Abstract
Background
The Fragility Index (FI) and Reverse Fragility Index are powerful tools to supplement the P value in evaluation of randomized clinical trial (RCT) outcomes. These metrics are defined as the number of patients needed to change the significance level of an outcome. The purpose of this study was to calculate these metrics for published RCTs in total joint arthroplasty (TJA).
Methods
We performed a systematic review of RCTs in TJA over the last decade. For each study, we calculated the FI (for statistically significant outcomes) or Reverse Fragility Index (for nonstatistically significant outcomes) for all dichotomous, categorical outcomes. We also used the Pearson correlation coefficient to evaluate publication-level variables.
Results
We included 104 studies with 473 outcomes; 92 were significant, and 381 were nonstatistically significant. The median FI was 6 overall and 4 and 7 for significant and nonsignificant outcomes, respectively. There was a positive correlation between FI and sample size (R = 0.14, P = .002) and between FI and P values (R = 0.197, P = .000012).
Conclusions
This study is the largest evaluation of FI in orthopedics literature to date. We found a median FI that was comparable to or higher than FIs calculated in other orthopedic subspecialties. Although the mean and median FIs were greater than the 2 recommended by the American Academy of Orthopaedic Surgeons Clinical Practice Guidelines to demonstrate strong evidence, a large percentage of studies have an FI < 2. This suggests that the TJA literature is on par or slightly better than other subspecialties, but improvements must be made.
Level of Evidence
Level I; Systematic Review.
Keywords: Total joint arthroplasty, Fragility index, Randomized controlled trials, Statistical significance
Introduction
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are 2 of the most commonly performed orthopedic surgeries in the world [[1], [2], [3], [4]]. Current data suggest an increase by 143% in TKAs performed annually in the United States by 2050, [4] with similar numbers for THA [2]. Given this scenario, researchers are constantly looking for ways to evaluate and improve techniques and outcomes in these patient populations, often in the form of randomized controlled trials (RCTs). Analyzing RCTs can, thus, facilitate establishing a standard for both clinical practice guidelines and future research.
In evaluating these studies, the P value is the most used tool. However, the P value provides information solely relevant to an outcome’s relation to the null hypothesis. It is unable to comment on sample size or strength of association. Thus, the Fragility Index (FI) and Reverse Fragility Index (RFI) have emerged as supplemental tools to assess clinical trial results. The FI and RFI are defined as the number of patients (or events) that would need to have an alternative outcome to convert an outcome from significant to nonsignificant or vice versa. A large FI suggests a robust outcome, as it would require many changed events to have a different outcome. Alternatively, a small FI suggests less confidence in an outcome, as very few events would be required to change its P value. The FI, thus, provides information on effect size, demonstrating how each event impacts the P value.
The FI has increasingly been used to evaluate orthopedic surgery clinical trials. The American Academy of Orthopaedic Surgeons (AAOS) published clinical practice guidelines for evaluating research, stating that an article with a median FI of 2 would be considered “strong research” [5]. The FI for orthopedic subspecialties is generally low, with reported FIs ranging from 2 to 5, with sports literature thus far being the most robust with an FI of 5 [[6], [7], [8], [9], [10]].
A recent study by Ekhtiari et al. described the FI of statistically significant outcomes in 34 RCTs in total joint arthroplasty (TJA) [11]. However, their sample was small, and this article seeks to expand that search. Research by Kahn et al. and McCormick et al. recently described the “reverse fragility index,” which determines FI in nonstatistically significant outcomes in general and orthopedic research, respectively [12,13]. This allows the FI to be applied to a much larger body of research. The aim of our study was to evaluate the quality of RCTs in the orthopedic subspecialty of adult reconstruction using FI and RFI as metrics.
Material and methods
Study design and eligibility
The authors performed a systematic review of all RCTs using methods akin to those described in previous analyses of statistical fragility [[5], [6], [7], [8], [9], [10],14]. The top 25 highest impact orthopedic surgery and arthroplasty journals were determined via Incites Journal Citation Reports. These journals were queried for all RCTs in knee or hip arthroplasty published in the last 10 years in English.
Inclusion criteria were articles written in English between January 1, 2010, and September 1, 2020, that investigated surgical interventions for primary TJA and required the use of a 1:1 parallel, 2-arm randomization procedure, with at least 1 dichotomous outcome. Articles were excluded if they did not meet any of these criteria. Titles and abstracts were screened independently by 2 different authors (K.L.M. and A.G.) to ensure studies met inclusion criteria. If there was disagreement, a third author (C.L.H.) read the article as well. All articles were reviewed in their entirety to record all dichotomous, categorical outcomes for further analysis. The following study characteristics were collected for analysis: study size, number of patients lost to follow-up, outcome type, reported P values, and journal of publication. We used PubMed, Embase, and Medline to search, and the specific search criteria are summarized in Table 1.
Table 1.
Search terms used for systematic review.
| Search category | Terms used |
|---|---|
| Keywords | “Arthroplasty” OR “knee arthroplasty” OR “hip arthroplasty” AND “orthopedics” OR “Orthopedic Surgery” OR “surgery” OR “surgical procedure” |
| Article type | “Randomized controlled trial” |
| Publication date | “2010/01/01” [PDAT]: “2020/09/01” [PDAT] |
| Language | “English” |
Calculation of FI
The FI is defined as the lowest number of outcomes that must be changed to reverse the statistical significance of a P value. FI scores were calculated for each categorical, dichotomous outcome using Fisher’s exact test as described by Walsh et al. [14]. For statistically significant outcomes, discrete outcome events were switched from the larger outcome group to the smaller group in a stepwise fashion until the P value was greater than 0.05. For statistically insignificant P values, events in the smaller outcome group were changed in a similar manner, until the P value was less than .05 and, thus, statistically significant.
Statistical analysis
As stated previously, all P values were recalculated using Fisher’s exact test. A Student’s t-test was used to calculate the difference between the aforesaid study variables. Finally, the Pearson Correlation Coefficient was used to evaluate associations between FI and P values of included studies, as well as the associations between publication-level variables. All statistical analyses were performed using Microsoft Excel 2016 (Microsoft, Redmond, WA) and SPSS Version 23 (IBM, Armonk, NY).
Results
Characteristics
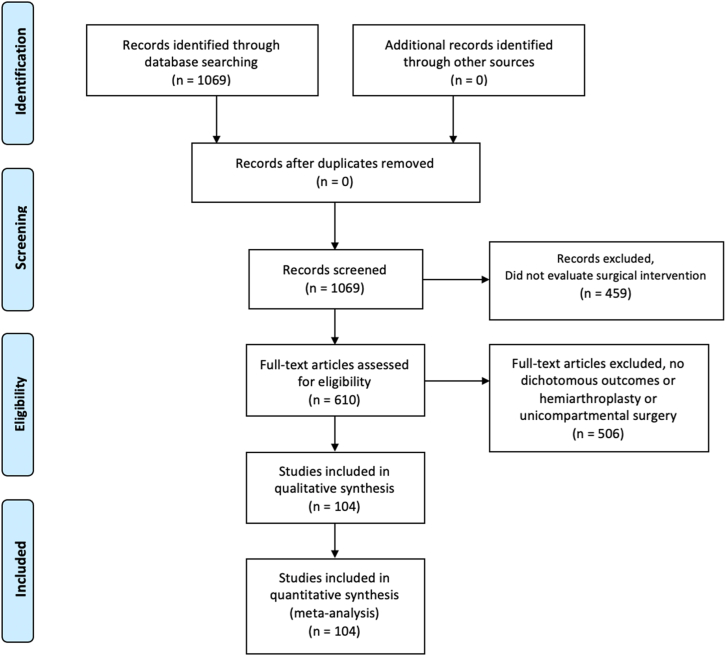
A total of 1069 articles were identified. After abstract review, 459 studies were excluded because they did not evaluate surgical interventions (eg, postoperative pain management, rehabilitation protocols). An additional 502 studies were excluded because they lacked dichotomous, categorical outcomes, and 5 studies for being focused on hemiarthroplasty and unicompartmental surgery. Ultimately, 104 studies were included for analysis with a total of 473 outcomes (Fig. 1). A full list of the included articles can be found in the appendices. The top 3 referenced journals were the Journal of Arthroplasty with 37 studies (35.6% of total articles), Clinical Orthopaedics and Related Research with 23 studies (22.1%), and Bone & Joint Journal with 14 articles (13.5%) (Table 2). The most often reported outcome type was postoperative complications (154 outcomes, 33%), as shown in Table 3.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
Table 2.
