Background:
Within plastic surgery, hematomas and seromas are frequently reported complications that can negatively impact wound healing and result in significant morbidity in patients. As a result, there has been considerable interest in hemostatic agents to complement traditional methods of hemostasis. The purpose of this study was to evaluate postoperative bleeding complications and duration of Jackson-Pratt (JP) drain use in general plastic surgery procedures with and without hemostatic agents.
Methods:
After obtaining institutional review board approval, a retrospective chart review was performed. Patients who underwent bilateral breast reduction, panniculectomy, or abdominoplasty were included. Data collected included indication for surgery, type of operation, use of hemostatic agent, specifically fibrin sealant (FS, EVICEL, Ethicon, USA) or combination powder (CP, HEMOBLAST Bellows, Biom’up, France), length of follow-up, time to JP drain removal, postoperative complications, and specimen weight. This was a consecutive experience where initially no hemostatic agent was used, followed by use of FS, and then CP.
Results:
The use of a hemostatic agent resulted in reduced time duration for JP drain use and overall fewer recorded complications in the hemostatic agent groups. Although not significant, the hemostatic agent group (FS and CP) experienced fewer hematomas and seromas compared with the nonhemostatic agent group. JP drain duration was significantly less among breast reduction (3.46 versus 6.92 days, P < 0.01) for CP when compared with FS.
Conclusion:
The use of hemostatic agents in general plastic surgery procedures may result in decreased postoperative complications and significantly reduce time to JP drain removal.
INTRODUCTION
Common postoperative complications after plastic surgery procedures include hematomas and seromas. These complications can negatively impact wound healing and result in significant morbidity in patients. This phenomenon is predominantly due to undermining of the soft tissue envelope, creating a new potential space with raw surface area.1,2 Adequate intraoperative hemostasis is one of the tenets of meticulous surgical practice; however, postoperative hematoma and seroma formation can often be inevitable regardless of surgical technique. For example, in breast reduction operations, seromas and hematomas occur at a rate of 1.2% and 3.7%, respectively,3 whereas in abdominoplasty/panniculectomy operations, seromas and hematomas occur at a rate of 10%4 and 1.1%,5 respectively. Multiple devices and products (such as FSs or glues, gelatin-based seals, adhesives, human fibrinogen, PEG hydrogel, adhesive dressings, and negative pressure wound vacuum systems) have been developed to enhance surgical hemostasis and to reduce the risk of hematoma and seroma formation through the perioperative period.6–9
A common method for early detection of hematoma formation and preventing seroma formation is via placement of closed-suction drains, such as Jackson Pratt (JP) or Blake drains, intraoperatively.10 The duration of time until drain removal depends upon the drain’s output and can range from a few days to weeks.11,12 The volume below which the suction drains are removed is typically 30 mL per day, although it can vary by surgeon’s preference. Earlier removal of drains can be thought to correlate with a lower volume of serosanguinous drainage. Using this concept, the time-to-removal of drains can be used as a surrogate to determine the efficacy of the hemostatic technique or product.
Given that the role of such hemostatic agents in these plastic surgery operations has been limited, the aim of this study was to (1) evaluate the frequency of postoperative bleeding complications, and (2) the duration of JP drain use in general plastic surgery procedures with and without the use of hemostatic agent.
METHODS
A retrospective chart review was performed from a single surgeon’s case database from January 2015 to September 2020. Patients were included who underwent bilateral breast reduction, panniculectomy, or abdominoplasty. Those on anticoagulation and not undergoing these procedures were excluded from the analysis.
Surgical Technique
We used the Caprini score to assess whether there was a need for deep vein thrombosis prophylactic anticoagulation. Breast reductions were performed using a Wise pattern incision and based upon either an inferior or superomedial pedicle depending on the degree of ptosis. Abdominoplasties and panniculectomies were done in the standard fashion without the use of progressive tension sutures to help eliminate deadspace.
