Abstract
Objective
Cognitive reserve (CR) is the capacity to adapt to (future) brain damage without any or only minimal clinical symptoms. The underlying neuroplastic mechanisms remain unclear. Electrocorticography (ECOG), electroencephalography (EEG), and magnetoencephalography (MEG) may help elucidate the brain mechanisms underlying CR, as CR is thought to be related to efficient utilization of remaining brain resources. The purpose of this systematic review is to collect, evaluate, and synthesize the findings on neural correlates of CR estimates using ECOG, EEG, and MEG.
Method
We examined articles that were published from the first standardized definition of CR. Eleven EEG and five MEG cross-sectional studies met the inclusion criteria: They concerned original research, analyzed (M)EEG in humans, used a validated CR estimate, and related (M)EEG to CR. Quality assessment was conducted using an adapted form of the Newcastle–Ottawa scale. No ECOG study met the inclusion criteria.
Results
A total of 1383 participants from heterogeneous patient, young and older healthy groups were divided into three categories by (M)EEG methodology: Eight (M)EEG studies employed event-related fields or potentials, six studies analyzed brain oscillations at rest (of which one also analyzed a cognitive task), and three studies analyzed brain connectivity. Various CR estimates were employed and all studies compared different (M)EEG measures and CR estimates. Several associations between (M)EEG measures and CR estimates were observed.
Conclusion
Our findings support that (M)EEG measures are related to CR estimates, particularly in healthy individuals. However, the character of this relationship is dependent on the population and task studied, warranting further studies.
Keywords: Cognitive reserve, Electroencephalography, Magnetoencephalography, Event-related potentials, Brain oscillations, P3, patients, healthy individuals
Introduction
According to a recent whitepaper that tried to reach a consensus on its definition, cognitive reserve (CR) refers to the adaptability of cognitive processes that may partially underlie the differences observed between individuals in the susceptibility of their cognitive abilities to (future) brain aging, pathology, or brain insult (Stern, Arenaza-Urquijo, et al., 2018). A higher level of CR is thought to be supported by more adaptable functional brain processes, that is, “the networks of brain regions associated with performing a task as well as the pattern of interactions between these networks” (Stern, Arenaza-Urquijo, et al., 2018). Differences in CR are accordingly determined by individual differences in these existing cognitive or functional brain processes. These processes can be influenced by both innate capacities as well as individual differences in experiences and exposures such as early-life general cognitive ability (e.g., intelligence), education, occupation, physical exercise, leisure activities, or social engagement (Stern, Arenaza-Urquijo, et al., 2018).
CR is therefore estimated in a variety of ways, using years of education (Jones et al., 2006; Roe et al., 2007; Farfel et al., 2013), premorbid-IQ (Starr & Lonie, 2008; Armstrong et al., 2012), leisure activities (Sumowski et al., 2010; Helzner et al., 2007), occupation (Ghaffar et al., 2012; Adam et al., 2013), or a questionnaire like the Cognitive Reserve Index questionnaire (CRIq, Nucci et al., 2012). The latter study also discusses the relation between the CRIq and two other estimates of CR (vocabulary tests of the Wechsler Adult Intelligence Scale and the Test di Intelligenza Breve).
The concept of CR originates from epidemiological studies (Katzman, 1993; Stern, 2002) and was introduced as a modulator that could explain the observed individual differences in the neuropathological state of patients with Alzheimer’s disease (AD) in relation to their degree of expressed symptoms. CR has since been investigated in other neurodegenerative conditions such as Parkinson’s disease (PD; e.g., Armstrong et al., 2012; Hindle et al., 2016) and in healthy cognitive aging (e.g., Jones et al., 2006; Singh-Manoux et al., 2011). More recent studies have found CR to be a predicting factor for recovery after traumatic brain injury (TBI; Schneider et al., 2014; Oldenburg et al., 2016; Stenberg et al., 2020) or stroke (Stenberg et al., 2020).
However, the neural basis underlying CR is not yet fully understood (Steffener & Stern, 2012). High CR is hypothesized to reflect active usage of remaining brain resources through efficient neural networks, thereby providing compensating neural mechanisms to cope with cognitive demands (Stern, 2009; Barulli & Stern, 2013). Earlier efforts to explore the neural basis of CR were primarily based on functional magnetic resonance imaging (fMRI; Habeck et al., 2003) in healthy elderly and (amnestic) mild cognitive impairment ([a]MCI) or AD patients (Colangeli et al., 2016; Anthony & Lin, 2018).
In a meta-analysis about neural correlates of CR in fMRI studies (Colangeli et al., 2016), which included 151 healthy elderly from 10 studies and 99 aMCI or AD patients from 7 studies, a significant correlation between brain activation and CR estimates was found. In the healthy elderly, the activated areas were the anterior cingulate and the precuneus in the left hemisphere as well as the cingulate gyrus and superior frontal gyrus in the right hemisphere. In both patients with aMCI or AD, activation in the anterior cingulate cortex in the left hemisphere correlated with estimates of their CR. Furthermore, in a systematic review of 13 studies of CR and fMRI (Anthony & Lin, 2018), stronger activations in the frontal regions and the dorsal attention network were related to neural compensation (i.e., better performance) in AD and MCI to a level comparable to that of healthy elderly. Across the whole age spectrum, activation in medial temporal regions and an anterior or posterior cingulate cortex-seeded default mode network were associated with neural reserve, in which certain brain regions or networks are resistant to the effect of neurodegeneration or AD pathology, which is considered a neural implementation supporting CR by Anthony and Lin (2018). More recent efforts to identify the neural underpinnings of CR focused on identifying task-invariant network activations (Stern, Gazes, et al., 2018; Van Loenhoud et al., 2020).
fMRI methodology is currently not routinely applied in individual patients and has logistic challenges. Therefore, it might be interesting to study the neural mechanisms underlying CR with the use of techniques that provide more direct measures of neural activity such as electroencephalography (EEG), electrocorticography (ECOG), or magnetoencephalography (MEG).
These electrophysiological neuroimaging techniques (Bunge & Kahn, 2009) are characterized by their superior temporal resolution (He et al., 2011) compared to fMRI (Stokes et al., 2015) and are particularly suited to perform frequency decomposition analysis (Ferris et al., 2019), model neural connectivity (Hagen et al., 2018), reconstruct spatiotemporal sources (Costa & Crini, 2011; van Mierlo et al., 2019) and build brain-computer interfaces (e.g., Bittencourt-Villalpando & Maurits, 2018; Angrick et al., 2019; Zubarev et al., 2019). EEG and ECOG both measure the electrical fluctuations originating from the postsynaptic potentials in the pyramidal cells in the cortex (Cohen, 2017; Dubey & Ray, 2019), while MEG measures the magnetic fields produced by these postsynaptic potentials (Baillet, 2017). The major difference between EEG and ECOG is in the position of their electrodes: EEG is a noninvasive technique that uses electrodes at the scalp, while ECOG electrodes are surgically placed directly on the cortex.
In addition, EEG has the advantage of lower costs (Schiff et al., 2016) and portable devices are available (Malcolm et al., 2017) that facilitate applicability. This was previously highlighted by Rajji (2018) who underlined the unexploited strengths of EEG to study CR. Although the literature on (M)EEG/ECOG results has continued to expand since their creation, there is no overview of (M)EEG/ECOG studies of CR, probably because the definition of CR varied over the years and until recently most authors used fMRI to study the neural basis of CR.
Since the concept of CR arose from epidemiological observations, CR is typically measured by indirect estimates that epidemiological studies have identified to be related to CR, inducing discrepancies in the way in which CR has been defined and measured over the years (Pettigrew & Soldan, 2019). We chose to employ the latest authorative conceptual definition and guidelines for research on CR published by the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup led by Stern and colleagues (2018) under the auspices of the Alzheimer Association. However, as the studies of CR and its underlying neural mechanisms used different CR definitions, we employed the previous most accepted definition of CR (Stern, 2002) in our inclusion criteria for our literature search taking into account the new CR research guidelines (Stern, Arenaza-Urquijo, et al., 2018).
In this study, we therefore summarize the literature on ECOG and (M)EEG studies that relate the neurophysiological brain measures to estimates of CR from the first landmark publication of the most accepted definition of CR (Stern, 2002) up-to-date of search.
Materials and Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (Moher et al., 2009) to assess the properties, advantages, and limitations of (M)EEG and ECOG methodologies to study CR. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), registration ID CRD42019115571 (Balart-Sánchez et al., 2019).
Search Strategy
On June 4, 2020, an electronic database search for potentially relevant studies was carried out in PubMed, Embase and Science Direct databases, targeting title, abstract, and keywords using the MESH and EMTREE terms ‘‘electroencephalography,” “magnetoencephalography,” “electrocorticography,” “event-related potentials,” and synonyms in logical conjunction with the term “cognitive reserve” (for details, see Appendix 1). The search was done from 2002 up to date of search. A reference check was done on the selected articles for potentially overlooked articles.
Selection Criteria and Data Extraction
The selection of eligible papers was carried out independently by two of the authors (SB and MB) who firstly removed all duplicates and secondly screened title and abstract to check whether (a) the paper concerned original research, (b) (M)EEG and/or ECOG measures were analyzed, (c) human subjects were considered, (d) a validated CR estimate was used, (e) the paper was written in English, and finally (f) (M)EEG and/or ECOG measures were related to a CR estimate.
Reviews and case reports were excluded. Finally, the remaining articles were fully read to verify the inclusion criteria. The reviewers’ interrater agreement for inclusion was calculated using Cohen’s Kappa statistic (Cohen, 1960) and disagreements between reviewers were resolved with discussion until consensus was reached.
The data extraction was undertaken by the first author and verified by the second author. The following information was extracted from the selected studies: year of publication, study type (e.g. cross-sectional, longitudinal), study sample characteristics (gender ratio, age, pathology), employed CR estimates, (M)EEG and/or ECOG technique/paradigm, used channels, and (M)EEG and/or ECOG outcome measure and results.
Quality Assessment
The quality of the included studies and the risk of bias were assessed with the Newcastle–Ottawa scale (NOS) modified for case–control and cross-sectional studies (Herzog et al., 2013). The operational definitions and assessment criteria are detailed in Appendix 2 based on the structure used by Cook and Reed (2015). We adopted the rating system described by McPheeters and colleagues (2012), which classifies the papers as good, fair, or poor. This approach was previously employed in a similar systematic review of CR and fMRI by Anthony and Lin (2018).
