Abstract
Background
Hyperphosphatemia has been related to the development of sarcopenia in aging mice. We describe the intracellular mechanisms involved in the impairment of the myogenic differentiation promoted by hyperphosphatemia and analyse these mechanisms in the muscle from older mice.
Methods
C2C12 cells were grown in 2% horse serum in order to promote myogenic differentiation, in the presence or absence of 10 mM beta‐glycerophosphate (BGP) for 7 days. Troponin T, paired box 7 (Pax‐7), myogenic factor 5 (Myf5), myogenic differentiation 1 (MyoD), myogenin (MyoG), myocyte enhancer factor 2 (MEF2C), P300/CBP‐associated factor (PCAF), histone deacetylase 1 (HDAC1), fibronectin, vimentin, and collagen I were analysed at 48, 72, and 168 h, by western blotting or by immunofluorescence staining visualized by confocal microscopy. Studies in mice were performed in 5‐ and 24‐month‐old C57BL6 mice. Three months before sacrifice, 21‐month‐old mice were fed with a standard diet or a low phosphate diet, containing 0.6% or 0.2% phosphate, respectively. Serum phosphate concentration was assessed by a colorimetric method and forelimb strength by a grip test. Fibrosis was observed in the tibialis anterior muscle by Sirius Red staining. In gastrocnemius muscle, MyoG, MEF2C, and fibronectin expressions were analysed by western blotting.
Results
Cells differentiated in the presence of BGP showed near five times less expression of troponin T and kept higher levels of Pax‐7 than control cells indicating a reduced myogenic differentiation. BGP reduced Myf5 about 50% and diminished MyoD transcriptional activity by increasing the expression of HDAC1 and reducing the expression of PCAF. Consequently, BGP reduced to 50% the expression of MyoG and MEF2C. A significant increase in the expression of fibrosis markers as collagen I, vimentin, and fibronectin was found in cells treated with BGP. In mice, serum phosphate (17.24 ± 0.77 mg/dL young; 23.23 ± 0.81 mg/dL old; 19.09 ± 0.75 mg/dL old with low phosphate diet) correlates negatively (r = −0.515, P = 0.001) with the muscular strength (3.13 ± 0.07 gf/g young; 1.70 ± 0.12 gf/g old; 2.10 ± 0.09 gf/g old with low phosphate diet) and with the expression of MyoG (r = −0.535, P = 0.007) and positively with the expression of fibronectin (r = 0.503, P = 0.001) in gastrocnemius muscle. The tibialis anterior muscle from old mice showed muscular fibrosis. Older mice fed with a low phosphate diet showed improved muscular parameters relative to control mice of similar age.
Conclusions
Hyperphosphatemia impairs myogenic differentiation, by inhibiting the transcriptional activity of MyoD, and enhances the expression of fibrotic genes in cultured myoblasts. Experiments carried out in older mice demonstrate a close relationship between age‐related hyperphosphatemia and the decrease in the expression of myogenic factors and the increase in factors related to muscle fibrosis.
Keywords: Hyperphosphatemia, Myogenic differentiation, Skeletal muscle, Muscular fibrosis
Introduction
Aging is characterized by the decline of physiological function of the body. 1 Aging is triggered by intrinsic and extrinsic factors, among which hyperphosphatemia, that is, elevated serum phosphate concentrations, seems to have a role in the development of some aging‐related pathologies. 2 The balance of serum phosphate (Pi) levels in the body depends on intestinal absorption and renal excretion, and dietary phosphate intake and is mainly regulated by parathyroid hormone (PTH), Klotho, the fibroblast growth factor 23 (FGF23), and vitamin D. 3 Mice lacking FGF23 or Klotho show abnormal calcium and phosphate metabolisms leading to hyperphosphatemia and hypercalcemia linked to a premature aging syndrome. 4 The reduction of serum phosphate concentration rescues the premature aging syndrome in these animals, so a primary role of hyperphosphatemia in premature aging can be suggested. In addition, Klotho knockout (KO) mice and FGF23 KO mice exhibit severe muscle atrophy. 4 These results suggested to us that hyperphosphatemia could directly alter the skeletal muscle cells and to induce sarcopenia. In humans, high phosphate levels are a common feature of several diseases including the Hutchinson–Gilford progeria syndrome, 5 euthyroid Graves' diseases, 6 and chronic kidney disease (CKD), where it is associated with vascular calcification, adverse cardiovascular events, and increased mortality. 7 Curiously, sarcopenia is present in patients who suffer from these diseases.
Sarcopenia is defined as the involuntary loss of muscular mass and strength linked to advanced age. 8 Some of the changes occurring in sarcopenic muscles are type II fibre atrophy, fibre size reduction, accumulation of connective tissue and fat between the fibres, increased inflammation, deterioration in mitochondrial metabolism, and its oxidative capacity. 9 , 10 It has also been reported that a decrease in the number of satellite cells (myogenic stem cells) and their function can compromise the muscle regenerative capacity and disturb the myogenic programme in sarcopenia. 11
The muscular regeneration process initiates when muscle is injured and starts with the activation of satellite cells, followed by the proliferation of myoblasts and subsequent myogenic differentiation, and finally, by the formation of mature myotubes. The main transcriptional regulator of the satellite cell differentiation process is paired box 7 (Pax‐7), which is critical for cell cycle progression of satellite cells and myoblasts. 12 The myogenic differentiation and the mature myotube formation are regulated by the family of skeletal muscle‐specific transcription factors termed myogenic regulatory factors (MRFs), as well as the myocyte enhancer factor 2 (MEF2C), which acting sequentially, control the expression of several muscle genes. 13
When an injury occurs, the differentiation programme is sometimes disrupted, and the activated myoblasts, instead of proceeding to myogenic differentiation, progress to a fibrogenic differentiation. This impaired regeneration, among other events, appears to be attributed to a dysfunction in muscular satellite cell proliferation 14 and to the divergence of satellite cells towards a fibrogenic lineage. 15 Muscular fibrosis profoundly affects muscular function and has been related to sarcopenia and muscular dystrophies. 16 In aged muscle, the modification from functional myofibre repair towards increased extracellular matrix deposition characterizes the regenerative cascade 17 induced by changes in the micro‐environment of the satellite cells niche. 18 In a previous work, we have reported that hyperphosphatemia induces senescence and reduces myogenic differentiation in cultured murine myoblasts. 19 The aim of the present work is to evaluate the direct effect of hyperphosphatemia on the factors involved in the myogenic differentiation process and in the appearance of the muscular fibrosis and whether these effects are involved in the presence of sarcopenia signs in old mice.
Experimental procedures
Cell culture
A mouse myoblast cell line (C2C12, CRL‐1772) was acquired from the American Type Culture Collection (Manassas, VA, USA). C2C12 cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/L glucose and supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 U/mL penicillin in an atmosphere of 95% O2, 5% CO2 and 37°C. Cells were used at Passages 3–10.
Experimental design of in vitro studies
The effect of high phosphate concentrations on the myogenic differentiation process was analysed in murine C2C12 cells. Myoblasts were cultured at a density of 6000 cells/cm2 and differentiated for 7 days using DMEM enriched with 2% horse serum (HS), in the presence or absence of 10 mM beta‐glycerophosphate (BGP), as extracellular phosphate donor. To determine the number of myotubes formed, desmin and myosin heavy chain (MHC) expressions were assessed by immunofluorescence after 7 days, and the fusion index was calculated as the percentage of the nuclei number in myotubes with two or more nuclei versus the total number of nuclei. To analyse the process of myotube formation, Pax‐7, myogenic factor 5 (Myf5), myogenic differentiation 1 (MyoD), myogenin (MyoG), MEF2C, histone deacetylase 1 (HDAC1), and P300/CBP‐associated factor (PCAF) expressions were evaluated at 48, 72, and 168 h. Fibrogenic differentiation was assessed analysing the expressions of collagen I, vimentin, and fibronectin at the same time.
