Abstract
Autonomously spiking dopaminergic neurons of the substantia nigra pars compacta (SNpc) are exquisitely specialized and suffer toxic iron-loading in Parkinson's disease (PD). However, the molecular mechanism involved remains unclear and critical to decipher for designing new PD therapeutics. The long-lasting (L-type) CaV1.3 voltage-gated calcium channel is expressed at high levels amongst nigral neurons of the SNpc, and due to its role in calcium and iron influx, could play a role in the pathogenesis of PD. Neuronal iron uptake via this route could be unregulated under the pathological setting of PD and potentiate cellular stress due to its redox activity. This Commentary will focus on the role of the CaV1.3 channels in calcium and iron uptake in the context of pharmacological targeting. Prospectively, the audacious use of artificial intelligence to design innovative CaV1.3 channel inhibitors could lead to breakthrough pharmaceuticals that attenuate calcium and iron entry to ameliorate PD pathology.
Keywords: Iron, Iron-loading, Iron transport, Iron redox cycling, Iron dyshomeostasis, Parkinson's disease
1. Parkinson's disease pathophysiology and the failure of current therapeutics
Regression of voluntary motor output is coupled with cognitive decline in Parkinson's disease (PD), imprisoning patients within a body they can no longer control. The current state of pharmacotherapies for major neurodegenerative diseases, namely Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis, and PD, remain at the level of masking symptoms rather than treating the disease process. Considering PD, levodopa (l-3,4-dihydroxyphenylalanine; l-DOPA) has been the gold-standard therapy since the 1960's, acting as a dopamine precursor to replace the depleted endogenous dopamine at post-synaptic dopamine receptors [1]. Unless PD patients are concurrently treated with the DOPA decarboxylase inhibitor, carbidopa, 99% of l-DOPA will be metabolized peripherally to its active metabolite, dopamine [2]. As this metabolism occurs before reaching the blood–brain barrier, it leads to low efficacy of l-DOPA and results in accentuated resting tremor [2]. Commonly, patients respond positively to the initial doses, but become refractory in >75% of patients within five years of the first treatment. Similarly, an alternative class of agents, namely the dopamine receptor agonists, are subject to the same fate and yield significantly more adverse effects in patients [3].
Currently, a variety of pharmacotherapies are under investigation and include calcium channel inhibitors (e.g., isradipine [4]), protein phosphatase modulators [5], and iron chelation therapy (e.g., deferiprone [6]). However, none of these pharmacological interventions have yet to become part of established clinical protocols.
2. Substantia nigra dopaminergic neurons could be susceptible to iron-loading and cytotoxic redox stress
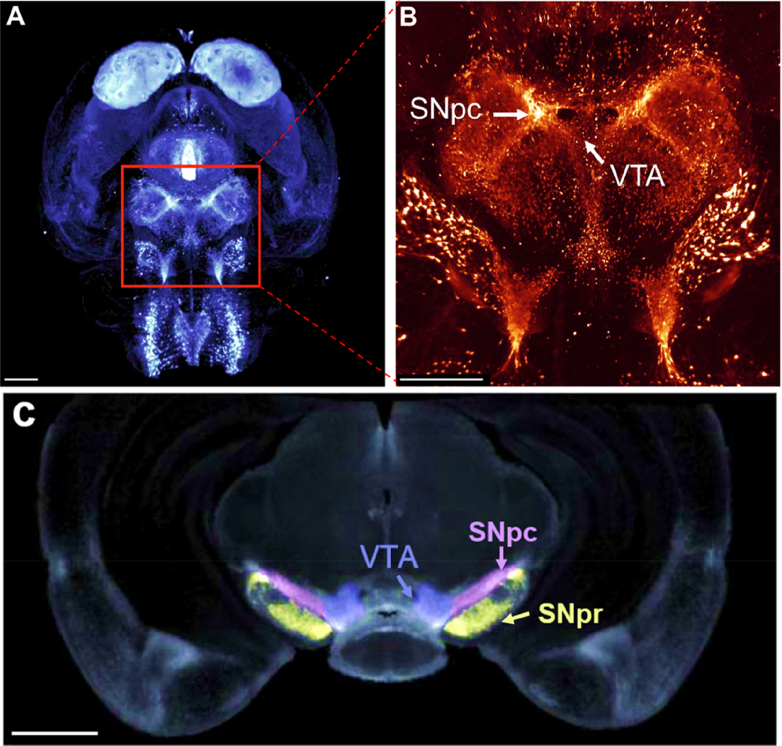
Considering the anatomical area of the midbrain affected in PD, the substantia nigra pars compacta (SNpc) is the most densely populated area of the brain in terms of dopaminergic neurons (nigral neurons) [7]. In fact, the SNpc consists of 250,000–400,000 dopaminergic nuclei, as demonstrated by tyrosine hydroxylase staining that identifies dopaminergic neurons (Fig. 1A, B) [7]. The SNpc relays synaptic inputs toward a multitude of structures, including the putamen, the GABAergic substantia nigra pars reticulata (SNpr), and external globus pallidus, to exert regulation over motor outputs and behavioral learning (Fig. 1C). However, the pathology of PD is hallmarked by a severe and specific loss of dopaminergic neurons in the SNpc. Dark pigmentation characterizes the SNpc, correlating with the expression of neuromelanin in dopaminergic neurons [8]. Neuromelanin is a neuroprotective pigment derived from dopamine oxidation [9]. This pigment binds iron and is thought to play a role in preventing iron's cytotoxicity mediated by redox cycling between the iron(II) and iron(III) states [10,11]. However, the iron storage protein, ferritin, probably also plays a key role in preventing iron-induced redox stress in neurons [12]. The SNpc demonstrates significant iron-loading relative to the adjacent SNpr [12], and this could be related to differences in calcium channel expression and function, which is discussed further below.