Results
Study Selection
The initial search yielded 268 studies. The selection process is illustrated in Fig. 1 with the PRISMA flow chart: 63 studies were extracted from PubMed, 138 from Embase, and 67 from Science Direct. After removing 68 duplicates, the remaining 200 articles were screened by title, abstract, and keywords, applying the inclusion and exclusion criteria. The following 154 articles were excluded: (a) 77 articles that did not include estimates of CR (25), ECOG/(M)EEG (30), or both (22); (b) 4 articles related to research on animal models; (c) 29 review articles; (d) 21 articles that did not include any original data (e.g., editorials, opinions, and one protocol article); (e) 22 grey literature that include conference or proceeding abstracts: 21 that did not fulfill the inclusion criteria and one that was excluded because the peer-reviewed article version was already in the search database; and (f) 1 case report. The remaining 46 articles passed to the third stage of analysis. They were fully read for the assessment of the fulfillment of inclusion criteria. Eleven of the excluded articles concerned CR-related studies that did not use a validated CR estimate. Nineteen articles were excluded for not relating (M)EEG outcomes to a CR estimate (e.g., instead controlling the CR levels between groups in an [M]EEG experiment that studies a different independent or dependent variable). The remaining articles were included giving a final set of 16 articles. The interrater agreement between reviewers was κ = .951.
Fig. 1.
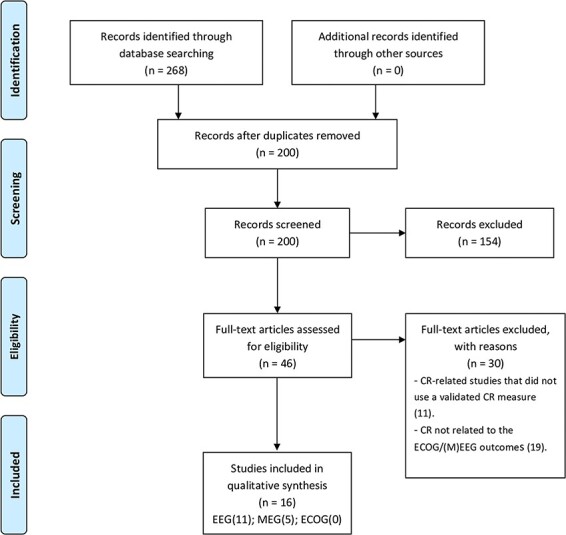
Flow chart of selected studies for qualitative synthesis according to the PRISMA statement. (Adapted from Moher et al., 2009).
Study Characteristics
The total sample of the 16 included studies consisted of 1383 participants constituting a heterogeneous and diverse population (Table 1). Nine studies investigated healthy elderly, comparing elderly women who learned to read in childhood or after their fifties (Nunes et al., 2009), comparing elderly with high or low CR (López et al., 2014; Martínez et al., 2018; Fleck et al., 2019; Yang & Lin, 2020), comparing musicians and non-musicians (Moussard et al., 2016), or comparing elderly to young participants (Speer & Soldan, 2015; Fleck et al., 2017; Gajewski et al., 2020). Two studies considered the moderator effect of CR on cognition in pathological states in younger adults, with human immunodeficiency virus infection, most commonly referred to as HIV (Bauer, 2008) or relapsing–remitting multiple sclerosis (RRMS; Sundgren et al., 2015). The five remaining studies did this in the elderly population: in patients with hepatic encephalopathy (Amodio et al., 2017), aMCI (Gu et al., 2018), MCI (López et al., 2016), subjective memory complaints (SMC; Babiloni et al., 2020), or elderly undergoing general anesthesia (Alonso et al., 2019).
Table 1.
Characteristics and results of studies on EEG, MEG, and CR
| Author/year | Sample characteristics | CR estimate | (M)EEG technique/task | (M)EEG channels | Outcome measure | Results |
|---|---|---|---|---|---|---|
| Bauer (2008) | 115 HIV-1 seropositive and 70 seronegative participants, mean age 39 years, in 8 groups based on VIQ and FH of substance abuse or dependence | VIQ as obtained from the KBIT | ERP/Stroop color-word interference task | EEG: 32 Channels tin electrodes (ElectroCap International, Eaton, Ohio) | Average P3 amplitude over anterior and posterior areas | There was no relationship between VIQ and P300 average amplitude over either area. However, HIV/AIDS and a positive FH both reduced anterior P300 average amplitude. Also, these effects of HIV/AIDS on anterior P300 average amplitude were reduced among subjects with a positive FH. |
| Nunes et al. (2009) | Seven women who learned to read after the age of 50 (ex-illiterates; mean age 70.86, SD 7.4) and five women with regular schooling (controls; mean age 73, SD 9.6). ` | Period (in life) of school education (regular or late-adulthood) | ERF; hemispheric asymmetry/auditory word recognition task | MEG: 148 Channels, whole head magnetometer (MAGNES, 2500 WH, 4D Neuroimaging, San Diego, CA). | Number of sources of activity over different brain areas | The ex-illiterates group exhibited an equivalent number of sources of activity between the LH and RH, due to an increased number of RH sources of activity in comparison to controls. Additionally, the ex-illiterates exhibited more sources of activity in the right inferior frontal gyrus in the timewindow of 150–400 ms after stimulus onset, in comparison to controls. |
| López et al. (2014) | 21 healthy elderly who did not differ in social, cognitive and physical activities, nine high-CR (mean age 67.3, SD 7.4 years) and 12 low-CR (mean age 69.7, SD 6.6 years). Groups were divided by CR level (High-CR cut-off score: CRI > 5) | CR index (years of education and occupational attainment) | Cortical brain connectivity/Memory task (modified Sternberg’s task) | MEG: 148 Channels, whole head magnetometer (MAGNES 2500WH, 4D Neuroimaging, San Diego, CA) | PLV and PLI in frequency bands of 4 Hz in the range between 4 and 44 Hz | There was increased connectivity (as measured by PLV and PLI) in the low-CR group compared to the high-CR group in the theta (4–8 Hz), alpha (8–12 Hz), beta1 (12–16 Hz), and beta2 (16–20 Hz) frequency bands. Differences for connectivity between groups in the theta band were found between fronto-occipital and parietal-occipital channels located in the RH. Differences for connectivity between groups in the alpha band were found between fronto-temporal channels located in the LH and within occipital channels. Differences for connectivity between groups in both beta bands were found between channels located in both LH and RH and within left temporal channels. In all cases, higher connectivity was found in the low-CR group. |
| Speer and Soldan (2015) | 25 healthy young adults (mean age 20.1, SD 2.3 years), 19 nondemented healthy older adults (mean age 70.2, SD 5.1 years) | Composite of NART, vocabulary subtest of WAIS-R and years of education | ERP/Verbal recognition memory task with 1, 4, or 7 letters | EEG:32 Channels with active electrodes (ActiveTwo electrodes, Biosemi, NL) | P3b amplitude and latency | Higher CR was associated with smaller changes in P3b amplitude and less slowing in P3b latency with increasing task difficulty. |
| Sundgren et al. (2015) | 71 RRMS patients (mean age 37.9, SD 10 years), 89 healthy subjects (mean age 38.2, SD 11.5 years) age, gender- and education-matched | Years of education and vocabulary knowledge based on Swedish Dureman–Salde test, separately | ERP/choice reaction time task (visual and auditory) | EEG: 23 Channels AG/AgCl (Nervus Digital Equipment Cephalon, DK). | P3 amplitude, latency, and reaction time | High P3 amplitude and short RT were associated with better cognitive performance, particularly in patients. In contrast, the association between cognitive scores and CR was similar in patients and controls. P3 amplitude and RT explained considerable variance in global cognitive performance in a hierarchical linear regression model. This effect was not modulated by CR. |
| López et al. (2016) | 33 MCI patients (17 women, mean age 73.8, SD 6.5) followed up during a 2 years period. Groups were divided by outcome: progressive MCI (pMCI, 12 patients) or stable MCI (sMCI,21 patients) | Two CR proxies: education level and occupational attainment | Brain oscillations; source reconstruction and spectral analysis/resting state, eyes-closed | MEG: 306 Channels, whole head magnetometer (Vectorview system, ElektaNeuromag) | Normalized spectral power in delta (1.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta1(12–20 Hz), and beta2 (20–30 Hz) frequency bands | The pMCI group showed higher theta and lower beta2 power in comparison to the sMCI group. Occipital theta power was a significant predictor for conversion to AD in a hierarchical linear regression model. CR proxies did not contribute to the prediction of conversion to AD. |
| Moussard et al. (2016) | 17 older musicians (mean age 69.9, range: 59–80 years) and 17 older non-musicians (mean age 69.2, range: 59–80 years) | Years of musical practice | ERP/visual go/no-go task | EEG: 64 Channels AG/AgCL Channel, Biosemi, NL | P2, N2, and P3 amplitudes and latencies | The P2 did not show any association with the group condition neither by amplitude nor latency. Musicians exhibited a larger N2-effect (no go minus go amplitude), which was due to a reduction in the go N2 amplitude and not to an increase in the no-go N2 amplitude. No effect was found for latency. Both groups exhibited a P3 effect, but the distribution of this effect was more frontal in musicians and more parietal in non-musicians. In the musicians, the anterior P3 effect correlated with years of musical practice. |
| Amodio et al. (2017) | 82 patients with HE (median age 62, interquartile range 54–68 years) | CR index as measured by CRIq | Brain oscillations; spectral analysis/eyes-closed resting state | EEG: 21 Channels Ag/AgCl, P3-P4 | Alterations in MDF (1–25.5 Hz), PHES outcome | The CRIq score correlated with the PHES as a measure of cognitive performance, but not with EEG speed as expressed in the MDF. The ratio between PHES and MDF did increase with the CRIq score. |
| Fleck et al. (2017) | 90 cognitively normal adults (mean age 58.51, SD 4.37 years), Groups were divided by age and CR level using a median split | Composite of VIQ as measured by NART-R and years of education | Brain oscillations; intra-hemispheric and mean coherence/resting-state (eyes open and closed) | EEG: 129 Channels HydroCel (Electrical Geodesic Inc.) | Coherence for bands: delta (1–4 Hz), theta (4–8 Hz), low alpha (8–10 Hz), high alpha (10–12 Hz), beta (12.5–25.5 Hz), and gamma (30–50 Hz) | Global coherence differences were found between age groups for left- versus right- hemisphere connectivity and between CR groups for eyes-closed versus eyes-open. Younger participants with lower CR exhibited greater EEG coherence than younger participants with higher CR, whereas older participants with higher CR demonstrated greater coherence than older participants with lower CR. |
