Abstract
Fungi are eukaryotes that inhabit various ecosystems worldwide and have a decomposing effect that other organisms cannot replace. Fungi are divided into two main groups depending on how their sexual spores are formed, viz. Ascomycota and Basidiomycota. The members of Botryosphaeriales (Dothideomycetes, Ascomycota) are ubiquitous. They are pathogenic on a wide range of hosts, causing diverse diseases including dieback, canker, leaf spots and root rots and are also reported as saprobes and endophytes worldwide. As an important fungal group, of which most are plant pathogens, it is necessary to organize data and information on Botryosphaeriales so that scientific literature can be used effectively. For this purpose, a new website, https://botryosphaeriales.org is established to gather all published data together with updates on the present taxonomy of Botryosphaeriales. The website consists of an easy-to-operate searching system and provides an up-to-date classification together with accounts of Botryosphaeriales taxa, including colour illustrations, descriptions, notes and numbers of species in each genus, as well as their classification. Thus, readers will be able to obtain information on botryosphaerialean taxa through this platform.
Database URL: https://botryosphaeriales.org/
Introduction
Botryosphaeriales (Dothideomycetes) was established in 2006, to accommodate a single family, Botryosphaeriaceae (1). Nine families—Aplosporellaceae, Botryosphaeriaceae, Endomelanconiopsisaceae, Melanopsaceae, Phyllostictaceae, Planistromellaceae, Pseudofusicoccumaceae, Saccharataceae and Septorioideaceae—were accepted in Botryosphaeriales by Wijayawardene et al. (2). However, based on morpho-molecular analyses and evolutionary divergence times, the number of families accepted in Botryosphaeriales was reduced to six, namely Aplosporellaceae, Botryosphaeriaceae, Melanopsaceae, Phyllostictaceae, Planistromellaceae and Saccharataceae (3, 4). Endomelanconiopsisaceae and Pseudofusicoccumaceae were synonymized under Botryosphaeriaceae and Phyllostictaceae, respectively, while Septorioideaceae was synonymized under Saccharataceae (3). Members of Botryosphaeriales are found worldwide, on many different host plants (5–17). They are endophytes, pathogens and saprobes and as opportunistic pathogens, they are of considerable importance to agriculture, horticulture and forestry (18, 19). They cause severe diseases of economically important crops and plants leading to huge economic losses (20, 21). Botryosphaeria, Diplodia, Dothiorella, Lasiodiplodia, Neofusicoccum and Phyllosticta are the major pathogenic genera in Botryosphaeriales. Botryosphaeria dothidea, Diplodia seriata, Lasiodiplodia theobromae and Neofusicoccum parvum are associated with grapevine dieback worldwide (27). Diplodia seriata has been reported as a pathogen on a wide range of hosts including eucalyptus, pine and stone fruits (22–26). Phyllosticta citricarpa and P. citriasiana cause freckle on banana and brown spots on pomelo, respectively (6, 9). One species can occur on several hosts in the same country, and pathogenicity of a species can vary from one region to another (19). This reflects the importance of gathering data on these fungal taxa to understand disease epidemiology.
Aplosporellaceae, Melanopsaceae and Planistromellaceae have immersed or semi-immersed multiloculate ascostromata, while those of Botryosphaeriaceae, Phyllostictaceae and Saccharataceae are uniloculate (3, 8, 28, 29). Asci are bitunicate, with a thick endotunica (3, 8, 28, 29). Ascospores are hyaline or pigmented, septate or aseptate, ellipsoid to ovoid. Conidiomata are pycnidial, uni- to multilocular, frequently embedded in stromatic tissue. Conidiogenous cells are hyaline. Conidia are hyaline or pigmented, septate or aseptate, thin- or thick-walled (3, 8, 28–32).
All newly published data, which usually provide detailed descriptions and illustrations of new records, new species, new genera or new families, will be used to update the database. None of the papers generally link data from all members of the order Botryosphaeriales. Up until now, some websites involving specific groups of fungi have been established, such as http://www.facesoffungi.org (33), https://onestopshopfungi.org (34), http://www.marinespecies.org (35), https://fungalgenera.org (36), https://www.dothideomycetes.org (37) and https://sordariomycetes.org (38). However, there is no database on Botryosphaeriales. Therefore, in this paper, we provide a search method, designed to gather all the information about Botryosphaeriales into one website.
