Abstract
Sarcopenia, which is characterized by a decrease in muscle quantity or quality, is commonly observed in patients with cancer. Recent research has reported contradictory results on the association between sarcopenia and the efficacy of immune checkpoint inhibitors (ICIs). We conducted a systematic review and meta‐analysis to investigate this discrepancy. We systematically searched three electronic databases to identify articles reporting on the association between sarcopenia and treatment outcomes in patients with solid cancers who received ICIs. The outcomes assessed were hazard ratios (HRs) for overall survival (OS) and progression‐free survival (PFS), and odds ratios (ORs) for objective response rate (ORR), disease control rate (DCR), and toxicity. Pooled estimates and their 95% confidence intervals (CIs) were calculated. A total of 2501 patients from 26 studies were analysed. Sarcopenia was observed in 44.7% (95% CI: 38.2–51.3) of the patients and was significantly associated with poor survival (HR = 1.55, 95% CI = 1.32–1.82 for OS and HR = 1.61, 95% CI = 1.35 to 1.93 for PFS). The HRs (95% CIs) for OS according to the diagnostic measures used were 1.97 (0.88–4.41) for psoas muscle index (PMI), 1.41 (0.87–2.28) for skeletal muscle density (SMD), and 1.43 (1.23–1.67) for skeletal mass index (SMI). The HRs (95% CIs) for PFS were 1.86 (1.08–3.21) for PMI, 1.27 (0.94–1.71) for SMD, and 1.38 (1.11–1.71) for SMI. Poor radiological response to ICI therapy was observed in patients with sarcopenia (OR = 0.52, 95% CI = 0.34–0.80 for ORR and OR = 0.45, 95% CI = 0.30–0.67 for DCR). The ORs for ORR (95% CIs) were 0.56 (0.15–2.05) for PMI and 0.78 (0.56–1.09) for SMI. The oncologic outcomes associated with melanoma and non‐small cell lung cancer (NSCLC) were comparable with those observed overall (HR for OS = 2.02, 95% CI = 1.26–3.24 for melanoma and HR for OS = 1.61, 95% CI = 1.19–2.18 for NSCLC). In contrast, the occurrence of severe toxicity was not associated with sarcopenia (OR = 1.13, 95% CI = 0.51–2.52). Poor survival and poor response in patients with sarcopenia indicate a negative association between sarcopenia and efficacy of ICIs. Sarcopenia's predictive ability is consistent across various tumour types. For the selection of patients who may respond to ICIs pre‐therapeutically, the presence of sarcopenia should be assessed in clinical practice.
Keywords: Immune checkpoint inhibitor, Sarcopenia, Solid cancer, Non‐small cell lung cancer, Melanoma
1. Background
Surgery, radiation, and chemotherapy have been the three main pillars of cancer treatment for decades. However, recent rapid progress in immunotherapy has changed this paradigm. 1 Immune checkpoint inhibitor (ICI) therapy is the most frequently used immunotherapy against various cancer types. ICIs are predominantly used for the treatment of recurrent and metastatic diseases that cannot be cured with conventional therapy; however, the indications for their use have been expanding. 2 The use of ICIs can significantly lengthen survival and sometimes result in a long duration of disease control even in patients with advanced disease and disease progression. So far, seven drugs—atezolizumab, avelumab, cemiplimab, durvalumab, ipilimumab, nivolumab, and pembrolizumab—have been approved for use in clinical practice. Although their clinical benefit is apparent, the use of ICIs is limited owing to the associated cost. To identify patients who may benefit the most from ICIs, companion and complementary diagnostics have been developed. 3 All ICIs, except ipilimumab, inhibit the binding between programmed death protein 1 (PD‐1) and programmed death ligand 1 (PD‐L1). Therefore, the immunohistochemical measurement of PD‐L1 expression is employed as a tool for companion diagnostics. 2 However, partly owing to the heterogeneous PD‐L1 expression in tumour tissues, its predictive ability is not satisfactory for use in clinical practice. 4 Other cancer immunity‐associated biomarkers used for companion diagnostics include tumour mutation burden and microsatellite instability. 3 However, when used alone, these biomarkers have limited predictive value. Efforts are underway for the identification of other biomarkers. 5
Sarcopenia is a skeletal muscle disorder characterized by reduced muscle strength and muscle quantity. 6 Recently, a meta‐analysis of various types of cancers demonstrated an association between sarcopenia and prognoses. 7 In addition, an increasing number of studies are focusing on the impact of sarcopenia on ICI treatment efficacy. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 However, most previous studies on the topic had a retrospective design and included a small number of patients in whom various methods were employed for the diagnosis of sarcopenia. Therefore, the predictive value of sarcopenia in ICI therapy requires elucidation.
Meta‐analyses have advantages in that they can generate a pooled effect size, as deduced from the results of previous studies and thus can yield more reliable conclusions using data from a larger number of patients. This study aimed to investigate, using a meta‐analysis, whether sarcopenia status is predictive of oncologic outcomes in patients treated with ICIs. Further, we also sought to determine the differences between various tools and tests for sarcopenia in the prediction of prognoses.
2. Methods
2.1. Search strategy
This study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. 34 We conducted a search for published studies focusing on the association between sarcopenia and ICI efficacy in the following electronic databases: PubMed www.ncbi.nlm.nih.gov/pubmed, Scopus www.elsevier.com/online‐tools/scopus, and Ichushi‐Web https://search.jamas.or.jp, which contains bibliographic information and abstracts of articles in Japanese journals (Japan Medical Abstracts Society) from inception to 4 May 2021. The search terms were (i) ‘CTLA‐4’ or ‘CTLA4’ or ‘cytotoxic T‐lymphocyte‐associated protein 4’ or ‘CD152’ or ‘PD‐1’ or ‘PD1’ or ‘programmed cell death protein 1’ or ‘CD279’ or ‘PD‐L1’ or ‘PDL1’ or ‘programmed death‐ligand 1’ or ‘CD274’ or ‘atezolizumab’ or ‘avelumab’ or ‘cemiplimab’ or ‘durvalumab’ or ‘ipilimumab’ or ‘nivolumab’ or ‘pembrolizumab’ and (ii) ‘sarcopenia’ or ‘sarcopenic’ or ‘muscle index’ or ‘muscle mass’ or ‘muscle depletion’ or ‘muscular atrophy’ or ‘muscle strength’ or ‘muscle quality’ or ‘muscle quantity’. The references in the retrieved articles were manually searched for associated studies.
2.2. Study selection
Articles in English or Japanese that met the following criteria were included in this study: (i) patients: patients with solid cancers treated with ICIs; (ii) exposure: sarcopenia was defined based on the diagnostic modalities recommended by consensus statements 6 , 35 ; (iii) comparison: non‐sarcopenia group; and (iv) outcome: overall survival (OS), progression‐free survival (PFS), objective response rate (ORR), and disease control rate (DCR), as defined by response evaluation criteria in solid tumours 36 and ICI‐induced toxicity. The exclusion criteria were as follows: (i) study design: animal study, review, case reports, and conference abstracts; (ii) articles written in languages other than English or Japanese; (iii) the hazard ratio (HR) or odds ratio (OR) for outcomes were neither described in the manuscript nor estimated from the published data. Two of the authors (Y. T. and R. O.) independently evaluated the electronically searched titles. All potentially relevant publications were retrieved. Disagreements were resolved by consensus.
2.3. Data extraction
The following data were extracted: name of first author, year of publication, institution and country, number of patients, number of outcomes according to sarcopenia status, disease stage, ICI drug names, toxicity, diagnostic measures for sarcopenia and their cut‐off methods and cut‐off values, and HRs and ORs and their 95% confidence intervals (CIs). The HRs, ORs, and 95% CIs were extracted preferentially from multivariate or univariate analyses. When HRs were not provided in the manuscript, survival data were extracted from Kaplan–Meier curves and estimated using the method proposed by Tierney et al. 37 The Newcastle–Ottawa Scale 38 was used to assess the quality of the included studies; those with a score ≥6 were considered high‐quality studies.