Animal studies
In vivo experiments were performed on C57BL6 male mice acquired from Janvier Laboratories. Mice were kept under standard conditions of temperature (22 ± 2°C), cycles of 12 h light/dark, and 54% relative humidity. They were provided with food ab libitum. Male C57BL6 mice were divided into three groups: 5‐month‐old mice (young group), 24‐month‐old mice (old group), and 24‐month‐old mice (old low Pi group) fed with a hypophosphatemic diet containing 0.2% phosphorous during the last 3 months of their life, whereas the other two groups were fed with a standard diet containing 0.6% phosphorus. Body weights were registered in all three groups just before changing the diet at 21 months in old mice and before sacrifice. One week before sacrifice, muscular strength was measured by a grip test. At the time of euthanasia, mice were anaesthetised, blood samples were collected by heart puncture exsanguinations, and serum was obtained after centrifugation (2200 g, 10 min). Gastrocnemius and tibialis anterior muscle samples were obtained and preserved in RNA later solution for protein and RNA extraction or stored in optimal cutting temperature compound at −80°C for histological analysis. Phosphate concentration of the serum was measured by the commercial kit QuantiChrom Phosphate Assay Kit (DIPI‐500) (BioAssay Systems; CA, USA).
The study design, as well as the experimental protocols, was developed by the guide for the care and use of laboratory animals, published by the US National Institute of Health (NIH) (Publication No. 85‐23, revised in 1996), as well as European and national regulations: European Directive 2010/63/EU, the Spanish State law 3/2007 on animal care, and the Royal Decrees RD1201/2005 and RD53/2013 on the protection of experimental animals and other scientific purposes. The study was reviewed and approved by Alcala University Research Ethics Committee (Madrid, Spain) and by the Community of Madrid (PROEX 210/17).
Protein extraction and immunoblot analysis
After cell treatment, cells were washed twice with cold phosphate‐buffered saline (PBS) and lysed in a buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X‐100, 0.1% sodium deoxycholate, 10 mM sodium pyrophosphate) containing a protease inhibitor cocktail. Then, cell lysate was centrifuged at 17 000 g for 30 min at 4°C. The protein concentration was determined using a BioRad protein assay kit (BioRad, CA, USA). Protein samples were run onto 8–12% sodium dodecyl sulfate‐polyacrylamide gels under reducing conditions and then transferred onto polyvinylidene fluoride membranes. Membranes were blocked with 5% non‐fat dry milk in Tween Tris‐buffered saline (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05% Tween‐20) for 1 h at room temperature (RT); then the corresponding primary antibodies Pax‐7 (1:1000), Myf5 (1:1000), MyoG (1:1000), MyoD (1:500), MEF2C (1:500), vimentin (1:1000), PCAF (1:500), collagen I (1:2000), and fibronectin (1:2000) were incubated overnight at 4°C; and, finally, blots were incubated with secondary antibodies 1 h at RT. The immunoreactive bands were visualized using the ECL western blotting detection system and analysed using ImageJ software 2.6 (http://rsbweb.nih.gov/ij/). Then, blots were reblotted with a mouse anti‐glyceraldehyde 3‐phosphate dehydrogenase (1:1000) or rabbit anti‐actin (1:2000) antibody to normalize the protein levels.
Immunofluorescence
Cells were blocked with 5% bovine serum albumin for 1 h at RT, before being incubated with each specific primary antibody: mouse anti‐MyoG (1:100), mouse anti‐HDAC1 (1:50), rabbit anti‐desmin (1:500), rabbit anti‐collagen I (1:200), or mouse anti‐α‐smooth muscle actin (1:200) overnight in a humidification chamber at 4°C or with mouse anti‐MHC (1:100) for 2 h at RT. After washing with PBS, cells were incubated for 1 h at RT with a mix of 200‐fold diluted goat anti‐rabbit IgG labelled with the antibody Alexa Fluor 488 (green), and 200‐fold diluted goat anti‐mouse IgG labelled with Alexa Fluor 647 (red), in order to detect rabbit antibodies in green and mouse antibodies in red in the same cover glass. Finally, cover glasses were mounted on ProLong Gold antifade reagent with 4′,6‐diamidino‐2‐phenylindole (DAPI) to stain the nuclei blue overnight. Preparations were visualized with a LEICA TCS‐SP5 confocal microscope (Leica Microsystems; GmbH, Mannheim, Germany) using the argon laser to detect green fluorescence, 405 nm diode laser to detect blue fluorescence, and the helium‐neon laser to detect red fluorescence. Pictures were taken and fluorescence intensity was measured using ImageJ software (http://rsbweb.nih.gov/ij/) and was calculated as the percentage of positive cells respect to the total number of cells in each picture.
For the proliferation assay, cover glasses were stained using the Click‐iT Plus EdU Cell Proliferation imaging kit (Thermo Fisher Scientific, Madrid, Spain) following the manufacturer's instructions. After treatments, cells were incubated with 10 μM 5‐ethynyl‐2′‐deoxyuridine (EdU), an analogue of 5‐bromo‐2′‐deoxyuridine, for 1 h. Afterwards, cells were fixed with 4% paraformaldehyde for 15 min at RT and permeabilizated with 0.5% Triton X‐100 for 20 min at RT. Next, cells were incubated with the Click‐iT Plus reaction cocktail (containing the antibody Alexa Fluor 488) for 30 min at RT. Finally, cover glasses were mounted on ProLong Gold antifade reagent with DAPI to stain the nuclei blue overnight. Preparations were visualized using a LEICA TCS‐SP5 confocal microscope as described earlier.
Immunoprecipitation
After treatments, cells were washed twice with cold PBS and lysed in an RIPA (radioimmunoprecipitation) lysis buffer. Cells were lysed on ice and incubated for 1 h at 4°C, then cells were centrifuged at 4000 g for 10 min at 4°C, and the supernatant was transferred to fresh tubes. The protein concentration was determined using a BioRad protein assay kit. Two micrograms of HDAC1 antibody (10E2) coupled to agarose beads (Santa Cruz Biotechnology; TX, USA) was added to 200 μg of protein sample, and they were incubated overnight at 4°C. Next, protein samples were centrifuged at 600 g for 5 min. Pellet was washed four times with PBS (600 g, 5 min). The immunodetection of protein samples was carried out using the western blot technique described earlier, and blots were incubated with anti‐HDAC1 (1:500) and anti‐MyoD (1:500). To check the efficacy of the technique, negative controls were carried out on samples without agarose.
Sirius Red staining
The connective tissues of the tibialis anterior muscle were stained using a Picro‐Sirius Red Stain Kit (Abcam; Cambridge, UK). Tissue sections stored at −80°C were hydrated in distilled water and incubated with Picro‐Sirius Red Solution for 20 min. Next, they were rinsed twice each with acetic acid solution and absolute ethanol. Finally, tissues were mounted with dibutylphthalate polystyrene xylene (DPX) solution to be observed with a microscope. Images were obtained under 20× magnification, and intensity of Sirius Red was measured using Image Pro Plus software (http://www.mediacy.com/imageproplus).
Materials
A complete list of the materials used is included as Supporting Information.
Statistical analysis
Results were expressed as the mean ± standard error of the mean (SEM) of an independent variable number of experiments detailed in the figure captions. GraphPad Prism 5 software (GraphPad Prism Software Inc.; San Diego, CA, USA) was used for statistical analysis. The following statistical tests were used: one‐way or two‐way analyses of variance (ANOVAs) followed by Dunnett's post‐tests for all experiments compared with control cells or followed by Bonferroni's post‐tests for multiple comparisons. Experiments performed on animals were analysed using one‐way ANOVA followed by Bonferroni's post‐tests for multiple comparisons, and t‐test followed by unpaired t‐test with Welch's correction for two groups' comparisons. Correlations were analysed using the non‐parametric test Spearman correlation. The level of statistically significance was defined as P < 0.05.
