Abstract
Tetrahydroquinoline (THQ) is an important structure for synthesizing multiple biologically active derivatives. Thus, we developed new quinoline derivatives and investigated them as anticancer agents. First, infrared spectroscopy, nuclear magnetic resonance spectroscopy, and other techniques were used to confirm the structure of synthesized compounds. Then, they were assessed in vitro against three human cancer cell lines. Consequently, four compounds, 10, 13, 15, and 16, were identified as promising anticancer agents with pyrazolo quinoline derivative (15) exhibiting the highest potential IC50 and a strong apoptotic effect on three cell lines.
Keywords: Tetrahydroquinoline, Quinolone, Pyrazole, Isooxazole, Isatin, Anticancer activity
Tetrahydroquinoline; Quinolone; Pyrazole; Isooxazole; Isatin; Anticancer activity.
1. Introduction
Cancer is a medical term that refers to a group of diseases characterized by uncontrolled cell division and destruction of healthy tissues in the body; however, the chances of being cured of most types of cancer are constantly improving because of advances in early cancer detection techniques, drug development, and cancer treatment options [1].
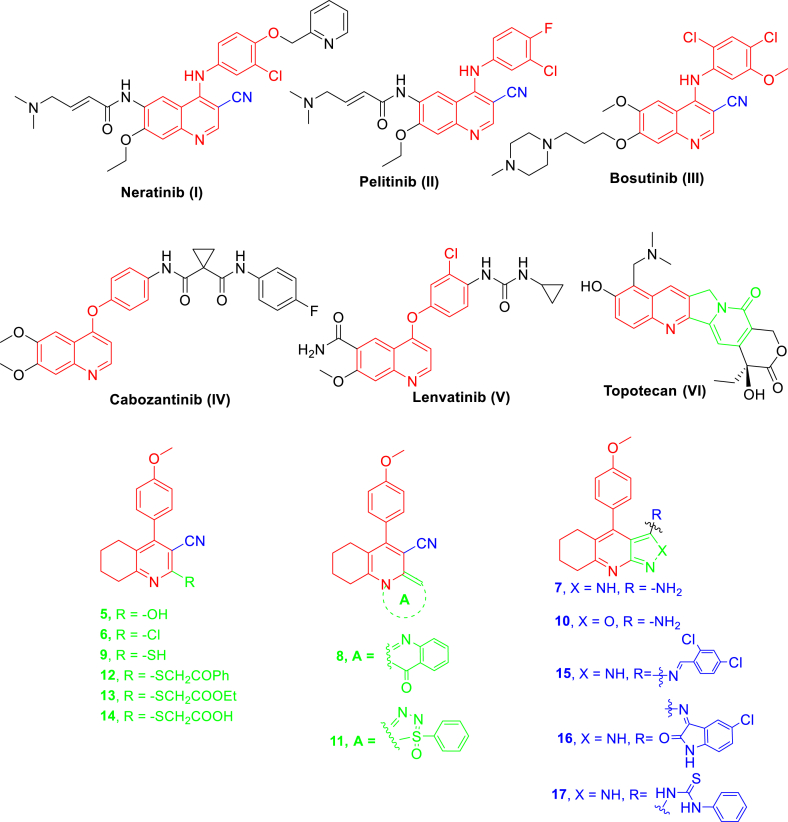
For decades, quinoline derivatives have been useful in various anticancer drug applications. For example, neratinib (I) is used to treat breast cancer [2]; pelitinib (II) has irreversible EGFR inhibitory activity [3]; bosutinib (III) is used to treat chronic myelogenous leukemia [4]; cabozantinib (IV) and lenvatinib (V) are used to treat medullary thyroid anticancer [5, 6]; and topotecan (VI) is used to treat cancer of the ovaries and certain types of lung cancers [7, 8] (Figure 1).
Figure 1.
Certain anticancer drug structures of quinoline derivatives I-VI and the structure of newly synthesized compounds 5–17.
Recently, quinoline derivatives have been demonstrated to have many biological activities [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19], including anticancer activity [20, 21, 22, 23]. Tetrahydroquinolines (THQs) are important building blocks in the chemical structure of a wide range of biologically active derivatives such as pyrazolo[3,4-b] quinolines (PQs) and have potent anticancer activity [24, 25]. PQs framework (PQF) has a high affinity and selectivity for electrophilic reagents because of its nucleophilic amino group. PQFs are excellent anticancer agents with a broad therapeutic range, high pharmaceutical potency, and anticancer activity [26, 27, 28, 29, 30].
In this study, THQ, chloroquinoline, pyrazolo quinoline, and mercapto quinoline moiety were constructed early at the sequence ring, allowing for a quick, flexible substitution and replacement route. Consequently, and in continuation of our research [31, 32]. The primary tetrahydroquinoline ring's substitution profile was designed to include various equivalents that would offer a different electronic, lipophilic, and steric environment, influencing the biological activities targeted. As a result, certain THQ fused-ring frameworks were synthesized as an interesting structural modification to improve the predicted chemotherapeutic character.
Because of previous data on the anticancer activity of drugs I-III, we aimed to synthesize the main core THQ, with the carbonitrile functional group in position 3 and 4-methoxyphenyl in position 4, and with different substitutions in position 2 as can be seen in compounds 5, 6, 9, 12, 13, and 14 (Figure 1). Moreover, because of the importance of fused quinoline anticancer drug Topotecan (VI), the development of fused quinoline heterocycles such as quinolino[2,1-b]quinazoline 8, thiatriazolo[5,4-a]quinoline 11, pyrazolo[3,4-b]quinolines (PQ) 7, 15, 16, 17 pyrazolo quinoline and isoxazolo[3,4-b]quinoline 10 was targeted (Figure 1). The newly synthesized compounds were tested on three solid tumor cell lines, MCF-7, HepG-2, and A549.
2. Results and discussion
2.1. Chemistry
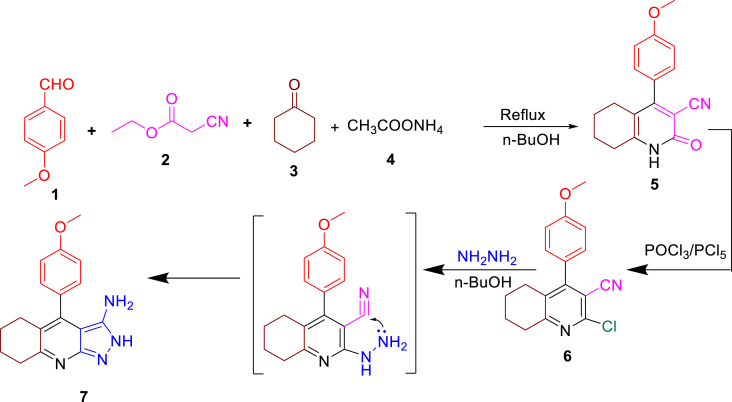
The synthetic strategy is to obtain quinoline derivative (5) from anisaldehyde (1), cyclohexanone (3), ethyl cyanoacetate (2), and an excess of CH3COONH4 (4) in a one-pot four-component reaction (Scheme 1). Infrared (IR) spectroscopy was used to characterize the quinolone carbonitrile (5), where 1685, 2225, and 3330 cm−1 can be attributed to C=O, C≡N, and NH, respectively. The 1H-NMR spectroscopy revealed 8H of quinoline in the aliphatic range of 1.52–2.65 ppm, 3H of the methoxy group at 3.88 ppm, and NH proton at 12.59 ppm. Both C=O and CN were detected by 13C-NMR at 116.92 and 162.77 ppm, respectively. Compound 5 served as a key intermediate structure in the synthesis of target compounds. Compound 6 was obtained by chlorinating compound 5 with POCl3 in the presence of a small amount of PCl5, the absence of C=O band in the IR spectrum, and the NH signal in the 1H-NMR spectrum confirmed its structure. Furthermore, the molecular ion has peaks at m/z 298 (35%) and 300 (12%) (3:1) based on two chlorine isotopes. By reacting with an excess of NH2–NH2·H2O (98%), compound 6 was cyclized to form pyrazolo quinoline (PQ) (7). The presence of NH2 and NH as singlet signals were demonstrated by 1H-NMR at 4.3 and 12.3 ppm, respectively (Scheme 1).
Scheme 1.
Synthesis of THQ derivatives.