The need for a Botryosphaeriales database
Many Botryosphaeriales species are associated with diseases in branches, leaves, fruits and seeds of aquatic and terrestrial plants (20, 31, 32, 39–43). In recent years, in-depth studies on Botryosphaeriales have resulted in changes in the genus/family-level classification (2–4). Moreover, the number of publications and research works related to Botryosphaeriales species are increasing all over the world (18, 20, 21, 44–48). With the increased number of studies on morphology, ecology and especially DNA-based phylogenetics, more and more new species are constantly being discovered. However, there are still many aspects needing clarification, such as naming new species from environmental samples, resolving the opportunistic pathogenic nature and how to define species boundaries in Botryosphaeriales.
Currently over 2300 species have been described in Botryosphaeriales in MycoBank based on morpho-molecular evidence (4). Most of the members of this order have been scientifically documented. These publications on Botryosphaeriales comprise various aspects such as taxonomy, morphology, pathogenicity or evolutionary studies. Information on Botryosphaeriales taxa are scattered in over 1000 publications mainly as books and research papers. Hence, the intention of this website is to gather all data regarding taxa accepted in Botryosphaeriales into a single entity that can be updated as new information becomes available.
The present website, https://botryosphaeriales.org/, focuses on the Botryosphaeriales with the following objectives: (i) gather all scattered data of accepted Botryosphaeriales taxa into a single platform, (ii) provide notes on the recent changes in genera and species of Botryosphaeriales with updated taxonomy and phylogeny and (iii) provide a list of all literature related to Botryosphaeriales. Hence, this website will be the best platform to access information on the botryosphaerialean taxa easily with simple searches, thus reducing the time spent on searching for information.
The Botryosphaeriales website
Botryosphaeriales.org is a website dedicated to Botryosphaeriales taxa by providing an up-to-date account with descriptions, colour illustrations, culture characteristics, associated hosts, distribution and notes for order, families, genera and species. The website, https://botryosphaeriales.org will be updated periodically, keeping abreast of current literature. It is convenient for all mycologists and pathologists who need information about the history and current classification status of botryosphaerialean taxa. The home page of the website has a mailbox so that mycologists, pathologists or anyone else who use this website can suggest ideas to improve it.
Construction
All fungi in the Botryosphaeriales.org are listed according to the latest classification (3, 4, 49). Each entry includes the accepted binominal name, Index Fungorum number, Faces of Fungi number, MycoBank number, ex-type culture collection number, dry culture collection and herbarium number and GenBank accession numbers of available DNA sequences. This website is handled and inspected regularly by the head curator, managing curator, senior curator and three other curators with expertise on botryosphaerialean taxonomy and phylogeny (Table 1).
Table 1.
List of curators for Botryosphaeriales webpage
| Position | Name | Affiliation | Contact details |
|---|---|---|---|
| Head Curator | Alan J.L. Phillips | Microbiology and Biotechnology Laboratory Biosystems and Integrative Sciences Institute Faculty of Science, University of Lisbon Campo Grande, 1749-016 Lisbon, Portugal | alan.jl.phillips@gmail.com |
| Managing Curator | Na Wu | Center of Excellence in Fungal Research, School of Science, Mae Fah Luang University, 333 Moo 1 Muang District, Chiang Rai 57100, Thailand | wuna220@gmail.com |
| Senior Curator | Asha J. Dissanayake | School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China | asha.janadaree@yahoo.com |
| Curators | Kevin D. Hyde | Center of Excellence in Fungal Research, School of Science, Mae Fah Luang University, 333 Moo 1 Muang District, Chiang Rai 57100, Thailand | kdhyde3@gmail.com |
| Ishara S. Manawasinghe | Innovative Institute for Plant Health, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, P.R. China | ishara.ishara@yahoo.com | |
| Achala R. Rathnayaka | Center of Excellence in Fungal Research, School of Science, Mae Fah Luang University, 333 Moo 1 Muang District, Chiang Rai 57100, Thailand | rathnayakaachala@gmail.com |
Database interface and visualization
The website can be accessed at URL https://botryosphaeriales.org and comprises eight main headings, i.e. Home, Taxa, Archives, Curators, History, References, Notes and Contact. The interface is user-friendly, and each section comprises the following information.
Home
The homepage briefly introduces the objectives of the website and has links to highlights of information. Colour photos of Botryosphaeriales are displayed representing the key morphological features of this order (Figure 1). The relevant information can be accessed easily using the search box located at the right top corner (Figure 2). Beneath the search box, there is quick access to recent notes, recent genera and recent species. Clicking on these options opens the link in a new window (Figure 3).
