Abstract
Background
Sarcopenia is an age‐related progressive and general skeletal muscle disease associated with negative consequences such as falls, disability, and mortality. An early‐stage diagnosis is important to enable adequate treatment, especially in geriatric psychiatry. However, there presently is little information about the feasibility of diagnostic procedures and the prevalence of sarcopenia in clinical geriatric psychiatry settings. The aim of this study is to implement a diagnostic process for sarcopenia in a geriatric psychiatry hospital, to investigate its feasibility and to analyse the prevalence rates.
Methods
A single‐centre cross‐sectional study over 3 months was conducted in a geriatric psychiatry hospital. All admitted patients with a diagnosis of dementia, depression, or delirium were screened regarding the clinical impression of frailty and sarcopenia according to the current diagnostic algorithm of the European Working Group on Sarcopenia in Older People 2 (EWGSOP2).
Results
We found that short physical performance tests, such as the handgrip strength testing (91%) or 4 m walking test (91%), were applicable in our sample. The original standardized instructions of longer tests could not be performed appropriately, for example, in the five‐times‐sit‐to‐stand‐test (32%), the timed‐up‐and‐go‐test (68%), and the 400 m walking test (38%). Muscle mass measurements using bioelectric impedance analysis were feasible in all patients (100%). The analysis revealed an estimated prevalence rate for sarcopenia of 65% for patients suffering from dementia and 36% for patients suffering from depression. In our final analysis, 15 patients suffering from dementia, 19 suffering from depression, and no patient suffering from delirium were included [22 female (64.7%) and twelve male (35.3%) patients]. The patients were on average 78.9 ± 7.7 years old, with the youngest patient being 61 years old and the oldest patient 93 years old. Out of the total sample, 14 patients suffering from dementia and eight patients suffering from depression were diagnosed with a severe stage of sarcopenia.
Conclusions
The EWGSOP2 algorithm seems to be applicable in the clinical routine of a geriatric psychiatry hospital. The high estimated prevalence rates of sarcopenia highlight the need for an early and comprehensive screening for sarcopenia in geriatric psychiatry.
Keywords: Sarcopenia, Geriatric psychiatry, Prevalence, Dementia, Depression
Introduction
The awareness as well as the relevance of sarcopenia has increased over the last years. Since January 2018, sarcopenia has been included in the German version of the International Classification of Diseases (ICD‐10‐GM) and is since then officially considered as a disease. 1 According to the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) guidelines, sarcopenia is defined as a progressive and general skeletal muscle disease associated with an increased likelihood of negative consequences such as falls, disability, and mortality. 2 The patient's muscle strength, muscle mass, and physical performance is relevant for a diagnosis of sarcopenia. If muscle strength is reduced, sarcopenia is suspected. To confirm this, muscle mass should be measured, and if muscle mass is below the recommended thresholds, sarcopenia is diagnosed. Consequently, physical performance tests are used to evaluate the severity of sarcopenia. 2 Besides the consequences and problems for the persons suffering from sarcopenia, this disease significantly challenges the health care system. 3 , 4 Five to 13% of older persons aged 60 to 70 years are affected by sarcopenia, and in persons aged 80 years and more, the numbers rise to 11–50%, depending on the sarcopenia definition, population, and thresholds used. 5 In addition, sarcopenia leads to more frequent and longer hospital stays, 6 and hospital stays increase the risk of developing sarcopenia. 7 For these reasons, sarcopenia is associated with increased costs for the health care system. 8 , 9 Furthermore, a published systematic review with meta‐analysis investigated the prevalence of sarcopenia as a comorbid disease. 10 Based on their results, the authors concluded that sarcopenia is highly prevalent in individuals with a diagnosis of cardiovascular disease, dementia, diabetes, and respiratory disease. With this in mind, identifying sarcopenia in the clinical setting, to enable a prevention and early treatment is highly relevant.
