Abstract
This study was designed to evaluate the effect of dietary Ser on performance, egg quality, serum indices, and ileal mucosal immunity in laying hens fed low crude protein (LCP), essential amino acids (EAA) balanced diets. A total of 480 Hy-Line Brown layers at 24 wk of age were randomly assigned into 5 dietary treatments with 8 replicates of 12 birds each. Treatments included a control diet (16.49% CP), and 4 LCP, EAA balanced diets (14.05% CP) supplemented with 0, 0.114%, 0.306%, 0.498% L-Ser, respectively. Dietary Ser supplementation linearly increased hen-day egg production (HDEP; P < 0.05) and decreased feed-to-egg ratio (P < 0.05) among LCP groups from wk 6 to 10, and the optimal HDEP of layers occurred at Ser level of 0.498%. At the end of wk 10, birds in the control had higher albumen height and thick white proportion than those fed the LCP diet without Ser addition (P < 0.05), and presented a lower yolk color score than all LCP groups (P < 0.05). Among LCP groups, serum total protein and globulin contents were significantly increased by dietary Ser addition at the levels of 0.306% and 0.498% (P < 0.05), and had a linear response to the supplemental Ser levels (P < 0.05). Furthermore, dietary 0.498% Ser supplementation significantly increased serum immunoglobulin G and immunoglobulin M contents (P < 0.05) and up-regulated the expression of mucin 2, secretory immunoglobulin A, and relevant glycosyltransferases of O-glycosylation in ileal mucosa (P < 0.05). The increased expression of proinflammatory cytokines IFN-γ and IL-1β induced by LCP diets (P < 0.05) was reversed following 0.498% Ser addition (P < 0.05). Collectively, dietary CP reduction by 2.44% could maintain the productive performance of layers when it was fortified with certain EAA, though poor albumen quality, and ileal inflammation were occurred. The addition of Ser to LCP diets improved performance probably through enhancing humoral and ileal mucosal immunity and attenuating the ileal inflammation of layers.
Key words: ileal mucosal immunity, laying hen, low crude protein, performance, serine
INTRODUCTION
Low crude protein (LCP) diets have been identified to potentially provide economic, environmental, healthy, and welfare benefits to poultry (Abou-Elkhair et al., 2020). Supplemental crystalline amino acids (AA) in LCP diets are supposed to be an effective strategy for reducing feed costs, nitrogen excretion, and increasing feed utilization (Ospina-Rojas et al., 2012; Hilliar et al., 2019). However, LCP diets fortified with essential AA (EAA) do not consistently result in layers with performance similar to those received adequate CP diets (Lieboldt et al., 2016; Parenteau et al., 2020). One possible reason is the lack of nonessential AA, resulting in a deficiency in nitrogen for the respective AA available and a consumption of EAA for the synthesis of nonessential AA (Dean et al., 2006; Siegert et al., 2015).
Gly and Ser are both nonessential AA for poultry that can be interconverted to each other by Ser hydroxymethyltransferase (Sugahara and Kandatsu, 1976). Meanwhile, it has been evidenced that dietary Ser is equally effective in fulfilling the functions of dietary Gly when considered on an equimolar basis (Sugahara and Kandatsu, 1976). Correspondingly, dietary concentration of Gly + Ser (or Glyequi) was suggested to be calculated to investigate the effect of Gly or Ser on performance of poultry. Several studies, in particular those investigating LCP diets, have observed the performance-enhancing effects of Gly + Ser (or Glyequi) supplementation in poultry, thus proposing that the existing recommendations for Gly + Ser are too low in LCP diets (Dean et al., 2006; Ospina-Rojas et al., 2013; Hofmann et al., 2019). In these studies, different concentrations of dietary Gly + Ser (or Glyequi) were all achieved by supplementing different levels of Gly. Since Gly is required for the metabolization of ammonia into uric acid (UA) in poultry (Namroud et al., 2008; Hilliar et al., 2019), it enhanced performance might through preventing against LCP-induced high level of blood ammonia that was identified to suppress appetite (Panksepp and Booth, 1971). Although Ser is considered as Gly equivalent in diet formulations, the complete supplementation of Ser to meet different dietary Gly + Ser (or Glyequi) were little investigated in laying hens.
As the major carbon donors of one-carbon metabolism, Ser helps coordinate synthesis of nucleotide, S-adenosyl-methionine, NADPH, and glutathione (Yang and Vousden, 2016). It has been confirmed that dietary Ser supplementation can prevent against oxidative stress because of its role in glutathione synthesis (Zhou et al., 2017; He et al., 2020). Ma et al. (2017) proved that Ser is a key immunometabolite that directly regulates adaptive immunity by controlling T cell proliferative capacity. Moreover, an anti-inflammatory effect of Ser was also observed in some recent studies conducted in certain mouse models. For example, it was reported that dietary Ser supplementation prevented LPS-induced intestinal inflammation via p53-dependent glutathione synthesis and AMPK activation (Zhou et al., 2017), while another study indicated that Ser could exert salutary effects on gut microbial composition and alleviate inflammatory responses induced by dextran sulfate sodium in mice (Zhang et al., 2018). These potential functions increase the feasibility of Ser application in poultry especially in those fed LCP diets, because the long-term use of a LCP diet has been identified to be associated with high levels of infections in laying hens (Fafournoux et al., 2000).
To develop a practical understanding of crystalline Ser use in LCP diets in laying hens, a study was hereby designed to assess the effects of L-Ser on performance, egg quality, serum indices, and ileal mucosal immunity in laying hens fed LCP diets fortified with crystalline AA. A recent review recommended 1.1 to 2.0% Glyequi in poultry diets based on existing literature (Siegert and Rodehutscord, 2019). Thr is an important endogenous precursor of Gly or Ser, can partly reduce the requirement of Glyequi in animals. Given the LCP diets were fortified with EAA inclusive of Thr in this study, the Ser addition levels were set to approach the lower range of recommendation. This integrative investigation may contribute to the extension of LCP diets in poultry production.
MATERIALS AND METHODS
Ethic Statement
All experimental protocols were approved by the Animal Care and Use Committee of the Institute of Feed Research of the Chinese Academy of Agricultural Sciences (ACE-CAAS-20200315), and the methods were carried out in accordance with the relevant guidelines and regulations.
Birds and Husbandry
A total of 480, 24-wk old Hy-Line Brown layers were randomly divided into 5 groups, with 8 replicates (12 birds in 4 adjacent cages as a replicate). Initial body weight and laying rate were similar across all the replicates. Layers in the trial were allocated to 3-tier battery cages of 3 laying hens each (cage size: 40 cm × 40 cm × 35 cm) and exposed to 16 h of light/d with an intensity of 20 lx. Temperature was between 26 and 29°C throughout the experiment. Diets and water were offered ad libitum in mash form and by nipple drinkers, respectively. The feeding trial lasted 10 wk after a 7-d adaption period. All hens remained in good health during the feeding period.
Diets
The AA contents of corn, soybean meal and wheat bran used in the current trial were analyzed before diet formulation. The standardized ileal digestible (SID) AA in these ingredients were calculated according to the SID coefficients provided by AminoDat 4.0 (2010). The ingredients and nutrient composition of experimental diets were presented in Table 1. The control diet based on corn and soybean meal contained 16.49% CP. The other 4 experimental diets were reduced dietary crude protein (14.05% CP) by decreasing the soybean meal content and partially increasing the corn content and wheat bran. All treatments were guaranteed to contain the same levels of SID Lys, Met, Met + Cys, Thr, Trp, Val, Ile, and Leu by supplementing crystalline AA. The 4 LCP diets were then supplemented with 0, 0.114%, 0.306%, and 0.498% L-Ser to satisfy the total SID Ser of 85%, 100%, 125%, and 150% of that in the control diet, respectively. All diets were formulated to meet or slightly exceed the NCR recommendations (National Research Council, 1994) for all nutrients (except for CP in LCP groups). The AA contents of each diet were reanalyzed using L-8900 Amino Acid Analyzer (Hitachi Limited, Tokyo, Japan) after formulation.
Table 1.