Number of included publications by journal.
| Journal | Number of publications |
|---|---|
| Journal of Arthroplasty | 37 |
| Clinical Orthopaedics and Related Research | 23 |
| Bone & Joint Journal | 14 |
| Journal of Bone and Joint Surgery | 12 |
| Knee Surgery Sports Traumatology Arthroscopy | 9 |
| Acta Orthopaedica | 6 |
| International Orthopedics | 3 |
Table 3.
Categorization of dichotomous recorded outcomes.
| Outcome | Count, N (%) |
|---|---|
| Postoperative complication | 154 (32.6) |
| Alignment: radiographic findings | 114 (24.1) |
| Patient pain/function | 86 (18.2) |
| Failure of surgery/required reoperation | 49 (10.4) |
| Other radiological findings | 44 (9.3) |
| Transfusion | 19 (4.0) |
| Patient satisfaction | 7 (1.5) |
Fragility index
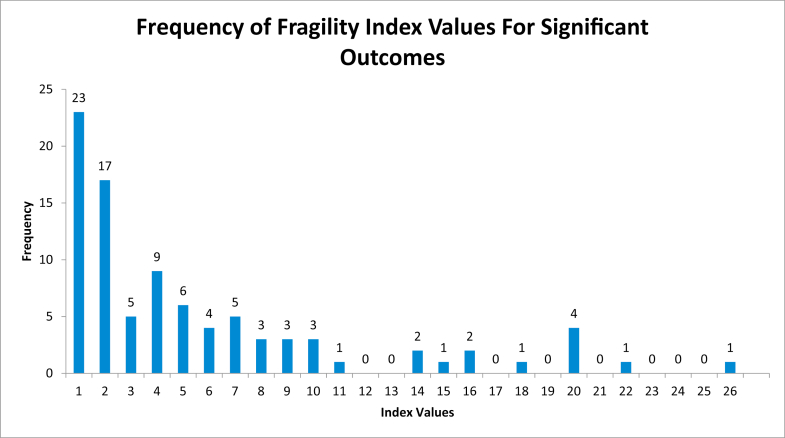
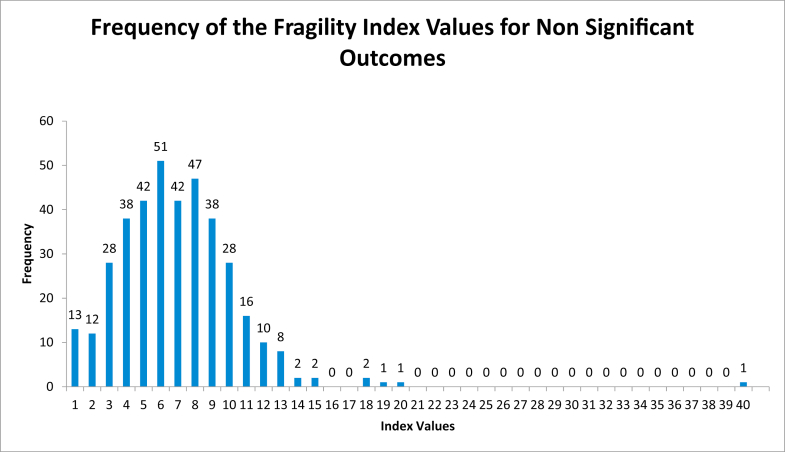
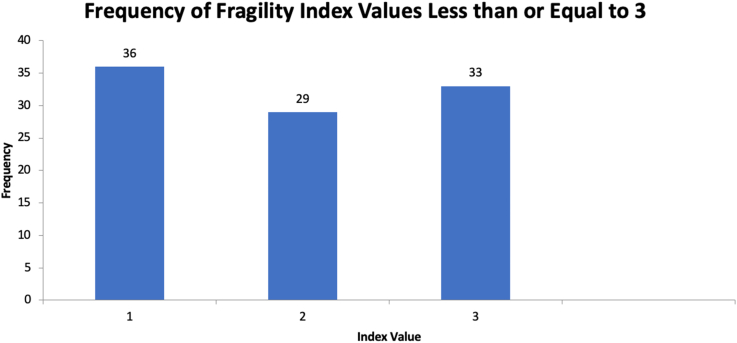
Among the 473 outcomes assessed, the median FI was 6 (mean 6.7, range 1-40). Of the 91 statistically significant outcomes, the median FI was 4 (mean 5.6, range 1-26) (Fig. 2). The median FI for the 382 nonstatistically significant outcomes was 7 (mean 7.0, range 1-40) (Fig. 3). The FI was less than or equal to 3 in 98 outcomes (Fig. 4). There was a statistically significant difference between statistically significant and statistically insignificant outcomes (P = .0007). The number of subjects lost to follow up can be seen in Appendices Tables 1-3. Number of patients lost to follow-up was found to be greater than FI for 181 outcomes (38.3%). There was a positive correlation between FI and sample size (R = 0.14, P = .002), and between FI and P values (R = 0.197, P = .000012). There was no, however, correlation between FI and number of patients lost to follow-up (R = 0.022, P = .62) (Table 4).
Figure 2.
Frequency of fragility index values of significant outcomes histogram.
Figure 3.
Frequency of fragility index values of nonsignificant outcomes histogram.
Figure 4.
Frequency of fragility index values less than or equal to 3 histogram.
Table 4.
Publication-level associations between fragility index and study variables.
| Study variables | Pearson correlation coefficient | P value |
|---|---|---|
| Patient sample size | 0.140 | .002a |
| Journal impact factor | -0.0263 | .56 |
| Number of journal citations | -0.096 | .035a |
| Patients lost to follow-up | 0.022 | .62 |
| All P values | 0.197 | .000012a |
| Significant P values | -0.028 | .78 |
| Nonsignificant P values | 0.177 | .000045a |
Statistically significant.
Discussion
We identified 104 studies and 473 outcomes in our systematic analysis. This is the largest study to date examining FI for surgical clinical trials in TJA and, moreover, in any orthopedic subspecialty, as well as the first study to evaluate nonstatistically significant outcomes in TJA literature through the use of the RFI. We found a median FI of 6 for all 473 outcomes assessed, with a median FI of 4 and RFI of 7 for statistically significant and nonstatistically significant outcomes, respectively. These median FIs are comparable to or greater than those reported for other orthopedic subspecialties, which ranged from 2 to 5 [[6], [7], [8], [9], [10]]. As stated previously, the AAOS released guidelines which consider an FI above 2 as “strong evidence” [5]. According to that guidance, the FI and RFI calculated here demonstrate strong evidence and robust P values. In this investigation, FI/RFI ranged from 1 to 40. The largest value was an RFI of 40, assigned to an RCT investigating the effect of triclosan-coated sutures on surgical site infection after TKA and THA [15].
In addition, there were positive correlations between FI and sample size (R = 0.14, P = .002), and between FI and P values (R = 0.197, P = .000012). We would expect to see these results, as it suggests that the larger a sample size is, the more confident one can be in the P value. The further the P value moves from the null hypothesis in either direction, the more changes in event are needed to change the significance level and the stronger the result.
This study contradicts that of Ekhtiari et al. that was recently published [11]. In it, the authors performed a literature search to identify RCTs performed for primary or revision surgery and ultimately included 34 RCTs from the past decade in TJA literature and found that the median FI was 1, meaning that reversing the outcome of just one subject would change any statistically significant outcome to not statistically significant. Furthermore, they found that the FI was lower than that in any other reported orthopedic subspecialty. In their discussion, they argue that as TJA is such a common procedure and has widely accepted indications and techniques, future trials should not be hampered by small sample sizes. Our data do corroborate their last point. Based on our calculations, FI does correlate strongly with increasing sample size. In evaluating the FI of both significant and nonsignificant outcomes, however, we found a much higher median FI of 6 overall, and 4 and 7 for significant and nonsignificant outcomes, respectively. Both these values are greater than what their study reported [11]. Our research evaluated different studies—we chose to evaluate solely primary TJA RCTs describing surgical interventions in the top 25 highest impact orthopedic journals, with manuscripts in English. However, we included more than triple the number of studies (104 rather than 34) by including insignificant outcomes and calculating the RFI, the number of patients needed to change outcomes in a study, to change a nonstatistically significant variable into one that is statistically significant. It is possible that this increased FI/RFI is in part due to using higher impact journals.
However, these data should be interpreted with caution. One hundred and eighty-one (38.3%) of the outcomes analyzed in this review had FIs greater than the number of patients lost to follow-up. Combining both FI and RFI, there are 65 outcomes with an FI or RFI ≤ 2, which represents 14% of the outcomes studied here (Fig. 4). We attempted to control for this by using median values rather than means, and by including more studies, we were able to show a strong overall median FI; but there is certainly still room for improvement. For comparison, a recent review of RCTs in cardiology showed that the median FI of 123 manuscripts was 13 [16].