The EWGSOP guidelines (2010) have already been implemented in a number of clinical studies. 10 One focus in former studies was to investigate the prevalence and feasibility of standardized tests for the diagnosis of sarcopenia in acutely ill older persons. 8 , 11 , 12 The prevalence of sarcopenia in the clinical setting ranged from 10% 9 to about 20% 13 , 14 , 15 and about 30% 16 , 17 , 18 up to 40%. 8 , 12 So far, the revised algorithm from 2019 has been implemented in a few studies so far. Here, the target groups were mainly community‐dwelling older adults 19 , 20 , 21 and outpatients. 22 Compared with the first algorithm (published in 2010), the prevalence of sarcopenia was significantly lower in the revised version, 22 , 23 and the overlap of cases was low. 24 But the new diagnostic criteria provide a relatively simple and applicable tool for screening among patients. 25 Only few studies in geriatric psychiatric institutions considering older adults suffering from dementia, depression, or delirium have been conducted yet. A recent review analysis included 11 studies with persons suffering from dementia or cognitive impairment and calculated a prevalence rate of sarcopenia of 26.4% (95% confidence interval: 13.6–44.8%) in individuals with dementia compared with 8.3% in their controls. 10 In other studies, patients with cognitive impairment or delirium have often been excluded in advance. 8 , 9 , 15 , 17 , 18 , 26
Adequate treatment of sarcopenia is an important issue in geriatric psychiatry; however, there presently is little information about the feasibility of diagnostic procedures and the prevalence of sarcopenia in a clinical geriatric psychiatry setting. Therefore, the aim of this study is to investigate the implementation of the EWGSOP2 sarcopenia screening algorithm in a geriatric psychiatry hospital, to investigate the feasibility of the algorithm and to analyse the estimated prevalence rates of sarcopenia.
Methods and materials
Study design
A cross‐sectional study was conducted over a period of 14 weeks in the Department of Geriatric Psychiatry at the LVR‐Hospital Cologne, Germany. The study was approved by the Ethics Committee of the North Rhine Medical Association Chamber (reference number 2018192).
Sample
Patients with a diagnosis of dementia (F00–F03), depression (F32 and F33), or delirium (F05) according to the ICD‐10 classification 1 were included based on a clinical impression of frailty, according to the criteria by Fried. 27 Patients with cardiac pacemakers and amputations were excluded, as the necessary bioelectric impedance analysis (BIA) is not approved by the producer for these persons. Immobile and bedridden patients were also excluded if they were unable to walk a distance of at least 4 m, as this would not allow implementing the physical performance tests. Written informed consent from the patient's legal guardian as well as from the patient, if possible, had to be given to include a patient. The patients' cognitive functioning was assessed via Mini‐Mental Status Evaluation (MMSE). 28
Implementation of the screening process and diagnostic algorithm
The approach of the EWGSOP2 guidelines is to find cases. Therefore, the impression of clinical frailty was used as a starting point for the screening process. Together with the clinical team of physicians, nursing staff, and therapists, frailty was assessed according to the criteria by Fried. 27 Therefore, an older person is rated as being frail, if three out of the following five criteria are given: unintentional weight loss, muscle weakness, exhaustion, slow walking speed, and low level of physical activity. 27 All patients rated as being ‘frail’ were preliminarily included in the project.
The diagnosis of sarcopenia starts with the first test. The EWGSOP2 guidelines propose different tests for the three components muscle strength, muscle mass, and physical performance. For a sarcopenia diagnosis, one of the tests for each component is sufficient. Nevertheless, because one aim of this project was to investigate the feasibility of the tests with patients in geriatric psychiatry, all proposed functional tests were carried out. With this procedure, statements concerning feasibility and future recommendations can be drawn. The algorithm for sarcopenia diagnostics is shown in Figure 1. Handgrip strength measurement (HGS) 29 and the five‐times‐sit‐to‐stand‐test (5×STS) 30 were used to determine muscle strength. Muscle mass was examined using the BIA model seca mbca525 (seca GmbH & Co.KG., Hamburg, Germany). The 4 m walking test, 31 the timed‐up‐and‐go‐test (TUG), 32 the short physical performance battery (SPPB), 33 and the 400 m walking test 34 were used to determine physical performance.
FIGURE 1.