| Gu et al. (2018) | 85 elderly, of which 39 aMCI (mean age 71.28, SD 5.98 years) and 46 controls (mean age 70.17, SD 5.63 years) | Composite of CRIq subscores and VIQ as measured by WAIS-RC | ERP/N-Back (0,1). | EEG: 64 Channels AG/AgCl (Brain Products, DE), CP1,CPz, CP2, P1, Pz, P2 | P3 mean amplitude, latency, reaction time, and accuracy | Higher CR levels reduced neural inefficiency (calculated as the ratio between task-related neural processing (P300 amplitude/latency change with respect to task load) and task performance) in controls only, which might be related to better performance (accuracy and reaction time) in this group. There was no correlation between CR and neural inefficiency in the aMCI group, but they did exhibit better performance with higher CR. |
| Martínez et al. (2018) | 20 healthy elderly who did not differ in social, cognitive and physical activities, 12 low-CR (mean age 69.7, SD 6.6 years) and eight high-CR (mean age 67.3, SD 7.4 years). Groups were divided by CR level (high-CR cut-off score: CRI > 5) | CR index (education level and occupational attainment) | Cortical brain connectivity/memory task (modified Sternberg’s task) | MEG: 148 Channels, whole head magnetometer, (MAGNES 2500WH, 4D Neuroimaging, San Diego, CA) | Graph metrics (six parameters) and dynamical connectivity metrics (entropy and complexity) derived from connectivity networks based on synchronization likelihood | The most important findings related to CR were the following: at the node-level, eigenvector centrality was higher in the low-CR group over left-temporal and occipital areas and in the high-CR group over the central area. Conversely, the within-module degree was higher in the high-CR group over temporal and occipital areas and in the low-CR group over central areas. At the network level, the low-CR group exhibited higher network outreach than the high-CR group, indicating more long-range connections for the low-CR group. Dynamically, higher CR was associated with lower entropy and higher complexity levels. |
| Alonso et al. (2019) | 54 elderly (mean age 69.5, SD 7.4 years) | Composite of word reading ability from WRAT and vocabulary subtest of WAIS | Brain oscillations/awake resting-state and under anesthesia | EEG: Two left-frontal channels AG/AgCl; Fz (Fpz or AFz) ground, F3 (or AF3) and F7 (FT9 or F9) | Bispectral index™ BIS score | The EEG intra-individual variability calculated as the squared deviation from the mean BIS value, correlated negatively with the CR estimate. |
| Fleck et al. (2019) | 104 healthy adults (mean age 56.59, SD 7.55 years; 76 women). Groups were divided by CR level using median split | CR based on principal factor analysis of GAMA, SNI, LPAQ and CRIq questionnaires resulting in cognitive, social, and exercise lifestyle factors | Cortical brain connectivity; source reconstruction using eLORETA/resting state (eyes open and closed) | EEG: 129 Channels HydroCel Geodesic Sensor Net (Electrical Geodesics Inc.) | Local and long-range LLC for delta (1–4 Hz), theta (4-8 Hz), low alpha (8–10.5 Hz), high alpha (10.5–13 Hz), low beta (13–20 Hz), high beta (20–30 Hz), and gamma (30–45 Hz) frequency bands | High social CR was related to greater local and long-range connectivity in theta and low alpha for eyes-open and eyes-closed conditions. In contrast, high cognitive CR was associated with greater eyes-closed low alpha long-range connectivity between the occipital lobe and other cortical regions. Additionally, in men with high cognitive CR, greater eyes-closed delta local LLC was found, while women exhibited higher low beta local and long-range LLC. |
| Babiloni et al. (2020) | 118 elderly with SMC and amyloid negative (SMCneg; mean age 75.7, SE 0.3 years) and 54 amyloid positive (SMCpos; mean age 76.6, SE 0.4 years). Subgroups were stratified by CR level (low–moderate/high) | Education level | Brain oscillations; spectral power analysis/resting state (eyes closed) | EEG: 256 Channels EGI system (Electrical Geodesics Inc. Eugene, OR). | Resting state EEG power density in individually determined alpha frequency bands, as well as beta 1 (13–20 Hz), beta 2 (20–30 Hz), and gamma (30–40 Hz) bands. | SMCneg, high-CR participants had higher amplitude of posterior alpha rhythms, while SMCpos, high-CR participants had higher amplitude of temporal alpha rhythms and lower amplitude of posterior alpha rhythms, both compared to low–moderate-CR participants. |
| Gajewski et al. (2020) | 246 healthy participants grouped by age (young, middle-aged and elderly). Elderly participants subdivided by performance at a Stroop task (old low, middle and high performers) | IQ measured by the MWT-B, education level, use of foreign language | ERP/Stroop task | EEG: 32 Channels AG/AgCL Channel, Biosemi, NL | CNV and P2/N2 amplitudes at FCz | The old high-performance group exhibited a larger CNV than old low- and old middle-performance groups. The old high-performance group also exhibited larger P2/N2 amplitudes than the old low-performance group. |
| Yang and Lin (2020) | 41 healthy participants between the ages of 48 and 76 years old, divided by groups of low-CR (20, 12 women, mean age 65.75, SD 6.43) and high-CR (21, 13 women, mean age 63.03, SD 7.71) | Modified CRIq | ERF and brain oscillations; machine learning/resting state (eyes closed) and n-back task (n = 0, 1, 2) | MEG: 306 Channels, whole-head magnetometer, MEG system (Neuromag TRIUX, Elekta, Stockholm, Sweden). | M300 intensity and latency, average power per frequency band, hemispheric asymmetry, accuracy, reaction time, and SVM classification accuracy | The high-CR group had higher accuracies and faster reaction times on the task. The low-CR group showed higher M300 intensities in the left occipital region but similar M300 latencies in comparison to the high-CR group. The high-CR group showed higher beta power in the parietal and occipital regions during the n-back task, and higher gamma power in the right temporal region during resting state. The low-CR group exhibited positive gamma asymmetry values in the occipital region in the resting state whereas the high-CR group exhibited negative values. Positive asymmetry values indicate higher activity level in the right hemisphere. The SVM classifier built using the MEG information as features for discriminating between the CR groups achieved a mean accuracy of 88.89%. |
Note: AD, Alzheimer’s disease; aMCI, amnestic mild cognitive impairment; AUC, area under the curve; BIS, bispectral index; CNV, contingent negative variation; CR, cognitive reserve; CRI, (composite) Cognitive Reserve Index; CRIq, Cognitive Reserve Index questionnaire; eLORETA, exact low-resolution brain electromagnetic tomography; ERF, event-related field; ERP, event-related potential; FH, familial risk; GAMA, general ability measure for adults; GFP, global field power; HE, hepathic encephalitis; HIV, human immunodeficiency virus; IQCODE, informant questionnaire of cognitive decline in the elderly; KBIT, Kaufman Brief Intelligence Test; LH, left hemisphere; LLC, lagged linear connectivity; LPAQ, Lifetime Physical Activity Questionnaire; MDF, mean dominant frequency; MWT-B, multiple-choice word test; NART(−R), National Adult Reading Task (−Revised); PLI, phase lag index; PLV, phase locking value; PHES: psychometric hepatic encephalopathy score; RH, right hemisphere; RRMS, relapsing–remitting multiple sclerosis; SCD, subjective cognitive decline; SMC, subjective memory complaint; SNI, Social Network Index; SVM, support vector machine; VIQ, verbal intelligence quotient; WAIS-R, Wechsler Adult Intelligence Scale—Revised; WAIS-RC, Wechsler Adult Intelligence Scale—Chinese revision; WASI, Wechsler Abbreviated Scale of Intelligence; WRAT, Wide Range Achievement Test.
Regarding the modality of acquisition, ECOG or (M)EEG, the most common modality was EEG (n = 11), followed by MEG (n = 5). No studies relating ECOG measures to a CR estimate were found. Regarding (M)EEG techniques, eight studies used event-related potential (ERP)/event-related field (ERF) techniques mainly focusing on the M/P2 or M/P3 components, as obtained from a go/no-go (Moussard et al., 2016), a Stroop color-word interference (Bauer, 2008; Gajewski et al., 2020), a verbal recognition memory (Speer & Soldan, 2015), a choice reaction (Sundgren et al., 2015), an auditory word recognition (Nunes et al., 2009), a 1-back (Gu et al., 2018), or an N-back task (Yang & Lin, 2020). Six studies investigated brain rhythms in continuous EEG (n = 4) or MEG (n = 2) that were obtained during eyes-closed resting state (López et al., 2016; Amodio et al., 2017; Babiloni et al., 2020; Yang & Lin, 2020) and eyes open (Fleck et al., 2017) or during anesthesia (Alonso et al., 2019). Note that one of the studies (Yang & Lin, 2020) used both ERFs and brain rhythms to identify features for a machine learning algorithm. The three remaining studies investigated brain connectivity in continuous EEG during eyes-open and eyes-closed resting states (Fleck et al., 2019) or in MEG data obtained during a modified Sternberg’s task (López et al., 2014; Martínez et al., 2018).
Concerning the used estimates of CR, most of the studies in this review used more than one measure of premorbid IQ either combined to form a single composite score (López et al., 2014; Sundgren et al., 2015; Fleck et al., 2017; Gu et al., 2018; Martínez et al., 2018; Alonso et al., 2019; Fleck et al., 2019) or non-combined (López et al., 2016; Gajewski et al., 2020), most frequently (different measures of) verbal IQ (VIQ) and years of education. VIQ is a score measuring acquired knowledge, verbal reasoning, and attention to verbal materials. One study exclusively used VIQ (Bauer, 2008) and one study exclusively used education level (Babiloni et al., 2020) as an estimate of CR. Only three studies (Amodio et al., 2017; Gu et al., 2018; Yang & Lin, 2020) employed a specific questionnaire for CR evaluation, namely the CRIq (Nucci, et al., 2012), which calculates a composite CR index (CRI). One study explored the specific effect of the period of school education (regular or late adulthood) on brain activity (Nunes et al., 2009). One final study (Moussard et al., 2016) explored the specific effect of years of musical practice as a special estimate of CR in elderly while controlling for age and years of education.
Neural Correlates of (M)EEG and CR
We summarize results by event-related and brain oscillation studies.
