Abstract
Standardized ileal digestibility coefficients (SIDC) of nitrogen (N) and amino acids (AA) in wheat and sorghum at 6 different ages (d 7, 14, 21, 28, 35, and 42) of broilers were determined. Two assay diets were formulated to contain 93.8% of each grain as the sole source of AA in the diet. Titanium dioxide (0.5%) was added as an indigestible marker. Each assay diet was fed to 6 replicate cages housing 14 (d 7), 12 (d 14), 10 (d 21), 8 (d 28), 8 (d 35), and 6 (d 42) birds per cage for 4 d prior to ileal digesta collection. The apparent ileal digestibility coefficients (AIDC) were standardized by using the age-appropriate basal endogenous AA losses. In the case of wheat, AIDC of N and all AA increased (linear or quadratic, P < 0.05 to 0.001) with advancing age. No age effect was noticed on the SIDC of N, average of indispensable (IAA) and dispensable AA (DAA), though the average of total AA (TAA) tended (linear, P = 0.09) to increase as birds grew older. In sorghum, the AIDC of N, average of IAA and DAA were unaffected (P > 0.05) by age. The SIDC of N, average SIDC of IAA, DAA and TAA were higher at d 7, reduced at d 14 and then plateaued. Among the IAA, the SIDC of Arg, His, Ile, Leu, Lys, Thr, Val, and the SIDC of all individual DAA (except Cys) decreased with age (linear or quadratic, P < 0.05 to 0.001) with higher values at d 7. The higher SIDC values determined at d 7 were due to higher EAA losses during wk 1. The results showed that broiler age influences AA digestibility and this may need be considered in practical feed formulations. The age effect is variable depending on the grain type and specific AA.
Key words: age, amino acid, broiler, sorghum, wheat
INTRODUCTION
Historically, amino acid (AA) digestibility in poultry has been measured at the excreta level (Fonolla et al., 1981; ten Doeschate et al., 1993; Angkanaporn et al., 1997) but this approach suffers from several limitations, including the modifying effects of hindgut microbiome, contamination with urine AA and contribution of microbial proteins to excreta AA (Ravindran and Bryden, 1999). These confounding issues are avoided when the digestibility is measured at the terminal ileal level. The ileal AA digestibility is expressed as either apparent or standardized. The calculation of standardized ileal digestibility (SID) involves the correction of apparent ileal digestibility (AID) values for basal endogenous AA (EAA) losses (Moughan et al., 1992; Lemme et al., 2004). The SID of AA is more additive than AID in feed formulations (Cowieson et al., 2019; An et al., 2020) and increasingly being used by the poultry industry.
The first week after hatch is the most challenging period in broilers because of the immaturity of the digestive tract and, the higher protein demand for organ and muscle development. The intestinal development in the neonatal chick is dynamic. During the first few weeks of life, significant morphological and developmental changes occur. The secretion and activity of different proteases (chymotrypsin, trypsin), intestinal peptidases and dipeptidases are low in the hatchling (Jin et al., 1998). Despite possible age effects on nutrient utilization (Zelenka, 1968; Murakami et al., 1992; Tarvid, 1995; Batal and Parsons, 2002), there are only sporadic data on the influence of age on the AID or SID of AA of ingredients in broilers (Fonolla et al., 1981; Wallis and Balnave, 1984; Huang et al., 2005; Adedokun et al., 2007; 2008; Szczurek et al., 2020) and the results are contradictory. Some studies have recorded improved protein or AA digestibility with advancing age (Wallis and Balnave, 1984; Huang et al., 2005), while others have reported reduced AA digestibility (Fonolla et al., 1981; Zuprizal et al., 1992).
Grains, because of their high inclusion levels in broiler feed formulations, can contribute up to 40% of the dietary protein supply. Wheat and sorghum are 2 common grains used in broiler diets. The digestibility of nutrients in wheat and sorghum depends, inter alia, on the antinutritive factors present in these grains. Wheat contains high concentrations of soluble nonstarch polysaccharides (NSP) that are not hydrolyzed in the intestinal tract of poultry (Choct et al., 1999; Bach Knudsen, 2014). The water-soluble fractions of NSP are viscous and have a negative impact on the digestion and absorption of nutrients (Choct and Annison, 1992a). Sorghum, a nonviscous grain, contains several antinutritive factors like kafirin, phenolic compounds, and phytate (Selle et al., 2018). The phytate content of sorghum is greater than most grains (Selle et al., 2003). Kafirin, a dominant protein fraction in sorghum endosperm, is present as discrete protein bodies in association with starch granules and may reduce the nutrient utilization because of starch-protein interactions (Rooney and Pflugfelder, 1986). The effects of antinutrients present in wheat and sorghum may vary with the age of birds and potentially influence AA digestion. Furthermore, the increased feed consumption and the resultant ingestion of greater amounts of antinutrients with advancing age can lower AA digestion (Szczurek et al., 2020).
Almost all published estimates of SID AA for feed ingredients have been derived using older broilers (22–35 d) and used to formulate diets for different growth stages of broiler chickens. To the authors’ knowledge, only scattered data are available on the age effect on the AA digestibility of grains, with no studies investigating the SID AA from hatch to the end of broiler growth cycle. The present study was, therefore, aimed to compare the standardized ileal digestibility coefficients (SIDC) of AA in wheat and sorghum at 6 different ages (d 7, 14, 21, 28, 35, and 42) of broilers.
MATERIALS AND METHODS
The experiment was conducted according to the New Zealand Revised Code of Ethical Conduct for the use of live animals for research, testing and teaching and approved by the Massey University Animal Ethics Committee.
Diets and Experimental Design
Two experimental diets with similar inclusions (93.8%) of either wheat or sorghum, as the sole source of AA in the diet, were developed (Table 1). The diets contained titanium dioxide (0.5%; Merck KGaA, Darmstadt, Germany) as an indigestible marker. Wheat and sorghum were of Australian origin, obtained from a commercial supplier and ground in a hammer mill to pass through a screen size of 3.0 mm. The diets were steam-conditioned at 70°C for 30 s and pelleted using a pellet mill (Model Orbit 15; Richard Size Limited Engineers, Kingston-upon-Hull, UK) capable of manufacturing 180 kg of feed/h and equipped with a die ring (3-mm apertures and 35-mm thickness). The pelleted diets were crumbled for feeding during the first 2 wk of the study.
Table 1.
Composition of the experimental diets (%, as fed basis) used in the ileal amino acid digestibility assay at different ages.
| Ingredient | Wheat | Sorghum |
|---|---|---|
| Wheat | 93.8 | - |
| Sorghum | - | 93.8 |
| Soybean oil | 2.0 | 2.0 |
| Dicalcium phosphate | 1.8 | 1.8 |
| Limestone | 1.3 | 1.3 |
| Titanium dioxide1 | 0.5 | 0.5 |
| Sodium chloride | 0.2 | 0.2 |
| Sodium bicarbonate | 0.2 | 0.2 |
| Trace mineral premix2 | 0.1 | 0.1 |
| Vitamin premix2 | 0.1 | 0.1 |
Merck KGaA, Darmstadt, Germany.
Supplied per kilogram of diet: antioxidant (ethoxyquin), 100 mg; biotin, 0.2 mg; calcium pantothenate, 12.8 mg; cholecalciferol, 0.06 mg; cyanocobalamin, 0.017 mg; folic acid, 5.2 mg; menadione, 4 mg; niacin, 35 mg; pyridoxine, 10 mg; trans-retinol, 3.33 mg; riboflavin, 12 mg; thiamine, 3.0 mg; dl-α-tocopheryl acetate, 60 mg; choline chloride, 638 mg; Co, 0.3 mg; Cu, 3.0 mg; Fe, 25 mg; I, 1 mg; Mn, 125 mg; Mo, 0.5 mg; Se, 0.2 mg; Zn, 60 mg.
Representative samples of each grain were analyzed, in duplicate, for DM, titanium, Nitrogen (N), starch, crude fat, crude fiber, neutral detergent fiber (NDF), gross energy (GE), calcium, phosphorus, and ash. The apparent ileal digestibility coefficients (AIDC) of N and AA in the grains were determined using the direct method.
Birds and Housing
Day-old male Ross 308 broilers were obtained from a commercial hatchery, raised in floor pens and fed a commercial crumbled broiler starter diet (AME, 2900 kcal/kg; CP, 22.5%) from d 1 to 21 and a broiler finisher diet (AME, 3030 kcal/kg; CP, 19.0%) from d 22 until d 42 in pelleted form (Table 2).
Table 2.
Composition and calculated analysis (%, as fed basis) of starter and finisher diets used prior to the allocation of broilers to the experimental periods.
| Ingredients | Starter diet (0–21 d) | Finisher diet (22–42 d) |
|---|---|---|
| Corn | 57.4 | 66.0 |
| Soybean meal, 46% | 38.1 | 29.6 |
| Soybean oil | 0.88 | 1.36 |
| Limestone | 1.13 | 0.99 |
| Dicalcium phosphate | 1.07 | 0.82 |
| DL-methionine | 0.33 | 0.30 |
| L-lysine HCl | 0.20 | 0.19 |
| L-threonine | 0.10 | 0.07 |
| Sodium bicarbonate | 0.27 | 0.25 |
| Sodium chloride | 0.25 | 0.25 |
| Trace mineral premix1 | 0.10 | 0.10 |
| Vitamin premix1 | 0.10 | 0.10 |
| Phytase | 0.01 | 0.01 |
| Calculated analysis | ||
| AME (kcal/kg) | 2900 | 3030 |
| CP | 22.5 | 19.0 |
| Digestible lysine | 1.10 | 0.92 |
| Digestible methionine | 0.62 | 0.56 |
| Digestible methionine + cysteine | 0.92 | 0.83 |
| Digestible threonine | 0.72 | 0.60 |
| Crude fat | 3.20 | 3.90 |
| Crude fiber | 2.93 | 2.75 |
| Calcium | 0.98 | 0.85 |
| Available phosphorus | 0.49 | 0.42 |
| Sodium | 0.22 | 0.21 |
| Chloride | 0.23 | 0.23 |
| Potassium | 1.15 | 0.97 |
Supplied per kilogram of diet: antioxidant (ethoxyquin), 100 mg; biotin, 0.2 mg; calcium pantothenate, 12.8 mg; cholecalciferol, 0.06 mg; cyanocobalamin, 0.017 mg; folic acid, 5.2 mg; menadione, 4 mg; niacin, 35 mg; pyridoxine, 10 mg; trans-retinol, 3.33 mg; riboflavin, 12 mg; thiamine, 3.0 mg; dl-α-tocopheryl acetate, 60 mg; choline chloride, 638 mg; Co, 0.3 mg; Cu, 3.0 mg; Fe, 25 mg; I, 1 mg; Mn, 125 mg; Mo, 0.5 mg; Se, 0.2 mg; Zn, 60 mg.
A total of 696 birds were used in this experiment. On d 1, 168 chicks were individually weighed and allocated to 12 battery brooders (n = 14 chicks per replicate) so that the average BW per replicate was similar. The remaining chicks were raised in floor pens and fed the standard diet (Table 2) until they were individually weighed and allocated to 12 replicate cages per age group on d 7 (n = 12 birds per replicate cage), d 14 (n = 10 birds per cage), d 21 (n = 8 birds per cage), d 28 (n = 8 birds per cage), and d 35 (n = 6 birds per cage). Once assigned to cages, the birds were fed broiler starter or finisher diets and the first 3 d were considered as the adaptation period. In each age group, the test diets were then fed for 4 d (d 3–7 and 10–14 [crumbled]; d 17–21, 24–28, 31–35, 38–42 [pelleted]) prior to ileal digesta collection on d 7, 14, 21, 28, 35, and 42 posthatch.
