Abstract
Background
Sarcopenia is a significant indicator of the severity of non‐alcoholic fatty liver disease. We investigated whether sarcopenia could identify subgroups with different risk of liver fibrosis and atherosclerotic cardiovascular disease (ASCVD) among subjects with metabolic dysfunction‐associated fatty liver disease (MAFLD).
Methods
Subjects from the Korea National Health and Nutrition Examination Survey 2008–2011 were selected (n = 8361). Sarcopenia was defined using the sarcopenia index. Hepatic steatosis was defined as a fatty liver index ≥30. Significant liver fibrosis was defined as a fibrosis‐4 index (FIB‐4) ≥2.67 or the highest quartile of non‐alcoholic fatty liver disease fibrosis score (NFS). High probability of ASCVD was defined as ASCVD risk score >10%.
Results
The mean age was 48.5 ± 15.6 years, and 42.6% of subjects were male. The prevalence of MAFLD was 37.3% (n = 3116 of 8361), and the proportion of sarcopenic subjects was 9.9% among those with MAFLD. After adjusting for confounders, the risk of significant liver fibrosis significantly increased from non‐sarcopenic subjects with MAFLD [odds ratio (OR) = 1.57 by FIB‐4 and 2.13 by NFS] to sarcopenic subjects with MAFLD (OR = 4.51 by FIB‐4 and 5.72 by NFS), compared with subjects without MAFLD (all P < 0.001). The risk for high probability of ASCVD significantly increased from non‐sarcopenic subjects with MAFLD (OR = 1.47) to sarcopenic subjects with MAFLD (OR = 4.08), compared with subjects without MAFLD (all P < 0.001).
Conclusions
The risks of significant liver fibrosis and ASCVD differed significantly according to sarcopenic status among subjects with MAFLD. An assessment of sarcopenia might be helpful in risk stratification among subjects with MAFLD.
Keywords: Metabolic dysfunction‐associated fatty liver disease, Sarcopenia, Liver fibrosis, Atherosclerotic cardiovascular disease, Risk stratification
Introduction
The new term metabolic dysfunction‐associated fatty liver disease (MAFLD) has been suggested to reflect current knowledge of fatty liver disease associated with metabolic dysfunction more accurately. 1 , 2 As compared with non‐alcoholic fatty liver disease (NAFLD), MAFLD is a diagnosis of inclusion, based on the presence of hepatic steatosis and the coexistence of a set of metabolic risk factors. 1 , 2 , 3 According to recent reports, MAFLD is more likely to capture patients at high risk of hepatic and extrahepatic complications, supporting the positive implications of the change from NAFLD to MAFLD. 4 , 5 , 6 However, there has been no study of stratifying patients with newly defined MAFLD according to disease severity.
Sarcopenia has been linked to fatty liver disease, because it shares the main potential pathophysiological mechanisms of insulin resistance and increased inflammation. 7 In line with these shared mechanisms, sarcopenia was significantly associated with liver fibrosis in patients with NAFLD, independently of obesity and insulin resistance. 8 Subsequent studies confirmed this finding, supporting a role for sarcopenia in the stratification of disease severity in patients with NAFLD. 8 , 9 , 10 Accordingly, the presence of sarcopenia is now regarded as an important indicator of the severity and prognosis of NAFLD, which might be extrapolated into MAFLD because of its substantial overlap with NAFLD.
Liver fibrosis is considered the most relevant predictor of poor prognosis in patients with NAFLD. 11 , 12 A recent meta‐analysis reported that the risks of all‐cause mortality, liver transplantation, and liver‐related events increased as the stage of fibrosis increased. 12 In addition, atherosclerotic cardiovascular disease (ASCVD) confers the most common cause of morbidity and mortality in patients with NAFLD. 13 , 14 The severity of NAFLD is associated with ASCVD risk, independently of other cardiovascular risk factors. 15 , 16 Based on these prognostic implications of liver fibrosis and ASCVD in patients with NALFD, the severities of these two factors might also differ among subgroups of patients according to MAFLD severity. 4 , 5 , 6
In this study, we investigated whether the stratification of patients with MAFLD using sarcopenia status could identify subgroups with different disease severity, in terms of the degree of liver fibrosis and the risk of ASCVD, using the Korea National Health and Nutrition Examination Survey (KNHANES) database.
Methods
Patients
The KNHANES involves a nationwide, population‐based, cross‐sectional health examination and survey that is annually conducted by the Division of Chronic Disease Surveillance of the Korea Centers for Disease Control and Prevention in the Ministry of Health and Welfare. 17 Each KNHANES is composed of independent datasets of subjects from the general population of South Koreans. These subjects are randomly selected from 600 randomly selected districts of cities and provinces in South Korea.
As depicted in Supporting Information, Figure S1, among a total of 37 753 subjects in the KNHANES 2008–2011, we included 28 071 subjects aged ≥20 years (12 160 men and 15 911 women). Of these, 19 710 subjects who met the following criteria were excluded: (i) missing data for appendicular skeletal muscle mass (ASM) (n = 9387); (ii) insufficient data to calculate the degree of fibrosis (n = 7627); (iii) insufficient data to diagnose MAFLD (n = 2352); and (iv) history of ASCVD before enrolment (n = 344). Finally, 8361 subjects were included in the analysis.
The use of the KNHANES data was conducted following the ethical approval by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (Nos. 2008‐04EXP‐01‐C, 2009‐01CON‐03‐2C, 2010‐02CON‐21‐C, and 2011‐02CON‐06C).
