Abstract
Ischemic stroke is a major cause of death and disability worldwide and is expected to increase in the future with the aging population. Currently, there are no clinically available treatments for damage sustained during an ischemic stroke, but much research is being conducted in this area. In this review, we will introduce current ischemic stroke treatments along with their limitations, as well as research on potential short and long-term future treatments. There are advantages and disadvantages in these potential treatments, but our understanding of these methods and their effectiveness in clinical trials are improving. We are confident that some future treatments introduced in this review will become commonly used in clinical settings in the future.
Keywords: Ischemic stroke, Stem cells, Exosomes, Regenerative medicine, Treatments
1. Introduction
Ischemic stroke is caused by clots in blood vessels supplying blood to the brain. The lack of oxygen leads to neuronal cell death during ischemia, while additional damage is caused by inflammatory reactions of innate immunity during reperfusion [1]. This inflammation in the brain persists for years after stroke, which hinders long-term recovery and can lead to later death [2]. Stroke, with over 13 million incidences per year, is one of the most common severe ailments worldwide today [3]. In particular, ischemic stroke was the most common, with over 9.5 million cases in 2016 [3]. Despite medical advances, there are still 5.5 million deaths annually, with over 50% of survivors being chronically disabled [3,4]. Stroke is the second most common cause of death and a major cause of disability globally [5]. The long-term side effects of ischemic stroke are often fatal. In a recent study in Japan (a country with an advanced healthcare system), the 5-year survival rate of non-fatal first-time ischemic stroke was only 63.5% [6].
The future aging population will contribute to an increased incidence of ischemic stroke, along with an increase in stroke risk factors associated with increased socioeconomic status in developing countries [4]. Across 169 causes of death from 1990 to 2017, stroke was the second most common cause of death associated with an aging population after ischemic heart disease [7]. Overall, the number of deaths from ischemic stroke per year increased from around 2.2 million in 1990 to 3.3 million in 2019 [8].
Given these statistics, it is imperative that advancements be made in ischemic stroke treatment, in terms of removal of the clot to limit both neuronal cell death and further damage caused by the immune system during reperfusion. Furthermore, treatments must also be devised to recover from the loss of neural function to improve chronic disability and long-term survival issues.
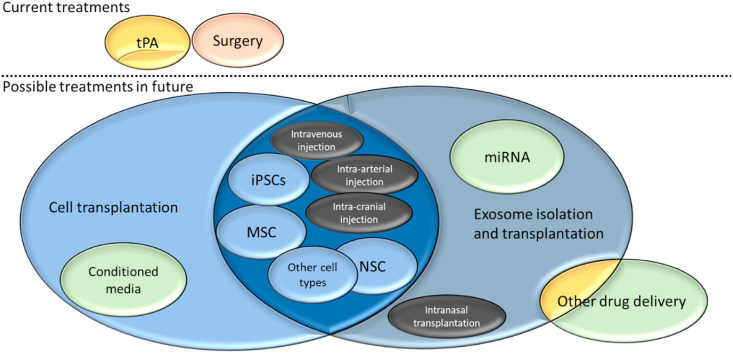
In this review, we will discuss the progress in ischemic stroke treatment, including the treatments currently available to the public, the research being conducted for potential future treatments in the short and long term, and their comparative efficacy (Fig. 1).
Fig. 1.
Outline of current and possible future treatments for stroke therapy.
2. Current treatments to stop ischemia
2.1. Thrombolysis
Currently, there are only two widely available treatments for ischemic stroke. The first is thrombolysis using tissue Plasminogen Activator (tPA), with alteplase being the most common form.
TPA replaced the earlier thrombolytic agent streptokinase and was shown to be effective in acute ischemic stroke in a 1995 study by the National Institute of Neurological Disorders and Stroke (NINDS) [9,10]. Later studies established the efficacy of tPA up to 4.5 h after the onset of ischemic stroke symptoms [9]. TPA is a 527 amino-acid chain containing three glycosylation sites and 17 disulfide bridges that can degrade fibrin in a plasminogen-dependent manner, thereby dissolving blood clots and inducing reperfusion following ischemic stroke [11]. Numerous studies have shown that tPA also has a neuroprotective effect; for example, tPA can attenuate zinc-induced cell death, protect neurons from oxygen-glucose deprivation (OGD), and protect cortical neurons by activating mTOR and JAK/STAT signaling pathways, which are linked to neuroprotection [11,12]. Further studies are needed to understand the effect of tPA on the brain, which will also improve the therapeutic window in which tPA should be administered for stroke recovery [12].
However, tPA also has drawbacks. First, tPA must be administered within 4.5 h of stroke onset, which is difficult in many situations. Second, it can result in severe side effects, the most common being hemorrhage, sometimes severe enough to be life-threatening [9]. The chances of hemorrhage increase with the delayed administration of tPA. Fortunately, the rate of symptomatic hemorrhage is less than 2% if alteplase is administered within 4.5 h [13]. Furthermore, a recent study showed no benefit for tPA administration in patients with a National Institute of Health Stroke Scale (NIHSS) score of <5 due to the risk of hemorrhage [9]. In addition to the neuroprotective effects listed above, tPA also has neurotoxic effects [11]. These include over-activation of N-methyl-d-aspartate receptors (NMDAR), promoting damage to Purkinje cells, and association with glutamate-promoted neural necrosis [11]. Additional studies are needed to determine whether tPA is ultimately more neurotrophic or neurotoxic.
These side effects have provided an impetus to find alternative thrombolytics, the most promising being Tenecteplase (TNK), which has a longer half-life, higher specificity, and lower risk of Hemorrhagic Transformation (HT) than tPA [9]. TNK may also have a longer window for administration, which is important because tPA needs to be administered within 4.5 h, resulting in only around 7% of ischemic stroke patients receiving tPA [9,13]. Alternatively, many “tPA helpers” are being researched to overcome the various side effects of tPA mentioned above, especially in cases of delayed tPA administration [14]. Many of these agents have shown a reduction in HT and infarct volume and induced stabilization of the blood–brain barrier (BBB) in animal stroke models; however, only some of these tPA helpers have entered clinical trials, and currently, none have been successful [14]. Moreover, endovascular thrombectomy after tPA administration has also entered common clinical practice, which will be elaborated on in the next section.
2.2. Mechanical Thrombectomy
The other widely available treatment is Mechanical Thrombectomy (MT), which was first accepted in 2015 after five clinical trials were published showing efficacy within 6–8 h of ischemic stroke onset. More recent studies have shown efficacy up to 24 h after onset [9,15]. In MT, instead of breaking down the clot through chemical means, it is physically removed using stent-retriever devices [16]. MT is especially recommended over thrombolysis in cases of large proximal vessel occlusions, especially in the carotid artery (in which thrombolysis is less than 10% effective) [16]. It not only provides a longer time window than thrombolysis after the onset of stroke, but also has fewer side effects if performed properly.
Initially, the main drawback of MT was that it could only be performed in more proximal occlusions. However, with the recent use of stent-retriever devices, they are able to reach more distal occlusions in smaller, higher branched arteries [9,17]. Another drawback was that MT was expected to be combined with thrombolysis in older generation devices, which was subject to major side effects that are worse than those associated with the use of either of the treatments alone [18]. However, recent clinical trials with newer generation devices have shown that MT alone is as effective for thrombolysis, and improves functional independence 90 days, 1 year, and 2 years post ischemic stroke compared to thrombolysis alone [19,20]. These results indicate that MT may completely replace thrombolysis in the future, unless thrombolytic agents with fewer side effects than tPA are discovered. Another advancement made with MT is their improved ability to achieve reperfusion; first-generation MT devices achieved reperfusion <50% of the time, compared with the almost 90% reperfusion achievement with newer devices [18,21]. This means that MT can achieve a nearly 90% reperfusion rate of tPA while having a wider efficacy window. With many new MTs currently being evaluated in clinical trials, this 90% reperfusion rate is expected to improve, surpassing the rate induced by thrombolysis with tPA [21].