The birds had ad libitum access to feed and water at all times. During the first week, the average room temperature was 32 ± 1°C and gradually reduced to 23°C by the end of the third week. The floor pens, battery brooders and grower cages were housed in an environmentally controlled room with 20 h of fluorescent illumination per day.
Growth Performance
Feed intake and BW were recorded on a cage basis during the 4-d experimental period in each week. Mortality was recorded daily.
Determination of the Coefficient of Apparent Ileal Digestibility
The birds were euthanized by intravenous injection (1 mL per 2 kg BW) of sodium pentobarbitone solution (Provet NZ Pty. Ltd., Auckland, New Zealand) at the end of respective experimental periods (d 7, 14, 21, 28, 35, and 42). Digesta were collected from the lower half of the ileum and processed as described by Ravindran et al. (2005). The ileum was defined as that portion of the small intestine extending from the Meckel's diverticulum to a point ∼40 mm proximal to the ileocecal junction. Briefly, the ileum was excised and divided into halves (proximal and distal ileum) and the digesta samples were collected from the lower half toward the ileocecal junction after gently flushing with distilled water into plastic containers. After the collection, the digesta from birds were pooled within a cage, frozen immediately and subsequently lyophilized (Model 0610, Cuddon Engineering, Blenheim, New Zealand). Diets and lyophilized digesta samples were ground to pass through a 0.5-mm sieve and stored in airtight plastic containers at 4°C until laboratory analysis.
Gizzard pH and Jejunal Digesta Viscosity
Two birds from each replicate cage, euthanized for ileal digesta collection, were used for the measurement of gizzard pH by a digital pH meter (pH spear, Oakton Instruments, Vernon Hill, IL). Briefly, the glass probe was inserted through an opening made in the gizzard, directly placed in the digesta and 3 values were taken from the proximal, middle, and distal regions. The average value was considered as the final pH value. The jejunal digesta viscosity was also measured in the same birds. Digesta were collected from the distal jejunum and centrifuged at 3000 × g at 20°C for 15 min. A 0.5 mL aliquot of the supernatant was used in a viscometer (Brookfield digital viscometer, Model DV2TLV; Brookfield Engineering Laboratories Inc., Stoughton, MA) fitted with CP-40 cone spindle with shear rates of 5 to 500/s to determine the viscosity.
Chemical Analysis
Dry matter was determined using the standard procedure (Method 930.15; AOAC International, 2016). Titanium was determined on a UV spectrophotometer following the method described by Short et al. (1996). Gross energy was measured by adiabatic bomb calorimeter (Gallenkamp autobomb, London, UK) standardized with benzoic acid. Starch was measured using the Megazyme Total Starch Assay kit (Megazyme International Ireland Ltd., Wicklow, Ireland) based on thermostable α-amylase and amyloglucosidase (McCleary et al., 1997; AOAC International, 2016). Nitrogen was determined by combustion (Method 968.06; AOAC International, 2016) using a carbon nanosphere-200 carbon, N and sulfur auto analyzer (LECO Corporation, St. Joseph, MI). The CP content was calculated as N × 6.25. Crude fat was measured using the Soxhlet extraction procedure (Method 2003.06; AOAC International, 2016). Neutral detergent fiber was measured (Method 2002.04; AOAC International, 2016) using Tecator Fibertec (FOSS Analytical AB, Höganäs, Sweden). Ash was determined by ashing in a muffle furnace at 550 °C for 16 h (Method 942.05; AOAC International, 2016). Calcium and phosphorus concentrations were measured by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) using a Thermo Jarrell Ash IRIS instrument (Thermo Jarrell Ash Corporation, Franklin, MA).
Amino acids were analyzed following standard procedures (Method 994.12; AOAC International, 2011). The samples were hydrolyzed with 6 N hydrochloric acid (HCl) (containing phenol) for 24 h at 110 ± 2°C in glass tubes in an oven. The AAs were determined using AA analyzer (ion exchange) with ninhydrin postcolumn derivatization. The chromatograms were integrated using a dedicated software (Agilent Open Lab software, Waldbronn, Baden-Württemberg, Germany) with AA simultaneously detected at 570 and 440 nm. For the sulfur containing AA, Cys was analyzed as cysteic acid and Met as methionine sulphone by oxidation with performic acid for 16 h at 0°C and neutralization with hydrobromic acid prior to hydrolysis.
For Trp analysis, the samples were saponified under alkaline conditions with barium hydroxide solution in the absence of air at 110°C for 20 h in an autoclave. Following hydrolysis, α-methyl Trp as internal standard, was added to the mixture. After adjusting the hydrolysate to pH 3.0 and diluting with 30% methanol, Trp and the internal standard were separated by reverse phase chromatography (RP-18) on a HPLC column. Detection was selectively done by means of a fluorescence detector to prevent interference by other AA and constituents.
Calculations
All data were expressed on a DM basis. The AIDC of AA was calculated from the dietary ratio of AA to Ti relative to the corresponding ratio in the ileal digesta using the following formula.
Where, (AA/Ti)d = ratio of AA to titanium in the diet, and (AA / Ti)i = ratio of AA to titanium in the ileal digesta.
Apparent digestibility data for N and AA were then converted to SIDC, using the age-appropriate basal endogenous N and AA estimates (EAA; g AA per kg DM intake [DMI]) determined at different ages (d 7, 14, 21, 28, 35, and 42) in a previous study (Barua et al., 2021a).
Where, SIDC = standardized ileal digestibility coefficient of the AA, AIDC = apparent ileal digestibility coefficient of the AA, Basal EAA = basal endogenous AA loss, and Ing. AA = concentration of the AA in the ingredient.
Data Analysis
Cage was considered as the experimental unit. For each grain, data were analyzed by the GLM procedure of SAS (version 9.4; 2015; SAS Institute, Cary, NC). Orthogonal polynomial contrasts were performed to determine the linear and quadratic effects of broiler age. Statistical significance was declared at P < 0.05.
RESULTS
Proximate and Nutrient Composition
The proximate and nutrient composition of the grains are presented in Table 3. The CP content in wheat and sorghum were 9.43% and 10.6%, respectively. The starch content in sorghum (60.6%) was higher than that in wheat (57.9%) whereas wheat had higher NDF content (8.38%) than sorghum (6.22%). Among the indispensable AA (IAA), the contents of Leu, Arg, Val, and Ile were the highest in both wheat and sorghum. Lower IAA contents were recorded for Trp, followed by Met, in both grains. In the case of dispensable AA (DAA), higher contents were recorded for Glu in both grains. The total IAA and total DAA contents in wheat were 2.92 and 5.37%, respectively. In sorghum, the total IAA and DAA contents were 3.91 and 6.00%, respectively. The total AA (TAA) content was lower in wheat (8.28%) than sorghum (9.87%).
Table 3.
Proximate, carbohydrate and amino acid composition of wheat and sorghum (%, as-received basis).
| Item | Wheat | Sorghum |
|---|---|---|
| DM | 89.9 | 88.1 |
| CP (N × 6.25) | 9.43 | 10.6 |
| Starch | 57.9 | 60.6 |
| Crude fat | 2.45 | 3.26 |
| NDF | 8.38 | 6.22 |
| Gross energy (kcal/kg) | 3,845 | 3,989 |
| Ash | 1.22 | 1.55 |
| Calcium | 0.022 | 0.010 |
| Phosphorus | 0.212 | 0.289 |
| Indispensable amino acids (IAA) | ||
| Arginine | 0.468 | 0.420 |
| Histidine | 0.217 | 0.242 |
| Isoleucine | 0.322 | 0.414 |
| Leucine | 0.631 | 1.44 |
| Lysine | 0.285 | 0.238 |
| Methionine | 0.168 | 0.170 |
| Threonine | 0.289 | 0.339 |
| Tryptophan | 0.125 | 0.126 |
| Valine | 0.412 | 0.525 |
| Total IAA | 2.92 | 3.91 |
| Dispensable amino acids (DAA) | ||
| Alanine | 0.354 | 0.993 |
| Aspartic acid | 0.499 | 0.719 |
| Cysteine1 | 0.213 | 0.192 |
| Glutamic acid | 2.58 | 2.26 |
| Glycine1 | 0.411 | 0.332 |
| Proline | 0.879 | 0.981 |
| Serine | 0.432 | 0.484 |
| Total DAA | 5.37 | 6.00 |
| Total AA2 | 8.28 | 9.87 |
Abbreviations: AA, amino acid; NDF, neutral detergent fiber.
Semi-indispensable amino acids for poultry.
Total AA = IAA + DAA.
Growth Performance, Gizzard pH, and Jejunal Digesta Viscosity
Table 4 presents weekly data on the performance, gizzard pH and jejunal digesta viscosity of birds fed wheat- or sorghum-based diets. Only 2 out of 696 birds died, and the deaths were not related to any specific treatment. Bird age had a significant (P < 0.001) effect on performance parameters. The daily feed intake and weight gain increased quadratically (P < 0.001) with advancing age in both wheat- and sorghum-based diets.
Table 4.
Daily feed intake (DFI; g/bird/d), daily weight gain (DWG; g/bird/d), gizzard pH, and viscosity (cP) in jejunal digesta of broilers fed wheat- and sorghum-based diets at different ages.1
| Age (day) |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| Wheat | |||||||||
| DFI2 | 14.2 | 49.4 | 88.8 | 127 | 160 | 178 | 3.4 | 0.001 | 0.001 |
| DWG2 | 13.6 | 32.2 | 41.7 | 58.1 | 62.6 | 63.5 | 1.88 | 0.001 | 0.001 |
| Gizzard pH3 | 2.68 | 2.39 | 3.54 | 3.20 | 3.40 | 3.61 | 0.105 | 0.001 | 0.174 |
| Viscosity3 | 3.31 | 5.60 | 6.38 | 8.29 | 8.01 | 7.77 | 0.470 | 0.001 | 0.001 |
| Sorghum | |||||||||
| DFI2 | 12.0 | 36.5 | 72.5 | 111 | 138 | 142 | 2.51 | 0.001 | 0.001 |
| DWG2 | 9.41 | 23.0 | 37.6 | 47.0 | 54.1 | 56.4 | 1.35 | 0.001 | 0.001 |
| Gizzard pH3 | 2.56 | 2.01 | 2.14 | 2.80 | 3.54 | 3.66 | 0.121 | 0.001 | 0.001 |
| Viscosity3 | 1.86 | 2.32 | 3.68 | 3.64 | 2.87 | 2.55 | 0.142 | 0.001 | 0.001 |
Each value represents the mean of 6 replicates (14, 12 and 10 birds per replicate for 7, 14 and 21-d old birds, respectively; 8 birds per replicate for 28 and 35-d old birds; and 6 birds per replicate for 42-d old birds).
Measured during 4-d feeding of experimental diets.
Calculated as the mean of 6 replicates (2 birds per replicate).
Gizzard pH increased linearly (P < 0.001) in wheat as the birds grew older. A quadratic (P < 0.001) effect was observed on the jejunal digesta viscosity with advancing age. The viscosity increased from d 7 to 35 and then decreased.
In the case of sorghum, both the gizzard pH and the jejunal digesta viscosity were affected quadratically (P < 0.001) by bird age. The gizzard pH decreased from d 7 to d 21 and then increased. The viscosity increased until d 21, plateaued to d 28 and then decreased.
