Abstract
Purpose
The present study aimed to compare treatment outcome of idarubicin versus doxorubicin in combination with Ara-C as induction therapy for untreated AML patients.
Patients and methods
This retrospective study included 143 patients with de novo AML. All patients received full dose of standard induction therapy (3 + 7) using anthracyclines (doxorubicin or idarubicin) and cytarabine.
Results
The studied groups had comparable CR. No significant differences were noted between the studied groups regarding DFS and OS. The DXR group had significantly lower cost in comparison to IDA group.
Conclusions
Idarubicin doesn't have a clear advantage over doxorubicin in treatment of AML.
Keywords: Doxorubicin, Idarubicin, Acute myeloid leukemia
1. Introduction
Acute myeloid leukemia (AML) is heterogeneous hematologic malignancy characterized by unregulated proliferation of the blood-forming cells in the bone marrow [1]. In Egypt, the incidence of AML in expected to increase by 114.0% in the year 2050. The highest incidence is expected to be in the age range of 50 to 70 years. It's higher in males than females till the age of 60, beyond which the incidence starts to be more in females [2].
The main goals of initial induction chemotherapy for AML are rapid disease control through achievement of complete remission (CR), long-term survival, and low relapse rates with minimal induction toxicity. The (7 + 3) regimen combining an anthracycline with cytarabine has formed the backbone of AML induction for decades. In the late 1980s, idarubicin was introduced into clinics, and 3 randomized studies comparing idarubicin with daunorubicin reported significantly higher complete remission (CR) rates with idarubicin treatment [3].
Modification to idarubicin (IDA) 12 mg/m2 has resulted in an improved curative effect and fewer adverse events. In 2010, the National Comprehensive Cancer Network (NCCN) recommended IDA 12 mg/m2as the first-line induction dosage [4].
In Egypt, doxorubicin was used till the end of 2014 where idarubicin started to be used in the AML protocol (3 + 7). However, it was noticed that there is no change in the number of refractory and replaced patients. We aimed in this retrospective study to compare patients’ response to idarubicin (12 mg/m2 for 3 days) versus doxorubicin (45 mg/m2 for 3 days), in combination with Ara-C (100 mg/m2 for 7 days), as induction therapy for previously untreated adult AML patients.
2. Patients and methods
The present retrospective study was conducted at the National Cancer Institute (NCI), Cairo University, Egypt. The study protocol was approved by the institutional review board. The study included 143 patients with de novo AML. All patients were subjected to careful history taking, thorough clinical examination and radiological assessment when indicated. Laboratory investigations included complete blood count, morphological examination of bone marrow aspirate, immunophenotyping, conventional karyotyping and molecular study for FLT3, NPM1 and CEBPA mutations as a part of the routine diagnostic work-up of a patient with suspected AML according to the WHO classification [5].
All patients received full dose of standard induction therapy (3 + 7) using anthracyclines (doxorubicin or idarubicin) and cytarabine. Cytarabine was given in a dose of 100 mg /m2 continuous IV infusion for 7 days. Anthracyclines were given IV for 3 days (doxorubicin (DXR): 45 mg /m2 or idarubicin (IDR): 12 mg /m2). Patients were excluded if they had promyelocytic leukemia, received different induction chemotherapy or if they were pregnant.
Patients’ medical records were thoroughly revised and relevant data were collected. These included baseline patient characteristics, pre-induction investigations, treatment response, adverse events, the time of death, the time of relapse and the last time of follow up.
2.1. Outcome parameters
The primary endpoint of the present study is achieving complete remission (CR). CR was defined as blasts <5% in bone marrow, no leukemic blasts in peripheral blood, recovery of peripheral neutrophil counts to more than 1.0 × 109/L, platelet counts to more than 100 × 109/L, and no evidence of extra-medullary disease. The secondary endpoint is patient relapse or death. Relapse after CR was defined as the presence of at least 1 of the following: reappearance of leukemic blasts in the peripheral blood and recurrence of more than 5% blasts in the bone marrow. Overall survival (OS) was calculated from the date of entry into the NCI until death due to any cause and was censored at the last follow-up. Disease-free survival (DFS) for patients who achieved CR was measured from the date of CR until the date of AML relapse or death of any cause and was censored at the last follow-up. Treatment costs per patient were reported until the time of induction.