The FI has inherent limitations. A major limitation of this metric is its inability to evaluate nondichotomous outcome variables. Many outcomes in TJA research are reported with continuous metrics including radiographic angles and patient-reported outcomes, which the FI is unable to assess. As a result, a significant portion of studies had to be excluded (Seventy-eight percent of studies evaluated were excluded for not having dichotomous outcomes.). Because of this the FI, while useful in the appropriate setting, has a relatively limited application. Previously, the FI was even more limited, only applicable to significant outcomes. By adding the RFI, we were able to include nonstatistically significant outcomes and expand the FI’s usefulness, but it is still limited by design as a statistical tool. Further work needs to be carried out to expand its use or to determine complementary tools.
TJAs remain some of the most common procedures in the world today [[1], [2], [3], [4]]. As of 2010, 0.83% of the population and 1.52% of the population have undergone THA and TKA, respectively. This number is growing, with estimations that THAs will grow by 71% by 2030 to 635,000 procedures annually and that TKAs will grow by 85% to 1.26 million procedures [1]. Given this, research is extremely important to ensure safe and accessible TJAs as demand increases. With RCTs being one of the strongest forms of clinical research, analyzing the robustness of their outcomes is of upmost importance in determining which treatment is safe and efficacious for our patients.
Despite its limitations, we believe the FI and RFI provide value in assessing outcomes in clinical research and holding our field accountable for the research we perform. Given the evidence shown here, although mean and median FI/RFI values were greater than the AAOS benchmark of 2, there are still a wide number of studies with numbers below that, and we must continue to be diligent in how we design trials evaluating TJA.
Conclusions
This study is the largest evaluation of FI in orthopedics literature to date. We found a median FI/RFI of 4 for recently published TJA literature, which is comparable to or higher than FIs calculated in other orthopedic subspecialties. Although, overall, these numbers suggest strong evidence, there is still a large minority of studies with poor methodology. These data should be interpreted with caution, and we must continue to demand more sound research designs from our subspecialty.
Conflicts of interest
C. L. Herndon is a board member in AAOS.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2021.08.018.
Appendix
Appendix Table 1.
Analyzed total hip arthroplasty articles.
| Journal | Author | Year | Comparison | Patients enrolled | Lost to follow-up | Outcomes (no.) | FI∗ |
|---|---|---|---|---|---|---|---|
| ACTA | Gustafson et al. [1] | 2014 | Metal-on-metal hip resurfacing vs metal-on-polyethylene THA | 54 | 10 | 14 | 6 |
| Flatøy et al. [2] | 2016 | Electrochemically deposited vs conventional plasma-sprayed hydroxyapatite femoral stem | 55 | 30 | 2 | 9 | |
| BJJ | Vendittoli et al. [3] | 2013 | Hybrid hip resurfacing vs metal-on-metal uncemented THA | 219 | 55 | 6 | 5 |
| Lee et al. [4] | 2014 | 28-mm vs 32-mm Ceramic heads | 120 | 107 | 1 | 13 | |
| van der Veen et al. [5] | 2015 | Metal-on-metal vs metal-on-polyethylene THA | 104 | 6 | 1 | 9 | |
| Schilcher et al. [6] | 2017 | Bisphosphonate solution vs saline | 60 | 2 | 3 | 5 | |
| Ando et al. [7] | 2018 | Large vs conventional femoral head | 185 | 69 | 1 | 2 | |
| Sköldenberg et al. [8] | 2019 | Argon-gas gamma-sterilized vs vitamin E-doped, highly crosslinked polyethylene | 42 | 4 | 1 | 2 | |
| CORR | Della Valle et al. [9] | 2010 | Mini-incision vs two-incision THA | 72 | 0 | 3 | 8 |
| Goosen et al. [10] | 2011 | Minimally invasive vs classic posterolateral approach | 120 | 0 | 10 | 7 | |
| Corten et al. [11] | 2011 | Cemented vs cementless | 250 | 0 | 5 | 6 | |
| Weber et al. [12] | 2014 | Fluoroscopy vs imageless navigation | 125 | 9 | 4 | 7 | |
| Engh et al. [13] | 2016 | Ceramic-on-metal vs metal-on-metal | 72 | 9 | 2 | 5 | |
| Parratte et al. [14] | 2016 | Computer-assisted vs conventional | 60 | 0 | 1 | 10 | |
| Kim et al. [15] | 2016 | Ultrashort vs conventional anatomic cementless femoral stem | 212 | 12 | 3 | 16 | |
| Hopper et al. [16] | 2018 | Crosslinked vs conventional polyethylene | 230 | 0 | 4 | 4 | |
| Nakamura et al. [17] | 2018 | Robot-assisted vs hand-rasped stem | 130 | 15 | 1 | 4 | |
| Taunton et al. [18] | 2018 | Direct anterior vs mini posterior THA | 116 | 15 | 1 | 4 | |
| Mjaaland et al. [19] | 2019 | Direct anterior vs direct lateral THA | 164 | 11 | 2 | 9 | |
| Int. Orthop. | Bascarevic et al. [20] | 2010 | Alumina-on-alumina ceramic vs metal on highly cross-linked polyethylene | 150 | 0 | 23 | 6 |
| JOA | Amanatullah et al. [21] | 2011 | Ceramic-ceramic vs ceramic-polyethylene | 357 | 45 | 19 | 6 |
| Beaupre et al. [22] | 2013 | Ceramic-on-ceramic vs ceramic-on-crossfire polyethylene | 92 | 14 | 1 | 3 | |
| Barrett et al. [23] | 2013 | Direct anterior vs posterolateral THA | 87 | 0 | 20 | 7 | |
| Gurgel et al. [24] | 2014 | Computer-assisted vs conventional THA | 40 | 0 | 1 | 9 | |
| Lass et al. [25] | 2014 | Imageless navigation system vs conventional THA | 130 | 5 | 1 | 7 | |
| Hamilton et al. [26] | 2015 | 28-mm vs 36-mm Femoral heads | 345 | 113 | 1 | 3 | |
| Wegrzyn et al. [27] | 2015 | Tantalum vs titanium cup | 111 | 25 | 2 | 4 | |
| Gao et al. [28] | 2015 | Tranexamic acid with epinephrine vs tranexamic acid alone | 110 | 3 | 11 | 7 | |
| Suarez et al. [29] | 2015 | Bipolar sealer vs standard electrocautery | 118 | 0 | 1 | 1 | |
| Sculco et al. [30] | 2016 | Perioperative corticosteroids vs placebo | 40 | 13 | 7 | 7 | |
| North et al. [31] | 2016 | Topical vs intravenous tranexamic acid | 139 | 0 | 1 | 1 | |
| Cheng et al. [32] | 2017 | Direct anterior vs posterior approach THA | 75 | 2 | 15 | 5 | |
| Guild et al. [33] | 2017 | Hybrid plasma scalpel vs bipolar sealer | 232 | 0 | 1 | 29 | |
| Abdel et al. [34] | 2017 | Two-incision vs mini-posterior approach THA | 72 | 1 | 4 | 8 | |
| Gielis et al. [35] | 2019 | Short vs wedge-shaped straight stem | 150 | 10 | 8 | 7 | |
| Brun et al. [36] | 2019 | Direct lateral vs minimal invasive anterior approach THA | 164 | 0 | 8 | 5 | |
| JBJS | Barsoum et al. [37] | 2011 | Bipolar sealer vs standard electrocautery | 140 | 0 | 2 | 9 |
| Howie et al. [38] | 2012 | 28-mm vs 36-mm Femoral heads | 645 | 30 | 1 | 2 | |
| Devane et al. [39] | 2017 | Highly cross-linked vs ultra-high-molecular-weight polyethylene | 122 | 31 | 1 | 5 | |
| Kayupov et al. [40] | 2017 | Oral vs intravenous tranexamic acid | 89 | 6 | 1 | 10 |
Acta, Acta Orthopaedica; BJJ, Bone & Joint Journal; CORR, Clinical Orthopaedics and Related Research; Int. Orthop., International Orthopedics; JBJS, Journal of Bone and Joint Surgery; JOA, Journal of Arthroplasty.
Average for all outcomes rounded to the nearest digit.
Appendix Table 2.
