Background:
Biopolymers consist of non-biocompatible allogeneic materials. They have been associated with autoimmune inflammatory syndrome induced by adjuvants, as described by Yehuda Shoenfeld and Nancy Agmon-Levin. Therefore, this study aimed to evaluate the clinical and immunological characteristics of patients with autoimmune inflammatory syndrome induced by adjuvants treated at a plastic surgery clinic in Colombia.
Methods:
This cross-sectional, descriptive observational study included 190 patients with biopolymers in the buttocks with no evidence of autoimmune disease who were diagnosed with autoimmune inflammatory syndrome induced by adjuvants and underwent treatment at a private plastic surgery clinic from 2017 to 2020. The clinical and paraclinical parameters were measured preoperatively, when the diagnosis of autoimmune inflammatory syndrome induced by adjuvants and the need for material removal were established, and postoperatively after 3 months.
Results:
The most frequent symptoms were myalgia (92%), arthralgia (77.9%), asthenia (77.9%), adynamia (77.9%), and neurological symptoms (55.8%). Preoperatively, patients were positive for antinuclear antibody, lactate dehydrogenase, complement proteins C3 and C4, and lupus anticoagulant. However, after removal of the biopolymer, there was a decrease in positivity or conversion to a negative status of paraclinical tests. Moreover, there was an association between LDH positivity and disease severity (odds ratio: 4.1, 95% confidence interval: 1.94–8.92).
Conclusions:
The removal of biopolymers using an open surgical technique in symptomatic or asymptomatic patients is crucial for functional and reconstructive purposes and to improve the quality of life. Therefore, this condition should be known as “human adjuvant disease caused by biopolymers.” Further, this condition mimics autoimmune diseases, with clinical and paraclinical manifestations that improve biopolymer removal.
INTRODUCTION
Autoimmune inflammatory syndrome induced by adjuvants (ASIA) was first described in 2011 by Yehuda Shoenfeld and Nancy Agmon-Levin as a condition characterized by an autoimmune response in humans after exposure to an adjuvant.1 Adjuvants enhance the immune response, but they do not act on their own; they help produce a more relevant reaction against the inoculated antigens. However, they can induce an immune response by themselves under certain conditions.2 (See Video [online], which displays the immunologic process of human adjuvant disease caused by biopolymers.) The list of these substances can be extensive, but the most relevant adjuvants include silica as a structural unit of polydimethylsiloxane, known as silicone, methacrylate compounds, aluminum hydroxide, squalene, oils of vegetable or animal origin, and aluminum salts3 (Figs. 1–3). It is occasionally impossible to identify the substance used in cosmetic procedures. However, the etiology of adjuvants involves a multifactorial interaction between the environment and genetic susceptibility because many autoimmune diseases share human leukocyte antigen (HLA) class II alleles, including DRB1.4
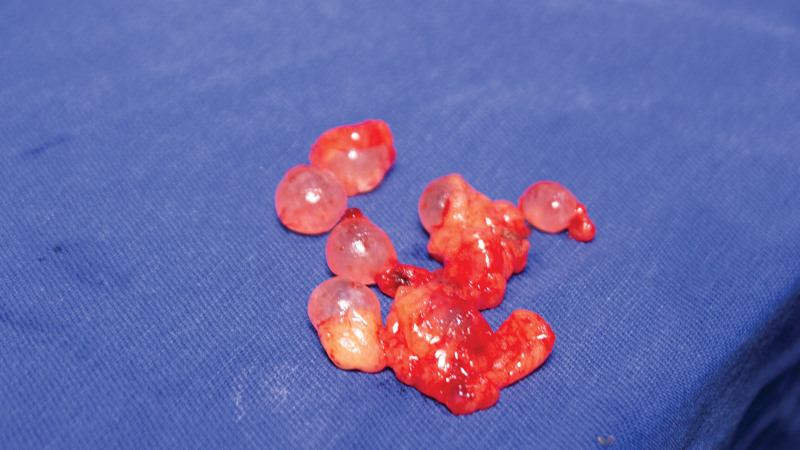
Fig. 1.
The most common and widely used biopolymer known as liquid silicone or polydimethylsiloxane.