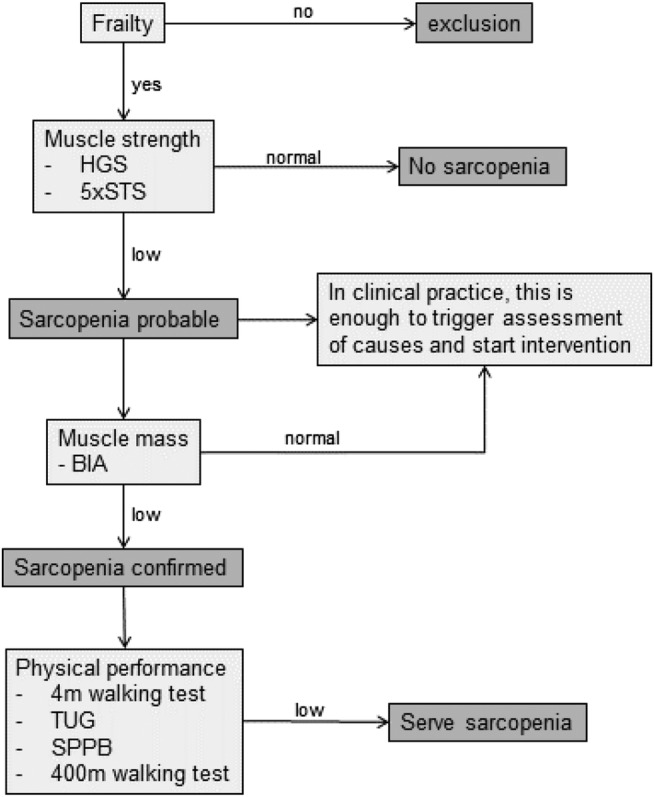
The EWGSOP2's screening process and diagnostic algorithm for sarcopenia and the used tests to determine muscle strength, muscle mass and physical performance 2 5×STS, five‐times‐sit‐to‐stand test; BIA, bioelectric impedance analysis; EWGSOP2, European Working Group on Sarcopenia in Older People 2 (2019); HGS, handgrip strength; SPPB, short physical performance battery; TUG, timed‐up‐and‐go‐test.
For the individual tests, the thresholds provided by the EWGSOP2 were used. 2 A value below or above the defined threshold are classified as low, and the diagnostic algorithm (Figure 1) was continued accordingly. One test below/above the limit value was sufficient to be classified as low in the specific factors muscle strength, muscle mass, and physical performance. In addition, it was documented in the measurement protocol to what reasons a test could not be performed according to the original and standardized instructions (physical, cognitive, motivational, and neuropsychiatric aspects).
Statistical analysis
The collected data were analysed using the IBM Statistical Package for the Social Sciences (SPSS) 25 for Windows (IBM Corporation, Route, Somers, NY, USA). In order to assess the feasibility of the tests, the reasons for failure were first divided into three categories (physical, cognitive, and motivational) and then further analysed as percentages. Due to various reasons, as reported in the Results section, a complete assessment was not possible in all patients who were considered ‘frail’ according to the Fried criteria. 27 Therefore, the overall prevalence of sarcopenia was estimated based on extrapolating the percentage of patients who tested positive to all patients who were rated as ‘frail’. This was calculated separately for each subgroup of patients.
Results
Out of 164 admitted patients, 104 patients were rated as being ‘frail’. Seventy persons had to be excluded based on early release (n = 20), health condition (n = 17), no legal guardian (n = 16), no interest (n = 13), or due to technical problems with the measurement equipment (n = 4). Finally, 34 patients were included. Out of these, n = 15 patients (44%) suffering from dementia were included: n = 12 with dementia in Alzheimer's disease (F00) and n = 3 with mixed type of dementia (F03). Further, n = 19 patients (56%) suffered from depression: n = 15 with recurrent depressive disorder, current episode severe without psychotic symptoms (F33.2), n = 2 with recurrent depressive disorder, current episode severe with psychotic symptoms (F33.3), n = 1 with moderate depressive episode (F32.1), and n = 1 with severe depressive episode without psychotic symptoms (F32.2). Within the final analysis, no patient with delirium was included (Figure 2).
FIGURE 2.
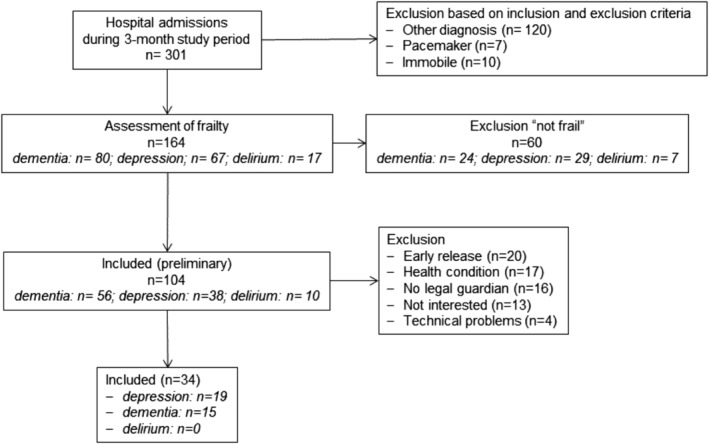
Flow chart for patient recruitment and reasons for exclusion.
