Abstract
Background & Aims
Fontan-associated liver disease (FALD) has emerged as an important morbidity following surgical palliation of single ventricle congenital heart disease. In this study, non-invasive biomarkers that may be associated with severity of FALD were explored.
Methods
A retrospective cohort of paediatric patients post-Fontan who underwent liver biopsy at a high volume at a paediatric congenital heart disease centre was reviewed.
Results
Among 106 patients, 66% were male and 69% were Hispanic. The mean age was 14.4 ± 3.5 years, and biopsy was performed 10.8 ± 3.6 years post-Fontan. The mean BMI was 20.8 ± 5 kg/m2, with 27.4% meeting obesity criteria. Bridging fibrosis was observed in 35% of patients, and 10.4% of all patients had superimposed steatosis. Bridging fibrosis was associated with lower platelet counts (168.3 ± 58.4 vs. 203.9 ± 65.8 K/μl for congestive hepatic fibrosis score [CHFS] 0–2b, p = 0.009), higher bilirubin (1.7 ± 2.2 vs. 0.9 ± 0.7 mg/dl, p = 0.0090), higher aspartate aminotransferase-to-platelet ratio index [APRI] and fibrosis-4 [FIB-4] scores (APRI: 0.5 ± 0.3 vs. 0.4 ± 0.1, p <0.01 [AUC: 0.69] and FIB-4: 0.6 ± 0.4 vs. 0.4 ± 0.2, p <0.01 [AUC: 0.69]), and worse overall survival (median 2 years follow-up post-biopsy, p = 0.027). Regression modelling of temporal changes in platelet counts before and after biopsy correlated with fibrosis severity (p = 0.005).
Conclusions
In this large, relatively homogeneous adolescent population in terms of age, ethnicity, and Fontan duration, bridging fibrosis was observed in 35% of patients within the first decade post-Fontan. Bridging fibrosis was associated with worse survival. Changes in platelet counts, even years before biopsy, and APRI/FIB-4 scores had modest discriminatory power in identifying patients with advanced fibrosis. Steatosis may represent an additional risk factor for disease progression in obese patients. Further prospective studies are necessary to develop strategies to screen for FALD in the adolescent population.
Lay summary
In this study, the prevalence of Fontan-associated liver disease (FALD) in the young adult population and clinical variables that may be predictive of fibrosis severity or adverse outcomes were explored. Several lab-based, non-invasive markers of bridging fibrosis in FALD were identified, suggesting that these values may be followed as a prognostic biomarker for FALD progression in the adolescent population.
Keywords: Congenital heart disease, Univentricular heart disease, Congestive hepatopathy
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CBC, complete blood count; CHFS, congestive hepatic fibrosis score; CHLT, combined heart–liver transplantation; CVP, central venous pressure; ECMO, extracorporeal membrane oxygenation; FALD, Fontan-associated liver disease; FIB-4, fibrosis-4; GFR, glomerular filtration rate; GGT, gamma-glutamyl transferase; INR, international normalised ratio; IQR, interquartile range; LVAD, left ventricular assist device; MELD, model of end-stage liver disease; MELD-Na, MELD-sodium; MELD-XI, MELD without INR; NAFLD, non-alcoholic fatty liver disease; PELD, paediatric end-stage liver disease; PT, prothrombin time; PTT, partial thromboplastin time; TTE, transthoracic echocardiograms
Graphical abstract
Highlights
-
•
FALD is universal within 10 years post-Fontan, with 35% of patients having bridging fibrosis.
-
•
Of our adolescent patient population, 10% had concomitant hepatic steatosis, which was associated with obesity.
-
•
Regression modelling demonstrates that temporal changes in platelet counts correlate with severity of fibrosis in FALD.
-
•
AST-to-platelet ratio index and FIB-4 scores correlate with bridging fibrosis with a high specificity.
-
•
Bridging fibrosis in FALD is associated with worse survival.
Introduction
Surgical palliation resulting in a total cavopulmonary connection and Fontan circulation is used to palliate patients with a spectrum of congenital cardiac lesions with univentricular physiology, including tricuspid atresia and hypoplastic left heart syndrome.1,2 Although the resulting passive venous return directly into the pulmonary circulation increases arterial blood oxygen saturation, there is an abrupt rise in central venous pressure (CVP), leading to several extracardiac effects.3 As this population survives into adulthood, Fontan-associated liver disease (FALD) has emerged as an unintended comorbidity of Fontan circulation.1,4 Multiple factors, including perioperative insults, hypoxia, low cardiac output, and elevated CVP, may all contribute to this variant of congestive hepatopathy, which is characterised by sinusoidal and portal fibrosis and is known to lead to cirrhosis and portal hypertension.1,5,6
Detection of FALD is limited by a lack of associated physical exam findings, relatively normal laboratory values, and no definitive imaging features.4 As a consequence, liver biopsy remains the most accepted approach to establish a diagnosis, and several surveillance biopsy series have confirmed that virtually 100% of young adult patients post-Fontan have histologic evidence of liver fibrosis, with a substantial subpopulation (35–68%) exhibiting bridging fibrosis.5,[7], [8], [9], [10], [11] Much effort has been dedicated to identifying laboratory values, imaging findings, or cardiac catheterisation variables that may be associated with severity of liver fibrosis, with limited success. FALD severity has been shown to be associated with duration of Fontan circulation, but it is unclear how fibrosis correlates with Fontan hemodynamics.1 The majority of these biopsy series describe a primarily Caucasian, young adult population, >15 years post-Fontan, although the prevalence and impact of FALD in the adolescent population has only recently begun to be explored.10 Furthermore, there are limited data on the relationship between the development of FALD and independent risk factors of chronic liver disease, such as obesity, or non-modifiable factors such as sex and ethnicity.[12], [13], [14] In recent years, it has become evident that FALD can progress to cirrhosis or hepatocellular carcinoma, which can further complicate management of young adult patients who may also require heart transplant for a failing Fontan.4,15
Over the past 15 years, our centre has performed >500 Fontan procedures in an ethnically and socioeconomically diverse patient population. As FALD has emerged as a common but underdiagnosed pathology in patients post-Fontan, liver biopsies have been added to cardiac catheterisation procedures being performed in patients followed at our centre at 10 years post-Fontan since 2015. We are increasingly considering timing and indications for combined heart–liver transplant (CHLT) in the adolescent population. The purpose of this study was to review our large single-centre experience of post-Fontan liver biopsies in a majority Hispanic adolescent population and to evaluate if patient demographics, histopathological findings, or clinical variables may be associated with severity of FALD.
Patients and methods
This study was approved by the Institutional Review Board (CHLA-20-00024) and was exempt, therefore not requiring consent.