Composition and nutrient levels of layer diets from 25 to 34 wk of age.
| Item1 | Control2 | LCP2 + L-Serine |
|||
|---|---|---|---|---|---|
| 0 | 0.114 | 0.306 | 0.498 | ||
| Ingredient, % | |||||
| Corn | 64.97 | 67.00 | 67.05 | 67.10 | 67.15 |
| Soybean meal | 24.10 | 16.50 | 16.22 | 15.75 | 15.29 |
| Wheat bran | 0 | 4.59 | 4.60 | 4.63 | 4.65 |
| Dicalcium phosphorate | 0.81 | 0.87 | 0.88 | 0.88 | 0.89 |
| Lime stone | 9.00 | 9.02 | 9.02 | 9.02 | 9.02 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| DL-Methionine, 98% | 0.170 | 0.193 | 0.194 | 0.197 | 0.199 |
| L-Lysine-HCl, 78% | 0.030 | 0.214 | 0.222 | 0.236 | 0.250 |
| L-Threonine, 98% | 0.050 | 0.129 | 0.133 | 0.140 | 0.146 |
| L-Tryptophan, 99% | 0.010 | 0.038 | 0.039 | 0.041 | 0.044 |
| L-Valine, 99% | 0.020 | 0.116 | 0.121 | 0.129 | 0.137 |
| L-Isoleucine, 98% | 0 | 0.105 | 0.110 | 0.118 | 0.126 |
| L-Cysteine•H2O, 68% | 0.180 | 0.208 | 0.210 | 0.213 | 0.217 |
| L-Leucine, 99% | 0 | 0.162 | 0.169 | 0.183 | 0.196 |
| L-Serine, 98% | 0 | 0 | 0.114 | 0.306 | 0.498 |
| Premix3 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| Choline chloride, 50% | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Phytase | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Zeolite | 0 | 0.195 | 0.263 | 0.393 | 0.527 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Nutrient level4, % | |||||
| Metabolizable energy, kcal/kg | 2688 (2719) | 2688 (2601) | 2688 (2640) | 2688 (2773) | 2688 (2711) |
| Crude protein | 16.49 (16.93) | 14.05 (14.07) | 14.05 (14.12) | 14.05 (14.04) | 14.05 (13.90) |
| Calcium | 3.526 (4.275) | 3.526 (4.013) | 3.526 (3.964) | 3.526 (3.520) | 3.526 (3.965) |
| Available phosphorus | 0.319 (0.345) | 0.319 (0.401) | 0.319 (0.382) | 0.319 (0.362) | 0.319 (0.337) |
| SID Lysine | 0.759 (0.739) | 0.759 (0.752) | 0.759 (0.760) | 0.759 (0.748) | 0.759 (0.766) |
| SID Methionine | 0.389 (0.397) | 0.389 (0.376) | 0.389 (0.403) | 0.389 (0.386) | 0.389 (0.401) |
| SID Methionine + cysteine | 0.746 (0.740) | 0.746 (0.721) | 0.746 (0.747) | 0.746 (0.731) | 0.746 (0.725) |
| SID Threonine | 0.565 (0.555) | 0.565 (0.511) | 0.565 (0.526) | 0.565 (0.561) | 0.565 (0.544) |
| SID Tryptophan | 0.173 (0.179) | 0.173 (0.167) | 0.173 (0.180) | 0.173 (0.174) | 0.173 (0.182) |
| SID Valine | 0.709 (0.706) | 0.709 (0.664) | 0.709 (0.691) | 0.709 (0.662) | 0.709 (0.708) |
| SID Isoleucine | 0.608 (0.597) | 0.608 (0.543) | 0.608 (0.560) | 0.608 (0.549) | 0.608 (0.614) |
| SID Leucine | 1.289 (1.319) | 1.289 (1.258) | 1.289 (1.300) | 1.289 (1.339) | 1.289 (1.327) |
| SID Phenylalanine | 0.734 (0.766) | 0.618 (0.598) | 0.612 (0.628) | 0.603 (0.685) | 0.594 (0.585) |
| SID Arginine | 0.963 (0.962) | 0.795 (0.763) | 0.787 (0.748) | 0.774 (0.768) | 0.760 (0.725) |
| SID Histidine | 0.406 (0.425) | 0.354 (0.344) | 0.351 (0.365) | 0.347 (0.347) | 0.342 (0.338) |
| SID Glycine | 0.567 (0.556) | 0.493 (0.512) | 0.489 (0.530) | 0.482 (0.520) | 0.475 (0.525) |
| SID Serine | 0.719 (0.749) | 0.613 (0.598) | 0.719 (0.709) | 0.899 (0.874) | 1.079 (1.084) |
| SID Glycine + serine | 1.286 (1.305) | 1.106 (1.110) | 1.208 (1.239) | 1.381 (1.394) | 1.554 (1.609) |
Abbreviations: HCl, hydrochloride; SID, standardized ileal digestible.
Control, the control diet (16.49% CP); LCP, the low crude protein diets (14.05% CP).
Premix supplied per kilogram of diet: vitamin A, 12,500 IU; vitamin D3, 4,125 IU; vitamin E, 15 IU; vitamin K3, 2 mg; thiamine, 1 mg; riboflavin, 8.5 mg; pyridoxine, 8 mg; vitamin B12, 0.04 mg; biotin, 0.1 mg; folic acid, 1.25 mg; Ca-pantothenate, 50 mg; niacin, 32.5 mg; copper, 8 mg; zinc, 65 mg; iron, 60 mg; manganese, 65 mg; selenium, 0.3 mg; iodine, 1 mg.
The values in parentheses indicate analyzed values. Others are calculated values. Data for the nutrients were calculated values. SID AAs data were calculated using SID coefficients of database from AminoDat 4.0 (2010).
Laying Performance Parameters
Mortality was recorded as it occurred. Daily egg number, total egg weight, irregular (soft, misshapen, oversized and subminiature) eggs, and weekly feed consumption were recorded and calculated as hen-day egg production (HDEP), average egg weight (AEW), average daily feed intake (ADFI), feed-to-egg ratio (F/E) on a weekly basis. The AEW was calculated as the mean weight of total eggs (without irregular eggs). HDEP and ADFI were adjusted for hen mortality and calculated for wk 1 to 5, wk 6 to 10, and wk 1 to 10.
Egg Interior Quality and Composition
Six eggs of each replicate with the weight close to replicate AEW were collected at each of the last 2 d of wk 5 and 10. Egg samples from the first day were tested for their interior quality. Albumen height, yolk color, and Haugh unit were measured using the Egg Analyzer (ORKA Food Technology Ltd, Ramat HaSharon, Israel).
Egg samples from the second day were collected for egg composition determination. The eggs were individually weighted, broken, and the albumen was separated from the yolk. The yolk was weighted after removing the chalaza, and the eggshell was weighted after washing and air-drying. Albumen weight was calculated by subtracting the eggshell and yolk weight from the whole egg weight. The albumen was further separated by passing through a 60-mesh sieve for 30 s. The portion entrapped on the sieve was weighted as thick egg white weight. The whole albumen was finally mixed, freeze-dried and weighted. The eggshell (yolk, albumen or thick egg white) portion (%) = the eggshell (yolk, albumen or thick egg white) weight/the whole egg weight × 100; The albumen solid content (%) = the freeze-dried albumen weight/the fresh albumen weight × 100.
Measurement of Serum Biochemistry and Immunoglobulin Contents
At the end of the feeding trial, one bird per replicate was randomly selected. Blood samples were collected from wing vein and then incubated in a 37°C water bath while tilted at a 45° angle for 8 h to harvest serum. The serum samples were stored at ‒20°C until analysis. Serum total protein (TP), albumin, aspartate transaminase (AST), alanine transaminase, glucose, and UA were determined using automatic biochemical analyzer (Zhuoyue 300, Kehua Bio. Co., Ltd. Shanghai, China). The globulin content in serum was calculated by subtracting the albumin content from the TP content. Serum immunoglobulin (Ig) including IgA, IgM, and IgG were determined only among 3 groups including the control, the LCP diet, and the LCP diet supplemented with 0.498% Ser, which was performed by assay kits for chickens (Shanghai Meilian Bio. Co., Ltd. Shanghai, China) following the manufacturer's instructions.