Event-related studies
ERPs and ERFs measure the brain response to a specific sensory, cognitive or motor event or stimulus using EEG and MEG, respectively. The resulting ERP/ERF waveforms are typically discussed and interpreted in terms of their constituting components. A component represents electrical activity that is generated by a cortical area engaged in a specific computational operation. The P2/M2 is an early EEG/MEG component that is thought to represent higher-order perceptual processing, modulated by attention, while the P3/M3 EEG/MEG component is related to higher-order cognitive functioning such as categorization, working memory, and integration (Gahni et al., 2020).
P2/N2 components
The P2 positive deflection is always followed by an N2 negative deflection and the two are typically discussed in conjunction as the P2/N2 complex. Two studies reported results on the P2/N2 complex (Moussard et al., 2016; Gajewski et al., 2020). The first study (Moussard et al., 2016) reported results on the P2 and N2 components as resulting from a go/no-go task performed by older (non)musicians. The go/no-go N2 effect is related to two cognitive functions: correct initiation of behavioral response and inhibition (Hoyniak, 2017). The P2 did not differ between musicians and non-musicians, neither by amplitude nor latency. However, musicians exhibited a larger so-called ‘‘N2-effect,” that is, the difference in the no go and the go N2 amplitude, which was due to a reduction in the go N2 amplitude and not to an increase in the no-go N2 amplitude. No effect was found for latency. The second study (Gajewski et al., 2020) reported P2/N2 and contingent negative variation (CNV) amplitudes as resulting from a Stroop task performed by healthy individuals. In the context of the Stroop task, the CNV is associated with preparation or “readiness” for task performance, perceptual optimization, and sensorimotor timing error correction (Jang et al., 2016; Kononowicz & Penney, 2016) and P2/N2 amplitudes are associated with response selection processes. In the scalp EEG, the CNV is seen as a slow negative deflection following a preparatory stimulus that resolves to baseline when the action or second stimulus occurs (Kononowicz & Penney, 2016). In this study, the participants were grouped by age (young, middle-aged, and elderly) and the elderly group was further subdivided by performance level in the Stroop task (old low, middle, and high performers). They found that both the CNV and P2/N2 complex were larger in the old high than low performers and similar to the younger groups. No effect for latency was found.
P3 component
From the five included studies that analyzed the P3, three studies found a significant association with CR estimates or CR group conditions (Speer & Soldan, 2015; Moussard et al., 2016; Gu et al., 2018). In the study eliciting a P3 from a verbal recognition memory task with three levels of difficulty (Speer & Soldan, 2015), higher estimated CR was associated with smaller changes in P3b amplitude and less slowing in P3b latency with increasing task difficulty. The P3 effect elicited from a go/no-go task in elderly (non)musicians (Moussard et al., 2016), differed between the groups. Although both groups exhibited a P3 effect (no-go relative to go amplitude), the distribution of this effect was more frontal in musicians and more parietal in non-musicians. In the musicians, the anterior P3 effect correlated with the total amount of musical instruction throughout life (as an estimate of CR). Finally, using an N-Back working memory task with two levels in aMCI elderly and age-matched controls, Gu and colleagues (2018) found that higher estimated CR reduced neural inefficiency scores in controls only, which might be related to better performance in this group. Here, neural inefficiency scores were calculated as the ratio between task-related neural processing (P300 amplitude/latency change with respect to task load) and task performance (accuracy slope, accuracy intercept, RT slope−1, and RT intercept−1). Even though there was no correlation between estimated CR and neural inefficiency in the aMCI group, they did exhibit better performance with higher CR.
Two of the five ERP studies that investigated the P3 component found no significant association with CR estimates or CR group conditions (Bauer, 2008; Sundgren et al., 2015). In the study of Bauer (2008), the frontal P3 that was elicited by a Stroop color-word interference task was lower in participants with a familial history of substance abuse independent of the effect of seropositivity. The frontal and parietal P3 amplitude did not depend on the CR estimate (VIQ), however. In the study of Sundgren and colleagues (2015), where RRMS patients and controls performing a choice reaction time task were compared, no association was found between P3 characteristics and the CR estimate. Also, when the P3 amplitude and reaction time were incorporated into a predictor model of cognitive performance, they constituted the strongest predictors, together explaining over 34% of the variance, but this effect was not modulated by estimated CR.
M3 component
Two MEG studies measured the M3 component as elicited by a 2-back task (Yang & Lin, 2020) or an auditory word recognition task (Nunes et al., 2009), respectively.
In the study of Nunes and colleagues (2009), women who learned to read at different stages of their lives (during childhood, defined as the control group and after their fifties, defined as ex-illiterates), performed an auditory word recognition task. A method employing equivalent current dipoles was used to identify the sources of activity underlying the observed ERFs. The ex-illiterates showed less brain functional asymmetry than controls, having an equivalent number of sources of activity in both hemispheres, whereas, also in comparison to controls, they presented a larger number of sources of activity in the right hemisphere between 150 and 400 ms after stimulus onset while performing similarly.
Yang and Lin (2020) studied healthy participants between the ages of 48 and 76 years old, grouped by estimated CR level (high or low). The high-CR group had better performance (higher accuracy and shorter reaction time) and lower M3 intensities in the left occipital region in comparison to their low-CR counterparts. However, no differences were found for M3 latencies. This study also analyzed brain oscillations measured during eyes-closed resting state and task performance as discussed in the next sections.
Brain oscillation studies
Brain oscillation studies typically measure aspects of ongoing brain activity, often during (eyes-open or -closed) resting state, but also during task execution. The most suited way to analyze brain oscillations is by spectral (or Fourier) analysis at sensor, source, or network level, the latter resulting in connectivity analyses. One such measure of connectivity is coherence, which, both in EEG and MEG, reflects the synchronization of neural brain rhythms at different frequencies (Bowyer, 2016). It is possible to analyze changes in brain oscillations in relation to specific events in time by so-called time-frequency analyses, but such studies were not identified in our search. For more details about best practices in (M)EEG data analysis, see Pernet and colleagues (2020).
Power spectral analyses
The four EEG studies on brain oscillations (Amodio et al., 2017; Fleck et al., 2017; Alonso et al., 2019; Babiloni et al., 2020) all exploited resting-state paradigms. In patients with hepatic encephalopathy (Amodio et al., 2017), the CR estimate (CRIq score) correlated with the psychometric hepatic encephalopathy score (PHES) as a measure of cognitive performance, but not with EEG speed as expressed in the mean dominant frequency (MDF). However, the ratio between PHES and MDF did increase with the CRIq score. In elderly with SMC grouped by amyloid status and stratified by CR level (Babiloni et al., 2020), having amyloid negative status and higher estimated CR was associated with higher amplitude of posterior alpha rhythms. In contrast, having amyloid positive status and higher estimated CR was associated with higher amplitude of temporal alpha rhythms and lower amplitude of posterior alpha rhythms. In healthy participants divided by median split based on age and estimated CR level (Fleck et al., 2017) a significant interaction between estimated CR and age with mean coherence was found for all frequency bands (delta, theta, low alpha, high alpha, beta, and gamma). Higher mean coherence was found in younger participants with lower estimated CR in comparison to younger participants with higher estimated CR, whereas in contrast mean coherence was higher in elderly with higher estimated CR than in elderly with lower estimated CR. Additionally, in healthy elderly under general anesthesia for knee surgery (Alonso et al., 2019), intra-individual EEG variability as obtained from the bispectral index (BIS) EEG value correlated negatively with premorbid estimated CR. The BIS is computed from the bispectrum (Sigl & Chamoun, 1994); it is defined as a proprietary nonlinear single variable that is based on a large volume of clinical data correlating behavioral and EEG assessments and gives a single dimensionless number, which ranges from 0 (equivalent to EEG silence) to 100, as an indicator of the anesthesia level (Kissin, 2000).
Both MEG studies on brain oscillations used eyes-closed resting state paradigms (López et al., 2016; Yang & Lin, 2020). Yang & Lin (2020) used an N-back paradigm to evaluate brain oscillations, as well. In the study of López and colleagues (2016), elderly MCI patients were followed up during 2 years and then divided into progressive MCI (pMCI) and stable MCI (sMCI) groups. The pMCI group showed higher theta and lower beta2 (20–30 Hz) power values in comparison to the sMCI group. However, no differences in CR estimates were found between groups and CR estimates did not correlate with beta power values. The best hierarchical linear regression model included occipital theta power as a predictor for conversion to AD, whereas estimated CR did not contribute to the prediction.
Finally, in healthy participants grouped by estimated CR level (Yang & Lin, 2020), higher estimated CR was associated with higher gamma MEG power in the right temporal region during eyes-closed resting state. Additionally, lower estimated CR was associated with positive gamma asymmetry in the occipital region, that is, higher activity level in the right occipital hemisphere, while higher estimated CR was associated with negative gamma asymmetry. While attending to a 2-back task, the high-CR group exhibited higher beta power intensity in the parietal and the occipital regions.
Connectivity analyses
One study investigated brain connectivity in continuous EEG during resting state (Fleck et al., 2019) in both eyes-open and eyes-closed conditions. They found that high estimated CR was associated with higher long-range lagged linear connectivity (LLC) between the occipital lobe and other cortical areas for low alpha in the eyes-closed condition. Additionally, men with high estimated CR had higher local LLC in the delta band than women with high estimated CR in the eyes-closed condition.
The remaining two studies studied brain connectivity during a modified Sternberg’s task using MEG (López et al., 2014; Martínez et al., 2018). López and colleagues (2014) investigated healthy elderly grouped by estimated CR level. Phase locking value (PLV) and phase lag index (PLI) were calculated to assess cortical brain connectivity in different frequency bands. They found increased connectivity in the low-CR group compared to the high-CR group in the theta (4–8 Hz), alpha (8–12 Hz), beta1 (12–16 Hz), and beta2 (16–20 Hz) frequency bands. Additionally, the low-CR group had higher theta band connectivity between fronto-occipital and parietal-occipital channels in the right hemisphere. In the left hemisphere, the low-CR group showed higher alpha connectivity between fronto-temporal channels, as well as within occipital channels. Furthermore, in both beta bands, the low-CR group exhibited higher connectivity between channels located in both hemispheres and within left temporal channels.