However, a critical problem in both thrombolysis and Mechanical Thrombectomy is that they only stop ischemia and do not limit additional damage caused by inflammation during reperfusion. They also do not induce the regeneration of any lost neurons. Because of this, even with the use of modern MT devices, only around 50% of ischemic stroke patients achieve functional independence in due time [21]. In the following sections, we introduce various methods that researchers have attempted to solve these problems.
3. Future treatments: limiting reperfusion damage and recovering from ischemic stroke
3.1. Cell transplantation
The first method that researchers are using to recover from ischemic stroke is stem cell transplantation into ischemic areas. Stem cells possess two fundamental properties that make them potentially beneficial following ischemic stroke. First is their ability to replace cells lost during ischemia-reperfusion (the replacement mechanism), and second is their secretion of many proteins and cytokines, which can provide a favorable environment for recovery (the paracrine mechanism), which is taking the lead [22]. Many types of cells have been studied for this transplantation, such as bone marrow-derived mesenchymal stem cells (MSCs) as neural stem cells (NSCs) [23]. Additionally, dental pulp stem cells (DPSCs), hair follicle stem cells (HFSCs), and genetically modified stem cells, among other cell types, have recently gained popularity [[23], [24], [25]]. These have generally been successful in animal stroke models; however, clinical trials have received erratic results so far [23,26,27]. Generally, they have been able to achieve safe transplantation, but there is a lack of functional improvement in many trials [28]. The advantages and disadvantages of each cell type are summarized in Table 1. Below, we discuss each of these cell types in more detail, highlighting the cells that are the most promising.
Table 1.
Advantages and disadvantages of prospective Ischemic Stroke treatments.
| Treatment | Advantages | Disadvantages |
|---|---|---|
| MSCs | Abundant, No Ethical Problems, Can Differentiate into Mature Neurons, Beneficial Cytokines, Most Studied Stem Cell Type | Vary from different sources and show different efficacy in different patients, risk for infection |
| NSCs | Cell Replacement and Paracrine Potential, Positive Results in Clinical Trials | Ethical Problems, Limited Cell Sources, risk for infection |
| Other Stem Cells (DPSCs, HFSCs) | DPSCs: Superior to MSCs in Pre-clinical Models, Easily Obtainable, Increased Homing to Ischemic Areas HFSCs: Easily Obtainable, Can Cross BBB Without BBB Permeabilizer |
Difficult to keep quality control, not fully explored yet, risk for infection |
| Modified Stem Cells | Better localization and can be used to address all the limitations from other sources | Safety issue of materials Risk for infection |
| Extracellular Vesicles | Smaller in size, can easily cross BBB, easy to modify | Difficult to characterize and monitor inside the system, limited numbers |
| Conditioned Media | Paracrine Effects, fewer side effects compared to cell transplantation | Risk for contamination of unknown components |
3.1.1. Mesenchymal stem cells
First, we discuss MSCs, with the most common type being derived from the bone marrow (BM). Some benefits of MSCs include their relative abundance (being present in everyone's BM), avoiding ethical problems (not having to be obtained from fetuses like NSCs and ESCs), and the lack of tumorigenicity when compared with induced pluripotent stem cells (iPSCs) [29,30]. A previous problem was the high cost due to the need to use early passage MSCs; however, researchers have shown that late-passage MSCs can be induced into glia-like cells (ghMSCs) [31]. These ghMSCs were effective in protecting neurons and restoring the brain, similar to early passage MSCs [31]. These results make clinical trials using MSCs much easier and cheaper [31]. Due to these and other factors, BM-MSCs are currently the leading transplantable cell type for treating ischemic stroke [29].
Even though they are widely studied, the mechanisms underlying the therapeutic effect of MSCs are still not well understood [32]. One aspect of ischemia-reperfusion injury is mitochondrial dysfunction, and research has demonstrated that MSCs can transfer their mitochondria into injured endothelial cells, which promote angiogenesis, reduce infarct volume, and improve functional recovery [32]. Carpenter et al. and Wang et al. showed that transplanted MSCs can reduce the expression of tumor necrosis factors, leading to anti-inflammatory and anti-apoptotic effects [30,33]. Another positive effect is possible endogenous neurogenesis through the secretion of many cytokines (such as BDNF, VEGF, and nerve growth factor), which provide a favorable environment [30]. Furthermore, MSCs have the potential to differentiate into endothelial cells [34,35], glial cells [36,37], and neurons [[38], [39], [40]], indicating that they may be able to replace lost neurons directly in the future.
As stated earlier, one problem with stem cell transplantation is its inconsistent results in clinical trials. For example, a phase II trial using BM-derived ALD-401 stem cells (which are composed of various types of stem cells taken from the BM, including MSCs) via internal carotid artery (ICA) injection showed no statistically significant modified Rankin Scale (mRS) improvements compared to the control, so the trial was stopped early [27]. In the only completed phase III clinical trial (STARTING-2), autologous intravenous (IV) transplantation of MSCs also did not significantly improve mRS scores after 90 days, but there was leg motor improvement [41]. Inversely, a phase I/II trial by Levy et al. using IV transplantation of allogenic MSCs was successful, showing significant behavioral improvement in their Barthel Index (BI) scores, which measures a patient's ability to function in daily life [42]. Fortunately, all these clinical trials were safe in patients, showing significant improvement from older clinical trials that had severe adverse effects [43]. The most likely reason for the inconsistent results is the small size of these trials, which leads to a risk of bias [43]. Two phase III clinical trials are currently being conducted (MASTERS-2 and TREASURE), so a better understanding of MSC efficacy may be reached soon [44].
3.1.2. Neural stem cells
The next stem cell type is Neural Stem Cells (NSCs). NSCs have not been studied as extensively as MSCs, mostly because of their rarity and ethical problems as they typically need to be obtained from fetuses [45]. Despite these problems, NSCs have potential for ischemic stroke treatment. First, NSCs can differentiate into neurons, astrocytes, and oligodendrocytes [45]. Preclinical research has shown that they can preserve the BBB, ameliorate inflammation, promote neurogenesis and angiogenesis, and promote functional recovery [46]. This gives them potential for both cell replacement and paracrine mechanisms, similar to MSCs. The mechanisms of their efficacy are less well understood than those of MSCs; however, it is known that CTX0E03 (an NSC line) expresses several angiogenic factors (VEGFA, EGF, ANGPT 1&2, etc.) to promote angiogenesis [47].
Given the lack of relevant studies, there is a lack of clinical trials with NSCs; however, the small size clinical trials completed so far have been promising. In a phase I clinical trial named PISCES, surgical intracerebral injection of CTX0E03 NSCs improved NIHSS by an average of 2 points in 2 years [48]. However, this clinical trial had limitations as only 11 patients received the injection of NSCs, and the cells were injected intracerebrally through surgery (which is highly invasive). Kalladka et al. also stated that other indices of neurological function (such as mRS) would be preferable in future clinical trials [48]. They were able to avoid adverse side effects related to the transplantation [48]. These results warrant further clinical trials with NSCs, so an open-label study titled PISCES-2 was undertaken [48,49] in which intracerebral injection was used again in 23 patients, with the main goal being improvement in Action Research Arm Test (ARAT) scores by more than 2 points. At 12 months, only 15% of the patients achieved targeted ARAT improvement; however, 70% of patients showed improvement in one or more of the ARAT, mRS, and BI scores. Similar to the previous study, a limitation was the small trial size; moreover, PISCES-2 was an open-label trial, whereas randomized, controlled clinical trials are needed [49]. As a result, a phase IIb trial (PISCES-3) is currently in progress [23,50]. PISCES-3 is a randomized, controlled clinical trial, unlike PISCES-2, with approximately 130 patients. Results showed that following intracerebral injection, mRS scores were improved after surgery [23]. This trial is promising but has not yet been completed. As with MSCs, there is still a lack of large, phase III clinical trials, which need to be conducted in the future.