Analyzed total knee arthroplasty articles.
| Journal | Author | Year | Comparison | Patients enrolled | Lost to follow-up | Outcomes (no.) | FI∗ |
|---|---|---|---|---|---|---|---|
| Acta | Meijerink et al. [41] | 2011 | CKS vs PFC TKA designs | 82 | 0 | 3 | 3 |
| Stilling et al. [42] | 2011 | High-porosity trabecular metal vs low-porosity titanium-pegged porous fiber-metal polyethylene backing tibial components | 50 | 4 | 1 | 6 | |
| Wilson et al. [43] | 2012 | Trabecular metal vs cemented tibial component | 70 | 25 | 1 | 11 | |
| Van Leeuwen et al. [44] | 2018 | Patient-specific positioning guides vs conventional method | 109 | 15 | 6 | 4 | |
| BJJ | Breeman et al. [45] | 2013 | Mobile vs fixed-bearing TKA | 539 | 7 | 14 | 8 |
| van Jonbergen et al. [46] | 2014 | Circumpatellar electrocautery vs no treatment | 300 | 98 | 1 | 1 | |
| Boonen et al. [47] | 2016 | Patient-matched positioning guides and conventional instruments | 180 | 17 | 1 | 2 | |
| Schotanus et al. [48] | 2016 | MRI vs CT patient-specific guides in TKA | 140 | 3 | 11 | 7 | |
| Powell et al. [49] | 2018 | Mobile vs fixed-bearing TKA | 167 | 82 | 2 | 3 | |
| Lachiewicz and O'Dell [50] | 2019 | Standard vs highly crosslinked polyethylene | 265 | 56 | 5 | 8 | |
| MacDessi et al. [51] | 2020 | Kinematic vs mechanical alignment | 128 | 0 | 21 | 9 | |
| CORR | Hernández-Vaquero et al. [52] | 2011 | Navigation vs jig-based TKA | 97 | 24 | 5 | 7 |
| Charoencholvanich et al. [53] | 2011 | Tranexamic acid vs placebo | 100 | 0 | 1 | 9 | |
| Laffosse et al. [54] | 2011 | Midline vs anterolateral skin incision | 64 | 2 | 3 | 5 | |
| Cip et al. [55] | 2013 | Autotransfusion vs control | 151 | 11 | 1 | 12 | |
| Roh et al. [56] | 2013 | Patient-specific instrumentation vs conventional method | 100 | 10 | 6 | 2 | |
| Fernandez-Fairen et al. [57] | 2013 | Porous tantalum cementless vs cemented tibial component | 145 | 13 | 3 | 6 | |
| Pongcharoen et al. [58] | 2013 | Medial parapatellar vs midvastus approach TKA | 59 | 0 | 13 | 8 | |
| Song et al. [59] | 2013 | Robot-assisted vs conventional TKA | 100 | 0 | 5 | 9 | |
| Pinsornsak et al. [60] | 2014 | Infrapatellar fat pad excision vs no excision | 90 | 13 | 3 | 2 | |
| Sah [61] | 2015 | Bidirectional barbed vs standard sutures | 50 | 0 | 3 | 7 | |
| Young et al. [62] | 2017 | Kinematic vs mechanical alignment | 114 | 0 | 3 | 8 | |
| Kim et al. [63] | 2018 | Navigation vs conventional TKA | 296 | 14 | 9 | 11 | |
| Int. Orthop. | Chen et al. [64] | 2014 | Whole vs half course tourniquet use | 64 | 0 | 1 | 8 |
| Ha et al. [65] | 2019 | Resurfacing vs nonresurfacing of the patella | 66 | 4 | 2 | 6 | |
| JOA | Hamilton et al. [66] | 2011 | High flex vs standard rotating platform TKA | 142 | 6 | 1 | 2 |
| Plymale et al. [67] | 2012 | Unipolar vs bipolar hemostasis in TKA | 113 | 0 | 1 | 9 | |
| Georgiadis et al. [68] | 2013 | Topical tranexamic acid vs placebo | 101 | 0 | 5 | 6 | |
| Kusuma et al. [69] | 2013 | Bovine thrombin vs no treatment | 80 | 0 | 1 | 4 | |
| Liow et al. [70] | 2014 | Robot-assisted vs conventional TKA | 60 | 0 | 3 | 4 | |
| Nam et al. [71] | 2014 | Extramedullary vs accelerometer navigational cutting guides | 100 | 6 | 4 | 5 | |
| Randelli et al. [72] | 2014 | Topical novel fibrin vs no treatment | 62 | 0 | 1 | 6 | |
| Patel et al. [73] | 2014 | Intravenous vs topical tranexamic acid | 100 | 0 | 1 | 7 | |
| Gao et al. [74] | 2015 | Tranexamic acid with epinephrine vs tranexamic acid alone in TKA | 103 | 0 | 7 | 7 | |
| Fricka et al. [75] | 2015 | Cemented vs cementless TKA | 100 | 3 | 3 | 5 | |
| Shi et al. [76] | 2016 | Fixed vs individualized valgus correction | 133 | 0 | 3 | 17 | |
| Ahn et al. [77] | 2016 | Reduction osteotomy vs pie-crusting for medial release | 106 | 0 | 1 | 4 | |
| Chan et al. [78] | 2017 | Bidirectional barbed vs traditional sutures in TKA | 117 | 0 | 6 | 5 | |
| Wang et al. [79] | 2017 | Tranexamic acid vs placebo | 200 | 0 | 4 | 4 | |
| Kim et al. [80] | 2017 | High flex vs standard TKA | 994 | 34 | 2 | 11 | |
| Teeter et al. [81] | 2017 | Measured resection vs gap balancing TKA | 23 | 0 | 1 | 3 | |
| Gharaibeh et al. [82] | 2017 | Navigation vs conventional TKA | 190 | 4 | 10 | 6 | |
| Tammachote et al. [83] | 2018 | Customized cutting block vs conventional TKA | 108 | 2 | 9 | 7 | |
| Cip et al. [84] | 2018 | Navigation vs conventional TKA | 200 | 141 | 11 | 5 | |
| Dong et al. [85] | 2018 | Patellar resurfacing and circumpatellar electrocautery vs circumpatellar electrocautery alone | 53 | 5 | 2 | 8 | |
| Thiengwittayaporn et al. [86] | 2019 | Patellar resurfacing vs nonresurfacing | 84 | 4 | 1 | 10 | |
| JBJS | Hui et al. [87] | 2011 | Oxidized zirconium vs cobalt-chromium femoral component | 40 | 6 | 1 | 9 |
| Huang et al. [88] | 2011 | Computer-assisted navigation vs conventional TKA | 113 | 0 | 4 | 2 | |
| Hinarejos et al. [89] | 2013 | Erythromycin and colistin cement vs standard cement | 3000 | 52 | 3 | 8 | |
| Schimmel et al. [90] | 2014 | Bicruciate substituting vs conventional posterior stabilizing implant | 124 | 0 | 1 | 4 | |
| Verburg et al. [91] | 2016 | Mini-midvastus vs conventional TKA | 100 | 0 | 3 | 5 | |
| Petursson et al. [92] | 2018 | Computer assisted vs conventional TKA | 190 | 23 | 11 | 4 | |
| Abdel et al. [93] | 2018 | Intravenous vs topical tranexamic acid | 664 | 24 | 2 | 13 | |
| Nam et al. [94] | 2019 | Cemented vs cementless TKA | 147 | 6 | 2 | 14 | |
| KSSTA | Demey et al. [95] | 2011 | Cemented vs uncemented femoral component | 130 | 9 | 5 | 6 |
| Pang et al. [96] | 2011 | Computer-assisted gap balancing vs conventional measures | 140 | 0 | 4 | 6 | |
| Jung et al. [97] | 2013 | Intramedullary vs extramedullary alignment | 91 | 0 | 3 | 6 | |
| Lee et al. [98] | 2013 | Tranexamic acid + indirect factor Xa inhibitor vs indirect factor Xa inhibitor alone | 72 | 0 | 4 | 6 | |
| Breugem et al. [99] | 2014 | Fixed vs mobile posterior stabilized design | 103 | 3 | 3 | 6 | |
| Izumi et al. [100] | 2015 | Transcutaneous electrical nerve stimulation vs control | 90 | 0 | 1 | 1 | |
| Chen et al. [101] | 2015 | Pin-less navigation vs conventional surgery | 100 | 0 | 3 | 1 | |
| Ollivier et al. [102] | 2016 | MRI-based vs computer-assisted TKA | 80 | 0 | 5 | 6 | |
| Collados-Maestre et al. [103] | 2017 | Single radius vs multiradius TKA | 240 | 3 | 3 | 2 |
Acta, Acta Orthopaedica; BJJ, Bone & Joint Journal; CORR, Clinical Orthopedics and Related Research; Int. Orthop., International Orthopedics; JOA, Journal of Arthroplasty; JBJS, Journal of Bone and Joint Surgery; CKS, continuum knee system; PFC, press fit condylar; MRI, magnetic resonance imaging; CT, computed tomography; KSSTA, knee surgery, sports traumatology, arthroscopy.
Average for all outcomes rounded to the nearest digit.
Appendix Table 3.