Inclusion criteria
Participants who had undergone liver biopsy between January 2011 and September 2020 were identified from an institutional congenital heart disease database. At our centre, approximately one-third of our Fontan population is followed up longitudinally within Children’s Hospital–Los Angeles, and cardiac catheterisation and surveillance transjugular liver biopsy (2 passes) have been performed in patients approximately 10 years post-Fontan since 2015. Before this time, liver biopsies were performed selectively (i.e. concern for chronic liver disease). The majority of biopsies have occurred since 2015 (Fig. S1), and all but 2 patients had transjugular biopsies performed during cardiac catheterisation (indications summarised in Table S1). Patients were excluded if they did not undergo liver biopsy or if biopsy slides were not available for re-assessment.
Clinical data
Charts were reviewed independently by 2 members of the research team. Demographics reviewed included age, sex, race and ethnicity (as reported during registration by the patient/family), biometrics, primary diagnosis, and resulting Fontan anatomy. Haemodynamic measurements from catheterisation procedures were reviewed. Transthoracic echocardiograms (TTE) were reviewed for qualitative assessment of ventricular systolic function and valvular insufficiency. Relevant medical history at the time of biopsy was collected, including medications and presence of comorbidities. Lab values (alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase [ALP], bilirubin, gamma-glutamyl transferase [GGT], blood urea nitrogen [BUN], creatinine, albumin, glomerular filtration rate [GFR, modified Schwartz formula], prothrombin time [PT], international normalised ratio [INR], complete blood count [CBC], and brain natriuretic peptide [BNP]) were reviewed at the time of biopsy (closest available labs within 6 months of biopsy).16 Platelets were further analysed by extracting all available platelet values for each patient from 5 years before biopsy and all time points post-biopsy from the electronic medical record. The model for end-stage liver disease (MELD), MELD-sodium (MELD-Na), MELD without INR (MELD-XI), and paediatric end-stage liver disease (PELD) scores were calculated using these labs according to published formulas, with MELD and MELD-Na excluding patients who were taking warfarin at the time of catheterisation.[17], [18], [19] For PELD calculations, no adjustments for ‘history of growth failure’ were applied. Imaging findings from abdominal ultrasounds were reviewed if obtained within 3 months before or any time following the liver biopsy. AST-to-platelet ratio index (APRI) was calculated using a reference upper limit AST of 46 U/L.20 Fibrosis-4 (FIB-4) was calculated using published formulas.21 Information pertaining to the biopsy procedure and procedural complications were recorded and scaled using Clavien–Dindo classification.22 For patients who died in the study period, causes of death were also reviewed.
Pathological data
Two pathologists were blinded to clinical data and independently reviewed and scored liver biopsies using the congestive hepatic fibrosis score (CHFS).23 The CHFS has been validated and is organised as follows: 0 (no significant fibrosis), 1 (central zone fibrosis only), 2a (centrizonal fibrosis and portal fibrosis with accentuation of central zone fibrosis), 2b (centrizonal and portal fibrosis with accentuation of portal zone fibrosis), 3 (bridging fibrosis), and 4 (cirrhosis). Discrepancies were rare and were reviewed together to determine a consensus score. Cases were also reviewed for evidence of macrosteatosis and microsteatosis, and the total percentage of steatosis was documented.
Statistical analysis
Because of limited sample sizes, categorical variables were compared using the Fisher exact test, and the Wilcoxon rank-sum test was used to compare continuous variables. Overall patient survival was calculated with the Kaplan–Meier method and compared via the log-rank test. For longitudinal analysis, platelet values were modelled over time using cubic splines by the CHFS subgroup, and repeated measures were accounted for with a random intercept for each patient in a mixed linear regression model. A p value of <0.05 was considered significant. Statistical analysis was performed with R (V.4.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
In the study period, 108 patients post-Fontan with a history of liver biopsy at our institution were identified. Two were excluded: only 1 biopsy yielded insufficient specimen to assess liver pathology, and another had records of biopsy, but the slides were unavailable for review because it was performed at an outside institution. Only 1 patient experienced a procedural complication, which involved bleeding from a small hepatic arterial branch that was controlled during the biopsy procedure with gel foam embolisation.
Overall, the mean age at biopsy was 14.4 ± 3.5 years, and 66% were male (Table 1). The majority (68.9%) of patients were of Hispanic ethnicity, and the mean duration in Fontan circulation at the time of biopsy was 10.8 ± 3.6 years. Hypoplastic left heart syndrome (38.7%) and tricuspid atresia (17%) were the most common underlying diagnoses related to congenital heart disease. Patients were further stratified by CHFS, which separates fibrosis alone (0–2b) from bridging fibrosis (3) or cirrhosis (4) and has been suggested as a branching point in algorithms for consideration of heart transplant alone vs. CHLT.4,23 There were no differences in patient demographics when stratified by CHFS.
Table 1.
Patient demographics.
| Characteristic, n (%) | Total N = 106 |
CHFS 0–2b n = 69 |
CHFS 3–4 n =37 |
p value |
|---|---|---|---|---|
| Patient age in years, mean ± SD | 14.4 ± 3.5 | 14 ± 3.4 | 14.9 ± 3.6 | 0.10 |
| Age at Fontan procedure in years, mean ± SD | 3.5 ± 1.7 | 3.6 ± 2.0 | 3.5 ± 0.9 | 0.28 |
| Duration of Fontan circulation in years, mean ± SD | 10.8 ± 3.6 | 10.5 ± 3.5 | 11.5 ± 3.7 | 0.07 |
| Male, N (%) | 70 (66.0) | 47 (68.1) | 23 (62.2) | 0.67 |
| Race, N (%) | ||||
| Asian | 6 (5.7) | 5 (7.2) | 1 (2.7) | 0.75 |
| Black | 8 (7.5) | 5 (7.2) | 3 (8.1) | |
| White | 35 (33) | 21 (30.4) | 14 (37.8) | |
| Other | 57 (53.8) | 38 (55.1) | 19 (51.4) | |
| Ethnicity, N (%) | ||||
| Hispanic | 73 (68.9) | 47 (68.1) | 26 (70.3) | 0.99 |
| Non-Hispanic | 33 (31.1) | 22 (31.9) | 11 (29.7) | |
| BMI (kg/m2), mean ± SD | 20.8 ± 5.0 | 20.5 ± 5.0 | 21.5 ± 5.0 | 0.16 |
| BMI percentile, mean ± SD | 52.8 ± 33.8 | 51.7 ± 34.3 | 54.9 ± 33.3 | 0.77 |
| Obese, N (%) | 29 (27.4) | 19 (27.5) | 10 (27.0) | 0.99 |
| Dominant ventricle, N (%) | ||||
| Left | 47 (44.3) | 33 (47.8) | 14 (37.8) | 0.51 |
| Right | 55 (51.9) | 34 (49.3) | 21 (56.8) | |
| Undetermined/2-ventricle | 4 (3.8) | 2 (2.9) | 2 (5.4) | |
| Fontan type, N (%) | ||||
| Extra cardiac | 99 (93.4) | 66 (95.7) | 33 (89.2) | 0.24 |
| Lateral tunnel | 7 (6.6) | 3 (4.3) | 4 (10.8) | |
| Fenestration present at biopsy, N (%) | 9 (8.5) | 7 (10.1) | 2 (5.4) | 0.49 |
| Protein-losing enteropathy, N (%) | 9 (8.5) | 6 (8.7) | 3 (8.1) | 0.99 |
| Plastic bronchitis, N (%) | 0 (0) | 0 (0) | 0 (0) | |
Descriptive statistics of the overall cohort and stratified-by-CHFS group. Means and standard deviations reported for continuous variables and tested using a t test. Counts and percentages reported for categorical variables and tested with a Chi-square test.