With the ethical and supply problems relating to NSCs, comparative studies with MSCs need to be conducted. If NSCs are not significantly superior to MSCs, MSCs will continue to be more prominently used in future clinical trials. The ethical and supply problems may be solved in the future, as NSCs (along with any other stem cell type) can be derived from iPSCs or ESCs, but the tumorigenicity of pluripotent stem cells (PSCs) must be solved before this can be done [45,46].
3.1.3. Other stem cells
In addition to the more common stem cells listed above, there are also other stem cell types showing promise in pre-clinical research; however, there have been few, if any, clinical studies performed using these cell types. Given that MSCs are currently the optimal cell type for cell transplantation following ischemic stroke, some studies have focused on demonstrating superiority to MSCs [25,29,51]. A couple of these other stem cell types are Dental Pulp Stem Cells (DPSCs) and Hair Follicle Stem Cells (HFSCs).
DPSCs are neural crest-derived stem cells, meaning they come from a neuronal lineage and can more readily differentiate into neural cells than MSCs [52]. They also avoid the ethical problems associated with other stem cell types such as NSCs as they can be obtained from extracted teeth [52]. Additionally, they are multipotent like MSCs, meaning they have a lower risk of tumor formation than do pluripotent stem cells such as iPSCs or ESCs [23].
In pre-clinical research using IV transplantation into middle cerebral artery occlusion (MCAO) rats, as with other stem cell types, DPSCs were effective in reducing infarct volume, inducing functional recovery, and inhibiting microglial activation and pro-inflammatory cytokines [52]. Furthermore, they believe that the neuroprotection of DPSCs is linked to this suppression of neuroinflammation [52]. In addition, a comparative study between DPSCs and BM-MSCs showed that DPSCs were more effective against MCAO in rats and in an in vitro ischemia model [51]. They showed that while both IV transplantation of BM-MSCs and DPSCs lead to a similar functional recovery, DPSCs cause an increased reduction in infarct size and were also more effective at homing to the ischemic stroke area and promoting angiogenesis [51].
Clinical trials with DPSCs will soon begin. A protocol for a future phase I clinical trial called TOOTH using DPSCs has already been designed with the planned selection of 27 ischemic stroke patients, who will undergo intracranial administration of DPSCs to determine the maximum tolerable dose in humans [53]. Moreover, a clinical trial in Japan has already begun entitled JTR-161, which will use IV injection of DPSCs [23].
Another stem cell type that has recently gained interest is HFSCs. Like DPSCs, HFSCs avoid ethical problems, as they are easily attainable from adult mammalian hair follicles [25]. Some of the advantages of HFSCs are that, compared to MSCs, it is easier to obtain the necessary numbers of these stem cells for clinical applications, they can differentiate into neurons (making them feasible for ischemic stroke treatment), and they can proliferate for longer periods, making autologous transplantation possible [25]. In a pre-clinical experiment using IV transplantation into MCAO rats, HFSCs crossed the BBB without a BBB permeabilizer, homed to ischemic areas, and then expressed neural cell markers. HFSCs were able to significantly enhance neurological functional recovery and reduce infarct size [25]. These results show that HFSCs are another potential alternative to MSCs. Clinical trials using HFSCs may begin once more studies are conducted to elucidate the mechanisms of efficacy.
3.1.4. Modified stem cells
As the previous sections have shown mixed results of clinical trials using stem cells, various methods are being undertaken to improve the results. One method is stem cell modification; experiments have shown that modified stem cells are more effective than natural or naïve stem cells [23]. The cell type most commonly modified are MSCs given that they are the most used in clinical and preclinical studies. In a study by Tobin et al., naïve MSCs (nMSCs) were activated with interferon-γ (IFN-γ), creating aMSCγ [54]. They showed that aMSCγ was superior to nMSCs in two main ways. First, aMSCγ was significantly more effective than nMSCs in increasing microglial anti-inflammatory cytokine secretion while decreasing pro-inflammatory cytokines, and second, aMSCγ more effectively induced oligodendrocyte differentiation from endogenous NPCs by upregulating neuron-glia antigen 254. In another experiment by Horita et al., MSCs were genetically modified by transfection with glial cell line-derived neurotrophic factor (GDNF) [55]. After IV transplantation in a rat MCAO model, GDNF-MSCs led to a significantly greater reduction in ischemic area compared to normal MSCs, partially due to an increase in the neurotrophin GDNF [55]. Other researchers have also transfected C–C motif chemokine ligand 2 (CCL2) using a plasmid into human umbilical cord MSCs, because CCL2 is associated with neurological repair and delivery of cells into the brain following stroke via CCL2/CCR2 (CCL2 receptor) interaction [56]. Lee et al. showed that IV administration of CCL2-MSCs significantly improved functional recovery, smaller stroke volume, and increased angiogenesis compared with nMSCs [56]. CCL2-overexpressing MSCs migrated to ischemic areas more effectively than nMSCs, showing that genetic modification is also a method to ensure transplanted cells home to ischemic areas [56].
Although not as common, modification of NSCs has also been attempted in animal models. For example, researchers have modified NSCs with plasmid-based transfection of brain-derived neurotrophic factor (BDNF). As with the other experiments, BDNF-NSCs had higher efficacy than NSCs; BDNF-NSCs significantly enhanced the survival, functional recovery, and infarct volume of MCAO mice compared with naïve NSCs [24].
These pre-clinical studies are promising; however, clinical trials are still needed. An alternative to MSCs may not need to be found if their efficacy can be improved through modification using methods such as those described above.
3.2. Extracellular vesicles transplantation
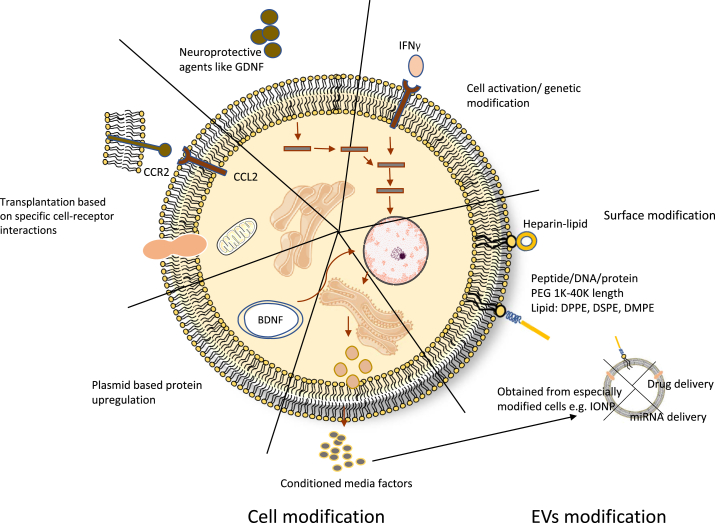
Extracellular vesicles (EVs) or exosomes are small membrane-covered vesicles, approximately 30–150 nm in size, are secreted by most cells, and function as mediators between cells [57,58]. As the terms have been used interchangeably, we will use EVs for the rest of the text [59]. Recently, EVs have gained a lot of interest in stroke therapy, since they have been found to cross the BBB and reach the central nervous system (CNS), usually as a component of conditioned media (CM) (Fig. 2) [58,60,61]. Being able to reach CNS and their small size make them highly biocompatible for targeted delivery of cell cargo [62]. EVs have been studied from various sources and have applications in the delivery of different drugs or microRNAs. Here, we will mention some of the emerging stroke therapies using EVs. They have been isolated from different cell types and have shown promising results after transplantation with or without artificial cargo.
Fig. 2.
Summary of cell/EVs modification methods being explored for ischemic stroke treatment.