Fig. 1.
(A, B) Expression map of murine tyrosine hydroxylase (TH) in the rodent midbrain, illustrating the dense dopaminergic population of the substantia nigra pars compacta (SNpc). Image A and B are light-sheet fluorescence images using an antibody against TH, with (B) being at 5x magnification. The scale bar in (A), (B), and (C) represents 1 mm. (C) A cross-sectional image of the midbrain demonstrating the anatomical relationship between the SNpc, substantia nigra pars reticulata (SNpr) and ventral tegmental area (VTA). Images are from [7] with permission.
The role of iron accumulation in PD pathogenesis has been a “hot” topic for some time, being demonstrated as a prospective disease biomarker through functional magnetic resonance imaging scans [13]. Iron overload is detrimental since Fenton and Haber-Weiss redox chemistry can spawn cytotoxic reactive oxygen species, such as the hydroxyl radical [14]. Neurons of the SNpc exhibit a unique form of autonomous pacemaking/spiking to maintain critical striatal dopamine levels, meaning that these neurons spontaneously fire without synaptic input [15]. Pacemaking is made robust via multiple channels working together, including high-voltage activated, long-lasting (L-type) calcium channels and hyper-polarization-activated cyclic nucleotide-gated (HCN channels), where inhibition of either has no effect, while inhibiting both suppresses pacemaking [16]. The “high-voltage activated” term means these channels are activated after strong depolarizing events, while “long-lasting” refers to the length of activation that enables substantial cation influx [17].
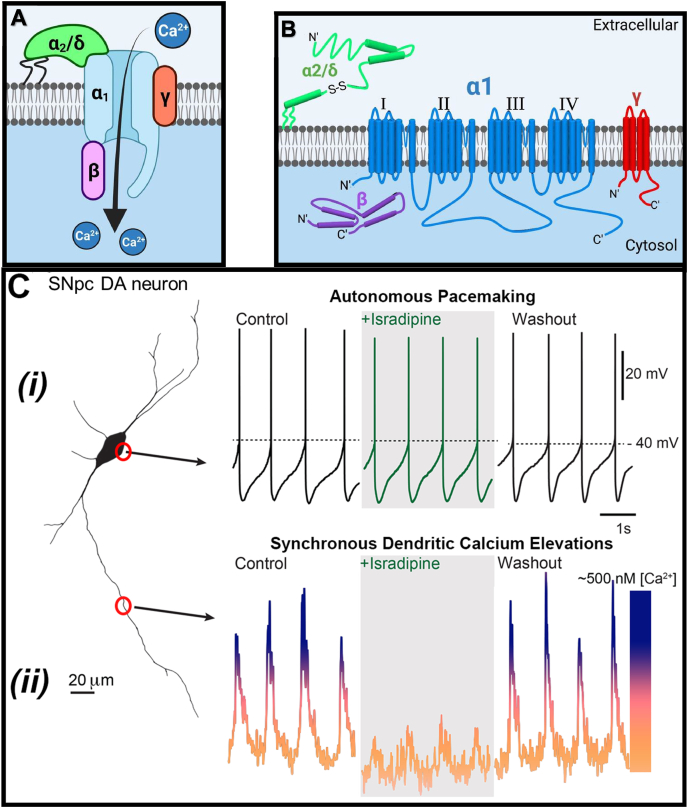
The structure of these channels consists of a primary pore-forming α1 subunit coupled with three auxiliary subunits, namely α2/δ, β, and γ (Fig. 2A) [18]. The pore-forming α1 subunit consists of four homologous domains (I-IV), each containing six transmembrane α-helices (S1–S6) [19]. In contrast, the α2/δ subunit is extracellular [20] and tethered to the plasma membrane via a glycosylphosphatidylinositol anchor [21] (Fig. 2B). Interestingly, the β subunit is the only cytoplasmic subunit, and modulates channel gating and trafficking from the endoplasmic reticulum via direct association with the α1 subunit between domains I and II [22] (Fig. 2B). The γ subunit is a transmembrane domain that modulates the activation and inactivation properties of the channel [23].
Fig. 2.
(A) The general structure of voltage-gated calcium channels, such as CaV1.3, which includes the pore-forming α1-subunit and auxiliary β, γ, and α2/δ subunits. (B) The α1-subunit is the primary component that facilitates calcium influx and is composed of four transmembrane domains (I, II, III, & IV). Commonly, these channels possess a long cytoplasmic C-terminal tail that is involved in regulating its activity (long isoform). The alternative spliced CaV1.3-short isoform lacks this region. (C) (i) Autonomous spiking is observed through whole-cell, patch-clamping recordings at the cell body (soma), where each spike indicates a neuronal action potential [4]. (ii) At distal dendritic sites, calcium concentrations spike synchronously with somatic firing. This elevated intracellular calcium concentration, but not the autonomous spiking, is blocked by the calcium channel inhibitor, isradipine, and is then restored after the washout of this agent. Fig. 2(C) sourced from [4] with permission.