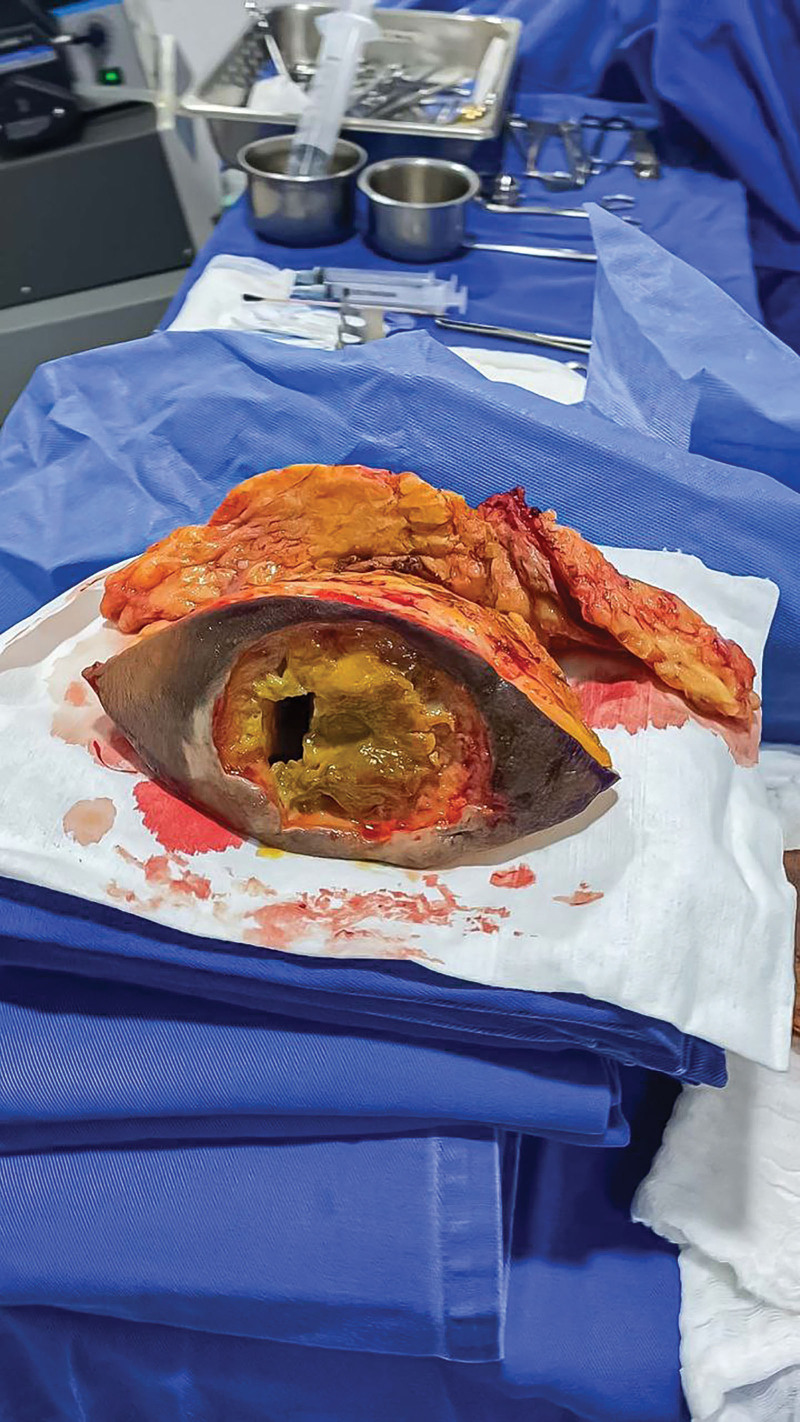
Fig. 3.
Specimen from gluteal biopolymer removal, polymethyl methacrylate, and polydimethylsiloxane.
Video 1. Biopolymers. Video 1 from “Clinical and Immunological Characteristics of Patients With Biopolymers and Autoimmune Inflammatory Syndrome Induced by Adjuvants.” This video displays the immunologic process of human adjuvant disease caused by biopolymers.
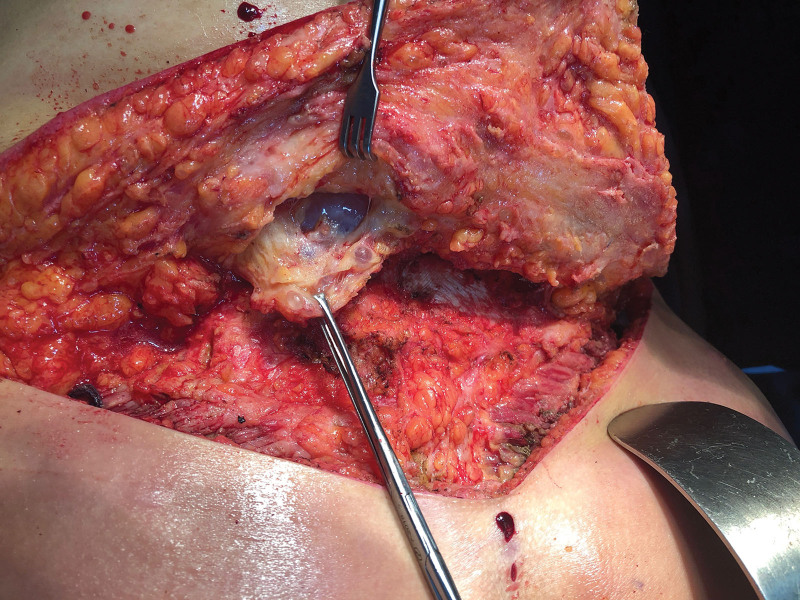
Fig. 2.