Results
Hyperphosphatemia declines myogenic differentiation of C2C12 cells
Cells were grown with 2% HS to drive to myogenic differentiation, in the presence or absence of 10 mM BGP for 48, 72, or 168 h, to assess the effect on the cell differentiation process. We analysed the expression of troponin T as a marker of myotube formation and the expression of Pax‐7 as a marker of satellite cells and non‐differentiated myoblasts. As expected, the expression of troponin T increased in control conditions as cells were differentiating, while in the presence of BGP, the expression of troponin T was lower after 168 h (Figure 1A). Myotube formation was also evaluated as the fusion index after immunofluorescence staining with MHC, BGP induced a significant reduction in the myotube formation after 7 days of culture in differentiation medium, reducing significantly the fusion index (Figure S1). In contrast, Pax‐7 expression, analysed by western blot and immunofluorescence, progressively decreased as cells entered the differentiation process in control conditions. In the presence of BGP, Pax‐7 expression remained higher, indicating that the differentiation process was being interrupted in some way (Figure 1B and 1C). Although the Pax‐7 expression remained high in the presence of BGP, myoblast proliferation, assessed by PCNA expression and EdU immunofluorescence, was significantly inhibited in this condition (Figure S2). Together, these results suggest that high extracellular phosphate partially inhibits the formation of mature myotubes.
Figure 1.
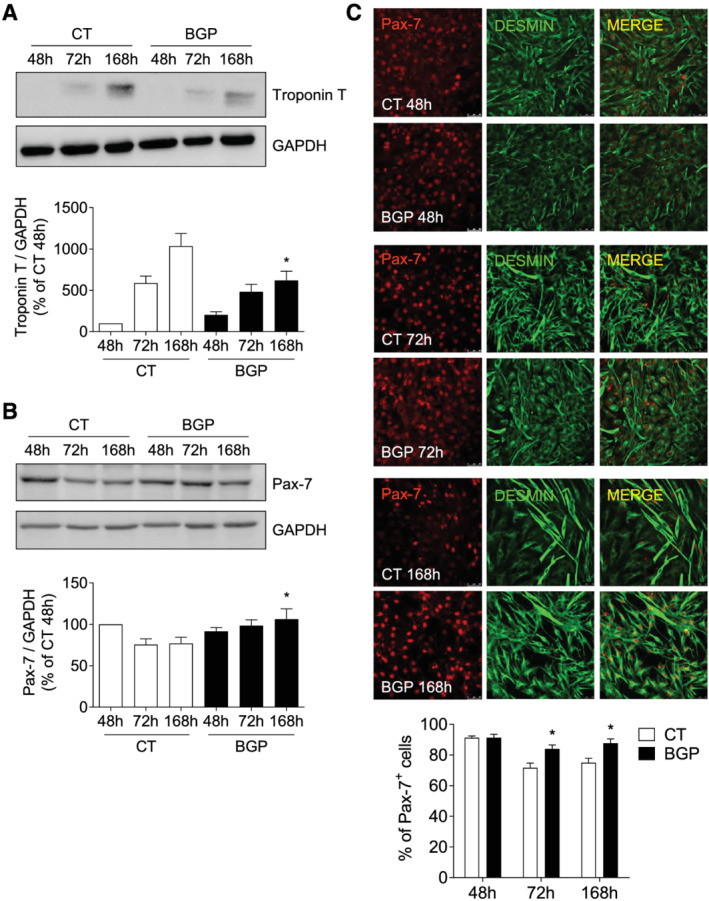
Hyperphosphatemia impairs myogenic differentiation of C2C12 cells. Myoblasts were differentiated with 2% HS in the presence or absence (CT) of 10 mM beta‐glycerophosphate (BGP) during 7 days. Troponin T (A) and Pax‐7 (B) expressions were evaluated by western blot at 48, 72, or 168 h. A representative blot is shown in each case. Bar graphs represent the densitometric analysis of the bands. The results are expressed as a percentage of control cells at 48 h (CT 48 h) and are the mean ± standard error of the mean from 10 (troponin T) or seven (Pax‐7) different experiments. *P < 0.05 versus CT at the same time. (C) Pax‐7 was evaluated by immunofluorescence with confocal microscopy. Pax‐7 (red) and desmin (green) to mark the cells. Pictures were obtained with 40× magnification. The bar graph represents the percentage of Pax‐7‐positive cells. Results are the mean ± standard error of the mean from eight different experiments. *P < 0.05 versus CT at the same time.
Hyperphosphatemia modifies the expression of some myogenic factors
To explore the intracellular mechanisms involved in the reduced myotube formation, the BGP effect on the different phases of myogenic differentiation was analysed by the evaluation of some of the MRF and MEF2C factors, implicated in these processes. Cells were treated with or without 10 mM BGP as explained earlier. First, we analysed Myf5 and MyoD expressions, which are the MRFs responsible for the specification of the skeletal muscle lineage. Myf5 expression was significantly reduced at 72 h in BGP‐treated cells with respect to control cells (Figure 2A). In contrast, no changes in MyoD expression were found between control and BGP‐treated cells (Figure 2B). Next, we studied the factors involved in early myogenesis, MyoG and MEF2C, which are essential to begin the myogenic differentiation process. MyoG expression was evaluated by western blot and immunofluorescence staining. The percentage of MyoG‐positive cells increased as the cells were differentiating under control conditions (Figure 3A), as well as MyoG expression (Figure 3B). By contrast, BGP‐treated cells had a lower MyoG expression compared with control cells (Figure 3A and 3B). The expression of MEF2C was maintained unchanged over time in cells differentiating in control conditions, whereas it was significantly reduced at 168 h in cells differentiating in the presence of BGP (Figure 3C). These results suggest that BGP modified the myogenic differentiation programme by reducing the expression of Myf5, MyoG, and MEF2C, which are implicated in the early phases of the myogenesis, without modifying the expression of MyoD. Given that MyoD is a transcriptional factor involved in the gene expression of MyoG and MEF2C, we next analysed whether BGP regulated the transcriptional activity of MyoD.
Figure 2.
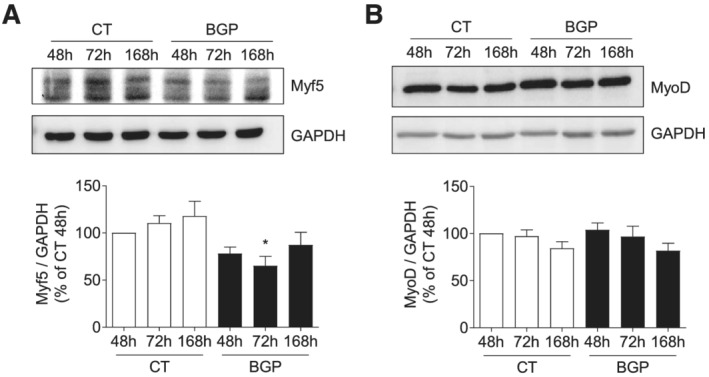
Hyperphosphatemia modifies the expression of some myogenic factors responsible for the specification of skeletal muscle lineage. Cells were differentiated with medium enriched by 2% HS during 7 days in the presence or absence of 10 mM BGP. Myf5 (A) and MyoD (B) expressions were assessed by western blot at 48, 72, or 168 h. A representative blot is shown in each case. Bar graphs represent the densitometric analysis of the bands. The results are expressed as a percentage of control cells at 48 h (CT 48 h) and are the mean ± standard error of the mean from five (Myf5) or 10 (MyoD) different experiments. *P < 0.05 versus CT at the same time.
Figure 3.