RNA Isolation and Real-Time Quantitative PCR
At the end of the trial, one bird of each replicate of 3 groups (the control, the LCP diet, and the LCP diet supplemented with 0.498% Ser) was randomly selected and slaughtered. The ileum was removed, opened longitudinally, and gently rinsed with PBS. The mucosa was scraped aseptically by sterile glass slides, frozen as aliquots in liquid nitrogen, and stored at ‒80°C for the quantification of gene expression. Total RNA of the ileal mucosa was extracted with the Trizol reagent (Tiangen Biotech Co., Ltd. Beijing, China) according to the manufacturer's instructions. RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and the integrity was evaluated using agarose-ethidium bromide electrophoresis. Reverse transcription (RT) reactions were immediately performed using the FastQuant RT Kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the instructions. Real-time quantitative PCR was conducted in duplicate in the Bio-Rad C1000 thermal cycler (CFX-96 real-time PCR detection systems; Bio-Rad). PCR programs for all genes were as follows: 95°C for 15 min; 40 cycles of 95°C for 10 s, 60°C for 30 s. The relative mRNA expression levels were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001), and avian β-actin was used as reference gene. The primer sequences for the target genes and β-actin are listed in Table 2.
Table 2.
Primers used for quantitative real-time PCR.
| Gene1 | Primer sequences2 (5’-3’) | Accession no. |
|---|---|---|
| MUC2 | F: CAGCACCAACTTCTCAGTTCC | NM_001318434.1 |
| R: TCTGCAGCCACACATTCTTT | ||
| sIgA | F: ACCACGGCTCTGACTGTACC | S40610.1 |
| R: CGATGGTCTCCTTCACATCA | ||
| IL-6 | F: CAAGGTGACGGAGGAGGAC | NM_204628.1 |
| R: ACTGTGGTGTGCTCAGAATCC | ||
| IL-10 | F: CGGGAGCTGAGGGTGAA | NM_001004414.2 |
| R: GTGAAGAAGCGGTGACAGC | ||
| IFN-γ | F: AGCTGACGGTGGACCTATTATT | NM_205427.1 |
| R: GGCTTTGCGCTGGATTC | ||
| IL-1β | F: CAGCCTCAGCGAAGAGACCTT | NM_204524.1 |
| R: ACTGTGGTGTGCTCAGAATCC | ||
| GALNT1 | F: GGCTGCCTGCTGGAGATGTTC | XM_015282326.1 |
| R: ACGCTTGTTGTGGGAAGGTTGTC | ||
| GALNT6 | F: GACGCCAGCACGGACGAATATC | XM_025145312.1 |
| R: CTCATCCTTCCTCCTCTGCCTCTC | ||
| OGT | F: TCGGCGGAAGGAGACGGATG | XM_001232518.5 |
| R: GGCTGAGGAGGCGGAGAAGG | ||
| ST3GAL1 | F: GCAGCAAGATGGTCACCGTCAG | NM_205217.1 |
| R: CAGTTTCAGCCACCACCTGTAGC | ||
| ST6GALNAC3 | F: TGAGCAACACCACGGATGAAGAAG | XM_025153111.1 |
| R: CTGTGATGTGCTGCTGGCTGTC | ||
| FUT6 | F: GCAGACACCCATCCCATTTCTCAG | XM_025144347.1 |
| R: GCAGGCGACAGCATACAGACAG | ||
| β-actin | F: ATGATATTGCTGCGCTCGTT | NM_205518.1 |
| R: TCTTTCTGGCCCATACCAACC |
Abbreviations: FUT6, fucosyltransferase 6; GALNT1, polypeptide N-acetylgalactosaminyltransferase 1; GALNT6, polypeptide N-acetylgalactosaminyltransferase 6; IFN-γ, interferon-gamma; IL-10, interleukin 10; IL-1β, interleukin 1beta; IL-6, interleukin 6; MUC2, mucin 2; OGT, O-linked N-acetylglucosamine transferase; sIgA, secretory immunoglobulin A; ST3GAL1, ST3 beta-galactoside alpha-2,3-sialyltransferase 1; ST6GALNAC3, ST6 N-acetylgalactosaminide alpha-2,6-sialyltransferase 3.
F, forward; R, reverse.
Statistical Analysis
All analyses were performed using SAS (SAS Institute Inc., 1999). The homogeneity of variances and normality of the data were tested first. The normality was tested using Shapiro-Wilk test. Then, differences among all groups and among LCP groups were analyzed using one-way ANOVA and means were compared using Duncan's Multiple Range Test. Student's t test was employed to explore the differences between the control and the LCP group without Ser addition. The linear and quadratic effects of Ser addition levels in LCP groups were assessed using regression analysis. Differences were considered statistically significant at P < 0.05. Data are expressed as mean and pooled SEM.
RESULTS
Laying Performance
The effect of dietary Ser supplementation on laying performance of layers (25–34 wk of age) fed different diets is presented in Table 3. No difference in laying performance was observed among all treatments during experimental period from wk 1 to 5 (P > 0.05). The HDEP of birds fed the LCP diet supplemented with 0.498% Ser was 4.84% higher when compared with those fed LCP diet without dietary Ser supplementation among all treatments (P < 0.05) and among LCP groups (P < 0.05) from wk 6 to 10. The addition of Ser to LCP diets linearly increased HDEP (P < 0.05) and decreased F/E (P < 0.05) from wk 6 to 10 and wk 1 to 10.
Table 3.
Effect of dietary L-serine supplementation on performance of laying hens fed the low crude protein diets.1
| Item | Hen-day egg production, % |
Average egg weight, g |
Average daily feed intake, g |
Feed-to-egg ratio, g/g |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period, wk | 1–5 | 6–10 | 1–10 | 1–5 | 6–10 | 1–10 | 1–5 | 6–10 | 1–10 | 1–5 | 6–10 | 1–10 |
| Treatments2 | ||||||||||||
| CON | 88.07 | 89.50AB | 88.69 | 57.20 | 59.79 | 58.46 | 107.44 | 113.95 | 110.21 | 2.20 | 2.25 | 2.22 |
| LCP | 86.76 | 86.62Bb | 86.70 | 56.23 | 58.34 | 57.14 | 109.87 | 114.20 | 111.32 | 2.23 | 2.29 | 2.25 |
| LCP + Ser0.114 | 87.00 | 86.94Bb | 86.92 | 56.68 | 59.04 | 57.69 | 108.00 | 115.43 | 110.69 | 2.20 | 2.30 | 2.24 |
| LCP + Ser0.306 | 87.44 | 88.73ABab | 87.94 | 56.55 | 58.46 | 57.37 | 108.80 | 111.07 | 109.70 | 2.20 | 2.16 | 2.18 |
| LCP + Ser0.498 | 88.28 | 91.46Aa | 89.63 | 56.21 | 58.68 | 57.27 | 109.03 | 115.91 | 111.67 | 2.20 | 2.16 | 2.18 |
| SEM | 0.619 | 0.578 | 0.453 | 0.239 | 0.263 | 0.235 | 0.569 | 0.643 | 0.493 | 0.011 | 0.025 | 0.013 |
| P-value | ||||||||||||
| All groups | 0.938 | 0.044 | 0.222 | 0.685 | 0.395 | 0.37 | 0.717 | 0.146 | 0.749 | 0.906 | 0.237 | 0.306 |
| CON vs. LCP | 0.564 | 0.066 | 0.201 | 0.307 | 0.119 | 0.158 | 0.231 | 0.880 | 0.501 | 0.518 | 0.716 | 0.556 |
| LCP groups | 0.863 | 0.045 | 0.166 | 0.911 | 0.863 | 0.909 | 0.774 | 0.115 | 0.637 | 0.789 | 0.111 | 0.098 |
| Linear | 0.384 | 0.005 | 0.025 | 0.878 | 0.934 | 0.963 | 0.850 | 0.898 | 0.924 | 0.422 | 0.026 | 0.016 |
| Quadratic | 0.684 | 0.364 | 0.074 | 0.789 | 0.966 | 0.882 | 0.751 | 0.301 | 0.461 | 0.622 | 0.085 | 0.050 |
n = 8 replicates per treatment.
CON, the control diet (16.49% CP); LCP, the low crude protein diets (14.05% CP); LCP + Ser0.114 (0.306 or 0.498), the low crude protein diet supplemented with 0.114% (0.306% or 0.498%) L-serine.
Within a column, means with no common capital letters (A, B) differ significantly (P < 0.05) among all groups, and with no common small letters (a, b) differ significantly (P < 0.05) among LCP groups.
Egg Interior Quality and Composition
Table 4 shows the egg interior quality indices of layers fed different diets. There were no differences in albumen height, egg yolk color, and Haugh unit among all groups at the end of wk 5 of the experimental period (P > 0.05). At the end of wk 10, birds fed the LCP diet without dietary Ser addition had a lower albumen height (approximately 5.91%) than those fed the control diet (P < 0.05), and all LCP treatments presented a higher yolk color score (P < 0.01).