Lastly, Martínez and colleagues (2018) used both graph metrics and dynamical connectivity metrics to investigate brain connectivity in healthy elderly performing the modified Stenberg’s task performance, grouped by estimated CR level (low/high). They found differences at the node level, at the network level and for dynamical connectivity between the CR groups. At the node level, eigenvector centrality was higher in the low-CR group over left-temporal and occipital areas and in the high-CR group over the central area. Conversely, the within-module degree was higher in the high-CR group over temporal and occipital areas and in the low-CR group over central areas. At the network level, more long-range connections were found in the low-CR compared to the high-CR group. Dynamically, higher estimated CR was associated with lower entropy and higher complexity.
Quality Assessment
The results of the quality assessment for all 16 included studies are shown in Table 2. Regarding the selection domain, all studies documented the selection process and the population under study. However, none described a prior power calculation for sample size estimation. Regarding the outcome domain, all studies extensively documented their experimental (M)EEG paradigms, measurement techniques, and pre-processing procedures in the (M)EEG pipeline, except for Alonso and colleagues (2019) who used the Bispectral index™ (BIS) monitor, which does not allow insight in detailed analysis procedures. However, the BIS is a validated neurophysiological measure to assess hypnotic components during anesthesia (Johansen, 2006). Overall, the statistical rationale and tests in the selected studies were well documented and transparent. Regarding the comparability domain, the most important factor to control was the demographics of the sampled groups, which was well done in the majority of the studies. In addition, most studies employed composite scores derived from more than one CR estimate, except Bauer (2008) who only used one validated form of estimated CR, and Babiloni et al. (2020), who only employed education level. Additionally, Moussard et al. (2016) studied the specific effect of years of musical practice as a special estimate of CR in elderly while trying to control for age and years of education, making it the first EEG study with this specific approach to estimating CR. The study quality was rated lowest (‘‘Fair”), mainly because the groups were not fully comparable on years of education. Finally, Nunes et al. (2009) compared two groups who differed in the period in life in which they received their education; this study also received a “Fair” assessment because of the small group sizes. The other 14 studies obtained a “Good” rating.
Table 2.
Modified Newcastle–Ottawa quality assessment scale for included studies
| Reference | Design | Selection (++++) | Comparability (++) | Outcome (++++) | Quality |
|---|---|---|---|---|---|
| Bauer (2008) | Case–control | ++++ | ++ | ++ | Good |
| Nunes et al. (2009) | Case–control | ++ | ++ | ++++ | Fair |
| López et al. (2014) | Cross-sectional | +++ | ++ | ++++ | Good |
| López et al. (2016) | Cross-sectional | +++ | ++ | ++++ | Good |
| Speer and Soldan (2015) | Case–control | +++ | ++ | +++ | Good |
| Sundgren et al. (2015) | Case–control | ++++ | ++ | ++ | Good |
| Moussard et al. (2016) | Case–control | +++ | + | ++ | Fair |
| Amodio et al. (2017) | Cross-sectional | +++ | ++ | +++ | Good |
| Fleck et al. (2017) | Cross-sectional | +++ | ++ | +++ | Good |
| Gu et al. (2018) | Case–control | ++++ | ++ | ++ | Good |
| Martínez et al. (2018) | Cross-sectional | +++ | ++ | ++++ | Good |
| Alonso et al. (2019) | Cross-sectional | +++ | ++ | +++ | Good |
| Fleck et al. (2019) | Cross-sectional | ++++ | ++ | ++++ | Good |
| Babiloni et al. (2020) | Cross-sectional | +++ | ++ | ++++ | Good |
| Gajewski et al. (2020) | Cross-sectional | +++ | ++ | ++++ | Good |
| Yang and Lin (2020) | Cross-sectional | +++ | + | +++ | Good |
Note: Each (+) represents one point. Good quality: Minimum of 3(+) on selection domain, 1(+) in comparability domain, and 2(+) in outcome domain. Fair quality: Minimum of 2(+) on selection domain, 1(+) on comparability domain, and 2(+) in outcome domain. Poor quality: Minimum of 1(+) point on selection domain. Rating system based on McPheeters and colleagues (2012).
Discussion
In this systematic review, we identified 11 EEG, 5 MEG, and 0 ECOG studies (from an original set of 268 articles) that relate (M)EEG or ECOG measures to CR estimates and summarized their results. With our approach, we add considerably to the knowledge obtained on the relation between EEG measures and CR estimates published in a recent rapid review (Šneidere et al., 2020). We found that although relations between (M)EEG measures and CR estimates have been identified, the presence and character of this relationship are highly variable and depend on the population and task that were studied and on the analysis technique that was employed. Even though ECOG measures were not yet related to CR estimates, ECOG still remains a potential candidate for future research in this domain as, among the techniques considered here, it measures electrical brain activity most directly. However, due to its invasive nature, it will always be limited to specific populations, such as epilepsy patients eligible for brain surgery.
To summarize results qualitatively, we distinguished between analyses of event-related (potentials, ERPs; fields, ERFs) and brain oscillatory measures (power spectral and connectivity analyses). Concerning the ERP studies, characteristics of the N2/P2 (Moussard et al., 2016; Gajewski et al., 2020) and the P3 (Speer & Soldan, 2015; Moussard et al., 2016; Gu et al., 2018) ERP components were found to be related to CR estimates, but only within healthy participants. Moussard and colleagues (2016) found that older musicians exhibited a larger N2-effect and a similar, but more anteriorly distributed P3-effect than older non-musicians during a go/no-go task. The larger N2-effect was due to a reduction in the go N2 amplitude and not to an increase in the no-go N2 amplitude. According to Moussard and colleagues (2016), this might mean that musicians are more efficient at deploying inhibitory control, with less “inappropriate” inhibition during go-trials. In the musicians, the anterior P3 effect correlated with total amount of practice, and the authors suggest that this more anterior shift in musicians could therefore reflect successful compensation. Similarly, Gajewski and colleagues (2020) found that healthy elderly who achieved high performance at a Stroop task exhibited larger P2/N2 and CNV amplitudes, in comparison to elderly in the lower performance groups. Additionally, the performance level of elderly high-performers was similar to the younger groups. Furthermore, higher task performance was associated to higher CR as reflected in higher level of education, usage of foreign languages and higher IQ. This suggests that elderly in the high performers group engaged more neural resources than elderly in other groups to perform the task, indicating a successful compensatory mechanism, fitting with the idea of higher levels of estimated CR co-existing with more adaptable functional brain processes (Stern, Arenaza-Urquijo, et al., 2018). Finally, higher estimated CR was also associated with smaller changes in P3 amplitude and latency with increasing task difficulty during a verbal recognition memory task in young and healthy older adults (Speer & Soldan, 2015) and with reduced neural inefficiency, as derived from P3 amplitude and latency during an N-back task in healthy elderly (Gu et al., 2018). Hence, these four studies all support that P2/N2 and P3 ERP component properties during inhibition and working memory tasks are related to estimates of CR in healthy adults.
However, no such relationship was found in the ERP studies with HIV-1 seropositive (Bauer, 2008), RRMS (Sundgren et al., 2015), or aMCI patients (Gu et al., 2018). It remains unclear why such a relationship was not found in these patient studies, where one may expect the pathology to affect brain functioning, making CR potentially more relevant to maintain adequate performance. Therefore, one would expect a similar and possibly even clearer relationship between CR estimates and ERP component amplitudes, in particular P3 amplitude (e.g., Polich, 2007), in patients compared to healthy persons. One explanation may be that other factors affect P3 characteristics to such an extent that the modulating effect of CR is no longer visible. For example, in the study by Bauer (2008), a history of substance abuse lowered the frontal P3 that was elicited by a Stroop color-word interference task independent of the effect of HIV-1 seropositivity. Another possibility is that the specific EEG measure is not sensitive to changes in CR in the patient group under study. For example, aMCI patients did exhibit better performance with higher CR, although there was no direct correlation between estimated CR and neural inefficiency (Gu et al., 2018). Finally, it may be that the patient populations under study had other general characteristics than the healthy participants, precluding finding any relationships between estimated CR and neural correlates. This could be due to, on the one hand, stricter inclusion criteria being applied in patient populations and, on the other hand, broader sampling of education level, where healthy volunteers are typically higher educated. That the absence of results is due to the studies being underpowered, seems unlikely, as some of the largest studies in this review are patient studies (Bauer, 2008 [N = 185]; Sundgren et al., 2015 [N = 160]; Babiloni et al., 2020 [N = 172]; with the latter study being the only patient study establishing a relationship).
Regarding the two studies relating ERF measures to CR estimates in healthy elderly (Nunes et al., 2009; Yang & Lin, 2020), both found differences in the interhemispheric distribution of brain activity when comparing high and low estimated CR participants. Nunes et al. (2009) found more symmetric brain activity in ex-illiterate women in comparison to women that learned to read during childhood (control group) during a word recognition task. In detail, the ex-illiterate group recruited resources from both hemispheres equally, effectively by increasing their activity in the right hemisphere, compared to the control group. Considering that both groups performed similarly on the task, these results were interpreted by the authors as a more efficient pattern of neural resource recruitment for task performance in the control group, whereas the ex-illiterates relied on compensatory recruitment of additional neural resources to maintain task performance. This is in line with the “hemispheric asymmetry reduction in older adults model” or HAROLD model (Cabeza, 2002). In the study of Yang and Lin (2020), the M3 component was elicited in healthy participants by an N-back task with three levels. Their higher-CR group achieved better performance in comparison to the lower-CR group, with lower M3 intensities in the left occipital region, while M3 latencies were similar. These findings support more efficient recruitment of neural resources for participants with higher CR.
Mostly, both in the ERP and ERF studies discussed above, the identified relationships between CR and neural correlates indicated that participants with higher CR recruited more brain resources resulting in better performance, evidencing successful compensation mechanisms. One study (Yang & Lin, 2020) provided evidence for more efficient recruitment of neural resources. Why these relationships were only observed in healthy participants and not in patients cannot be fully explained from the studies discussed here.