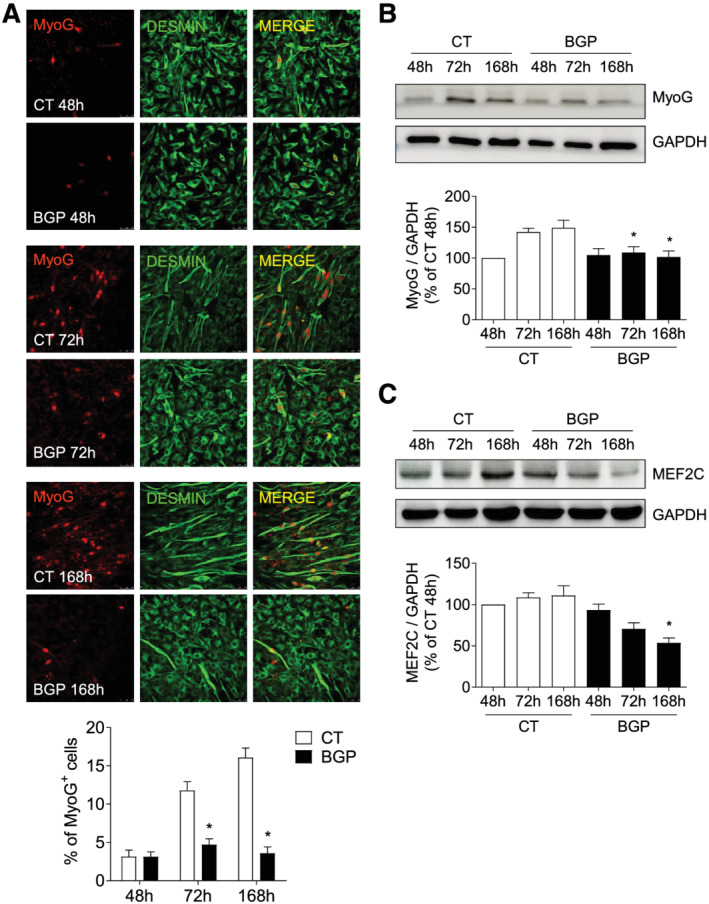
Hyperphosphatemia modifies the expression of some myogenic factors implicated in early myogenesis. Myoblasts were differentiated with 2% HS for 7 days in the presence or absence of 10 mM BGP. (A) Cellular localization of MyoG (red) and desmin (green) were measured by immunofluorescence with confocal microscopy. Pictures were obtained with 40× magnification. The bar graph represents the percentage of MyoG‐positive cells. Results are the mean ± standard error of the mean from eight different experiments. *P < 0.05 versus CT at the same time. MyoG (B) and MEF2C (C) expressions were analysed by western blot at 48, 72, and 168 h. A representative blot is shown in each case. Bar graphs represent the densitometric analysis of the bands. The results are expressed as a percentage of control cells at 48 h (CT 48 h) and are the mean ± standard error of the mean from 11 (MyoG) or three (MEF2C) different experiments. *P < 0.05 versus CT at the same time.
Hyperphosphatemia modifies the expression of the regulator proteins of MyoD transcriptional activity
To analyse whether BGP was modifying the expression of some of the epigenetic regulators of MyoD activity, we measured the expression of HDAC1, a histone deacetyltransferase responsible for inhibiting the transcriptional activity of MyoD, and the expression of PCAF, a histone acetyltransferase necessary for the transcriptional activity of MyoD.
For that purpose, C2C12 cells were differentiated as indicated earlier in the presence or absence of 10 mM BGP. The expressions of HDAC1 and PCAF were evaluated by immunofluorescence and western blot, respectively. It was observed that the number of cells expressing HDAC1 in the nucleus at 48 h was significantly higher in cells treated with BGP compared with cells without treatment (Figure 4A and 4B). In turn, it was observed that PCAF expression levels were significantly decreased in cells treated with BGP when compared with those levels expressed by cells without treatment for the same times (Figure 4C).
Figure 4.
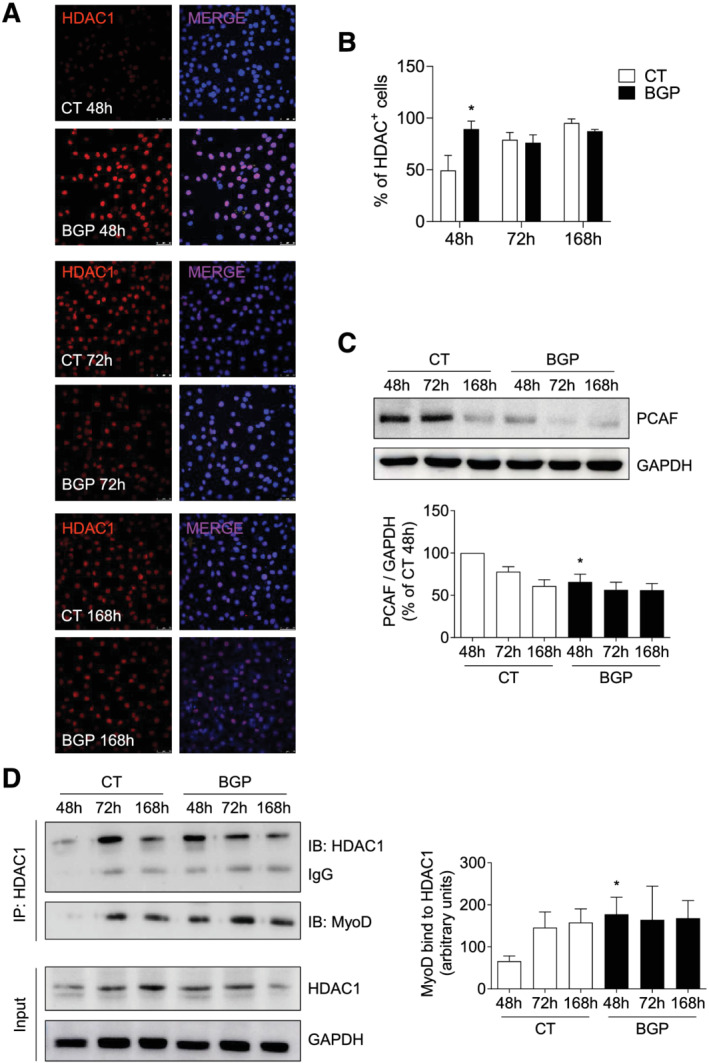
Hyperphosphatemia modifies the expression of the regulator proteins of the MyoD transcriptional activity. Myoblasts were differentiated with 2% HS for 7 days in the presence or absence of 10 mM BGP. (A and B) HDAC1 expression (red) was evaluated by immunofluorescence by confocal microscopy. Pictures were obtained with 40× magnification. The bar graph represents the percentage of HDAC1‐positive cells. Results are expressed as a percentage of control cells at 48 h (CT 48 h) and are the mean ± standard error of the mean from six different experiments. *P < 0.05 versus CT at the same time. (C) PCAF expression was analysed by western blot at 48, 72, and 168 h. A representative blot is shown. The bar graph represents the densitometric analysis of the bands. The results are the mean ± standard error of the mean from six different experiments. *P < 0.05 versus CT at the same time. (D) HDAC1 was immunoprecipitated and protein levels of MyoD bound to HDAC1 were evaluated by western blot. As input, HDAC1 and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) were used. A representative experiment is shown. Bar graph represents the densitometric analysis of MyoD band. Results are the mean ± standard error of the mean from five different experiments. *P < 0.05 versus CT at the same time.
Next, it was determined whether the high expression levels of HDAC1 presented by the BGP‐treated cells were preventing the transcriptional activity of MyoD; for this, the levels of MyoD expression bound to HDAC1 were assessed, because these would be the levels of inactive MyoD. Differentiation and treatment of C2C12 myoblasts were done as explained earlier. HDAC1 was immunoprecipitated and protein levels of MyoD bound to HDAC1 were analysed by western blot. At 48 h, a greater expression of the MyoD–HDAC1 complex was found in BGP‐treated cells compared with control cells (Figure 4D). This result demonstrates that high phosphate levels regulate MyoD by inhibiting its transcriptional activity and disrupting the myogenic programme.
Hyperphosphatemia promotes fibrogenic differentiation in myoblasts
To analyse whether hyperphosphatemia promoted fibrogenic differentiation on myoblasts, cells were grown in a 2% HS‐enriched medium for 7 days, in the presence or absence of BGP, and vimentin, fibronectin, and collagen I expressions were assessed after different exposure times by western blot. Collagen I was also analysed by immunofluorescence staining. Results showed that the expressions of fibronectin, vimentin, and collagen I were elevated in cells differentiated under BGP treatment (Figure 5A–5D), indicating that BGP increased the fibrogenic differentiation of myoblasts.
