quantify the added predictive value offered by the new biomarker. However, the AUC is relatively insensitive to small improvements in model performance when the AUC of the baseline model is well-discriminated (33).
Calibration is also an important measure of model accuracy. It measures the ability of the model to accurately predict the proportion of individuals in a group who will develop the disease events. A risk prediction model is well-calibrated when the predicted probabilities agree with the observed frequencies of an event. Statistical metric of Hosmer-Lemeshow χ2 test is commonly used for assessing the calibration of a risk prediction model (34). A P < 0.05 for Hosmer-Lemeshow test indicates poor calibration of the model.
Reclassification refers to the ability to reclassify individuals into different risk categories. The reclassification measures including net reclassification index (NRI) and integrated discrimination improvement (IDI) have been proposed to quantify how well a new biomarker improves risk classification and as alternatives to the AUC (35). NRI is the net proportion of individuals with the event correctly reclassified “upward” (i.e., moving up to higher risk category) and the net proportion of individuals without the event correctly reclassified “downward” (i.e., moving down to lower risk category). This category-based NRI is highly sensitive to the number of risk categories and the choice of risk thresholds. Pencina et al., therefore, proposed a category-free version of the NRI to overcome the problem of selecting categories (36). Positive values of NRI indicate improved reclassification and negative values indicate worsened reclassification. On the other hand, IDI is independent of risk category and defined as the difference in discrimination slopes between models with and without the new marker (35). Discrimination slope is calculated as the difference between the average predicted probabilities for events and non-events.
In summary, there is no single statistical method can be used for evaluating the incremental value of a new biomarker. The metrics that used should be depending on the needs and objectives.
Methods
Search Strategy
A literature search was conducted using PubMed to identify all relevant studies. Research articles were also selected manually from the reference lists of articles. The search strategy used the terms “biomarker,” “coronary artery disease,” “cardiovascular disease,” “metabolic disease,” “cardiac troponin,” “natriuretic peptide,” “heart-type fatty acid-binding protein,” “adipokines,” “adiponectin,” “fibroblast growth factor,” “fatty acid binding protein,” “lipocalin 2,” “neutrophil gelatinase-associated lipocalin,” “retinol binding protein,” “plasminogen activator inhibitor,” “vitamin D,” “PCSK9,” and “risk prediction” in several combinations. Duplicated studies were identified and removed using Endnote duplicate function. The abstracts and titles of article retrieved were screened to exclude the irrelevant studies. Full-text articles were then examined to determine whether they met the inclusion criteria.
Inclusion and Exclusion Criteria
Inclusion criteria were: (1) studies investigating the association of a biomarker with metabolic and cardiovascular diseases, and adverse clinical outcomes such as cardiovascular events and death; (2) studies using blood serum or plasma for biomarker analysis; and (3) peer-reviewed articles and all types of reviews published in English between January 1980 and November 2020. Unpublished theses, reports, and conference proceedings were excluded. Animal studies were also excluded.
Data Extraction and Quality Assessment
Due to the heterogeneity of focus and results from the refined studies, we did not perform a meta-analysis as part of the review process. Data were extracted using a standardized form by one reviewer and verified by a second reviewer. The following data were extracted from eligible studies: first author, year of publication, country, study design, population characteristics and sample size, specimen type, follow-up duration, and main findings. The Newcastle-Ottawa Scale was used to assess the quality of the selected cohort and case-control studies, with a maximum score of nine points (37). The quality of the cross-sectional studies was assessed using the adapted version of the Newcastle-Ottawa Scale that awards a maximum score of 10 points (38). The Newcastle-Ottawa Scale assesses three main domains: selection, comparability, and outcome assessment. The AMSTAR-2 (A Measurement Tool to Assess Systematic Reviews-2) was used to evaluate the methodological quality of systematic reviews (39).
Results
Study Identification
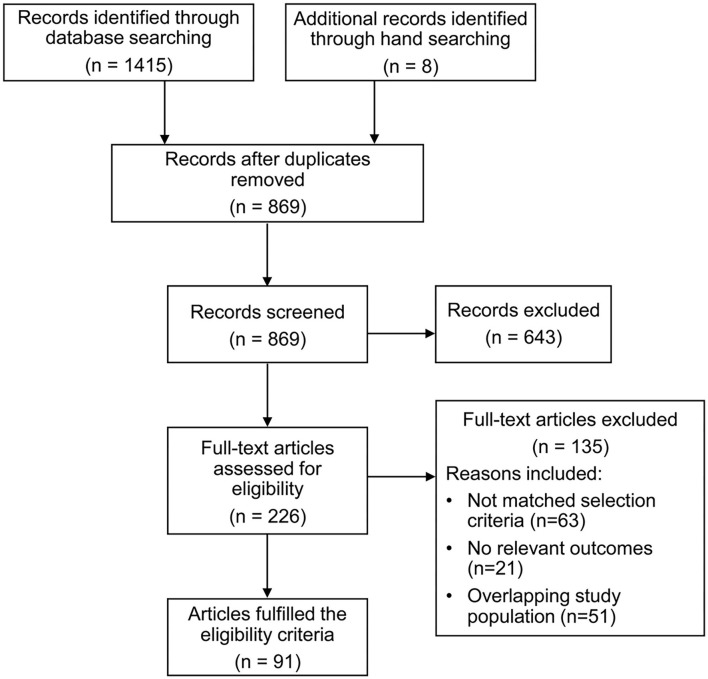
The study selection process is summarized in Figure 1. A total of 1,423 records were identified through the initial literature search. After removing 554 duplicates, the remaining 869 articles were screened, and 643 articles were excluded. The remaining 226 full-text articles were retrieved for detailed assessment. Ninety-one articles were identified to fulfill the eligibility criteria and were included in the final analysis.
Figure 1.
Flowchart of study selection process.
Study Characteristics
The 91 included studies were conducted in 24 countries and were published from 1986 to 2020. There were 43 cohort studies, 36 cross-sectional studies, 7 case-control studies, and 5 meta-analyses. The sample size of these observational studies ranged from 22 to 41,504, with a total of 135,811 participants. The following biomarkers were studies: 6 studies investigated cardiac troponin I, 10 investigated BNP or NT-proBNP, 9 reported on adiponectin, 8 reported on A-FABP, 5 reported on H-FABP, 12 on lipocalin-2, 12 on FGF-19 and/or FGF-21, 7 assessed RBP4, 7 assessed PAI-1, 8 reported on vitamin D, and 7 on PCSK9.
Quality Assessment
The results of study quality assessment are presented in Supplementary Table 1 for the cohort studies, in Supplementary Table 2 for the case-control studies, in Supplementary Table 3 for the cross-sectional studies, and in Supplementary Table 4 for the meta-analyses. According to the Newcastle-Ottawa Scale, 84 studies scored 7 or more points (good quality) and 2 studies scored 6 points (fair quality). Of the five included reviews, according to the AMSTAR-2 rating, three were rated as moderate or high quality and two were rated as low or critically low quality.