Definition of metabolic dysfunction‐associated fatty liver disease and non‐alcoholic fatty liver disease
Metabolic dysfunction‐associated fatty liver disease was defined as the presence of hepatic steatosis with any one of the following three conditions: overweight/obesity [body mass index (BMI) ≥23 kg/m2], diabetes mellitus, or ≥2 metabolic dysregulations. 1 Metabolic dysregulations were defined as follows: (i) waist circumference ≥90 cm in men and ≥80 cm in women; (ii) blood pressure ≥130/85 mmHg or specific drug treatment; (iii) triglyceride ≥150 mg/dL or specific drug treatment; (iv) high‐density lipoprotein cholesterol <40 mg/dL for men and <50 mg/dL for women or specific drug treatment; (v) prediabetes (fasting glucose levels 100–125 mg/dL); and (vi) homeostasis model assessment of insulin resistance (HOMA‐IR) score ≥2.5. 1 , 18 NAFLD was defined as the presence of hepatic steatosis in the absence of significant alcohol consumption and concomitant liver disease. 19
Definition of sarcopenia
Appendicular skeletal muscle mass was measured using dual‐energy X‐ray absorptiometry (QDR 4500A; Hologic, Inc., Bedford, MA, USA). 8 The sarcopenia index (SI) was calculated using the following formula: SI = total ASM (kg)∕BMI (kg/m2). Sarcopenia was defined as SI < 0.789 in men and <0.521 in women, based on the recent recommendation. 20
Definitions of hepatic steatosis and significant liver fibrosis
Hepatic steatosis and significant liver fibrosis were defined using well‐validated prediction models. Hepatic steatosis was assessed using the fatty liver index, and the comprehensive NAFLD score was used to validate the main finding. 21 Hepatic steatosis was defined as a fatty liver index ≥30 or a comprehensive NAFLD score ≥40 (Table S1). 21 The fibrotic burden of the liver was assessed using the fibrosis‐4 index (FIB‐4) or NAFLD fibrosis score (NFS). 22 Significant liver fibrosis was defined as either FIB‐4 ≥ 2.67 23 or the highest quartile of NFS (Table S1). Because NFS could not be calculated because of the absence of serum albumin data in the KNHANES cohort, we defined significant liver fibrosis as the highest quartile of NFS, which was similarly used in previous studies. 8 , 24
Definition of atherosclerotic cardiovascular risk
The atherosclerotic cardiovascular risk was calculated using the 10 year ASCVD risk prediction model of the 2013 American College of Cardiology/American Heart Association Guideline. 25 High probability of ASCVD was defined as ASCVD score >10%. 26
Definition of covariates
Demographic and lifestyle data were obtained using a self‐reported questionnaire. Smoking status was classified as non‐smoker or current smoker. Data from health examinations and blood specimens were also provided. Blood specimens were collected after overnight fasting for at least 8 h and analysed within 24 h of collection. 27 The HOMA‐IR score was assessed as previously described. 28
Significant alcohol consumption was defined as alcohol intake ≥30 g/day for men or ≥20 g/day for women. Regular exercise was defined as moderate or vigorous physical activity for ≥20 min at least three times per week. Obesity was defined as BMI ≥ 25 kg/m2, and central obesity was defined as waist circumference ≥90 cm in men and ≥80 cm in women. Diabetes was defined as a fasting plasma glucose level ≥126 mg/dL, or glycated haemoglobin ≥6.5%, or the use of insulin or oral hypoglycaemic agents. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medications. Chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2. 29
Statistical analysis
Data are expressed as the mean ± standard deviation or n (%), as appropriate. Differences between continuous and categorical variables were compared using Student's t‐test (or the Mann–Whitney U test, as appropriate) and the χ 2 test (or Fisher's exact test, as appropriate).
Multivariate logistic regression analysis was used to examine the independent risks of significant liver fibrosis and ASCVD, according to the status of MAFLD/sarcopenia. Adjustments for variables were as follows: Model 1, age and sex; Model 2, variables in Model 1 plus alcohol consumption, smoking status, and exercise; and Model 3, variables in Model 2 plus chronic kidney disease and malignancy. Because there were significant correlations among age, SI, and FIB‐4 or NFS (Table S2), an interaction term between age and SI (age × SI) was additionally included in all multiple regression models for significant liver fibrosis. The adjusted odds ratio (aOR) and its 95% confidence interval were calculated for each model. All statistical analyses were performed using SPSS Version 25.0 (IBM, Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of the study population are described in Table 1. The mean age was 48.5 years, and 42.6% of subjects were men. The mean ASM and SI were 18.7 kg and 0.80, respectively. Among the 8361 subjects in this study, MAFLD was identified in 3116 (37.3%). Subjects with MAFLD were significantly older and predominantly male (all P < 0.001). They were more likely to have unfavourable cardiovascular risk factors, consume significant amounts of alcohol, be current smokers, have significant liver fibrosis, and have a high probability of ASCVD, compared with subjects without MAFLD (n = 5245, 62.7%) (all P < 0.001). In addition, liver, metabolic, and kidney function were more unfavourable in subjects with MAFLD than in those without MAFLD (all P < 0.05) (Table 1).
Table 1.
Baseline characteristics of the study population
| Variables | Total | Non‐MAFLD | MAFLD | P value* | P value** | |||
|---|---|---|---|---|---|---|---|---|
| All | Without sarcopenia | With sarcopenia | P value | |||||
| (n = 8361) | (n = 5245, 62.7%) | (n = 3116, 37.3%) | (n = 2806, 90.1%) | (n = 310, 9.9%) | ||||
| Demographic variables | ||||||||