Figure 5.
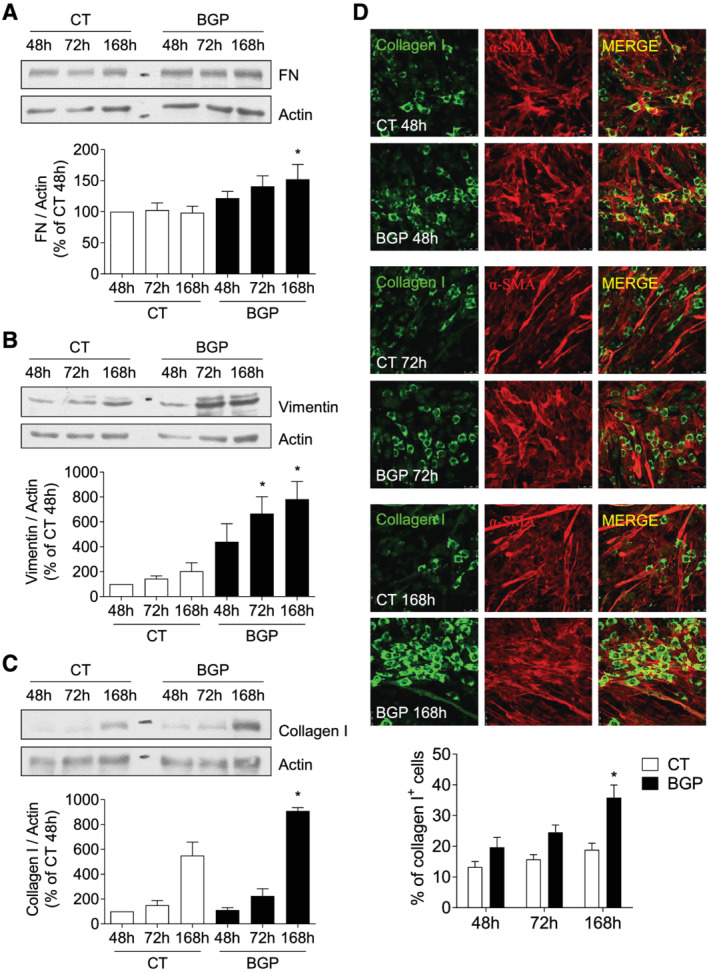
Hyperphosphatemia promotes fibrogenic differentiation in myoblast. Cells were differentiated with 2% HS for 7 days and treated with or without 10 mM BGP. Fibronectin (FN) (A), vimentin (B), and collagen I (C) expressions were evaluated by western blot at 48, 72, and 168 h. A representative blot is shown in each case. Bar graphs represent the densitometric analysis of the bands. The results are expressed as a percentage of control cells at 48 h (CT 48 h) and are the mean ± standard error of the mean from seven (FN and vimentin) or four (collagen I) different experiments. *P < 0.05 versus CT at the same time. (D) Collagen I (Col I, green) and α‐smooth muscle actin (α‐SMA, red) expressions were analysed by immunofluorescence. Pictures were obtained with 40× magnification. The bar graph represents the percentage of collagen I‐positive cells. Results are expressed as a percentage of CT 48 h and are the mean ± standard error of the mean from eight different experiments. *P < 0.05 versus CT at the same time.
Hyperphosphatemia correlates with reduced myogenic factor expression and increased fibrosis in muscle isolated from old mice
Finally, to establish whether there is some relationship between the serum phosphate concentration and the muscular expression of myogenic and fibrotic factors in vivo, some in vivo studies were done in old and young mice.
First, it was detected that phosphate concentration of the serum significantly rose in older mice compared with young mice (Figure 6A). Similarly, old mice display a significant loss in the muscular strength respect to young mice as measured by the grip test (Figure 6B). The expressions of MyoG and MEF2C were evaluated in the gastrocnemius muscle isolated from 24‐month‐old mice and compared with muscle from 5‐month‐old mice. Results showed a decreased expression of both MyoG (Figure 6C) and MEF2C (Figure 6D) in old mice. The expression of MyoG was negatively correlated with serum phosphate concentration (Table 1) and positively correlated with muscular strength (Table 1). Fibronectin expression was also increased in muscle isolated from old mice, assessed by western blot (Figure 6E) and was positively correlated with phosphate concentration of the serum and negatively correlated with muscular strength (Table 1). In addition, we analysed the collagen content in the tibialis anterior muscle isolated from mice by Sirius Red staining, finding a significant increase in old mice (Figure 6F).
Figure 6.
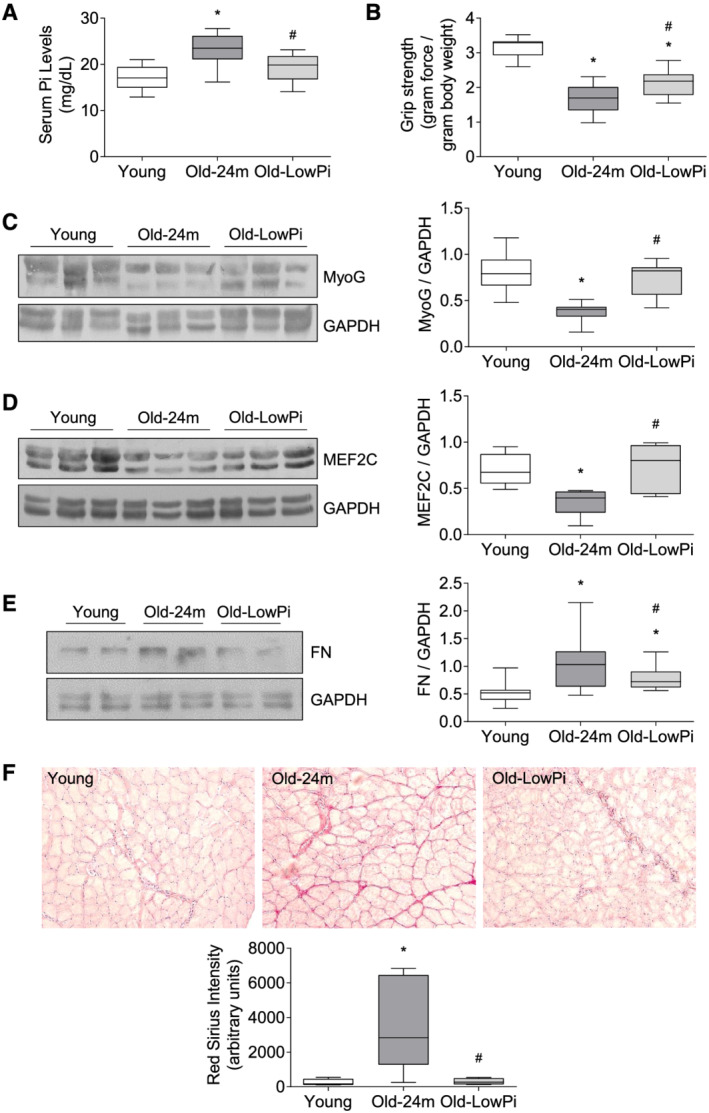
Hyperphosphatemia reduces myogenic factor expression and increases fibrosis in muscle isolated from old mice. Five‐month‐old mice (Young), 24‐month‐old mice fed with normal diet (Old‐24m), and 24‐month‐old mice fed with a low phosphate diet for the last 3 months (Old‐Low Pi) were evaluated. (A) Serum phosphate concentrations were assessed by a colorimetric method. (B) Forelimb grip strength test was performed in all mice. MyoG (C), MEF2C (D), and fibronectin (FN) (E) expressions were evaluated by western blot in protein extracts isolated from gastrocnemius muscle. A representative blot is shown in each case. (F) Collagen I content in tibialis anterior muscle was analysed by Sirius Red staining. Pictures were obtained with 20× magnification. Results are presented by Box and Whisker plot, where boxes represent the upper and lower quartiles, vertical lines represent the lowest and highest value, and the line inside the box represents the median of values obtained from 10 animals per group. *P < 0.05 versus Young, # P < 0.05 versus Old‐24m.
