Abstract
The host defence of insects includes a combination of cellular and humoral responses. The cellular arm of the insect innate immune system includes mechanisms that are directly mediated by haemocytes (e.g., phagocytosis, nodulation and encapsulation). In addition, melanization accompanying coagulation, clot formation and wound healing, nodulation and encapsulation processes leads to the formation of cytotoxic redox‐cycling melanin precursors and reactive oxygen and nitrogen species. However, demarcation between cellular and humoral immune reactions as two distinct categories is not straightforward. This is because many humoral factors affect haemocyte functions and haemocytes themselves are an important source of many humoral molecules. There is also a considerable overlap between cellular and humoral immune functions that span from recognition of foreign intruders to clot formation. Here, we review these immune reactions starting with the cellular mechanisms that limit haemolymph loss and participate in wound healing and clot formation and advancing to cellular functions that are critical in restricting pathogen movement and replication. This information is important because it highlights that insect cellular immunity is controlled by a multilayered system, different components of which are activated by different pathogens or during the different stages of the infection.
Keywords: autophagy and apoptosis, cytotoxic intermediates, haematopoiesis, haemocytes, insect cellular immunity, prophenoloxidase
Here, we review insect cellular immune mechanisms that limit haemolymph loss and participate in wound healing and clot formation. We also cover haemocyte‐based functions that are critical in restricting pathogen movement and replication.

Abbreviations
- AMP
Antimicrobial peptide
- ApoLp
apolipophorin
- ATG
Autophagy‐related genes
- Col
Collier
- DCV
Drosophila C virus
- EBF
B‐cell factor
- FGF
Fibroblast growth factor
- FHV
Flock House virus
- GALE
Galectin
- GBP
Growth‐blocking peptide
- Hml
Hemolectin
- IAP
Inhibitor of apoptosis
- IMD
Immune deficiency
- JAK/STAT
Janus Kinase‐Signal Transducer and Activator of Transcription
- JNK
c‐Jun N‐terminal kinase
- LPS
Lipopolysaccharide
- Mys
Myospheroid
- NO
Nitric oxide
- PAP
Prophenoloxidase‐activating peptidase
- PDGF/VEGF
Platelet‐derived growth factors/vascular endothelial growth factors
- PGN
peptidoglycan
- PGRP
Peptidoglycan recognition proteins
- PHT
Posterior haematopoietic tissue
- PI3K
Phosphatidylinositol‐3‐kinase
- PLA2
phospholipase A2
- PLC
Phospholipase C
- PO
Phenoloxidase
- PPAE
Prophenoloxidase‐activating enzyme
- PPAF
Prophenoloxidase‐activating factor
- PPO
Prophenoloxidase
- PRR
Pattern Recognition Receptor
- PSC
Posterior signalling centre
- PSP
Plasmatocyte‐spreading peptide
- RNA
reactive nitrogen species
- ROS
reactive oxygen species
- RVFV
Rift Valley Fever Virus
- SOD
Superoxide dismutase
- SPH
Serine‐peptidase homolog
- TOR
Target of rapamycin
- VSV
Vesicular stomatitis virus
- βGRP
β‐1,3‐glucan recognition protein
HEMOLYMPH COAGULATION, CLOT FORMATION AND WOUND HEALING
The frontline of insect defence against pathogens is the cuticular coverage of epidermal body wall that acts as an effective physical barrier, and cuticle (intima) lining structures of ectodermal origin like the epithelial wall of the gut (stomatedeum and proctodeum), tracheae and reproductive ducts [1, 2, 3. These epithelia are normally in contact with microbes, and they serve as natural routes of microbial infection. Upon breaching or wounding of these walls, haemolymph (an aqueous medium in which the haemocytes are suspended and circulating) coagulates in the injured area and a haemolymph clot appears in this region [4, 5. Damaged epithelial cells release chemotactic cytokines like hemokinin [6] and haemocyte chemotactic peptide [7], which may be signalling molecules and/or adhesion peptides that induce rapid aggregation of haemocytes. The process involves rupturing and degranulation of granulocytes into the haemolymph, and consequently, an extracellular matrix appears and forms a soft clot sealing the wound [8, 9. Prophenoloxidase (PPO) cascade/transglutaminase activation subsequently leads to cross‐linking and melanization of the clot to form hard clot [10]. Phenoloxidase (PO) is not necessary for initial soft clot formation in larval Drosophila. However, it is involved in cross‐linking and melanization of soft clot, forming the hard clot [11]. In larval Anopheles gambiae, on the other side, PO may be involved at an early state of coagulation [12]. The c‐Jun N‐terminal kinase (JNK) pathway, small GTPases and TNF homolog Eiger were all required for the induction and lysis of crystal cells and the release of PPO precursors in the haemolymph at clot formation in Drosophila larvae [13, 14. Haemolymph coagulation requires Ca2+ for the activation of protein cross‐linking enzymes like transglutaminases as suggested in Leucophaea maderae [15]. Behaviour of plasmatocytes changes, possibly under the control of plasmatocyte‐spreading peptide (PSP) [16], and they aggregate around the injured area to tightly cover the underside of the hard clot, and finally, a scab is developed. Coagulants, haemocytes and melanization components form multi‐functional immune complexes, which are recruited to a wound surface or a nonself [17]. The clotting reaction is very fast, and the clot becomes completely insoluble after less than 3 min. Regeneration of the injured cells takes place, and the new cells grow across the wound site, replacing the scab [8].
Hemolectin (Hml, a multi‐domain protein containing domains that are observed in vertebrate and arthropod clotting factors with two discoidin domains that are most likely responsible for the protein's lectin activity) and Fondue are abundant clotting factors, which are induced upon injury in Drosophila larvae [18, 19, 20. Fondue in particular is a key transglutaminase substrate in the clot due to its multiple repeats with high ratios of alanine, glycine and glutamine [20, 21. A proteomic comparison of larval haemolymph proteins before and after clotting identified two sets: (i) those with increased levels after clotting include a ferritin‐subunit and two members of the immunoglobulin family and (ii) proteins present in lower amounts after clotting, which include two POs, lipophorin, a secreted gelsolin, a PPO‐activating peptidase and a mucin‐type domain containing protein [22]. Hml homologs were also reported from other insects, like the hemimetabolous triatomines [23]. Other clotting factors have been identified in insects. Lipophorins and other lipoproteins have been shown to participate in haemolymph clotting and melanization response in Galleria mellonella, Locusta migratoria and Periplaneta americana [8, 24. Two apolipoproteins, the apolipophorin apoLp‐I and apoLp‐I/II, have been reported from D. melanogaster and A. gambiae clots, respectively [12, 25. Also, G. mellonella apoLp‐III in sync with other apolipoproteins was reported as a component of neutrophil extracellular trap (net)‐like coagulation structures containing endogenous extracellular nucleic acids and acts in extracellular RNA‐mediated immune response [26]. Altogether, clot formation represents an important component of the overall insect immune defence. Aside from wound healing and limiting loss of haemolymph, a clot entraps microbes at the wound site thus preventing them entering the haemocoel and spreading to other tissues. Also, upon activation of the PO system, killing and elimination of the entrapped microbes are reinforced.
CELLULAR IMMUNE RESPONSES
These refer to the immune reactions mediated by haemocytes. The haemocytes and the plasma represent the two components of insect haemolymph. Haemocytes originate from mesodermally derived stem cells that differentiate into specific lineages. Certain types of haemocytes are responsible for a number of cellular defence responses and also for the production of important molecules in the humoral immune defences (Figure 1). In the latter context, haemocytes are actively involved in synthesizing a repertoire of antimicrobial peptides (AMPs) and proteins, which are released into the haemolymph following microbial invasion or integument damage. Haemocytes express the major classes of AMPs, subgrouped on the basis of their sequence composition and the secondary structures, including the linear amphipathic alpha‐helical AMPs like cecropins [27, 28, the defensins [29], and the proline‐ and glycine‐rich AMPs [30, 31. Different antimicrobial proteins are also expressed by haemocytes in response to microbial stimulation, including lysozymes [32], transferrins [33], an array of soluble forms of microbial pattern recognition receptors like C‐type lectins, peptidoglycan recognition proteins (PGRPs), β‐1,3‐glucan recognition proteins (βGRPs) and galectins (GALEs), among others [34, 35. Therefore, aspects of haemocyte biology will firstly be emphasized below, then followed by cell‐mediated immune responses.
FIGURE 1.

A simplified overview of haemocytes and their contribution to insect immunity and wound healing. Haemocytes include plasmatocytes, granulocytes, lamellocytes and oenocytoids/crystal cells. Cellular immune responses to microbial or parasitic infection are graphically depicted. These include encapsulation by lamellocytes, nodulation, phagocytosis and haemolymph clotting by plasmatocytes, and melanization by crystal cells and oenocytoids. The contribution of haemocytes and fat body in the prophenoloxidase (PPO) cascade activation is also portrayed, in addition to role of epithelia in immunity. Cytotoxic species released in epithelia include Reactive Oxygen Species (ROS), Reactive Nitrogen Species (RNS) and quinones. Upon infection, the fat body induces the production of clotting factors and serine proteases. The latter regulates the melanization response. Microbial recognition induces the expression of peptidoglycan recognition protein (PGRP), which regulate the activity of proteases that control the prophenoloxidase (PPO) cascade and melanization response
Haemocyte types
The terminology used to designate haemocytes by different authors is inconstant among insect species and differ from order to order and species to species. Morphological features of haemocytes are often a function of the media in which haemocytes are fixed and examined or developmental stage. Hence, different terms used for the same cell type may arise due to variations in cell structure, artefacts of fixation or spurious preparations, or different stages during the development of a single cell. Commonly, the classification of haemocytes is based on two major criteria: cell morphology [36, 37 and expression of marker genes [38, 39. So far, seven types of haemocytes have been identified in various insects: prohaemocytes, plasmatocytes, granulocytes, oenocytoids, spherulocytes, coagulocytes and adipohemocytes [40, 41. However, most insects do not have all seven haemocyte types. Prohaemocytes are small, rounded cells with a high nucleus:cytoplasm ratio and no specialized cytoplasmic organelles. Plasmatocytes have more cytoplasm than prohaemocytes, and their membrane forms filopodia and ruffles. Granulocytes have electron‐dense, regularly sized, acidophilic granules. Oenocytoids are large, oval cells whose cytoplasm contains agglomerates of crystalline material and/or microtubules. Spherulocytes or adipohaemocytes are blood cells with basophilic, variably sized and shaped granules. The range of blood cells characterized above is simpler, or modified, in some cases. For example, in Drosophila, no granulocytes or spherulocytes are encountered. Other Dipterans, such as Calliphora, possess granulocytes [42].
In lepidopterans, such as Anticarsia gemmatalis, Bombyx mori, Manduca sexta, as in several other models, five cell types are present: prohaemocytes, plasmatocytes, granulocytes, spherulocytes and oenocytoids [43, 44, 45, 46, 47. In these insects, granulocytes are the most abundant haemocyte type in circulation as a major phagocyte. They are morphologically distinguished by the granules in their cytoplasm, their ability to strongly adhere and spread symmetrically on foreign surfaces. Plasmatocytes are usually larger than granulocytes, spread asymmetrically on foreign surfaces and are the main capsule‐forming haemocytes. Non‐adhesive haemocytes in larval Lepidoptera include oenocytoids that contain PO precursors [47] and spherule cells that are potential sources of cuticular components. Prohaemocytes are non‐adhesive haemocytes and are present as a small proportion of haemocytes in circulation. In haematopoietic organs, prohaemocytes, as progenitor cells, mature and differentiate primarily into plasmatocytes and partly into the other types of haemocytes. In circulation, the different types of haemocytes are able to proliferate. In Coleoptera also, there are at least five types of haemocytes, namely prohaemocytes, oenocytoids, plasmatocytes, granulocytes and spherulocytes [48, 49 comparable to certain Cyclorrhapha [50, 51, 52. In some basal insects, like the cricket Gryllus bimaculatus [53] and the migratory locust L. migratoria [54], six types were recognized, namely granulocytes, plasmatocytes, prohaemocytes, coagulocytes, oenocytoids and spherulocytes. However, others used a different terminology to describe the different morphological types of haemocytes in six triatomines (Hemipteran) [55]. They recognized seven types: prohaemocytes, plasmatocytes, granular cells, cystocytes, oenocytoids, adipohaemocytes and giant cells.
In Drosophila larvae, plasmatocytes, crystal cells and lamellocytes as well as prohaemocytes were observed [28, 56, 57. However, these types are morphologically and functionally similar to granulocytes (= plasmatocytes), oenocytoids (= crystal cells) and plasmatocytes (= lamellocytes). Plasmatocytes represent a major percentage of all mature haemocytes and make up ca. 90%–95% of circulating haemocytes, they are strongly adhesive in vitro, and function as the major phagocytes most similar to the mammalian monocyte/macrophage lineage [56, 57. Crystal cells are non‐adhesive cells that account for a small proportion of approximately 5% of the haemocyte population found in circulation. Crystal cells are recognized by their rounded morphology and the expression of PO cascade components and release of chemicals that encase microbes in a hardened gel [57]. Lamellocytes are identified as large, flat, adhesive cells; their main function is encapsulation of parasitoids and other large foreign targets. Lamellocytes are virtually absent in naïve Drosophila larvae, but rapidly differentiate from prohaemocytes following attack by parasitoid wasps and during metamorphosis [56]. Prohaemocytes mainly reside in the lymph gland where they originate from pre‐prohaemocytes; however, a small number is in circulation. The three haemocyte types are thought to be differentiated from prohemocytes [58]. However, recent developments suggest that Drosophila haematopoiesis enfold a striking plasticity [57]. For example, new crystal cells in third instar Drosophila larvae originate through a Notch‐dependent process of plasmatocyte transdifferentiation [59].
In mosquitoes, comparative studies of haemocyte types based on antigenic, functional and morphological approaches revealed that both Aedes aegypti and A. gambiae have in circulation the three haemocyte types of granulocytes, oenocytoids and prohemocytes [60, 61. Granulocytes are strongly adhesive and phagocytic. They are also the most abundant circulating cell type in both mosquito species, comprising 80%–95% of the circulating haemocyte population [62]. Oenocytoids and prohaemocytes comprise a small percentage of the total haemocyte population, both ≤10%. Oenocytoids are non‐adhesive and constitutively express PO; however, granulocytes express inducible PO following immune challenge. In contrast to Drosophila or lepidopterans, mosquitoes apparently do not produce any haemocyte type specialized for capsule formation [62, 63. Haemocytes kill pathogens via phagocytosis, melanization and lysis. No haematopoietic organs have been identified in adult mosquitoes. Prohaemocytes likely are progenitor cells with unclear haematopoiesis characteristics [62].
The number of circulating haemocytes in insects varies according to the age/developmental stage, physiological conditions and infection status [64]. The density of circulating haemocytes reported in different insect species is variable. For instance, adult D. melanogaster contain between 1000 and 2000 circulating hemocytes [65], adult mosquitoes have a range of 500–4000 circulating hemocytes [62], adult Glossina morsitans contain 500–900 haemocytes/μl of hemolymph [66] comparable to the fruit fly Anastrepha obliqua [50]. Lepidopteran [67, 68, 69, 70 and orthopteroid species contain haemocyte densities in the same range [71, 72, 73, 74.
Haematopoiesis
Haematopoiesis and haemocytes population
In D. melanogaster, the haemocytes were found to arise during the embryonic and post‐embryonic development [57, 59, 75. During embryogenesis, a first population of haemocytes is produced from head or dorsal mesoderm, while during the post‐embryonic development, a second population is produced in mesodermally derived haematopoietic organs. Some mature blood cells have the ability to divide while circulating. Nevertheless, most of blood cell proliferation and differentiation occurs in the haematopoietic organs [76]. These organs provide the proper cellular and molecular environment for the control of cell proliferation and differentiation, viz. in the so‐called stem cell niches [77].
The haematopoietic organs in lepidopterans are prominent single‐lobed structures that are located as paired organs in each of the meso‐ and metathorax, but smaller organs also occur along the segmentally reiterated tracheal spiracles (Figure 2a) [78, 79. The number of haemocytes in the haematopoietic organs upsurges significantly during larval development to a maximum of 300 000 cells/organ just prior the onset of metamorphosis in Spodoptera frugiperda [80]. Prohaemocytes and plasmatocytes are the major cell type and reside in an inner medullary region and the outer cortical region of the haematopoietic organ, respectively. Also, prohaemocytes and plasmatocytes are abundantly contained in haematopoietic organs of M. sexta and B. mori [47, 79, 81. These findings may suggest that lepidopteran plasmatocytes are derived basically from progenitor cells of haematopoietic organs, whereas the greater part of granulocytes, oenocytoids and spherule cells is derived from proliferating haemocytes in circulation [64]. Extirpation of the haematopoietic organs in M. sexta significantly reduces the prohaemocyte and plasmatocyte number, inferring a true larval haematopoietic function [81].
FIGURE 2.
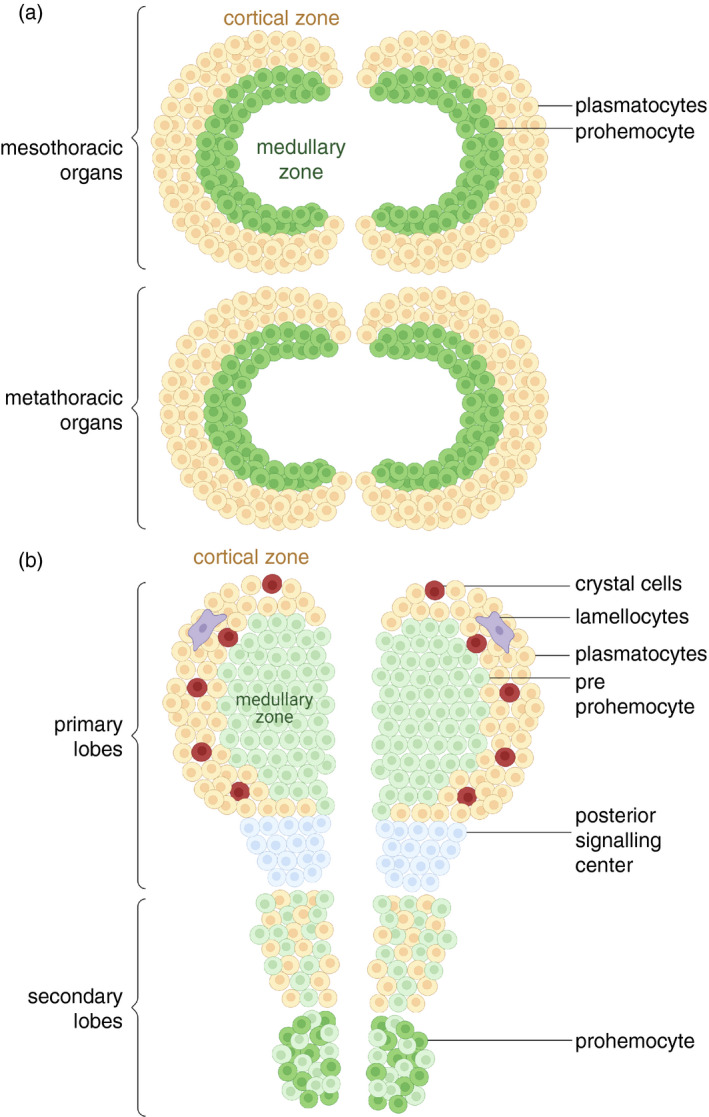
Structure of haematopoietic organs. (a) The haematopoietic organs of the 6th instar Spodoptera frugiperda. These are equal‐sized semi‐circular paired organs located in the mesothorax and metathorax. The inner medullary and outer cortical zones of each organ contain prohaemocytes and plasmatocytes, respectively (see Gardiner and Strand, 2000). (b) Drosophila melanogaster larval lymph gland. Each lymph gland consists of (i) a primary lobe, which is subdivided into a posterior signalling centre (PSC) that contains PSC cells (blue), a medullary zone (MZ) containing mainly prohaemocytes (green) and a cortical zone (CZ) containing primarily plasmatocytes (brown), crystal cells (red) and lamellocytes (violet), and (ii) two or more secondary lobes. Haemocytes that proliferate and differentiate during the larval stages reside in the primary lobe. Cells in between the CZ and the MZ are differentiating blood cells that show features of both progenitors and mature haemocytes
In Drosophila, the haematopoietic organs are the lymph glands (Figure 2b), which are formed bilaterally along the anterior part of the dorsal vessel during embryogenesis. The structure of Drosophila larval lymph gland and the differentiation of haemocytes have been well‐documented [38, 82. Each lymph gland appears to consist of an anterior primary lobe and posterior secondary lobes. The primary lobe consists of: (i) a posterior signalling centre (PSC) that contains a unique population of cells and (ii) two anterior zones; one is a medullary zone that contains a relatively uniform population of pluripotent prohaemocytes (sometimes called stem‐like cells), and the other is a cortical zone that contains plasmatocytes, crystal cells and lamellocytes (the latter type can be observed following parasitoid attack) [85]. Secondary lobes contain pre‐prohaemocytes, prohaemocytes and some plasmatocytes. The balance between prohaemocytes and differentiated blood cells is moderated by the PSC [82].