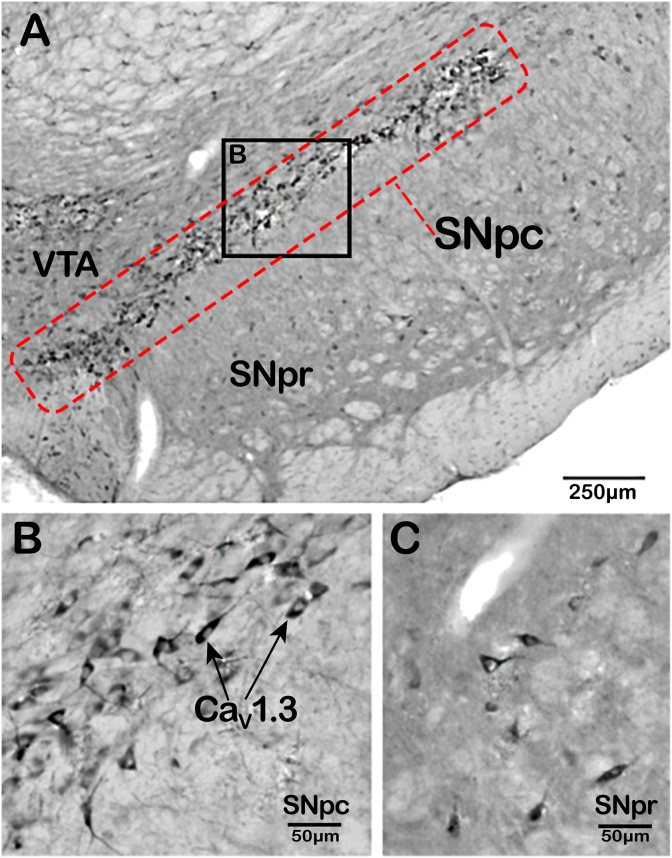
Upon electrophysiological recording, the SNpc neurons exhibit robust, slow, broad spikes (i.e., action potentials; Fig. 2Ci) that indicates: (1) consistent, low-frequency firing; and (2) that they remain open for long periods to enable considerable calcium influx (Fig. 2Cii) [4]. Furthermore, neurons within the ventral compartment of the SNpc demonstrate low expression of the calcium-binding protein, calbindin-D-28k, which safely sequesters calcium [24]. Unfortunately, these low levels of calbindin-D-28k increase susceptibility of nigral neurons to calcium-induced stress. In contrast, dopaminergic neurons of the proximally located ventral tegmental area (VTA; Fig. 1B, C), which are far less susceptible to PD neurodegeneration, also demonstrate a similar slow pacemaking phenotype of nigral neurons [25]. However, these neurons in the VTA demonstrate lower CaV1.3 channel density (Fig. 3A), less calcium uptake, and maintain calbindin-D-28k expression, all of which appear to result in a neuroprotective phenotype [24]. Indeed, comparing the expression profile of CaV1.3 in the midbrain, the SNpc demonstrates markedly greater levels relative to either the VTA or SNpr (Fig. 3A–C) [25].
Fig. 3.
(A) Immunohistochemistry staining for CaV1.3 (α1D) amongst midbrain structures. CaV1.3 positive staining is abundant across the substantia nigra pars compacta (SNpc; (B)). In contrast, the expression of this channel is sparse amongst other surrounding cortical structures, namely the substantia nigra pars reticulata (SNpr); (C)) and the ventral tegmental area (VTA). (A) Scale bar represents 250 μm. (B, C) Represents a 5x magnification of the SNpc and SNpr shown in (A). The scale bar in (B, C) represents 50 μm. Sourced from [25].
The autonomous pacemaking process is extremely energy-depleting, in part because of sizeable calcium influx and subsequently, efflux to pump out excess calcium [26]. The metabolic depletion of ATP in nigral neurons and coupling of high calcium levels is a critical event in accentuating mitochondrial dysfunction and cellular stress indicative of PD pathogenesis. In fact, dopaminergic neurons in the SNpc are unique, especially in terms of the tremendous length of their axons (⁓470,000 μm) and massive axonal field [27], with each axon supporting ⁓370,000 synapses [28]. This characteristic potentiates cellular stress due to the marked axonal trafficking and mitochondrial activity necessary to maintain this massive axonal field [15,29]. Considering this, age is the primary risk factor in the pathogenesis of PD, and as dopaminergic neurons are non-mitotic entities, the effects of stress are cumulative and more apparent in the older population [30]. The progressive neuronal degeneration is an early event that precedes clinical symptoms, yet dysfunction continues and spreads across the cortex until death of the PD patient [30].
The calcium influx observed during autonomous spiking (Fig. 2Cii) does not appear to be essential for the pacemaking process (Fig. 2Ci) [15]. This conclusion has been derived from studies with L-type calcium channel inhibitors, such as the dihydropyridine, isradipine, that spares autonomous pacemaking, but diminishes “calcium transients” (Fig. 2Cii) [15]. This latter term refers to the oscillation in cytoplasmic calcium observed due to calcium influx via these channels, followed by calcium being pumped out of the neuron. The efflux of calcium is achieved by the calcium ATPase pump and the sodium/calcium exchanger, which expend large amounts of energy to function [31]. Of interest, the ability of isradipine to maintain autonomous pacemaking while depressing cytosolic calcium oscillation has been mimicked by selectively silencing CaV1.3 channel subunit expression [15].
The results in Fig. 2C suggested that dihydropyridines could be potential therapeutics for PD [32]. However, calcium uptake resulting from CaV1.3 activity is used for multiple critical physiological processes, such as promotion of mitochondrial respiration and activation of synaptic proteins involved in dopamine release [33]. Considering the essential functions of calcium influx, it could be suggested that CaV1.3 inhibitors that attenuate this function could lead to detrimental side effects. However, treatment of mice with isradipine had the beneficial effects of suppressing mitochondrial oxidative stress, decreasing mitophagy, and normalizing mitochondrial mass [4]. These results suggest that the CaV1 channels drive mitochondrial oxidant stress and turnover in vivo and that isradipine decreases the vulnerability of SNpc neurons to mitochondrial challenges and cytopathic stress [15].