Role of Biomarkers in Cardiovascular Risk Assessment
Cardiac Troponin I
cTnI is one of the subunits of troponin regulatory complex that exclusively expressed in cardiac muscle, and released into the bloodstream after cardiac injury. cTnI is an established biomarker and clinically used as gold standard for the detection of myocardial injury (10). Increased levels of cTnI can be found in a variety of cardiac and non-cardiac conditions, including myocardial infarction, HF, pulmonary embolism, myocarditis, sepsis, and renal failure (40). Several studies have demonstrated that elevated high-sensitivity cardiac troponin I (hs-cTnI) levels in patients with HF were associated with poor prognosis and increased risk of mortality (41, 42). The addition of hs-cTnI to a traditional risk factor model improved the AUC by 0.05 for subsequent HF and cardiac death (43). Moreover, levels of hs-cTnI independently predicted adverse cardiovascular events in type 2 diabetes mellitus (T2DM) patients with ACS. Patients with hs-cTnI levels >99th percentile demonstrated a 4-fold higher risk of major cardiovascular events (44). Among patients with stable CAD, hs-cTnI has been shown to predict subsequent myocardial infarction and cardiovascular death during a median follow-up of 6 years (45). In a prospective study of patients with CAD, elevated hs-cTnI levels were higher in patients with more severe CAD, and were independently associated with adverse cardiovascular events and mortality. Addition of hs-cTnI improved the AUC by 0.03 and an NRI of 25% (46). These findings showed that hs-cTnI levels had an additive prognostic value for future cardiovascular outcomes over a conventional model with clinical risk factors. Supplementary Table 5 summarizes the studies on the predictive value of cTnI.
B-Type Natriuretic Peptide
BNP is a protein secreted by the cardiac ventricles in response to increased ventricular stretch or wall stress. It is also involved in regulating volume homeostasis and cardiovascular remodeling (47). BNP is synthesized as proBNP and is cleaved into active BNP and more stable NT-proBNP within cardiomyocytes. NT-proBNP has a longer half-life and lower variation than BNP. The clinical utility of BNP and NT-proBNP is largely similar (11). BNP and NT-proBNP are widely used for the diagnosis and risk stratification in patients with HF (48). Circulating BNP levels are lower in obese than in non-obese patients, and inversely correlated with body mass index (49). Higher levels of BNP have been found in patients with left ventricular hypertrophy and myocardial infarction (50). It has been proven that BNP level provides important prognostic information in patients with CAD, T2DM, and hypertension (51–53). Among patients with ACS and T2DM, BNP has been shown to be a powerful predictor of cardiovascular death, regardless of prior history or HF or any prior CVD (54). Another study has demonstrated that HF patients with elevated levels of BNP and cardiac troponin were at particularly high risk for mortality (55). Previous studies have also found that elevated BNP levels were associated with increased risk of adverse cardiovascular events and mortality in patients with CAD. The addition of BNP to a traditional risk factor model improved the AUC by 0.02 for prediction of adverse cardiovascular events (51, 56). Multi-marker approach based on NT-proBNP and cardiac troponin was associated with adverse events after adjustment for cardiovascular risk factors. The model incorporating a combination of NT-proBNP and cardiac troponin resulted in increases in the AUC, NRI, and IDI, suggesting that these biomarkers may serve as independent prognostic markers for CVD risk prediction (57). Supplementary Table 6 summarizes the studies on the predictive value of BNP/NT-proBNP.
Adiponectin
Adiponectin is an adipokine secreted by adipose tissues and exhibits anti-inflammatory, anti-atherogenic, and cardioprotective effects (12, 13). Adiponectin expression is reduced in obesity, insulin resistance, and T2DM, and the plasma level is inversely related to body mass index and components of metabolic syndrome such as triglycerides and insulin levels (58, 59). Lower adiponectin levels are associated with endothelial dysfunction, increased carotid intima-media thickness (IMT) and severity of CAD (60–62). Several studies have demonstrated that adiponectin could serve as a risk factor for CVD and had moderate accuracy for the identification of metabolic syndrome, with AUC ranged from 0.67 to 0.89 (63). Circulating adiponectin has also been shown to predict cardiovascular and all-cause mortality risk in patients with prevalent CVD (64). In patients with ACS, adiponectin was associated with higher risk of adverse cardiovascular outcomes (65). Another prospective study of patients with stable CAD also reported that higher level of adiponectin was associated with a 6-fold increased risk of all-cause mortality, with good discrimination ability (AUC, 0.78) (66). Supplementary Table 7 summarizes the studies on the predictive value of adiponectin.
Adipocyte Fatty Acid-Binding Protein
A-FABP is mainly expressed in adipocytes and macrophages, and has an important role in regulating glucose and lipid metabolism (14). Circulating A-FABP levels are closely linked to the development of obesity, insulin resistance, diabetes, hypertension, cardiac dysfunction, and atherosclerosis (15, 67). Elevated A-FABP levels are found in patients with CAD, and are positively correlated with metabolic syndrome and severity of coronary atherosclerosis (68, 69). Recent studies have shown that increased A-FABP concentrations were independently associated with increased risk of adverse cardiovascular events and cardiovascular mortality in patients with CAD (70–72). The association between A-FABP levels and cardiovascular events has also been observed in a prospective study with median follow-up of 9.4 years (73). Subjects with elevated A-FABP levels showed a 1.6-fold increased risk of cardiovascular events. The NRI and IDI were significantly improved by adding A-FABP to a traditional risk factor model (NRI, 18.6%; IDI, 0.25%). In another prospective study of patients with ACS, A-FABP was associated with a higher risk of adverse events, and demonstrated that the model with a combination of A-FABP and NT-proBNP may provide a better predictive performance than A-FABP alone, with the AUC increased from 0.65 to 0.68 (74). Supplementary Table 8 summarizes the studies on the predictive value of A-FABP.
Heart-Type Fatty Acid-Binding Protein
H-FABP is a low molecular-weight cytoplasmic protein that is abundant in the myocardium. H-FABP is released rapidly into the circulation in response to myocardial injury, and is therefore used as an early and sensitive diagnostic marker for myocardial infarction (16). It has been reported that serum H-FABP levels are elevated in patients with metabolic syndrome and pre-diabetic patients, and positively correlated with carotid IMT (75, 76). Circulating H-FABP level has also been shown to be a strong predictor of major cardiac events and mortality in patients with ACS, suggesting that H-FABP may provide incremental information for cardiovascular risk stratification that was independent of traditional risk factors, troponin I, and BNP (77). In patients with chronic heart failure, high H-FABP was associated with 5.4-fold higher risk cardiac events, and had a higher predictive value than BNP (AUC, 0.79 vs. 0.67) (78). A recent prospective study comprising of 4,594 patients with stable CAD showed that high levels of H-FABP were associated with increased risk of adverse cardiovascular events, and found a greater risk in CAD patients with impaired glucose metabolism (79). Supplementary Table 9 summarizes the studies on the predictive value of H-FABP.