Maintenance of circulating haemocytes or ‘the blood cell development’ in Drosophila larvae involves two phases, that is derive from two lineages. In the first, ‘primitive’ phase – the embryonic lineage – haemocytes develop from the early embryo head mesoderm and differentiate mainly into self‐renewing plasmatocytes that will continue to proliferate in circulation during the larval stage. This lineage is capable of producing crystal cells and lamellocytes during inflammatory conditions. The embryo‐derived plasmatocytes and crystal cells are found circulating in the haemolymph and form resident (sessile) clusters in subepidermal locations in ‘sessile pockets’, aka haematopoietic pockets [59, 86, 87. This event appears to be more important during early instars. The second phase – the lymph gland lineage – gives rise to adult haemocytes, produced in a small organ, the lymph gland, and occur primarily in the late third instar before their degeneration during metamorphosis [38, 57, 82, 83. Prohaemocytes can give rise to all mature haemocytes in the lymph gland. An essential aspect of the Drosophila larval haematopoiesis is that the lymph gland produces plasmatocytes and crystal cells that are not released/disperse until pupariation or upon injury such as parasitoid wasp egg infection. Yet, throughout larval development, both haemocyte types increase in number [57, 88.
A rapid increase in the circulating haemocytes can occur in response to infection, stress, or wounding. This may partly, be due to, for example in Drosophila, rapid differentiation and release of lamellocytes from its lymph glands ensuing parasitoid infestation [89, 90. Also, differentiated haemocytes are often sessile and weakly adhere to the surface of internal organs, but in response to immune challenge, they can rapidly enter circulation and increase their number in the hemolymph [91]. In Drosophila, cellular and molecular events involving actin stabilization, the GTPases Rac1 and Rac2, the JNK Basket are required for successful encapsulation of parasitoids eggs by lamellocytes and for recruitment of plasmatocytes into circulation [92, 93.
In hemimetabolous species, the process of haemocyte recruitment and replenishment is not well understood. Haemocyte count declines rapidly after infection mostly due to terminal events such as nodule/capsule formation and removing the plasmatocytes from circulation, or due to lyses to release pathogen toxic compounds into the haemocoel, or simply by haemocyte coagulation during injury and wound healing [94, 95, 96. However, within a very few hours this returns to its basal level and may then become elevated above the control level for a day. In L. migratoria, the circulating haemocytes undergo active proliferation to replenish themselves following infection, and they are the primary sites of haemocyte production in adult locusts, not the haematopoietic tissue [72].
Drosophila sessile haemocyte clusters and transdifferentiation
The Drosophila larval haemocytes are distributed in three major compartments: the circulating haemocytes, the lymph gland and a population of sessile cells attached to epithelial tissues [57]. Haemocytes in the sessile clusters outside the lymph gland are densely packed in a banded pattern (i.e., linked through interdigitations) under the epidermis and within muscle tissue, but many are also found attached to the imaginal discs, in a structure that has been called haematopoietic pockets [65, 75. In the last two abdominal larval segments, groups of sessile haemocytes are concentrated in two denser organ‐like clusters, that is the putative posterior haematopoietic tissue (PHT) [38]. Sessile haemocytes in clusters constitute the biggest compartment of haemocytes in the larva [75]. As a larva develops, the number of blood cells in the larva increases. However, differentiated haemocytes in the lymph gland do not contribute to the circulating and sessile haemocyte population [88]. Plasmatocytes are mitotically active cells increasing during larval development by self‐renewal [65, 75. On the contrary, mature crystal cells do not divide during larval stages [65, 97, although they have been shown to proliferate during embryogenesis [98]. The stationary clusters of blood cells are the main source of lamellocytes and shown recently to produce new crystal cells in Drosophila larvae constituting a novel haematopoietic compartment [59, 99. The sessile plasmatocytes in such clusters have a higher division rate than those in circulation [75]. The EGF‐like repeat transmembrane receptor Eater is required in the formation of the haemocyte sessile compartment. Deletion of eater causes the absence of the sessile haemocyte state, both plasmatocyte and crystal cell types [100]. Haemocytes leave the sessile patches and enter the circulation upon wasp infestation or mechanical stimulation of the cuticle by brushing [75, 99. Within the clusters, plasmatocytes transdifferentiate into crystal cells via a Notch signalling pathway [59], which is known to be essential for forming crystal cells [101, 102. Drosophila sessile haemocyte clusters are true haematopoietic tissues that regulate larval blood cell differentiation [59, 99.
Regulation of haematopoiesis
Factors controlling/mediating haematopoiesis in the lymph gland ‐ Interactions between the GATA factor (globin transcription factor 1) Serpent, the Runx factor Lozenge, the FOG gene U‐shaped and two zinc‐finger trans‐activators, the transcription factors glial cell missing (gcm) and gcm2, are needed for regulating haematopoietic cell lineage commitment [98, 103. For example, Gcm/Gcm2 plays a key role in controlling the density of the crystal cell population by inhibiting lozenge activation [106]. Following parasitization, the number of plasmatocytes increases greatly while the number of crystal cells remains unchanged, suggesting precise regulation of the progenitor cell lineages [20]. The fibroblast growth factor (FGF) ligand branchless and receptor breathless are expressed by rare subsets within crystal cells and lamellocytes in larval Drosophila, respectively, and required for mediating effective immune responses against parasitoid wasp eggs [20]. This is highlighting a novel role for FGF signalling in inter‐haemocyte crosstalk.
Collier (Col) [the Drosophila orthologue of the vertebrate gene encoding early B‐cell factor (EBF)] is acting in specification of lamellocytes [107, 108. A complete lack of lamellocytes was observed in parasitized larvae mutant for Col [107]. Expression of Col during ontogeny of the lymph gland defines the PSC [107]. In wild‐type larvae, Col expression is restricted to the PSC following parasitization, despite the massive production of lamellocytes. Communication between the PSC and haematopoietic progenitors strictly depends on this PSC‐restricted expression. PSC cells act, in a non‐cell autonomous manner, to maintain Janus Kinase (JAK)‐Signal Transducer and Activator of Transcription (STAT) signalling activity in prohaemocytes, preventing their premature differentiation [107, 108. Two independent sites of Collier/early B‐cell factor expression, haematopoietic progenitors and the PSC, achieve control of hematopoiesis [108].
The JAK‐STAT signal transduction pathway controls haemocyte proliferation in Drosophila larvae, together with the Toll signalling pathway [91, 109, 110. For example, the lamellocyte‐active enhancer located within the misshapen (encodes a protein kinase within the Jun N‐terminal kinase signalling pathway that functions to form an active AP‐1 complex) likely serves as a transcriptional target within an activated JNK auto‐regulatory circuit that functions to continuously promote the differentiation of lamellocytes from a progenitor haemocyte population [111]. Changes in expression of JAK‐STAT and Toll genes have been observed post parasitization by Leptopilina parasitoids. Also, overproliferation and differentiation of haemocytes following parasitization were observed [109]. Mutations in different genes in these pathways lead to over proliferation of lamellocytes and the development of tumor [112]. The lesswright mutation leads to activation of the Dorsal and Dif proteins that are key transcription factors in haematopoiesis; Dorsal primarily stimulates plasmatocyte production while Dif controls both plasmatocyte and lamellocyte production [113]. PDVs (bracoviruses and ichnoviruses) that are symbiotically associated with parasitoid wasps encodes inhibitor B‐like proteins that inhibit NF‐kB activation and suppress the insect immune response [114]. These observations also emphasize the importance of the Toll pathway in the Drosophila immune response. Several other genes have been shown to be involved in haemocyte proliferation. One of these genes is Pvr, coding for the single Drosophila homolog of the mammalian PDGF/VEGF (platelet‐derived growth factors/vascular endothelial growth factors receptor). Pvr receptor together with one of its putative ligands PVF2 plays a crucial role in the control of haemocyte proliferation in larvae. It is worthy to note that PVF1 and PVF2 are up‐regulated following parasitization by Leptopilina parasitoids [115]. Another example is the multi‐sex combs gene involved in controlling normal rates of proliferation as well as the timing of haemocyte differentiation [116].
Genes from the Ras‐MAPK pathway regulate the number of hemocytes [117], while the JAK‐STAT and Jun kinase pathways strongly affect lamellocyte production [110]. Constitutive expression of some signals, such as those mediated by aop(ACT), Toll(10b), or Rac1, cause a simultaneous increase in both lamellocytes and total haemocyte numbers [118]. The effect of aop is remarkable since this gene is regulated by edl/mae, a candidate for the resistance gene to the wasp Leptopilina boulardi [118].
Notch signalling controls lineage specification during larval haematopoiesis in Drosophila [59, 101. Notch is activated by two different ligands: Serrate and Delta [119]. In the sessile haemocyte clusters, Serrate (the ligand necessary to induce Notch signalling in plasmatocytes) is expressed in plasmatocytes themselves and is essential for crystal cell differentiation [59]. Notch activation is essential also to induce lozenge expression in larval haemocytes that will mature into crystal cells [59, 101, 102. Plasmatocytes are responsible for Serrate signalling to activate Notch in other plasmatocytes and start the differentiation of crystal cells from plasmatocytes within sessile clusters [59].
Insect cytokines
Cytokines are small, secreted proteins (~5–20 kDa) released by cells to synchronize the interaction and promote communication between different cell types bearing the respective receptor(s) [120]. Cytokines regulate haematopoiesis, immunity and inflammation [120]. They act synergistically or antagonistically and are often pleiotropic; that is, one single cytokine can act on several different cell types or have multiple effects on growth and differentiation. The cellular and humoral arms of an insect immune system are not separate as often depicted but interconnected [35]. Insect immune responses are regulated by a complex network of signalling pathways mediating haemocyte‐to‐fat body, fat body‐to‐haemocyte, muscle‐to‐haemocyte and gut‐to‐fat body immune interactions that activate local and systemic immune responses at the onset of infection [110, 121. Part of this process is performed by cytokines. A brief outline of cytokines and cytokine‐like molecules acting in Drosophila innate immunity, accentuating cellular events and phenomena, is shown in Table 1.
TABLE 1.
Cytokines in Drosophila immunity
| Cytokine/cytokine‐like | Tissue | Receptor | Pathway(s) affected/Immunomodulatory action | Mammalian or vertebrate homologue | References |
|---|---|---|---|---|---|
| Diedel (Die) | Fat body, induced and secreted upon infection | ND | Imd (a systemic negative regulator); Die mutant flies have reduced viability and succumb more rapidly than controls when infected with the RNA Sindbis virus | ND a | [252, 253 |
| Edin (elevated during infection) | Fat body, secreted into the haemolymph upon infection | ND | Edin mediates plasmatocyte self‐renew and proliferation; mobilization of sessile haemocytes; lamellocyte generation; induces encapsulation | ND | [89, 254, 255 |
| Eiger (eda‐like cell death trigger) | Imaginal discs, fat body, haemocytes, released in the haemolymph | Wengen | JNK; Eiger is a proinflammatory cytokine that strongly induces apoptotic cell death; drive apoptosis‐induced proliferation of imaginal discs epithelia via resident plasmatocytes; triggers activation of Toll signalling in the fat body when expressed in imaginal disc tumours and haemocytes; induces crystal cell rupture post injury and mediates melanization; activates phagocytosis to aid clearance of extracellular pathogens | A homolog of the mammalian tumour necrosis factor (TNF‐α) | [122, 256 |
| GBP (Growth‐blocking peptide) | Fat body, central nervous system, haemocytes, integument | Methuselah‐like 10 (Mthl10) d | JNK; inositol phosphate (IP3)/Ca2+ signalling cascade b and in turn activate phospholipase C; PVR/ERK pathway c , co‐ordinate switch between humoral and cellular immunity: activating phagocytosis and encapsulation, while inhibiting production and release of AMPs | The consensus GBP motif ‐C‐x(2)‐G‐x(4,6)‐G‐x(1,2)‐C‐[KR] shares a significant similarity with a motif in the mammalian epidermal growth factor (EGF) family | [172, 260, 261 |
| Pvfs (1–3) e | Embryonic haemocytes, expressed in many tissues partly redundantly | Pvr | PVR; inhibit humoral immune responses, while stimulating haemocytes spreading; Pvf‐1 activates resident plasmatocytes to recover the eye imaginal disc epithelium from tissue damage; Pvf‐2 is required for haemocyte proliferation and completion of normal lymph gland size; Pvf2 and Pvf3 control haemocyte viability and invasive migration | Drosophila Pvfs are akin to the mammalian PDGF/VEGF receptors | [172, 262 |
| Spätzle (Spz) | Haemocytes, other tissues? secreted into the haemolymph | The dimeric Toll | Spz binding to the Toll receptor activates the Toll pathway crucial for humoral immunity against Gram+ bacteria and fungi; Toll pathway activation leads to formation of lamellocytes during cellular immune response; Toll signalling in the fat body activates cellular immune defence events, like mobilization of sessile haemocytes, melanotic nodules formation, haemocyte proliferation; encapsulation and killing of the wasp larvae | Vertebrate NGF, mammalian IL‐17F | [121, 265, 266 |
| Unpaired (Upd) (Upd1, Upd2, Upd3) | Secreted from haemocytes, fat body (Upd2), expressed in larval lymph glands | Domeless (Dome) | JAK/STAT; Upd2 and Upd3 co‐ordinate different tissues for the cellular immune response, e.g., activate JAK/STAT signalling in somatic muscle which is necessary for lamellocyte formation and wasp egg encapsulation; Upd control haemocyte proliferation during wounding and restricting tumour growth; Upd released by haemocytes co‐ordinate the wound‐healing program in the gut via stimulating intestinal stem cell proliferation and immune response | Mammalian type I cytokines and vertebrate leptin‐like (interleukin 6 (IL‐6) family) | [110, 123, 267, 268 |
| WntD (Wnt inhibitor of Dorsal) | Early embryo | Frizzled (Fz)4 | WntD negatively regulates both the Toll (a feedback inhibitor of Dorsal/NF‐κB in embryos during both embryonic patterning and immune response) and the Imd pathway in adult flies; WntD‐deficiency rendered flies are more susceptible to Listeria monocytogenes infection | Vertebrate Wnt8 proteins | [253, 254, 269 |
Abbreviation: ND, not determined.
Die belongs to a larger family that includes homologs that can be found in genomes of members of three unrelated families of large DNA viruses that infect lepidopterans, the Entomopoxviridae, Baculoviridae and Ascoviridae. Die homologs are also present in the venom of two related parasitoid wasp species, Leptopilina boulardi and L. heterotoma [252].
GBP also acts through the phospholipase C (PLC)/Ca2+ signalling cascade to mediate the secretion of Pvf [260].
A receptor‐regulated kinase cascade.
G protein‐coupled receptor.
Pvf: a ligand for platelet‐derived growth factor (PDGF)‐ and vascular endothelial growth factor (VEGF)‐receptor (Pvr) homologue.
Haemocyte‐mediated immune responses
Haemocytes possess a variety of immune functions and their responses occur once an intruder is recognized as a non‐self in the insect body. The most obvious of the haemocyte‐mediated immune responses is phagocytosis, nodulation and encapsulation [90, 124, 125. These processes depend upon recognition of the target followed by activation of downstream signalling and effector responses [125, 126. Some foreign entities are recognized by humoral pattern recognition receptors (PRRs) that after binding to a target enhance its recognition by other receptors on the surface of haemocytes, or what is known as opsonization process, that is opsonins that tag foreign pathogens for phagocytic clearance [125]. Other targets, in contrast, are recognized directly by haemocyte surface receptors. In general, granulocytes appear to be involved in the initial stages of recognition of nonself. Degranulation by these cells certainly occurs in the initial stages of nodule formation and encapsulation [127, 128. It seems possible that PGRPs, GNBP and βGRP are present in granules of the granulocytes [129]. As a consequence of the interaction of the granulocytes with the invading organism, plasmatocytes are attracted to the site and both are commonly observed in capsules in lepidopterans, while in Drosophila, lamellocytes were observed most frequently in capsules [90, 129, 130. Haemocytes also secrete a variety of opsonins, enzymes, PPO cascade components, lysozymes, nitric oxide (NO), etc [125, 129. In addition to mediating bacterial killing, it is also possible that secreted molecules may be involved in coordinating the immune response [125, 126.
Phagocytosis
Phagocytosis is a widely conserved and ancient immune defence response performed by single‐celled organisms and different metazoan cell types in which individual cells recognize, internalize and ingest invading microbial pathogens and apoptotic cells (≥0·5 μm) [125, 131. Phagocytic haemocytes can function individually in the process of phagocytosis and the phagocytic ability declines with age [132]. A small number of bacteria, fungal spores or protozoans are phagocytosed by the phagocytes [125, 133. The phagocytic haemocytes in Lepidoptera are granulocytes and plasmatocytes, whereas the latter is the key component in Drosophila [64, 133. Phagocytosis involves several receptors that have been well‐documented in Drosophila and many of which have mammalian homologs (Table 2) [134]. Recognition of the target object is either direct by binding of the haemocyte to its surface or mediated via opsonic ligands marking the object to be phagocytosed. Microbial elicitors such as glucans, peptidoglycans (PGNs) or lipopolysaccharides (LPS) can enhance the phagocytic rate of hemocytes [135, 136, 137, 138, 139. Lectins act also in opsonization and the molecular interaction between carbohydrates on the microbial cell surfaces (PAMPs) and haemolymph lectins regulate/and or promote phagocytosis [140, 141.
TABLE 2.