Ileal Digestibility Coefficients of N and AA in Wheat
The influence of broiler age on AIDC, SIDC and SID content of N and AA in wheat is summarized in Tables 5, 6 and 7, respectively. Age quadratically influenced the AIDC of N (P < 0.001) and average digestibility of IAA, DAA and TAA of wheat (P < 0.01; Table 5). The average AIDC of IAA, DAA and TAA increased from d 7 to 14, then plateaued up to d 21, and then increased to d 42. The AIDC of Arg, Glu and Gly increased linearly (P < 0.001) with age, while the increases in AIDC of other AA were quadratic (P < 0.05–0.001).
Table 5.
Apparent ileal digestibility coefficients1 of nitrogen (N) and amino acids of wheat at different ages of broilers.1
| Age (day) |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| N | 0.737 | 0.793 | 0.825 | 0.841 | 0.840 | 0.856 | 0.0088 | 0.001 | 0.001 |
| Indispensable amino acids | |||||||||
| Arg | 0.823 | 0.844 | 0.854 | 0.864 | 0.867 | 0.875 | 0.0090 | 0.001 | 0.275 |
| His | 0.784 | 0.831 | 0.838 | 0.852 | 0.858 | 0.872 | 0.0085 | 0.001 | 0.033 |
| Ile | 0.755 | 0.809 | 0.817 | 0.844 | 0.846 | 0.865 | 0.0105 | 0.001 | 0.045 |
| Leu | 0.796 | 0.842 | 0.851 | 0.876 | 0.876 | 0.892 | 0.0088 | 0.001 | 0.032 |
| Lys | 0.638 | 0.730 | 0.751 | 0.784 | 0.771 | 0.808 | 0.0186 | 0.001 | 0.022 |
| Met | 0.745 | 0.803 | 0.855 | 0.875 | 0.870 | 0.886 | 0.0103 | 0.001 | 0.001 |
| Thr | 0.577 | 0.664 | 0.690 | 0.699 | 0.711 | 0.739 | 0.0159 | 0.001 | 0.021 |
| Trp | 0.705 | 0.777 | 0.812 | 0.825 | 0.831 | 0.848 | 0.0127 | 0.001 | 0.002 |
| Val | 0.723 | 0.782 | 0.800 | 0.818 | 0.823 | 0.840 | 0.0107 | 0.001 | 0.012 |
| IAA | 0.727 | 0.787 | 0.808 | 0.826 | 0.828 | 0.847 | 0.0108 | 0.001 | 0.008 |
| Dispensable amino acids | |||||||||
| Ala | 0.694 | 0.765 | 0.773 | 0.784 | 0.779 | 0.801 | 0.0131 | 0.001 | 0.017 |
| Asp | 0.561 | 0.649 | 0.727 | 0.752 | 0.753 | 0.775 | 0.0162 | 0.001 | 0.001 |
| Cys2 | 0.697 | 0.765 | 0.809 | 0.823 | 0.828 | 0.839 | 0.0086 | 0.001 | 0.001 |
| Glu | 0.922 | 0.942 | 0.935 | 0.943 | 0.941 | 0.948 | 0.0039 | 0.001 | 0.232 |
| Gly2 | 0.725 | 0.766 | 0.781 | 0.799 | 0.802 | 0.819 | 0.0104 | 0.001 | 0.090 |
| Pro | 0.879 | 0.902 | 0.908 | 0.917 | 0.918 | 0.922 | 0.0043 | 0.001 | 0.007 |
| Ser | 0.728 | 0.789 | 0.809 | 0.820 | 0.826 | 0.845 | 0.0102 | 0.001 | 0.007 |
| DAA | 0.744 | 0.797 | 0.820 | 0.834 | 0.835 | 0.849 | 0.0092 | 0.001 | 0.002 |
| TAA | 0.734 | 0.791 | 0.813 | 0.829 | 0.831 | 0.848 | 0.0101 | 0.001 | 0.005 |
Each value represents the mean of 6 replicates (14, 12 and 10 birds per replicate for 7, 14 and 21-d old birds, respectively; 8 birds per replicate for 28 and 35-d old birds; and 6 birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: DAA = average digestibility of dispensable amino acids; IAA = average digestibility of indispensable amino acids; TAA = average digestibility of all amino acids.
Table 6.
Standardized ileal digestibility coefficients1 of nitrogen (N) and amino acids of wheat at different ages of broilers.2
| Age (day) |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| N | 0.950 | 0.904 | 0.931 | 0.949 | 0.947 | 0.933 | 0.0088 | 0.393 | 0.586 |
| Indispensable amino acids | |||||||||
| Arg | 0.953 | 0.909 | 0.914 | 0.933 | 0.928 | 0.914 | 0.0089 | 0.197 | 0.162 |
| His | 0.904 | 0.896 | 0.899 | 0.919 | 0.919 | 0.913 | 0.0085 | 0.079 | 0.975 |
| Ile | 0.929 | 0.899 | 0.907 | 0.939 | 0.929 | 0.921 | 0.0105 | 0.333 | 0.718 |
| Leu | 0.934 | 0.910 | 0.920 | 0.953 | 0.944 | 0.937 | 0.0088 | 0.051 | 0.938 |
| Lys | 0.839 | 0.824 | 0.840 | 0.884 | 0.859 | 0.863 | 0.0186 | 0.097 | 0.705 |
| Met | 0.886 | 0.868 | 0.921 | 0.949 | 0.933 | 0.923 | 0.0103 | 0.001 | 0.017 |
| Thr | 0.965 | 0.891 | 0.911 | 0.905 | 0.931 | 0.900 | 0.0139 | 0.083 | 0.066 |
| Trp | 0.852 | 0.859 | 0.898 | 0.915 | 0.918 | 0.911 | 0.0127 | 0.001 | 0.070 |
| Val | 0.899 | 0.876 | 0.894 | 0.917 | 0.914 | 0.905 | 0.0108 | 0.075 | 0.903 |
| IAA | 0.910 | 0.881 | 0.900 | 0.924 | 0.919 | 0.909 | 0.0108 | 0.141 | 0.987 |
| Dispensable amino acids | |||||||||
| Ala | 0.881 | 0.857 | 0.865 | 0.887 | 0.873 | 0.863 | 0.0131 | 0.862 | 0.922 |
| Asp | 0.813 | 0.779 | 0.855 | 0.885 | 0.882 | 0.864 | 0.0162 | 0.001 | 0.119 |
| Cys3 | 0.896 | 0.879 | 0.917 | 0.928 | 0.939 | 0.926 | 0.0085 | 0.001 | 0.282 |
| Glu | 0.981 | 0.969 | 0.963 | 0.974 | 0.969 | 0.967 | 0.0039 | 0.047 | 0.144 |
| Gly3 | 0.894 | 0.853 | 0.868 | 0.892 | 0.889 | 0.881 | 0.0104 | 0.448 | 0.334 |
| Pro | 0.972 | 0.949 | 0.957 | 0.966 | 0.966 | 0.957 | 0.0043 | 0.712 | 0.372 |
| Ser | 0.948 | 0.905 | 0.926 | 0.931 | 0.943 | 0.928 | 0.0101 | 0.819 | 0.273 |
| DAA | 0.912 | 0.885 | 0.907 | 0.923 | 0.923 | 0.912 | 0.0092 | 0.101 | 0.950 |
| TAA | 0.909 | 0.882 | 0.903 | 0.923 | 0.921 | 0.911 | 0.0099 | 0.092 | 0.902 |
Apparent digestibility values were standardized using the following basal ileal endogenous flow values (g/kg DM intake), determined by the feeding N-free diet at different ages (Barua et al., 2021a):
D7: N, 3.59; Arg, 0.68; His, 0.29; Ile, 0.63; Leu, 0.97; Lys, 0.64; Met, 0.27; Thr, 1.35; Trp, 0.21; Val, 0.81; Ala, 0.75; Asp, 0.75; Cys, 0.47; Glu, 1.71; Gly, 0.78; Pro, 0.91; and Ser, 1.06.
D14: N, 1.87; Arg, 0.30; His, 0.16; Ile, 0.33; Leu, 0.49; Lys, 0.30; Met, 0.12; Thr, 0.73; Trp, 0.12; Val, 0.43; Ala, 0.37; Asp, 0.73; Cys, 0.27; Glu, 0.80; Gly, 0.39; Pro, 0.48; and Ser, 0.55.
D21: N, 1.79; Arg, 0.31; His, 0.15; Ile, 0.33; Leu, 0.49; Lys, 0.28; Met, 0.12; Thr, 0.71; Trp, 0.12; Val, 0.43; Ala, 0.36; Asp, 0.72; Cys, 0.26; Glu, 0.80; Gly, 0.40; Pro, 0.48; and Ser, 0.56.
D28: N, 1.82; Arg, 0.36; His, 0.16; Ile, 0.34; Leu, 0.55; Lys, 0.32; Met, 0.14; Thr, 0.67; Trp, 0.13; Val, 0.45; Ala, 0.41; Asp, 0.74; Cys, 0.25; Glu, 0.89; Gly, 0.43; Pro, 0.48; and Ser, 0.54.
D35: N, 1.81; Arg, 0.32; His, 0.15; Ile, 0.30; Leu, 0.49; Lys, 0.28; Met, 0.12; Thr, 0.71; Trp, 0.12; Val, 0.42; Ala, 0.37; Asp, 0.72; Cys, 0.26; Glu, 0.81; Gly, 0.40; Pro, 0.47; and Ser, 0.56.
D42: N, 1.29; Arg, 0.20; His, 0.10; Ile, 0.20; Leu, 0.31; Lys, 0.18; Met, 0.07; Thr, 0.52; Trp, 0.09; Val, 0.29; Ala, 0.25; Asp, 0.49; Cys, 0.21; Glu, 0.53; Gly, 0.28; Pro, 0.34; and Ser, 0.40.
Each value represents the mean of 6 replicates (14, 12 and 10 birds per replicate for 7, 14 and 21-d old birds, respectively; 8 birds per replicate for 28 and 35-d old birds; and 6 birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: DAA = average digestibility of dispensable amino acids; IAA = average digestibility of indispensable amino acids; TAA = average digestibility of all amino acids.
Table 7.
Influence of age on standardized ileal digestible protein (CP) and amino acid contents1 of wheat (%; as-received basis).
| Age (day) |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| CP | 8.96 | 8.52 | 8.78 | 8.95 | 8.93 | 8.79 | 0.083 | 0.393 | 0.584 |
| Indispensable amino acids | |||||||||
| Arg | 0.446 | 0.422 | 0.428 | 0.437 | 0.434 | 0.428 | 0.0043 | 0.213 | 0.169 |
| His | 0.196 | 0.195 | 0.195 | 0.199 | 0.199 | 0.198 | 0.0018 | 0.074 | 0.984 |
| Ile | 0.298 | 0.289 | 0.292 | 0.302 | 0.299 | 0.297 | 0.0034 | 0.306 | 0.722 |
| Leu | 0.589 | 0.574 | 0.581 | 0.602 | 0.596 | 0.591 | 0.0055 | 0.048 | 0.974 |
| Lys | 0.239 | 0.235 | 0.239 | 0.252 | 0.245 | 0.246 | 0.0053 | 0.097 | 0.715 |
| Met | 0.149 | 0.146 | 0.155 | 0.159 | 0.157 | 0.155 | 0.0018 | 0.001 | 0.021 |
| Thr | 0.288 | 0.257 | 0.263 | 0.262 | 0.269 | 0.260 | 0.0046 | 0.013 | 0.012 |
| Trp | 0.106 | 0.107 | 0.112 | 0.114 | 0.115 | 0.114 | 0.0016 | 0.001 | 0.069 |
| Val | 0.370 | 0.361 | 0.368 | 0.378 | 0.377 | 0.373 | 0.0044 | 0.069 | 0.913 |
| IAA | 2.67 | 2.59 | 2.63 | 2.70 | 2.69 | 2.66 | 0.0303 | 0.221 | 0.862 |
| Dispensable amino acids | |||||||||
| Ala | 0.312 | 0.304 | 0.306 | 0.314 | 0.309 | 0.305 | 0.0046 | 0.814 | 0.904 |
| Asp | 0.406 | 0.389 | 0.427 | 0.441 | 0.440 | 0.431 | 0.0081 | 0.001 | 0.123 |
| Cys2 | 0.191 | 0.187 | 0.195 | 0.198 | 0.199 | 0.198 | 0.0018 | 0.001 | 0.334 |
| Glu | 2.53 | 2.49 | 2.48 | 2.51 | 2.49 | 2.49 | 0.010 | 0.045 | 0.144 |
| Gly2 | 0.367 | 0.350 | 0.357 | 0.367 | 0.365 | 0.362 | 0.0043 | 0.461 | 0.341 |
| Pro | 0.854 | 0.834 | 0.841 | 0.849 | 0.849 | 0.841 | 0.0038 | 0.722 | 0.342 |
| Ser | 0.409 | 0.391 | 0.399 | 0.402 | 0.407 | 0.401 | 0.0044 | 0.773 | 0.277 |
| DAA | 5.07 | 4.95 | 5.01 | 5.08 | 5.07 | 5.03 | 0.036 | 0.488 | 0.735 |
| Total AA | 7.74 | 7.54 | 7.64 | 7.78 | 7.76 | 7.69 | 0.066 | 0.347 | 0.790 |
Standardized digestible amino acid content (%) = [Ingredient amino acid content (%) × standardized ileal digestibility (%)] × 100.