2.4. Statistical analysis
Pooled HRs, ORs, and their 95% CIs were estimated with both a random effect model and a fixed effect model using Comprehensive Meta‐Analysis Version 2 (Biostat, Englewood, NJ, USA). First, we investigated the predictive impact of sarcopenia on OS, PFS, objective response, disease control, and toxicity. The mean HR was used as the representative of the study in a meta‐analysis when more than one diagnostic procedure for sarcopenia was used. 12 , 16 , 25 , 26 , 29 Second, we conducted meta‐analyses according to each diagnostic procedure. Sensitivity analyses were performed by the sequential omission of each individual study. Subgroup analyses were conducted for primary tumour sites and ICIs. Publication bias was assessed using the funnel plot and tested with Egger's regression intercept test. Heterogeneity was assessed using Cochran's Q test and I 2 statistics. All statistical tests were two‐sided, and significance was defined by a P‐value <0.05. The included studies differed in the tumour sites, prior treatment, ICIs used, institutions, and diagnostic measures for sarcopenia and their cut‐off values. Owing to the heterogeneity among the studies, a random effect model was preferred in this manuscript.
The protocol for this meta‐analysis is available in UMIN (registration code: UMIN000042621).
3. Results
3.1. Literature search results
The electronic database search for articles from the inception of each database to 4 May 2021 led to the retrieval of 597 records (Figure 1). We excluded duplicate entries and articles written in languages other than English and Japanese and then screened for titles and abstracts. The full texts of the 49 studies selected were then inspected according to the inclusion and exclusion criteria; finally, 26 studies 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 comprising 2501 patients were included in the systematic review. Two studies by Cortellini et al. contain overlapping data. 11 , 16 Newer and more detailed data were used when the same outcome data were provided in both studies. All 26 articles were written in English.
Figure 1.
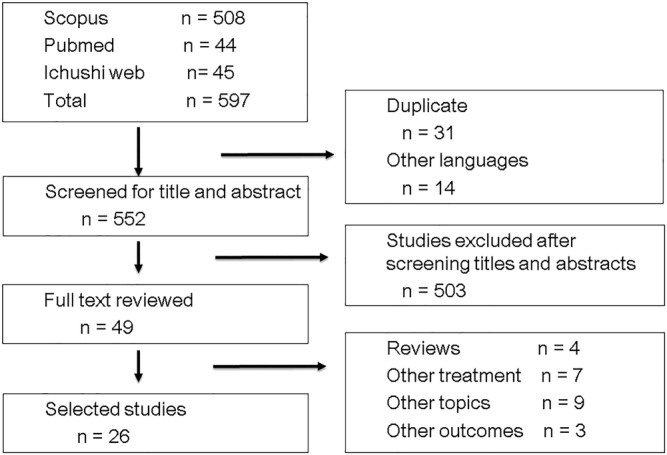
Flow diagram of article selection.
3.2. Diagnosis and prevalence of sarcopenia
Table 1 shows the characteristics of the included studies. Nine studies each were conducted in Europe and Japan, and three were performed in the USA. All the studies used computed tomography (CT) as a modality to diagnose sarcopenia. None of the included studies used questionnaires, dual‐energy X‐ray absorptiometry (DXA), or bioelectrical impedance assay (BIA). Of the diagnostic methods, the skeletal mass index (SMI) was the most commonly used, 9 , 10 , 11 , 16 , 18 , 19 , 22 , 23 , 25 , 26 , 27 , 29 , 30 , 31 , 33 followed by the psoas muscle index (PMI) 14 , 17 , 20 , 21 , 24 , 26 , 28 and skeletal muscle density (SMD). 15 , 16 , 29 Of the 15 articles that employed SMI, five 10 , 11 , 18 , 19 , 29 used the cut‐off value described by Martin et al., 39 while of the seven that employed PMI, four 14 , 24 , 26 , 28 used the cut‐off value for Asian adults. 40 The prevalence of sarcopenia ranged from 21.9% to 75.0%, and the pooled prevalence of sarcopenia was 44.7% (95% CI: 38.2–51.3) (Supporting Information, Figure S1).
Table 1.
Study characteristics
| Year | Author | Country | Site | Treatment | Diagnostic method | Cut‐off value | Outcome | No. of patients | Age median [range] {interquartile range} mean ± SD | Gender (male/female) | Newcastle–Ottawa scale |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | Sabel | USA | Melanoma | Ipilimumab | Psoas density | Highest quartile | DCR, ORR, and OS | 44 | 55.1 [15–90] | 84/49 | 4 |
| 2016 | Dercle | France | Melanoma, lung cancer, bladder cancer, RCC | Anti‐PD1 and anti‐PDL1 | SMI | 53 | OS | 251 | 56 ± 13 | 131/120 | 7 |
| 2017 | Daly | Ireland | Melanoma | Ipilimumab | Muscle loss at L3 | 7.5% (lowest quartile) | OS | 84 | 54 [22–85] | 52/32 | 7 |
| SMI | Male, 43 for BMI < 25, 53 for BMI ≥ 25; Female, 41 | Toxicity | |||||||||
| 2019 | Cortellini | Italy | NSCLC | Nivolumab | SMI | Male, 43 for BMI < 25, 53 for BMI ≥ 25; Female, 41 | ORR and toxicity | 22 | 67 [41–82] | 18/5 | 4 |
| 2019 | Deike‐Hofmann | Germany | Melanoma | Ipilimumab | Mean psoas density | 45 (lower quartile) | PFS | 147 | 60 {49.5–66.5} | 90/57 | 7 |
| 2019 | Nishioka | Japan | NSCLC | Nivolumab and pemblolizumab | Decrease of the psoas major muscle area | 10% | DCR, ORR, and PFS | 38 | 68.7 [46–85] | 26/12 | 4 |
| 2019 | Shiroyama | Japan | NSCLC | Nivolumab and pemblolizumab | PMI | Male, 6.36; Female, 3.92 | DCR, ORR, and PFS | 42 | Sarcopenia group: 72 [51–87]; Non‐sarcopenia group: 69 [37–78] | 26/16 | 6 |
| 2020 | Chu | Canada | Melanoma | Ipilimumab | SMD | BMI > 25; 20 HU; BMI < 25; 42 | DCR, ORR, OS, PFS, and toxicity | 97 | 56 [25–91] | 58/39 | 6 |