Cell‐surface pattern recognition receptors and opsonins acting in phagocytosis in Drosophila
| Receptors | Ligands | Mammalian homologues | References |
|---|---|---|---|
| Scavenger receptors | |||
| Croquemort a | Apoptotic cells | CD36 a | [270, 271 |
| Gm−: Erwinia carotovora (Ecc15) | [272] | ||
| Gm−: Escherichia coli | [272] | ||
| Gm+: Staphylococcus aureus | [272, 273 | ||
| Yeast: Candida albicans | [272] | ||
| Protozoa: Leishmania amazonensis | [274] | ||
| dSR‐CI | Gm−: E. coli | Complement control proteins and mucins | [275, 276, f ] |
| Gm+: S. aureus | [275, 276, 277 | ||
| Peste a | Intracellular Gm+: Listeria monocytogenes | SR‐BI a | [278] |
| Gm+: Mycobacterium fortuitum | [279] | ||
| EGF‐like‐repeat‐containing Nimrods | |||
| Draper | Apoptotic cells | MEGF10 and MEGF11 | [280, 281 |
| Gm−: E. coli | [281] | ||
| Gm+: S. aureus | [281, 282, 283 | ||
| Eater b | Gm−: E. coli | CD91 (LRP); MEGF10 and MEGF11; SREC; Stabilin 1,2 | [100, 284 |
| Gm+: Enterococcus faecalis | [285] | ||
| Gm+: S. aureus | [125, 284, 285 | ||
| Zygomycetes | [286] | ||
| Nimrod B (1,2) c | Opsonin (E. coli) | ND | [287] |
| Nimrod C1 | Gm−: E. coli | ND | [287] |
| Gm+: S. aureus | [38] | ||
| Latex beads and zymosan particles | [125] | ||
| SIMU | Apoptotic cells | ND | [288] |
| Thioester‐containing protein (TEP) | |||
| TEP2 | Gm−: E. coli | α2‐macroglobulins (complement components) | [289, 290 |
| TEP3 | Gm−: Photorhabdus luminescens | [290] | |
| Gm+: S. aureus | [289] | ||
| TEP4 | Gm−: P. luminescens and P. asymbiotica | [291] | |
| Gm−: Pseudomonas aeruginosa | [292] | ||
| TEP6 (MCR) | Gm−: P. asymbiotica | [290] | |
| Yeast: C. albicans | [289] | ||
| TEP1‐4 | Several Gm+, several fungi, parasitic wasps | [293, g ] | |
| Others | |||
| Dscam a | Gm−: E. coli | Ig‐CAMs (Dscam) a | [294, 295 |
| Integrin (βν) a | Apoptotic cells | Integrin a | [296] |
| Gm+: S. aureus | [282] | ||
| PGRP‐LC | Gm−: E. coli | Mammalian PGRPs d | [297] |
| PGRP‐SC1 (soluble) e | Gm+: S. aureus | [298] | |
Dscam, Down syndrome cell adhesion molecule; dSR‐CI, Drosophila Scavenger receptor class C, type I (ScrCI); IgSF CAMs, immunoglobulin‐like cell adhesion molecules, belonging to immunoglobulin (Ig) superfamily; LRP, lipoprotein receptor‐related protein; MCR, macroglobulin complement‐related (=Tep VI or Tep 6); MEGF, multiple epidermal growth factor (EGF)‐like domain; ND, not determined; PGRP, peptidoglycan‐recognition protein; SIMU, Six‐Microns‐Under; SR, scavenger receptor; SREC, scavenger receptor expressed by endothelial cells.
Direct mammalian orthologues.
Eater is required for the attachment of haemocytes to the sessile compartment [100].
NimB proteins lack the transmembrane domain and are most likely to be secreted in the haemolymph [287].
Soluble molecules with no role in phagocytosis.
PGRP‐SC1a mutant defects in phagocytosis of S. aureus [298].
Genome‐wide analysis using microarray hybridization. dSR‐CI was strongly upregulated post immune challenges.
Using Tep1‐4 quadruple mutants.
Fundamental scenarios of phagocytosis by insect haemocytes can be envisioned as a specialized form of receptor‐mediated endocytosis and may be assumed to be similar to those in other animals, differences may occur in some steps [125, 131, 133. Detailed steps, dynamics and molecular regulators of phagocytosis in different animal models were reviewed elsewhere [125, 142. This multiple step process involves chemotaxis, activation of haemocyte cell‐surface receptors, attachment of the intruder to these receptors, recognition, signal transduction and activation of pseudopodia formation via an actin polymerization‐dependent mechanism modifying the cytoskeleton. A phagocytic cup is then formed and develops into a membrane‐bound phagosome, and intracellular vesicular transport to phagosomes internalize the invading intruder. Phagosomes fuse with lysosomes to create phagolysosomes, and the low pH environment in these compartments enhances killing of living intruders. There is some evidence that insect haemocytes generate reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are released into the phagosome or extracellularly and are toxic to a variety of microbes [145].
Nodulation
Nodules are multicellular haemocyte aggregates recruited to the site of infection that entrap large number of bacteria or fungal spores in an extracellular material [124]. Nodule formation can be induced by certain PAMPs of the invader [146]. The process involves entrapping bacteria or foreign objects in the coagulum formed from granules and released by granulocytes, with subsequent melanization of necrotic haemocytes. Nodules develop with time and consist of a cluster of granulocytes and rosetting plasmatocytes with fan‐shaped lamellipodia [147, 148, 149, 150. Such nodular aggregates may adhere to host tissues and the larger nodules may eventually be encapsulated [151]. Nodulation reactions clear infecting bacteria from the haemocoel in insects, generally [146]. Cytokines like PSP [68], eicosanoids [152], NO [153], various PPO cascade‐activating components [150, 154, apolipophorins [155], and biogenic amines signal and/or control the aggregation of plasmatocytes [156]. The intensity of the response varies, depending on the pathogenicity of the invading organisms. A reeler domain (found in some extracellular matrix proteins)‐containing protein, Noduler, regulates nodule formation in Antheraea mylitta [157]. Certain entomopathogenic bacteria like Xenorhabdus nematophila, X. bovienni, X. poinarii and Photorhabdus luminescens induce host immunosuppression and inhibit nodule formation via inhibiting phospholipase A2 (PLA2) acting in eicosanoid biosynthesis [158, 159. Killing of the entrapped micro‐organisms may be due to a suite of melanin precursors, and antimicrobial oxidizing (·OH, O2 −) and nitrosative (ONOO−) free radicals produced as intermediates during melanization reactions [145, 160.
Encapsulation
When foreign invading objects, like metazoan parasites, protozoan, eggs or larvae and foreign invading organisms or abiotic particles are too large to be phagocytosed, they become encapsulated by multiple layers of haemocytes and/or a melanin coat [131, 146. Two types of encapsulation are recognized in insects: cellular encapsulation, mainly described in Lepidoptera, and melanotic humoral encapsulation, more typical in some dipterans like Drosophila [64, 161. Cellular encapsulation can occur without any sign of melanization. In contrast, melanotic encapsulation involves PO activity and has been described to occur with or without participation of haemocytes. In cellular encapsulation, granulocytes and plasmatocytes of Lepidoptera, and plasmatocytes and lamellocytes of Drosophila, are the main haemocyte types involved in encapsulation [162, 163. In Lepidoptera, an inner layer of granulocytes and an outer one of plasmatocytes were reported to surround the encapsulated objects [164, 165. Taking the nomenclature of Drosophila haemocyte types into account, the same sequence of plasmatocytes (= granulocytes) followed by lamellocytes (=plasmatocytes) may occur in capsule formation [162]. Crystal cells of Drosophila and oenocytoids of other insects also play a role in melanization of the capsule [91, 146. Melanotic encapsulation, for example in chironomids and some mosquitoes, can occur with or apparently without haemocytes, and is always associated with phenoloxidase [166, 167, 168. Threads of material accumulate around a foreign particle within 2–15 min after it enters the haemolymph. Extra material is added until the invader is completely ensheathed in a capsule and the capsule becomes melanized. ROS levels contribute to the melanotic encapsulation of Plasmodium in A. gambiae. ROS induced a chronic state of oxidative stress, which favour melanization of parasites as well as Sephadex beads [169].
Effectors and Regulators — The cytokine PSP released by granulocytes may acts in cellular encapsulation process by inducing spreading and migration of large numbers of plasmatocytes, to bind to the target and form a capsule [127, 170. After its release into the haemolymph, it is activated via proteolytic cleavage by haemolymph peptidases. Upon binding of PSP to its cognate receptor on plasmatocytes, it causes a change in their morphology and behaviour. PSP induces plasmatocytes to adhere and spread on foreign surfaces within minutes at concentrations ≥100 pM [171]. PSP induces plasmatocytes to export cytoplasmically stored adhesion molecules to their surface; consequently, they aggregate and adhere to foreign surfaces within seconds. PSP is a member of the large Lepidopteran‐derived ENF‐peptide family named for their invariable N‐terminal ENF sequences [170]. Cytokine‐induced plasmatocyte spreading in Drosophila functions by growth‐blocking peptides (GBP) [172]. Both PSP and GBP are multifunctional homologs [170]. GBP acts through the phospholipase C (PLC)/Ca2+ signalling cascade to mediate the secretion of Pvf, a ligand for platelet‐derived growth factor‐ and vascular endothelial growth factor‐receptor (Pvr) homolog. Activated Pvr recruits extracellular signal‐regulated protein kinase to inhibit humoral immune responses, while stimulating plasmatocyte spreading. Putative ENF homologs have been identified in mosquitos, but they have not yet been functionally characterized [173]. In a related context, the transformation of circulating/non‐adherent haemocytes to adherent haemocytes with homophilic or heterophilic interactions during encapsulation reactions in M. sexta involve transmembrane proteins like integrin, neuroglian, tetraspanins and lacunin on granulocyte surfaces [174, 175, 176.
Humoral factors may act in encapsulation, particularly in the early recognition and binding of PAMPs. Different C‐type lectins may act as opsonin or recognition receptor to bind larger biotic or abiotic objects promoting encapsulation and melanization [177, 178, 179, 180. Also, other humoral PRRs and haemolymph proteins stimulate haemocyte aggregation on the surface of the invaders or exert modulatory effects on cell attachment and spreading during cellular encapsulation [181, 182. Evasive endoparasitoids inject certain venom component(s) or virulence factors during deposition of their egg(s) into haemocoel of larval Drosophila triggering overproduction of lamellocytes [183, 184. The larval host wounded or ruptured basal membrane is also a key recognition factor activating subsequent haemocyte proliferation and lamellocyte production [91, 185. The cooperation between these humoral factors and immunocompetent cells results in a multicellular layered thick capsule that segregates the foreign organism, avoiding trophic exchanges with the host body environment.
Encapsulation of parasitoid eggs (in Figure 3 the cellular immune response of Drosophila larvae against this type of infection/infestation is depicted) requires intercellular signalling for recruitment and cooperation of different immunocompetent haemocyte, and for adhesion and cell shape [161]. The Rac GTPases protein family is central to the processes involved in cell shape [186]. Upon activation, Racs act in many cell processes including cytoskeletal rearrangement and organization, regulation of cell adhesion, cell polarity and transcriptional activation. Both Drosophila Rac1 and Rac2 genes are required for proper encapsulation of L. boulardi eggs [92, 187. Rac1 promotes haemocyte number, induces lamellocyte production and is required for proper encapsulation of parasitoid eggs in Drosophila larvae [187]. Rac2 is necessary for haemocyte spreading and cell‐cell contact formation. In parasitized Rac2‐mutant Drosophila larvae, plasmatocytes adhere to the egg but fail to undergo transformation from round cells to spread form, and septate junctions are not formed. Rac2 mutants also showed a disrupted melanization [92]. Other genes implicated in immune responses of D. melanogaster against parasitoids include Hemese, modulating the activation or recruitment of hemocytes [188], and the myospheroid (Mys) integrin that interacts with its ligand to cause haemocyte capsules to surround L. boulardi eggs [187]. Rac1 is needed for Mys localization to the haemocyte periphery and Mys mislocalization induced in a Rac1 knockout line was rescuable by hyperthermia and involved heat shock protein 83 (Hsp83). Therefore, Hsp83 acts in encapsulation [187]. Other genes acting in encapsulation include Eph (an erythropoietin‐producing hepatocellular receptor; a tyrosine kinase) and an ephrin ligand act in cell‐to‐cell interactions (Howell et al., 2012), and Mgat1 is involved in the N‐glycosylation of membrane proteins [162, 189.
FIGURE 3.
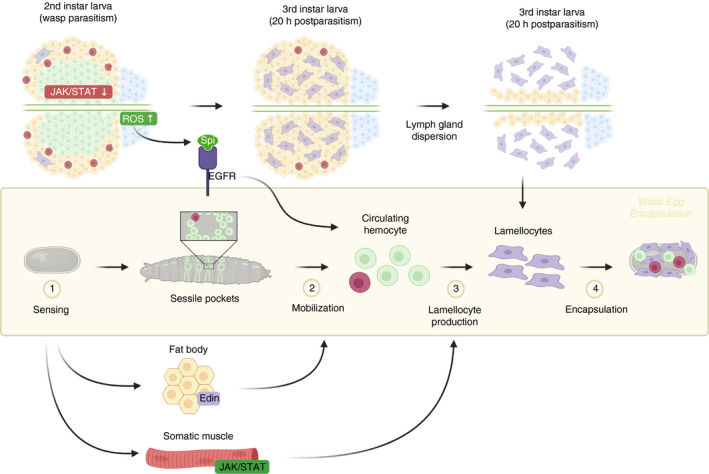
Cellular immune response of Drosophila larvae against parasitoid infection. Parasitization by the parasitoid wasp Leptopilina boulardi triggers changes in haemocyte activity and number of circulating haemocytes as well as the potential to produce additional haemocytes following oviposition of a wasp egg in the larval Drosophila melanogaster. This process involves the release of sessile haemocytes following the expression of Edin in the fat body and the differentiation of plasmatocytes into lamellocytes, which are then dispersed from the lymph gland. Both the posterior signalling centre (PSC) and the Janus Kinase (JAK)‐Signal Transducer and Activator of Transcription (STAT) (JAK/STAT) pathway participate in lemellocyte production and release. Lamellocyte differentiation occurs in the lymph gland when ROS concentrations are elevated in PSC and the latter subsequently produce the epidermal growth factor receptor (EGFR) Spitz (Spi) to trigger lamellocyte differentiation from plasmatocytes. Also, lamellocyte appearance depends on JAK/STAT signalling pathway activity in somatic muscles or abolishment in medullary zone (MZ) haematopoietic progenitors. Modified after reference [91]
Termination of an encapsulation response has been well studied in the mosquito A. quadrimaculatus [190]. Cellular encapsulation of microfilariae was ceased by the formation of a basement membrane‐like structure, formed by a monolayer of granulocytes, on the outermost surface of the cellular capsule. The termination structure appears to be laid down by releasing the vesicle inclusions of haemocytes. Once a capsule has been formed, the encapsulated invader almost always dies. Several factors are implicated in the invader‐killing. These include asphyxiation, production of cytotoxic ROS and RNS, which may result from redox cycling processes of certain melanin metabolites, and antimicrobial peptides (AMPs) have been suggested to function as killing agents [191, 192, 193, 194.
Autophagy and apoptosis
Autophagy and apoptosis are two effector mechanisms insects marshal for pathogen defence. These processes are of key importance during development and shape adult organs at metamorphosis [195, 196, 197. Both are involved in the response to stress.
Autophagy is an evolutionary conserved mechanism that breaks down cytoplasmic material through the lysosomal degradation pathway. Basal levels of autophagy are important in maintaining cellular homeostasis by removal of damaged organelles and in degradation of protein aggregates and protein turnover. Autophagy acts as an adaptive response to nutrient deprivation, and autophagic recycling of macromolecules is critical for energy homeostasis and survival during starvation [198, 199, 200. The protein kinase target of rapamycin (TOR) couples nutrient sensing with autophagy regulation. TOR is activated by the phosphatidylinositol‐3‐kinase (PI3K)‐Akt‐signalling pathway, keeping autophagy blocked, but during starvation TOR is inhibited, relieving repression on downstream autophagy‐related (ATG) genes that triggers autophagy (Figure 4a) [201].
FIGURE 4.
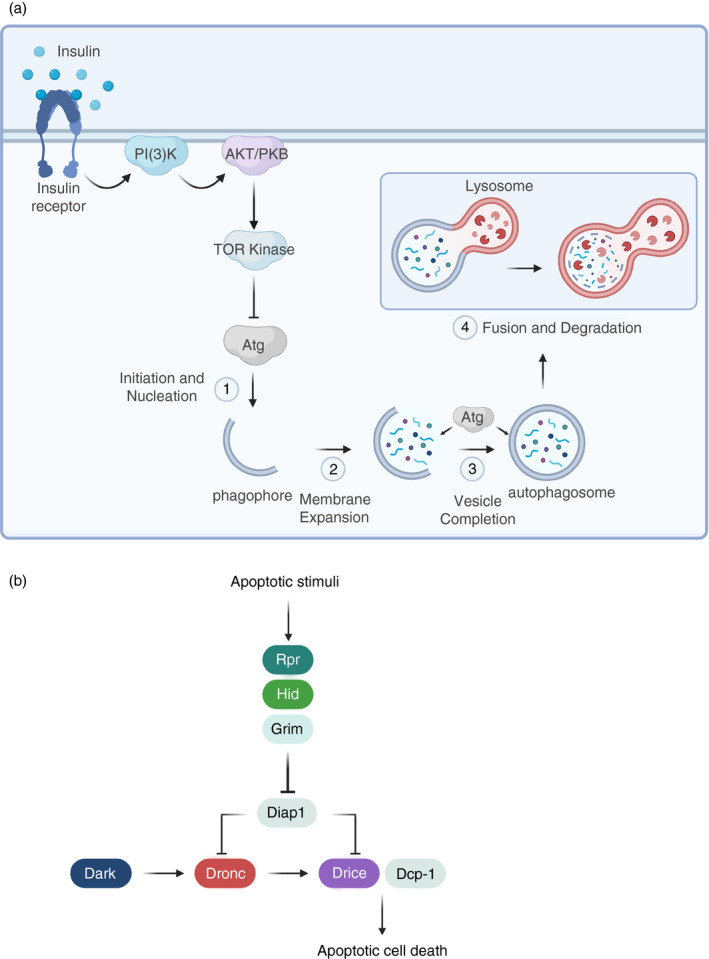
Schematic representation of the autophagic and apoptotic machinery in Drosophila. (a) Under growth conditions, the activation of TOR (target of rapamycin) kinase through the phosphatidylinositol 3‑kinase (PI3K)/protein kinase B (AKT) signalling pathway keeps autophagy inhibited. In the absence of nutrients, TOR suppression is relieved, and autophagy is set in motion through the recruitment of different Atg (autophagy‐related) proteins. After the nucleation of a double membrane phagophore (1), this progressively expands (2) to form an autophagosome that engulfs the target material for degradation (3). The autophagosome fuses with lysosomes and hydrolytic enzymes break down the cargo (4). (b) The initiator caspase Dronc (Nedd2‐like caspase) and its activator Dark (aka Drosophila Apaf‐1‐related killer [Ark]) form the apoptosome complex, which activates downstream effector caspases Drice (Drosophila ice) and Dcp‐1 (death caspase‐1), thus triggering the ordered dismantling of the cell through apoptotic cell death. Both Dronc and Drice are subjected to negative regulation by Diap1 (Drosophila inhibitor of apoptosis protein 1). Rpr (reaper), Hid (head involution defective) and Grim proteins function as IAP‐agonists to relieve caspase inhibition by Diap1
Autophagy has a role in antiviral defence and can be rapidly activated in response to virus infection, restricting viral replication and pathogenesis. In Drosophila, the activation of autophagy by vesicular stomatitis virus (VSV) is mediated by Toll‐7 recognition of VSV‐G, a glycoprotein on the virion surface. This PAMP‐PRR interaction leads to autophagy initiation by acting on the (PI3K)‐Akt‐signalling pathway. In this case, autophagy induction occurs independently of viral replication because UV‐inactivated VSV and VSV‐G‐containing vesicular particles devoid of any other viral component are able to induce autophagy [202, 203. A similar protective autophagic response has been reported for other viruses, such as rift valley fever virus (RVFV) [204]. The connection of a Toll receptor to antiviral autophagy in flies suggests an evolutionary conserved requirement for PRR in triggering autophagy between insects and mammals. Autophagy may in some cases promote virus infection as shown for Sindbis virus, where the activation of the (PI3K)‐Akt‐signalling pathway enhances the infection of insect cells [205]. A separate study provided another piece of information to unravel the true role of autophagy in response to viruses. By impairing the Drosophila autophagic pathway through mutation of ATG7 and comparing the effects of the infection with a panel of six different viruses, the investigators demonstrated that autophagy can impact positively or negatively on viral replication depending on the virus [206]. The authors also showed that autophagy has a pro‐viral effect for flock house virus (FHV), probably due to its action in assuring the turnover of mitochondria where this virus replicates, removing damaged ones. The antiviral response towards VSV is limited and does not seem to depend on Toll‐7, in contrast to what was found in Drosophila [202]. Thus, autophagy is probably not a broad antiviral mechanism and does not show general antiviral pathways, but virus‐specific functions.