Each value represents the mean of 6 replicates (14, 12 and 10 birds per replicate for 7, 14 and 21-d old birds, respectively; 8 birds per replicate for 28 and 35-d old birds; and 6 birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: DAA, total digestible dispensable amino acid contents; IAA, total digestible indispensable amino acid contents; Total AA = total digestible content of all amino acids.
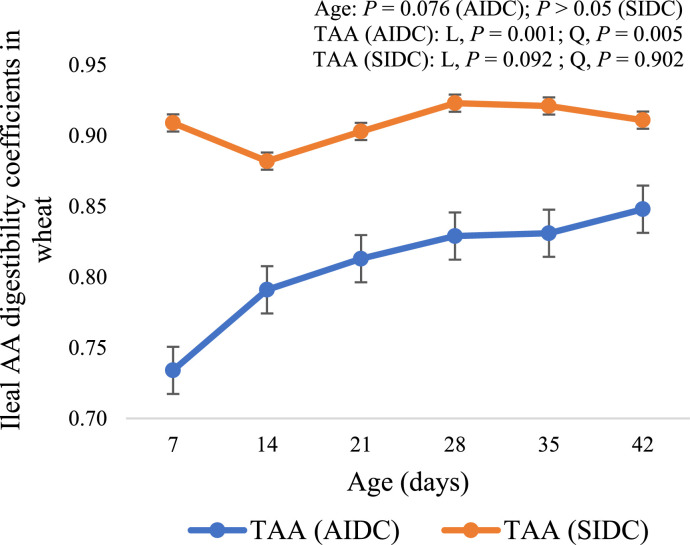
No age effect was observed on SIDC of N and, average digestibility of IAA and DAA in wheat (P > 0.05; Table 6). The broiler age, however, tended (P = 0.092) to linearly influence the average of TAA. Among IAA, the SIDC of Met (quadratic; P < 0.05) was higher on d 7, increased from d 14 to 21, and then plateaued. The SIDC of Trp increased linearly (P < 0.001) with advancing age. Among the DAA, a linear effect of age was observed for SIDC of Asp (P < 0.001), Cys (P < 0.001), and Glu (P < 0.05).
No age effects (P > 0.05) were observed for the SID contents of protein, total IAA, total DAA, and TAA of wheat (Table 7). Among the IAA, the SID contents of Leu and Met increased (P < 0.05) by broiler age in linear and quadratic manner, respectively. However, the SID content of Thr showed quadratic decline (P < 0.05) as birds grew older. The SID Trp content increased linearly from 0.106% at d 7 to 0.114% at d 42. Among the DAA, the SID content of Asp and Cys increased linearly (P < 0.001) with age. A linear effect (P = 0.045) of bird age was observed for the SID content of Glu.
Ileal Digestibility Coefficients of N and AA in Sorghum
The influence of broiler age on AIDC, SIDC and SID content of N and AA in sorghum are summarized in Tables 8, 9, and 10, respectively. No effect of age (P > 0.05) was recorded for the AIDC of N and, average digestibility of IAA, DAA and of TAA (Table 8). Among the IAA, the AIDC of Met (P < 0.001) and Trp (P < 0.05) increased linearly with advancing age. The AIDC of Cys increased linearly (P < 0.001) from 0.580 at d 7 to 0.681 at d 42. A linear effect of bird age was also observed for Ala (P < 0.05) and Glu (P < 0.01). The AIDC of Asp tended (P = 0.066) to linearly increase from 0.739 at d 7 to 0.781 at d 42.
Table 8.
Apparent ileal digestibility coefficients1 of nitrogen (N) and amino acids of sorghum at different ages of broilers.1
| Age (day) |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| N | 0.745 | 0.762 | 0.748 | 0.776 | 0.753 | 0.777 | 0.0164 | 0.242 | 0.990 |
| Indispensable amino acids | |||||||||
| Arg | 0.815 | 0.805 | 0.789 | 0.837 | 0.817 | 0.835 | 0.0173 | 0.216 | 0.436 |
| His | 0.727 | 0.733 | 0.693 | 0.735 | 0.701 | 0.733 | 0.0156 | 0.850 | 0.286 |
| Ile | 0.783 | 0.807 | 0.769 | 0.797 | 0.772 | 0.801 | 0.0169 | 0.917 | 0.617 |
| Leu | 0.845 | 0.875 | 0.842 | 0.844 | 0.819 | 0.846 | 0.0115 | 0.098 | 0.862 |
| Lys | 0.677 | 0.727 | 0.649 | 0.748 | 0.696 | 0.744 | 0.0335 | 0.227 | 0.762 |
| Met | 0.737 | 0.779 | 0.791 | 0.823 | 0.792 | 0.824 | 0.0164 | 0.001 | 0.152 |
| Thr | 0.637 | 0.627 | 0.607 | 0.650 | 0.633 | 0.664 | 0.0252 | 0.359 | 0.355 |
| Trp | 0.719 | 0.764 | 0.733 | 0.770 | 0.754 | 0.786 | 0.0192 | 0.045 | 0.978 |
| Val | 0.753 | 0.766 | 0.737 | 0.772 | 0.749 | 0.775 | 0.0182 | 0.550 | 0.586 |
| IAA | 0.744 | 0.765 | 0.734 | 0.775 | 0.748 | 0.778 | 0.0182 | 0.289 | 0.716 |
| Dispensable amino acids | |||||||||
| Ala | 0.845 | 0.879 | 0.838 | 0.835 | 0.811 | 0.836 | 0.0115 | 0.014 | 0.816 |
| Asp | 0.739 | 0.747 | 0.754 | 0.780 | 0.759 | 0.781 | 0.0173 | 0.066 | 0.789 |
| Cys2 | 0.580 | 0.501 | 0.605 | 0.679 | 0.658 | 0.681 | 0.0318 | 0.001 | 0.975 |
| Glu | 0.858 | 0.892 | 0.841 | 0.842 | 0.816 | 0.841 | 0.0114 | 0.002 | 0.608 |
| Gly2 | 0.702 | 0.688 | 0.615 | 0.683 | 0.657 | 0.679 | 0.0234 | 0.493 | 0.094 |
| Pro | 0.781 | 0.788 | 0.753 | 0.777 | 0.743 | 0.764 | 0.0146 | 0.126 | 0.596 |
| Ser | 0.748 | 0.736 | 0.724 | 0.758 | 0.739 | 0.767 | 0.0209 | 0.438 | 0.386 |
| DAA | 0.750 | 0.747 | 0.733 | 0.765 | 0.741 | 0.764 | 0.0176 | 0.586 | 0.566 |
| TAA | 0.747 | 0.758 | 0.735 | 0.771 | 0.745 | 0.772 | 0.0177 | 0.385 | 0.664 |
Each value represents the mean of 6 replicates (14, 12 and 10 birds per replicate for 7, 14 and 21-d old birds, respectively; 8 birds per replicate for 28 and 35-d old birds; and 6 birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: DAA, average digestibility of dispensable amino acids; IAA, average digestibility of indispensable amino acids; TAA, average digestibility of all amino acids.
Table 9.
Standardized ileal digestibility coefficients1 of nitrogen (N) and amino acids of sorghum at different ages of broilers.2
| Age |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| N | 0.933 | 0.860 | 0.842 | 0.872 | 0.848 | 0.845 | 0.0164 | 0.003 | 0.037 |
| Indispensable amino acids | |||||||||
| Arg | 0.961 | 0.869 | 0.857 | 0.913 | 0.885 | 0.879 | 0.0173 | 0.043 | 0.031 |
| His | 0.837 | 0.793 | 0.748 | 0.795 | 0.757 | 0.771 | 0.0156 | 0.005 | 0.034 |
| Ile | 0.915 | 0.875 | 0.837 | 0.869 | 0.836 | 0.843 | 0.0170 | 0.004 | 0.109 |
| Leu | 0.906 | 0.905 | 0.872 | 0.878 | 0.849 | 0.865 | 0.0115 | 0.001 | 0.345 |
| Lys | 0.934 | 0.847 | 0.841 | 0.877 | 0.808 | 0.814 | 0.0252 | 0.003 | 0.353 |
| Met | 0.874 | 0.842 | 0.855 | 0.895 | 0.852 | 0.859 | 0.0164 | 0.988 | 0.866 |
| Thr | 0.967 | 0.817 | 0.792 | 0.824 | 0.818 | 0.799 | 0.0246 | 0.001 | 0.003 |
| Trp | 0.869 | 0.848 | 0.821 | 0.862 | 0.842 | 0.849 | 0.0192 | 0.637 | 0.339 |
| Val | 0.891 | 0.839 | 0.810 | 0.849 | 0.819 | 0.826 | 0.0183 | 0.031 | 0.009 |
| IAA | 0.906 | 0.849 | 0.826 | 0.862 | 0.829 | 0.834 | 0.0167 | 0.011 | 0.089 |
| Dispensable amino acids | |||||||||
| Ala | 0.912 | 0.913 | 0.870 | 0.872 | 0.844 | 0.859 | 0.0115 | 0.001 | 0.232 |
| Asp | 0.916 | 0.839 | 0.845 | 0.874 | 0.851 | 0.844 | 0.0173 | 0.048 | 0.149 |
| Cys3 | 0.810 | 0.725 | 0.729 | 0.800 | 0.786 | 0.781 | 0.0207 | 0.554 | 0.095 |
| Glu | 0.927 | 0.924 | 0.874 | 0.878 | 0.848 | 0.863 | 0.0113 | 0.001 | 0.116 |
| Gly3 | 0.911 | 0.795 | 0.723 | 0.797 | 0.765 | 0.756 | 0.0234 | 0.001 | 0.003 |
| Pro | 0.873 | 0.837 | 0.802 | 0.825 | 0.792 | 0.799 | 0.0146 | 0.001 | 0.105 |
| Ser | 0.943 | 0.838 | 0.827 | 0.857 | 0.843 | 0.841 | 0.0209 | 0.012 | 0.014 |
| DAA | 0.899 | 0.826 | 0.810 | 0.843 | 0.819 | 0.820 | 0.0176 | 0.014 | 0.045 |
| TAA | 0.903 | 0.844 | 0.819 | 0.854 | 0.825 | 0.828 | 0.0164 | 0.007 | 0.059 |
Apparent digestibility values were standardized using the following basal ileal endogenous flow values (g/kg DM intake), determined by the feeding N-free diet at different ages (Barua et al., 2021a); see Table 6.
