Abstract
The severe respiratory consequences of the coronavirus disease 2019 (COVID-19) pandemic have prompted the urgent need for novel therapies. Cell-based therapies, primarily using mesenchymal stromal cells (MSCs), have demonstrated safety and potential efficacy in the treatment of critical illness, particularly sepsis and acute respiratory distress syndrome (ARDS). However, there are limited preclinical data for MSCs in COVID-19. Recent studies have shown that MSCs could decrease inflammation, improve lung permeability, enhance microbe and alveolar fluid clearance, and promote lung epithelial and endothelial repair. In addition, MSC-based therapy has shown promising effects in preclinical studies and phase 1 clinical trials in sepsis and ARDS. Here, we review recent advances related to MSC-based therapy in the context of sepsis and ARDS and evaluate the potential value of MSCs as a therapeutic strategy for COVID-19.
Keywords: COVID-19, MSCs therapies, ARDS, sepsis, SARS-CoV-2
Introduction
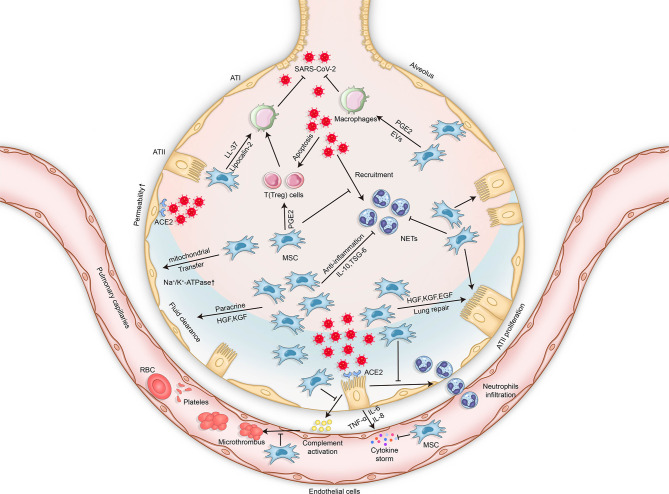
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is rapidly and continuously spreading globally and can result in serious significant respiratory morbidity and mortality (1, 2). Although most patients with COVID-19 present with mild respiratory tract infection, severe pneumonia and acute respiratory distress syndrome (ARDS) have been described in 19% of reported cases, and the overall mortality is approximately 49% (3). The most common symptoms of SARS-CoV-2 infection are cough, fever, fatigue, headache, myalgia, and diarrhea. Approximately 1 week after the onset of symptoms, patients become severely ill when dyspnea and hypoxemia appear, and progressive respiratory failure subsequently develops (4). The main reason for severe COVID-19 disease is aberrant immune host response accompanied by a “cytokine storm.” More precisely, it involves the excessive activation and dysregulation of CD8+ T cells and immoderate pulmonary recruitment of immune cells, as well as the overproduction of pro-inflammatory cytokines such as interleukin 2 (IL-2), IL-6, IL-7, and tumor necrosis factor (TNF) (5). Additionally, endothelial dysfunction and immunothrombosis are also regarded as critical pathogenic mechanisms leading to severe COVID-19 (6) ( Figure 1 ).
Figure 1.
Mechanisms of lung injury in COVID-19 and potential therapeutic effects of MSCs in COVID-19-related respiratory lung injury. The mechanisms of lung injury in COVID-19 include, but are not limited to, aberrant immune host responses accompanied by a “cytokine storm,” excessive neutrophil activation, dysregulation of immune cells, endothelial dysfunction, and immunothrombosis formation. The potential therapeutic effects of MSCs in COVID-19 involve multiple mechanisms via their secretion of soluble paracrine factors and possible mitochondrial transfer. MSCs can enhance microbe clearance, resolve inflammation, modulate immunity, improve lung permeability, increase alveolar fluid clearance, and promote lung epithelial and endothelial repair. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; MSCs, mesenchymal stromal cells; ATI, alveolar type 1 cells; ATII, alveolar type 2 cells; TNF, tumor necrosis factor; IL, interleukin; ACE2, angiotensin-converting enzyme 2; PGE2, prostaglandin E2; KGF, keratinocyte growth factor; TSG-6, TNF-stimulated gene 6; HGF, hepatocyte growth factor; EGF, epidermal growth factor; EVs, extracellular vesicles; NETs, neutrophil extracellular traps; RBCs, red blood cells.
Without effective antiviral medications, current therapeutic approaches are limited to aggressive standard supportive care and treatment of any other co-infections. More recently, a growing number of clinical investigations of cell-based therapies, primarily involving mesenchymal stromal cells (MSCs), have demonstrated safety and possible efficacy in the treatment of critical illness, particularly in sepsis and ARDS (7–15). Recent preclinical data in models of sepsis/ARDS and relevant related clinical studies of MSC administration in patients with ARDS may contribute to a better understanding of the potential MSC-based cell therapy approaches for COVID-19. Therefore, our review focused on MSCs as a potential therapeutic agent for COVID-19 on the basis of the current evidence from sepsis and ARDS.
Mesenchymal Stromal Cells
MSCs were originally characterized in bone marrow stromal cells and are now the best-described and most widely used cells for cell-based therapies. According to the International Society of Cellular Therapy, MSCs are defined by the following minimal criteria: 1) must be adherent to plastic; 2) must positively present CD105, CD90, and CD73 and must be lacking CD45, CD34, CD14, CD11b, CD79 alpha, CD19, and HLA-DR surface makers; and 3) must have a differentiation potential in in vitro conditions (16).
Sources of MSCs
MSCs were originally identified in the bone marrow, and the most recent studies have focused on the effects of bone marrow-derived MSCs. However, MSCs can also be isolated from several tissues such as umbilical cord/fetal blood, placenta, amniotic fluid, and adipose tissue (17–21). Recent studies on rodent models have shown that adipose tissue-derived MSCs minimized ischemia–reperfusion lung injury by suppressing oxidative stress and inflammatory reaction (22); improved phagocytosis and the bactericidal functions of macrophages in a model of Pseudomonas aeruginosa pneumonia (23); attenuated radiation-induced lung injury through anti-inflammation, anti-fibrosis, and anti-apoptosis mechanisms (24); and abrogated bleomycin-induced lung fibrosis by significantly reducing collagen deposition at an early stage (25). Moreover, it was also reported that umbilical cord-derived MSCs can ameliorate hyperoxia-induced lung injury in newborn rats with bronchopulmonary dysplasia and improve the bacterial clearance and survival in neonatal septic rats (26, 27).
Attractive Therapeutic Candidates Based on MSCs
There are many advantages of MSCs as an attractive therapeutic candidate. Firstly, they express low levels of human leukocyte antigen (HLA)-associated proteins, which lead to the absence of immunosuppression by allogeneic MSCs as stimulators (28). Secondly, MSCs can be easily isolated from host tissue and expanded stably and rapidly in vitro, which enables sufficient quantities for clinical use (29). Thirdly, the homing capacity of MSCs to the injury sites contributes to their persistence in the target tissues (30). Moreover, MSCs can be trapped in the lungs by systemic administration, thus prolonging their persistence in the lungs, which may be of most benefit in lung disease treatment (30). Fourthly, the safety profile of MSCs was demonstrated by phase 1 clinical trials in patients with ARDS and sepsis (7–15). Finally, growing evidence shows that MSCs exert anti-inflammatory and anti-apoptotic effects in response to injury (31).
Therapeutic Mechanisms of MSCs
Paracrine Cytokines
Paracrine secretion is a well-known mechanism for MSCs to exhibit their potential benefits (32). A paracrine mechanism of the small mediators secreted by MSCs was suggested to exert the effects of immune modulation, inflammation resolution, and injury restoration. Subsequent studies revealed that several paracrine factors are involved in MSC secretomes, including LL-37 (antimicrobial peptides) (33), keratinocyte growth factor (KGF, enhanced alveolar fluid clearance) (34, 35), angiopoietin-1 (Ang-1, restored epithelial cell permeability) (36), hepatocyte growth factor (HGF, restored endothelial cell permeability) (37–39), and lipoxin A4 (LXA4, alleviated inflammation) (36). For example, in an in vitro experiment, conditioned medium (CM) from Escherichia coli-stimulated MSCs, compared with unstimulated MSCs, showed remarkable antimicrobial activity against E. coli and P. aeruginosa, followed by a significantly higher level of LL-37 being observed in MSCs after bacterial challenge. However, E. coli-stimulated MSCs pre-incubated with the anti-LL-37 antibody resulted in a decrease in bacterial clearance. A subsequent in vivo experiment also displayed a consistent result, suggesting that the antimicrobial activity of MSCs is mainly attributed to the secretion of the antimicrobial peptide LL-37 (33). In addition, a study by Wang et al. (38) reported that the ability of bone marrow-derived MSCs to stabilize the endothelial barrier was partly affected by HGF. Lipopolysaccharide (LPS)-induced endothelial paracellular and transcellular permeabilities were improved after treatment with MSC macrovesicles (MSC-MVs), accompanied by more expressions of the endothelial intercellular junction proteins VE-cadherin and occludin and less endothelial apoptosis. However, these effects were removed after HGF gene knockdown in MSC-MVs. Carrying plenty of cytokines with specific function, MSCs were shown to be of great benefit to the resolution of inflammation and the repair of acute lung injury (ALI).
Mitochondrial Transfer
Mitochondrial transfer is also considered as one of the important mechanisms underlying the protective effects of MSCs (40). MSCs can aid the repair of lung injury through the transfer of mitochondria, which results in increased alveolar ATP concentration (41). Moreover, mitochondrial transfer from MSCs to alveolar macrophages leads to the enhancement of phagocytic activity, thus exerting antimicrobial effects in ARDS (42). Additionally, MSCs can promote an anti-inflammatory and phagocytic macrophage phenotype via extracellular vesicle-mediated mitochondrial transfer (43). Finally, MSCs can donate cytoplasmic content and mitochondria to repair damaged bronchial epithelial cells (44). However, the microenvironment is crucial for effective MSC therapy. It was reported that hypercapnic acidosis induces mitochondrial dysfunction and impairs the ability of MSCs to promote distal lung epithelial repair (45, 46), which may lead to the invalidation of MSCs in patients who develop hypercapnia. Another study further illustrated that the administration of MSC extracellular vesicles with dysfunctional mitochondria had no effect in attenuating LPS-induced ALI, whereas normal MSC extracellular vesicles were able to restore mitochondrial respiration and improve barrier integrity (47).
MSCs in Sepsis Treatment
In COVID-19 patients who develop acute respiratory failure requiring intubation and mechanical ventilation, sepsis and sepsis shock can also develop. Sepsis is a systemic inflammatory response to infection that is characterized by the imbalance between pro-inflammation and anti-inflammation (48, 49). Recently, MSCs have been shown to be effective in modulating such processes, which may be applicable to COVID-19.