Of note, before operative closure of any incision site, lidocaine with epinephrine was injected for postoperative analgesia, and the surgeon made sure that the patient was not hypotensive so as to minimize the chances of missing vessels that would potentially bleed when the patient became normotensive postoperatively. No tumescent fluid was used.
Data were collected including the indication for surgery, type of operation, use of hemostatic agent, specifically FS (EVICEL, Ethicon, USA) or combination powder (CP, HEEMOBLAST Bellows, Biom’up, France), length of follow-up, duration of time to JP drain removal, postoperative complications [seroma, hematoma, or operating room (OR) takeback], and specimen weight. Specimen weight was used to standardize the amount of deadspace among patients with differing BMI.
This was a consecutive experience where initially no hemostatic agent was used, followed by use of FS (beginning June 2017), and then CP (beginning August 2019). These changes were motivated by an observed increase in bleeding complications.
JP drains were removed in the clinic when drain output was less than 30 cm3 per day for 2 consecutive days. JP drain output was compared between groups using Welch t-test. Postoperative complications were compared using Fisher exact test. Statistical significance was defined as a P value less than 0.05.
Institutional review board approval was obtained. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki.
RESULTS
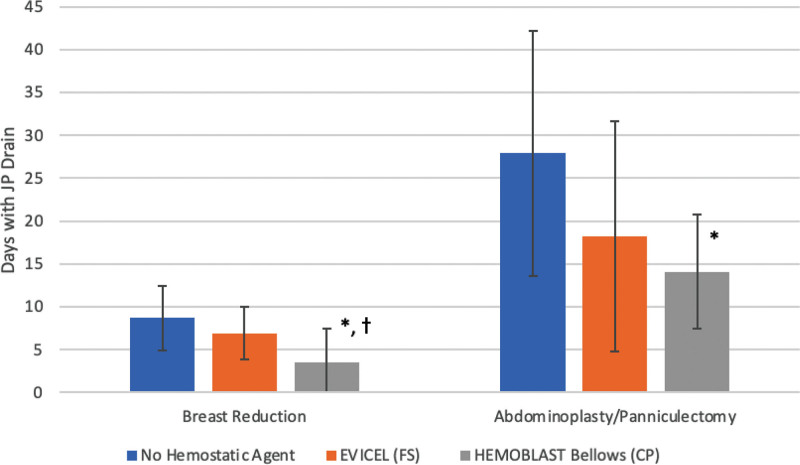
The use of a hemostatic agent resulted in reduced time duration for JP drain use and overall fewer recorded bleeding complications when compared with the nonhemostatic agent group (Table 1). For the nonhemostatic agent group, there was a 6.6% incidence of seroma, 1.6% incidence of hematoma, and 1.6% incidence of cases for return to OR for breast reductions (n = 61). The CP group experienced no hematomas, seromas, or return to OR for breast reduction (n = 26) procedures in contrast to the FS group (n = 66) with a 4.5% seroma and 1.5% hematoma rate. These were without statistical significance. JP drain duration was statistically significantly decreased among breast reduction (3.46 versus 6.92 days, P < 0.01) for CP when compared with FS (Fig. 1).
Table 1.