Granulomas and siliconomas present in the lumbosacral region.
Thus far, the following five main conditions have been reported in the literature as part of ASIA: silicosis, Gulf War syndrome, macrophagic myofasciitis syndrome, sick building syndrome, and postvaccination phenomena. However, recent studies have reported the occurrence of Sjögren’s syndrome and autoimmune thyroiditis in ASIA.5–7 The above conditions share clinical characteristics with conditions such as chronic fatigue syndrome and fibromyalgia, chronic pain, cognitive dysfunction, fever, arthralgia, myalgia, and autoantibody positivity.8,9
Limited information is available on the causes of ASIA because only a few cases of ASIA have been reported thus far due to the unawareness of the treating physician. Many treating physicians are unaware of this condition because it has been recently characterized and is not included in the diagnostic workup or medical history taking and patients often do not relate the symptoms with the presence of adjuvants in their body, leading to no or delayed diagnosis. Further, the presence of multiple suspicious adjuvants to which the individual may be exposed make it difficult to establish a precise temporal–causal association.
This study aimed to evaluate the clinical and immunological characteristics of patients with ASIA treated at a private plastic surgery clinic in Bogotá, Colombia from 2017 to 2020 to provide scientific evidence regarding avoiding adjuvant use and facilitating a timely diagnosis.
MATERIALS AND METHODS
This cross-sectional, descriptive observational study included 196 patients with biopolymers in buttocks, without a previous diagnosis of autoimmune disease and who were diagnosed with ASIA between January 1, 2017 and December 31, 2020 according to the 2011 criteria proposed by Shoenfeld and Agmon-Levin5,10—patients with three major criteria, those with two major criteria and one minor criterion, or those with one major criterion and one minor criterion, with the essential major criterion being exposure to an adjuvant before the onset of symptoms. Patients with uncontrolled diabetes mellitus and arterial hypertension; active infection at the site of biopolymer administration; human immunodeficiency virus infection; or a diagnosis of disseminated infiltration in the organ, device, or biopolymer system (stage V) were excluded.11,12 Thus, 190 patients were included in the study. Measurements of frequencies and values were performed twice, preoperatively, when the diagnosis of ASIA and need for material removal were established, and postoperatively after 3 months after medical consultation or videoconsultation in foreign patients. In both cases the paraclinical results were considered. The signs and symptoms of the disease and the results of laboratory tests, including the immunological profile, were assessed. All patients underwent simple nuclear magnetic resonance imaging or computed tomography of the gluteal region with three-dimensional reconstruction preoperatively to evaluate the extent of the procedure and identify the non-biocompatible material. However, this is not one of the reasons for this assessment in the context of this article because the diagnostic images should be assessed 1 year after the product removal to minimize inflammatory findings or immature scar tissue that may lead to diagnostic uncertainty due to product persistence after removal. This study was considered a risk-free study based on Resolution 8430 of the Ministry of Health of Colombia, which establishes the scientific, technical, and administrative standards for health research in its title II chapter 1 article 11, because it used data from a database and clinical history records; informed consent was obtained from the patients to use their images in scientific publications. Patients were not administered any intervention before inclusion in the study.13 Qualitative variables are presented as frequencies and percentages, and quantitative variables are presented as measures of central tendency and dispersion. If there is a loss more than 15% in a variable data, this one will be eliminated and reported from analysis of the study. Odds ratio with its respective 95% confidence interval was used to evaluate associations between high LDH levels and severity of disease. The chi-square test was used for hypothesis testing for categorical variables, and a P value of less than 0.05 was considered significant. Epi Info Version 7.2.4, a free-access software by the U.S. Department of Health and Human Services Centers for Disease Control and Prevention, was used for all statistical analyses.
RESULTS
This study included 190 patients with biopolymers in their buttocks who were diagnosed with ASIA and underwent a surgical procedure. It was found that 171 (90%) patients were users of polydimethylsiloxane, 13 (6.8%) patients polymethylmethacrylate, four (2.1%) patients paraffin (Fig. 4), and three (1.5%) patients with oils whose physicochemical composition (vegetable or industrial) could not be defined.