Enhancement of Microbe Clearance
Antibacterial Effect of MSCs
Sepsis is a multifaceted host response to an infecting pathogen. The clearance of bacteria with antibiotics is one of the most important treatments for sepsis (50, 51). Beyond antibiotics, MSCs were recently reported to play a crucial role in clearing bacteria in sepsis (52, 53). In in vivo experimental models of sepsis, MSC delivery was shown to be significantly greater in the clearance of bacteria via enhancing the phagocytotic activity of neutrophils, monocytes, and macrophages (54–56). In addition, MSCs could secrete antimicrobial factors such as LL-37 (33, 57) and lipocalin 2 (53) to reduce bacterial growth (in colony forming units). This bactericidal effect may be the result of the enhancement of NOX2-dependent reactive oxygen species (ROS) production by MSCs (55). Furthermore, MSC extracellular vesicles suppressed the activity of multidrug resistance-associated protein 1 (MRP1) through the transfer of miR-145, thereby resulting in antimicrobial activity in E. coli-induced pneumonia in C57BL/6 mice through LTB4/BLT1 signaling (58).
Antiviral Effect of MSCs
Aside from bacterial infection in sepsis, viral infection is an independent risk factor for sepsis and septic shock (59, 60). However, the antiviral effect of MSCs remains controversial in preclinical studies. MSCs are susceptible to H1N1, H5N1, and H9N5 avian influenza virus, which may lead to cell death and induce the immune dysregulation of MSCs by releasing high levels of TNF-α, IL-6, MCP-1, and MIP-1β (61–63). Moreover, MSC therapy was shown to prolong the disruption of the alveolar–capillary barrier and failed to improve outcomes in experimental severe influenza in mice (64, 65). In contrast, more recent studies of MSCs have reported their protective effects in influenza virus-induced ALI in mice (66–69). The inconsistent results on the antiviral effects of MSCs may have been dependent on the different types of influenza virus and MSCs, animal models, ages, and the methods of administration. A study by Michael et al. showed that MSC treatment was beneficial in aged mice (8–12 months of age), but not in young mice (6–8 weeks of age) with H5N1 influenza virus infection (66). To avoid infection of MSCs by the influenza virus, Mahesh et al. applied MSCs derived from extracellular vesicles for the treatment of influenza-infected pigs, which showed great benefits in attenuating ALI and may provide a cell-free therapeutic strategy for influenza in humans (68). Loy et al. reported that umbilical cord MSCs (UC-MSCs) with greater growth factor secretion of Ang-1 and HGF outperformed bone marrow-derived MSCs in restoring the impaired alveolar fluid clearance and protein permeability of H5N1-infected alveolar epithelial cells (69). Although SARS-CoV-2, a coronavirus, may differ from influenza virus, the use of MSCs in COVID-19 should be fully considered according to the double-edged sword of MSC application in influenza.
Antifungal Effect of MSCs
Fungal causes of sepsis have increased rapidly worldwide (59, 70). However, there remains a lack of information regarding MSC treatment in fungal infection. It was reported that a subset of single colony-derived MSCs producing IL-17 was capable of inhibiting the growth of Candida albicans both in vitro and in vivo (71). Thus, the potential antifungal effect of MSCs for sepsis and ALI warrants rapid investigation.
Resolution of Inflammation
The reduction and resolution of inflammation is of vital importance in sepsis. MSCs seem to be promising in this respect. It was reported that MSC administration contributed to a reduction in the pro-inflammatory responses (TNF-α, MIP-2, and IL-6) to endotoxins while upregulating the anti-inflammatory cytokine IL-10 (72–78). A network analysis of transcriptional responses showed that more than 4,000 genes were significantly altered in MSC-treated mice, which indicated that the protective effect of MSCs in sepsis was not limited to a single mediator (79). Of these, TNF-α stimulated gene 6 (TSG-6) may serve as a potential biomarker to predict the efficacy of MSCs in modulating inflammation because MSCs with high levels of TSG-6 exert an enhanced anti-inflammatory efficacy (80–82). However, MSCs are highly sensitive to their microenvironment (46). Under excessive inflammatory circumstances, they may undergo apoptosis through inflammation-induced autophagy (83). Conversely, MSC-conditioned media with an anti-IL6 antibody was more effective in augmenting the promotion of an anti-inflammatory monocyte phenotype (84). In addition, MSCs could restrict NLRP3 inflammasome activation, which suppressed the generation of mitochondrial ROS (85). Moreover, MSC-conditioned media contributed to a reduction in the activity of NF-kB and MMP-9 in neutrophils from patients with sepsis-related ARDS, thus exhibiting great potential in the treatment of inflammatory disorders (86).
Modulation of Immunity
Immunological dysfunction is also an important clinical manifestation of sepsis and septic shock (87, 88). MSCs were shown to possess immunomodulatory functions in sepsis. MSCs expressing toll-like receptor 4 (TLR4) and myeloid differentiation primary response gene-88 (MyD88) are essential for releasing prostaglandin E2 (PGE2), which can reprogram macrophages to increase the production of IL-10 (89, 90). Along with the prostaglandin E2 receptor, PGE2 is indispensable to optimal CD4+ T-cell activation and T-cell-mediated inflammatory responses (91). When PGE2 synthesis of human MSCs was inhibited, the MSC-mediated immunosuppressive effects on T cells were also negated (92). In this respect, MSCs may be useful in COVID-19 management where aberrant immune activation plays an important role in the disease progression (5). Moreover, circulating CD3+CD4+CD25+ regulatory T cells (Tregs) were significantly increased when MSCs were administered in a cecal ligation and puncture (CLP)-induced sepsis rat model (93). Furthermore, the immunosuppressive capacity of Tregs can inhibit the production of pro-inflammatory cytokines such as IL-6 and TNF-α (93, 94). In addition, MSCs exhibited immunosuppressive effects by reducing the level of immunoglobulin production and chemokines that are related to recruiting neutrophils in lung B cells (95). When IL-1β was pretreated, the immunomodulatory properties of MSCs could be effectively enhanced (96). MSC-mediated immunomodulation during sepsis was likely to be associated with the MyD88–NFκB signaling pathway (74).
MSCs in ARDS Treatment
Characterized by the acute onset of non-cardiogenic pulmonary edema, hypoxemia, and diffuse alveolar–capillary membrane damage, ARDS is a common cause of respiratory failure in critically ill patients (97). Recently, a growing number of studies have suggested that MSCs may be a potential therapy for ARDS by improving lung permeability, increasing alveolar fluid clearance, and promoting lung epithelial and endothelial repair.
Improvement of Lung Permeability
Epithelial injury is critical in the development of ALI. Alveolar epithelial cells may be the first to be affected by pulmonary ARDS (caused by pneumonia, acid aspiration, and toxic gas inhalation), which may contribute to the loss of the alveolar epithelial barrier integrity and thus increase lung permeability (98). MSCs were reported to show benefits in improving lung epithelial permeability, such as restoring the permeability of alveolar type II epithelial cells through the secretion of Ang-1 (36). Additionally, sodium transport and epithelial permeability were recovered by conditioned media from MSCs via the secretion of KGF in an in vitro model of acute alveolar injury (99), while the intrapulmonary delivery of MSCs contributed to significantly decreasing the levels of bronchoalveolar lavage protein and improving the alveolar epithelial permeability in endotoxin-induced ALI in mice (72). The improvement in alveolar epithelial permeability by MSCs may be a result of the activation of Wnt3a-induced Wnt/β-catenin signaling (100).
Similarly, pulmonary vascular endothelial cells may be the first to be injured by extrapulmonary ARDS (resulting from sepsis, trauma, or hemorrhagic shock), which may lead to dysfunction of the pulmonary vascular endothelial barrier and, thus, increase lung permeability (101). It was reported that MSCs inhibit pulmonary vascular endothelial permeability by preserving critical vascular endothelial barrier proteins (VE-cadherin, occludin-1, and claudin-1) in the lungs after hemorrhagic shock (102, 103). Adipose-derived stem cells also attenuated pulmonary microvascular hyperpermeability in a sheep model of smoke inhalation-induced ALI (104). MSC treatment for endotoxin-induced lung injury improves the lung endothelial barrier permeability partly by secreting KGF and HGF (37, 38, 105). The capacity of MSCs to inhibit vascular permeability is likely through the modulation of VE-cadherin/β-catenin signaling (106).
Increment of Alveolar Fluid Clearance
Alveolar fluid clearance is usually impaired in patients with ARDS (107) and contributes to lung edema and hypoxemia. MSC treatments were reported to play a significant role in clearing alveolar fluid. In an in vivo mouse model of E. coli endotoxin-induced ALI, bone marrow-derived MSCs were shown to reduce extravascular lung water by 60% and total protein levels by 66% (108). In a sheep model of ALI, MSCs also attenuated the lung water content and edema (104, 109). In an ex vivo perfused human lung endotoxin-induced ALI model, MSCs increased the alveolar fluid clearance partly by restoring sodium transport (105). The above benefits of MSC treatments may have originated from the paracrine signaling of KGF, which contributed to restoring the effects of the α-epithelial sodium channel (α-ENaC) (99, 110, 111).
Promotion of Lung Epithelial and Endothelial Repair
The promotion of lung epithelial and endothelial cell repair is of crucial importance in ARDS treatment. MSC-based therapy may be the breaking point in lung repair and regeneration. MSC therapy enhances pulmonary epithelial wound healing and restores lung structure by secreting KGF in ventilator-induced lung injury (34). MSCs can also improve the viability of BEAS-2B cells and inhibit LPS-induced apoptosis, which may be due to the increased expression of proliferating cell nuclear antigen (PCNA) and KGF and the reduced expression of caspase-3 (112). In addition, receptor tyrosine kinase-like orphan receptor 2 (ROR2)- (113), β-catenin- (114), and KGF-overexpressing (115) MSCs significantly promoted their proliferation and differentiation into type II alveolar epithelial cells, which demonstrated much better therapeutic effects than did native MSCs alone. Furthermore, MSCs can mitigate LPS-induced injury in murine lung epithelial cells and reverse the epithelial–mesenchymal transition by blocking the activation of the NF-κB and Hedgehog pathways (116). Finally, the repair and regeneration potential of MSCs may be induced by the stimulation of pro-inflammatory cytokines (i.e., TNF-α and IL-1β) and the activation of Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) (117, 118).
MSCs in COVID-19-Related ARDS
According to statistical data generated early in the COVID-19 pandemic, 14% of cases were classified as severe COVID-19 and 5% were determined as critical with organ failure (3). Severe pneumonia and ARDS have been described in the reported COVID-19 cases. In parallel with proper ventilation management, dexamethasone, antiviral agents, convalescent plasma, and IL-1/6 inhibitors, MSCs are also considered a promising candidate therapy for COVID-19-related ARDS (4, 119). It was reported that MSCs from the bone marrow, amniotic fluid, and adipose tissue were all resistant to SARS-CoV-2 infection due to the low expressions of angiotensin-converting enzyme 2 (ACE-2) and transmembrane protease serine subtype 2 (TMPRSS2) on the cell surface under both steady-state and inflammatory conditions (120, 121), which supported the potential applicability of MSCs for COVID-19 treatment ( Figure 1 ).