| 2020 | Cortellini | Italy | NSCLC, melanoma, RCC, and others | Atezolizumab, nivolumab, pemblolizumab, and others | SMD | Male, 24.2 for BMI < 25; 35.6 for BMII ≥ 25; Female, 27.9 for BMI < 25, 37.4 for BMII ≥ 25 | ORR, OS, and PFS | 100 | 66 [25–88] | 67/33 | |
| SMI | Male, 48.4 for BMI < 25, 50.2 for BMII ≥ 25; Female, 36.9 for BMI < 25, 59.6 for BMII ≥ 25 | ORR, OS, and PFS | 5 | ||||||||
| 2020 | Crombe | France | Metastatic solid cancers | Anti‐PD1, anti‐PDL1, and anti‐PDL1/CTLA4 | PMI decrease | Lowest tertile | PFS | 117 | 63 [33.9–84.3] | 62/55 | 6 |
| 2020 | Fukushima | Japan | Urothelial carcinoma | Pemblolizumab | SMI | Male, 43 for BMI < 25, 53 for BMI ≥ 25; Female, 41 | ORR, OS, PFS, and toxicity | 28 | 74 [70–82] | 19/9 | 5 |
| 2020 | Hirsch | France | Solid cancer | Nivolumab | SMI | Male, 43 for BMI < 25, 53 for BMI ≥ 25; Female, 41 | Toxicity | 87 | N/A | N/A | 8 |
| 2020 | Hu | USA | Melanoma | Pemblolizumab | PMI | Bottom tertile | ORR and toxicity | 156 | 66 [21–93] | 91/65 | 5 |
| 2020 | Kano | Japan | Gastric cancer | Nivolumab | PMI | Male, 3.6; Female, 2.9 | DCR, ORR, PFS, and toxicity | 31 | 70 [35–83] | 21/10 | 5 |
| 2020 | Kim N | Korea | HCC | Nivolumab | SMI | Male, 42; Female, 38 | DCR, ORR, PFS, and OS | 102 | 61.3 [54–69] | 87/15 | 7 |
| 2020 | Kim Y | Korea | Gastric cancer | Pembrolizumab and nivolumab | SMI | Male, 49; Female, 31 | DCR, ORR, OS, and PFS | 147 | 57.0 ± 12.3 | 93/54 | 8 |
| 2020 | Minami | Japan | NSCLC | Nivolumab, pemblolizumab, and atezolizumab | PMI | Male, 6.36; Female, 3.92 | DCR, ORR, OS, and PFS | 74 | Sarcopenia group: 69 {63–74}; non‐sarcopenia group: 70 {61–73} | 48/26 | 7 |
| 2020 | Roch | France | NSCLC | Nivolumab and pemblolizumab | SMI decrease | 5% | DCR, PFS, and OS | 142 | 63.54 ± 10.58 | 93/49 | 5 |
| SMI | Male, 52.4; Female, 38.5 | DCR, PFS, and OS | |||||||||
| 2020 | Shimizu | Japan | Urothelial carcinoma | Pembrolizumab | PMI | Male, 6.36; Female, 3.92 | OS, PFS, and toxicity | 27 | 73 [52–82] | 23/4 | 5 |
| PMI decrease(1 month from baseline) | 5% | OS and PFS | |||||||||
| SMI decrease | 5% | OS and PFS | |||||||||
| 2020 | Takada | Japan | NSCLC | Nivolumab and pemblolizumab | SMI | Male, 25.63; Female, 21.73 | DCR, ORR, OS, and PFS | 103 | 67 [36–88] | 84/19 | 5 |
| 2020 | Tsukagoshi | Japan | NSCLC | Nivolumab | PMI | Male, 6.36; Female, 3.92 | DCR, ORR, OS, and PFS | 30 | 67 [47–82] | 23/7 | 6 |
| 2020 | Young | USA | Melanoma | Ipilimumab + nivolumab, pembrolizumab, nivolumab, and atezolizumab | SMD | 41 for BMI < 25, 33 for BMI ≥ 25 | ORR, OS, and PFS | 287 | 63 [20–89]; 61 ± 14.4 | 184/103 | 7 |
| SMG | 1475 | ORR, OS, and PFS | |||||||||
| SMI | Male, 43 for BMI < 25, 53 for BMI ≥ 25; Female, 41 | ORR, OS, and PFS | |||||||||
| 2021 | Akce | Canada | HCC | Anti‐PD‐1 antibody | SMI | Male, 43; Female, 39 | OS and PFS | 57 | Median 66 | 44/13 | 5 |
| 2021 | Loosen | Germany | NSCLC, melanoma, urothelial cancer, GI cancer, head and neck cancer, and others | Nivolumab, pembrolizumab, nivolumab + ipilimumab, and others | SMD decrease | −0.4 | OS | 88 | 67 [34–87] | 58/30 | 6 |
| SMI decrease | −6.18 | ||||||||||
| 2021 | Nishioka | Japan | NSCLC | Nivolumab, pembrolizumab, and atezolizumab | SMD | 33 for BMI ≧ 25, 41 for BMI < 25 | ORR, OS, and PFS | 156 | 67 [33–85] | 101/55 | 7 |
| SMI | Male, 53 for BMI ≧ 25, 43 for BMI < 25; Female, 41 | ||||||||||
| 2021 | Youn | Canada | Melanoma | Nivolumab or nivolumab + ipilimumab | SMD | 25.65 | OS | 44 | 57 [29–79] | 25/19 | 6 |
DCR, disease control rate; GI, gastrointestinal; HCC, hepatocellular carcinoma; NSCLC, non‐small cell lung cancer; N/A, not available; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; PMI, psoas muscle index; SMD, skeletal muscle density; SMG, skeletal muscle gauge; SMI, skeletal muscle index.
3.3. Overall survival and sarcopenia
Eighteen studies investigated the association between sarcopenia and OS. 8 , 13 , 14 , 15 , 16 , 18 , 20 , 21 , 22 , 23 , 24 , 27 , 28 , 29 , 30 , 31 , 32 , 33 The HRs for OS ranged from 0.76 to 6.21. Multivariate analyses were performed in 13 studies. 9 , 10 , 15 , 16 , 22 , 24 , 25 , 26 , 27 , 29 , 30 , 32 , 33 HRs were estimated using the Kaplan–Meier curve in three studies. 8 , 18 , 31 The meta‐analysis demonstrated the significant predictive ability of sarcopenia for OS (HR [95% CI] 1.55 [1.32–1.82]) (Figure 2A). The results of the sensitivity analysis are shown in the Supporting Information, Table S1.
Figure 2.
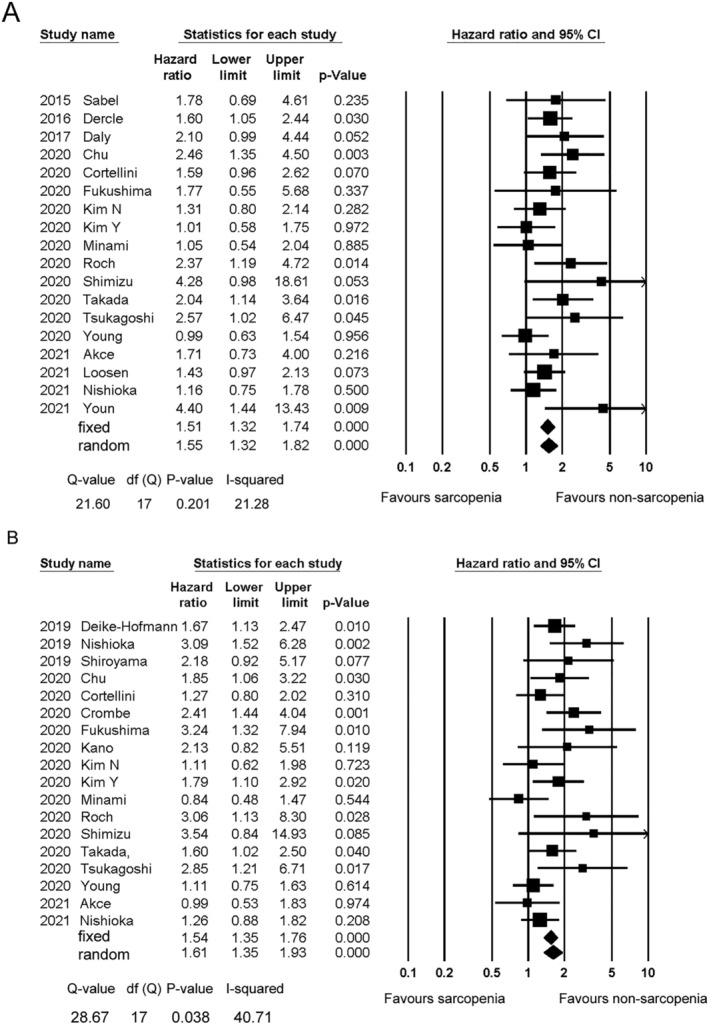
Forest plot showing the hazard ratios for overall survival (A) and progression‐free survival (B) between the sarcopenia and non‐sarcopenia patients. The squares represent the hazard ratios for each study. The sizes of the squares and the horizontal lines crossing the squares represent the weight of the study in the random effect model and the 95% confidence intervals, respectively.