Table 4.
Effect of dietary L-serine supplementation on egg interior quality of laying hens fed the low crude protein diets.1
| Item2 | Albumen height, mm | Egg yolk color | Haugh unit |
|---|---|---|---|
| Wk 5 | |||
| CON | 8.12 | 5.11 | 89.50 |
| LCP | 7.64 | 5.47 | 86.74 |
| LCP + Ser0.114 | 7.71 | 5.28 | 88.07 |
| LCP + Ser0.306 | 7.72 | 5.50 | 87.49 |
| LCP + Ser0.498 | 7.78 | 5.33 | 88.29 |
| SEM | 0.115 | 0.086 | 0.612 |
| P-value | |||
| All groups | 0.689 | 0.626 | 0.698 |
| CON vs. LCP | 0.253 | 0.241 | 0.170 |
| LCP groups | 0.981 | 0.822 | 0.857 |
| Linear | 0.678 | 0.842 | 0.530 |
| Quadratic | 0.918 | 0.976 | 0.816 |
| Wk 10 | |||
| CON | 8.02 | 4.47A | 88.09 |
| LCP | 7.32 | 5.04BC | 85.12 |
| LCP + Ser0.114 | 7.57 | 4.87BC | 86.34 |
| LCP + Ser0.306 | 7.66 | 4.76B | 87.08 |
| LCP + Ser0.498 | 7.51 | 5.11C | 86.79 |
| SEM | 0.093 | 0.058 | 0.492 |
| P-value | |||
| All groups | 0.133 | <0.001 | 0.367 |
| CON vs. LCP | 0.031 | 0.001 | 0.095 |
| LCP groups | 0.601 | 0.118 | 0.576 |
| Linear | 0.831 | 0.702 | 0.866 |
| Quadratic | 0.385 | 0.054 | 0.365 |
n = 8 replicates (6 eggs per replicate) per treatment.
CON, the control diet (16.49% CP); LCP, the low crude protein diets (14.05% CP); LCP + Ser0.114 (0.306 or 0.498), the low crude protein diet supplemented with 0.114% (0.306% or 0.498%) L-serine.
Within a column, means with no common capital letters (A-C) differ significantly (P < 0.05) among all groups.
There were no differences in egg weight, egg proportions (eggshell, yolk, albumen, and thick egg white), and albumen solid content among all groups at the end of wk 5 and 10 (P > 0.05, Table 5). Among 4 LCP groups, dietary Ser supplementation linearly increased the eggshell proportion at the end of wk 5 (P < 0.05). At the end of wk 10, birds received the LCP diet presented a 2.04% decreased egg thick white proportion as compared with those in the control (P < 0.05).
Table 5.
Effect of dietary L-serine supplementation on egg composition of laying hens fed the low crude protein diets.1
| Item2 | Egg weight, g | Egg composition, % |
Albumen solid, % | |||
|---|---|---|---|---|---|---|
| Eggshell | Egg yolk | Albumen | Thick white | |||
| Wk 5 | ||||||
| CON | 58.20 | 10.45 | 23.80 | 66.03 | 36.54 | 12.44 |
| LCP | 57.93 | 10.53 | 23.41 | 66.22 | 34.50 | 12.15 |
| LCP + Ser0.114 | 57.19 | 10.42 | 23.60 | 66.32 | 34.50 | 12.51 |
| LCP + Ser0.306 | 57.34 | 10.53 | 23.72 | 65.75 | 34.83 | 12.60 |
| LCP + Ser0.498 | 58.15 | 10.80 | 23.45 | 65.75 | 35.17 | 12.27 |
| SEM | 0.394 | 0.066 | 0.169 | 0.174 | 0.495 | 0.096 |
| P-value | ||||||
| All groups | 0.903 | 0.406 | 0.944 | 0.806 | 0.666 | 0.612 |
| CON vs. LCP | 0.803 | 0.500 | 0.339 | 0.583 | 0.210 | 0.381 |
| LCP groups | 0.896 | 0.084 | 0.956 | 0.706 | 0.967 | 0.506 |
| Linear | 0.795 | 0.035 | 0.942 | 0.290 | 0.609 | 0.779 |
| Quadratic | 0.747 | 0.067 | 0.849 | 0.576 | 0.876 | 0.305 |
| Wk 10 | ||||||
| CON | 60.60 | 10.02 | 24.15 | 65.83 | 38.99 | 12.42 |
| LCP | 59.48 | 10.24 | 25.08 | 64.68 | 35.47 | 12.68 |
| LCP + Ser0.114 | 59.56 | 10.31 | 25.03 | 64.66 | 37.14 | 12.50 |
| LCP + Ser0.306 | 58.63 | 10.37 | 24.96 | 64.67 | 37.06 | 12.56 |
| LCP + Ser0.498 | 59.41 | 10.30 | 25.35 | 64.35 | 38.28 | 12.68 |
| SEM | 0.280 | 0.064 | 0.167 | 0.209 | 0.447 | 0.068 |
| P-value | ||||||
| All groups | 0.334 | 0.410 | 0.145 | 0.133 | 0.101 | 0.688 |
| CON vs. LCP | 0.118 | 0.325 | 0.129 | 0.119 | 0.005 | 0.384 |
| LCP groups | 0.707 | 0.715 | 0.588 | 0.628 | 0.256 | 0.792 |
| Linear | 0.789 | 0.212 | 0.410 | 0.433 | 0.065 | 0.832 |
| Quadratic | 0.764 | 0.289 | 0.415 | 0.670 | 0.177 | 0.650 |
n = 8 replicates (6 eggs per replicate) per treatment.
CON, the control diet (16.49% CP); LCP, the low crude protein diets (14.05% CP); LCP + Ser0.114 (0.306 or 0.498), the low crude protein diet supplemented with 0.114% (0.306% or 0.498%) L-serine.
Serum Biochemistry and Immunoglobulin Contents
No statistical significance of comparison in serum biochemistry was observed among all groups and between the control and the LCP group (P > 0.05, Table 6). However, among LCP groups, dietary 0.306% and 0.498% Ser addition showed higher contents in TP than 0.114% Ser supplemental level (P < 0.05) and in globulin than 0 and 0.114% Ser supplemented groups (P < 0.05). The addition of Ser to LCP diets linearly increased TP and globulin contents (P < 0.05) and decreased AST activity (P < 0.05) in serum.
Table 6.
Effect of dietary L-serine supplementation on serum biochemistry of laying hens fed the low crude protein diets.1
| Item2 | TP (g/L) | ALB (g/L) | GLOB (g/L) | AST (U/L) | ALT (U/L) | GLU (mmol/L) | UA (U/L) |
|---|---|---|---|---|---|---|---|
| Treatments3 | |||||||
| CON | 50.28 | 19.81 | 30.47 | 205.86 | 4.43 | 4.04 | 216.29 |
| LCP | 43.78ab | 20.14 | 23.64b | 226.50 | 3.00 | 3.45 | 175.00 |
| LCP + Ser0.114 | 42.13b | 18.74 | 23.39b | 220.33 | 4.17 | 4.02 | 156.17 |
| LCP + Ser0.306 | 51.75a | 21.21 | 30.54a | 217.67 | 3.00 | 4.47 | 165.50 |
| LCP + Ser0.498 | 49.88a | 20.79 | 29.09a | 193.50 | 4.50 | 3.76 | 169.67 |
| SEM | 1.586 | 0.414 | 1.330 | 4.889 | 0.362 | 0.195 | 11.313 |
| P-value | |||||||
| All groups | 0.216 | 0.406 | 0.207 | 0.230 | 0.506 | 0.605 | 0.476 |
| CON vs. LCP | 0.282 | 0.818 | 0.200 | 0.257 | 0.261 | 0.376 | 0.323 |
| LCP groups | 0.047 | 0.318 | 0.036 | 0.085 | 0.431 | 0.429 | 0.963 |
| Linear | 0.026 | 0.271 | 0.016 | 0.016 | 0.386 | 0.575 | 0.999 |
| Quadratic | 0.075 | 0.551 | 0.040 | 0.401 | 0.645 | 0.248 | 0.828 |
n = 8 replicates (6 eggs per replicate) per treatment.
Abbreviations: ALB, albumin; ALT, alanine transaminase; AST, aspartate transaminase; GLOB, globulin; GLU, glucose; TP, total protein; UA, uric acid.