Bone marrow derived mesenchymal stem cells EVs have been reported to home in the infarct area after transplantation and have therapeutic effects such as endogenous neurogenesis, increase the number of axons, and improve vital functions of animals [63,64]. When incorporated with different miRNAs, MSC-derived EVs were able to promote a neuroprotective response [[65], [66], [67]]. In some studies, MSCs overexpressing specific miRNAs were chosen and were able to be passed on to the daughter EVs. These EVs also showed a similar response to the artificially incorporated miRNAs [68]. Some research groups have developed novel methods of delivery or administration, which are worth mentioning here. Kim et al. isolated magnetic EVs from MSCs by harboring them with magnetic nanoparticles called iron oxide nanoparticles (IONPs) [69]. These are FDA-approved biocompatible particles that are stored in the body by ionization into iron ions and help to increase the production of growth factors [70,71]. These magnetic extracellular vesicles were prepared and guided for targeted delivery using a magnet and exhibited significant therapeutic effects. Another study by Long et al. used intranasal delivery of EVs for epilepticus therapy and observed localization in the necrotic area [72].
Neural progenitor cell-derived EVs have been shown to have anti-inflammatory properties. MiRNAs isolated from these EVs were sequenced and found to be MAPK inhibitors [73]. Many studies have reported the use of EVs from iPSC-derived cells as the most promising approach for treating ischemic disorders. Ye et al. showed that EVs from iPSC-derived endothelial cells were enriched with miR-199 b-5p and promoted neuro-vascularization [74]. EVs have been used for targeted delivery of drugs as well as biocompatible nano-carriers [75,76]. In one of the earlier reports, exosomes were loaded with curcumin and showed reduced inflammation in the mouse brain [77,78]. Yang et al. were able to promote neuronal recovery by using EVs for enkephalin rapid delivery [79]. Many other groups have reported drug delivery using EVs [80,81].
For a more targeted delivery, surface modification techniques have also been used with EVs which is further explained in further sections. The most common type of modification is the use of an RGD (arginine-glycine-aspartic acid) peptide which has a high affinity for integrins expressed in the ischemic brain [76,82]. Kotaro et al. identified an LRP1 binding peptide L57 by phage display to modify the EVs so that they cross the blood brain barrier [83]. EVs are slowly gaining acceptance for clinical trials and have a potential for future therapies for ischemia reperfusion injury [84].
3.3. Conditioned media
As stated earlier, the paracrine mechanism takes the lead as a method for stem cell transplantation to ameliorate ischemic stroke [22]. It is still not adequately understood which cytokines/proteins are responsible for this; conditioned media experiments are a method to further understand the paracrine mechanism. We will now introduce experiments using CM, demonstrating which stem cell types induce functional recovery after ischemic stroke.
Given that MSCs are the most prevalent cell type for transplantation, their CM has naturally been studied. Tsai et al. compared CM-mediated effects of CM derived from normal (NormBM-MSC) and cerebral ischemia (IschBM-MSC) rats [85]. They found that there were no statistically significant differences between NormBM- and IschBM-MSCs as they both significantly induced functional recovery, enhanced neurogenesis, and attenuated microglial/macrophage infiltration into the ischemic area [85]. This suggests that either autologous or allogeneic MSCs exhibit the desired paracrine effects. In another study by Asgari Taei et al., ESC-derived CM of MSCs (ESC-MSC-CM) led to increased functional recovery, reduced infarction volume, and reduced mortality [86]. They identified upregulation of GAP-43, SYP, and p-CREB in ischemic areas as possible mediators for recovery [86]. Other experiments have also attempted to isolate which cytokines are responsible for this recovery. BDNF is a leading neurotrophin, and research has shown that it is associated with increased functional recovery, decreased apoptosis, and increased angiogenesis; however, removing BDNF does not completely ameliorate these benefits from MSCs, suggesting that other proteins are also important for recovery [87]. VEGF has mixed effects as it induces the positive effects of BDNF, but it can also cause BBB leakage [87]. Furthermore, G-CSF has been shown to not improve functional outcomes after stroke [87]. Some cytokines from MSCs also prevent inflammation, such as IL-10 and TSG-6 [87]. Ultimately, more pre-clinical experiments need to be undertaken, but progress is being made in understanding the paracrine effects of MSCs.
Along with cell transplantation, CM of NSCs (NSC-CM) is also being studied. Research has shown that NSC-CM promotes angiogenesis, which is an important method by which cell therapy induces functional recovery [47]. Specifically, they identified ANGPT 1&2, VEGFA, bFGF, EGF, etc. as important cytokines that induce angiogenesis [47]. Another experiment by Yang et al. also showed that NSC-CM significantly improved neurological scores and decreased infarct volume following a rat MCAO model [88]. They also identified B-cell lymphoma-2 (Bcl-2) as an important protein upregulated by NSC-CM in the ischemic hemisphere, which can attenuate apoptosis, and that NSC-CM can preserve mitochondria to further contribute to neuroprotection [88]. Furthermore, Salikhova et al. generated NSCs from iPSCs, which were further differentiated into glial and neuronal progenitor cells (GPCs and NPCs) to compare their CM [22]. They completely mapped out the protein secretory profiles of NPCs and GPCs and identified 304 proteins in NPC-CM and 243 proteins in GPC-CM, among which 168 proteins were common between the two [22]. GPC-CM also showed higher neurotrophin content (BDNF, GDNF, CTNF, and NGF). Surprisingly, GPC-CM induced blood vessel formation and neurological recovery, and reduced inflammation and microglia/macrophage infiltration; however, NPC-CM exerted no significant positive effects. Many cytokines (such as neurotrophins) excreted exclusively by GPCs and not NPCs are possibly responsible for functional recovery [22].
For cell therapy, DPSCs are less studied than other stem cell types, but comparative research has shown that DPSC-CM is more efficacious than BM-MSC-CM [89,90]. For example, Inoue et al. demonstrated that DPSC-CM was significantly more effective than BM-MSC-CM in reducing infarct volume and improving motor disability scores in MCAO model rats [89]. Moreover, in another experiment, DPSC-CM conferred more cytoprotection against astrocyte cell death compared to BM-MSC-CM in an in vitro Oxygen Glucose Deprivation (OGD) model [90]. DPSC-CM is not yet well understood, but these results are promising as a prospective new source of CM.
These pre-clinical experiments show that conditioned media is effective in ischemic stroke and can avoid issues such as tumor formation and immune rejection that are associated with cell transplantation [89]. However, the secretome of stem cells is still not well understood [87]. Before CM can be used in clinical trials, and especially in common clinical use, hundreds of cytokines/proteins excreted by stem cells must be studied in stroke models to determine which proteins are responsible for their efficacy in ischemic stroke [87]. A summary of these prospective ischemic stroke treatments is presented in Table 1. A major limitation with cell transplantation is that the optimum time after the onset of stroke, at which cells should be transplanted is unknown [28,91]. Most of the studies agree that the earlier transplantation the better, but transplantations as late as 4.5 years after the onset have been carried out as well [[92], [93], [94]]. Optimization studies for transplantation conditions according to the timing are required.
4. Routes of administration
As previously discussed clinical trials have shown, there are various routes of administration for transplanting these stem cells: the most common being intravenous (IV), intra-arterial (IA), and intracerebral or intracranial. Each route has its advantages and disadvantages. An advantage of IV transplantation is that it is completely non-invasive, and the cells will circulate throughout the cerebral vasculature to respond to global cerebral ischemia [30,50]. However, drawbacks include the limited ability to cross the BBB and limited homing to ischemic areas [30,50]. One result of this is the need to transplant a much higher number of cells in IV administration; for example, an IV clinical trial (MASTERS) administered 1.2 billion cells to patients, while intracranial studies administered only 2–20 million cells [50]. For IA transplantation, an advantage is that a smaller number of cells is needed and accumulation in the lungs and peripheral immune organs can be prevented, among other things [50]. However, IA transplantation is invasive and can result in increased side effects as it has resulted in higher mortality rates due to microvascular occlusions caused by clumping of transplanted cells [30]. In addition, similar to IV, crossing the BBB is challenging for IA administration and sometimes requires BBB permeabilizers [50].