The HRs for OS according to the diagnostic measures used are shown in the Supporting Information, Table S2. PMI, SMD, and SMI were employed for dichotomization in three, 24 , 26 , 28 five, 15 , 16 , 29 , 31 , 32 and 10 studies, 9 , 16 , 18 , 22 , 23 , 25 , 27 , 29 , 31 , 33 respectively. The HRs (95% CIs) were 1.97 (0.88–4.41) for PMI, 1.41 (0.87–2.28) for SMD, and 1.43 (1.23–1.67) for SMI. There were no significant differences among the different diagnostic measures (P = 0.507).
3.4. Progression‐free survival and sarcopenia
Eighteen studies 12 , 13 , 14 , 15 , 16 , 17 , 18 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 31 , 33 investigated the association between sarcopenia and PFS. Multivariate analysis was performed in 14 studies. 14 , 15 , 16 , 17 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 31 , 33 The HRs for PFS were estimated using the Kaplan–Meier curve analysis in two studies. 13 , 21 The HRs for PFS ranged from 0.84 to 12.80. Sarcopenia was significantly associated with worse PFS values (random effect model, HR [95% CI] 1.61 [1.35–1.93]) (Figure 2B). The results of the sensitivity analysis are shown in the Supporting Information, Table S3. The result was similar when any individual study was removed from the analysis.
The HRs for PFS according to the diagnostic measures employed are shown in the Supporting Information, Table S4. PMI, SMD, and SMI were employed for dichotomization in five, 14 , 21 , 24 , 26 , 28 four, 15 , 16 , 29 , 31 and nine studies, 16 , 18 , 22 , 23 , 25 , 27 , 29 , 31 , 33 respectively. SMI and PMI were predictors of PFS (HR = 1.38, 95% CI = 1.11–1.71; and HR = 1.86, 95% CI = 1.08–3.21, respectively). In contrast, SMD was not associated with PFS (HR = 1.27, 95% CI = 0.94–1.71). There were no significant differences among the different diagnostic measures (P = 0.207).
3.5. Objective response and sarcopenia
Objective response rate was investigated in 15 studies. 8 , 13 , 14 , 15 , 16 , 18 , 20 , 21 , 22 , 23 , 24 , 27 , 28 , 29 , 31 Only one study used multivariate analyses. 29 The ORs for ORR ranged from 0.03 to 5.26. Sarcopenia was significantly associated with worse response (OR = 0.52, 95% CI = 0.34–0.80) (Figure 3A). The results of the sensitivity analysis are shown in the Supporting Information, Table S5. The result was similar when any individual study was removed from the analysis.
Figure 3.
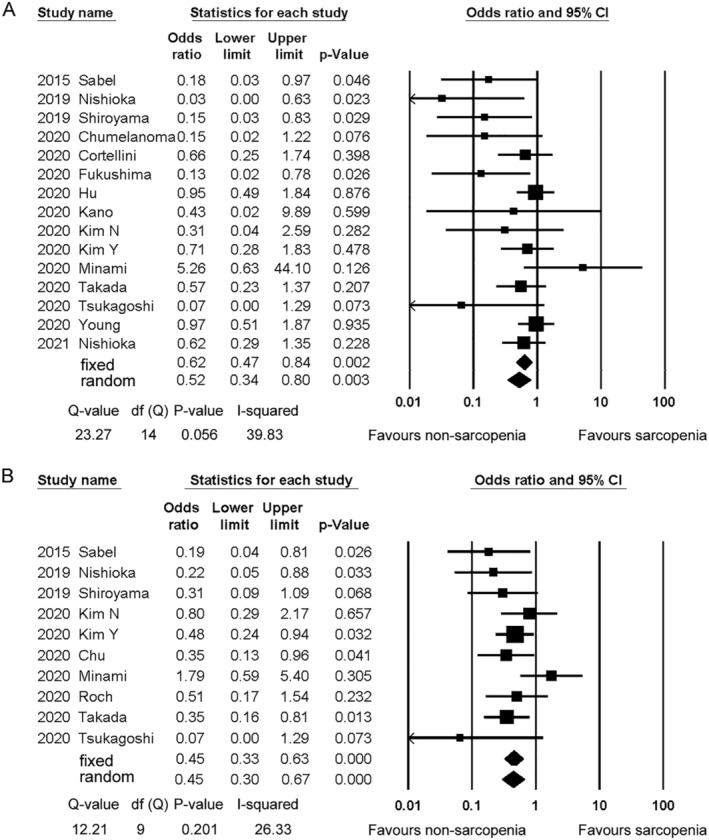
Forest plot showing the odds ratios for objective response rate (A) and disease control rate (B) between the sarcopenia and non‐sarcopenia patients. The squares represent the hazard ratios for each study. The sizes of the squares and the horizontal lines crossing the squares represent the weight of the study in the random effect model and the 95% confidence intervals, respectively.
PMI, SMD, and SMI were employed for dichotomization in five, 14 , 20 , 21 , 24 , 28 four, 15 , 16 , 29 , 31 and seven studies, 16 , 18 , 22 , 23 , 27 , 29 , 31 respectively. The ORs for each procedure showed a tendency for worse response in sarcopenia patients. The pooled ORs (95% CIs) were 0.56 (0.15–2.05) for PMI, 0.51 (0.22–1.17) for SMD, and 0.78 (0.56–1.09) for SMI (Supporting Information, Table S6). There were no significant differences among the different diagnostic measures (P = 0.153). The ORs and 95% CIs for other diagnostic procedures are also shown in the Supporting Information, Table S6.
3.6. Disease control and sarcopenia
Disease control rate was investigated in 10 studies. 8 , 13 , 14 , 15 , 22 , 23 , 24 , 25 , 27 , 28 None of the 10 studies performed multivariate analyses for DCR. The ORs for DCR ranged from 0.07 to 1.79. The pooled OR (95% CI) in the 10 studies was 0.45 (0.30–0.67) (Figure 3B). Although the studies by Minami and Tsukagoshi seemed to be outliers, the exclusion of either study did not change the results significantly (Supporting Information, Table S7).
Psoas muscle index and SMI were employed for dichotomization in three studies each 14 , 24 , 28 . 22 , 25 , 27 The pooled ORs (95% CIs) were 0.47 (0.09–2.52) for PMI and 0.51 (0.34–0.78) for SMI (Supporting Information, Table S8). There were no significant differences among the different diagnostic measures (P = 0.754).
3.7. Subgroup analysis
Subgroup analyses using a random effect model were performed according to the primary tumour site (Table 2). Melanoma and non‐small cell lung cancer (NSCLC) were the most commonly investigated tumours; other tumours were included only in two or fewer studies. The pooled HRs and ORs for melanoma and NSCLC showed a statistically significant association between sarcopenia and worse OS, worse PFS, and worse DCR. Similar results were obtained with other types of tumours, although some failed to show a significant result.
Table 2.