CON, the control diet (16.49% CP); LCP, the low crude protein diets (14.05% CP); LCP + Ser0.114 (0.306 or 0.498), the low crude protein diet supplemented with 0.114% (0.306% or 0.498%) L-serine.
Within a column, means with no common small letters (a, b) differ significantly (P < 0.05) among LCP groups.
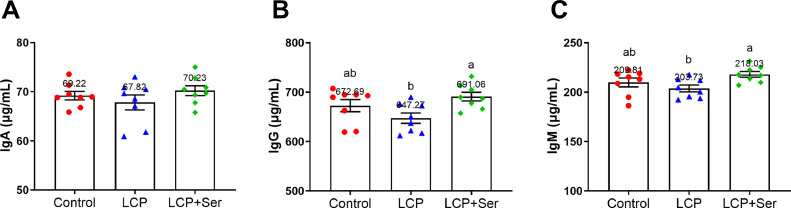
Figure 1 illustrates the serum immunoglobulin contents of layers fed the control diet, the LCP diet, and the LCP diet supplemented with 0.498% Ser. Serum IgG and IgM contents of birds fed the LCP diet supplemented with 0.498% Ser were respectively 6.77% and 6.56% higher than those received the LCP diet without dietary Ser supplementation (P < 0.05).
Figure 1.
Effect of dietary L-serine supplementation on serum immunoglobulins of laying hens fed the low crude protein diet. A, B and C respectively show the serum immunoglobulin A, immunoglobulin G and immunoglobulin M contents of layers. Control, the control diet (16.49% CP); LCP, the low crude protein diet (14.05% CP); LCP + Ser, the low crude protein diet supplemented with 0.498% L-serine. Data are represented with the means ± SEM (n = 8). Bars with no common small letters (a, b) indicate statistical differences among 3 treatments (P < 0.05).
Relative mRNA Expression in Ileal Mucosa
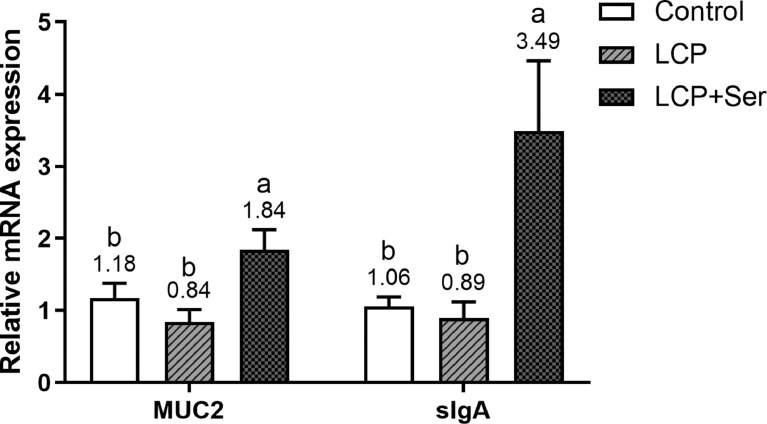
The relative mRNA expression of MUC2 and sIgA in the ileal mucosa of layers after feeding 10 wk is shown in Figure 2. There were significant increases (P < 0.05) in the relative expression of ileal mucosal MUC2 and sIgA of layers fed the LCP diet supplemented with 0.498% Ser, compared with the other 2 groups. The relative expressions of ileal mucosal MUC2 and sIgA in laying hens fed the LCP diet supplemented with 0.498% Ser were respectively 119.05% and 292.13% higher than the LCP without Ser addition group.
Figure 2.
Effect of dietary L-serine supplementation on the relative mRNA expression of mucin 2 (MUC2) and secretory immunoglobulin A (sIgA) in the ileal mucosa of laying hens fed the low crude protein diet. Control, the control diet (16.49% CP); LCP, the low crude protein diet (14.05% CP); LCP + Ser, the low crude protein diet supplemented with 0.498% L-serine. Data are represented with the means ± SEM (n = 8). Bars with no common small letters (a, b) indicate statistical differences among 3 treatments (P < 0.05).
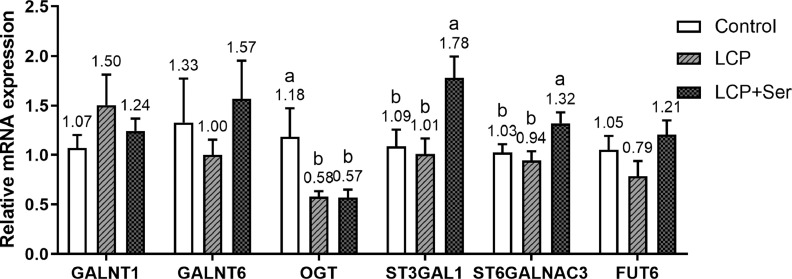
Figure 3 shows the relative expression of ileal mucosal glycosyltransferases related to O-glycosylation among the 3 treatments including the control diet, the LCP diet and the LCP diet supplemented with 0.498% Ser. No differences were found in the relative expression of polypeptide N-acetylgalactosaminyltransferase 1, polypeptide N-acetylgalactosaminyltransferase 6, and fucosyltransferase 6 among these treatments (P > 0.05). Compared with the control, the LCP diet with or without 0.498% Ser supplementation led to a down-regulated mRNA expression of O-linked N-acetylglucosamine transferase (OGT) in the ileal mucosa (P < 0.05). The supplementation of 0.498% Ser to LCP diet up-regulated the mRNA expression for ST3 beta-galactoside alpha-2,3-sialyltransferase 1 (ST3GAL1) and ST6 N-acetylgalactosaminide alpha-2,6-sialyltransferase 3 (ST6GALNAC3) as compared with the other 2 diets (P < 0.05).
Figure 3.
Effect of dietary L-serine supplementation on the relative mRNA expression of ileal mucosal glycosyltransferases related to O-glycosylation of laying hens fed the low crude protein diet. Control, the control diet (16.49% CP); LCP, the low crude protein diet (14.05% CP); LCP + Ser, the low crude protein diet supplemented with 0.498% L-serine. Data are represented with the means ± SEM (n = 8). Bars with no common small letters (a, b) indicate statistical differences among 3 treatments (P < 0.05).
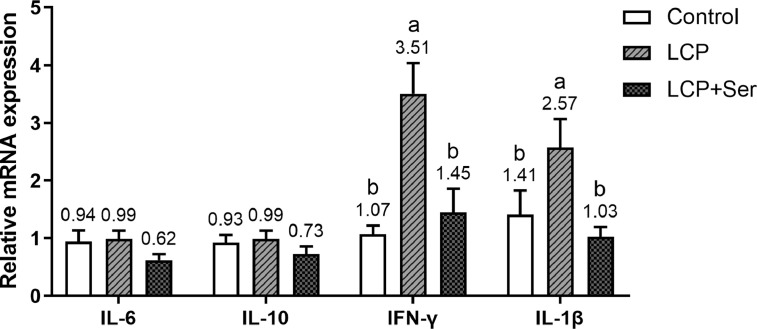
No differences in the relative expression of ileal mucosal IL-6 and IL-10 (P > 0.05) were observed among the 3 groups including the control diet, the LCP diet and the LCP diet supplemented with 0.498% Ser (Figure 4). However, the relative expression of ileal mucosal IFN-γ and IL-1β of birds fed the LCP diet were 228.04% and 82.27% higher than those in the control, respectively (P < 0.05), these up-regulations were reversed with the addition of 0.498% Ser in LCP diet (P < 0.05).
Figure 4.
Effect of dietary L-serine supplementation on the relative mRNA expression of ileal mucosal cytokines of laying hens fed the low crude protein diet. Control, the control diet (16.49% CP); LCP, the low crude protein diet (14.05% CP); LCP + Ser, the low crude protein diet supplemented with 0.498% L-serine. Data are represented with the means ± SEM (n = 8). Bars with no common small letters (a, b) indicate statistical differences among 3 treatments (P < 0.05).