CHFS, congestive hepatic fibrosis score.
Liver assessment, cardiac function, and medications
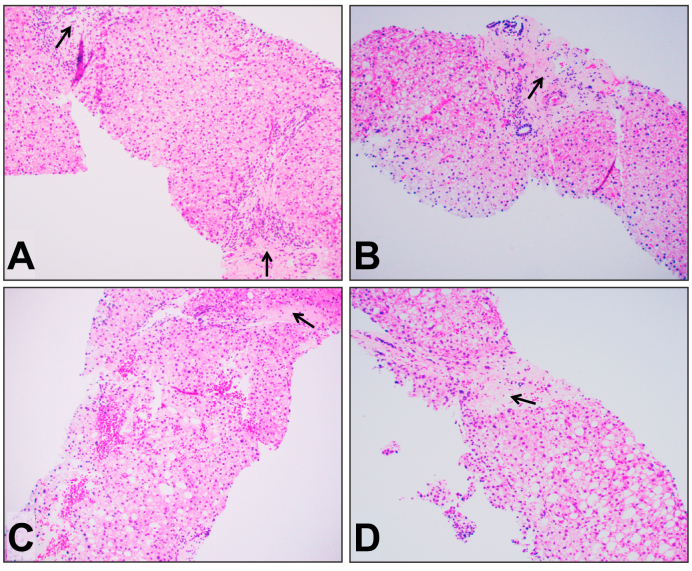
All but 1 patient (99.1%) had evidence of liver fibrosis on biopsy. The median CHFS was 2b (IQR 2a–3; N = 55 with CHFS 2a/b); bridging fibrosis was seen in 35% of cases, and cirrhosis was documented in 5.7% (N = 31 CHFS 3, N = 6 CHFS 4). A subset of 9 patients (10.4%) also had hepatic steatosis (range: 1–50%; Fig. 1), and this did not differ between CHFS subgroups. A subset of 17 patients had undergone liver ultrasonography around the time of liver biopsy, and although 38.5% exhibited evidence of hepatomegaly or hepatosplenomegaly, there were no differences between CHFS groups.
Fig. 1.
Representative examples of variable degree of fatty change/steatosis in patients with FALD.
(A) Two portal areas show marked fibrosis (arrow), consistent with CHFS 2b, and liver parenchyma shows rare macrovesicular fatty change (about 1–5%). (B) One expanded portal tract with fibrosis (arrow), consistent with CHFS 2b, and there is focal macrovesicular fatty change (5–10%). (C) One portal vein with marked fibrosis (arrow), consistent with CHFS 2b–3, and there is mild diffuse macrovesicular fatty change (10–20%). (D) One portal vein with bridging fibrosis (arrow), consistent with CHFS 3, and there is mild to moderate diffuse macrovesicular fatty change (30–40%). H&E stain. Original magnification: 100x for all. CHFS, congestive hepatic fibrosis score; FALD, Fontan-associated liver disease.
Cardiac evaluation by TTE revealed 12.3% had a mild decrease and 6.6% had moderate or severe decrease in ventricular systolic function (Table S2). At cardiac catheterisation, mean Fontan pressure was 12.9 ± 3.1 mmHg. There were no differences in cardiac catheterisation values when stratifying patients by CHFS. Common medications prescribed in the Fontan population were also reviewed (Table S2). In the CHFS 0–2b group, 88% were on aspirin and 6% on warfarin compared with 68% and 14%, respectively, in the CHFS 3–4 group (p = 0.05 for aspirin). Otherwise, no differences in medication profiles were identified.
Evaluation of laboratory values and clinical scores by severity of fibrosis
There were no differences in most liver-associated laboratory values (ALT, AST, ALP, GGT, protein, or albumin), renal function (BUN, creatinine, GFR), or BNP when comparing patients post-Fontan by CHFS (Table 2). Patients with CHFS 3–4 had a higher total bilirubin (1.7 ± 2.2 vs. 0.9 ± 0.7 mg/dl, p = 0.009). Furthermore, patients with CHFS 3–4 had modest elevations in PT (p = 0.03) and INR (p = 0.04). Examination of the hemogram revealed that patients with CHFS 3–4 had lower platelet counts when compared with patients with CHFS 0–2b (168.3 ± 58.4 vs. 203.9 ± 65.8 K/μl, p <0.01). The MELD and subsequent MELD-Na have been widely utilised to stratify risk of death in patients ≥12 years of age with end-stage liver disease.18 MELD-XI, which represents the MELD excluding INR, was developed to risk stratify patients with chronic liver disease who are being pharmacologically anticoagulated, which includes a substantial subset of the Fontan population.17,24 MELD-XI scores were higher in patients with more advanced fibrosis (CHFS 3–4: 11.6 ± 3.8 vs. 10.4 ± 2.1 for CHFS 0–2, p = 0.02). The APRI and FIB-4 scores were developed to predict fibrosis in patients with chronic hepatitis C infection and have since been validated via meta-analysis.20,21,25,26 In our cohort, patients with CHFS 3–4 had higher APRI and FIB-4 scores when compared with those with CHFS 0–2b (APRI: 0.5 ± 0.3 vs. 0.4 ± 0.1, p <0.01, and FIB-4: 0.6 ± 0.4 vs. 0.4 ± 0.2, p <0.01). Examination of both APRI and FIB-4 in predicting CHFS severity using a logistic regression model showed a moderate AUC of 0.69 for APRI and 0.69 for FIB-4 (Fig. S2). Further, using an APRI score cut-off of ≥0.6 optimally predicted bridging fibrosis (CHFS 3–4) with a sensitivity of 27.3% and specificity of 93.5%. Similarly, using a cut-off of 0.74, FIB-4 predicted bridging fibrosis with a sensitivity of 24.2% and specificity of 90.3%.
Table 2.