Analyzed total hip and total knee arthroplasty articles.
| Journal | Author | Year | Comparison | Patients enrolled | Lost to follow-up | Outcomes (no.) | FI∗ |
|---|---|---|---|---|---|---|---|
| BJJ | Sprowson et al. [104] | 2018 | Triclosan-coated vs standard sutures | 2546 | 109 | 20 | 9 |
BJJ, Bone & Joint Journal.
Average for all outcomes rounded to the nearest digit.
Supplementary data
References
- 1.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Jt Surg Am. 2018;100(17):1455. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 2.Kremers M.H., Larson D.R., Crowson C.S. Prevalence of total hip and knee replacement in the United States. J Bone Jt Surg Am. 2015;97(17):1386. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SJ A., Yu S., Chen L., Cleveland J.D. Rates of total joint replacement in the United States: future Projections to 2020-2040 using the National Inpatient sample. J Rheumatol. 2019;46(9):1134. doi: 10.3899/jrheum.170990. [DOI] [PubMed] [Google Scholar]
- 4.Inacio M.C.S., Paxton E.W., Graves S.E., Namba R.S., Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthr Cartil. 2017;25(11):1797. doi: 10.1016/j.joca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Checketts J.X., Scott J.T., Meyer C., Horn J., Jones J., Vassar M. The robustness of trials that Guide evidence-based Orthopaedic surgery. J Bone Jt Surg Am. 2018;100(12):e85. doi: 10.2106/JBJS.17.01039. [DOI] [PubMed] [Google Scholar]
- 6.Khormaee S., Choe J., Ruzbarsky J.J. The fragility of statistically significant results in Pediatric Orthopaedic randomized controlled trials as Quantified by the fragility index: a systematic review. J Pediatr Orthop. 2018;38(8):e418. doi: 10.1097/BPO.0000000000001201. [DOI] [PubMed] [Google Scholar]
- 7.Nathan E., Files C., Smith C. The fragility of statistically significant findings from randomized trials in spine surgery: a systematic survey. Spine J. 2015;15(10):2188. doi: 10.1016/j.spinee.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Khan M., Evaniew N., Gichuru M. The fragility of statistically significant findings from randomized trials in sports surgery: a systematic survey. Am J Sports Med. 2017;45(9):2164. doi: 10.1177/0363546516674469. [DOI] [PubMed] [Google Scholar]
- 9.Parisien R.L., Trofa D.P., Dashe J. Statistical fragility and the Role of P values in the sports Medicine literature. J Am Acad Orthop Surg. 2019;27(7):e324. doi: 10.5435/JAAOS-D-17-00636. [DOI] [PubMed] [Google Scholar]
- 10.RJ J., Khormaee S., Daluiski A. The fragility index in Hand surgery randomized controlled trials. J Hand Surg. 2019;44(8):698.e1. doi: 10.1016/j.jhsa.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Ekhtiari S., Gazendam A.M., Nucci N.W., Kruse C.C., Bhandari M. The fragility of statistically significant findings from randomized controlled trials in hip and knee arthroplasty. J Arthroplasty. 2021;36(6):2211. doi: 10.1016/j.arth.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Khan M.S., Fonarow G.C., Friede T. Application of the reverse fragility index to statistically Nonsignificant randomized clinical trial results. JAMA Netw Open. 2020;3(8):e2012469. doi: 10.1001/jamanetworkopen.2020.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick K.L., Tedesco L.J., Swindell H.W., Forrester L.A., Jobin C.M., Levine W.N. Statistical fragility of randomized clinical trials in shoulder arthroplasty. J Shoulder Elbow Surg. 2021;30(8):1787. doi: 10.1016/j.jse.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Walsh M., Srinathan S.K., McAuley D.F. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol. 2014;67(6):622. doi: 10.1016/j.jclinepi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Sprowson A.P., Jensen C., Parsons N. The effect of triclosan-coated sutures on the rate of surgical site infection after hip and knee arthroplasty: a double-blind randomized controlled trial of 2546 patients. Bone Joint J. 2018;100-b(3):296. doi: 10.1302/0301-620X.100B3.BJJ-2017-0247.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan M.S., Ochani R.K., Shaikh A. Fragility index in cardiovascular randomized controlled trials. Circ Cardiovasc Qual Outcomes. 2019;12(12):e005755. doi: 10.1161/CIRCOUTCOMES.119.005755. [DOI] [PMC free article] [PubMed] [Google Scholar]
References for Appendices
- 1.Gustafson K., Jakobsen S.S., Lorenzen N.D. Metal release and metal allergy after total hip replacement with resurfacing versus conventional hybrid prosthesis. Acta Orthop. 2014;85(4):348. doi: 10.3109/17453674.2014.922730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flatøy B., Röhrl S.M., Bøe B., Nordsletten L. No medium-term advantage of electrochemical deposition of hydroxyapatite in cementless femoral stems. 5-year RSA and DXA results from a randomized controlled trial. Acta Orthop. 2016;87(1):42. doi: 10.3109/17453674.2015.1084768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vendittoli P.A., Rivière C., Roy A.G., Barry J., Lusignan D., Lavigne M. Metal-on-metal hip resurfacing compared with 28-mm diameter metal-on-metal total hip replacement: a randomised study with six to nine years' follow-up. Bone Joint J. 2013;95-b(11):1464. doi: 10.1302/0301-620X.95B11.31604. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y.K., Ha Y.C., Koo K.H. Comparison between 28 mm and 32 mm ceramic-on-ceramic bearings in total hip replacement. Bone Joint J. 2014;96-b(11):1459. doi: 10.1302/0301-620X.96B11.34358. [DOI] [PubMed] [Google Scholar]
- 5.van der Veen H.C., Reininga I.H., Zijlstra W.P., Boomsma M.F., Bulstra S.K., van Raay J.J. Pseudotumour incidence, cobalt levels and clinical outcome after large head metal-on-metal and conventional metal-on-polyethylene total hip arthroplasty: mid-term results of a randomised controlled trial. Bone Joint J. 2015;97-b(11):1481. doi: 10.1302/0301-620X.97B11.34541. [DOI] [PubMed] [Google Scholar]
- 6.Schilcher J., Palm L., Ivarsson I., Aspenberg P. Local bisphosphonate reduces migration and formation of radiolucent lines adjacent to cemented acetabular components. Bone Joint J. 2017;99-b(3):317. doi: 10.1302/0301-620X.99B3.BJJ-2016-0531.R1. [DOI] [PubMed] [Google Scholar]
- 7.Ando W., Yasui H., Yamamoto K. A comparison of the effect of large and small metal-on-metal bearings in total hip arthroplasty on metal ion levels and the incidence of pseudotumour: a five-year follow-up of a previous report. Bone Joint J. 2018;100-b(8):1018. doi: 10.1302/0301-620X.100B8.BJJ-2018-0414.R1. [DOI] [PubMed] [Google Scholar]
- 8.Sköldenberg O.G., Rysinska A.D., Chammout G. A randomized double-blind noninferiority trial, evaluating migration of a cemented vitamin E-stabilized highly crosslinked component compared with a standard polyethylene component in reverse hybrid total hip arthroplasty. Bone Joint J. 2019;101-b(10):1192. doi: 10.1302/0301-620X.101B10.BJJ-2019-0456.R2. [DOI] [PubMed] [Google Scholar]
- 9.Della Valle C.J., Dittle E., Moric M., Sporer S.M., Buvanendran A. A prospective randomized trial of mini-incision posterior and two-incision total hip arthroplasty. Clin Orthop Relat Res. 2010;468(12):3348. doi: 10.1007/s11999-010-1491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goosen J.H., Kollen B.J., Castelein R.M., Kuipers B.M., Verheyen C.C. Minimally invasive versus classic procedures in total hip arthroplasty: a double-blind randomized controlled trial. Clin Orthop Relat Res. 2011;469(1):200. doi: 10.1007/s11999-010-1331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corten K., Bourne R.B., Charron K.D., Au K., Rorabeck C.H. What works best, a cemented or cementless primary total hip arthroplasty?: minimum 17-year followup of a randomized controlled trial. Clin Orthop Relat Res. 2011;469(1):209. doi: 10.1007/s11999-010-1459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber M., Woerner M., Springorum R. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150. doi: 10.1007/s11999-014-3740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engh C.A., Jr., Sritulanondha S., Korczak A. No difference in Reoperations at 2 Years between ceramic-on-metal and metal-on-metal THA: a randomized trial. Clin Orthop Relat Res. 2016;474(2):447. doi: 10.1007/s11999-015-4424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parratte S., Ollivier M., Lunebourg A., Flecher X., Argenson J.N. No Benefit after THA performed with computer-assisted Cup Placement: 10-year results of a randomized controlled study. Clin Orthop Relat Res. 2016;474(10):2085. doi: 10.1007/s11999-016-4863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y.H., Park J.W., Kim J.S. Ultrashort versus conventional Anatomic cementless femoral stems in the same patients Younger than 55 Years. Clin Orthop Relat Res. 2016;474(9):2008. doi: 10.1007/s11999-016-4902-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopper R.H., Jr., Ho H., Sritulanondha S., Williams A.C., Engh C.A., Jr. Otto Aufranc award: Crosslinking reduces THA wear, Osteolysis, and revision Rates at 15-year followup compared with Noncrosslinked polyethylene. Clin Orthop Relat Res. 2018;476(2):279. doi: 10.1007/s11999.