Research is also being conducted to improve BBB crossing to improve the efficacy of IV or IA transplantation. Of benefit to ischemic stroke therapies is the fact that the BBB becomes compromised following ischemic stroke, making cellular trafficking across the BBB easier [95]. In fact, Chen et al. demonstrated that human embryonic kidney (HEK293T) cell-derived exosomes were able to cross the BBB in stroke-like conditions, but not in normal conditions [96]. However, artificial BBB permeation is required as it is more difficult for stem cells to cross the BBB. Borlongan et al. demonstrated that shrinking endothelial cells hyperosmotically by using mannitol can allow transplanted stem cells to cross the BBB [97]. A problem is that this also allows inflammatory responses to cross the BBB, so Borlongan et al. proposed to inhibit nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) by combining mannitol with an NF-kB decoy in order to limit the inflammatory response [97]. Another method by Burgess et al. included using MRI-guided focused ultrasound (MRIgFUS) to transiently open the BBB to allow NSCs to cross in rats with limited tissue damage [98]. Burgess et al. also demonstrated that MRIgFUS can be used to deliver stem cells to a single brain structure, assisting homing to ischemic areas [98]. Such BBB permeation experiments may improve transplanted stem cell delivery in IV or IA administration.
Finally, in intracranial transplantation, the advantages are that it requires the smallest number of cells as the product is delivered directly to the target area, and cells do not have to cross the BBB, similar to IV and IA [99,100]. Hattori et al. represented a clinically relevant rodent model of monocyte derived multipotential cells intracranial transplantation. They showed how, relative to IV or IA transplantation, there was minimal loss of cells attributed to apoptosis or homing [99]. However, this method is the most invasive, as it requires surgery [50]. For example, in the PISCES-2 trial, serious adverse events (SAEs) were observed due to the surgical procedure required for intracerebral transplantation [49].
Currently, clinical trials are being conducted using all these methods, as discussed in the previous sections. However, considering the BBB permeation research introduced earlier, stem cell modification or DPSCs result in increased homing to ischemic areas, and HFSCs can cross the BBB without a permeabilizer. Moreover, the disadvantages of IV transplantation can be solved, resulting in IV transplantation being the most prominent in the future, especially in acute ischemic stroke [25,51]. However, chronic stroke is more challenging with IV transplantation, as chemotactic signaling is weaker in the chronic stage [50]. Therefore, intracranial transplantation may be required in patients with chronic stroke.
5. Future perspectives
So far, we have covered future treatments that are closer to becoming clinically available. Now, we will introduce a couple of methods that show promise but are further from reaching common use, with no clinical trials planned or performed yet. These methods include cell transplantation using iPSCs and cell therapy with cell surface modifications.
5.1. Induced pluripotent stem cells
Pluripotent Stem Cells (PSCs) such as ESCs can differentiate into all cell types, including neurons, which give them limitless therapeutic potential. However, there are ethical problems associated with ESCs, since they must be obtained from fetuses [101]. Induced Pluripotent Stem Cells (iPSCs) avoid these ethical problems, as they are somatic cells which are reprogrammed to be pluripotent [101]. Although cells such as DPSCs are easily obtainable, iPSCs can be obtained in virtually limitless quantities since they can be reprogrammed from any somatic cell from any individual, and they endlessly proliferate [23,52]. Being generated from a patient's own cells also avoids immune rejection, giving iPSCs another advantage over ESCs [102].
Another advantage of iPSCs is that they have been shown to be effective in animal models. For example, Jiang et al. generated iPSCs from adult human fibroblasts and transplanted them intracerebrally into MCAO model rats [101]. The iPSCs migrated to ischemic areas, differentiated into neurons, significantly decreased infarct volume, and improved neurological scores [101]. This means that iPSCs can achieve the same pre-clinical results as other stem cell types, and these results have been replicated in other animal experiments. Other experiments have also demonstrated that iPSCs can induce vascular regeneration, similar to the previously mentioned stem cell types [103].
However, there are problems with iPSCs that prevent clinical trials from being performed. The endless proliferation of iPSCs can result in tumor formation, a problem that also presents with other PSCs [23,29,103]. To combat this, researchers are attempting to sub-differentiate iPSCs into other stem cell types and then transplant them into animal stroke models. In a study by Chau et al., iPSCs were differentiated into NPCs (iPSC-NPCs) and transplanted intracerebrally [104]. The transplanted cells were able to differentiate into mature neurons, increase endogenous neurogenesis/angiogenesis, and improve neurological scores. The researchers identified increased expression of SDF-1α and VEGF in the peri-infarct region as possible mediators for this recovery [104]. In addition, Oh et al. generated iPSCs from Peripheral Blood Mononuclear Cells (PBMC), which were then differentiated into NPCs using Episomal Plasmids (EPs) containing reprogramming factors (EP-iPSC-NPCs) [102]. Once injected intracerebrally into MCAO rats, EP-iPSC-NPCs differentiated into neural and glial cells, induced functional recovery, and decreased infarct size [102]. These and other pre-clinical models are promising, but given the tumorigenicity of iPSCs, there are many hurdles to overcome before clinical trials can begin. EPs, as shown earlier, may be able to solve problems with the generation of iPSCs [102,103]. Another requirement is the removal of undifferentiated iPSCs by magnetic/fluorescence-activated cell sorting (MACS/FACS) [103]. Even though iPSCs cannot be directly transplanted, iPSC-derived multipotent stem cells (such as iPSC-MSC/NSCs) could lead to virtually infinite amounts of any stem cell type, making clinical trials easier and cheaper to perform. As shown earlier, iPSC-derived cells can also be used to study conditioned media if the full removal of un-differentiated iPSCs can be achieved [22].
5.2. Cell surface modification
As mentioned in the routes of administration section, IV transplantation is the least invasive transplantation option for ischemic stroke; however, a major problem is the death of transplanted cells, as the innate immune system will kill most cells transplanted intravenously [24,86,105]. This is because the stem cells mentioned earlier are not normally present in human blood, so they express tissue factors that activate thromboinflammation, resulting in cell death. This contributes to less than 0.01% of transplanted cells reaching the brain [106]. Cell surface modification is an emerging method to solve this problem (Fig. 2). The genetic modification of stem cells discussed earlier can also be performed to improve cell survival, but cell surface modification can be performed much more rapidly and effectively [105].
Asawa et al. demonstrated in vitro that cell surface modification of BM-hMSCs with heparin-conjugated lipids (fHep-lipid) can prevent coagulation activation and platelet aggregation, suggesting that fHep-lipid can prevent cell death after transplantation [107]. This is because fHep is structurally similar to heparan sulfate proteoglycan, a component of the endothelial cell glycocalyx that regulates coagulation and complement activation [107]. Cell surface modification can also be performed to increase cell homing to ischemic areas. Given that activated endothelium (such as after ischemic stroke) expresses E-selectin receptors, research by Teramura et al. showed that conjugating E-selectin-binding oligopeptide-conjugated PEG-lipids to MSCs causes these cells to home to activated endothelial cells in vitro [106]. However, these results need to be replicated in vivo before they can be used in clinical trials. In another study by Kusamori et al., longer-term stable modification of MSC membranes was achieved by developing an avidin–biotin complex (ABC) technique [105]. However, they have not yet evaluated whether this long-term modification improves the results in animal stroke models [105].
Cell surface modification can also be applied to targeted drug delivery. Lv et al. demonstrated that a stroke-homing peptide (SHp)-conjugated PEG-lipid (SHp-PEG-DSPE) attached to red blood cell membranes containing a neuroprotective agent (NR2B9) was able to cause the drug to target ischemic areas in a rat MCAO model, which in turn caused a significant reduction in infarct size and neurological score improvement [108]. Further studies can be performed to determine if SHp could also target MSCs in ischemic stroke areas.
As with iPSC transplantation, cell surface modification still needs more pre-clinical studies conducted before it could be used in a clinical trial; however, cell surface modification has the potential to drastically reduce the number of cells needed for IV clinical trials by increasing survival and improving targeted delivery to ischemic stroke areas, while being quicker and more reliable than genetic modification [105,106]. If cell surface modification eventually shows efficacy in clinical trials, it could lead to IV becoming the leading route of administration in cell therapies.