Lipocalin-2
Lipocalin-2, also known as neutrophil gelatinase-associated lipocalin, belongs to the lipocalin superfamily, and was first identified in the specific granules of neutrophils (80). Lipocalin-2 is expressed in a various tissues including liver, kidney, lung, adipose tissue, stomach, and small intestine (81). There is also evidence to suggest that lipocalin-2 may play a role in vascular remodeling and plaque instability in atherosclerosis (17). Circulating lipocalin-2 levels are elevated in obese patients and patients with T2DM, and positively correlated with insulin resistance index and inflammatory markers (18, 82, 83). It has been reported that high levels of lipocalin-2 are associated with markers of atherosclerosis, presence and severity of CAD (84–86). In a population-based cohort study, lipocalin-2 level was an independent predictor of cardiovascular events in male subjects. The addition of lipocalin-2 to traditional risk factors improved the AUC from 0.77 to 0.81 (87). Serum lipocalin-2 levels were higher in patients with CAD or chronic HF compared with the healthy individuals (88, 89). Several studies have reported that elevated lipocalin-2 level was associated with increased risk of cardiovascular and all-cause mortality in patients with ST-segment elevation myocardial infarction after adjustment for conventional risk factors, with AUC ranging from 0.76 to 0.85, indicating a good predictive ability for prediction of mortality in these patients (90, 91). Elevated level of lipocalin-2 has also been found to be associated with a 4-fold higher risk of mortality in a 2-year follow-up study of patients with HF (92). Supplementary Table 10 summarizes the studies on the predictive value of lipocalin-2.
Fibroblast Growth Factor 19 and 21
FGF-19 and FGF-21 belong to the same subfamily of endocrine FGFs. The FGF family comprises of 22 members, which are classified into seven subfamilies based on the structural characteristics and mechanisms of action (93). FGF-19 is primarily secreted by the small intestine during feeding, and FGF-21 is secreted by the liver during fasting, with both FGF-19 and FGF-21 share similar functions in regulating lipid, glucose and energy metabolism (19). It has been shown that circulating levels of FGF-19 are decreased in obese patients and T2DM patients with metabolic syndrome, and are inversely correlated with fasting glucose levels (20, 21, 94). In a study of 315 patients, serum FGF-19 levels were significantly lower in patients with CAD than those in the control group, and were independently associated with severity of CAD (95). On the other hand, levels of FGF-21 are elevated in patients with T2DM and those with established CAD, and are strongly associated with body mass index, triglycerides, insulin resistance, and serum A-FABP levels (96, 97). High FGF-21 level has also been reported to be an independent predictor of the development of T2DM and metabolic syndrome (98, 99). A prior study recruited individuals who underwent carotid IMT assessment demonstrated that elevated FGF-21 levels were associated with the presence of carotid atherosclerosis (100). Serum FGF-21 level was increased in patients with acute myocardial infarction compared to the control group, and associated with a higher risk of adverse cardiovascular event after follow-up of 24 months. The predictive performance of FGF-21 level was modest with an AUC of 0.67 (101). In patients with CAD, elevated FGF-21 level was associated with increased risk of cardiovascular events and mortality after adjustment for traditional cardiovascular risk factors (102, 103). Supplementary Table 11 summarizes the studies on the predictive value of FGF-19 and FGF-21.
Retinol-Binding Protein 4
RBP4 is a member of the lipocalin family and the sole retinol transporter in blood. It is mainly secreted by the human liver and adipose tissue (104). Previous studies have revealed that RBP4 concentrations were elevated in patients with obesity and T2DM, and were associated with insulin resistance (105). Other studies have also demonstrated strong correlations of increased RBP4 levels with carotid IMT and components of the metabolic syndrome including hypertension, hypertriglyceridemia, and waist circumference, suggesting that RBP4 may serve as a marker of metabolic complications and atherosclerosis (22, 23, 106). Moreover, circulating RBP4 levels have been shown to be correlated with CVD. A recent study reported that RBP4 levels were higher in patients with CAD than those in control subjects, and were positively correlated with the prevalent and severity of CAD (107). Elevated RBP4 level was associated with an increased risk of CAD in a 16-year follow-up study of women subjects (108). It has also been reported that serum RBP4 level is an independent predictor of adverse cardiovascular events in patients with chronic HF after adjustment for cardiovascular risk factors, and shows good prognostic performance with an AUC of 0.74 (109). Supplementary Table 12 summarizes the studies on the predictive value of RBP4.
Plasminogen Activator Inhibitor-1
PAI-1, a member of the serine protease inhibitor (serpin) family, is the primary inhibitor of both the tissue-type and the urinary-type plasminogen activator (110). PAI-1 is mainly secreted by endothelial cells and various tissue types such as liver and adipose tissue. It is also involved in various physiological and pathological processes including fibrinolysis, tissue modeling, cancer, inflammation and CVD (24, 111, 112). Circulating levels of PAI-1 are increased in obesity, insulin resistance, and T2DM (113, 114). Elevated plasma PAI-1 levels have been reported to be an independent predictor of CVD in patients with myocardial infarction (115). Recently, a study revealed that elevated PAI-1 level was causally associated with incident CAD, suggesting that PAI-1 may have a role in the pathogenesis of CAD (116). Several studies have also demonstrated that elevated PAI-1 levels were associated with adverse cardiovascular events in patients with established CAD (117). In a prospective study of patients with ST-elevation myocardial infarction, high PAI-1 level was associated with a 5.5-fold increased risk of 5-year mortality, with an AUC of 0.75 (118). Furthermore, in the study of the Framingham Offspring study, Tofler et al. showed that both baseline and serial changes in PAI-1 levels were associated with subsequent risk of CVD, but only modest improvement in the AUCs were observed when adding PAI-1 to the traditional risk factor model (119). Supplementary Table 13 summarizes the studies on the predictive value of PAI-1.
Vitamin D
Vitamin D is a secosteroid hormone that involves in maintaining calcium and phosphorus homeostasis, and promoting bone mineralization. 25-hydroxyvitamin D concentrations is the best indicator of vitamin D status (120). Vitamin D deficiency is often associated with bone disorders such as rickets and osteoporosis. Vitamin D has also been linked to non-skeletal diseases, including cancer, CVDs, obesity, diabetes and hypertension (25). Low vitamin D level has been found to be independently associated with increased carotid IMT and presence of carotid plaque, suggesting a potential role of vitamin D in the development of atherosclerosis (121). In addition, vitamin D deficiency was found to be associated with the prevalence and severity of CAD (122). Several studies have demonstrated that low vitamin D level was associated with increased risk of cardiovascular events including myocardial infarction (123–125). In a prospective study of 41,504 individuals, vitamin D deficiency was associated with higher prevalence of diabetes, hypertension, hyperlipidemia, and peripheral vascular disease. Patients with vitamin D level below 15 ng/mL demonstrated a 2-fold higher risk of adverse outcomes than those with normal level (126). Another large prospective study also reported that low vitamin D levels were associated with increased risk of ischemic heart disease, myocardial infarction and early death (127). More recently, a study showed that serum vitamin D levels on admission were associated with in-hospital mortality in patients with acute pulmonary embolism. A cut-off level of vitamin D ≤6.47 ng/mL was optimum for the prediction of in-hospital mortality with an AUC of 0.81, suggesting that vitamin D may be a potential prognostic biomarker for pulmonary embolism (128). Supplementary Table 14 summarizes the studies on the predictive value of 25-hydroxyvitamin D.