Figure 1.
The homepage of Botryosphaeriales webpage.
Figure 2.
Red arrow indicates search tool to enter the taxon name.
Figure 3.
The recent notes, genus and species to enable easy access.
Taxa
Under the ‘Taxa’ heading, the recent taxonomic classification as families, genera and species of Botryosphaeriales is provided (Figure 4). In addition, an updated phylogenetic tree for the order is given at the bottom. The search box at the top left corner will facilitate quick access to each entry on the website. Clicking a listed species name will direct to the full description and details in the archives section (Figure 5).
Figure 4.
The ‘Taxa’ view of the Botryosphaeriales webpage.
Figure 5.
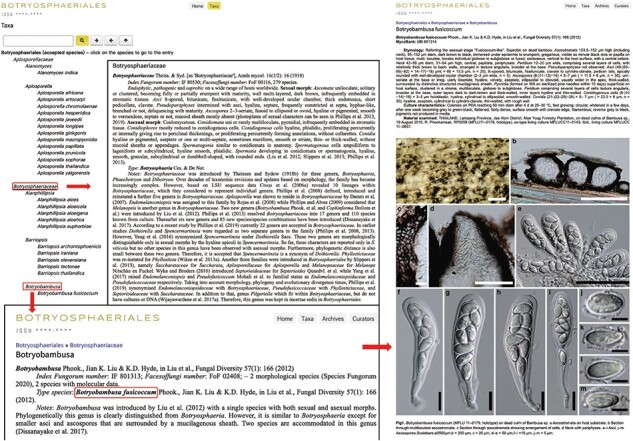
Details of the genus Botryobambusa.
Archives
The ‘Archives’ is the main section containing all information on every entry in the website (Figure 6). It consists of six tabs representing each family in Botryosphaeriales. Clicking on a particular family will automatically generate a dropdown list including all the genera belonging to the particular family (Figure 7). This can be further expanded into genus and species levels (Figure 7). By clicking the link for each species will open a new window containing all information on that species (Figure 8).
Figure 6.
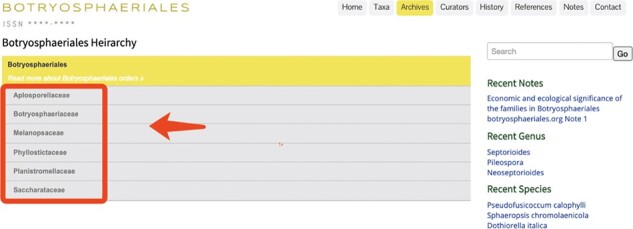
Appearance of the ‘Archives’ section in Botryosphaeriales webpage.
Figure 7.
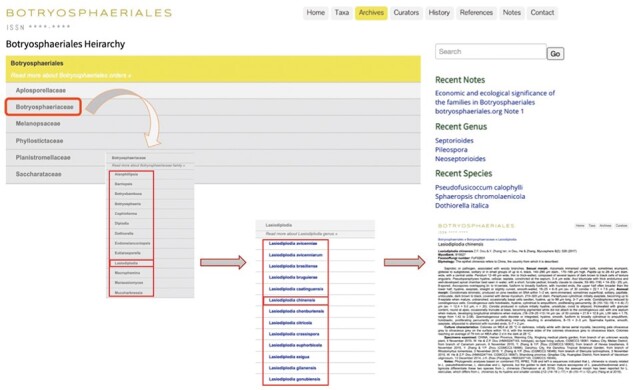
User-friendly interface of the ‘Archives’ section in Botryosphaeriales webpage.
Figure 8.
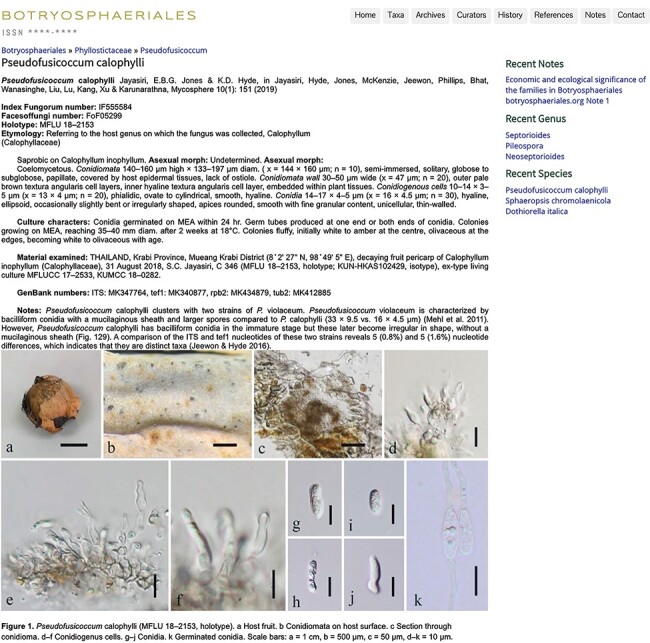
An entry with description, notes and plate (15).