Rates of Postoperative Complications, Time to JP Drain Removal, and Specimen Weight for General Plastic Surgery Procedures Using No Hemostatic Agent versus EVICEL (FS) and HEMOBLAST Bellows (CP)
| No Hemostatic Agent | HEMOBLAST Bellows (CP) | |||
|---|---|---|---|---|
| Breast Reduction | Abdominoplasty/Panniculectomy | Breast Reduction | Abdominoplasty/Panniculectomy | |
| N | 61 | 61 | 26 | 14 |
| Avg F/u (wk) | 11.3 | 13.17 | 6.27 | 7.21 |
| Avg time with JP (d) | 8.67 | 27.89 | 3.46 | 14.1 |
| SD | 3.75 | 14.27 | 3.96 | 6.63 |
| P < 0.01 | P < 0.01 | |||
| Average specimen weight (kg) | 1.54 | 2.58 | 1.25 | 2.02 |
| SD | 0.96 | 1.68 | 0.54 | 0.98 |
| P = 0.08 | P = 0.16 | |||
| Seroma (%) | 4 (6.6) | 4 (6.6) | 0 (0) | 0 (0) |
| P = 0.31 | P = 0.67 | |||
| Hematoma (%) | 1 (1.6) | 5 (8.2) | 0 (0) | 0 (0) |
| P = 1 | P = 1 | |||
| OR takeback (%) | 1 (1.6) | 3 (4.9) | 0 (0) | 0 (0) |
| P = 1 | P = 0.23 | |||
| EVICEL (FS) | HEMOBLAST Bellows (CP) | |||
| Breast Reduction | Abdominoplasty/Panniculectomy | Breast Reduction | Abdominoplasty/Panniculectomy | |
| N | 66 | 4 | 26 | 14 |
| Avg F/u (wk) | 8.95 | 12.75 | 6.27 | 7.21 |
| Avg time with JP (d) | 6.92 | 18.25 | 3.46 | 14.1 |
| SD | 3.05 | 13.45 | 3.96 | 6.63 |
| P < 0.01 | P = 0.25 | |||
| Average specimen weight (kg) | 1.58 | 3.28 | 1.25 | 2.02 |
| SD | 0.84 | 2.23 | 0.54 | 0.98 |
| P = 0.028 | P = 0.43 | |||
| Seroma (%) | 3 (4.5) | 1 (25) | 0 (0) | 0 (0) |
| P = 0.56 | P = 0.22 | |||
| Hematoma (%) | 1 (1.5) | 1 (25) | 0 (0) | 0 (0) |
| P = 1 | P = 0.22 | |||
| OR takeback (%) | 0 (0) | 1 (25) | 0 (0) | 0 (0) |
| P = 1 | P = 0.22 | |||
Fig. 1.
Duration of time to JP drain removal. *Statistically significant when compared with no hemostatic agent. †Statistically significant when compared with EVICEL (FS).
For abdominoplasty and panniculectomy procedures in the nonhemostatic agent group, there was a 6.6% incidence of seroma, 8.2% incidence of hematoma, and 4.9% incidence of cases for return to OR (n = 61). The CP group experienced no hematomas, seromas, or return to OR for abdominoplasty/panniculectomy (n = 14) when compared with the FS group (n = 4) with one instance each of hematoma, seroma, and return to OR. Again, these were without statistical significance. In general, the vast majority of patients had a low Caprini score and did not receive deep vein thrombosis chemoprophylaxis.
DISCUSSION
Similar to most surgical disciplines, plastic surgery procedures are not without associated complications. Seromas and hematomas are a relatively common complication due to increased dead space created from the mobilization of the overlying soft tissues. Therefore, the use of hemostatic agents can greatly reduce these complications. Studies looking at hemostatic agents in these types of common plastic surgeries are rare, and our results are among the first to examine these agents within general plastic surgery and to evaluate their efficacy. The importance of decreasing complications cannot be understated as it not only aligns with the goals of the surgeon in doing no harm, but it also improves a patient’s experience and satisfaction13 and decreases financial cost burden to both patients and society.14,15
This study evaluated two distinct categories of hemostatic agents. The first of these is EVICEL, a fibrin-sealant-based product, which consists of BAC2 (human fibrinogen) and thrombin, and is FDA approved for surgical hemostasis.16 The other product is HEMOBLAST Bellows, which has recently gained traction as a newer intraoperative hemostatic agent. It contains human-derived thrombin that works within the coagulation cascade and activates the conversion of fibrinogen to fibrin. Additionally, the porcine-derived collagen and the chondroitin sulfate in its formulation provide cohesion to the wound, which has been shown to further enhance its hemostatic effect with a similar safety profile.16 Previous studies have shown that the combined powder used in the HEMOBLAST Bellows has a superior role in immediate hemostasis in cardiothoracic, abdominal, and orthopedic surgery compared with the traditional hemostatic matrix.17–19 Therefore, based upon the multiple mechanisms of HEMOBLAST Bellows, it may be preferable to EVICEL for use in surgical hemostasis.