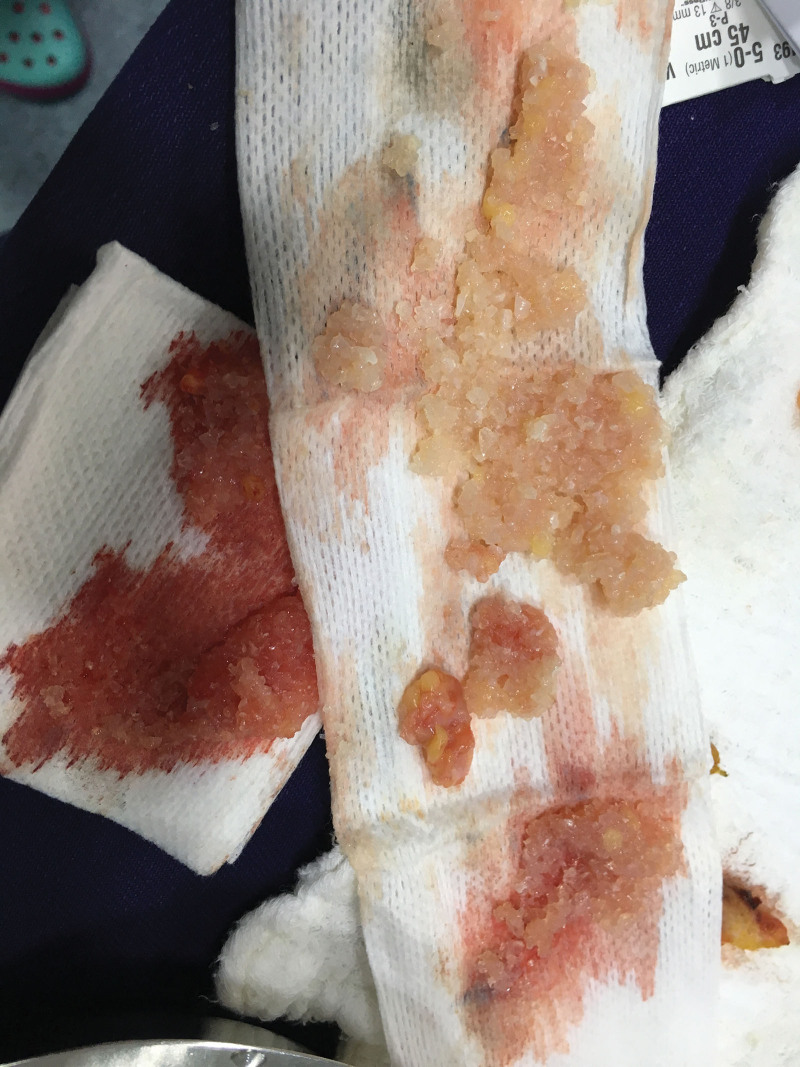
Fig. 4.
Paraffin named as kerosene obtained after open biopolymer removal.
The prevalence of ASIA period found in this cohort between January 1, 2017 and December 31, 2020 is 5.3%, which is calculated taking into account as numerator 190 patients with the diagnosis and as denominator 3550 patients seen in the clinic with any diagnosis or procedure during the same period in the clinic, and the prevalence of ASIA period calculated for patients with biopolymer injection is 4.8% taking into account as numerator 171 cases and the same denominator reported above. There was no loss of data in any of the variables evaluated in people. There were 98.4% female patients, and the mean patient age was 41 ± 10.3 years. Although most patients were Colombians, some patients were of various nationalities (Table 1).
Table 1.
Distribution of Patients with ASIA by Nationality
| Country | Number of Patients (%) |
|---|---|
| Colombia | 120 (63.16) |
| United States | 12 (6.32) |
| Peru | 8 (4.21) |
| Mexico | 6 (3.16) |
| Venezuela | 6 (3.16) |
| Dominican Republic | 5 (2.63) |
| Ecuador | 5 (2.63) |
| Panama | 5 (2.63) |
| Chile | 4 (2.11) |
| Spain | 3 (1.58) |
| Brazil | 2 (1.05) |
| Bolivia | 2 (1.05) |
| Cuba | 2 (1.05) |
| Canada | 2 (1.05) |
| Aruba | 2 (1.05) |
| Costa Rica | 1 (0.53) |
| Curazao | 1 (0.53) |
| Germany | 1 (0.53) |
| Lituania | 1 (0.53) |
| Rumania | 1 (0.53) |
| Sint Maarten | 1 (0.53) |
| Total | 190 |
Table 2 shows the symptoms reported by the patients at the time of the preoperative assessment. The first five most frequent symptoms were myalgia (92.1%), arthralgia (77.9%), asthenia (77.9%), adynamia (77.9%), and neurological findings (55.8%). The number and proportion of patients who reported symptoms persistence at the 3-month follow-up assessment are also shown in Table 2. There was a significant decrease in the prevalence of symptoms at the 3-month follow-up.
Table 2.