Hazard ratios and odds ratios according to the primary tumour site
| No. of studies | No. of patients | Estimates | Lower limit | Upper limit | P‐value | |
|---|---|---|---|---|---|---|
| OS | HR | |||||
| Gastric cancer | 1 | 149 | 1.01 | 0.58 | 1.75 | 0.972 |
| HCC | 2 | 159 | 1.40 | 0.91 | 2.14 | 0.121 |
| Melanoma | 6 | 583 | 2.02 | 1.26 | 3.24 | 0.003 |
| NSCLC | 6 | 551 | 1.61 | 1.19 | 2.18 | 0.002 |
| Urothelial cancer | 2 | 55 | 2.49 | 1.00 | 6.20 | 0.051 |
| PFS | HR | |||||
| Gastric cancer | 2 | 180 | 1.86 | 1.20 | 2.87 | 0.005 |
| HCC | 2 | 159 | 1.05 | 0.69 | 1.60 | 0.813 |
| Melanoma | 4 | 558 | 1.53 | 1.13 | 2.07 | 0.006 |
| NSCLC | 8 | 631 | 1.69 | 1.24 | 2.31 | 0.001 |
| Urothelial cancer | 2 | 55 | 3.32 | 1.55 | 7.11 | 0.002 |
| ORR | OR | |||||
| Gastric cancer | 2 | 178 | 0.68 | 0.27 | 1.69 | 0.406 |
| HCC | 1 | 102 | 0.31 | 0.04 | 2.59 | 0.282 |
| Melanoma | 4 | 584 | 0.63 | 0.30 | 1.31 | 0.295 |
| NSCLC | 7 | 465 | 0.49 | 0.20 | 1.22 | 0.127 |
| Urothelial cancer | 1 | 28 | 0.13 | 0.02 | 0.78 | 0.026 |
| DCR | OR | |||||
| Gastric cancer | 1 | 147 | 0.48 | 0.24 | 0.94 | 0.032 |
| HCC | 1 | 102 | 0.80 | 0.29 | 2.17 | 0.657 |
| Melanoma | 2 | 141 | 0.28 | 0.12 | 0.66 | 0.003 |
| NSCLC | 6 | 429 | 0.43 | 0.22 | 0.87 | 0.019 |
CI, confidence interval; DCR, disease control rate; HCC, hepatocellular carcinoma; HR, hazard ratio; NSCLC, non‐small cell lung cancer; OR, odds ratio; ORR, objective response rate; OS, overall survival; PFS; progression‐free survival.
Next, we conducted a subgroup analysis for the ICI drugs (Table 3). Data on ICI monotherapy were investigated in four studies on Ipilimumab, 8 , 10 , 12 , 15 five on Nivolumab, 11 , 19 , 21 , 23 , 28 and three on pembrolizumab. 18 , 20 , 26 HR for OS and PFS, OR for ORR, and DCR favoured non‐sarcopenia in all drugs. The difference among the drugs was not significant with respect to any outcomes (P = 0.670 for OS, P = 0.291 for PFS, P = 0.107 for ORR, and P = 0.876 for DCR).
Table 3.
Hazard ratios and odds ratios according to immune checkpoint inhibitors
| No. of studies | No. of patients | Estimates | 95% CI | P‐value | ||
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| OS | HR | |||||
| Ipilimumab | 3 | 225 | 2.20 | 1.44 | 3.35 | 0.000 |
| Nivolumab | 2 | 132 | 1.63 | 0.88 | 3.03 | 0.121 |
| Pembrolizumab | 2 | 55 | 2.49 | 1.00 | 6.20 | 0.051 |
| PFS | HR | |||||
| Ipilimumab | 2 | 244 | 1.73 | 1.25 | 2.38 | 0.001 |
| Nivolumab | 3 | 163 | 1.74 | 0.95 | 3.20 | 0.072 |
| Pembrolizumab | 2 | 55 | 3.32 | 1.55 | 7.11 | 0.002 |
| ORR | OR | |||||
| Ipilimumab | 2 | 141 | 0.16 | 0.04 | 0.62 | 0.008 |
| Nivolumab | 4 | 185 | 0.44 | 0.11 | 1.72 | 0.239 |
| Pembrolizumab | 2 | 184 | 0.43 | 0.06 | 2.82 | 0.375 |
| DCR | OR | |||||
| Ipilimumab | 2 | 141 | 0.28 | 0.12 | 0.66 | 0.003 |
| Nivolumab | 2 | 132 | 0.34 | 0.03 | 3.48 | 0.367 |
CI, confidence interval; DCR, disease control rate; HR, hazard ratio; OR, odds ratio; ORR, objective response rate.
3.8. Severe toxicity and sarcopenia
The incidence of severe toxicity was assessed in seven studies. 8 , 11 , 15 , 17 , 19 , 23 , 26 Of them, two performed multivariate analyses17,19. The ORs for severe toxicity ranged from 0.26 to 5.34. The pooled OR (95% CI), irrespective of the diagnostic procedure, was 1.13 (0.51–2.52) (Figure 4).
Figure 4.
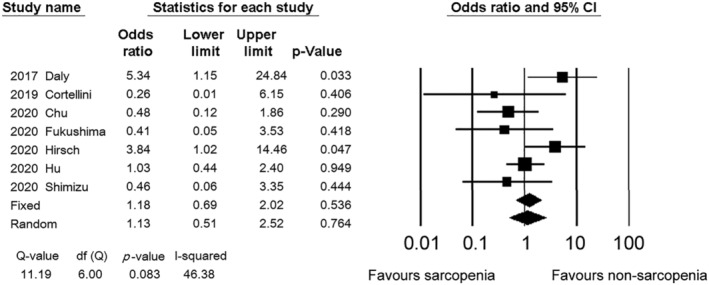
Forest plot showing the odds ratios for severe toxicity between the sarcopenia and non‐sarcopenia patients. The squares represent the hazard ratios for each study. The sizes of the squares and the horizontal lines crossing the squares represent the weight of the study in the random effect model and the 95% confidence intervals, respectively.
3.9. Publication bias
Figure 5 shows funnel plots of the HRs and ORs for the relationship between sarcopenia and OS, PFS, DCR, ORR, and toxicity. These funnel plots showed apparent asymmetry towards higher HRs and asymmetry towards lower ORs. The P values derived from the Egger's test of the intercept were 0.006 for OS, 0.013 for PFS, 0.008 for ORR, 0.263 for DCR, and 0.592 for severe toxicity.
Figure 5.
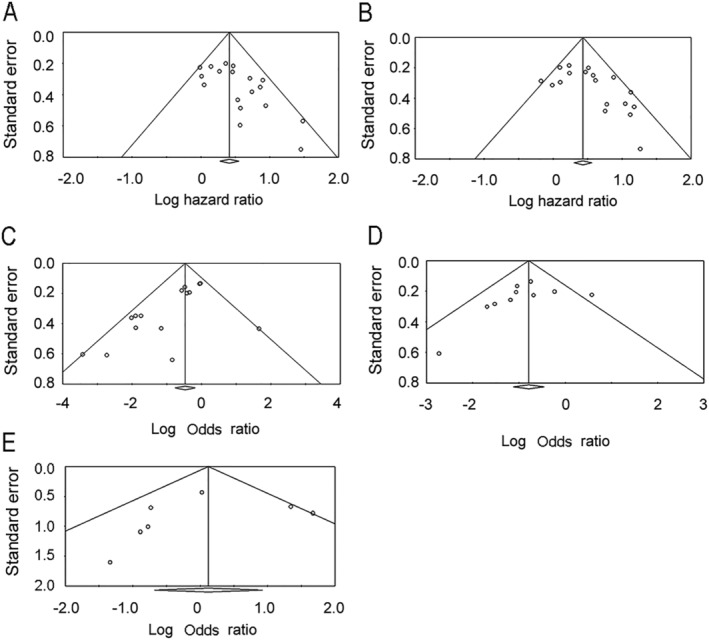
Funnel plot of the hazard ratios for overall survival (A) and progression‐free survival (B), and funnel plot of the odds ratio for objective response rate (C), disease control rate (D), and severe toxicity (E).