It was reported that MSCs were well tolerated in ARDS patients without severe adverse events in phase 1 and phase 2a clinical trials (13, 15). Even though adverse effects such as shivering, muscle contraction, and increased lactic acid dehydrogenase arose after the infusion of MSCs in a small minority of patients (122–124), multiple clinical studies have identified the tolerability and efficacy of MSCs in the treatment of COVID-19-related ARDS. Current clinical trials advise that it is safe to administer MSCs extracted from the umbilical cord, placenta, or menstrual blood to severely and critically ill COVID-19 patients (122–132), always intravenously, with one to three infusions and 106–108 cells per dose.
Leng et al. (128) reported the earliest clinical trial (ChiCTR2000029990) of MSC therapy against COVID-19. In one dose of 1 × 106 cells/kg, clinical grade MSCs were intravenously transplanted to seven patients with COVID-19 pneumonia (one of the critical type, four of the severe type, and two of the common type), and three patients in the control group received placebo treatment. Without any observed adverse effects, the symptoms (high fever, weakness, and shortness of breath) and oxygen saturations significantly improved within 2–4 days after MSC transplantation in all patients. More importantly, it was suggested that MSCs were valuable in suppressing virus-induced cytokine storms. Compared with the placebo control group, the overactivated cytokine-secreting immune cells disappeared in 3–6 days and regulatory dendritic cells dramatically increased in the group that received MSC treatment. Meanwhile, the serum pro-inflammatory cytokine TNF-α was significantly decreased and the anti-inflammatory cytokine IL-10 was remarkably increased after MSC transplantation. Of note is that all seven patients were put forward for this pilot study after no improvements were observed under standard treatment, and thus, it is not surprising that patients in the placebo group showed negative results. Therefore, the functions of MSCs should be identified in a larger population.
In a multicenter, open-label, non-randomized, parallel controlled phase 1 exploratory trial in China (ChiCTR2000029606) (125), 44 severe or critical COVID-19 patients were enrolled. Among them, 26 patients received allogeneic, menstrual blood-derived MSC therapy (three infusions totaling 9 × 107 MSCs, one infusion every other day), while 18 patients received only concomitant medication. After 1 month of follow-up, it was observed that the mortality between the two groups was significantly different (7.69% in the MSC group vs. 33.33% in the control group, p = 0.048). Compared with the control group, the symptoms of patients in the experimental group were quickly alleviated, with expiratory dyspnea significantly improving during MSC infusion on day 1 (p = 0.016), day 3 (p = 0.040), and day 5 (p = 0.031). Chest imaging results also showed improvements in the experimental group in the first month after MSC infusion. In addition, Lanzoni et al. (132) reported a double-blind, phase 1/2a, randomized controlled trial of UC-MSC therapy against COVID-19. A total of 24 patients who were diagnosed with COVID-19-related ARDS within 24 h were randomized 1:1 to either a UC-MSC treatment group (two intravenous infusions of 100 ± 20 × 106 UC-MSCs, on days 0 and 3) or a control group (two infusions of vehicle solution). The results reflected that UC-MSC treatment resulted in significant reductions of inflammatory cytokines on day 6 and improved survival (91% vs. 42%, p = 0.015) during a 31-day follow-up. In another double-blind, multicenter, phase 1 randomized controlled trial (NCT04457609) (130) in 40 critically ill COVID-19 patients, 20 patients received a single intravenous infusion of 1 × 106/kg UC-MSCs in 100 ml saline (0.9%) solution (SS) and 20 patients received 100 ml 0.9% SS as the control group. The median time from intensive care unit (ICU) enrollment to MSC treatment was 8 days (range = 2–30 days). During 30 days of observation after MSC administration, the results revealed that UC-MSC treatment was significantly associated with a higher survival rate, with particular benefit in patients with comorbidities. In conclusion, MSC therapy is a safe approach against COVID-19 disease and shows promising benefits for severely ill COVID-19 patients at a progression stage. However, the observation periods of these studies were short and the sample size was small; thus, whether MSC therapy has long-term benefits warrants further research.
In a study (NCT04288102) by Shi et al. (131), a randomized, double-blind, placebo-controlled phase 2 trial was performed in 100 severe COVID-19 patients who were randomly assigned to receive either UC-MSCs (4 × 107 cells per infusion) or placebo on days 0, 3, and 6. According to the analyses of both radiologists and lung imaging artificial intelligence software, the results of high-resolution chest CT imaging revealed that the proportions of solid component lesion volumes were significantly reduced in the MSC treatment group, but not in the placebo group, after 28 days of follow-up. The results of a 6-min walk test also indicated better restoration in patients treated with UC-MSCs. However, with a median time from symptom onset to study baseline of approximately 45 days, most of these patients were at the convalescent stage when joining this trial, which might account for the lack of significant reduction in the duration of oxygen therapy, dyspnea scores, cytokine levels, or chemokine levels. Nonetheless, COVID-19 patients might benefit from MSC therapy, even in the convalescent stage.
Several clinical studies were performed to assess the safety and effectiveness of MSCs in COVID-19 disease ( Table 1 ). However, these preclinical trials seemed to be less convincing due to the small sample sizes ranging from 5 to 100. Because of the emergency nature of the COVID-19 outbreak and the ethical limitations, some of these studies failed to strictly follow the principles of standard clinical trials, such as randomization, blindness, and comparisons. In addition, the patients enrolled in these clinical studies were at different stages of severe COVID-19 disease, which resulted in greater heterogeneity and different results. In particular, given that intravascular coagulation and thromboembolism are considered as leading causes of fatality in COVID-19, the application of MSCs remains controversial because variable levels of highly procoagulant tissue factor (TF/CD142) are expressed by MSCs (133). Furthermore, the time to starting MSC treatment, the cell dosage, and the interval duration have not been fully determined. Hence, further high-quality randomized clinical trials are needed to provide evidence regarding the application of MSCs in COVID-19-related ARDS, and hundreds of clinical trials registered on (https://clinicaltrials.gov/) may offer assistance with this.
Table 1.
Clinical trials of mesenchymal stromal cells (MSCs) in acute respiratory distress syndrome (ARDS) and sepsis.
| First author (reference) | Journal (publication year) | Study design | MSC (n) | Control (n) | MSC source | Dose of MSC | Main outcome |
|---|---|---|---|---|---|---|---|
| MSC therapy in sepsis | |||||||
| Perlee (10) | Stem Cells (2018) | Single-blind phase 1 RCT | 24 | 8 | Adipose tissue | Dose escalation: 1/5/10 × 106 cells/kg | ASCs are safe and exerted a variety of time-dependent pro-inflammatory and anti-inflammatory effects, as well as procoagulant features. |
| McIntyre (11) | Am J Respir Crit Care Med (2018) | Open-label phase 1 clinical trail | 9 | 21 | Bone marrow | Dose escalation: 0.3/1/3 × 106 cells/kg | BM-MSCs are safe for patients with septic shock and appeared to attenuate the levels of several pro-inflammatory cytokines. |
| He (12) | Transl Res (2018) | Open-label phase 1 clinical trail | 15 | 15 | Umbilical cord | Dose escalation: 0.25/1/4 × 106 cells/kg | A single intravenous MSC infusion of up to 3 × 106 cells/kg was well tolerated and is safe in patients with severe sepsis. |
| MSC therapy in ARDS | |||||||
| Zheng (14) | Respir Res (2014) | Double-bind phase 1 RCT | 6 | 6 | Adipose tissue | 1 × 106 cells/kg IBW | Safe and feasible. The length of hospital stay, ventilator-free days, and ICU-free days at 28 days were similar between groups. |
| Wilson (13) | Lancet Respir Med (2015) | Open-label phase 1 clinical trial | 9 | 0 | Bone marrow | Dose escalation: 1/5/10 × 106 cells/kg IBW | An infusion of up to 10 million cells/kg IBW was well tolerated without serious adverse events. |
| Matthay (15) | Lancet Respir Med (2019) | Double-blind randomized phase 2a RCT | 40 | 20 | Bone marrow | 1 × 107 cells/kg IBW | Patients in the MSC group had numerically higher disease severity scores than those in the placebo group at baseline, but mortality did not differ significantly between groups. |
| Chen (8) | Engineering (Beijing) (2020) | Open-label clinical trail | 17 | 44 | Menstrual blood | 100 ml | MSCs significantly improved the survival rate of H7N9-induced ARDS. |
| Yip (7) | Crit Care Med (2020) | Prospective phase 1 clinical trial | 9 | 0 | Umbilical cord | Dose escalation: 1/5/10 × 106 cells/kg | No serious adverse events were identified in any patient, and circulating inflammatory biomarkers were notably progressively reduced. |
| MSC therapy in COVID-19-associated ARDS | |||||||
| Leng (128) | Aging Dis (2020) | Open-label non-randomized pilot trial | 7 | 3 | Not applicable | One dose of 1 × 106/kg | MSCs could cure or significantly improve the functional outcomes of seven patients without observed adverse effects. |
| Shu (124) | Stem Cell Res Ther (2020) | Open-label controlled cohort study | 12 | 29 | Umbilical cord | 2 × 106 cells/kg IBW | MSC treatment is safe and effective at preventing deterioration in severe COVID-19. |
| Sengupta (129) | Stem Cells Dev (2020) | Prospective non-randomized open-label cohort study | 25 | 2 | Exosomes derived from BM-MSCs | A total of 15 ml exosomes | MSC exosomes exhibited capacity to restore oxygenation, downregulate cytokine storm, and reconstitute immunity. |
| Meng (126) | Signal Transduct Target Ther (2020) | Controlled non-randomized phase 1 clinical trial | 9 | 9 | Umbilical cord | Three infusions of 3 × 107 MSCs | Intravenous MSC infusion in patients with moderate and severe COVID-19 is safe and well tolerated. |
| Feng (127) | Cell Prolif (2020) | Single-arm pilot trial | 16 | 0 | Umbilical cord | Four infusions of 1 × 108 MSCs | Intravenous transplantation of UC-MSCs is safe and feasible for the treatment of patients with severe and critically severe COVID-19 pneumonia. |
| Dilogo (130) | Stem Cells Transl Med (2021) | Multicenter double-blind RCT | 20 | 20 | Umbilical cord | 1 × 106 cells/kg | Intravenous infusion MSCs increased the survival rate by modulating the immune system toward an anti-inflammatory state. |
| Shi (131) | Signal Transduct Target Ther (2021) | Double-blind phase 2 RCT | 65 | 35 | Umbilical cord | Three infusions of 4 × 107 MSCs | UC-MSC administration is safe and accelerated the resolution of lung solid component lesions and improvement in the integrated reserve capability. |
| Xu (125) | Clin Transl Med (2021) | Multicenter open-label non-randomized trial | 26 | 18 | Menstrual blood | Three infusions of 3 × 107 MSCs | Patients in the MSC group showed significantly lower mortality and improvements in dyspnea, SpO2, and chest images. |
| Lanzoni (132) | Stem Cells Transl Med (2021) | Double-blind phase 1/2a RCT | 12 | 12 | Umbilical cord | Two infusions of 1 × 108 MSCs | Inflammatory cytokines were significantly decreased in MSC-treated subjects, and treatment was associated with significantly improved patient survival. |
| Iglesias (122) | Aging Dis (2021) | Open-label longitudinal pilot trial | 5 | 0 | Umbilical cord | l × l06 cells/kg | Three patients survived and were extubated on the ninth day post-infusion. Two patients died 13 and 15 days after infusion. |
| Hashemian (123) | Stem Cell Res Ther (2021) | Open-label two-center phase 1 single-arm trial | 11 | 0 | Umbilical cord; placenta | Three infusions of 2 × 108 MSCs | Treatment of MSCs could rapidly improve respiratory distress and reduce inflammatory biomarkers. |
Treatments in the control arm are not shown because they are always mentioned as “placebo” in the original text.