Autophagy has also an antibacterial role, triggered by the recognition of PAMPs, similarly to antiviral protection. Lysteria monocytogenes invades and replicates in haemocytes, and is recognized by PGRP‐LE (a PRR which senses a DAP‐type PGN), eliciting an autophagic response that prevents intracellular growth of the bacterium and promotes host survival. Because the involvement of the Toll and immune deficiency (IMD) pathways in this process has been excluded, a distinct innate immune pathway could be responsible for induction of autophagy [207].
Apoptosis is a form of programmed cell death that removes unwanted or damaged cells. In Drosophila, the initiator caspase Dronc can activate the effector caspases DrICE and DCP‐1, leading to apoptotic induction. Dronc and DrICE are negatively regulated by binding of DIAP1, an inhibitor of apoptosis (IAP). Reaper, Hid and Grim, three DIAP‐antagonists collectively referred to as RHG proteins, are able to relieve caspases from inhibition by DIAP (Figure 4b) [208].
In insects, apoptosis can be triggered by a variety of stimuli and the scenario is more complex than for autophagy. Apoptosis represents a basic mechanism widely used by pathogens to inhibit the host immune response. For example, the entomopathogenic nematode Ovomermis sinensis impairs Helicoverpa armigera immunity by triggering apoptosis in larval hemocytes [209]. Similarly, the infection of the silkworm, Bombyx mori, with Serratia marcescens and Porphyromonas gingivalis leads to caspase activation in the immune cells and suppression of insect immunity [210, 211. The venom of parasitoids contains cytotoxic proteins that are capable of inducing apoptosis in host hemocytes [212]. Apoptosis, however, can have an active role in the host immune response by helping the insect cope with the immune challenge. In this regard, apoptosis is a basic frontline defence mechanism against viral infection in both Lepidoptera and Diptera and this antiviral strategy, as well as the related regulatory pathways, apparently seem to be conserved among insects and do not strictly depend on the virus, unlike autophagy. The rapid induction of apoptosis, triggered by viral replication, is effective when the organism is exposed to relatively small amounts of virus and, by removing infected cells, it contributes to reduce viral infectivity and prevent virus spread within the insect body [213]. After infection, the expression levels of pro‐apoptotic RHG proteins increase, leading to the activation of Dronc and DrICE [213, 214, cell dismantling and exposure of phosphatidylserine that is recognized by phagocytes through Draper receptor, with consequent engulfment of virus‐infected cells [206, 213. Together with this increased production of pro‐apoptotic factors by infected cells, a key role in the induction of apoptosis is played by cellular DIAP, a critical regulator of cell survival whose presence in the cell usually keeps apoptosis shut down. DIAP acts as a key sensor of viral invasion because it is rapidly depleted after infection as a result of the downregulation of host protein synthesis by the virus [214], resulting in caspase‐mediated apoptosis, which, in the absence of virus‐encoded apoptosis inhibitors, limits virus multiplication [215]. Because virus DNA replication is required to trigger apoptosis by IAP turnover, it is likely that the cell interprets this phenomenon as DNA damage, a known apoptotic signal, and activates the apoptotic pathway through the tumour suppressor p53 [215, 216, although a p53‐indipendent pathway has been reported, too [217].
The antiviral mechanism of apoptosis can be suppressed by the virus, indicating an arms race between the insect and the virus. For example, all baculoviruses express antiapoptotic proteins, namely p35 or IAP, that interfere with caspase activity and block apoptosis in the host cells [218], and there are additional strategies to inhibit host antiviral apoptosis [219]. Drosophila C virus (DCV) is able to suppress the degradation of DIAP, thus inhibiting apoptosis and enhancing viral replication [220]. While viruses usually delay or evade apoptosis in successful infection, they can induce apoptosis to assist their dissemination. In this regard, it has been shown that Spodoptera frugiperda ascovirus encodes a caspase, which is similar to human caspase 3, that induces the formation of apoptotic bodies needed for virion assembly, thus facilitating viral reproduction and transmission [221].
There is some evidence about additional roles of apoptosis in the insect response to pathogens and mounting an efficient immune response, although information is fragmentary. For example, apoptosis, accompanied by secondary necrosis [222], is involved in the demise of Drosophila crystal cells leading to the release of endogenous elicitors able to trigger/assemble the PPO cascade [13]. These promoting effects towards PPO system are superinduced by the exposure of phosphatidylserine [223]. Apoptosis is also involved in preventing pathogen entrance into the organism, as observed for baculovirus that enters the insect body through the gut. In this case, apoptosis leads to the loss of cells from the midgut epithelium (known as sloughing), getting rid of infected cells before the virus can penetrate the basal lamina and move into the hemolymph [218].
PHENOLOXIDASE, MELANIN BIOSYNTHESIS AND CYTOTOXIC INTERMEDIATES
Insect PPOs are metallated zymogens belonging to the family of type‐3 copper proteins having two copper ions and three histidine residues in each active site pocket, which includes arthropod and mollusc haemocyanins and oxidoreductases that occur in almost all organisms [224, 225. Insects contain two types of phenoloxidases: (i) Enzymes in haemolymph that have tyrosinase‐like activity that o‐hydroxylate monophenols (e.g., L‐tyrosine) into o‐diphenols (e.g., L‐DOPA) and their subsequent two‐electron oxidation to the corresponding o‐quinones (e.g., L‐DOPAquinone); these are referred as POs [225, 226. The quinones produced by PO undergo a series of further enzymatic and non‐enzymatic reactions leading to polymerization and melanin biosynthesis; (ii) the laccase‐type enzymes, which are able to oxidize o‐ or p‐diphenols to quinones and function in sclerotization and tanning of the cuticle [227, 228. The latter are multicopper oxidases rather than a PO sensu stricto [225].
The melanization reaction is a principal humoral immune response in insects, and various PPOs have important role in the survival to infection with different microbes and eukaryotic parasites [14, 229. Upon wounding or infection, phenoloxidase is activated, as part of the innate immune response, from its zymogen form PPO to the active form PO. The PPO‐activation system may be elicited by the PAMPs PGN and β‐1,3‐glucan. This system is a proteolytic cascade comprising several serine peptidases and their inhibitors. Microbial PGN and β‐1,3‐glucan react at first with PRRs [235], PGRP or βGRP, which consequently induce activation of serine peptidases of the PPO‐activation system [236]. This active form has a prominent role in melanin biosynthesis, and the formed melanin is deposited in specific sites of haemolymph clots of wounds, nodules and capsules. This process appears to be important in insect immune defences through its action in wound healing and pathogen sequestration in wounds, nodules and capsules and in their killing by resulting ROS and RNS [225, 236.
PPO biosynthesis, release and activation
The PPOs are biosynthesized mainly by haemocytes. In lepidopterans, oenocytoids are identified as the cell‐type producing PPO [47], some granulocytes and plasmatocytes also have PPO as in B. mori [237], whereas it is produced in the crystal cells of D. melanogaster [229], suggesting that crystal cells and oenocytoids may be functionally homologous cell types, but the latter do not exhibit obvious crystalline cytoplasmic inclusions [225]. In mosquitoes, PPO is synthesized in both oenocytoids and granular hemocytes [62]. The PPOs contain two atoms of copper per protein molecule [225, 227. Insect PPO may exist predominantly as dimers, either homodimers such as PPO8 from A. gambiae [238] or heterodimers (~160 kDa) such as PPO1/2 (isozymes) from M. sexta and B. mori [239], although monomers [240] as well as higher multimers can be formed, depending on ionic strength [241].
Oenocytoids from different species vary in stability; some are spontaneously ruptured upon injury of the body or infection, and perhaps at a low rate, even in the absence of these enhancers [242]. Also, oenocytoid lysis may occur in vivo in the absence of injury or infection, to maintain a steady state level of PPO in plasma. In lepidopterans, the bulk of PPO appears in plasma [236, 243, but in hemimetabolous insects such as locusts and cockroaches, PPO appears to be mostly retained in haemocytes until microbial exposure or injury stimulates its release [242]. Lysis of PPO‐producing haemocytes at site of wounding or infection could result in a local high concentration of PPO [243]. A signal transduction pathway stimulating crystal cell rupture may be operating in D. melanogaster [13].
The zymogen PPO can be activated by specific proteolysis or interaction with amphipathic molecules [227, 228. Both types of activation must involve a conformational change in the protein, making the active site accessible to substrate, or rearranging the active site to a structure required for catalysing oxidation reactions [225, 226, 236. Cleavage of a conserved Arg–Phe bond about 50 residues from the N‐terminus results in the activation of insect PPOs. The PPO from Holotrichia diomphalia undergoes a second cleavage after Arg [68] during its activation [244]. The activation of B. mori and M. sexta PPO occurs by specific proteolysis, i.e., by PPO‐activating peptidases (PAPs). PAPs cut the polypeptide at the carboxyl end of arginine to yield active PO [129, 225. These PAPs are synthesized as zymogens and must be activated by another peptidase as part of a serine peptidase cascade. B. mori PAP is expressed in haemocytes, integument and salivary glands, but not in fat body. Its putative M. sexta ortholog, PAP‐1, is expressed in larval fat body, tracheae and nerve tissue, and is upregulated in fat body and haemocytes after injection of bacteria. PAP‐2 expression was detected in fat body and haemocytes only after the larvae were injected with bacteria [129]. A purified PAP from M. sexta could not efficiently activate PPO, but required participation of a non‐proteolytic protein cofactor. This protein cofactor was identified in B. mori [245] and M. sexta [246] as a protein with a clip domain and a serine peptidase domain in which the active site serine residue is changed to glycine. These serine‐peptidase homologs (SPHs) interact with POs forming ‘activation complexes’. SPHs lack peptidase activity due to the incomplete catalytic triad; the conserved His, Asp, Ser residues form the catalytic triad of serine peptidases [243]. The active form of the SPHs that function as cofactors for PPO activation is themselves activated through specific cleavage by a serine peptidase in haemolymph. B. mori PAPs, however, do not require SPH cofactors for activating PPO. The SPHs from M. sexta that stimulate PPO activation bind to a haemolymph lectin and to PPO and PAP [247]. See Figure 5 outlining haemolymph protease cascades in activation of immune‐induced PPO cascade in selected insect models.
FIGURE 5.
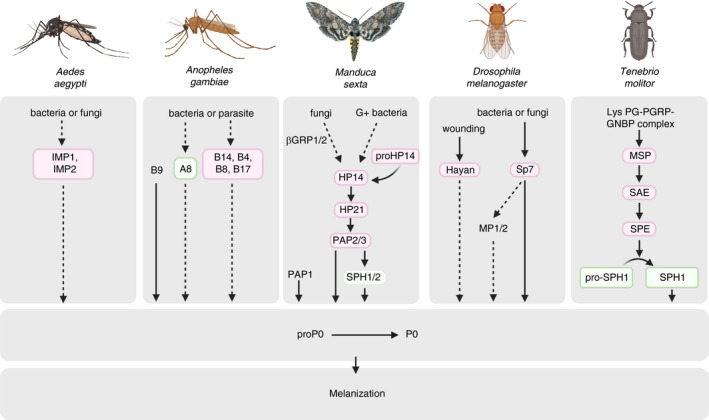
Haemolymph protease cascades in activation of immune‐induced prophenoloxidase (PPO) cascade in different insect species. Upon microbial stimulation, haemolymph pattern recognition proteins (PRRs) like β‐1,3‐glucan recognition proteins (βGRP), peptidoglycan recognition proteins (PGRPs), and c‐type lectins, bind to molecular motifs conserved within surface of microbes, known as pathogen‐associated molecular patterns (PAMPs). This interaction leads to activation of modular serine proteases via a not well‐known mechanism which triggers activation of clip‐domain serine proteases (CLIP proteases) and serine proteinase homologs (SPHs) organized in pathways that eventually lead to activation of PPO to form active phenoloxidase (PO). In Manduca sexta for example, activation of proHP21 by HP14 leads to activation of PPO‐activating proteinase (PAP). SPHs (are also activated by an unknown mechanism) function together with PAPs to form a functional PPO activator to form PO which cleaves PPO to form PO. PO catalyses the oxidation of haemolymph catecholic phenols to corresponding quinones, which can undergo further reactions to form melanin (see Figure 6 for an overall view of this process). For protease names shown in boxes, genetic evidence indicates participation in an immune pathway, but the activating protease and the protease's substrate are not yet known. Proforms (zymogens) of putatively involved proteases are not shown (e.g., M. sexta proHP14 → HP14). Similarly, regulation of proteases by inhibitors known as serpins is not indicted. Arrows indicate activation of downstream components or steps. Steps that have not been verified experimentally are indicated by dashed arrows. Modified after Kanost and Jiang (2015). This Figure summarizes data from various literature, cited parenthetically in the caption of the source diagram (# 2 in reference [247])
Triggering of PPO activation cascade is thought to depend on auto‐activation of zymogen of initiating peptidase proHP14, which is stimulated by infection or wounding through its interaction with PGN and β‐1,3‐glucans. Certain plasma proteins, lectins, PGRPs, and βGRP that bind to these pathogen PAMPs may stimulate this auto‐activation [236]. In M. sexta, and perhaps in many insect species, PGN and β‐1,3‐glucans are more potent stimulators of PPO activation than is LPS [248]. HP14 activates proHP21 which then activates proPAP2 and proPAP3 where the resulting PAP2 and PAP3 then cleave PPO to form active PO in the presence of SPH1 and SPH2. Activation of PPO can also be catalysed by PAP1 in presence of SPH complex. PAP1 also activates proSPH2 directly and can indirectly lead to activation of proHP6.
Briefing the pathway for PPO activation (Figure 5): in response to microbial challenge, haemolymph pattern‐recognition proteins like C‐type lectins, PGRP and ßGRP bind to the micro‐organism's surface polysaccharides, inducing initiator protease(s) activation. Next, the initiator protease triggers a protease cascade, activating terminal serine proteases such as PPO‐activating enzyme (PPAE), PAPs or PPO‐activating factor (PPAF) to cleave PPO and form active PO [236, 243.
Melanin biosynthesis and production of ROS and RNS
Melanin biosynthesis is a multiple‐step process (Figure 6) involving a number of intermediate metabolites and reactions initiated by the hydroxylation of phenylalanine to tyrosine, followed by formation of DOPA, and subsequently of o‐dopaquinone. These latter three metabolites result from reactions catalysed by phenylalanine hydroxylase (tyrosine), PO (DOPA) and also PO (o‐dopaquinone). After o‐dopaquinone formation, a series of enzymatic and spontaneous nonenzymatic redox reactions take place [228]. In this context, o‐dopaquinone undergoes a spontaneous intramolecular cyclization to form cyclodopa, which produces dopachrome on redox exchange between cyclodopa and o‐dopaquinone and leads ultimately to the biosynthesis of eumelanins via 5,6‐dihydroxyindole intermediates and their respective indole quinones. In insects, dopachrome is converted to dihydroxyindole in a reaction catalysed by dopachrome conversion enzyme. Dihydroxyindole can also be produced by action of dopa decarboxylase, which converts DOPA to dopamine, and then, it is converted by PO to o‐dopaminequinone, cyclized to form cyclodopamine, then through redox exchange to form dopaminechrome, a precursor for dihydroxyindole. This pathway may be a principal route for the production of pigment precursors, for example, in Drosophila against eggs of certain parasitoids. In the oxidation pathway that leads to the formation of pheomelanin, o‐dopaquinone readily forms intermediate conjugates with thiol compounds. In the melanization pathway occurring in D. melanogaster haemocoel against parasitoid eggs [249], the participation of dopamine and then o‐dopaminequinone was indicated. It was observed that dopa decarboxylase catalyses this reaction and the upregulation of dopa decarboxylase gene expression in response to infection in several insect species was observed. Since dopa decarboxylase converts DOPA to dopamine, it seems likely that during an immune response, dopamine is formed and then oxidized by PO to o‐dopaminequinone, as mentioned above. It is also possible that its derivatives such as N‐acetyldopamine and N‐β‐alanyldopamine form the corresponding quinones.
FIGURE 6.
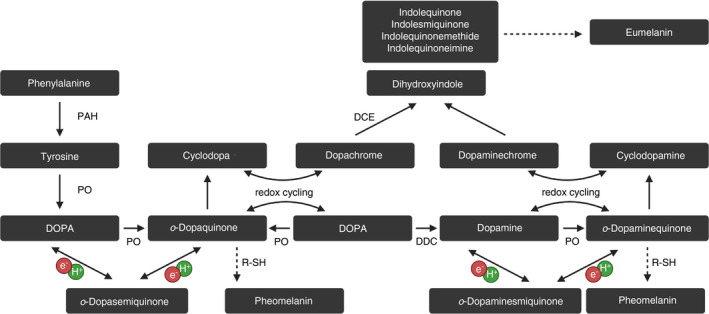
A simplified overview of insect melanogenesis via phenoloxidase (PO). Phenylalanine is initially hydroxylated to tyrosine via phenylalanine hydroxylase (PAH), a step aided by tetrahydrobiopterin (BH4) synthesized by dihydropteridine reductase and guanosine triphosphate cyclohydrolase (not shown). Tyrosine can be transformed into DOPA either by PO or tyrosine hydroxylase. Dopa is converted to dopaquinone via PO or to dopamine via dopa decarboxylase (DDC). The reactive intermediate dopaquinone undergoes spontaneous intramolecular cyclization to form cyclodopa. Redox cycling of dopaquinone with cyclodopa produces dopa and dopachrome. Dopaquinone also reacts with thiol compounds to form cysteinyl and glutathionyl conjugates and benzothiazinylalanine compounds that produce pheomelanins (yellow to reddish‐brown pigments, alkali soluble). 5,6‐dihydroxyindole (DHI) can be formed as the major product from both synthesis pathways by dopachrome conversion enzyme (DCE) which promotes the decarboxylation and rearrangement of dopachrome to DHI. Following some reaction steps involving the formation of indole‐5,6‐quinone via PO, dopamine and DHI are finally converted into melanin. Both the brown to black eumelanin and the lighter yellow to reddish‐brown pheomelanin pigments are redox‐active polymers produced in insects and both are derived from the common precursor o‐dopaquinone. R‐SH, thiol or thiol derivatives
The ROS are readily produced from univalent reactions of O2 to form H2O (Figure 7, equations below). These ROSs include superoxide anion radical () and hydroxyl radical ( · OH). The major intracellular source of ROS is the electron transport chain. The is produced during normal cell aerobic metabolism by transfer of one electron to O2 from the formed semiquinone (UQH•) produced during reduction of ubiquinone (UQ) by complex I and II of this chain.
FIGURE 7.