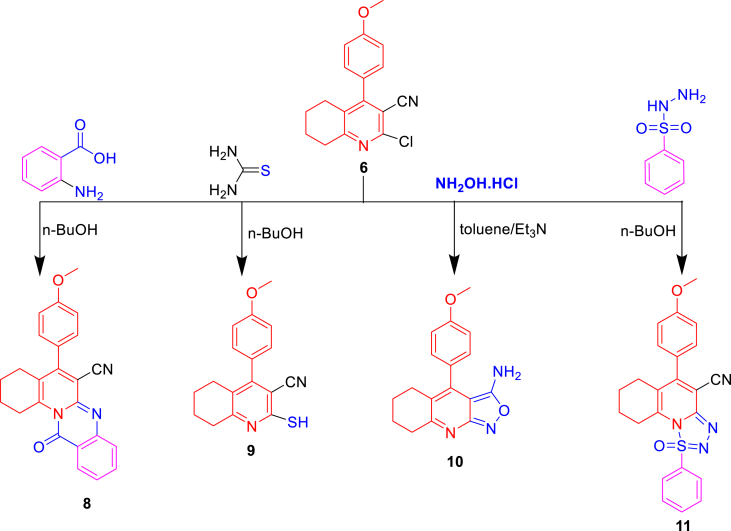
Next, chloroquinoline (6) (Scheme 2) was subjected to a nucleophilic substitution reaction by reacting with anthranilic acid to yield compounds 8 (Scheme 2). The IR spectrum of compound 8 revealed C=O and C≡N groups at 1665 and 2235 cm−1, respectively. Furthermore, 1H-NMR exhibited 8H in the aliphatic range of 1.61–2.88 ppm and 8H in the aromatic range of 6.46–7.64 ppm. Compound 9 was synthesized by replacing Cl in position 2 with the SH group during the reaction of thiourea with compound 6. The mass spectrum demonstrated a molecular ion peak at m/z was 296, and 1H-NMR spectra showed the SH group at 13.5 ppm to confirm the presence of compound 9.
Scheme 2.
Nucleophilic substitutions of 2-chloroquinoline.
Isoxazolo[3,4-b] quinoline derivatives (10) were obtained by reacting compound 6 with NH2OH·HCl in dry toluene in the presence of Et3N. 1H-NMR detected NH2 at 5.2 ppm; moreover, compound 6 was reacted with benzenesulfono hydrazide in n-BuOH to produce compound 11. The IR spectrum detected S=O and C≡N groups at 1320 and 2230 cm−1, respectively. Moreover, the 1H-NMR detected 8H in the aliphatic range (THQ) at 1.69–2.88 ppm and nine protons in the aromatic range of 6.93–7.54 ppm of two phenyl rings.
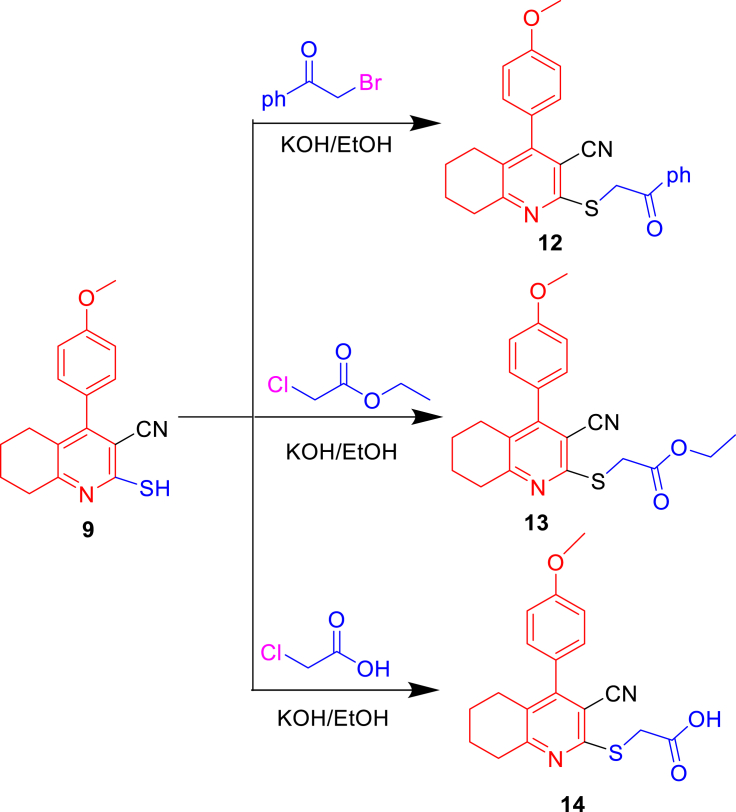
Similarly, the 2-mercapto quinoline derivatives [9] crystals were subjected to nucleophilic reactions, as shown in Scheme 3. Here, compound 9 was treated with alcoholic KOH and then reacted with electrophilic reagents such as phenacyl bromide, chloroethylacetate, and chloroacetic acid to produce compounds 12, 13, and 14, respectively. The IR spectrum of these compounds exhibited C=O at 1665–1675 cm−1 and C≡N at 2225–2230 cm−1. The 1H-NMR spectra revealed two protons of S–CH2 at the range of 4.12–4.25 ppm. In addition to the proton, OH was observed at 11.54 ppm for compound 14. Moreover, the 13C-NMR spectra showed C=O at the range of 165.5–170.5 ppm for compounds 12–14.
Scheme 3.
Nucleophilic reactions of 2-mercapto quinoline derivatives.
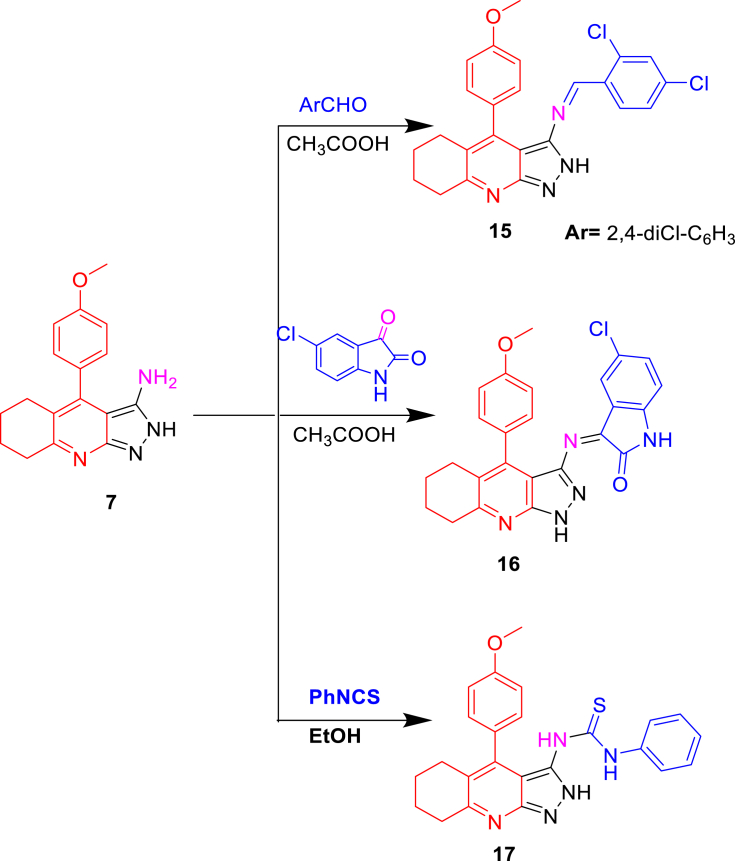
However, pyrazolo[3,4-b]quinolin-3-amine (7) allows for a flexible nucleophilic reaction by reacting with 2,4-dichlorobenzaldehyde, 5-chloroisatin, and PhNCS to produce compounds 15, 16, and 17, respectively (Scheme 4). The 1H-NMR spectra of compound 15 indicated the presence of CH = N and NH at 8.61 and 12.32 ppm, respectively. The IR spectrum of compound 16 revealed C=O and 2NH groups at 1660, 3350, and 3360 cm−1, respectively. The 1H-NMR of compound 16 revealed 2NH groups at 10.15 and 13.5 ppm. The final compound 17 was detected in the IR spectrum to contain C=S and 3NH groups at 2110 and 3340–3360 cm−1, respectively. Furthermore, the 1H-NMR revealed 3NH at 9.76, 13.16, and 13.61 ppm where 13C-NMR exhibited C=S at 180 ppm (Scheme 4).