Laboratory values at the time of liver biopsy.
| Measured value, mean ± SD | Total N = 106 |
CHFS 0–2b n = 69 |
CHFS 3–4 n = 37 |
p value |
|---|---|---|---|---|
| Liver function panel | ||||
| Aspartate aminotransferase (U/L) | 34 ± 10.0 | 32.7 ± 8.4 | 36.6 ± 12.5 | 0.17 |
| Alanine aminotransferase (U/L) | 35.1 ± 10.6 | 34.9 ± 9.7 | 35.6 ± 12.5 | 0.97 |
| Gamma-glutamyl transferase (U/L), median [IQR] | 63 [36–85] | 64 [37–81.5] | 56.5 [35–88.8] | 0.98 |
| Protein (g/dl) | 6.6 ± 0.9 | 6.5 ± 0.8 | 6.9 ± 0.8 | 0.09 |
| Albumin (g/dl) | 3.7 ± 0.6 | 3.7 ± 0.6 | 3.8 ± 0.6 | 0.11 |
| Total bilirubin (mg/dl) | 1.2 ± 1.4 | 0.9 ± 0.7 | 1.7 ± 2.2 | <0.01 |
| Liver disease scores | ||||
| MELD | 10.7 ± 3.4 | 10.4 ± 3.2 | 11.6 ± 3.8 | 0.10 |
| MELD-XI | 10.7 ± 2.8 | 10.4 ± 2.1 | 11.6 ± 3.8 | 0.02 |
| MELD-Na | 11.4 ± 3.5 | 11.1 ± 3.3 | 12.3 ± 4.2 | 0.24 |
| PELD | −4.1 ± 5.3 | −4.6 ± 5.4 | −2.6 ± 5.0 | 0.06 |
| APRI | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.3 | <0.01 |
| FIB-4 | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.6 ± 0.4 | <0.01 |
| Coagulation | ||||
| PT (s), median [IQR] | 14.2 [13.5–16.1] | 14.1 [13.2–15.7] | 14.5 [14.1–17.1] | 0.03 |
| INR | 1.6 ± 0.9 | 1.6 ± 1.0 | 1.7 ± 0.7 | 0.04 |
| PTT (s), median [IQR] | 36 [33–42.5] | 36 [33–39] | 37 [33.2–48.8] | 0.41 |
| Renal function | ||||
| Blood urea nitrogen (mg/dl) | 14 ± 6.2 | 13.7 ± 5.2 | 14.8 ± 7.8 | 0.76 |
| Creatinine (mg/dl) | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.7 ± 0.2 | 0.10 |
| GFR (ml/min/1.73 m2) | 93.2 ± 21.8 | 94.6 ± 22.3 | 90.1 ± 20.9 | 0.39 |
| Haemogram | ||||
| White blood cells (K/μl) | 5.8 ± 2.3 | 5.7 ± 2.0 | 5.9 ± 2.8 | 0.93 |
| Haemoglobin (g/dl) | 13.9 ± 1.7 | 13.9 ± 1.6 | 14.1 ± 1.8 | 0.37 |
| Platelets (K/μl) | 191.5 ± 65.3 | 203.9 ± 65.8 | 168.3 ± 58.4 | <0.01 |
| Miscellaneous | ||||
| Brain natriuretic peptide (pg/ml), median [IQR] | 16 [6.5–35] | 14 [1.2–18.8] | 28.5 [8.8–121.8] | 0.07 |
Clinical statistics of the overall cohort and stratified-by-CHFS group. Means and standard deviations reported for continuous variables and tested using a t test. For highly non-normal distributions, medians and interquartile ranges reported and tested using the Mann–Whitney U test. Counts and percentages reported for categorical variables and tested with a Chi-square test.
APRI, aspartate aminotransferase-to-platelet ratio index; CHFS, congestive hepatic fibrosis score; FIB-4, fibrosis-4; GFR, glomerular filtration rate; INR, international normalised ratio; MELD, model of end-stage liver disease; MELD-Na, MELD-sodium; MELD-XI, MELD without INR; PELD, paediatric end-stage liver disease; PT, prothrombin time; PTT, partial thromboplastin time.
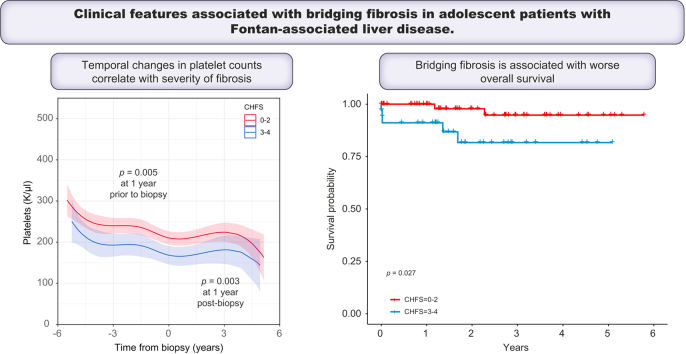
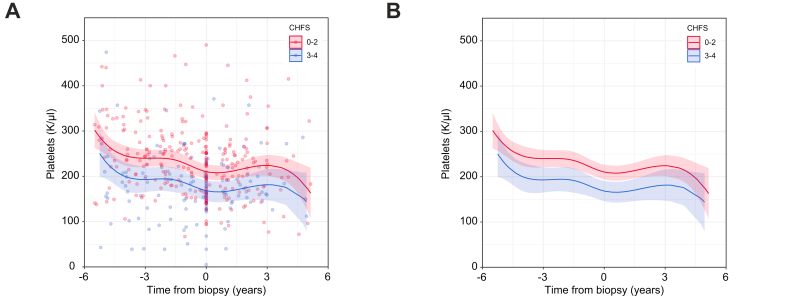
After observing significant differences in platelet counts and APRI scores between CHFS subgroups, we conducted a deeper analysis of temporal changes in platelet counts, as haemograms were often performed in this patient population. Analysis using mixed linear regression modelling of >400 discrete values across the entire cohort demonstrated that starting 1 year before liver biopsy, patients with CHFS 3–4 had lower platelet levels (184.6 K/μl [95% CI, 160.2–208.9]) than patients with CHFS 0–2b (227.6 K/μl [95% CI, 210.2–244.9], p = 0.005; Fig. 2). This difference persisted at 1-year post-biopsy, with lower platelets in patients with CHFS 3–4 (166.8 K/μl [95% CI, 143.7–189.9]) when compared with patients with CHFS 0–2b (209.3 K/μl [95% CI, 192.8–225.9], p = 0.003). Analysis of the difference of differences pre- and post-biopsy indicated that the mean difference over time was highly consistent (p = 0.98).
Fig. 2.
Temporal analysis of changes in platelet counts for patients post-Fontan, stratified by severity of fibrosis.
(A) Values for individual patients were plotted in relationship to the time of the liver biopsy, colour coded by CHFS 0–2 (red) and CHFS 3–4 (blue). (B) Mixed nonlinear regression modelling demonstrated significant differences in platelet counts in the years leading up to biopsy (p = 0.003) and post-biopsy (p = 0.005) (t test). Assessment of the difference of differences pre- and post-biopsy indicated that the mean difference was highly consistent (p = 0.98, t test). CHFS, congestive hepatic fibrosis score.