Distribution of Signs and Symptoms Preoperatively and at the 3-month Postoperative Follow-up
| Clinical Characteristics | Preoperative (%) | Persistence of Symptoms at the 3-month Follow-up (%) |
|---|---|---|
| Myalgias | 175 (92.1) | 9 (5.14) |
| Arthralgias | 148 (77.9) | 6 (4.05) |
| Asthenia | 148 (77.9) | 5 (3.38) |
| Adynamia | 148 (77.9) | 5 (3.38) |
| Neurological symptoms | 106 (55.8) | 14 (13.21) |
| Abdominal distension | 76 (40) | 5 (6.58) |
| Depression | 71 (37.4) | 5 (7.04) |
| Headache | 71 (37.4) | 5 (7.04) |
| Anxiety | 66 (34.8) | 1 (1.52) |
| Photophobia | 54 (28.4) | 2 (1.85) |
| Hyperacusis | 51 (26.8) | 2 (1.96) |
| Hair loss | 48 (25.3) | 2 (4.17) |
| Fever | 21 (11.1) | 3 (14.29) |
| Dry mouth | 21 (11.1) | 2 (9.52) |
| Dry eye | 20 (10.5) | 2 (10) |
| Memory loss | 15 (7.89) | 2 (6.67) |
The antinuclear antibodies (ANAs) were measured in all patients with ASIA during the preoperative assessment as previous studies have reported an increase in the expression of ANAs in ASIA (14). In total, 60% of patients tested positive for ANAs, and the most frequent dilution in the ANA tests was 1:160. The monitoring of this paraclinical characteristic at 3 months postoperatively showed conversion to a negative result in 89 patients, increasing the negativity rate to 46%. There was a similar decrease in the postoperative monitoring of lactate dehydrogenase (LDH), lupus anticoagulant, complement proteins C3 and C4, and rheumatoid factor (Table 3).
Table 3.
Preoperative and 3-month Postoperative Laboratory Results
| Laboratory Tests | Preoperative | Three-month Follow-up | ||
|---|---|---|---|---|
| Dilution | n (%) (total = 190) | Dilution | n (%) (total = 190) | |
| Antinuclear antibodies | Negative | 76 (40) | Negative | 165 (86.8) |
| 1/40 | 2 (1) | 0 | 0 | |
| 1/80 | 4 (2.1) | 1/80 | 9 (4.7) | |
| 1/160 | 78 (41) | 1/160 | 9 (4.7) | |
| 1/320 | 27 (14.2) | 1/320 | 4 (2.1) | |
| 1/640 | 0 | 1/640 | 2 (1) | |
| 1/1280 | 3 (1.6) | 1/1280 | 1 (0.5) | |
| Lactic dehydrogenase | 38 (16.13) | 0 (0.0) | ||
| Lupus anticoagulant | 27 (14.2) | 4 (14) | ||
| Serum complement C3 and C4 | 8 (4.2) | 0 (0.0) | ||
| Rheumatoid factor | 4 (2.1) | 1 (25) | ||
Similarly, when classifying patients into two groups based on disease severity,12 there was an association between LDH positivity and disease severity (odd ratio: 4.16, 95% confidence interval: 1.94–8.92, P < 0.0001); there was a four-fold higher probability of having severe disease when LDH was positive, with statistical significance.
DISCUSSION
The major histocompatibility complex is the most polymorphic group of genes in the human genome and is located on the short arm of chromosome 6. This system encodes genes associated with the immune system that produce proteins that ensure correct antigen recognition and presentation. HLA I (HLA A, B, and C) gene products present endogenous peptides from intracellular infections; HLA II (HLA-DR, DP, and DQ) gene products present exogenous peptides, such as bacterial antigens; and HLA III encodes gene products present in immune regulatory molecules, such as tumor necrosis factor and proteins of the complement system.15
The mechanism linking HLA polymorphisms to autoimmune diseases is not well understood, but it is commonly accepted that there is a loss of immunological tolerance of HLA class II antigens, resulting in the presentation of self or foreign peptides that mimic self-molecules to autoreactive T lymphocytes. This theory is associated with rheumatoid arthritis, Sjögren’s syndrome, systemic lupus erythematosus, multiple sclerosis, type 1 diabetes mellitus, and autoimmune thyroid disease.15
Shoenfeld et al proposed an immunological mimicking model that identifies the immunological mosaic and disease presentation based on different types of adjuvants and triggering substances.3,16 ASIA occurs due to non-biocompatible allogeneic products, leading to pathogen-associated activation at the molecular level, thereby activating inflammatory receptors and cytokines, such as interleukin (IL)-1b, IL-6, and interferon-alpha and -gamma, which stimulate dendritic cells, monocytes, cell adhesion molecules, and major histocompatibility complex expression, which concomitantly stimulates immunoglobulin M and immunoglobulin G antibody production.10
Local tissue damage stimulates the processes of fat necrosis and liquefaction, protein denaturation, and local granulomatous reactions, which favor the appearance of epitopes known as antigens, thereby promoting and amplifying the inflammatory response from a local stage to a systemic stage, recognizing the lymph nodes of the peri-regional chains as the center of presentation and expression of those cells.10
It is not possible to objectively quantify the amount of product administered to the patients because they do not know it or do not remember it; however, according to the author’s protocol, an approximate measurement is made considering the weight of the specimen removed, which does not allow establishing a direct correlation with the immunologic response because it is confused with the amount of tissue compromised by biopolymer at the time of surgery. Considering the above, it is not possible to conclude a direct association between serological positivity and the amount of product, but it coincides with the response to the product and the autoimmunity mosaic theory described by Schoenfeld.1,10,14,15
The allogeneic materials can act as superantigens and favor cellular reactions and, to a greater extent, the antigen-antibody and granulomatous response, which leads to myalgia, arthralgia, asthenia, adynamia, memory loss, dry eyes, dry mouth, photophobia, hyperacusis, abdominal distension, neuropathic pain, headache, and hair loss. Additionally, more severe signs and symptoms, such as loss of consciousness or neurological manifestations of demyelination, can also occur.1,8 These symptoms and signs are part of the major criteria for the diagnosis of ASIA, and this study shows their decrease, and, in some cases, their absence, after biopolymer removal via an open surgical technique.