Curators
This section provides information about the curators who handle and update the website (Table 1; Figure 9).
Figure 9.
Curators of the Botryosphaeriales webpage.
History
This section provides a brief account of the classification of Botryosphaeriales.
References
A list of the citations used in the entries and history as well as other related information are provided under this heading.
Notes
Economic and ecological significance of families in Botryosphaeriales are given in this section. In addition, trends and current applications of the species of Botryosphaeriales will be added regularly to this section.
Contact
This section provides the contact details of the website and allows users to communicate any comments and suggestions.
Funding
This work was supported by the National Natural Science Foundation of China “Characterization of roots and their associated rhizosphere microbes in agroforestry systems: ecological restoration in high-phosphorus environment” (Grant No: 31861143002).
Contributor Information
N a Wu, CAS, Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, P.R. China; Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand; School of Life Science and Technology, Center for Informational Biology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China.
Asha J Dissanayake, School of Life Science and Technology, Center for Informational Biology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China.
Ishara S Manawasinghe, Innovative Institute for Plant Health, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, P.R. China.
Achala R Rathnayaka, Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand.
Jian-Kui Liu, School of Life Science and Technology, Center for Informational Biology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China.
Alan j.l Phillips, Biosystems and Integrative Sciences Institute (BioISI), Universidade de Lisboa, Lisbon, 1749-016, Portugal.
Itthayakorn Promputtha, Department of Biology, Chiang Mai University, Chiang Mai 50200, Thailand.
Kevin D Hyde, CAS, Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, P.R. China; Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand; Innovative Institute for Plant Health, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, P.R. China; Department of Biology, Chiang Mai University, Chiang Mai 50200, Thailand.
Acknowledgements
Kevin D. Hyde thanks Chiang Mai University for the award of Visiting Professor. Alan J.L. Phillips acknowledges the support from UIDB/04046/2020 and UIDP/04046/2020 Centre grants from FCT, Portugal (to BioISI). Na Wu acknowledges the Mae Fah Luang University for financial support and the Mushroom Research Foundation for a grant for living costs.
Conflict of interest
None declared.
References
- 1. Schoch C.L., Shoemaker R.A., Seifert K.A.. et al. (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia, 98, 1041–1052. [DOI] [PubMed] [Google Scholar]
- 2. Wijayawardene N.N., Hyde K.D., Lumbsch H.T.. et al. (2018) Outline of ascomycota: 2017. Fungal Divers., 88, 167–263. [Google Scholar]
- 3. Phillips A.J.L., Hyde K.D., Alves A.. et al. (2019) Families in Botryosphaeriales: a phylogenetic, morphological and evolutionary perspective. Fungal Divers., 94, 1–22. [Google Scholar]
- 4. Wijayawardene N.N., Hyde K.D., Al-Ani L.K.T.. et al. (2020) Outline of Fungi and fungus-like taxa. Mycosphere, 11, 1060–1456. [Google Scholar]
- 5. Phillips A.J.L., Alves A., Pennycook S.R.. et al. (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia, 21, 29–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wulandari N., To-Anun C., Hyde K.D.. et al. (2009) Phyllosticta citriasiana sp. nov., the cause of Citrus tan spot of Citrus maxima in Asia. Fungal Divers., 34, 23–39. [Google Scholar]
- 7. Liu J.K., Chomnunti P., Cai L.. et al. (2010) Phylogeny and morphology of Neodeightonia palmicola sp. nov. from palms. Sydowia, 62, 261–276. [Google Scholar]