Scheme 4.
Nucleophilic substitution reactions of pyrazolo quinoline derivatives.
2.2. Cytotoxicity effects
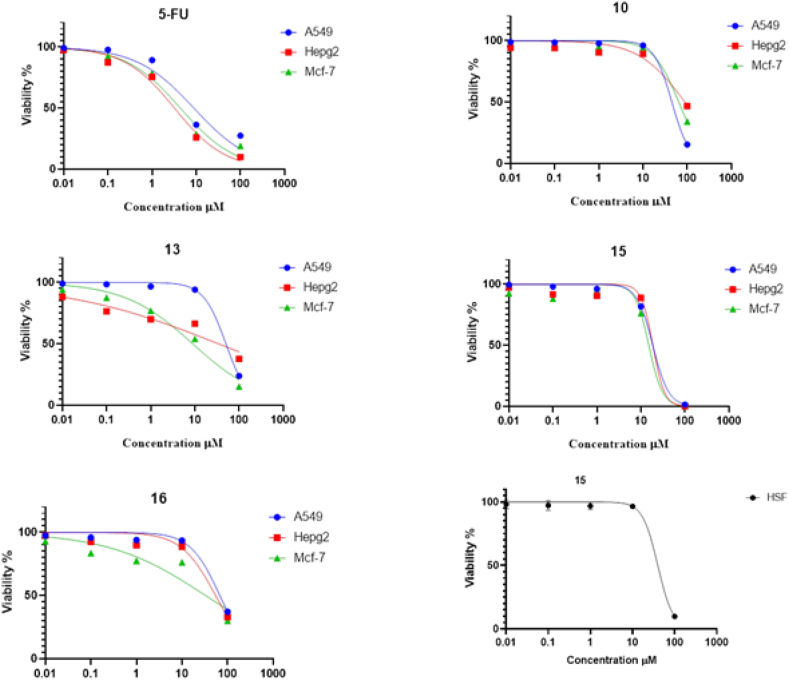
The SRB assay was employed to examine the cytotoxicity of 13 different compounds on three different solid tumor cell lines ((MCF-7, HepG-2, and A549). Five concentrations (0.01, 0.1, 1.0, 10.0 and 100 μM) were used to plot the dose–response curve for compounds on different cell lines using 5-fluorouracil (5-FU) as a standard drug.
Four compounds (10, 13, 15, and 16) out of 13 compounds yielded IC50 < 100 μM on all cell lines employed, except for compound 17, which was cytotoxic to only the A549 cell line. However, other compounds exhibited an IC50 > 100 μM. The IC50 values for the four compounds against the tested cell lines ranged from 9 to 68.02 μM, with compound 15 (15.16, 18.74, and 18.68 μM) having the strongest cytotoxic effects on MCF-7, HepG-2, and A549 (Table 1, Figure 2). Moreover, compound 15 afforded an IC50 of 39.74 μM when tested on the HSF normal cell line. The selectivity index (SI) (IC50 value normal cell/IC50 value cancer cell) was 2.6, 2.1, and 2.1 on MCF-7, HepG-2, and A549, respectively. The SI values indicate that compound 15 is more cytotoxic on cancer cell lines than normal cell lines, where the higher the SI values, the safer the compound. Accordingly, compound 15 was selected for additional investigation.
Table 1.
The anticancer IC50 values of synthesized compounds.
|
Compounds |
IC50 μM |
||
|---|---|---|---|
| MCF-7 | HepG-2 | A549 | |
| 5 | >100 | >100 | >100 |
| 6 | >100 | >100 | >100 |
| 7 | >100 | >100 | >100 |
| 8 | >100 | >100 | >100 |
| 9 | >100 | >100 | >100 |
| 10 | 64.75 ± 2.20 | 89.54 ± 2.50 | 45.28 ± 1.65 |
| 11 | >100 | >100 | >100 |
| 12 | >100 | >100 | >100 |
| 13 | 9.02 ± 0.22 | 34.89 ± 1.45 | 50.68 ± 1.75 |
| 14 | >100 | >100 | >100 |
| 15 | 15.16 ± 0.25 | 18.74 ± 0.50 | 18.68 ± 0.75 |
| 16 | 33.22 ± 1.25 | 54.93 ± 2.5 | 68.02 ± 2.25 |
| 17 | >100 | >100 | 84.04 ± 3.15 |
| 5-FU | 8.91 | 3.24 | 4.40 |
Figure 2.
Cytotoxicity of compounds was assessed in vitro against three cell lines using 5-FU as a standard drug.
2.3. Assessment of cell cycle distribution
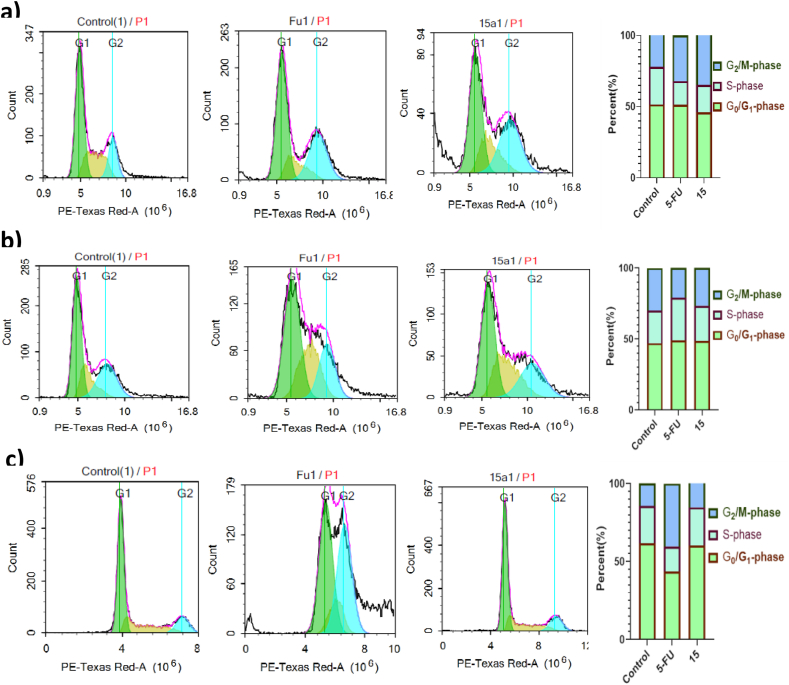
DNA flow cytometry was employed to examine compound 15's cell cycle progression.
In HepG2 cells, both 5-Fu and compound 15 increased the G2/M phase population, where the G2/M phase increased from 22.04% (untreated cells) to 32.12% and 34.78% for 5-FU and compound 15, respectively. Thus, an indication for arresting the cell cycle at the G2/M phase and a dramatic increase in the Pre-G phase population was noticed in HepG2 cells treated with compound 15 (Figure 3, HepG-2,MCF-7 , and A549, respectively).
Figure 3.
Effect of 5-FU and compound 15 (3.24, 8.91 and 4.40 µM) and compound 15 (18.74, 15.16 and 18.68 µM) on the cell cycle distribution of HepG-2, MCF-7, and A549 (a, b, and c) for 48 hours.
On the MCF-7 cell line, only compound 15 significantly increase the G1 population from 46.91% (untreated cells) to 54.41%, indicating that compound 15 arrests the cell cycle at the G1 phase. However, for the A549 cell cycle analysis, 5-FU arrests the cell cycle at the G2/M phase with a substantial increase in the G2/M phase population from 14.52% (untreated cells) to 35.71% (Figure 3, MCF-7, HepG-2, and A549, respectively).