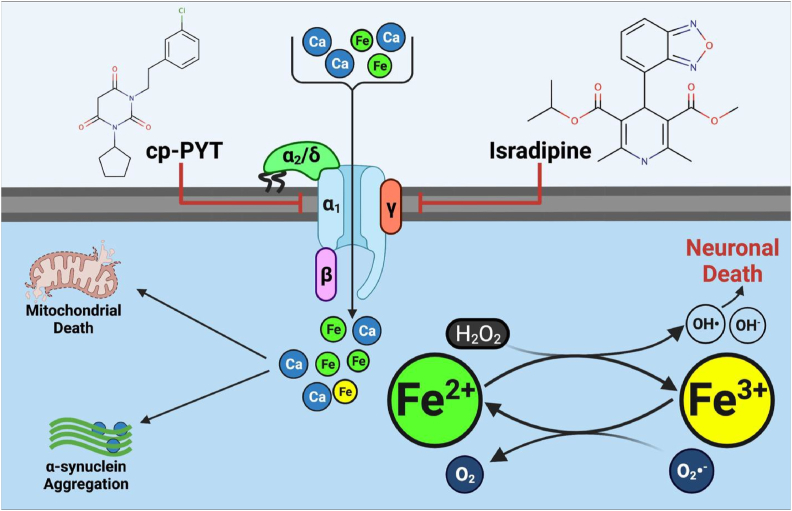
The extended opening of L-type calcium channels (e.g., the CaV1.3 channel) induced by pacemaking leads to a sizeable calcium influx. However, channel opening also appears to facilitate low molecular weight iron uptake into SNpc DA neurons after incubation with various forms of non-transferrin-bound, low molecular weight (Mr) iron [[34], [35], [36]] (Fig. 4). Due to the inherent redox activity of iron, its unregulated uptake by the CaV1.3 channel activity could facilitate cytopathic events by accentuating stress in already overstressed neurons. The transport of iron by these calcium channels is possible in the brain due to a significant fraction of extracellular low Mr iron that is not transferrin-bound [37]. The high concentration of ascorbate in the mammalian brain, which is 4–8 times (200–400 μM) that in the blood (50 μM), suggests non-transferrin-bound iron will be in the iron(II) state [38].
Fig. 4.
Voltage-gated calcium channels such as CaV1.3 facilitate iron-loading in nigral neurons to exacerbate cytopathic iron redox-cycling. Neurons burdened with high intracellular iron and calcium concentrations exhibit significant mitochondrial dysfunction and α-synuclein aggregation, propagating their demise. Developing novel inhibitors that supersede the activity of current agents, such as isradipine and cp-PYT, is now essential.
This iron(II) has the potential to be transported by the CaV1.3 channel, as its atomic radius (ionic radius: 0.92 Å) is significantly less than that of calcium(II) (ionic radius: 1.14 Å) [39]. Of interest, non-transferrin-bound iron(II) has been demonstrated to permeate the CaV3.1 calcium channel, which is a homolog of the CaV1.3 channel [40]. Currently, the precise molecular mechanism of how the CaV1.3 channel mediates iron influx remains unclear, as the X-ray crystal structure of this protein has yet to be elucidated. It is also unknown what precise molecular form of Fe(II) can be transported in terms of its hydration shell and coordination state with endogenous ligands. Furthermore, a lack of in-depth electrophysiological studies specifically examining CaV1.3 channels and iron uptake have impaired interpretation, with a major aim of this article being to stimulate further research.
Not only iron, but other metal ions, including redox-active copper as copper (II) (ionic radius: 0.87 Å) [39], may potentially be transported by calcium channels, which could aggravate redox stress that is known to exist in nigral neurons [4]. Furthermore, it is established that copper ions ligate with α-synuclein fibrils and remain redox-active [41]. This factor is significant, as α-synuclein aggregation is a major triad hallmark of PD, along with mitochondrial dysfunction and iron accumulation [12,42].
3. Voltage-gated calcium channels are culprits in neuronal iron-loading
Contrary to their long-lasting opening to mediate marked calcium uptake, L-type channels do inactivate after calcium ion influx, a process known as calcium-dependent inactivation. This regulatory mechanism is thought to be mediated by the binding of calcium to calmodulin, which is bound to L-type calcium channels [43]. This latter homeostatic process is not mimicked by other divalent ions such as barium [44]. As such, since the cerebrospinal fluid contains significant ferrous iron, particularly in PD patients [45], non-specific iron influx could potentially aberrate the inactivation kinetics of these L-type channels. It is well known that iron enters nigral neurons through L-type channels, with channel inhibitors (e.g., dihydropyridines) being capable of restoring cellular viability [46,47], potentially by blocking iron uptake, and thus, oxidative stress [47]. Hence, calcium channel inhibitors potentially provide a unique pharmacological strategy of preventing calcium uptake, but also redox-active, iron-loading, which is thought to be detrimental in PD [12,35]. The paramount issue is to develop novel CaV1.3 inhibitors that demonstrate high-affinity and selectivity towards this channel relative to other related channels (e.g., CaV1.2), which are vital for other critical processes such as heart function.
As described above, L-type Cav channels associate with calmodulin, which inhibits their activity upon rising intracellular calcium concentrations [48]. It is also of interest that increasing cellular calcium results in the binding of calmodulin to neuronal nitric oxide synthase, leading to increased nitric oxide (NO) production [49]. There is evidence that glutaminergic, serotonergic, and dopaminergic signaling occur more frequently in response to elevated NO [[50], [51], [52]]. As NO is known to directly bind to iron [53,54] and considering the increased iron-loading observed in SNpc dopaminergic neurons [11,12,55], the complex interplay between all these molecules is likely to be disturbed under PD, which could further aggravate the pathology.