| Age (years) | 48.5 ± 15.6 | 46.4 ± 15.9 | 52.1 ± 14.4 | 50.8 ± 14.1 | 63.3 ± 12.6 | <0.001 | <0.001 | <0.001 |
| Male gender | 3558 (42.6) | 1725 (32.9) | 1833 (58.8) | 1703 (60.7) | 130 (41.9) | <0.001 | <0.001 | <0.001 |
| Body mass index (kg/m2) | 23.5 ± 3.3 | 21.9 ± 2.4 | 26.2 ± 2.8 | 26.1 ± 2.7 | 27.6 ± 3.1 | 0.027 | <0.001 | <0.001 |
| Waist circumference (cm) | 80.9 ± 9.9 | 75.7 ± 7.3 | 89.5 ± 7.2 | 89.3 ± 7.0 | 91.9 ± 7.9 | 0.001 | <0.001 | <0.001 |
| Appendicular skeletal muscle mass (kg) | 18.7 ± 4.9 | 17.3 ± 4.3 | 21.1 ± 5.1 | 21.6 ± 4.9 | 16.3 ± 3.8 | <0.001 | <0.001 | <0.001 |
| Sarcopenia index | 0.80 ± 0.19 | 0.79 ± 0.19 | 0.81 ± 0.19 | 0.83 ± 0.18 | 0.59 ± 0.13 | <0.001 | <0.001 | <0.001 |
| Central obesity | 2918 (34.9) | 912 (17.4) | 2006 (64.4) | 1753 (62.5) | 253 (81.6) | <0.001 | <0.001 | <0.001 |
| Obesity | 2579 (30.8) | 540 (10.3) | 2039 (65.4) | 1786 (63.6) | 253 (81.6) | <0.001 | <0.001 | <0.001 |
| Hypertension | 2193 (26.2) | 900 (17.2) | 1293 (41.5) | 1114 (39.7) | 179 (57.7) | <0.001 | <0.001 | <0.001 |
| Diabetes | 762 (9.1) | 268 (5.1) | 494 (15.9) | 419 (14.9) | 77 (24.8) | <0.001 | <0.001 | <0.001 |
| Metabolic syndrome | 2437 (29.1) | 568 (10.8) | 1869 (60.0) | 1634 (58.2) | 234 (75.8) | <0.001 | <0.001 | <0.001 |
| Smoking (current) | 1725 (20.6) | 854 (16.3) | 871 (28.0) | 824 (29.4) | 47 (15.2) | <0.001 | <0.001 | <0.001 |
| Significant alcohol consumption | 1329 (15.9) | 625 (11.9) | 704 (22.6) | 671 (23.9) | 33 (10.6) | <0.001 | <0.001 | <0.001 |
| Regular exercise | 4414 (52.8) | 2762 (52.7) | 1652 (53.0) | 1501 (53.5) | 151 (48.7) | 0.109 | 0.752 | 0.476 |
| Malignancy | 214 (2.6) | 145 (2.8) | 69 (2.2) | 57 (2.0) | 12 (3.9) | 0.037 | 0.124 | 0.045 |
| Laboratory variables | ||||||||
| Platelet count (109/L) | 254.1 ± 57.3 | 254.2 ± 57.2 | 253.9 ± 57.5 | 252.7 ± 57.1 | 264.8 ± 60.5 | 0.001 | 0.822 | 0.262 |
| Aspartate aminotransferase (IU/L) | 22.4 ± 14.2 | 20.1 ± 8.8 | 26.4 ± 19.6 | 26.4 ± 20.2 | 26.0 ± 12.1 | 0.588 | <0.001 | <0.001 |
| Alanine aminotransferase (IU/L) | 21.4 ± 18.4 | 16.8 ± 10.5 | 29.3 ± 25.1 | 29.5 ± 25.7 | 26.8 ± 18.7 | 0.023 | <0.001 | <0.001 |
| γ‐Glutamyltransferase (mg/dL) | 41.2 ± 48.9 | 28.4 ± 31.9 | 62.7 ± 63.1 | 63.1 ± 63.8 | 59.6 ± 56.5 | 0.318 | <0.001 | <0.001 |
| Serum creatinine (mg/dL) | 0.84 ± 0.23 | 0.81 ± 0.24 | 0.89 ± 0.20 | 0.89 ± 0.19 | 0.86 ± 0.23 | 0.001 | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 87.5 ± 17.6 | 89.5 ± 17.7 | 84.2 ± 17.0 | 84.5 ± 16.7 | 81.2 ± 19.3 | 0.001 | 0.045 | 0.006 |
| Total cholesterol (mg/dL) | 188.6 ± 36.1 | 181.4 ± 33.0 | 200.8 ± 37.6 | 200.2 ± 37.2 | 206.5 ± 41.2 | 0.005 | <0.001 | <0.001 |
| Triglyceride (mg/dL) | 133.2 ± 110.4 | 92.4 ± 47.0 | 201.8 ± 146.5 | 203.1 ± 149.0 | 189.6 ± 121.6 | 0.071 | <0.001 | <0.001 |
| HDL cholesterol (mg/dL) | 48.2 ± 10.9 | 51.0 ± 10.8 | 43.6 ± 9.2 | 43.6 ± 9.1 | 43.8 ± 10.2 | 0.010 | <0.001 | <0.001 |
| LDL cholesterol (mg/dL) | 114.8 ± 31.8 | 111.3 ± 29.8 | 121.0 ± 34.3 | 120.2 ± 33.7 | 127.9 ± 38.5 | 0.003 | <0.001 | <0.001 |
| Fasting glucose (mg/dL) | 97.3 ± 21.5 | 93.0 ± 16.8 | 104.5 ± 26.0 | 104.1 ± 26.0 | 107.8 ± 26.0 | 0.018 | <0.001 | <0.001 |
| Fasting insulin (μIU/mL) | 9.9 ± 4.5 | 8.9 ± 3.5 | 11.7 ± 5.5 | 11.5 ± 5.3 | 13.0 ± 6.5 | 0.001 | <0.001 | <0.001 |
| HOMA‐IR | 2.4 ± 1.4 | 2.0 ± 1.0 | 3.0 ± 1.8 | 3.0 ± 1.7 | 3.5 ± 2.2 | <0.001 | <0.001 | <0.001 |
| Liver steatosis assessment | ||||||||
| Fatty liver index | 28.7 ± 24.9 | 12.4 ± 9.0 | 56.1 ± 18.0 | 55.7 ± 17.9 | 60.3 ± 18.9 | <0.001 | <0.001 | <0.001 |
| Comprehensive NAFLD score | 30.8 ± 30.9 | 11.9 ± 14.2 | 62.6 ± 24.5 | 61.6 ± 24.6 | 71.8 ± 22.0 | <0.001 | <0.001 | <0.001 |
| Liver fibrosis assessment | ||||||||
| FIB‐4 | 1.06 ± 0.80 | 1.01 ± 0.73 | 1.14 ± 0.90 | 1.11 ± 0.92 | 1.33 ± 0.67 | <0.001 | 0.001 | 0.001 |
| NAFLD fibrosis score | 0.539 ± 1.288 | 0.303 ± 1.220 | 0.936 ± 1.302 | 0.871 ± 1.289 | 1.519 ± 1.272 | <0.001 | <0.001 | <0.001 |
| Cardiovascular disease risk assessment | ||||||||
| ASCVD risk score (%) | 6.9 ± 10.5 | 5.2 ± 9.6 | 9.7 ± 11.3 | 8.8 ± 10.3 | 17.7 ± 15.5 | <0.001 | <0.001 | <0.001 |
ASCVD, atherosclerotic cardiovascular disease; eGFR, estimated glomerular filtration rate; FIB‐4, fibrosis‐4 index; HDL, high‐density lipoprotein; HOMA‐IR, homoeostatic model assessment of insulin resistance; LDL, low‐density lipoprotein; MAFLD, metabolic dysfunction‐associated fatty liver disease; NAFLD, non‐alcoholic fatty liver disease.
Variables are expressed as mean ± SD or n (%).
P value indicates the comparison between non‐MAFLD and MAFLD.
P value indicates the comparison between non‐MAFLD and MAFLD without sarcopenia.
Among subjects with MAFLD, 310 (9.9%) had sarcopenia. Sarcopenic subjects were older than non‐sarcopenic subjects (n = 2806, 90.1%), and female sex was predominant (all P < 0.001). The proportions of subjects with obesity, central obesity, hypertension, diabetes, metabolic syndrome, and malignancy were significantly higher among sarcopenic subjects, whereas the proportions of subjects who were current smokers and consumed significant amounts of alcohol were significantly lower among sarcopenic subjects (all P < 0.05). In addition, platelet count, total cholesterol level, high‐density lipoprotein cholesterol level, low‐density lipoprotein cholesterol level, fasting glucose level, fasting insulin level, and HOMA‐IR score were significantly higher in sarcopenic subjects than in non‐sarcopenic subjects (all P < 0.05), whereas alanine aminotransferase levels, serum creatinine levels, and estimated glomerular filtration rate were significantly lower in sarcopenic subjects (all P < 0.05). Moreover, sarcopenic subjects had higher FIB‐4, NFS, and ASCVD risk score than those without sarcopenia (Table 1) (all P < 0.001).