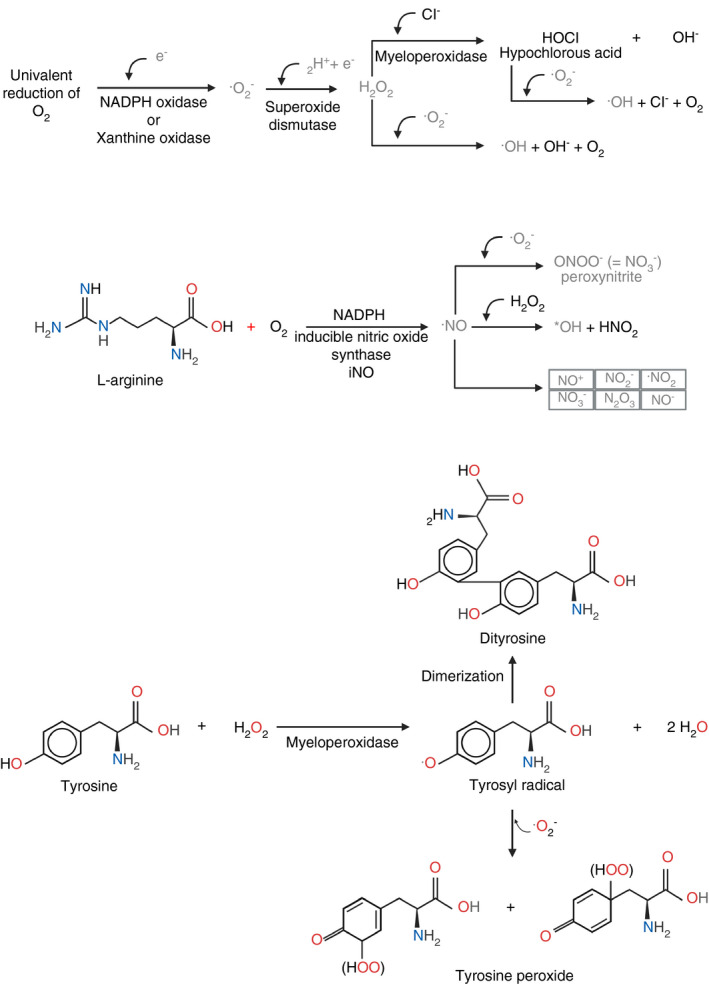
Examples of potential reaction pathways generating reactive species or free radicals of oxygen (ROS) and nitrogen (RNS) and melanin during insect immune responses. ROS: , superoxide anion; •OH, hydroxyl radical. RNS: •NO, nitric oxide; NO+, nitrosonium cation; ; ; N2O3, dinitrogen trioxide; NO−, nitroxyl anion; •NO2, nitrogen dioxide; ONOO−, peroxynitrite. ROS/RNS mutual interactions as well as with CI−, Cu+ of phenoloxidase (PO) and Fe2+ of peroxidase (PER) are outlined. Fe2+ or Cu+ speed up lipid hydroperoxides autoxidation and the subsequent cytotoxic •OH production. Coordinated activities of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, superoxide dismutase (SOD), inducible nitric oxide synthase (NOS), and myeloperoxidase generate different cytotoxic species like , H2O2, •NO and its derivatives, and HOCl−. For example, H2O2 is formed by the SOD‐mediated dismutation of . Several ROS and RNS generated during the course of immune challenge are likely acting in pathogen killing by modifying the function and structural integrity and of various target molecules. PO‐mediated melanogenesis is typically associated with phagocytosis and encapsulation of pathogens. Certain melanin intermediates cooperate with ROS and RNS and augment cytotoxic activity. Tyrosine oxidation mediated by myeloperoxidase produces the tyrosyl radical, tyrosine peroxide and dityrosine. Other melanogenic intermediates produced during melanotic responses like semiquinone (SQ−) function like and can increase the oxidative damage events like lipid peroxidation, initiated by ROS and RNS, by forming the reduced states of the metals (see equation sets in the text). Modified after reference [251]
The can also be produced during activity of certain enzyme systems, for example xanthine oxidase, NADPH oxidase, cytochrome P‐450 oxidase, lipo‐oxygenase and cyclooxygenase. The formed can be dismutated spontaneously or in an enzyme reaction catalysed by superoxide dismutase (SOD) to H2O2, which can subsequently be hydrolysed to H2O by catalase (Figure 7, equations below).
During melanotic encapsulation reactions of immune‐reactive D. melanogaster larvae, the univalent oxidations of the redox‐cycling o‐diphenols (QH2), such as L‐DOPA and dopamine, form QH¯• and then Q, with the unpaired electron transferred to O2 generating . These reactions can be enhanced in presence of Fe3+ (Cu2+) (see equations, below). At these conditions, elevated levels of , H2O2 and •NO were reported. The can be dismutated to H2O2 and, subsequently, in insects and/or non‐insect forms, can react with each other, with certain redox‐active transition metal ions such as iron or copper, or with Cl¯ or NO generating highly reactive potentially cytotoxic molecules such as the •OH, ONOO¯, HOCl and nitrogen‐dioxide radical (•NO2). Nitric oxide itself is generated by inducible nitric oxide synthase (iNOS) in a reaction that uses NADPH, oxygen and L‐arginine (Figure 7). The •OH is the most reactive ROS, and the ONOO¯ is a strong oxidant and nitrating agent that is able to nitrate aromatic compounds such as tyrosine. Other products of interactions of with •NO may be produced; for example, peroxynitrous acid (ONOOH), nitrogen dioxide radical (•NO2), nitroxyl anion (NO¯), nitrosonium cation (NO+), nitronium cation (), nitrite () and nitrate (). Because melanins are redox‐cycling polymers that can readily interact with electron donors and acceptors, melanogenic responses during infection may serve certain roles in producing ROS and RNS (Figure 7). They are also important in sequestering and localizing these and other reactive molecules to the surface of the parasitoid, thereby preventing adverse systemic reactions from occurring in the open circulatory system.
Equation sets 1
Examples of reactions generating cytotoxic reactive intermediates [145, 249, 250. The first set outlines the production of quinone and semiquinone species from diphenols such as dopa and their o‐quinone derivatives as well as interactions with ROS. Reactions initiated by •SQ− and include the reduction of Fe3+ or Cu2+. Experimentally, super oxide anion () can reduce Q and oxidize QH2 and exists in equilibrium with these two molecules, with •SQ− as an intermediary. The second sets brief reactions generating some ROS like hydroxyl radical and superoxide anion involving iron of peroxidase and copper of PO. The oxidized states of these metals readily react with H2O2 to form •OH. The third set outlines the generation of different RNS outlined in the text.
This shows that both melanin precursors and ROS and RNS generated during melanin biosynthesis in immune‐reactive D. melanogaster larvae may participate as cytotoxic molecules for the invading parasitoids. The cytotoxic effects may include, for example, oxidative damage to the macromolecule lipids, proteins and DNA, and nitration of aromatic compounds, in biological systems. However, it was observed that melanin precursors alone may not be the killing agent since successful development of the parasitoid inside the host was observed to occur in presence of fully formed melanotic capsule, and dead parasitoid was observed to accompany no visible host melanotic reaction.
CONCLUDING REMARKS
In the past 20 years, significant strides of progress have been made in deciphering the molecular and mechanistic basis of insect cellular immunity. These efforts have been substantially facilitated by the incorporation and integration of insect models into the research as well as by the technical advances to explore the gene regulation responsible for the production and activation of immune cells, which is critical for providing efficient antimicrobial host defence and maintaining insect homeostasis. Future challenges include the better understanding of the functional cooperation between the different types of insect haemocytes not only in response to a single microbial enemy, but also against polymicrobial infections. An important aspect for further investigation could include the interpretation of the role of insect haemocytes and cellular immune components in immune priming effects and how the presence of various kinds of endosymbiotic microbes influence these processes. It should be noted that most research to identify insect cellular immune activities has been conducted in a laboratory setting; therefore, confirming the existing knowledge in the natural environment would provide crucial information on extrinsic factors that could promote or impede insect cell‐mediated immune reactions. Analysing the dynamics of the insect cellular immune system will considerably increase the capacity of practices for confronting infectious diseases and contribute towards elucidating the functional properties of immune effector cells in vertebrates.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
IE, TB, GT, WZ and AM designed and conceptualized the review. IE, TB, GT, WZ, AM and CH prepared the original draft. CH and AM conceived and prepared the graphical designs of the review. AM and WZ conceived and prepared the tables. IE, AM, GT and TB edited and reviewed the final manuscript. All authors approved the final version of the manuscript.
ETHICAL APPROVAL
Not required.
PATIENT CONSENT STATEMENT
Not required.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
All information related to the produced figures is mentioned in the figure captions.
CLINICAL TRIAL REGISTRATION
Not required.
ACKNOWLEDGEMENTS
We thank members of the Department of Biological Sciences at The George Washington University for critical reading of the manuscript. AM is indebted to Professors James L. Nation (Department of Entomology and Nematology, University of Florida, Florida, USA), David Stanley (USDA ARS, Missouri, USA) and Bernard Duvic (Université Montpellier, INRAE, DGIMI, Montpellier, France) for their continuous help and support.
Eleftherianos I, Heryanto C, Bassal T, Zhang W, Tettamanti G, Mohamed A. Haemocyte‐mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology. 2021;164:401–432. 10.1111/imm.13390
Christa Heryanto, Taha Bassal and Wei Zhang contributed equally.
Funding information
This work was supported by a start‐up fund from the Columbian College of Arts and Sciences at The George Washington University to IE. The work was also supported by The Research Support Fund Program of the Faculty of Science, Cairo University, Egypt, to AM and by FAR 2019–2020 (Fondi di Ateneo per la Ricerca, University of Insubria) and Fondazione Cariplo (grant number 2020‐0900) to GT and by National Natural Science Foundation of China (32001961) to WZ.
Contributor Information
Ioannis Eleftherianos, Email: ioannise@gwu.edu.
Amr Mohamed, Email: mamr@sci.cu.edu.eg.
REFERENCES
- 1. Moret Y, Moreau J. The immune role of the arthropod exoskeleton. Invertebr Surviv J. 2012;9:200–6. http://www.isj.unimo.it/index.php/ISJ/article/view/274. [Google Scholar]
- 2. Pradeep AN, Anitha J, Awasthi AK, Babu MA, Geetha MN, Arun HK, et al. Activation of autophagic programmed cell death and innate immune gene expression reveals immuno‐competence of integumental epithelium in Bombyx mori infected by a dipteran parasitoid. Cell Tissue Res. 2013;352:371–85. 10.1007/s00441-012-1520-7. [DOI] [PubMed] [Google Scholar]
- 3. Li H, Sun CY, Fang Y, Carlson CM, Xu H, Ješovnik A, et al. Biomineral armor in leaf‐cutter ants. Nat Commun. 2020;11:5792. 10.1038/s41467-020-19566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haine ER, Rolff J, Siva‐Jothy MT. Functional consequences of blood clotting in insects. Dev Comp Immunol. 2007;31:456–64. 10.1016/j.dci.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 5. Dziedziech A, Shivankar S, Theopold U. Drosophila melanogaster responses against entomopathogenic nematodes: focus on hemolymph clots. Insects. 2020;11:62. 10.3390/insects11010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cherbas L. The induction of an injury reaction in cultured hemocytes from saturniid pupae. J Insect Physiol. 1973;19:2011–23. 10.1016/0022-1910(73)90195-9. [DOI] [Google Scholar]
- 7. Nakatogawa S, Oda Y, Kamiya M, Kamijima T, Aizawa T, Clark KD, et al. A novel peptide mediates aggregation and migration of hemocytes from an insect. Curr Biol. 2009;19:779–85. 10.1016/j.cub.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 8. Dushay MS. Insect hemolymph clotting. Cell Mol Life Sci. 2009;66:2643–50. 10.1007/s00018-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aprelev P, Bruce TF, Beard CE, Adler PH, Kornev KG. Nucleation and formation of a primary clot in insect blood. Sci Rep. 2019;9:3451. 10.1038/s41598-019-40129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li D, Scherfer C, Korayem AM, Zhao Z, Schmidt O, Theopold U. Insect hemolymph clotting: evidence for interaction between the coagulation system and the prophenoloxidase activating cascade. Insect Biochem Mol Biol. 2002;32:919–28. 10.1016/s0965-1748(02)00030-9. [DOI] [PubMed] [Google Scholar]
- 11. Bidla G, Lindgren M, Theopold U, Dushay MS. Hemolymph coagulation and phenoloxidase in Drosophila larvae. Dev Comp Immunol. 2005;2005:669–79. 10.1016/j.dci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 12. Agianian B, Lesch C, Loseva O, Dushay MS. Preliminary characterization of hemolymph coagulation in Anopheles gambiae larvae. Dev Comp Immunol. 2007;31:879–88. 10.1016/j.dci.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 13. Bidla G, Dushay MS, Theopold U. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci. 2007;120:1209–15. 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- 14. Dudzic JP, Kondo S, Ueda R, Bergman CM, Lemaitre B. Drosophila innate immunity: regional and functional specialization of prophenoloxidases. BMC Biol. 2015;13:81. 10.1186/s12915-015-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohn H, Barwig B. Hemolymph clotting in the cockroach Leucophaea maderae (Blattaria). J Comp Physiol B. 1984;154:457–67. 10.1007/BF02515150. [DOI] [Google Scholar]
- 16. Strand MR, Clark KD. Plasmatocyte spreading peptide induces spreading of plasmatocytes but represses spreading of granulocytes. Arch Insect Biochem Physiol. 1999;42:213–23. . [DOI] [PubMed] [Google Scholar]
- 17. Phillips DR, Clark KD. Bombyx mori and Aedes aegypti form multi‐functional immune complexes that integrate pattern recognition, melanization, coagulants, and hemocyte recruitment. PLoS One. 2017;12:e0171447. 10.1371/journal.pone.0171447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scherfer C, Qazi MR, Takahashi K, Ueda R, Dushay MS, Theopold U, et al. The Toll immune‐regulated Drosophila protein Fondue is involved in hemolymph clotting and puparium formation. Dev Biol. 2006;295:156–63. 10.1016/j.ydbio.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 19. Lesch C, Goto A, Lindgren M, Bidla G, Dushay MS, Theopold U. A role for Hemolectin in coagulation and immunity in Drosophila melanogaster . Dev Comp Immunol. 2007;31:1255–63. 10.1016/j.dci.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 20. Lindgren M, Riazi R, Lesch C, Wilhelmsson C, Theopold U, Dushay MS. Fondue and transglutaminase in the Drosophila larval clot. J Insect Physiol. 2008;54:586–92. 10.1016/j.jinsphys.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 21. Bajzek C, Rice AM, Andreazza S, Dushay MS. Coagulation and survival in Drosophila melanogaster fondue mutants. J Insect Physiol. 2012;58:1376–81. 10.1016/j.jinsphys.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 22. Karlsson C, Korayem AM, Scherfer C, Loseva O, Dushay MS, Theopold U. Proteomic analysis of the Drosophila larval hemolymph clot. J Biol Chem. 2004;279:52033–41. 10.1074/jbc.M408220200. [DOI] [PubMed] [Google Scholar]
- 23. Zumaya‐Estrada FA, Martínez‐Barnetche J, Lavore A, Rivera‐Pomar R, Rodríguez MH. Comparative genomics analysis of triatomines reveals common first line and inducible immunity‐related genes and the absence of Imd canonical components among hemimetabolous arthropods. Parasit Vectors. 2018;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duvic B, Brehélin M. Two major proteins from locust plasma are involved in coagulation and are specifically precipitated by laminarin, a β‐1,3‐glucan. Insect Biochem Mol Biol. 1998;28:959–67. 10.1016/S0965-1748(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 25. Scherfer C, Karlsson C, Loseva O, Bidla G, Goto A, Havemann J, et al. Isolation and characterization of hemolymph clotting factors in Drosophila melanogaster by a pullout method. Curr Biol. 2004;14:625–9. 10.1016/j.cub.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 26. Altincicek B, Stötzel S, Wygrecka M, Preissner KT, Vilcinskas A. Host‐derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J Immunol. 2008;181:2705–12. 10.4049/jimmunol.181.4.2705. [DOI] [PubMed] [Google Scholar]
- 27. Ding JL, Hou J, Feng MG, Ying SH. Transcriptomic analyses reveal comprehensive responses of insect hemocytes to mycopathogen Beauveria bassiana, and fungal virulence‐related cell wall protein assists pathogen to evade host cellular defense. Virulence. 2020;11:1352–65. 10.1080/21505594.2020.1827886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tattikota SG, Cho B, Liu Y, Hu Y, Barrera V, Steinbaugh MJ, et al. A single‐cell survey of Drosophila blood. Elife. 2020;9:e54818. 10.7554/eLife.54818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Contreras G, Shirdel I, Braun MS, Wink M. Defensins: transcriptional regulation and function beyond antimicrobial activity. Dev Comp Immunol. 2020;104:103556. 10.1016/j.dci.2019.103556. [DOI] [PubMed] [Google Scholar]
- 30. Yadav S, Daugherty S, Shetty AC, Eleftherianos I. RNAseq analysis of the Drosophila response to the entomopathogenic nematode Steinernema . G3. 2017;7:1955–67. 10.1534/g3.117.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang LL, Zhan MY, Zhuo YL, Pan YM, Xu Y, Zhou XH, et al. Antimicrobial activities of a proline‐rich proprotein from Spodoptera litura . Dev Comp Immunol. 2018;87:137–46. 10.1016/j.dci.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 32. Ma H, Abbas MN, Zhang K, Hu X, Xu M, Liang H, et al. 20‐Hydroxyecdysone regulates the transcription of the lysozyme via Broad‐Complex Z2 gene in silkworm, Bombyx mori . Dev Comp Immunol. 2019;94:66–72. 10.1016/j.dci.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 33. Dutta A, Dandapat J, Mohanty N. First report on transferrin in the silkworm, Antheraea mylitta, with a putative role in antioxidant defense: Insights from proteomic analysis and immunodetection. Comp Biochem Physiol B Biochem Mol Biol. 2019;233:23–34. 10.1016/j.cbpb.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 34. Lin Z, Wang JL, Cheng Y, Wang JX, Zou Z. Pattern recognition receptors from lepidopteran insects and their biological functions. Dev Comp Immunol. 2020;108:103688. 10.1016/j.dci.2020.103688. [DOI] [PubMed] [Google Scholar]
- 35. Zhang W, Tettamanti G, Bassal T, Heryanto C, Eleftherianos I, Mohamed A. Regulators and signalling in insect antimicrobial innate immunity: functional molecules and cellular pathways. Cell Signal. 2021;84:110003. 10.1016/j.cellsig.2021.110003. [DOI] [PubMed] [Google Scholar]
- 36. Rizki TM. Experimental analysis of hemocyte morphology in insects. Am Zool. 1962;2:247–56. 10.1093/icb/2.2.247. [DOI] [Google Scholar]
- 37. Shrestha R, Gateff E. Ultrastructure and cytochemistry of the cell types in the larval hematopoietic organs and hemolymph of Drosophila melanogaster . Dev Growth Differ. 1982;24:65–82. 10.1111/j.1440-169X.1982.00065.x. [DOI] [PubMed] [Google Scholar]
- 38. Kurucz E, Váczi B, Márkus R, Laurinyecz B, Vilmos P, Zsámboki J, et al. Definition of Drosophila hemocyte subsets by cell‐type specific antigens. Acta Biol Hung. 2007;58(Suppl):95–111. 10.1556/ABiol.58.2007.Suppl.8. [DOI] [PubMed] [Google Scholar]
- 39. Evans CJ, Liu T, Banerjee U. Drosophila hematopoiesis: markers and methods for molecular genetic analysis. Methods. 2014;68:242–51. 10.1016/j.ymeth.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jones JC. Current concepts concerning insect hemocytes. Am Zool. 1962;2:209–46. 10.1093/icb/2.2.209. [DOI] [Google Scholar]
- 41. Gupta AP. Cellular elements in the hemolymph. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology, vol. 3. Elmsford, New York: Pergamon Press; 1985. p. 402–44. [Google Scholar]
- 42. Kaaya GP, Ratcliffe NA. Comparative study of hemocytes and associated cells of some medically important dipterans. J Morphol. 1982;173:351–65. 10.1002/jmor.1051730310. [DOI] [PubMed] [Google Scholar]
- 43. Horohov DW, Dunn PE. Changes in the circulating hemocyte population of Manduca sexta larvae following injection of bacteria. J Invertebr Pathol. 1982;40:327–39. 10.1016/0022-2011(82)90171-9. [DOI] [Google Scholar]
- 44. Ling E, Shirai K, Kanekatsu R, Kiguchi K. Hemocyte differentiation in the hematopoietic organs of the silkworm, Bombyx mori: prohemocytes have the function of phagocytosis. Cell Tissue Res. 2005;320:535–43. 10.1007/s00441-004-1038-8. [DOI] [PubMed] [Google Scholar]
- 45. Beetz S, Holthusen TK, Koolman J & Trenczek T. Correlation of hemocyte counts with different developmental parameters during the last larval instar of the tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol. 2008;67:63–75. 10.1002/arch.20221. [DOI] [PubMed] [Google Scholar]
- 46. De Andrade FG, De Negreiro MCC, Levy SM, De Batista Fonesca IC, Moscardi F, Falleiros ÂMF. Hemocyte quantitative changes in Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae infected by AgMNPV. Braz Arch Biol Technol. 2010;53:279–84. 10.1590/S1516-89132010000200005. [DOI] [Google Scholar]