Utilizing results from hundreds of patients, the use of hemostatic agents (CP and FS) had a statistically significant reduction in time to JP drain removal and overall fewer bleeding complications, including seroma, hematoma, and OR takeback. This effect on time before JP drain removal was more pronounced in the CP group. It should be noted that the size of tissue excised was similar between groups, increasing the robustness of the results. Therefore, the use of the agents may potentially improve cosmetic outcomes and patient experience due to a reduction in complications and overall time with JP drains.
Earlier removal of drains decreases patient discomfort and theoretically prevents the chances of drain infections. It is interesting to note that even with statistically decreased drain times, the hemostatic agent groups showed a decrease in overall seromas supporting a mechanism for decreased postoperative seroma complications other than the presence of a drain. This is thought to be secondary to the hemostatic agent collapsing and decreasing deadspace.20
It should be noted that the American Society of Plastic Surgeons guidelines recommend against the routine use of drains in standard reduction mammaplasty (Level 1 evidence). In our practice, drain use has begun to be phased out in most breast reductions. This is largely due to the routine use of hemostatic agents leading to decreased bleeding complications and decreased length of drain times.
Although this study is novel, it is not without limitations. Although this study incorporated many patients, all procedures were performed at a single institution, by a single surgeon. First, it is important to note that even experienced surgeons experience growth and refinement in their technique over time that may confound the decrease in bleeding complications attributed to the hemostatic agent. Moreover, there were insufficient patients in the panniculectomy and abdominoplasty group for EVICEL to make a meaningful comparison. Therefore, larger studies at multiple sites with differing patient populations would be better able to address the reproducibility and generalizability of the results. In addition, it is important to mention that this study was retrospective and not blinded, which could potentially introduce bias. However, it is not feasible to blind the surgeon to which product is being used due to the differences in product preparation. Furthermore, while promising outcomes were noted with hemostatic agents, the lack of statistical significance favoring hemostatic agents could be related to sample size with larger samples possibly showing statistical significance with avoidance of a potential Type II statistical error. Lastly, the cost of the hemostatic agent itself is an important consideration that contributes to the overall cost of the operation. A cost-effectiveness analysis examining the use of these two hemostatic agents compared with no hemostatic agent would be worthwhile to rationalize the routine use of hemostatic agents in general plastic surgery procedures.
Therefore, it is imperative that future studies be performed, preferably randomized trials that will evaluate long-term outcomes to greater assess the utility of hemostatic agents. In the end, we hope to provide a more detailed and standardized data set incorporating many institutions to analyze outcomes more precisely and accurately.
CONCLUSION
The use of hemostatic agents in general plastic surgery procedures significantly shorten time to JP drain removal and may decrease postoperative complications, including seroma, hematoma, and OR takeback.
ACKNOWLEDGMENT
The authors thank all who helped make this project possible.
Footnotes
Published online 19 August 2021.
Disclosure: Dr. Chatterjee is a consultant for Biom’Up. All the other authors have no financial interest to declare in relation to the content of this article. This study was partially supported by an Investigator Initiated Study grant from Biom’Up.