2.2. Statistical analysis
Data obtained from the present study were statistically analyzed using the Statistical Package for Social Science (SPSS version 21.0, IBM, USA). Statistical significance was considered at p values < 0.05. Continuous data were presented as median and range while categorical data were expressed as number and percent. Comparison between continuous data were was achieved using Mann-Whitney U test while categorical data were compared using chi-square test or Fisher's exact test. The Kaplan–Meier method and life table were performed to estimate the survival probabilities, and a log-rank test was used for univariate comparison. Binary logistic regression was used to identify predictors of CR and Cox hazard regression was used to identify predictors of DFS and OS.
3. Results
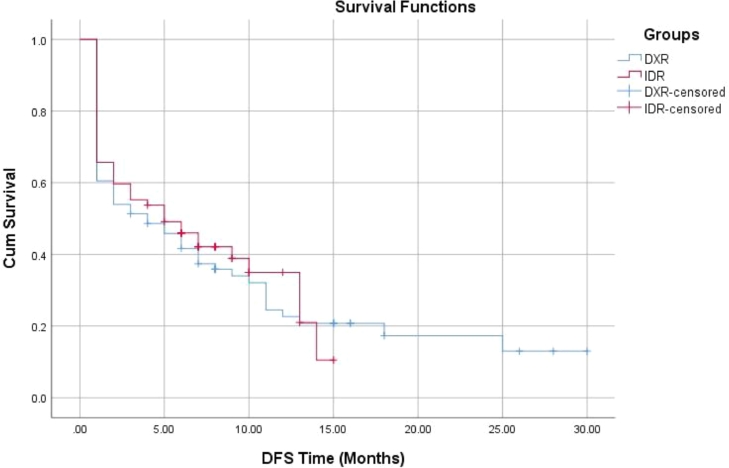
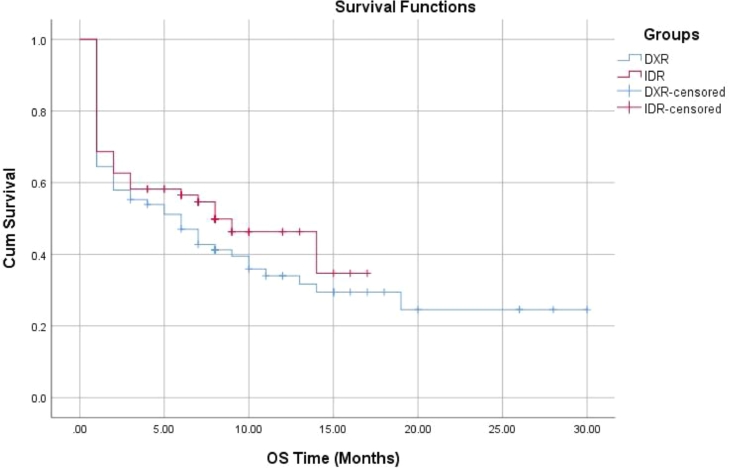
The present study was conducted on 143 adult AML patients. They comprised 76 patients in the DXR group and 67 patients in the IDR group. Comparison between the studied groups regarding the baseline data revealed significantly higher frequency of diabetes in the IDR group. Also, it was noted that patients in the IDR group had significantly higher hemoglobin levels (Table 1). The studied groups had comparable CR (67.1% and 68.7% in DXR and IDR groups respectively, p = 0.84). While we noted that IDR group patients experienced significantly lower percent of overall non-survivors when compared with DXR group (50.7% versus 67.1%, p = 0.047). However, no significant differences were noted between DXR and IDR groups regarding the percent of non-survivors at 30 (35.5% versus 31.3% respectively, p = 0.6) and 60 days (41.4% versus 37.3% respectively, p = 0.56). Moreover, the reported causes of death were comparable between groups (Table 1). Also, no significant differences were noted between the studied groups regarding DFS and OS (Table 1, Figs. 1, 2). Interestingly, The DXR group had significantly lower cost in comparison to IDA group (1021.5 ± 254.6 versus 4603.8 ± 1196.9 EGP, p < 0.001) (Table 1).
Table 1.