The sample consisted of 22 (64.7%) female and 12 (35.3%) male patients. The patients were on average 78.9 ± 7.7 years old, with the youngest patient being 61 years old and the oldest patient 93 years old. The analysis of the cognitive functioning revealed on average 19.6 ± 8.3 points on the MMSE for all patients, with a mean of 11.0 ± 6.8 points for the group of patients suffering from dementia and a mean of 24.1 ± 4.3 points for the group of patients suffering from depression.
Feasibility
The results on the feasibility of the functional tests of the diagnostic algorithm are presented in Table 1. Short tests, such as the HGS measurement and the 4 m walking test, showed to be feasible. Especially, the 5×STS, the TUG, and the 400 m walking test could not be performed according to the original and standardized instructions. Although the patients were able to walk 4 m without help, most of the patients were not able to stand up from a chair without the help of their arms in the 5×STS. This phenomenon occurred in both, patients suffering from dementia (n = 13, 73.3%) and patients suffering from depression (n = 10, 57.9%). Due to the cognitive impairment, the TUG could not be implemented as instructed by patients suffering from dementia: n = 10 (66.7%) of the patients could not remember the test procedure or did not understand the whole task. The application of the 400 m walking test also proved to be difficult for a geriatric psychiatric target group: n = 9 (26.5%) patients could not walk such a long distance. In addition, n = 5 (17.6%) of the dementia patients tended to either only follow the instructor, to leave the test area, or to stop because the task could not be sufficiently processed. The BIA was well accepted by the patients in this study and the measurement could be performed with all patients.
TABLE 1.
Feasibility of functional tests with geriatric psychiatric patients, indicated in per cent (number of patients)
| Test | Possible | Not possible (reason) | ||
|---|---|---|---|---|
| physical | cognitive | motivational | ||
| HGS | 91.2% (31) | 2.9% (1) | 5.9% (2) | — |
| 5×STS | 32.4% (11) | 64.7% (22) | 2.9% (1) | — |
| 4 m walking test | 91.2% (31) | — | 5.9% (2) | 2.9% (1) |
| TUG | 67.6% (23) | — | 29.4% (10) | 2.9% (1) |
| SPPB (balance tasks) | 82.4% (28) | — | 14.7% (5) | 2.9% (1) |
| 400 m walking test | 38.2% (13) | 26.5% (9) | 17.6% (6) | 17.6% (6) |
5×STS, five‐times‐sit‐to‐stand test; HGS, handgrip strength; SPPB, Short Physical Performance Battery; TUG, timed‐up‐and‐go‐test.
Sarcopenia prevalence
In total n = 34 patients have been included for the muscle mass diagnostic and physical performance assessment. From n = 15 included patients suffering from dementia, n = 14 were diagnosed with sarcopenia—all in the severe stage of sarcopenia (Figure 3). Based on this ratio, n = 52 out of the n = 56 patients rated as being ‘frail’ would have been tested positive for sarcopenia. Taking all n = 80 admitted patients suffering from dementia into account, the analysis revealed an overall estimated prevalence rate for sarcopenia of 65%.
FIGURE 3.
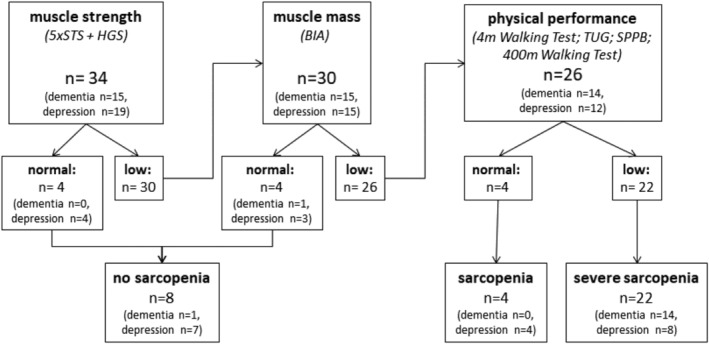
Classification of the patients according to the EWGSOP2 screening process and diagnostic algorithm into ‘no sarcopenia’, ‘sarcopenia’ and ‘severe sarcopenia’. 5×STS, five times sit to stand test; BIA, bioelectric impedance analysis; HGS, handgrip strength; SPPB, short physical performance battery; TUG, timed‐up‐and‐go‐test.