6. Discussion
In this review, we described the status of ischemic stroke treatment, which is summarized in Fig. 1. Currently, there are only two clinically available ischemic stroke treatments: thrombolysis and thrombectomy. These are only targeted at stopping ischemia and do not limit reperfusion or help patients recover from neuronal damage sustained during stroke. We show in this paper that there are many methods with the potential to aid in the recovery of damage sustained from ischemic stroke; however, none of these methods are ready for common clinical use, nor have any large-scale phase III clinical trials been performed even though stem cell clinical trials have been conducted for approximately 20 years now [44]. It is known that cells generally do not survive transplantation, and most (if not all) benefits from cell transplantation in clinical trials are from the paracrine mechanism [22,92,106].
Although this review article mostly covers stem cell or stem cell derivatives (such as CM or EVs) transplantation, there are additional methods of stem cell treatment that are useful. For example, as damage/loss of endothelial glycocalyx contributes to additional brain damage during reperfusion; perhaps artificial glycocalyx could be transplanted to ameliorate this in the future [106]. Additionally, in vitro disease modeling, such as brain/body-on-a-chip methods, is also useful, especially in determining the effect of cytokines and the efficacy of conditioned media [47,109]. IPSCs are particularly useful for this purpose, given their endless proliferative abilities and their ability to tailor disease modeling to any patient [109].
As stated before, ischemic stroke is a leading cause of death and disability worldwide, and it will continue to become more common in the future [4]. Therefore, we hope that some of the treatments presented here will gain common clinical applicability in the future.
Declaration of competing interest
The authors (Mason Daniel Hurd, Isha Goel, Yasuyuki Sakai and Yuji Teramura) declare no conflict of interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Eltzschig H.K., Eckle T. Ischemia and reperfusion-from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamel H., Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol. 2012;69:576–581. doi: 10.1001/archneurol.2011.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsay P.M., Norrving Bo, Sacco Ralph L., Brainin M., Hacke W., Martins S. World stroke organization (WSO): global stroke fact sheet 2019. Int J Stroke. 2019;14:806–817. doi: 10.1177/1747493019881353. [DOI] [PubMed] [Google Scholar]
- 4.Paul S., Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335 doi: 10.1016/j.expneurol.2020.113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katan M., Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 6.Takashima N., Arima H., Kita Y., Fujii T., Tanaka-Mizuno S., Shitara S. Long-term survival after stroke in 1.4 million Japanese population: shiga stroke and heart attack registry. J Stroke. 2020;22:336–344. doi: 10.5853/jos.2020.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng X., Yang Y., Schwebel D., Liu Z., Li L., Cheng P. Population ageing and mortality during 1990-2017: a global decomposition analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebeskind D., Liaw N. Emerging therapies in acute ischemic stroke. F1000Research. 2020;9(546):1–8. doi: 10.12688/f1000research.21100.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rich M.W., Louis S. TPA: Is it worth the price? Am Heart J. 1987;114:1259–1261. doi: 10.1016/0002-8703(87)90211-0. [DOI] [PubMed] [Google Scholar]
- 11.Chevilley A., Lesept F., Lenoir S., Ali C., Parcq J., Vivian D. Impacts of tissue-type plasminogen activator(tPA) on neuronal survival. Front Cell Neurosci. 2015;9 doi: 10.3389/fncel.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grummisch J.A., Jadavji N.M., Smith P.D. TPA promotes cortical neuron survival via mTOR-dependent mechanisms. Mol Cell Neurosci. 2016;74:25–33. doi: 10.1016/j.mcn.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Campbell B.C.V. Thrombolysis and thrombectomy for acute ischemic stroke: strengths and synergies. Semin Thromb Hemost. 2017;43:185–190. doi: 10.1055/s-0036-1585078. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.S. tPA helpers in the treatment of acute ischemic stroke: are they ready for clinical use? J Stroke. 2019:21 160–174. doi: 10.5853/jos.2019.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jovin T.G., Chamorro A., Cobo E., DeMiquel M.A., Molina C.A., Rovira A. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 16.Derex L., Cho T.H. Mechanical thrombectomy in acute ischemic stroke. Rev Neurol (Paris) 2017;173:106–113. doi: 10.1016/j.neurol.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Jayaraman M.V., McTaggart R.A., Goyal M. Unresolved issues in thrombectomy. Curr Neurol Neurosci Rep. 2017;17 doi: 10.1007/s11910-017-0776-4. [DOI] [PubMed] [Google Scholar]
- 18.Smith W.S., Sung G., Saver J., Budzik R., Duckwiler G., Liebeskind D.S. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 19.Zi W., Qiu Z., Li F., Sang H., Wu D., Luo W. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA, J Am Med Assoc. 2021;325:234–243. doi: 10.1001/jama.2020.23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy D.J., Diaz A., Sheinberg D., Snelling B., Luther E.M., Chen S.H. Long-term outcomes of mechanical thrombectomy for stroke: a meta-analysis. Sci World J. 2019;2019 doi: 10.1155/2019/7403104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samaniego E.A., Roa J.A., Limaye K., Adams H.P. Mechanical thrombectomy: emerging technologies and techniques. J Stroke Cerebrovasc Dis. 2018;27:2555–2571. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Salikhova D., Bukharova T., Cherkashova E., Namestnikova D., Leonov G., Nikitina M. Therapeutic effects of hipsc-derived glial and neuronal progenitor cells-conditioned medium in experimental ischemic stroke in rats. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22094694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suda S., Nito C., Yokobori S., Sakamoto Y., Nakajima M., Sowa K. Recent advances in cell-based therapies for ischemic stroke. Int J Mol Sci. 2020;21:1–24. doi: 10.3390/ijms21186718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X.C., Xiang J.J., Wu H.H., Jhang T.Y., Jhang D.P., Xu Q.H. Neural stem cells transfected with reactive oxygen species–responsive polyplexes for effective treatment of ischemic stroke. Adv Mater. 2019;31 doi: 10.1002/adma.201807591. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X., Tang H., Mao S., Li B., Zhou Y., Yue H. Transplanted hair follicle stem cells migrate to the penumbra and express neural markers in a rat model of cerebral ischaemia/reperfusion. Stem Cell Res Ther. 2020;11 doi: 10.1186/s13287-020-01927-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao L.-Y., Huang F., Zhao M., Xie J., Shi J., Wang J. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 2014;23:65–72. doi: 10.3727/096368914X684961. [DOI] [PubMed] [Google Scholar]
- 27.Savitz S.I., Yavagal D., Rappard G., Likosky W., Rutledge N., Graffagnino C. A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow-derived ALD-401 cells in patients with recent stable ischemic stroke (RECOVER-Stroke) Circulation. 2019;139:192–205. doi: 10.1161/CIRCULATIONAHA.117.030659. [DOI] [PubMed] [Google Scholar]
- 28.Boncoraglio G.B., Ranieri M., Bersano A., Parati E.A., Giovane C. Del. Stem cell transplantation for ischemic stroke. Cochrane Database Syst Rev. 2019;2019:1–45. doi: 10.1002/14651858.CD007231.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stonesifer C., Corey S., Ghanekar S., Diamandis Z., Acosta S.A., Borlongan C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017:158 94–158131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenter A., Ahsan H., Kong A., Regunathan A. Understanding the therapeutic potential of bone marrow stem cell therapy in ischemic stroke. Georg Med Rev. 2018;2:1–18. 3417. [Google Scholar]
- 31.Son J.W., Park J., Kim Y.E., Ha J., Park D.W., Chang M.S. Glia-like cells from late-passage human MSCs protect against ischemic stroke through IGFBP-4. Mol Neurobiol. 2019;56:7617–7630. doi: 10.1007/s12035-019-1629-8. [DOI] [PubMed] [Google Scholar]