Clinical and outcome data in the studied groups.
| DXR groupN = 76 | IDR groupN = 67 | p | |
|---|---|---|---|
| Age (years) median (range) | 34.0 (19.0–57.0) | 35.0 (18.0–58.0) | 0.13 |
| Male/female n | 42/34 | 38/29 | 0.86 |
| BMI (Kg/m2) median (range) | 25.2 (17.3–43.3) | 26.8 (16.5–41.6) | 0.15 |
| Comorbidities n (%) | |||
| Hypertension | 1 (1.3) | 3 (4.5) | 0.25 |
| Diabetes | – | 5 (7.5) | 0.015 |
| Hepatitis C | 2 (2.6) | 5 (7.5) | 0.18 |
| Ischemic heart disease | – | 3 (4.5) | 0.062 |
| Lymphadenopathy n (%) | 32 (42.1) | 29 (43.3) | 0.9 |
| Hepatosplenomegaly n (%) | 34 (44.7) | 35 (52.2) | 0.37 |
| FAB classification n (%) | |||
| M0 | 2 (2.6) | 2 (3.0) | 0.26 |
| M1 | 29 (38.2) | 21 (31.3) | |
| M2 | 18 (23.7) | 25 (37.3) | |
| M4 | 18 (23.7) | 14 (20.9) | |
| M5 | 8 (10.5) | 3 (4.5) | |
| M6 | 1 (1.3) | – | |
| M7 | – | 2 (3.0) | |
| WBCs (× 103/ml) median (range) | 33.65 (1.3–214.0) | 17.0 (1.8–231.0) | 0.28 |
| Hb (gm/dl) median (range) | 7.0 (2.9–12.3) | 7.7 (4.0–11.6) | 0.03 |
| Platelets (× 103/ml) median (range) | 35.0 (2.0–525.0) | 45 (4.0–257.0) | 0.64 |
| Blasts (%) median (range) | 55.5 (10.0 - 99.0) | 48.5 (6.0–95.0) | 0.26 |
| Cytogenetic risk n (%) | |||
| Favorable | 9 (11.8) | 15 (22.4) | 0.17 |
| Intermediate | 44 (57.9) | 38 (56.7) | |
| Adverse | 23 (30.3) | 14 (20.9) | |
| Treatment side effects n (%) | |||
| Neutropenic fever | 76 (100.0) | 67 (100.0) | NA |
| Severe septicemia | 76 (100) | 67 (100.0) | NA |
| Cardiotoxicity | 18 (23.7) | 20 (29.9) | 0.76 |
| Liver dysfunction | 14 (18.4) | 13 (19.4) | 0.86 |
| Renal dysfunction | 10 (13.1) | 3 (4.5) | 0.096 |
| Respiratory dysfunction | 10 (13.1) | 6 (9.0) | 0.38 |
| Complete response n (%) | 51 (67.1) | 46 (68.7) | 0.84 |
| Allogenic stem cell transplantation n (%) | 3 (3.9) | 4 (6.0) | 0.58 |
| Overall non-survivors n (%) | 51 (67.1) | 34 (50.7) | 0.047 |
| 30-day non-survivors | 27 (35.5) | 21 (31.3) | 0.6 |
| 60-day non-survivors | 32 (41.4) | 25 (37.3) | 0.56 |
| DFS (months) median (95.0% CI) | 4.0 (1.02–7.0) | 5.0 (1.34–8.7) | 0.69 |
| OS (months) median (95.0% CI) | 6.0 (2.4–9.6) | 8.0 (3.3–12.7) | 0.29 |
| Cause of death n (%) | |||
| Uncontrolled infection | 32 (42.1) | 20 (29.9) | 0.65 |
| Uncontrolled hemorrhage | 14 (18.4) | 9 (13.4) | |
| Multiple organ failure | 4 (5.3) | 5 (7.5) | |
| Sudden unexpected death | 1 (1.3) | – | |
| Treatment cost/patient (EGP) mean ± SD | 1021.5 ± 254.6 | 4603.8 ± 1196.9 | <0.001 |
Fig. 1.
DFS in the studied groups.
Fig. 2.
OS in the studied groups.