- 47. Liu F, Xu Q, Zhang Q, Lu A, Beerntsen BT, Ling E. Hemocytes and hematopoiesis in the silkworm, Bombyx mori . Invertebr Surviv J. 2013;10:102–9. https://www.isj.unimore.it/index.php/ISJ/article/view/292. [Google Scholar]
- 48. Kwon H, Bang K, Cho S. Characterization of the hemocytes in Larvae of Protaetia brevitarsis seulensis: involvement of granulocyte‐mediated phagocytosis. PLoS One. 2014;9:e103620. 10.1371/journal.pone.0103620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hwang S, Bang K, Lee J, Cho S. Circulating hemocytes from larvae of the Japanese rhinoceros beetle Allomyrina dichotoma (Linnaeus) (Coleoptera: Scarabaeidae) and the cellular immune response to microorganisms. PLoS One. 2015;10:e0128519. 10.1371/journal.pone.0128519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silva JE, Boleli IC, Simoes ZL. Hemocyte types and total and differential counts in unparasitized and parasitized Anastrepha obliqua (Diptera, Tephritidae) larvae. Braz J Biol. 2002;62:689–99. 10.1590/S1519-69842002000400017. [DOI] [PubMed] [Google Scholar]
- 51. Pal R, Kumar K. A comparative study of haemocytes in three cyclorrhaphous dipteran flies. Int J Trop Insect Sci. 2014;34:207–16. 10.1017/S1742758414000332. [DOI] [Google Scholar]
- 52. Dorrah MA, Mohamed AA, Shaurub EH. Immunosuppressive effects of the limonoid azadirachtin, insights on a nongenotoxic stress botanical, in flesh flies. Pestic Biochem Physiol. 2019;153:55–66. 10.1016/j.pestbp.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 53. Cho Y, Cho S. Hemocyte‐hemocyte adhesion by granulocytes is associated with cellular immunity in the cricket, Gryllus bimaculatus . Sci Rep. 2019;9:18066. 10.1038/s41598-019-54484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duressa TF, Huybrechts R. Development of primary cell cultures using hemocytes and phagocytic tissue cells of Locusta migratoria: an application for locust immunity studies. In Vitro Cell Dev Biol Anim. 2016;52:100–6. 10.1007/s11626-015-9952-5. [DOI] [PubMed] [Google Scholar]
- 55. de Azambuja P, Garcia ES, Ratcliffe NA. Aspects of classification of Hemiptera hemocytes from six triatomine species. Mem Inst Oswaldo Cruz. 1991;86:1–10. 10.1590/s0074-02761991000100002. [DOI] [PubMed] [Google Scholar]
- 56. Williams MJ. Drosophila hemopoiesis and cellular immunity. J Immunol. 2007;178:4711–6. 10.4049/jimmunol.178.8.4711. [DOI] [PubMed] [Google Scholar]
- 57. Banerjee U, Girard JR, Goins LM, Spratford CM. Drosophila as a genetic model for hematopoiesis. Genetics. 2019;211:367–417. 10.1534/genetics.118.300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–26. 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 59. Leitão AB, Sucena É. Drosophila sessile hemocyte clusters are true hematopoietic tissues that regulate larval blood cell differentiation. Elife. 2015;4:e06166. 10.7554/eLife.06166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Castillo JC, Robertson AE, Strand MR. Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti . Insect Biochem Mol Biol. 2006;36:891–903. 10.1016/j.ibmb.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pondeville E, Puchot N, Parvy JP, Carissimo G, Poidevin M, Waterhouse RM, et al. Hemocyte‐targeted gene expression in the female malaria mosquito using the hemolectin promoter from Drosophila . Insect Biochem Mol Biol. 2020;120:103339. 10.1016/j.ibmb.2020.103339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hillyer JF, Strand MR. Mosquito hemocyte‐mediated immune responses. Curr Opin Insect Sci. 2014;3:14–21. 10.1016/j.cois.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hillyer JF, Christensen BM. Characterization of hemocytes from the yellow fever mosquito, Aedes aegypti . Histochem Cell Biol. 2002;117:431–40. 10.1007/s00418-002-0408-0. [DOI] [PubMed] [Google Scholar]
- 64. Strand MR. Insect hemocytes and their role in immunity. In: Beckage N, editor. Insect immunology. San Diego: Academic Press; 2008. p. 25–47. 10.1016/B978-012373976-6.50004-5. [DOI] [Google Scholar]
- 65. Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila . Dev Biol. 2001;230:243–57. 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- 66. Weiss BL, Wang J, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011;9:e1000619. 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ibrahim AM, Kim Y. Parasitism by Cotesia plutellae alters the hemocyte population and immunological function of the diamondback moth, Plutella xylostella . J Insect Physiol. 2006;52:943–50. [DOI] [PubMed] [Google Scholar]
- 68. Eleftherianos I, Xu M, Yadi H, Ffrench‐Constant RH, Reynolds SE. Plasmatocyte‐spreading peptide (PSP) plays a central role in insect cellular immune defenses against bacterial infection. J Exp Biol. 2009;212:1840–8. 10.1242/jeb.026278. [DOI] [PubMed] [Google Scholar]
- 69. Stoepler TM, Castillo JC, Lill JT, Eleftherianos I. Hemocyte density increases with developmental stage in an immune‐challenged forest caterpillar. PLoS One. 2013;8:e70978. 10.1371/journal.pone.0070978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vogelweith F, Moret Y, Monceau K, Thiéry D, Moreau J. The relative abundance of hemocyte types in a polyphagous moth larva depends on diet. J Insect Physiol. 2016;88:33–9. 10.1016/j.jinsphys.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 71. Islam A, Roy S. Diurnal rhythm of hemocyte population in an insect, Schizodactylus monstresus Drury. Experientia. 1982;38:567–9. 10.1007/BF02327052. [DOI] [Google Scholar]
- 72. Duressa TF, Vanlaer R, Huybrechts R. Locust cellular defense against infections: sites of pathogen clearance and hemocyte proliferation. Dev Comp Immunol. 2015;48:244–53. 10.1016/j.dci.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 73. Kolundžić E, Kovacević G, Špoljar M, Sirovina D. A comparison of hemocytes in Phasmatodea and Blattodea species. Entomol News. 2018;127:471–7. 10.3157/021.127.0510. [DOI] [Google Scholar]
- 74. Lubawy J, Słocińska M. Characterization of Gromphadorhina coquereliana hemolymph under cold stress. Sci Rep. 2020;10:12076. 10.1038/s41598-020-68941-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Makhijani K, Alexander B, Tanaka T, Rulifson E, Brückner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–91. 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grigorian M, Hartenstein V. Hematopoiesis and hematopoietic organs in arthropods. Dev Genes Evol. 2013;223:103–15. 10.1007/s00427-012-0428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martinez‐Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21:3044–60. 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- 78. Hinks C, Arnold J. Haemopoiesis in Lepidoptera. II. The role of the haemopoietic organs. Can J Zool. 1977;55:1740–55. 10.1139/z77-225. [DOI] [Google Scholar]
- 79. Nardi JB, Pilas B, Ujhelyi E, Garsha K, Kanost MR. Hematopoietic organs of Manduca sexta and hemocyte lineages. Dev Genes Evol. 2003;213:477–91. 10.1007/s00427-003-0352-6. [DOI] [PubMed] [Google Scholar]
- 80. Gardiner E, Strand M. Hematopoiesis in larval Pseudoplusia includens and Spodoptera frugiperda . Arch Insect Biochem Physiol. 2000;43:147–64. . [DOI] [PubMed] [Google Scholar]
- 81. von Bredow CR, von Bredow YM, Trenczek TE. The larval haematopoietic organs of Manduca sexta (Insecta, Lepidoptera): an insight into plasmatocyte development and larval haematopoiesis. Dev Comp Immunol. 2021;115:103858. 10.1016/j.dci.2020.103858. [DOI] [PubMed] [Google Scholar]
- 82. Mandal L, Martinez‐Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog‐ and Antennapedia‐dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–4. 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sinenko SA, Mandal L, Martinez‐Agosto JA, Banerjee U. Dual role of wingless signaling in stem‐like hematopoietic precursor maintenance in Drosophila . Dev Cell. 2009;16:756–63. 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Minakhina S, Steward R. Hematopoietic stem cells in Drosophila. Development. 2010;137:27–31. 10.1242/dev.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;2005:2521–33. 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 86. Tepass U, Fessler LI, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila . Development. 1994;120:1829–37. [DOI] [PubMed] [Google Scholar]
- 87. de Velasco B, Mandal L, Mkrtchyan M, Hartenstein V. Subdivision and developmental fate of the head mesoderm in Drosophila melanogaster . Dev Genes Evol. 2006;216:39–51. 10.1007/s00427-005-0029-4. [DOI] [PubMed] [Google Scholar]
- 88. Honti V, Csordás G, Márkus R, Kurucz E, Jankovics F, Andó I. Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in Drosophila melanogaster . Mol Immunol. 2010;47:1997–2004. 10.1016/j.molimm.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 89. Anderl I, Vesala L, Ihalainen TO, Vanha‐Aho LM, Andó I, Rämet M, et al. Transdifferentiation and proliferation in two distinct hemocyte lineages in Drosophila melanogaster larvae after wasp infection. PLoS Pathog. 2016;12:e1005746. 10.1371/journal.ppat.1005746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kim‐Jo C, Gatti J‐L, Poirié M. Drosophila cellular immunity against parasitoid wasps: a complex and time‐dependent process. Front Physiol. 2019;10:603. 10.3389/fphys.2019.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Letourneau M, Lapraz F, Sharma A, Vanzo N, Waltzer L, Crozatier M. Drosophila hematopoiesis under normal conditions and in response to immune stress. FEBS Lett. 2016;590:4034–51. 10.1002/1873-3468.12327. [DOI] [PubMed] [Google Scholar]
- 92. Williams MJ, Andó I, Hultmark D. Drosophila melanogaster Rac2 is necessary for a proper cellular immune response. Genes Cells. 2005;10:813–23. 10.1111/j.1365-2443.2005.00883.x. [DOI] [PubMed] [Google Scholar]
- 93. Williams MJ, Wiklund ML, Wikman S, Hultmark D. Rac1 signalling in the Drosophila larval cellular immune response. J Cell Sci. 2006;119:2015–24. 10.1242/jcs.02920. [DOI] [PubMed] [Google Scholar]
- 94. Da Silva CCA. Activation of the prophenoloxidase and removal of Bacillus subtilis from the hemolymph of Acheta domesticus (L.) (Orthoptera: Gryllidae). Neotrop Entomol. 2002;31:487–91. 10.1590/S1519-566X2002000300024. [DOI] [Google Scholar]
- 95. Schmitz A, Anselme C, Ravallec M, Rebuf C, Simon JC, Gatti JL, et al. The cellular immune response of the pea aphid to foreign intrusion and symbiotic challenge. PLoS One. 2012;7:e42114. 10.1371/journal.pone.0042114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang X, Zhang K. Cellular response to bacterial infection in the grasshopper Oxya chinensis . Biol Open. 2019;8:bio045864. 10.1242/bio.045864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol. 2010;346:310–9. 10.1016/j.ydbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 98. Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–9. 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 99. Márkus R, Laurinyecz B, Kurucz E, Honti V, Bajusz I, Sipos B, et al. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci USA. 2009;106:4805–9. 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bretscher AJ, Honti V, Binggeli O, Burri O, Poidevin M, Kurucz É, et al. The Nimrod transmembrane receptor Eater is required for hemocyte attachment to the sessile compartment in Drosophila melanogaster . Biol Open. 2015;4:355–63. 10.1242/bio.201410595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Duvic B, Hoffmann JA, Meister M, Royet J. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr Biol. 2002;12:1923–7. 10.1016/s0960-9822(02)01297-6. [DOI] [PubMed] [Google Scholar]
- 102. Lebestky T, Jung SH, Banerjee U. A Serrate‐expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–53. 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Waltzer L, Ferjoux G, Bataillé L, Haenlin M. Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. EMBO J. 2003;22:6516–25. 10.1093/emboj/cdg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fossett N, Hyman K, Gajewski K, Orkin SH, Schulz RA. Combinatorial interactions of serpent, lozenge, and U‐shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc Natl Acad Sci USA. 2003;100:11451–6. 10.1073/pnas.1635050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Muratoglu S, Hough B, Mon ST, Fossett N. The GATA factor Serpent cross‐regulates lozenge and u‐shaped expression during Drosophila blood cell development. Dev Biol. 2007;311:636–49. 10.1016/j.ydbio.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Waltzer L, Bataillé L, Peyrefitte S, Haenlin M. Two isoforms of Serpent containing either one or two GATA zinc fingers have different roles in Drosophila haematopoiesis. EMBO J. 2002;21:5477–86. 10.1093/emboj/cdf545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Crozatier M, Ubeda J‐M, Vincent A, Meister M. Cellular immune response to parasitization in Drosophila requires the EBF orthologue Collier. PLoS Biol. 2004;2:e196. 10.1371/journal.pbio.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Oyallon J, Vanzo N, Krzemień J, Morin‐Poulard I, Vincent A, Crozatier M. Two independent functions of Collier/Early B Cell Factor in the control of Drosophila blood cell homeostasis. PLoS One. 2016;11:e0148978. 10.1371/journal.pone.0148978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Morin‐Poulard I, Vincent A, Crozatier M. The Drosophila JAK‐STAT pathway in blood cell formation and immunity. JAKSTAT. 2013;2:e25700. 10.4161/jkst.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang H, Kronhamn J, Ekström JO, Korkut GG, Hultmark D. JAK/STAT signaling in Drosophila muscles controls the cellular immune response against parasitoid infection. EMBO Rep. 2015;16:1664–72. 10.15252/embr.201540277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tokusumi T, Sorrentino RP, Russell M, Ferrarese R, Govind S, Schulz RA. Characterization of a lamellocyte transcriptional enhancer located within the misshapen Gene of Drosophila melanogaster . PLoS One. 2009;4:e6429. 10.1371/journal.pone.0006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ponrathnam T, Saini R, Banu S, Mishra RK. Drosophila Hox genes induce melanized pseudo‐tumors when misexpressed in hemocytes. Sci Rep. 2021;11:1838. 10.1038/s41598-021-81472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Huang L, Ohsako S, Tanda S. The lesswright mutation activates Rel‐related proteins, leading to overproduction of larval hemocytes in Drosophila melanogaster . Dev Biol. 2005;280:407–20. 10.1016/j.ydbio.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 114. Strand MR, Burke GR. Polydnaviruses: from discovery to current insights. Virology. 2015;479–480:393–402. 10.1016/j.virol.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Munier AI, Doucet D, Perrodou E, Zachary D, Meister M, Hoffmann JA, et al. PVF2, a PDGF/VEGF‐like growth factor, induces hemocyte proliferation in Drosophila larvae. EMBO Rep. 2002;3:1195–200. 10.1093/embo-reports/kvf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Remillieux‐Leschelle N, Santamaria P, Randsholt NB. Regulation of larval hematopoiesis in Drosophila melanogaster: a role for the multi sex combs gene. Genetics. 2002;162:1259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ragab A, Buechling T, Gesellchen V, Spirohn K, Boettcher AL, Boutros M. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2011;30:1123–36. 10.1038/emboj.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, et al. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci USA. 2004;101:14192–7. 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fiúza UM, Arias AM. Cell and molecular biology of Notch. J Endocrinol. 2007;194:459–74. 10.1677/JOE-07-0242. [DOI] [PubMed] [Google Scholar]
- 120. Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol. 2010;125:S53–72. 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 121. Schmid MR, Anderl I, Vesala L, Vanha‐aho LM, Deng XJ, Rämet M, et al. Control of Drosophila blood cell activation via Toll signaling in the fat body. PLoS One. 2014;9:e102568. 10.1371/journal.pone.0102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Parisi F, Stefanatos RK, Strathdee K, Yu Y, Vidal M. Transformed epithelia trigger non‐tissue‐autonomous tumor suppressor response by adipocytes via activation of Toll and Eiger/TNF signaling. Cell Rep. 2014;6:855–67. 10.1016/j.celrep.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 123. Yang H, Hultmark D. Tissue communication in a systemic immune response of Drosophila . Fly. 2016;10:115–22. 10.1080/19336934.2016.1182269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Satyavathi VV, Minz A, Nagaraju J. Nodulation: an unexplored cellular defense mechanism in insects. Cell Signal. 2014;26:1753–63. 10.1016/j.cellsig.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 125. Melcarne C, Lemaitre B, Kurant E. Phagocytosis in Drosophila: From molecules and cellular machinery to physiology. Insect Biochem Mol Biol. 2019;109:1–12. 10.1016/j.ibmb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 126. Lu Y, Su F, Li Q, Zhang J, Li Y, Tang T, et al. Pattern recognition receptors in Drosophila immune responses. Dev Comp Immunol. 2020;102:103468. 10.1016/j.dci.2019.103468. [DOI] [PubMed] [Google Scholar]
- 127. Pech LL, Strand MR. Granular cells are required for encapsulation of foreign targets by insect haemocytes. J Cell Sci. 1996;109:2053–60. [DOI] [PubMed] [Google Scholar]
- 128. Fallon JP, Reeves EP, Kavanagh K. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology. 2011;157:1481–8. 10.1099/mic.0.043786-0. [DOI] [PubMed] [Google Scholar]
- 129. Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol. 2010;708:181–204. 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mohamed AA, Ali MM, Dorrah MA, Bassal TTM. Mediation of inducible nitric oxide and immune‐reactive lysozymes biosynthesis by eicosanoid and biogenic amines in flesh flies. Int J Trop Insect Sci. 2018;38:93–104. 10.1017/S1742758417000315. [DOI] [Google Scholar]
- 131. Browne N, Heelan M, Kavanagh K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence. 2013;1:597–603. 10.4161/viru.25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Horn L, Leips J, Starz‐Gaiano M. Phagocytic ability declines with age in adult Drosophila hemocytes. Aging Cell. 2014;13:719–28. 10.1111/acel.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol. 2008;8:131–41. 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 134. Melcarne C, Ramond E, Dudzic J, Bretscher AJ, Kurucz É, Andó I, et al. Two Nimrod receptors, NimC1 and Eater, synergistically contribute to bacterial phagocytosis in Drosophila melanogaster . FEBS J. 2019;286:2670–91. 10.1111/febs.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rittig MG, Kuhn KH, Dechant CA, Gauckler A, Modolell M, Ricciardi‐Castagnoli P, et al. Phagocytes from both vertebrate and invertebrate species use “coiling” phagocytosis. Dev Comp Immunol. 1996;20:393–406. 10.1016/s0145-305x(96)00023-7. [DOI] [PubMed] [Google Scholar]
- 136. Tojo S, Naganuma F, Arakawa K, Yokoo S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella . J Insect Physiol. 2000;46:1129–35. 10.1016/s0022-1910(99)00223-1. [DOI] [PubMed] [Google Scholar]