Proprotein Convertase Subtilisin/Kexin Type 9
PCSK9, a member of the proprotein convertase family, is predominantly produced in the liver and plays a key role in cholesterol homeostasis. It reduces the low-density lipoprotein intake from circulation by enhancing the degradation of hepatic low-density lipoprotein receptor (26). Circulating PCSK9 concentrations are elevated in patients with metabolic syndrome, T2DM, and obesity (129–131). In a study of 126 with hypertensive patients, serum PCSK9 was associated with carotid IMT (132). Several studies have reported that PCSK9 levels were associated with the severity of coronary stenosis in patients with ACS, after adjustment for established risk factors (133). In a prospective study of 1,225 patients with stable CAD, elevated PCSK9 levels were related to cardiovascular metabolic markers such as total cholesterol and hemoglobin A1c, and independently associated with increased risk of adverse cardiovascular events. Patients with T2DM and high PCSK9 levels demonstrated a 5-fold increased risk of adverse cardiovascular events compared with non-diabetic patients with low PCSK9 levels (134). The association of PCSK9 levels with cardiovascular events was also observed in patients with CAD on statin treatment (135). Supplementary Table 15 summarizes the studies on the predictive value of PCSK9.
Discussion
Accurate risk stratification tools are important for clinical risk prediction and treatment strategy, particularly for individuals in higher risk groups. The selected biomarkers in this review are closely linked with CVD and have shown promise in improving the prediction of adverse cardiovascular events for primary and secondary prevention. However, validation of potential biomarkers on a larger scale remains challenging and their clinical utility in stable CAD patients is still to be determined. There is some controversy regarding which biomarker is more suitable for the prognosis of CAD. The multi-biomarker approach may help overcome some of the limitations of individual markers and improve the prognostic accuracy. It has been suggested that the strategy of combining biomarkers from different pathways is more likely to be clinically useful than biomarkers in the same pathway, and may provide greater discriminative ability than individual biomarker. For example, Hillis et al. demonstrated that the combined model of NT-proBNP and cardiac troponin provided better prognostic information with regard to the risk for future cardiovascular events than the use of a single biomarker (57). Reiser et al. also reported that the combination of NT-proBNP and A-FABP yielded a more accurate predictive value for adverse outcomes in patients with ACS (74). These findings provide new insights into the potential use of multiple biomarkers related to cardiovascular and metabolic pathways to improve strategies for secondary prevention of CVD.
Cost is also an important consideration when selecting biomarkers for risk prediction models. Some biomarkers can be expensive to measure and other practical issues such as collection, storage and handling of samples may affect the cost of a biomarker model. Moreover, the economic burden on healthcare system after implementation of biomarker prediction tools may include the costs of: (i) additional biomarker tests; (ii) detailed assessments for risk estimation; and (iii) new therapies or interventions for treating high-risk patients to reduce risk. Although the overall costs may be increased, it may be cost-effective if health outcomes are improved sufficiently. Further evaluation of the cost-effectiveness of using biomarker prediction tools is needed to inform health policy as well as to guide clinical decisions.
In conclusion, our study revealed that these biomarkers representing different pathophysiological pathways could help to improve risk stratification for CVD. Further work is warranted to identify optimal combination of biomarkers for risk stratification of secondary prevention patients. In addition, validation studies are still required to confirm the applicability of these biomarkers in CVD risk prediction.
Author Contributions
Y-KW wrote the manuscript. H-FT reviewed the manuscript. Both authors read and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- ACS
acute coronary syndrome
- A-FABP
adipocyte fatty acid-binding protein
- AUC
area under the curve
- CAD
coronary artery disease
- hs-cTnI
high-sensitivity cardiac troponin I
- CVD
cardiovascular disease
- BNP
B-type natriuretic peptide
- FGF
fibroblast growth factor
- HF
heart failure
- H-FABP
heart-type fatty acid-binding protein
- IDI
integrated discrimination improvement
- IMT
intima-media thickness
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- NRI
net reclassification index
- PAI
plasminogen activator inhibitor
- PCSK9
proprotein convertase subtilisin/kexin type 9
- RBP
retinol-binding protein
- T2DM
type 2 diabetes mellitus.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.713191/full#supplementary-material
References
- 1.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart study. Circulation. (2008) 117:743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 2.Omland T, White HD. State of the art: blood biomarkers for risk stratification in patients with stable ischemic heart disease. Clin Chem. (2017) 63:165–76. 10.1373/clinchem.2016.255190 [DOI] [PubMed] [Google Scholar]
- 3.Nadar SK, Shaikh MM. Biomarkers in routine heart failure clinical care. Card Fail Rev. (2019) 5:50–6. 10.15420/cfr.2018.27.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvagno GL, Pavan C. Prognostic biomarkers in acute coronary syndrome. Ann Transl Med. (2016) 4:258. 10.21037/atm.2016.06.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindholm D, Lindback J, Armstrong PW, Budaj A, Cannon CP, Granger CB, et al. Biomarker-based risk model to predict cardiovascular mortality in patients with stable coronary disease. J Am Coll Cardiol. (2017) 70:813–26. 10.1016/j.jacc.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 6.Kleber ME, Goliasch G, Grammer TB, Pilz S, Tomaschitz A, Silbernagel G, et al. Evolving biomarkers improve prediction of long-term mortality in patients with stable coronary artery disease: the BIO-VILCAD score. J Intern Med. (2014) 276:184–94. 10.1111/joim.12189 [DOI] [PubMed] [Google Scholar]
- 7.Rusnak J, Fastner C, Behnes M, Mashayekhi K, Borggrefe M, Akin I. Biomarkers in stable coronary artery disease. Curr Pharm Biotechnol. (2017) 18:456–71. 10.2174/1389201018666170630120805 [DOI] [PubMed] [Google Scholar]
- 8.Onda T, Inoue K, Suwa S, Nishizaki Y, Kasai T, Kimura Y, et al. Reevaluation of cardiac risk scores and multiple biomarkers for the prediction of first major cardiovascular events and death in the drug-eluting stent era. Int J Cardiol. (2016) 219:180–5. 10.1016/j.ijcard.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 9.Busch A, Eken SM, Maegdefessel L. Prospective and therapeutic screening value of non-coding RNA as biomarkers in cardiovascular disease. Ann Transl Med. (2016) 4:236. 10.21037/atm.2016.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasic S, Kiseljakovic E, Jadric R, Radovanovic J, Winterhalter-Jadric M. Cardiac troponin I: the gold standard in acute myocardial infarction diagnosis. Bosn J Basic Med Sci. (2003) 3:41–4. 10.17305/bjbms.2003.3527 [DOI] [PubMed] [Google Scholar]