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura N., Sugano N., Sakai T., Nakahara I. Does Robotic milling for Stem Implantation in cementless THA result in improved outcomes scores or Survivorship compared with Hand Rasping? Results of a randomized trial at 10 Years. Clin Orthop Relat Res. 2018;476(11):2169. doi: 10.1097/CORR.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taunton M.J., Trousdale R.T., Sierra R.J., Kaufman K., Pagnano M.W. John Charnley award: randomized clinical trial of direct anterior and Miniposterior approach THA: which provides better functional recovery? Clin Orthop Relat Res. 2018;476(2):216. doi: 10.1007/s11999.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mjaaland K.E., Kivle K., Svenningsen S., Nordsletten L. Do postoperative results differ in a randomized trial between a direct anterior and a direct lateral approach in THA? Clin Orthop Relat Res. 2019;477(1):145. doi: 10.1097/CORR.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bascarevic Z., Vukasinovic Z., Slavkovic N. Alumina-on-alumina ceramic versus metal-on-highly cross-linked polyethylene bearings in total hip arthroplasty: a comparative study. Int Orthop. 2010;34(8):1129. doi: 10.1007/s00264-009-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amanatullah D.F., Landa J., Strauss E.J., Garino J.P., Kim S.H., Di Cesare P.E. Comparison of surgical outcomes and implant wear between ceramic-ceramic and ceramic-polyethylene articulations in total hip arthroplasty. J Arthroplasty. 2011;26(6 Suppl):72. doi: 10.1016/j.arth.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Beaupre L.A., Manolescu A., Johnston D.W. A randomized trial of ceramic-on-ceramic bearing versus ceramic-on-crossfire-polyethylene bearing in total hip arthroplasty: five-year outcomes. J Arthroplasty. 2013;28(3):485. doi: 10.1016/j.arth.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Barrett W.P., Turner S.E., Leopold J.P. Prospective randomized study of direct anterior vs postero-lateral approach for total hip arthroplasty. J Arthroplasty. 2013;28(9):1634. doi: 10.1016/j.arth.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 24.Gurgel H.M., Croci A.T., Cabrita H.A., Vicente J.R., Leonhardt M.C., Rodrigues J.C. Acetabular component positioning in total hip arthroplasty with and without a computer-assisted system: a prospective, randomized and controlled study. J Arthroplasty. 2014;29(1):167. doi: 10.1016/j.arth.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Lass R., Kubista B., Olischar B., Frantal S., Windhager R., Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786. doi: 10.1016/j.arth.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton W.G., McAuley J.P., Blumenfeld T.J., Lesko J.P., Himden S.E., Dennis D.A. Midterm results of Delta ceramic-on-ceramic total hip arthroplasty. J Arthroplasty. 2015;30(9 Suppl):110. doi: 10.1016/j.arth.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Wegrzyn J., Kaufman K.R., Hanssen A.D., Lewallen D.G. Performance of porous Tantalum vs. Titanium Cup in total hip arthroplasty: randomized trial with minimum 10-year follow-up. J Arthroplasty. 2015;30(6):1008. doi: 10.1016/j.arth.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Gao F., Sun W., Guo W., Li Z., Wang W., Cheng L. Topical application of tranexamic acid Plus Diluted Epinephrine reduces postoperative Hidden blood loss in total hip arthroplasty. J Arthroplasty. 2015;30(12):2196. doi: 10.1016/j.arth.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Suarez J.C., Slotkin E.M., Szubski C.R., Barsoum W.K., Patel P.D. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953. doi: 10.1016/j.arth.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Sculco P.K., McLawhorn A.S., Desai N., Su E.P., Padgett D.E., Jules-Elysee K. The effect of Perioperative Corticosteroids in total hip arthroplasty: a prospective double-blind placebo controlled Pilot study. J Arthroplasty. 2016;31(6):1208. doi: 10.1016/j.arth.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 31.North W.T., Mehran N., Davis J.J., Silverton C.D., Weir R.M., Laker M.W. Topical vs intravenous tranexamic acid in primary total hip arthroplasty: a double-blind, randomized controlled trial. J Arthroplasty. 2016;31(5):1022. doi: 10.1016/j.arth.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Cheng T.E., Wallis J.A., Taylor N.F. A prospective randomized clinical trial in total hip arthroplasty-comparing early results between the direct anterior approach and the posterior approach. J Arthroplasty. 2017;32(3):883. doi: 10.1016/j.arth.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 33.Guild G.N., 3rd, Runner R.P., Castilleja G.M., Smith M.J., Vu C.L. Efficacy of hybrid Plasma Scalpel in Reducing blood loss and Transfusions in direct anterior total hip arthroplasty. J Arthroplasty. 2017;32(2):458. doi: 10.1016/j.arth.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 34.Abdel M.P., Chalmers B.P., Trousdale R.T., Hanssen A.D., Pagnano M.W. Randomized clinical trial of 2-incision vs mini-posterior total hip arthroplasty: differences persist at 10 Years. J Arthroplasty. 2017;32(9):2744. doi: 10.1016/j.arth.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Gielis W.P., van Oldenrijk J., Ten Cate N., Scholtes V.A.B., Geerdink C.H., Poolman R.W. Increased Persistent mid-Thigh pain after Short-Stem compared with Wedge-Shaped Straight-Stem uncemented total hip arthroplasty at medium-term follow-up: a randomized double-Blinded cross-Sectional study. J Arthroplasty. 2019;34(5):912. doi: 10.1016/j.arth.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Brun O.L., Sund H.N., Nordsletten L., Röhrl S.M., Mjaaland K.E. Component Placement in direct lateral vs minimally invasive anterior approach in total hip arthroplasty: radiographic outcomes from a prospective randomized controlled trial. J Arthroplasty. 2019;34(8):1718. doi: 10.1016/j.arth.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Barsoum W.K., Klika A.K., Murray T.G., Higuera C., Lee H.H., Krebs V.E. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513. doi: 10.2106/JBJS.J.00036. [DOI] [PubMed] [Google Scholar]
- 38.Howie D.W., Holubowycz O.T., Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095. doi: 10.2106/JBJS.K.00570. [DOI] [PubMed] [Google Scholar]
- 39.Devane P.A., Horne J.G., Ashmore A., Mutimer J., Kim W., Stanley J. Highly cross-linked polyethylene reduces wear and revision Rates in total hip arthroplasty: a 10-year double-Blinded randomized controlled trial. J Bone Joint Surg Am. 2017;99(20):1703. doi: 10.2106/JBJS.16.00878. [DOI] [PubMed] [Google Scholar]
- 40.Kayupov E., Fillingham Y.A., Okroj K. Oral and intravenous tranexamic acid are equivalent at Reducing blood loss following total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2017;99(5):373. doi: 10.2106/JBJS.16.00188. [DOI] [PubMed] [Google Scholar]
- 41.Meijerink H.J., Verdonschot N., van Loon C.J., Hannink G., de Waalmalefijt M.C. Similar TKA designs with differences in clinical outcome: a randomized, controlled trial of 77 knees with a mean follow-up of 6 years. Acta Orthop. 2011;82(6):685. doi: 10.3109/17453674.2011.636677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stilling M., Madsen F., Odgaard A. Superior fixation of pegged trabecular metal over screw-fixed pegged porous titanium fiber mesh: a randomized clinical RSA study on cementless tibial components. Acta Orthop. 2011;82(2):177. doi: 10.3109/17453674.2011.566139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson D.A., Richardson G., Hennigar A.W., Dunbar M.J. Continued stabilization of trabecular metal tibial monoblock total knee arthroplasty components at 5 years-measured with radiostereometric analysis. Acta Orthop. 2012;83(1):36. doi: 10.3109/17453674.2011.645196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Leeuwen J., Snorrason F., Röhrl S.M. No radiological and clinical advantages with patient-specific positioning guides in total knee replacement. Acta Orthop. 2018;89(1):89. doi: 10.1080/17453674.2017.1393732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breeman S., Campbell M.K., Dakin H. Five-year results of a randomised controlled trial comparing mobile and fixed bearings in total knee replacement. Bone Joint J. 2013;95-b(4):486. doi: 10.1302/0301-620X.95B4.29454. [DOI] [PubMed] [Google Scholar]