- 32.Liu K., Guo L., Zhou Z., Pan M., Yan C. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res. 2019;123:74–80. doi: 10.1016/j.mvr.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Wang F., Tang H., Zhu J., Zhang J.H. Transplanting mesenchymal stem cells for treatment of ischemic stroke. Cell Transplant. 2018;27:1825–1834. doi: 10.1177/0963689718795424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C., Lin Y., Liu Q., He J., Xiang P., Wang D. Growth differentiation factor 11 promotes differentiation of MSCs into endothelial-like cells for angiogenesis. J Cell Mol Med. 2020;24:8703–8717. doi: 10.1111/jcmm.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oswald J., Boxberger S., Jørgensen B., Feldmann S., Ehninger G., Bornhäuser M. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cell. 2004;22:506. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 36.Elgamal A., Althani A., Abd-Elmaksoud A., Kassab M., Farag A., Lashen S. Xeno-free trans-differentiation of adipose tissue-derived mesenchymal stem cells into glial and neuronal cells. AJSC. 2019;8(2):38–51. [PMC free article] [PubMed] [Google Scholar]
- 37.Bossolasco P., Cova L., Calzarossa C., Rimoldi S.G., Borsotti C., Lambertenghi Deliliers G. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005;193:312–325. doi: 10.1016/j.expneurol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Yim E.K.F., Pang S.W., Leong K.W. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J., Zhang N., Zhu Y., Jin R., Wu F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials. 2021;265 doi: 10.1016/j.biomaterials.2020.120448. [DOI] [PubMed] [Google Scholar]
- 40.Nandy S.B., Mohanty S., Singh M., Behari M., Airan B. Fibroblast Growth Factor-2 alone as an efficient inducer for differentiation of human bone marrow mesenchymal stem cells into dopaminergic neurons. J Biomed Sci. 2014;21 doi: 10.1186/s12929-014-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung J.W., Chang W.H., Bang O.Y., Moon G.J., Kim S.J., Kim S.K. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021;96:e1012–e1023. doi: 10.1212/WNL.0000000000011440. [DOI] [PubMed] [Google Scholar]
- 42.Levy M.L., Crawford J.R., Dib N., Verkh L., Tankovich N., Cramer S.C. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50:2835–2841. doi: 10.1161/STROKEAHA.119.026318. [DOI] [PubMed] [Google Scholar]
- 43.Li Z., Dong X., Tian M., Liu C., Wang K., Li L. Stem cell-based therapies for ischemic stroke: a systematic review and meta-analysis of clinical trials. Stem Cell Res Ther. 2020;11(252):1–13. doi: 10.1186/s13287-020-01762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krause M., Phan T.G., Ma H., Sobey C.G., Lim R. Cell-based therapies for stroke: are we there yet? Front Neurol. 2019;10(656):1–13. doi: 10.3389/fneur.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang G.L., Zhu Z.H., Wang Y.Z. Neural stem cell transplantation therapy for brain ischemic stroke: review and perspectives. World J Stem Cell. 2019;11:817–830. doi: 10.4252/wjsc.v11.i10.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boese A.C., Le Q.S.E., Pham D., Hamblin M.H., Lee J.P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res Ther. 2018;9 doi: 10.1186/s13287-018-0913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hicks C., Stevanato L., Stroemer R., Tang E., Richardson S., Sinden J.D. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 2013;22:1541–1552. doi: 10.3727/096368912X657936. [DOI] [PubMed] [Google Scholar]
- 48.Kalladka D., Sinden J., Pollock K., Haig C., McLean J., Smith W. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 49.Muir K.W., Bulters D., Willmot M., Sprigg N., Dixit A., Ward N. Intracerebral implantation of human neural stem cells and motor recovery after stroke: multicentre prospective single-arm study (PISCES-2) J Neurol Neurosurg Psychiatry. 2020;91:396–401. doi: 10.1136/jnnp-2019-322515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wechsler L.R., Bates D., Stroemer P., Andrews-Zwilling Y.S., Aizman I. Cell therapy for chronic stroke. Stroke. 2018;49:1066–1074. doi: 10.1161/STROKEAHA.117.018290. [DOI] [PubMed] [Google Scholar]
- 51.Song M., Lee J.H., Bae J., Bu Y., Kim E.C. Human dental pulp stem cells are more effective than human bone marrow-derived mesenchymal stem cells in cerebral ischemic injury. Cell Transplant. 2017;26:1001–1016. doi: 10.3727/096368916X694391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nito C., Sowa K., Nakajima M., Sakamoto Y., Suda S., Nishiyama Y. Transplantation of human dental pulp stem cells ameliorates brain damage following acute cerebral ischemia. Biomed Pharmacother. 2018;108:1005–1014. doi: 10.1016/j.biopha.2018.09.084. [DOI] [PubMed] [Google Scholar]
- 53.Nagpal A., Kremer K.L., Hamilton-Bruce M., Kaidonis X., Milton A.G., Levi C. TOOTH (The Open study of dental pulp stem cell Therapy in Humans): study protocol for evaluating safety and feasibility of autologous human adult dental pulp stem cell therapy in patients with chronic disability after stroke. Int J Stroke. 2016;11:575–585. doi: 10.1177/1747493016641111. [DOI] [PubMed] [Google Scholar]
- 54.Tobin M.K., Stephen T.K.L., Lopez K.L., Pergande M.R., Bartholomew A.M., Cologna S.M. Activated mesenchymal stem cells induce recovery following stroke via regulation of inflammation and oligodendrogenesis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horita Y., Honmou O., Harada K., Houkin K., Hamada H., Kocsis J.D. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S., Kim O.J., Lee K.O., Jung H., Oh S.H., Kim N.K. Enhancing the therapeutic potential of ccl2- overexpressing mesenchymal stem cells in acute stroke. Int J Mol Sci. 2020;21:1–19. doi: 10.3390/ijms21207795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002:2 569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 58.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 59.Witwer K.W., Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles. 2019;8 doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang X., Zuo Z., Hong W., Tang H., Geng W. Progress of research on exosomes in the protection against ischemic brain injury. Front Neurosci. 2019;13 doi: 10.3389/fnins.2019.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Tang Y., Yang G.Y. Therapeutic application of exosomes in ischaemic stroke. Stroke Vasc Neurol. 2021 doi: 10.1136/svn-2020-000419. 0 svn-2020-000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z.G., Chopp M. Exosomes in stroke pathogenesis and therapy. J Clin Invest. 2016;126:1190–1197. doi: 10.1172/JCI81133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu R., Bai Y., Min S., Xu X., Tang T., Ju S. Vivo monitoring and assessment of exogenous mesenchymal stem cell-derived exosomes in mice with ischemic stroke by molecular imaging. Int J Nanomed. 2020;15:9011–9023. doi: 10.2147/IJN.S271519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dabrowska S., Andrzejewska A., Lukomska B., Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16 doi: 10.1186/s12974-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ai Z., Cheng C., Zhou L., Yin S., Wang L., Liu Y. Bone marrow mesenchymal stem cells-derived extracellular vesicles carrying microRNA-221-3p protect against ischemic stroke via ATF3. Brain Res Bull. 2021;172:220–228. doi: 10.1016/j.brainresbull.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 66.Guo D., Ma J., Li T., Yan L. Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-κB pathway by targeting FOXO3. Exp Cell Res. 2018;369:34–42. doi: 10.1016/j.yexcr.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 67.He X.W., Shi Y.H., Zhao R., Liu Y.S., Li G.F., Hu Y. Plasma levels of miR-125b-5p and miR-206 in acute ischemic stroke patients after recanalization treatment: a prospective observational study. J Stroke Cerebrovasc Dis. 2019;28:1654–1661. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 68.Deng Y., Chen D., Gao F., LV H., Zhang G., Sun X. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng. 2019;13 doi: 10.1186/s13036-019-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim H.Y., Kim T.J., Kang L., Kim Y.J., Kang M.K., Kim J. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243 doi: 10.1016/j.biomaterials.2020.119942. [DOI] [PubMed] [Google Scholar]