Survival and risk factor analysis
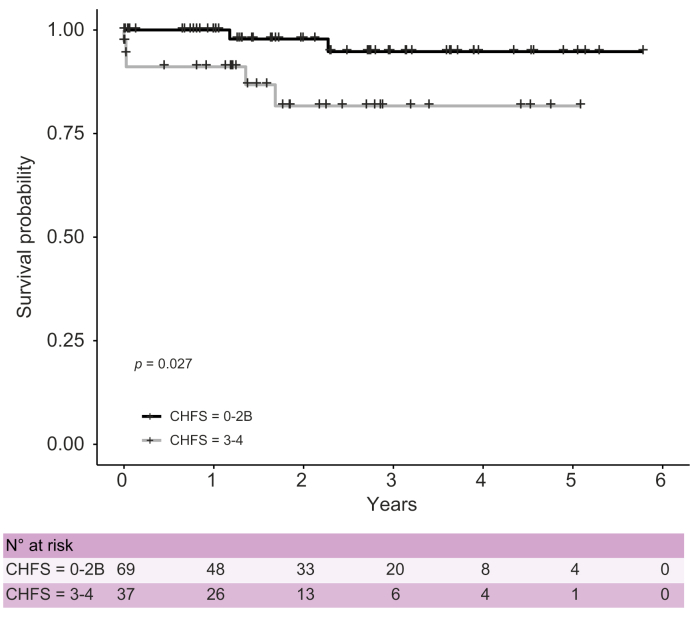
From the time of biopsy to the end of the study period, patients with bridging fibrosis (CHFS 3-4) had worse survival when compared with those scoring 0–2b (Fig. 3, p = 0.027). The median follow-up was 2.0 years in the CHFS 0–2b group and 1.5 years for the CHFS 3–4 group. Causes of death in the liver biopsy population are summarised in Table 3. Two patients with CHFS 1 and 2b died as a result of an acute catastrophic event with a mean MELD-Na of 12, whereas 5 patients with bridging fibrosis died as a result of multiorgan failure, including liver failure, with a mean MELD-Na of 30 (p = 0.006 vs. CHFS 0–2).
Fig. 3.
Overall survival by the CHFS group.
Kaplan–Meier survival plot of patients with CHFS 0–2b vs. CHFS 3–4 from the time of biopsy to end of the study period, showing a significant increased survival over time in patients with lower CHFS (p = 0.027, Log-rank test). CHFS, congestive hepatic fibrosis score.
Table 3.
Causes of death in liver biopsy population.
| Patient | CHFS biopsy score | Age at death (years) | MELD-Na at death | Cause of death |
|---|---|---|---|---|
| 1 | 1 | 14.5 | 10 | Cerebral air embolism during epicardial lead placement Anoxic brain injury |
| 2 | 2b | 14.4 | 14 | Cardiopulmonary arrest secondary to pulmonary haemorrhage |
| 3 | 3 | 16.6 | 25 | Heart failure exacerbation requiring Impella LVAD placement Acute renal failure, liver failure |
| 4 | 3 | 5.5 | 25∗ | Cardiac arrest, septic shock, disseminated intravascular coagulation Heart failure, renal failure, liver failure |
| 5 | 3 | 10.6 | 32 | Influenza A Heart failure, renal failure, liver failure |
| 6 | 3 | 20.6 | 31 | Acute cardiogenic shock following replacement of epicardial pacing system, ECMO, lower extremity ischaemic necrosis Heart failure, renal failure, liver failure |
| 7 | 4 | 20.5 | 37 | Progressive Fontan failure Heart failure, respiratory failure, renal failure, liver failure |
Liver failure was defined as INR >2 with or without hepatic encephalopathy.
CHFS, congestive hepatic fibrosis score; ECMO, extracorporeal membrane oxygenation; INR, international normalised ratio; LVAD, left ventricular assist device; MELD-Na, MELD-sodium; PELD, paediatric end-stage liver disease.
PELD and MELD-Na were equivalent.
Duration of Fontan circulation, Fontan pressure, female sex, obesity, and Hispanic ethnicity were explored as risk factors for bridging fibrosis in FALD. Examination of potential relationships between these variables and significant lab values including platelet count and bilirubin did not identify strong correlations (data not shown). Although neither presence of steatosis nor BMI were found to be associated with CHFS, they were associated with each other. Patients with steatosis had a higher mean BMI of 25.3 ± 4.8 kg/m2 when compared with those without (BMI 20.3 ± 4.8; p <0.01). A similar finding existed for BMI percentile, where patients with steatosis had a higher mean BMI percentile (80.2 ± 22.6) compared with those without (49.6 ± 33.5, p <0.01).
Discussion
FALD continues to present a diagnostic and management enigma for both cardiologists and hepatologists following the Fontan patient population. Within the context of an adult patient with a failing Fontan, it has been established that liver fibrosis is nearly universal, but manifestations of chronic liver disease are often difficult to detect until a clinical decompensating event occurs. Our study provides important insight into the prevalence of FALD in a relatively homogeneous population of patients in terms of age, ethnicity, and duration of Fontan, confirming that fibrosis is nearly universal as soon as 10 years post-Fontan, with a substantial subset showing evidence of bridging fibrosis. Comprehensive review of clinical data demonstrated that these patients are well compensated, without detectable changes in routine liver function tests with the exception of slight increases in total bilirubin and INR. The large sample size presented the opportunity to detect significant differences in platelet counts for patients with more severe FALD, and modelling of temporal changes, even in the years leading up to biopsy, suggests that decreasing values can serve as an indicator of severity of fibrosis. We also observed that an APRI score of ≥0.6 and an FIB-4 score of ≥0.74 could predict CHFS 3–4 with high specificity. Importantly, we confirmed that adolescent patients with severe FALD experienced worse overall survival. Collectively, these findings establish that FALD is highly prevalent in adolescent patients post-Fontan, and despite being clinically ‘silent’, bridging fibrosis may represent a risk factor for early death.