- 11.Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. (2006) 92:843–9. 10.1136/hrt.2005.071233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanayakkara G, Kariharan T, Wang L, Zhong J, Amin R. The cardio-protective signaling and mechanisms of adiponectin. Am J Cardiovasc Dis. (2012) 2:253–66. [PMC free article] [PubMed] [Google Scholar]
- 13.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. (2007) 380:24–30. 10.1016/j.cca.2007.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. (2013) 17:768–78. 10.1016/j.cmet.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuhashi M, Saitoh S, Shimamoto K, Miura T. Fatty Acid-Binding Protein 4 (FABP4): pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin Med Insights Cardiol. (2014) 8:23–33. 10.4137/CMC.S17067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glatz JF, van der Vusse GJ, Simoons ML, Kragten JA, van Dieijen-Visser MP, Hermens WT. Fatty acid-binding protein and the early detection of acute myocardial infarction. Clin Chim Acta. (1998) 272:87–92. 10.1016/s0009-8981(97)00255-6 [DOI] [PubMed] [Google Scholar]
- 17.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. (2006) 26:136–42. 10.1161/01.ATV.0000193567.88685.f4 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. (2007) 53:34–41. 10.1373/clinchem.2006.075614 [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Yu L, Lin X, Cheng P, He L, Li X, et al. Minireview: roles of fibroblast growth factors 19 and 21 in metabolic regulation and chronic diseases. Mol Endocrinol. (2015) 29:1400–13. 10.1210/me.2015-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barutcuoglu B, Basol G, Cakir Y, Cetinkalp S, Parildar Z, Kabaroglu C, et al. Fibroblast growth factor-19 levels in type 2 diabetic patients with metabolic syndrome. Ann Clin Lab Sci. (2011) 41:390–6. [PubMed] [Google Scholar]
- 21.Wang D, Zhu W, Li J, An C, Wang Z. Serum concentrations of fibroblast growth factors 19 and 21 in women with gestational diabetes mellitus: association with insulin resistance, adiponectin, and polycystic ovary syndrome history. PLoS ONE. (2013) 8:e81190. 10.1371/journal.pone.0081190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobbert T, Raila J, Schwarz F, Mai K, Henze A, Pfeiffer AF, et al. Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis. (2010) 213:549–51. 10.1016/j.atherosclerosis.2010.07.063 [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Wang D, Li D, Sun R, Xia M. Associations of retinol-binding protein 4 with oxidative stress, inflammatory markers, and metabolic syndrome in a middle-aged and elderly Chinese population. Diabetol Metab Syndr. (2014) 6:25. 10.1186/1758-5996-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. (2010) 28:e72–91. 10.1111/j.1755-5922.2010.00171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kheiri B, Abdalla A, Osman M, Ahmed S, Hassan M, Bachuwa G. Vitamin D deficiency and risk of cardiovascular diseases: a narrative review. Clin Hypertens. (2018) 24:9. 10.1186/s40885-018-0094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. (2003) 100:928–33. 10.1073/pnas.0335507100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. (2001) 69:89–95. 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- 28.Carlomagno N, Incollingo P, Tammaro V, Peluso G, Rupealta N, Chiacchio G, et al. Diagnostic, predictive, prognostic, and therapeutic molecular biomarkers in third millennium: a breakthrough in gastric cancer. Biomed Res Int. (2017) 2017:7869802. 10.1155/2017/7869802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. (2007) 115:949–52. 10.1161/CIRCULATIONAHA.106.683110 [DOI] [PubMed] [Google Scholar]
- 30.Koenig W. Cardiovascular biomarkers: added value with an integrated approach? Circulation. (2007) 116:3–5. 10.1161/CIRCULATIONAHA.107.707984 [DOI] [PubMed] [Google Scholar]
- 31.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. (2009) 119:2408–16. 10.1161/CIRCULATIONAHA.109.192278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. (1982) 143:29–36. 10.1148/radiology.143.1.7063747 [DOI] [PubMed] [Google Scholar]
- 33.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. (2007) 115:928–35. 10.1161/CIRCULATIONAHA.106.672402 [DOI] [PubMed] [Google Scholar]
- 34.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A. comparison of goodness-of-fit tests for the logistic regression model. Stat Med. (1997) 16:965–80. [DOI] [PubMed] [Google Scholar]
- 35.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. (2008) 27:157–72; discussion 207–12. 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 36.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. (2011) 30:11–21. 10.1002/sim.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed July 15, 2021).
- 38.Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. (2013) 13:154. 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest. (2004) 125:1877–84. 10.1378/chest.125.5.1877 [DOI] [PubMed] [Google Scholar]
- 41.You JJ, Austin PC, Alter DA, Ko DT, Tu JV. Relation between cardiac troponin I and mortality in acute decompensated heart failure. Am Heart J. (2007) 153:462–70. 10.1016/j.ahj.2007.01.027 [DOI] [PubMed] [Google Scholar]
- 42.Myhre PL, O'Meara E, Claggett BL, de Denus S, Jarolim P, Anand IS. Cardiac troponin I and risk of cardiac events in patients with heart failure and preserved ejection fraction. Circ Heart Fail. (2018) 11:e005312. 10.1161/CIRCHEARTFAILURE.118.005312 [DOI] [PubMed] [Google Scholar]
- 43.Stelzle D, Shah ASV, Anand A, Strachan FE, Chapman AR, Denvir MA, et al. High-sensitivity cardiac troponin I and risk of heart failure in patients with suspected acute coronary syndrome: a cohort study. Eur Heart J Qual Care Clin Outcomes. (2018) 4:36–42. 10.1093/ehjqcco/qcx022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavender MA, White WB, Jarolim P, Bakris GL, Cushman WC, Kupfer S, et al. Serial measurement of high-sensitivity troponin I and cardiovascular outcomes in patients with type 2 diabetes mellitus in the EXAMINE trial (Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care). Circulation. (2017) 135:1911–21. 10.1161/CIRCULATIONAHA.116.024632 [DOI] [PubMed] [Google Scholar]
- 45.White HD, Tonkin A, Simes J, Stewart R, Mann K, Thompson P, et al. Association of contemporary sensitive troponin I levels at baseline and change at 1 year with long-term coronary events following myocardial infarction or unstable angina: results from the LIPID Study (Long-Term Intervention With Pravastatin in Ischaemic Disease). J Am Coll Cardiol. (2014) 63:345–54. 10.1016/j.jacc.2013.08.1643 [DOI] [PubMed] [Google Scholar]