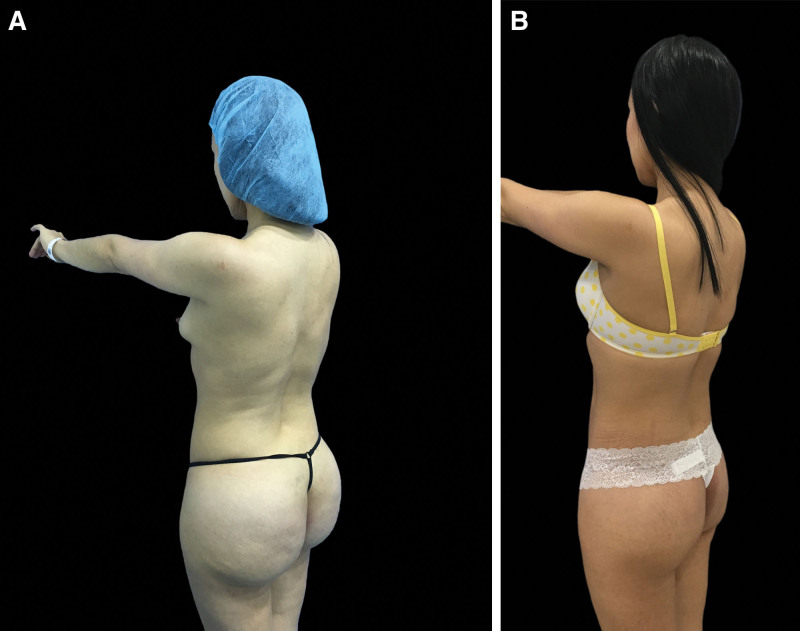
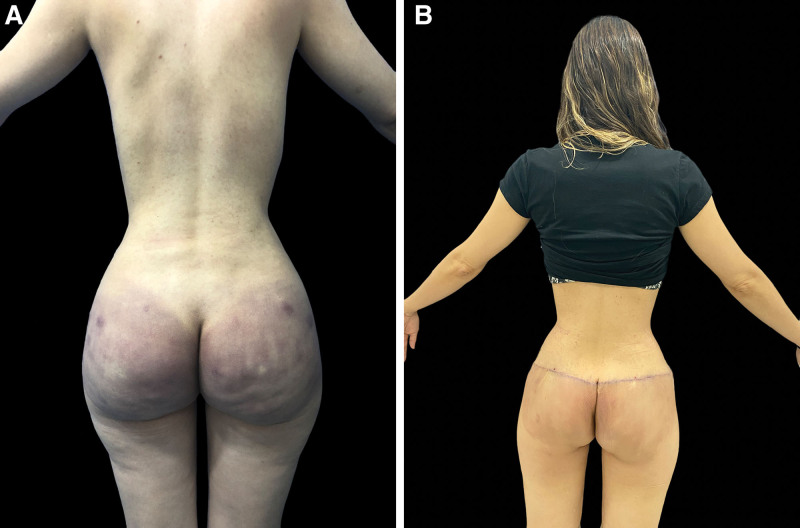
The open Meticulous Approach Safer and Keeper technique created and developed by Jaime Eduardo Pachón Suárez, author of this article, has six main objectives: (1) to remove as much product as possible, depending on the location and the material that is contained in bloc resection of the tissues, including fasciectomies, (2) to perform a reconstructive procedure to minimize esthetic sequelae secondary to the inflammatory process and the surgical procedure, (3) to resect and release the affected tissues to improve the local compartment syndrome produced by chronic inflammation and the damage with lymphatic involvement produced by non-biocompatible allogeneic materials, (4) to manage the migration pattern to the lumbar region, infragluteal folds, and fascia lata, (5) to improve local and systemic symptoms with consequent recovery of functionality and positively impact the patient’s quality of life, and (6) to provide the best possible esthetic result with the bilateral gluteal pexy with a mask design that allows hiding the scar tissue in the esthetic subunit of the underwear and provide firmness to the gluteal fasciocutaneous flaps (Figs. 5, 6).