Data in the present study confirm that the histopathological changes associated with FALD occur earlier than previously reported, with a similar distribution of bridging fibrosis. The first series of patients with FALD were reported in the mid-2000s, primarily as a new histologic entity in adult patients examined at autopsy.[27], [28], [29] Subsequently, congenital heart disease programmes have implemented surveillance liver biopsy programmes and reported their experience. The Boston Children’s Hospital group reported on 68 patients in 2015, with a mean age of 23.2 years, who underwent biopsy at a mean of 18.1 years post-Fontan.7 They observed fibrosis in 100% of liver biopsies, with 78% showing bridging fibrosis. Shortly thereafter, the Children’s Hospital of Philadelphia group reported on 74 patients, mean age 17.7 years, who underwent biopsy at a mean of 14.9 (range 3.1–25.3) years post-Fontan.5 This study also reported 100% fibrosis, with 44.6% bridging fibrosis. This group was the first to attempt to risk stratify patients based on CHFS subgroups, but there were no differences in laboratory values or cardiac catheterisation variables. A follow-up study in this population examined the utility of Sirius Red staining for fibrosis and showed an association between degree of fibrosis and time from Fontan.1 Similar single-centre studies from San Diego, Nevada, and the United Kingdom have reported on liver biopsy findings in young adult patients 12.9–21.4 years post-Fontan, each confirming nearly universal presence of fibrosis with a subset of patients (up to 68%) exhibiting bridging fibrosis and limited associations between fibrosis and clinical variables.8,9,30 Only 1 prior study demonstrated an association between Fontan pressure and more advanced fibrosis, but our results do not support this (Table S2, p = 0.23).30
There has been controversy surrounding the role for and procedural risk of surveillance liver biopsy in the Fontan population, with a recent survey of US congenital heart disease centres indicating only 45% routinely perform liver biopsy.31 In our experience, the risk for procedural complications using the transvenous approach, even with routine use of a 2-pass technique, is minimal, even among the subset of patients who had histologic evidence of cirrhosis. Given the observation that more advanced fibrosis was associated with worse overall survival even in the setting of compensated liver function tests, our data provide evidence that liver biopsy offers important prognostic value to the clinical team, particularly in patients who may be at risk for future decompensating events, such as infection or Fontan failure. Our observation of associations between degree of fibrosis and patient survival in the pre-transplant, adolescent population is novel and may reflect both the strength of our sample size and a dropout effect in prior retrospective adult studies, where liver biopsies were not obtained before mortalities and thus could not be stratified by severity of fibrosis.7 Another possibility is that underlying fibrotic liver disease represents a greater clinical risk in adolescent patients who experience decompensating events when compared with the adult Fontan population. Among all of the deaths in the bridging fibrosis group, the MELD-Na was elevated, confirming that liver failure was a contributing factor (Table 3). If this is indeed true, consideration for heart transplant alone versus CHLT in the adolescent population becomes even more complex, as FALD has been shown to stabilise but not regress at 1 year post-transplant in children post-Fontan undergoing heart transplant alone.32 In practice, CHLT for paediatric patients for all causes has been infrequent, with only 15 cases performed in the entire USA since 2010, and it is possible that paediatric patients who require heart transplant for a failing Fontan in the setting of severe FALD are considered too high risk for this procedure.33 Further studies are necessary to gain a deeper understanding of the impact of advanced FALD and role for CHLT in the paediatric population.
Laboratory values have thus far provided little predictive value as to the degree of fibrosis in FALD (summarised in Keung et al.34). Our examination of liver function tests in adolescent patients confirm that FALD rarely results in abnormalities, which is consistent with prior adult studies.5,8,9,30 Patients with bridging fibrosis in our study had a mild elevation in total bilirubin (1.7 ± 2.2 vs. 0.9 ± 0.7 mg/dl, p <0.02) when compared with patients with CHFS 0–2. This finding suggests that fasting serum bile acids, a more sensitive marker of liver disease than bilirubin, should be examined as a biomarker of disease severity in FALD.35 Attempts to model the degree of liver dysfunction using various iterations of the MELD score in patients with FALD has generated largely negative results, with only MELD-XI showing a mild association with total hepatic fibrosis scores in our study and a prior study.24 Nonetheless, among patients with bridging fibrosis who died, the MELD-Na was highly elevated, which suggests that decompensated chronic liver disease contributed to mortality in these patients (Table 3).
Analysis of the CBC revealed that patients with bridging fibrosis have lower platelet counts when compared with patients with CHFS 0–2 (168.3 ± 58.4 vs. 203.9 ± 65.8 K/μl, p = 0.009). The platelet count for patients with CHFS 3–4 in our study is consistent with prior studies (range 155–185 K/μl), although this lower value for patients with advanced FALD is still in the ‘normal range’ for platelets (>150 K/μl).5,7,8,30 Based on our observations, we analysed the APRI and FIB-4 scores, which integrate platelet counts and were developed as surrogate markers for degree of fibrosis in hepatitis C.20 Although FALD is not a classical inflammatory disease process, which is what these scoring systems were designed to address, hepatic venous outflow tract pressure-related hepatic lobular congestion is a well-recognised but poorly understood pathophysiology post-Fontan. We may hazard to say that seemingly inconsequential increases in right-sided heart pressures may lead to a significant zone 3-focused, hepatic fibro-inflammatory process. Indeed, a recent study examined both APRI and FIB-4 in relation to non-invasive measures of liver fibrosis in patients post-Fontan, demonstrating limited relationship to severity of fibrosis.36 In a large meta-analysis, APRI scores >1.0 were shown to have a sensitivity of 76% and specificity of 72% for predicting cirrhosis.25 Our analysis demonstrated that APRI ≥0.6 was predictive of bridging fibrosis, with high specificity. Nonetheless, for lower APRI scores, prior studies have suggested that it should be combined with other modalities, such as elastography, to increase predictive value.37 By focusing on platelet count alone, we were able to detect significant differences in platelet counts leading up to and after biopsy for patients post-Fontan with bridging fibrosis (Fig. 2). This modelling suggests an early divergence for patients who go on to develop bridging fibrosis and hints at the pathophysiology of FALD. Our data support monitoring trends in APRI scores and progressive thrombocytopenia, a recognised and well-described manifestation of chronic liver disease, as part of a surveillance strategy in the adolescent Fontan population.38
No study thus far has examined the cumulative risk of FALD and chronic liver disease of other aetiologies in patients post-Fontan. Male sex, Hispanic ethnicity, and obesity are all recognised risk factors for the development of non-alcoholic fatty liver disease (NAFLD), the most prevalent form of chronic liver disease in both children and adults in the USA.39,40 Relationships between obesity, race, and ethnicity and poor long-term outcomes in the Fontan population have been reported.41,42 Prior studies have not investigated hepatic steatosis in the context of FALD, although 1 reported it on histology.5 We observed that 10.4% of patients had hepatic steatosis on biopsy, with a range of involvement and severity (Fig. 1). Although no associations existed between sex, race, ethnicity, BMI, or obesity and severity of FALD, we did observe that patients with higher BMI and BMI percentile were more likely to have steatosis (p <0.01 for both). These data suggest that adolescent patients post-Fontan at risk for obesity should receive close follow-up and be counselled regarding the risks of malnutrition, high-fat diet, metabolic syndrome, lifestyle modification (including alcohol avoidance), and hepatotoxic medications. Interestingly, patients with less severe fibrosis were more likely to be taking aspirin, for unclear reasons (Table S2, p = 0.05). Although this has not been explored in the paediatric population, aspirin use has been associated with reduced progression of fibrosis in NAFLD and following liver transplantation.[43], [44], [45], [46] Further studies are warranted to investigate the physiologic mechanism of Fontan-associated steatohepatitis, including associated risk factors, complications, and survival outcomes.