- 46.Samman Tahhan A, Sandesara P, Hayek SS, Hammadah M, Alkhoder A, Kelli HM, et al. High-sensitivity troponin i levels and coronary artery disease severity, progression, and long-term outcomes. J Am Heart Assoc. (2018) 7:e007914. 10.1161/JAHA.117.007914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. (2006) 69:318–28. 10.1016/j.cardiores.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 48.Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol. (2008) 52:997–1003. 10.1016/j.jacc.2008.04.069 [DOI] [PubMed] [Google Scholar]
- 49.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. (2004) 109:594–600. 10.1161/01.CIR.0000112582.16683.EA [DOI] [PubMed] [Google Scholar]
- 50.Morita E, Yasue H, Yoshimura M, Ogawa H, Jougasaki M, Matsumura T, et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation. (1993) 88:82–91. [DOI] [PubMed] [Google Scholar]
- 51.Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R, et al. Prognostic value of B-Type natriuretic peptides in patients with stable coronary artery disease: the PEACE trial. J Am Coll Cardiol. (2007) 50:205–14. 10.1016/j.jacc.2007.03.038 [DOI] [PubMed] [Google Scholar]
- 52.Bhalla MA, Chiang A, Epshteyn VA, Kazanegra R, Bhalla V, Clopton P, et al. Prognostic role of B-type natriuretic peptide levels in patients with type 2 diabetes mellitus. J Am Coll Cardiol. (2004) 44:1047–52. 10.1016/j.jacc.2004.05.071 [DOI] [PubMed] [Google Scholar]
- 53.Paget V, Legedz L, Gaudebout N, Girerd N, Bricca G, Milon H, et al. N-terminal pro-brain natriuretic peptide: a powerful predictor of mortality in hypertension. Hypertension. (2011) 57:702–9. 10.1161/HYPERTENSIONAHA.110.163550 [DOI] [PubMed] [Google Scholar]
- 54.Wolsk E, Claggett B, Pfeffer MA, Diaz R, Dickstein K, Gerstein HC, et al. Role of B-type natriuretic peptide and N-terminal prohormone BNP as predictors of cardiovascular morbidity and mortality in patients with a recent coronary event and type 2 diabetes mellitus. J Am Heart Assoc. (2017) 6:e004743. 10.1161/JAHA.116.004743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fonarow GC, Peacock WF, Horwich TB, Phillips CO, Givertz MM, Lopatin M, et al. Usefulness of B-type natriuretic peptide and cardiac troponin levels to predict in-hospital mortality from ADHERE. Am J Cardiol. (2008) 101:231–7. 10.1016/j.amjcard.2007.07.066 [DOI] [PubMed] [Google Scholar]
- 56.Mishra RK, Beatty AL, Jaganath R, Regan M, Wu AH, Whooley MA. B-type natriuretic peptides for the prediction of cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. J Am Heart Assoc. (2014) 3:e000907. 10.1161/JAHA.114.000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hillis GS, Welsh P, Chalmers J, Perkovic V, Chow CK, Li Q, et al. The relative and combined ability of high-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide to predict cardiovascular events and death in patients with type 2 diabetes. Diabetes Care. (2014) 37:295–303. 10.2337/dc13-1165 [DOI] [PubMed] [Google Scholar]
- 58.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. (2000) 20:1595–9. 10.1161/01.atv.20.6.1595 [DOI] [PubMed] [Google Scholar]
- 59.Ng TW, Watts GF, Farvid MS, Chan DC, Barrett PH. Adipocytokines and VLDL metabolism: independent regulatory effects of adiponectin, insulin resistance, and fat compartments on VLDL apolipoprotein B-100 kinetics? Diabetes. (2005) 54:795–802. 10.2337/diabetes.54.3.795 [DOI] [PubMed] [Google Scholar]
- 60.Inoue T, Kotooka N, Morooka T, Komoda H, Uchida T, Aso Y, et al. High molecular weight adiponectin as a predictor of long-term clinical outcome in patients with coronary artery disease. Am J Cardiol. (2007) 100:569–74. 10.1016/j.amjcard.2007.03.062 [DOI] [PubMed] [Google Scholar]
- 61.Gardener H, Sjoberg C, Crisby M, Goldberg R, Mendez A, Wright CB, et al. Adiponectin and carotid intima-media thickness in the northern Manhattan study. Stroke. (2012) 43:1123–5. 10.1161/STROKEAHA.111.641761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. (2003) 88:3236–40. 10.1210/jc.2002-021883 [DOI] [PubMed] [Google Scholar]
- 63.Liu Z, Liang S, Que S, Zhou L, Zheng S, Mardinoglu A. Meta-analysis of adiponectin as a biomarker for the detection of metabolic syndrome. Front Physiol. (2018) 9:1238. 10.3389/fphys.2018.01238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L, Li B, Zhao Y, Zhang Z. Prognostic value of adiponectin level in patients with coronary artery disease: a systematic review and meta-analysis. Lipids Health Dis. (2019) 18:227. 10.1186/s12944-019-1168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliveira GB, Franca JI, Piegas LS. Serum adiponectin and cardiometabolic risk in patients with acute coronary syndromes. Arq Bras Cardiol. (2013) 101:399–409. 10.5935/abc.20130186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pratesi A, Di Serio C, Orso F, Foschini A, Bartoli N, Marella A, et al. Prognostic value of adiponectin in coronary artery disease: role of diabetes and left ventricular systolic dysfunction. Diabetes Res Clin Pract. (2016) 118:58–66. 10.1016/j.diabres.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 67.Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. (2006) 52:405–13. 10.1373/clinchem.2005.062463 [DOI] [PubMed] [Google Scholar]
- 68.Bao Y, Lu Z, Zhou M, Li H, Wang Y, Gao M, et al. Serum levels of adipocyte fatty acid-binding protein are associated with the severity of coronary artery disease in Chinese women. PLoS ONE. (2011) 6:e19115. 10.1371/journal.pone.0019115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu BG, Chen YC, Lee RP, Lee CC, Lee CJ, Wang JH. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ J. (2010) 74:327–31. 10.1253/circj.cj-09-0568 [DOI] [PubMed] [Google Scholar]
- 70.Huang IC, Hsu BG, Chang CC, Lee CJ, Wang JH. High levels of serum adipocyte fatty acid-binding protein predict cardiovascular events in coronary artery disease patients. Int J Med Sci. (2018) 15:1268–74. 10.7150/ijms.25588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takagi W, Miyoshi T, Doi M, Okawa K, Nosaka K, Nishibe T, et al. Circulating adipocyte fatty acid-binding protein is a predictor of cardiovascular events in patients with stable angina undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. (2017) 17:258. 10.1186/s12872-017-0691-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.von Eynatten M, Breitling LP, Roos M, Baumann M, Rothenbacher D, Brenner H. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol. (2012) 32:2327–35. 10.1161/ATVBAHA.112.248609 [DOI] [PubMed] [Google Scholar]
- 73.Chow WS, Tso AWK, Xu AM, Yuen MMA, Fong CHY, Lam TH, et al. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc. (2013) 2:e004176. 10.1161/JAHA.112.004176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reiser H, Klingenberg R, Hof D, Cooksley-Decasper S, Fuchs N, Akhmedov A, et al. Circulating FABP4 is a prognostic biomarker in patients with acute coronary syndrome but not in asymptomatic individuals. Arterioscler Thromb Vasc Biol. (2015) 35:1872–9. 10.1161/ATVBAHA.115.305365 [DOI] [PubMed] [Google Scholar]
- 75.Akbal E, Ozbek M, Gunes F, Akyurek O, Ureten K, Delibasi T. Serum heart type fatty acid binding protein levels in metabolic syndrome. Endocrine. (2009) 36:433–7. 10.1007/s12020-009-9243-6 [DOI] [PubMed] [Google Scholar]