- 46.van Jonbergen H.P., Scholtes V.A., Poolman R.W. A randomised, controlled trial of circumpatellar electrocautery in total knee replacement without patellar resurfacing: a concise follow-up at a mean of 3.7 years. Bone Joint J. 2014;96-b(4):473. doi: 10.1302/0301-620X.96B4.32118. [DOI] [PubMed] [Google Scholar]
- 47.Boonen B., Schotanus M.G., Kerens B., van der Weegen W., Hoekstra H.J., Kort N.P. No difference in clinical outcome between patient-matched positioning guides and conventional instrumented total knee arthroplasty two years post-operatively: a multicentre, double-blind, randomised controlled trial. Bone Joint J. 2016;98-b(7):939. doi: 10.1302/0301-620X.98B7.37274. [DOI] [PubMed] [Google Scholar]
- 48.Schotanus M.G., Sollie R., van Haaren E.H., Hendrickx R.P., Jansen E.J., Kort N.P. A radiological analysis of the difference between MRI- and CT-based patient-specific matched guides for total knee arthroplasty from the same manufacturer: a randomised controlled trial. Bone Joint J. 2016;98-b(6):786. doi: 10.1302/0301-620X.98B6.36633. [DOI] [PubMed] [Google Scholar]
- 49.Powell A.J., Crua E., Chong B.C. A randomized prospective study comparing mobile-bearing against fixed-bearing PFC Sigma cruciate-retaining total knee arthroplasties with ten-year minimum follow-up. Bone Joint J. 2018;100-b(10):1336. doi: 10.1302/0301-620X.100B10.BJJ-2017-1450.R1. [DOI] [PubMed] [Google Scholar]
- 50.Lachiewicz P.F., O'Dell J.A. Prospective randomized trial of standard versus highly crosslinked tibial polyethylene in primary posterior-stabilized total knee arthroplasty: clinical and radiological follow-up at 2 to 11 years. Bone Joint J. 2019;101-b(7_Supple_C):33. doi: 10.1302/0301-620X.101B7.BJJ-2018-1126.R2. [DOI] [PubMed] [Google Scholar]
- 51.MacDessi S.J., Griffiths-Jones W., Chen D.B. Restoring the constitutional alignment with a restrictive kinematic protocol improves quantitative soft-tissue balance in total knee arthroplasty: a randomized controlled trial. Bone Joint J. 2020;102-b(1):117. doi: 10.1302/0301-620X.102B1.BJJ-2019-0674.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernández-Vaquero D., Suarez-Vazquez A., Iglesias-Fernandez S. Can computer assistance improve the clinical and functional scores in total knee arthroplasty? Clin Orthop Relat Res. 2011;469(12):3436. doi: 10.1007/s11999-011-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charoencholvanich K., Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res. 2011;469(10):2874. doi: 10.1007/s11999-011-1874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laffosse J.M., Potapov A., Malo M., Lavigne M., Vendittoli P.A. Hypesthesia after anterolateral versus midline skin incision in TKA: a randomized study. Clin Orthop Relat Res. 2011;469(11):3154. doi: 10.1007/s11999-011-1973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cip J., Widemschek M., Benesch T., Waibel R., Martin A. Does single use of an autologous transfusion system in TKA reduce the need for allogenic blood?: a prospective randomized trial. Clin Orthop Relat Res. 2013;471(4):1319. doi: 10.1007/s11999-012-2729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roh Y.W., Kim T.W., Lee S., Seong S.C., Lee M.C. Is TKA using patient-specific instruments comparable to conventional TKA? A randomized controlled study of one system. Clin Orthop Relat Res. 2013;471(12):3988. doi: 10.1007/s11999-013-3206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez-Fairen M., Hernández-Vaquero D., Murcia A., Torres A., Llopis R. Trabecular metal in total knee arthroplasty associated with higher knee scores: a randomized controlled trial. Clin Orthop Relat Res. 2013;471(11):3543. doi: 10.1007/s11999-013-3183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pongcharoen B., Yakampor T., Charoencholvanish K. Patellar tracking and anterior knee pain are similar after medial parapatellar and midvastus approaches in minimally invasive TKA. Clin Orthop Relat Res. 2013;471(5):1654. doi: 10.1007/s11999-012-2778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song E.K., Seon J.K., Yim J.H., Netravali N.A., Bargar W.L. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118. doi: 10.1007/s11999-012-2407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinsornsak P., Naratrikun K., Chumchuen S. The effect of infrapatellar fat pad excision on complications after minimally invasive TKA: a randomized controlled trial. Clin Orthop Relat Res. 2014;472(2):695. doi: 10.1007/s11999-013-3321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sah A.P. Is there an advantage to Knotless Barbed suture in TKA Wound Closure? A randomized trial in Simultaneous bilateral TKAs. Clin Orthop Relat Res. 2019;473(6):2015. doi: 10.1007/s11999-015-4157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young S.W., Walker M.L., Bayan A., Briant-Evans T., Pavlou P., Farrington B. The Chitranjan S. Ranawat award : No difference in 2-year functional outcomes using kinematic versus mechanical alignment in TKA: a randomized controlled clinical trial. Clin Orthop Relat Res. 2017;475(1):9. doi: 10.1007/s11999-016-4844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y.H., Park J.W., Kim J.S. 2017 Chitranjan S. Ranawat award: does computer navigation in knee arthroplasty improve functional outcomes in Young patients? A randomized study. Clin Orthop Relat Res. 2018;476(1):6. doi: 10.1007/s11999.0000000000000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen S., Li J., Peng H., Zhou J., Fang H., Zheng H. The influence of a half-course tourniquet strategy on peri-operative blood loss and early functional recovery in primary total knee arthroplasty. Int Orthop. 2014;38(2):355. doi: 10.1007/s00264-013-2177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ha C., Wang B., Li W., Sun K., Wang D., Li Q. Resurfacing versus not-resurfacing the patella in one-stage bilateral total knee arthroplasty: a prospective randomized clinical trial. Int Orthop. 2019;43(11):2519. doi: 10.1007/s00264-019-04361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamilton W.G., Sritulanondha S., Engh C.A., Jr. Prospective randomized comparison of high-flex and standard rotating platform total knee arthroplasty. J Arthroplasty. 2011;26(6 Suppl):28. doi: 10.1016/j.arth.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 67.Plymale M.F., Capogna B.M., Lovy A.J., Adler M.L., Hirsh D.M., Kim S.J. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133. doi: 10.1016/j.arth.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 68.Georgiadis A.G., Muh S.J., Silverton C.D., Weir R.M., Laker M.W. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):78. doi: 10.1016/j.arth.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 69.Kusuma S.K., Sheridan K.C., Wasielewski R.C. Use of bovine thrombin to reduce blood loss in primary total knee arthroplasty: a controlled randomized trial. J Arthroplasty. 2013;28(8):1278. doi: 10.1016/j.arth.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 70.Liow M.H., Xia Z., Wong M.K., Tay K.J., Yeo S.J., Chin P.L. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373. doi: 10.1016/j.arth.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Nam D., Cody E.A., Nguyen J.T., Figgie M.P., Mayman D.J. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP Paul award. J Arthroplasty. 2014;29(2):288. doi: 10.1016/j.arth.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Randelli F., D'Anchise R., Ragone V., Serrao L., Cabitza P., Randelli P. Is the newest fibrin sealant an effective strategy to reduce blood loss after total knee arthroplasty? A randomized controlled study. J Arthroplasty. 2014;29(8):1516. doi: 10.1016/j.arth.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 73.Patel J.N., Spanyer J.M., Smith L.S., Huang J., Yakkanti M.R., Malkani A.L. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528. doi: 10.1016/j.arth.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Gao F., Sun W., Guo W., Li Z., Wang W., Cheng L. Topical administration of tranexamic acid Plus Diluted-Epinephrine in primary total knee arthroplasty: a randomized double-Blinded controlled trial. J Arthroplasty. 2015;30(8):1354. doi: 10.1016/j.arth.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Fricka K.B., Sritulanondha S., McAsey C.J. To cement or not? Two-Year results of a prospective, randomized study comparing cemented Vs. Cementless total knee arthroplasty (TKA) J Arthroplasty. 