Fig. 5.
A, A patient with intragluteal allogenosis due to polydimethylsiloxane with 8 years of evolution. B, The patient reported improvement of symptoms and gluteal appearance at the 3-month postoperative follow-up.
Fig. 6.
A, A patient with gluteal biopolymer and lumbar migration with extensive superficial and deep tissue damage. B, The patient reported improvement of symptoms and diminished inflammatory involvement at the 1-month postoperative follow-up after an open Meticulous Approach Safer and Keeper surgery.
Unlike the studies published in the scientific literature on biopolymers, which are mostly case studies or case series,17,18 this study found that the prevalence of ASIA induced by biopolymers is 5.3% in a plastic surgery clinic. Moreover, although biopolymers were removed in all patients in this study, Dr. Pachón’s clinical experience indicates that this material (polydimethylsiloxane) corresponds to 90% of the patients’ postsurgical specimens, and the remaining 10% is composed of polymethylmethacrylate, paraffin, airplane oil, intradermal fat-soluble vitamins, mineral oils (isopropyl myristate) or vegetable oils, which agrees with the findings of other investigators, such as Olga Vera Lastra et al, who reported findings with similar substances (mineral oils, liquid paraffin, liquid silicone, methacrylate compounds).8
This study found that the mean time that biopolymers take to produce symptoms after their administration is 10.4 ± 4 years and that patients showed elevated levels of ANA, LDH, complement proteins C3 and C4, and rheumatoid factor preoperatively. The patients agree on two relevant statements: (1) they have undergone assessment for autoimmune diseases, but have not had a conclusive diagnosis; however, three patients were receiving immunosuppressive treatment and (2) neither they nor their treating physicians had previously associated the presence of the biopolymers with their symptoms and laboratory results. Considering this, it was not possible to determine how long the autoimmune markers were positive because patients could not remember the information, and it was difficult to access their complete medical history.
As evidenced by the results, LDH is positive when the patient presents with severe disease. This can be explained by the physiology of this acute phase reactant as it is produced by an anaerobic metabolic reaction secondary to cell lysis due to chronic inflammation, tissue damage, and production of free radicals by biopolymers. This finding agrees with that reported by Aibek E Mirrakhimov et al, who also reported that LDH is produced by anaerobic glucose metabolism that causes cell destruction in cell lysis syndrome, and this biomarker has also been used for staging risk of tissue damage.19,20
Although this study does not have the best epidemiological design to show causality, it shows an increase in the levels of biomarkers in the blood of patients with a clinical diagnosis of ASIA and a significant decrease in their levels after biopolymer removal via an open surgical technique. Based on these findings, this condition is believed to mimic autoimmune pathologies as it resolves after surgical removal of the adjuvant. However, some patients may develop similar pathologies despite an adequate removal technique as it is not possible to remove the entire product.
At the 3-month postoperative assessment, the patients reported a significant improvement in the symptoms (Table 2), functionality, and quality of life, with increased self-esteem, decreased feeling of guilt, and satisfaction with the esthetic result obtained as a secondary gain.
As a research group, considering the clinical and paraclinical characteristics before and after surgery, we suggest naming this condition “human adjuvant disease caused by biopolymers.” We also suggest defining patients with this condition as follows: patients with a history of having biopolymers in the previous 6–14 years who present one or more of the following symptoms: myalgia, arthralgia, asthenia, adynamia, and predominantly sensitive neurological symptoms, with positivity of any of the following: ANAs, complement protein C3 and C4, LDH, lupus anticoagulant, or rheumatoid factor and showing improvement on removal of the material. The above information will help the treating physician who suspects this condition to provide the best management to the patient, thus improving the chances of disease control and reducing the complications.
This study is susceptible to selection bias due to the nature of the epidemiological design because the ASIA diagnostic criteria, despite being well established, are applied by the treating physician, making the study susceptible to observer errors, but it is clarified that the diagnosis was established by a professional with extensive experience and paraclinical supports and medical history. It is susceptible to confusion because people may have had administration of other substances as part of aesthetic procedures, in other parts of the body. A suitable prevention questionnaire was used for exclusion. It is important to read the study in the context of the population studied when extrapolating the information.
In conclusion, every patient who is a user of biopolymers, whether symptomatic or asymptomatic, should undergo a procedure to remove the said material, considering that it is not possible to make an association between the persistence of the biopolymer and the positivity of the biomarkers. However, we conclude that the greater the amount of material removed, the greater the possibility of having negative markers, and patients should be monitored for at least 2 years to better characterize ASIA. Considering the described results and the evidence of paraclinical variation, we propose, as a strong hypothesis, that ASIA is caused because of having biopolymers. However, further studies are needed to verify the generalizability of our findings. Similarly, this syndrome mimics autoimmune diseases, with improvement in clinical and paraclinical manifestations after the substance is removed from the body.