DISCUSSION
Previous studies reported that dietary CP reduction was associated with poor egg production and feed efficiency of layers (Roberts et al., 2007; Azzam et al., 2017; Alagawany et al., 2020), which can be imputed to the imbalance of AA composition in LCP diets (Keshavarz and Jacson, 1992). In the current study, the unobvious differences in HDEP and F/E between the LCP treatment (14.05% CP) and the control (16.49% CP) might because that the LCP diet was well fortified with EAA, including Lys, Met, Met + Cys, Thr, Trp, Val, Ile, and Leu. Similarly, it was reported that the performance of layers fed a 13% CP diet supplemented with Lys, Met, and Try could all be maintained as compared with those fed 16% CP diet (Keshavarz and Austic, 2004). Reduced egg weight was also reported in birds fed LCP diets (Lieboldt et al., 2016; Dong et al., 2017), which was believed to result from an inadequate level of total nitrogen (Fariborz et al., 2007). Sohail et al. (2002) emphasized the importance of EAA in LCP diets for egg weight maintenance that removing synthetic Lys, Ile, Thr, and Try resulted in reduced egg weight within 2 wk. Thus, a comparable AEW between the control and the LCP treatment in the current study suggests the adequate levels of total nitrogen and EAA in LCP diet. A study with similar percentage point's reduction of CP in the diet balanced with Lys, Met, Trp, Val, and Ile also illustrated that the AEW was not affected by dietary CP reduction (Azzam et al., 2017). Taken together, dietary CP reduction from 16.49% to 14.05% can maintain a comparable productive performance to the control within a certain period when it was fortified with the EAA, including Lys, Met, Met + Cys, Thr, Trp, Val, Ile, and Leu.
Limited information can be found regarding the effect of the Ser supplementation on the performance of layers fed LCP diets. Dietary Ser has been reported to fulfill equal functions as dietary Gly fed in equimolar amounts (Sugahara and Kandatsu, 1976). Therefore, Ser and Gly are usually considered together in the form of Gly + Ser (or Glyequi) when the effect on poultry performance of varying dietary Ser and Gly supplementation is investigated (Siegert et al., 2015). In the current study, dietary Gly + Ser was increased with the Ser addition since the concentration of Gly in the LCP diets was almost equal. Ser addition linearly increased HDEP and decreased F/E of layers fed LCP diets, resulting in an optimal HDEP with Ser level of 0.498% (1.554% Gly + Ser). Similarly, it was reported that the growth performance of broilers fed LCP diets (16.21% CP) was increased linearly as the concentration of dietary Gly + Ser was increased, and 2.07% Gly + Ser supplemented group performed no difference from the control (22.21% CP) (Dean et al., 2006). Although the HDEP of hens fed the LCP diet was not different from that of the control in the current study, it was still improved by dietary Gly + Ser at a higher concentration than the control. This would support the view that the ideal dietary Gly + Ser is higher in poultry given LCP diets (Dean et al., 2006; Ospina-Rojas et al., 2013; Siegert et al., 2015).
Albumen height is an important indicator of protein quality. Deteriorations in this value also happen based on freshness, but the main factor is protein quality in the egg white. In the current study, albumen height was decreased by dietary CP reduction. Similar results were also reported by Parenteau et al. (2020) that birds given a 2% CP reduction diet exhibited the lower albumen height and Haugh unit than those in the control. Ovomucin is generally accepted as a key component in maintaining the natural viscosity of albumen, and consequently the albumen height (Toussant and Latshaw, 1999). Thus, the LCP diet might be detrimental to the production of ovomucin. In support of this view, the proportion of thick white that contains the most ovomucin (about 2–4 times that in thin white) of the albumen was also significantly lower in the LCP group than that in the control. Egg yolk color is another important criterion for consumers, and high yolk color score eggs are usually considered more attractive because it is believed that dark colored eggs evoke natural eggs (Esfahani-Mashhour et al., 2009). In the current study, all the LCP diets increased the yolk color score compared with the control. As all known, corn contains higher contents of xanthophylls that are a primary contributor of yolk pigmentation (Roberts et al., 2007). The LCP diets contained a higher percentage of corn than the control diet. Thus, the deepened egg yolk color in LCP groups can be understandable. There were no effects of dietary Ser on egg interior quality and egg composition except that a linearly increased eggshell proportion was observed with Ser addition in LCP diets. Limited information is available regarding the dietary effect of Ser on the eggshell parameters of layers fed LCP diets. Ser is the most abundant AA of osteopontin which is crucial for eggshell formation (Chien et al., 2008). We speculated that the influence of dietary Ser on eggshell proportion might be related to osteopontin. Further studies are needed to elucidate the potential mechanism.
Little attention has been paid to the influence of dietary Ser supplementation or its deficiency on serum biochemistry or features of the immune system of laying hens, although its anti-inflammatory and anticancer effects are well documented in vitro or in vivo (Stiuso et al., 2016; Newman and Maddocks, 2017; He et al., 2020). Serum biochemical indices can partly reflect the metabolism and health status of the organism. Serum TP, albumin, and UA reflect the metabolic status of protein in the body to a certain extent, while serum globulin is closely related to the immune level of the body (Geng et al., 2021). In the current study, serum TP and globulin contents were significantly increased following Ser addition at the levels of 0.306% and 0.498%, and had a linear response to the Ser supplemental levels in LCP diets. As seen from Table 6, the increased serum TP was mainly caused by the increased serum globulin, indicating a possible reinforcement of immunity of birds (Attia et al., 2020). Immunoglobulins that produced by lymphocytes are viewed as crucial indicators of humoral immunity (Schroeder and Cavacini, 2010). IgA, IgG and IgM belong to natural antibodies in avian species, which have broad reactivity against foreign antigens and play multiple roles in health and disease (Sun et al., 2013). It has been evidenced that dietary intervention could improve the immune function by increasing serum IgG and IgM in laying hens (Sun et al., 2020). Herein, serum IgG and IgM contents were significantly increased by supplementing 0.498% Ser to the LCP diet, which further proved that Ser supplementation in a CP-reduction diet enhanced the immunity of layers.
In attempt to better understand the positive effects of dietary Ser addition on the performance of birds fed LCP diets, the expression of some important genes related to ileal mucosal immunity was further investigated. Mucins are the main component of the intestinal mucus layer, whose main function is to protect the intestine from the hostile external elements (chemical, physical, or bacterial) as a form of innate immunity in poultry (Erf, 2004; Lee et al., 2020). MUC2 is a typical secreted mucin, and any dietary changes may influence its expression and function in the intestinal mucus layer (Linden et al., 2008; van der Sluis et al., 2009). In the current study, the mRNA expression of ileal mucosal MUC2 was significantly increased by supplementing 0.498% Ser to the LCP diet. Analogously, it was reported that dietary L-Thr supplementation could up-regulate the ileal MUC2 gene expression of laying hens fed a LCP diet (Dong et al., 2017). Since Ser and Thr are both Gly equivalents and can transform to each other in vivo via glycine metabolism (Hilliar et al., 2019), they might affect MUC2 mRNA expression with a similar undiscovered mechanism. Notably, the protein cores of mucins domains are characterized by abundant Ser and Thr, which are crucial for the initiation of O-glycosylation of mucin as the attachment sites for O-glycans (Arike and Hansson, 2016). Mucin-type O-glycosylation plays important roles in protein stability, processing, and function and MUC2 is the major O-glycosylated protein of the small intestine (Kidani et al., 2016; Arike et al., 2017). Thus, we further determined the relative mRNA expression of relevant glycosyltransferases of O-glycosylation in ileal mucosa, since the most glycosyltransferases found in the intestinal epithelial mucosa are involved in MUC2 O-glycosylation (Arike et al., 2017). A decreased mRNA expression of OGT was observed in LCP groups, while dietary 0.498% Ser addition up-regulated the mRNA expression of ST3GAL1 and ST6GALNAC3, implying a possible regulation of Ser on the O-glycosylation of ileal MUC2. Thus, it can be concluded that dietary Ser supplementation may enhance the ileal mucosal immunity by simultaneously regulating the transcription and modification of MUC2 gene in the ileum of laying hens fed a LCP diet.
One main way of mucins to enhance the mucosal immune system is by the accumulation of sIgA (Dong et al., 2017). SIgA secreted by epithelial cells is the principal immunoglobulin in the intestinal mucus, which was served as a key indicator of intestinal immune system (Zhou et al., 2021). It is important in preventing bacterial or viral infection and pathogenesis, maintaining the maintenance of mucosal homeostasis and intestinal barrier integrity, and improving the intestinal immune response by uptaking antigen to dendritic cells (Lammers et al., 2010; Wu et al., 2014). In the current study, supplementing 0.498% Ser to the LCP diet significantly up-regulated the mRNA expression of ileal mucosal sIgA in this study, proving the improvement of ileal mucosal immunity. The enhancement of intestinal immune barrier would benefit to the nutrients absorption, this may contribute to the improvement of performance of layers following Ser addition.