Only adverse cytogenetic risk was found to be independent predictor of CR in the studied patients [OR (95% CI): 16.1 (3.29–79.06), p = 0.001] (Table 2). Independent predictors of DFS included patients’ age [HR (95% CI): 1.03 (1.0–1.05), p = 0.01] and achievement of CR [HR (95% CI): 0.32 (0.2–0.53), p<0.001] (Table 3) while independent predictors of OS were patients’ age [HR (95% CI): 1.03 (1.01–1.06), p = 0.004], sex [HR (95% CI): 0.64 (0.42–0.99), p = 0.046], adverse cytogenetics [OR (95% CI): 2.94 (1.23–7.0), p = 0.015] and achievement of CR [HR (95% CI): 0.27 (0.16–0.46), p < 0.001] (Table 4).
Table 2.
Predictors of complete response.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.02 | 0.98–1.05 | 0.33 | – | – | – |
| Sex | 1.04 | 0.51–2.1 | 0.92 | – | – | – |
| Cytogenetic risk | ||||||
| Favorable | Ref. | – | – | – | ||
| Intermediate | 4.03 | 0.88–18.6 | 0.074 | 4.03 | 0.88–18.6 | 0.074 |
| Adverse | 16.1 | 3.29–79.06 | 0.001 | 16.1 | 3.29–79.06 | 0.001 |
| Treatment group | 1.07 | 0.53–2.17 | 0.84 | – | – | – |
Table 3.
Predictors of DFS.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.02 | 1.0–1.04 | 0.02 | 1.03 | 1.0–1.05 | 0.01 |
| Sex | 0.79 | 0.54–1.17 | 0.25 | – | – | – |
| Cytogenetic risk | ||||||
| Favorable | Ref. | – | – | – | – | – |
| Intermediate | 1.4 | 0.77–2.5 | 0.28 | 1.16 | 0.64–2.09 | 0.64 |
| Adverse | 2.69 | 1.41–5.13 | 0.003 | 1.76 | 0.89–3.48 | 0.1 |
| CR | 3.48 | 2.2–5.5 | <0.001 | 0.32 | 0.2–0.53 | <0.001 |
| Treatment group | 1.07 | 0.72–1.6 | 0.73 | – | – | – |
Table 4.
Predictors of OS.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.03 | 1.01–1.05 | 0.004 | 1.03 | 1.01–1.06 | 0.004 |
| Sex | 0.64 | 0.41–0.97 | 0.037 | 0.64 | 0.42–0.99 | 0.046 |
| Cytogenetic risk | ||||||
| Favorable | Ref. | – | – | – | – | – |
| Intermediate | 2.37 | 1.07–5.26 | 0.033 | 1.83 | 0.82–4.09 | 0.14 |
| Adverse | 4.56 | 1.98–10.52 | <0.001 | 2.94 | 1.23–7.0 | 0.015 |
| CR | 4.17 | 2.57–6.78 | <0.001 | 0.27 | 0.16–0.46 | <0.001 |
| Treatment group | 1.23 | 0.8–1.91 | 0.35 | – | – | – |
4. Discussion
Doxorubicin is the first liposomal encapsulated drug approved for management of AML [3]. However, its use was hampered by its association with significant side effects particularly the long-term and potentially fatal cardiotoxicity [6]. IDA has recently replaced DXR to be with daunorubicin the most commonly used formulations of anthracyclines in management of AML [4].
Remarkably, the wide use of IDA instead of DXR in management of AML wasn't adequately discussed in the literature in spite of the clinical notion that IDA mayn't add significant benefits to the treatment outcome and survival rates. The present retrospective study aimed to document the Egyptian experience with these two drugs.
In our study, both drugs were comparable regarding the achieved CR, reported side effects, DFS and OS. However it was noted that IDA-treated patients had significantly lower mortality rate. Findings the current study cover an important gap in the published literature to compare the short and long-term effects of both drugs. In comparison, the randomized clinical trial of Bezwoda and Dansey [7] found that better CR and fewer side effects including cardiotoxicity and longer DFS in IDR-treated patients in comparison to DXR-treated counterparts. In agreement with our study, the differences between the studied groups weren't statistically significant. Notably, the study didn't report patients’ OS.
In another randomized clinical study, Intragumtornchai et al. [8] reported that IDR-treated group had significantly better CR rate in comparison to DXR-treated group (80.4% versus 56.1%, p = 0.014). Both groups were comparable regarding the reported side effects. The study didn't report patients DFS and OS.