Out of n = 19 included patients suffering from depression, n = 12 were diagnosed with sarcopenia—n = 8 in the severe stage of sarcopenia. Based on this ratio, n = 24 out of the n = 38 patients rated as being ‘frail’ would have been tested positive for sarcopenia. Taking all n = 67 admitted patients suffering from depression into account, the analysis indicated an overall estimated prevalence rate for sarcopenia of 36%.
As no patient suffering from delirium could have been included in the final analysis, no results can be presented for this patient group.
Discussion
The aim of this study was to investigate the implementation of the EWGSOP2 sarcopenia screening algorithm in a geriatric psychiatry hospital, to investigate the feasibility of the algorithm, and to analyse the prevalence rates for sarcopenia.
The results of this study indicate that the implementation of sarcopenia diagnosis with patients suffering from dementia and depression in a geriatric psychiatry hospital is possible. Short and simple tests such as the HGS test and the 4 m walking test have shown to be more feasible as compared with longer and more complex tests like the 5×STS test, the TUG test, and the 400 m walking test. The analysis revealed an overall estimated prevalence for sarcopenia of 65% in patients suffering from dementia and 36% in patients suffering from depression. These results indicate that a screening and diagnostic procedure for sarcopenia is very important in geriatric psychiatry and sarcopenia as a comorbid muscular disease should not be underestimated.
Functional performance tests are a key aspect of the diagnostic procedure. The feasibility analysis revealed that the patients were not able to perform all tests due to their cognitive and physical impairment. Original standardized test instructions were often not sufficient to realize a correct test implementation without subjective influence by the researcher conducting the test. Only by repeating, further explaining, and demonstrating the tests again, persons were able to perform the tests. However, the analysis exposed at least one test for each of the components muscle strength, muscle mass, and physical performance, which the included patients were able to perform according to the original standardized instructions.
Within the functional tests, the measurement of HGS was carried out with only three exceptions according to the original and standardized instructions. The 5×STS could only be performed by n = 11 (32.4%) patients. The main reason was insufficient leg strength. Studies have shown that the test is usually not easy to perform for frail persons. 35 The 5×STS should therefore not be the first choice for assessing physical performance in geriatric patients. The BIA was well accepted by the patients in this study. Concerning physical fitness, the 4 m walking test was the most adequate test for the sample. This is in line with previous studies, where the 4 m walking test was performed with the highest feasibility rate in a clinical setting to assess physical performance. 35 The balance tasks of the SPPB showed limited feasibility, especially in patients suffering from dementia. Jacobsen et al. indicated that applying the SPPB should not be recommended for acutely ill persons due to floor effects. 17 Furthermore, Trumpf et al. reported a low feasibility for the SPPB for patients suffering from dementia (8.7%) and patients suffering from depression (46.4%) in geriatric psychiatry. 36 With only five persons (33.3%) of the patients being able to perform the TUG according to the original and standard instructions in our study, the TUG also proved to be unsuitable, especially for patients suffering from dementia. This is in line with Trumpf et al., who showed a low feasibility rate for the TUG of 8.7% in patients suffering from dementia. 36 The 400 m walking test proved to be inappropriate for the whole sample. The walking distance was too long, resulting in 26.5% (n = 9) of the patients having to stop the test due to pain, shortness of breath, or not being able to walk at all. Another explanation for the low feasibility rate for the 400 m test could be due to the fact that persons had to leave the ward for the test resulting in increased effort and expense, and thus, the motivation of the participants played a significant role.