Associations of metabolic dysfunction‐associated fatty liver disease/sarcopenia status with fibrotic burden and atherosclerotic cardiovascular disease risk by quartile stratification
When FIB‐4 and NFS were stratified by quartiles, the degree of fibrotic burden significantly increased from subjects without MAFLD to non‐sarcopenic subjects with MAFLD and sarcopenic subjects with MAFLD (all P for trend <0.001) (Figure 1). The ASCVD risk score significantly increased from subjects without MAFLD to non‐sarcopenic subjects with MAFLD and sarcopenic subjects with MAFLD, when ASCVD risk score was stratified by quartiles (all P for trend <0.001) (Figure 2).
Figure 1.
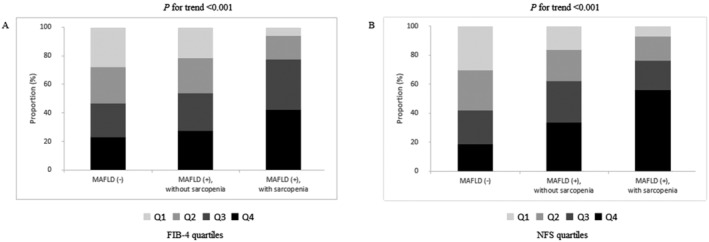
Association between the status of metabolic dysfunction‐associated fatty liver disease (MAFLD)/sarcopenia and fibrotic burden by quartile stratification. When (A) fibrosis‐4 index (FIB‐4) and (B) non‐alcoholic fatty liver disease fibrosis score (NFS) were stratified by quartiles, the degree of fibrotic burden significantly increased from subjects without MAFLD to non‐sarcopenic subjects with MAFLD and sarcopenic subjects with MAFLD (all P for trend <0.001).
Figure 2.
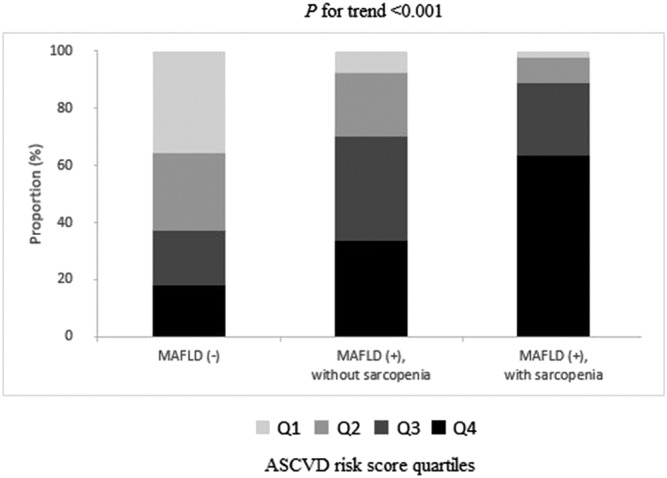
Associations between the status of metabolic dysfunction‐associated fatty liver disease (MAFLD)/sarcopenia and atherosclerotic cardiovascular disease (ASCVD) risk by quartile stratification. When ASCVD risk score was stratified by quartiles, the ASCVD risk significantly increased from subjects without MAFLD to non‐sarcopenic subjects with MAFLD and sarcopenic subjects with MAFLD (all P for trend <0.001).
When we assessed the associations of MAFLD status with SI, fibrotic burden, and ASCVD risk by quartile stratification, the degree of sarcopenia, fibrotic burden (by FIB‐4 or NFS), and ASCVD risk were significantly higher in subjects with MAFLD than in those without MAFLD (P for trend <0.001) (Figure S2). The prevalence of significant liver fibrosis was significantly higher in subjects with MAFLD (29.7% by FIB‐4 and 35.8% by NFS) than in those without MAFLD (22.6% by FIB‐4 and 18.6% by NFS) (all P < 0.001). Subjects with MAFLD had significantly higher prevalence of high probability of ASCVD than those without MAFLD (all P < 0.001). Compared with subjects without MAFLD, those with MAFLD showed significantly increased relative risks of significant liver fibrosis [odds ratio (OR) = 1.94 by FIB‐4; OR = 2.54 by NFS] and high probability of ASCVD (OR = 3.74) (all P < 0.001) (Figure S3).
Risk of significant liver fibrosis according to the status of metabolic dysfunction‐associated fatty liver disease/sarcopenia
The prevalence of significant liver fibrosis was highest in sarcopenic subjects with MAFLD (6.1% by FIB‐4 and 56.1% by NFS), followed by non‐sarcopenic subjects with MAFLD (3.0% by FIB‐4 and 33.5% by NFS), and then subjects without MAFLD (1.7% by FIB‐4 and 18.6% by NFS) (Figure 3). When subjects without MAFLD were considered as a reference group, the risk of significant liver fibrosis significantly increased from non‐sarcopenic subjects with MAFLD (OR = 1.36 by FIB‐4; OR = 2.44 by NFS) to sarcopenic subjects with MAFLD (OR = 2.44 by FIB‐4; OR = 5.60 by NFS) (all P < 0.001) (Figure 3).
Figure 3.

Prevalence and relative risk of significant liver fibrosis according to the status of metabolic dysfunction‐associated fatty liver disease (MAFLD)/sarcopenia. The prevalence and relative risk of significant liver fibrosis [(A) by fibrosis‐4 index (FIB‐4) and (B) by non‐alcoholic fatty liver disease fibrosis score (NFS)] significantly increased from non‐sarcopenic subjects with MAFLD to sarcopenic subjects with MAFLD, compared with those without MAFLD (all P < 0.001). CI, confidence interval; OR, odds ratio.
After multistep adjustments for confounders that might affect significant liver fibrosis, MAFLD subjects, regardless of sarcopenia, showed significantly higher aOR for significant liver fibrosis, by both FIB‐4 and NFS, in all adjustment models, compared with subjects without MAFLD (all P < 0.001). The aOR was higher in sarcopenic subjects with MAFLD (aOR = 4.51 by FIB‐4; aOR = 5.72 by NFS) than in non‐sarcopenic subjects with MAFLD (aOR = 1.57 by FIB‐4; aOR = 2.13 by NFS) after sufficient adjustment (Model 3). In an analysis of subjects with MAFLD, sarcopenic subjects showed significantly increased risk of significant liver fibrosis (aOR = 3.17 by FIB‐4; aOR = 3.13 by NFS), compared with non‐sarcopenic subjects (Table 2).