Table 1.
Spearman correlation coefficients (r), and its P value (P), between serum concentrations of phosphate (Pi), muscular strength, and myogenin (MyoG) and fibronectin expressions
| Serum Pi concentration | Muscular strength | MyoG expression | Fibronectin expression | |
|---|---|---|---|---|
| Serum Pi concentration | — | r = −0.515 | r = −0.535 | r = 0.503 |
| P = 0.001 | P = 0.007 | P = 0.001 | ||
| Muscular strength | r = −0.515 | — | r = 0.536 | r = −0.701 |
| P = 0.001 | P = 0.004 | P ≤ 0.001 | ||
| MyoG expression | r = −0.535 | r = 0.536 | — | r = −0.508 |
| P = 0.007 | P = 0.004 | P = 0.006 | ||
| Fibronectin expression | r = 0.503 | r = −0.701 | r = −0.508 | — |
| P = 0.001 | P ≤ 0.001 | P = 0.006 |
In order to establish a direct relationship between the increased serum phosphate levels found in old mice and the changes found in all the parameters measured in old mice, a group of 21‐month‐old mice were fed for the last 3 months of their life with a low phosphate (Pi) diet containing 0.2% Pi respect to the standard diet with 0.6% Pi. Hypophosphatemic diet did not modify the body weight of the mice during the 3 months of treatment. At the end of the treatment, the average body weight was 31.99 ± 1.4 for young mice, 39.64 ± 2.81 for old mice, and 39.24 ± 2.38 for old mice fed with low phosphate diet. The old mice submitted to low Pi diet showed improvement in all parameters analysed: improved muscular strength, increased expression of MyoG and MEF2C, and lower collagen staining and fibronectin expression, apart from a significant reduction in phosphate concentration of the serum (Figure 6A–6F). Together, these results would be pointing to a direct role of hyperphosphatemia in the age‐associated deterioration of muscle in mice.
Discussion
Mechanisms involved in the development of sarcopenia are not fully understood. We demonstrate, by a mechanistic study, that hyperphosphatemia, an aging‐related condition, is implicated in the reduction of the regenerative capacity of aged muscle and in the appearance of muscular fibrosis, which are events related to sarcopenia.
We present here new results exploring the effect of hyperphosphatemia on the myogenic differentiation process and demonstrating that hyperphosphatemia changes the expression pattern of some of the factors involved in the regulation of myogenesis.
We demonstrated that myoblasts, when differentiating in the presence of a high concentration of extracellular phosphate, showed a lower expression of troponin T, a marker of mature myotube, 20 which is consistent with hyperphosphatemia reducing the expression of MHC, as previously reported by us. 19 Moreover, in these conditions, cells maintained a high expression level of Pax‐7, which is usually used as a reliable indicator for identifying the total pool of quiescent and proliferating satellite cells. 21 Although cells that differentiate in the presence of BGP maintain a high expression of Pax‐7, BGP partially inhibited myoblasts proliferation. Pax‐7 has been reported to play a central role in the myogenic specification of satellite cells 22 and in promoting their proliferation and self‐renewal. 12 However, the effect of Pax‐7 on myogenic differentiation remains controversial. A reciprocal inhibition between Pax‐7 and MRF has been described; Pax‐7 expression must be low to allow myogenic differentiation, although Pax‐7 is unable to repress muscle differentiation by itself. 23 Altogether, these data suggest that hyperphosphatemia avoids partly the myogenic differentiation process of cultured myoblasts.
The mechanisms involved in this effect were evaluated, determining the expression of some of the MRFs involved in myogenesis during the differentiation process of cultured myoblasts. In this work, we demonstrated that cells treated with high concentrations of phosphate showed a decreased expression of Myf5 and MyoG, which would lead to a lower myogenic differentiation rate, whereas no changes were found in MyoD expression.
Myf5 and MyoD are both essential factors for the specification of the myogenic lineage of undifferentiated myoblasts, but they contribute differently to the myogenic differentiation programme. 24 Myf5 seems to have an important role in enabling the proliferation of activated transient myoblasts, although mice lacking Myf5 can regenerate skeletal muscle as well. 25 Moreover, Myf5 has a weak transcriptional activity compared with MyoD. 24 Although we found that Myf5 expression was diminished by hyperphosphatemia, this diminished expression was not enough to inhibit myogenic differentiation.
In contrast, MyoD is considered the key regulator of myogenic differentiation, 26 as its activity triggers the entire myogenic programme when ectopically expressed in non‐muscle cell types. 27 Experiments performed in a MyoD‐null adult mouse showed that satellite cells have a lesser proliferative capacity and a delayed transition to myogenic differentiation even when they were cultured under myogenic conditions. 28 MyoD expression is low in undifferentiated myoblasts and increases during the early stages of the differentiation process. 26 It acts as a transcriptional factor that enhances the transcription of MyoG and MEF2C genes, among others. 29 In our experiments, hyperphosphatemia did not change the protein expression of MyoD but reduced MyoG and MEF2C, suggesting that hyperphosphatemia could reduce the transcriptional activity of MyoD rather than its protein levels. The transcriptional activity of MyoD is regulated by epigenetic mechanisms involving several chromatin modifiers, among others, histone deacetylases and histone acetyltransferases. Therefore, we analysed the expression of two of these proteins, HDAC1 and PCAF, closely related to the inhibition or activation of the transcriptional activity of MyoD, respectively. 30 , 31 We found significant changes in their expressions when myoblasts were differentiating in the presence of a high concentration of phosphate. HDAC1 expression remained elevated during the whole incubation time, whereas PCAF diminished.
HDAC1 is a histone deacetylase, which inhibits MyoD activity when it is recruited by SNAIL, a transcription factor family involved in the regulation of the epithelial to mesenchymal transition. The repressive complex of SNAIL with histone deacetylases binds and then excludes MyoD from G/C‐rich E‐box motifs in murine satellite cells and inhibits myogenic differentiation. 32 HDAC1 is bound to MyoD in undifferentiated myoblasts, while its expression is reduced during differentiation. 30 HDAC1 overexpression inhibits myogenic differentiation because it reduces acetylation of histones in late differentiation promoters such as the MHC gene and keeps MyoD deacetylated and inactive. 30 , 32 Immunoprecipitation of HDAC1 confirmed an increase of HDAC1 bound to MyoD in the presence of BGP during the early stages of myogenic differentiation, suggesting that transcriptional activity of MyoD was partially inhibited.
On the other hand, PCAF is a histone acetyltransferase that also participates in the regulation process of MyoD. The acetyltransferase activity of PCAF is required for MyoD transcriptional activity and muscle differentiation. MyoD is acetylated by PCAF, giving rise to conformational changes in DNA that allow the interaction of MyoD with the E‐box of the genes it regulates. 31 Taken together, the present results suggest that MyoD activity could be inhibited by hyperphosphatemia. Because of the reduction in the transcriptional activity of MyoD, MyoG and MEF2C decreased in cells differentiating under hyperphosphatemic conditions. According to previous studies, the diminished expression of MyoG and MEF2C could explain by itself the reduction in myotube formation.
MyoG acts later to regulate myoblast terminal differentiation, the size, and myofibre maturation. 32 The lack of MyoG allows myoblast terminal differentiation, expression of many muscle‐specific markers, and generation of functional contractile muscle but prevents fusion of most myocytes and leads to excess mononucleated muscle fibres. 33 MyoG is required for the expression of membrane proteins involved in cell fusion, such as Myomaker. 34 In addition, MyoG also regulates the expression of some MRFs. The lack of MyoG reduces the expression of Myf5, and, in contrast, the increased expression of MyoG lowers Pax‐7 expression. 23 The myogenic differentiation process is also regulated by members of the family of MEF2 proteins that play a pivotal role in the activation of muscle‐specific genes. 35 MEF2 proteins bind to a consensus A/T‐rich DNA sequence present in the promoters of most muscle‐specific genes and can act as a transcriptional cofactor increasing expression of MyoD and MyoG. 29 , 35 Loss of MEF2C has been also related to the loss of differentiated muscle cells in Drosophila, demonstrating the requirement of MEF2C protein in skeletal muscle differentiation. 36 Our results show that MEF2C expression decreases when cells are differentiating in the presence of high phosphate concentrations; this prevents MEF2C from acting as a cofactor for MyoG and MyoD, and consequently myogenic differentiation.