- 137. Ling E, Yu XQ. Hemocytes from the tobacco hornworm Manduca sexta have distinct functions in phagocytosis of foreign particles and self dead cells. Dev Comp Immunol. 2006;30:301–9. 10.1016/j.dci.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 138. Kim CH, Shin YP, Noh MY, Jo YH, Han YS, Seong YS, et al. An insect multiligand recognition protein functions as an opsonin for the phagocytosis of microorganisms. J Biol Chem. 2010;285:25243–50. 10.1074/jbc.M110.134940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. De Lerma Barbaro A, Gariboldi MB, Mastore M, Brivio MF, Giovannardi S. In vivo effects of a Pro‐PO system Inhibitor on the phagocytosis of Xenorhabdus nematophila in Galleria mellonella larvae. Insects. 2019;10:263. 10.3390/insects10090263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wilson R, Chen C, Ratcliffe NA. Innate immunity in insects: the role of multiple, endogenous serum lectins in the recognition of foreign invaders in the cockroach, Blaberus discoidalis . J Immunol. 1999;162:1590–6. [PubMed] [Google Scholar]
- 141. Tian YY, Liu Y, Zhao XF, Wang JX. Characterization of a C‐type lectin from the cotton bollworm, Helicoverpa armigera . Dev Comp Immunol. 2009;33:772–9. 10.1016/j.dci.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 142. Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev. 2014;262:193–215. 10.1111/imr.12212. [DOI] [PubMed] [Google Scholar]
- 143. Rosales C, Uribe‐Querol E. Phagocytosis: a fundamental process in immunity. Biomed Res Int. 2017;2017:9042851. 10.1155/2017/9042851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lee HJ, Woo Y, Hahn TW, Jung YM, Jung YJ. Formation and maturation of the phagosome: a key mechanism in innate immunity against intracellular bacterial infection. Microorganisms. 2020;8:1298. 10.3390/microorganisms8091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Nappi AJ, Christensen BM. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem Mol Biol. 2005;35:443–59. 10.1016/j.ibmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 146. Dubovskiy IM, Kryukova NA, Glupov VV, Ratcliffe NA. Encapsulation and nodulation in insects. Invertbr Surv J. 2016;13:229–46. [Google Scholar]
- 147. Ratcliffe NA, Gagen SJ. Studies on the in vivo cellular reactions of insects: an ultrastructural analysis of nodule formation in Galleria mellonella . Tissue Cell. 1977;9:73–85. 10.1016/0040-8166(77)90050-7. [DOI] [PubMed] [Google Scholar]
- 148. Lapointe JF, Dunphy GB, Mandato CA. Hemocyte‐hemocyte adhesion and nodulation reactions of the greater wax moth, Galleria mellonella are influenced by cholera toxin and its B‐subunit. Results Immunol. 2012;2:54–65. 10.1016/j.rinim.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Arai I, Ohta M, Suzuki A, Tanaka S, Yoshizawa Y, Sato R. Immunohistochemical analysis of the role of hemocytin in nodule formation in the larvae of the silkworm, Bombyx mori . J Insect Sci. 2013;13:125. 10.1673/031.013.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tokura A, Fu GS, Sakamoto M, Endo H, Tanaka S, Kikuta S, et al. Factors functioning in nodule melanization of insects and their mechanisms of accumulation in nodules. J Insect Physiol. 2014;60:40–9. 10.1016/j.jinsphys.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 151. Marmaras VJ, Charalambidis ND. Certain hemocyte proteins of the Medfly, Ceratitis capitata, are responsible for nonself recognition and immobilization of Escherichia coli in vitro . Arch Insect Biochem Physiol. 1992;21:281–8. 10.1002/arch.940210405. [DOI] [Google Scholar]
- 152. Shrestha S, Kim Y. Various eicosanoids modulate the cellular and humoral immune responses of the beet armyworm, Spodoptera exigua . Biosci Biotech Biochem. 2009;73:2077–84. 10.1271/bbb.90272. [DOI] [PubMed] [Google Scholar]
- 153. Sadekuzzaman M, Stanley D, Kim Y. Nitric oxide mediates insect cellular immunity via phospholipase A2 activation. J Innate Immun. 2018;10:70–81. 10.1159/000481524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sakamoto M, Ohta M, Suzuki A, Takase H, Yoshizawa Y, Kitami M, et al. Localization of the serine protease homolog BmSPH‐1 in nodules of E. coli‐injected Bombyx mori larvae and functional analysis of its role in nodule melanization. Dev Comp Immunol. 2011;35:611–9. 10.1016/j.dci.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 155. Stączek S, Zdybicka‐Barabas A, Wiater A, Pleszczyńska M, Cytryńska M. Activation of cellular immune response in insect model host Galleria mellonella by fungal α‐1,3‐glucan. Pathog Dis. 2020;78:ftaa062. 10.1093/femspd/ftaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Diehl‐Jones W, Mandato CA, Whent G, Downer RGH. Monoaminergic regulation of hemocyte activity. J Insect Physiol. 1996;42:13–9. 10.1016/0022-1910(95)00078-X. [DOI] [Google Scholar]
- 157. Gandhe AS, John SH, Nagaraju J. Noduler, a novel immune up‐regulated protein mediates nodulation response in insects. J Immunol. 2007;179:6943–51. 10.4049/jimmunol.179.10.6943. [DOI] [PubMed] [Google Scholar]
- 158. Park Y, Kim Y, Putnam SM, Stanley DW. The bacterium Xenorhabdus nematophilus depresses nodulation reactions to infection by inhibiting eicosanoid biosynthesis in tobacco hornworms, Manduca sexta . Arch Insect Biochem Physiol. 2003;52:71–80. 10.1002/arch.10076. [DOI] [PubMed] [Google Scholar]
- 159. Kim Y, Ji D, Cho S, Park Y. Two groups of entomopathogenic bacteria, Photorhabdus and Xenorhabdus, share an inhibitory action against phospholipase A2 to induce host immunodepression. J Invertebr Pathol. 2005;89:258–64. 10.1016/j.jip.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 160. Zhao P, Li J, Wang Y, Jiang H. Broad‐spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochem Mol Biol. 2007;37:952–9. 10.1016/j.ibmb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yang L, Qiu LM, Fang Q, Stanley DW, Ye GY. Cellular and humoral immune interactions between Drosophila and its parasitoids. Insect Sci. 2020. 10.1111/1744-7917.12863. [DOI] [PubMed] [Google Scholar]
- 162. Mortimer NT, Kacsoh BZ, Keebaugh ES, Schlenke TA. Mgat1‐dependent N‐glycosylation of membrane components primes Drosophila melanogaster blood cells for the cellular encapsulation response. PLoS Pathog. 2012;8:e1002819. 10.1371/journal.ppat.1002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ono M, Arimatsu C, Kakinoki A, Matsunaga K, Yoshiga T. Comparison of cellular encapsulation with nematodes in two lepidopteran insects. Appl Entomol Zool. 2020;55:337–44. 10.1007/s13355-020-00687-6. [DOI] [Google Scholar]
- 164. Akai H, Sato S. Ultrastructure of the larval hemocytes of the silkworm, Bombyx mori L. (Lepidoptera: Bombycidae). Int J Insect Morphol Embryol. 1973;2:207–31. 10.1016/0020-7322(73)90029-9. [DOI] [Google Scholar]
- 165. Takahashi S, Enomoto G. The initial phase of encapsulation of silicone oil injected in Samia cynthia ricini (Lepidoptera, Saturniidae): The innermost structure of the developing capsule. Zool Sci. 1995;12:303–9. 10.2108/zsj.12.303. [DOI] [Google Scholar]
- 166. Infanger LC, Rocheleau TA, Bartholomay LC, Johnson JK, Fuchs J, Higgs S, et al. The role of phenylalanine hydroxylase in melanotic encapsulation of filarial worms in two species of mosquitoes. Insect Biochem Mol Biol. 2004;34:1329–38. 10.1016/j.ibmb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 167. Doherty JF, Chai X, Poulin R. Varying levels of melanotic encapsulation of gordiid hairworm cysts (Nematomorpha) by aquatic insect larvae: seasonal and host effects. J Invertebr Pathol. 2019;168:107258. 10.1016/j.jip.2019.107258. [DOI] [PubMed] [Google Scholar]
- 168. Liu WT, Chen TL, Hou RF, Chen CC, Tu WC. The invasion and encapsulation of the entomopathogenic nematode, Steinernema abbasi, in Aedes albopictus (Diptera: Culicidae) larvae. Insects. 2020;11:832. 10.3390/insects11120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, et al. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae . Proc Natl Acad Sci USA. 2003;100:14139–44. 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Strand MR, Hayakawa Y, Clark KD. Plasmatocyte spreading peptide (PSP1) and growth blocking peptide (GBP) are multifunctional homologs. J Insect Physiol. 2000;46:817–24. 10.1016/s0022-1910(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 171. Clark KD, Garczynski SF, Arora A, Crim JW, Strand MR. Specific residues in plasmatocyte‐spreading peptide are required for receptor binding and functional antagonism of insect immune cells. J Biol Chem. 2004;279:33246–52. 10.1074/jbc.M401157200. [DOI] [PubMed] [Google Scholar]
- 172. Tsuzuki S, Matsumoto H, Furihata S, Ryuda M, Tanaka H, Sung EJ, et al. Switching between humoral and cellular immune responses in Drosophila is guided by the cytokine GBP. Nat Commun. 2014;5:4628. 10.1038/ncomms5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Matsumoto H, Tsuzuki S, Date‐Ito A, Ohnishi A, Hayakawa Y. Characteristics common to a cytokine family spanning five orders of insects. Insect Biochem Mol Biol. 2012;42:446–54. 10.1016/j.ibmb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 174. Nardi JB, Pilas B, Bee CM, Zhuang S, Garsha K, Kanost MR. Neuroglian‐positive plasmatocytes of Manduca sexta and the initiation of hemocyte attachment to foreign surfaces. Dev Comp Immunol. 2006;30:447–62. 10.1016/j.dci.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 175. Zhuang S, Kelo L, Nardi JB, Kanost MR. An integrin‐tetraspanin interaction required for cellular innate immune responses of an insect, Manduca sexta . J Biol Chem. 2007;282:22563–72. 10.1074/jbc.M700341200. [DOI] [PubMed] [Google Scholar]
- 176. Zhuang S, Kelo L, Nardi JB, Kanost MR. Neuroglian on hemocyte surfaces is involved in homophilic and heterophilic interactions of the innate immune system of Manduca sexta . Dev Comp Immunol. 2007;31:1159–67. 10.1016/j.dci.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 177. Ao J, Ling E, Yu XQ. Drosophila C‐type lectins enhance cellular encapsulation. Mol Immunol. 2007;44:2541–8. 10.1016/j.molimm.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Fang Q, Wang F, Gatehouse JA, Gatehouse AM, Chen XX, Hu C, et al. Venom of parasitoid, Pteromalus puparum, suppresses host, Pieris rapae, immune promotion by decreasing host C‐type lectin gene expression. PLoS One. 2011;6:e26888. 10.1371/journal.pone.0026888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ishihara T, Maruyama Y, Furukawa S. Gene expression and molecular characterization of a novel C‐type lectin, encapsulation promoting lectin (EPL), in the rice armyworm, Mythimna separata . Insect Biochem Mol Biol. 2017;89:51–7. 10.1016/j.ibmb.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 180. Yu XQ, Kanost MR. Immulectin‐2, a pattern recognition receptor that stimulates hemocyte encapsulation and melanization in the tobacco hornworm, Manduca sexta . Dev Comp Immunol. 2004;28:891–900. 10.1016/j.dci.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 181. Levin DM, Breuer LN, Zhuang S, Anderson SA, Nardi JB, Kanost MR. A hemocyte‐specific integrin required for hemocytic encapsulation in the tobacco hornworm, Manduca sexta . Insect Biochem Mol Biol. 2005;35:369–80. 10.1016/j.ibmb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 182. Whitten MM, Tew IF, Lee BL, Ratcliffe NA. A novel role for an insect apolipoprotein (apolipophorin III) in beta‐1,3‐glucan pattern recognition and cellular encapsulation reactions. J Immunol. 2004;172:2177–85. 10.4049/jimmunol.172.4.2177. [DOI] [PubMed] [Google Scholar]
- 183. Asgari S, Rivers DB. Venom proteins from endoparasitoid wasps and their role in host‐parasite interactions. Annu Rev Entomol. 2011;56:313–35. 10.1146/annurev-ento-120709-144849. [DOI] [PubMed] [Google Scholar]
- 184. Russo J, Brehélin M, Carton Y. Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp Leptopilina boulardi . J Insect Physiol. 2001;47:167–72. 10.1016/S0022-1910(00)00102-5. [DOI] [PubMed] [Google Scholar]
- 185. Márkus R, Kurucz E, Rus F, Andó I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster . Immunol Lett. 2005;101:108–11. 10.1016/j.imlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 186. Bustelo XR, Sauzeau V, Berenjeno IM. GTP‐binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays. 2007;29:357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Xavier MJ, Williams MJ. The Rho‐family GTPase Rac1 regulates integrin localization in Drosophila immunosurveillance cells. PLoS One. 2011;6:e19504. 10.1371/journal.pone.0019504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kurucz E, Zettervall CJ, Sinka R, Vilmos P, Pivarcsi A, Ekengren S, et al. Hemese, a hemocyte‐specific transmembrane protein, affects the cellular immune response in Drosophila . Proc Natl Acad Sci USA. 2003;100:2622–7. 10.1073/pnas.0436940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Howell L, Sampson CJ, Xavier MJ, Bolukbasi E, Heck MM, Williams MJ. A directed miniscreen for genes involved in the Drosophila anti‐parasitoid immune response. Immunogenetics. 2012;64:155–61. 10.1007/s00251-011-0571-3. [DOI] [PubMed] [Google Scholar]
- 190. Liu CT, Hou RF, Chen CC. Formation of basement membrane‐like structure terminates the cellular encapsulation of microfilariae in the haemocoel of Anopheles quadrimaculatus . Parasitology. 1998;116:511–8. 10.1017/s0031182098002595. [DOI] [PubMed] [Google Scholar]
- 191. Nappi AJ, Vass E, Frey F, Carton Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide. 2000;4:423–30. 10.1006/niox.2000.0294. [DOI] [PubMed] [Google Scholar]
- 192. Kraaijeveld AR, Elrayes NP, Schuppe H, Newland PL. L‐arginine enhances immunity to parasitoids in Drosophila melanogaster and increases NO production in lamellocytes. Dev Comp Immunol. 2011;35:857–964. 10.1016/j.dci.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 193. Arefin B, Kucerova L, Krautz R, Kranenburg H, Parvin F, Theopold U. Apoptosis in hemocytes induces a shift in effector mechanisms in the Drosophila immune system and leads to a pro‐inflammatory state. PLoS One. 2015;10:e0136593. 10.1371/journal.pone.0136593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Negri P, Quintana S, Maggi M, Szawarski N, Lamattina L, Eguaras M. Apis mellifera hemocytes generate increased amounts of nitric oxide in response to wounding/encapsulation. Apidologie. 2014;45:610–7. 10.1007/s13592-014-0279-0. [DOI] [Google Scholar]
- 195. Romanelli D, Casati B, Franzetti E, Tettamanti G. A molecular view of autophagy in Lepidoptera. Biomed Res Int. 2014;2014:902315. 10.1155/2014/902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Romanelli D, Casartelli M, Cappellozza S, de Eguileor M, Tettamanti G. Roles and regulation of autophagy and apoptosis in the remodelling of the lepidopteran midgut epithelium during metamorphosis. Sci Rep. 2016;6:32939. 10.1038/srep32939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Tettamanti G, Casartelli M. Cell death during complete metamorphosis. Philos Trans R Soc Lond B Biol Sci. 2019;374:20190065. 10.1098/rstb.2019.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Casati B, Terova G, Cattaneo AG, Rimoldi S, Franzetti E, de Eguileor M, et al. Molecular cloning, characterization and expression analysis of ATG1 in the silkworm, Bombyx mori . Gene. 2012;511:326–37. 10.1016/j.gene.2012.09.086. [DOI] [PubMed] [Google Scholar]
- 199. Tettamanti G, Malagoli D, Marchesini E, Congiu T, de Eguileor M, Ottaviani E. Oligomycin A induces autophagy in the IPLB‐LdFB insect cell line. Cell Tissue Res. 2006;326:179–86. 10.1007/s00441-006-0206-4. [DOI] [PubMed] [Google Scholar]
- 200. Tettamanti G, Cao Y, Feng Q, Grimaldi A, de Eguileor M. Autophagy in Lepidoptera: more than old wine in new bottle. Inverteb Surviv J. 2011;8:5–14. https://www.isj.unimore.it/index.php/ISJ/article/view/230. [Google Scholar]
- 201. Denton D, Xu T, Kumar S. Autophagy as a pro‐death pathway. Immunol Cell Biol. 2015;93:35–42. 10.1038/icb.2014.85. [DOI] [PubMed] [Google Scholar]
- 202. Nakamoto M, Moy RH, Xu J, Bambina S, Yasunaga A, Shelly SS, et al. Virus recognition by Toll‐7 activates antiviral autophagy in Drosophila . Immunity. 2012;36:658–67. 10.1016/j.immuni.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Shelly S, Lukinova N, Bambina S, Berman A, Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–98. 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Moy RH, Gold B, Molleston JM, Schad V, Yanger K, Salzano MV, et al. Antiviral autophagy restricts Rift Valley fever virus infection and is conserved from flies to mammals. Immunity. 2014;40:51–65. 10.1016/j.immuni.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Patel RK, Hardy RW. Role for the phosphatidylinositol 3‐kinase‐Akt‐TOR pathway during sindbis virus replication in arthropods. J Virol. 2012;86:3595–604. 10.1128/jvi.06625-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lamiable O, Arnold J, de Faria IJDS, Olmo RP, Bergami F, Meignin C, et al. Analysis of the contribution of hemocytes and autophagy to Drosophila antiviral immunity. J Virol. 2016;90:5415–26. 10.1128/jvi.00238-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, et al. Autophagic control of Listeria through intracellular innate immune recognition in Drosophila . Nat Immunol. 2008;9:908–16. 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Denton D, Aung‐Htut MT, Kumar S. Developmentally programmed cell death in Drosophila . Biochim Biophys Acta. 2013;1833:3499–506. 10.1016/j.bbamcr.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 209. Jiao Z, Wen G, Tao S, Wang J, Wang G. Induction of hemocyte apoptosis by Ovomermis sinensis: implications for host immune suppression. J Invertebr Pathol. 2018;159:41–8. 10.1016/j.jip.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 210. Ishii K, Hamamoto H, Imamura K, Adachi T, Shoji M, Nakayama K, et al. Porphyromonas gingivalis peptidoglycans induce excessive activation of the innate immune system in silkworm larvae. J Biol Chem. 2010;285:33338–47. 10.1074/jbc.m110.112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Ishii K, Adachi T, Imamura K, Takano S, Usui K, Suzuki K, et al. Serratia marcescens induces apoptotic cell death in host immune cells via a lipopolysaccharide‐ and flagella‐dependent mechanism. J Biol Chem. 2012;287:36582–92. 10.1074/jbc.m112.399667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wan B, Yang L, Zhang J, Qiu L, Fang Q, Yao H, et al. The venom of the ectoparasitoid wasp Pachycrepoideus vindemiae (Hymenoptera: Pteromalidae) induces apoptosis of Drosophila melanogaster hemocytes. Insects. 2020;11:363. 10.3390/insects11060363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Liu B, Behura SK, Clem RJ, Schneemann A, Becnel J, Severson DW, et al. P53‐mediated rapid induction of apoptosis conveys resistance to viral infection in Drosophila melanogaster . PLoS Pathog. 2013;9:e1003137. 10.1371/journal.ppat.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Settles EW, Friesen PD. Flock house virus induces apoptosis by depletion of Drosophila inhibitor‐of‐apoptosis protein DIAP1. J Virol. 2008;82:1378–88. 10.1128/jvi.01941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Vandergaast R, Schultz KL, Cerio RJ, Friesen PD. Active depletion of host cell inhibitor‐of‐apoptosis proteins triggers apoptosis upon baculovirus DNA replication. J Virol. 2011;85:8348–58. 10.1128/jvi.00667-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Schultz KL, Friesen PD. Baculovirus DNA replication‐specific expression factors trigger apoptosis and shutoff of host protein synthesis during infection. J Virol. 2009;83:11123–32. 10.1128/jvi.01199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Vandergaast R, Mitchell JK, Byers NM, Friesen PD. Insect inhibitor‐of‐apoptosis (IAP) proteins are negatively regulated by signal‐induced N‐terminal degrons absent within viral IAP proteins. J Virol. 2015;89:4481–93. 10.1128/jvi.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Clem RJ. The role of apoptosis in defense against baculovirus infection in insects. Curr Top Microbiol Immunol. 2005;289:113–29. 10.1007/3-540-27320-4_5. [DOI] [PubMed] [Google Scholar]