- 76.Ramesh P, Chauhan A, Goyal P, Singh A. H-FABPA. beacon of hope for prediabetic heart disease. J Family Med Prim Care. (2020) 9:3421–28. 10.4103/jfmpc.jfmpc_296_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Donoghue M, de Lemos JA, Morrow DA, Murphy SA, Buros JL, Cannon CP, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. (2006) 114:550–7. 10.1161/CIRCULATIONAHA.106.641936 [DOI] [PubMed] [Google Scholar]
- 78.Niizeki T, Takeishi Y, Arimoto T, Takahashi T, Okuyama H, Takabatake N, et al. Combination of heart-type fatty acid binding protein and brain natriuretic peptide can reliably risk stratify patients hospitalized for chronic heart failure. Circ J. (2005) 69:922–7. 10.1253/circj.69.922 [DOI] [PubMed] [Google Scholar]
- 79.Zhang HW, Jin JL, Cao YX, Liu HH, Zhang Y, Guo YL, et al. Prognostic utility of heart-type fatty acid-binding protein in patients with stable coronary artery disease and impaired glucose metabolism: a cohort study. Cardiovasc Diabetol. (2020) 19:15. 10.1186/s12933-020-0992-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. (1994) 83:799–807. [PubMed] [Google Scholar]
- 81.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. (1997) 45:17–23. 10.1006/geno.1997.4896 [DOI] [PubMed] [Google Scholar]
- 82.De la Chesnaye E, Manuel-Apolinar L, Oviedo-de Anda N, Revilla-Monsalve MC, Islas-Andrade S. Gender differences in lipocalin 2 plasmatic levels are correlated with age and the triglyceride/high-density lipoprotein ratio in healthy individuals. Gac Med Mex. (2016) 152:612–17. [PubMed] [Google Scholar]
- 83.Elkhidir AE, Eltaher HB, Mohamed AO. Association of lipocalin-2 level, glycemic status and obesity in type 2 diabetes mellitus. BMC Res Notes. (2017) 10:285. 10.1186/s13104-017-2604-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zografos T, Haliassos A, Korovesis S, Giazitzoglou E, Voridis E, Katritsis D. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am J Cardiol. (2009) 104:917–20. 10.1016/j.amjcard.2009.05.023 [DOI] [PubMed] [Google Scholar]
- 85.Ni J, Ma X, Zhou M, Pan X, Tang J, Hao Y, et al. Serum lipocalin-2 levels positively correlate with coronary artery disease and metabolic syndrome. Cardiovasc Diabetol. (2013) 12:176. 10.1186/1475-2840-12-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao Y, Xu A, Hui X, Zhou P, Li X, Zhong H, et al. Circulating lipocalin-2 and retinol-binding protein 4 are associated with intima-media thickness and subclinical atherosclerosis in patients with type 2 diabetes. PLoS ONE. (2013) 8:e66607. 10.1371/journal.pone.0066607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu G, Li H, Fang Q, Jiang S, Zhang L, Zhang J, et al. Elevated circulating lipocalin-2 levels independently predict incident cardiovascular events in men in a population-based cohort. Arterioscler Thromb Vasc Biol. (2014) 34:2457–64. 10.1161/ATVBAHA.114.303718 [DOI] [PubMed] [Google Scholar]
- 88.Choi KM, Lee JS, Kim EJ, Baik SH, Seo HS, Choi DS, et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol. (2008) 158:203–7. 10.1530/EJE-07-0633 [DOI] [PubMed] [Google Scholar]
- 89.Yndestad A, Landro L, Ueland T, Dahl CP, Flo TH, Vinge LE, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. (2009) 30:1229–36. 10.1093/eurheartj/ehp088 [DOI] [PubMed] [Google Scholar]
- 90.Akcay AB, Ozlu MF, Sen N, Cay S, Ozturk OH, Yalcn F, et al. Prognostic significance of neutrophil gelatinase-associated lipocalin in ST-segment elevation myocardial infarction. J Investig Med. (2012) 60:508–13. 10.2310/JIM.0b013e31823e9d86 [DOI] [PubMed] [Google Scholar]
- 91.Avci A, Ozturk B, Demir K, Akyurek F, Altunkeser BB. The prognostic utility of plasma NGAL levels in ST segment elevation in myocardial infarction patients. Adv Prev Med. (2020) 2020:4637043. 10.1155/2020/4637043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bolignano D, Basile G, Parisi P, Coppolino G, Nicocia G, Buemi M. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res. (2009) 12:7–14. 10.1089/rej.2008.0803 [DOI] [PubMed] [Google Scholar]
- 93.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. (2004) 20:563–9. 10.1016/j.tig.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 94.Fang Q, Li H, Song Q, Yang W, Hou X, Ma X, et al. Serum fibroblast growth factor 19 levels are decreased in Chinese subjects with impaired fasting glucose and inversely associated with fasting plasma glucose levels. Diabetes Care. (2013) 36:2810–4. 10.2337/dc12-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hao YP, Zhou J, Zhou M, Ma XJ, Lu ZG, Gao MF, et al. Serum levels of fibroblast growth factor 19 are inversely associated with coronary artery disease in chinese individuals. PLoS ONE. (2013) 8:e72345. 10.1371/journal.pone.0072345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. (2008) 57:1246–53. 10.2337/db07-1476 [DOI] [PubMed] [Google Scholar]
- 97.Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, et al. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS ONE. (2010) 5:e15534. 10.1371/journal.pone.0015534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bobbert T, Schwarz F, Fischer-Rosinsky A, Pfeiffer AF, Mohlig M, Mai K, et al. Fibroblast growth factor 21 predicts the metabolic syndrome and type 2 diabetes in Caucasians. Diabetes Care. (2013) 36:145–9. 10.2337/dc12-0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen C, Cheung BM, Tso AW, Wang Y, Law LS, Ong KL, et al. High plasma level of fibroblast growth factor 21 is an Independent predictor of type 2 diabetes: a 54-year population-based prospective study in Chinese subjects. Diabetes Care. (2011) 34:2113–5. 10.2337/dc11-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, et al. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol. (2013) 33:2454–9. 10.1161/ATVBAHA.113.301599 [DOI] [PubMed] [Google Scholar]
- 101.Chen H, Lu N, Zheng M. A high circulating FGF21 level as a prognostic marker in patients with acute myocardial infarction. Am J Transl Res. (2018) 10:2958–66. [PMC free article] [PubMed] [Google Scholar]
- 102.Shen Y, Zhang X, Xu Y, Xiong Q, Lu Z, Ma X, et al. Serum FGF21 is associated with future cardiovascular events in patients with coronary artery disease. Cardiology. (2018) 139:212–18. 10.1159/000486127 [DOI] [PubMed] [Google Scholar]
- 103.Li Q, Zhang Y, Ding D, Yang YN, Chen Q, Su DF, et al. Association between serum fibroblast growth factor 21 and mortality among patients with coronary artery disease. J Clin Endocr Metab. (2016) 101:4886–94. 10.1210/jc.2016-2308 [DOI] [PubMed] [Google Scholar]
- 104.Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. (2011) 165:703–11. 10.1530/EJE-11-0431 [DOI] [PubMed] [Google Scholar]
- 105.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. (2005) 436:356–62. 10.1038/nature03711 [DOI] [PubMed] [Google Scholar]