REFERENCES
- 1.Jordan SW, Khavanin N, Kim JYS. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg. 2016;137:1104–1116. [DOI] [PubMed] [Google Scholar]
- 2.Seth AK, Hirsch EM, Kim JY, et al. Hematoma after mastectomy with immediate reconstruction: an analysis of risk factors in 883 patients. Ann Plast Surg. 2013;71:20–23. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham BL, Gear AJ, Kerrigan CL, et al. Analysis of breast reduction complications derived from the BRAVO study. Plast Reconstr Surg. 2005;115:1597–1604. [DOI] [PubMed] [Google Scholar]
- 4.Di Martino M, Nahas FX, Kimura AK, et al. Natural evolution of seroma in abdominoplasty. Plast Reconstr Surg. 2015;135:691e–698e. [DOI] [PubMed] [Google Scholar]
- 5.Hood K, Ganesh Kumar N, Kaoutzanis C, et al. Hematomas in aesthetic surgery. Aesthet Surg J. 2018;38:1013–1025. [DOI] [PubMed] [Google Scholar]
- 6.Galanakis I, Vasdev N, Soomro N. A review of current hemostatic agents and tissue sealants used in laparoscopic partial nephrectomy. Rev Urol. 2011;13:131–138. [PMC free article] [PubMed] [Google Scholar]
- 7.Tompeck AJ, Gajdhar AUR, Dowling M, et al. A comprehensive review of topical hemostatic agents: the good, the bad, and the novel. J Trauma Acute Care Surg. 2020;88:e1–e21. [DOI] [PubMed] [Google Scholar]
- 8.Moore M, Burak WE, Jr, Nelson E, et al. Fibrin sealant reduces the duration and amount of fluid drainage after axillary dissection: a randomized prospective clinical trial. J Am Coll Surg. 2001;192:591–599. [DOI] [PubMed] [Google Scholar]
- 9.Foroutanjazi S, Jonczyk M, Chen L, et al. Closed incision negative pressure therapy: indications and adherence to protocol. Am Surg. 2021;87:760–764. [DOI] [PubMed] [Google Scholar]
- 10.Baker E, Piper J. Drainless mastectomy: is it safe and effective? Surgeon. 2017;15:267–271. [DOI] [PubMed] [Google Scholar]
- 11.Phillips BT, Wang ED, Mirrer J, et al. Current practice among plastic surgeons of antibiotic prophylaxis and closed-suction drains in breast reconstruction: experience, evidence, and implications for postoperative care. Ann Plast Surg. 2011;66:460–465. [DOI] [PubMed] [Google Scholar]
- 12.Chen CF, Lin SF, Hung CF, et al. Risk of infection is associated more with drain duration than daily drainage volume in prosthesis-based breast reconstruction: a cohort study. Medicine (Baltimore). 2016;95:e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkowitz R, Vu J, Brummett C, et al. The impact of complications and pain on patient satisfaction. Ann Surg. 2019;273:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee A, Asban A, Jonczyk M, et al. A cost-utility analysis comparing large volume displacement oncoplastic surgery to mastectomy with free flap reconstruction in the treatment of breast cancer. Am J Surg. 2019;218:597–604. [DOI] [PubMed] [Google Scholar]
- 15.Asban A, Homsy C, Chen L, et al. A cost-utility analysis comparing large volume displacement oncoplastic surgery to mastectomy with single stage implant reconstruction in the treatment of breast cancer. Breast. 2018;41:159–164. [DOI] [PubMed] [Google Scholar]
- 16.Dhillon S. Fibrin sealant (Evicel [Quixil/Crosseal]): a review of its use as supportive treatment for haemostasis in surgery. Drugs. 2011;71:1893–1915. [DOI] [PubMed] [Google Scholar]
- 17.Ardehali A, Spotnitz WD, Hoffman RW, et al. ; Advanced Powder Investigators Group (APIG). Evaluation of the safety and efficacy of a new hemostatic powder using a quantitative surface bleeding severity scale. J Card Surg. 2019;34:50–62. [DOI] [PubMed] [Google Scholar]
- 18.Dang NC, Ardehali A, Bruckner BA, et al. Prospective, multicenter, randomized, controlled trial evaluating the performance of a novel combination powder vs hemostatic matrix in cardiothoracic operations. J Card Surg. 2020;35:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruckner BA, Ngo U, Ramchandani M, et al. Application techniques of a novel hemostat in cardiac operations: HEMOBLAST. J Card Surg. 2019;34:849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bullocks J, Basu CB, Hsu P, Singer R. Prevention of hematomas and seromas. Semin Plast Surg. 2006;20:233–240. [Google Scholar]