It has been reported that prolonged duration of a LCP diet will decrease plasma AA concentration and is associated with high level of infections in laying hens (Fafournoux et al., 2000). In concert with a previous study (Abou-Elkhair et al., 2020), this study showed that dietary CP reduction induced intestinal inflammation in laying hens, evidenced by the elevated mRNA expression of ileal proinflammatory cytokines inclusive of IFN-γ and IL-1β. They increased by 228.04% and 82.27%, respectively. IFN-γ could induce the protective immune responses against intracellular pathogens, whereas IL-1β is related to the initiation of inflammatory responses (Abbas et al., 1996; Eckmann and Kagnoff, 2001; Kogut et al., 2005). However, the expressions of ileal mucosal IFN-γ and IL-1β of birds fed LCP diet were respectively reduced 58.69% and 59.92% by 0.498% Ser addition, providing a clue for the role of Ser in relieving ileal inflammation of laying hens. Based on some previous studies, the inflammation was considered occurred or alleviated when the mRNA expression of inflammatory cytokines changed by 30% to 50% (Wang et al., 2016; Su et al., 2018). Thus, the magnitude of the expression changes of IFN-γ and IL-1β could be enough to validate the proinflammatory response of LCP diet and anti-inflammatory effect by Ser addition in this study. Similar results were also described by some previous studies, in which dietary Ser supplementation prevented intestinal inflammation against challenge in poultry (Zhou et al., 2017; Zhang et al., 2018). Notably, although the rapid increases of proinflammatory cytokines might cause intestinal damage and result in high consumption of nutrients (Dinarello, 2000), it does not seem to trigger the impaired performance in LCP group. However, the performance-enhancing effect of Ser in LCP diets might still be related to its role in alleviating intestinal inflammation.
CONCLUSION
This study revealed that lowering the dietary crude protein from 16.49% to 14.05% could maintain the performance of layers from 25 to 34 wk of age, when it was fortified with essential amino acids. However, poor albumen quality and ileal inflammation were observed following crude protein reduction. Dietary Ser supplementation linearly increased hen-day egg production and decreased feed-to-egg ratio of layers fed low crude protein diets, leading to an optimal egg production with Ser level of 0.498% in this trial. The improved performance may be attributed to the enhancement of serum and ileal mucosal immunity and the alleviation of ileal inflammation induced by low crude protein diets. In future, long-term studies with more graded levels of Ser should be conducted to investigate the optimal dietary Ser in laying hens fed low crude protein diets.
Acknowledgments
ACKNOWLEDGMENTS
This study was financially supported by National Natural Science Foundation of China (32072774), Beijing Innovation Consortium of Agriculture Research System (BAIC04-2018), the earmarked fund for Modern Agro-industry Technology Research System (CARS-40-K12), and the Agricultural Science and Technology Innovation Program (ASTIP) of the Chinese Academy of Agricultural Sciences.
DISCLOSURES
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with this work submitted.
REFERENCES
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Abou-Elkhair R., Ahmed H., Ketkat S., Selim S. Supplementation of a low-protein diet with tryptophan, threonine, and valine and its impact on growth performance, blood biochemical constituents, immune parameters, and carcass traits in broiler chickens. Vet. World. 2020;13:1234–1244. doi: 10.14202/vetworld.2020.1234-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., El-Hindawy M.M., El-Hack M.E.A., Arif M., El-Sayed S.A. Influence of low-protein diet with different levels of amino acids on laying hen performance, quality and egg composition. Ann. Acad. Bras. Cienc. 2020;92 doi: 10.1590/0001-3765202020180230. [DOI] [PubMed] [Google Scholar]
- AminoDat 4.0 . Evonik Industries AG; Hanau, Germany: 2010. Pages 0–50 in Year Amino Acid Analysis. [Google Scholar]
- Arike L., Hansson G.C. The densely O-glycosylated MUC2 mucin protects the intestine and provides food for the commensal bacteria. J. Mol. Biol. 2016;428:3221–3229. doi: 10.1016/j.jmb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arike L., Holmén-Larsson J., Hansson G.C. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology. 2017;27:318–328. doi: 10.1093/glycob/cww134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y.A., Al-Harthi M.A., Abo El-Maaty H.M. Calcium and cholecalciferol levels in late-phase laying hens: effects on productive traits, egg quality, blood biochemistry, and immune responses. Front. Vet. Sci. 2020;7:389. doi: 10.3389/fvets.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam M.M.M., Dong X.Y., Zou X.T. Effect of dietary threonine on laying performance and intestinal immunity of laying hens fed low-crude-protein diets during the peak production period. J. Anim. Physiol. Anim. Nutr. 2017;101:e55–e66. doi: 10.1111/jpn.12559. [DOI] [PubMed] [Google Scholar]
- Chien Y.C., Hincke M.T., Vali H., McKee M.D. Ultrastructural matrix-mineral relationships in avian eggshell, and effects of osteopontin on calcite growth in vitro. J. Struct. Biol. 2008;163:84–99. doi: 10.1016/j.jsb.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Dean D.W., Bidner T.D., Southern L.L. Glycine supplementation to low crude protein, amino acid-supplemented diets supports optimal performance of broiler chicks. Poult. Sci. 2006;85:288–296. doi: 10.1093/ps/85.2.288. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Dong X.Y., Azzam M.M.M., Zou X.T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017;96:3654–3663. doi: 10.3382/ps/pex185. [DOI] [PubMed] [Google Scholar]
- Eckmann L., Kagnoff M.F. Cytokines in host defense against Salmonella. Microbes Infect. 2001;3:1191–1200. doi: 10.1016/s1286-4579(01)01479-4. [DOI] [PubMed] [Google Scholar]
- Erf G.F. Cell-mediated immunity in poultry. Poult. Sci. 2004;83:580–590. doi: 10.1093/ps/83.4.580. [DOI] [PubMed] [Google Scholar]
- Esfahani-Mashhour M., Moravej H., Mehrabani-Yeganeh H., Razavi S.H. Evaluation of coloring potential of Dietzia natonolinaea biomass as source of canthaxanthin for egg yolk pigmentation. Asian-Aust. J. Anim. Sci. 2009;22:254–259. [Google Scholar]
- Fariborz K., Faraji M., Dehkordi S.K. Effects of reduced-protein diets at constant total sulfur amino acids: lysine ratio on pullet development and subsequent laying hen performance. Am. J. Anim. Vet. Sci. 2007;2:89–92. [Google Scholar]
- Fafournoux P., Bruhat A., Jousse C. Amino acid regulation of gene expression. Biochem. J. 2000;351:1–12. doi: 10.1042/0264-6021:3510001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng A.L., Zhang Y., Zhang J., Zeng L.C., Chang C., Wang H.H., Yan Z.X., Chu Q., Liu H.G. Effects of light regime on the hatching performance, body development and serum biochemical indexes in Beijing You Chicken. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Long J., Zhou X., Liu Y., Li T., Wu X. Serine is required for the maintenance of redox balance and proliferation in the intestine under oxidative stress. FASEB J. 2020;34:4702–4717. doi: 10.1096/fj.201902690R. [DOI] [PubMed] [Google Scholar]
- Hilliar M., Huyen N., Girish C.K., Barekatain R., Wu S., Swick R.A. Supplementing glycine, serine, and threonine in low protein diets for meat type chickens. Poult. Sci. 2019;98:6857–6865. doi: 10.3382/ps/pez435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann P., Siegert W., Kenéz Á., Naranjo V.D., Rodehutscord M. Very low crude protein and varying glycine concentrations in the diet affect growth performance, characteristics of nitrogen excretion, and the blood metabolome of broiler chickens. J. Nutr. 2019;149:1122–1132. doi: 10.1093/jn/nxz022. [DOI] [PubMed] [Google Scholar]
- Keshavarz K., Jackson M.E. Performance of growing pullets and laying hens fed low-protein, amino acid-supplemented diets. Poult. Sci. 1992;71:905–918. doi: 10.3382/ps.0710905. [DOI] [PubMed] [Google Scholar]