RCT, randomized controlled trial; IBW, ideal body weight; BM-MSCs, bone marrow-derived mesenchymal stromal cells; ASCs, allogeneic adipose MSCs.
Gene Engineering Studies of MSCs
The homing capacity and the systemic distribution of MSCs contribute to the persistence of MSCs in the lungs (26). Moreover, interesting work has been undertaken on therapeutic factors, such as Ang-1, KGF, and HGF, in ARDS and sepsis. However, MSCs alone or “protected mediators” alone may not be sufficient to rectify the disorder of the microenvironment, particularly in severe ARDS and septic shock. Therefore, there is a strong rationale for gene-modified MSCs with critical molecules to “rescue” lung injury and sepsis. It was reported that Ang-1-transfected MSCs could further improve both alveolar inflammation and permeability in septic mice (134, 135). In addition, ROR2-overexpressing MSCs led to more significant effects than did the native MSC treatment in ARDS mice, including the retention of MSCs in the lung, differentiation into alveolar type 2 (ATII) cells, and promotion of lung repair (113). Moreover, gene-modified MSCs also showed further benefits in LPS-induced sepsis and ALI treatment than did native MSCs alone, including overexpressing β-catenin, KGF, HGF, IL-10, the E-prostanoid 2 receptor, CXCR4 receptor, soluble IL-1 receptor-like-1 (sST2), ACE2, TGFβ1, CXCR7, LL-37, heme oxygenase-1, angiotensin II type 2 receptor (AT2R), Miro1, and 7ND (dominant-negative inhibitor of CCL2) (114, 115, 136–148) ( Table 2 ). Aside from the above-mentioned coding genes, the modification of non-coding genes, microRNAs, is an alternative approach. For instance, Wei et al. (150) reported that miR-377-3p released by UC-MSCs suppressed the expression of the target gene RPTOR, and RPTOR silencing played a pivotal role in enhancing autophagy-related proteins. Administration of UC-MSCs with overexpression of miR-377-3p activated the process of autophagy in alveolar epithelial cells under LPS treatment and finally dramatically ameliorated inflammation both in vitro and in vivo. Besides, miR-124-3p transferred by MSC-derived exosomes was observed to improve oxidative stress injury and suppress inflammatory response, thus was beneficial to attenuate traumatic ALI in rats (151). It was also reported that the transduction of miRNA let-7d (with anti-fibrotic effects) promoted recovery from bleomycin-associated lung injury (149). However, there are no reports on which genes should be overexpressed or knocked out in different models of sepsis and ARDS, and it is not clear whether double or triple knockouts of specific genes overexpressed in MSCs are better than a single gene. There is also no report of whether genetically modified MSCs are safe or effective for use in the human body. Therefore, further preclinical studies are required to elucidate the exact role of gene-modified MSCs in different microenvironments.
Table 2.
Genetically modified MSCs in treatment-based studies using models of ALI and sepsis.
| Gene/RNA | Model | MSC source | Dose | Administration | Reference |
|---|---|---|---|---|---|
| ROR2 | LPS-induced ALI | Bone marrow from mice | 5 × 105 cells | i.t. | (113) |
| β-catenin | LPS-induced ALI | Bone marrow from mice | 5 × 105 cells | i.t. | (114) |
| KGF | LPS-induced ALI | Bone marrow from mice | 5 × 105 cells | i.v. | (115) |
| ANGPT1 | LPS-induced ALI | Murine MSCs | 2.5 × 105 cells | i.v. | (135) |
| IL-10 | LPS-induced ALI | Bone marrow from mice | 1 × 106 cells | i.t. | (136) |
| EP2 | LPS-induced ALI | Bone marrow from mice | 2.5 × 105 cells | i.v. | (137) |
| TGFβ1 | LPS-induced ALI | Bone marrow from mice | 2 × 105 cells | i.t. | (138) |
| CXCR7 | Phosgene-induced ALI | Bone marrow from rats | 1 × 106 cells | i.v. | (139) |
| BPI21/LL-37 | CLP-induced sepsis; LPS-induced sepsis | Human umbilical cords | 2 × 105 cells | i.v. | (140) |
| HO-1 | LPS-induced ALI | Bone marrow from rats | 5 × 105 cells | i.v. | (141) |
| ACE2 a | LPS-induced endothelial injury (in vitro) | Bone marrow from mice | 1 × 104 cells | Co-culture with HPMEC | (142) |
| AT2R | LPS-induced ALI | Bone marrow from human | 1 × 106 cells | i.v. | (145) |
| CXCR4 | LPS-induced ALI | Bone marrow from rats | 1 × 106 cells | i.v. | (143) |
| MIRO-1 | Rotenone-induced airway injury | Bone marrow from mice | 1 × 106 cells | i.t. | (146) |
| 7ND | Bleomycin-induced ALI | Bone marrow and adipose tissue from mice | 5 × 105 cells | i.v. | (148) |
| HGF | Radiation-induced lung injury | Bone marrow from human | 1 × 106 cells | i.v. | (147) |
| Let-7d | Bleomycin-induced ALI | Bone marrow from human | 5 × 105 cells | i.v. | (149) |
| miR-154 | Bleomycin-induced ALI | Bone marrow from human | 5 × 105 cells | i.v. | (149) |
| miR-377-3p | LPS-induced ALI | Human umbilical cords | 2 × 105 cells | i.t. | (150) |
| P130 | LPS-induced ALI | Bone marrow from mice | 5 × 105 cells | i.t. | (144) |
| E2F4 | LPS-induced ALI | Bone marrow from mice | 5 × 105 cells | i.t. | (144) |
| miR-124-3p | Traumatic ALI | Bone marrow from rats | 25 μg exosomes from 2 × 107 MSCs | i.v. | (151) |
MSCs, mesenchymal stromal cells; ALI, acute lung injury; LPS, lipopolysaccharide; CLP, cecal ligation and puncture; PBS, phosphate-buffered saline; NS, normal saline; HMPECs, human pulmonary microvascular endothelial cells; i.v., intravenous injection; i.t., intratracheal administration.
All of the above studies were performed in animal models, except for the in vitro experiment.
Adverse Effects of MSCs
Despite the promising effects of MSCs in both preclinical and phase 1 clinical trials, the adverse effects of MSC treatment should not be ignored. MSCs are highly adherent to membrane oxygenators, which led to a rapid decline in oxygenator performance during extracorporeal membrane oxygenation (ECMO) in a sheep model of ALI (152, 153). In addition, human MSC administration showed a lack of efficacy in influenza-mediated lung injury in mice (64) and in improving the survival of mice with staphylococcal toxic shock syndrome (154). Senescent MSCs were also unable to show protection in a murine model of LPS-induced lethal endotoxemia (155). In some reports, MSCs were indicated to enhance neutrophil recruitment and its bactericidal functions through TNF-α and IL-1β signaling (156, 157). However, these findings highlight MSCs as a potential risk factor because aberrant neutrophil activation contributes to disease severity and local tissue damage in COVID-19 through the amplification of hypercytokinemia and the formation of neutrophil extracellular traps (NETs) (158). Furthermore, the encouraging findings from animal experiments do not guarantee their efficacy in clinical trials. It was reported that biomarkers of inflammation (IL-6 and IL-8) at 5 days and the length of hospital stay, ventilator-free days, and ICU-free days at 28 days were similar between the MSC treatment and saline placebo groups in a randomized controlled trial of MSCs for ARDS (14).
Challenges of Future Studies
Clinical trials of MSCs for ARDS and COVID-19 treatment demonstrated safety and tolerance (see Table 1 ). However, both published trials contained very few patients, and much work remains to be undertaken before MSCs can be used in the clinic and even for COVID-19. Firstly, there are various sources of MSCs (including tissues and donors), and the best source needs to be identified. Secondly, the preparation of MSCs (including the culture conditions, passage, and storage) should be standardized and consistent. Thirdly, the best MSC dose, timing, and delivery remain to be further clarified. Finally, the etiologies of sepsis and ARDS vary; thus, optimal treatment with MSCs may depend on different microenvironments. Future studies should take the above suggestions into full consideration and carefully investigate them.
Conclusions and Perspectives
A growing number of preclinical studies have shown encouraging findings for MSC-based therapy in sepsis and ARDS treatment. Phase 1 clinical trials have also reported that MSCs are safe and well tolerated in ARDS patients. Nevertheless, experimental studies and clinical trials are still needed to fully understand the optimal MSC application in sepsis/ARDS and COVID-19.
Author Contributions
ZX, YH, and JZ prepared the original draft and reviewed and edited the manuscript. WH, XL, YL, NZ, and LS reviewed and edited the manuscript. XD prepared the tables and figures. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the National Natural Science Foundation of China (81870069, 81970071, and 82070084), the Natural Science Foundation of Guangdong Province (2020A1515011459 and 2021A1515012565), the Science and Technology Program of Guangzhou (202102010157), the State Key Laboratory of Respiratory Disease Independent Program (SKLRD-Z-202108), Emergency Key Program of Guangzhou Laboratory (EKPG21-17) and Natural Science Foundation of Guangdong Province (2020A1515011459).