Table 2.
The risk of significant liver fibrosis according to the status of MAFLD/sarcopenia
| Groups | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Liver fibrosis assessment by FIB‐4 | |||||||||
| Entire study population | |||||||||
| Non‐MAFLD | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||||
| MAFLD without sarcopenia | 1.62 | 1.18–2.23 | 0.003 | 1.56 | 1.14–2.15 | 0.006 | 1.57 | 1.14–2.16 | 0.006 |
| MAFLD with sarcopenia | 4.20 | 2.41–7.33 | <0.001 | 4.50 | 2.57–7.87 | <0.001 | 4.51 | 2.58–7.90 | <0.001 |
| Subgroup with MAFLD | |||||||||
| MAFLD without sarcopenia | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||||
| MAFLD with sarcopenia | 2.80 | 1.59–4.94 | <0.001 | 3.17 | 1.78–5.63 | <0.001 | 3.17 | 1.78–5.63 | <0.001 |
| Liver fibrosis assessment by NFS | |||||||||
| Entire study population | |||||||||
| Non‐MAFLD | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||||
| MAFLD without sarcopenia | 2.13 | 1.88–2.41 | <0.001 | 2.12 | 1.87–2.40 | <0.001 | 2.13 | 1.88–2.42 | <0.001 |
| MAFLD with sarcopenia | 5.69 | 4.36–7.43 | <0.001 | 5.70 | 4.37–7.45 | <0.001 | 5.72 | 4.38–7.47 | <0.001 |
| Subgroup with MAFLD | |||||||||
| MAFLD without sarcopenia | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||||
| MAFLD with sarcopenia | 3.04 | 2.30–4.01 | <0.001 | 3.12 | 2.36–4.13 | <0.001 | 3.13 | 2.36–4.13 | <0.001 |
CI, confidence interval; CKD, chronic kidney disease; FIB‐4, fibrosis‐4 index; MAFLD, metabolic dysfunction‐associated fatty liver disease; NFS, non‐alcoholic fatty liver disease fibrosis score; OR, odds ratio; SI, sarcopenia index.
Model 1 = age (applied as a categorical variable with a median cut‐off value of 47), age × SI, and gender. Model 2 = Model 1 + alcohol, smoking, and exercise. Model 3 = Model 2 + CKD and malignancy.
When comprehensive NAFLD score was used to define hepatic steatosis, similar findings were observed (Table S3). In addition, when we excluded subjects with malignancy, similar findings were obtained (Table S4).
High probability of atherosclerotic cardiovascular disease according to the status of metabolic dysfunction‐associated fatty liver disease/sarcopenia
We assessed the relative risk for high probability of ASCVD according to the status of MAFLD/sarcopenia. The prevalence of high probability of ASCVD increased from subjects without MAFLD (16.3%) to non‐sarcopenic subjects with MAFLD (29.2%) and sarcopenic subjects with MAFLD (60.6%). When subjects without MAFLD were considered as a reference group, the risk for high probability of ASCVD significantly increased from non‐sarcopenic subjects with MAFLD (OR = 2.12) to sarcopenic subjects with MAFLD (OR = 7.91) (all P < 0.001) (Figure 4).
Figure 4.
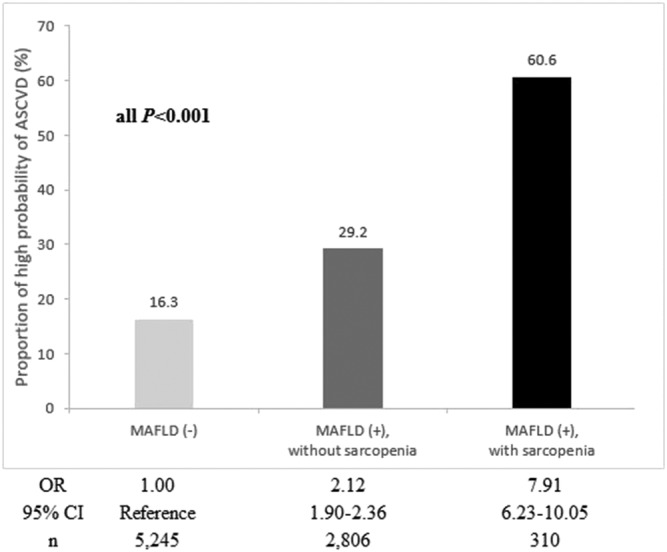
Prevalence and relative risk of high probability of atherosclerotic cardiovascular disease (ASCVD) risk according to the status of metabolic dysfunction‐associated fatty liver disease (MAFLD)/sarcopenia. The prevalence and relative risk of high probability of ASCVD significantly increased from non‐sarcopenic subjects with MAFLD to sarcopenic subjects with MAFLD, compared with those without MAFLD (P < 0.001). CI, confidence interval; OR, odds ratio.
The risk for high probability of ASCVD after multistep adjustments is shown in Table 3. Compared with subjects without MAFLD, MAFLD subjects, regardless of sarcopenia, showed significantly increased aOR for high probability of ASCVD in all adjustment models (all P < 0.001). In addition, sarcopenic subjects with MAFLD exhibited higher aOR (aOR = 4.08) than non‐sarcopenic subjects with MAFLD (aOR = 1.47) after sufficient adjustment (Model 3) (all P < 0.001). Among subjects with MAFLD, sarcopenic subjects showed significantly increased risk for high probability of ASCVD (aOR = 2.78), compared with non‐sarcopenic subjects (Table 3).
Table 3.
The risk for high probability of ASCVD risk according to the status of MAFLD/sarcopenia
| Groups | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Entire study population | |||||||||
| Non‐MAFLD | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||||
| MAFLD without sarcopenia | 1.44 | 1.26–1.65 | <0.001 | 1.46 | 1.27–1.67 | <0.001 | 1.47 | 1.28–1.68 | <0.001 |
| MAFLD with sarcopenia | 3.98 | 3.04–5.22 | <0.001 | 4.06 | 3.09–5.33 | <0.001 | 4.08 | 3.11–5.36 | <0.001 |
| Subgroup with MAFLD | |||||||||
| MAFLD without sarcopenia | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||||
| MAFLD with sarcopenia | 2.71 | 2.06–3.56 | <0.001 | 2.77 | 2.10–3.66 | <0.001 | 2.78 | 2.10–3.67 | <0.001 |
ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CKD, chronic kidney disease; MAFLD, metabolic dysfunction‐associated fatty liver disease; OR, odds ratio.