- 8. Liu J.K., Phookamsak R., Doilom M.. et al. (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers., 57, 149–210. [Google Scholar]
- 9. Wong M.H., Crous P.W., Henderson J.. et al. (2012) Phyllosticta species associated with freckle disease of banana. Fungal Divers., 56, 173–187. [Google Scholar]
- 10. Alves A., Linaldeddu B.T., Deidda A.. et al. (2014) The complex of Diplodia species associated with Fraxinus and some other woody hosts in Italy and Portugal. Fungal Divers., 67, 143–156. [Google Scholar]
- 11. Trakunyingcharoen T., Cheewangkoon R., To-anun C.. et al. (2014) Botryosphaeriaceae associated with diseases of mango (Mangifera indica). Australas. Plant Pathol., 43, 425–438. [Google Scholar]
- 12. Dissanayake A.J., Phillips A.J.L., Li X.H.. et al. (2016) Botryosphaeriaceae: current status of genera and species. Mycosphere, 7, 1001–1073. [Google Scholar]
- 13. Dou Z.P., Lu M., Wu J.R.. et al. (2017) A new species and interesting records of Aplosporella from China. Sydowia, 69, 1–7. [Google Scholar]
- 14. Tibpromma S., Hyde K.D., McKenzie E.H.C.. et al. (2018) Fungal diversity notes 840–928: micro-fungi associated with Pandanaceae. Fungal Divers., 93, 1–160. [Google Scholar]
- 15. Jayasiri S.C., Hyde K.D., Jones E.B.G.. et al. (2019) Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere, 10, 1–186. [Google Scholar]
- 16. Wang Y., Lin S., Zhao L.. et al. (2019) Lasiodiplodia spp. associated with Aquilaria crassna in Laos. Mycol. Prog., 18, 683–701. [Google Scholar]
- 17. Berraf-Tebbal A., Mahamedi A.E., Aigoun-Mouhous W.. et al. (2020) Lasiodiplodia mitidjana sp. nov. and other Botryosphaeriaceae species causing branch canker and dieback of Citrus sinensis in Algeria. PLoS One, 15, e0232448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chethana K.W.T., Li X., Zhang W.. et al. (2016) Trail of decryption of molecular research on Botryosphaeriaceae in woody plants. Phytopathol. Mediterr., 55, 147–171. [Google Scholar]
- 19. Manawasinghe I.S., Phillips A.J.L., Hyde K.D.. et al. (2016) Mycosphere essays 14: assessing the aggressiveness of plant pathogenic Botryosphaeriaceae. Mycosphere, 7, 883–892. [Google Scholar]
- 20. Slippers B. and Wingfield M.J. (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol. Rev., 21, 90–106. [Google Scholar]
- 21. Mehl J.W., Slippers B., Roux J.. et al. (2017) Overlap of latent pathogens in the Botryosphaeriaceae on a native and agricultural host. Fungal Biol., 121, 405–419. [DOI] [PubMed] [Google Scholar]
- 22. Kaliterna J., Milicevic T., Ivic D.. et al. (2012) First report of Diplodia seriata as causal agent of olive dieback in Croatia. Plant Dis., 96, 290. [DOI] [PubMed] [Google Scholar]
- 23. Cáceres M., Lolas M., Gutierrez M.. et al. (2016) Severe outbreak of black rot in apple fruit cv. Fuji caused by Diplodia seriata during pre-harvest in Maule region, Chile. Plant Dis., 100, 2333. [Google Scholar]
- 24. Zhang M., Zhang Y.K., Geng Y.H.. et al. (2017) First report of Diplodia seriata causing twig dieback of English walnut in China. Plant Dis., 101, 1036. [Google Scholar]
- 25. Abbas M.F. and Naz F. (2018) First report of Diplodia seriata causing fruit rot of loquat in Pakistan. J. Plant Pathol., 100, 325. [Google Scholar]
- 26. Besoain X., Guajardo J., Larach A.. et al. (2019) First report of Diplodia seriata causing gummy canker in Araucaria araucana wild populations in South-Central Chile. Plant Dis., 103, 2684. [Google Scholar]
- 27. Yan J.Y., Xie Y., Zhang W.. et al. (2013) Species of Botryosphaeriaceae involved in grapevine dieback in China. Fungal Divers., 61, 221–236. [Google Scholar]
- 28. Monkai J., Liu J.K., Boonmee S.. et al. (2013) Planistromellaceae (Botryosphaeriales). Cryptogam. Mycol., 34, 45–77. [Google Scholar]
- 29. Ekanayaka A.H., Dissanayake A.J., Jayasiri S.C.. et al. (2016) Aplosporella thailandica; a novel species revealing the sexual-asexual connection in Aplosporellaceae (Botryosphaeriales). Mycosphere, 7, 440–447. [Google Scholar]