Conflict of Interest
YL is an editor of Frontiers in Immunology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med (2020) 382(18):1708–20. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phelan AL, Katz R, Gostin LO. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA (2020) 323(8):709–10. doi: 10.1001/jama.2020.1097 [DOI] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA (2020) 323(13):1239–42. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4. Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med (2020) 383(25):2451–60. doi: 10.1056/NEJMcp2009575 [DOI] [PubMed] [Google Scholar]
- 5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat Rev Immunol (2020) 20(6):363–74. doi: 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial Dysfunction and Immunothrombosis as Key Pathogenic Mechanisms in COVID-19. Nat Rev Immunol (2021) 21(5):319–29. doi: 10.1038/s41577-021-00536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yip HK, Fang WF, Li YC, Lee FY, Lee CH, Pei SN, et al. Human Umbilical Cord-Derived Mesenchymal Stem Cells for Acute Respiratory Distress Syndrome. Crit Care Med (2020) 48(5):e391–e9. doi: 10.1097/ccm.0000000000004285 [DOI] [PubMed] [Google Scholar]
- 8. Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, et al. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering (Beijing) (2020) 6(10):1153–61. doi: 10.1016/j.eng.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schlosser K, Wang JP, Dos Santos C, Walley KR, Marshall J, Fergusson DA, et al. Effects of Mesenchymal Stem Cell Treatment on Systemic Cytokine Levels in a Phase 1 Dose Escalation Safety Trial of Septic Shock Patients. Crit Care Med (2019) 47(7):918–25. doi: 10.1097/CCM.0000000000003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perlee D, van Vught LA, Scicluna BP, Maag A, Lutter R, Kemper EM, et al. Intravenous Infusion of Human Adipose Mesenchymal Stem Cells Modifies the Host Response to Lipopolysaccharide in Humans: A Randomized, Single-Blind, Parallel Group, Placebo Controlled Trial. Stem Cells (2018) 36(11):1778–88. doi: 10.1002/stem.2891 [DOI] [PubMed] [Google Scholar]
- 11. McIntyre LA, Stewart DJ, Mei SHJ, Courtman D, Watpool I, Granton J, et al. Cellular Immunotherapy for Septic Shock. A Phase I Clinical Trial. Am J Respir Crit Care Med (2018) 197(3):337–47. doi: 10.1164/rccm.201705-1006OC [DOI] [PubMed] [Google Scholar]
- 12. He X, Ai S, Guo W, Yang Y, Wang Z, Jiang D, et al. Umbilical Cord-Derived Mesenchymal Stem (Stromal) Cells for Treatment of Severe Sepsis: Aphase 1 Clinical Trial. Transl Res (2018) 199:52–61. doi: 10.1016/j.trsl.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 13. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, et al. Mesenchymal Stem (Stromal) Cells for Treatment of ARDS: A Phase 1 Clinical Trial. Lancet Respir Med (2015) 3(1):24–32. doi: 10.1016/s2213-2600(14)70291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, et al. Treatment of Acute Respiratory Distress Syndrome With Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Randomized, Placebo-Controlled Pilot Study. Respir Res (2014) 15(1):39. doi: 10.1186/1465-9921-15-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment With Allogeneic Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome (START Study): A Randomised Phase 2a Safety Trial. Lancet Respir Med (2019) 7(2):154–62. doi: 10.1016/s2213-2600(18)30418-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy (2006) 8(4):315–7. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 17. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol Biol Cell (2002) 13(12):4279–95. doi: 10.1091/mbc.E02-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of Mesenchymal Stem/Progenitor Cells in Human First-Trimester Fetal Blood, Liver, and Bone Marrow. Blood (2001) 98(8):2396–402. doi: 10.1182/blood.V98.8.2396 [DOI] [PubMed] [Google Scholar]
- 19. In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, et al. Amniotic Fluid as a Novel Source of Mesenchymal Stem Cells for Therapeutic Transplantation. Blood (2003) 102(4):1548–9. doi: 10.1182/blood-2003-04-1291 [DOI] [PubMed] [Google Scholar]
- 20. Chamberlain G, Fox J, Ashton B, Middleton J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells (2007) 25(11):2739–49. doi: 10.1634/stemcells.2007-0197 [DOI] [PubMed] [Google Scholar]
- 21. Nazarov I, Lee JW, Soupene E, Etemad S, Knapik D, Green W, et al. Multipotent Stromal Stem Cells From Human Placenta Demonstrate High Therapeutic Potential. Stem Cells Trans Med (2012) 1(5):359–72. doi: 10.5966/sctm.2011-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun CK, Leu S, Hsu SY, Zhen YY, Chang LT, Tsai CY, et al. Mixed Serum-Deprived and Normal Adipose-Derived Mesenchymal Stem Cells Against Acute Lung Ischemia-Reperfusion Injury in Rats. Am J Trans Res (2015) 7(2):209–31. [PMC free article] [PubMed] [Google Scholar]
- 23. Mao YX, Xu JF, Seeley EJ, Tang XD, Xu LL, Zhu YG, et al. Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Pulmonary Infection Caused by Pseudomonas Aeruginosa via Inhibiting Overproduction of Prostaglandin E2. Stem Cells (2015) 33(7):2331–42. doi: 10.1002/stem.1996 [DOI] [PubMed] [Google Scholar]
- 24. Jiang X, Jiang X, Qu C, Chang P, Zhang C, Qu Y, et al. Intravenous Delivery of Adipose-Derived Mesenchymal Stromal Cells Attenuates Acute Radiation-Induced Lung Injury in Rats. Cytotherapy (2015) 17(5):560–70. doi: 10.1016/j.jcyt.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 25. Reddy M, Fonseca L, Gowda S, Chougule B, Hari A, Totey S. Human Adipose-Derived Mesenchymal Stem Cells Attenuate Early Stage of Bleomycin Induced Pulmonary Fibrosis: Comparison With Pirfenidone. Int J Stem Cells (2016) 9(2):192–206. doi: 10.15283/ijsc16041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hou C, Peng D, Gao L, Tian D, Dai J, Luo Z, et al. Human Umbilical Cord-Derived Mesenchymal Stem Cells Protect From Hyperoxic Lung Injury by Ameliorating Aberrant Elastin Remodeling in the Lung of O2-Exposed Newborn Rat. Biochem Biophys Res Commun (2018) 495(2):1972–9. doi: 10.1016/j.bbrc.2017.12.055 [DOI] [PubMed] [Google Scholar]
- 27. Zhu Y, Xu L, Collins JJP, Vadivel A, Cyr-Depauw C, Zhong S, et al. Human Umbilical Cord Mesenchymal Stromal Cells Improve Survival and Bacterial Clearance in Neonatal Sepsis in Rats. Stem Cells Dev (2017) 26(14):1054–64. doi: 10.1089/scd.2016.0329 [DOI] [PubMed] [Google Scholar]
- 28. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA Expression and Immunologic Properties of Differentiated and Undifferentiated Mesenchymal Stem Cells. Exp Hematol (2003) 31(10):890–6. doi: 10.1016/S0301-472X(03)00110-3 [DOI] [PubMed] [Google Scholar]
- 29. Laffey JG, Matthay MA. Fifty Years of Research in ARDS. Cell-Based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am J Respir Crit Care Med (2017) 196(3):266–73. doi: 10.1164/rccm.201701-0107CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Becker A, Riet IV. Homing and Migration of Mesenchymal Stromal Cells: How to Improve the Efficacy of Cell Therapy? World J Stem Cells (2016) 8(3):73–87. doi: 10.4252/wjsc.v8.i3.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walter J, Ware LB, Matthay MA. Mesenchymal Stem Cells: Mechanisms of Potential Therapeutic Benefit in ARDS and Sepsis. Lancet Respir Med (2014) 2(12):1016–26. doi: 10.1016/s2213-2600(14)70217-6 [DOI] [PubMed] [Google Scholar]
- 32. Qin H, Zhao A. Mesenchymal Stem Cell Therapy for Acute Respiratory Distress Syndrome: From Basic to Clinics. Protein Cell (2020) 11(10):707–22. doi: 10.1007/s13238-020-00738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, et al. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part From Secretion of the Antimicrobial Peptide LL-37. Stem Cells (2010) 28(12):2229–38. doi: 10.1002/stem.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, et al. Mesenchymal Stem Cells Enhance Recovery and Repair Following Ventilator-Induced Lung Injury in the Rat. Thorax (2012) 67(6):496–501. doi: 10.1136/thoraxjnl-2011-201059 [DOI] [PubMed] [Google Scholar]
- 35. Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic Effects of Human Mesenchymal Stem Cells in Ex Vivo Human Lungs Injured With Live Bacteria. Am J Respir Crit Care Med (2013) 187(7):751–60. doi: 10.1164/rccm.201206-0990OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic Human Mesenchymal Stem Cells Restore Epithelial Protein Permeability in Cultured Human Alveolar Type II Cells by Secretion of Angiopoietin-1. J Biol Chem (2010) 285(34):26211–22. doi: 10.1074/jbc.M110.119917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu S, Li J, Xu X, Liu A, He H, Xu J, et al. The Hepatocyte Growth Factor-Expressing Character Is Required for Mesenchymal Stem Cells to Protect the Lung Injured by Lipopolysaccharide In Vivo. Stem Cell Res Ther (2016) 7(1):66. doi: 10.1186/s13287-016-0320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang H, Zheng R, Chen Q, Shao J, Yu J, Hu S. Mesenchymal Stem Cells Microvesicles Stabilize Endothelial Barrier Function Partly Mediated by Hepatocyte Growth Factor (HGF). Stem Cell Res Ther (2017) 8(1):211. doi: 10.1186/s13287-017-0662-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu Z, Chang W, Meng S, Xu X, Xie J, Guo F, et al. Mesenchymal Stem Cells Induce Dendritic Cell Immune Tolerance via Paracrine Hepatocyte Growth Factor to Alleviate Acute Lung Injury. Stem Cell Res Ther (2019) 10(1):372. doi: 10.1186/s13287-019-1488-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fan XL, Zhang Y, Li X, Fu QL. Mechanisms Underlying the Protective Effects of Mesenchymal Stem Cell-Based Therapy. Cell Mol Life Sci CMLS (2020) 77(14):2771–94. doi: 10.1007/s00018-020-03454-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial Transfer From Bone-Marrow-Derived Stromal Cells to Pulmonary Alveoli Protects Against Acute Lung Injury. Nat Med (2012) 18(5):759–65. doi: 10.1038/nm.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, et al. Mitochondrial Transfer via Tunneling Nanotubes Is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells (2016) 34(8):2210–23. doi: 10.1002/stem.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med (2017) 196(10):1275–86. doi: 10.1164/rccm.201701-0170OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sinclair KA, Yerkovich ST, Hopkins PM, Chambers DC. Characterization of Intercellular Communication and Mitochondrial Donation by Mesenchymal Stromal Cells Derived From the Human Lung. Stem Cell Res Ther (2016) 7(1):91. doi: 10.1186/s13287-016-0354-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fergie N, Todd N, McClements L, McAuley D, O'Kane C, Krasnodembskaya A. Hypercapnic Acidosis Induces Mitochondrial Dysfunction and Impairs the Ability of Mesenchymal Stem Cells to Promote Distal Lung Epithelial Repair. FASEB J (2019) 33(4):5585–98. doi: 10.1096/fj.201802056R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Islam D, Huang Y, Fanelli V, Delsedime L, Wu S, Khang J, et al. Identification and Modulation of Microenvironment Is Crucial for Effective Mesenchymal Stromal Cell Therapy in Acute Lung Injury. Am J Respir Crit Care Med (2019) 199(10):1214–24. doi: 10.1164/rccm.201802-0356OC [DOI] [PubMed] [Google Scholar]