4. Discussion
In the present study, we found that sarcopenia could predict the response to ICIs and survival after ICI treatment for solid cancers and that its presence was not associated with severe toxicity incidence. The increased mortality observed in the sarcopenia patients was consistent across various cancer types.
Immune checkpoint inhibitors exhibit dramatic and long‐term effects in some patients, while imposing immune‐related adverse events (irAEs) without survival benefits in others. To personalize treatment, facilitate the cost‐effective use of ICIs, and avoid unnecessary irAEs, predictive and prognostic biomarkers have been sought. Some predictive factors for ICI treatment include PDL‐1 expression, haematologic markers, tissue infiltration lymphocytes, metastatic site, inflammatory cytokines, T cell markers, and irAEs. 3 , 5 , 41 , 42 Sarcopenia has been shown to be a prognostic marker of cancer 7 and a predictive marker of toxicity during chemotherapy. 43 A recent meta‐analysis on NSCLC showed that the loss of CT‐defined skeletal muscle mass affected the efficacy of ICIs. 44 However, the predictive role of sarcopenia in other types of cancer remains to be elucidated. Moreover, although several diagnostic procedures for sarcopenia have been used in the oncologic field, it remains to be elucidated which procedure best predicts the efficacy of ICIs.
Sarcopenia is a muscle disease defined by muscle quantity or quality. 6 A variety of diagnostic tests and tools are used to detect and diagnose sarcopenia. These include the SARC‐F questionnaire, physical performance tests, muscle strength tests, anthropometric measures, and skeletal muscle measurements. 45 Among them, muscle measurements using CT, dual‐energy X‐ray, and BIA are popular in the oncology research field. DXA requires special equipment, and the accuracy of BIA is affected by dehydration, which is commonly observed in patients with advanced cancer. In contrast, patients with cancer routinely undergo CT for tumour assessment. Thus, CT is the modality of choice for the diagnosis of sarcopenia in the oncologic field. SMI is the most commonly used index in the literature and is calculated as the total skeletal muscle area at the third lumbar vertebra level divided by the height squared. This index has been shown to be closely correlated with whole body muscle 46 and is associated with various health‐related outcomes. 6 PMI is frequently used in research from Japan 14 , 24 , 26 , 28 ; it uses the psoas major muscle area instead of the total skeletal muscle area. PMI is easier to calculate, and a cut‐off value has been proposed for Asian adults. 40 However, some argue that PMI is not a good indicator of sarcopenia. 47 When PMI and SMI as continuous variables were applied to the same cohort, their HRs for PFS showed comparable values. 17 Similarly, our meta‐analysis showed that the HRs for OS and PFS were comparable between the two indices, although statistical significance in OS for PMI was not reached owing to the statistical power. Therefore, both SMI and PMI could be used as predictive factors for ICIs.
Previous meta‐analyses on cancer and sarcopenia incorporated only SMI or other muscle mass evaluations as a requirement for inclusion. 7 , 44 However, we allowed the inclusion of other methods, such as SMD, muscle mass decrease, and skeletal muscle gauge (SMG). The European consensus statement notes that low muscle quantity or quality is required for the confirmation of sarcopenia diagnoses. 6 On CT images, the muscle mass area represents muscle quantity, while the muscle density reflects muscle quality. The impairment of muscle quality and infiltration of fat into the skeletal muscle can be indicative of muscle density decrease. SMD is a widely used index for muscle quality and has been shown to be a prognosticator in cancer. 48 Moreover, SMD, but not SMI, was shown to be associated with physical function, 49 indicating that it may be a better marker for severe sarcopenia. However, the results of the present meta‐analysis demonstrated that SMD could not predict the survival in patients treated with ICIs. In addition, SMG, an index in which the quantity and quality of skeletal muscle are integrated, was not a predictor of ICI therapy. 29 Patients with cancer lose weight due to decreased food intake, a catabolic state induced by cancer, and anti‐cancer treatment. Weight loss is a well‐established prognostic factor in patients with cancer. 39 Similarly, patients with cancer experience loss of skeletal muscle after diagnosis and a decline in gait speed even before diagnosis. 49 A decrease in skeletal muscle before or during ICI therapy, in other words, the progression of sarcopenia, was associated with adverse outcomes in patients treated with ICIs. 13 , 17 , 25 , 26 Owing to the small number of studies and differences in the diagnostic procedures, we did not synthesize HRs pertaining to the progression of sarcopenia in the present meta‐analysis. Collectively, of the various sarcopenia measures, muscle mass or its change can be a predictive factor for the efficacy of ICIs.
It may be argued that sarcopenia is reflective of a person's advanced disease status and deteriorated physical condition, resulting in a worse survival. However, our ORR and DCR results suggest that sarcopenia is not a mere prognostic factor but also a predictive factor. Skeletal muscle is known to release myokines, which are muscle‐derived cytokines that exert their effects through the autocrine, paracrine, and endocrine routes. 50 Among the myokines, interleukin (IL)‐15 increases the proportion of circulating natural killer cells and CD8+ T cells. 51 More importantly, the administration of IL‐15 in combination with ICIs prolonged the survival of tumour‐bearing mice. 52 Thus, changes in the myokine levels as a result of sarcopenia may affect the efficacy of ICI treatment, indicating the predictive value of sarcopenia in this therapy.
Skeletal muscle decrease after the initiation of ICIs treatment; that is, PMI and SMI decrease showed higher HRs than pretreatment sarcopenia did (Supporting Information, Tables S2 and S4). There are several causes for sarcopenia associated with cancer treatment, which include impaired food intake, reduced activity secondary to fatigue, and a direct effect of drugs on muscle. 53 Cytotoxic anti‐cancer drugs, including cisplatin, irinotecan, doxorubicin, and etoposide, increase proteolysis through NF‐κB and inflammatory cytokines, resulting in sarcopenia. 53 Mammalian target of rapamycin (mTOR) is one of the key enzymes involved in the maintenance of skeletal muscle. 54 Activation of mTOR pathway induces muscle hypertrophy, while blockade of the pathway leads to muscle atrophy. 54 Everolimus and temsirolimus, mTOR inhibitors used for renal cancer, induced a marked loss of muscle mass in clinical settings. 55 In vitro experiments demonstrated that pembrolizumab activated mTOR pathway. 56 Therefore, ICIs could affect skeletal muscle directly. Several studies have reported change in skeletal mass after ICIs therapy. 10 , 17 , 23 , 25 , 26 , 30 , 57 Supporting Information, Table S9 summarises the results of these studies. Six out of seven studies assessed skeletal muscle change from 3 weeks to 3 months after baseline and showed reduced muscle mass or muscle attenuation. 10 , 17 , 23 , 25 , 26 , 30 On the contrary, long‐term survivors treated with ICIs showed increased SMI and SMG. 57 This discrepancy between short‐term and long‐terms might indicate that the direct effect of ICIs on skeletal muscle is minimal and that skeletal muscle loss in short‐term reflects cancer progression and resultant cachexia in non‐responders. Therefore, higher HRs associated with progressive muscle loss could suggest worse survival in non‐responders.
This study has several strengths. First, we investigated a large number of patients using a meta‐analysis. The studies included in the present meta‐analysis were small‐scale retrospective studies. By combining the results, we obtained more reliable estimates of the predictive impact of sarcopenia. Till this date, only one published meta‐analysis has focused on the effect of sarcopenia on ICI efficacy. 44 However, while the previous meta‐analysis included 576 patients with NSCLC, the present study enrolled 2501 patients with solid cancers, providing a more comprehensive understanding of the predictive ability of sarcopenia. Another strong point is the broad inclusion criteria for muscle measurement. This enabled us to decide which method would be suitable for the prediction of ICI efficacy.