2.4. Apoptosis assessment
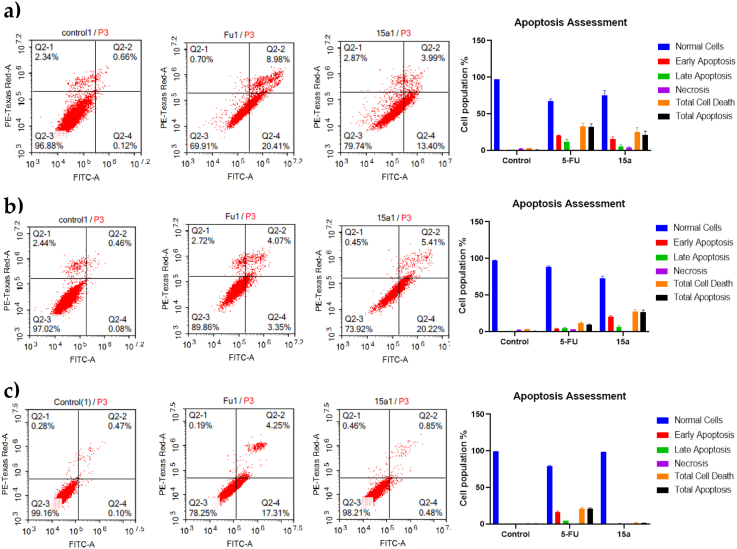
The three cell lines were treated with IC50 values of 5-FU (standard) and compound 15 to determine the apoptosis and necrosis populations using annexin-v/FITC-PI where the apoptosis was measured using flow cytometry (Figure 4). Both 5-FU and compound 15 induced significant apoptosis in the HepG2 cell line. Total apoptosis increased from 1% (untreated cell line) to 32% and 21% for 5-FU and compound 15, respectively.
Figure 4.
Apoptosis assessment of 5-FU (3.24, 8.91 and 4.40 µM) and 15 (18.74, 15.16 and 18.68 µM) on three cell lines (a, b, and c respectively) for 48 hours.
On the MCF-7 cell line, compound 15 exerted a selective apoptotic effect compared with untreated cells and treated cells with 5-FU. The apoptosis increased from 0.6% (untreated cells) to 8.8% and 26.6% for 5-FU and compound 15, respectively.
For the lung cancer A549 cell line, compound 15 weakly increased apoptosis with the least percentage compared with other cell lines. The total apoptosis increased from 0.6% to 20.6% and 1.3% for 5-Fu and 15, respectively (Figure 4).
Consequently, compound 15 induced apoptosis on the treated cell lines with a strong apoptotic effect on MCF-7 and HepG2 cell lines and selectivity for the MCF-7 cell line.
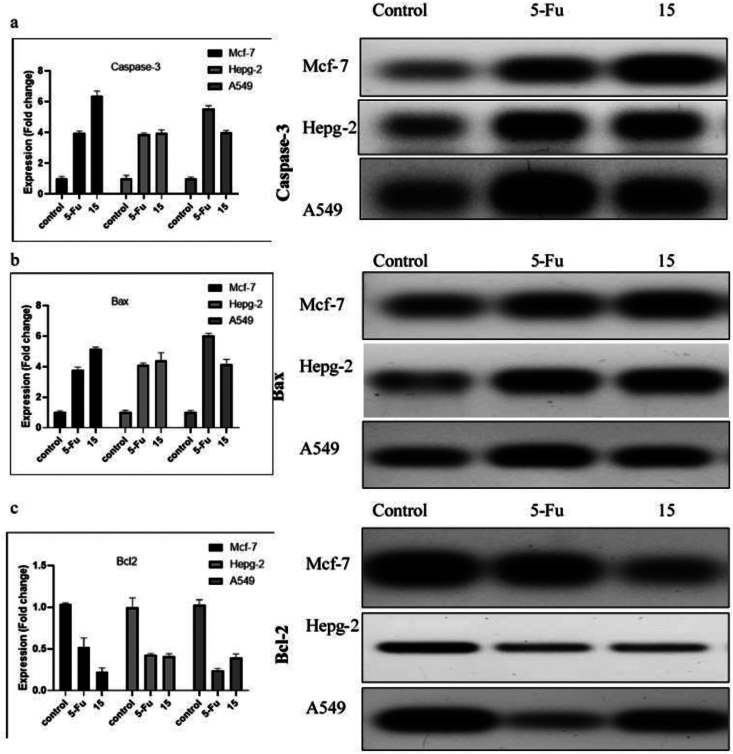
2.5. Western immunoblotting
Compound 15 was tested against the three cell lines using Western immunoblotting to investigate essential apoptotic proteins. The expression of various pro-and anti-apoptotic proteins, BAX and caspase-3 as pro-apoptotic proteins and Bcl-2 as anti-apoptotic proteins, was conducted. The results demonstrated that compound 15 increased pro-apoptotic proteins (Bax and caspase-3) and decreased anti-apoptotic protein Bcl-2 on three cell lines tested compared to untreated cells (Figure (5). Furthermore, compound 15 significantly increased the expression of Caspase-3 protein compared to untreated cells by six fold on MCF-7 and by four fold on HepG-2 and A549. Furthermore, compound 15 significantly increased Bax by 5-, 4.5-, and 4-fold on MCF-7, HepG2, and A549, respectively. However, compound 15 significantly decreased the anti-apoptotic protein Bcl-2 by 4.5-, 2.4-, and 2.5-fold compared to untreated cells on the three cell lines. Based on these results, we discovered that compound 15 exhibited the highest expression of pro-apoptotic proteins on MCF-7 among the tested cell lines. The results were consistent with the flow cytometry assay results.
Figure 5.
Effect of 5-Fu (8.91, 3.24 and 4.40 µM) and compound 15 (15.16, 18.74, and 18.68 µM) for 48 hours on the expression level of Caspase-3 (a), Bax (b), and Bcl-2 (c) on MCF-7, Hepg-2, and A549 cell lines using western blot analysis. Data represented as average ± SD; n = 3.
Apoptosis is the preferred mechanism in cancer therapy. In apoptosis, Caspase-3 is the executioner protein, whereas Bcl-2 and Bax are the regulatory proteins. Therefore, our results provided additional evidence for the potential anticancer activity of compound 15 on target cell lines.
In the structure-activity relationship studies, THQ bearing isoxazolo (compound 10) or thiano thioacetate (compound 13) moiety showed moderate significance against the three cell lines. However, THQ containing a pyrazole moiety and methanimine (compound 15) or isatin derivatives (compound 16) were effective against the three cell lines. The pyrazolo [3,4-b] quinoline framework was previously reported as an excellent substance with a broad therapeutic spectrum and high anticancer activity [32, 33, 34].
3. Conclusion
THQ was synthesized in a one-pot four-component reaction and served as a key structure for synthesizing new derivatives of THQs, which had previously been successfully synthesized via nucleophilic substitution reactions. The compounds were tested for anticancer activity against three cell lines (MCF-7, HepG2, and A549) using different concentrations. Four compounds (10, 13, 15, 16) exhibited significant anticancer activity (IC50 < 100 μM on all cell lines used) compared with 5-FU as the standard drug. Compound 15 has a significant cytotoxic effect, and thus was selected for further investigation. Consequently, compound 15 induces apoptosis in treated cell lines while having a significant effect on decreasing anti-apoptotic protein. The results showed that compound 15 increased pro-apoptotic proteins (Bax and caspase-3) and decreased anti-apoptotic protein Bcl-2 on the three cell lines tested compared to untreated cells. Furthermore, compound 15 has the highest expression of pro-apoptotic proteins on MCF-7 among the cell lines tested.
4. Experimental
4.1. Chemistry
The melting points were determined using an Electro-thermal IA 9100 instrument without correction (Shimadzu, Japan). On a Perkin-Elmer 1650 Spectrophotometer at the National Research Centre in Cairo, Egypt, IR spectra were captured as KBr pellets using the KBr disc technique. Chemical shifts were measured using a Varian-500 MHz in deuterated DMSO-d6 and measured as ppm against TMS as an internal reference at the National Research Centre, Cairo, Egypt. Mass spectra were recorded on Shimadzu GCMS-QP-1000EX mass spectrometer at 70 eV.
4.1.1. Synthesis of 4-(4-methoxyphenyl)-2-oxo-1,2,5,6,7,8-hexahydroquinoline-3-carbonitrile (5)
This compound was synthesized using a one-pot four-component reaction where a mixture of 4-methoxybenzaldehyde (13.6 g, 0.1 mol) (1), ethylcyanoacetate (11.3 g, 0.1 mol) (2), cyclohexanone (9.8 g, 0.1 mol) (3), and an excess of anhydrous ammonium acetate (4) (35 g, 0.5 mol) in n-butanol was stirred and refluxed for 6 h Then, the reaction mixture was allowed to cool, filtered, washed, dried, and crystallized from ethanol to produce the yellow compound 5. Measurement results for this compound are listed below.