- 106.Majerczyk M, Kocelak P, Choreza P, Arabzada H, Owczarek AJ, Bozentowicz-Wikarek M, et al. Components of metabolic syndrome in relation to plasma levels of retinol binding protein 4 (RBP4) in a cohort of people aged 65 years and older. J Endocrinol Invest. (2018) 41:1211–19. 10.1007/s40618-018-0856-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lambadiari V, Kadoglou NP, Stasinos V, Maratou E, Antoniadis A, Kolokathis F, et al. Serum levels of retinol-binding protein-4 are associated with the presence and severity of coronary artery disease. Cardiovasc Diabetol. (2014) 13:121. 10.1186/s12933-014-0121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun Q, Kiernan UA, Shi L, Phillips DA, Kahn BB, Hu FB, et al. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the nurses' health study. Circulation. (2013) 127:1938–47. 10.1161/CIRCULATIONAHA.113.002073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li XZ, Zhang KZ, Yan JJ, Wang L, Wang Y, Shen XY, et al. Serum retinol-binding protein 4 as a predictor of cardiovascular events in elderly patients with chronic heart failure. ESC Heart Fail. (2020) 7(2):542–50. 10.1002/ehf2.12591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sui GC, Mangs H, Wiman B. The role of His(143) for the pH-dependent stability of plasminogen activator inhibitor-1. Biochim Biophys Acta. (1999) 1434:58–63. 10.1016/s0167-4838(99)00157-0 [DOI] [PubMed] [Google Scholar]
- 111.Gils A, Declerck PJ. The structural basis for the pathophysiological relevance of PAI-I in cardiovascular diseases and the development of potential PAI-I inhibitors. Thromb Haemost. (2004) 91:425–37. 10.1160/TH03-12-0764 [DOI] [PubMed] [Google Scholar]
- 112.Zorio E, Gilabert-Estelles J, Espana F, Ramon LA, Cosin R, Estelles A. Fibrinolysis: the key to new pathogenetic mechanisms. Curr Med Chem. (2008) 15:923–9. 10.2174/092986708783955455 [DOI] [PubMed] [Google Scholar]
- 113.Vague P, Juhan-Vague I, Aillaud MF, Badier C, Viard R, Alessi MC, et al. Correlation between blood fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level, and relative body weight in normal and obese subjects. Metabolism. (1986) 35:250–3. 10.1016/0026-0495(86)90209-x [DOI] [PubMed] [Google Scholar]
- 114.Yarmolinsky J, Bordin Barbieri N, Weinmann T, Ziegelmann PK, Duncan BB, Ines Schmidt M. Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep. (2016) 6:17714. 10.1038/srep17714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamsten A, de Faire U, Walldius G, Dahlen G, Szamosi A, Landou C, et al. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. (1987) 2:3–9. 10.1016/s0140-6736(87)93050-9 [DOI] [PubMed] [Google Scholar]
- 116.Song C, Burgess S, Eicher JD, O'Donnell CJ, Johnson AD. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J Am Heart Assoc. (2017) 6:e004918. 10.1161/JAHA.116.004918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung RG, Motazedian P, Ramirez FD, Simard T, Di Santo P, Visintini S, et al. Association between plasminogen activator inhibitor-1 and cardiovascular events: a systematic review and meta-analysis. Thromb J. (2018) 16:12. 10.1186/s12959-018-0166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pavlov M, Nikolic-Heitzler V, Babic Z, Milosevic M, Kordic K, Celap I, et al. Plasminogen activator inhibitor-1 activity and long-term outcome in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention: a prospective cohort study. Croat Med J. (2018) 59:108–17. 10.3325/cmj.2018.59.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tofler GH, Massaro J, O'Donnell CJ, Wilson PWF, Vasan RS, Sutherland PA, et al. Plasminogen activator inhibitor and the risk of cardiovascular disease: the Framingham Heart Study. Thromb Res. (2016) 140:30–5. 10.1016/j.thromres.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holick MF. Vitamin D deficiency. N Engl J Med. (2007) 357:266–81. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Zhang H. Serum 25-hydroxyvitamin D3 levels are associated with carotid intima-media thickness and carotid atherosclerotic plaque in type 2 diabetic patients. J Diabetes Res. (2017) 2017:3510275. 10.1155/2017/3510275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verdoia M, Schaffer A, Sartori C, Barbieri L, Cassetti E, Marino P, et al. Vitamin D deficiency is independently associated with the extent of coronary artery disease. Eur J Clin Invest. (2014) 44:634–42. 10.1111/eci.12281 [DOI] [PubMed] [Google Scholar]
- 123.Roy A, Lakshmy R, Tarik M, Tandon N, Reddy KS, Prabhakaran D. Independent association of severe vitamin D deficiency as a risk of acute myocardial infarction in Indians. Indian Heart J. (2015) 67:27–32. 10.1016/j.ihj.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. (2008) 168:1174–80. 10.1001/archinte.168.11.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. (2008) 117:503–11. 10.1161/CIRCULATIONAHA.107.706127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. (2010) 106:963–8. 10.1016/j.amjcard.2010.05.027 [DOI] [PubMed] [Google Scholar]
- 127.Brondum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. (2012) 32:2794–802. 10.1161/ATVBAHA.112.248039 [DOI] [PubMed] [Google Scholar]
- 128.Tanik VO, Cinar T, Simsek B. The prognostic value of vitamin D level for in-hospital mortality in patients with acute pulmonary embolism. Turk Kardiyol Dern Ars. (2020) 48:20–5. 10.5543/tkda.2019.69256 [DOI] [PubMed] [Google Scholar]
- 129.Paquette M, Luna Saavedra YG, Chamberland A, Prat A, Christensen DL, Lajeunesse-Trempe F, et al. Association between plasma proprotein convertase subtilisin/kexin type 9 and the presence of metabolic syndrome in a predominantly rural-based Sub-Saharan African population. Metab Syndr Relat Disord. (2017) 15:423–29. 10.1089/met.2017.0027 [DOI] [PubMed] [Google Scholar]
- 130.Mba CM, Mbacham W, Sobngwi E, Mbanya JC. Is PCSK9 associated with plasma lipid levels in a Sub-Saharan African population of patients with obesity and type 2 diabetes? Diabetes Metab Syndr Obes. (2019) 12:2791–97. 10.2147/DMSO.S234243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Toth S, Fedacko J, Pekarova T, Hertelyova Z, Katz M, Mughees A, et al. Elevated circulating PCSK9 concentrations predict subclinical atherosclerotic changes in low risk obese and non-obese patients. Cardiol Ther. (2017) 6:281–89. 10.1007/s40119-017-0092-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee CJ, Lee YH, Park SW, Kim KJ, Park S, Youn JC, et al. Association of serum proprotein convertase subtilisin/kexin type 9 with carotid intima media thickness in hypertensive subjects. Metabolism. (2013) 62:845–50. 10.1016/j.metabol.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 133.Bae KH, Kim SW, Choi YK, Seo JB, Kim N, Kim CY, et al. Serum levels of PCSK9 are associated with coronary angiographic severity in patients with acute coronary syndrome. Diabetes Metab J. (2018) 42:207–14. 10.4093/dmj.2017.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peng J, Liu MM, Jin JL, Cao YX, Guo YL, Wu NQ, et al. Association of circulating PCSK9 concentration with cardiovascular metabolic markers and outcomes in stable coronary artery disease patients with or without diabetes: a prospective, observational cohort study. Cardiovasc Diabetol. (2020) 19:167. 10.1186/s12933-020-01142-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Werner C, Hoffmann MM, Winkler K, Bohm M, Laufs U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol. (2014) 62:94–102. 10.1016/j.vph.2014.03.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.