Each value represents the mean of 6 replicates (14, 12 and 10 birds per replicate for 7, 14 and 21-d old birds, respectively; 8 birds per replicate for 28 and 35-d old birds; and 6 birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: DAA, average digestibility of dispensable amino acids; IAA, average digestibility of indispensable amino acids; TAA, average digestibility of all amino acids.
Table 10.
Influence of age on standardized ileal digestible protein (CP) and amino acid contents1 of sorghum (%; as-received basis).
| Age |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| CP | 9.78 | 9.45 | 8.82 | 9.14 | 8.88 | 8.86 | 0.158 | 0.001 | 0.048 |
| Indispensable amino acids | |||||||||
| Arg | 0.394 | 0.369 | 0.351 | 0.375 | 0.363 | 0.360 | 0.0060 | 0.003 | 0.021 |
| His | 0.196 | 0.209 | 0.175 | 0.186 | 0.177 | 0.180 | 0.0036 | 0.001 | 0.138 |
| Ile | 0.382 | 0.376 | 0.349 | 0.363 | 0.349 | 0.353 | 0.0066 | 0.001 | 0.119 |
| Leu | 1.28 | 1.28 | 1.23 | 1.24 | 1.19 | 1.22 | 0.016 | 0.001 | 0.354 |
| Lys | 0.206 | 0.181 | 0.185 | 0.193 | 0.178 | 0.179 | 0.0054 | 0.006 | 0.297 |
| Met | 0.149 | 0.148 | 0.146 | 0.153 | 0.146 | 0.147 | 0.0028 | 0.558 | 0.732 |
| Thr | 0.327 | 0.301 | 0.268 | 0.278 | 0.277 | 0.270 | 0.0073 | 0.001 | 0.002 |
| Trp | 0.105 | 0.104 | 0.099 | 0.104 | 0.102 | 0.103 | 0.0023 | 0.492 | 0.375 |
| Val | 0.459 | 0.452 | 0.418 | 0.438 | 0.423 | 0.426 | 0.0084 | 0.002 | 0.105 |
| IAA | 3.49 | 3.42 | 3.22 | 3.33 | 3.21 | 3.24 | 0.055 | 0.001 | 0.106 |
| Dispensable amino acids | |||||||||
| Ala | 0.887 | 0.834 | 0.847 | 0.848 | 0.822 | 0.836 | 0.0111 | 0.004 | 0.091 |
| Asp | 0.639 | 0.544 | 0.589 | 0.609 | 0.593 | 0.588 | 0.0114 | 0.391 | 0.060 |
| Cys2 | 0.147 | 0.157 | 0.132 | 0.145 | 0.142 | 0.142 | 0.0038 | 0.079 | 0.332 |
| Glu | 2.01 | 2.10 | 1.89 | 1.91 | 1.84 | 1.87 | 0.024 | 0.001 | 0.241 |
| Gly2 | 0.297 | 0.278 | 0.236 | 0.259 | 0.249 | 0.247 | 0.0067 | 0.001 | 0.002 |
| Pro | 0.754 | 0.819 | 0.692 | 0.713 | 0.683 | 0.689 | 0.0115 | 0.001 | 0.362 |
| Ser | 0.450 | 0.431 | 0.394 | 0.409 | 0.402 | 0.401 | 0.0078 | 0.001 | 0.006 |
| DAA | 5.19 | 5.17 | 4.79 | 4.89 | 4.73 | 4.77 | 0.074 | 0.001 | 0.090 |
| Total AA | 8.69 | 8.59 | 7.99 | 8.22 | 7.94 | 8.01 | 0.132 | 0.001 | 0.088 |
Standardized digestible amino acid content (%) = [Ingredient amino acid content (%) × standardized ileal digestibility (%)] × 100.
Each value represents the mean of 6 replicates (14, 12 and 10 birds per replicate for 7, 14 and 21-d old birds, respectively; 8 birds per replicate for 28 and 35-d old birds; and 6 birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: DAA, total digestible dispensable amino acid contents; IAA, total digestible indispensable amino acid contents; Total AA, total digestible content of all amino acids.
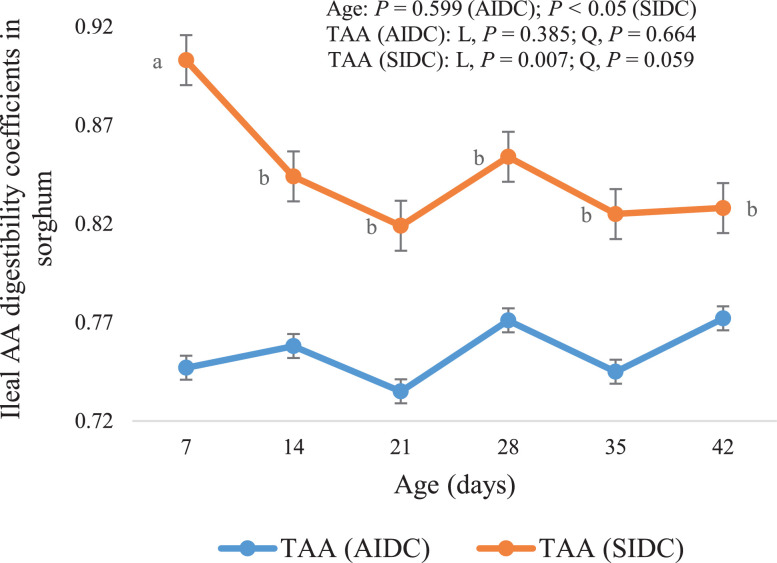
The highest SIDC of N, average SIDC of IAA, DAA and TAA were determined at d 7, declining at d 14 and then plateauing (Table 9). Among the IAA, a quadratic decline (P < 0.05–0.01) was observed for the SIDC of Arg, His, Thr, and Val with advancing age. The SIDC of Ile, Leu, Lys, and the average of IAA were linearly decreased (P < 0.05–0.001) with advancing age, with the highest SIDC values being recorded on d 7. The SIDC of individual DAA, except Cys, and the average of DAA was influenced by bird age either linearly or quadratically (P < 0.05–0.001; Table 9). A linear decrease (P < 0.05–0.001) was observed in SIDC of Ala, Asp, Glu, and Pro as birds grew older. The SIDC of Gly, Ser and average DAA reduced with advancing age, but the decline was greater between d 7 and 14 resulting in a quadratic effect (P <0.05–0.01). The average SIDC of TAA declined linearly (P < 0.01) with advancing age, from 0.903 at d 7 to 0.828 at d 42.
The SID protein content of sorghum declined quadratically (P < 0.05) from 9.78% on d 7 to 8.86% on d 42 (Table 10). With the exception of Met and Trp, the SID contents of individual IAA declined either in quadratic (Arg and Thr; P < 0.05–0.01) or linear (His, Ile, Leu, Lys and Val; P < 0.01–0.001) manner with age, with the highest values recorded mostly on d 7. Except Asp and Cys, the SID contents of all individual DAA and TAA of sorghum declined either linearly or quadratically (P < 0.01–0.001) with advancing age. Similar to IAA, the greatest SID AA contents were observed on d 7.
Uplift in Digestibility Coefficients Due to Correction for Endogenous Amino Acid Losses as Influenced by Age
The percentage increase in the digestibility coefficients of N and AA in wheat and sorghum after correction of AIDC for age-appropriate basal endogenous N and AA losses is shown in Table 11.
Table 11.
Percentage increase in digestibility coefficients of nitrogen and amino acids in wheat and sorghum after correction of apparent ileal digestibility coefficients for age-appropriate basal endogenous amino acid losses of broilers.
| Wheat |
Sorghum |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (day) | Age (day) | |||||||||||
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | 7 | 14 | 21 | 28 | 35 | 42 |
| N | 28.9 | 13.9 | 12.9 | 12.8 | 12.7 | 8.99 | 25.2 | 12.9 | 12.6 | 12.4 | 12.6 | 8.75 |
| Indispensable amino acids | ||||||||||||
| Arg | 15.8 | 7.70 | 7.03 | 7.99 | 7.04 | 4.46 | 17.9 | 7.95 | 8.62 | 9.08 | 8.32 | 5.27 |
| His | 15.3 | 7.82 | 7.28 | 7.86 | 7.11 | 4.70 | 15.1 | 8.19 | 7.94 | 8.16 | 7.99 | 5.18 |
| Ile | 23.0 | 11.1 | 11.0 | 11.3 | 9.81 | 6.47 | 16.9 | 8.43 | 8.84 | 9.03 | 8.29 | 5.24 |
| Leu | 17.3 | 8.08 | 8.11 | 8.79 | 7.76 | 5.04 | 7.22 | 3.43 | 3.56 | 4.03 | 3.66 | 2.25 |
| Lys | 31.5 | 12.9 | 11.9 | 12.8 | 11.4 | 6.81 | 37.9 | 16.5 | 29.6 | 17.3 | 16.1 | 9.41 |
| Met | 18.9 | 8.09 | 7.72 | 8.46 | 7.24 | 4.18 | 18.6 | 8.09 | 8.09 | 8.75 | 7.58 | 4.25 |
| Thr | 67.2 | 34.2 | 32.0 | 29.5 | 30.9 | 21.8 | 51.8 | 30.3 | 30.5 | 26.8 | 29.2 | 20.3 |
| Trp | 20.9 | 10.6 | 10.6 | 10.9 | 10.5 | 7.43 | 20.9 | 11.0 | 12.0 | 11.9 | 11.7 | 8.02 |
| Val | 24.3 | 12.0 | 11.8 | 12.1 | 11.1 | 7.74 | 18.3 | 9.53 | 9.91 | 9.97 | 9.35 | 6.58 |
| IAA | 25.2 | 11.9 | 11.4 | 11.9 | 10.9 | 7.32 | 21.8 | 11.0 | 12.5 | 11.2 | 10.8 | 7.19 |
| Dispensable amino acids | ||||||||||||
| Ala | 26.9 | 12.0 | 11.9 | 13.1 | 12.1 | 7.74 | 7.93 | 3.87 | 3.82 | 4.40 | 4.07 | 2.75 |
| Asp | 44.9 | 18.6 | 17.6 | 17.7 | 17.1 | 11.5 | 24.0 | 12.3 | 12.1 | 12.1 | 12.1 | 8.07 |
| Cys1 | 28.6 | 14.9 | 13.4 | 12.8 | 13.4 | 10.4 | 39.7 | 44.7 | 20.5 | 17.8 | 19.5 | 14.7 |
| Glu | 6.39 | 2.87 | 2.99 | 3.29 | 2.98 | 2.00 | 8.04 | 3.59 | 3.92 | 4.28 | 3.92 | 2.62 |
| Gly1 | 23.3 | 11.4 | 11.1 | 11.6 | 10.9 | 7.57 | 29.8 | 15.6 | 17.6 | 16.7 | 16.4 | 11.3 |
| Pro | 10.6 | 5.21 | 5.40 | 5.34 | 5.23 | 3.79 | 11.8 | 6.22 | 6.51 | 6.18 | 6.59 | 4.58 |
| Ser | 30.2 | 14.7 | 14.5 | 13.5 | 14.2 | 9.82 | 26.1 | 13.9 | 14.2 | 13.1 | 14.1 | 9.65 |
| DAA | 22.6 | 11.0 | 10.6 | 10.7 | 10.5 | 7.42 | 19.9 | 10.6 | 10.5 | 10.2 | 10.5 | 7.33 |
| TAA | 23.8 | 11.5 | 11.1 | 11.3 | 10.8 | 7.43 | 20.9 | 11.3 | 11.4 | 10.8 | 10.7 | 7.25 |
Semi-indispensable amino acids for poultry.