Yield 90–95%; m.p. ˃ 300 °C, IR (KBr, ν cm−1): 1685 (C=O), 2225 (C≡N), 3330 (NH); 1H NMR (DMSO-d6, ppm) δ 1.52 (m, 2H, quinoline C-7), 1.66 (m, 2H, quinoline C-6), 2.03 (m, 2H, quinoline C-8), 2.57 (m, 2H, quinoline C-5), 3.88 (s, 3H, –OCH3), 6.90 (d, J = 8.5 Hz, 2H, (m) di-OCH3 phenyl), 7.31 (d, J = 9 Hz, 2H (o), di-OCH3 phenyl), 12.59 (s, 1H, D2O-exchangeable, NH); 13C NMR (DMSO-d6, ppm) 21.18, 22.42, 25.52, 27.75, 55.72 (-OCH3), 101.02, 113.15, 114.51 (2C), 114.59, 116.92, 128.02, 129.88 (2C), 150.47, 160.25, 160.50, 162.28 (C=O); MS (C17H16N2O2), (m/z, %) = 280 (M+).
4.1.2. Synthesis of 2-chloro-4-(4-methoxyphenyl)-5,6,7,8-tetrahydroquinoline-3-carbonitrile (6)
Compound 5 (5.5 g 0.02 mol) with excess POCl3 (50 mL) and a small amount of PCl5 (1 g) were refluxed for 6 h. The reaction mixture was left to cool. Care must be taken in the fume hood when pouring the mixture on ice-cold water. The yellowish-white precipitate was filtered, washed, dried, and recrystallized from EtOH to form pale yellowish compounds 6. The compound's measured results are provided below.
Yield 65%; m.p. = 285–288 °C, IR (KBr, ν cm−1): 2230 (C≡N); 1H NMR (DMSO-d6, ppm) δ 1.60 (m, 2H, quinoline-C-6), 1.79 (m, 2H, quinoline-C-7), 2.41 (m, 2H, quinoline-C-5), 2.88 (m, 2H, quinoline-C-8), 3.73 (s, 3H, –OCH3), 6.86 (d, J = 6.5 Hz, 2H (m), di-OCH3 phenyl), 7.07 (d, J = 9 Hz, 2H (o), di-OCH3 phenyl); 13C NMR (DMSO-d6, ppm) 22.08, 22.15, 27.01, 33.20, 56.28(-OCH3), 107.96, 112.29, 112.49, 114.52 (2C), 121.42, 127.18, 129.85 (2C), 131.48, 149.22, 162.93 (-C=N); MS (C17H15ClN2O), (m/z, %) = 298 (M+, 35%), 300 (M+ + 2, 12%).
4.1.3. Synthesis of 4-(4-methoxyphenyl)-5,6,7,8-tetrahydro-2H-pyrazolo[3,4-b]quinolin-3-amine(7)
A mixture of compound 6 (0.1 mmol, 0.3 g) with an excess of NH2–NH2·H2O (25 mL) in n-butanol (20 mL) was refluxed for 4 h. Then, the product was filtered off, washed, dried, and crystallized from EtOH to give yellow compound 7. The analytical results are provided below.
Yield 75%, m.p. 278–280 °C; IR (KBr, ν cm−1): 3350–3360 (NH + NH2); 1H NMR (DMSO-d6, ppm) δ 1.51 (m, 2H, quinoline C-6), 1.64 (m, 2H, quinoline-C-7), 2.02 (m, 2H, quinoline-C-8), 2.58 (m, 2H, quinoline-C-5), 3.78 (s, 3H, –OCH3), 4.03 (s, 2H, D2O-exchangeable, NH2), 7.01 (d, J = 8 Hz, 2H (m), di-OCH3 phenyl), 7.20 (d, 2H (o), di-OCH3 phenyl), 12.30 (s, 1H, D2O-exchangeable, NH); 13C NMR (DMSO-d6, ppm) 22.41, 22.99, 25.52, 26.64, 55.70, 100.02, 114.53 (2C), 129.71 (2C), 142.70, 147.28, 150.04, 157.58, 159.40, 160.28, 162.35; MS (C17H18N4O), m/z = 294 (M+). .
4.1.4. Synthesis of 5-(4-methoxyphenyl)-12-oxo-2,3,4,12-tetrahydro-2H-quinolino[2,1-b]quinazo-line-6-carbonitrile (8)
This compound was synthesized by reacting compound 6 (0.1 mmol, 0.3 g) with (0.2 mmol, 0.28 g) anthranilic acid in n-butanol (25 mL) and refluxed for 6 h. The reaction was filtered, dried, and crystallized from xylene to yield a brownish-yellow solid 8. Its measured values are listed below.
Yield 70%–75%, m.p. 168–170 °C; IR (KBr, ν cm−1): 1665 (C=O), 2235 (C≡N); 1H NMR (DMSO-d6, ppm) δ 1.61 (m, 2H, quinoline C-6), 1.75 (m, 2H, quinoline-C-7), 2.40 (m, 2H, quinoline-C-8), 2.88 (m, 2H, quinoline-C-5), 3.73 (s, 3H, –OCH3), 6.46 (d, J = 8 Hz, 2H (m), di-OCH3 phenyl), 6.69 (d, J = 8 Hz, 2H (o), di-OCH3 phenyl), 6.88 (t, J = 8 Hz, 1H of quinazolin), 7.06 (d, J = 8 Hz, 1H of quinazolin), 7.17 (t, J = 8 Hz, 1H of quinazolin), 7.64 (d, J = 8 Hz, 1H of quinazolin); 13C NMR (DMSO-d6, ppm) 21.99, 22.13, 27.0, 33.18, 56.04, 107.94, 110.22, 112.25, 115.09 (2C), 116.85, 121.42, 127.17, 130.1 (2C), 131.69, 134.21, 142. 65, 145.5, 152.02, 158.9, 159.8, 165. 2, 170.14; MS (C24H19N3O2), m/z = 381 (M+).
4.1.5. Synthesis of 2-mercapto-4-(4-methoxyphenyl)-5,6,7,8-tetrahydroquinoline-3-carbonitrile (9)
A mixture of compound 6 (0.1 mmol, 0.3 g) and thiourea (0.3 mmol, 0.228) in n-butanol (25 mL) was refluxed for 6 h. The reaction was filtered off, washed, dried, and crystallized from EtOH to obtain a white compound 9. Its analytical values are provided below.
Yield 80%–85%, m.p. 180–183 °C; IR (KBr, ν cm−1): 2225 (C≡N), 2550 (SH); 1H NMR (DMSO-d6, ppm) δ 1.59 (m, 2H, quinoline C-6), 1.74 (m, 2H, quinoline-C-7), 2.38 (m, 2H, quinoline-C-8), 2.88 (m, 2H, quinoline-C-5), 3.71 (s, 3H, –OCH3), 6.46 (d, J = 8 Hz, 2H (m), di-OCH3 phenyl), 6.69 (d, J = 8 Hz, 2H (o), di-OCH3 phenyl), 13.50 (s, 1H, D2O-exchangeable, SH); 13C NMR (DMSO-d6, ppm) 21.99, 22.14, 27.01, 33.20, 56.07, 107.90, 112.25, 112.44, 115.61, 121.42, 127.16, 131.46, 148.41, 149.99, 156.24, 162.97, 18434 (C-SH); MS (C17H16N2OS), m/z = 296 (M+).
4.1.6. Synthesis of 4-(4-methoxyphenyl)-5,6,7,8-tetrahydroisoxazolo[3,4-b]quinolin-3-amine (10)
A mixture of compound 6 (0.1 mmol, 0.3 g) and NH2OH·HCl (0.2 mmol, 0.14) was refluxed for 6 h in dry toluene (25 mL) in the presence of TEA (0.5 mL). The precipitate was collected and recrystallized from xylene to afford white crystals. The analytical results of the crystal are presented.