However, our study also has some limitations that must be considered. First, the studies included were of a retrospective nature. A majority of the enrolled studies retrospectively collected patient data. For the precise determination of the response rate and PFS, predefined protocols are mandatory. Second, the methods used for the calculation of the HRs and ORs differed across the studies. Although the use of data from multivariate analyses was desirable, we also included HRs from univariate analyses and estimated HRs from Kaplan–Meier curves. Moreover, the ORs for ORR were adjusted in only one study, 29 and those for DCR were not adjusted in any of the studies. Even when the HRs were adjusted for confounders, the adjustment was not sufficient owing to the limited number of events. In the investigation of the factors predictive of ICI efficacy, adjustment with established predictive factors, such as PD‐L1 expression or tumour mutation burden, is required. In addition, when investigating the effect of sarcopenia, adjustment with relevant factors, such as body mass index, performance status, and nutritional parameters should be conducted. Third, the cut‐off values associated with the same diagnostic measure varied across the studies. Seven and three cut‐off values were used for PMI and SMI, respectively. The effect of cut‐off values should be investigated using meta‐regression analyses in future studies. Finally, there existed significant publication bias, as shown in Figure 5. To reduce the degree of publication bias, we attempted to include non‐English articles. Researchers from non‐English‐speaking countries tend to publish studies of a weaker impact in their local journals and those with positive results in international journals. To retrieve non‐English articles and English articles, we searched Ichushi‐Web, but no Japanese article pertaining to our study topic was identified.
5. Conclusions
The number of patients who respond to ICIs is limited. Additionally, ICI treatment imposes a huge financial burden and is associated with irAEs. The identification of responders pre‐therapeutically or in the early phase of the treatment course is critically important. Unfortunately, current companion and complementary diagnostics are insufficient. In the present study, we demonstrated the predictive impact of sarcopenia in patients treated with ICIs. However, sarcopenia alone as a predictor would not be sufficiently useful. Indices comprising the combination of predictive factors are warranted. Further research is required to elaborate on the effective use of ICIs.
Ethics approval
The approval of the institutional review board was not required because this study was conducted using only previously published data. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 58
6. Conflict of interests
There are no conflicts of interest to declare.
Funding
This work was supported by JSPS KAKENHI Grant Number 19K09868. This work was partially supported by a grant awarded to B Gagnon and M. L. Tremblay from the Terry Fox Research Institute, Canada.
Author contributions
Y.T. conceived and designed the study and wrote the paper. Y.T. and R.O. collected and analysed the data. N.T., R.O., and H.I. reviewed and revised the manuscript.
7.
Supporting information
Data S1. PRISMA Checklist
Figure S1. Forest plot showing the prevalence of sarcopenia. The squares represent the hazard ratios for each study. The sizes of the squares and the horizontal lines crossing the squares represent the weight of the study in the random effect model and the 95% confidence intervals, respectively.
Table S1. Sensitivity analysis for overall survival
Table S2. Hazard ratios for overall survival according to diagnostic measures for sarcopenia
Table S3. Sensitivity analysis for progression‐free survival
Table S4. Hazard ratios for progression‐free survival according to diagnostic measures for sarcopenia
Table S5. Sensitivity analysis for objective response rate
Table S6. Odds ratios for objective response rate according to diagnostic measures for sarcopenia
Table S7. Sensitivity analysis for disease control rate
Table S8. Odds ratios for disease control rate according to diagnostic measures for sarcopenia
Table S9. Skeletal muscle change after treatment initiation
Acknowledgement
We thank Editage for the English editing.
Takenaka Y., Oya R., Takemoto N., and Inohara H. (2021) Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 12, 1122–1135, 10.1002/jcsm.12755
7.1. Data availability statement
All the data generated during this study are included in this published article and supporting information. All the original data were obtained from the published articles listed in the references.
References
- 1. Hunter P. The fourth pillar. EMBO Rep 2017;18:1889–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018;50:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jørgensen JT. Companion and complementary diagnostics: clinical and regulatory perspectives. Trends Cancer 2016;2:706–712. [DOI] [PubMed] [Google Scholar]
- 4. Zhou KI, Peterson BF, Serritella A, Thomas J, Reizine N, Moya S, et al. Spatial and temporal heterogeneity of PD‐L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res 2020;26:6453–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Y, Xu J, Du C, Wu Y, Xia D, Lv W, et al. The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: a systematic review and meta‐analysis. Front Oncol 2019;9: 10.3389/fonc.2019.01161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 8. Sabel MS, Lee J, Wang A, Lao C, Holcombe S, Wang S. Morphomics predicts response to ipilimumab in patients with stage IV melanoma. J Surg Oncol 2015;112:333–337. [DOI] [PubMed] [Google Scholar]
- 9. Dercle L, Ammari S, Champiat S, Massard C, Ferte C, Taihi L, et al. Rapid and objective CT scan prognostic scoring identifies metastatic patients with long‐term clinical benefit on anti‐PD‐1/‐L1 therapy. Eur J Cancer 2016;65:33–42. [DOI] [PubMed] [Google Scholar]
- 10. Daly LE, Power DG, O'Reilly Á, Donnellan P, Cushen S, O'Sullivan K, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer 2017;116:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cortellini A, Verna L, Porzio G, Bozzetti F, Palumbo P, Masciocchi C, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non‐small cell lung cancer patients: a “hypothesis‐generator” preliminary report. Thorac Cancer 2019;10:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deike‐Hofmann K, Gutzweiler L, Reuter J, Paech D, Hassel J, Sedlaczek O, et al. Macroangiopathy is a positive predictive factor for response to immunotherapy. Sci Rep 2019;9:9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, et al. Association of sarcopenia with and efficacy of anti‐PD‐1/PD‐L1 therapy in non‐small‐cell lung cancer. J Clin Med 2019;8:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, et al. Impact of sarcopenia in patients with advanced non–small cell lung cancer treated with PD‐1 inhibitors: a preliminary retrospective study. Sci Rep 2019;9:2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu MP, Li Y, Ghosh S, Sass S, Smylie M, Walker J, et al. Body composition is prognostic and predictive of ipilimumab activity in metastatic melanoma. J Cachexia Sarcopenia Muscle 2020;11:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cortellini A, Bozzetti F, Palumbo P, Brocco D, Di Marino P, Tinari N, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD‐1/PD‐L1 checkpoint inhibitors: a multicenter real‐life study. Sci Rep 2020;10:1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crombé A, Kind M, Toulmonde M, Italiano A, Cousin S. Impact of CT‐based body composition parameters at baseline, their early changes and response in metastatic cancer patients treated with immune checkpoint inhibitors. Eur J Radiol 2020;133:109340. [DOI] [PubMed] [Google Scholar]
- 18. Fukushima H, Fukuda S, Moriyama S, Uehara S, Yasuda Y, Tanaka H, et al. Impact of sarcopenia on the efficacy of pembrolizumab in patients with advanced urothelial carcinoma: a preliminary report. Anticancer Drugs. Published online 2020;31:866–871. [DOI] [PubMed] [Google Scholar]
- 19. Hirsch L, Bellesoeur A, Boudou‐Rouquette P, Arrondeau J, Thomas‐Schoemann A, Kirchgesner J, et al. The impact of body composition parameters on severe toxicity of nivolumab. Eur J Cancer 2020;124:170–177. [DOI] [PubMed] [Google Scholar]
- 20. Hu JB, Ravichandran S, Rushing C, Beasley GM, Hanks BA, Jung SH, et al. Higher BMI, but not sarcopenia, is associated with pembrolizumab‐related toxicity in patients with advanced melanoma. Anticancer Res 2020;40:5245–5254. [DOI] [PubMed] [Google Scholar]