In the four studies relating oscillatory EEG measures to CR estimates (Amodio et al., 2017; Fleck et al., 2017; Alonso et al., 2019; Babiloni et al., 2020), significant relationships were again mainly established in healthy participants. Higher mean EEG coherence was found in younger participants with lower estimated CR compared those with higher estimated CR, whereas in contrast mean coherence was higher in healthy elderly with higher estimated CR compared to those with lower estimated CR (Fleck et al., 2017). This suggests a shift in the relationship between brain connectivity and estimated CR with age. In healthy elderly under general anesthesia for knee surgery (Alonso et al., 2019) intra-individual EEG variability as obtained from the bispectral index (BIS) EEG value correlated negatively with premorbid estimated CR. As higher EEG variability according to this measure is associated with brain pathology and degeneration, this relationship confirms that higher premorbid estimated CR reflects better brain functioning. Amodio and colleagues (2017) investigated a population of patients with HE and did not find a direct association between estimated CR and EEG measures. However, the ratio between the cognitive performance (PHES score) and EEG speed (MDF) did increase with estimated CR (CRIq score), indicating that the mismatch between cognitive and neurophysiological measures increased with estimated CR. The only EEG study on brain oscillations that found a significant relationship with estimated CR in patients was performed by Babiloni and colleagues (2020) in elderly with SMC. In this study, individuals with negative amyloid status and higher estimated CR exhibited higher posterior alpha amplitudes whereas individuals with positive amyloid status and higher estimated CR exhibited higher temporal and lower posterior alpha amplitudes, compared to individuals with lower estimated CR. The posterior EEG alpha in resting state progressively reduces with aging, which is linked to fiber deterioration of the cholinergic projections (Babiloni et al., 2009; Wan et al., 2019). According to Babiloni et al. (2020), this suggests that compensatory and neuroprotective mechanisms are in place in the positive amyloid status, high-CR group, where additional neural resources seem to be recruited in the temporal regions.
In the two studies relating oscillatory MEG measures to CR estimates (López et al., 2016; Yang & Lin, 2020), a significant relationship between CR estimates and neural correlates was again only established in healthy participants. Yang & Lin (2020) investigated healthy elderly and found that the high-CR group exhibited higher gamma power in right temporal regions during resting state, and higher beta power in the parietal and occipital regions during an N-back task, while performing better than the low-CR group. This suggests that increased beta activity reflects compensatory brain activity in healthy elderly. In the study of López and colleagues (2016), CR estimates did not correlate with beta power neither in stable nor progressive MCI patients.
The three remaining M(EEG) studies on brain oscillations used brain connectivity analysis on EEG (Fleck et al., 2019) and MEG data (López et al., 2014; Martínez et al., 2018). All found significant associations between brain connectivity measures and CR estimates. Higher estimated CR was associated with higher long-range LLC in low alpha between the occipital lobe and different cortical regions during eyes-closed resting state (Fleck et al., 2019), confirming the role of alpha oscillations in CR identified by Babiloni et al., (2020). In the same study and condition, men in the high-CR group exhibited increased local and long-range LLC in the delta band. Given that delta activity during eyes closed progressively reduces with aging (Barry et al., 2007; Barry & De Blasio, 2017), this suggests that the delta activity level in men might represent a suitable marker for aging brain functioning.
The other two MEG studies on brain connectivity used a modified Sternberg’s task in healthy elderly (López et al., 2014; Martínez et al., 2018). López and colleagues (2014) suggested that the low-CR group had to make a greater “effort” than the high-CR group to perform the same task, as reflected by increased brain connectivity in the alpha and beta bands in the left hemisphere and in the theta band in the right hemisphere. This apparent compensation mechanism was further explored in a follow-up study from the same group, by Martínez et al. (2018), who used a similar task and population to investigate topological and dynamical properties of brain networks. They found that higher estimated CR was associated with lower entropy and higher complexity levels. According to the authors, this may suggest that the low-CR group exhibited a dual pattern of, again, compensation and furthermore network impairment, since performing the task was more energetically costly for them than for the high-CR group.
Although EEG and MEG have better temporal than spatial resolution, it is still interesting to compare (global) localization of neural correlates of CR for these neurophysiological techniques to localization of fMRI activations as described in Colangeli and colleagues (2016) and Anthony and Lin (2018; see Introduction). Of the 16 studies reviewed here, four MEG studies (Nunes et al., 2009; López et al., 2014; Martínez et al., 2018; Yang & Lin, 2020) and three EEG studies (Moussard et al., 2016; Fleck et al., 2019; Babiloni et al., 2020) provide some form of regional localization of the (M)EEG measures that are related with CR estimates. As may be expected given the variety of results described in these studies, localizations also vary, but there are some communalities across studies. Refraining from reiterating all results, differences in (M)EEG measures between high- and low-CR groups seem to be more often found in occipital/posterior areas, for all oscillation and connectivity frequency bands, except the delta band (López et al., 2014; Martínez et al., 2018; Fleck et al., 2019; Babiloni et al., 2020, Yang & Lin, 2020). Also the right hemisphere seems to be implicated more often than the left hemisphere (Nunes et al., 2009, López et al., 2014; Yang & Lin, 2020). Only one study (Moussard et al., 2016) mentions that frontal EEG activity is related to estimated CR, whereas in the fMRI studies frontal activation is more often implicated to be related to estimated CR. It should be kept in mind, however, that localization is not a strong suit of (M)EEG.
To conclude, the findings of the current review support that (M)EEG measures are related to CR estimates, particularly in healthy individuals. The presence and character of this relationship is highly variable and depends on the population and task that were studied and on the analysis technique that was employed. It should also be noted that some of these relationships were reflected in differences in (M)EEG measures between groups with high or low estimated CR, without establishing a direct relationship such as a correlation or in a predictive model, between (M)EEG measures and CR estimates. It remains unclear why such a relationship was only found in one patient study using EEG oscillations. To elucidate this issue and avoid the variability in populations and tasks that we encountered in our review, a sufficiently powered study in neurologically afflicted patients, which compares the correlation between different (M)EEG measures and different CR estimates, within this one group, might help.
Funding
This project was partially supported by a grant of the National Council of Science and Technology of Mexico (CONACyT): Fellowship No. 709126 (awarded to the first author).
Appendix 1
Appendix Table 1.
Search strategies for PubMed, Embase, and Science Direct databases
| PubMed |
| (("Electroencephalography"[Mesh] OR "Evoked Potentials"[Mesh] OR "Magnetoencephalography"[Mesh] OR "Electrocorticography"[Mesh] OR eeg[tiab] OR electroencephalog*[tiab] OR magnetoencephalog*[tiab] OR electrocorticog*[tiab] OR erp[tiab] OR event-related potential*[tiab] OR evoked-related potential*[tiab]) AND ("Cognitive Reserve"[Mesh] OR “cognitive reserve”[tiab] OR “brain reserve”[tiab] OR “neural reserve” [tiab] OR “neural compensation” [tiab])) AND ("2002/01/01"[PDAT] : "2020/6/4"[PDAT]) |
| Embase |
| (('electroencephalogram'/exp OR 'electroencephalography'/exp OR 'event related potential'/exp OR 'magnetoencephalography'/exp OR 'electrocorticography'/exp) OR (eeg OR electroencephalog* OR erp OR ‘event-related potential*’ OR ‘evoked-related potential*’ OR ECOG OR MEG OR 'magnetoencephalog*' OR 'electrocorticog*' ):ab,ti) AND ('cognitive reserve'/exp OR (‘cognitive reserve’):ab,ti OR (‘brain reserve’):ab,ti OR (‘neural reserve’):ab,ti OR (‘neural compensation’):ab,ti) AND [2002-2020]/py |
| Science Direct |
| title-abs-key((cognitive reserve OR brain reserve OR neural reserve OR neural compensation) AND (eeg OR erp OR magnetoencephalography OR electrocorticography OR event-related potential )-Years(2002-2020) |
Appendix 2
Appendix Table 2.
Adapted NOS for analysis of case-control studies on M/EEG or ECOG and CR Rating system based on McPheeters and colleagues (2012).
| Adapted NOS for analysis of case–control studies on M/EEG or ECOG and CR | |||
|---|---|---|---|
| Item | Response options: score | Operational definitions | |
| (1) Is the case definition adequate? | (a) Yes, with independent validation: 1 (b) Yes, for example, record linkage or based on self-reports: 0 (c) No description: 0 |
• (a) Independent validation: case/control classification can be independently verified (i.e., test, independent clinician diagnosis). • (b) Case/control classification is based on self-report from the participant. • (c) No description |
|
| (2) Representativeness of the cases | (a) Consecutive or obviously representative series of cases: 1 (b) Potential for selection biases or not stated: 0 |
• (a) Cases and controls were included in consecutive order or (b) is there a possible (undesirable) interference in the inclusion of participants? | |
| (3) Selection of controls | (a) Community controls: 1 (b) Hospital controls: 0 (c) No description: 0 |
• Were the controls recruited (a) from the whole community (e.g., with public advertisements) or (b) inside the hospital/no description? | |
| Selection | (4) Definition of controls | (a) No history of disease (endpoint): 1 (b) No description of source: 0 |
• The controls would be considered as the natural trajectory of the subjects in comparisons without CR. |
| Comparability | (5) Comparability of cases and controls on the basis of the design or analysis | (a) Study controls for age: 1 (b) Study controls for gender and/or education level: 1 |
• Controlling for subject characteristic covariates (e.g., including age, gender, and/or educational levels) |
| (6) Ascertainment of outcome | (a) Secure record (e.g., measured M/EEG or ECOG, CR questionnaire): 1 (b) Structured interview, blind to case/control status: 1 (c) Interview not blinded to case/control status: 0 (d) Written self-report or medical record only: 0 (e) No description: 0 |
• Described tool for CR and M/EEG or ECOG • The assessment must report the demographic measures providing the data (e.g., years of education, composite score). • Controlling for signal-to-noise ratio of M/EEG or ECOG measures |
|
| (7) Same method of ascertainment for cases and controls | (a) Yes: 1 (b) No: 0 |
• The method applied to cases and controls is explicitly detailed as being the same. | |
| Outcome | (8) Non-response rate | (a) Same rate for both groups: 1 (b) Non-respondents described: 0 (c) Rate different and no designation: 0 |
• Non-responders can also be considered when they are excluded due to low-performance on task or noise records on M/EEG or ECOG. |
Contributor Information
Sebastián A Balart-Sánchez, Department of Neurology, University Medical Center Groningen, University of Groningen, Groningen, 9700 RB, Netherlands; Research School of Behavioural and Cognitive Neurosciences, University of Groningen, Groningen, 9713 AV, Netherlands.
Mayra Bittencourt-Villalpando, Department of Neurology, University Medical Center Groningen, University of Groningen, Groningen, 9700 RB, Netherlands; Research School of Behavioural and Cognitive Neurosciences, University of Groningen, Groningen, 9713 AV, Netherlands.
Joukje van der Naalt, Department of Neurology, University Medical Center Groningen, University of Groningen, Groningen, 9700 RB, Netherlands; Research School of Behavioural and Cognitive Neurosciences, University of Groningen, Groningen, 9713 AV, Netherlands.