Yield 75%–80%, m.p. 192–195 °C; IR (KBr, ν cm−1): 3420 (NH2); 1H NMR (DMSO-d6, ppm) δ 1.63 (m, 2H, quinoline C-6), 1.78 (m, 2H, quinoline-C-7), 2.43 (m, 2H, quinoline-C-8), 2.89 (m, 2H, quinoline-C-5), 3.74 (s, 3H, –OCH3), 5.20 (s, 2H, D2O-exchangeable, NH2), 6.91 (d, J = 9 Hz, 2H (m), di-OCH3 phenyl), 7.07 (d, J = 8 Hz, 2H (o), di-OCH3 phenyl); 13C NMR (DMSO-d6, ppm) 21.98, 22.00, 27.01, 33.20, 56.07, 107.93, 112.27, 112.47, 121.41, 127.16, 131.46, 148.43, 149.22, 150.05, 156.29, 158.55, 162.94 (C–OCH3); MS (C17H17N3O2), m/z = 295 (M+).
4.1.7. Synthesis of 5-(4-methoxyphenyl)-1-phenyl-6,7,8,9-tetrahydro-1,4-[1,2,3,5]thiatriazolo[5,4-a]quinoline-4-carbonitrile 1-oxide (11)
A mixture of compound 6 (0.1 mmol, 0.3 g) and PhSO2NHNH2 (0.2 mmol, 0.34 g) was refluxed for 10 h in n-butanol (25 mL). After cooling, the product was collected and recrystallized from xylene to produce a yellow compound 11.
Yield 65%, m. p. 220–225 °C; IR (KBr, ν cm−1): 1320 S=O, 2230 (C≡N); 1H NMR (DMSO-d6, ppm) δ 1.69 (m, 2H, quinoline C-6), 1.75 (m, 2H, quinoline-C-7), 2.43 (m, 2H, quinoline-C-8), 2.88 (m, 2H, quinoline-C-5), 3.79 (s, 3H, –OCH3), 6.93 (d, J = 8 Hz, 2H (m), di-OCH3 phenyl), 7.20–7.54 (m, 7H, 2H (o) di-OCH3 phenyl+5H of benzene ring); 13C NMR (DMSO-d6, ppm) 21.78, 22.07, 27.15, 33.55, 56.08, 107.20, 112.45, 112.48, 116.81, 118.30, 119.50, 125.41, 128.16, 128.20, 130.01, 130.55, 131.12, 146.70, 149.53, 152.05, 155.78, 159.26, 168.89; MS (C23H20N4O2S), m/z = 416 (M+).
4.1.8. Synthesis of compounds 12-14
A mixture of 2-mercapto compound 9 (1 mmol, 0.3 g) reacted with phenacyl bromide, ethyl chloroacetate, and chloroacetic acid (1.5 mmol) in the presence of alcoholic KOH (0.01 mol, 0.56 g). The reaction was refluxed in EtOH for 2–4 h. It was then cooled, poured into ice/water, and the obtained precipitate was filtered off, washed, dried, and crystallized from glacial acetic acid to produce compounds 12–14, respectively. Their analytical results are presented below.
4.1.9. 4-(4-Methoxyphenyl)-2-((2-oxo-2-phenylethyl)thio)-5,6,7,8-tetrahydroquinoline-3-carbonitrile (12)
Yield 68%–70%, m.p. 197–200 °C; IR (KBr, ν cm−1): 1675 (C=O), 2230 (C≡N); 1H NMR (DMSO-d6, ppm) δ 1.40 (m, 2H, quinoline C-6), 1.74 (m, 2H, quinoline-C-7), 1.99 (m, 2H, quinoline-C-8), 3.01 (m, 2H, quinoline-C-5), 3.83 (s, 3H, –OCH3), 5.20 (s, 2H, –CH2-S), 6.97 (d, J = 8 Hz, 2H (m), di-OCH3 phenyl), 7.04 (d, J = 8 Hz, 2H (o) di-OCH3 phenyl), 7.22 (d, J = 8 Hz, 2H (m) of benzene ring H-3/5), 7.31 (t, J = 8 Hz, 1H (p) of benzene ring H-4), 7.76 (d, J = 8 Hz, 2H (o) of benzene ring H-2/6); 13C NMR (DMSO-d6, ppm) 21.50, 22.70, 24.54, 27.80, 36.31, 55.80, 107.80, 112.12, 112.48, 112.50, 125.41, 126.40 (2C), 128.60 (2C), 130.01 (2C), 131.55, 131.89, 146.70, 149.80, 160.54, 165.21, 187.13; MS (C25H22N2O2S), m/z = 414 (M+).
4.1.10. Ethyl 2-((3-cyano-4-(4-methoxyphenyl)-5,6,7,8-tetrahydroquinolin-2-yl)thio)acetate (13)
Yield 60%–65%, m.p. 145–148 °C; IR (KBr, ν cm−1): 1665 (C=O), 2225 (C≡N); 1H NMR (DMSO-d6, ppm) δ 1.49 (t, J = 8 Hz, 3H, CH2CH3), 1.70 (m, 4H, quinoline-C-6/7), 2.04 (m, 2H, quinoline-C-8), 2.13 (m, 2H, quinoline-C-5), 3.76 (s, 3H, –OCH3), 4.25 (s, 2H, –CH2), 4.90 (q, 2H, –CH2CH3), 7.04 (d, J = 8 Hz, 2H (m), di-OCH3 phenyl), 7.23 (d, J = 8.5 Hz, 2H (o) di-OCH3 phenyl); 13C NMR (DMSO-d6, ppm) 14.61, 21.81, 22.90, 24.87, 27.85, 32.50, 55.77, 61.50, 102.80, 113.55 (2C), 120.18, 124.80, 128.41, 130.35 (2C), 151.54, 156.21, 156.50, 160.36, 169.50; MS (C21H22N2O3S), m/z = 382 (M+).
4.1.11. 2-((3-Cyano-4-(4-methoxyphenyl)-5,6,7,8-tetrahydroquinolin-2-yl)thio)acetic acid (14)
Yield 60%–62%, m.p. 175–178 °C; IR (KBr, ν cm−1): 1665 (C=O), 2230 (C≡N), 3150 (OH); 1H NMR (DMSO-d6, ppm) δ 1.71 (m, 2H, quinoline C-6), 1.75 (m, 2H, quinoline-C-7), 2.50 (m, 2H, quinoline-C-8), 2.90 (m, 2H, quinoline-C-5), 3.80 (s, 3H, –OCH3), 4.12 (s, 2H, –CH2), 7.04 (d, J = 8.5 Hz, 2H (m), di-OCH3 phenyl), 7.23 (d, J = 9 Hz, 2H (o) di-OCH3 phenyl), 11.54 (s, 1H, D2O-exchangeable, OH); 13C NMR (DMSO-d6, ppm) 21.75, 23.01, 24.42, 27.75, 34.62, 55.72, 106.50, 112.42, 115.80 (2C), 124.41, 129.50 (2C),133.90, 151.54, 160.75, 161.23, 163.75, 170.50 (C=O); MS (C19H18N2O3S), m/z = 354 (M+).
4.1.12. Synthesis of compounds 15-17
A mixture of compound 7 (0.3 g; 0.1 mmol) was reacted with the appropriate aldehyde (1.5 mmol, 0.25 g), 5-chloroisatin (1.5 mmol, 0.27 g), and phNSC (1.5 mmol, 0.20 g). The reactions were refluxed for 4–6 h in absolute EtOH (25 mL) in the presence of glacial acetic acid (1 mL), and then left to cool. The obtained product was filtered off, washed, dried, and crystallized from EtOH to produce compounds 15, 16, and 17, respectively. Their analytical findings are provided.
4.1.13. 1-(2,4-Dichlorophenyl)-N-(4-(4-methoxyphenyl)-5,6,7,8-tetrahydro-2H-pyrazolo[3,4-b] quino-line-3-yl)methanimine (15)
Yield 72%, m.p. 270–273 °C; IR (KBr, ν cm−1): 3350 (NH); 1H NMR (DMSO-d6, ppm) δ 1.52 (m, 2H, quinoline-C-6), 1.64 (m, 2H, quinoline-C-7), 2.03 (m, 2H, quinoline-C-8), 2.14 (m, 2H, quinoline-C-5), 3.78 (s, 3H, –OCH3), 7.02 (m, 2H (m), di-OCH3 phenyl), 7.04–7.25 (m, 5H, 3H 2,4-diCl phenyl + 2H o-di-OCH3 phenyl), 8.61 (s, 1H 1H, –N=CH-), 12.32 (s, 1H, D2O-exchangeable, NH); 13C NMR (DMSO-d6, ppm) 22.22, 22.67, 26.48, 29.55, 55.47, 111.46, 113.48 (2C), 120.57, 120.70. 121.74, 127.32, 128.00, 129.04, 129.35, 131.65, 135.58, 141.89, 144.86, 147.25, 148.24, 153.92, 158.39(-CH = N-), 164.89; MS (C24H20Cl2N4O),(m/z) = 450 (M+, 21), 452 (M+ + 2, 12), 454 (M+ + 4, 2.5).