- 21. Kano M, Hihara J, Tokumoto N, Kohashi T, Hara T, Shimbara K, et al. Association between skeletal muscle loss and the response to nivolumab immunotherapy in advanced gastric cancer patients. Int J Clin Oncol Published online 2020;26:523–531. [DOI] [PubMed] [Google Scholar]
- 22. Kim YY, Lee J, Jeong WK, Kim ST, Kim JH, Hong JY, et al. Prognostic significance of sarcopenia in microsatellite‐stable gastric cancer patients treated with programmed death‐1 inhibitors. Gastric Cancer 2020;24:457–466. 10.1007/s10120-020-01124-x [DOI] [PubMed] [Google Scholar]
- 23. Kim N, Yu J II, Park HC, Yoo GS, Choi C, Hong JY, et al. Incorporating sarcopenia and inflammation with radiation therapy in patients with hepatocellular carcinoma treated with nivolumab. Cancer Immunol Immunother Published online November 2020;24:1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Minami S, Ihara S, Tanaka T, Komuta K. Sarcopenia and visceral adiposity did not affect efficacy of immune‐checkpoint inhibitor monotherapy for pretreated patients with advanced non‐small cell lung cancer. World J Oncol 2020;11:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roch B, Coffy A, Jean‐Baptiste S, Palaysi E, Daures JP, Pujol JL, et al. Cachexia ‐ sarcopenia as a determinant of disease control rate and survival in non‐small lung cancer patients receiving immune‐checkpoint inhibitors. Lung Cancer 2020;143:19–26. [DOI] [PubMed] [Google Scholar]
- 26. Shimizu T, Miyake M, Hori S, Ichikawa K, Omori C, Iemura Y, et al. Clinical impact of sarcopenia and inflammatory/nutritional markers in patients with unresectable metastatic urothelial carcinoma treated with pembrolizumab. Diagnostics (Basel, Switzerland) 2020;10. 10.3390/diagnostics10050310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takada K, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, Wakasu S, et al. Clinical impact of skeletal muscle area in patients with non‐small cell lung cancer treated with anti‐PD‐1 inhibitors. J Cancer Res Clin Oncol 2020;146:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsukagoshi M, Yokobori T, Yajima T, Maeno T, Shimizu K, Mogi A, et al. Skeletal muscle mass predicts the outcome of nivolumab treatment for non‐small cell lung cancer. Med (United States) 2020;99:e19059, 10.1097/MD.0000000000019059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Young AC, Quach HT, Song H, Davis EJ, Moslehi JJ, Ye F, et al. Impact of body composition on outcomes from anti‐PD1 +/−anti‐CTLA‐4 treatment in melanoma. J Immunother Cancer 2020;8:e000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loosen SH, van den Bosch V, Gorgulho J, Schulze‐Hagen M, Kandler J, Jordens M, et al. Progressive sarcopenia correlates with poor response and outcome to immune checkpoint inhibitor therapy. J Clin Med 2021;10:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishioka N, Naito T, Notsu A, Mori K, Kodama H, Miyawaki E, et al. Unfavorable impact of decreased muscle quality on the efficacy of immunotherapy for advanced non‐small cell lung cancer. Cancer Med 2021;10:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Youn S, Reif R, Chu MP, Smylie M, Walker J, Eurich D, et al. Myosteatosis is prognostic in metastatic melanoma treated with nivolumab. Clin Nutr ESPEN 2021;42:348–353. [DOI] [PubMed] [Google Scholar]
- 33. Akce M, Liu Y, Zakka K, Martini D, Draper A, Alese O, et al. Impact of sarcopenia, BMI, and inflammatory biomarkers on survival in advanced hepatocellular carcinoma treated with anti‐PD‐1 antibody. Am J Clin Oncol 2021;44. Lippincott Williams and Wilkins:74–81. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097, 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 36. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 37. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 39. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 40. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016;32:1200–1205. [DOI] [PubMed] [Google Scholar]
- 41. Ouwerkerk W, Van Den Berg M, Van Der Niet S, Limpens J, Luiten RM. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Melanoma Res 2019;29:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zou Y, Zou X, Zheng S, Tang H, Zhang L, Liu P, et al. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta‐analysis. Ther Adv Med Oncol 2020;12:175883592094092, 10.1177/1758835920940928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre‐therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 2018;37:1101–1113. [DOI] [PubMed] [Google Scholar]
- 44. Wang J, Cao L, Xu S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non‐small cell lung cancer patients: a systematic review and meta‐analysis. Int Immunopharmacol 2020;88:106907. [DOI] [PubMed] [Google Scholar]
- 45. Miller J, Wells L, Nwulu U, Currow D, Johnson MJ, Skipworth RJE. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: a systematic review. Am J Clin Nutr 2018;108:1196–1208. [DOI] [PubMed] [Google Scholar]
- 46. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 47. Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle 2017;8:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee CM, Kang J. Prognostic impact of myosteatosis in patients with colorectal cancer: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2020;11:1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams GR, Deal AM, Muss HB, Weinberg MS, Sanoff HK, Nyrop KA, et al. Skeletal muscle measures and physical function in older adults with cancer: sarcopenia or myopenia? Oncotarget 2017;8:33658–33665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dalamaga M. Interplay of adipokines and myokines in cancer pathophysiology: emerging therapeutic implications. World J Exp Med 2013;3:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Waldmann TA, Dubois S, Miljkovic MD, Conlon KC. IL‐15 in the combination immunotherapy of cancer. Front Immunol 2020;11:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin‐15 in a murine metastatic colon carcinoma model. Clin Cancer Res 2010;16:6019–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davis MP, Panikkar R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. Ann Palliat Med 2019;8:86–101. [DOI] [PubMed] [Google Scholar]
- 54. Blaauw B, Mccarthy JJ, Yoon M‐S. mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol 2017;8:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gyawali B, Shimokata T, Honda K, Kondoh C, Hayashi N, Yoshino Y, et al. Muscle wasting associated with the long‐term use of mTOR inhibitors. Mol Clin Oncol 2016;5:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sasidharan Nair V, Toor SM, Taouk G, Phister G, Ouararhni K, Alajez NM, et al. Pembrolizumab interferes with the differentiation of human FOXP3 + –induced T regulatory cells, but not with FOXP3 stability, through activation of mTOR. J Immunol 2020;204:199–211. [DOI] [PubMed] [Google Scholar]
- 57. Patrinely JR, Young AC, Quach H, Williams G, Ye F, Fan R, et al. Survivorship in immune therapy: assessing toxicities, body composition and health‐related quality of life among long‐term survivors treated with antibodies to programmed death‐1 receptor and its ligand. Eur J Cancer 2020;135:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. PRISMA Checklist
Figure S1. Forest plot showing the prevalence of sarcopenia. The squares represent the hazard ratios for each study. The sizes of the squares and the horizontal lines crossing the squares represent the weight of the study in the random effect model and the 95% confidence intervals, respectively.
Table S1. Sensitivity analysis for overall survival
Table S2. Hazard ratios for overall survival according to diagnostic measures for sarcopenia
Table S3. Sensitivity analysis for progression‐free survival
Table S4. Hazard ratios for progression‐free survival according to diagnostic measures for sarcopenia
Table S5. Sensitivity analysis for objective response rate
Table S6. Odds ratios for objective response rate according to diagnostic measures for sarcopenia
Table S7. Sensitivity analysis for disease control rate
Table S8. Odds ratios for disease control rate according to diagnostic measures for sarcopenia
Table S9. Skeletal muscle change after treatment initiation
Data Availability Statement
All the data generated during this study are included in this published article and supporting information. All the original data were obtained from the published articles listed in the references.