Natasha M Maurits, Department of Neurology, University Medical Center Groningen, University of Groningen, Groningen, 9700 RB, Netherlands; Research School of Behavioural and Cognitive Neurosciences, University of Groningen, Groningen, 9713 AV, Netherlands.
References
- Adam, S., Bonsang, E., Grotz, C., & Perelman, S. (2013). Occupational activity and cognitive reserve: Implications in terms of prevention of cognitive aging and Alzheimer’s disease. Clinical Interventions in Aging, 8, 377–390. doi: 10.2147/CIA.S39921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, C. H., Tanner, J. J., Wiggins, M. E., Sinha, P., Parvataneni, H. K., Ding, M.et al. (2019). Proof of principle: Preoperative cognitive reserve and brain integrity predicts intra-individual variability in processed EEG (bispectral index monitor) during general anesthesia. PLoS One, 14(5), e0216209. doi: 10.1371/journal.pone.0216209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio, P., Montagnese, S., Spinelli, G., Schiff, S., & Mapelli, D. (2017). Cognitive reserve is a resilience factor for cognitive dysfunction in hepatic encephalopathy. Metabolic Brain Disease, 32(4), 1287–1293. doi: 10.1007/s11011-017-0032-2. [DOI] [PubMed] [Google Scholar]
- Angrick, M., Herff, C., Mugler, E., Tate, M. C., Slutzky, M. W., Krusienski, D. J.et al. (2019). Speech synthesis from ECoG using densely connected 3D convolutional neural networks. Journal of Neural Engineering, 16(3), 036019. doi: 10.1088/1741-2552/ab0c59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, M., & Lin, F. (2018). A systematic review for functional neuroimaging studies of cognitive reserve across the cognitive aging spectrum. Archives of Clinical Neuropsychology, 33(8), 937–948. doi: 10.1093/arclin/acx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, M. J., Naglie, G., Duff-Canning, S., Meaney, C., Gill, D., Eslinger, P. J.et al. (2012). Roles of education and IQ in cognitive reserve in Parkinson’s disease-mild cognitive impairment. Dementia and Geriatric Cognitive Disorders Extra, 2(1), 343–352. doi: 10.1159/000341782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni, C., Pievani, M., Vecchio, F., Geroldi, C., Eusebi, F., Fracassi, C.et al. (2009). White-matter lesions along the cholinergic tracts are related to cortical sources of EEG rhythms in amnesic mild cognitive impairment. Human Brain Mapping, 30(5), 1431–1443. doi: 10.1002/hbm.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni, C., Lopez, S., del Percio, C., Noce, G., Pascarelli, M. T., Lizio, R.et al. (2020). Resting-state posterior alpha rhythms are abnormal in subjective memory complaint seniors with preclinical Alzheimer's neuropathology and high education level: The INSIGHT-preAD study. Neurobiology of Aging, 90, 43–59. doi: 10.1016/j.neurobiolaging.2020.01.012. [DOI] [PubMed] [Google Scholar]
- Baillet, S. (2017). Magnetoencephalography for brain electrophysiology and imaging. Nature Neuroscience, 20(3), 327–339. doi: 10.1038/nn.4504. [DOI] [PubMed] [Google Scholar]
- Balart-Sánchez, S. A., Bittencourt-Villalpando, M., van der Naalt, J., & Maurits, N. (2019). Electroencephalography (EEG) & cognitive reserve (CR)—a systematic review. PROSPERO 2019 CRD42019115571. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019115571 [DOI] [PMC free article] [PubMed]
- Bauer, L. O. (2008). The effects of HIV on P300 are moderated by familial risk for substance dependence: Implications for a theory of brain reserve. Drug and Alcohol Dependence, 94(1–3), 92–100. doi: 10.1016/j.drugalcdep.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, R. J., Clarke, A. R., Johnstone, S. J., Magee, C. A., & Rushby, J. A. (2007). EEG differences between eyes-closed and eyes-open resting conditions. Clinical Neurophysiology, 118(12), 2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Barry, R. J., & De Blasio, F. M. (2017). EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biological Psychology, 129, 293–304. doi: 10.1016/j.biopsycho.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Barulli, D., & Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17(10), 502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt-Villalpando, M., & Maurits, N. M. (2018). Stimuli and feature extraction algorithms for brain-computer interfaces: A systematic comparison. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 26(9), 1669–1679. doi: 10.1109/tnsre.2018.2855801. [DOI] [PubMed] [Google Scholar]
- Bowyer, S. M. (2016). Coherence a measure of the brain networks: Past and present. Neuropsychiatric Electrophysiology, 2, 1. doi: 10.1186/s40810-015-0015-7. [DOI] [Google Scholar]
- Bunge, S. A., & Kahn, I. (2009). Cognition: An overview of neuroimaging techniques. Encyclopedia of Neuroscience, 1063–1067. doi: 10.1016/b978-008045046-9.00298-9. [DOI] [Google Scholar]
- Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement, 20(1), 37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- Cohen, M. X. (2017). Where does EEG come from and what does it mean? Trends in Neurosciences, 40(4), 208–218. doi: 10.1016/j.tins.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Colangeli, S., Boccia, M., Verde, P., Guariglia, P., Bianchini, F., & Piccardi, L. (2016). Cognitive reserve in healthy aging and Alzheimer’s disease: A meta-analysis of fMRI studies. American Journal of Alzheimer's Disease and Other Dementias, 31(5), 443–449. doi: 10.1177/1533317516653826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D. A., & Reed, D. A. (2015). Appraising the quality of medical education research methods: The medical education research study quality instrument and the Newcastle–Ottawa scale-education. Academic Medicine, 90(8), 1067–1076. doi: 10.1097/ACM.0000000000000786. [DOI] [PubMed] [Google Scholar]
- Costa, T., & Crini (2011). Basic emotions: Differences in time sequence and functional imaging with low resolution brain electrical tomography (LORETA). Nature Precedings. doi: 10.1038/npre.2011.5566.1. [DOI] [Google Scholar]
- Dubey, A., & Ray, S. (2019). Cortical electrocorticogram (ECoG) is a local signal. The Journal of Neuroscience, 39(22), 4299–4311. doi: 10.1523/jneurosci.2917-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfel, J. M., Nitrini, R., Suemoto, C. K., Grinberg, L. T., Ferretti, R. E. L., Leite, R. E. P.et al. (2013). Very low levels of education and cognitive reserve: A clinicopathologic study. Neurology, 81(7), 650–657. doi: 10.1212/WNL.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, D. P., McArthur, K., Mehrkanoon, S., Liley, D., & Mehrkanoon, S. (2019). Guest editorial special issue on neural systems engineering and mathematical modeling of brain dynamics using ECoG/EEG/MEG oscillations and machine learning methods. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 27(3), 335–336. doi: 10.1109/tnsre.2019.2902696. [DOI] [Google Scholar]
- Fleck, J. I., Kuti, J., Mercurio, J., Mullen, S., Austin, K., & Pereira, O. (2017). The impact of age and cognitive reserve on resting-state brain connectivity. Frontiers in Aging Neuroscience, 9, 392. doi: 10.3389/fnagi.2017.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck, J. I., Arnold, M., Dykstra, B., Casario, K., Douglas, E., & Morris, O. (2019). Distinct functional connectivity patterns are associated with social and cognitive lifestyle factors: Pathways to cognitive reserve. Frontiers in Aging Neuroscience, 11, 310. doi: 10.3389/fnagi.2019.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski, P. D., Falkenstein, M., Thönes, S., & Wascher, E. (2020). Stroop task performance across the lifespan: High cognitive reserve in older age is associated with enhanced proactive and reactive interference control. NeuroImage, 207(116430). doi: 10.1016/j.neuroimage.2019.116430. [DOI] [PubMed] [Google Scholar]
- Ghaffar, O., Fiati, M., & Feinstein, A. (2012). Occupational attainment as a marker of cognitive reserve in multiple sclerosis. PLoS One, 7(10), e47206. doi: 10.1371/journal.pone.0047206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani, U., Signal, N., Niazi, I., & Taylor, D. (2020). ERP based measures of cognitive workload: A review. Neuroscience and Biobehavioral Reviews, 118, 18–26. doi: 10.1016/j.neubiorev.2020.07.020. [DOI] [PubMed] [Google Scholar]
- Gu, L., Chen, J., Gao, L., Shu, H., Wang, Z., Liu, D.et al. (2018). Cognitive reserve modulates attention processes in healthy elderly and amnestic mild cognitive impairment: An event-related potential study. Clinical Neurophysiology, 129(1), 198–207. doi: 10.1016/j.clinph.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Habeck, C., Hilton, H. J., Zarahn, E., Flynn, J., Moeller, J., & Stern, Y. (2003). Relation of cognitive reserve and task performance to expression of regional covariance networks in an event-related fMRI study of nonverbal memory. NeuroImage, 20(3), 1723–1733. doi: 10.1016/j.neuroimage.2003.07.032. [DOI] [PubMed] [Google Scholar]
- Hagen, E., Næss, S., Ness, T. V., & Einevoll, G. T. (2018). Multimodal modeling of neural network activity: Computing LFP, ECoG, EEG, and MEG signals with LFPy 2.0. Frontiers in Neuroinformatics, 12, 92. doi: 10.3389/fninf.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, B., Yang, L., Wilke, C., & Yuan, H. (2011). Electrophysiological imaging of brain activity and connectivity-challenges and opportunities. IEEE Transactions on Biomedical Engineering, 58(7), 1918–1931. doi: 10.1109/TBME.2011.2139210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner, E. P., Scarmeas, N., Cosentino, S., Portet, F., & Stern, Y. (2007). Leisure activity and cognitive decline in incident Alzheimer disease. Archives of Neurology, 64(12), 1749–1754. doi: 10.1001/archneur.64.12.1749. [DOI] [PubMed] [Google Scholar]
- Herzog, R., Álvarez-Pasquin, M. J., Díaz, C., Del Barrio, J. L., Estrada, J. M., & Gil, Á. (2013). Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health, 13(1), 154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle, J. V., Hurt, C. S., Burn, D. J., Brown, R. G., Samuel, M., Wilson, K. C.et al. (2016). The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson's disease—A longitudinal cohort study. International Journal of Geriatric Psychiatry, 31(1), 13–23. doi: 10.1002/gps.4284. [DOI] [PubMed] [Google Scholar]