4.1.14. 5-Chloro-3-((4-(4-methoxyphenyl)-5,6,7,8-tetrahydro-1H-pyrazolo[3,4-b] quinoline-3-yl)-imino)indolin-2-one (16)
Yield 60%, m.p. 220–225 °C; IR (KBr, ν cm−1): 1660 (C=O), 3350, 3360 (2NH); 1H NMR (DMSO-d6, ppm) δ 1.55 (m, 2H, quinoline-C-7), 1.65 (m, 2H, quinoline-C-6), 2.35 (m, 2H, quinoline-C-5), 2.72 (m, 2H, quinoline-C-8), 3.78 (s, 3H, –OCH3), 6.98 (m, 2H (m), di-OCH3 phenyl), 7.04–7.75 (m, 4H, 2H o-di-OCH3 phenyl + 2H of isatin), 8.11 (d, J = 9 Hz, 1H of isatin), 10.15 (s, 1H, D2O-exchangeable, NH of isatin), 13.50 (s, 1H, D2O-exchangeable, NH of pyrazol); 13C NMR (DMSO-d6, ppm) 22.45, 22.78, 25.55, 29.40, 55.45, 110.22, 115.80 (2C), 120.12, 122.58, 124.50, 128.85, 130.25 (2C), 132.45, 135.22, 135.75, 138.30, 142.32, 145.55, 147.38, 152.35, 155.50, 165.01 (C=O), 168.90 (-CH = N-); MS (C25H20ClN5O2), (m/z) = 457 (M+, 23), 459 (M + 2, 7).
4.1.15. 1-(4-(4-Methoxyphenyl)-5,6,7,8-tetrahydro-2H-pyrazolo[3,4-b]quinolin-3-yl)-3-phenylthiourea (17)
Yield 75%, m.p. ˃ 300 °C; IR (KBr, ν cm−1): 2110 (C=S), 3340, 3355, 3360 (3NH); 1H NMR (DMSO-d6, ppm) δ 1.80 (m, 2H, quinoline-C-7), 1.82 (m, 2H, quinoline-C-6), 2.95 (m, 2H, quinoline-C-5), 2.97 (m, 2H, quinoline-C-8), 3.74 (s, 3H, –OCH3), 6.98–7.29 (m, 5H, (m), di-OCH3 phenyl+3H of NH phenyl ring), 7.28–7.44 (m, 3H, of ph ring), 7.44–7.46 (m, 4H, (o), di-OCH3 phenyl+2H of NH phenyl ring 2/6), 9.76, 13.16, 13.61 (s, 3H, D2O-exchangeable, 3NH); 13C NMR (DMSO-d6, ppm) 22.75, 23.21, 26.85, 34.06, 56.17, 98.65, 112.67 (2C), 114.32, 124.19 (2C), 124.58, 126.65, 128.96 (2C), 132.45, 135.17, 139.99, 142.61, 142.62, 149.41, 155.12, 162.81, 180.23 (C=S); MS (C24H23N5OS), (m/z) = 429 (M+).
4.2. Biological evaluation
4.2.1. Cell culture
A-549 Lung Cancer, HepG-2: Hepatocellular carcinoma, and MCF-7: were obtained from Nawah Scientific (Mokatam, Cairo, Egypt). The cells were cultured in Dulbecco's modified Eagle medium (DMEM) fortified with 10% inactivated fetal bovine serum, 100 units/mL of penicillin, and 100 mg/mL of streptomycin. The cells were kept in a humidified atmosphere at 37 °C with 5% CO2.
4.2.2. Cytotoxicity assay
To investigate the cytotoxic effect of the compounds and the safety of compound 15, an SRB assay was performed on the tested cell lines. About 3000–5000 Cells were seeded in 96-well plates contained in a 100 μl complete growth medium. After 24 h, the cells were attached to one another in the 100 μl containing the tested compounds with a serial concentration of (0.01, 0.1, 1, 10, and 100 μM). After 72 h of incubating the cells with the treated compounds, the growth medium was discarded, and the cells were fixed by adding TCA (10%W/V) to each well and incubated for 1 h at 4 °C. After washing, 70 μL SRB solution (0.4% w/v) was added to each well and incubated at 20 °C. After 10 min, the plates were washed with acetic acid (1%V/V) and allowed to dry. The absorbance of the bounded SRB was measured at 540 nm using a BMG LABTECH®-FLUOstar Omega microplate reader (Ortenberg, Germany) after adding TRIS buffer (10 mM) to dissolve protein-bound SRB stain. The dose–response curve was fitted for each compound using non-linear regression. And the SI = IC50 value normal cell/IC50 value cancer cell [35, 36].
4.3. Statistical analysis
All experiments were independently conducted at least three times as mean ± SD. All IC50 values were computed using GraphPad Prism version 8.0.1., San Diego, California USA.
4.4. Assessment of protein expression using western blot analysis
Whole-cell lysates from treated cell lines (MCF-7, HepG-2, and A549) were collected after 48 h incubation with IC50 values of 5-Fu and compound 15. The whole-cell proteins were separated using SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (PVDF). The membranes were then consecutively probed with antibodies against the following proteins (Bcl-2, caspase-3, and BAX) and incubated at 4 °C overnight. Next, the membranes were washed and incubated with secondary antibodies for 1 h at room temperature. Anti-β-actin antibody was used for loading correction. Band densities were estimated using Bio-Rad Chemi Doc Touch Imaging System.
Declarations
Author contribution statement
Usama Fathy: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision (Corresponding author).
Hayam A. Abd El Salam: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – original draft.
Eman A. Fayed: Conceptualization, Investigation, Resources, Methodology, Writing – original draft.
Abdelbaset M. Elgamal: Methodology, Resources, Formal analysis, Investigation.
Ahmed Gouda: Conceptualization, Methodology, Resources, Formal analysis, Writing – original draft, Writing – review & editing.
Funding statement
This work was supported by the National Research Centre.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Usama Fathy, Email: usamafathy06@gmail.com, usamafathy2000@yahoo.com.
Eman A. Fayed, Email: alfayed_e@azhar.edu.eg, alfayed_e@yahoo.com.