- 47. Silva JD, Su Y, Calfee CS, Delucchi KL, Weiss D, McAuley DF, et al. MSC Extracellular Vesicles Rescue Mitochondrial Dysfunction and Improve Barrier Integrity in Clinically Relevant Models of ARDS. Eur Respir J (2020) 58(1):2002978. doi: 10.1183/13993003.02978-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Crit Care Med (1992) 20(6):864–74. [PubMed] [Google Scholar]
- 50. Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, et al. Early Combination Antibiotic Therapy Yields Improved Survival Compared With Monotherapy in Septic Shock: A Propensity-Matched Analysis. Crit Care Med (2010) 38(9):1773–85. doi: 10.1097/CCM.0b013e3181eb3ccd [DOI] [PubMed] [Google Scholar]
- 51. Howell MD, Davis AM. Management of Sepsis and Septic Shock. JAMA (2017) 317(8):847–8. doi: 10.1001/jama.2017.0131 [DOI] [PubMed] [Google Scholar]
- 52. Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, et al. Mesenchymal Stem Cells Reduce Inflammation While Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am J Respir Crit Care Med (2010) 182(8):1047–57. doi: 10.1164/rccm.201001-0010OC [DOI] [PubMed] [Google Scholar]
- 53. Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, et al. Mesenchymal Stem Cells Enhance Survival and Bacterial Clearance in Murine Escherichia Coli Pneumonia. Thorax (2012) 67(6):533–9. doi: 10.1136/thoraxjnl-2011-201176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, et al. Human Mesenchymal Stem Cells Reduce Mortality and Bacteremia in Gram-Negative Sepsis in Mice in Part by Enhancing the Phagocytic Activity of Blood Monocytes. Am J Physiol Lung Cell Mol Physiol (2012) 302(10):L1003–13. doi: 10.1152/ajplung.00180.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rabani R, Volchuk A, Jerkic M, Ormesher L, Garces-Ramirez L, Canton J, et al. Mesenchymal Stem Cells Enhance NOX2 Dependent ROS Production and Bacterial Killing in Macrophages During Sepsis. Eur Respir J (2018) 51(4):1702021. doi: 10.1183/13993003.02021-2017 [DOI] [PubMed] [Google Scholar]
- 56. Hall SR, Tsoyi K, Ith B, Padera RF, Jr., Lederer JA, Wang Z, et al. Mesenchymal Stromal Cells Improve Survival During Sepsis in the Absence of Heme Oxygenase-1: The Importance of Neutrophils. Stem Cells (2013) 31(2):397–407. doi: 10.1002/stem.1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Devaney J, Horie S, Masterson C, Elliman S, Barry F, O'Brien T, et al. Human Mesenchymal Stromal Cells Decrease the Severity of Acute Lung Injury Induced by E. Coli in the Rat. Thorax (2015) 70(7):625–35. doi: 10.1136/thoraxjnl-2015-206813 [DOI] [PubMed] [Google Scholar]
- 58. Hao Q, Gudapati V, Monsel A, Park JH, Hu S, Kato H, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Decrease Lung Injury in Mice. J Immunol (2019) 203(7):1961–72. doi: 10.4049/jimmunol.1801534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martin GS. Sepsis, Severe Sepsis and Septic Shock: Changes in Incidence, Pathogens and Outcomes. Expert Rev Anti-Infective Ther (2012) 10(6):701–6. doi: 10.1586/eri.12.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miggins M, Hasan A, Hohmann S, Southwick F, Casella G, Schain D, et al. The Potential Influence of Common Viral Infections Diagnosed During Hospitalization Among Critically Ill Patients in the United States. PLoS One (2011) 6(4):e18890. doi: 10.1371/journal.pone.0018890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khatri M, O'Brien TD, Goyal SM, Sharma JM. Isolation and Characterization of Chicken Lung Mesenchymal Stromal Cells and Their Susceptibility to Avian Influenza Virus. Dev Comp Immunol (2010) 34(4):474–9. doi: 10.1016/j.dci.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thanunchai M, Kanrai P, Wiboon-Ut S, Puthavathana P, Hongeng S, Thitithanyanont A. Tropism of Avian Influenza A (H5N1) Virus to Mesenchymal Stem Cells and CD34+ Hematopoietic Stem Cells. PLoS One (2013) 8(12):e81805. doi: 10.1371/journal.pone.0081805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Khatri M, Saif YM. Influenza Virus Infects Bone Marrow Mesenchymal Stromal Cells In Vitro: Implications for Bone Marrow Transplantation. Cell Transplant (2013) 22(3):461–8. doi: 10.3727/096368912x656063 [DOI] [PubMed] [Google Scholar]
- 64. Gotts JE, Abbott J, Matthay MA. Influenza Causes Prolonged Disruption of the Alveolar-Capillary Barrier in Mice Unresponsive to Mesenchymal Stem Cell Therapy. Am J Physiol Lung Cell Mol Physiol (2014) 307(5):L395–406. doi: 10.1152/ajplung.00110.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Darwish I, Banner D, Mubareka S, Kim H, Besla R, Kelvin DJ, et al. Mesenchymal Stromal (Stem) Cell Therapy Fails to Improve Outcomes in Experimental Severe Influenza. PLoS One (2013) 8(8):e71761. doi: 10.1371/journal.pone.0071761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, et al. Human Mesenchymal Stromal Cells Reduce Influenza A H5N1-Associated Acute Lung Injury In Vitro and In Vivo. Proc Natl Acad Sci U S A (2016) 113(13):3621–6. doi: 10.1073/pnas.1601911113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li Y, Xu J, Shi W, Chen C, Shao Y, Zhu L, et al. Mesenchymal Stromal Cell Treatment Prevents H9N2 Avian Influenza Virus-Induced Acute Lung Injury in Mice. Stem Cell Res Ther (2016) 7(1):159. doi: 10.1186/s13287-016-0395-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khatri M, Richardson LA, Meulia T. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Influenza Virus-Induced Acute Lung Injury in a Pig Model. Stem Cell Res Ther (2018) 9(1):17. doi: 10.1186/s13287-018-0774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Loy H, Kuok DIT, Hui KPY, Choi MHL, Yuen W, Nicholls JM, et al. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus-Associated Acute Lung Injury. J Infect Dis (2019) 219(2):186–96. doi: 10.1093/infdis/jiy478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Martin GS, Mannino DM, Eaton S, Moss M. The Epidemiology of Sepsis in the United States From 1979 Through 2000. N Engl J Med (2003) 348(16):1546–54. doi: 10.1056/NEJMoa022139 [DOI] [PubMed] [Google Scholar]
- 71. Yang R, Liu Y, Kelk P, Qu C, Akiyama K, Chen C, et al. A Subset of IL-17(+) Mesenchymal Stem Cells Possesses Anti-Candida Albicans Effect. Cell Res (2013) 23(1):107–21. doi: 10.1038/cr.2012.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. J Immunol (2007) 179(3):1855–63. doi: 10.4049/jimmunol.179.3.1855 [DOI] [PubMed] [Google Scholar]
- 73. Luo CJ, Zhang FJ, Zhang L, Geng YQ, Li QG, Hong Q, et al. Mesenchymal Stem Cells Ameliorate Sepsis-Associated Acute Kidney Injury in Mice. Shock (2014) 41(2):123–9. doi: 10.1097/shk.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 74. Wu KH, Wu HP, Chao WR, Lo WY, Tseng PC, Lee CJ, et al. Time-Series Expression of Toll-Like Receptor 4 Signaling in Septic Mice Treated With Mesenchymal Stem Cells. Shock (2016) 45(6):634–40. doi: 10.1097/shk.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 75. Asami T, Ishii M, Namkoong H, Yagi K, Tasaka S, Asakura T, et al. Anti-Inflammatory Roles of Mesenchymal Stromal Cells During Acute Streptococcus Pneumoniae Pulmonary Infection in Mice. Cytotherapy (2018) 20(3):302–13. doi: 10.1016/j.jcyt.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 76. Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, et al. Mesenchymal Stem Cells Attenuate Myocardial Functional Depression and Reduce Systemic and Myocardial Inflammation During Endotoxemia. Surgery (2010) 148(2):444–52. doi: 10.1016/j.surg.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 77. Pedrazza L, Cubillos-Rojas M, de Mesquita FC, Luft C, Cunha AA, Rosa JL, et al. Mesenchymal Stem Cells Decrease Lung Inflammation During Sepsis, Acting Through Inhibition of the MAPK Pathway. Stem Cell Res Ther (2017) 8(1):289. doi: 10.1186/s13287-017-0734-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human Adult Stem Cells Derived From Adipose Tissue Protect Against Experimental Colitis and Sepsis. Gut (2009) 58(7):929–39. doi: 10.1136/gut.2008.168534 [DOI] [PubMed] [Google Scholar]