Model 1 = age (applied as a categorical variable with a median cut‐off value of 47) and gender. Model 2 = Model 1 + alcohol, smoking, and exercise. Model 3 = Model 2 + CKD and malignancy.
Similar findings were observed when hepatic steatosis was defined using the comprehensive NAFLD score (Table S5) and after the exclusion of subjects with malignancy (Table S6).
Risk of sarcopenia according to the metabolic dysfunction‐associated fatty liver disease diagnostic criteria
Our subjects with MAFLD (n = 3116) were differentially diagnosed according to the MAFLD diagnostic criteria (Table S7). The proportion of subjects with MAFLD was 10.4% (n = 324 of 3116), 75.7% (n = 2358 of 3116), and 13.9% (n = 434 of 3116) in each group who met one, two, and three criteria, respectively. The risk of sarcopenia increased as the number of diagnostic criteria met increased: 2.5%, 9.6%, and 17.3% in each group who met one, two, and three criteria, respectively.
Risk of significant liver fibrosis and high probability of atherosclerotic cardiovascular disease according to the status of non‐alcoholic fatty liver disease/sarcopenia
Of the study population, 2344 (28.0%) had NAFLD, which was lower than the prevalence of subjects with MAFLD (n = 3116, 37.3%). The proportion of subjects who met both MAFLD and NAFLD criteria was 27.4% (n = 2292). Using the MAFLD definition, 824 (9.9%) subjects who were previously not defined as NAFLD were classified into the population with MAFLD. Conversely, 52 (0.6%) subjects who had been previously defined as NAFLD were not classified into the population with MAFLD (Figure S4).
The risk of significant liver fibrosis significantly increased from non‐sarcopenic subjects with NAFLD (aOR = 1.41 by FIB‐4; aOR = 1.51 by NFS) to sarcopenic subjects with NAFLD (aOR = 3.03 by FIB‐4; aOR = 3.71 by NFS) (all P ≤ 0.001). Among subjects with NAFLD, sarcopenic subjects showed significantly increased risk of significant liver fibrosis (aOR = 2.48 by FIB‐4; aOR = 3.01 by NFS), compared with non‐sarcopenic subjects (Table S8) (all P ≤ 0.05).
The risk for high probability of ASCVD significantly increased from non‐sarcopenic subjects with NAFLD (aOR = 1.47) to sarcopenic subjects with NAFLD (aOR = 3.92) (all P ≤ 0.001). Among subjects with NAFLD, sarcopenic subjects showed significantly increased risk for high probability of ASCVD (aOR = 2.60), compared with non‐sarcopenic subjects (P ≤ 0.001) (Table S9).
Discussion
In this large, population‐based cohort study, subjects without MAFLD showed more favourable characteristics than those with MAFLD. In addition, when stratified according to sarcopenia status, non‐sarcopenic subjects with MAFLD had more favourable characteristics than sarcopenic subjects with MAFLD. The degree of fibrotic burden and the risk of ASCVD significantly increased from subjects without MAFLD to non‐sarcopenic subjects with MAFLD and sarcopenic subjects with MAFLD (all P for trend <0.001). When compared with subjects without MAFLD, non‐sarcopenic subjects with MAFLD had a 1.8‐fold (by FIB‐4) and 2.2‐fold (by NFS) increased risk of significant liver fibrosis, respectively, whereas sarcopenic subjects with MAFLD had a 3.7‐fold (by FIB‐4) and 5.6‐fold (by NFS) increased risk, respectively. Similarly, when compared with subjects without MAFLD, non‐sarcopenic subjects with MAFLD had a 2.1‐fold increased high probability of ASCVD risk, whereas sarcopenic subjects with MAFLD had a 7.9‐fold increased probability. This trend persisted despite comprehensive adjustments for confounding factors.
Our study has several clinical implications. First, we used a representative population‐based database, which strengthens the results. The selected cohort was large (n > 8000) to ensure statistical reliability and to investigate the fibrotic burden and ASCVD risk in subjects with MAFLD stratified according to sarcopenia status. This large cohort enabled us to validate our main findings with several sensitivity analyses. Furthermore, the proportion of patients with MAFLD (37.3%) was similar to that of other studies, 5 , 6 suggesting the validity of our results and appropriate selection of our study population.
Second, our study first demonstrated that the assessment of sarcopenia status might be helpful in identifying high‐risk populations among subjects with MAFLD, who might have unfavourable long‐term prognoses because of higher fibrotic burden and higher risk of ASCVD. To date, a few studies have reported worse metabolic profiles among subjects with MAFLD, when compared with those with NAFLD. 4 , 5 All these findings imply that the MAFLD definition can identify subjects with higher risk of disease progression, compared with the NAFLD definition. 30 However, heterogeneous disease severity and prognosis might exist even among subjects with the same disease category of MAFLD, which is strongly supported by our results of risk stratification according to sarcopenia status. In addition, the risk of sarcopenia increased as the number of MAFLD diagnostic criteria that patients met increased. These results suggest that the increasing burden of metabolic risk factors was associated with higher risk of sarcopenia.
Third, over the past few years, the association of sarcopenia with fatty liver has been widely studied, based on the common pathophysiology of insulin resistance, chronic inflammation, myokine changes, vitamin D deficiency, and physical inactivity. 7 , 31 Epidemiological studies have demonstrated the positive association of sarcopenia with NAFLD severity. 8 , 9 , 10 , 32 A recent meta‐analysis involving 19 studies found that sarcopenic subjects had higher risks of non‐alcoholic steatohepatitis (OR = 2.42) and significant liver fibrosis (OR = 1.56), compared with non‐sarcopenic subjects, among patients with NAFLD. 32 Our findings were consistent with those of previous studies. The risk of significant liver fibrosis and high probability of ASCVD significantly increased from non‐sarcopenic subjects with NAFLD to sarcopenic subjects with NAFLD (all P ≤ 0.001). Therefore, we hypothesized that sarcopenia could also be used to distinguish subgroups with different liver fibrotic burdens among subjects with MAFLD, and demonstrated the clinical implications of the assessment of skeletal muscle mass in subjects with MAFLD, as in previous studies concerning NAFLD. 8 , 9 , 10
Fourth, in addition to fibrotic burden, our study used the risk of ASCVD to validate the rationale of using sarcopenia status for the risk stratification of subjects with MAFLD, because it has been well established that ASCVD is the leading cause of morbidity and mortality in patients with NAFLD. 11 , 13 , 15 For MAFLD, recent two studies using large nationwide cohorts from the UK and South Korea reported that subjects with MAFLD also have a significantly increased risk of ASCVD. 6 , 33 Furthermore, subjects with NAFLD, but not MAFLD indicating no metabolic derangements, were at lower risk of ASCVD than those with MAFLD, implying the superiority of MAFLD criteria in identifying subjects with metabolically complicated fatty liver with higher ASCVD risk. 6
However, we are also aware of several limitations that remain unresolved. First, we used non‐invasive calculation‐based diagnostic surrogates to define liver steatosis and fibrosis. In addition, as the KNHANES data lack serum albumin levels, the highest quartile of NFS was used to define significant liver fibrosis as in previous studies, 8 , 24 instead of using the well‐known cut‐off of 0.676. 34 Further studies using imaging surrogates or histological data might be required for validation. However, because non‐invasive surrogates used in our study for liver steatosis 21 and fibrosis 35 have been widely validated and shown acceptable diagnostic accuracy, the use of these non‐invasive surrogates might be sufficient in our large‐scale cohort study. The independent associations between these surrogates and the long‐term outcomes in patients with NAFLD 36 also support the rationale of our study.