Reported prevalence rates for sarcopenia in the clinical geriatric setting ranged from 10% 9 to about 20% 13 , 14 , 15 and about 30% 16 , 17 , 18 up to 40% 8 , 12 in other studies. When comparing our estimated prevalence rates for sarcopenia with the current literature, it must be considered that the pre‐selection process of our study only allows to indicate a statement for a subset of patients based on the results measured and does not represent a general prevalence rate for all patients being admitted to the geriatric psychiatry hospital. The study sample can be classified as a geriatric sample with an average age of 79 years and more female patients. Included patients suffering from depression were primarily diagnosed with recurrent major depressive disorders in severe stages and no accompanying cognitive impairment. According to the mean MMSE result of 11.0 points, the patients suffering from dementia had moderate to severe cognitive impairments. These group characteristics indicate predominantly severe stages of the diseases that link to a long disease course of the included patients. Furthermore, most of the available trials have applied a different algorithm for sarcopenia diagnosis. One study showed that the new algorithm of EWGSOP2 leads to a different classification of the cases ‘sarcopenia’ and ‘no sarcopenia’. 24 The prevalence rate in two other studies, comparing the sarcopenia algorithms by EWGSOP and EWGSOP2, was significantly lower by using the new algorithm and the overlap of individual cases was low. 22 , 23 A further key difference between this study and previously reported trials was that patients with cognitive impairments were especially addressed and included in our study. Dementia as a geriatric disease also carries a higher risk of developing sarcopenia. A systematic review published by Pacifico et al. in 2020 reported a prevalence rate of 26.4% for patients suffering from dementia. 10 As compared with these results, our estimated prevalence rate for sarcopenia of 65% in patients suffering from dementia in geriatric psychiatry care is considered as very high. Depression was also linked to the development of sarcopenia, as a high level of inactivity is typical for depression, which can promote the development of sarcopenia. 2 Our estimated sarcopenia prevalence rate of 36% in patients suffering from depression underlines this pathophysiological link.
As most of the patients were diagnosed with a severe stage of sarcopenia, the necessity of awareness for muscular weakness and physical constitution in geriatric psychiatry care is to be addressed. From our point of view, this high number of severe stages of the disease is due to the screening procedure using the frailty‐syndrome for the inclusion of patients at risk. Frailty is a well‐defined geriatric syndrome 27 that nevertheless rather reflects a subjective impression of the patients. In this project, the frailty screening process was mainly applied by the caretakers, as they have the most contact to the patients. However, it became obvious that many caretakers tended to overestimate the physical capacity of their patients. Another problem with the aspect of frailty was the time component. Patients first had to spend a few days on the ward before an assessment by the caretakers was possible. For the implementation in regular care, it is therefore recommended to use a different screening method or to promote a better awareness of muscular weakness and physical constitution in the context of the frailty‐syndrome.
It is important to note that out of the 104 patients rated as being ‘frail’, 70 patients (67%) could not be included in further investigations of the study—mainly due to early release, health condition, no legal guardian or no interest in taking part within the study. Therefore, more than half of the patients classified as being ‘frail’ could not be included in the feasibility analysis (Figure 2). This may result in an overestimation or underestimation of the sarcopenia prevalence calculated here. Furthermore, out of the 10 patients with delirium, no patient could be included in this study. The main reasons were no legal guardians or an authorized representative, or the health condition of the patients. Therefore, the project could only provide information on the diagnostic procedure and prevalence rate of sarcopenia for patients suffering from dementia and depression. As a methodological limitation, the missing information on the patients' diseases duration should be considered, as the prevalence of sarcopenia could be related to the dementia and depression progression.
In summary, this study shows that an implementation of sarcopenia diagnostic according to the algorithm of EWGSOP2 in a geriatric psychiatric hospital is possible and relevant. For this specific target group and stage of care, it appears to be important to develop a more sensitive screening procedure allowing to identify frailty and sarcopenia in an initial stage of the disease. Particularly, the assessment of physical performance was only relevant to evaluate the severity of sarcopenia. However, the treatment and intervention options do not change by the classification of the severity of sarcopenia. Therefore, the measurements of muscle strength by using the HGS test, as well as the measurement of muscle mass by BIA, are meaningful. Within the EWGSOP2 definition, muscle strength is up to date the first determining parameter to start the algorithm. In a second step, muscle mass using a BIA could be investigated. Based on these results, appropriate intervention strategies can already be determined. One possibility for an adapted screening procedure would be to perform the HGS measurement as part of the comprehensive geriatric assessment at admission to the hospital. This short test provides objective feedback instead of a subjective assessment of the patients. The BIA would then be performed on patients with low‐rated muscle strength in the HGS test.
As this study is one of the first studies with geriatric psychiatry patients implementing the new EWGSOP2 guidelines, further studies are needed that consider the limitations mentioned earlier and confirm the results obtained in this study. However, future research is needed in order to identify persons suffering from sarcopenia at an initial stage to be able to counteract the course of the disease.