On the other side, skeletal muscle fibrosis is considered a major cause of muscle weakness and has been related to muscular dystrophies and sarcopenia. Fibrosis impairs muscle function, negatively affecting muscle regeneration after injury and increasing muscle susceptibility to re‐injury. 37 It has been described that muscle satellite cells from aged mice convert from a myogenic to a fibrogenic lineage as they begin to proliferate, which is mediated by factors from the systemic environment of the old animals. 15 We tested whether hyperphosphatemia induced fibrogenic differentiation in cultured myoblasts and found an increased expression of fibronectin, collagen I, and vimentin. Our results indicate that high phosphate concentration is not only diminishing myogenic differentiation but also favouring fibrogenic differentiation, and this mechanism could be related to sarcopenia.
In order to translate the results obtained in cultured myoblasts to an animal experimentation model, we analysed the muscle composition and the expression of MRF in gastrocnemius muscle isolated from 24‐month‐old mice to compare with 5‐month‐old mice. First, we found that old mice had higher serum phosphate concentration and lower muscular strength than young mice, as previously published. 19 , 38 In parallel, gastrocnemius isolated from old mice showed less expression of MyoG and MEF2C whereas the expression of fibronectin and collagen staining was higher as compared with muscles isolated from young mice. The serum concentration of phosphate positively correlated with muscular fibrosis signs and negatively with the expression of MRF. On the contrary, muscular strength correlated negatively with fibrotic signs and positively with MRF expression. In agreement with this, it has been described that the expression of MRFs decreases in aging muscle, inhibiting their myogenic differentiation, 39 and that muscular fibrosis increased in old mice. 40 The mechanism involved in the muscle fibrosis induced by hyperphosphatemia was not assessed in the present work, but what we previously described as muscular fibrosis was induced by an excess of circulating endothelin‐1 in old mice, 41 and that hyperphosphatemia induces endothelin‐1 production in endothelial cells by up‐regulation of endothelin‐converting enzyme 1 through reactive oxygen species‐induced activator protein 1 factor. 41
Finally, we tested whether reducing the phosphate concentration of the serum in old mice could improve the muscular function and composition. For this purpose, a group of 21‐month‐old mice were fed with a low phosphate diet for the last 3 months of their life. Old mice receiving the low phosphate diet had lower serum phosphate levels linked to higher muscular strength than its coetaneous fed with a standard diet. Also, these animals showed higher expression of MyoG and MEF2C, and lower muscular fibrosis, so the dietary restriction of phosphate would improve the expression of MRF in the muscle from old mice and would reduce the appearance of fibrosis. These results suggest a direct role of hyperphosphatemia in the aging‐related changes in composition and muscular strength. In accordance with these outcomes, poor phosphate diets rescue the aging phenotype in animal models with hyperphosphatemia such as the Klotho or FGF‐deficient mice. 2 Because hyperphosphatemia has been related to human aging and several diseases 5 , 6 , 7 associated to sarcopenia, the reduction of the phosphate intake could be a useful tool to reduce serum phosphate levels and to improve muscle function and composition. In this sense, a recent analysis of the Scottish pSoBid cohort showed a positive correlation between dietary phosphate intake, frequency of red meat consumption, and some accelerated aging signs, such as telomere shortening and DNA methylation. 42
In conclusion, we demonstrated, by a mechanistic study, that high levels of phosphate reduce myogenic differentiation by inhibiting the activity of MyoD and enhance the expression of fibrotic genes in cultured myoblasts. Additionally, the experiments carried out in old mice show a close relationship between age‐related hyperphosphatemia and the decrease in the expression of myogenic factors, the increase in factors related to muscle fibrosis, and the loss of muscular strength. Further experiments will be necessary to demonstrate whether the reduction of serum phosphate levels prevents the development of sarcopenia in old mice and to translate these results to the hyperphosphatemia‐associated human diseases.
Funding
This work was supported by grants from the Fondo de Investigaciones Sanitarias from Instituto de Salud Carlos III and FEDER funds (grants PI19/01339, PI19/00502, PI16/02082, PI16/01619), a grant from Comunidad de Madrid NOVELREN‐CM (grant B2017/BMD‐3751), Networks Program REDinREN from Instituto de Salud Carlos III and FEDER funds (grant RETIC REDinREN: RD016/0009/0018; RD16/0009/0017), IRYCIS group 3.07, and FRIAT.
E.A.‐E. holds a predoctoral contract from Ministerio de Educación, cultura y deportes (FPU16/01450), A.A.‐B. and P.P. held contracts from Programa de Garantía Juvenil of CAM as a predoctoral fellow (PEJ‐2018‐PRE/BMD‐8289) and as technician (PEJ‐2017‐TL/BMD‐5956), respectively, A.A.‐B. holds a project‐associated contract from the Fondo de Investigaciones Sanitarias from Instituto de Salud Carlos III (PI16/01619), P.S. held a predoctoral contract from Alcala University (FPI16‐UAH), and S.L.‐O. holds a contract from the Research Stabilization program from Instituto de Salud Carlos III (CES07/032). Carrillo‐LópezN has been supported by Plan de Ciencia, Tecnología e Innovación 2018‐2022 del Principado de Asturias (IDI‐2018‐000152).
Ethical guidelines statement
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 43
Author contributions
M.P.R.‐T. and S.L.‐O. designed research; E.A.‐E., A.A.‐B., P.S., and P.P. performed research; P.S., E.A.‐E., A.A.‐B., N.C.‐L., G.O., and S.L.‐O. analysed data; and G.O., M.P.R.‐T., and S.L.O. wrote the paper. All authors have read and approved the final version of the manuscript.
Conflict of interest
All authors declare that they have no conflict of interest.
Supporting information
Figure S1. Hyperphosphatemia impairs myogenic differentiation of C2C12 cells. Myoblasts were differentiated with 2% HS in the presence or absence (CT) of 10 mM BGP (BGP) during 7 days. a) MHC expression was evaluated by immunofluorescence with confocal microscopy. MHC (red) and desmin (green) to mark the cells. Pictures were obtained with 40X magnification. The bar graph represents the fusion index. Results are the mean ± standard error of the mean from ten different experiments. *p<0.05 vs CT.
Figure S2. Hyperphosphatemia reduced the proliferation capacity of differentiating C2C12 cells. Myoblasts were differentiated with 2% HS in the presence or absence of 10 mM BGP during 7 days. a) 5‐ethynyl‐2´‐deoxyuridine (EdU) expression was evaluated by immunofluorescence with confocal microscopy. Edu (green) and DAPI (blue) to stain the nuclei. Pictures were obtained with 40X magnification. b) The bar graph represents the percentage of EdU positive cells. Results are the mean ± standard error of the mean from two experiments. *p<0.05 vs CT at the same time. c) PCNA expression was analysed by western‐blot at 48, 72 and 168 hours. A representative blot is shown. The bar graph represents the densitometric analysis of the bands. The results are the mean ± standard error of the mean from four different experiments. *p<0.05 vs CT at the same time.
Acknowledgements
Immunofluorescence analyses were visualized using a Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany), through the Confocal Microscopy Service (ICTS ‘NANBIOSIS’ U17) of the Biomedical Research Networking Centre on Bioengineering, Biomaterials and Nanomedicine (CIBER‐BBN at the Alcalá University, Madrid, Spain) (https://www.uah.es/enlaces/investigacion.shtm). We acknowledge Prof. Raymond Stallings for English grammar and spelling revision.