Abbreviations: DAA, average digestibility of dispensable amino acids; IAA, average digestibility of indispensable amino acids; TAA, average digestibility of all amino acids.
Although the correction of AIDC for endogenous losses increased the SIDC of N and AA at all ages, the magnitude of increase was remarkably higher on d 7 and declined with advancing age. Standardization of AIDC estimates for EAA losses increased the average TAA digestibility coefficients in wheat by 23.8% (d 7), 11.5% (d 14), 11.1% (d 21), 11.3% (d 28), 10.8% (d 35), and 7.43% (d 42), respectively. The corresponding uplifts in digestibility coefficients in sorghum were 20.9% (d 7), 11.3% (d 14), 11.4% (d 21), 10.8% (d 28), 10.7% (d 35), and 7.25 % (d 42), respectively.
DISCUSSION
The main aim of this study was to investigate whether age affects the SIDC of AA in wheat and sorghum in broilers. Whilst there are sporadic data on age-related digestibility of AA in different ingredients for broilers (Fonolla et al., 1981; Wallis and Balnave, 1984; Adedokun et al., 2007; Szczurek et al., 2020), these studies determined the digestibility only at 2 or 3 specific ages of broilers using assay diets fed in mash form. Previous attempts to establish a link between the age and AA digestibility have been inconclusive (Fonolla et al., 1981; Wallis and Balnave, 1984; Zelenka and Liska, 1986; Huang et al., 2005). The unique features of the present experiment are that 1) this is the first study reporting the SIDC of AA in wheat and sorghum from hatch to the end of growth cycle, 2) age-appropriate EAA losses (Barua et al., 2021a) were used to standardize the AIDC and 3) the assay diets were pelleted so the physical form of diet resembled the industry practice.
Nutrient Composition
The proximate and amino acid concentrations of test cereals were, in general, within the range reported in the literature (Huang et al., 2005; Leeson and Summers, 2005; Ravindran et al., 2005; Bryden et al., 2009a,b; Selle et al., 2012). Some variation in ingredient nutrient composition is to be expected due to differences in cultivar, geographical location, agronomy, growing season, and analytical techniques (Gutierrez-Alamo et al., 2008; Mateos et al., 2019).
Performance, Gizzard pH, and Jejunal Digesta Viscosity
As could be expected, the daily feed intake and weight gain values increased as the birds grew older, regardless of the grain type. The gizzard pH in birds fed wheat- (2.68) and sorghum-based diets (2.56) on d 7 in the current study was close to the findings of Angel et al. (2010) that analyzed 15 published studies and Morgan et al. (2014), in a study with corn-, wheat- and sorghum-based diets, reporting gizzard pH of 2.37 and 2.42 on d 7, respectively. Gizzard pH increased from 2.68 in d 7 to 3.61 in d 42 in wheat-based diets and from 2.56 in d 7 to 3.66 in d 42 in sorghum-based diets. Although the digestive secretions and enzyme activities (Nitsan et al., 1991), and concurrent secretion of HCl, increase with broiler age, the increase in gizzard pH with age in present study could be, partially, due to increased consumption of feed with neutral pH (Ravindran, 2013), which could dilute the HCl concentration and increase the pH.
In birds fed wheat-based diets, the jejunal digesta viscosity increased with advancing age until d 35, with lower viscosities recorded on d 7 and 14. The positive relationship between the soluble NSP content and digesta viscosity is known (Choct and Annison, 1992b). Higher viscosity with advancing age could be a reflection of increased feed intake and intake of soluble NSP. The measured viscosities, however, were lower in birds fed sorghum diets, which is an expected result for a grain with low soluble NSP contents (Bryden et al., 2009a). Viscosity is correlated with the antinutritive effects of NSP on nutrient digestion and absorption (Fengler and Marquardt, 1988; Choct et al., 1999). In the present study, however, despite increased viscosity, the AIDC of AA in wheat increased with age, whereas no influence was observed in sorghum. No explanation could be provided for the lack of relationship between viscosity and AA digestibility.
Ileal Digestibility Coefficients of Nitrogen and Amino Acids
It is widely accepted that poultry feed formulations based on digestible AA are superior to those based on total AA (Rostagno et al., 1995; Ravindran and Bryden, 1999). To ensure adequate supply of available AA, therefore, better understanding of AA digestibility in birds at different ages of growth is fundamental.
In the present study, the AIDC of N, IAA, DAA, and TAA of wheat increased with advancing age in broilers. Several earlier studies have found that the AIDC of AA differs between broilers of different ages (Fonolla et al., 1981; Wallis and Balnave, 1984; Zuprizal et al., 1992; Noy and Sklan, 1995; Tarvid, 1995; Uni et al., 1995; Batal and Parsons, 2002; Huang et al., 2005). Wallis and Balnave (1984) reported an increased ileal AA digestibility in broilers from d 30 to 50 posthatch fed finisher diets containing a range of grain and protein sources. Batal and Parsons (2002) reported an increase in the total tract digestibility of AA in a corn-soybean diet from 0 to 10 d of age. Huang et al. (2005) determined AIDC AA of 8 feed ingredients (wheat, sorghum, corn, soybean meal, canola meal, cottonseed meal, mill run and, meat and bone meal) at 3 different ages (d 14, 28 and 42) of broiler and, observed a variable age effects depending on the ingredient. They concluded a general increase in digestibility with age. The AIDC of AA in corn, canola meal, soybean meal and, meat and bone meal were higher at d 28 and 42 than d 14. In contrast, the AIDC of most AA in wheat was found to be higher at d 14 than at d 28 and 42. No age effect was observed in the case of cottonseed meal. The ileal N digestibility of a corn-soybean meal diet was reported to be low in broilers at d 4 posthatch and increased at d 14 or 21 (Noy and Sklan, 1995; Uni et al., 1995). Adedokun et al. (2008) reported the ileal AA digestibility of 5 plant-based feed ingredients (light and dark distiller's dried grains with solubles, canola meal, soybean meal and corn) in broilers at d 5 and 21 of age. Though the AID AA increased with age in all feed ingredients, the SID AA increased only in distiller's dried grains with solubles and corn. In the case of soybean meal and canola meal, there were no differences in SID AA between the 2 age groups.
Increased apparent AA digestibility of wheat with bird age may be, partly, explained by the age effect on feed passage rate. Food transit time through the gastrointestinal tract plays an important role in the digestion of nutrients by determining the contact time among nutrients, absorptive surfaces, digestive enzymes and microbiome (Clemens et al., 1975; Mateos et al., 1982). The nutrient absorption is facilitated by increased digesta retention time as the contact time between the digesta and absorptive surface is increased (Washburn, 1991). It has been reported that feed passage rates decrease with bird age (Wilson et al., 1980). Hence, the digesta is exposed longer to digestive and absorptive processes, thus increasing nutrient digestibility (Wallis and Balnave, 1984). Besides, the gizzard of older birds is more developed than the young birds with heavy musculature. Increased digesta retention time in a well-developed gizzard enhances nutrient digestibility, including AA, by increasing intestinal refluxes and re-exposing the digesta to pepsin (Abdollahi and Ravindran, 2013).
Intestinal microbiome is known to play a key role in the digestive process by competing for nutrients (Stanley et al., 2013; Mancabelli et al., 2016). The colonization of microbiota in avian gut occurs naturally either at hatching or even before hatching by passing the microbes through the eggshell pores (Roto et al., 2016; Lee et al., 2019). Strict hygienic practices in the hatchery prevent any colonization and the gastrointestinal tract of newly hatched chick is sterile. Instead of natural maternal source, the hatchlings receive the initial microbial load from the farm environment (Donaldson et al., 2017). Initial colonization, followed by species richness and complexity of population structure, and finally maturation and stabilization of microbiota is noticed as the birds grow older. This process of development and stabilization of gut microbiota normally takes about 3 wk in commercial broilers (Oakley et al., 2014; Johnson et al., 2018; Lee et al., 2019). Gut bacteria are consumers of protein and AA, potentially influencing the digestibility estimates in the host (Yadav and Jha, 2019) and may partly contribute to the age effects.
While the AIDC of N and most AA in wheat increased with advancing age, only the SIDC of Met, Trp, Asp, Cys and Glu were influenced. The SIDC of Met, Trp, Asp and Cys followed the same pattern as AIDC and increased with broiler age. In contrast, Szczurek et al. (2020) measured the ileal AA digestibility in wheat, triticale and barley at 2 ages (d 14 and 28) of broilers and reported no age effect on the SID of AA in wheat.
In the case of sorghum, only the AIDC of Met, Trp and Cys increased with broiler age. Broiler age had no effect on the average AIDC of IAA, DAA and TAA. Huang et al. (2005) reported that the AIDC AA in sorghum was higher at d 42 than d 28, but similar to that at d 14. In contrast to the trends observed for AIDC, the highest SIDC of N, average of IAA, DAA and TAA were determined at d 7, which declined at d 14 and then plateaued. The SIDC of TAA in sorghum at d 7 was 8.3% higher than those from average SIDC of d 14 to 42. Among the IAA, higher values were recorded on d 7 for the SIDC of Arg, His, Thr and Val. The SIDC of most individual IAA and all individual DAA, except that of Cys, reduced with advancing age.
A number of early studies (Hakansson and Eriksson, 1974; Fonolla et al., 1981; Hassan and Delpech, 1986; Zelenka and Liska, 1986; Carré et al., 1991; ten Doeschate et al., 1993) have investigated the influence of age on the AA digestibility in poultry and, found that the protein and AA digestibility is lower in older birds. These studies, however, determined the digestibility over the total tract. Carré et al. (1991) reported that the apparent protein digestibility of field pea was higher in 3-wk-old broilers than in adult roosters. Fonolla et al. (1981) observed a reduction in apparent protein digestibility of broilers with advancing age (d 21 vs. d 52) by feeding 4 diets (starter, finisher, finisher diet with 1.39% urea, finisher diet with 0.25% Lys, 0.10% Met and 0.22% Gly). These researchers suggested that this reduction was caused by increased excretion of metabolic N. ten Doeschate et al. (1993) also observed decreased protein digestibility coefficients with increasing broiler age, with the values of 0.849 (d 13 to 15), 0.830 (d 27 to 29), 0.840 (d 41 to 43). Zuprizal et al. (1992) reported that the true digestibility of protein and AA in soybean meal or rapeseed meal for broilers decreased from wk 3 to 6. The total tract digestibility findings, however, are not comparable to the ileal digestibility values determined in the current study, because of the possible microbial fermentation and alteration of AA composition in the hindgut (Salter and Coats, 1971; Ravindran and Bryden, 1999), and AA contamination from urine (Sykes, 1971; Terpstra, 1979).
Some reports suggest that the ileal digestibility of major nutrients, including AA, in feed ingredients declines with advancing age. Adedokun et al. (2007) reported a reduction in the AIDC of TAA in meat and bone meal for broilers from 0.705 on d 5 to 0.595 on d 21. Decreased digestibility with age was observed even after the standardization of AIDC using EAA losses determined by feeding a N-free diet (0.760 on d 5 vs. 0.632 on d 21). Reduction in the digestibility of other nutrients with bird age has also been reported. Moss et al. (2020) observed a reduction in starch digestibility from 0.935 on d 10 to 0.885 on d 35 in broilers fed a corn-based diet. The corresponding starch digestibility values on d 10 and 35 reported to be 0.901 and 0.845 for red sorghum, and 0.987 and 0.904 for wheat, respectively. David et al. (2020) reported a decrease in apparent ileal calcium digestibility with advancing age in broilers from 0.51 on d 7 to 0.36 on d 21, and 0.27 on d 42. According to Li et al. (2018), the AIDC of calcium was higher in 9-d old broiler chickens than 21 d, regardless of the dietary calcium level.