- 79. dos Santos CC, Murthy S, Hu P, Shan Y, Haitsma JJ, Mei SH, et al. Network Analysis of Transcriptional Responses Induced by Mesenchymal Stem Cell Treatment of Experimental Sepsis. Am J Pathol (2012) 181(5):1681–92. doi: 10.1016/j.ajpath.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 80. Lee RH, Yu JM, Foskett AM, Peltier G, Reneau JC, Bazhanov N, et al. TSG-6 as a Biomarker to Predict Efficacy of Human Mesenchymal Stem/Progenitor Cells (hMSCs) in Modulating Sterile Inflammation In Vivo. Proc Natl Acad Sci U S A (2014) 111(47):16766–71. doi: 10.1073/pnas.1416121111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs Improve Myocardial Infarction in Mice Because Cells Embolized in Lung Are Activated to Secrete the Anti-Inflammatory Protein TSG-6. Cell Stem Cell (2009) 5(1):54–63. doi: 10.1016/j.stem.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, et al. Kynurenic Acid, an IDO Metabolite, Controls TSG-6-Mediated Immunosuppression of Human Mesenchymal Stem Cells. Cell Death Differ (2018) 25(7):1209–23. doi: 10.1038/s41418-017-0006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dang S, Yu ZM, Zhang CY, Zheng J, Li KL, Wu Y, et al. Autophagy Promotes Apoptosis of Mesenchymal Stem Cells Under Inflammatory Microenvironment. Stem Cell Res Ther (2015) 6:247. doi: 10.1186/s13287-015-0245-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Abreu SC, Rolandsson Enes S, Dearborn J, Goodwin M, Coffey A, Borg ZD, et al. Lung Inflammatory Environments Differentially Alter Mesenchymal Stromal Cell Behavior. Am J Physiol Lung Cell Mol Physiol (2019) 317(6):L823–l31. doi: 10.1152/ajplung.00263.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li S, Wu H, Han D, Ma S, Fan W, Wang Y, et al. A Novel Mechanism of Mesenchymal Stromal Cell-Mediated Protection Against Sepsis: Restricting Inflammasome Activation in Macrophages by Increasing Mitophagy and Decreasing Mitochondrial ROS. Oxid Med Cell Longevity (2018) 2018:15. doi: 10.1155/2018/3537609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Su VY, Lin CS, Hung SC, Yang KY. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated With Inhibition of the NF-kappaB Pathway in Endotoxin-Induced Acute Lung Injury. Int J Mol Sci (2019) 20(9):2208. doi: 10.3390/ijms20092208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Deutschman CS, Tracey KJ. Sepsis: Current Dogma and New Perspectives. Immunity (2014) 40(4):463–75. doi: 10.1016/j.immuni.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 88. Hotchkiss RS, Monneret G, Payen D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat Rev Immunol (2013) 13(12):862–74. doi: 10.1038/nri3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone Marrow Stromal Cells Attenuate Sepsis via Prostaglandin E(2)-Dependent Reprogramming of Host Macrophages to Increase Their Interleukin-10 Production. Nat Med (2009) 15(1):42–9. doi: 10.1038/nm.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhu H, Xiong Y, Xia Y, Zhang R, Tian D, Wang T, et al. Therapeutic Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Lung Injury Mice. Sci Rep (2017) 7:39889. doi: 10.1038/srep39889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sreeramkumar V, Hons M, Punzón C, Stein JV, Sancho D, Fresno M, et al. Efficient T-Cell Priming and Activation Requires Signaling Through Prostaglandin E2 (EP) Receptors. Immunol Cell Biol (2016) 94(1):39–51. doi: 10.1038/icb.2015.62 [DOI] [PubMed] [Google Scholar]
- 92. Aggarwal S, Pittenger MF. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood (2005) 105(4):1815–22. doi: 10.1182/blood-2004-04-1559 [DOI] [PubMed] [Google Scholar]
- 93. Chao YH, Wu HP, Wu KH, Tsai YG, Peng CT, Lin KC, et al. An Increase in CD3+CD4+CD25+ Regulatory T Cells After Administration of Umbilical Cord-Derived Mesenchymal Stem Cells During Sepsis. PLoS One (2014) 9(10):e110338. doi: 10.1371/journal.pone.0110338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhu J, Feng B, Xu Y, Chen W, Sheng X, Feng X, et al. Mesenchymal Stem Cells Alleviate LPS-Induced Acute Lung Injury by Inhibiting the Proinflammatory Function of Ly6C(+) CD8(+) T Cells. Cell Death Dis (2020) 11(10):829. doi: 10.1038/s41419-020-03036-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Feng B, Zhu J, Xu Y, Chen W, Sheng X, Feng X, et al. Immunosuppressive Effects of Mesenchymal Stem Cells on Lung B Cell Gene Expression in LPS-Induced Acute Lung Injury. Stem Cell Res Ther (2020) 11(1):418. doi: 10.1186/s13287-020-01934-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1beta-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells (2017) 35(5):1208–21. doi: 10.1002/stem.2564 [DOI] [PubMed] [Google Scholar]
- 97. Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute Respiratory Distress Syndrome. Nat Rev Dis Primers (2019) 5(1):1–18. doi: 10.1038/s41572-019-0069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bhattacharya J, Matthay MA. Regulation and Repair of the Alveolar-Capillary Barrier in Acute Lung Injury. Annu Rev Physiol (2013) 75:593–615. doi: 10.1146/annurev-physiol-030212-183756 [DOI] [PubMed] [Google Scholar]
- 99. Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T, et al. Conditioned Media From Mesenchymal Stromal Cells Restore Sodium Transport and Preserve Epithelial Permeability in an In Vitro Model of Acute Alveolar Injury. Am J Physiol Lung Cell Mol Physiol (2014) 306(11):L975–85. doi: 10.1152/ajplung.00242.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang J, Shao Y, He D, Zhang L, Xu G, Shen J. Evidence That Bone Marrow-Derived Mesenchymal Stem Cells Reduce Epithelial Permeability Following Phosgene-Induced Acute Lung Injury via Activation of Wnt3a Protein-Induced Canonical Wnt/Beta-Catenin Signaling. Inhal Toxicol (2016) 28(12):572–9. doi: 10.1080/08958378.2016.1228720 [DOI] [PubMed] [Google Scholar]
- 101. Pelosi P, D'Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, et al. Pulmonary and Extrapulmonary Acute Respiratory Distress Syndrome Are Different. Eur Respir J Suppl (2003) 42:48s–56s. doi: 10.1183/09031936.03.00420803 [DOI] [PubMed] [Google Scholar]
- 102. Pati S, Gerber MH, Menge TD, Wataha KA, Zhao Y, Baumgartner JA, et al. Bone Marrow Derived Mesenchymal Stem Cells Inhibit Inflammation and Preserve Vascular Endothelial Integrity in the Lungs After Hemorrhagic Shock. PLoS One (2011) 6(9):e25171. doi: 10.1371/journal.pone.0025171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Potter DR, Miyazawa BY, Gibb SL, Deng X, Togaratti PP, Croze RH, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Pulmonary Vascular Permeability and Lung Injury Induced by Hemorrhagic Shock and Trauma. J Trauma Acute Care Surg (2018) 84(2):245–56. doi: 10.1097/TA.0000000000001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ihara K, Fukuda S, Enkhtaivan B, Trujillo R, Perez-Bello D, Nelson C, et al. Adipose-Derived Stem Cells Attenuate Pulmonary Microvascular Hyperpermeability After Smoke Inhalation. PLoS One (2017) 12(10):e0185937. doi: 10.1371/journal.pone.0185937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic Human Mesenchymal Stem Cells for Treatment of E. Coli Endotoxin-Induced Acute Lung Injury in the Ex Vivo Perfused Human Lung. Proc Natl Acad Sci U S A (2009) 106(38):16357–62. doi: 10.1073/pnas.0907996106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, et al. Human Mesenchymal Stem Cells Inhibit Vascular Permeability by Modulating Vascular Endothelial Cadherin/Beta-Catenin Signaling. Stem Cells Dev (2011) 20(1):89–101. doi: 10.1089/scd.2010.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ware LB, Matthay MA. Alveolar Fluid Clearance Is Impaired in the Majority of Patients With Acute Lung Injury and the Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med (2001) 163(6):1376–83. doi: 10.1164/ajrccm.163.6.2004035 [DOI] [PubMed] [Google Scholar]
- 108. Hao Q, Zhu YG, Monsel A, Gennai S, Lee T, Xu F, et al. Study of Bone Marrow and Embryonic Stem Cell-Derived Human Mesenchymal Stem Cells for Treatment of Escherichia Coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells Trans Med (2015) 4(7):832–40. doi: 10.5966/sctm.2015-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Asmussen S, Ito H, Traber DL, Lee JW, Cox RA, Hawkins HK, et al. Human Mesenchymal Stem Cells Reduce the Severity of Acute Lung Injury in a Sheep Model of Bacterial Pneumonia. Thorax (2014) 69(9):819–25. doi: 10.1136/thoraxjnl-2013-204980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Li JW, Wu X. Mesenchymal Stem Cells Ameliorate LPS-Induced Acute Lung Injury Through KGF Promoting Alveolar Fluid Clearance of Alveolar Type II Cells. Eur Rev Med Pharmacol Sci (2015) 19(13):2368–78. [PubMed] [Google Scholar]
- 111. McAuley DF, Curley GF, Hamid UI, Laffey JG, Abbott J, McKenna DH, et al. Clinical Grade Allogeneic Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am J Physiol Lung Cell Mol Physiol (2014) 306(9):L809–15. doi: 10.1152/ajplung.00358.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. Transplantation of Menstrual Blood-Derived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. Int J Mol Sci (2017) 18(4):689. doi: 10.3390/ijms18040689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cai SX, Liu AR, Chen S, He HL, Chen QH, Xu JY, et al. The Orphan Receptor Tyrosine Kinase ROR2 Facilitates MSCs to Repair Lung Injury in ARDS Animal Model. Cell Transplant (2016) 25(8):1561–74. doi: 10.3727/096368915x689776 [DOI] [PubMed] [Google Scholar]
- 114. Cai SX, Liu AR, Chen S, He HL, Chen QH, Xu JY, et al. Activation of Wnt/beta-Catenin Signalling Promotes Mesenchymal Stem Cells to Repair Injured Alveolar Epithelium Induced by Lipopolysaccharide in Mice. Stem Cell Res Ther (2015) 6:65. doi: 10.1186/s13287-015-0060-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115. Chen J, Li C, Gao X, Li C, Liang Z, Yu L, et al. Keratinocyte Growth Factor Gene Delivery via Mesenchymal Stem Cells Protects Against Lipopolysaccharide-Induced Acute Lung Injury in Mice. PLoS One (2013) 8(12):e83303. doi: 10.1371/journal.pone.0083303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xiao K, He W, Guan W, Hou F, Yan P, Xu J, et al. Mesenchymal Stem Cells Reverse EMT Process Through Blocking the Activation of NF-kappaB and Hedgehog Pathways in LPS-Induced Acute Lung Injury. Cell Death Dis (2020) 11(10):863. doi: 10.1038/s41419-020-03034-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Broekman W, Amatngalim GD, de Mooij-Eijk Y, Oostendorp J, Roelofs H, Taube C, et al. TNF-Alpha and IL-1beta-Activated Human Mesenchymal Stromal Cells Increase Airway Epithelial Wound Healing In Vitro via Activation of the Epidermal Growth Factor Receptor. Respir Res (2016) 17:3. doi: 10.1186/s12931-015-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chen J, Li Y, Hao H, Li C, Du Y, Hu Y, et al. Mesenchymal Stem Cell Conditioned Medium Promotes Proliferation and Migration of Alveolar Epithelial Cells Under Septic Conditions In Vitro via the JNK-P38 Signaling Pathway. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol (2015) 37(5):1830–46. doi: 10.1159/000438545 [DOI] [PubMed] [Google Scholar]