Footnotes
Published online 24 September 2021.
Disclosure: The authors have no financial interest in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Shoenfeld Y, Agmon-Levin N. ‘ASIA’ – autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36:4–8. [DOI] [PubMed] [Google Scholar]
- 2.Scanzi F, Andreoli L, Martinelli M, et al. Are the autoimmune/inflammatory syndrome induced by adjuvants (ASIA) and the undifferentiated connective tissue disease (UCTD) related to each other? A case-control study of environmental exposures. Immunol Res. 2017;65:150–156. [DOI] [PubMed] [Google Scholar]
- 3.Vera-Lastra O, Medina G, Cruz-Domínguez MP, et al. Autoimmune/inflammatory syndrome induced by mineral oil: a health problem. Clin Rheumatol. 2018;37:1441–1448. [DOI] [PubMed] [Google Scholar]
- 4.Perricone C, Colafrancesco S, Mazor RD, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects. J Autoimmun. 2013;47:1–16. [DOI] [PubMed] [Google Scholar]
- 5.Ameratunga R, Gillis D, Gold M, et al. Evidence refuting the existence of autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA). J Allergy Clin Immunol Pract. 2017;5:1551–1555.e1. [DOI] [PubMed] [Google Scholar]
- 6.Colafrancesco S, Perricone C, Priori R, et al. Sjögren’s syndrome: another facet of the autoimmune/inflammatory syndrome induced by adjuvants (ASIA). J Autoimmun. 2014;51:10–16. [DOI] [PubMed] [Google Scholar]
- 7.Watad A, David P, Brown S, et al. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol (Lausanne). 2016;7:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vera-Lastra O, Medina G, Cruz-Dominguez Mdel P, et al. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): clinical and immunological spectrum. Expert Rev Clin Immunol. 2013;9:361–373. [DOI] [PubMed] [Google Scholar]
- 9.Hawkes D, Benhamu J, Sidwell T, et al. Revisiting adverse reactions to vaccines: A critical appraisal of autoimmune syndrome induced by adjuvants (ASIA). J Autoimmun. 2015;59:77–84. [DOI] [PubMed] [Google Scholar]
- 10.Denisse L. Síndrome de ASIA, la creciente enfermedad inducida por adyuvantes. 2018:1–6. Available at https://www.elsevier.com/es-es/connect/medicina/asia,-el-extrano-sindrome-inducido-por-adyuvantes. Accessed April 9, 2021.
- 11.Blancas RP, Lozano RR, Serrano A, Tame JLH. Clasificación y tratamiento de la enfermedad mamaria por modelantes. Cirugía Plástica. 2010;20:112–119. [Google Scholar]
- 12.Stanford N, Montealegre G. Alogenosis iatrogénica, hallazgos de una enfermedad reumática. Revista Colombiana de Cirugía Plástica y Reconstructiva. 2013;19:28–38. [Google Scholar]
- 13.Colombia M de S de. Resolución 8430 DE 1993 Por la cual se establecen las normas científicas, técnicas y administrativas para la investigación en salud. Vol. 1, Ministerio de salud de Colombia. 1993:19. Available at: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/RESOLUCION-8430-DE-1993.PDF [Google Scholar]
- 14.Alijotas-Reig J. Human adjuvant-related syndrome or autoimmune/inflammatory syndrome induced by adjuvants. Where have we come from? Where are we going? A proposal for new diagnostic criteria. Lupus. 2015;24:1012–1018. [DOI] [PubMed] [Google Scholar]
- 15.Arango MT, Perricone C, Kivity S, et al. HLA-DRB1 the notorious gene in the mosaic of autoimmunity. Immunol Res. 2017;65:82–98. [DOI] [PubMed] [Google Scholar]
- 16.Goren I, Segal G, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvant (ASIA) evolution after silicone implants. Who is at risk? Clin Rheumatol. 2015;34:1661–1666. [DOI] [PubMed] [Google Scholar]
- 17.Watad A, Quaresma M, Brown S, et al. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome) – An update. Lupus. 2017;26:675–681. [DOI] [PubMed] [Google Scholar]
- 18.Watad A, Quaresma M, Bragazzi NL, et al. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA)/Shoenfeld’s syndrome: descriptive analysis of 300 patients from the international ASIA syndrome registry. Clin Rheumatol. 2018;37:483–493. [DOI] [PubMed] [Google Scholar]
- 19.Wells R. Tumour lysis syndrome. Med J Aust. 2009;30:123–126. [Google Scholar]
- 20.Rahmani B, Patel S, Seyam O, et al. Current understanding of tumor lysis syndrome. Hematol Oncol. 2019;37:537–547. [DOI] [PubMed] [Google Scholar]