Second, the cross‐sectional nature of the study design could not explain causal relationships between sarcopenia and the risks of significant liver fibrosis and ASCVD. Further prospective studies focusing on determining the prognostic implications of sarcopenia are warranted to determine whether a change in sarcopenia through therapeutic intervention can change the long‐term outcomes in patients with MAFLD. Third, we only included subjects of East Asian ethnicity, which compromises the generalizability of our conclusions to subjects of other ethnicities. Fourth, a pooled cohort risk equation was used to assess ASCVD risk, so we could not examine the risk of real cardiovascular events during follow‐up. However, the risk equation was validated in several cohorts from the USA comprising Caucasians and African Americans. 37 , 38 In addition, the predicted risk score was clearly associated with the risk of ASCVD events in a Korean cohort. 39 Finally, a substantial number of subjects (n = 19 710) were excluded because of lack of sufficient clinical information (Table S10). Accordingly, our study may not be completely free of selection bias. Indeed, several variables were significantly different between enrolled and excluded subjects, including age, obesity, co‐morbidities, and laboratory values (all P ≤ 0.05). However, the skeletal muscle mass and SI, which are the main exposures of our study, were statistically similar (both P ≥ 0.05). Thus, exclusion of subjects for whom data were lacking may not significantly affect our main results.
In conclusion, our study showed that the degree of liver fibrosis and the risk of ASCVD differed significantly according to sarcopenia status in subjects with MAFLD, which strongly suggests that the amount of skeletal muscle mass needs to be assessed for long‐term risk stratification in subjects with MAFLD.
Funding
This study was supported by the Research Supporting Program of the Korean Association for the Study of the Liver and the Korean Liver Foundation, and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019R1A2C4070136). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
S.U.K. and M.N.K. conceptualized the study. S.U.K., M.N.K., and H.S.C. designed the study. H.S.C., M.N.K., S.U.K., D.Y.K., and S.H.A. analysed and interpreted the data. H.S.C., M.N.K., S.U.K., J.S.L., H.W.L., B.K.K., J.Y.P., D.Y.K., and S.H.A. reviewed the results. M.N.K., H.S.C., and S.U.K. drafted the manuscript. S.U.K. and M.N.K. contributed in the overall study oversight and are the guarantors of the manuscript. All authors reviewed the paper and approved the final version.
Conflict of interest
None declared.
Supporting information
Table S1. Prediction models for determining hepatic steatosis and significant liver fibrosis.
Table S2. Pearson's correlation analysis.
Table S3. The risk of significant liver fibrosis according to the status of MAFLD and sarcopenia, when comprehensive NAFLD score was used to define fatty liver.
Table S4. The risk of significant liver fibrosis according to the status of MAFLD and sarcopenia in patients without malignancy.
Table S5. The risk for high probability of ASCVD risk according to the status of MAFLD and sarcopenia, when comprehensive NAFLD score was used to define fatty liver.
Table S6. The risk for high probability of ASCVD risk according to the status of MAFLD and sarcopenia in patients without malignancy.
Table S7. The risk of sarcopenia in MAFLD patients according to the diagnostic criteria they met.
Table S8. The risk of significant liver fibrosis according to the status of NAFLD/sarcopenia.
Table S9. The risk for high probability of ASCVD risk according to the status of NAFLD/sarcopenia.
Table S10. Comparison of baseline characteristics between enrolled and excluded study population.
Figure S1. Flow chart of the study population from the Korea National Health and Nutrition Examination Surveys (KNHANES IV and V). Of the total subjects (n = 37,753), 8,361 (5,245 without MAFLD and 3,115 with MAFLD) were selected for the statistical analysis after excluding 29,392 subjects according to our exclusion criteria.
Figure S2. Associations of the status of MAFLD with sarcopenia index, fibrotic burden, and cardiovascular disease risk by quartile stratification. When we assessed the associations of MAFLD status with SI, fibrotic burden, and ASCVD risk by quartile stratification, the degree of sarcopenia, fibrotic burden (by FIB‐4 or NFS), and ASCVD risk were significantly higher in subjects with MAFLD than in those without MAFLD (P for trend <0.001).
Figure S3. Prevalence and relative risk of significant liver fibrosis and high probability of ASCVD according to the status of MAFLD. The prevalence and relative risk of significant liver fibrosis and high probability of ASCVD were significantly higher in subjects with MAFLD than those without MAFLD (all P < 0.001).
Figure S4. Venn diagram for the proportion of NAFLD and MAFLD in the study population.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
Chun H. S., Kim M. N., Lee J. S., Lee H. W., Kim B. K., Park J. Y., Kim D. Y., Ahn S. H., and Kim S. U. (2021) Risk stratification using sarcopenia status among subjects with metabolic dysfunction‐associated fatty liver disease, Journal of Cachexia, Sarcopenia and Muscle, 12, 1168–1178, 10.1002/jcsm.12754
Contributor Information
Mi Na Kim, Email: mdminakim@gmail.com.
Seung Up Kim, Email: ksukorea@yuhs.ac.