- 119. Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current Status of Cell-Based Therapies for Respiratory Virus Infections: Applicability to COVID-19. Eur Respir J (2020) 55(6):2000858. doi: 10.1183/13993003.00858-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Schafer R, Spohn G, Bechtel M, Bojkova D, Baer PC, Kuci S, et al. Human Mesenchymal Stromal Cells Are Resistant to SARS-CoV-2 Infection Under Steady-State, Inflammatory Conditions and in the Presence of SARS-CoV-2-Infected Cells. Stem Cell Rep (2021) 16(3):419–27. doi: 10.1016/j.stemcr.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Avanzini MA, Mura M, Percivalle E, Bastaroli F, Croce S, Valsecchi C, et al. Human Mesenchymal Stromal Cells do Not Express ACE2 and TMPRSS2 and Are Not Permissive to SARS-CoV-2 Infection. Stem Cells Transl Med (2021) 10(4):636–42. doi: 10.1002/sctm.20-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Iglesias M, Butrón P, Torre-Villalvazo I, Torre-Anaya EA, Sierra-Madero J, Rodriguez-Andoney JJ, et al. Mesenchymal Stem Cells for the Compassionate Treatment of Severe Acute Respiratory Distress Syndrome Due to COVID 19. Aging Dis (2021) 12(2):360–70. doi: 10.14336/ad.2020.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hashemian SR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE, et al. Mesenchymal Stem Cells Derived From Perinatal Tissues for Treatment of Critically Ill COVID-19-Induced ARDS Patients: A Case Series. Stem Cell Res Ther (2021) 12(1):91. doi: 10.1186/s13287-021-02165-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, et al. Treatment of Severe COVID-19 With Human Umbilical Cord Mesenchymal Stem Cells. Stem Cell Res Ther (2020) 11(1):361. doi: 10.1186/s13287-020-01875-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, et al. Evaluation of the Safety and Efficacy of Using Human Menstrual Blood-Derived Mesenchymal Stromal Cells in Treating Severe and Critically Ill COVID-19 Patients: An Exploratory Clinical Trial. Clin Trans Med (2021) 11(2):e297. doi: 10.1002/ctm2.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, et al. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy in Patients With COVID-19: A Phase 1 Clinical Trial. Signal Transduct Target Ther (2020) 5(1):172. doi: 10.1038/s41392-020-00286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Feng Y, Huang J, Wu J, Xu Y, Chen B, Jiang L, et al. Safety and Feasibility of Umbilical Cord Mesenchymal Stem Cells in Patients With COVID-19 Pneumonia: A Pilot Study. Cell Prolif (2020) 53(12):e12947. doi: 10.1111/cpr.12947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients With COVID-19 Pneumonia. Aging Dis (2020) 11(2):216–28. doi: 10.14336/ad.2020.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes Derived From Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev (2020) 29(12):747–54. doi: 10.1089/scd.2020.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, et al. Umbilical Cord Mesenchymal Stromal Cells as Critical COVID-19 Adjuvant Therapy: A Randomized Controlled Trial. Stem Cells Transl Med (2021) 10(9):1279–87. doi: 10.1002/sctm.21-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, et al. Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells on Lung Damage in Severe COVID-19 Patients: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. Signal Transduct Target Ther (2021) 6(1):58. doi: 10.1038/s41392-021-00488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-Blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl Med (2021) 10(5):660–73. doi: 10.1002/sctm.20-0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. George MJ, Prabhakara K, Toledano-Furman NE, Gill BS, Wade CE, Cotton BA, et al. Procoagulant In Vitro Effects of Clinical Cellular Therapeutics in a Severely Injured Trauma Population. Stem Cells Transl Med (2020) 9(4):491–8. doi: 10.1002/sctm.19-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-Induced Acute Lung Injury in Mice by Mesenchymal Stem Cells Overexpressing Angiopoietin 1. PLoS Med (2007) 4(9):e269. doi: 10.1371/journal.pmed.0040269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Florian M, Wang JP, Deng Y, Souza-Moreira L, Stewart DJ, Mei SHJ. Gene Engineered Mesenchymal Stem Cells: Greater Transgene Expression and Efficacy With Minicircle vs. Plasmid DNA Vectors in a Mouse Model of Acute Lung Injury. Stem Cell Res Ther (2021) 12(1):184. doi: 10.1186/s13287-021-02245-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang C, Lv D, Zhang X, Ni ZA, Sun X, Zhu C. Interleukin-10-Overexpressing Mesenchymal Stromal Cells Induce a Series of Regulatory Effects in the Inflammatory System and Promote the Survival of Endotoxin-Induced Acute Lung Injury in Mice Model. DNA Cell Biol (2018) 37(1):53–61. doi: 10.1089/dna.2017.3735 [DOI] [PubMed] [Google Scholar]
- 137. Han J, Lu X, Zou L, Xu X, Qiu H. E-Prostanoid 2 Receptor Overexpression Promotes Mesenchymal Stem Cell Attenuated Lung Injury. Hum Gene Ther (2016) 27(8):621–30. doi: 10.1089/hum.2016.003 [DOI] [PubMed] [Google Scholar]
- 138. Chen J, Zhang X, Xie J, Xue M, Liu L, Yang Y, et al. Overexpression of TGFbeta1 in Murine Mesenchymal Stem Cells Improves Lung Inflammation by Impacting the Th17/Treg Balance in LPS-Induced ARDS Mice. Stem Cell Res Ther (2020) 11(1):311. doi: 10.1186/s13287-020-01826-0 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139. Shao Y, Zhou F, He D, Zhang L, Shen J. Overexpression of CXCR7 Promotes Mesenchymal Stem Cells to Repair Phosgene-Induced Acute Lung Injury in Rats. BioMed Pharmacother (2019) 109:1233–9. doi: 10.1016/j.biopha.2018.10.108 [DOI] [PubMed] [Google Scholar]
- 140. Li Z, Song Y, Yuan P, Guo W, Hu X, Xing W, et al. Antibacterial Fusion Protein BPI21/LL-37 Modification Enhances the Therapeutic Efficacy of hUC-MSCs in Sepsis. Mol Ther (2020) 28(8):1806–17. doi: 10.1016/j.ymthe.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chen X, Wu S, Tang L, Ma L, Wang F, Feng H, et al. Mesenchymal Stem Cells Overexpressing Heme Oxygenase-1 Ameliorate Lipopolysaccharide-Induced Acute Lung Injury in Rats. J Cell Physiol (2019) 234(5):7301–19. doi: 10.1002/jcp.27488 [DOI] [PubMed] [Google Scholar]
- 142. He HL, Liu L, Chen QH, Cai SX, Han JB, Hu SL, et al. MSCs Modified With ACE2 Restore Endothelial Function Following LPS Challenge by Inhibiting the Activation of RAS. J Cell Physiol (2015) 230(3):691–701. doi: 10.1002/jcp.24794 [DOI] [PubMed] [Google Scholar]
- 143. Yang JX, Zhang N, Wang HW, Gao P, Yang QP, Wen QP. CXCR4 Receptor Overexpression in Mesenchymal Stem Cells Facilitates Treatment of Acute Lung Injury in Rats. J Biol Chem (2015) 290(4):1994–2006. doi: 10.1074/jbc.M114.605063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang X, Chen J, Xue M, Tang Y, Xu J, Liu L, et al. Overexpressing P130/E2F4 in Mesenchymal Stem Cells Facilitates the Repair of Injured Alveolar Epithelial Cells in LPS-Induced ARDS Mice. Stem Cell Res Ther (2019) 10(1):74. doi: 10.1186/s13287-019-1169-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145. Xu XP, Huang LL, Hu SL, Han JB, He HL, Xu JY, et al. Genetic Modification of Mesenchymal Stem Cells Overexpressing Angiotensin II Type 2 Receptor Increases Cell Migration to Injured Lung in LPS-Induced Acute Lung Injury Mice. Stem Cells Transl Med (2018) 7(10):721–30. doi: 10.1002/sctm.17-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, et al. Miro1 Regulates Intercellular Mitochondrial Transport & Enhances Mesenchymal Stem Cell Rescue Efficacy. EMBO J (2014) 33(9):994–1010. doi: 10.1002/embj.201386030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wang H, Yang YF, Zhao L, Xiao FJ, Zhang QW, Wen ML, et al. Hepatocyte Growth Factor Gene-Modified Mesenchymal Stem Cells Reduce Radiation-Induced Lung Injury. Hum Gene Ther (2013) 24(3):343–53. doi: 10.1089/hum.2012.177 [DOI] [PubMed] [Google Scholar]
- 148. Saito S, Nakayama T, Hashimoto N, Miyata Y, Egashira K, Nakao N, et al. Mesenchymal Stem Cells Stably Transduced With a Dominant-Negative Inhibitor of CCL2 Greatly Attenuate Bleomycin-Induced Lung Damage. Am J Pathol (2011) 179(3):1088–94. doi: 10.1016/j.ajpath.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Huleihel L, Sellares J, Cardenes N, Álvarez D, Faner R, Sakamoto K, et al. Modified Mesenchymal Stem Cells Using miRNA Transduction Alter Lung Injury in a Bleomycin Model. Am J Physiol Lung Cell Mol Physiol (2017) 313(1):L92–l103. doi: 10.1152/ajplung.00323.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wei X, Yi X, Lv H, Sui X, Lu P, Li L, et al. MicroRNA-377-3p Released by Mesenchymal Stem Cell Exosomes Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Targeting RPTOR to Induce Autophagy. Cell Death Dis (2020) 11(8):657. doi: 10.1038/s41419-020-02857-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Li QC, Liang Y, Su ZB. Prophylactic Treatment With MSC-Derived Exosomes Attenuates Traumatic Acute Lung Injury in Rats. Am J Physiol Lung Cell Mol Physiol (2019) 316(6):L1107–L17. doi: 10.1152/ajplung.00391.2018 [DOI] [PubMed] [Google Scholar]
- 152. Millar JE, von Bahr V, Malfertheiner MV, Ki KK, Redd MA, Bartnikowski N, et al. Administration of Mesenchymal Stem Cells During ECMO Results in a Rapid Decline in Oxygenator Performance. Thorax (2018) 74(2):194–6. doi: 10.1136/thoraxjnl-2017-211439 [DOI] [PubMed] [Google Scholar]
- 153. Millar JE, Bartnikowski N, Passmore MR, Obonyo NG, Malfertheiner MV, von Bahr V, et al. Combined Mesenchymal Stromal Cell Therapy and Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome. A Randomized Controlled Trial in Sheep. Am J Respir Crit Care Med (2020) 202(3):383–92. doi: 10.1164/rccm.201911-2143OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kim H, Darwish I, Monroy MF, Prockop DJ, Liles WC, Kain KC. Mesenchymal Stromal (Stem) Cells Suppress Pro-Inflammatory Cytokine Production But Fail to Improve Survival in Experimental Staphylococcal Toxic Shock Syndrome. BMC Immunol (2014) 15:1. doi: 10.1186/1471-2172-15-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sepulveda JC, Tome M, Fernandez ME, Delgado M, Campisi J, Bernad A, et al. Cell Senescence Abrogates the Therapeutic Potential of Human Mesenchymal Stem Cells in the Lethal Endotoxemia Model. Stem Cells (2014) 32(7):1865–77. doi: 10.1002/stem.1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hackel A, Aksamit A, Bruderek K, Lang S, Brandau S. TNF-α and IL-1β Sensitize Human MSC for IFN-γ Signaling and Enhance Neutrophil Recruitment. Eur J Immunol (2021) 51(2):319–30. doi: 10.1002/eji.201948336 [DOI] [PubMed] [Google Scholar]
- 157. Wang LT, Wang HH, Chiang HC, Huang LY, Chiu SK, Siu LK, et al. Human Placental MSC-Secreted IL-1β Enhances Neutrophil Bactericidal Functions During Hypervirulent Klebsiella Infection. Cell Rep (2020) 32(13):108188. doi: 10.1016/j.celrep.2020.108188 [DOI] [PubMed] [Google Scholar]
- 158. Vanderbeke L, Van Mol P, Van Herck Y, De Smet F, Humblet-Baron S, Martinod K, et al. Monocyte-Driven Atypical Cytokine Storm and Aberrant Neutrophil Activation as Key Mediators of COVID-19 Disease Severity. Nat Commun (2021) 12(1):4117. doi: 10.1038/s41467-021-24360-w [DOI] [PMC free article] [PubMed] [Google Scholar]