Currently broiler feeds are being formulated based on SIDC AA because of the recognition that SIDC values for individual feed ingredients are additive when mixed into a complete diet. On the other hand, the AIDC AA undervalues the digestibility of several nutritionally critical AA and is less additive in feed formulations than SIDC (Cowieson et al., 2019; An et al., 2020). The only difference between AIDC and SIDC values is the correction for basal EAA flow (Ravindran, 2021). Despite the shortcomings of apparent digestibility estimates, the AIDC data are also presented in this paper to understand what proportion of the age effect on SIDC could be attributed to EAA losses. The data demonstrated that the observed differences in patterns between AIDC and SIDC (Figure 1, Figure 2) was due to the use of age-appropriate EAA flows which were substantially higher at d 7, almost similar from d 14 to 35, and much lower at d 42. The basal ileal endogenous flow of TAA, used for standardization of AIDC values (Barua et al., 2021a), in d 7 (12.93 g/kg DMI) was 96% higher than the average endogenous loss of TAA from d 14 to 35 (6.61 g/kg DMI), and 189% higher than the endogenous loss of TAA in d 42 (4.481g/kg DMI). Once corrected for age-related endogenous AA flows, substantial differences were observed for the percentage increase in SIDC of AA over AIDC values at different ages (Table 11). Though a large number of studies (Lemme et al., 2004; Bryden et al., 2009b; Szczurek, 2009; Bandegan et al., 2011; Kim et al., 2012; Ravindran et al., 2014, 2017; Barua et al., 2020, 2021b) exist on the SIDC of AA of a wide range of poultry feed ingredients, only few studies (Zuprizal et al., 1992, Garcia et al., 2007; Adedokun et al., 2007; 2008; Szczurek et al., 2020) have determined the SIDC at different broiler ages. To the authors’ knowledge, apart from those by Adedokun et al. (2008) and Szczurek et al. (2020), all previous studies have used a single EAA flow value, derived from older birds. As the EAA loss is highest in d 7 and decreases with advancing age, the use of a single EAA value for correction at different ages underestimates the SIDC in early life and overestimates the SIDC in birds older than d 35.
Figure 1.
Apparent and standardized ileal digestibility coefficients of total amino acids (TAA) in wheat (bars represent means ± SE) as influenced by broiler age. Abbreviations: L, linear; Q, quadratic.
Figure 2.
Apparent and standardized ileal digestibility coefficients of total amino acids in sorghum (bars represent means ± SE) as influenced by broiler age. a,bValues with different superscripts differ significantly (P < 0.05). Abbreviations: L, linear; Q, quadratic.
Correction of AIDC values using age-appropriate EAA losses resulted in an increase of 23.8% in the SIDC of average TAA in wheat in d 7, which was almost twice than the increase in d 14 to 35 (10.8–11.5%), and 3 times more than d 42 (7.43%). Similar trends were recorded for the average SIDC of TAA in sorghum at d 7 being 20.9% higher than the average AIDC of TAA. The magnitude of uplift in the average SIDC of TAA at d 14 to 35 and d 42 were 10.7 to 11.4% and 7.25%, respectively. In essence, the trends in age effects on SIDC were reflective of those on EAA flow.
Overall, the exact reasons for the observed age effects on AA digestibility are intricate and complicated by the interactions among plethora of factors including digestion, absorption, transport, endogenous losses, feed intake, digesta passage rate and intestinal microbiome. The present data also showed that the EAA flow is the prime contributing factor contributing to SIDC, especially during wk 1.
CONCLUSIONS
The present study, for the first time, provides information on the SIDC of AA in wheat and sorghum from hatching to the end of growth cycle of broilers. These results suggest that the age effect on AA digestibility is variable depending on the grain type and specific AA. Though the apparent AA digestibility in wheat increased by age, the SIDC was not affected in most of the AA. In the case of sorghum, except for a few AA, the AIDC of most AA was unaffected by age. However, the highest SIDC AA of sorghum were recorded at d 7, reduced at d 14, and then plateaued. The correction of apparent AA digestibility values using age-appropriate EAA flows resulted in substantial increases in SIDC over AIDC at different ages in both wheat and sorghum. The EAA value used for correction of apparent values in this study was notably higher at d 7 compared to other ages. Because the use of a single EAA value for birds of different ages might underestimate the SIDC in early life and overestimate the SIDC in older birds, application of age-appropriate EAA values for standardization should be considered to improve the precision of feed formulations.
Acknowledgments
ACKNOWLEDGMENTS
The authors express their gratitude to the “AgriFutures Australian Chicken Meat Program” for funding the project, and to Preethi Ramesh, AMINOLab EMSEA, Singapore for AA analyses.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Abdollahi M.R., Ravindran V. Influence of pellet length on pellet quality and performance of broiler starters. J. Appl. Poult. Res. 2013;22:516–522. [Google Scholar]
- Adedokun S.A., Parsons C.M., Lilburn M.S., Adeola O., Applegate T.J. Standardized ileal amino acid digestibility of meat and bone meal from different sources in broiler chicks and turkey poults with a nitrogen-free or casein diet. Poult. Sci. 2007;86:2598–2607. doi: 10.3382/ps.2007-00164. [DOI] [PubMed] [Google Scholar]
- Adedokun S.A., Adeola O., Parsons C.M., Lilburn M.S., Applegate T.J. Standardized ileal amino acid digestibility of plant feedstuffs in broiler chickens and turkey poults using a nitrogen-free or casein diet. Poult. Sci. 2008;87:2535–2548. doi: 10.3382/ps.2007-00387. [DOI] [PubMed] [Google Scholar]
- An S.H., Sung J.Y., Kang H-K., Kong C. Additivity of ileal amino acid digestibility in diets containing corn, soybean meal, and corn distillers dried grains with solubles for male broilers. Animals. 2020;10:933–943. doi: 10.3390/ani10060933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel R., Humphreys B., Saylor W. Age changes in gastrointestinal pH in broilers. Poult. Sci. 2010;89(Suppl. E):650. (Abstr.) [Google Scholar]
- Angkanaporn K., Ravindran V., Bryden W.L. Influence of cecectomy and dietary protein level on apparent excreta amino acid digestibility in adult cockerels. Br. Poult. Sci. 1997;38:270–276. doi: 10.1080/00071669708417985. [DOI] [PubMed] [Google Scholar]
- AOAC International . 18th ed. Association of Official Analytical Chemists; Washington, DC: 2011. Official Methods of Analysis. [Google Scholar]
- AOAC International . 20th ed. Association of Official Analytical Chemists; Washington, DC: 2016. Official Methods of Analysis. [Google Scholar]
- Bach Knudsen K.E. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014;93:2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- Bandegan A., Golian A., Kiarie E., Payne R.L., Crow G.H., Guenter W., Nyachoti C.M. Standardized ileal amino acid digestibility in wheat, barley, pea and flaxseed for broiler chickens. Can. J. Anim. Sci. 2011;91:103–111. [Google Scholar]
- Barua M., Abdollahi M.R., Zaefarian F., Wester T.J., Girish C.K., Ravindran V. Standardized ileal amino acid digestibility of protein sources for broiler chickens is influenced by the feed form. Poult. Sci. 2020;99:6925–6934. doi: 10.1016/j.psj.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua M., Abdollahi M.R., Zaefarian F., Wester T.J., Girish C.K., Chrystal P.V., Ravindran V. Basal ileal endogenous amino acid flow in broiler chickens as influenced by age. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua M., Abdollahi M.R., Zaefarian F., Wester T.J., Girish C.K., Ravindran V. Influence of feed form on the standardized ileal amino acid digestibility of common grains for broiler chickens. Anim. Feed Sci. Technol. 2021;272 doi: 10.1016/j.psj.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batal A., Parsons C. Effects of age on nutrient digestibility of chicks fed different diets. Poult. Sci. 2002;81:400–407. doi: 10.1093/ps/81.3.400. [DOI] [PubMed] [Google Scholar]
- Bryden, W. L., P. H. Selle, D. J. Cadogan, X. Li, N. D. Muller, D. R. Jordan, M. J. Gidley, and W. D. Hamilton. 2009a. A Review of the Nutritive Value of Sorghum for Broilers. Rural Industries Research and Development Corporation. RIRDC Publication No: 09/077, Canberra, Australia.
- Bryden, W. L., X. Li, G. Ravindran, Li, Hew, and V. Ravindran. 2009b. Ileal Digestible Amino Acid Values in Feedstuffs for Poultry. Rural Industries Research and Development Corporation. RIRDC Publication No: 09/071, Canberra, Australia.