- Keshavarz K., Austic R.E. The use of low-protein, low-phosphorus, amino acid- and phytase-supplemented diets on laying hen performance and nitrogen and phosphorus excretion. Poult. Sci. 2004;83:75–83. doi: 10.1093/ps/83.1.75. [DOI] [PubMed] [Google Scholar]
- Kidani S., Kaneoka H., Okuzaki Y., Asai S., Kojima Y., Nishijima K., Iijima S. Analyses of chicken sialyltransferases related to O-glycosylation. J. Biosci. Bioeng. 2016;122:379–384. doi: 10.1016/j.jbiosc.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., He H., Kaiser P. Lipopolysaccharide binding protein/CD14/TLR4-dependent recognition of Salmonella LPS induces the functional activation of chicken heterophils and up-regulation of pro-inflammatory cytokine and chemokine gene expression in these cells. Anim. Biotechnol. 2005;16:165–181. doi: 10.1080/10495390500264896. [DOI] [PubMed] [Google Scholar]
- Lammers A., Wieland W.H., Kruijt L., Jansma A., Straetemans T., Schots A., den Hartog G., Parmentier H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010;34:1254–1262. doi: 10.1016/j.dci.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Lee C.Y., Song A.A., Loh T.C., Abdul Rahim R. Effects of lysine and methionine in a low crude protein diet on the growth performance and gene expression of immunity genes in broilers. Poult. Sci. 2020;99:2916–2925. doi: 10.1016/j.psj.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieboldt M.A., Halle I., Frahm J., Schrader L., Weigend S., Preisinger R., Dänicke S. Effects of long-term graded L-arginine supply on growth development, egg laying and egg quality in four genetically diverse purebred layer lines. J. Poult. Sci. 2016;53:8–21. doi: 10.2141/jpsa.0150067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden S.K., Sutton P., Karlsson N.G., Korolik V., McGuckin M.A. Mucins in the mucosal barrier to infection. Mucosal. Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma E.H., Bantug G., Griss T., Condotta S., Johnson R.M., Samborska B., Mainolfi N., Suri V., Guak H., Balmer M.L., Verway M.J., Raissi T.C., Tsui H., Boukhaled G., Henriques da Costa S., Frezza C., Krawczyk C.M., Friedman A., Manfredi M., Richer M.J., Hess C., Jones R.G. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 2017;25:345–357. doi: 10.1016/j.cmet.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Namroud N.F., Shivazad M., Zaghari M. Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks. Poult. Sci. 2008;87:2250–2258. doi: 10.3382/ps.2007-00499. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Newman A.C., Maddocks O.D.K. Serine and functional metabolites in cancer. Trends Cell Biol. 2017;27:645–657. doi: 10.1016/j.tcb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Eyng C., Nunes R.V., Duarte C.R.A., Vargas M.D. Commercially available amino acid supplementation of low-protein diets for broiler chickens with different ratios of digestible glycine+serine:lysine. Poult. Sci. 2012;91:3148–3155. doi: 10.3382/ps.2012-02470. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Moreira I., Picoli K.P., Rodrigueiro R.J.B., Furlan A.C. Dietary glycine+serine responses of male broilers given low-protein diets with different concentrations of threonine. Br. Poult. Sci. 2013;54:486–493. doi: 10.1080/00071668.2013.794257. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Booth D.A. Decreased feeding after injections of amino-acids into the hypothalamus. Nature. 1971;233:341–342. doi: 10.1038/233341a0. [DOI] [PubMed] [Google Scholar]
- Parenteau I.A., Stevenson M., Kiarie E.G. Egg production and quality responses to increasing isoleucine supplementation in Shaver white hens fed a low crude protein corn-soybean meal diet fortified with synthetic amino acids between 20 and 46 weeks of age. Poult. Sci. 2020;99:1444–1453. doi: 10.1016/j.psj.2019.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.A., Xin H., Kerr B.J., Russell J.R., Bregendahl K. Effects of dietary fiber and reduced crude protein on nitrogen balance and egg production in laying hens. Poult. Sci. 2007;86:1716–1725. doi: 10.1093/ps/86.8.1716. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS Proprietary Software. Release 8.2. SAS Inst. Inc.; Cary, NC: 1999. [Google Scholar]
- Schroeder H.W., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immun. 2010;125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert W., Ahmadi H., Rodehutscord M. Meta-analysis of the influence of dietary glycine and serine, with consideration of methionine and cysteine, on growth and feed conversion of broilers. Poult. Sci. 2015;94:1853–1863. doi: 10.3382/ps/pev129. [DOI] [PubMed] [Google Scholar]
- Siegert W., Rodehutscord M. The relevance of glycine and serine in poultry nutrition: a review. Br. Poult Sci. 2019;60:579–588. doi: 10.1080/00071668.2019.1622081. [DOI] [PubMed] [Google Scholar]
- Sohail S.S., Bryant M.M., Roland D.A. Influence of supplemental lysine, isoleucine, threonine, tryptophan and total sulfur amino acids on egg weight of Hy-line W-36 hens. Poult. Sci. 2002;81:1038–1044. doi: 10.1093/ps/81.7.1038. [DOI] [PubMed] [Google Scholar]
- Stiuso P., Bagarolo M.L., Ilisso C.P., Vanacore D., Martino E., Caraglia M., Porcelli M., Cacciapuoti G. Protective effect of tyrosol and S-adenosylmethionine against ethanol-induced oxidative stress of Hepg2 cells involves Sirtuin 1, p53 and Erk1/2 signaling. Int. J. Mol. Sci. 2016;17:622. doi: 10.3390/ijms17050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Zhang X., Xin H., Li S., Li J., Zhang R., Li X., Li J., Bao J. Effects of prior cold stimulation on inflammatory and immune regulation in ileum of cold-stressed broilers. Poult. Sci. 2018;97:4228–4237. doi: 10.3382/ps/pey308. [DOI] [PubMed] [Google Scholar]
- Sugahara M., Kandatsu M. Glycine serine interconversion in the rooster. Agric. Biol. Chem. 1976;40:833–837. [Google Scholar]
- Sun X., Yue S.Z., Qiao Y.H., Sun Z.J., Wang C., Li H.F. Dietary supplementation with selenium-enriched earthworm powder improves antioxidative ability and immunity of laying hens. Poult. Sci. 2020;99:5344–5349. doi: 10.1016/j.psj.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Biscarini F., Bovenhuis H., Parmentier H.K., van der Poel J.J. Genetic parameters and across-line SNP associations differ for natural antibody isotypes IgM and IgG in laying hens. Anim. Genet. 2013;44:413–424. doi: 10.1111/age.12014. [DOI] [PubMed] [Google Scholar]
- Toussant M.J., Latshaw J.D. Ovomucin content and composition in chicken eggs with different interior quality. J. Sci. Food Agric. 1999;79:1666–1670. [Google Scholar]
- van der Sluis M., Schaart M.W., de Koning B.A., Schierbeek H., Velcich A., Renes I.B., van Goudoever J.B. Threonine metabolism in the intestine of mice: loss of mucin 2 induces the threonine catabolic pathway. J. Pediatr. Gastroenterol. Nutr. 2009;49:99–107. doi: 10.1097/MPG.0b013e3181a23dbe. [DOI] [PubMed] [Google Scholar]
- Wang W., Li Z., Ren W., Yue Y., Guo Y. Effects of live yeast supplementation on lipopolysaccharide-induced inflammatory responses in broilers. Poult. Sci. 2016;95:2557–2564. doi: 10.3382/ps/pew191. [DOI] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Huang J. Toxicological effects of nickel chloride on IgA+ B Cells and sIgA, IgA, IgG, IgM in the intestinal mucosal immunity in broilers. Int. J. Environ. Res. Public Health. 2014;11:8175–8192. doi: 10.3390/ijerph110808175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Vousden K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- Zhang H., Hua R., Zhang B., Zhang X., Yang H., Zhou X. Serine alleviates dextran sulfate sodium-induced colitis and regulates the gut microbiota in mice. Front. Microbiol. 2018;9:3062. doi: 10.3389/fmicb.2018.03062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.M., Zhang H.J., Wu S.G., Qiu K., Fu Y., Qi G.H., Wang J. Supplemental xylooligosaccharide modulates intestinal mucosal barrier and cecal microbiota in laying hens fed oxidized fish oil. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.635333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zhang Y., He L., Wan D., Liu G., Wu X., Yin Y. Serine prevents LPS-induced intestinal inflammation and barrier damage via p53-dependent glutathione synthesis and AMPK activation. J. Funct. Foods. 2017;39:225–232. [Google Scholar]