Conflict of interest
Esther Sperlich, Tim Fleiner, Wiebren Zijlstra, Peter Haussermann, and Tobias Morat declare that they have no conflict of interest.
Acknowledgements
The authors thank all patients and legal guardians for their participation in the study. Special thanks belong to the caretakers of the LVR‐Hospital for their support in this study. In addition, we acknowledge the support of Axel Megerle and Simon Niemeyer in data acquisition. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 37
Sperlich E., Fleiner T., Zijlstra W., Haussermann P., and Morat T. (2021) Sarcopenia in geriatric psychiatry: feasibility of the diagnostic process and estimation of prevalence within a hospital context, Journal of Cachexia, Sarcopenia and Muscle, 12, 1153–1160, 10.1002/jcsm.12748.
Esther Sperlich and Tim Fleiner shared first authorship.
References
- 1. Deutsches Institut für Medizinische Dokumentation und Information . DIMDI—ICD‐10‐GM version 2018, 2018. https://www.dimdi.de/static/de/klassifikationen/icd/icd‐10‐gm/kode‐suche/htmlgm2018/ (accessed February 3, 2021).
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang S‐F, Lin P‐L. Systematic literature review and meta‐analysis of the association of sarcopenia with mortality. Worldviews Evid Based Nurs 2016;13:153–162. [DOI] [PubMed] [Google Scholar]
- 4. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012;31:652–658. [DOI] [PubMed] [Google Scholar]
- 5. Morley J. Sarcopenia in the elderly. Fam Pract 2012;29:i44–i48. [DOI] [PubMed] [Google Scholar]
- 6. Bianchi L, Ferrucci L, Cherubini A, Maggio M, Bandinelli S, Savino E, et al. The predictive value of the EWGSOP definition of sarcopenia: results from the InCHIANTI study. J Gerontol Ser A Biol Sci Med Sci 2016;71:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martone AM, Bianchi L, Abete P, Bellelli G, Bo M, Cherubini A, et al. The incidence of sarcopenia among hospitalized older patients: results from the Glisten study. J Cachexia Sarcopenia Muscle 2017;8:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Hufthammer KO, et al. Does sarcopenia predict change in mobility after hip fracture? A multicenter observational study with one‐year follow‐up. BMC Geriatr 2018;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antunes AC, Araújo DA, Veríssimo MT, Amaral TF. Sarcopenia and hospitalisation costs in older adults: a cross‐sectional study. Nutr Diet 2017;74:46–50. [DOI] [PubMed] [Google Scholar]
- 10. Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: a systematic review and meta‐analysis. Exp Gerontol 2020;131:110801. [DOI] [PubMed] [Google Scholar]
- 11. Bianchi L, Abete P, Bellelli G, Bo M, Cherubini A, Corica F, et al. Prevalence and clinical correlates of sarcopenia, identified according to the EWGSOP definition and diagnostic algorithm, in hospitalized older people: the GLISTEN study. J Gerontol Ser A 2017;72:1575–1581. [DOI] [PubMed] [Google Scholar]
- 12. Pérez‐Zepeda MU, Sgaravatti A, Dent E. Sarcopenia and post‐hospital outcomes in older adults: a longitudinal study. Arch Gerontol Geriatr 2017;69:105–109. [DOI] [PubMed] [Google Scholar]
- 13. Cerri AP, Bellelli G, Mazzone A, Pittella F, Landi F, Zambon A, et al. Sarcopenia and malnutrition in acutely ill hospitalized elderly: prevalence and outcomes. Clin Nutr 2015;34:745–751. [DOI] [PubMed] [Google Scholar]
- 14. González‐Montalvo JI, Alarcón T, Gotor P, Queipo R, Velasco R, Hoyos R, et al. Prevalence of sarcopenia in acute hip fracture patients and its influence on short‐term clinical outcome. Geriatr Gerontol Int 2016;16:1021–1027. [DOI] [PubMed] [Google Scholar]
- 15. Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc 2014;15:267–272. [DOI] [PubMed] [Google Scholar]
- 16. Bellelli G, Zambon A, Volpato S, Abete P, Bianchi L, Bo M, et al. The association between delirium and sarcopenia in older adult patients admitted to acute geriatrics units: results from the GLISTEN multicenter observational study. Clin Nutr 2018;37:1498–1504. [DOI] [PubMed] [Google Scholar]