This study has limitations. Our dataset was obtained retrospectively, from 1 centre, with variations in the timing and availability of clinical data. There were too few studies available to correlate biopsy findings with abdominal imaging modalities, and not all lab values, particularly longitudinal ALT and AST values, were available for all patients. Thus, regression modelling of temporal changes in APRI and FIB-4 was not possible. The frequency of obesity and hepatic steatosis was <30%, which limited the power of risk factor analysis in these patients. Also, liver biopsy data represent a single time point and may not reflect severity of FALD because of sampling variation even with 2 biopsy passes, although a recent retrospective analysis demonstrated that liver biopsy never overestimated and often underestimated severity of fibrosis at the time of CHLT.47
Conclusion
This study provides insight into the prevalence of FALD in a large, relatively homogeneous, and predominantly ethnic minority adolescent population. We establish that fibrosis is universally present within the first decade post-Fontan, with substantial rates of bridging fibrosis. There was an association between bridging fibrosis and worse survival in this population. Higher APRI scores and lower platelet counts were observed in patients with more severe fibrosis, suggesting that these values may be followed as a marker for FALD progression. Further prospective studies are required to determine the likelihood of progression of fibrosis, and the possible susceptibility to early steatosis in post-Fontan patients. A consensus-based approach to screening for FALD, including non-invasive liver disease monitoring, in the adolescent population is required.
Financial support
JE is supported by a career development award from the National Cancer Institute (K08 CA245220-01), and SRK is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121191).
Authors’ contributions
Involved in the conception or design of the work: JE, GY, RK, SB, NP. Literature screen and review: JE, SK, NP. Data acquisition and statistical analysis: CW, NP, PS, CT, JC, RS, SZ, NS. Analysis and interpretation of data: CG, GY, RK, JE, NP. Drafted the article: JE, SK, CG, NP. Critically revised the article: all contributing authors. Finally approved the version to be published: all contributing authors.
Data availability statement
Data may be made available upon request, subject to USC institutional review board policies.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100362.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Goldberg D.J., Surrey L.F., Glatz A.C., Dodds K., O’Byrne M.L., Lin H.C. Hepatic fibrosis is universal following Fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roeleveld P.P., Axelrod D.M., Klugman D., Jones M.B., Chanani N.K., Rossano J.W. Hypoplastic left heart syndrome: from fetus to Fontan. Cardiol Young. 2018;28:1275–1288. doi: 10.1017/S104795111800135X. [DOI] [PubMed] [Google Scholar]
- 3.Gordon-Walker T.T., Bove K., Veldtman G. Fontan-associated liver disease: a review. J Cardiol. 2019;74:223–232. doi: 10.1016/j.jjcc.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Emamaullee J., Zaidi A.N., Schiano T., Kahn J., Valentino P.L., Hofer R.E. Fontan-associated liver disease: screening, management, and transplant considerations. Circulation. 2020;142:591–604. doi: 10.1161/CIRCULATIONAHA.120.045597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surrey L.F., Russo P., Rychik J., Goldberg D.J., Dodds K., O’Byrne M.L. Prevalence and characterization of fibrosis in surveillance liver biopsies of patients with Fontan circulation. Hum Pathol. 2016;57:106–115. doi: 10.1016/j.humpath.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Rychik J., Veldtman G., Rand E., Russo P., Rome J.J., Krok K. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol. 2012;33:1001–1012. doi: 10.1007/s00246-012-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu F.M., Jonas M.M., Opotowsky A.R., Harmon A., Raza R., Ukomadu C. Portal and centrilobular hepatic fibrosis in Fontan circulation and clinical outcomes. J Heart Lung Transplant. 2015;34:883–891. doi: 10.1016/j.healun.2015.01.993. [DOI] [PubMed] [Google Scholar]
- 8.Munsterman I.D., Duijnhouwer A.L., Kendall T.J., Bronkhorst C.M., Ronot M., van Wettere M. The clinical spectrum of Fontan-associated liver disease: results from a prospective multimodality screening cohort. Eur Heart J. 2019;40:1057–1068. doi: 10.1093/eurheartj/ehy620. [DOI] [PubMed] [Google Scholar]
- 9.Evans W.N., Acherman R.J., Mayman G.A., Galindo A., Rothman A., Winn B.J. The rate of hepatic fibrosis progression in patients post-Fontan. Pediatr Cardiol. 2020;41:905–909. doi: 10.1007/s00246-020-02331-0. [DOI] [PubMed] [Google Scholar]
- 10.Patel N.D., Sullivan P.M., Sabati A., Hill A., Maedler-Kron C., Zhou S. Routine surveillance catheterization is useful in guiding management of stable Fontan patients. Pediatr Cardiol. 2020;41:624–631. doi: 10.1007/s00246-020-02293-3. [DOI] [PubMed] [Google Scholar]
- 11.Atz A.M., Zak V., Mahony L., Uzark K., D’agincourt N., Goldberg D.J. Longitudinal outcomes of patients with single ventricle after the Fontan procedure. J Am Coll Cardiol. 2017;69:2735–2744. doi: 10.1016/j.jacc.2017.03.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen A.M., Hay J.E. Review article: the management of cirrhosis in women. Aliment Pharmacol Ther. 2014;40:1146–1154. doi: 10.1111/apt.12974. [DOI] [PubMed] [Google Scholar]
- 13.Lau-Corona D., Kershenobich D., Gutierrez-Reyes G. The impact of genetic variability on liver disease in the Hispanic/Latin-American population. Autoimmunity. 2011;44:549–554. doi: 10.3109/08916934.2011.592883. [DOI] [PubMed] [Google Scholar]
- 14.Streba L.A.M., Vere C.C., Rogoveanu I., Streba C.T. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World J Gastroenterol. 2015;21:4103–4110. doi: 10.3748/wjg.v21.i14.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenway S.C., Crossland D.S., Hudson M., Martin S.R., Myers R.P., Prieur T. Fontan-associated liver disease: implications for heart transplantation. J Heart Lung Transplant. 2016;35:26–33. doi: 10.1016/j.healun.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz G.J., Work D.F. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 17.Heuman D.M., Mihas A.A., Habib A., Gilles H.S., Stravitz R.T., Sanyal A.J. MELD-XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2007;13:30–37. doi: 10.1002/lt.20906. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y., Qi X., Guo X. Child–Pugh versus MELD score for the assessment of prognosis in liver cirrhosis. Medicine (Baltimore) 2016;95:e2877. doi: 10.1097/MD.0000000000002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barshes N.R., Lee T.C., Udell I.W., O’Mahoney C.A., Karpen S.J., Carter B.A. The pediatric end-stage liver disease (PELD) model as a predictor of survival benefit and posttransplant survival in pediatric liver transplant recipients. Liver Transpl. 2006;12:475–480. doi: 10.1002/lt.20703. [DOI] [PubMed] [Google Scholar]