Interestingly, the pooled analysis of data obtained from both studies concluded that IDR treatment was associated with significantly better CR in spite of fact that the authors of this meta-analysis noted the low qualities of evidence derived from both studies [9].
In the present study, it's remarkable that DXR-treated patients had lower rate of cardiotoxicity in spite of lack of statistical significance. This contradicts other findings reported by comparative experimental [10] and clinical studies [7] that indicated a higher prevalence of cardiotoxicity in DXR-treated patients.
Noteworthy, the present study highlighted the significantly lower cost associated with DXR use. Considering the comparable effects of both drugs on DFS and OS in our study and in other studies, the differences in treatment cost should be considered in the treatment choice. Superiority of IDA over DXR isn't disputed and a clear clinical evidence derived from randomized clinical studies is strongly advocated.
In conclusion, our study found that idarubicin doesn't have a clear advantage over doxorubicin in treatment of AML patients particularly in terms of DFS and OS. In addition, doxorubicin use was associated with lower rate of cardiotoxicity and lower cost. Our conclusions, however, are limited by the retrospective nature of the study.
Declaration of Competing Interest
Authors of the present study entitled “Treatment Outcome of Doxorubicin versus Idarubicin in Adult Acute Myeloid Leukemia” declares no conflict of interest.
References
- 1.Narayanan D., Weinberg O.K. How I investigate acute myeloid leukemia. Int. J. Lab. Hematol. 2020;42(1):3–15. doi: 10.1111/ijlh.13135. FebEpub 2019 Dec 10. PMID: 31820579. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim A.S., Khaled H.M., Mikhail N.H., Baraka H. Cancer incidence in Egypt: results of the National Population-Based Cancer Registry Program. J. Cancer Epidemiol. 2014:18. doi: 10.1155/2014/437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivankar S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014;10(4):853–858. doi: 10.4103/0973-1482.139267. PMID: 25579518. [DOI] [PubMed] [Google Scholar]
- 4.Owattanapanich W., Owattanapanich N., Kungwankiattichai S., Ungprasert P., Ruchutrakool T. Efficacy and toxicity of idarubicin versus high-dose daunorubicin for induction chemotherapy in adult acute myeloid leukemia: a systematic review and meta-analysis. Clin. Lymphoma Myeloma Leuk. 2018;18(12):814–821. doi: 10.1016/j.clml.2018.08.008. Dece3Epub 2018 Aug 22. PMID: 30241991. [DOI] [PubMed] [Google Scholar]
- 5.Arber D.A., Orazi A., Hasserjian R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. May 19Epub 2016 Apr 11. PMID: 27069254. [DOI] [PubMed] [Google Scholar]
- 6.Kalyanaraman B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: have we been barking up the wrong tree? Redox Biol. 2020;29 doi: 10.1016/j.redox.2019.101394. JanEpub 2019 Nov 26. PMID: 31790851; PMCID: PMC6909145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezwoda W.R., Dansey R.D. Idarubicin plus cytarabine versus doxorubicin plus cytarabine in induction therapy for acute non- lymphoid leukaemia: a randomized trial. Leuk. Lymphoma. 1990;1(3–4):221–225. doi: 10.3109/10428199009042483. PMID: 27463989. [DOI] [PubMed] [Google Scholar]
- 8.Intragumtornchai T., Lekhakula A., Dhamaprasit T., Sutchartichan P., Swasdikul D. Idarubicin versus doxorubicin in combination with cytarabine as first course induction therapy in acute myelogenous leukemia: a randomized controlled trial. Asian Pac. J. Allergy Immunol. 1999:23. [Google Scholar]
- 9.Li X., Xu S., Tan Y., Chen J. The effects of idarubicin versus other anthracyclines for induction therapy of patients with newly diagnosed leukaemia. Cochrane Database Syst. Rev.. 2015 Jun 3;(6):CD010432. doi: 10.1002/14651858.CD010432.pub2. PMID: 26037486. [DOI] [PMC free article] [PubMed]
- 10.Platel D., Pouna P., Bonoron-Adèle S., Robert J. Comparative cardiotoxicity of idarubicin and doxorubicin using the isolated perfused rat heart model. Anticancer Drugs. 1999;10(7):671–676. doi: 10.1097/00001813-199908000-00007. AugPMID: 10507317. [DOI] [PubMed] [Google Scholar]