4. The potential benefits of effective and selective CaV1.3 channel inhibitors – the failure of dihydropyridines
Dihydropyridines that antagonize CaV1.3 calcium channels (Fig. 4) can rescue cell culture and murine PD models to attenuate oxidative stress [4,56]. The National Institutes of Health has registered three clinical trials examining the clinical effectiveness of isradipine against early-onset PD (ClinicalTrials.gov Identifier: NCT00753636, NCT00909545, NCT02168842). Results from these investigations have been eagerly anticipated for over a decade, with the STEADY-PD III trial published in 2020 reporting that isradipine demonstrated no significant benefit against PD progression [57]. The low specificity of dihydropyridines towards Cav1.3 channels, their inability to precisely target the SNpc, and an insufficient isradipine dose, probably contributed to their clinical failure [57,58]. This lack of response was not due to the inability of isradipine to permeate the blood-brain barrier, as it is well known to cross this barrier and have anticonvulsant activity [59]. Moreover, during the clinical trials of early PD patients, isradipine was administered intravenously for maximal blood-brain barrier permeation and resulted in neurological alterations demonstrating its effective entry into the brain [57]. The development of more effective CaV1.3 channel inhibitors remains an area of active research.
Silverman's team [32] has attempted to develop novel, specific inhibitors against CaV1.3 and has synthesized the pyrimidine-2,4,6-trione class of agents (cp-PYT; Fig. 4) that target the same binding pocket on the calcium channel as the dihydropyridines [60]. The lead compound (1-(3-chlorophenethyl)-3-cyclopentylpyrimidine-2,4,6-(1H,3H, 5H)-trione) demonstrated a ∼612-fold greater specificity against CaV1.3 than CaV1.2 channels. However, patch clamping of CaV1.3 transfected HEK293T cells illustrated that cp-PYT demonstrates a relatively low affinity for CaV1.3 (IC50: 24.3 ± 0.7 μM), which is markedly lower than isradipine (IC50: 5.8–19.9 nM).
The pertinent issue with isradipine is the lack of selectivity and high-affinity for CaV1.2 (IC50: 2.2–3.9 nM) [61]. To be clinically relevant, a significantly lower IC50 value may be necessary to preclude the failure observed with isradipine in clinical trials. Breakthroughs in the pharmacological inhibition of CaV1.3 are now critical to investigate and could be facilitated through the extensive processing offered by artificial intelligence (AI). The pinnacle discovery would be an innovative, high-affinity CaV1.3 channel inhibitor that effectively and selectively inhibits cellular stress induced by calcium and iron to ameliorate PD pathology.
5. Innovative pharmacotherapies for treating iron-loading: The new world order of implementing AI in drug design and development
AI has progressively been implemented into the laborious process of drug design and discovery over the last decade, recently being used to identify novel therapies for the human immune-deficiency virus [62]. The advantage of AI approaches such as deep learning neural networks, is that they comprehend very complex patterns amongst data for almost an unlimited number of variables. Such AI networks include the use of supervised learning models, where algorithms have been specifically designed to predict unknown pharmacophores based upon large quantities of input data that constitute the “training sets”. These training data sets can be pharmacological properties, such as 2- and 3- dimensional structures of candidate compounds, IC50 values, hydrogen-bond acceptor/donor coefficients, Log P values, and many others [63]. When these parameters are combined and analysed by AI, this can result in valuable predictions that aid drug design and discovery.
Incorporating specific deep learning strategies into the complex field of neuropharmacology may unlock the therapeutic potential for otherwise difficult to treat diseases such as PD. With no pharmaco-intervention superseding l-DOPA in its sixty-year reign as the gold standard therapeutic for PD, drug design strategies for this condition now require a novel strategy to break new ground. AI has already aided the clinical diagnosis of PD and assisted in monitoring disease progression. In fact, AI can diagnose early PD with greater than 95% accuracy [64]. However, unfortunately, the promise of AI has yet to be fully implemented in the design of drugs for PD treatment.
The Renslow group is amongst the few to have already incorporated AI into neurodegenerative drug discovery [65]. These investigators described twelve unique compounds that possibly antagonize the phencyclidine (PCP)-binding domain of the N-methyl-d-aspartate (NMDA) receptor. The agent, PCP, is a drug of abuse that causes psychosis resembling the positive and negative signs of schizophrenia [66,67]. The goal of the investigation by Renslow et al. was to develop a PCP-site antagonizing agent that does not induce dissociative effects, therefore bypassing illicit misuse. The agents generated were unique and chemically distinct from the training dataset.
Considering the design of new CaV1.3 inhibitors for PD, the argument detailed above indicates that dihydropyridines are suboptimal, with all analogues of this class targeting the same allosteric binding pocket [32,60]. Despite numerous modification attempts, no current dihydropyridine demonstrates high selectivity for the CaV1.3 channel without inhibiting CaV1.2. Similarly, as described above, although the more recently designed pyrimidine-2,4,6-triones have significantly improved selectivity over the dihydropyridines, they show a limited affinity for the same dihydropyridine target site on the CaV1.3 channel [60]. Thus, new classes of CaV1.3 inhibitors with different targets on this molecule could be important for PD drug discovery.
Regarding the possible use of AI as part of this quest, negative training sets of data consisting of the structures and pharmacological properties of the dihydropyridines and the pyrimidine-2,4,6-triones could be used. This could provide initial examples to an AI processing platform of what is not structurally appropriate in a new drug for selectively antagonizing CaV1.3 channels. These examples would be combined with positive training sets of new agents. Such drug candidates could be identified from the screening of large drug libraries for agents demonstrating antagonistic effects against CaV1.3 channels. By using these data to train AI algorithms, predictions of new pharmacophores could be generated and subsequently assessed in vitro and in vivo their ability to antagonize the CaV1.3 channel. Such a unique approach could lead to the discovery of a novel pathology attenuating therapy for PD patients.