- Carré B., Beaufils E., Melcion J. Evaluation of protein and starch digestibility and energy value of pelleted or unpelleted pea seed from winter or spring cultivars in adult and young chicks. J. Agric. Food Chem. 1991;39:468–472. [Google Scholar]
- Choct M., Annison G. The inhibition of nutrient digestion by wheat pentosans. Br. J. Nutr. 1992;67:123–132. doi: 10.1079/bjn19920014. [DOI] [PubMed] [Google Scholar]
- Choct M., Annison G. Antinutritive activity of wheat arabinoxylans: role of viscosity and gut microflora. Br. Poult. Sci. 1992;33:821–834. doi: 10.1080/00071669208417524. [DOI] [PubMed] [Google Scholar]
- Choct M., Hughes R.J., Bedford M.R. Effects of a xylanase on individual bird variation, starch digestion throughout the intestine, and ileal and cecal volatile fatty acid production in chickens fed wheat. Br. Poult. Sci. 1999;40:419–422. doi: 10.1080/00071669987548. [DOI] [PubMed] [Google Scholar]
- Clemens E., Stevens C., Southworth M. Sites of organic acid production and pattern of digesta movement in the gastrointestinal tract of geese. J. Nutr. 1975;105:1341–1350. doi: 10.1093/jn/105.10.1341. [DOI] [PubMed] [Google Scholar]
- Cowieson A., Sorbara J.O., Pappenberger G., Abdollahi M.R., Roos F.F., Ravindran V. Additivity of apparent and standardized ileal amino acid digestibility of corn and soybean meal in broiler diets. Poult. Sci. 2019;98:3722–3728. doi: 10.3382/ps/pez060. [DOI] [PubMed] [Google Scholar]
- David L.S., Abdollahi M.R., Bedford M.R., Ravindran V. Effect of age and dietary crude protein content on the apparent ileal calcium digestibility of limestone in broiler chickens. Anim. Feed Sci. Technol. 2020;263 [Google Scholar]
- Donaldson E.E., Stanley D., Hughes R.J., Moore R.J. The time-course of broiler intestinal microbiota development after administration of cecal contents to incubating eggs. Peer J. 2017;5:3587. doi: 10.7717/peerj.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fengler A.I., Marquardt R.R. Water-soluble pentosans from rye. 1. Isolation, partial-purification, and characterization. Cereal Chem. 1988;65:291–297. [Google Scholar]
- Fonolla J., Prirto C., Sanz R. Influence of age on nutrient utilization of diets for broilers. Anim. Feed Sci. Technol. 1981;6:405–411. [Google Scholar]
- Garcia A.R., Batal A.B., Dale N.M. A comparison of methods to determine amino acid digestibility of feed ingredients for chickens. Poult. Sci. 2007;86:94–101. doi: 10.1093/ps/86.1.94. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Alamo A., Perez de Ayala P., Verstegen M.W.A., Den Hartog L.A., Villamide M.J. Variability in wheat: factors affecting its nutritional value. World's Poult. Sci. J. 2008;64:20–39. [Google Scholar]
- Hakansson J., Eriksson S. Digestibility, nitrogen retention and consumption of metabolizable energy by chickens on feeds of low and high concentration. Swed. J. Agri. Res. 1974;4:195–207. [Google Scholar]
- Hassan A.S., Delpech P. Metabolizable energy value and protein digestibility in chickens: influence of genotype, age and diet. Genet. Sel. Evol. 1986;18:225–236. doi: 10.1186/1297-9686-18-2-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.H., Ravindran V., Li X., Bryden W.L. Influence of age on the apparent ileal amino acid digestibility of feed ingredients for broiler chickens. Br. Poult. Sci. 2005;46:236–245. doi: 10.1080/00071660500066084. [DOI] [PubMed] [Google Scholar]
- Jin S.H., Corless A., Sell J.L. Digestive system in post-hatch poultry. World's Poult. Sci. J. 1998;54:335–345. [Google Scholar]
- Johnson T.J., Youmans B.P., Noll S., Cardona C., Evans N.P., Karnezos T.P., Ngunjiri J.M., Abundo M.C., Lee C.W. A consistent and predictable commercial broiler chicken bacterial microbiota in antibiotic-free production displays strong correlations with performance. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00362-18. e00362-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Utterback P.L., Parsons C.M. Comparison of amino acid digestibility coefficients for corn, corn gluten meal, and corn distillers dried grains with solubles among 3 different bioassays. Poult. Sci. 2012;91:3141–3147. doi: 10.3382/ps.2012-02418. [DOI] [PubMed] [Google Scholar]
- Lee S., La T.M., Lee H.J., Choi I.S., Song C.S., Park S.Y., Lee J.B., Lee S.W. Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Sci. Rep. 2019;9:6838. doi: 10.1038/s41598-019-43280-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson S., Summers J.D. Pages 15–23 in Commercial Poultry Nutrition. 3rd ed., University Books; Guelph, Ontario, Canada: 2005. Ingredient evaluation and diet formulation. [Google Scholar]
- Lemme A., Ravindran V., Bryden W.L. Ileal digestibility of amino acids in feed ingredients for broilers. World's Poult. Sci. J. 2004;60:423–438. [Google Scholar]
- Li W., Angel R., Kim S.-W., Jiménez-Moreno E., Proszkowiec-Weglarz M., Plumstead P.W. Impacts of age and calcium on phytase efficacy in broiler chickens. Anim. Feed Sci. Technol. 2018;238:9–17. [Google Scholar]
- Mancabelli L., Ferrario C., Milani C., Mangifesta M., Turroni F., Duranti S., Lugli G.A., Viappiani A., Ossiprandi M.C., van Sinderen D., Ventura M. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016;18:4727–4738. doi: 10.1111/1462-2920.13363. [DOI] [PubMed] [Google Scholar]
- Mateos G.G., Sell J.L., Eastwood J.A. Rate of food passage (transit time) as influenced by level of supplemental fat. Poult. Sci. 1982;61:94–100. doi: 10.3382/ps.0610094. [DOI] [PubMed] [Google Scholar]
- Mateos G.G., Ca´mara L., Fondevila G., La´zaro R.P. Critical review of the procedures used for estimation of the energy content of diets and ingredients in poultry. J. Appl. Poult. Res. 2019;28:506–525. [Google Scholar]
- McCleary B.V., Gibson T.S., Mugford D.C. Measurement of total starch in cereals products by amyloglucosidase-α-amylase method: collaborative study. J. AOAC Int. 1997;80:571–579. [Google Scholar]
- Morgan N.K., Walk C.L., Bedford M.R., Burton E.J. The effect of dietary calcium inclusion on broiler gastrointestinal pH: quantification and method optimization. Poult. Sci. 2014;93:354–363. doi: 10.3382/ps.2013-03305. [DOI] [PubMed] [Google Scholar]
- Moss A.F., Khoddami A., Chrystal P.V., Sorbara J.B., Cowieson A.J., Selle P.H., Liu S.Y. Starch digestibility and energy utilization of maize- and wheat-based diets is superior to sorghum-based diets in broiler chickens offered diets supplemented with phytase and xylanase. Anim. Feed Sci. Technol. 2020;264 [Google Scholar]
- Moughan P.J., Buttery P.J., Essex C.P., Soar J.B. Evaluation of the isotope dilution technique for determining ileal endogenous nitrogen excretion in the rat. J. Sci. Food Agric. 1992;58:165–172. [Google Scholar]
- Murakami H., Akiba Y., Horiguchi M. Growth and utilization of nutrients in newly-hatched chick with or without removal of residual yolk. Growth Dev. Aging. 1992;56:75–84. [PubMed] [Google Scholar]
- Nitsan Z., Ben-Avraham G., Zoref Z., Nir I. Growth and development of the digestive organs and some enzymes in broiler chicks after hatching. Br. Poult. Sci. 1991;32:515–523. doi: 10.1080/00071669108417376. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Digestion and absorption in the young chicks. Poult. Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Bryden W.L. Amino acid availability in poultry in vitro and in vivo measurements. Aust. J. Agric. Res. 1999;50:889–908. [Google Scholar]
- Ravindran V. Feed enzymes: the science, practice, and metabolic realities. J. Appl. Poult. Res. 2013;22:628–636. [Google Scholar]
- Ravindran V. Progress in ileal endogenous amino acid flow research in poultry. J. Anim. Sci. Biotechnol. 2021;12:5. doi: 10.1186/s40104-020-00526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran V., Hew L.I., Ravindran G., Bryden W.L. Apparent ileal digestibility of amino acids in dietary ingredients for broiler chickens. Anim. Sci. 2005;81:85–97. [Google Scholar]
- Ravindran V., Abdollahi M.R., Bootwalla S. Nutrient analysis, metabolizable energy, and digestible amino acids of soybean meals of different origins for broilers. Poult. Sci. 2014;93:2567–2577. doi: 10.3382/ps.2014-04068. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Adeola O., Rodehutscord M., Kluth H., van der Klis J.D., van Eerden E., Helmbrecht A. Determination of ileal digestibility of amino acids in raw materials for broiler chickens - results of collaborative studies and assay recommendations. Anim. Feed Sci. Technol. 2017;225:62–72. [Google Scholar]
- Rooney L.W., Pflugfelder R.L. Factors affecting starch digestibility with special emphasis on sorghum and corn. J. Anim. Sci. 1986;63:1607–1623. doi: 10.2527/jas1986.6351607x. [DOI] [PubMed] [Google Scholar]
- Rostagno H.S., Pupa J.M.R., Pack M. Diet formulation for broilers based on total versus digestible amino acids. J. Appl. Poult. Res. 1995;4:293–299. [Google Scholar]
- Roto S.M., Kwon Y.M., Ricke S.C. Applications of in ovo technique for the optimal development of the gastrointestinal tract and the potential influence on the establishment of its microbiome in poultry. Front. Vet. Sci. 2016;3:63. doi: 10.3389/fvets.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D.N., Coates M.E. The influence of the microflora of the alimentary tract on protein digestion in chicks. Br. J. Nutr. 1971;26:55–69. doi: 10.1079/bjn19710008. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Moss A.F., Truong H.H., Khoddami A., Cadogan D.J., Godwin I.D., Liu S.Y. Outlook: sorghum as a feed grain for Australian chicken-meat production. Anim. Nutr. 2018;4:17–30. doi: 10.1016/j.aninu.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle P.H., Huang K.H., Muir W.I. Effects of nutrient specifications and xylanase plus phytase supplementation of wheat-based diets on growth performance and carcass traits of broiler chicks. Asian-Australas. J. Anim. Sci. 2003;16:1501–1509. [Google Scholar]
- Selle P.H., Liu S.Y., Cai J., Cowieson A.J. Steam-pelleting and feed form of broiler diets based on three coarsely ground sorghums influences growth performance, nutrient utilization, starch and nitrogen digestibility. Anim. Prod. Sci. 2012;52:842–852. [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Stanley D., Geier M.S., Denman S.E., Haring V.R., Crowley T.M., Hughes R.J., Moore R.J. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet. Microbiol. 2013;164:85–92. doi: 10.1016/j.vetmic.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Sykes A.H. In: Pages 233–276 in Physiology and Biochemistry of the Domestic Fowl. Bell D.J., Freeman B.M., editors. Acad. Press; New York, NY: 1971. Formation and composition of urine. [Google Scholar]
- Szczurek W. Standardized ileal digestibility of amino acids from several cereal grains and protein-rich feedstuffs in broiler chickens at the age of 30 days. J. Anim. Feed Sci. 2009;18:662–676. [Google Scholar]
- Szczurek W., Szymczyk B., Arczewska-Włosek A., Świątkiewicz S. Apparent and standardized ileal digestibility of amino acids in wheat, triticale and barley for broiler chickens at two different ages. Br. Poult. Sci. 2020;61:63–69. doi: 10.1080/00071668.2019.1673317. [DOI] [PubMed] [Google Scholar]
- Tarvid I. The development of protein digestion in poultry. Poult. Avian Biol. Rev. 1995;6:35–54. [Google Scholar]
- ten Doeschate R.A.H.M., Scheele C.W., Schreurs V.V.A.M., van der Klis J.D. Digestibility studies in broiler chickens: influence of genotype, age, sex and method of determination. Br. Poult. Sci. 1993;34:131–146. [Google Scholar]
- Terpstra, K. 1979. Total and digestible amino acids. Pages 1–5 in Proc. 2nd Eur. Symp. Poult. Nutr., Beekbergen, The Netherlands.
- Uni Z., Noy Y., Sklan D. Posthatch changes in morphology and function of the small intestines in heavy and light strain chicks. Poult. Sci. 1995;74:1622–1629. doi: 10.3382/ps.0741622. [DOI] [PubMed] [Google Scholar]
- Wallis I.R., Balnave D. The influence of environmental temperature, age and sex on the digestibility of amino acids in growing broiler chickens. Br. Poult. Sci. 1984;25:401–407. doi: 10.1080/00071668408454880. [DOI] [PubMed] [Google Scholar]
- Washburn K.W. Efficiency of feed utilization and rate of feed passage through the digestive system. Poult. Sci. 1991;70:447–452. doi: 10.3382/ps.0700447. [DOI] [PubMed] [Google Scholar]
- Wilson E.K., Pierson F.W., Hester P.Y., Adams R.L., Stadelman W.J. The effects of high environmental temperature on feed passage time and performance traits of White Pekin Ducks. Poult. Sci. 1980;59:2322–2330. [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenka J. Influence of the age of chickens on the metabolizable energy values of poultry diets. Br. Poult. Sci. 1968;9:135–142. doi: 10.1080/00071666808415703. [DOI] [PubMed] [Google Scholar]
- Zelenka, J., and I. Liska. 1986. Effect of sex and age on amino acids digestibility in a feed mixture. Pages 298–302 in Proc. 7th Eur. Poult. Conf., August 24-28. M. Larbier, ed. Vol. 1. World's Poult. Sci. Assoc., Paris, France.
- Zuprizal ., Larbier M., Chagneau A.M. Effect of age and sex on true digestibility of amino acids of rapeseed and soybean meals in growing broilers. Poult. Sci. 1992;71:1486–1492. doi: 10.3382/ps.0711486. [DOI] [PubMed] [Google Scholar]