Sosa P., Alcalde‐Estévez E., Asenjo‐Bueno A., Plaza P., Carrillo‐López N., Olmos G., López‐Ongil S., and Ruiz‐Torres M. P. (2021) Aging‐related hyperphosphatemia impairs myogenic differentiation and enhances fibrosis in skeletal muscle, Journal of Cachexia, Sarcopenia and Muscle, 12, 1266–1279, 10.1002/jcsm.12750
References
- 1. Kirkwood TB. Understanding the odd science of aging. Cell 2005;120:437–447. [DOI] [PubMed] [Google Scholar]
- 2. Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J 2010;24:3562–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, et al. In vivo genetic evidence for klotho‐dependent, fibroblast growth factor 23 (Fgf23)‐mediated regulation of systemic phosphate homeostasis. FASEB J 2009;23:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. John GB, Cheng CY, Kuro‐o M. Role of Klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis 2011;58:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, et al. Phenotype and course of Hutchinson‐Gilford progeria syndrome. N Engl J Med 2008;358:592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin CH, Chang CK, Shih CW, Li HY, Chen KY, Yang WS, et al. Serum fibroblast growth factor 23 and mineral metabolism in patients with euthyroid Graves' diseases: a case‐control study. Osteoporos Int 2019;30:2289–2297. [DOI] [PubMed] [Google Scholar]
- 7. Cozzolino M, Ciceri P, Galassi A. Hyperphosphatemia: a novel risk factor for mortality in chronic kidney disease. Ann Transl Med 2019;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verdijk LB, Snijders T, Beelen M, Savelberg HH, Meijer K, Kuipers H, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc 2010;58:2069–2075. [DOI] [PubMed] [Google Scholar]
- 10. Nair KS. Aging muscle. Am J Clin Nutr 2005;81:953–963. [DOI] [PubMed] [Google Scholar]
- 11. Sousa‐Victor P, Munoz‐Canoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med 2016;50:109–117. [DOI] [PubMed] [Google Scholar]
- 12. von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A 2013;110:16474–16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet 2000;57:16–25. [DOI] [PubMed] [Google Scholar]
- 14. Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch‐mediated restoration of regenerative potential to aged muscle. Science 2003;302:1575–1577. [DOI] [PubMed] [Google Scholar]
- 15. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007;317:807–810. [DOI] [PubMed] [Google Scholar]
- 16. Sakuma K, Aoi W, Yamaguchi A. The intriguing regulators of muscle mass in sarcopenia and muscular dystrophy. Front Aging Neurosci 2014;6:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stearns‐Reider KM, D'Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 2017;16:518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–764. [DOI] [PubMed] [Google Scholar]
- 19. Sosa P, Alcalde‐Estévez E, Plaza P, Troyano N, Alonso C, Martínez‐Arias L, et al. Hyperphosphatemia promotes senescence of myoblasts by impairing autophagy through Ilk overexpression, a possible mechanism involved in sarcopenia. Aging Dis 2018;9:769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gahlmann R, Kedes L. Tissue‐specific restriction of skeletal muscle troponin C gene expression. Gene Expr 1993;3:11–25. [PMC free article] [PubMed] [Google Scholar]
- 21. Allouh MZ, Yablonka‐Reuveni Z, Rosser BW. Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem 2008;56:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seale P, Sabourin LA, Girgis‐Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell 2000;102:777–786. [DOI] [PubMed] [Google Scholar]
- 23. Olguin HC, Yang Z, Tapscott SJ, Olwin BB. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol 2007;177:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conerly ML, Yao Z, Zhong JW, Groudine M, Tapscott SJ. Distinct activities of Myf5 and MyoD indicate separate roles in skeletal muscle lineage specification and differentiation. Dev Cell 2016;36:375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ustanina S, Carvajal J, Rigby P, Braun T. The myogenic factor Myf5 supports efficient skeletal muscle regeneration by enabling transient myoblast amplification. Stem Cells 2007;25:2006–2016. [DOI] [PubMed] [Google Scholar]
- 26. Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 2005;132:2685–2695. [DOI] [PubMed] [Google Scholar]
- 27. Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, et al. Activation of muscle‐specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci USA 1989;86:5434–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yablonka‐Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol 1999;210:440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Black BL, Molkentin JD, Olson EN. Multiple roles for the MyoD basic region in transmission of transcriptional activation signals and interaction with MEF2. Mol Cell Biol 1998;18:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mal A, Sturniolo M, Schiltz RL, Ghosh MK, Harter ML. A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: inhibition of the myogenic program. EMBO J 2001;20:1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sartorelli V, Puri PL, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, et al. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell 1999;4:725–734. [DOI] [PubMed] [Google Scholar]
- 32. Venuti JM, Morris JH, Vivian JL, Olson EN, Klein WH. Myogenin is required for late but not early aspects of myogenesis during mouse development. J Cell Biol 1995;128:563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ganassi M, Badodi S, Ortuste Quiroga HP, Zammit PS, Hinits Y, Hughes SM. Myogenin promotes myocyte fusion to balance fibre number and size. Nat Commun 2018;9:4232–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Millay DP, O'Rourke JR, Sutherland LB, Bezprozvannaya S, Shelton JM, Bassel‐Duby R, et al. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013;499:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 1995;83:1125–1136. [DOI] [PubMed] [Google Scholar]
- 36. Bour BA, O'Brien MA, Lockwood WL, Goldstein ES, Bodmer R, Taghert PH, et al. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev 1995;9:730–741. [DOI] [PubMed] [Google Scholar]
- 37. Mahdy MAA. Skeletal muscle fibrosis: an overview. Cell Tissue Res 2019;375:575–588. [DOI] [PubMed] [Google Scholar]
- 38. Alcalde‐Estévez E, Asenjo‐Bueno A, Sosa P, Olmos G, Plaza P, Caballero‐Mora MA, et al. Endothelin‐1 induces cellular senescence and fibrosis in cultured myoblasts. A potential mechanism of aging‐related sarcopenia. Aging (Albany NY) 2020;12:11200–11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arthur ST, Cooley ID. The effect of physiological stimuli on sarcopenia; impact of Notch and Wnt signaling on impaired aged skeletal muscle repair. Int J Biol Sci 2012;8:731–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Munoz‐Canoves P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol 2011;96:167–201. [DOI] [PubMed] [Google Scholar]
- 41. Olmos G, Martínez‐Miguel P, Alcalde‐Estévez E, Medrano D, Sosa P, Rodríguez‐Manas L, et al. Hyperphosphatemia induces senescence in human endothelial cells by increasing endothelin‐1 production. Aging Cell 2017;16:1300–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McClelland R, Christensen K, Mohammed S, McGuinness D, Cooney J, Bakshi A, et al. Accelerated ageing and renal dysfunction links lower socioeconomic status and dietary phosphate intake. Aging (Albany NY) 2016;8:1135–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Hyperphosphatemia impairs myogenic differentiation of C2C12 cells. Myoblasts were differentiated with 2% HS in the presence or absence (CT) of 10 mM BGP (BGP) during 7 days. a) MHC expression was evaluated by immunofluorescence with confocal microscopy. MHC (red) and desmin (green) to mark the cells. Pictures were obtained with 40X magnification. The bar graph represents the fusion index. Results are the mean ± standard error of the mean from ten different experiments. *p<0.05 vs CT.
Figure S2. Hyperphosphatemia reduced the proliferation capacity of differentiating C2C12 cells. Myoblasts were differentiated with 2% HS in the presence or absence of 10 mM BGP during 7 days. a) 5‐ethynyl‐2´‐deoxyuridine (EdU) expression was evaluated by immunofluorescence with confocal microscopy. Edu (green) and DAPI (blue) to stain the nuclei. Pictures were obtained with 40X magnification. b) The bar graph represents the percentage of EdU positive cells. Results are the mean ± standard error of the mean from two experiments. *p<0.05 vs CT at the same time. c) PCNA expression was analysed by western‐blot at 48, 72 and 168 hours. A representative blot is shown. The bar graph represents the densitometric analysis of the bands. The results are the mean ± standard error of the mean from four different experiments. *p<0.05 vs CT at the same time.