2015;30(9 Suppl):55. doi: 10.1016/j.arth.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 76.Shi X., Li H., Zhou Z., Shen B., Yang J., Pei F. Comparison of postoperative alignment using fixed vs Individual Valgus Correction angle in primary total knee arthroplasty with lateral Bowing Femur. J Arthroplasty. 2016;31(5):976. doi: 10.1016/j.arth.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 77.Ahn J.H., Yang T.Y., Lee J.Y. Reduction Osteotomy vs Pie-Crust technique as possible alternatives for medial release in total knee arthroplasty and compared in a prospective randomized controlled trial. J Arthroplasty. 2016;31(7):1470. doi: 10.1016/j.arth.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 78.Chan V.W.K., Chan P.K., Chiu K.Y., Yan C.H., Ng F.Y. Does Barbed suture lower Cost and improve outcome in total knee arthroplasty? A randomized controlled trial. J Arthroplasty. 2017;32(5):1474. doi: 10.1016/j.arth.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 79.Wang J.W., Chen B., Lin P.C., Yen S.H., Huang C.C., Kuo F.C. The efficacy of Combined Use of Rivaroxaban and tranexamic acid on blood Conservation in minimally invasive total knee arthroplasty a double-blind randomized, controlled trial. J Arthroplasty. 2017;32(3):801. doi: 10.1016/j.arth.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 80.Kim Y.H., Park J.W., Kim J.S. Do high-flexion total knee designs increase the Risk of femoral component Loosening? J Arthroplasty. 2017;32(6):1862. doi: 10.1016/j.arth.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 81.Teeter M.G., Perry K.I., Yuan X., Howard J.L., Lanting B.A. Contact kinematic differences between gap Balanced vs measured resection techniques for single radius posterior-stabilized total knee arthroplasty. J Arthroplasty. 2017;32(6):1834. doi: 10.1016/j.arth.2016.12.054. [DOI] [PubMed] [Google Scholar]
- 82.Gharaibeh M.A., Solayar G.N., Harris I.A., Chen D.B., MacDessi S.J. Accelerometer-Based, portable navigation (KneeAlign) vs conventional instrumentation for total knee arthroplasty: a prospective randomized comparative trial. J Arthroplasty. 2017;32(3):777. doi: 10.1016/j.arth.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 83.Tammachote N., Panichkul P., Kanitnate S. Comparison of Customized Cutting Block and conventional Cutting Instrument in total knee arthroplasty: a randomized controlled trial. J Arthroplasty. 2018;33(3):746. doi: 10.1016/j.arth.2017.09.055. [DOI] [PubMed] [Google Scholar]
- 84.Cip J., Obwegeser F., Benesch T., Bach C., Ruckenstuhl P., Martin A. Twelve-Year follow-up of Navigated computer-assisted versus conventional total knee arthroplasty: a prospective randomized comparative trial. J Arthroplasty. 2018;33(5):1404. doi: 10.1016/j.arth.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 85.Dong Y., Li T., Zheng Z., Xiang S., Weng X. Adding patella resurfacing after circumpatellar electrocautery did not improve the clinical outcome in bilateral total knee arthroplasty in Chinese population: a prospective randomized study. J Arthroplasty. 2018;33(4):1057. doi: 10.1016/j.arth.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 86.Thiengwittayaporn S., Srungboonmee K., Chiamtrakool B. Resurfacing in a posterior-stabilized total knee arthroplasty reduces patellar Crepitus complication: a randomized, controlled trial. J Arthroplasty. 1969;34(9):2019. doi: 10.1016/j.arth.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 87.Hui C., Salmon L., Maeno S., Roe J., Walsh W., Pinczewski L. Five-year comparison of oxidized zirconium and cobalt-chromium femoral components in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93(7):624. doi: 10.2106/JBJS.I.01753. [DOI] [PubMed] [Google Scholar]
- 88.Huang T.W., Hsu W.H., Peng K.T., Hsu R.W., Weng Y.J., Shen W.J. Total knee arthroplasty with use of computer-assisted navigation compared with conventional guiding systems in the same patient: radiographic results in Asian patients. J Bone Joint Surg Am. 2011;93(13):1197. doi: 10.2106/JBJS.J.00325. [DOI] [PubMed] [Google Scholar]
- 89.Hinarejos P., Guirro P., Leal J. The use of erythromycin and colistin-loaded cement in total knee arthroplasty does not reduce the incidence of infection: a prospective randomized study in 3000 knees. J Bone Joint Surg Am. 2013;95(9):769. doi: 10.2106/JBJS.L.00901. [DOI] [PubMed] [Google Scholar]
- 90.Schimmel J.J., Defoort K.C., Heesterbeek P.J., Wymenga A.B., Jacobs W.C., van Hellemondt G.G. Bicruciate substituting design does not improve maximal flexion in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(10):e81. doi: 10.2106/JBJS.M.00277. [DOI] [PubMed] [Google Scholar]
- 91.Verburg H., Mathijssen N.M., Niesten D.D., Verhaar J.A., Pilot P. Comparison of mini-midvastus and conventional total knee arthroplasty with clinical and radiographic evaluation: a prospective randomized clinical trial with 5-year follow-up. J Bone Joint Surg Am. 2016;98(12):1014. doi: 10.2106/JBJS.15.00654. [DOI] [PubMed] [Google Scholar]
- 92.Petursson G., Fenstad A.M., Gøthesen Ø. Computer-Assisted compared with conventional total knee replacement: a Multicenter parallel-group randomized controlled trial. J Bone Joint Surg Am. 2018;100(15):1265. doi: 10.2106/JBJS.17.01338. [DOI] [PubMed] [Google Scholar]
- 93.Abdel M.P., Chalmers B.P., Taunton M.J. Intravenous versus topical tranexamic acid in total knee arthroplasty: both effective in a randomized clinical trial of 640 patients. J Bone Joint Surg Am. 2018;100(12):1023. doi: 10.2106/JBJS.17.00908. [DOI] [PubMed] [Google Scholar]
- 94.Nam D., Lawrie C.M., Salih R., Nahhas C.R., Barrack R.L., Nunley R.M. Cemented versus cementless total knee arthroplasty of the same Modern design: a prospective, randomized trial. J Bone Joint Surg Am. 2019;101(13):1185. doi: 10.2106/JBJS.18.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Demey G., Servien E., Lustig S., Aït Si Selmi T., Neyret P. Cemented versus uncemented femoral components in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1053. doi: 10.1007/s00167-010-1347-2. [DOI] [PubMed] [Google Scholar]
- 96.Pang H.N., Yeo S.J., Chong H.C., Chin P.L., Ong J., Lo N.N. Computer-assisted gap balancing technique improves outcome in total knee arthroplasty, compared with conventional measured resection technique. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1496. doi: 10.1007/s00167-011-1483-3. [DOI] [PubMed] [Google Scholar]
- 97.Jung W.H., Chun C.W., Lee J.H., Ha J.H., Jeong J.H. The accuracy of the extramedullary and intramedullary femoral alignment system in total knee arthroplasty for varus osteoarthritic knee. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):629. doi: 10.1007/s00167-012-1994-6. [DOI] [PubMed] [Google Scholar]
- 98.Lee S.H., Cho K.Y., Khurana S., Kim K.I. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2611. doi: 10.1007/s00167-012-2213-1. [DOI] [PubMed] [Google Scholar]
- 99.Breugem S.J., van Ooij B., Haverkamp D., Sierevelt I.N., van Dijk C.N. No difference in anterior knee pain between a fixed and a mobile posterior stabilized total knee arthroplasty after 7.9 years. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):509. doi: 10.1007/s00167-012-2281-2. [DOI] [PubMed] [Google Scholar]
- 100.Izumi M., Ikeuchi M., Aso K. Less deep vein thrombosis due to transcutaneous fibular nerve stimulation in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3317. doi: 10.1007/s00167-014-3141-z. [DOI] [PubMed] [Google Scholar]
- 101.Chen J.Y., Chin P.L., Li Z. Radiological outcomes of pinless navigation in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3556. doi: 10.1007/s00167-014-3226-8. [DOI] [PubMed] [Google Scholar]
- 102.Ollivier M., Tribot-Laspiere Q., Amzallag J., Boisrenoult P., Pujol N., Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using "MRI-based patient-specific" compared to "computer assisted" instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2016;24(11):3441. doi: 10.1007/s00167-015-3645-1. [DOI] [PubMed] [Google Scholar]
- 103.Collados-Maestre I., Lizaur-Utrilla A., Gonzalez-Navarro B. Better functional outcome after single-radius TKA compared with multi-radius TKA. Knee Surg Sports Traumatol Arthrosc. 2017;25(11):3508. doi: 10.1007/s00167-016-4273-0. [DOI] [PubMed] [Google Scholar]
- 104.Sprowson A.P., Jensen C., Parsons N. The effect of triclosan-coated sutures on the rate of surgical site infection after hip and knee arthroplasty: a double-blind randomized controlled trial of 2546 patients. Bone Joint J. 2018;100-b(3):296. doi: 10.1302/0301-620X.100B3.BJJ-2017-0247.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.