6. Perspectives and conclusions
This Commentary provides oversight regarding the ineffective attempts to identify neuroprotective therapeutics currently available to PD patients. Considering the serious shortcomings of L-type CaV channel inhibitors, innovative approaches are desperately required. The implementation of AI remains a growing accomplice to drug discovery research, analysing incomprehensibly large datasets that can provide unique compound outputs. Attenuating intraneuronal iron and calcium accumulation in PD patients should be a forefront priority for small molecule development, particularly as no therapy is yet available to slow PD progression and prevent neuronal death. The dream of a novel high-affinity and selective inhibitor against the CaV1.3 channels might become a reality by incorporating deep learning neural networks into drug design and discovery.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
D.R.R appreciates career support provided by a Senior Principal Research Fellowship [APP1159596] from the National Health and Medical Research Council of Australia. We thank Professor D.J. Surmeier (Department of Physiology, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA) for his excellent suggestions on the manuscript during revision.
References
- 1.Tolosa E., Marti M.J., Valldeoriola F., Molinuevo J.L. History of levodopa and dopamine agonists in Parkinson's disease treatment. Neurology. 1998;50(6):2–10. doi: 10.1212/wnl.50.6_suppl_6.s2. 44-48. [DOI] [PubMed] [Google Scholar]
- 2.Fahn S., Jankovic J., Hallett M. Elsevier Saunders; Edinburgh, New York: 2011. Principles and Practice of Movement Disorders. [Google Scholar]
- 3.Antonini A., Tolosa E., Mizuno Y., Yamamoto M., Poewe W.H. A reassessment of risks and benefits of dopamine agonists in Parkinson's disease. Lancet Neurol. 2009;8(10):929–937. doi: 10.1016/S1474-4422(09)70225-X. [DOI] [PubMed] [Google Scholar]
- 4.Guzman J.N., Ilijic E., Yang B., Sanchez-Padilla J., Wokosin D., Galtieri D., Kondapalli J., Schumacker P.T., Surmeier D.J. Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J. Clin. Invest. 2018;128(6):2266–2280. doi: 10.1172/JCI95898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braithwaite S.P., Voronkov M., Stock J.B., Mouradian M.M. Targeting phosphatases as the next generation of disease modifying therapeutics for Parkinson's disease. Neurochem. Int. 2012;61(6):899–906. doi: 10.1016/j.neuint.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Bastida A., Ward R.J., Newbould R., Piccini P., Sharp D., Kabba C., Patel M.C., Spino M., Connelly J., Tricta F., Crichton R.R., Dexter D.T. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson's disease. Sci. Rep. 2017;7(1):1398. doi: 10.1038/s41598-017-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roostalu U., Salinas C.B.G., Thorbek D.D., Skytte J.L., Fabricius K., Barkholt P., John L.M., Jurtz V.I., Knudsen L.B., Jelsing J., Vrang N., Hansen H.H., Hecksher-Sorensen J. Quantitative whole-brain 3D imaging of tyrosine hydroxylase-labeled neuron architecture in the mouse MPTP model of Parkinson's disease. Dis. Model Mech. 2019;12(11) doi: 10.1242/dmm.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shima T., Sarna T., Swartz H.M., Stroppolo A., Gerbasi R., Zecca L. Binding of iron to neuromelanin of human substantia nigra and synthetic melanin: an electron paramagnetic resonance spectroscopy study. Free Radic. Biol. Med. 1997;23(1):110–119. doi: 10.1016/s0891-5849(96)00623-5. [DOI] [PubMed] [Google Scholar]
- 9.Vila M., Laguna A., Carballo-Carbajal I. Intracellular crowding by age-dependent neuromelanin accumulation disrupts neuronal proteostasis and triggers Parkinson disease pathology. Autophagy. 2019;15(11):2028–2030. doi: 10.1080/15548627.2019.1659621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zecca L., Shima T., Stroppolo A., Goj C., Battiston G.A., Gerbasi R., Sarna T., Swartz H.M. Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience. 1996;73(2):407–415. doi: 10.1016/0306-4522(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 11.Ma L., Gholam Azad M., Dharmasivam M., Richardson V., Quinn R.J., Feng Y., Pountney D.L., Tonissen K.F., Mellick G.D., Yanatori I., Richardson D.R. Parkinson's disease: alterations in iron and redox biology as a key to unlock therapeutic strategies. Redox Biol. 2021;41:101896. doi: 10.1016/j.redox.2021.101896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dexter D.T., Carayon A., Javoy-Agid F., Agid Y., Wells F.R., Daniel S.E., Lees A.J., Jenner P., Marsden C.D. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- 13.Sulzer D., Cassidy C., Horga G., Kang U.J., Fahn S., Casella L., Pezzoli G., Langley J., Hu X.P., Zucca F.A., Isaias I.U., Zecca L. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson's disease. NPJ Parkinsons Dis. 2018;4(1):11. doi: 10.1038/s41531-018-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abeyawardhane D.L., Lucas H.R. Iron redox chemistry and implications in the Parkinson's disease brain. Oxid. Med. Cell Longev. 2019:4609702. doi: 10.1155/2019/4609702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzman J.N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P.T., Surmeier D.J. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468(7324):696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzman J.N., Sanchez-Padilla J., Chan C.S., Surmeier D.J. Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci. 2009;29(35):11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipscombe D., Helton T.D., Xu W. L-type calcium channels: the low down. J. Neurophysiol. 2004;92(5):2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- 18.Felix R., Calderon-Rivera A., Andrade A. Regulation of high-voltage-activated Ca2+ channel function, trafficking, and membrane stability by auxiliary subunits. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2013;2(5):207–220. doi: 10.1002/wmts.