- 30. Wikee S., Udayanga D., Crous P.W.. et al. (2011) Phyllosticta—an overview of current status of species recognition. Fungal Divers., 51, 43–61. [Google Scholar]
- 31. Phillips A.J.L., Alves A., Abdollahzadeh J.. et al. (2013) The Botryosphaeriaceae: genera and species known from culture. Stud. Mycol., 76, 51–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hyde K.D., de Silva N., Jeewon R.. et al. (2020) AJOM new records and collections of fungi: 1–100. Asian J. Mycol., 3, 22–294. [Google Scholar]
- 33. Jayasiri S.C., Hyde K.D., Ariyawansa H.A.. et al. (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers., 74, 3–18. [Google Scholar]
- 34. Jayawardena R.S., McKenzie E.H.C., Chen Y.J.. et al. (2019) https://onestopshopfungi.org, a database to enhance identification of phytopathogenic genera. Asian J. Mycol., 2, 281–286. [Google Scholar]
- 35. Jones E.G.Pang K.L., Abdel-Wahab M.A.. et al. (2019) An online resource for marine fungi. Fungal Divers., 96, 347–433. [Google Scholar]
- 36. Monkai J., McKenzie E.H.C., Phillips A.J.L.. et al. (2019) https://fungalgenera.org/: a comprehensive database providing webbased information for all fungal genera. Asian J. Mycol., 2, 298–305. [Google Scholar]
- 37. Pem D., Hongsanan S., Doilom M.. et al. (2019) https://www.dothideomycetes.org: an online taxonomic resource for the classification, identification, and nomenclature of Dothideomycetes. Asian J. Mycol., 2, 287–297. [Google Scholar]
- 38. Bundhun D., Maharachchikumbura S.S.N., Jeewon R.. et al. (2020) https://sordariomycetes.org/, a platform for the identification, ranking and classification of taxa within Sordariomycetes. Asian J. Mycol., 3, 13–21. [Google Scholar]
- 39. McDonald V. and Eskalen A. (2011) Botryosphaeriaceae species associated with avocado branch cankers in California. Plant Dis., 95, 1465–1473. [DOI] [PubMed] [Google Scholar]
- 40. Ismail A.M., Cirvilleri G., Polizzi G.. et al. (2012) Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas. Plant Pathol., 41, 649–660. [Google Scholar]
- 41. Marques M.W., Lima N.B., de Morais M.A.. et al. (2013) Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers., 61, 181–193. [Google Scholar]
- 42. Trakunyingcharoen T., Cheewangkoon R. and To-anun C. (2013) Phylogeny and pathogenicity of fungal species in the family Botryosphaeriaceae associated with mango (Mangifera indica) in Thailand. J. Agric. Technol., 9, 1535–1543. [Google Scholar]
- 43. Zhao L., Wang Y., He W.. et al. (2019) Stem blight of blueberry caused by Lasiodiplodia vaccinii sp. nov. in China. Plant Dis., 103, 2041–2050. [DOI] [PubMed] [Google Scholar]
- 44. Desprez-Loustau M.L., Marcais B., Nageleisen L.M.. et al. (2006) Interactive effects of drought and pathogens in forest trees. Ann. For. Sci., 63, 597–612. [Google Scholar]
- 45. Sturrock R.N., Frankel S.J., Brown A.V.. et al. (2011) Climate change and forest diseases. Plant Pathol., 60, 133–149. [Google Scholar]
- 46. Sarr M.P., Ndiaye M.B., Groenewald J.Z.. et al. (2014) Genetic diversity in Macrophomina phaseolina, the causal agent of charcoal rot. Phytopathol. Mediterr., 53, 250–268. [Google Scholar]
- 47. Wyka S.A. and Broders K.D. (2016) The new family Septorioideaceae, within the Botryosphaeriales and Septorioides strobi as a new species associated with needle defoliation of Pinus strobus in the United States. Fungal Biol., 120, 1030–1040. [DOI] [PubMed] [Google Scholar]
- 48. Zlatkovic M., Keca N., Wingfield M.J.. et al. (2016) Botryosphaeriaceae associated with the die-back of ornamental trees in the western balkans. Antonie Van Leeuwenhoek, 109, 543–564. [DOI] [PubMed] [Google Scholar]
- 49. Li G., Slippers B., Wingfield M.J.. et al. (2020) Variation in Botryosphaeriaceae from Eucalyptus plantations in YunNan Province in southwestern China across a climatic gradient. IMA Fungus, 11, 1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