- 70.Han J., Kim B., Shin J.Y., Ryu S., Noh M., Woo J. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano. 2015;9:2805–2819. doi: 10.1021/nn506732n. [DOI] [PubMed] [Google Scholar]
- 71.Shi L., Chang Y., Yang Y., Zhang Y., Yu F., Wu X. Activation of jnk signaling mediates connective tissue growth factor expression and scar formation in corneal wound healing. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Longa Q., Upadhya D., Hattiangady B., Kim D.K., An S.Y., Shuai B. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci USA. 2017;114:E3536–E3545. doi: 10.1073/pnas.1703920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian T., Cao L., He C., Ye Q., Liang R., You W. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics. 2021;11:6507–6521. doi: 10.7150/thno.56367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye M., Ni Q., Qi H., Qian X., Chen J., Guo X. Exosomes derived from human induced pluripotent stem cells-endothelia cells promotes postnatal angiogenesis in mice bearing ischemic limbs. Int J Biol Sci. 2019;15:158–168. doi: 10.7150/ijbs.28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Contr Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian T., Zhang H., He C.P., Fan S., Zhu Y.L., Qi C. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhuang X., Xiang X., Grizzle W., Sun D., Zhang S., Axtell R.C. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y., Fu N., Su J., Wang X., Li X. Rapid enkephalin delivery using exosomes to promote neurons recovery in ischemic stroke by inhibiting neuronal p53/caspase-3. BioMed Res Int. 2019;2019 doi: 10.1155/2019/4273290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo L., Huang Z., Huang L., Liang J., Wang P., Zhao L. Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. J Nanobiotechnol. 2021;19 doi: 10.1186/s12951-021-00879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bunggulawa E.J., Wang W., Yin T., Wang N., Durkan C., Wang Y. Recent advancements in the use of exosomes as drug delivery systems 06 Biological Sciences 0601 Biochemistry and Cell Biology. J Nanobiotechnol. 2018;16 81 doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J., Li W., Lu Z., Zhang L., Hu Y., Li Q. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis. Nanoscale. 2017;9:15598–15605. doi: 10.1039/c7nr04425a. [DOI] [PubMed] [Google Scholar]
- 83.Sakamoto K., Shinohara T., Adachi Y., Asami T., Ohtaki T. A novel LRP1-binding peptide L57 that crosses the blood brain barrier. Biochem Biophys Rep. 2017;12:135–139. doi: 10.1016/j.bbrep.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mendt M., Rezvani K., Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54:789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 85.Tsai M.J., Tsai M.K., Hu B.R., Liou D.Y., Huang S.L., Huang M.C. Journal of Bio Med Sci; 2014. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asgari Taei A., Dargahi L., Nasoohi S., Hassanzadeh G., Kadivar M., Farahmandfar M. The conditioned medium of human embryonic stem cell-derived mesenchymal stem cells alleviates neurological deficits and improves synaptic recovery in experimental stroke. J Cell Physiol. 2021;236:1967–1979. doi: 10.1002/jcp.29981. [DOI] [PubMed] [Google Scholar]
- 87.Cunningham C.J., Redondo-Castro E., Allan S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cerebr Blood Flow Metabol. 2018;38:1276–1292. doi: 10.1177/0271678X18776802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang H.N., Wang C., Chen H., Li L., Ma S., Wang H. Neural stem cell-conditioned medium ameliorated cerebral ischemia-reperfusion injury in rats. Stem Cell Int. 2018;2018 doi: 10.1155/2018/4659159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inoue T., Sugiyama M., Hattori H., Wakita H., Wakabayashi T., Ueda M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng. 2013;19:24–29. doi: 10.1089/ten.tea.2011.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song M., Jue S.S., Cho Y.A., Kim E.C. Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. J Neurosci Res. 2015;93:973–983. doi: 10.1002/jnr.23569. [DOI] [PubMed] [Google Scholar]
- 91.Wang L.Q., Lin Z.Z., Zhang H.X., Shao B., Xiao L., Jiang H.G. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci Ther. 2014;20:317–326. doi: 10.1111/cns.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bersano A., Ballabio E., Lanfranconi S., Boncoraglio G.B., Corti S., Locatelli F. Clinical studies in stem cells transplantation for stroke: a review. Curr Vasc Pharmacol. 2010;8 doi: 10.2174/157016110790226570. [DOI] [PubMed] [Google Scholar]
- 93.Kondziolka D., Steinberg G.K., Wechsler L., Meltzer C.C., Elder E., Gebel J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 94.Honmou O., Houkin K., Matsunaga T., Niitsu Y., Ishiai S., Onodera R. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. J Neurol. 2021 doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu L., Eckert M.A., Riazifar H., Kang D.K., Agalliu D., Zhao W. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cell Int. 2013 doi: 10.1155/2013/435093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen C.C., Liu L., Ma F., Wong C.W., Guo X.E., Chacko J.V. Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell Mol Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borlongan C.V., Glover L.E., Sanberg P.R., Hess D.C. 2012. Permeating the blood brain barrier and abrogating the inflammation in stroke: implications for stroke therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burgess A., Ayala-Grosso C.A., Ganguly M., Jordão J.F., Aubert I., Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hattori H., Suzuki S., Okazaki Y., Suzuki N., Kuwana M. Intracranial transplantation of monocyte-derived multipotential cells enhances recovery after ischemic stroke in rats. J Neurosci Res. 2012;90:479–488. doi: 10.1002/jnr.22755. [DOI] [PubMed] [Google Scholar]
- 100.Lee J., Chang W.S., Shin J., Seo Y., Kong C., Song B.W. Non-invasively enhanced intracranial transplantation of mesenchymal stem cells using focused ultrasound mediated by overexpression of cell-adhesion molecules. Stem Cell Res. 2020;43 doi: 10.1016/j.scr.2020.101726. [DOI] [PubMed] [Google Scholar]
- 101.Jiang M., LV L., Ji H., Yang X., Zhu W., Cai L. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem. 2011;354:67–75. doi: 10.1007/s11010-011-0806-5. [DOI] [PubMed] [Google Scholar]
- 102.Oh S.H., Jeong Y.W., Choi W., Noh J.E., Lee S., Kim H.S. Multimodal therapeutic effects of neural precursor cells derived from human-induced pluripotent stem cells through episomal plasmid-based reprogramming in a rodent model of ischemic stroke. Stem Cell Int. 2020;2020 doi: 10.1155/2020/4061516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu Y., Wan S., Zhan R.Y. Inducible pluripotent stem cells for the treatment of ischemic stroke: current status and problems. Rev Neurosci. 2012;23:393–402. doi: 10.1515/revneuro-2012-0042. [DOI] [PubMed] [Google Scholar]
- 104.Chau M.J., Deveau T.C., Song M., Gu X., Chen D., Wei L. IPSC transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cell. 2014;32:3075–3087. doi: 10.1002/stem.1802. [DOI] [PubMed] [Google Scholar]
- 105.Kusamori K., Takayama Y., Nishikawa M. Stable surface modification of mesenchymal stem cells using the avidin-biotin complex technique. Curr Protoc Stem Cell Biol. 2018;47 doi: 10.1002/cpsc.66. [DOI] [PubMed] [Google Scholar]
- 106.Teramura Y., Ekdahl K.N., Fromell K., Nilsson B., Ishihara K. Potential of cell surface engineering with biocompatible polymers for biomedical applications. Langmuir. 2020;36:12088–12106. doi: 10.1021/acs.langmuir.0c01678. [DOI] [PubMed] [Google Scholar]
- 107.Asawa K., Ishihara K., Ekdahl K.N., Nilsson B., Teramura Y. Cell surface functionalization with heparin-conjugated lipid to suppress blood activation. Adv Funct Mater. 2021;31 [Google Scholar]
- 108.Lv W., Xu J., Wang X., Li X., Xu Q., Xin H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12:5417–5426. doi: 10.1021/acsnano.8b00477. [DOI] [PubMed] [Google Scholar]
- 109.Zierold R., Harberts J., Fendler C., Teuber J., Siegmund M., Silva A. Toward brain-on-a-chip: human induced pluripotent stem cell-derived guided neuronal networks in tailor-made 3d nanoprinted microscaffolds. ACS Nano. 2020;14:13091–13102. doi: 10.1021/acsnano.0c04640. [DOI] [PubMed] [Google Scholar]