- 219. Jiang L, Goldsmith MR, Xia Q. Advances in the arms race between silkworm and baculovirus. Front Immunol. 2021;12:628151. 10.3389/fimmu.2021.628151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Wang Z, Xia X, Yang X, Zhang X, Liu Y, Wu D, et al. A picorna‐like virus suppresses the N‐end rule pathway to inhibit apoptosis. Elife. 2017;6:e30590. 10.7554/elife.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Bideshi DK, Tan Y, Bigot Y, Federici BA. A viral caspase contributes to modified apoptosis for virus transmission. Genes Dev. 2005;19:1416–21. 10.1101/gad.1300205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Franzetti E, Romanelli D, Caccia S, Cappellozza S, Congiu T, Rajagopalan M, et al. The midgut of the silkmoth Bombyx mori is able to recycle molecules derived from degeneration of the larval midgut epithelium. Cell Tissue Res. 2015;361:509–28. 10.1007/s00441-014-2081-8. [DOI] [PubMed] [Google Scholar]
- 223. Bidla G, Hauling T, Dushay MS, Theopold U. Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J Innate Immun. 2009;1:301–8. 10.1159/000168009. [DOI] [PubMed] [Google Scholar]
- 224. Aguilera F, McDougall C, Degnan BM. Origin, evolution and classification of type‐3 copper proteins: lineage‐specific gene expansions and losses across the Metazoa. BMC Evol Biol. 2013;13:96. 10.1186/1471-2148-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Whitten MMA, Coates CJ. Re‐evaluation of insect melanogenesis research: Views from the dark side. Pigment Cell Melanoma Res. 2017;30:386–401. 10.1111/pcmr.12590. [DOI] [PubMed] [Google Scholar]
- 226. Decker H, Schweikardt T, Nillius D, Salzbrunn U, Jaenicke E, Tuczek F. Similar enzyme activation and catalysis in hemocyanins and tyrosinases. Gene. 2007;398:183–91. 10.1016/j.gene.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 227. Dittmer NT, Kanost MR. Insect multicopper oxidases: diversity, properties, and physiological roles. Insect Biochem Mol Biol. 2010;40:179–88. 10.1016/j.ibmb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 228. Sugumaran M, Barek H. Critical analysis of the melanogenic pathway in insects and higher animals. Int J Mol Sci. 2016;17:1753. 10.3390/ijms17101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Binggeli O, Neyen C, Poidevin M, Lemaitre B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila . PLoS Pathog. 2014;10:e1004067. 10.1371/journal.ppat.1004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yokoi K, Hayakawa Y, Kato D, Minakuchi C, Tanaka T, Ochiai M, et al. Prophenoloxidase genes and antimicrobial host defense of the model beetle, Tribolium castaneum . J Invertebr Pathol. 2015;132:190–200. 10.1016/j.jip.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 231. Cooper D, Wuebbolt C, Heryanto C, Eleftherianos I. The prophenoloxidase system in Drosophila participates in the anti‐nematode immune response. Mol Immunol. 2019;109:88–98. 10.1016/j.molimm.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 232. Özbek R, Mukherjee K, Uçkan F, Vilcinskas A. Reprograming of epigenetic mechanisms controlling host insect immunity and development in response to egg‐laying by a parasitoid wasp. Proc Biol Sci. 2020;287:20200704. 10.1098/rspb.2020.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Wang Y, Yang F, Cao X, Huang R, Paskewitz S, Hartson SD, et al. Inhibition of immune pathway‐initiating hemolymph protease‐14 by Manduca sexta serpin‐12, a conserved mechanism for the regulation of melanization and Toll activation in insects. Insect Biochem Mol Biol. 2020;116:103261. 10.1016/j.ibmb.2019.103261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Zhang X, Li M, El Moussawi L, Saab S, Zhang S, Osta MA, et al. CLIPB10 is a terminal protease in the regulatory network that controls melanization in the African malaria mosquito Anopheles gambiae . Front Cell Infect Microbiol. 2021;10:585986. 10.3389/fcimb.2020.585986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Satyavathi VV, Mohamed AA, Kumari S, Mamatha DM, Duvic B. The IMD pathway regulates lysozyme‐like proteins (LLPs) in the silkmoth Antheraea mylitta . J Invertebr Pathol. 2018;154:102–8. 10.1016/j.jip.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 236. Lu A, Zhang Q, Zhang J, Yang B, Wu K, Xie W, et al. Insect prophenoloxidase: the view beyond immunity. Front Physiol. 2014;5:252. 10.3389/fphys.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ling E, Shirai K, Kanehatsu R, Kiguchi K. Reexamination of phenoloxidase in larval circulating hemocytes of the silkworm, Bombyx mori . Tissue Cell. 2005b;37:101–7. 10.1016/j.tice.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 238. Hu Y, Wang Y, Deng J, Jiang H. The structure of a prophenoloxidase (PPO) from Anopheles gambiae provides new insights into the mechanism of PPO activation. BMC Biol. 2016;14:2. 10.1186/s12915-015-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Li Y, Wang Y, Jiang H, Deng J. Crystal structure of Manduca sexta prophenoloxidase provides insights into the mechanism of type 3 copper enzymes. Proc Natl Acad Sci USA. 2009;106:17002–6. 10.1073/pnas.0906095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Chase MR, Raina K, Bruno J, Sugumaran M. Purification, characterization and molecular cloning of prophenoloxidases from Sarcophaga bullata . Insect Biochem Mol Biol. 2000;30:953–67. 10.1016/s0965-1748(00)00068-0. [DOI] [PubMed] [Google Scholar]
- 241. Jiang H, Wang Y, Ma C, Kanost MR. Subunit composition of pro‐phenol oxidase from Manduca sexta: molecular cloning of subunit ProPO‐P1. Insect Biochem Mol Biol. 1997;27:835–50. 10.1016/s0965-1748(97)00066-0. [DOI] [PubMed] [Google Scholar]
- 242. Cerenius L, Kawabata S, Lee BL, Nonaka M, Söderhäll K. Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem Sci. 2010;35:575–83. 10.1016/j.tibs.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 243. Kanost MR, Gorman MJ. Phenoloxidases in insect immunity. In: Beckage NE, editor. Insect immunology. San Diego: Academic Press; 2008. p. 69–96. 10.1016/B978-012373976-6.50006-9. [DOI] [Google Scholar]
- 244. Kwon TH, Kim MS, Choi HW, Joo CH, Cho MY, Lee BL. A masquerade‐like serine proteinase homologue is necessary for phenoloxidase activity in the coleopteran insect, Holotrichia diomphalia larvae. Eur J Biochem. 2000;267:6188–96. 10.1046/j.1432-1327.2000.01695.x. [DOI] [PubMed] [Google Scholar]
- 245. Clark KD, Strand MR. Hemolymph melanization in the silkmoth Bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine. J Biol Chem. 2013;288:14476–87. 10.1074/jbc.M113.459222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Gupta S, Wang Y, Jiang H. Manduca sexta prophenoloxidase (proPO) activation requires proPO‐activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem Mol Biol. 2005;35:241–8. 10.1016/j.ibmb.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Kanost MR, Jiang H. Clip‐domain serine proteases as immune factors in insect hemolymph. Curr Opin Insect Sci. 2015;11:47–55. 10.1016/j.cois.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Jiang H, Ma C, Lu ZQ, Kanost MR. Beta‐1,3‐glucan recognition protein‐2 (betaGRP‐2) from Manduca sexta; an acute‐phase protein that binds beta‐1,3‐glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem Mol Biol. 2004;34:89–100. [DOI] [PubMed] [Google Scholar]
- 249. Nappi A, Poirié M, Carton Y. The role of melanization and cytotoxic by‐products in the cellular immune responses of Drosophila against parasitic wasps. Adv Parasitol. 2009;70:99–121. 10.1016/S0065-308X(09)70004-1. [DOI] [PubMed] [Google Scholar]
- 250. Halliwell B, Gutteridge JMC. Free radicals in biology and medicine, 3rd ed. Oxford: Oxford University Press; 1999. [Google Scholar]
- 251. Carton Y, Poirié M, Nappi AJ. Insect immune resistance to parasitoids. Insect Sci. 2008;15:67–87. 10.1111/j.1744-7917.2008.00188.x. [DOI] [Google Scholar]
- 252. Lamiable O, Kellenberger C, Kemp C, Troxler L, Pelte N, Boutros M, et al. Cytokine Diedel and a viral homologue suppress the IMD pathway in Drosophila . Proc Natl Acad Sci USA. 2016a;113:698–703. 10.1073/pnas.1516122113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lamiable O, Meignin C, Imler JL. WntD and Diedel: two immunomodulatory cytokines in Drosophila immunity. Fly. 2016b;10:187–94. 10.1080/19336934.2016.1202387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Gordon MD, Ayres JS, Schneider DS, Nusse R. Pathogenesis of Listeria‐Infected Drosophila wntD mutants is associated with elevated levels of the novel immunity gene edin . PLoS Pathog. 2008;4:e1000111. 10.1371/journal.ppat.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Vanha‐Aho LM, Anderl I, Vesala L, Hultmark D, Valanne S, Rämet M. Edin expression in the fat body is required in the defense against parasitic wasps in Drosophila melanogaster . PLoS Pathog. 2015;11:e1004895. 10.1371/journal.ppat.1004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Kauppila S, Maaty WS, Chen P, Tomar RS, Eby MT, Chapo J, et al. Eiger and its receptor, Wengen, comprise a TNF‐like system in Drosophila . Oncogene. 2003;22:4860–7. 10.1038/sj.onc.1206715. [DOI] [PubMed] [Google Scholar]
- 257. Schneider DS, Ayres JS, Brandt SM, Costa A, Dionne MS, Gordon MD, et al. Drosophila eiger mutants are sensitive to extracellular pathogens. PLoS Pathog. 2007;3:e41. 10.1371/journal.ppat.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Mabery EM, Schneider DS. The Drosophila TNF ortholog eiger is required in the fat body for a robust immune response. J Innate Immun. 2010;2:371–8. 10.1159/000315050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Fogarty CE, Diwanji N, Lindblad JL, Tare M, Amcheslavsky A, Makhijani K, et al. Extracellular reactive oxygen species drive apoptosis‐induced proliferation via Drosophila macrophages. Curr Biol. 2016;26:575–84. 10.1016/j.cub.2015.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Sung EJ, Ryuda M, Matsumoto H, Uryu O, Ochiai M, Cook ME, et al. Cytokine signaling through Drosophila Mthl10 ties lifespan to environmental stress. Proc Natl Acad Sci USA. 2017;114:13786–91. 10.1073/pnas.1712453115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Zhou Y, Wu S, Wang H, Hayakawa Y, Bird GS, Shears SB. Activation of PLC by an endogenous cytokine (GBP) in Drosophila S3 cells and its application as a model for studying inositol phosphate signalling through ITPK1. Biochem J. 2012;448:273–83. 10.1042/BJ20120730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Kelsey EM, Luo X, Brückner K, Jasper H. Schnurri regulates hemocyte function to promote tissue recovery after DNA damage. J Cell Sci. 2012;125:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Parsons B, Foley E. The Drosophila platelet‐derived growth factor and vascular endothelial growth factor‐receptor related (Pvr) protein ligands Pvf2 and Pvf3 control hemocyte viability and invasive migration. J Biol Chem. 2013;288:20173–83. 10.1074/jbc.m113.483818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Ferguson GB, Martinez‐Agosto JA. The TEAD family transcription factor Scalloped regulates blood progenitor maintenance and proliferation in Drosophila through PDGF/VEGFR receptor (Pvr) signaling. Dev Biol. 2017;425:21–32. 10.1016/j.ydbio.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 265. Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125:1909–20. [DOI] [PubMed] [Google Scholar]
- 266. Shia AK, Glittenberg M, Thompson G, Weber AN, Reichhart JM, Ligoxygakis P. Toll‐dependent antimicrobial responses in Drosophila larval fat body require Spätzle secreted by haemocytes. J Cell Sci. 2009;122:4505–15. 10.1242/jcs.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Pastor‐Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila . Dis Model Mech. 2008;1:144–54. 10.1242/dmm.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Chakrabarti S, Dudzic JP, Li X, Collas EJ, Boquete J‐P, Lemaitre B. Remote control of intestinal stem cell activity by haemocytes in Drosophila . PLoS Genet. 2016;12:e1006089. 10.1371/journal.pgen.1006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Gordon MD, Dionne MS, Schneider DS, Nusse R. WntD is a feedback inhibitor of Dorsal/NF‐kappaB in Drosophila development and immunity. Nature. 2005;437:746–9. 10.1038/nature04073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–43. 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- 271. Franc NC, Heitzler P, Ezekowitz RA, White K. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila . Science. 1999;284:1991–4. 10.1126/science.284.5422.1991. [DOI] [PubMed] [Google Scholar]
- 272. Guillou A, Troha K, Wang H, Franc NC, Buchon N. The Drosophila CD36 homologue Croquemort is required to maintain immune and gut homeostasis during development and aging. PLoS Pathog. 2016;12:e1005961. 10.1371/journal.ppat.1005961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, et al. Response to Staphylococcus aureus requires CD36‐mediated phagocytosis triggered by the COOH‐terminal cytoplasmic domain. J Cell Biol. 2005;170:477–85. 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Okuda K, Tong M, Dempsey B, Moore KJ, Gazzinelli RT, Silverman N. Leishmania amazonensis engages CD36 to drive parasitophorous vacuole maturation. PLoS Pathog. 2016;12:e1005669. 10.1371/journal.ppat.1005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Rämet M, Pearson A, Manfruelli P, Li X, Koziel H, Göbel V, et al. Drosophila scavenger receptor CI is a pattern recognition receptor for bacteria. Immunity. 2001;15:1027–38. 10.1016/s1074-7613(01)00249-7. [DOI] [PubMed] [Google Scholar]
- 276. Irving P, Ubeda JM, Doucet D, Troxler L, Lagueux M, Zachary D, et al. New insights into Drosophila larval haemocyte functions through genome‐wide analysis. Cell Microbiol. 2005;7:335–50. 10.1111/j.1462-5822.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 277. Pearson A, Lux A, Krieger M. Expression cloning of dSR‐CI, a class C macrophage‐specific scavenger receptor from Drosophila melanogaster . Proc Natl Acad Sci USA. 1995;92:4056–60. 10.1073/pnas.92.9.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Agaisse H, Burrack LS, Philips JA, Rubin EJ, Perrimon N, Higgins DE. Genome‐wide RNAi screen for host factors required for intracellular bacterial infection. Science. 2005;309:1248–51. 10.1126/science.1116008. [DOI] [PubMed] [Google Scholar]
- 279. Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–3. 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 280. Manaka J, Kuraishi T, Shiratsuchi A, Nakai Y, Higashida H, Henson P, et al. Draper‐mediated and phosphatidylserine‐independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages. J Biol Chem. 2004;279:48466–76. 10.1074/jbc.m408597200. [DOI] [PubMed] [Google Scholar]
- 281. Cuttell L, Vaughan A, Silva E, Escaron CJ, Lavine M, Van Goethem E, et al. Undertaker, a Drosophila Junctophilin, links Draper‐mediated phagocytosis and calcium homeostasis. Cell. 2008;135:524–34. 10.1016/j.cell.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 282. Shiratsuchi A, Mori T, Sakurai K, Nagaosa K, Sekimizu K, Lee BL, et al. Independent recognition of Staphylococcus aureus by two receptors for phagocytosis in Drosophila . J Biol Chem. 2012;287:21663–72. 10.1074/jbc.m111.333807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Hashimoto Y, Tabuchi Y, Sakurai K, Kutsuna M, Kurokawa K, Awasaki T, et al. Identification of lipoteichoic acid as a ligand for draper in the phagocytosis of Staphylococcus aureus by Drosophila hemocytes. J Immunol. 2009;183:7451–60. 10.4049/jimmunol.0901032. [DOI] [PubMed] [Google Scholar]
- 284. Kocks C, Cho JH, Nehme N, Ulvila J, Pearson AM, Meister M, et al. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila . Cell. 2005;123:335–46. 10.1016/j.cell.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 285. Chung YS, Kocks C. Recognition of pathogenic microbes by the Drosophila phagocytic pattern recognition receptor Eater. J Biol Chem. 2011;286:26524–32. 10.1074/jbc.m110.214007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Chamilos G, Lewis RE, Hu J, Xiao L, Zal T, Gilliet M, et al. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci USA. 2008;105:9367–72. 10.1073/pnas.0709578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zsámboki J, Csordás G, Honti V, Pintér L, Bajusz I, Galgóczy L, et al. Drosophila Nimrod proteins bind bacteria. Cent Eur J Biol. 2013;8:633–45. 10.2478/s11535-013-0183-4. [DOI] [Google Scholar]
- 288. Kurant E, Axelrod S, Leaman D, Gaul U. Six‐microns‐under acts upstream of Draper in the glial phagocytosis of apoptotic neurons. Cell. 2008;133:498–509. 10.1016/j.cell.2008.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Stroschein‐Stevenson SL, Foley E, O'Farrell PH, Johnson AD. Identification of Drosophila gene products required for phagocytosis of Candida albicans . PLoS Biol. 2006;4:e4. 10.1371/journal.pbio.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Shokal U, Kopydlowski H, Eleftherianos I. The distinct function of Tep2 and Tep6 in the immune defense of Drosophila melanogaster against the pathogen Photorhabdus . Virulence. 2017a;8:1668–82. 10.1080/21505594.2017.1330240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Shokal U, Eleftherianos I. Thioester‐containing protein‐4 regulates the Drosophila immune signaling and function against the pathogen Photorhabdus . J Innate Immun. 2017b;9:83–93. 10.1159/000450610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Haller S, Franchet A, Hakkim A, Chen J, Drenkard E, Yu S, et al. Quorum‐sensing regulator RhlR but not its autoinducer RhlI enables Pseudomonas to evade opsonization. EMBO Rep. 2018;19:e44880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Dostálová A, Rommelaere S, Poidevin M, Lemaitre B. Thioester‐containing proteins regulate the Toll pathway and play a role in Drosophila defence against microbial pathogens and parasitoid wasps. BMC Biol. 2017;15:79. 10.1186/s12915-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Watson FL, Püttmann‐Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, et al. Extensive diversity of Ig‐superfamily proteins in the immune system of insects. Science. 2005;309:1874–8. 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- 295. Vlisidou I, Dowling AJ, Evans IR, Waterfield N, ffrench‐Constant RH, Wood W. Drosophila embryos as model systems for monitoring bacterial infection in real time. PLoS Pathog. 2009;5:e1000518. 10.1371/journal.ppat.1000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Nagaosa K, Okada R, Nonaka S, Takeuchi K, Fujita Y, Miyasaka T, et al. Integrin βν‐mediated phagocytosis of apoptotic cells in Drosophila embryos. J Biol Chem. 2011;286:25770–7. 10.1074/jbc.m110.204503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Rämet M, Manfruelli P, Pearson A, Mathey‐Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli . Nature. 2002;416:644–8. 10.1038/nature735. [DOI] [PubMed] [Google Scholar]
- 298. Garver LS, Wu J, Wu LP. The peptidoglycan recognition protein PGRP‐SC1a is essential for Toll signaling and phagocytosis of Staphylococcus aureus in Drosophila . Proc Natl Acad Sci USA. 2006;103:660–5. 10.1073/pnas.0506182103. [DOI] [PMC free article] [PubMed] [Google Scholar]