- 17. Jacobsen EL, Brovold T, Bergland A, Bye A. Prevalence of factors associated with malnutrition among acute geriatric patients in Norway: a cross‐sectional study. BMJ Open 2016;6:e011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Murthy L, Grill V, et al. High parathyroid hormone levels are associated with osteosarcopenia in older individuals with a history of falling. Maturitas 2018;113:21–25. [DOI] [PubMed] [Google Scholar]
- 19. Yang M, Liu Y, Zuo Y, Tang H. Sarcopenia for predicting falls and hospitalization in community‐dwelling older adults: EWGSOP versus EWGSOP2. Sci Rep 2019;9:17636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang L, Yao X, Shen J, Sun G, Sun Q, Tian X, et al. Comparison of revised EWGSOP criteria and four other diagnostic criteria of sarcopenia in Chinese community‐dwelling elderly residents. Exp Gerontol 2020;130:110798. [DOI] [PubMed] [Google Scholar]
- 21. Bachettini NP, Bielemann RM, Barbosa‐Silva TG, Menezes AMB, Tomasi E, Gonzalez MC. Sarcopenia as a mortality predictor in community‐dwelling older adults: a comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur J Clin Nutr 2019;74:573–580. [DOI] [PubMed] [Google Scholar]
- 22. de Freitas MM, de Oliveira VLP, Grassi T, Valduga K, Miller MEP, Schuchmann RA, et al. Difference in sarcopenia prevalence and associated factors according to 2010 and 2018 European consensus (EWGSOP) in elderly patients with type 2 diabetes mellitus. Exp Gerontol 2020;132:110835. [DOI] [PubMed] [Google Scholar]
- 23. Petermann‐Rocha F, Chen M, Gray SR, Ho FK, Pell JP, Celis‐Morales C. New versus old guidelines for sarcopenia classification: what is the impact on prevalence and health outcomes? Age Ageing 2019;19:300–304. [DOI] [PubMed] [Google Scholar]
- 24. Reiss J, Iglseder B, Alzner R, Mayr‐Pirker B, Pirich C, Kässmann H, et al. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing 2019;48:713–718. [DOI] [PubMed] [Google Scholar]
- 25. Vrbova P, Smaha J, Stepan J, Tobias D, Kuzma M, Payer J, et al. Cross‐sectional pilot study: prevalence of sarcopenia among hospitalized internal medicine patients: a cross‐sectional single‐center pilot study according to EWGSOP2 criteria. Bratislava Med J 2019;120:717–722. [DOI] [PubMed] [Google Scholar]
- 26. Verlaan S, Van Ancum JM, Pierik VD, Van Wijngaarden JP, Scheerman K, Meskers CGM, et al. Muscle measures and nutritional status at hospital admission predict survival and independent living of older patients—the EMPOWER study. J Frailty Aging 2017;6:161–166. [DOI] [PubMed] [Google Scholar]
- 27. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 28. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 29. Hamilton GF, McDonald C, Chenier TC. Measurement of grip strength: validity and reliability of the sphygmomanometer and Jamar grip dynamometer. J Orthop Sport Phys Ther 1992;16:215–219. [DOI] [PubMed] [Google Scholar]
- 30. Goldberg A, Chavis M, Watkins J, Wilson T. The five‐times‐sit‐to‐stand test: validity, reliability and detectable change in older females. Aging Clin Exp Res 2012;24:339–344. [DOI] [PubMed] [Google Scholar]
- 31. Maggio M, Ceda GP, Ticinesi A, De Vita F, Gelmini G, Costantino C, et al. Instrumental and non‐instrumental evaluation of 4‐meter walking speed in older individuals. PLoS ONE 2016;11:e0153583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 33. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 34. Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M. Reliability of the 400‐m usual‐pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc 2004;52:972–976. [DOI] [PubMed] [Google Scholar]
- 35. Bodilsen AC, Juul‐Larsen HG, Petersen J, Beyer N, Andersen O, Bandholm T. Feasibility and inter‐rater reliability of physical performance measures in acutely admitted older medical patients. PLoS ONE 2015;10:e0118248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trumpf R, Morat T, Zijlstra W, Haussermann P, Fleiner T. Assessment of functional performance in acute geriatric psychiatry—time for new strategies? J Geriatr Psychiatry Neurol 2020;33:316–323. [DOI] [PubMed] [Google Scholar]
- 37. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