References
- 1. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero‐Gomez M, et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–209. [DOI] [PubMed] [Google Scholar]
- 2. Eslam M, Sanyal AJ, George J. MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999–2014.e1991. [DOI] [PubMed] [Google Scholar]
- 3. Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020;14:889–919. [DOI] [PubMed] [Google Scholar]
- 4. Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int 2020;40:3018–3030. [DOI] [PubMed] [Google Scholar]
- 5. Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int 2020;40:2082–2089. [DOI] [PubMed] [Google Scholar]
- 6. Lee H, Lee YH, Kim SU, Chang KH. Metabolic dysfunction‐associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol 2020. 10.1016/j.cgh.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 7. Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055–2065. [DOI] [PubMed] [Google Scholar]
- 8. Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008–2011). Hepatology 2016;63:776–786. [DOI] [PubMed] [Google Scholar]
- 9. Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, et al. Sarcopenia is associated with severe liver fibrosis in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;45:510–518. [DOI] [PubMed] [Google Scholar]
- 10. Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non‐alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123–131. [DOI] [PubMed] [Google Scholar]
- 11. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology 2020;158:1611–1625.e1612. [DOI] [PubMed] [Google Scholar]
- 13. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- 14. Wijarnpreecha K, Aby ES, Ahmed A, Kim D. Evaluation and management of extrahepatic manifestations of nonalcoholic fatty liver disease. Clin Mol Hepatol 2020;27:221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2020;51:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyersohn NM, Mayrhofer T, Corey KE, Bittner DO, Staziaki PV, Szilveszter B, et al. Association of hepatic steatosis with major adverse cardiovascular events, independent of coronary artery disease. Clin Gastroenterol Hepatol 2020;19:1480–1488.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim Y, Choi S, Chun C, Park S, Khang YH, Oh K. Data resource profile: the Korea Youth Risk Behavior Web‐based Survey (KYRBS). Int J Epidemiol 2016;45:1076–1076e. [DOI] [PubMed] [Google Scholar]
- 18. Kang SH, Cho Y, Jeong SW, Kim SU, Lee JW. From nonalcoholic fatty liver disease to metabolic‐associated fatty liver disease: big wave or ripple? Clin Mol Hepatol 2021;27:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 20. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee YH, Bang H, Park YM, Bae JC, Lee BW, Kang ES, et al. Non‐laboratory‐based self‐assessment screening score for non‐alcoholic fatty liver disease: development, validation and comparison with other scores. PLoS ONE 2014;9:e107584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castera L. Diagnosis of non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis: non‐invasive tests are enough. Liver Int 2018;38:67–70. [DOI] [PubMed] [Google Scholar]
- 23. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Use of the FIB4 index for non‐invasive evaluation of fibrosis in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol: Off Clin Practice J Am Gastroenterol Assoc 2009;7:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han E, Lee YH, Kim BK, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with the risk of significant liver fibrosis in metabolically unhealthy subjects with chronic hepatitis B. Aliment Pharmacol Ther 2018;48:300–312. [DOI] [PubMed] [Google Scholar]
- 25. Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol 2014;63:2886. [DOI] [PubMed] [Google Scholar]
- 26. Yang X, Li J, Hu D, Chen J, Li Y, Huang J, et al. Predicting the 10‐year risks of atherosclerotic cardiovascular disease in Chinese population: the China‐PAR project (prediction for ASCVD risk in China). Circulation 2016;134:1430–1440. [DOI] [PubMed] [Google Scholar]
- 27. Lee YH, Kim JE, Roh YH, Choi HR, Rhee Y, Kang DR, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008–2011. J Clin Endocrinol Metab 2014;99:3879–3888. [DOI] [PubMed] [Google Scholar]
- 28. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 29. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 30. Ratziu V, Rinella M, Beuers U, Loomba R, Anstee QM, Harrison S, et al. The times they are a‐changin' (for NAFLD as well). J Hepatol 2020;73:1307–1309. [DOI] [PubMed] [Google Scholar]
- 31. Zhai Y, Xiao Q. The common mechanisms of sarcopenia and NAFLD. Biomed Res Int 2017;2017:6297651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cai C, Song X, Chen Y, Chen X, Yu C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Hepatol Int 2020;14:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Z, Suo C, Shi O, Lin C, Zhao R, Yuan H, et al. The health impact of MAFLD, a novel disease cluster of NAFLD, is amplified by the integrated effect of fatty liver disease related genetic variants. Clin Gastroenterol Hepatol 2020; 10.1016/j.cgh.2020.12.033 [DOI] [PubMed] [Google Scholar]
- 34. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 35. Vallet‐Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin‐Venier V, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–36. [DOI] [PubMed] [Google Scholar]
- 36. Oh H, Jun DW, Saeed WK, Nguyen MH. Non‐alcoholic fatty liver diseases: update on the challenge of diagnosis and treatment. Clin Mol Hepatol 2016;22:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yadlowsky S, Hayward RA, Sussman JB, McClelland RL, Min YI, Basu S. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med 2018;169:20–29. [DOI] [PubMed] [Google Scholar]
- 38. Muntner P, Colantonio LD, Cushman M, Goff DC Jr, Howard G, Howard VJ, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA 2014;311:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jung HH. Statin use and outcome risks according to predicted CVD risk in Korea: a retrospective cohort study. PLoS ONE 2021;16:e0245609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Prediction models for determining hepatic steatosis and significant liver fibrosis.
Table S2. Pearson's correlation analysis.
Table S3. The risk of significant liver fibrosis according to the status of MAFLD and sarcopenia, when comprehensive NAFLD score was used to define fatty liver.
Table S4. The risk of significant liver fibrosis according to the status of MAFLD and sarcopenia in patients without malignancy.
Table S5. The risk for high probability of ASCVD risk according to the status of MAFLD and sarcopenia, when comprehensive NAFLD score was used to define fatty liver.
Table S6. The risk for high probability of ASCVD risk according to the status of MAFLD and sarcopenia in patients without malignancy.
Table S7. The risk of sarcopenia in MAFLD patients according to the diagnostic criteria they met.
Table S8. The risk of significant liver fibrosis according to the status of NAFLD/sarcopenia.
Table S9. The risk for high probability of ASCVD risk according to the status of NAFLD/sarcopenia.
Table S10. Comparison of baseline characteristics between enrolled and excluded study population.
Figure S1. Flow chart of the study population from the Korea National Health and Nutrition Examination Surveys (KNHANES IV and V). Of the total subjects (n = 37,753), 8,361 (5,245 without MAFLD and 3,115 with MAFLD) were selected for the statistical analysis after excluding 29,392 subjects according to our exclusion criteria.
Figure S2. Associations of the status of MAFLD with sarcopenia index, fibrotic burden, and cardiovascular disease risk by quartile stratification. When we assessed the associations of MAFLD status with SI, fibrotic burden, and ASCVD risk by quartile stratification, the degree of sarcopenia, fibrotic burden (by FIB‐4 or NFS), and ASCVD risk were significantly higher in subjects with MAFLD than in those without MAFLD (P for trend <0.001).
Figure S3. Prevalence and relative risk of significant liver fibrosis and high probability of ASCVD according to the status of MAFLD. The prevalence and relative risk of significant liver fibrosis and high probability of ASCVD were significantly higher in subjects with MAFLD than those without MAFLD (all P < 0.001).
Figure S4. Venn diagram for the proportion of NAFLD and MAFLD in the study population.