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simms B.A., Zamponi G.W. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 2014;82(1):24–45. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Davies A., Hendrich J., Van Minh A.T., Wratten J., Douglas L., Dolphin A.C. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007;28(5):220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Buraei Z., Yang J. Structure and function of the beta subunit of voltage-gated Ca2+ channels. Biochim. Biophys. Acta. 2013;1828(7):1530–1540. doi: 10.1016/j.bbamem.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zurzolo C., Simons K. Glycosylphosphatidylinositol-anchored proteins: membrane organization and transport. Biochim. Biophys. Acta. 2016;1858(4):632–639. doi: 10.1016/j.bbamem.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Tuluc P., Theiner T., Jacobo-Piqueras N., Geisler S.M. Role of high voltage-gated Ca2+ channel subunits in pancreatic beta-cell insulin release. From Structure to Function. Cells. 2021;10(8) doi: 10.3390/cells10082004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.German D.C., Manaye K.F., Sonsalla P.K., Brooks B.A. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann. N. Y. Acad. Sci. 1992;648(1 Neurotoxins a):42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- 25.Takada M., Kang Y., Imanishi M. Immunohistochemical localization of voltage-gated calcium channels in substantia nigra dopamine neurons. Eur. J. Neurosci. 2001;13(4):757–762. doi: 10.1046/j.1460-9568.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 26.Zaichick S.V., McGrath K.M., Caraveo G. The role of Fe2+ signaling in Parkinson’s disease. Dis. Model Mech. 2017;10(5):519–535. doi: 10.1242/dmm.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda W., Furuta T., Nakamura K.C., Hioki H., Fujiyama F., Arai R., Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 2009;29(2):444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbuthnott G.W., Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30(2):62–69. doi: 10.1016/j.tins.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Surmeier D.J., Guzman J.N., Sanchez-Padilla J., Goldberg J.A. Elsevier; 2010. What Causes the Death of Dopaminergic Neurons in Parkinson's Disease? Recent Advances in Parkinson's Disease: Basic Research; pp. 59–77. [DOI] [PubMed] [Google Scholar]
- 30.Reeve A., Simcox E., Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14(100):19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagur R., Hajnoczky G. Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol. Cell. 2017;66(6):780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang S., Cooper G., Dunne S.F., Dusel B., Luan C.H., Surmeier D.J., Silverman R.B. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat. Commun. 2012;3(1):1146. doi: 10.1038/ncomms2149. [DOI] [PubMed] [Google Scholar]
- 33.Surmeier D.J., Schumacker P.T. Calcium, bioenergetics, and neuronal vulnerability in Parkinson's disease. J. Biol. Chem. 2013;288(15):10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y.Y., Wan W.P., Zhao S., Ma Z.G. L-type calcium channels are involved in iron-induced neurotoxicity in primary cultured ventral mesencephalon neurons of rats. Neurosci. Bull. 2020;36(2):165–173. doi: 10.1007/s12264-019-00424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bostanci M.O., Bagirici F. Blocking of L-type calcium channels protects hippocampal and nigral neurons against iron neurotoxicity. The role of L-type calcium channels in iron-induced neurotoxicity. Int. J. Neurosci. 2013;123(12):876–882. doi: 10.3109/00207454.2013.813510. [DOI] [PubMed] [Google Scholar]
- 36.Gaasch J.A., Geldenhuys W.J., Lockman P.R., Allen D.D., Van der Schyf C.J. Voltage-gated calcium channels provide an alternate route for iron uptake in neuronal cell cultures. Neurochem. Res. 2007;32(10):1686–1693. doi: 10.1007/s11064-007-9313-1. [DOI] [PubMed] [Google Scholar]
- 37.Moos T., Morgan E.H. Evidence for low molecular weight, non-transferrin-bound iron in rat brain and cerebrospinal fluid. J. Neurosci. Res. 1998;54(4):486–494. doi: 10.1002/(SICI)1097-4547(19981115)54:4<486::AID-JNR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 38.Covarrubias-Pinto A., Acuna A.I., Beltran F.A., Torres-Diaz L., Castro M.A. Old things new view: ascorbic acid protects the brain in neurodegenerative disorders. Int. J. Mol. Sci. 2015;16(12):28194–28217. doi: 10.3390/ijms161226095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A. 1976;32(5):751–767. [Google Scholar]
- 40.Lopin K.V., Gray I.P., Obejero-Paz C.A., Thevenod F., Jones S.W. Fe2+ block and permeation of CaV3.1 (alpha1G) T-type calcium channels: candidate mechanism for non-transferrin-mediated Fe2+ influx. Mol. Pharmacol. 2012;82(6):1194–1204. doi: 10.1124/mol.112.080184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okita Y., Rcom-H'cheo-Gauthier A.N., Goulding M., Chung R.S., Faller P., Metallothionein D.L. Pountney. Copper and alpha-synuclein in alpha-synucleinopathies. Front. Neurosci. 2017;11:114. doi: 10.3389/fnins.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cookson M.R., Parkinson's disease O. Bandmann. Insights from pathways. Hum. Mol. Genet. 2010;19(R1):R21–R27. doi: 10.1093/hmg/ddq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben Johny M., Yang P.S., Bazzazi H., Yue D.T. Dynamic switching of calmodulin interactions underlies Ca2+ regulation of CaV1.3 channels. Nat. Commun. 2013;4(1):1717. doi: 10.1038/ncomms2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haack J.A., Rosenberg R.L. Calcium-dependent inactivation of L-type calcium channels in planar lipid bilayers. Biophys. J. 1994;66(4):1051–1060. doi: 10.1016/S0006-3495(94)80886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shahandeh A., Bui B.V., Finkelstein D.I., Nguyen C.T.O. Therapeutic applications of chelating drugs in iron metabolic disorders of the brain and retina. J. Neurosci. Res. 2020;98(10):1889–1904. doi: 10.1002/jnr.24685. [DOI] [PubMed] [Google Scholar]