- Hoyniak, C. (2017). Changes in the NoGo N2 event-related potential component across childhood: A systematic review and meta-analysis. Developmental Neuropsychology, 42(1), 1–24. doi: 10.1080/87565641.2016.1247162. [DOI] [PubMed] [Google Scholar]
- Jang, J., Jones, M., Milne, E., Wilson, D., & Lee, K.-H. (2016). Contingent negative variation (CNV) associated with sensorimotor timing error correction. NeuroImage, 127, 58–66. doi: 10.1016/j.neuroimage.2015.11.071. [DOI] [PubMed] [Google Scholar]
- Johansen, J. W. (2006). Update on bispectral index monitoring. Best Practice & Research. Clinical Anaesthesiology, 20(1), 81–99. doi: 10.1016/j.bpa.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jones, R. N., Yang, F. M., Zhang, Y., Kiely, D. K., Marcantonio, E. R., & Inouye, S. K. (2006). Does educational attainment contribute to risk for delirium? A potential role for cognitive reserve. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(12), 1307–1311. doi: 10.1093/gerona/61.12.1307. [DOI] [PubMed] [Google Scholar]
- Katzman, R. (1993). Education and the prevalence of dementia and Alzheimer's disease. Neurology, 43(1), 13–20. doi: 10.1212/WNL.43.1_Part_1.13. [DOI] [PubMed] [Google Scholar]
- Kissin, I. (2000). Depth of anesthesia and bispectral index monitoring. Anesthesia and Analgesia, 90(5), 1114–1117. doi: 10.1097/00000539-200005000-00021. [DOI] [PubMed] [Google Scholar]
- Kononowicz, T. W., & Penney, T. B. (2016). The contingent negative variation (CNV): Timing isn’t everything. Current Opinion in Behavioral Sciences, 8, 231–237. doi: 10.1016/j.cobeha.2016.02.022. [DOI] [Google Scholar]
- López, M. E., Aurtenetxe, S., Pereda, E., Cuesta, P., Castellanos, N. P., Bruña, R.et al. (2014). Cognitive reserve is associated with the functional organization of the brain in healthy aging: A MEG study. Frontiers in Aging Neuroscience, 6, 125. doi: 10.3389/fnagi.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López, M. E., Turrero, A., Cuesta, P., López-Sanz, D., Bruña, R., Marcos, A.et al. (2016). Searching for primary predictors of conversion from mild cognitive impairment to Alzheimer’s disease: A multivariate follow-up study. Journal of Alzheimer's Disease, 52(1), 133–143. doi: 10.3233/jad-151034. [DOI] [PubMed] [Google Scholar]
- Van Loenhoud, A. C., Habeck, C., van der Flier, W. M., Ossenkoppele, R., & Stern, Y. (2020). Identifying a task-invariant cognitive reserve network using task potency. NeuroImage, 210, 1106593. doi: 10.1016/j.neuroimage.2020.116593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm, B. R., Foxe, J. J., Butler, J. S., Mowrey, W. B., Molholm, S., & De Sanctis, P. (2019). Long-term test-retest reliability of event-related potential (ERP) recordings during treadmill walking using the mobile brain/body imaging (MoBI) approach. Brain Research, 1716, 62–69. doi: 10.1016/j.brainres.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez, J. H., López, M. E., Ariza, P., Chavez, M., Pineda-Pardo, J. A., López-Sanz, D.et al. (2018). Functional brain networks reveal the existence of cognitive reserve and the interplay between network topology and dynamics. Scientific Reports, 8(1), 1–11. doi: 10.1038/s41598-018-28747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters, M. L., Kripalani, S., Peterson, N. B., Idowu, R. T., Jerome, R. N., Potter, S. A.et al. (2012). Closing the quality gap: Revisiting the state of the science (vol. 3: Quality improvement interventions to address health disparities). Evidence Report/Technology Assessment, 208(3), 1–475. doi: 10.1037/e676532012-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mierlo, P., Höller, Y., Focke, N. K., & Vulliemoz, S. (2019). Network perspectives on epilepsy using EEG/MEG source connectivity. Frontiers in Neurology, 10, 721. doi: 10.3389/fneur.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Moussard, A., Bermudez, P., Alain, C., Tays, W., & Moreno, S. (2016). Life-long music practice and executive control in older adults: An event-related potential study. Brain Research, 1642, 146–153. doi: 10.1016/j.brainres.2016.03.028. [DOI] [PubMed] [Google Scholar]
- Nucci, M., Mapelli, D., & Mondini, S. (2012). The cognitive reserve questionnaire (CRIq): A new instrument for measuring the cognitive reserve. Aging Clinical and Experimental Research, 24, 218–126. doi: 10.1037/t53917-000. [DOI] [PubMed] [Google Scholar]
- Nunes, M. V. S., Castro-Caldas, A., Del Rio, D., Maestú, F., & Ortiz, T. (2009). The ex-illiterate brain: The critical period, cognitive reserve and HAROLD model. Dementia & Neuropsychologia, 3(3), 222–227. doi: 10.1590/s1980-57642009dn30300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg, C., Lundin, A., Edman, G., Nygren-de Boussard, C., & Bartfai, A. (2016). Cognitive reserve and persistent post-concussion symptoms—A prospective mild traumatic brain injury (mTBI) cohort study. Brain Injury, 30(2), 146–155. doi: 10.3109/02699052.2015.1089598. [DOI] [PubMed] [Google Scholar]
- Pernet, C., Garrido, M. I., Gramfort, A., Maurits, N., Michel, C. M., Pang, E.et al. (2020). Issues and recommendations from the OHBM COBIDAS MEEG committee for reproducible EEG and MEG research. Nature Neuroscience, 23(12), 1473–1483. doi: 10.1038/s41593-020-00709-0. [DOI] [PubMed] [Google Scholar]
- Pettigrew, C., & Soldan, A. (2019). Defining cognitive reserve and implications for cognitive aging. Current Neurology and Neuroscience Reports, 19(1), 1. doi: 10.1007/s11910-019-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji, T. K. (2018). Neurophysiology and cognitive reserve: A promising path. Clinical Neurophysiology, 129(1), 286–287. doi: 10.1016/j.clinph.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Roe, C. M., Xiong, C., Miller, J. P., & Morris, J. C. (2007). Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology, 68(3), 223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Schneider, E. B., Sur, S., Raymont, V., Duckworth, J., Kowalski, R. G., Efron, D. T.et al. (2014). Functional recovery after moderate/severe traumatic brain injury: A role for cognitive reserve? Neurology, 82(18), 1636–1642. doi: 10.1212/WNL.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff, S., Casa, M., di Caro, V., Aprile, D., Spinelli, G., de Rui, M.et al. (2016). A low-cost, user-friendly electroencephalographic recording system for the assessment of hepatic encephalopathy. Hepatology, 63(5), 1651–1659. doi: 10.1002/hep.28477. [DOI] [PubMed] [Google Scholar]
- Sigl, J. C., & Chamoun, N. G. (1994). An introduction to bispectral analysis for the electroencephalogram. Journal of Clinical Monitoring, 10(6), 92–404. doi: 10.1007/BF01618421. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux, A., Marmot, M. G., Glymour, M., Sabia, S., Kivimäki, M., & Dugravot, A. (2011). Does cognitive reserve shape cognitive decline? Annals of Neurology, 70(2), 296–304. doi: 10.1002/ana.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šneidere, K., Mondini, S., & Stepens, A. (2020). Role of EEG in measuring cognitive reserve: A rapid review. Frontiers in Aging Neuroscience, 12, 249. doi: 10.3389/fnagi.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer, M. E., & Soldan, A. (2015). Cognitive reserve modulates ERPs associated with verbal working memory in healthy younger and older adults. Neurobiology of Aging, 36(3), 1424–1434. doi: 10.1016/j.neurobiolaging.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr, J. M., & Lonie, J. (2008). Estimated pre-morbid IQ effects on cognitive and functional outcomes in Alzheimer disease: A longitudinal study in a treated cohort. BMC Psychiatry, 8(1), 27. doi: 10.1186/1471-244X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener, J., & Stern, Y. (2012). Exploring the neural basis of cognitive reserve in aging. Biochimica et Biophysica Acta, 1822(3), 467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg, J., Håberg, A. K., Follestad, T., Olsen, A., Iverson, G. L., Terry, D. P.et al. (2020). Cognitive reserve moderates cognitive outcome after mild traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 101(1), 72–80. doi: 10.1016/j.apmr.2019.08.477. [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Arenaza-Urquijo, E. M., Bartrés-Faz, D., Belleville, S., Cantilon, M., Chetelat, G.et al. (2018). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, S1552–5260(18), 33491–33495. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Gazes, Y., Razlighi, Q., Steffener, J., & Habeck, C. (2018). A task-invariant cognitive reserve network. NeuroImage, 178, 36–45. doi: 10.1016/j.neuroimage.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes, M. G., Wolff, M. J., & Spaak, E. (2015). Decoding rich spatial information with high temporal resolution. Trends in Cognitive Sciences, 19(11), 636–638. doi: 10.1016/j.tics.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski, J. F., Wylie, G. R., Gonnella, A., Chiaravalloti, N., & Deluca, J. (2010). Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology, 75(16), 1428–1431. doi: 10.1212/WNL.0b013e3181f881a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundgren, M., Wahlin, Å., Maurex, L., & Brismar, T. (2015). Event related potential and response time give evidence for a physiological reserve in cognitive functioning in relapsing–remitting multiple sclerosis. Journal of the Neurological Sciences, 356(1–2), 107–112. doi: 10.1016/j.jns.2015.06.025. [DOI] [PubMed] [Google Scholar]
- Wan, L., Huang, H., Schwab, N., Tanner, J., Rajan, A., Lam, N. B.et al. (2019). From eyes-closed to eyes-open: Role of cholinergic projections in EC-to-EO alpha reactivity revealed by combining EEG and MRI. Human Brain Mapping, 40(2), 566–577. doi: 10.1002/hbm.24395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C.-Y., & Lin, C.-P. (2020). Classification of cognitive reserve in healthy older adults based on brain activity using support vector machine. Physiological Measurement, 41(6), 065009. doi: 10.1088/1361-6579/ab979e. [DOI] [PubMed] [Google Scholar]
- Zubarev, I., Zetter, R., Halme, H.-L., & Parkkonen, L. (2019). Adaptive neural network classifier for decoding MEG signals. NeuroImage, 197, 425–434. doi: 10.1016/j.neuroimage.2019.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]