References
- 1.Aly R.M., Serya R.A., El-Motwally A.M., Esmat A., Abbas S., Abou El Ella D.A. Novel quinoline-3-carboxamides (Part 2): design, optimization and synthesis of quinoline based scaffold as EGFR inhibitors with potent anticancer activity. Bioorg. Chem. 2017;75:368–392. doi: 10.1016/j.bioorg.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Cherian M.A., Ma C.X. The role of neratinib in HER2-driven breast cancer. Future Oncol. 2017;13:1931–1943. doi: 10.2217/fon-2017-0186. [DOI] [PubMed] [Google Scholar]
- 3.Wissner A., Overbeek E., Reich M.F., Floyd M.B., Johnson B.D., Mamuya N., Tsou H.R. Synthesis and structure−activity relationships of 6, 7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2) J. Med. Chem. 2003;46:49–63. doi: 10.1021/jm020241c. [DOI] [PubMed] [Google Scholar]
- 4.Vultur A., Buettner R., Kowolik C., Liang W., Smith D., Boschelli F., Jove R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol. Cancer Therapeut. 2008;7:1185–1194. doi: 10.1158/1535-7163.MCT-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viola D., Cappagli V., Elisei R. Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid cancer. Future Oncol. 2013;9:1083–1092. doi: 10.2217/fon.13.128. [DOI] [PubMed] [Google Scholar]
- 6.Matsui J., Funahashi Y., Uenaka T., Watanabe T., Tsuruoka A., Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008;14:5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- 7.Mekheimer R.A., Al-Sheikh M.A., Medrasi H.Y., Sadek K.U. Advancements in the synthesis of fused tetracyclic quinoline derivatives. RSC Adv. 2020;10:19867–19935. doi: 10.1039/d0ra02786c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aly A.A., El-Sheref E.M., Mourad A.F.E., Bakheet M.E., Bräse S. 4-Hydroxy-2-quinolones: syntheses, reactions and fused heterocycles. Mol. Divers. 2020;24:477–524. doi: 10.1007/s11030-019-09952-5. [DOI] [PubMed] [Google Scholar]
- 9.Gouhar R.S., Fathy U., El-Zahar M.I., Kamel M.M., Awad G.E. Synthesis of novel 1,4,5,6,7,8-hexahydroquinolines of potential antimicrobial activity. Acta Pol. Pharm. 2017;74:147–159. [PubMed] [Google Scholar]
- 10.Musiol R., Serda M., Hensel-Bielowka S., Polanski J. Quinoline-based antifungals. Curr. Med. Chem. 2010;17:1960–1973. doi: 10.2174/092986710791163966. [DOI] [PubMed] [Google Scholar]
- 11.Carta A., Briguglio I., Piras S., Corona P., Boatto G., Nieddu M., La Colla P. Quinoline tricyclic derivatives. Design, synthesis and evaluation of the antiviral activity of three new classes of RNA-dependent RNA polymerase inhibitors. Bioorg. Med. Chem. 2011;19:7070–7084. doi: 10.1016/j.bmc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y.Q., Gao C., Zhang S., Xu L., Xu Z., Feng L.S., Zhao F. Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2017;139:22–47. doi: 10.1016/j.ejmech.2017.07.061. [DOI] [PubMed] [Google Scholar]
- 13.Hayat F., Salahuddin A., Umar S., Azam A. Synthesis, characterization, antiamoebic activity and cytotoxicity of novel series of pyrazoline derivatives bearing quinoline tail. Eur. J. Med. Chem. 2010;45:4669–4675. doi: 10.1016/j.ejmech.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Keri R.S., Patil S.A. Quinoline: a promising antitubercular target. Biomed. Pharmacother. 2014;68:1161–1175. doi: 10.1016/j.biopha.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Mroueh M., Faour W.H., Shebaby W.N., Daher C.F., Ibrahim T.M., Ragab HM Synthesis. Biological evaluation and modeling of hybrids from tetrahydro-1H-pyrazolo [3, 4-b] quinolines as dual cholinestrase and COX-2 inhibitors. Bioorg. Chem. 2020;100:103895. doi: 10.1016/j.bioorg.2020.103895. [DOI] [PubMed] [Google Scholar]
- 16.Kumar Gupta S., Mishra A. Synthesis, characterization and screening for anti-inflammatory and analgesic activity of quinoline derivatives bearing azetidinones scaffolds. Antiinflamm. Antiallergy Agents Med. Chem. 2016;15:31–43. doi: 10.2174/1871523015666160210124545. [DOI] [PubMed] [Google Scholar]
- 17.Hao Q., Cai Z., Pan J., Li Y., Xia Y., Min Y., Zhou W. Synthesis and anti-hypercholesterolemic evaluations of calcium salts of 4-substituted quinoline-based mevalonic acid. Chem. Biol. Drug Des. 2011;78:730–733. doi: 10.1111/j.1747-0285.2011.01198.x. [DOI] [PubMed] [Google Scholar]
- 18.Vandekerckhove S., D’hooghe M. Quinoline-based antimalarial hybrid compounds. Bioorg. Med. Chem. 2015;23:5098–5119. doi: 10.1016/j.bmc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Danel A., Gondek E., Kityk I. 1H-pyrazolo [3, 4-b] quinoline and 1H-pyrazolo [3, 4-b] quinoxaline derivatives as promising materials for optoelectronic applications. Opt. Mater. 2009;32:267–273. [Google Scholar]
- 20.George Boyle R., Travers S. Hypoxia: targeting the tumour. Anti Cancer Agents Med. Chem. 2006;6:281–286. doi: 10.2174/187152006777698169. [DOI] [PubMed] [Google Scholar]
- 21.Shi A., Nguyen T.A., Battina S.K., Rana S., Takemoto D.J., Chiang P.K., Hua D.H. Synthesis and anti-breast cancer activities of substituted quinolines. Bioorg. Med. Chem. Lett. 2008;18:3364–3368. doi: 10.1016/j.bmcl.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y.L., Chen Y.L., Chang F.S., Tzeng C.C. Synthesis and cytotoxic evaluation of certain 4-anilino-2-phenylquinoline derivatives. Eur. J. Med. Chem. 2005;40:792–797. doi: 10.1016/j.ejmech.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y.H., Shin K.J., Lee T.G., Kim E., Lee M.S., Ryu S.H., Suh P.G. G2 arrest and apoptosis by 2-amino-N-quinoline-8-yl-benzenesulfonamide (QBS), a novel cytotoxic compound. Biochem. Pharmacol. 2005;69:1333–1341. doi: 10.1016/j.bcp.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Alqasoumi S.I., Al-Taweel A.M., Alafeefy A.M., Noaman E., Ghorab M.M. Novel quinolines and pyrimido[4,5-b]quinolines bearing biologically active sulfonamide moiety as a new class of antitumor agents. Eur. J. Med. Chem. 2010;45:738–744. doi: 10.1016/j.ejmech.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Faidallah H.M., Rostomb S.A. Synthesis, in vitro antitumor evaluation and DNA-binding study of novel tetrahydroquinolines and some derived tricyclic and tetracyclic ring systems. Eur. J. Med. Chem. 2013;63:133–143. doi: 10.1016/j.ejmech.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Cappelli A., Bini G., Valenti S., Giuliani G., Paolino M., Anzini M., Biggio G. Synthesis and structure–activity relationship studies in translocator protein ligands based on a pyrazolo [3, 4-b] quinoline scaffold. J. Med. Chem. 2011;54:7165–7175. doi: 10.1021/jm200770f. [DOI] [PubMed] [Google Scholar]
- 27.Gaurav A., Gautam V., Singh R. An overview on synthetic methodologies and biological activities of pyrazoloquinolines. Mini Rev. Med. Chem. 2010;10:1194–1210. doi: 10.2174/13895575110091194. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Aziz H.A., El-Zahabi H.S., Dawood K.M. Microwave-assisted synthesis and in-vitro anti-tumor activity of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides. Eur. J. Med. Chem. 2010;45:2427–2432. doi: 10.1016/j.ejmech.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Opoku-Temeng C., Dayal N., Sooreshjani M.A., Sintim HO 3H-pyrazolo [4, 3-f] quinoline haspin kinase inhibitors and anticancer properties. Bioorg. Chem. 2018;78:418–426. doi: 10.1016/j.bioorg.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y.R., Luo J.Z., Duan P.P., Shao J., Zhao B.X., Miao J.Y. Synthesis of pyrazole peptidomimetics and their inhibition against A549 lung cancer cells. Bioorg. Med. Chem. Lett. 2012;22:6882–6887. doi: 10.1016/j.bmcl.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 31.Fathy U., Azzam M.A., Mahdy F., El-Maghraby S., Allam R.M. Synthesis and in vitro anticancer activity of some novel tetrahydroquinoline derivatives bearing pyrazol and hydrazide moiety. J. Heterocycl. Chem. 2020;57:2108–2120. [Google Scholar]
- 32.Fathy U., Abu-Hashem A.A., Gouhar R.S., Awad H.M., Elgamal A.M. Synthesis, structural characterization of some pyrazolo [1-5a] pyrimidine and imidazo [1, 2-b]-pyrazole derivatives as anticancer activity. Rasyan J. Chem. 2021;14:741–750. [Google Scholar]
- 33.Karthikeyan C., Amawi H., Viana A.G., Sanglard L., Hussein N., Saddler M., Tiwari A.K. lH-Pyrazolo [3, 4-b] quinolin-3-amine derivatives inhibit growth of colon cancer cells via apoptosis and sub G1 cell cycle arrest. Bioorg. Med. Chem. Lett. 2018;28:2244–2249. doi: 10.1016/j.bmcl.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 34.Hamdy R., Elseginy S.A., Ziedan N.I., Jones A.T., Westwell A.D. New quinoline-based heterocycles as anticancer agents targeting bcl-2. Molecules. 2019;24:1274. doi: 10.3390/molecules24071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.