- 46.Knutson M.D. Non-transferrin-bound iron transporters. Free Radic. Biol. Med. 2019;133:101–111. doi: 10.1016/j.freeradbiomed.2018.10.413. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Zhao X., Chang Y., Zhang Y., Chu X., Zhang X., Liu Z., Guo H., Wang N., Gao Y., Zhang J., Chu L. Calcium channel blockers ameliorate iron overload-associated hepatic fibrosis by altering iron transport and stellate cell apoptosis. Toxicol. Appl. Pharmacol. 2016;301:50–60. doi: 10.1016/j.taap.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Sang L., Vieira D.C.O., Yue D.T., Ben-Johny M., Dick I.E. The molecular basis of the inhibition of CaV1 calcium dependent inactivation by the distal carboxy tail. J. Biol. Chem. 2021:100502. doi: 10.1016/j.jbc.2021.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su Z., Blazing M.A., Fan D., George S.E. The calmodulin-nitric oxide synthase interaction. Critical role of the calmodulin latch domain in enzyme activation. J. Biol. Chem. 1995;270(49):29117–29122. doi: 10.1074/jbc.270.49.29117. [DOI] [PubMed] [Google Scholar]
- 50.Picon-Pages P., Garcia-Buendia J., Munoz F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta. 2019;1865(8):1949–1967. doi: 10.1016/j.bbadis.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Hanbauer I., Wink D., Osawa Y., Edelman G.M., Gally J.A. Role of nitric oxide in NMDA-evoked release of [3H]-dopamine from striatal slices. Neuroreport. 1992;3(5):409–412. doi: 10.1097/00001756-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Lorrain D.S., Hull E.M. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport. 1993;5(1):87–89. doi: 10.1097/00001756-199310000-00024. [DOI] [PubMed] [Google Scholar]
- 53.Watts R.N., Ponka P., Richardson D.R. Effects of nitrogen monoxide and carbon monoxide on molecular and cellular iron metabolism: mirror-image effector molecules that target iron. Biochem. J. 2003;369(Pt 3):429–440. doi: 10.1042/BJ20021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lok H.C., Sahni S., Jansson P.J., Kovacevic Z., Hawkins C.L., Richardson D.R. A nitric oxide storage and transport system that protects activated macrophages from endogenous nitric oxide cytotoxicity. J. Biol. Chem. 2016;291(53):27042–27061. doi: 10.1074/jbc.M116.763714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C., Liang M.C., Soong T.W. Nitric oxide, iron and neurodegeneration. Front. Neurosci. 2019;13(114):114. doi: 10.3389/fnins.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortner N.J. Voltage-gated Ca2+ channels in dopaminergic substantia nigra neurons: therapeutic targets for neuroprotection in Parkinson’s disease? Front. Synaptic Neurosci. 2021;13:636103. doi: 10.3389/fnsyn.2021.636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parkinson's disease study group, STEADY-PD III investigators. Isradipine versus placebo in early Parkinson disease: a randomized trial. Ann. Intern. Med. 2020;172(9):591–598. doi: 10.7326/M19-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maiti B., Perlmutter J.S. A clinical trial of isradipine: what went wrong? Ann. Intern. Med. 2020;172(9):625–626. doi: 10.7326/M20-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozyazgan S., Senses V., Utkan T., Yildiran G., Ulak G., Gacar N., Ozüner Z., Akkan A.G. The effect of isradipine on maximal electroshock seizures in mice. Gen. Pharmacol. 1998;31(1):133–135. doi: 10.1016/s0306-3623(97)00390-x. [DOI] [PubMed] [Google Scholar]
- 60.Cooper G., Kang S., Perez-Rosello T., Guzman J.N., Galtieri D., Xie Z., Kondapalli J., Mordell J., Silverman R.B., Surmeier D.J. A single amino acid determines the selectivity and efficacy of selective negative allosteric modulators of CaV1.3 L-type calcium channels. ACS Chem. Biol. 2020;15(9):2539–2550. doi: 10.1021/acschembio.0c00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ortner N.J., Bock G., Dougalis A., Kharitonova M., Duda J., Hess S., Tuluc P., Pomberger T., Stefanova N., Pitterl F., Ciossek T., Oberacher H., Draheim H.J., Kloppenburg P., Liss B., Striessnig J. Lower affinity of isradipine for L-type Ca2+ channels during substantia nigra dopamine neuron-lke activity: implications for neuroprotection in Parkinson’s disease. J. Neurosci. 2017;37(28):6761–6777. doi: 10.1523/JNEUROSCI.2946-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zorn K.M., Lane T.R., Russo D.P., Clark A.M., Makarov V., Ekins S. Multiple machine learning comparisons of HIV cell-based and reverse transcriptase data sets. Mol. Pharm. 2019;16(4):1620–1632. doi: 10.1021/acs.molpharmaceut.8b01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vamathevan J., Clark D., Czodrowski P., Dunham I., Ferran E., Lee G., Li B., Madabhushi A., Shah P., Spitzer M., Zhao S. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019;18(6):463–477. doi: 10.1038/s41573-019-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belic M., Bobic V., Badza M., Solaja N., Duric-Jovicic M., Kostic V.S. Artificial intelligence for assisting diagnostics and assessment of Parkinson's disease-A review. Clin. Neurol. Neurosurg. 2019;184:105442. doi: 10.1016/j.clineuro.2019.105442. [DOI] [PubMed] [Google Scholar]
- 65.Schultz K.J., Colby S.M., Yesiltepe Y., Nunez J.R., McGrady M.Y., Renslow R.S. Application and assessment of deep learning for the generation of potential NMDA receptor antagonists. Phys. Chem. Chem. Phys. 2021;23(2):1197–1214. doi: 10.1039/d0cp03620j. [DOI] [PubMed] [Google Scholar]
- 66.Javitt D.C., Zukin S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatr. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 67.Moghadam A.A., Vose L.R., Miry O., Zhang X.L., Stanton P.K. Pairing of neonatal phencyclidine exposure and acute adolescent stress in male rats as a novel developmental model of schizophrenia. Behav. Brain Res. 2021;409:113308. doi: 10.1016/j.bbr.2021.113308. [DOI] [PubMed] [Google Scholar]