- 20.Wai C.-T., Greenson J.K., Fontana R.J., Kalbfleisch J.D., Marrero J.A., Conjeevaram H.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 21.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., Nalpas A., Dhalluin-Venier V. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 22.Dindo D., Demartines N., Clavien P.-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosch D.E., Koro K., Richards E., Hoch B.L., Jalikis F., Koch L.K. Validation of a congestive hepatic fibrosis scoring system. Am J Surg Pathol. 2019;43:766–772. doi: 10.1097/PAS.0000000000001250. [DOI] [PubMed] [Google Scholar]
- 24.Evans W.N., Acherman R.J., Ciccolo M.L., Carrillo S.A., Galindo A., Rothman A. MELD-XI scores correlate with post-Fontan hepatic biopsy fibrosis scores. Pediatr Cardiol. 2016;37:1274–1277. doi: 10.1007/s00246-016-1428-1. [DOI] [PubMed] [Google Scholar]
- 25.Lin Z.-H., Xin Y.-N., Dong Q.-J., Wang Q., Jiang X.-J., Zhan S.-H. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 26.Li Y., Chen Y., Zhao Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: a meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiesewetter C.H., Sheron N., Vettukattill J.J., Hacking N., Stedman B., Millward-Sadler H. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–584. doi: 10.1136/hrt.2006.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendall T.J., Stedman B., Hacking N., Haw M., Vettukattill J.J., Salmon A.P. Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol. 2008;61:504–508. doi: 10.1136/jcp.2007.052365. [DOI] [PubMed] [Google Scholar]
- 29.Ghaferi A.A., Hutchins G.M. Progression of liver pathology in patients undergoing the Fontan procedure: chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348–1352. doi: 10.1016/j.jtcvs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Silva-Sepulveda J.A., Fonseca Y., Vodkin I., Vaughn G., Newbury R., Vavinskaya V. Evaluation of Fontan liver disease: correlation of transjugular liver biopsy with magnetic resonance and hemodynamics. Congenit Heart Dis. 2019;14:600–608. doi: 10.1111/chd.12770. [DOI] [PubMed] [Google Scholar]
- 31.Di Maria M.V., Brown D.W., Cetta F., Ginde S., Goldberg D., Menon S.C. Surveillance testing and preventive care after Fontan operation: a multi-institutional survey. Pediatr Cardiol. 2019;40:110–115. doi: 10.1007/s00246-018-1966-9. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez D.S., Mao C., Mahle W.T., Kanter K.R., Alazraki A., Braithwaite K. Pretransplantation and post-transplantation liver disease assessment in adolescents undergoing isolated heart transplantation for Fontan failure. J Pediatr. 2021;229:P78–P85.E2. doi: 10.1016/j.jpeds.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 33.Menteer J., Goldbeck C., Herrington C., Yanni G., Emamaullee J.A. Immunologic and survival benefits of combined heart-liver transplantation in children. Transplantation. 2021 Sep 1;105(9):e107–e108. doi: 10.1097/TP.0000000000003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keung C.Y., Zentner D., Gibson R.N., Phan D.-K.H., Grigg L.E., Sood S. Fontan-associated liver disease: pathophysiology, investigations, predictors of severity and management. Eur J Gastroenterol Hepatol. 2020:907–915. doi: 10.1097/MEG.0000000000001641. [DOI] [PubMed] [Google Scholar]
- 35.Azer S.A., Coverdale S.A., Byth K., Farrell G.C., Stacey N.H. Sequential changes in serum levels of individual bile acids in patients with chronic cholestatic liver disease. J Gastroenterol Hepatol. 1996;11:208–215. doi: 10.1111/j.1440-1746.1996.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 36.An H.S., Choi Y.H., Song M.K., Lee S.Y., Kim G.B., Bae E.J. Early development of hepatic fibrosis after Fontan procedure: a non-invasive study of a subclinical liver disease. Int J Cardiol. 2020;320:64–69. doi: 10.1016/j.ijcard.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Chou R., Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807–820. doi: 10.7326/0003-4819-158-11-201306040-00005. [DOI] [PubMed] [Google Scholar]
- 38.Afdhal N., McHutchison J., Brown R., Jacobson I., Manns M., Poordad F. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–1007. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Schwimmer J.B., Deutsch R., Kahen T., Lavine J.E., Stanley C., Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 40.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karamlou T., Peyvandi S., Federman M., Goff D., Murthy R., Kumar S.R. Resolving the Fontan paradox: addressing socioeconomic and racial disparities in patients with a single ventricle. J Thorac Cardiovasc Surg. 2018;155:1727–1731. doi: 10.1016/j.jtcvs.2017.11.103. [DOI] [PubMed] [Google Scholar]
- 42.Martinez S.C., Byku M., Novak E.L., Cedars A.M., Eghtesady P., Ludbrook P.A. Increased body mass index is associated with congestive heart failure and mortality in adult Fontan patients. Congenit Heart Dis. 2016;11:71–79. doi: 10.1111/chd.12296. [DOI] [PubMed] [Google Scholar]
- 43.Simon T.G., Henson J., Osganian S., Masia R., Chan A.T., Chung R.T. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:2776–2784.e4. doi: 10.1016/j.cgh.2019.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poujol-Robert A., Boëlle P.-Y., Conti F., Durand F., Duvoux C., Wendum D. Aspirin may reduce liver fibrosis progression: evidence from a multicenter retrospective study of recurrent hepatitis C after liver transplantation. Clin Res Hepatol Gastroenterol. 2014;38:570–576. doi: 10.1016/j.clinre.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Jiang Z.G., Feldbrügge L., Tapper E.B., Popov Y., Ghaziani T., Afdhal N. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther. 2016;43:734–743. doi: 10.1111/apt.13515. [DOI] [PubMed] [Google Scholar]
- 46.Iqbal U., Dennis B.B., Li A.A., Cholankeril G., Kim D., Khan M.A. Use of anti-platelet agents in the prevention of hepatic fibrosis in patients at risk for chronic liver disease: a systematic review and meta-analysis. Hepatol Int. 2019;13:84–90. doi: 10.1007/s12072-018-9918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaikunth S.S., Higgins J.P., Concepcion W., Haeffele C., Wright G.E., Chen S. Does liver biopsy accurately measure fibrosis in Fontan-associated liver disease? A comparison of liver biopsy pre–combined heart and liver transplant and liver explant post-transplant. Clin Transplant. 2020;34 doi: 10.1111/ctr.14120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be made available upon request, subject to USC institutional review board policies.